Translate this page into:
Chemical constituents, antioxidant and hepatoprotective properties of ethanol extract from Artemisia japonica Thumb. Leaves
⁎Corresponding authors. lmy@126.com (Mei-ya Li), jfs1020@163.com (Fu-sheng Jiang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The tender leaves of Artemisia japonica Thumb. are often used as vegetables and tea in China. They also used as a traditional Chinese medicine for the treatment of liver disease. However, the identity of components responsible for health benefits remains unclear. The present study aimed to investigate the antioxidant and hepatic protective effects of a 60 % ethanol extract of Artemisia japonica Thumb. leaves (AJLEE). Total phenol content of AJLEE was determined using Folin Ciocalteau Method and it was calculated to be 123.142 ± 8.441 mg GAE/g. AJLEE shows remarkable antioxidant activity in vitro; pretreatment at 150 µg/mL can almost completely eliminate H2O2-induced ALM12 cell death. Moreover, AJLEE at a dosage of 400 mg/kg exhibits a strong hepatic protective effect against α-naphthylisothiocyanate (ANIT)-induced liver injury though the upregulation of genes for the antioxidant enzymes Sod1, Cat, Gpx4 and Ho-1, while decreasing the level of MDA. These properties of AJLEE may be attributed to the existence of abundant phenolic acids, flavonoids and coumarins, identified by UPLC/QTOF-MS as chlorogenic acid, isochlorogenic acid A, 7-methoxycoumarin and quercetin, apigenin and luteolin glycoside derivatives. Our data support the use of Artemisia japonica leaves as functional tea and food additives to improve human health.
Keywords
Artemisia japonica Thumb.
Polyphenols
Antioxidant
Reactive oxygen species
Hepatic protection
1 Introduction
Intrahepatic cholestasis (IC) is a syndrome characterized by the partial or complete obstruction of bile outflow due to the obstruction of bile excretion in hepatocytes and/or bile capillaries. Primary biliary cirrhosis, primary sclerosing cholangitis, viral hepatitis, alcohol and drug-induced liver damage may lead to intrahepatic cholestasis. The exact pathogenesis of IC is still unclear, but studies have shown that accumulated bile acids trigger oxidative stress and an inflammatory response, leading to the development and progression of hepatocyte injury, liver fiber cirrhosis and eventually liver failure (De Vries and Beuers, 2017). Experimental animal liver injury induced by hepatotoxic compound α-naphthylisothiocyanate (ANIT) is a useful model to study the mechanism of cholestasis caused by drug-induced liver injury (Ohta et al., 1999). It has been reported that ANIT injection can lead to imbalance between hepatocyte oxidation and the antioxidant system, which can manifest as a significant decrease in superoxide dismutase (SOD), catalase (CAT) and glutathione (GSH) and a significant increase in reactive oxygen species (ROS) (Ohta et al., 1999). Numerous lines of evidence recommend that treatments can directly eliminate ROS or upregulate antioxidant enzymes in alleviating ANIT-induced liver injury (Ohta et al., 2001; Wang et al., 2019). Therefore, researchers believe that nutritional application relying on medicinal plants may help to reduce oxidative stress, especially by preventing lipid oxidation. Polyphenols are the most abundant antioxidants in plants, and numerous studies have provided evidence of the beneficial health effects resulting from consumption of these phenolic compounds (Simon et al., 2020; Sobeh et al., 2020). In this line, traditional Chinese herbal medicine clearly represents a valuable resource.
Artemisia japonica Thumb. is a member of the Artemisia genus; its tender leaves are widely used as a vegetable and tea in China, Korea and Japan (Zhang, 2008; Trendafilova et al., 2020). It contains high levels of carotenes, vitamins, minerals, polyphenols and dietary fibers. Phytochemical investigations on Artemisia japonica show the presence of terpenoids, phenolic acids, flavonoids and essential oils, most of which have been identified as hydrophobic components, although bioactive evaluation is lacking (Kwon and Lee, 2001; Giang et al., 2014). According to the Xian Dai Ben Cao Gang Mu (Compendium of modern materia medica), the whole herb of Artemisia japonica can be used as a medicine for treating cold, fever, strain, cough, hot flashes, heatstroke, malaria, hypertension, aphthous, scabies and eczema (Huang et al., 2001). Pharmacological studies of Artemisia japonica demonstrate that its aqueous extract has high anti-inflammatory, antioxidant and hemostatic activity (Huang et al., 2010; Zhang et al., 2011). Genotoxicity and maximum tolerance tests in mice have revealed that the water extract of Artemisia japonica is associated with low toxicity and high safety (Huang et al., 2010; Zhang et al., 2011). These results, at least in part, confirm the effectiveness of Artemisia japonica as a traditional Chinese medicine and its safety as an edible plant.
Artemisia japonica is widely used as a vegetable and tea in Fujian, Chongqing and other areas of China and its consumption has been declared beneficial for liver diseases such as viral hepatitis and jaundice. However, to the best of our knowledge, there are no systematic studies reporting on the chemical profile of Artemisia japonica or demonstrating that this plant is effective against hepatic injury. Therefore, we sought to address this in the present study, wherein Artemisia japonica leaves were extracted with 60 % ethanol, and their chemical compositions, total phenolic content and in vitro antioxidant and hepatic cell protective activities were evaluated. Additionally, an ANIT-induced mice liver injury model was utilized to uncover the hepatic protective properties and potential in vivo mechanism of Artemisia japonica from the perspective of oxidative stress. We believe that the results from this study will provide sufficient evidence for the further study and utilization of Artemisia japonica as a tea and functional food.
2 Materials and methods
2.1 Reagents
The reagents 2,2-azinobis (3-ethylbenzothiazoline-6-sulfonic acid ammonium salt) (ABTS) and chlorogenic acid (3-O-caffeoylquinic acid, CA) were purchased from Bide Pharmatech ltd. (Shanghai, China). Isochlorogenic acid A (3,5-dicaffeoylquinic acid, IAA), isochlorogenic acid B (3,4-dicaffeoylquinic acid, IAB), isochlorogenic acid C (4,5-dicaffeoylquinic acid, IAC) and 7-methoxycoumarin (7-MC) were purchased from Chengdu Push Biotechnology Co., ltd. (Chengdu, China). Rutin was purchased from the National Institutes for Food and Drug Control. Folin–Ciocalteu’s phenol reagent was purchased from Solarbio Science & Technology Co., ltd. (Beijing, China). Gallic acid, 2,2-diphenyl-1-picrylhydrazyl radical (DPPH•), vitamin C (Vc) and 2′,7′-dichlorofluorescin diacetate (DCFH-DA) were obtained from Meryer Chemical Technology Co., ltd. (Shanghai, China). ABTS Diammonium salt, α-naphthylisothiocyanate (ANIT) and Ursodeoxycholic acid (UDCA) were purchased from Shanghai Macklin Biochemical Co., ltd. (Shanghai, China). Tripyridyltriazine was purchased from Shanghai yuanye Bio-Technology Co.,ltd (Shanghai, China). HPLC-grade acetonitrile and formic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA). Ultrapure water was prepared using a Milli-Q purification system (Millipore Laboratory, Bedford, MA, USA). High-glucose Dulbecco’s modified Eagle’s medium (DMEM), penicillin and streptomycin were purchased from HyClone Co. (Logan, Utah). Fetal bovine serum (FBS) (Gibco, USA) was also used. Other chemicals or solvents were of analytical grade and used without further purification.
2.2 Preparation of AJLEE
The leaves of Artemisia japonica were cultivated and collected in Shouning County, Ningde City (Fujian Province, China), in May 2019, and their identity confirmed by Professor Ding Zhishan of Zhejiang Chinese Medical University. The fresh leaves were cleaned and dried in a constant-temperature oven at 60 °C and then powdered and sieved through an 80-mesh screen. Finally, the powder was sealed and stored in a drier at − 40 °C. A voucher specimen (20190510-ND) was deposited in the laboratory of the College of Life Science, Zhejiang Chinese Medical University. The powder of Artemisia japonica leaves (10.0 g) was ultrasonically extracted with 100 mL 60 % ethanol according to the following conditions: ultrasonic power of 250 W, ultrasonic frequency of 53 kHz and ultrasonic time of 20 min at room temperature. The mixture was then filtered under reduced pressure, and the residue was re-extracted following the same conditions. The supernatants were combined and concentrated under vacuum at 45 °C to remove ethanol, and the mixture was extracted with petroleum ether (3 × 80 mL) to eliminate lipids. It was then concentrated under vacuum and lyophilized to obtain AJLEE, which was stored at − 40 °C for further research.
2.3 Chemical profile characterization
AJLEE was dissolved in 50 % acetonitrile, filtered through a 0.22 μm membrane and analyzed by injection into the UPLC/QTOF-MS system, which consisted of an ACQUITY ultraperformance liquid chromatography instrument (Waters Corporation, Milford, MA, USA) and a SYNAPT G2-Si QTOF Mass Spectrometer (Waters Corporation, Manchester, UK). The separation was performed using a Waters ACQUITY UPLC Cortecs T3 column (2.10 mm × 100 mm, 1.70 µm, Waters, UK) with Cortecs T3 Van Guard (2.10 mm × 50 mm, 1.70 μm). A binary solvent system of A) acetonitrile and B) 0.1 %(V/V) formic acid in deionized water was used. The gradient elution program was 0–10 min, 95–75 % B; 10–20 min, 75–36 % B, with a constant flow rate of 0.30 mL/min. The column temperature was maintained at 40 °C. In electrospray ionization (ESI)–MS analysis, the source was operated under positive and negative ion mode, and the capillary voltage was set as 3.0 kV. The ionization temperature was 120 °C, and the desolvation temperature was 500 °C. Argon was used as a collision gas for CID in both MSE and MS2 mode. The full scan mass range was 50–1200 Da.
