Translate this page into:
Effect of functionalization of iron oxide nanoparticles on the physical properties of poly (aniline-co-pyrrole) based nanocomposites: Experimental and theoretical studies
⁎Corresponding author. e.nazarzadeh@du.ac.ir (Ehsan Nazarzadeh Zare)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Poly(aniline-co-pyrrole)@functionalized Fe3O4 (PACP@f-Fe3O4) nanocomposites were prepared by a two-step method. In the first step, the Fe3O4-OH and Fe3O4-NH2 nanoparticles were synthesized by the solvothermal and co-precipitation techniques, respectively. In the second step, the PACP@f-Fe3O4 nanocomposites were synthesized by an in-situ microemulsion polymerization technique. The synthesized materials were characterized by Fourier transform infrared (FTIR) spectroscopy, X-ray diffraction (XRD), scanning electron microscopy (SEM), and thermogravimetric analysis (TGA). Based on the SEM, the iron oxide changed the morphology of the PACP copolymer from being completely nanospheres. The TGA showed the higher thermal stability of the PACP@Fe3O4-OH nanocomposite in comparison to the PACP@Fe3O4-NH2, and the density functional theory (DFT) successfully confirmed this fact by calculating the binding energies between the PACP copolymer and functionalized nanoparticles. Also, the HOMO − LUMO energy gap ( ) values were determined by the DFT to investigate the electrical conductivities, which are in accord with the experimental electrical conductivities in the order PACP@Fe3O4-OH > PACP@Fe3O4-NH2 > PACP.
Keywords
Poly(aniline-co-pyrrole)
Functionalized Fe3O4
Nanocomposite
Electrical conductivity
Density functional theory
1 Introduction
Conducting polymers including polyaniline (PAni), polypyrole (PPy), polythiophene (PTh), and polyfuran (PFu) have been attractive for academic centers due to their unique properties, such as environmental stability, electrical conductivity, electronic, optical, and redox properties (De Souza, 2007; Lu et al., 2007; Roković et al., 2007; Segal et al., 2005; Xiao et al., 2005). However, they suffer from the disadvantages of low solubility or insolublility in most common solvents, poor processability, and the decrease of electrical conductivity over a long period of time (Rao and Sathyanarayana, 2002). A number of attempts, including copolymerization of conducting monomers with substituted or unsubstituted monomers, have been so far made to overcome these disadvantages and enhance the solubility and processability (Pandey et al., 1993). A number of polymer based nanocomposites have been introduced for the applications in the various fields such as, EMI shielding, biomedical, sensing and etc. (Ding et al., 2018; Guan et al., 2016; Hu et al., 2018, 2017; Li et al., 2018; Lin et al., 2018; Liu et al., 2016, 2014; Zhang et al., 2017).
On the other hand, another approach for improving the processability and physical properties of the conducting polymers is the preparation of composites based on conducting polymers (Laska et al., 1997). For instance, Li et al. reported the facile synthesis and intrinsic conductivity of novel pyrrole copolymer nanoparticles with inherent self-stability (Li et al., 2007). Moghadam et al. studied the sonochemical synthetic methods to produce functionalized conducting copolymers of aniline (Moghadam et al., 2010). Bijleveld et al. synthesized the copolymers of diketopyrrolopyrrole and thienothiophene for photovoltaic cells (Bijleveld et al., 2011). Tanne et al. synthesized the poly [(3-aminobenzoicacid)-co-(3-aminobenzenesulfonic acid)-co-aniline] terpolymer for the immobilization of redox active cytochrome c (Tanne et al., 2014). Varshney et al. prepared the polypyrrole-γ-Fe2O3-Fly ash nanocomposites for the protection against EMI pollution (Varshney et al., 2014). Rossetto et al. reported synthesis, characterization and toxicological evaluation of a core–shell copper oxide/polyaniline nanocomposite (de OF Rossetto et al., 2014). Ruecha et al. reported a sensitive electrochemical sensor using a graphene–polyaniline nanocomposite for simultaneous detection of Zn(II), Cd(II), and Pb(II) cations (Ruecha et al., 2015). Kumar et al. synthesized the polyaniline/f-MWCNT nanocomposite coatings on mild steel for the corrosion protection in 3.5% NaCl solution (Madhan Kumar and Gasem, 2015). Liu et al. synthesized polypyrrole nanocomposites decorated with silver nanoparticles with electrocatalytic and antibacterial properties (Liu et al., 2015). Wang et al. reported a facile method for the synthesis of polypyrrole decorated reduced graphene oxide–Fe3O4 magnetic composites for the removal of Cr(VI) (Wang et al., 2015). Our research group has also developed extensive research works to improve the application of conducting polymers in biosensors, anti-corrosion coatings, medicine, removal of heavy metal ions and catalyst (Baghayeri et al., 2015; Fard et al., 2017; Lakouraj et al., 2014; Moghadam et al., 2012; Zare and Lakouraj, 2014; Zare et al., 2016b, 2015a, 2015b, 2016a).
Iron oxide magnetic nanoparticles have received a great deal of research attention because of their facile synthesis routes and their unique applications in physics, materials science, biology and specially in biomedical applications including magnetic enzyme immobilization, drug targeting, and magnetic resonance imaging contrasting agent (Wu et al., 2015). Furthermore, according to our previous publications, iron oxide can greatly enhance the electrical conductivity and thermal stability of composites (Lakouraj et al., 2014; Zare et al., 2015a).
