Translate this page into:
Pre-column derivatization HPLC method for rapid and sensitive determination of free and total formaldehyde in hair straightening products
⁎Corresponding author at: College of Pharmacy, King Saud University, P.O. Box 22452, Riyadh 11495, Saudi Arabia. malshihri@ksu.edu.sa (Mona M. AlShehri)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
This paper describes development and validation of rapid and sensitive pre-column derivatization HPLC method to determine free and total formaldehyde content in five hair straightening products purchased from the local market. None of these products was labeled to contain formaldehyde and all of them were labeled with specific formaldehyde releasers. The formaldehyde derivative was separated using a µBondapak™ C18 column with mobile phase consisted of acetonitrile: water (70:30, v/v) and detected at 350 nm. The method was linear over the concentration range of 8–80 ng mL−1 (r = 09997) with detection limit of 0.8 ng mL−1. The formaldehyde derivative was stable for 30 days at room temperature. The results showed that all the samples were formaldehyde positive. The concentrations of free and total formaldehyde were 0.015–3.336 g% and 1.462–3.877 g%, respectively. The method showed high precision as the values of relative standard deviations (RSD) did not exceed 2.20%.
Keywords
Formaldehyde
Formaldehyde-releaser
Pre-column derivatization
HPLC
Hair straightening products
1 Introduction
Cosmetic products are widely used by consumers on a daily bases. Due to its bactericidal and fungicidal properties, formaldehyde is used as a preservative in these products. Formaldehyde-releasers, which slowly release formaldehyde under usage conditions, are also commonly used to preserve cosmetics. The antimicrobial activity of these releasers is possibly due to the released formaldehyde (De Groot et al., 2009; Wu et al., 2003; Sweetman, 2011; Golden and Valentini, 2014), which increased with increasing the pH, the temperature of the solution, and the storage period (Lv et al., 2015). The EU working party (WP) on Methods of Chemical Analysis of Cosmetic Products has observed that formaldehyde-releasers release formaldehyde, and they do not remain as a single compound, and suggested that they may be regulated based on their formaldehyde content (European Commission, 22nd plenary meeting, 2002).
Exposure to formaldehyde through using these products can be harmful to consumers because it affects human eyes, skin, and respiration system (Salthammer, 2015). A positive relation between exposure to formaldehyde and cancer has been observed, and it has been classified as Group 1 human carcinogen by the International Agency for Research on Cancer (IARC) (IARC Monographs, 2012). Different regulations have been established to regulate the use of formaldehyde in cosmetics. The US Cosmetic Ingredient Review (CIR) Expert Panel stated that formaldehyde concentration in cosmetics should not exceed 0.2% (w/w), and products containing more than 0.05% formaldehyde must be labeled ‘contains formaldehyde’ (Boyer et al., 2013).
One of the most widely used cosmetics is hair straightening product. It is used to straighten curly hair by using products containing formaldehyde or formaldehyde-releasers. This process leads to permanent breakage of the disulfide bonds that maintain the dimensional structure of the hair (Miranda-Vilela et al., 2014; Da Gama et al., 2017).
HPLC method based on pre-column derivatization was adopted as one of the most frequently used methods for determination of formaldehyde. Pre-column derivatization with 2,4-dinitrophenylhydrazine reagent has been shown to be a powerful technique for selectively enhancing the detectability of formaldehyde using HPLC analysis. 2,4-dinitrophenylhydrazine reagent selectively condenses with formaldehyde to produce a stable hydrazone derivatives. This method is one of the most reliable methods to determine formaldehyde in cosmetic products (Rivero, and Topiwala, 2004; Maneli et al., 2014).
A lot of hair straighteners containing a high unlabeled amount of formaldehyde or formaldehyde-releasers are available in the market. Therefore it is very important to human health and to the environment to develop a method for determination of formaldehyde in these products. The present work was intended to develop and validate a simple, rapid, and sensitive analytical method to determine the free and total formaldehyde in 5 hair straightening products collected from the local market using HPLC method after pre-column derivatization with 2,4-dinitrophenylhydrazine reagent.