2.4 Determination of total antioxidant compounds
2.4.1 Total phenolic content (TPC) assay
The TPC in AJLEE was determined using Folin–Ciocalteu’s method (Zhang et al., 2015). Briefly, 60 μL AJLEE 50 % ethanol solution (0.50 mg/mL) was mixed with equal volume of Folin–Ciocalteu reagent. After 3 min, 60 μL 10 % (w/v) sodium bicarbonate solution was added to the mixture, and following incubation at room temperature for 60 min, then absorbance was measured at 765 nm by a microplate reader (PE enpire, United States) with gallic acid as a standard (Y = 9.2522X + 0.0048, R2 = 0.9998). The TPC was expressed as milligrams of gallic acid equivalent per gram of extract (mg GAE/g). All samples were tested in triplicate.
2.4.2 Total Flavonoid content assay
The total flavonoid content (TFC) in AJLEE was measured as reported by Tsai et al. (Tsai et al., 2022). In brief, 100 μL AJLEE 50 % ethanol solution (0.50 mg/mL) was mixed with 100 μL sodium nitrite solution (5 %) and 100 μL aluminum chloride solution (10 %), and incubated for 6 min at room temperature. Then 600 μL of sodium hydroxide solution (10 %) was added and incubated for 30 min. Absorbance at 510 nm was measured using a microplate reader (PE enpire, United States) with 50 % ethanol as a blank. Rutin was used as a standard (Y = 1.9418X + 0.0066, R2 = 0.9993), and the TFC was expressed as milligrams of rutin equivalent per gram of extract (mg RUT/g). All the tests were performed in triplicate.
2.5 Determination of total antioxidant Capacity of AJLEE
2.5.1 DPPH radical scavenging activity assay
The DPPH• scavenging activity was measured following the procedure of (Wu et al., 2022) with slight modification. Briefly, 100 μL of diluted sample was mixed with 100 μL of freshly prepared DPPH• solution (0.1 mM in ethanol). After incubation in darkness for 30 min under room temperature, absorbance was determined at 510 nm. Vc was used as a positive control. The IC50 value (the concentration needed to scavenge 50 % of DPPH•) was calculated, and all samples were tested in triplicate.
2.5.2 ABTS radical scavenging activity assay
The ABTS+• scavenging activity was measured as described by (Zhang et al., 2015) with modification. Briefly, 5 mL ABTS (7.4 mM) stock solution and 88 μL K2S2O8 (2.6 mM) stock solution was mixed to obtain ABTS+• solution and kept in the dark for 16 h before use. Then 20 μL of sample was mixed with 180 μL of prepared ABTS+• solution. The absorbance was measured at 734 nm after 6 min. Using Vc as a positive control, the IC50 value (the concentration needed to clear 50 % of ABTS+•) was calculated.
2.5.3 Ferric reducing antioxidant power assay (FRAP)
The reducing power activity was evaluated following the procedures reported by (Wu et al., 2022). Briefly, 20 μL of sample was mixed with 180 μL of freshly prepared FRAP reagent (contained 2.5 mL of 20 mM FeCl3 and 2.5 mL of 10 mM tripyridyltriazine solution in 40 mM HCl and 25 mL of pH3.6, 0.3 M acetate buffer). Then, absorbance was recorded at 700 nm against a sample blank (with all reagents except sample), and the OD0.5 (the concentration of sample absorbance of 0.50) value was calculated. Vc was used as a positive control and all the tests were performed in triplicate.
2.6 Protective effect of AJLEE on H2O2-Induced cytotoxicity in AML12 cells
2.6.1 Cytoprotective assay
AML12 hepatocyte cells were obtained from the American Type Culture Collection and cultured in DMEM/F12 (Gibco, Grand Island, NY) containing 10 % heat-inactivated fetal bovine serum (FBS), 1 g/L insulin, 0.55 g/L transferrin, 0.67 mg/L sodium pyruvate, 102 nM dexamethasone (SV30010, HyClone, USA), 100 U/mL penicillin and 100 µg/mL streptomycin at 37 ℃ in a humidified incubator containing 5 % CO2. Cells were subcultured every 3 days. Cells (1 × 104 cells) were seeded in 96-well plates and incubated overnight. For the cytotoxicity assay, AML12 cells were treated with a broad range of AJLEE concentrations. To examine the protective effects of AJLEE on H2O2, cells were pre-exposed to different safe doses of AJLEE for 1 h and co-incubated with 0.60 mM H2O2 for 24 h. Three hours before final culture, 20 μL of CCK8 solution was added into each well, and the optical density at 450 nm was then measured using a microplate reader. Moreover, the supernatants were collected after treatment with H2O2 for 24 h with or without AJLEE, and malondialdehyde (MDA) levels were measured according to the instructions of the kit (Beijing Biodee Biotechnology Co., ltd., Beijing, China). All procedures were performed in triplicate.
2.6.2 Determination of intracellular ROS
AML12 cells (1 × 104 cells) were seeded in 96-well plates and incubated overnight. Cells were subsequently subjected to various concentrations of AJLEE or NAC (10 mM) and cultured for 6 h, followed by exposure to 0.6 mM H2O2 for 30 min under cell culture conditions. After being washed with PBS, cells were stained with 2′,7′-dichlorofluorescin diacetate (DCFH-DA, 10 μM) for 30 min at 37 °C. The medium was then removed and samples washed with PBS three times. The levels of intracellular ROS were photographed using an ImageXpress Micro XLS system (Molecular Devices LLC, CA, USA), and the fluorescence intensities were analyzed using IPP software (Version 6.0, Media Cybernetics, USA) (Miao et al., 2020).
2.7 Animals and treatments
Male ICR mice (8 weeks old, 20 ± 2 g) (SPF, SYXK(Zhe)2018–0012) were purchased from Sina-British Sippr/Bk Lab Animal ltd. (Shanghai, China). All animals were kept under standard conditions of a 12 h light/dark cycle, 40–60 % humidity and temperature of 25 ± 2 ℃. Mice were allowed free access to water and food. The experiment was carried out in accordance with the Guidelines for the Care and Use of Laboratory Animals and approved by the Animal Ethical Committee of Zhejiang Chinese Medical University (IACUC-20191202–04). Forty ICR mice were randomly divided into five groups, with eight mice in each group: control group—mice were orally gavaged daily for seven consecutive days with 0.10 mL/10 g body weight of water; model group—mice were orally gavaged daily for seven consecutive days with 0.10 mL/10 g body weight of water; low-dose group (200 mg/kg AJLEE)—mice were orally gavaged daily for seven consecutive days with 0.10 mL/10 g body weight of AJLEE (20 mg/mL, dissolved in water); high-dose group (400 mg/kg AJLEE)—mice were orally gavaged daily for seven consecutive days with 0.10 mL/10 g body weight of AJLEE (40 mg/mL, dissolved in water) and positive group (ursodeoxycholic acid, UDCA, 100 mg/kg)—mice were orally gavaged daily for seven consecutive days with 0.10 mL/10 g body weight of UDCA (10 mg/mL, dissolved with 1 mg/mL sodium hydroxide aqueous solution) (Liu et al., 2022). One hour after the fifth day of administration, ANIT (112 mg/kg) was orally administered to all groups except the control group (gavaged with 0.10 mL/10 g of corn oil). ANIT was diluted in corn oil to prepare 11.20 mg/mL, and administered orally with 0.10 mL/10 g body weight (Lin et al., 2012).. All mice were fasted for 12 h after last administration on day 7. The body weight was then recorded, and blood samples and liver tissues were collected for further biochemical and histological analysis.
2.8 Serum biochemistry
Animals were anesthetized with pentobarbital sodium (50 mg/kg), and blood samples were harvested from the abdominal aorta. After coagulation, serum samples were obtained at 3000 rpm for 10 min. Serum levels of total bilirubin (TBIL), direct bilirubin (DBIL), alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured according to the instructions of the kits (Nanjing Jiancheng Biotechnology Company, Nanjing, China).
2.9 Histological examination
The liver was quickly removed, rinsed with normal saline and weighed. The liver tissue was then frozen in liquid nitrogen or fixed in 4 % paraformaldehyde solution. Thick liver sections were cut and stained with hematoxylin–eosin (HE). The degree of liver lesions was observed and recorded on an optical microscope (Axio Scope.A1, ZEISS, Germany).
2.10 Liver antioxidant enzyme activities
Liver samples were homogenized with ice-cold PBS (9 %, W/V), and then centrifuged at 4000 rpm for 10 min at 4 ℃. The supernatant was collected and the levels of SOD, CAT, GSH and MDA were measured according to the protocols of commercial assay kits (Nanjing Jiancheng Biotechnology Company, Nanjing, China).
2.11 Liver antioxidant enzyme mRNA expression
Around 90 to 100 mg of liver tissue in 1 mL TRIzol reagent (Invitrogen, USA) was homogenized using the MagNA Lyser (Roche, Germany) at 6000 rpm for 20 s. Total RNA was extracted using TRIzol reagent according to the manufacturer's instructions and quantified by spectrophotometry. cDNA was obtained using the High-Capacity cDNA Reverse Transcription Kit (Cwbio, China). Quantitative RT-PCR (qRT-PCR) was performed on the LC480 Real-Time PCR Detection System (Roche, Germany) using SYBR Green PCR Master Mix (Cwbio, China) and the specific primers (Sangon Biotech, China) listed in Table 1. GAPDH was taken as an endogenous reference, and the data were normalized accordingly. All the reactions were assessed in triplicate, and the results are expressed as the fold change relative to the control group (=1).
