Translate this page into:
Synthesis and characterization of surface-active antimicrobial hyperbranched polyurethane coatings based on oleo-ethers of boric acid
⁎Corresponding author. sharifahmad_jmi@yahoo.co.in (Sharif Ahmad)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
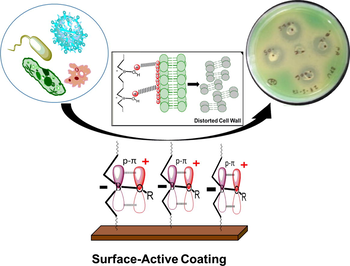
Present study reports a facile synthesis of surface-active antimicrobial hyperbranched polyurethane coatings using oleo-ethers of boric acid (BA) as branching and biocidal moiety (BHPU). The antimicrobial branching center was synthesized via polycondensation reaction of BA and vegetable oil-based diol. The structural characterization of synthesized BHPU and its linear counterpart was investigated using Fourier-transform infrared (FTIR) and nuclear magnetic resonance (1H, 13C, and 11B NMR) spectroscopy techniques. The cured coatings were examined by physico-mechanical, thermogravimetric (TG) analysis and differential scanning calorimetry (DSC). The antimicrobial behavior of these polymers against Gram-positive and Gram-negative bacteria was carried out by well diffusion technique. The appearance of zone of inhibition (ZOI) in case of BHPU confirmed its antimicrobial activity, which arisen due to the presence of cationic moiety in its structure. These investigations showed that the utilization of oleo-ethers of BA as branching agent in synthesis of BHPU coatings induced prominent effect on its physico-mechanical, thermal, and biocidal properties. In addition, soil burial study for 210 days was conducted on BHPU film to confirm its contact-killing mechanism against soil-borne bacteria. These results suggest the potential scope of BHPU in various applications such as long-term antimicrobial surface-active coatings for medical devices, packaging industry, paints, etc.
Keywords
Boric acid
Hyperbranched
Polyurethane
Antimicrobial coating
Sustainable polymers
1 Introduction
The increasing awareness about healthcare and severe losses caused by pathogenic bacteria (food born and hospital-acquired infections) and microbes has forced the scientists to investigate the mechanism of biological damage and its protection (Habibullah et al., 2016). Among various protection techniques, application of antimicrobial coatings has played a significant role in the prevention of growth as well as accumulation of harmful microbes and bacteria on the substrate surfaces in medical and other industries (Nigel et al., 2016). Generally antimicrobial coating materials are developed by the incorporation of bactericidal agents, like antibiotics, silver ions, etc., which results in the formation of hybrid or composite materials (Klassen, 2000; Sun et al, 2005; Potta et al., 2014, Irfan et al., 2017). The antimicrobial activity of such systems is mainly governed by the slow release/leaching of antimicrobial agents into the surrounding environment over certain interval of time (Worley et al., 2010). The approach has proved to be effective, but the continuous application of such systems found to be one of the main cause of bacterial resistance as leached bactericides can get accumulated in environment which is undesirable from environmental point of view (Sun et al., 2010; Zhao et al., 2016).
Recently, these problems could be resolved to an extent by development of surface-active antimicrobial polymers via introducing cationic moieties in the polymer backbone through covalent bonding (Popa et al., 2003; Coad et al., 2016). This technique controls the growth of bacteria mostly by contact killing mechanism. The concept of contact killing by the introduction of cationic moiety has attracted more attention to overcome above-mentioned issues due to their fast biocidal action with much broader spectrum than that of composites (Hancock1 and Sahl, 2006; Mukherjee et al., 2008). The cationic and amphiphilic properties of these materials attract the anionic bacterial cell wall by electrostatic force of attraction and disrupts their cell membrane that eventually results in bacterial death (Bastarrachea and Goddard, 2015). This method of contact killing mechanism by cell wall disruption does not induce the bacteria and microbes to development resistance towards these materials (Mukherjee et al., 2008). But the drawbacks associated with this method are the complexity of the preparation process, the presence of limited compounds, and their availability which inhibits their large-scale production (Rajbir and Song, 2016). Therefore, the development of new antimicrobial materials with enhanced biocidal properties and ease of production is on demand.
Boron and its derivatives have attracted interest of scientists and industrialists because of their biocidal properties, low toxicity toward mammalian cells and their availability (Haque et al., 2004; Sezen et al., 2016). Boron is capable of forming complexes rapidly with aromatic and aliphatic —OH containing compounds by interaction with their hydroxyl groups (Donmez Cavdar et al., 2015). Literature reveals that the incorporation of boron in the synthesis of petro-based polymers having linear structure improves the thermal, mechanical, flame retardancy, and biocidal activities than that of their counterparts, resulting in the formulation of inorganic-organic hybrid polymers (Kuo et al., 2016; Ali et al., 2014).
The application of highly branched polymers using natural based precursors (like vegetable oils) induces unique properties to the polymer compared to their linear ones (Deniz and Baozhong, 2017; Obaid et al., 2017). Hyperbranched polymers (HBPs) possess unique characteristics due to their special 3D structures like low viscosity, high solubility and large number of terminal groups which can be functionalized to enhance the polymer properties (Enciso et al., 2015). Vegetable oils (linseed, castor, sunflower, etc.) are extensively utilized in the synthesis of different types of HBPs such as epoxies, alkyds, polyurethanes, etc. that improves their biodegradability, biocompatibility and flexibility (Obaid et al., 2017; Suman and Niranjan, 2013). Among these, hyperbranched polyurethanes (HPUs) are widely used in various applications, due to their high adhesion, good biocompatibility, mechanical and physical properties and ease of chemical modification (Pion et al., 2010).
Literature survey reveals that HBPs are synthesized via various techniques, such as step-growth polycondensation of AB2 monomers, self-condensing vinyl polymerization (SCVP), self-condensing ring-opening polymerization (SC-ROP), A2 + Bn (n ≥ 2), proton-Transfer polymerization (PTP), couple-monomer methodology, etc. (Cook et al., 2016, Sudo et al., 2018, Tundo et al., 2016, Gadwal, et al., 2014). For instance, Kantheti et al., have fabricated hyperbranched polyurethane-urea (HPUU) coatings via click chemistry approach (Kantheti et al., 2014). They initially utilized ring-opening polymerization technique to develop different chlorinated polyols, which were converted to triazole-rich hyperbranched polyethers upon treatment with sodium azide and propargyl alcohol. These triazole-rich compounds were further reacted with isocyanate containing moieties to attain the final product. Later, Thakur et al. have reported a self-healable smart HPU nanocomposite using vegetable oil (castor oil) branching center and nano-hybrid (sulfur nanoparticles decorated reduced graphene oxide) as fillers via A2 + B3 methodology (Thakur et al., 2015). These HPU nanocomposites exhibited high flexibility, mechanical, thermal and antimicrobial properties due to the presence of oil based moiety and bioactive nano-hybrid materials. On the other hand, Sathiyaraj et al., described the synthesis of antimicrobial HPU using two synthetic AB2 blocked monomers, where A and B contain one –OH and two isocyanate groups, respectively. Based on their investigations, the synthesized HPUs showed less activity in contradiction of Gram-negative bacteria (Sathiyaraja et al., 2017). Recently, Bayan et al. reported the synthesis of bio-based HPU nanocomposites via A2 + B3 approach using castor oil as branching agent and silica functionalized graphene oxide as nano-fillers (Bayan and Karak, 2018). These nanocomposites were fabricated by in situ incorporation of nano-fillers, which enhanced their properties, while exhibiting hydrophobic surface along with inherent self-healing behavior.
