Translate this page into:
1,3,4-Oxadiazole, 1,3,4-thiadiazole and 1,2,4-triazole derivatives as potential antibacterial agents
⁎Corresponding author. adelaliothman@gmail.com (Adil A. Othman)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Since the introduction of the first antibiotic (penicillin, 1942) into medical practice, to date, there has been an ongoing “race” between scientists creating new drugs and pathogenic bacteria. Antibiotic-bacteria are becoming progressively common, and to make matters worse, more and more bacteria are becoming resistant to all known antibiotics. The traditional method for this problem is to introduce new antibiotics that kill the resistant mutants. This specific “arms race” resulted into thousands of potentially active chemicals are synthesized in laboratories around the world every day.
1,3,4-Oxadiazole; 1,3,4-thiadiazole; 1,2,4-triazole and some of their derivatives are involved in modifications at the following axes: First, attaching a thio-group into heterocyclic rings. Second, introducing different substitutions at position 5 which often are the residuals of the synthetic starting materials such as simple aliphatic, substituted aliphatic chains, aromatic carbocyclic and heterocyclic residues.
Keywords
Mercapto-1,3,4-oxadiazole
1,3,4-Thiadiazole
1,2,4-Triazole
Antibacterial agents
0 Introduction
Deciding whether any bacterium should be considered susceptible or resistant to any antimicrobial involves an integrated assessment of in vitro activity, pharmacologic characteristics, and clinical evaluation. Any agent approved for clinical use has demonstrated in vitro its potential to inhibit the growth of some target group of bacteria at concentrations that can be achieved with acceptable risks of toxicity. That is, the minimum inhibitory concentration (MIC) can be comfortably exceeded by doses tolerated by the patient. Use of the antimicrobial in animal models and then human infections must have also demonstrated a therapeutic response. Because the influence of antimicrobials on the natural history of different categories of infection (e.g., pneumonia, meningitis, and diarrhea) varies, extensive clinical trials must include both a range of bacterial species and infected sites (e.g., lung, bone, CSF). The clinical studies are important to determine whether what should work actually does work and, if so, to define the parameters of success and failure. MICs must be below achievable blood level. Clinical experience must validate in vitro data.
Once these factors are established, the routine selection of therapy can be based on known or expected characteristics of organisms and pharmacologic features of antimicrobial agents. With regard to organisms, use of the term susceptible (sensitive) implies that their MIC is at a concentration attainable in the blood or other appropriate body fluids (e.g., urine) using the usually recommended doses. Resistant, the converse of susceptible implies that the MIC is not exceeded by normally attainable levels. As in all biological systems, the MIC of some organisms lies in between the susceptible and resistant levels. Borderline strains are called intermediate, moderately sensitive, or moderately resistant, depending on the exact values and conventions of the reporting system. The antimicrobial in question may still be used to treat these organisms but at increased doses. For example, nontoxic antibiotics such as the penicillins and cephalosporins can be administered in massive doses and may thereby inhibit some pathogens that would normally be considered resistant in vitro. Furthermore, in urinary infections, urine levels of some antimicrobial agents may be very high, and organisms that are seemingly resistant in vitro may be eliminated (Cole et al., 2011).
For example sulfonamides and azoles, sulfonamides are structural analogs of para-aminobenzoic acid (PABA) and compete with it for the enzyme (dihydropteroate synthetase), which combines PABA and pteridine in the initial stage of folate synthesis. This blockage has multiple effects on the bacterial cells; the most important of these is disruption of nucleic acid synthesis. The effect is bacteriostatic, and the addition of PABA to a medium that contains sulfonamide neutralizes the inhibitory effect and allows growth to resume. When introduced in the 1940s, sulfonamides had a very broad spectrum (staphylococci, streptococci and many Gram-negative bacteria), but resistance developed quickly, and this has restricted their use for systemic infections. Now their primary use is for uncomplicated urinary tract infections caused by members of the Enterobacteriaceae family, particularly Escherichia coli. Sulfonamides are convenient for this purpose because they are inexpensive, well absorbed by the oral route, and excreted in high levels in the urine (Shegam, 2006; Wheelis, 2007; Dimova and Perisic-Janjic, 2009; Lindsay et al., 1994). Five membered ring sulfonamides are closely related to azasolone and similar related structures such as 1,3,4-oxadiazole, 1,3,4-thiadiazole, and 1,2,4-triazoles.
A survey of literature reveals that 1,4-disubstituted thiosemicarbazides as well as 1,3,4-oxadiazole, 1,3,4-thiadiazole, 1,2,4-triazoles and their amino derivatives are known as promising antimicrobial agents (Dimova and Perisic-Janjic, 2009; Lindsay et al., 1994; Salgin-Goksen et al., 2007; Tehranchian et al., 2005; Turan-Zitouni et al., 2005; Ahoya et al., 2011; Belkadi and Othman, 2006, 2011; Khiati et al., 2007; Benhammadi et al., 2010). Compounds of the said structure often exhibit higher activity than standard antibiotics, penicillin G (Salgin-Goksen et al., 2007), ampicillin and gentamicin (Shafiee et al., 2002). On the other hand, various reports reveal that the introduction of halogen atoms into the pharmacophore structure can be beneficial for antimicrobial activity so far as this improves the lipid-solubility of the active ingredients (Kitani et al., 1997; Tang et al., 1998).
In spite of a large number of antibiotics and chemotherapeutics available for medical use, the antimicrobial resistance created a substantial need for a new class of antimicrobial agents in the last decades (Clinical and Laboratory Standards Institute, 2006). Hydrazide hydrazones (are synthetic intermediates in the synthesis of 1,3,4-oxadiazole, 1,3,4-thiadiazole, and 1,2,4-triazoles) form a class of compounds possessing a wide range of biological activities viz. antimicrobial (Baluja et al., 2007) and antimycobacterial (Foroumadi et al., 2006).
Non-steroidal antimicrobial drugs (NSAMDs) represent a heterogeneous family of pharmacologically active compounds. A literature survey revealed that substances liked to 1,3,4-oxadiazole (Bhatia and Gupta, 2011), 1,3,4-thiadiazole (Badran et al., 2007) and 1,2,4-triazole (Plech et al., 2011) moieties have occupied a unique position in the design and synthesis of biologically active agents with remarkable antibacterial, analgesic and anti-inflammatory activities.
1 1,3,4-oxadiazole and its derivatives
1.1 Introduction
The urgent need for new antibiotics is mainly due to the increase in the frequency of bacterial infections with resistant strains, especially Gram-positive organisms, in both the hospital and community settings. Oxazolidinones are a relatively new class of antibiotics that inhibit bacterial protein synthesis by preventing binding of the aminoacyl-tRNA to the A site of the ribosome (Locke et al., 2010).
1,3,4-Oxadiazole (1) is a thermally stable neutral aromatic molecule. Out of its four possible isomers 1–4, 1,3,4-oxadiazole (1) is widely exploited for various applications (Nagaraj et al., 2011). Other aromatic related systems are 1,3,4-oxadiazolines (2), 1,3,4-oxadiazolium cations (3), and the exocyclic-conjugated mesoionic 1,3,4-oxadiazole (4) (Nagaraj et al., 2011) (Fig. 1).