A literature survey shows that there is no theoretical investigation dealing with the conductivity of poly (aniline-co-pyrrole) (PACP), and its nanocomposites containing functionalized iron oxide. Therefore, in this study, at first the poly(aniline-co-pyrrole)@functionalized Fe3O4 (PACP@f-Fe3O4) nanocomposites were synthesized by microemulsion technique and characterized by transform infrared spectroscopy (FT-IR), X-ray diffraction (XRD), scanning electron microscopy (SEM), and thermogravimetric analysis (TGA). Then, a DFT study was performed on the systems to have an insight on the electrical conductivity and interactions between the constituent components. The theoretical obtained data were in agreement with the experimental results. DFT calculations have been also used for other applications (Sun et al., 2017; Zhao et al., 2017).
2 Methodology
2.1 Experimental
2.1.1 Materials
Aniline (Ani) and pyrrole (Py) were purchased from Merck-Germany and purified by double distillation under the reduced pressure and stored in a refrigerator before use. Triton X-100 (TX-100) was purchased from Aldrich (St. Louis, USA). Ammonium persulfate (APS), ammonium hydroxide, ferric chloride hexahydrate, ferric chloride tetrahydrate, anhydrous sodium acetate, ethylene glycol, ethylenediamine and the used solvents were all purchased from Merck (Darmstadt, Germany). All these chemicals were of analytical grade and used as received without further purification.
2.1.2 Characterizations
FTIR spectra of the synthesized materials were taken by Bruker Tensor 27 spectrometer (Bruker, Karlsrohe, Germany) in 400–4000 cm−1 region using KBr pellets. The XRD patterns were recorded on an X’pert Philips X-ray photoelectron spectrometer (Netherlands) with non-monochromated Mg Kα radiation as the excitation source. The surface morphology of the materials was examined by scanning electron microscopy (SEM) (Hitachi S4160 instrument). Thermogravimetric analysis (TGA) of the prepared materials was investigated using a LENSES STAPT-1000 calori-meter (Germany) by scanning up to 750 °C at a heating rate of 10 °C/min under N2 atmosphere. The electrical conductivity of the prepared PACP@f-Fe3O4 nanocomposites was measured by a standard four-probe method (Azar Electrode Comp., Tabriz, Iran) under laboratory conditions.
2.1.3 Synthesis of nanoparticles
The Fe3O4-OH nanoparticles were synthesized by the co-precipitation technique using ferric chloride hexahydrate and ferric chloride tetrahydrate (molar ratio of 2:1), according to our previous reported method (Zare et al., 2015a).
Amine-functionalized iron oxide magnetite nanoparticles (Fe3O4-NH2) were synthesized by a solvothermal method (Jin et al., 2012). FeCl3·6H2O (1 g), ethylenediamine (6 g), and anhydrous sodium acetate (2 g) were added to ethylene glycol (40 mL) to give a clear solution via vigorous stirring. This mixture was then transferred to a Teflon-lined autoclave (50 mL) and treated at 200 °C for 6 h. The precipitate was collected by an external magnetic field and washed with deionized water. Finally, the nanoparticles were stored in distilled water for further processing.
2.1.4 Synthesis of poly (aniline-co-pyrrole) based nanocomposites containing functionalized iron oxide
The poly (aniline-co-pyrrole)@hydroxyl functionalized iron oxide (PACP@Fe3O4-OH) and poly (aniline-co-pyrrole)@amine functionalized iron oxide (PACP@Fe3O4-NH2) nanocomposites were synthesized by an in-situ microemulsion polymerization technique as follows. At first, the functionalized iron oxide nanoparticles (Fe3O4-OH or Fe3O4-NH2) (0.5 g) were dispersed in TX-100 solution (25 mL) under ultrasonication for 1 h to obtain a uniform magnetic fluid. Then, a mixture of Py and Ani with feed molar ratio of 1:1 was added to the above mixture and the suspension was ultrasonicated for 30 min. The mixture was kept at the temperature range of 0–5 °C for 30 min, and then 30 mL of APS solution (0.4 M) was added to initiate the polymerization, which can complete without agitation at room temperature for 24 h. The reaction mixture was filtered, rinsed with distilled water and acetone. Finally, the obtained magnetic dark powder was dried in a vacuum oven at 50 °C for 24 h. For a comparative study, the PACP copolymer was also synthesized via an in-situ microemulsion polymerization according to our previous report (Zare et al., 2015b).
2.2 Computational details
Geometry optimization was carried out using a high quantum level of DFT-D approach implemented in the DMOL3 program. Effective core potential (ECP) calculations were performed by a generalized gradient approximation (GGA) with Perdew, Burke and Enzerhof (PBE) functional (Perdew et al., 1996, 1992) and the double numerical plus polarization function (DNP) basis set (Benedek et al., 2005) with global cutoff radius of orbital at 4.5 Å under spin-unrestricted condition and automatic option for multiplicity. All electrons were used to treat the core electrons and the tolerance of the energy change was 10−6 Hartree. The DMOL3 numerical frequency analysis was performed to ensure reaching the global minimum. For all the interacting systems, the basis set superposition error (BSSE) was evaluated by means of the counterpoise method (Boys and de Bernardi, 1970).