2 Experimental
2.1 Chemicals and reagents
Formaldehyde 37% standard solution was purchased from PACEGROVE LIMITED (UK). The 2,4-Dinitrophenylhydrazine reagent was purchased from BDH Chemicals Ltd (UK). HPLC grade acetonitrile and ethanol were purchased from SIGMA-ALDRICH (France). Sulphuric acid 98% was purchased from S.D. Fine Chem. Ltd. (India). Hair straightener products were purchased from the local market. Deionized water and a Millipore membrane filter (0.2 mm) from Nihon, Millipore were used throughout the experiments.
2.2 Instrumentation and chromatographic conditions
The development and validation of the method was performed on Waters HPLC instrument equipped with Waters 1525 Binary Pump, Waters 2489 Ultraviolet/Visible detector (UV/Visible), and Waters 2707 Autosampler (WATERS, USA). The data handling system comprised of a Dell personal computer and Breeze 2 software. The stationary phase used was µBondapak™ C18 (3.9 × 150 mm, 10 µm) column. The mobile phase consisted of acetonitrile: Water (70:30, v/v). The mobile phase was filtered through MS®-nylon membrane filter (pore size 0.45 μm, diameter 4.7 cm, Membrane solution, USA) and degassed before use. The detection wavelength was 350 nm with a flow rate of 1 mL/min.
2.3 Preparation of reagent (Brady’s reagent)
Brady’s reagent was prepared by dissolving 8.0 g of 2,4-dinitrophenylhydrazine in 40 mL of sulfuric acid (98%), then 60 mL of water was added gradually. The mixture was stirred, and then 200 mL of ethanol was added to it and stored until use.
2.4 Preparation of hydrazone derivative
In a test tube 0.5 mL of 37% formaldehyde solution was transferred with 2 mL of ethanol, then 3 mL of the 2,4-dinitrophenylhydrazine reagent was added. A yellow precipitate was formed immediately, and kept at room temperature for 15–20 min. The precipitate was filtered, and then washed with 3 × 1 mL ethanol. After complete dryness, the precipitate weighed then dissolved in 100 mL acetonitrile.
2.5 Preparation of stock solution
An exactly weighed amount (0.1 g) of the hydrazone derivative was transferred into a 100-ml volumetric flask, dissolved in 20 mL acetonitrile, completed to volume with the same solvent to obtain a stock solution of 1 mg mL−1. The stock solution could be diluted with acetonitrile as needed.
2.6 Preparation of quality control samples
The quality control (QC) samples at three concentrations i.e. 20, 40, 60 ng mL−1 were prepared from the stock solution by suitable dilutions with acetonitrile.
2.7 Preparation of sample solutions
2.7.1 Free formaldehyde
One milliliter of each sample was transferred to test tube and mixed with 2 mL of ethanol and 3 mL of 2,4-dinitrophenylhydrazine reagent. A large quantity of yellow precipitate was formed immediately. The mixture kept at room temperature for 15–20 min before collecting the solid product. The precipitate was filtered then washed with 3 × 1 mL ethanol. After the precipitate has been dried completely, it was dissolved in 100 mL acetonitrile. The sample solutions were diluted to suitable concentrations and injected into the HPLC system in triplicate under the optimized chromatographic conditions.
2.7.2 Total formaldehyde
To confirm the presence of formaldehyde-releasers and determine the total formaldehyde present in the hair straightener samples, 1 mL of each sample was mixed with 2 mL of ethanol and heated for 30 min at 95 °C. After cooling, 3 mL of 2,4-dinitrophenylhydrazine reagent was added and the resulted precipitate was treated as described above. The sample solutions were diluted to suitable concentrations and injected into the HPLC system in triplicate under the optimized chromatographic condition.
3 Result and discussion
The initial method development was conducted using standard solutions of the hydrazone derivative. The chromatographic parameters were preliminarily optimized to develop an HPLC method with pre-column derivatization for the determination of free and total formaldehyde in its standard solution and in hair straightening products.