Some aromatic systems of 1,3,4-oxadiazole.
X-Linked substituents to aromatic moieties normally appearing at positions C2, C5 and N4 may be represented by following structural features 5–8 (Hill, 1984) (Fig. 2).
Different positions of substitution in 1,3,4-oxadiazoles.
Also known are derivatives of the non-aromatic reduced systems, 2,3-dihydro-1,3,4-oxadiazole (9), 2,5-dihydro-1,3,4-oxadiazole (10), and 2,3,4,5-tetrahydro-1,3,4-oxadiazole (11) (Hill, 1984) (Fig. 3).
Some non-aromatic systems of hydro-1,3,4-oxadiazoles.
The electronic distribution in 1,3,4-oxadiazole has been calculated by versious SCF-MO methods (Kakitani and Kakitani, 1977). Other structural parameters, dipole moment and data to its Ultraviolet-visible (γmax calculated to be in the region 193–203 nm), NMR, NQR and microwave spectra have been derived. Studies on 1,3,4-oxadiazole indicate a maximum positive charge in the 2-position (Ha, 1979). Molecular diagrams for 1,3,4-oxadiazole, 2-phenyl- and 2,5-diphenyl-1,3,4-oxadiazole, and oligomeric oxadiazoles have been derived and conjugation between the rings is found to be similar to that in polyphenyls (Kosobutskii et al., 1972). Calculated ionization potentials for 1,3,4-oxadiazole (1) and 1,3,4-oxadiazoline-5-one (2, X⚌O) have been compared with values from PE spectra (Paine and Werstiuk, 1978).
1.2 Synthesis of 1,3,4-oxadiazole and derivatives
Various methods were reported in the literature for the synthesis of 1,3,4-oxadiazole and its derivatives (Wang et al., 2007). The most widely applicable route to the synthesis of 1,3,4-oxadiazole and its 2,5-disubstituted derivatives is the thermal, acid and base catalyzed cyclization of their corresponding carbonylhydrazides (Hill, 1984). The hydrazide prepared from carboxylic acid via an ester can be regarded as the real starting material for the synthesis of 1,3,4-oxadiazole by treating the latter with isocyanide dichlorides (Ollis and Ramsden, 1971) (see Scheme 1).
General synthetic method for 1,3,4-oxadiazole.
Several carbonylhydrazide derivatives have been synthesized in our laboratory starting from the appropriate carboxylic acids. The common method is described by converting the carboxylic acid to corresponding ester which on treatment with hydrazine gives the corresponding hydrazides. The hydrazide derivatives (1,2-diacylhydrazines and related compounds) are thermally on acid catalysis give 1,3,4-oxadiazole (Hill, 1984) as shown in Scheme 2.
General synthetic method for 1,3,4-oxadiazole.
In another way when hydrazides were treated with CS2 in basic medium it resulted in 2-R-5-mercaptyl derivatives as illustrated in Scheme 3 (Ollis and Ramsden, 1971; Gaetano et al., 1991).
General synthetic method for 1,3,4-oxadiazole-5-thiones.
1.3 Tautomerism in 1,3,4-oxadiazole
2-Hydroxy (12a), 2-mercapto (12b) and 2-amino-oxadiazoles (12c) are in equilibrium with the tautomeric oxadiazolines (13a), (13b) and (13c) respectively (Aydogan et al., 2002; Giudicelli et al., 1969) (Scheme 4).
Tautomerism of thiol thione forms.
Evidence from U.V, IR, H1-NMR and C13-NMR spectra supports structure 13b for 1,3,4-oxadiazoline-5-thione. The U.V and IR spectra, fluorescence and pK values of 2-amino-1,3,4-oxadiazoles indicate that the amine tautomer (12c or 12d) rather than the imine tautomer (13c or 13d) predominates (Khiati et al., 2007).
1.4 Antibacterial activity
A variety of Mannich bases derived from 1,3,4-oxadiazole-5-thiones show fungicidal activity and in some cases also act as bactericides and insecticides. Various derivatives for 1,3,4-oxadiazole-5-thiones with R substituents distributed over positions C2,N4 and C5 were synthesized and assigned for antibacterial activity (Ollis and Ramsden, 1971; Kumar et al., 2011).
1.4.1 From gluconic acid
The C-nucleoside possessing 1,3,4-oxadiazolo-2-thiol (14) ring derived from gluconic acid was found to have appreciable effects in antibacterial activity against: Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa and E. coli using ampicillin as standard.
The oxadiazole residues were found to exhibit higher inhibition effects against these bacteria (Belkadi and Othman, 2011).
1.4.2 From salicylic acid
5-(2-Hydroxyphenyl)-1,3,4-oxadiazole-2-thione (15) has been synthesized from salicylic acid. Antibacterial activity was investigated in vitro against E. coli and S. aureus using ampicillin and gentamycin as references.
The screening results indicate that the compound showed a moderate to slight activity against other tested bacteria (Khiati et al., 2007).
1.4.3 From pyridine carboxylic acids
5-(2-Pyridyl)-1,3,4-oxadiazole-2-thione (16) has been synthesized from the corresponding 2-pyridine carboxylic acid (picolinic acid) and tested in vitro against the following microorganisms: E. coli, P. aeruginosa, Enterococcus faecalis, S. aureus, P. aeruginosa and compared with the known antibiotics cephalosporin and gentamycin.
Oxadiazole derivative 16 has relatively lower inhibition effect on S. aureus and E. coli but exhibited more effect on P. aeruginosa (Belkadi and Othman, 2006).
1.4.4 From pyridin-2-amine
1-(5-Mercapto-1,3,4-oxadiazol-2-yl)-2-(pyridine-2-ylamino)ethanone (17) has been synthesized from 2-(pyridine-2-ylamino) acetohydrazide. This compound was found to have significant antimicrobial activity. The findings of the study indicate that cyclization of the hydrazide acid group of 2-(pyridine-2-ylamino) acetohydrazide into 1,3,4-oxadiazole nucleus resulted in increased antimicrobial activity.
The antibacterial activities of the compound 17 were tested against some pathogenic microorganisms: S. aureus, Streptococcus viridians and E. coli and found to have good antimicrobial activity with higher value of MIC. From structure-activity relationships, introduction of the 1,3,4-oxadiazole ring into compound significantly increases their biological activity. The oxadiazole ring system could be incorporated into many more ring systems which have their own activity and could lead to more potent and highly active compounds (Salimon et al., 2011).
1.4.5 From acylhydrazine-S-derivatives
Some novel derivatives of acylhydrazine such as; 5-substituted-2-mercapto-1,3,4-oxadiazoles 18(a–g), their corresponding S-esters 19(a–g) and amides 20(a–g), have been synthesized.