Gene Name
Forward primer (5′-3′)
Revers primer (5′-3′)
Sod1
GAGCATTCCATCATTGGCCG
ACTGCGCAATCCCAATCACT
Sod2
GTGTGGGAGCACGCTTACTA
CTATAAACCAGCCCGGAGCC
Cat
CACTGACGAGATGGCACACT
TGTGGAGAATCGAACGGCAA
Gpx4
CCTTCCCCTGCAACCAGTTT
GTGGGCATCGTCCCCATTTA
Ho-1
CAGAAGAGGCTAAGACCGCC
TCTGACGAAGTGACGCCATC
Gapdh
CATCACTGCCACCCAGAAGACT
GACACATTGGGGGTAGGAACAC
2.12 Statistical analysis
All in vitro tests were conducted in triplicate in at least three independent experiments. Data obtained in the current study are expressed as the mean ± standard deviation (SD). One-way ANOVA followed by Dunnett’s test was performed to calculate the significance of differences between the groups, which were considered statistically significant at P < 0.05.
3 Results
3.1 Chemical profile characterization of AJLEE
The 60 % ethanol extract of Artemisia japonica leaves (AJLEE) was concentrated, frozen and vacuum-dried at a low temperature to obtain a light-yellow solid with a yield of (24.32 ± 1.35) % of the dry leaves. Then, UPLC/QTOF-MS was applied to profile AJLEE. The structures were identified by carefully analyzing various types of information, such as the retention time (Rt) and precise molecular weight, and by matching the MS/MS fragment ions generated in positive (Fig. 1A) and negative (Fig. 1B) ion modes with reference compounds as well as the data archived in the literature. A total of 23 compounds in AJLEE were tentatively identified, as indicated in Table 2 and Fig. 1. The majority were characterized as three types, namely phenolic acids, flavonoids and coumarins. Phenolic acids are one of the most abundant groups in AJLEE, and the nine phenolic acid compounds were identified as caffeic acid (6) and its derivatives (2, 3, 4, 8, 11, 18, 19 and 21). AJLEE is also rich in flavonoids, and all the identified flavonoids are present in the form of glycosides. Seven of the compounds were identified as quercetin derivatives (5, 7, 9, 10, 13, 14 and 15), two as luteolin derivatives (16 and 17) and one as an apigenin derivative (20). In addition, there are three coumarin derivatives (12, 22 and 23) and one organic acid (1). Rt represents for retention time; the formulas were calculated using Masslynx 4.1 software with mass accuracy of<5 ppm between experimental and theoretical m/z.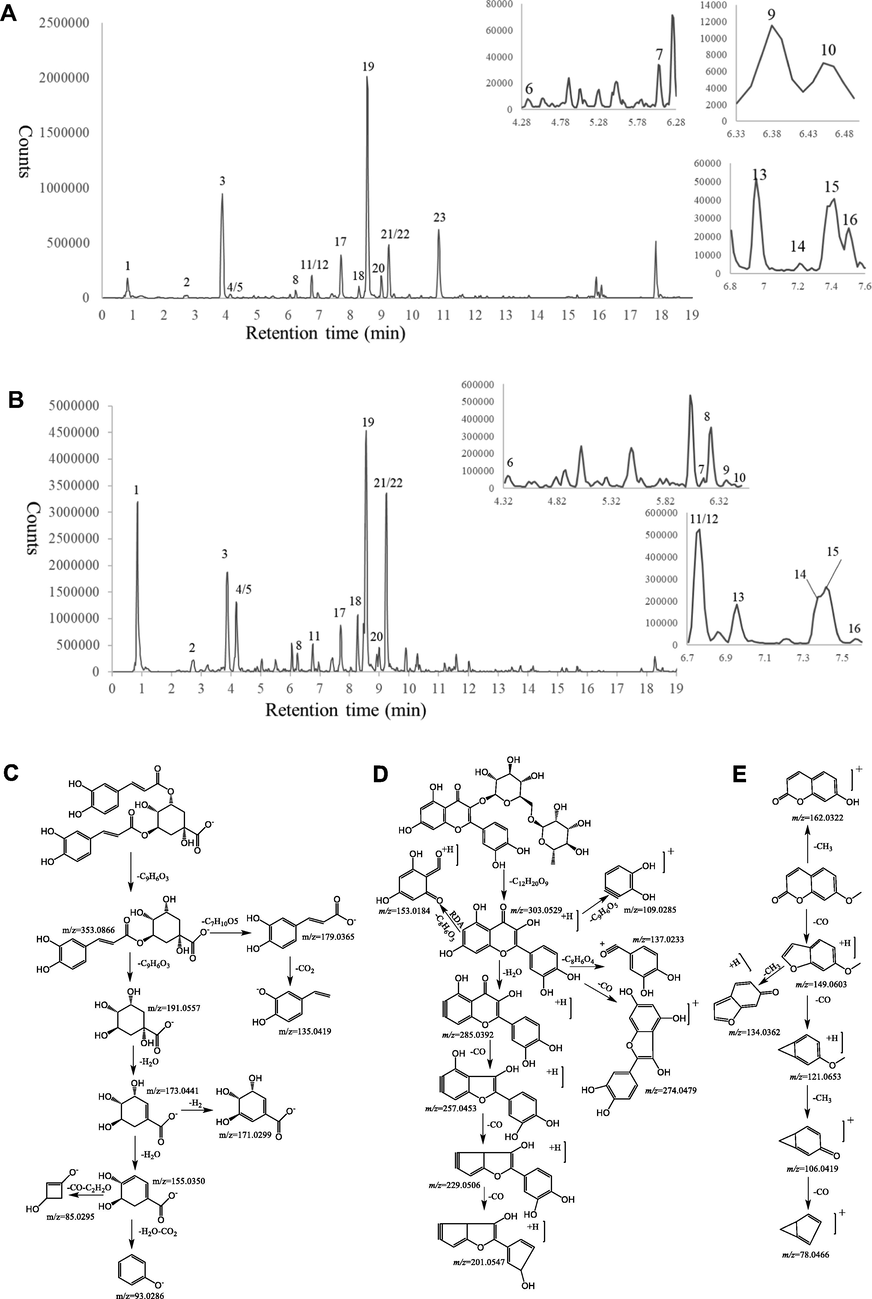
Chemical characterization of AJLEE. The base peak ion chromatograms of AJLEE corresponding to (A) Positive ion mode and (B) negative ion mode. Mass spectrometric fragmentation of (C) chlorogenic acids, (D) flavonoids and (E) coumarins.
Peak
Rt(min)
Measured ion
Selected ion
Error
(ppm)MS/MS fragmentations
Chemical
formulaTentative Identification
Family
1
0.85
191.0547
[M−H]-
−4.7
85.0287(M−H−2H2O−CO−C2H2O),108.9013(M−H−2H2O−2H−CO2−H),173.0445(M−H−H2O)
C7H12O6
Quinic acid
Organic acid
2
2.73
353.0871
[M−H]-
−0.6
191.0549 (M−H−C9H6O3),179.0337 (M−H−C7H10O5),135.0440(M−H−C7H10O5−CO2),173.0450, (M−H−C9H6O3−H2O)
C16H18O9
5-O-Caffeoylquinic acid
Phenolic acid
3
3.88
353.0865
[M−H]-
−2.3
191.0548(M−H−C9H6O3)
C16H18O9
4-O-Caffeoylquinic acid
Phenolic acid
4
4.10
353.0883
[M−H]-
2.8
191.0557(M−H−C9H6O3),179.0344(M−H−C7H10O5),135.0446(M−H−C7H10O5−CO2),173.0441(M−H−C9H6O3−H2O)
C16H18O9
3-O-Caffeoylquinic acid 1
Phenolic acid
5
4.16
479.0832
[M + H]+
1.3
303.0499(M + H-C6H8O6),229.0503(M + H-C6H8O6-H2O-2CO),153.0192(M + H-C6H8O6-C8H6O3),201.0534(M + H-C6H8O6-H2O-3CO),257.0453(M + H-C6H8O6-H2O-CO),137.0240(M + H- C6H8O6-C8H6O4)
C21H18O13
Quercetin-7-O-β-D- glucuronide
Flavonoid
6
4.36
179.0339
[M−H]-
−2.8
135.0442(M−H−CO2),134.0372(M−H−CO2−H)
C9H8O4
Caffeic acid
Phenolic acid
7
6.17
611.1619
[M + H]+
1.1
303.0520(M + H-C12H20O9),257.0400(M + H-C12H20O9-H2O-CO),229.0512(M + H-C12H20O9-H2O-2CO),201.0593 (M + H-C12H20O9-H2O-3CO)
C27H30O16
isorutin
Flavonoid
8
6.22
367.1024
[M−H]-
−1.4
173.0444(M−H−C10H10O4),193.0506(M−H−C7H10O5),134.0364(M−H−C7H10O5−CO2−CH3),93.0338(M−H−C10H10O4−2H2O−CO2),111.0446(M−H−C10H10O4−H2O−CO2)
C17H20O9
3-O-Feruloylquinic acid
Phenolic acid
9
6.40
611.1613
[M + H]+
0.2
303.0505(M + H-C12H20O9),285.0424(M + H-C12H20O9-H2O),257.0410(M + H-C12H20O9-H2O-CO),229.0495(M + H-C12H20O9-H2O-2CO),201.0505(M + H-C12H20O9-H2O-3CO),153.0139(M + H-C12H20O9-C8H6O3)
C27H30O16
Quercetin-7-O-rutinoside
Flavonoid
10
6.45
479.0826
[M + H]+
0.0
303.0503(M + H-C6H8O6),285.0405(M + H-C6H8O6-H2O),123.0085(M + H-C6H8O6-C8H5O2-CO-H2O),169.0145(M + H-C6H8O6-C8H6O2),257.0432(M + H- C6H8O6-H2O-CO)
C21H18O13
Quercetin-7-O-β-d-glucuronide isomers
Flavonoid
11
6.75
193.0496
[M−H]-
−2.6
149.0596(M−H−CO2),134.0359 (M−H−CO2−CH3)
C10H10O4
Ferulic acid2
Phenolic acid
12
6.76
177.0549
[M + H]+
−1.7
121.0650(M + H-2CO),134.0686(M + H-CO-CH3),78.0466(M + H-3CO-CH3),162.0316(M + H-CH3)
C10H8O3
7-methoxy coumarin derivative
Coumarin
13
6.95
465.1025
[M + H]+
−1.7
303.00534(M + H-C6H10O5),229.0492(M + H-C6H10O5-H2O-2CO),153.0184(M + H-C6H10O5-C8H6O3), 257.0443(M + H-C6H10O5-H2O-CO),201.0555(M + H-C6H10O5-H2O-3CO)
C21H20O12
Quercetin-7-O-β-d-glucoside
Flavonoid
14
7.37
611.1613
[M + H]+
0.2
465.1033 (M + H-C6H10O4),303.0500(M + H-C6H10O4-C6H10O5),229.0504(M + H-C6H10O4-C6H10O5-H2O-2CO),153.0182(M + H-C6H10O4-C6H10O5-C8H6O3),257.0464(M + H-C6H10O4-C6H10O5-H2O-CO)
C27H30O16
Rutin1
Flavonoid
15
7.43
465.1035
[M + H]+
0.4
303.0499(M + H-C6H10O5),153.0181(M + H-C6H10O5-C8H6O3)
C21H20O12
Hyperoside1
Flavonoid
16
7.57
595.1664
[M + H]+
0.2
287.0548(M + H-C12H20O9),153.0195 (M + H-C12H20O9-C8H6O2)
C27H30O15
Luteolin-3′-rutinoside
Flavonoid
17
7.69
463.0875
[M + H]+
−0.4
287.0549(M + H-C6H8O6),153.0184(M + H-C6H8O6-C8H6O2),135.0437(M + H-C6H8O6-C8H6O2-H2O),241.0508(M + H-C6H8O6-CO-H2O)
C21H18O12
Luteolin-3′-d-glucuronide