In view of this, present article reports a facile synthesis of HPU using oleo-ethers of BA (polyol) as branching and antimicrobial agent for the first time following A2 + Bn (n ≥ 2) methodology. The series of BHPUs were synthesized via polycondensation reaction between different mole ratio of BA and sunflower (SF) oil monoglyceride (SMG) for the generation of branching moieties followed by addition polymerization using toluene diisocyanate (TDI). In addition, linear polyurethane (LPU) was also synthesized (without BA) to examine the effect of BA on the various properties of BHPUs. The structural studies were performed by FT-IR and NMR (1H, 13C and 11B) spectroscopic techniques. The effect of BA loading on physico-chemical, physico-mechanical, thermal stability (TG analysis and DSC) and antibacterial properties against both class of bacteria (Gram positive and Gram negative) was examined. The functionalization of BA with SMG to prepare a multifunctional core increased the degree of cross-linking in BHPU structure. This induced hydrophobic characteristic to the polymer, providing a good barrier property against moisture that prevented the growth of bacteria and fungi. These features make BHPU a suitable candidate, having high potential as an inherent antimicrobial coating material in coatings of medical devices, paints and packaging industries. It is worth stating that to the best of our knowledge, the synthesis of HPU based on oleo-ethers of BA has not been reported till date.
2 Materials and testing
Materials
SF oil was purchased from the local market, sodium hydroxide (NaOH, Merck, India), sodium sulphate (Na2SO4, Merck, India), sodium bicarbonate (NaHCO3), glycerol (98% purified), BA (Merck, India), xylene, TDI (Merck, Germany) and dimethyl formamide (DMF). The chemicals obtained were used as such without any further purification.
2.1 Characterization
The structural determination of LPU and BHPU was investigated by FT-IR and NMR spectroscopic (1H, 13C and 11B) techniques. FT-IR spectra of SMG, LPU and BHPU were analyzed using Perkin Elmer1750 FT-IR spectrometer (PerkinElmer Cetus Instrument, Norwalk, CT) by means of NaCl cell. The NMR (1H and 13C) spectra of LPU and BHPU were recorded on A JEOL GSX 300 MHz FX-100, while the 11B NMR spectra was analyzed using Bruker AVANCE III 500 MHz (AV 500) using deuterated dimethyl sulphoxide (DMSO) as solvent in all the NMR spectroscopies. The BHPU solutions of different mole ratios (1:0.5, 1:1, 1:2 and 1:3) were applied by brush technique on glass slides and polished metal strips (MS) having size of 70 mm × 25 mm × 1 mm using SiC papers of fine grade (120–500 G) to prepare the polymer coatings. These coated specimens were dried at room temperature and used for physico-mechanical and contact angle measurements by Drop Shape Analysis System (model DSA10MK2, Krüss GmbH, Germany). Specific gravity (SG) [ASTM D891-09], refractive index (RI) [ASTM D1218, by Abbe’s refractometer via yellow sodium light at 25 °C], bending test (BT) [Conical Mendrel, ASTM-D3281-84], impact resistance (IRt) [IS; 101 part 5/sec-3, 1998], scratch hardness (SH) [BS 3900], thickness [Elcometer, model 345, Manchester, UK) specular gloss (at 45°), solvent resistance test using methyl ethyl ketone(MEK) [ASTM D5402], drying time (DT), cross-hatch test [ASTM D3359], inherent viscosity (IV) of LPU and BHPU [(1.0 g/100 ml) at 25 °C using a Ubbelhode viscometer taking MEK as solvent] were recorded. The degree of cross-linking (Ct) of polymers was evaluated by solution extraction method using chloroform as the solvent. The Ct (%) was calculated using [Ct (%) = Wt/W0 × 100%] equation, where Wt is the weight of dry films extracted in chloroform after 12 h and W0 is the initial weight of polymers (Zhang et al., 2018). The thermal properties of LPU and BHPU-0.5 was evaluated by TG analysis using EXSTAR TG/DTA 6000 under a N2 atmosphere at 10 °C/min up to 900 °C. The glass transition temperature (Tg) of these polymers was studied by DSC on SII EXSTAR 6000, DSC620, Japan under N2 atmosphere at temperature range of 25–900 °C with heating and cooling rate of 10 °C/min.
The antimicrobial properties of synthesized LPU and BHPU were investigated against two classes of bacteria viz, Gram positive (Staphylococcus aureus, MTCC 902) and Gram negative (Pseudomonas aeruginosa, MTCC 2453, Klebsiella pneumoniae, ATCC 700603 and Escherichia coli, ATCC 25922) by well diffusion method. In brief, suspensions of these bacteria were prepared and spread on plates containing Mueller Hinton Agar (MHA). On these plates wells of 6 mm diameter were made by a cork borer and polymer samples were loaded. The different dilution of LPU and BHPU polymer samples in DMF were prepared by ultra-sonication followed by continuous agitation. The different concentrations of LPU and BHPU-0.5 solutions (60, 120, 250 and 500 µg) were made on dilution by DMF to increase the diffusion of polymer samples in the media and then loaded into each well and incubated overnight at 37 °C. At the same time sample-free solvent (DMF alone) was used as negative control. The bactericidal behavior of polymers reported by the appearance of clear ZOI around well after overnight incubation. The soil burial degradability test was established on LPU and BHPU-0.5 films for period of 210 days to evaluate environmental stability and surface-active character of fabricated films. The soil with pH: 6.7 was used during the course of investigation collected from Indian Agricultural Research Institute (IARI, PUSA, New Delhi, India). The soil characteristics were as: Organic Carbon: 33 g kg−1, Ultisol (loamy type), average phosphorus: 5 mg kg−1, CEC: 16.2c mol kg−1, average nitrogen: 151.4 mg kg−1. The test specimens (2cm × 1 cm) of LPU and BHPU-0.5 were buried in separate petri dishes containing soil. The soil moisture was maintained at approximately 30% by spraying the doubled distilled water at regular interval of time throughout the course of study. The weight loss of the samples was carefully measured using Sartorius balance of ±0.0001 sensitivity. The average weight change was calculated. The petri dishes containing samples were placed in a well-ventilated place at room temperature (30 °C).
3 Experimental section
3.1 Synthesis and purification of sunflower oil monoglyceride (SMG) via trans-esterification reaction
SMG was synthesized following the previously reported procedure (Scheme 1) (Obaid et al., 2017). In brief, SF oil and glycerol in 1:2 ratios were taken in a three necked round bottom (r.b) flask which was fitted with thermometer, nitrogen gas inlet and magnetic stirrer. NaOH (0.1 wt% of sunflower oil) was used to catalyze the reaction and temperature of the reaction vessel was raised to 180 °C, which was maintained for 2 h under continuous stirring. The reaction progress was monitored by solubility test of the reaction product in 1:3 SMG with methanol at room temperature. Completion of the reaction was further confirmed by determining the solubility of the reaction product in 1:3 SMG with methanol. The synthesized SMG, was cooled to room temperature and taken for the purification step.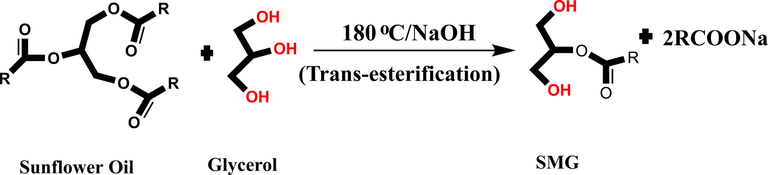
Synthesis of sunflower monoglyceride (SMG) via trans-esterification.
