Translate this page into:
Porous polyurethane-polystyrene composites produced in a co-expansion process
⁎Corresponding author. Fax: +48 12 628 29 47. eporada@chemia.pk.edu.pl (Elżbieta Malewska)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Porous polyurethane (PUR) and polystyrene (PS) composites can be obtained by co-expansion process. This method involves simultaneous foaming of PUR and expanding PS beads (PSB), what is possible by utilizing exothermic heat of PUR formation. In this study, PUR-PS porous composites were obtained by using different types of commercial PS beads having various initial sizes. Co-expansion processes were characterized using Foamat device. The following parameters of co-expanded materials were measured: the rise profile, the reaction temperature, the rise pressure and the dielectric polarization. The results analyzes allowed to divide the co-expansion process into 3 stages: PUR foaming below the PS softening temperature, expansion of PUR and PS in the same time, PS expansion after PUR gelling. The addition of PS filler causes: a reduction of the core temperature, increasing the pressure, slowing the PUR reaction formation and the slowdown in the rise velocity of reaction mixture. It also causes the elongation of start, gelling, rise and curing times.
Keywords
Polymer composites
Polyurethane foams
Expanded polystyrene
Co-expansion
Foaming process
1 Introduction
Rigid polyurethane foams (RPURFs) are now considered as an irreplaceable insulating material in many industries, primarily in refrigeration, construction and automotive industries due to their beneficial physical and mechanical properties. RPURFs are characterized by the most preferred functional properties such as low thermal conductivity, low apparent density, excellent dimensional stability and low moisture absorption. Due to these preferred qualities RPURFs are commonly used as thermal insulation materials (Thirumal et al., 2008). However, RPURFs are still relatively rarely used, what is generally caused by their high price. Currently, the most popular thermal insulating materials in building are expanded polystyrene (EPS), what is mainly the result of its low price and satisfactory properties of the final product as well as mineral wool due to its incombustibility (Jelle, 2011).
There is an enormous interest in the development of new heat insulating materials in the construction industry. Such materials should meet the criteria of sustainable development, have physical and mechanical properties within required standards, and be economically viable.
One of the ways to reduce the cost of polyurethane (PUR) materials is the addition of fillers. PUR compositions for the synthesis of foamed materials are very sensitive to changes in the type and content of used components and production parameters. Although it is possible to obtain a porous composite, wherein the matrix is a PUR material (Czupryński et al., 2008; Hatakeyema et al., 2005; Saint-Michel et al., 2006), the addition of fillers complicates the manufacturing process but it can have a positive impact on physical and mechanical properties of final foams. For porous composites obtained on the basis of PUR matrix, nano, micro and macro fillers can be added. The main purpose of the use of fillers is to improve the dimensional stability of the final product and its mechanical properties (Lee et al., 2002). Mechanical properties of the porous PUR composites depend on the amount of additives and the interaction between the used filler and polymer matrix (Saint-Michel et al., 2006).
Co-expansion is a method of preparing composites in which one component is expanded by using heat of the exothermic reaction of the second component formation (Patent CA762531, 1967; Patent USA 3607797, 1971; Patent RP 379672, 2006; Patent USA 6605650, 2003; Patent USA 6727290, 2004; Patent USA 20030181536, 2003; Patent KR 20060071009, 2006; Patent KR 20060071440, , 2006). The idea of the co-expansion is balancing the energy effects of the exothermic process of PUR foam creating and the endothermic process of expanding thermoplastic granules, for example polystyrene (PS) within the PUR matrix (Malewska et al., 2012; Malewska et al., 2011, 2013). Producers offer a large selection of polystyrene beads (PSB) for expansion, which differ in size fraction, content and the type of blowing agent and flame retardants. So called expanded PS (EPS) is commonly used in construction industry as a heat insulating material. EPS is cheaper comparing to PUR foams, however its thermal conductivity is considerably higher. Generally, thermal conductivity of RPUFs is in the range of 20–23 mW/m K, comparing to 37–38 mW/m K for typical EPS used on industrial scale (Al-Homoud, 2005). Therefore, the first choice for the production of composites by the co-expansion is PSB due to its widespread access on the market.
Currently, low-boiling, non-solvent polymer liquid having a high vapor pressure, such as isomers of pentane and hexane, are the most commonly used for the preparation of EPS. Blowing agents are added in an amount of approx. 6% by mass. EPS is produced in expansion processes that need an energy to evaporate the blowing agent contained in PSB (Shen et al., 2006).