3 Results and discussion
3.1 FTIR analysis
Fig. 1 shows the FTIR spectra of PACP@Fe3O4-NH2, and PACP@Fe3O4-OH nanocomposites. In the FTIR spectrum of the PACP copolymer (not shown here) the peaks at 1575, 1564, 1160, 1110 and 690 cm−1 were given to the C—C stretching vibration for the quinonoid phenyl rings of polyaniline, C⚌C stretching mode of polypyrrole, existence of aromatic NH groups, C—N stretching vibration and C—H in-plane bending in the 1,3-substituted benzene ring, respectively (Zare et al., 2015b). The characteristic peaks of Fe3O4-NH2 and Fe3O4-OH were not observed in the PACP@Fe3O4-NH2 and PACP@Fe3O4-OH nanocomposites, indicating that the Fe3O4 nanoparticles were fully encapsulated in the PACP nanospheres. On the other hand, the existence of main peaks corresponding to the PACP, with slightly shift to lower wavenumbers in the PACP@Fe3O4-NH2 and PACP@Fe3O4-OH nanocomposites confirmed the successful synthesis of nanocomposites. A reasonable explanation for this issue may be attributed to the formation of hydrogen bonding between the N atoms of pyrrole or aniline in the PACP and hydroxyl or amine groups in the Fe3O4. These results are in agreement with the previous reported composites containing Fe3O4-NH2 and Fe3O4-OH (Zare et al., 2015b; Jin et al., 2012).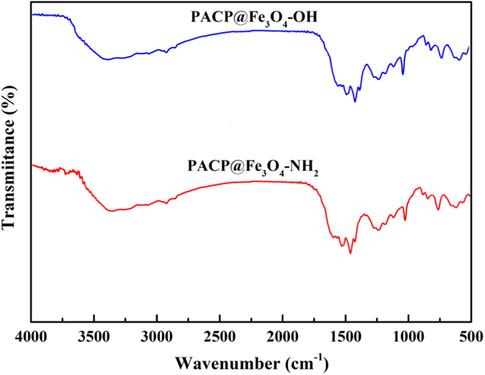
FTIR spectra of PACP@Fe3O4-NH2 and PACP@Fe3O4-OH nanocomposites.
3.2 XRD analysis
In the pattern of the typical X-ray diffraction (XRD) of Fe3O4-OH and Fe3O4-NH2 magnetic nanoparticles, the peaks match well with pure magnetite reflections, which are consistent with the results of Zare and Jin, respectively (Jin et al., 2012; Zare et al., 2015a). On the other hand, the XRD pattern of PACP exhibited an amorphous nature (Zhu et al., 2012). The presence of the characteristic peaks of Fe3O4-OH, Fe3O4-NH2 and PACP were clear in the XRD patterns of the synthesized PACP@Fe3O4-NH2 and PACP@Fe3O4-OH nanocomposites (Fig. 2). Hence, it can be concluded that PACP@Fe3O4-NH2 and PACP@Fe3O4-OH nanocomposites were successfully synthesized.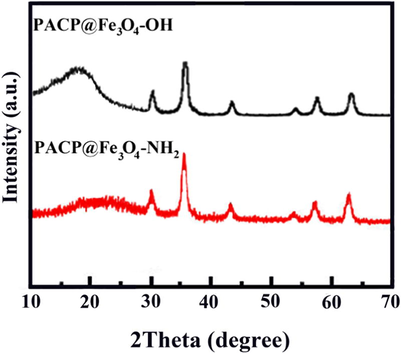
XRD patterns of the PACP@Fe3O4-NH2 and PACP@Fe3O4-OH nanocomposites.
3.3 SEM analysis
According to the reported works, the SEM images of the Fe3O4-OH and Fe3O4-NH2 nanoparticles showed structures almost uniform in shape and size (average diameter of 50 nm) (Jin et al., 2012; Lakouraj et al., 2014). Our previous work revealed that PACP had a well-defined nanosphere structure with an average diameter of 90 nm (Zare et al., 2015b). Also, in our pervious experiments, we showed that TX-100, as a non-anionic surfactant, and the concentration of co-monomers can play an important role on the formation of PACP nanospheres. In the absence of TX-100, the polymerization of co-monomers produced irregular particulates. The optimum molar ratios for Py: Ani: TX-100 was 1:1: 0.00003 (Zare et al., 2015b).