3.2 Synthesis of hydroxyl terminated SMG-ethers of boric acid (BSMG polyol)
Synthesis of BSMG involves the polycondensation reaction between BA and SMG without using catalyst, resulted in the formation of oleo-ethers of BA containing terminal hydroxyl groups. Different mole percent of SMG and BA (1:1, 1:0.5 and 1:0.25) were charged in four-necked r.b flask (Table 1) equipped with a dean stark apparatus, thermometer and nitrogen gas inlet. The temperature of the reaction mixture was raised up to 120–140 °C under continuous stirring in an inert atmosphere until the expected amount of water was trapped in dean stark apparatus. The reaction progress was recorded by FT-IR spectroscopy by observing the increase in intensity of —OH peak at 3417.86 cm−1 and appearance of peaks at 1386.81 and 650.01 cm−1 for —B—O stretching and bending vibrations respectively. The increase in intensity of color and viscosity was observed, the end product obtained was a viscous, dark russet color solution at room temperature.
3.3 Synthesis of linear polyurethane and antimicrobial BHPUs
The prepared BSMG polyol was cooled and dissolved in 10 ml xylene, transferred in to a four-necked r.b flask fitted with thermometer, nitrogen gas inlet, dropping funnel and mechanical stirrer on ice bath. To this exact required amount of TDI solution (Table 1) was added drop wise under an inert atmosphere. After the addition, the reaction mixture was allowed to react at 70 ± 2 °C for 4 h until a viscous mass was obtained under a controlled reaction condition to prevent the gelation of the final product. The same procedure was followed in the absence of BA for the synthesis of LPU. During the course of reaction the NCO/OH ratio was maintained as 1 in the synthesis of LPU and BHPU. Small portion of these products was precipitated in water separately for NMR analysis.
4 Results and discussion
Series of BHPUs were successfully synthesized via A2 + Bn methodology by varying the mole ratio of BA: SMG (BHPU-1, BHPU-0.5 and BHPU-0.25) in two steps (Scheme 2). The first step involves the synthesis of biocidal multifunctional BSMG pre-polymer (polyol) by polycondensation reaction between BA and SMG. Here the —OH groups of SMG reacts with the —OH functionalities of BA and forms C-O-B linkage that results in the formation of multifunctional branching center. The second step involves the addition polymerization of BSMG polyol with TDI (maintaining 1:1 OH/NCO ratio). In this step the formation of urethane linkage (—NH—COO—) takes place by the reaction between —OH groups of BSMG polyol and —NCO moiety of TDI that ultimately results in the formation of BHPU. In addition, LPU was synthesized without BA to examine the effect of inorganic branching unit in various properties of fabricated BHPU.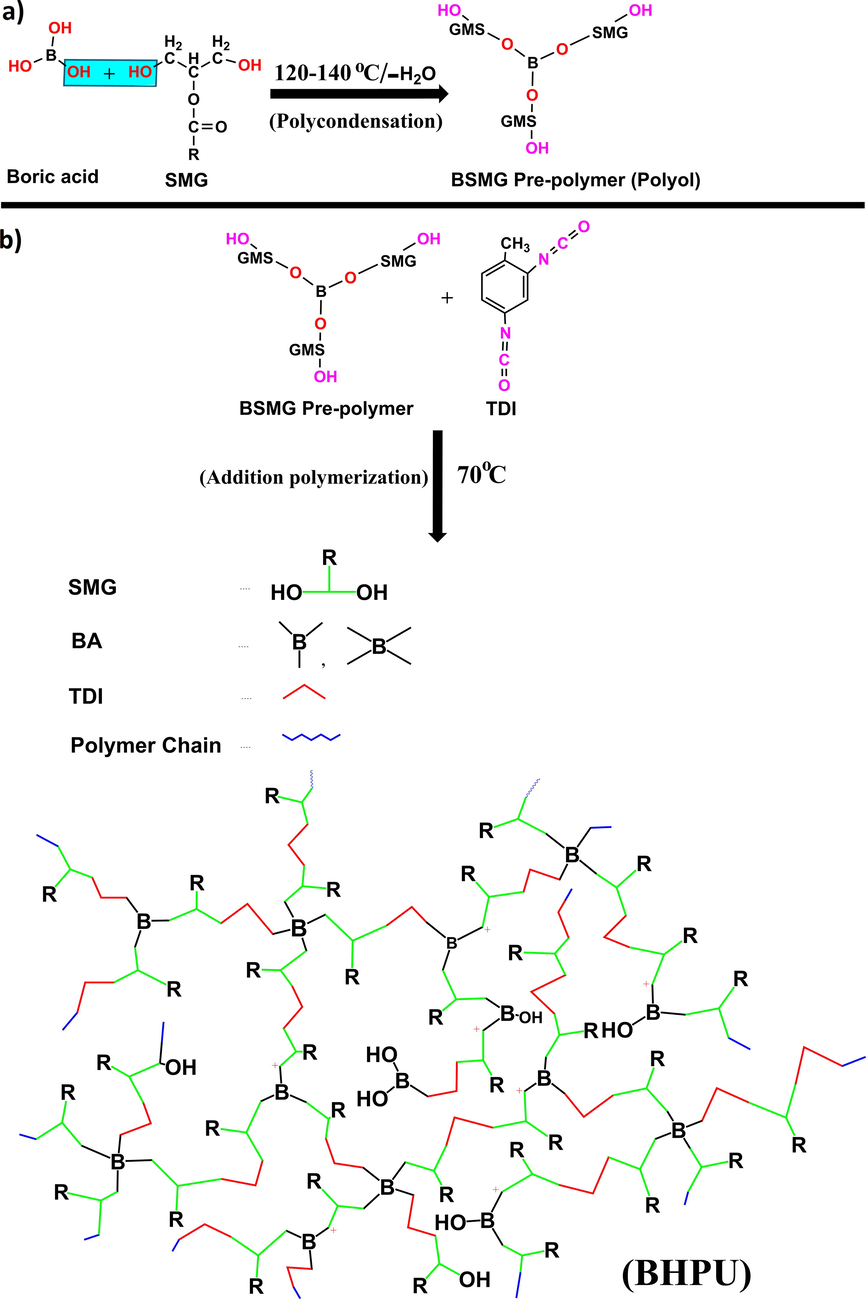
Synthesis of (a) hydroxyl terminated BSMG pre-polymer polyol via polycondensation reaction; and (b) BHPU through addition polymerization.
4.1 Physico-chemical analysis of LPU and BHPUs
The physico-chemical tests were carried out to investigate the effect of BA on the synthesized HPUs (Table 2). According to this study, LPU and various BHPU exhibits a continuous increase in InV and RI values, while a linear decrease in SG values. The increase in InV can be attributed to the increase in molecular weight of the resin with the increase in the concentration of BA (branching agent) while a linear decrease in SG suggests that due to the increase in the branching of resin with the increased concentration of branching agent lower mass occupies the higher volume which revealed in the lowering of SG and also suggests the branching of polyurethane (Martin et al., 2006).
Code
IV (dL/g)
RI
Specific gravity
LPU
0.585
1.303
0.994
BHPU-0.25
0.655
1.502
0.965
BHPU-0.5
0.674
1.502
0.957
BHPU-1
1.450
1.506
0.946
4.2 Physico-mechanical performance and degree of crosslinking (Ct) of LPU and BHPU
The polymer coatings having thickness of ≈130 µm were prepared by applying LPU and BHPUs on cleaned MS with 70 mm × 25 mm × 1 mm dimensions using brush technique which further subjected to various physico-mechanical tests (Table 3) after drying at room temperature. Their corresponding films were also prepared by solution casting method using Teflon sheet. These films were further used to determine the degree of cross-linking (Ct). The tests were conducted in triplicate to ensure the reproducibility of the results.
Code
DT (min)
Gloss (45°)
SH (kg)
IRt (150 lb/in.)