An interesting porous composite for heat insulating applications can be produced in the co-expansion process of PUR system containing thermoplastic PSB. The PSB may be added in an amount up to 200% by weight relative to the weight of PUR. Such fillers should have a softening point below 120 °C and a particle size between 0.2 mm and 4 mm (Patent RP 189498, 2000).
The addition of EPS as a filler to RPURF made it possible to obtain products that differ in apparent density, dimensional stability, mechanical properties and thermal conductivity. Malewska et al. (2012) have synthesized rigid polyurethane-polystyrene composite (RPURF-EPS) contained different amount of EPS beads (38–50%). Core density of the RPURF-EPS composites was about 50 kg/m3. Introduction of the EPS beads as a filler to the RPURF affected composites mechanical properties and was related to the concentration of the EPS and it was about 100–250 kPa. Thermal conductivity coefficient of the RPURF-EPS composites was ca. 30 [mW/m K)] (Malewska et al., 2012). RPURF-EPS had a relatively high thermal conductivity measured after 24 h. Thermal conductivity of foams changed gradually during long ageing. After the 50 days period of ageing, thermal conductivity of the reference foam raised above the RPURF-EPS composite value. The value of water absorption was similar to the reference foam (Malewska et al., 2011).
Understanding the foaming process is important because it determines the properties received foams. The process begins when isocyanate are mixed with so called polyol premix containing components with active hydrogen, catalysts, surfactant and blowing agents. In a short time the reaction mixture changes color and consistency, which indicates progress of the reaction (1). This reaction leads to the formation of PUR matrix (Kurańska and Prociak, 2016).
Water is often added into PUR formulation as a chemical blowing agent. Water reacts with isocyanate generating carbon dioxide (2). This reaction is a competitive reaction to the reaction of isocyanate and polyol (Falke et al., 1999).
The foaming process changes an initially homogeneous reaction mixture in the two-phase system consisting of PUR matrix which is solid and the gas in the foam cells (Falke et al., 1999).
In this paper the co-expansion process of PUR and PSB is analyzed in details, which have not previously been described in the literature. The results allow to get new knowledge and to understand better competitive processes, which take place during the co-expansion of two different polymers. Moreover, the influence of PSB size on the selected parameters of co-expansion process and final structure of obtained porous PUR-PS composites is discussed.
2 Experimental part
2.1 Materials
PUR systems consisted of the following raw materials:
Rokopol RF-551 is polyether obtained on the basis of modified sorbitol having a molecular weight ca. 600 g/mol, hydroxyl number LOH = 430 mg KOH/g. The polyol is produced by PCC Rokita SA,
Petol PM 500 3F is a polyether-type Mannich base, LOH = 480 mg KOH/g, which is produced by Oltchim SA,
Purocyn B polymeric methylene diphenydiisocyanate (PMDI) containing 31 wt.% of free isocyanate groups. Supplier: Alfa Systems Sp. z o.o.
DMCHA (dimethylcyclohexylamine) is a catalyst produced by Texaco Chemical Deutschland GmbH,
Kosmos 75 is a catalyst (potassium octoate) produced by Evonik Goldschmidt GmbH,
NIAX Silicone L-6900 is silicone oil that is not subject to hydrolysis. The surfactant is produced by Momentive Performance,
Solkane 365/227 is a physical blowing agent. It is a mixture of 1,1,1,3,3-pentafluorobutane and 1,1,1,2,3,3,3-heptafluoropropane in a mass ratio of 93 to 7, which is produced by Solvay Fluor GmbH,
Owipian® FS is expandable polystyrene, which is in the form of beads. It is produced by Synthos Sp. z o.o. from Poland. Owipian FS contains a flame-retardant (hexabromocyclododecane in an amount up to 0.5 wt.%) and a mixture of isomers of pentane as a blowing agent. Table 1 shows size of polystyrene beads (PSBs) of commercially available fractions. Table 2 shows the quantitative composition of the RPURF-EPS.