The SEM images of the PACP@Fe3O4-NH2 and PACP@Fe3O4-OH nanocomposites (Fig. 3) showed that the particles, with the average diameter of about 100–150 nm, were not completely nanospheres; and were rather rougher than the PACP particles.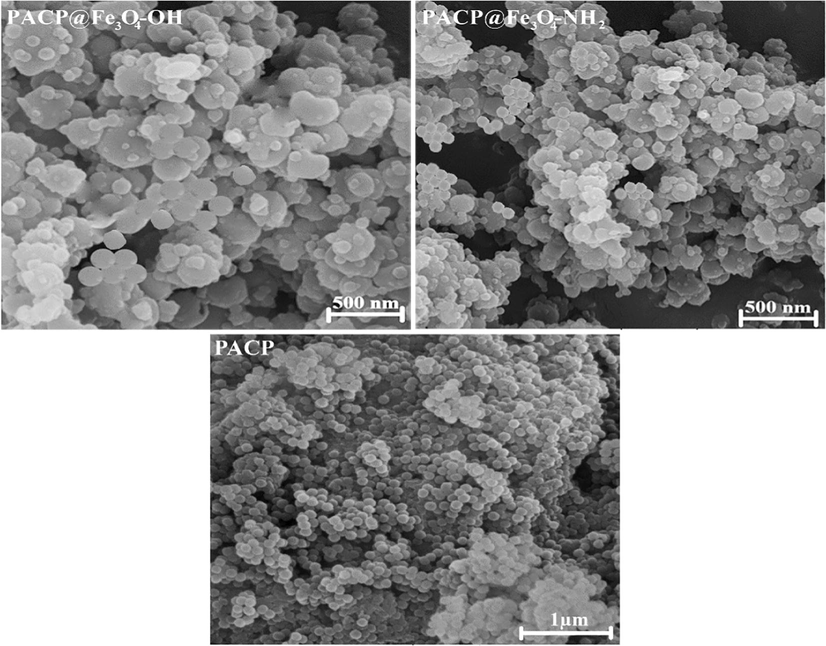
SEM micrographs of the PACP, PACP@Fe3O4-NH2 and PACP@Fe3O4-OH nanocomposites.
3.4 Thermal stability
TGA curves of the PACP nanospheres, PACP@Fe3O4-OH and PACP@Fe3O4-NH2 nanocomposites are shown in Fig. 4. The PACP thermogram displayed three stages of weight loss. The first weight loss at 100 °C was due to the evaporation of moisture trapped in the copolymer. The second weight loss at 280 °C was attributed to the loss of low molecular weight oligomer. The third stage beginning at 400 °C was related to the oxidative thermal degradation of the copolymer chains. In the PACP@Fe3O4-OH and PACP@Fe3O4-NH2 curves, the weight loss was mainly divided into three regions: below 300 °C, at 300–600 °C and above 600 °C. The weight loss during heating from 25 to 300 °C is assigned to the loss of loosely bound water. The large weight loss at temperature between 300 and 600 °C is due to the decomposition of OH or NH2— functionalized surface of Fe3O4. Generally, the thermal stability of the studied systems are in the order PACP@Fe3O4-OH > PACP@Fe3O4-NH2 > PACP.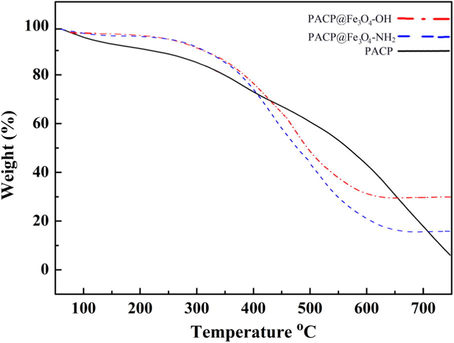
TGA curves of the PACP nanospheres, PACP@Fe3O4-OH and PACP@Fe3O4-NH2 nanocomposites.
3.5 Electrical conductivity
The electrical conductivities of the PACP copolymer, PACP@Fe3O4-OH and PACP@Fe3O4-NH2 nanocomposites were measured by a four probe technique (Table 1). It was found that the electrical conductivity of the PACP@Fe3O4-OH and PACP@Fe3O4-NH2 nanocomposites were higher than that of the PACP copolymer. Moreover, the electrical conductivity of the nanocomposites increased to some extent with the content of Fe3O4 (Zare et al., 2015a). Therefore, we carried out synthesis of PACP@Fe3O4-OH and PACP@Fe3O4-NH2 nanocomposites at the optimized content of Fe3O4. On the other hand, the electrical conductivity of PACP@Fe3O4-OH was higher than that of the PACP@Fe3O4-NH2 nanocomposites. It is well known that the electrical conductivity of materials may be not only affected by the microscopic conductivity but could also be affected by macroscopic conductivity. Microscopic conductivity depends on doping level, conjugation length and polymer chain length, which should not vary widely in samples prepared under identical conditions. Our PACP@Fe3O4-OH and PACP@Fe3O4-NH2 nanocomposites were synthesized in identical conditions, so the microscopic conductivities should be nearly the same. Though, the macroscopic electrical conductivity depends on certain external factors, such as compactness of the samples and molecular orientation, which in turn, significantly depend on the type of Fe3O4 in the nanocomposites. In fact, the Fe3O4-NH2 particles are poorly compacted in the corresponding nanocomposites, and therefore, result in their relatively lower electrical conductivities. These statements can be verified based on the theoretical investigation, which will be discussed below.