Those compounds have been tested in vitro for their antibacterial activity against E. coli bacteria by the agar well diffusion method using roxithromycin and cefixime as standard drugs. The results showed that all compounds were active against E. coli except 20f (Zareef et al., 2008).
1.4.6 From β-aroyl propionic acids
A series of 5-{3-oxo-6-(substituted aryl)-2,3,4,5-tetrahydropyridazin-2-ylmethyl}-2-substituted 1,3,4-oxadiazole (21) have been synthesized from β-aroyl propionic acids.
All the compounds are evaluated for their antibacterial activity against E. coli, S. aureus, Micrococcus luteus and Klebsiella pneumoniae by using the cup plate technique in the nutrient agar. Antitubercular activity was determined using the BACTEC 460 system. All the synthesized compounds were screened against Mycobacterium tuberculosis H37 Rv comparable with that of standard rifampicin and isoniazid. From the results, it was observed that most of the compounds were active against the microorganism having significant activity against these bacteria comparable to standard drugs, ampicillin and chloramphenicol.
The above synthesized compounds showed the percentage inhibition ranging from 48 to 91%. Compound 21a was a highly active analog in this series with 91% inhibition against M. tuberculosis H37 Rv comparable with that of standard rifampicin and isoniazid (Islam et al., 2008).
1.4.7 From phenylpropionohydrazides
The newly synthesized compounds 22(a–e) from phenyl propionohydrazides were screened for antibacterial activity against freshly cultured strains of S. aureus and P. aeruginosa using ampicillin as standard.
Among newly synthesized derivatives, compounds 22(a–b) were found to be equipotent to ampicillin when tested against the strains of S. aureus, and P. aeruginosa, whereas some of the newly synthesized compounds like 22a, 22d and 22e were found to possess good antibacterial activity when tested against S. aureus and P. aeruginosa.
After comparing the antimicrobial results of compounds 22(a–e), it was concluded that the incorporation of an oxadiazole moiety in phenylpropionyl derivatives enhances their antimicrobial activity and also para-substitution in the R2 group of the oxadiazoles was found to enhance their potency, especially in compounds 22(a–b) (Fuloria et al., 2009).
1.4.8 Alkyl, alkenyl, sulfonyl, thiocarbamates and Mannich derivatives
Alkyl, alkenyl, sulfonyl, thiocarbamates and Mannich derivatives 23–26 were synthesized.
All tested compounds were assayed for their antimicrobial activity against three standard bacterial strains, S. aureus, E. coli and P. aeruginosa.
The lowest concentration which inhibited growth was considered as the MIC.
The MIC for the synthesized compounds indicates that the conversion of the sulfhydryl group in 1,3,4-oxadiazole into alkyl 23a, allene 23b, sulfonyl 24 derivatives and mannich product 26 showed a weak antimicrobial activity. However, thiocarbamates 25a and 25b were effective against Gram positive and Gram negative bacteria (Muhi-eldeen et al., 2008).
1.4.9 From glucaric acid
This bis(1,3,4-oxadiazole-2-thiol) (27) derivative of glucaric acid was essayed for antibacterial activity against S. aureus, B. subtilis, P. aeruginosa and E. coli using ampicillin as reference drug.
The oxadiazole exhibited higher inhibition effects against these bacteria as compared with drug reference (Belkadi and Othman, 2011).
1.4.10 From terephthalic acid
5,5′-benzene-1,4-diylbis(1,3,4-oxadiazole-2-thione) (28) was synthesized from terephthalic acid and tested in vitro against E. faecalis and E. coli and compared with known antibiotics cephalosporin and gentamycin.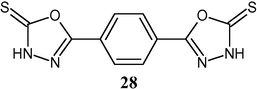
The compound showed an intermediate effect on E. faecalis and E. coli (Datoussaid et al., 2012).
1.4.11 From pyridine carboxylic acids
The bis-5-(2,6-pyridyl)-1,3,4-oxadiazole-2-thione (29) has been synthesized from 2,5-pyridine dicarboxylic acid, and tested in vitro against the following microorganisms: E. coli, P. aeruginosa, E. faecalis, S. aureus and P. aeruginosa and compared with the known antibiotics cephalosporin and gentamycin.
The results have shown that the synthesized compound 29 has very high effect in comparison to pyridine mono oxadiazole ring 16 on the gram-negative bacteria P. aeruginosa in particular, where its effect exceeded that of the well-known cephalosporin (Benhammadi et al., 2010).
1.4.12 Conclusions
1,3,4-oxadiazoles and their thione derivatives proved to be effective against different microorganisms as summarized in Table 1.
Compounds
Gram positive bacteria
Gram negative bacteria
14
+
+
15
+/−
+/−
16
+/−
+
17
+
+
18
/
+
19
/
+
20
/
+
21
+
+
22
+
+
23
+/−
+/−
24
+/−
+/−
25
+
+
26
+/−
+/−
27
+
+
28
+/−
+/−
29
−
+
2 1,3,4-Thiadiazole and its derivatives
2.1 Introduction
1,3,4-Thiadiazoles (30) and (31) were first described in 1882 by Fischer and further developed by Busch. Thiadiazoles carrying mercapto, hydroxyl and amino substituents can exist in many tautomeric forms and this property is being intensively studied (Kornis, 1984).
1,3,4-Thiadiazoles are conveniently divided into three subclasses:
-
Aromatic systems which include the neutral thiadiazoles 30.
-
Mesoionic systems 32 which are defined as five-membered heterocycles which are non-covalent or polar and possess a sextet of electrons in association with the five atoms comprising the ring.

-
Non-aromatic systems such as 1,3,4-thiadiazolines (33) and (34) and the tetrahydro-1,3,4-thiadiazolidines (35).

2.2 Synthesis of thiadiazoles and their derivatives
The syntheses of thiadiazoles are discussed in terms of the number of bonds being formed and by ring transformation. Thiadiazole synthesis by one-bond formation is exemplified by cyclization of an acylated thiosemicarbazide as shown in Scheme 5 (Treppendahl and Jackobsen, 1977):
Synthesis of thiadiazoles.
The synthesis of other simple mercapto-thiadiazoles is outlined in Scheme 6: (El-Sayed and Wasfy, 2005):
Synthesis of mercapto-thiadiazoles.
The most common two bond formation takes place via 1,3-dipolar cycloaddition presented in Scheme 7 (Dickore and Wegler, 1966):
Synthesis of mercapto-thiadiazoles by 1,3-dipolar cycloaddition reaction.
1,3,4-Thiadiazoles can easily be obtained from 1,3,4-oxadiazoles thus in refluxing 43 in ethanolic HCl rearranges to 44 (Giammanco, 1965).
2.3 Tautomerism
The 1,3,4-thiadiazole ring system, with three heteroatoms, does not exhibit tautomerism in its fully conjugated form 30. However, when certain substituents are present, tautomerism is possible. 1,3,4-Thiadiazolin-2-ones (45a, X⚌O) and -2-thione (45b, X⚌S) exist in the oxo and thione forms, respectively, as shown by spectroscopic and LCAO-MO calculations.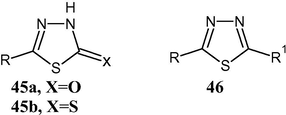
2-Amino-1,3,4-thiadiazoles exist in the amino form 46 (R1⚌NH2) in solution and in the solid state (Elguero et al., 1976).