Flavonoid
18
8.26
515.1190
[M−H]-
0.0
173.0443(M−H−2C9H6O3−H2O),179.0339(M−H−C9H6O3−C7H10O5),191.0549(M−H−2 C9H6O3),135.0438(M−H−C9H6O3−C7H10O5−CO2),353.0667(M−H−C9H6O3),335.0759(M−H−C9H6O5−H2O)
C25H24O12
3,4-O-Dicaffeoylquinic acid12
Phenolic acid
19
8.54
515.1188
[M−H]-
−0.4
191.0565(M−H−2C9H6O3),179.0353(M−H−C9H6O3−C7H10O5),135.0365(M−H−C9H6O3−C7H10O5−CO2),353.0667(M−H−C9H6O3)
C25H24O12
3,5-O-Dicaffeoylquinic acid12
Phenolic acid
20
9.0
447.0923
[M + H]+
−0.9
271.0621(M + H-C6H8O6),153.0194(M + H-C6H8O6-C8H6O),243.0671(M + H-C6H8O6-CO)
C21H18O11
Apigenin 7-O-glucuronide
Flavonoid
21
9.23
515.1190
[M−H]-
0.0
173.0348(M−H−2C9H6O3−H2O),179.0345(M−H−C9H6O3−C7H10O5),191.0555(M−H−2 C9H6O3),135.0440(M−H−C9H6O3−C7H10O5−CO2),353.0872(M−H−C9H6O3)
C25H24O12
4,5-O-Dicaffeoylquinic acid12
Phenolic acid
22
9.24
177.0546
[M + H]+
−1.1
121.0648(M + H-2CO),133.0646(M + H-CO-CH3-H),78.0468(M + H-3CO-CH3),134.0365(M + H-CO-CH3)
C10H8O3
Methoxy Coumarin
Coumarin
23
10.86
177.0546
[M + H]+
−3.4
121.0648(M + H-2CO),134.0365(M + H-CO-CH3),106.0418((M + H-2CO-CH3),78.0468(M + H-3CO-CH3),162.0313(M + H-CH3), 133.0646(M + H-CO2)
C10H8O3
7-Methoxy Coumarin1
Coumarin
3.1.1 Identification of chlorogenic acids
In mass spectrometry, the major diagnostic fragmentations for chlorogenic acids identification are those fragmentations involving the neutral loss of CO, CO2 and H2O with the major fragment ion at m/z 191.0557, 173.0441 and 93.0286 (Fig. 1C). Nine compounds were thereby categorized into chlorogenic acids according to their retention time as well as the MS/MS fragment ions. Compound 2, 3 and 4 shared the same molecular ion ([M−H]-) at m/z 353.0883, major fragment at m/z 191.0557 [M−H−C9H6O3]-, 179.0344 [M−H−C7H10O5]-, 135.0446 [M−H−C7H10O5−CO2]- and 173.0449 [M−H−C9H6O3−H2O]-. By matching retention time and fragments of reference compound, compound 4 tentatively was elucidated as 3-O-caffeoylquinic acid and the other two compounds were also assumed as caffeoylquinic acid. In addition, Zhang et al. (Zhang et al., 2020) showed that the order of peaks of 3-OH, 4-OH and 5-OH substitutes of quinic acid on reversed-phase column was 5-OH, 4-OH and 3-OH substitutes. Therefore, compound 2 and 3 were tentatively characterized as 5-O-caffeoylquinic acid and 4-O-caffeoylquinic acid. Compound 18, 19 and 21 had the same molecular ion ([M−H]-) at m/z 515.1188 and ([M−H2O + H]+) at m/z 499.1286, major fragments at m/z 173.0348 [M−H−2C9H6O3−H2O]-, 179.0242 [M−H−C9H6O3−C7H10O5]-, 191.0445 [M−H−2C9H6O3]-, 135.0365 [M−H−C9H6O3- C7H10O5-CO2]- and 353.0667 [M−H−C9H6O3]- as the authenticated standard 3,4-O-dicaffeoylquinic acid, 3,5-O-dicaffeoylquinic acid and 4,5-O-dicaffeoylquinic acid. Another example, that compound 8 gave a [M−H]- ion at m/z 367.1024, and with a typical MS/MS fragment at m/z 193.0367 ([Ferulic acid-H]-), indicated as 3-O-feruloylquinic acid. Compound 11 showed [M−H]- ion at m/z 193.0497 ([Ferulic acid-H]-), major fragments at m/z 149.0596(M−H−CO2) and 134.0359 (M−H−CO2−CH3), thus compound 11 was identified as Ferulic acid.
3.1.2 Identification of flavonoids
The major diagnostic fragmentations for flavonoid identification are those ions resulted from the retro-Diels-Alder (RDA) dissociation of the C-ring and the neutral loss of CO (Fig. 1D). These ions provide information on the number and type of substituents in A- and B-rings. The MS/MS spectrums of compounds 5, 7, 9, 10, 13, 14 and 15 (5, 9, 11, 12, 15, 16 and 17) showed the presence of fragmentation at m/z 303.0520 (ESI + ) similar to quercetin. The MS/MS fragments at m/z 257.0400, 229.0512 and 201.0593 showed the neutral loss of CO. Other fragments signals at m/z 153.0139 was also observed and corresponded to the RDA dissociation of the C-ring of quercetin, except for compound 10 which presented a fragment ion at m/z 169.0145 without m/z 153.0139. Thus, compounds 5, 7, 9, 13, 14 and 15 were proposed as quercetin derivatives, and compound 10 was proposed as isoquercetin derivative which is facilitated by three hydroxyl groups of A ring. Compound 14 produced the same molecular ion at m/z 611.1613[M + H]+ and retention time as reference rutin, and the proposed fragmentation patterns was showed in Fig. 1D. Compound 15 produced the same molecular ion at m/z 465.1035 [M + H]+ and retention time as reference hyperoside. Compound 13 showed mass ions at m/z 465.1025[M + H]+ and 303.0534 [M + H-C6H10O5]+ indicating to the loss of glucose residue. Consequently, compound 13 was concluded to be quercetin-7-O-β-d-glucoside. Thus, compounds 5, 7 and 9 were unambiguously assigned as quercetin-7-O-β-d-glucuronide, isorutin and quercetin-7-O-rutinoside. Compounds 16 and 17 exhibited significantly similar fragmentations of m/z 153.0181, corresponding to the RDA dissociation of quercetin. Compound 17 showed parent ions at m/z 463.0875 [M + H]+ owing to a glucuronide to 3′-OH. In ESI(+)-MS/MS of compound 17, we could observe several fragment ions, such as m/z 153.0184 [M + H-C6H8O6-C8H6O2]+, 135.0437 [M + H-C6H8O6-C8H6O2-H2O]+, 241.0508 [M + H-C6H8O6-CO-H2O]+ originated from the parent ion at m/z 463.0875. Luteolin-3′-rutinoside and luteolin-3′-d-glucuronide were proposed to compounds 16 and 17. Compound 20 presented a molecular ion at m/z 447.0923 [M + H]+. The MS/MS data showed a fragment signal at m/z 271.0621[M + H-C6H8O6]+, corresponding to the loss of an O-glucuronide. Other fragment signals were observed at m/z 153.0194 [M + H-C6H8O6-C8H6O]+, 243.0671 [M + H-C6H8O6-CO]+. These fragmentations were in agreement with the presence of apigenin. Finally, compound 20 was plausibly established as apigenin-7-O-glucuronide.