3.1 Method optimization
The effects of changing the type of the mobile phase as well as the ratio were studied. First the analyte was eluted with methanol: water (80:20, v/v). The retention time was 2.13 min, but with very broad and tailed peak. When a mobile phase consisting of acetonitrile: water (60:40, v/v) was used, the peak was sharp and eluted at 2.84 min. The percentage of acetonitrile added to the mobile phase was also studied over the range 50–70%. Late retention time was observed at 50% acetonitrile. As the percentage increased, the analyte eluted earlier, therefore acetonitrile: water (70:30, v/v) was chosen as the optimum mobile phase.
The detection wavelength was 350 nm as the formaldehyde derivative showed maximum intensity at this wavelength, and the flow rate was 1 mL/min.
Finally separation of formaldehyde derivative was carried out under the above optimized conditions and the retention time was 2.17 ± 0.04 min (Fig. 1).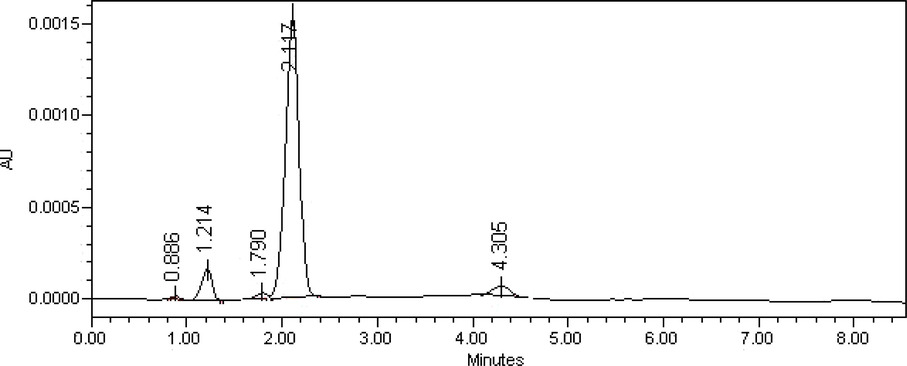
Representative chromatogram of derivatized formaldehyde standard solution (80 ng mL−1) obtained under the optimized HPLC conditions.
3.2 Method validation
The main objective of validation of an analytical method is to prove that this method is suitable for its proposed purpose. Therefore, the HPLC method developed for the determination of formaldehyde in this study was validated according to the ICH guidelines (ICH, 2005).
3.2.1 Linearity and range
The linearity of the method was assessed by constructing five points calibration curve. Excellent linearity was observed over the concentration range of 8–80 ng mL−1. The peak areas of the standard versus concentrations were plotted and a least-square regression analysis was conducted to prove the linearity of the method. The correlation coefficient (r) was found to be 0.9997. The linear regression analysis data are summarized in Table 1.
Parameters
Formaldehyde derivative
Linear range (ng mL−1)
8–80
Regression equation, Ya = BXb + A
Y = 149.67 X + 3982.9
Correlation coefficient (r)
0.9997
Sy/x
103.53
Sa
87.07
Sb
1.77
Limit of detection, LOD (ng mL−1)c
0.8
Limit of quantitation, LOQ (ng mL−1)d
8
3.2.2 Accuracy and precision
Accuracy and precision should be reported as percentage error (% E) and relative standard deviation (% RSD), respectively, and should be established across the range of the developed method. They were determined by the analysis of the three QC samples over a period of 3 days. The within-day accuracy and precision were calculated from the results of the analysis of the three concentrations on one day. The between-day accuracy and precision were determined from the same three concentrations analyzed on three successive days. All accuracy and precision values were within the limits considered acceptable. The within-day and between-day relative standard deviation ranged between 0.34% and 2.20%, while the % recovery ranged from 100.90% to 101.95% (Table 2).