2.4 Antibacterial activity
1,3,4-Thiadiazoles have activity on many biological systems. Cefazolin (47) is a thiadiazole analog of cephalosporanic acid useful as antibacterial (Chabbert and Lutz, 1978).
Another compound (48) is a patent as bactericidal (Omprakash et al., 2011).
2.4.1 From semicarbazide hydrochloride
A bioactive ligand, 2,5-diamino-1,3,4-thiadiazole (49), derived from semicarbazide hydrochloride, and its metal complexes were prepared and characterized (Obaleye et al., 2011).
In vivo evaluation of the antimicrobial activities of the metal complexes and the ligands showed greater activity against some micro-organisms when compared to the parent compounds (Obaleye et al., 2011).
2.4.2 Thiadiazole phenyl oxazolidinone analogs
Replacement of the morpholine C-ring of Linezolid with a 1,3,4-thiadiazolyl ring leads to oxazolidinone analogs 50(a–d) having potent antibacterial activity against both gram-positive and gram-negative organisms.
All of the analogs 50(a–d) were tested in vitro against a panel of gram-positive and fastidious gram-negative bacteria. Selected compounds were also evaluated for in vivo effect against S. aureus in a mouse bacteremia model. All of these analogs exhibited good to excellent antibacterial activity, including good activity against fastidious gram-negative organisms. In many cases, the compounds had superior activity to linezolid. The in vitro activity of the 1,3,4-thiadiazolyl phenyl oxazolidinones is relatively insensitive to the nature of the substituent at the 2-position. As expected, the thioacetamide analogs 50(a–d) are extremely potent against both gram-positive and fastidious gram-negative organisms. Various 1,3,4-thiadiazole analogs had in vivo activity comparable to linezolid, but the thioamides lacked oral activity presumably due to metabolism of the thioamide.
Oxazolidinone analogs that contain a 1,3,4-thiadiazole C-ring represent a new class of oxazolidinone antibacterial agents having excellent activity against both gram-positive and fastidious gram-negative organisms.
1,3,4-Thiadiazolyl phenyl oxazolidinones are useful extensions of the quantitative structure-activity relationship of the oxazolidinone class of antibacterial agents (Thomasco et al., 2003).
2.4.3 Sulfonyl-1,3,4-thiadiazoles derivatives
Bahram et al. reported the synthesis and antibacterial activity of a new series of 2-(1-methyl-4-nitro-1H-imidazol-5-ylsulfonyl)-1,3,4-thiadiazoles 51(a–c) (Bahram et al., 2011). The three compounds were tested in vitro by the conventional agar dilution method against a panel of microorganisms including gram-negative and gram-positive bacteria.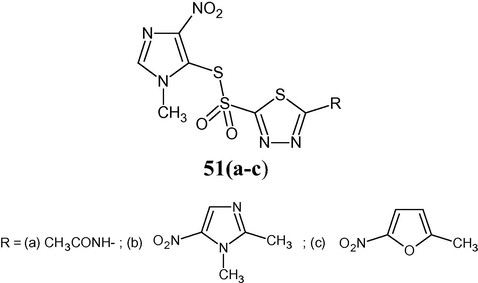
Compound 51c with 5-(5-nitrofuran-2-yl)-residue on 1,3,4-thiadiazole scaffold had shown promising antibacterial activities against gram-positive bacteria including S. aureus, Staphylococcus epidermidis and B. subtilis (Bahram et al., 2011).
2.4.4 Newly synthesized cephalosporins
New arylideneamino-(1,3,4-thiadiazol-5-yl)-dithioacetamido-cephalosporanic acids 52(a–d) have been synthesized and tested in vitro antimicrobial activities of the prepared cephalosporins were investigated using a panel of selected microorganisms.
The antimicrobial activities of the newly synthesized cephalosporins as in compounds 52(a–d) were determined by the agar diffusion method using representative Gram (+) and Gram (−) bacteria on tryptic soya agar media.
The test microorganisms used to evaluate the potential antimicrobial activity of the newly synthesized cephalosporins were: S. aureus, E. coli, P. aeruginosa and M. luteus. Cephalexin was used as a reference.
Cephalosporins containing 1,3,4-thiadiazole moiety linked through a disulfide bond in the acyl side chain compounds 52(a–d) were the most potent and were found to be equipotent to cephalexin, especially compounds 52c and 52d. This finding was expected and was supported when compared with antibiotics containing disulfide bonds which showed marked activities (Alwan, 2012).
2.4.5 From 6-methyl-1,3-benzothiazol-2-amine
A series of 2-aryl-5-(6′-chloro-1′,3′-benzothoazole-2-yl-amino)-1,3,4-thiadiazoles 53(a–j) have been synthesized from 6-methyl-1,3-benzothiazol-2-amine and screened for both antibacterial activities using ofloxacin as a standard drug. The compounds were screened against S. aureus, E. coli and P. aeruginosa in nutrient agar medium.
The thiadiazole derivative 53i having an acetoxy-phenyl group showed potent activity against S. aureus, whereas compound 53g having the 2-napthyl-methyl group showed maximum inhibition against E. coli, when compared with standard drug ofloxacin. Compound 53c having the 2,4-dichloro-phenyl group also showed significant antibacterial activity against S. aureus, E. coli and P. aeruginosa. Rest of the compounds showed moderate to good antibacterial activity (Amir et al., 2009).
2.4.6 Derivatives of 5-amino-2-hydroxybenzoic acid
Variously substituted 4-amino-2-{5-[(4-substituted phenyl)amino]-1,3,4-thiadiazole-2-yl} phenol 54(a–g) were synthesized and evaluated for their antibacterial activity.
These compounds showed significant antibacterial activity against S. aureus and E. coli bacteria using the cup plate technique (Hussain et al., 2008).
2.4.7 Conclusions
1,3,4-Thiadiazole and its derivatives proved to be effective against different microorganisms as summarized in Table 2.
3 1,2,4-Triazole and its derivatives
3.1 Introduction
Triazoles are two basic aromatic heterocycle isomers, 1,2,3-triazole (55) and its isomer 1,2,4-triazole (56).
1,2,4-Triazole derivatives find uses in a wide variety of applications mostly antifungals such as fluconazole and intraconazole. 4-Amino-5-mercapto-3-substituted-1,2,4-triazole showed antifungal, anti-inflammatory and antitubercular properties that have made them important chemotherapeutic agents (Kurtzer et al., 1965). Some 3,6-disubstituted-1,2,4-triazol[3,4-b]-1,2,4-thiadiazole derivatives (57) showed anti HIV-1 activity at concentrations slightly below cytotoxic levels.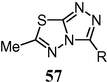
Partially or fully reduced triazoles (thiazolines and thiazolidine respectively) with monovalent substituents are respectively unstable and of little interest. Triazolines and thiazolidines with exocyclic bonds such as ⚌O, ⚌S, ⚌NR′R″, ⚌CR′R″, etc. are also aromatic (Polya, 1984).