| Fraction symbol | ||||
|---|---|---|---|---|
| 0308 | 0513 | 0816 | 1325 | |
| Beads diameter (mm) | 0.4–0.7 | 0.7–1.0 | 1.0–1.6 | 1.6–2.4 |
| Ingredients | Weight (g) |
|---|---|
| Rokopol RF 551 | 70 |
| Petol PM 500 3F | 30 |
| Water | 2.0 |
| Kosmos 75 | 1.2 |
| DMCHA | 0.8 |
| NIAX Silicone L-6900 | 1.8 |
| Solkane HFC 365/227 | 30 |
| PSB | 130 |
| Purocyn B | 158 |
2.2 Preparation of the RPURF-EPS composites
All of the RPURF-EPS composite samples and reference RPURF were synthesized with a one-shot method at room temperature. The formulation used for composites preparation differed by the size of PSBs. The tests were performed with fractions 0308, 0513, 0816 and 1325, which were added in the amount of 44 mass% (resulted from previous experiments (Malewska et al., 2012; Malewska et al., 2011, 2013). Firstly, so called polyol premix was prepared using the following components: polyols, catalysts, water, blowing agent and surfactant which were mixed in a polypropylene cup with PSB. Then, an appropriate amount of isocyanate component was added to the polyol premix and vigorously stirred at 1 200 rpm for 10 s. After mixing, such composition was poured into a cardboard tube which was used as a mold.
2.3 Measurement methods
The traditional method characterizing PUR foaming process involves a determination of foam height in the cup or mold. In our research there was used a measuring device FOAMAT that allows conducting the analysis of parameters of the foaming process.
The foam qualification system FOAMAT consists of the ultrasonic fan sensor, the thermocouple, the pressure measurement and curing monitor devices (Fig. 1). The measurement sequence and the data processing are controlled by the software “FOAM”.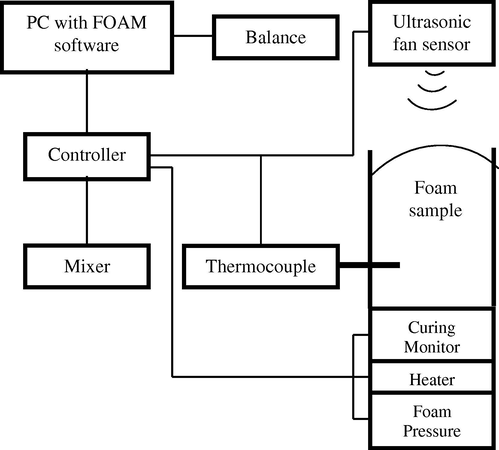
Scheme of Foamat device.
In order to analyze, polyurethane system is introduced to the cardboard tube about 25 cm high and 10 cm in diameter, placed on a heated base. Thermocouple is entered to the bottom of the tube to measure the core temperature of the foam material. The dielectric polarization sensor is located in the bottom also. Dielectric polarization is a measurement parameter that gives insight into the electrochemical processes occurring during foam formation. Dielectric polarization is essentially determined by chain-like molecules with a large dipole moment due to their polar ends (OH, NCO). Chain formation precedes the crosslinking reaction that ultimately suppresses all dipole mobility during curing. At the same time it is possible to measure changes in the height and speed of growth expanding polyurethane system. For this purpose, the cardboard tube is placed under ultrasonic sensor. Furthermore, it shows the conditions of temperature and pressure in which analysis of the foaming process was carried out (Hofmann, 2004).
The selected data allow to calculate the viscosity and rise velocity of reaction mixture, its contraction, as well as start, rise, gelling and curing times. These times are defined as follows: start time is the time after which the rise velocity increased to 15% of maximum rise velocity value, rise time is defined as the time after which the foam reached 98% of its final height, gel time is the time after which the pressure reaches 10% of its maximum value, curing time is the time after which the dielectric polarization is reduced to 10% of its initial value. The stages according to the special procedure during the composite synthesis and result analysis allow avoiding most errors caused by human factors, such as errors associated with weighing, mixing and measuring. The device is coupled to a computer with special software FOAM, which stores and processes the measurement results. Shrinkage was calculated as the ratio of the maximum and final height of the foam.
Additionally structure of prepared porous materials was evaluated using scanning electron microscopy (SEM). The volume of the EPS phase in the RPURF-EPS composites was assessed by the IMAGE programme, created especially for this task. The picture for that task were taken using a digital camera.