3.1.3 Identification of coumarins
Compounds 22 and 23 exhibited the same ion at m/z 177.0546[M + H]+ which was observed as base peak. The MS/MS data showed fragment ions at m/z 121.0648 [M + H-2CO]+ and 134.0365 [M + H-CO-CH3]+ which are characteristics of methoxy coumarin fragmentations of loss of CO and CH3 (Fig. 1E). The signal of m/z 106.0418 [M + H-2CO-CH3]+, 78.0468 [M + H-3CO-CH3]+ and 162.0313 [M + H-CH3]+, was observed with a low relative intensity. Compared with reference 7-methoxy coumarin, compound 23 showed same retention time, molecular ion and MS/MS fragment ions, which was identified to 7-methoxy coumarin. In comparison to compound 23, the MS/MS data of compound 22 showed different intensities of m/z 133.0646 [M + H-CO-CH3-H]+ and 134.0365 [M + H-CO-CH3]+, which was assumed to be iso7-methoxy coumarin. Compound 12 eluted at 6.76 min which showed same fragment ions with compound 25 at m/z 177.0549, 121.0650, 134.0686, 78.0466 and 162.0316 considered as the 7-methoxy coumarin derivative.
All of the identified compounds are present in many plants; however, only five of the above identified compounds (4, 11, 18, 19 and 21) have been reported in Artemisia japonica (Gu and Tu, 1993; Wang, 2008; Ou Yang et al., 2021), while the remaining compounds have been reported in other Artemisia plants (Gu et al., 2012; Lu et al., 2014; Lu et al., 2015; Zhang et al., 2017; Yu and Gao, 2019; Zhang et al., 2020). In general, these results provide an adequate basis for further quantitative and structural analysis of the active phenolic compounds responsible for the beneficial health effects of Artemisia japonica.
3.2 Antioxidant activity of AJLEE
Free radicals are one of the main factors leading to oxidative cell injury. Polyphenol compounds usually contain multiple hydroxyl groups, which can directly capture free radicals and terminate free radical chain reactions (Wu et al., 2021). Therefore, radical scavenging ability assays (DPPH• and ABTS+•) and the FRAP assay were used to evaluate the antioxidant activity of AJLEE. To provide context for the measured antioxidant activity values of AJLEE, Vc was used as a positive control. The results are shown in Fig. 2. In the range of 20.00–100.00 μg/mL, AJLEE effectively scavenges DPPH• radicals in a dose-dependent manner. Similarly, in the range of 31.25–500.00 μg/mL, it scavenges ABTS+• radicals and promotes the reduction of Fe3+ to Fe2+ in a dose-dependent manner. The IC50 values of AJLEE for DPPH• and ABTS+• and the OD50 values of AJLEE for FRAP were determined as 49.49 ± 1.90, 172.37 ± 25.72 and 95.88 ± 5.79 μg/mL, respectively.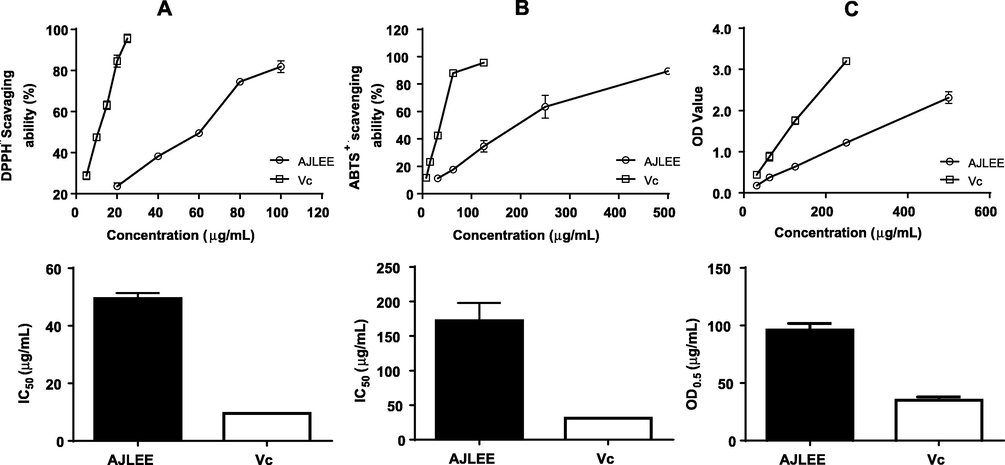
Antioxidant activity of AJLEE. Free radical scavenging activity of AJLEE as determined by (A) DPPH• and (B) ABTS+•scavenging assays. (C) Reducing power as estimated via a ferric-reducing antioxidant power assay. Values are expressed as means ± standard deviation (SD) from three experiments.
3.3 Protective effect of AJLEE on H2O2-Induced cytotoxicity in AML12 cells
H2O2 can induce cell oxidative stress, resulting in cell damage or even death. Antioxidants exhibiting protective effects against H2O2-induced cell damage act by either directly scavenging reactive oxygen species (ROS) or indirectly upregulating the antioxidant defense system, and they are beneficial for health (Liu et al., 2018). AJLEE is rich in polyphenolics and has shown remarkable DPPH• and ABTS+• free radical scavenging activity and reducing potential based on FRAP, and it also displayed low cytotoxicity toward AML12 cells, as no significant cell-growth-inhibitory activity of AJLEE was observed in the range of 25–200 μg/mL (Fig. 3A). This result is consistent with the literature (Huang et al., 2010; Zhang et al., 2011), indicating that Artemisia japonica is a low-toxicity herb.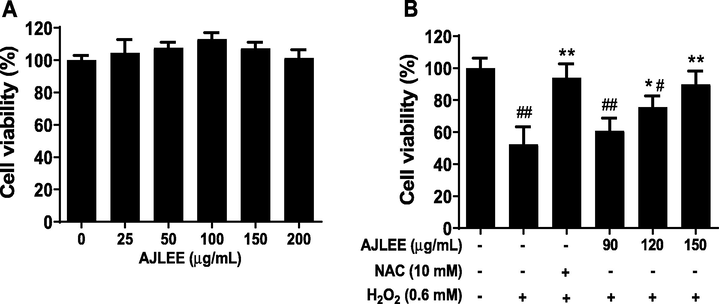
Protective effect of AJLEE toward H2O2-induced AML12 cell injury: (A) influence of AJLEE on AML12 cell proliferation; (B) cell-protective effect of AJLEE toward H2O2-induced cell injury. Values are expressed as means ± standard deviation (SD) from three experiments. #P < 0.05, ##P < 0.01 compared with the control group; *P < 0.05, **P < 0.01 compared with the model group.
To evaluate the cytoprotective effect of AJLEE, a H2O2-induced cell injury model was utilized. As shown in Fig. 3B, 0.60 mM H2O2 treatment dramatically reduced viability of AML12 cells, which was significantly attenuated by 10 mM NAC pretreatment. Compared with the H2O2 treatment group, the low-dose treatment group (90 μg/mL) failed to effectively protect cells from damage, but, in general, the protective effect of AJLEE was dose-dependent. In particular, the high-dose treatment group (150 μg/mL), AJLEE almost completely abolished H2O2 cytotoxicity.
3.4 Effect of AJLEE on H2O2-Induced oxidative stress in AML12 cells
H2O2 can induce the production of a large number of ROS, placing AML12 cells in a state of extreme oxidative stress. As depicted in Fig. 4A, the intracellular fluorescence intensity, indicative of ROS activity, significantly increased after 0.60 mM H2O2 exposure, and pretreatment with antioxidant NAC and various doses of AJLEE significantly mitigated this increase in intracellular fluorescence intensity. IPP6.0 software calculation results showed that, compared with the control group, the average fluorescence intensity in the H2O2 exposure group increased by 123.14 ± 30.80 times, and AJLEE pretreatment could effectively eliminate ROS in a dose-dependent manner in the range of 90–150 μg/mL (Fig. 4B). H2O2-induced ROS production in cells increases the level of the lipid peroxidation product MDA, which serves as a reliable marker of oxidative stress (Pinya et al., 2021). As expected, the MDA levels in AML12 cells treated with 0.6 mM H2O2 increased significantly, from 4.92 ± 0.33 to 7.72 ± 0.40 μM (Fig. 4C). Pretreatment with 90, 120 and 150 μg/mL of AJLEE, resulted in lower MDA content of 6.26 ± 0.29, 5.73 ± 0.46 and 4.37 ± 0.22 μM, respectively. These results suggest that AJLEE may play a protective role against H2O2-induced AML12 cell injury by regulating oxidative stress.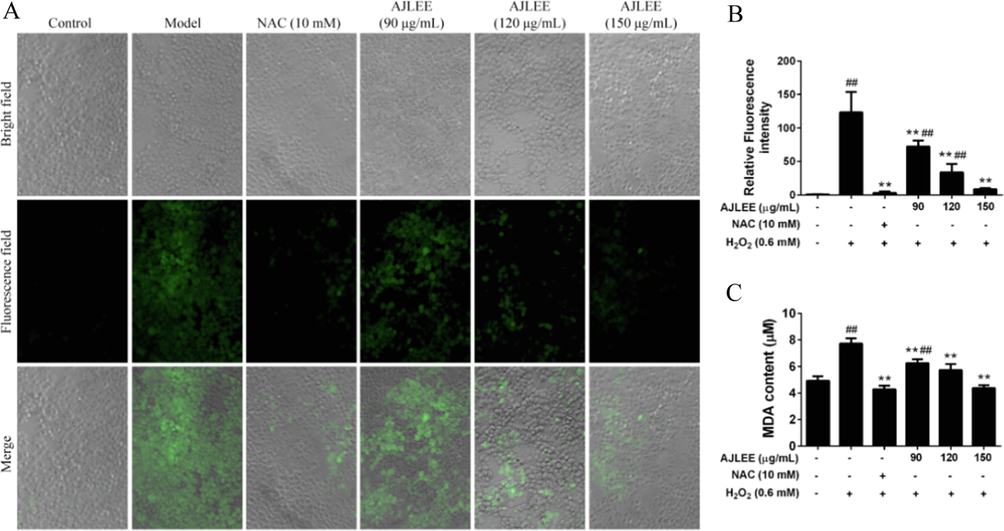
Antioxidant effect of AJLEE on H2O2-induced AML12 cells. (A) Images of AML12 cells treated with NAC (10 mM) or different concentrations of AJLEE (90, 120 and 150 μg/mL) for 6 h and then stimulated with 0.6 mM H2O2 for 30 min, followed by staining with 10 μM DCFH-DA for 30 min. After washing with PBS three times, cells were photographed using the ImageXpress Micro XLS system. (B) The fluorescence intensities as analyzed using IPP6.0 and expressed as a percentage of relative ROS level versus control cells. (C) The MDA content in the supernatant of AML12 cells pretreated with NAC (10 mM) or different concentrations of AJLEE (90, 120 and 150 μg/mL) for 1 h and then exposed to 0.6 mM H2O2 for 24 h. Values are expressed as means ± standard deviation (SD) from three experiments. ##P < 0.01 compared with the control group; **P < 0.01 compared with the model group.