Substituents to aromatic and non-aromatic 1,2,4-triazoles can be situated in all positions of the molecule due to multiple valency of carbon and nitrogen atoms.
Bridgehead nitrogen-heterocyclic compounds obtained by fusion of the 4,5-dihydroimidazole and [1,2,4]triazole nuclei, have identified one compound containing the methylthio group at position 3 and with a 4-methylphenyl substituent at position 7 (e.g., 7-(4-methylphenyl)-3-methylthio-5H-6,7-dihydroimidazo[2,1-c][1,2,4]triazole) with a significant antibacterial activity. This heterocycle was strongly active against S. aureus and showed superior antibacterial activity to ampicillin (Sztanke et al., 2006).
1,2,4-Triazole and its amino derivatives, tetrazole, thiadiazole, pyrazole, imidazole and the corresponding derivatives have been studied as corrosion inhibitors of copper based material such as bronzes (Khiati et al., 2011).
1,2,4-Triazole (56) may exist in equilibrium between three forms: 1H-form(i), 1H-form(ii) and 4H-form as following,
The calculated energy differences between azole tautomers support preference for the 1Hover 4H tautomer. Similarly, the usual tautomeric preference for triazolines over hydroxytriazoles and aminotriazoles over triazolinimines is supported on thermochemical evidence (Dewar and Morita, 1969).
The molecular structure of 1,2,4-triazole (56) was determined by gas phase electron diffraction. The internuclear distances and bond angles were obtained by applying a least-squares analysis to the experimental intensity. The bond distances (rg) and bond angles suggested that the 1,2,4-triazole exists in an 1H-form. The bond distances N1–N2 = 1.377 ± 0.010 Å, N2–C3 = 1.329 ± 0.009 Å, C3–N4 = 1.348 ± 0.009 Å, N1–C5 = 1.377 ± 0.004 Å, N4 = C5 = 1.305 Å (calculated value), N1–H = 0.990 Å, C3–H and C5–H = 1.054 Å. The bond angles ∠N1N2C3 = 102.7 ± 0.5°, ∠N2C3N4 = 113.8 ± 01.3°, ∠N2N1C5 = 108.9 ± 0.8°, ∠H1N1N2 = 110.9°, ∠H2C3N4 = 119.2°, ∠H3C5N1 = 121.0°, ∠C3N4C5 = 105.7° (calculated value), and ∠N4C5N1 = 108.7° (calculated value).
Whereas the substituted C3-S-preferred the 4H-form based on the crystal structure of 4H-1,2,4-triazole-3-mercapto acetic acid (58) which showed that atoms C3,C5,N1,N2,N4 are coplanar and form a conjugated plane with a mean deviation of 0.002 Å.
The bond lengths of C3-N2, C3-N4, C5-N4, C5-N1 and N1-N2 are 1.317(2) Å, 1.364(2) Å, 1.328(2) Å, 1.317(2) Å, and 1.362 Å respectively, being in accordance with 4H-form (Chiang and Lu, 1977).
3.2 Synthesis of 1,2,4-triazoles and their derivatives
During the last few decades, considerable attention has been paid to synthesize 1,2,4-triazole derivatives possessing comprehensive bioactivities as antibacterial and antimycobacterial (Klimesová et al., 2004).
Several methods for the synthesis of 1,2,4-triazole and its derivatives were reported (Zaharia et al., 2001). One of these methods follows cyclization of aminoacylhydrazines (60) and acyl halides as shown in Scheme 8. Sometimes this method may be used into formation of some 1,3,4-oxadiazole derivatives (61,62) (Gehlen and Blankenstein, 1960).
Tautomeric structures of substituted 1,2,4-triazole.
The most common procedure to synthesize the 1,2,4-triazole-5-thiol derivatives (64) is also involving a base catalyzed cyclization of thiosemicarbazides (63) or its thio derivative as shown in Scheme 9 (Wheelis, 2007; Zhang et al., 2002) (see Scheme 10).
Synthesis of 1,2,4-triazole.
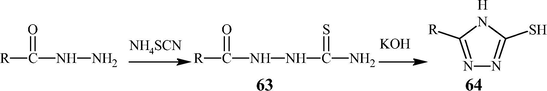
Synthesis of 1,2,4-triazole-5-thiole.
3.3 Antibacterial activity
It has been shown that the antiviral and antibacterial activities of thiourea derivatives are due to the presence of the –NH–C(S)–NH– function in the molecule and that changes in this activity depend on the nature of the substituents (Cansiz et al., 2001). Thus, the substitute groups present in various compounds have different effects against different bacteria.
Regarding antimicrobial activity, triazole is structurally similar to imidazole molecule. Although triazole and imidazole act by the same mechanism of action, triazoles possess advantages over imidazoles, which have slow metabolic rate, oral bioavailability, and less effect on human sterol synthesis. For these reasons imidazoles are slowly being replaced by triazole molecules (Palekar et al., 2009).
3.3.1 From gluconic acid
New acyclo C-nucleosides bearing 1,2,4-triazole-3-thiol (65)moieties derived from gluconic acid were synthesized and tested against S. aureus, E. faecalis, P. aeruginosa and E. coli using ampicillin as standard.
The triazole exhibited weaker inhibition effects than ampicillin against these bacteria (Belkadi and Othman, 2011).
3.3.2 From salicylic acid
3-(2-hydroxyphenyl)-1H-1,2,4-triazol-5-thiol (66) has been synthesized starting from salicylic acid and tested in vitro against E. coli, P. aeruginosa and S. aureus using ampicillin and gentamycin as references.
The screening results indicate that the compound 66 showed a moderately active effect against all bacteria tested (Khiati et al., 2007).
3.3.3 From substituted aniline
Hussain et al. synthesized a series of 1-[(1,2,4-triazole-4-yl) carbothioamide]-3,5-dimethyl-4-[(substituted phenyl) diazenyl] pyrazoles 67(a–d). These compounds were investigated for their antibacterial activities.
Antibacterial activities of the synthesized compounds were determined in vitro against S. aureus and E. coli. Standard antibiotic ofloxacin was used as reference drug.
These compounds showed moderate antimicrobial activity against tested bacterial strains (Hussain et al., 2010).
3.3.4 From glucaric acid
This bis(1,2,4-triazole-3-thiol) (68) derivative of glucaric acid was essayed for antibacterial activity against P. aeruginosa and E. coli using ampicillin as reference drug.
The triazole exhibited an important antibacterial activity against these bacteria (Belkadi and Othman, 2011).
3.3.5 From terephthalic acid
5,5′-Benzene-1,4-diylbis(1H-1,2,4-triazole-3-thiol) (69a) and its derivatives 69(b-c) were synthesized from terephthalic acid and tested in vitro against P. aeruginosa and E. coli and compared with known antibiotics cephalosporin and gentamycin.