3 Result and discussion
3.1 The measurement of core temperature
The measurements of the temperature in reaction mixture cores of synthesized materials were carried out using a thermocouple that was placed at a height of 3 cm from the bottom of the mold. Changes in temperature recorded by Foamat are shown in Fig. 2. The highest maximum temperature (ca. 155 °C) was observed for RPURF without PS filler. In the case of RPURF-EPS the temperature achieved was ca. 95 °C, what was the effect of the heat from the polyurethane-forming reaction being partially used for PSBs expansion. This heat was consumed in order to melt PS and to evaporate a blowing agent contained within the PSBs. Additionally, it was observed that the temperature maxima were reached at different times depending on the type of the synthesized material. The temperature increase was significantly faster in the case of reference PUR foam in relation to the composite materials. After a period of ca. 200 s the temperature began to decrease. However, in the case of composites, the increase of temperature was slower and took place in ca 300 s.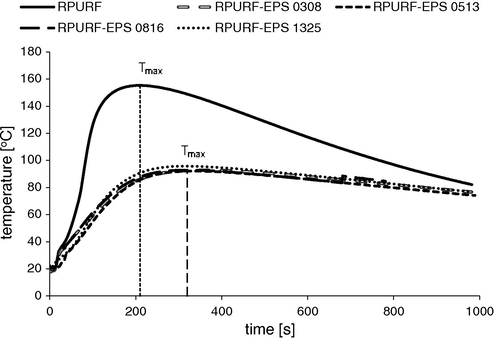
Changes of core temperatures during the synthesis of RPURF and RPURF-EPS.
3.2 The pressure measurement
During the foam rising the pressure exerted on the bottom of the mold by components of the PUR mixture was measured. The fastest pressure increase took place for PUR reference foam (Fig. 3). The maximum pressure of RPURF was obtained after 200 s, while for composite materials after ca. 300 s. These times correspond to the times of the maximum temperature of the proper reaction mixtures. Furthermore, the maximum pressure exerted on the bottom of the mold by composite materials which contained PS with beads of higher sizes, was higher than in the case of composites with the smallest PSBs (fraction 0308). The maximum pressure shift in time is caused by the presence of PSBs and a lower temperature effect slowing-down the exothermic reactions of RPURF synthesis. Fig. 2 shows also a characteristic momentary reduction of the pressure. Such pressure decrease can be caused by the partial opening PUR cells. A balance between the gelling and blowing reaction is extremely important during preparing PUR foams. This can be achieved by choosing a suitable amount of the catalysts. Pressure within the foam cells is increased during foaming reaction. At the same time the viscosity of the mixture increases because the polymer chains are extended. If the pressure is too large in relation to the viscosity of the reaction mixture walls of forming cells are broken and blowing agent running away outside, thereby reducing the pressure. Partial opening of cells can protect the material from shrinkage, however, it is not desirable because of possible deterioration of the thermal conductivity (Kurańska and Prociak, 2016; Choe et al., 2004).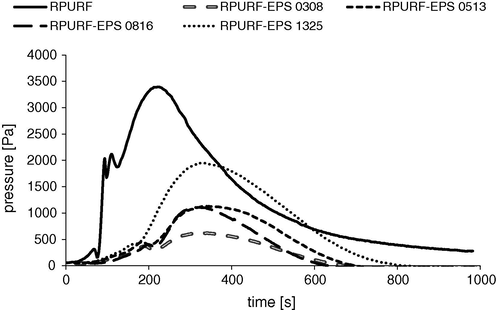
Pressure changes during the synthesis of RPURF and RPURF-EPS.
3.3 Measurement of the foam rise velocity
Foamat measuring device is equipped with an ultrasonic sensor, which makes it possible to record changes of the foam height vs. time. The foam rise velocity changes are presented in Fig. 4. The foam rising was the fastest for reference material and exceeded 3.5 mm/s. Composite materials regardless of the PSBs size fraction rose slower at a rate of ca. 1.5 mm/s. In addition, the PUR foam rising also ended in the shortest time. After 120 s the rise velocity decreased to zero, while for composite materials it ended after ca. 200–250 s. There were no significant differences in the rise velocity between the composites containing different fractions of the PSBs. This means that the addition of PSBs slows dynamics of the forming process of the porous material. However, the size of the PSBs does not significantly change the foam rise velocity.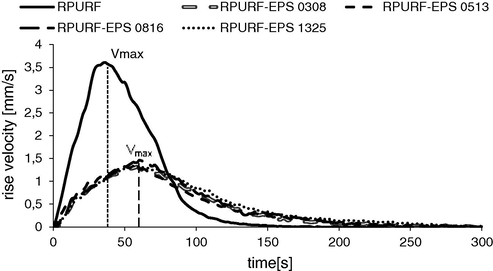
RPURF and RPUR-EPS rise velocity vs. time.