Sample
Ratio of Ani:Py:Fe3O4 (v:v: w)
Conductivity (S/cm)
PACP
0.98: 0.74: 0
6.35 × 10−3
PACP@Fe3O4-OH
0.98: 0.74: 0.5
4.20 × 10−2
PACP@Fe3O4-NH2
0.98: 0.74: 0.5
3.88 × 10−2
3.6 Theoretical results
At first, to obtain a model for the PACP copolymer different number of monomer units (n = 3–8) were optimized at the PBE-D/DNP-ECP level of theory (Fig. 5). The calculations show that increasing the number of monomer units leads to decreasing the HOMO–LUMO energy gap (
) which is defined as
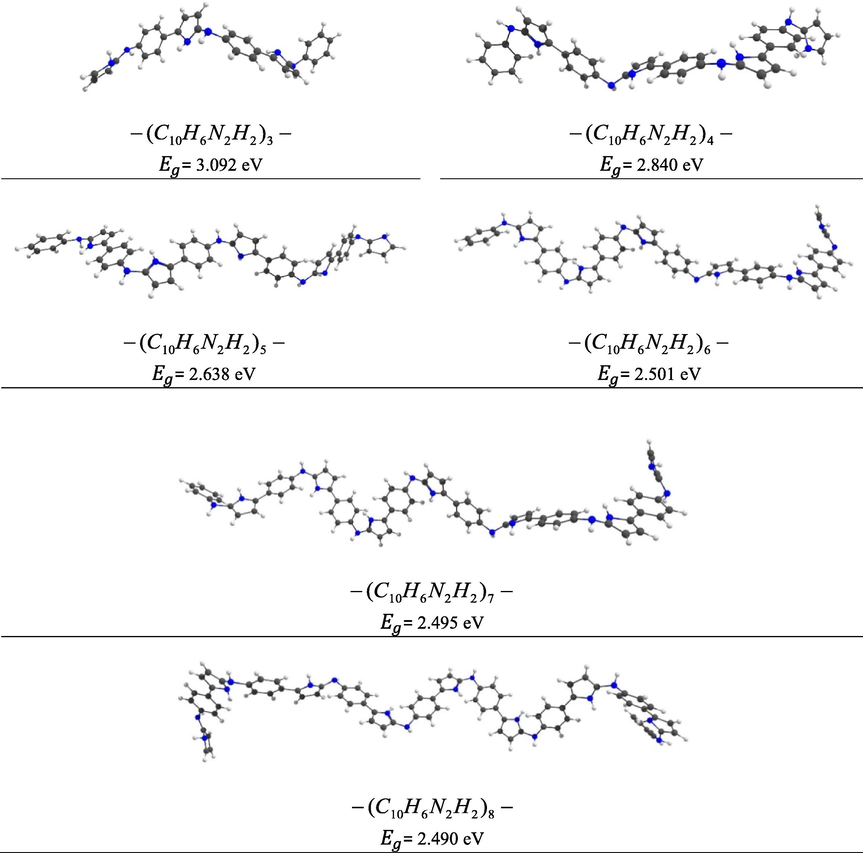
The optimized structures representatives of PACP copolymer (n = 3–8) at the PBE-D/DNP-ECP level.
The iron oxide containing OH groups on its surface and also the amine-functionalized iron oxide were at first optimized at the PBE-D/DNP-ECP level to find the most stable geometries for further investigations on their interactions with the oligomer. Study on the structural and electronic properties of large superstructures requires state of art software and supercomputers. Also, these computations are so costly and time consuming. Therefore, researchers model the superstructures by lowering their constituting number of atoms. Hence, in this work, we worked on an array of Fe3O4 containing 272 oxygen (O) and iron (Fe) atoms, in which the oxygen atoms were terminated with the hydrogen atoms. Fig. 6 shows the optimized structures from two views.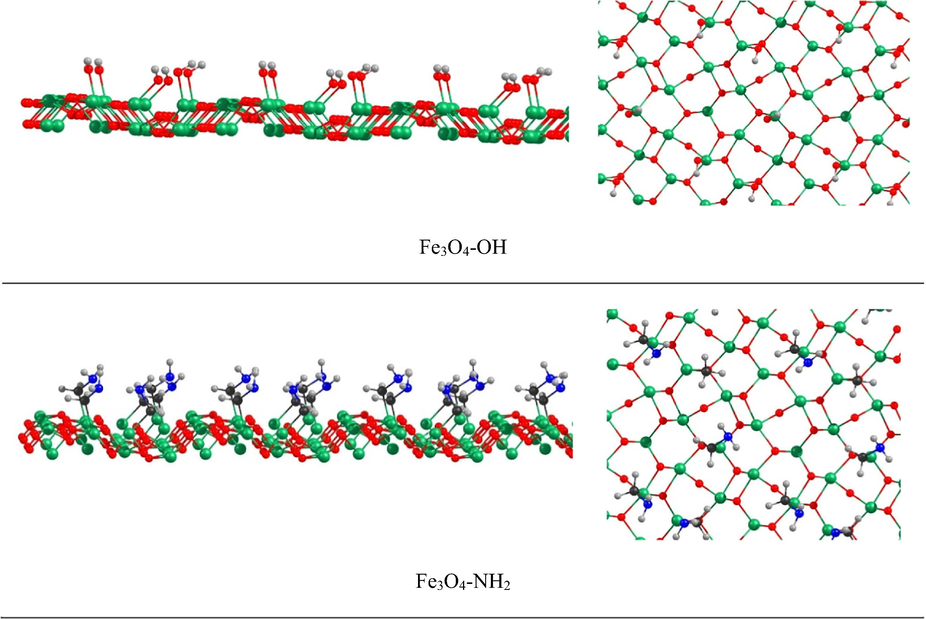
The optimized functionalized iron oxide structures (side view on the left and top view on the right) at the PBE-D/DNP-ECP level. The green, red, blue, gray, and black colors are representative of iron, oxygen, nitrogen, hydrogen, and carbon atoms, respectively.