Triazole 69a exhibited an intermediate effect on P. aeruginosa while methyl triazole 69b showed a similar effect on the same bacteria. The highest effect was observed by dimethyl triazole 69c upon E. coli at the lowest concentration (Datoussaid et al., 2012).
3.3.6 Conclusions
1,2,4-Triazole and its derivatives proved to be effective against different microorganisms as summarized in Table 3.
4 C–R and N–R derivatives of 1,2,4-triazol-5-thiol
4.1 Introduction
Three categories of derivatives may be observed for 1,2,4-triazoles. The first category is the C–R derivatives, they existed in two types, either C3–R or C5–R and C3–R and C5–R derivatives. The second category involves Cx–R and Ny–R derivatives. The third category involves N1–R derivatives, N2–R derivatives and N1 and N2 derivatives. The C3–R or C5–R and C3–R and C5-R derivatives may be chosen from the beginning of the synthesis by selecting the appropriate starting material such as the wanted carboxylic acid.
4.2 Synthesis
The Nx–R derivatives were either made up by substitution on N– atom or by selecting the appropriate hydrazine derivatives. The 71, N4–R and 72, N4–NH2 can be derived from reaction of 1,3,4-oxadiazoles (70) with R3NH2 or NH2NH2 respectively (Palekar et al., 2009) (see Scheme 11).
Synthesis of 71, N4–R and 72, N4–NH2 from oxadiazoles 70.
Another method for preparation of N–NH2 derivative is by treating the triazoles with methyl iodide.
4.3 Antibacterial activity
4.3.1 From sodium salt of α-sulphonated fatty acid hydrazide
Sodium 1-[4-amino-5-mercapto-4H-(1,2,4)triazol-3-yl]heptadecane-1-sulfonate (73) has been synthesized.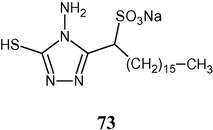
The antibacterial activity of the compound was determined in vitro against various pathogenic bacteria such as gram positive bacteria (B. subtilis, S. aureus) and gram negative bacteria (E. coli). The results indicated that the compound was highly active against selected pathogens (El-Sayed, 2006).
4.3.2 From gluconic acid
An acyclo C-nucleoside bearing 4-amino-1,2,4-triazole-3-thiol moieties (74) derived from gluconic acid was synthesized and tested against S. aureus, and E. coli using ampicillin as standard.
The activity of the compound is equal to that of ampicillin in terms of antibacterial activity against S. aureus. The compound 74 has a moderately active effect on E. coli bacteria (Belkadi and Othman, 2011).
4.3.3 From salicylic acid
3-(2-Hydroxyphenyl)-4-amino-1,2,4-triazol-5-thiol (75) has been synthesized starting from salicylic acid and tested in vitro against E. coli and S. aureus using ampicillin and gentamycin as references.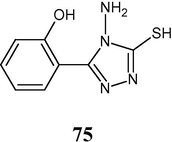
The screening results indicate that the compound showed a moderate to slight activity against bacteria tested (Khiati et al., 2007).
4.3.4 From glucaric acid
The bis (4-amino-1,2,4-triazole-3-thiol) (76) derivative of glucaric acid was essayed for antibacterial activity against P. aeruginosa and E. coli using ampicillin as reference drug.
The bis-amino-triazole 76 exhibited an important antibacterial activity against these bacteria (Belkadi and Othman, 2011).
4.3.5 From isoniazid
Synthesized 5-substituted-3-pyridine-1,2,4-triazole 77(a–g) has been tested for antibacterial activity.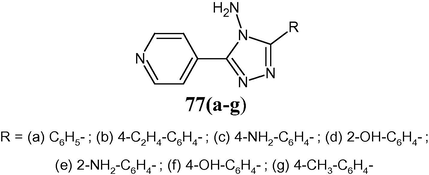
A cup plate method was employed for the in vitro study of antibacterial effect against B. subtilis, S. aureus, Proteus mirabilis and Salmonella typhi.
The screening result indicates that all compounds exhibited moderate to good antibacterial activities. It was reported that compounds with free NH2 in the 4th position C 77(a–g) showed inhibitory effect against one or more types of bacteria and also due to the presence of the triazole ring system in the synthesized compounds, that exhibited antimicrobial activity. Compounds 77(b–d) showed moderate antibacterial activity. Among the synthesized compounds, compound 77f showed good antibacterial activity (Muthal et al., 2010).
4.3.6 Triazole containing Thiophene moieties
Some 3-(thenylmethyl)-4-substituted-4,5-dihydro-1H-1,2,4-triazol-5-one 78(a–e) derivatives were synthesized by the cyclization reaction of 1-(thiophen-2-ylacetyl)-4-substituted semicarbazide derivatives and were evaluated in vitro against several species of aerobic bacteria.
Some of them showed activity against K. pneumoniae, S. aureus, Streptococcus pyogenes and P. aeruginosa. Among tested compounds, the most effective was 78c. The highest susceptibility to tested derivative was detected in S. pyogenes, P. aeruginosa and S. aureus (Pitucha et al., 2010).
4.3.7 Triazole, amino triazole and thiadiazole derivatives of 5-Amino-2-hydroxybenzoic acid
4-amino-2-[4-(4-methylphenyl)-5-sulfanyl-4H-1,2,4-triazol-3-yl]phenol (79), 4-amino-2-{4-amino-5-[(4-chlorophenyl)amino]-4H-1,2,4-triazol-3-yl}phenol (80) and 4-amino-2-{5-[(4-substituted phenyl)amino]-1,3,4-thiadiazole-2-yl} phenol (81a–c) were synthesized and evaluated for their antibacterial activity against bacterial strain S. aureus and E. coli. Ofloxacin was used as standard drugs.
Compounds 79 and 80 showed significant antibacterial activity against S. aureus (gram-positive) and E. coli (gram-negative) bacteria using the cup plate technique.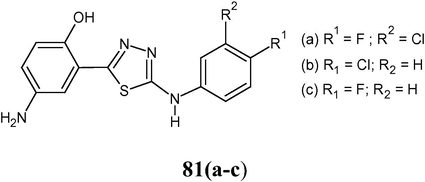
The 5-amino-2-hydroxybenzohydrazide derivative (81a) having the 3-chloro-4-fluorophenyl amino group at the 2nd position of the thiadiazole ring was found to have MIC 25 μg/mL against S. aureus and E. coli. 1,3,4-thiadiazole derivatives 81b and 81c also exhibited promising antibacterial activity (MIC 25 μg/mL) against S. aureus (Hussain et al., 2008).
4.3.8 Conclusions
C–R and N–R derivatives of 1,2,4-triazol-5-thiol proved to be effective against different microorganisms as summarized in Table 4.
References
- Synthesis and antibacterial activity of new spiro[thiadiazolinequinoxaline] derivatives. ARKIVOC. 2011;ii:217.