Actual conc. (ng mL−1)
Experimental conc. (ng ml−1)
Recovery (%)
RSD (%)a
Error (%)b
Intradayc
20
20.33 ± 0.07
101.65
0.34
1.65
40
40.40 ± 0.66
101.00
1.63
1.00
60
60.54 ± 0.49
100.90
0.81
0.90
Interdayd
20
20.39 ± 0.12
101.95
0.59
1.95
40
40.39 ± 0.45
100.98
1.11
0.98
60
60.78 ± 1.34
101.30
2.20
1.30
3.2.3 Limit of quantitation and limit of detection
The limit of detection (LOD) and the limit of quantification (LOQ) were determined as 3 and 10 times the baseline noise, respectively. The LOD obtained was 0.8 ng mL−1 and the LOQ was 8 ng mL−1 (Table 1).
3.2.4 Stability of standard solution
The stability of the standard solution of the hydrazone derivative was tested by the proposed HPLC method over a period of 30 days. A freshly prepared solution at room temperature and a 30-day-stored sample at room temperature were analyzed by the proposed HPLC method. The concentration of the hydrazone derivative in the stored sample were calculated and compared to that in the freshly prepared sample.
The hydrazone derivative was stable under this condition. No additional peaks were found in the chromatogram throughout the analysis time, indicating the stability of the derivative in standard solution.
3.3 Determination of formaldehyde in hair straightening products
Five hair straightening products of different international brands collected from the local market in Riyadh, Saudi Arabia, were analyzed to determine formaldehyde in their contents. These hair straighteners were suspected to contain concentrations of formaldehyde higher than those allowed by regulation, because it was highly recommended for their good straightening effect. None of them were labeled ‘formaldehyde free’ or ‘contain formaldehyde’.
To all the samples, 2,4-dinitrophenylhydrazine reagent was added and a yellow precipitates (hydrazone derivative) were formed, which confirm the presence of formaldehyde. The precipitates were treated as prescribed under the experimental section. The final solutions were analyzed by the validated HPLC method under the optimized conditions. At high temperatures, such as that occur during hair treatment, formaldehyde is released. Therefore, to evaluate the release of formaldehyde from formaldehyde-releasers present in the tested samples, aliquots from these samples were heated at 95 °C for 30 min. After cooling, the samples were treated as prescribed under the experimental section, and then analyzed.
The results indicate the presence of free formaldehyde, and total formaldehyde which released from the tested samples after heating (Fig. 2). Formaldehyde concentrations in the samples were obtained by substituting their peak areas into the regression equation. The concentration of formaldehyde was <0.2% in sample no. 1 and 4, while sample no. 2, 3, and 5 contained very high concentration. After heating, all samples released a high concentration of formaldehyde that exceeded the allowed limit (Table 3).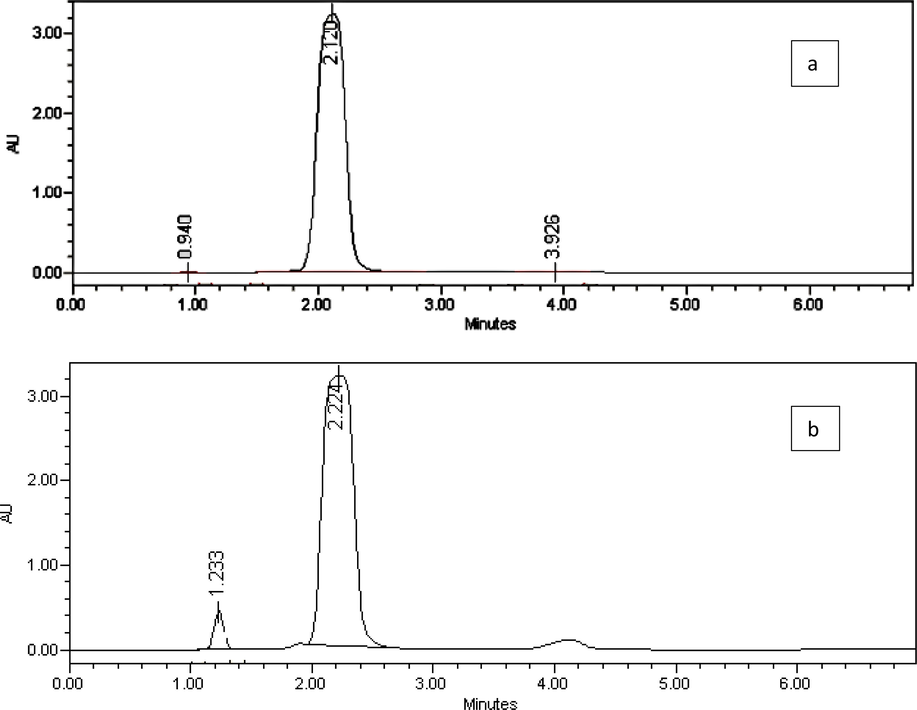
Representative chromatograms of derivatized formaldehyde in sample no. 3 before heating (a), and after heating (b) obtained under the optimized HPLC conditions.