3.5 Effects of AJLEE on liver index (Liver Weight/Body Weight, mg/g)
Liver cell injury will lead to liver tissue edema and increased liver index values. As depicted in Fig. 5A, ANIT exposure dramatically increased the liver index value compared with the control group (7.38 ± 3.94 mg/g versus 51.58 ± 3.40 mg/g; P < 0.01). AJLEE pretreatment at the dosage of 400 mg/kg resulted in a significantly reduced liver index value (62.95 ± 3.37 mg/g; P < 0.01). However, AJLEE (200 mg/kg) and positive control UDCA (100 mg/kg) failed to effectively reduce the measured liver index.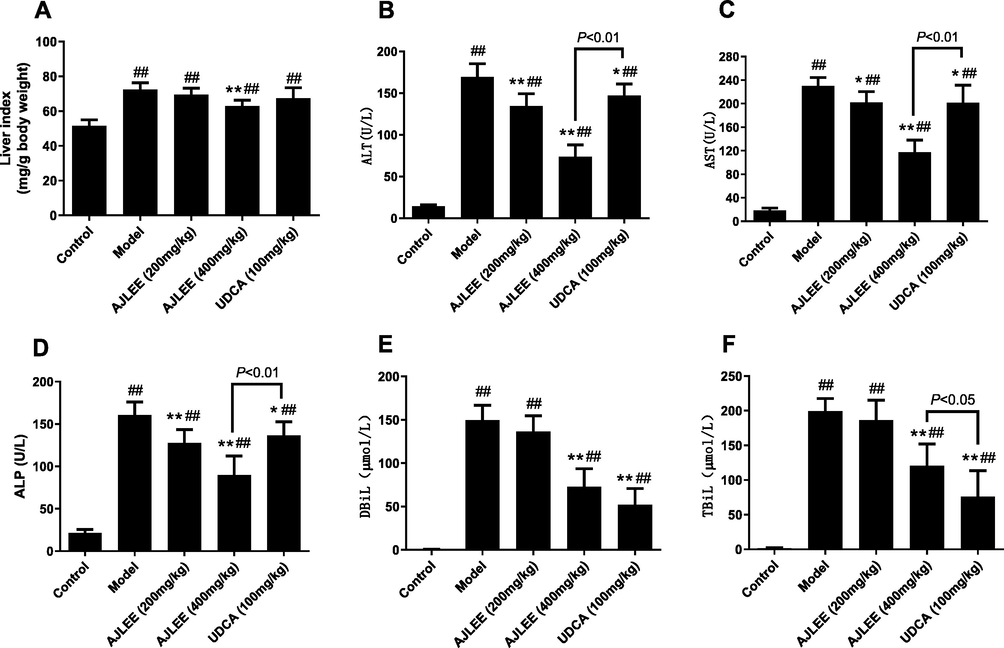
Effects of AJLEE on liver index and serum biochemical indicators: (A) liver index; (B) alanine transaminase (ALT); (C) aspartate transaminase (AST); (D) alkaline phosphatase (ALP); (E) total bilirubin (TBiL) and (F) direct bilirubin (DBiL). Data are expressed as the mean ± SD (n = 8 in each group). ##P < 0.01 compared with the control group; *P < 0.05, **P < 0.01 compared with the model group.
3.6 Effects of AJLEE on serum levels of hepatic injury biochemical indicators
Consistent with the liver index, the levels of serum ALT, AST, ALP, TBiL and DBiL in the model group significantly increased after ANIT treatment (Fig. 5B-F), indicating that ANIT caused liver injury in the mice. Both AJLEE and UDCA treatment can effectively attenuate the increases in the biochemical indicators of liver injury. Compared with the model group, high-dose AJLEE (400 mg/kg) treatment showed a remarkable hepatic protective effect, and the levels of serum ALT, AST, ALP, TBiL and DBiL were significantly reduced (P < 0.01). Compared with the high-dose AJLEE treatment group, the low-dose AJLEE (200 mg/kg) and UDCA treatment groups showed a weaker effect regarding the reduction in ALT, AST and ALP levels. However, UDCA showed excellent results in reducing TBiL and DBiL levels, which may be attributed to its cholagogic properties (Gregory, 2000).
3.7 Effects of AJLEE on hepatic levels of GSH, SOD, CAT and MDA
An imbalance in the oxidation and antioxidation system is one of the most important causes of liver injury. Therefore, the levels of GSH, SOD, CAT and MDA in liver tissues were measured using commercial assay kits. ANIT exposure significantly elevated liver MDA levels (Fig. 6A); in contrast, AJLEE and UDCA treatment at different doses dramatically reduced the hepatic content of MDA compared with the model group. Obviously, the increase in MDA caused by the intake of ANIT is closely related to reduced levels of the antioxidant-related enzymes GSH, SOD and CAT (Fig. 6B, C, D), and AJLEE and UDCA administration partially reversed the adverse effects of ANIT. In addition, the effect of high-dose AJLEE in increasing the levels of these antioxidants was superior to that of UDCA.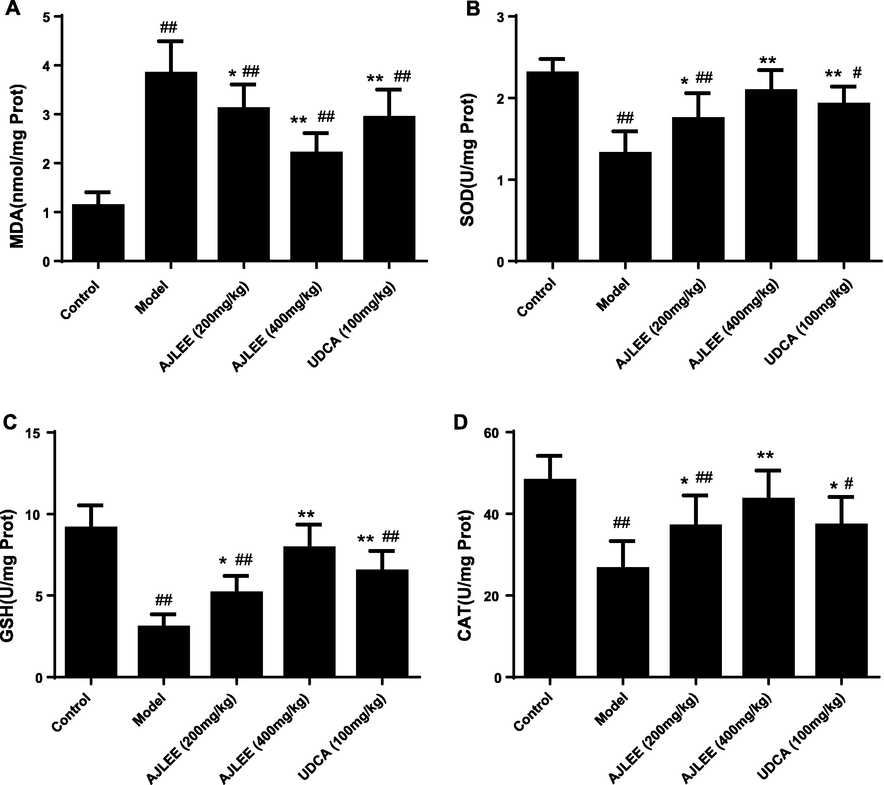
Effects of AJLEE on the levels of hepatic (A) MDA, (B) SOD, (C) GSH and (D) CAT. Data are expressed as the mean ± SD (n = 8 in each group). #P < 0.05, ##P < 0.01 compared with the control group; *P < 0.05, **P < 0.01 compared with the model group.