- [Google Scholar]
- Synthesis and preliminary antimicrobial activities of new arylideneamino-1,3,4-thiadiazole-(thio/dithio)-acetamido cephalosporanic acids. Molecules. 2012;17:1025.
- [Google Scholar]
- Synthesis of pharmaceutically important 1,3,4-thiadiazole and imidazolinone derivatives as antimicrobials. Indian J. Chem.. 2009;48B:1288.
- [Google Scholar]
- Synthesis and electronic structure of new aryl- and alkyl-substituted 1,3,4-oxadiazole-2-thione derivatives. Turk. J. Chem.. 2002;26:159.
- [Google Scholar]
- Synthesis and antimicrobial activity of novel quinoxaline derivatives. J. Chin. Chem. Soc.. 2007;54:469.
- [Google Scholar]
- Synthesis and in vitro antibacterial activity of new 2-(1-methyl-4-nitro-1H-imidazol-5-ylsulfonyl)-1,3,4-thiadiazoles. Eur. J. Chem.. 2011;8:1120.
- [Google Scholar]
- A facile synthesis and the antimicrobial activity of some 4-aryltriazoles. J. Serb. Chem. Soc.. 2007;72(6):539.
- [Google Scholar]
- A common route to the synthesis of 1,3,4-oxadiazole -2-thione and 1,2,4-triazole -3-thiols derivatives of trioses and pentoses as models for acyclic C-nucleosides. ARKIVOC. 2006;xi:183.
- [Google Scholar]
- Regioselective glycosylation: Synthesis, characterization and biological evaluation of new acyclo C-nucleosides bearing 5-(substituted)-1,3,4-oxadiazole-2-thione, 5-(substituted)-4-amino-1,2,4-triazole-3-thiol and 5-(substituted)-1,2,4-triazole-3-thiones moieties. Trends Appl. Sci. Res.. 2011;6(1):19.
- [Google Scholar]
- Synthesis and antimicrobial evaluation of 1,2,3-oxadiazole-2-thione from some pyridine carboxylic acids. Asian J. Chem.. 2010;22(7):5535.
- [Google Scholar]
- 1,3,4-Oxadiazole as antimicrobial agents: an overview. J. Chem. Pharm. Res.. 2011;3(3):137.
- [Google Scholar]
- 5-Furan-2yl[1,3,4]oxadiazole-2-thiol, 5-Furan-2yl-4H [1,2,4] triazole-3-thiol and their thiol-thione tautomerism. J. Chem. Soc. Pak.. 2001;23:237.
- [Google Scholar]
- HR 756, the syn isomer of a new methoxyimino cephalosporin with unusual antibacterial activity. Antimicrob. Agents Chemother.. 1978;14(5):749.
- [Google Scholar]
- Clinical and Laboratory Standards Institute, 2006. Performance Standards for Antimicrobial Susceptibility Testing, Sixteenth International Supplement, 26, 11
- Investigation of antibiotic and antibacterial susceptibility and resistance in staphylococcus from the skin of users and nonusers of antibacterial wash products in home environments. Inter. J. Microbiol. Res.. 2011;3(2):90.
- [Google Scholar]
- Synthesis and antibacterial activity of some 5,5′-(1,4-phenylene)-bis-1,3,4-oxadiazole and bis-1,2,4-triazole derivatives as precursors of new S-nucleosides. S. Afr. J. Chem.. 2012;65:30.
- [Google Scholar]
- Ground states of conjugated molecules 12. Improved calculations for compounds containing nitrogen or oxygen. J. Am. Chem. Soc.. 1969;91(4):796.
- [Google Scholar]
- Formation of 1,3,4-thiadiazoles and 1,4,2-oxathiazoles from thioketenes. Angew. Chem. Int. Ed. Engl.. 1966;5:970.
- [Google Scholar]
- QSAR study by 1,2,4-triazoles using several physicochemical descriptors. Maced. J. Chem. Chem. Eng.. 2009;28:79.
- [Google Scholar]
- Synthesis, antibacterial and surface activity of 1,2,4-triazole derivatives. Indian J. Chem.. 2006;45B:738.
- [Google Scholar]
- Synthesis of heterocycles having double characters: as antimicrobial and surface active agents. J. Chin. Chem. Soc.. 2005;52:129.
- [Google Scholar]
- Synthesis and antimycobacterial activity of some alkyl [5-(nitroaryl)-1,3,4-thiadiazol-2-ylthio]propionates. Bioorg. Med. Chem. Lett.. 2006;16(5):1164.
- [Google Scholar]
- Synthesis and antimicrobial evaluation of some new oxadiazoles derived from phenylpropionohydrazides. Molecules. 2009;14:1898.
- [Google Scholar]
- Reactions of azoesters and dimethyl acetylenedicarboxylate with 3-methyl-1,2,4-triazole-5-thione. J. Heterocycl. Chem.. 1991;28:325.
- [Google Scholar]
- Zur Kenntnis der Nβ-Cyan-carbonsäurehydrazide, VI Über die Identität von Nβ-Cyan-carbonsäurehydraziden mit substituierten 2-Amino-oxdiazolen. Leibigs Ann. Chem.. 1960;638:136.
- [Google Scholar]
- Giammanco, L., 1965. 2,5,6-Triphenyl-4-hydrazinopyrimidine and its derivatives. Atti. Acad. Sci. Lett. Arti. Palermo. Part 1, 66, 20, 313.
- Sur la tautomérie phenyl-5 amino-2 sélénazolinones-4-phenyl-5 inimo-2 sélénazolidinones-4. Bull. Soc. Chim. Fr.. 1969;870
- [Google Scholar]
- A theoretical study of the electronic structure and properties of some five-membered heterocyclic compounds: pyrazole, imidazole, furan, isoxazole, 1,2,5-oxadiazole and 1,3,4-oxadiazole. J. Mol. Struct.. 1979;51:87.
- [Google Scholar]
- Katritzky A.R., Rees C.W., eds. Comprehensive Heterocyclic Chemistry. Vol 6. Oxford: Pergamon Press; 1984. p. :428.
- Synthesis and biological evaluation of some new 1-substituted-3,5-dimethyl-4-[(substituted phenyl) diazenyl] pyrazole derivatives. Am. Eurasian J. Sci. Res.. 2010;5(4):257.
- [Google Scholar]
- Synthesis and antimicrobial activities of 1,2,4-triazole and 1,3,4-thiadiazole derivatives of 5-amino-2-hydroxybenzoic acid. Eur. J. Chem.. 2008;5(4):963.
- [Google Scholar]
- Synthesis and antimicrobial activity of some novel oxadiazole derivatives. ActaPoloniae Pharm. Drug Res.. 2008;65(4):441.
- [Google Scholar]
- Application of self-consistent HMO theory to heteroconjugated molecules. Theor. Chim. Acta. 1977;46:259.
- [Google Scholar]
- Synthesis and antibacterial activity of 1,3,4-oxadiazole and 1,2,4-triazole derivatives of salicylic acid and its synthetic intermediates. S. Afr. J. Chem.. 2007;60:20.