Bending (1/8 in)
CHT
Ct (%)
LPU
45
50
6
Pass
Pass
Pass
13
BHPU-0.25
50
60
8
Pass
Pass
Pass
52
BHPU-0.5
30
69
12
Pass
Pass
Pass
71
BHPU-1
25
65
Fail
Fail
Fail
Fail
93
These tests were performed to ascertain the optimal amount of BA required for the formulation of BHPU coatings, which revealed that the BHPU-0.5 showed superior SH performance as it increases from 6 to 12 kg by the incorporation of BA up to the load ratio of 1:0.5 SMG:BA, beyond this ratio, causing bristliness within the coatings. This can be attributed to the increase of inorganic moiety that causes reduction in the flexibility of polymer coatings. The DT decreases upon increasing the BA content, while gloss value increases up to BHPU-0.5 beyond which decreases, this can be due to decrease in opaqueness of the coating surface. The increase in gloss value from LPU to BHPU-0.5 can be attributed to increase in branching/cross linking, which results in the increase in reflection of light by BHPU-0.5 coating surface. The physico-mechanical analysis exhibited improved performance on comparison with our earlier reported work (Alam et al., 2004) that the incorporation of BA as branching moiety in synthesis of HPUs induces remarkable increase in IRt (15 kg/m) and BT (1/18″ of Conical Mendrel) values for BHPU-0.5 (Fig. 1c and d). The excellent physico-mechanical performance of BHPU-0.5 coatings can be attributed to the presence of flexible long fatty acid chains of SF oil (Uday and Niranjan, 2011) that can bear the stress loaded on coating surface due to the presence of strong covalent linkages with BA. The enhancement in SH values (Fig. 1b) from LPU to BHPU-0.5 can be elucidated by the increase in the functionality that improves the adhesion of BHPU-0.5 to metal substrate, limiting the indentation of polymer coatings. Subsequently, the CH test (Fig. 1a) also confirmed the outstanding adhesion of polymer coatings. In addition, the increase in Ct upon incorporation of BA further confirmed the formation of compact and dense structure along with chain entanglement within BHPU structure. Although, extend of cross-linking determines the flexibility and barrier property of coatings (Zhang et al., 2018). This study revealed that the higher Ct of BHPU-1 is correlated with its brittle nature and least adhesion property as it is obtained by IRt test. Furthermore, it was found that BHPU-1 exhibited highest Ct (%) of 93%, while BHPU-0.25 and BHPU-0.5 showed lesser Ct (%) of 52% and 71%, respectively. Moreover, the insoluble potion of BHPU-0.5 in chloroform (solvent) was increased upon incorporation of BA as branching unit, which supports the formation of compact and branched structure, having some extent of crosslinking.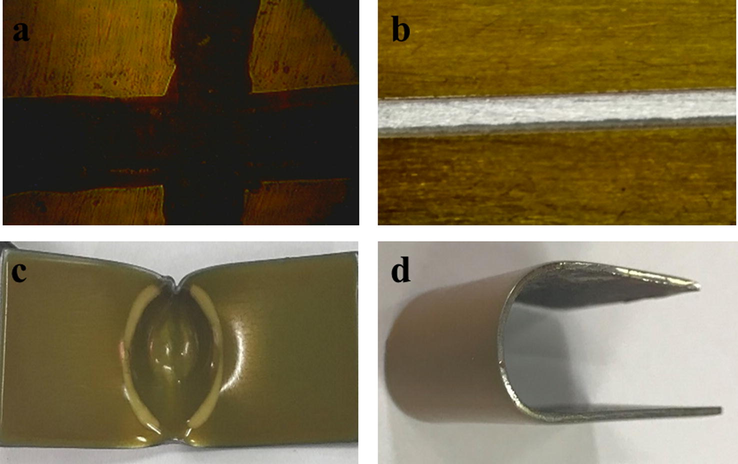
Digital images of BHPU-0.5 after physico-mechanical tests; (a) cross hatch, (b) scratch hardness, (c) impact resistant, and (d) bending tests.
4.3 Structural analysis
The structural investigation of fabricated LPU and BHPU-0.5 was carried out using FT-IR and NMR (1H, 13C, and 11B) spectroscopy techniques. The FT-IR spectra (Fig. 2) of SMG, BSMG, and BHPU-0.5 shows band at 3429.43 cm−1 which is ascribes to hydroxyl group in monoglyceride. The appearance of same band with higher intensity at 3454.35 cm−1 for BSMG was observed due to the increase in —OH functionality, while in case of BHPU-0.5 the peak at 3323.34 cm−1 confirms the formation of —N—H bond of urethane linkage. The broadening of this peak in case of BHPU-0.5 and LPU may be due to the overlapping of stretching vibrations of hydroxyl (—OH) and —NH functionality. The higher intensity of broadening in case of BHPU-0.5 may be due to the occurrence of large number of these functional groups (Uday and Niranjan, 2011). Bands at 1747.50 cm−1, 2924.08 cm−1 and 2852.71 cm−1 ascribe to ester linkages, symmetric and asymmetric stretching of —C—H functionalities present in long chain of fatty acid in SMG respectively (Obaid et al., 2017). The appearance of typical bands at, 1386.81 cm−1 (—B—O stretching) and 650.01 cm−1 (—B—O bending) are evidence for the presence of BA in backbone of BSMG pre-polymer and BHPU-0.5 polymer. Besides, bands at 1556.55 cm−1 and 1068.56 cm−1 are due to —NH bending/—CN stretching and —O—C⚌O stretching vibrations of BHPU-0.5 respectively. In this investigation, shifting of absorption band 1745.04 cm−1 in BHPU-0.5 and 1727.54 cm−1 in LPU FT-IR spectra indicates an increase in —C⚌O group of BHPU-0.5 polymer (Beauty et al., 2013). On the basis of these data synthesis of BHPU-0.5 by the reaction of —OH groups of BSMG pre-polymer and —NCO groups of TDI was conformed.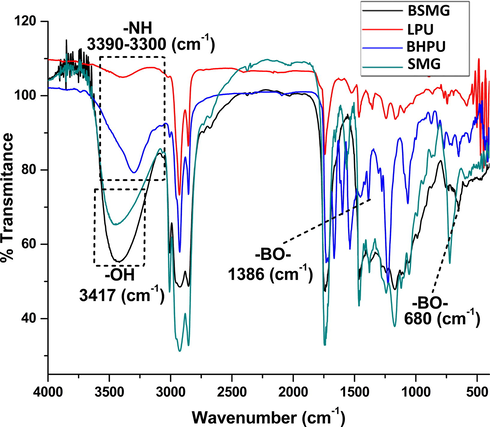
FTIR spectra of SMG, LPU, BSMG, and BHPU-0.5.