3.8 Effects of AJLEE on liver antioxidant enzyme expression
To further clarify the possible antioxidant mechanism of AJLEE against ANIT- induced liver injury, the mRNA levels of detoxification enzymes Sod1, Sod2, Cat, Gpx4 and Ho-1 were determined using qRT-PCR. Compared with the control group, mRNA levels of all the above antioxidant enzymes, except Sod2, were significantly downregulated in the model group (Fig. 7). All the groups of treatments could efficiently upregulate the Sod1, Cat, Gpx4 and Ho-1 mRNA levels. In general, the mRNA levels of antioxidant enzymes in the high-dose AJLEE treatment group (400 mg/kg) increased more significantly than those in other treatment groups; in particular, the Ho-1 gene’s mRNA level increased to 3.27 ± 0.93 times that in the control group and was significantly higher than that in the UDCA (100 mg/kg) treatment group (Fig. 7E).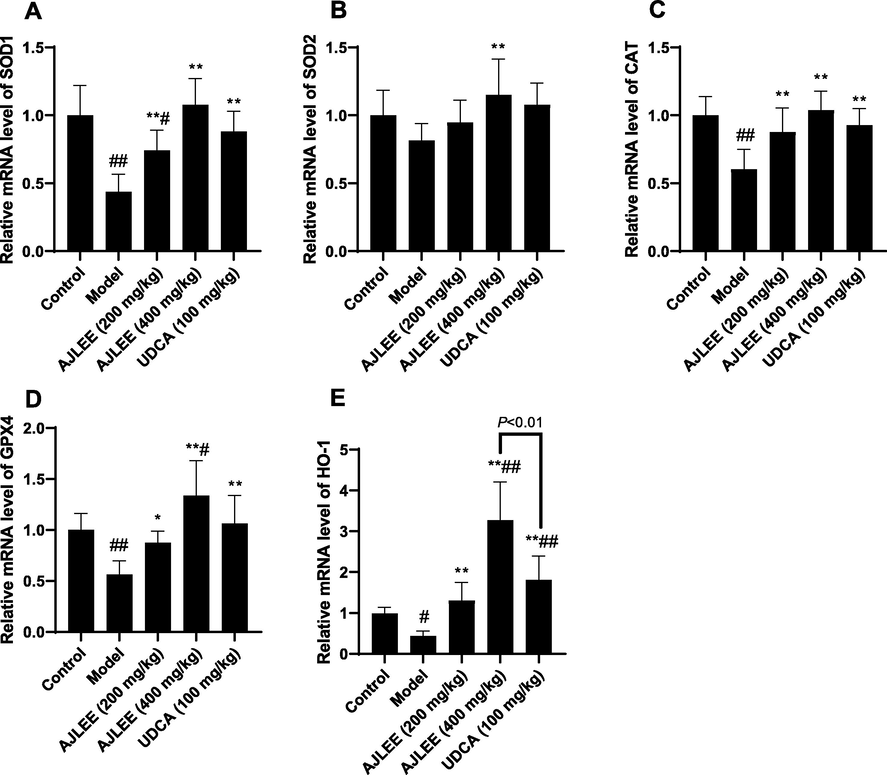
Relative mRNA levels of detoxification enzymes in liver: (A) SOD1; (B) SOD2; (C) CAT; (D) GPX4; (E) HO-1. Data are expressed as the mean ± SD (n = 8 in each group). #P < 0.05, ##P < 0.01 compared with the control group; *P < 0.05, **P < 0.01 compared with the model group.
3.9 Effects of AJLEE on histopathological changes in liver tissue
Liver sections stained with HE was applied to determine changes in liver histology. The liver tissue structure of the control group was normal, and no abnormal morphological changes were observed (Fig. 8A). On the contrary, hepatocytes in the model group were significantly swollen and degenerated, and a large amount of hepatocyte necrosis and inflammatory cell infiltration was observed. Mice treated with AJLEE and UDCA exhibited significantly reduced liver injury. In particular, high-dose AJLEE treatment remarkably ameliorated the hepatocyte necrosis score (Fig. 8B), accompanied by less inflammatory cell infiltration.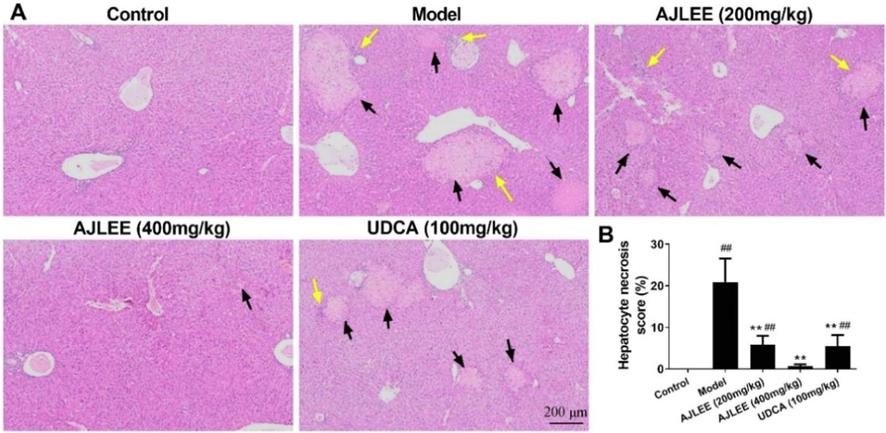
Effect of AJLEE on histological changes in liver tissue. (A) HE-stained liver sections of different treatment groups. Black arrows indicate necrosis of hepatocytes, and yellow arrows indicate infiltration of inflammatory cells. (B) Hepatocyte necrosis score: Three visual fields were randomly selected for each sample. The necrosis area in each field was measured with Image pro plus 6.0 (Media Cybernetics Co., USA), and the proportion of necrosis area in the field was calculated.. Data are expressed as the mean ± SD (n = 8 in each group). ##P < 0.01 compared with the control group; **P < 0.01 compared with the model group.
4 Discussion
For centuries, Artemisia japonica leaves have been used not only as food and tea, but also as traditional Chinese medicine. However, their chemical composition in terms of compounds responsible for the observed health benefits is still unknown. Natural plant polyphenols have antioxidant, anti-inflammatory, antibacterial and other types of biological activity and show broad application prospects in food, health products and the pharmaceutical industry. As expected, AJLEE is abundant in polyphenols, and the total phenolic and flavonoid contents reached 123.14 ± 8.44 mg GAE/g and 49.37 ± 2.24 mg RUT/g, respectively, in this study. It also showed strong DPPH• and ABTS+• radical scavenging activity and FRAP reduction ability (Fig. 2).
To better uncover the chemical profile of AJLEE, UPLC/QTOF-MS qualitative analyses was conducted. The results showed that AJLEE is rich in phenolic acids, flavonoids and coumarins. From the signal intensity of MS peaks, the content of chlorogenic acids was particularly high (Fig. 1), and these compounds are often used as quality control indicators in the same genus of plant Artemisia capillaris Thunb., which is a well-known traditional Chinese medicine, commonly used to treat hepatitis (Tian et al., 2020). Chlorogenic acid is a well-known natural plant polyphenol with significant free radical scavenging activity (Miao and Xiang, 2020). Compared with chlorogenic acid, isochlorogenic acid A contains two caffeic acyl groups, has more phenolic hydroxyl groups and also shows significant antioxidant activity according to indicators such as DPPH•, ABTS+• and FRAP (Wang et al., 2020). The scavenging activity of these two compounds toward some free radicals is even superior to that of butyl hydroxytoluene and vitamin C, which are usually used as positive controls (Miao and Xiang, 2020; Wang et al., 2020). Considerable research has indicated that both chlorogenic acid and isochlorogenic acid A exert strong antioxidant and anti-inflammatory activity and show potential beneficial effects in treatment of diabetes and chronic liver and cardiovascular diseases (Miao and Xiang, 2020; Wang et al., 2020). Consequently, they have been used as bioactive markers for the quality control of some important Chinese herbal medicines, such as Erycibe obtusifolia Benth. stem (Xu et al., 2015), the flowers of Lonicera japonica Thunb. (He et al., 2015) and Tussilago farfara L. (He et al., 2013), as well as a series of prescriptions containing these herbs. Regarding flavonoids, another major polyphenol component in AJLEE, 7 of the 10 tentatively identified compounds are quercetin glycoside derivatives. Studies have shown that quercetin has significant free radical scavenging activity, whereby the antioxidant activity is closely related to the catechol group in the B ring, the 2,3-double bond in conjugation with a 4-oxo function in the C ring and the –OH groups at positions 3 and 5 in the heterocyclic ring (Wang et al., 2016). Quercetin, luteolin and apigenin glycoside derivatives identified in AJLEE contain the above antioxidant-associated structural characteristics; therefore, they all possess strong antioxidant activity, which has been confirmed by numerous publications (Meng et al., 2008; Wu et al., 2012; Wang et al., 2019). These polyphenols therefore clearly and significantly contribute to the DPPH• and ABTS+• radical scavenging activity and FRAP reduction power of AJLEE.
In addition to free radical scavenging activity in vitro, antioxidant activity at the level of cellular and in vivo animals is also critical. Hence, a H2O2-induced AML12 cell injury model and ANIT-induced mice liver injury model were adopted to evaluate the effects of AJLEE on aspect of oxidative stress regulation. The results indicated that H2O2 exposure dramatically reduces AML12 cell viability, which could be dose-dependently reversed by pretreatment with AJLEE in the range of 90–150 µg/mL (Fig. 3B). Correspondingly, the ROS burst and MDA increase induced by H2O2 also decrease in a dose-dependent manner in response to AJLEE pretreatment (Fig. 4). More importantly, AJLEE can effectively reduce the liver injury caused by ANIT in mice at intragastric doses of 200 and 400 mg/kg. Especially in the high-dose treatment group, the hepatocyte necrosis scores in liver tissue sections stained with HE (Fig. 8) and the levels of serum biochemical indicators of liver injury—namely, ALT, AST and ALP—were significantly lower than those in the positive control group treated with UDCA (100 mg/kg) (Fig. 5). Chlorogenic acid and its derivatives (such as isochlorogenic acid A, isochlorogenic acid B and isochlorogenic acid C) have long been proved to have protective effects on cell damage caused by oxidative stress (Jiang et al., 2014; Miao and Xiang, 2020; Wang et al., 2020; Wu et al., 2012), and also showed remarkable protective effects on liver injury induced by various insults, such as carbon tetrachloride, acetaminophen and lipopolysaccharide (Chen et al., 2019; Jiang et al., 2014; Sun et al., 2014; Wang et al., 2020; Zheng et al., 2015). Therefore, the protective effect of AJLEE on AML12 cells against oxidative damage and ANIT-induced liver injury in mice may be partially attributed to the high content of chlorogenic acids.