3.4 The dielectric polarization measurement
The results of dielectric polarization measurement are correlated with the presented results of core temperature changes and the foam rise velocity. The dielectric polarization is a parameter that gives information about the chemical processes occurring during the preparation of foams. The presence of free —OH and —NCO groups affects the high dipole moment value of the raw materials used for the preparation of PUR. After complete curing of the foam (when chemical reactions are close to finish) the dielectric polarization reaches a value close to zero. The comparison of dielectric polarization changes in time for RPURF and RPURF-EPS is presented in Fig. 5. The fastest decrease of dielectric polarization was observed for the reference foam. Dielectric polarization curve reached values close to zero after time of ca 80 s. In the case of reference PUR system foam rise was ended after a period of about 120 s, what means that a large majority of functional groups able to react with isocyanate is already bounded.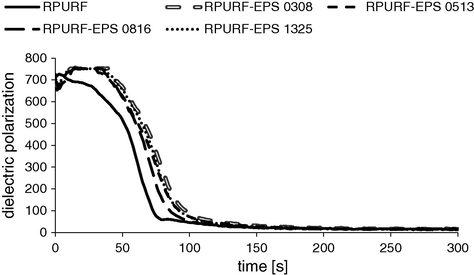
Dielectric polarization of RPURF and RPURF-EPS vs. time.
Dielectric polarization of the composite foams achieved the minimum value after ca. 100 s. This time was distinct from the time in which velocity rise of the composite material decreased to zero (250 s). This is caused by the presence of PSBs. PS is a non-polar material and during its expansion there are no chemical reactions that would affect the value of dielectric polarization. Therefore, PSBs dielectric polarization during the process of creating the composite has a constant value – zero. Consequently, the changes of dielectric polarization recorded during the preparation of the composite foams are only an effect of PUR matrix-forming reactions as well as the reactions of isocyanate with water. While, RPURF-EPS rise velocity changes are caused by the both chemical and physical effects in PUR and PS phases respectively.
It was concluded that in the porous composites, the finish rising PUR phase takes place when the PS phase is still expanded as an effect of elevated temperature of reaction mixture. PSBs increase their volume even after the end of PUR rise. Taking this into account, the composite expansion process was divided into three steps:
PUR phase rise – below the softening temperature of PSBs,
The simultaneous increase of the two phases - above the softening temperature of PSBs, but before the PUR rise end,
PS phase rise only – after the PUR rise end.
From a practical point of view, to achieve the composites with the possibly lowest apparent density, the step 2 in which both phases at the same time expands should be maximized. One method of extending the second step could be heating the polyol or isocyanate before the synthesis of PUR. This procedure should allow for faster expansion start of PSBs, which in the heated polyol or isocyanate be able reach their softening temperature faster.
Another possibility to extend the second step of co-expansion could be achieved by applying PSBs with lower softening temperature. Such PSBs will rapidly expand due to the start of expansion process at a lower temperature. Selection of a suitable catalyst system is also important. A suitable catalyst system allows proper temperature rise in the reaction mixture core, so that the PSBs had time to heat up to a temperature above the softening temperature before crosslinking PUR. The replacement of a chemical blowing agent with a physical blowing agent may also be considered.
3.5 Viscosity of reaction mixture measurement
Pressure measurement data allowed calculating the viscosity of expanding reaction mixture. This can be achieved by using the model of Hagen-Poiseuille viscosity (Hoffmann, 2000). The model is based on the idea that the viscosity is determined by the force required for moving the foam at a determined speed along the cylindrical mold. Pressure and rise data are sufficient to determine the change in viscosity as a function of time.
Fig. 6 shows the viscosity changes of the reaction mixtures. According to the observations, the viscosity of reference composition increases fastest comparing to composites PUR systems with PSBs, because the PUR reactions occur in the shortest time. The presence of PSBs in the reaction mixture affects the rate of chemical reactions slowing gelling ones. Reducing the viscosity of the RPUFR reaction mixture at time of ca. 100 s is a result of lower pressure in the bottom of the mold. It was caused by a partial opening of the cells in the foamed material. The viscosity of the reaction mixture was calculated directly from experimental data provided by Foamat. Obtaining data of pressure and velocity of the foam rise are sufficient to determine the changes of material viscosity as a function of time.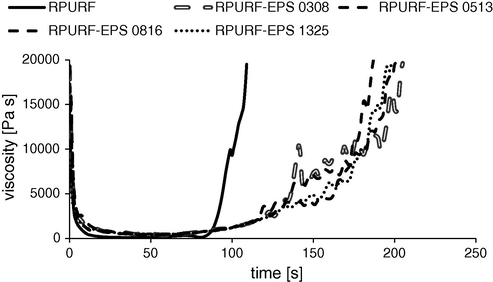
Viscosity of the reaction mixture during the creation of RPURF and RPURF-EPS.