1H NMR (Fig. 3a) characteristic peaks of BHPU-0.5 are mentioned. The peaks at δ = 0.852 ppm and δ = 1.516 ppm are ascribed to the protons of methyl group present at terminal position and protons attached directly to this group in the fatty acid chains, while the peaks at δ = 1.241 ppm and δ = 5.315 ppm are assigned to the protons of all internal —CH2— groups and protons of unsaturated carbon in the fatty acid chains respectively. The peak at δ = 7.046–7.509 ppm are due to the presence of aromatic protons present in TDI (Cao and Liu, 2006). In addition, the signals at δ = 5.924–5.951 ppm corresponded to the —B—OH proton which are absent in Fig. 3b and peaks at 5.208 ppm represents unsubstituted —OH proton of SMG present in the backbone of the BHPU-0.5 polymer, which is an evidence for successful incorporation of BA in synthesis of BHPU through covalent bond formation with SMG. The presence of an intense peak was observed at 3.324 ppm which corresponds to the protons of —CH2 group attached to unsubstituted –OH group of monoglyceride while the peak at δ = 3.293 ppm in 1H NMR spectra of LPU (Fig. 3b) corresponds to the same protons with low intensity which indicates low presence of these type of protons (Beauty et al., 2013).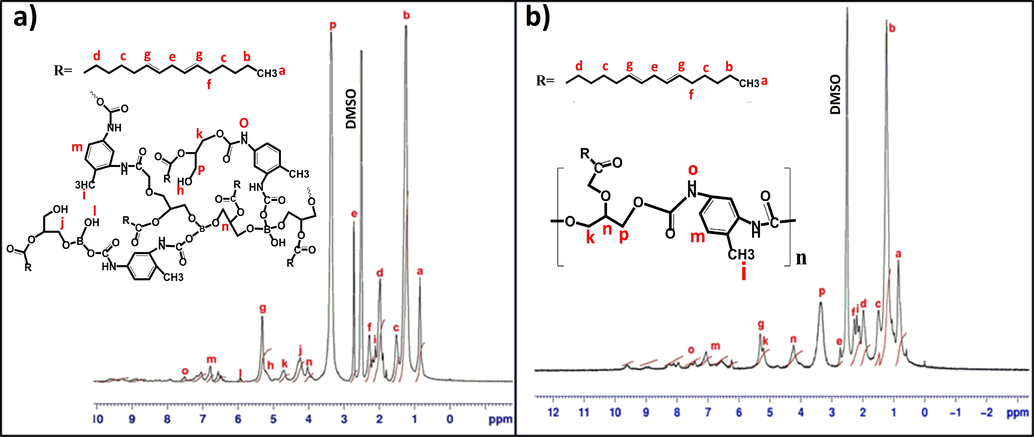
1H NMR spectra of (a) BHPU and (b) LPU.
Fig. 4 shows the 13C NMR spectrum of BHPU-0.5. The terminal methyl carbon and all internal —CH2 groups of SMG segment appeared at 14.35 ppm and 17.285 ppm respectively. Peaks at 24.840 ppm, 67.699 ppm and 68.650 ppm are associated with methyl group of TDI, —CH2— groups that are adjacent to urethane linkage and BA respectively, while the peak at 61.066 ppm is assigned to those soft-segment carbons that are adjacent to —OH group. The peak at 40.254–40.808 ppm are attributed to methylene carbons of monoglyceride. Peaks present for aromatic group of TDI are at 128.18–137.20 ppm. The peaks appeared at 144.25 ppm and 168.65 ppm for carbonyl carbon of urethane linkage and ester linkage of SMG respectively (Cao and Liu, 2006). These information evidenced the presence of BA as building-block in the structure of BHPU-0.5 through covalent bonding.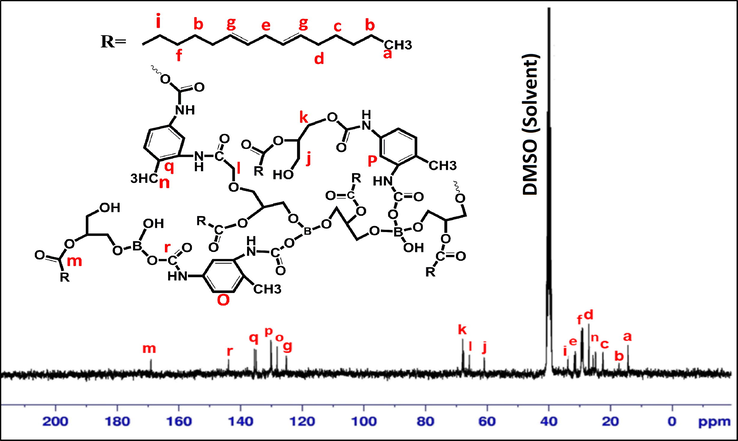
13C NMR spectra of BHPU.
Fig. 5 shows 11B NMR spectra of BHPU-0.5 with all the characteristic peaks that confirmed the formation of branching centers in synthesized polymers. The appearance of the peak at 20.728 ppm is due to the B atom in tri-coordinated state with sp2 hybridization in which BA formed at least one urethane linkage (Wrackmeyer, 1988). The peak at 1.675 ppm corresponds to the presence of B-O-C (BA-ether) containing residual —OH groups substituted with an alkoxy group of SMG in which B atom is in sp2 hybridization. Shielding of this species arises due to the presence of strong p-π interaction between empty p orbital of B atom and free electron pair of the substituent (oxygen atom) which makes orbital electrons to resemble the D3h symmetry (Hermanek, 1992). Thus, tri-coordinate species approached the signal of related anions of tetra-coordinate species. This p-π interaction can further be reinforced due to the presence of electron releasing fatty acid chain of SMG, which results in the formation of partial positive charge on O atom and negative charge on B atom (—B−—O+—). The peak at −3.559 ppm ascribed for the tetrahedral structure of B atom having sp3 hybridization with tetra-coordinate state. Shielding of this species arises due to presence of four electron rich substituents, which increases the electron density around the B atom and produces a negatively charged moiety in BHPU-0.5 structure (Wrackmeyer, 1988). The presence of tri- and tetra-coordinated B atoms in BHPU-0.5 backbone confirms the formation of branching sites, favoring the successful incorporation of BA as branching center in development of BHPU. Further, the occurrence of ionic moieties in the BHPU-0.5 structure suggests the formation of biocidal sites that induces the antimicrobial property to these polymers.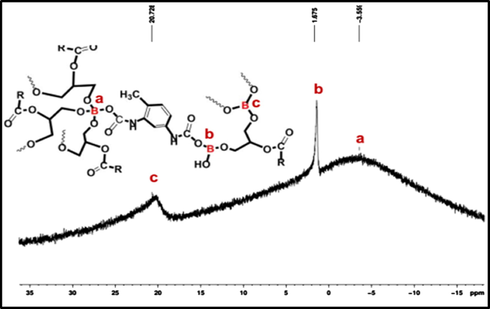
11B NMR spectra of BHPU.
4.4 Thermal properties of BHPU-0.5 and LPU
Thermal stability of synthesized BHPU-0.5 and LPU was studied under N2 atmosphere with heating rate of 10 °C/min up to 900 °C. These results revealed that the utilization of BA in fabrication of BHPU-0.5 has direct impact on improvement of its thermal stability. Fig. 6a shows the three steps thermal degradation of BHPU-0.5, and onset degradations of LPU after the first degradation in 220–290 °C, which is attributed to the process of depolymerization in its structure (Patel and Patel, 2015).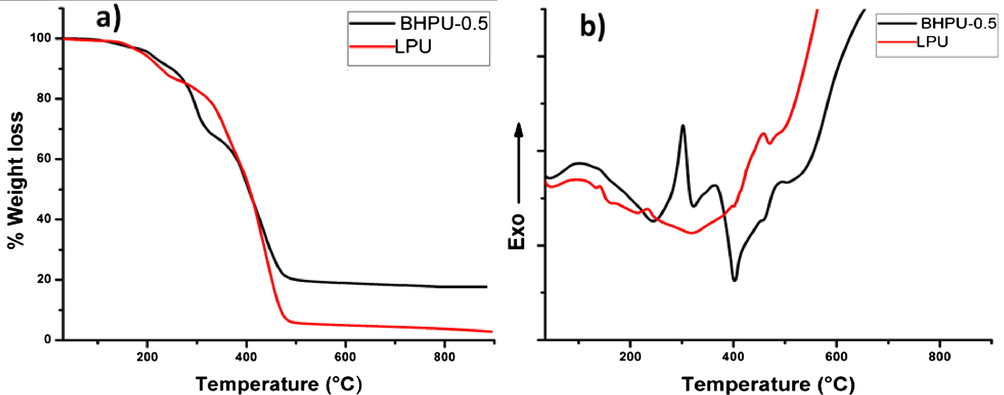
(a) TG analysis thermogram and (b) DSC plots of BHPU-0.5 and LPU under N2 atmosphere.