- [Google Scholar]
- Corrosion inhibition of copper in neutral chloride media by a novel derivative of 1,2,4-triazole. Corros. Sci.. 2011;53(10):3092.
- [Google Scholar]
- Synthesis and structural optimization of 7-(3,3-disubstituted-1-pyrrolidinyl)-1-cyclopropyl-6-fluoro-1,4-dihydro-8-methoxy-4-oxo-3-quinolinecarboxylic acids as antibacterial agents. Bioorg. Med. Chem. Lett.. 1997;7:515.
- [Google Scholar]
- Synthesis and antimycobacterial activity of 1,2,4-triazole 3-benzylsulfanyl derivatives. Farmaco. 2004;59(4):279.
- [Google Scholar]
- Katritzky A.R., Rees C.W., eds. Comprehensive Heterocyclic Chemistry. Vol 6. Oxford: Pergamon Press; 1984. p. :546.
- Asian J. Pharm. Res.. 2011;1(2):23.
- Advances in Heterocyclic Chemistry. New York: Academic Press; 1965. 5, 165
- Examination of the activities of 43 chemotherapeutic agents against Neospora caninum tachyzoites in cultured cells. Am. J. Vet. Res.. 1994;55(7):976.
- [Google Scholar]
- Structure-activity relationships of diverse oxazolidinones for linezolid-resistant Staphylococcus aureus strains possessing the cfr methyltransferase gene or ribosomal mutations. Antimicrob. Agents Chemother.. 2010;54(12):5337.
- [Google Scholar]
- Antimicrobial activity of some new oxadiazole derivatives. Jordan J. Chem.. 2008;3(3):233.
- [Google Scholar]
- Synthesis, antimicrobial and anti-inflammatory activity of some 5-substituted-3-pyridine-1, 2, 4-triazoles. Int. J. PharmTech Res.. 2010;2(4):2450.
- [Google Scholar]
- 1,3,4-Oxadiazole: a potent drug candidate with various pharmacological activities. Int. J. Pharm. Pharm. Sci.. 2011;3(3):9.
- [Google Scholar]
- Synthesis and biological activities on metal complexes of 2,5-diamino-1,3,4-thiadiazole derived from semicarbazide hydrochloride. Molecules. 2011;16(7):5861.
- [Google Scholar]
- Synthesis of meso-ionic anhydro-2-arylamino-1,3,4-oxadiazolium hydroxides. Chem. Commun. 1971:1223.
- [Google Scholar]
- Synthesis, characterization and anti-microbial screening of novel heterocyclic system containing bridgehead nitrogen atom. Res. J. Pharm. Biol. Chem. Sci.. 2011;2(1):410.
- [Google Scholar]
- SCF molecular orbitals and the photoelectron spectrum of 5,5-dimethyl-Δ3-1,3,4-oxadiazolin-2-one. Can. J. Chem.. 1978;56:1319.
- [Google Scholar]
- Synthesis and antibacterial activity of some novel bis-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazoles and bis-4-thiazolidinone derivatives from terephthalic dihydrazide. Eur. J. Med. Chem.. 2009;44:5112.
- [Google Scholar]
- Synthesis and antibacterial evaluation of some semicarbazides and 1,2,4-triazol-5-ones containing thiophene moieties. J. Chin. Chem. Soc.. 2010;57(2):260.
- [Google Scholar]
- Synthesis and antimicrobial activity of thiosemicarbazides, s-triazoles and their Mannich bases bearing 3-chlorophenyl moiety. Eur. J. Med. Chem.. 2011;46:241.
- [Google Scholar]
- Katritzky A.R., Rees C.W., eds. Comprehensive Heterocyclic Chemistry. Vol 5. Oxford: Pergamon Press; 1984. p. :734.
- 1-Acylthiosemicarbazides, 1,2,4-triazole-5(4H)-thiones, 1,3,4-thiadiazoles and hydrazones containing 5-methyl-2-benzoxazolinones: synthesis, analgesic-anti-inflammatory and antimicrobial activities. Bioorg. Med. Chem.. 2007;15(17):5738.
- [Google Scholar]
- Synthesis and antimicrobial activity of 1-(5-mercapto-1,3,4-oxadiazol-2-yl)-2-(pyridine-2-ylamino)ethanone. Sains Malaysiana. 2011;40(5):445.
- [Google Scholar]
- Synthesis and in vitro antimicrobial evaluation of 5-(1-methyl-5-nitro-2-imidazolyl)-4H-1,2,4-triazoles. Arch. Pharm. Pharm. Med. Chem.. 2002;335(10):495-499.
- [Google Scholar]
- Introduction to Microbiology. Pearson Education Limited; 2006.
- Synthesis of imidazoline and imidazo[2,1-c][1,2,4]triazole aryl derivatives containing the methylthio group as possible antibacterial agents. Bioorg. Med. Chem.. 2006;14(11):3635.
- [Google Scholar]
- QSAR/QSTR of fluoroquinolones: an example of simultaneous analysis of multiple biological activities using neural network method. Eur. J. Med. Chem.. 1998;33:647.
- [Google Scholar]
- Synthesis and antibacterial activity of 1-[1,2,4-triazol-3-yl] and 1-[1,3,4-thiadiazol-2-yl]-3-methylthio-6,7-dihydrobenzo[c]thiophen-4(5H)ones. Bioorg. Med. Chem. Lett.. 2005;15(4):1023.
- [Google Scholar]
- The synthesis and antibacterial activity of 1,3,4-thiadiazole phenyl oxazolidinone analogues. Bioorg. Med. Chem. Lett.. 2003;13:4193.
- [Google Scholar]
- The reaction of 4-substituted thiosemicarbazides with phenyl isocyanide, 1,3,4-thiadiazoles and 1,2,4-triazoles. Acta Chem. Scand. Ser. B. 1977;31:264.
- [Google Scholar]
- Synthesis and antimicrobial activity of 4-phenyl/cyclohexyl-5-(1-phenoxyethyl)-3-[N-(2-thiazolyl)acetamido]thio-4H-1,2,4-triazole derivatives. Eur. J. Med. Chem.. 2005;40:607.
- [Google Scholar]
- Radius-dependent assembly of complexes with the rigid unsymmetric ligand 5-(2-pyridyl)-1,3,4-oxadiazole-2-thione: syntheses, structures and luminescence properties. Polyhedron. 2007;26:4542.
- [Google Scholar]
- Principles of Microbiology. Jones and Bartlett Publishers; 2007.
- Synthesis and characterisation of some 1,4-phenylene-bisheterocyclic compounds. Farmacia. 2001;49(4):54.
- [Google Scholar]
- Synthesis and antimicrobial activity of some derivatives of acylhydrazine including novel benzenediazasulfonamides. ARKIVOC. 2008;ii:141.
- [Google Scholar]
- Synthesis and biological activity of 3-(2-furanyl)-6-aryl-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazoles. Molecules. 2002;7:681.
- [Google Scholar]