In order to find the active sites of the oligomer for the interaction with nanoparticles the charge distribution over the —(C10H6N2H2)6— was visualized through electrostatic potential surface (ESP) map (Fig. 7). This map was generated at PBEPBE/6-31G∗∗ level, with the isosurfaces of 0.01 electrons au−3 through Gauss View package. The sites with the lowest and highest electrostatic potential energy values are in red and blue color, respectively. In fact, the red and blue regions are indicative of the relative abundance and absence of electrons in these regions, respectively.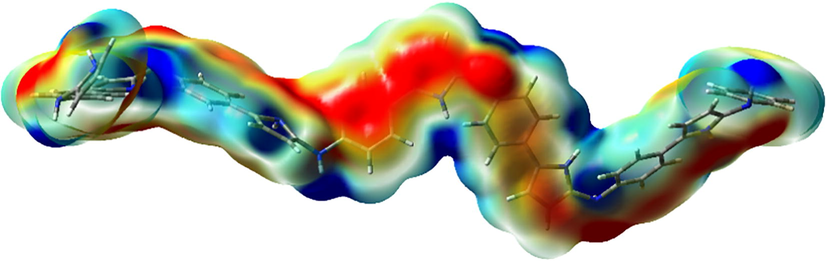
The ESP map of —(C10H6N2H2)6— generated with the isosurfaces of 0.01 electrons au−3, scaled between −0.6 (red) and 35.5 (blue) kcal/mol.
The initial geometry for the interacting PACP@f-Fe3O4 system was designed using the ESP map and optimized at the PBE-D/DNP-ECP level of theory. The most stable structures of these systems are shown in Fig. 8.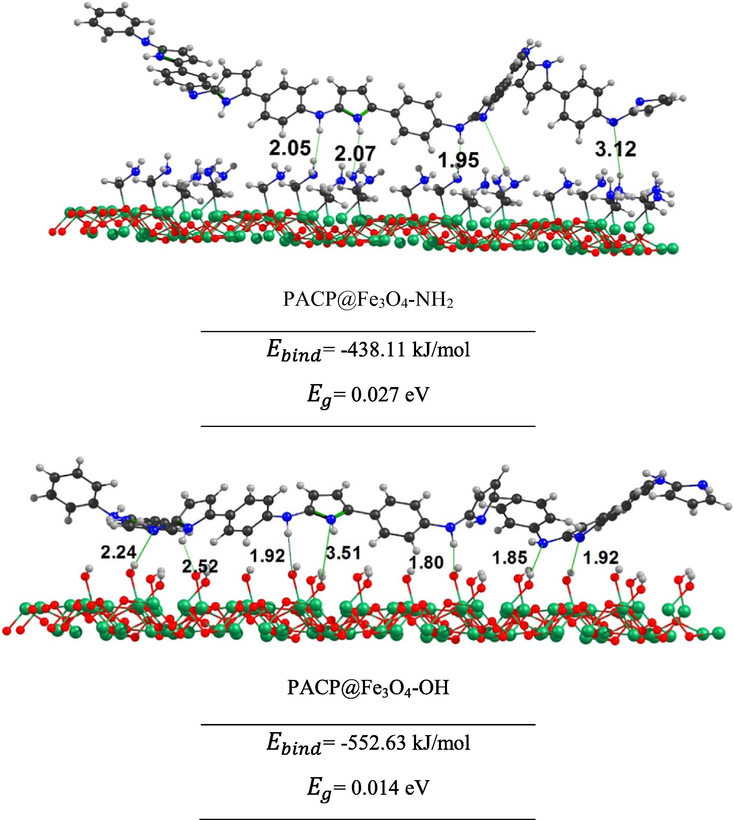
The optimized structures of interacting PACP@f-Fe3O4 systems at the PBE-D/DNP-ECP level. The distances are in Å.