Sample no.
Free formaldehyde derivative conc. (g%)
Total formaldehyde derivative conc. (g%)
1
0.015
1.462
2
3.271
3.877
3
3.336
3.810
4
0.034
1.465
5
3.302
3.742
4 Conclusion
A simple, rapid, and sensitive HPLC method for the determination of free and total formaldehyde in hair straightening products was developed and validated. The developed HPLC method proved to be linear, accurate, precise, sensitive, and stable at room temperature for at least one month. It was successfully applied for the determination of free formaldehyde and total formaldehyde that released from heated hair straightening products. The presence of hair straightening products that release high amounts of formaldehyde in the local market was confirmed. These products could create a potential risk on consumers, and need to be regulated.
Acknowledgements
This research project was supported by a grant from the “Research Center of the Female Scientific and Medical Colleges”, Deanship of Scientific Research, King Saud University.
References
- Amended safety assessment of formaldehyde and methylene glycol as used in cosmetics. Int. J. Toxicol.. 2013;32(Suppl. 6):5S-32S.
- [Google Scholar]
- In vitro methodologies to evaluate the effects of hair care products on hair fiber. Cosmetics. 2017;4:2-10.
- [Google Scholar]
- Formaldehyde-releasers: relationship to formaldehyde contact allergy. Contact allergy to formaldehyde and inventory of formaldehyde-releasers. Contact Dermatitis. 2009;61:63-85.
- [Google Scholar]
- European Commission, 2002. The Determination of Certain Formaldehyde Releasers in Cosmetic Products. Scientific commIttee on Cosmetic Products and Non-food Products Intended for Consumers, 22nd Plenary Meeting.
- Formaldehyde and methylene glycol equivalence: critical assessment of chemical and toxicological aspects. Regul. Toxicol. Pharmacol.. 2014;69:178-186.
- [Google Scholar]
- Chemical agents and related occupations, a review of human carcinogens. IARC Monogr. Eval. Carcinogen. Risks Hum.. 2012;100F:401-435.
- [Google Scholar]
- ICH Q2 (R1), 2005. Validation of analytical procedures: text and methodology. In: Proceeding of the International Conference on Harmonization, Geneva.
- Investigation on formaldehyde release from preservatives in cosmetics. Int. J. Cosmet. Sci.. 2015;37:474-478.
- [Google Scholar]
- Elevated formaldehyde concentration in “Brazilian keratin type” hair-straightening products: a cross-sectional study. J. Am. Acad. Dermatol.. 2014;70:276-280.
- [Google Scholar]
- An overview of chemical straightening of human hair: technical aspects, potential risks to hair fiber and health and legal issues. Int. J. Cosmet. Sci.. 2014;36:2-11.
- [Google Scholar]
- Quantitative determination of formaldehyde in cosmetics using a combined solid-phase microextraction–isotope dilution mass spectrometry method. J. Chromatogr. A. 2004;1029:217-222.
- [Google Scholar]
- Martindale: The Complete Drug Reference (37th ed.). London: Pharmaceutical Press; 2011.
- Determination of formaldehyde in cosmetics by HPLC method and acetylacetone method. J. Food Drug Anal.. 2003;11:8-15.
- [Google Scholar]