3.6 Measurement of mass loss
Another parameter measured by Foamat is loss of mass. The mass changes of expanding reaction mixtures are shown in Fig. 7 as the percent of the total weight of the reactants. The loss of mass for various composite materials is comparable, while for the reference foam is significantly higher. However, taking into account the fact that for obtaining of the reference foam the used mass of PUR raw material (no addition of PSBs), was ca. two times higher it can be concluded that the loss of mass is generated mainly by the evaporation of volatile components of the PUR system, but not from PSBs.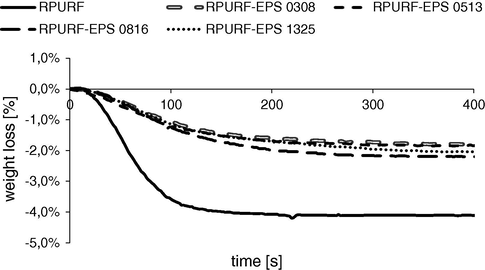
The loss of mass during the creation of RPURF and RPURF-EPS.
3.7 Measurement of the foaming process characteristic times and basic properties of the obtained porous materials
The analysis of the data from the Foamat measuring device allows determining the characteristic times of the expansion process. The values of such times are shown in Table 3. All times – start, rise, gelling and curing of composite materials are longer than of the reference foam. Start time is extended by a few seconds. The rise and gelling time increases twice. Extending these times confirms the fact that the addition of PSBs significantly slowed down the reaction of PUR creation.
Foam symbol
RPURF
RPURF-EPS 0308
RPURF-EPS 0513
RPURF-EPS 0816
RPURF-EPS 1325
Time (s)
Start
22
29
27
25
28
Rise
125
235
230
212
260
Gelling
59
59
116
114
128
Curing
126
132
121
122
133
Shrinkage (%)
0.45
0.4
0.35
0.27
0.25
Apparent density (kg/m3)
24.8
47.9
47.4
48.6
45.9
Through a detailed measuring the profile of materials rise vs. co-expansion process time it was also possible to estimate the shrinkage. All materials are characterized by low shrinkage value. However, it was observed that the presence of PSBs in the foamed compositions reduces the tendency of the final porous products to shrink.
However, the result presented show that the apparent density of RPURF-EPS composites is higher than in the case of RPURF. The composites with the addition of the largest PSBs (1325) were characterized by a little bit lower apparent density among RPURF-EPS materials. The composites apparent densities were considerably larger than in the case of reference material, what is mainly the thermal effect shown in Fig. 2.
3.8 Structure of RPURF-EPS
For the preparation of composites the same amount of PSBs differing from each other only by the size of beads was used. Therefore, it was suspected that the volume fraction of PS in each phase of the composite should be the same. However, it was found, that the PSBs fraction size affects the efficiency of expansion of beads. Fig. 8 presents cross-sections of the composites obtained using PSBs of different sizes.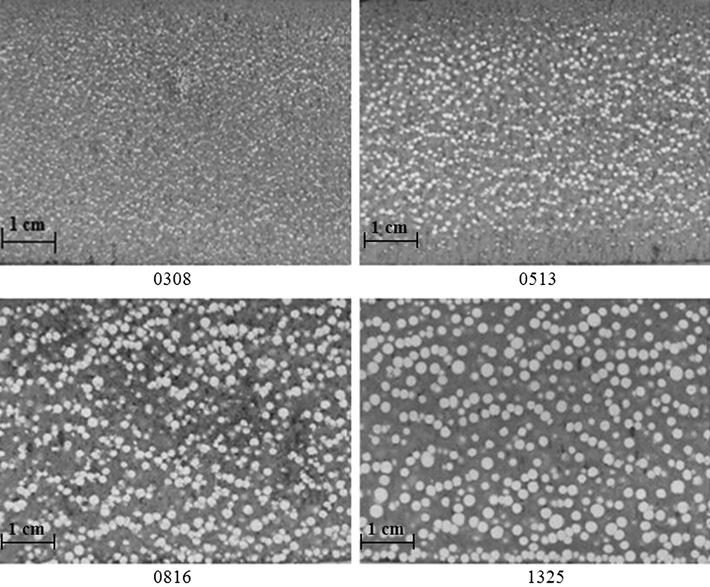
The RPURF-EPS cross-section structure.