The binding energy (
) for the interacting PACP@f-Fe3O4 systems was calculated by:
The binding energies for the PACP@Fe3O4-NH2 and PACP@Fe3O4-OH are −438.11 and −552.63 kJ/mol, respectively (Fig. 8). Therefore, the interaction of PACP with Fe3O4-OH is higher than that with Fe3O4-NH2. The reported bond distances, shown in Fig. 8, also reveals the interactions in these two systems. Herein, the calculated binding energies are in accord with the higher thermal stability of the PACP@Fe3O4-OH than PACP@Fe3O4-NH2 discussed in Section 3.4. In fact, due to the shorter bond distances and higher interactions, a more compact nanocomposite is prepared when using Fe3O4-OH than Fe3O4-NH2 nanoparticles, and hence, its thermal stability increases. Also, the lower binding energy of the PACP@Fe3O4-NH2 can be related to the fact that the Fe3O4-NH2 nanoparticles are poorly compacted in the nanocomposite and its electrical conductivity decreases (Section 3.5). To have an insight on the electrical conductivity using the DFT study we calculate the
(Eq. (1)). The population of conduction electrons, responsible for the electrical conductivity, can be related to
by (Blakemore, 1974)
4 Conclusions
The functionalization of iron oxide nanoparticles changes the physical properties of poly (aniline-co-pyrrole) based nanocomposites, synthesized by the microemulsion polymerization. The presence of the Fe3O4-OH and Fe3O4-NH2 nanoparticles enhances the electrical conductivity of the prepared nanocomposites, and the electrical conductivity of the PACP@Fe3O4-OH nanocomposite is higher than that of the PACP@Fe3O4-NH2. Based on the TGA, the PACP@f-Fe3O4 nanocomposites have higher thermal stability than the PACP copolymer. The SEM micrographs showed that the particles of nanocomposites, with the average diameter of about 100–150 nm, are rather rougher than those of the PACP nanospheres. The binding energies between the PACP copolymer and the functionalized nanoparticles, calculated by the density functional theory, confirmed the higher thermal stability of the PACP@Fe3O4-OH nanocomposite than that of the PACP@Fe3O4-NH2. Also, the calculated HOMO–LUMO energy gap ( ) values were in agreement with the trend of experimental electrical conductivities, which are in the order PACP@Fe3O4-OH > PACP@Fe3O4-NH2 > PACP.
References
- Monitoring of hydrogen peroxide using a glassy carbon electrode modified with hemoglobin and a polypyrrole-based nanocomposite. Microchim. Acta. 2015;182:771-779.
- [Google Scholar]
- Application of numerical basis sets to hydrogen bonded systems: a density functional theory study. J. Chem. Phys.. 2005;122:144102.
- [Google Scholar]
- Copolymers of diketopyrrolopyrrole and thienothiophene for photovoltaic cells. J. Mater. Chem.. 2011;21:9224-9231.
- [Google Scholar]
- The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys.. 1970;19:553-566.
- [Google Scholar]
- Synthesis, characterization and toxicological evaluation of a core–shell copper oxide/polyaniline nanocomposite. Chemosphere. 2014;108:107-114.
- [Google Scholar]
- Smart coating based on polyaniline acrylic blend for corrosion protection of different metals. Surf. Coat. Technol.. 2007;201:7574-7581.
- [Google Scholar]
- FePt-Au ternary metallic nanoparticles with the enhanced peroxidase-like activity for ultrafast colorimetric detection of H2O2. Sensors Actuators, B Chem.. 2018;259:775-783.
- [Google Scholar]
- Poly (pyrrole-co-aniline) hollow nanosphere supported Pd nanoflowers as high-performance catalyst for methanol electrooxidation in alkaline media. Energy. 2017;127:419-427.
- [Google Scholar]
- Carbon nanotubes-adsorbed Electrospun PA66 nanofiber bundles with improved conductivity and robust flexibility. ACS Appl. Mater. Interfaces. 2016;8:14150-14159.
- [Google Scholar]
- Nondestructive functionalization of graphene by surface-initiated atom transfer radical polymerization: an ideal nanofiller for poly(p-phenylene benzobisoxazole) Fibers. Macromolecules. 2017;50:1422-1429.
- [Google Scholar]
- Light-triggered C60 release from graphene/cyclodextrin nanoplatform for the protection of cytotoxicity induced by nitric oxide. J. Mater. Chem. B. 2018;6:518-526.
- [Google Scholar]
- 2, 2′-(Phenylazanediyl) diacetic acid modified Fe3O4@PEI for selective removal of cadmium ions from blood. Nanoscale. 2012;4:733-736.
- [Google Scholar]
- Lakouraj, M.M., Zare, E.N., Moghadam, P.N., 2014. Synthesis of novel conductive poly(p-phenylenediamine)/Fe3O4 nanocomposite via emulsion polymerization and investigation of antioxidant activity. Adv. Polym. Technol. 33, 21385(1–7).
- Conducting blends of polyaniline with conventional polymers. Synth. Met.. 1997;84:117-118.
- [Google Scholar]
- Facile synthesis and intrinsic conductivity of novel pyrrole copolymer nanoparticles with inherent self-stability. J. Phys. Chem. B. 2007;111:5829-5836.
- [Google Scholar]
- Continuously prepared highly conductive and stretchable SWNT/MWNT synergistically composited electrospun thermoplastic polyurethane yarns for wearable sensing. J. Mater. Chem. C. 2018;6:2258-2269.
- [Google Scholar]
- Durably antibacterial and bacterially antiadhesive cotton fabrics coated by cationic fluorinated polymers. ACS Appl. Mater. Interfaces. 2018;10:6124-6136.
- [Google Scholar]
- Synthesis of polypyrrole nanocomposites decorated with silver nanoparticles with electrocatalysis and antibacterial property. Compos. Part B Eng.. 2015;69:232-236.
- [Google Scholar]
- Electrically conductive thermoplastic elastomer nanocomposites at ultralow graphene loading levels for strain sensor applications. J. Mater. Chem. C. 2016;4:157-166.
- [Google Scholar]
- NiO nanoparticles modified with 5,10,15,20-tetrakis(4-carboxyl pheyl)-porphyrin: promising peroxidase mimetics for H2O2and glucose detection. Biosens. Bioelectron.. 2014;64:147-153.