The weight loss of samples at 100–155.8 °C is due to evaporation of water and solvent molecules trapped in polymer coatings. The first thermal degradation of BHPU-0.5 at 188.11–298.11 °C is attributed to the removal of ether, ester and water molecules by decomposition of unreacted —OH groups of B atom and SMG in polymer structure. The final decomposition of BHPU-0.5 occurs at 330.90–510.18 °C, which does not degrade further on higher temperature with final weight loss of 80.4% that proves the higher stability of fabricated BHPU-0.5. The higher weight loss at 330.90 °C in BHPU-0.5 is due to presence of large number of –OH groups that result in formation of water molecules on heating. The TG analysis of LPU revealed its rapid weight loss, which indicated its low thermal stability with total weight loss of 93.6%. The DSC results (Fig. 6b) reveals that the utilization of BA in synthesis of BHPU-0.5 increases the crosslinking/branching in polymer matrix that inhibits the chain mobility, which leads to an increase in Tg value (Deewan et al., 2008). The DSC thermogram of BHPU-0.5 shows three endotherm, the first arises between 139 °C and 300 °C, the second one in 300–380 °C range and the third sharp endotherm occurs at 480–510 °C where TG thermogram shows weight loss in same temperature ranges. These endotherms could be ascribed to the melting of polymer, the release of internal strain by decomposition of —B—O—C— linkages, resulting in relaxation of polymer chains and decomposition of BHPU-0.5 (Deewan et al., 2008). The broad endotherms occurred above this range could be due to the decomposition of other components present in BHPU-0.5. Over all the thermal stability of BHPU-0.5 has increased compared to LPU and our previously reported systems (Haque et al., 2004), which is because of increase in chain entanglement in BHPU-0.5 structure revealing in its compact structure. These results are in well agreement with those of physico-mechanical studies and crosslinking density.
4.5 Contact angle measurement
The contact angle measurements of BHPU-0.5 and LPU coatings (Fig. 7) revealed that presence of BA as branching agent in BHPU-0.5 enhances the contact angle values from 50.4° to 90o. This can be assigned to the increase in degree of crosslinking and branching in polymer chains that introduces hydrophobic character to the polymer coatings resulting in low wettability. The intermediate hydrophobicity of BHPU-0.5 might be due to the presence of ionic moieties in polymer structure that has reduced the hydrophobic effect induced by crosslinking/and or branching of BA. The improved hydrophobic nature of the BHPU-0.5 coating minimizes the growth and accumulation of bacteria on the surface of the polymer coating as the main criteria for the growth of bacteria is water. According to literatures most of the antimicrobial polymers are hydrophilic in nature, as a result water gets attracted on their surface, providing a suitable media for the growth of bacteria and fungi. Subsequently, the steric hindrance formed on the surface of the coatings led to the adsorption of microorganisms or proteins to the coatings (Chamsaz et al., 2017). Hence, the hydrophobicity of BHPU-0.5 increases the barrier property of polymer against the diffusion of electrolytes and bacteria through the polymer matrix, improving the antimicrobial activity of coatings.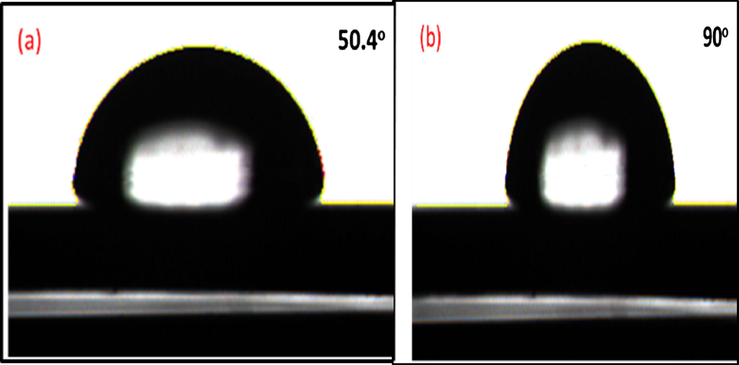
Contact angle measurements of; (a) LPU and (b) BHPU-0.5.
4.6 Antimicrobial property and action mechanism of BHPU-0.5
The antimicrobial activities of synthesized LPU and BHPU-0.5 were examined in contradiction of both Gram negative and Gram-positive bacteria by well diffusion method. After overnight incubation of polymer solutions samples with different dilution (60, 120, 250, 500 µg) in MHA culture media a clear ZOI (Fig. 8) around the sample wells of BHPU-0.5 was observed while LPU did not show any activity. This indicates significant antimicrobial activity of BHPU-0.5 against Pseudomonas aeruginosa, MTCC 2453, Escherichia coli, ATCC 25922, and Klebsiella pneumoniae ATCC 700603, Staphylococcus aureus, MTCC 902 bacteria even at low concentration.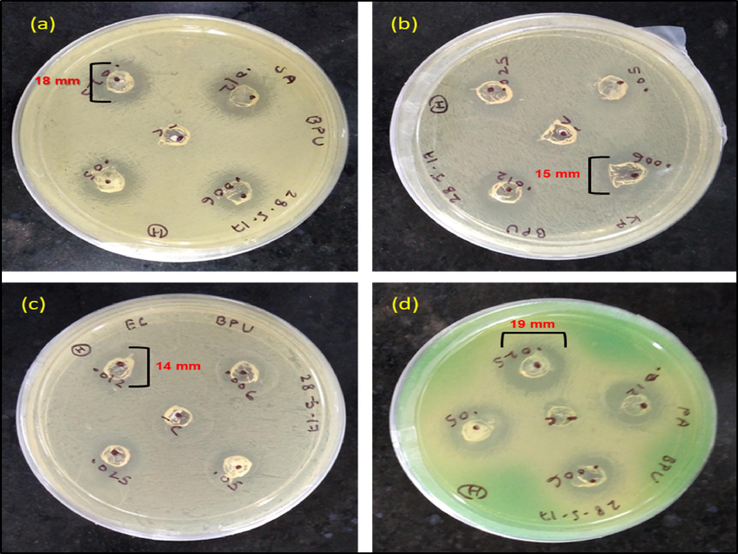
Antibacterial activity of BHPU-0.5 against (a) Staphylococcus aureus (b) Klebsiella pneumoniae (c) Escherichia coli (d) Pseudomonas aeruginosa.