Fig. 9 shows the influence of PSBs fraction size on the percentage contribution of the EPS fraction in the cross-section area. In all composites the same mass fraction of the PSBs was placed, but the different areas of the EPS were achieved depending on the initial size of used PSBs. The largest area fraction of the EPS is in the composite obtained using PSBs of the largest size (1325). Therefore, it can be concluded that the degree of expansion of PSBs in RPURF depends on the initial size of PSBs.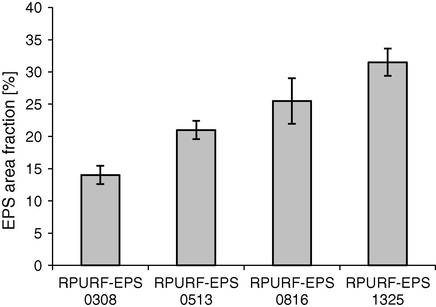
EPS area fraction in composites with PSB with different size.
Fig. 10 shows SEM images of expanded in hot water PSB morphology. It has a similar cell structure as beads contained in the composite, as shown in Fig. 11.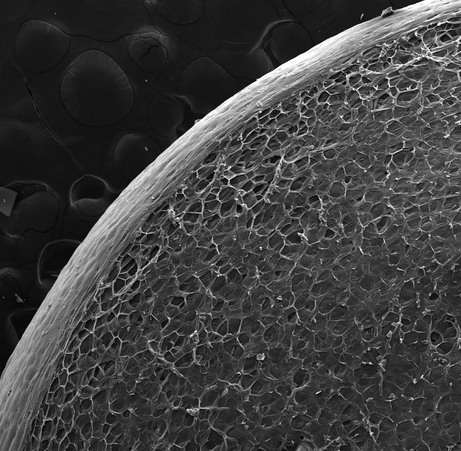
The SEM image expanded PSB.
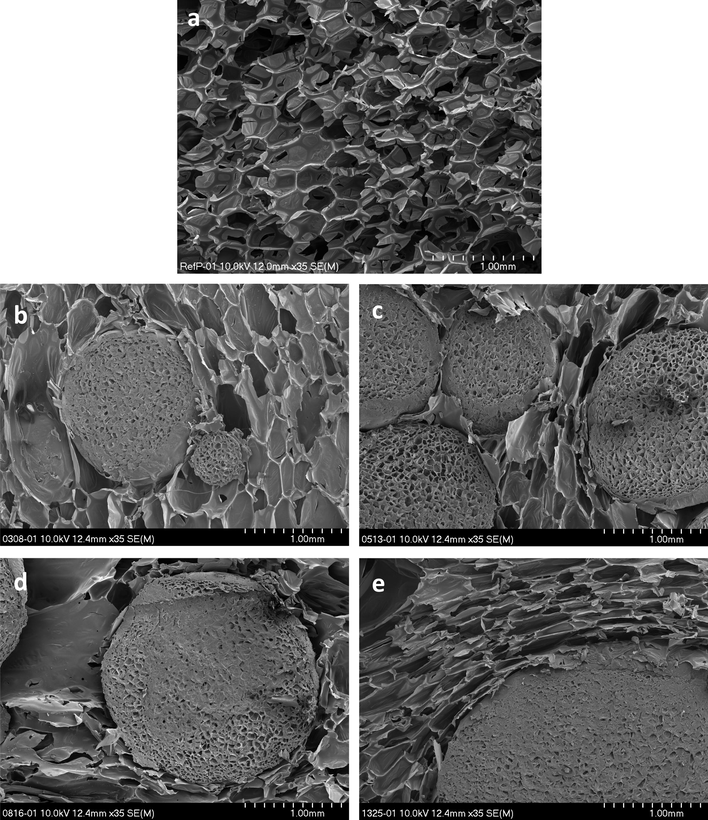
SEM microphotographs of the cross-sections of RPURF (a) and RPURF-EPS composites obtained using following fractions 0308 (b), 0513 (c), 0816 (d) and 1325 (e).
The cell structure of the reference foam containing no filler is shown in Fig. 11a. Generally, the cells have a regular structure and roughly spherical shape. Fig. 11b shows the porous composite obtained using the PSBs of the smallest sizes. It was observed that the smallest fraction of PSBs (0308) only slightly affects the surrounding of PUR cellular structure. PUR phase cells in RPURF-EPS composite with PSBs of 0308 fraction have a size and shape similar to the cells in RPURF. Cellular structure of composites containing the largest fraction of PSBs – 0816 and 1325 are shown in Fig. 11d and e. It was found that in these composites, fillers have a considerable influence on the cell structure of PUR phase. Cells near the EPS have elongated shape and are arranged along the edge of beads.