- [Google Scholar]
- High dielectric constant polyaniline/epoxy composites via in situ polymerization for embedded capacitor applications. Polymer (Guildf). 2007;48:1510-1516.
- [Google Scholar]
- In situ electrochemical synthesis of polyaniline/f-MWCNT nanocomposite coatings on mild steel for corrosion protection in 3.5% NaCl solution. Prog. Org. Coat.. 2015;78:387-394.
- [Google Scholar]
- Sonochemical synthetic methods to produce functionalized conducting copolymers. Polym. Adv. Technol.. 2010;21:235-243.
- [Google Scholar]
- Preparation of conductive nanocomposites based on poly (aniline-co-butyl 3-aminobenzoate) and poly (aniline-co-ethyl 3-aminobenzoate) by solution blending method. Compos. Interfaces. 2012;19:475-488.
- [Google Scholar]
- Synthesis and characterization of poly (aniline-co-o-anisidine). A processable conducting copolymer. Macromolecules. 1993;26:3190-3193.
- [Google Scholar]
- Atoms, molecules, solids, and surfaces: applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B. 1992;46:6671.
- [Google Scholar]
- Synthesis of electrically conducting copolymers of aniline with o/m-amino benzoic acid by an inverse emulsion pathway. Polymer (Guildf). 2002;43:5051-5058.
- [Google Scholar]
- Poly (ortho-ethoxyaniline) in corrosion protection of stainless steel. Corros. Sci.. 2007;49:2567-2580.
- [Google Scholar]
- Sensitive electrochemical sensor using a graphene–polyaniline nanocomposite for simultaneous detection of Zn (II), Cd (II), and Pb (II) Anal. Chim. Acta. 2015;874:40-48.
- [Google Scholar]
- Polystyrene/polyaniline nanoblends for sensing of aliphatic alcohols. Sensors Actuators B Chem.. 2005;104:140-150.
- [Google Scholar]
- Experimental and simulation-based understanding of morphology controlled barium titanate nanoparticles under co-adsorption of surfactants. CrystEngComm. 2017;19:3288-3298.
- [Google Scholar]
- Nanohybrid materials consisting of poly [(3-aminobenzoic acid)-co-(3-aminobenzenesulfonic acid)-co-aniline] and multiwalled carbon nanotubes for immobilization of redox active cytochrome c. Electroanalysis. 2014;26:732-738.
- [Google Scholar]
- In situ synthesis of polypyrrole-γ-Fe2O3-fly ash nanocomposites for protection against EMI pollution. Ind. Eng. Chem. Res.. 2014;53:14282-14290.
- [Google Scholar]
- Facile synthesis of polypyrrole decorated reduced graphene oxide–Fe3O4 magnetic composites and its application for the Cr (VI) removal. Chem. Eng. J.. 2015;262:597-606.
- [Google Scholar]
- Wei, Y., Shuxi, L., Jia, X., Cheng, M., Mathai, M.W., Yeh, J., Wei, L., Jansen, S.A, Wang, Z.Y., Yang, C., Gao, J.P., Narkis, M., Siegmann, A., Hsieh, B.R., 1999. Electroactive Aniline Oligomers of Well-Defined Structures and Their Polymeric Derivatives, 384–398.
- A composite polymer membrane with reversible overcharge protection mechanism for lithium ion batteries. Electrochem. Commun.. 2005;7:589-592.
- [Google Scholar]
- Synthesis of conductive polyaniline/epoxy resin composites: doping of the interpenetrating network. Synth. Met.. 2004;142:57-61.
- [Google Scholar]
- Biodegradable polyaniline/dextrin conductive nanocomposites: synthesis, characterization, and study of antioxidant activity and sorption of heavy metal ions. Iran. Polym. J.. 2014;23:257-266.
- [Google Scholar]
- Electro-magnetic polyfuran/Fe3O4 nanocomposite : synthesis, characterization, antioxidant activity, and its application as a biosensor. Int. J. Polym. Mater. Polym. Biomater.. 2015;64:175-183.
- [Google Scholar]
- Multilayered electromagnetic bionanocomposite based on alginic acid: characterization and biological activities. Carbohydr. Polym.. 2015;130:372-380.
- [Google Scholar]
- Poly (3-aminobenzoic acid)@MWCNTs hybrid conducting nanocomposite: preparation, characterization, and application as a coating for copper corrosion protection. Compos. Interfaces. 2016;23:571-583.
- [Google Scholar]
- Efficient sorption of Pb (ii) from an aqueous solution using a poly (aniline-co-3-aminobenzoic acid)-based magnetic core–shell nanocomposite. New J. Chem.. 2016;40:2521-2529.
- [Google Scholar]
- Ultralow percolation threshold and enhanced electromagnetic interference shielding in poly(L-lactide)/multi-walled carbon Na notubes nanocomposites with electrically conductive segregated networks. J. Mater. Chem. C. 2017;5:9359-9369.
- [Google Scholar]
- Overview of polymer nanocomposites: computer simulation understanding of physical properties. Polym. (United Kingdom). 2017;133:272-287.
- [Google Scholar]
- Hollow poly(aniline-co-pyrrole)–Fe3O4 composite nanospheres: synthesis and characterization. J. Nanoelectron. Optoelectron.. 2012;7:292-296.
- [Google Scholar]