The mechanism of action of BA on bacteria is not clearly understood and it is still hypothetical (Becker et al., 2011). However, the antimicrobial studies evidences that, after the successful synthesis of HPU using BA as branching agent in the polymeric backbone, antimicrobial BHPU was fabricated. The exhibition of biocidal activity by BHPU-0.5 may be governed by the cationic moiety present in the BHPU matrix (Mukherjee et al., 2008; Chamsaz et al., 2017). This cationic moiety is generated as a result of strong p-π interaction of lone pair of electrons of O-atom with empty p-orbital of B-atom (Fig. 9), which is confirmed by 11B NMR (Fig. 5) that results in formation of Oδ+. The partial donation of lone pair of electrons by oxygen atom is further enhanced by the increase in electron density around the oxygen atom due to the presence of electron releasing alkyl groups in the fatty acid chains of SMG. The appearance of ZOI by BHPU-0.5 may be due to the rupturing of the bacterial cell wall by the action of these cationic moieties. The presence of this moiety at peripheral position of the BHPU-0.5 chains can attract the bacteria towards itself by the electrostatic force of attraction because of the negatively charged bacterial cell wall, which causes the death of bacteria by rupturing its cell wall (Mukherjee et al., 2008).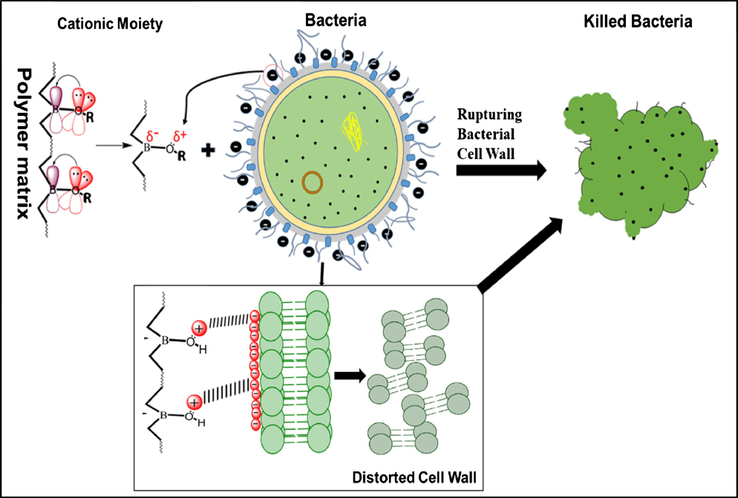
The proposed action mechanism of BHPU-0.5 leading to distortion of bacterial cell wall.
The advantage associated with the present system over other reported antimicrobial composite coatings (governed by leaching mechanism) is the contact killing of bacteria through inherent antibacterial activity of BHPU-0.5 in which the antibacterial active compound (BA) does not leach out to the environment, thus enables the polymer to be used as long-term coating material. Fig. 8 represents the probable action mechanism of BHPU-0.5 on bacteria by cell wall disruption.
The antimicrobial activity of various reported antimicrobial agents against Gram positive and Gram negative bacteria are given in Table 4. The comparison of the size of ZOI in present manuscript with the reported systems revealed that the BHPU-0.5 coatings exhibits superior antimicrobial activity towards both classes of bacteria.
Name of bacteria
Antimicrobial agent
ZOI (mm)
References
S. aureus
BA
<8
Haque et al. (2004)
E. coli
BA
<14
P. aeruginosa
BA
<8
S. aureus
Ag NPs
12
Peter et al. (2015)
P. aeruginosa
Ag NPs
7
E. coli
Ag NPs
8
S. aureus
20
No significant effect on Gram negative bacteria
Hyaluronic acid
Isabelle et al. (2014)
E. coli
Essential oils mixtures (formulations A and B)
10.7
Afia et al. (2013)
E. coli
Coccinia grandis extract
<10
Giriprasath et al. (2015)
S. aureus
<18
S. aureus
Boric Acid
18
This work
K. pneumoniae
15
E. coli
14
P. aeruginosa
19
4.7 Soil burial test (environmental stability study)
The stability of LPU and BHPU-0.5 films against soil-borne bacteria was determined by soil burial method for 210 days under humid condition. This technique performed to examine the environmental stability and the actual effect of bacteria present in soil on these coatings. Bacteria and fungi present in the soil are mainly responsible for the degradation of these polymer films (Sullivan et al., 2017). Fig. 10 shows the negative effect of these microorganisms on the LPU film during the period of 210 days, which caused the loss of its physical and chemical properties. However, in case of BHPU-0.5 soil media either did not show any effect/ negligible effect on its film. The weight loss of LPU and BHPU-0.5 samples were recorded every 30 days to monitor the effect of microorganisms present in soil media on their structure. Fig. 10 insets show the physical changes caused by these microorganisms to LPU and BHPU-0.5 after completion of study. These results showed that the weight loss of BHPU-0.5 film was only −0.05% compared to that of LPU films −23.17%. It can be attributed to the antimicrobial effect of cationic moiety present at peripheral site of the chain which causes the leakage in bacterial cell membrane and results in inherent antimicrobial activity of BHPU-0.5 coating. This study ascertained the inhibitory effect of BHPU-0.5 on the growth of soil-borne bacteria and fungi, which prevents their negative effects on chemical and physical properties of these films, enabling long-term application of such coatings.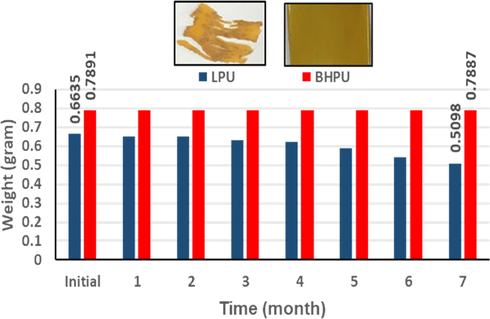
Biodegradability of LPU and BHPU-0.5 after 210 days (7 months). The inserts show the images of LPU and BHPU-0.5 films after completion of the course of study.
5 Conclusion
The nonleaching surface-active antimicrobial HPU was successfully formulated via one-pot A2 + Bn methodology using low-cost oil based precursor and BA as branching and antimicrobial agent via polycondensation reaction followed by addition polymerization. The LPU and BHPUs were characterized for structural, physico-mechanical and thermal (TG analysis and DSC) properties that revealed outstanding results about bending ability (flexibility), adhesion, CHT and thermal stability of BHPU-0.5. The antimicrobial activity of LPU and BHPU-0.5 was examined against Staphylococcus aureus, MTCC 902, Pseudomonas aeruginosa, MTCC 2453, Escherichia coli, ATCC 25922 and Klebsiella pneumoniae, ATCC 700603, where BHPU-0.5 showed remarkable biocidal action against these bacteria via contact killing, while LPU did not show any antimicrobial activity. The biocidal activity of the synthesized BHPU-0.5 might be due to the presence of charged (cationic) moieties within /or at peripheral site of the polymer that ruptures the bacterial cell wall on coming in contact with polymer surface. The soil burial test conducted for 210 days revealed that the antimicrobial agent (BA) present in the BHPU-0.5 backbone do not leach out through the polymer matrix to the surrounding environment, which enables the polymer for its long-term application. These studies suggest the potential scope for applications in the field of antimicrobial surface-active coatings for medical instruments and devices, packaging industry, etc.
Acknowledgment
Authors would like to thank the Department of Chemistry, Jamia Millia Islamia, New Delhi, India for providing the facility and space to carry out this work.
References
- Characterization of trilayer antimicrobial diffusion films (ADFs) based on methylcellulose-polycaprolactone composites. J. Agric. Food Chem.. 2013;6:811-821.
- [Google Scholar]
- Newly developed urethane modified polyetheramide-based anticorrosive coatings from a sustainable resource. Prog. Org. Coat.. 2004;50:224-230.
- [Google Scholar]
- Mitochondrial dysfunction is involved in the toxic activity of boric acid against Saprolegnia. PLoS ONE 2014:9.
- [Google Scholar]
- Bio-derived aliphatic hyperbranched polyurethane nanocomposites with inherent self-healing tendency and surface hydrophobicity: towards creating high performance smart materials. Compos. A Appl. Sci. Manuf.. 2018;110:142-153.
- [Google Scholar]
- Sunflower oil based biodegradable hyperbranched polyurethane as a thin film material. Ind. Crops Prod.. 2013;44:396-404.
- [Google Scholar]
- Effects of boric acid on various microbes, plants, and soil invertebrates. J. Soils Sediments. 2011;11:238-248.