The ideal structure of polyurethane and polystyrene composite obtained by co-expansion, is one in which the EPS are expanded as much as possible (too low core temperature may cause the insufficient expansion of PSB). In addition, the expanding PSB should not affect the PUR cellular structure. Moreover, EPS should not be melted inside the PUR matrix, which may occur when the core temperature of the material is too high (Malewska et al., 2012).
4 Conclusions
The process of PUR and PSBs co-expansion is complicated. The addition of PSBs to a PUR composition influences on the foaming process. The addition of PS filler causes: a reduction of the core temperature, increasing the pressure, slowing the PUR reaction formation and the slowdown in the rise velocity of reaction mixture. It also causes the elongation of start, gelling, rise and curing times.
The presence of PSBs in the synthesis of porous composites beneficially influence on dimensional stability of such low density materials reducing a tendency to their shrinkage.
In contrast, the fraction size of PSBs does not significantly affect the foaming process. However, the size of PSBs influences on the structure of the final porous composites. The application of larger beads allows expanding PSBs more effectively, while the smallest beads achieve the smallest volume fraction in the co-expanded composite. Additionally, PSBs affect the structure of the PUR-PS porous composites. The larger initial size of PSBs, the greater disturbance of PUR structure takes place.
References
- Performance characteristics and practical applications of common building thermal insulation materials. Build. Environ.. 2005;40:353-366.
- [Google Scholar]
- Polym. J.. 2004;36(5):368.
- Modification of rigid polyurethane-polyisocyanurate foam with selected powder fillers. Polimery. 2008;2008(53):133-137.
- [Google Scholar]
- Some aspects of matrix formation of flexible polyurethane foams. J. Cell. Plast.. 1999;35:43-68.
- [Google Scholar]
- Bio-based polyurethane composite foams with inorganic fillers studied by thermogravimetry. Thermochim. Acta. 2005;431:155-160.
- [Google Scholar]
- Hoffmann, B.H.W., 2000. Viscosity Determination form rise profiles and pressure data. In: Polyurethane Conference 2000, Conference Proceedings, 8–11 October 2000, Boston, USA.
- Hofmann, B.H.W., Dec 2004/Jan2005. Meeting Product Quality Demands by Monitoring PU Foam Formation, vol. 21, Urethanes Technology, pp. 18–21.
- Traditional, state-of-the-art and future thermal building insulation materials and solutions – properties, requirements and possibilities. Energy Build.. 2011;43:2549-2563.
- [Google Scholar]
- The influence of rapeseed oil-based polyols on the foaming process of rigid polyurethane foams. Ind. Crops Prod.. 2016;89:182-187.
- [Google Scholar]
- Processing of polyurethane/polystyrene hybrid foam and numerical simulation. Fibers Polym.. 2002;3:159-168.
- [Google Scholar]
- The influence of expandable polystyrene fillers on properties of water-blown rigid polyurethane foams. Polimery. 2011;56:865-868.
- [Google Scholar]
- Physical and mechanical properties of rigid polyurethane foams modified with polystyrene beads. e-Polymers. 2012;055:1-10.
- [Google Scholar]
- The influence of apparent density of polyurethane matrix on selected properties of its foamed composites with expanded polystyrene. Modern Polym. Mater. Environ. Appl.. 2013;5:57-62.
- [Google Scholar]
- Effect of foam density on the properties of water blown rigid polyurethane foam. J. Appl. Polym. Sci.. 2008;108:1810-1817.
- [Google Scholar]
- Patent CA762531, 1967.
- Patent KR 20060071009, 2006.
- Patent KR 20060071440, 2006.
- Patent RP 189498, 2000.
- Patent RP 379672, 2006.
- Patent USA 20030181536, 2003.
- Patent USA 3607797, 1971.
- Patent USA 6605650, 2003.
- Patent USA 6727290, 2004.
- Mechanical properties of high density polyurethane foams: II effect of the filler size. Compos. Sci. Technol.. 2006;66:2709-2718.
- [Google Scholar]
- Synthesis and foaming of water expandable polystyrene–clay nanocomposites. Polymer. 2006;47:6303.
- [Google Scholar]