- [Google Scholar]
- Anti-infective surface coatings: design and therapeutic promise against device associated infections. PLoS Pathog.. 2016;12(6):e1005598
- [Google Scholar]
- Hyperbranched polyurethane as novel solid-solid phase change material for thermal energy storage. Eur. Polym. J.. 2006;42:2931-2939.
- [Google Scholar]
- Non-toxic cationic coumarin polyester coatings prevent pseudomonas aeruginosa biofilm formation non-toxic cationic coumarin polyester coatings prevent pseudomonas aeruginosa. Biofilm Form.. 2017;9:6704-6711.
- [Google Scholar]
- Hyperbranched polymers with high degrees of branching and low dispersity values: pushing the limits of thiol-yne chemistry. Macromolecules. 2016;49:1296-1304.
- [Google Scholar]
- Synthesis, characterization and corrosion protective properties of boron-modified polyurethane from natural polyol. Prog. Org. Coat.. 2008;63:25-32.
- [Google Scholar]
- Facile synthesis of novel soluble cellulose-grafted hyperbranched polymers as potential natural antimicrobial materials. Carbohydr. Polym.. 2017;157:1913-1921.
- [Google Scholar]
- Effect of boric acid and borax on mechanical, fire and thermal properties of wood flour filled high density polyethylene composites. Meas.: J. Int. Meas. Confed.. 2015;60:6-12.
- [Google Scholar]
- Influence of linker groups on the solubility of triazine dendrimers. New J. Chem.. 2015;39:1247-1252.
- [Google Scholar]
- Dual-reactive hyperbranched polymer synthesis through proton transfer polymerization of thiol and epoxide groups. Macromolecules. 2014;47:5070-5080.
- [Google Scholar]
- Fabrication and characterization of collagen coated electrospun Poly (3-hydroxybutyric acid)-gelatin nanofibrous scaffold as a soft bio-mimetic material for skin tissue engineering applications. RSC Adv.. 2015;00:1-9.
- [Google Scholar]
- Economic evaluation of interventions for prevention of hospital acquired infections: a systematic review. PLoS ONE. 2016;11(1):e0146381
- [Google Scholar]
- Urethane modified boron filled polyesteramide: a novel anti-microbial polymer from a sustainable resource. Eur. Polym. J.. 2004;40:2097-2104.
- [Google Scholar]
- 11B NMR spectra of boranes, main-group heteroboranes, and substituted derivatives factors influencing chemical shifts of skeletal atoms. Chem. Rev.. 1992;92:325-362.
- [Google Scholar]
- Addition of antimicrobial properties to hyaluronic acid by grafting of antimicrobial peptide. Eur. Polym. J.. 2014;51:182-190.
- [Google Scholar]
- Click chemistry engineered hyperbranched polyurethane−urea for functional coating applications. Ind. Eng. Chem. Res.. 2014;53:8357-8365.
- [Google Scholar]
- A historical review of the use of silver in the treatment of burns. Burns. 2000;26:117-130.
- [Google Scholar]
- Practical aspects of hydrophobic polycationic bactericidal “Paints”. Appl. Biochem. Biotechnol.. 2008;151:61-70.
- [Google Scholar]
- Photodegradation of aqueous bisphenol A using boric acid-doped titanium dioxide. Water Sci. Technol. Water Supp.. 2016;16:1410-1416.
- [Google Scholar]
- Antimicrobial coatings with dual cationic and n-halamine character: characterization and biocidal efficacy. J. Agric. Food Chem.. 2015;63:4243-4251.
- [Google Scholar]
- Synthesis, crosslinking and flame retardance of polymers of boron-containing difunctional styrenic monomers. React. Funct. Polym.. 2006;66:1047-1054.
- [Google Scholar]
- Antimicrobial functionalization of cotton fabric with silver nanoclusters/silica composite coating via RF co-sputtering technique. Cellulose. 2017;24:2331-2345.
- [Google Scholar]
- Highly efficient F, Cu doped TiO2 anti-bacterial visible light active photocatalytic coatings to combat hospital-acquired infections. Sci. Rep.. 2016;6:24770.
- [Google Scholar]
- Hyperbranched soya alkyd nanocomposite: a sustainable feedstock-based anticorrosive nanocomposite coatings. ACS Sustain. Chem. Eng.. 2017;5:9725-9734.
- [Google Scholar]
- Synthesis of silver nanoparticles using plants extract and analysis of their antimicrobial property. J. Saudi Chem. Soc.. 2015;19:311-317.
- [Google Scholar]
- Preparation and characterization of waterborne hyperbranched polyurethane-urea and their hybrid coatings. Ind. Eng. Chem. Res.. 2010;49(10):4517-4527.
- [Google Scholar]
- Study of quaternary “onium” salts grafted on polymers: antibacterial activity of quaternary phosphonium salts grafted on “gel-type” styrene-divinylbenzene copolymers. React. Funct. Polym.. 2003;55:151-158.
- [Google Scholar]
- Discovery of antibiotics-derived polymers for gene delivery using combinatorial synthesis and cheminformatics modeling. Biomaterials. 2014;35:1977-1988.
- [Google Scholar]
- Synthesis and characterization of flame retardant hyperbranched polyurethanes for nano-composite and nano-coating applications. Prog. Org. Coat.. 2015;88:283-292.
- [Google Scholar]
- Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol.. 2006;24:1551-1557.
- [Google Scholar]
- The first example of bis (indolyl)methane based hyperbranched polyurethanes: synthesis, solar cell application and anti-bacterial and anti-oxidant properties. Eur. Polym. J.. 2017;95:216-231.
- [Google Scholar]
- Protective effect of boric acid on oxidative DNA damage in chinese hamster lung fibroblast V79 cell lines. Cell J.. 2016;17(4):748-754.
- [Google Scholar]
- Self-condensing vinyl polymerization of a switchable chain transfer monomer for facile synthesis of star-shaped block copolymers. Appl. Surf. Sci. 2018
- [CrossRef] [Google Scholar]
- Cellulose coating and chelation of antibacterial compounds for the protection of flax yarns against natural soil degradation. Polym. Degrad. Stab.. 2017;138:12-17.
- [Google Scholar]
- Castor oil-based hyperbranched polyurethanes as advanced surface coating materials. Prog. Org. Coat.. 2013;76:157-164.
- [Google Scholar]
- Electrospun composite nanofiber fabrics containing uniformly dispersed antimicrobial agents as an innovative type of polymeric materials with superior antimicrobial efficacy. ACS Appl. Mater. Interf.. 2010;2:952-956.
- [Google Scholar]
- Preparation and physical and antimicrobial properties of a cellulose-supported chloromelamine derivative. Ind. Eng. Chem. Res.. 2005;44:7916-7920.
- [Google Scholar]
- Self-healable castor oil based tough smart hyperbranched polyurethane nanocomposite with antimicrobial attributes. RSC Adv.. 2015;5:2167.
- [Google Scholar]
- Hyperbranched polyether core containing vegetable oil-modified polyester and its clay nanocomposites. Polym. J.. 2011;43:565-576.
- [Google Scholar]
- Acyclic N-halamine polymeric biocidal films. J. Bioact. Compat. Polym.. 2010;25:392-405.
- [Google Scholar]
- Nuclear magnetic resonance spectroscopy of boron compounds containing two-, three- and four-coordinate boron. Annu. Rep. NMR Spectrosc.. 1988;20:61-203.
- [Google Scholar]
- Antibacterial and biocompatible cross-linked waterborne polyurethanes containing Gemini quaternary ammonium salts. Biomacromolecules. 2018;19:279-287.
- [Google Scholar]
- Dual-functional antifogging/antimicrobial polymer coating. ACS Appl. Mater. Interf.. 2016;8:8737-8742.
- [Google Scholar]