The endogenous defense system of organisms is mainly composed of antioxidant enzymes such as SOD, CAT and glutathione peroxidase, which are responsible for maintaining the oxidative balance. However, ANIT exposure significantly reduced the levels of these antioxidant enzymes (Figs. 6 and 7), leading to an imbalance in the oxidation–antioxidant system. In addition to the direct free radical scavenging activity, the hepatoprotective activity of AJLEE may be further attributed to the induced expression of antioxidant enzymes, which can be confirmed by the increased activity of SOD, CAT and GSH in liver tissue (Fig. 6) and the elevated mRNA levels of the Sod1, Sod2, Gpx4, Cat and Ho-1 genes in AJLEE-treated (400 mg/kg) liver tissue (Fig. 7). These results were consistent with those reported by (Zhang et al., 2011) on the protective effect of Artemisia japonica extract on immunological liver injury in mice. In particular, the Ho-1 mRNA levels were significantly higher than in the UDCA treatment group (Fig. 7E). It is well known that Ho-1 gene expression is regulated by nuclear factor erythroid 2-related factor 2 (Nrf2), which is one of the most important transcription factors regulating the expression of antioxidant enzymes (Kensler et al., 2007), suggesting that AJLEE may play an important role in the regulation of the Nrf2 signaling pathway. There is sufficient evidence that chlorogenic acid (Miao and Xiang, 2020), isochlorogenic acid A (Wang et al., 2020) and quercetin, luteolin and apigenin glycoside derivatives (Tumova et al., 2019; Cho et al., 2020; Ha et al., 2021; Xu et al., 2021), the main components of AJLEE, can effectively prevent oxidative stress-related disease by regulating the Nrf2 signaling pathway and promoting the expression of the above antioxidant enzymes. Consequently, the results of the current work are consistent with the relevant literature.
Moreover, three coumarins were also detected in AJLEE. Extensive studies have shown that plant-derived coumarins largely occur in vegetables and fruits and are beneficial for health as dietary antioxidants (Chang et al., 2021). Moreover, 7-methoxycoumarin widely exists in many common edible plants, such as celery, carrot and coriander, and it shows promising antigenotoxicity against H2O2-induced DNA damage in human blood lymphocytes (Rezaee et al., 2014). Therefore, coumarins represent one of the most important beneficial health ingredients in AJLEE.
5 Conclusions
In summary, our results show that AJLEE is rich in polyphenols, and the total phenol content reached 123.14 ± 8.44 mg GAE/g. AJLEE polyphenols at a dose of 400 mg/kg were found to remarkably alleviate liver injury induced by ANIT, and AJLEE can also effectively block H2O2-induced AML12 cell death in a concentration-dependent manner in the range of 90–150 µg/mL. The protective effect of AJLEE polyphenols was suggested to be mediated through its excellent antioxidant properties, including the direct scavenging of free radicals and the increased mRNA and protein levels of antioxidant enzymes. These properties may be attributed to the abundant phenolic acids, flavonoids and coumarins in AJLEE identified by UPLC/QTOF-MS, such as chlorogenic acid, isochlorogenic acid A, 7-methoxycoumarin and quercetin, apigenin and luteolin glycoside derivatives. Herein, it was shown that AJLEE polyphenols have the potential to protect against liver injury and are beneficial for human health, and these properties are likely to contribute to the plant’s future application in food, tea, cosmetics and the pharmaceutical industry. AJLEE poly-phenols show significant hepatoprotective activity and promote the expression of antioxidant enzymes; thus, AJLEE is worthy of further study regarding its active components and their corresponding molecular mechanisms.
Funding
The present research was carried out with the financial support of the Science Foundation of Zhejiang Chinese Medical University (Project NO. 2020ZG31 and 2020ZG35).
Acknowledgements
We thank MDPI (www.mdpi.com) for its linguistic assistance during the preparation of this manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- An update of prenylated phenolics: Food sources, chemistry and health benefits. Trends Food Sci. Tech.. 2021;108:197-213.
- [CrossRef] [Google Scholar]
- Hepatoprotective effect of chlorogenic acid against chronic liver injury in inflammatory rats. J. Funct Foods. 2019;62:103540
- [CrossRef] [Google Scholar]
- Anti-Inflammatory and Anti-Oxidative Effects of luteolin-7-O-glucuronide in LPS-Stimulated Murine Macrophages through TAK1 Inhibition and Nrf2 Activation. Int. J. Mol. Sci.. 2020;21:2007.
- [CrossRef] [Google Scholar]
- Management of cholestatic disease in 2017. Liver Int.. 2017;37(Suppl 1):123-129.
- [CrossRef] [Google Scholar]
- Three new eudesmanes from Artemisia japonica. Nat. Prod. Res.. 2014;28:631-635.
- [CrossRef] [Google Scholar]
- Mechanisms of cell injury and death in cholestasis and hepatoprotection by ursodeoxycholic acid. J. Hepatol.. 2000;32:11-13.
- [CrossRef] [Google Scholar]
- Gu, Y. C., Tu, Y. Y., 1993. Studies on the chemical constituents of Japanese Wormwood (Artemisia japonica). Chin. Tradit. Herb. Drugs. 24, 122-124+166. http://www.cnki.com.cn/Article/CJFDTOTAL-ZCYO199303002.htm.
- Characterization and identification of chemical compositions in the extract of Artemisia rupestris L. by liquid chromatography coupled to quadrupole time-of-flight tandem mass spectrometry. Rapid Commun. Mass Spectrom.. 2012;26:83-100.
- [CrossRef] [Google Scholar]
- Anti-Inflammatory, Antioxidant, Moisturizing, and Antimelanogenesis Effects of Quercetin 3-O-beta-D-Glucuronide in Human Keratinocytes and Melanoma Cells via Activation of NF-kappaB and AP-1 Pathways. Int. J. Mol. Sci.. 2021;23:433.
- [CrossRef] [Google Scholar]
- Determination of 10 Active Constituents in Farfarae Flos by Quantitative Analysis of Multi-Components by Single Marker. Chin. J. Pharm. Anal.. 2013;33:1518-1524.
- [Google Scholar]
- Quality Control of Lonicerae Japonicae Flos by Multi-Marker Components Quantitative Fingerprint. J. Chin. Pharm. Sci.. 2015;50:1237-1242.
- [Google Scholar]
- Xian Dai Ben Cao Gang Mu:. 2001;Volume I:1325.
- Huang, T. H., Xian-Ming, L. U., Chen, S. L., 2010. Pharmacodynamics Research and safety evaluation of the Folk Herb-Artemisia japonica Thumb. J. Changchun Univ. Tradit. Chin. Med. 33, 77-79. https://doi.org/ 10.13593/j.cnki.51-1501/r.2010.02.023.
- Protection of Flos Lonicerae against acetaminophen-induced liver injury and its mechanism. Environ. Toxicol. Pharmacol.. 2014;38:991-999.
- [CrossRef] [Google Scholar]
- Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol.. 2007;47:89-116.
- [CrossRef] [Google Scholar]
- Phytochemical constituents of Artemisia japonica ssp. littoricola. Arch. Pharm. Res.. 2001;24:194-197.
- [CrossRef] [Google Scholar]
- Effects and mechanisms of α-naphthalene isothiocyanate on liver in mice. J. Toxicol.. 2012;26:340-343.
- [Google Scholar]
- Role of ROS and Nutritional Antioxidants in Human Diseases. Front. Physiol.. 2018;9:477.
- [CrossRef] [Google Scholar]
- Effect of Hirudin on farnesol X receptor pathway during acute intrahepatic cholestasis. Gastroenterol. Hepatol. Res.. 2022;4:29-35.
- [Google Scholar]
- Solvent optimization, antioxidant activity, and chemical characterization of extracts from Artemisia selengnesis Turcz. Ind. Crops Prod.. 2014;56:223-230.
- [CrossRef] [Google Scholar]
- Metabolic profiling of antioxidants constituents in Artemisia selengensis leaves. Food Chem.. 2015;186:123-132.
- [CrossRef] [Google Scholar]
- Antioxidant activities of polyphenols extracted from Perilla frutescens varieties. Molecules.. 2008;14:133-140.
- [CrossRef] [Google Scholar]
- The impact of flavonoids-rich Ziziphus jujuba Mill. Extract on Staphylococcus aureus biofilm formation. BMC Complement Med. Ther.. 2020;20:187.
- [CrossRef] [Google Scholar]
- Pharmacological action and potential targets of chlorogenic acid. Adv. Pharmacol.. 2020;87:71-88.
- [CrossRef] [Google Scholar]
- Change in hepatic antioxidant defense system with liver injury development in rats with a single alpha-naphthylisothiocyanate intoxication. Toxicology.. 1999;139:265-275.
- [CrossRef] [Google Scholar]
- Effect of melatonin on changes in hepatic antioxidant enzyme activities in rats treated with alpha-naphthylisothiocyanate. J. Pineal. Res.. 2001;31:370-377.
- [CrossRef] [Google Scholar]
- Ou Yang, Y. T., Tan, Z. B., Chen, L. M., 2021. Simultaneous Determination of Three Phenolic Acids in Artemisia Japonica by QAMS. Pharmacy. Today. 31, 23-26+31. https://doi.org/ 10.12048 /j.issn.1674-229X.2021.01.004.
- Physiological biomarkers in loggerhead turtles (Caretta caretta) as a tool for monitoring sanitary evolution in marine recovery centres. Sci. Total Environ.. 2021;757:143930
- [CrossRef] [Google Scholar]
- Antigenotoxic activities of the natural dietary coumarins umbelliferone, herniarin and 7-isopentenyloxy coumarin on human lymphocytes exposed to oxidative stress. Drug Chem. Toxicol.. 2014;37:144-148.
- [CrossRef] [Google Scholar]
- Nutraceutical Properties of Polyphenols against Liver Diseases. Nutrients.. 2020;12:3517.
- [CrossRef] [Google Scholar]
- A Polyphenol-Rich Fraction from Eugenia uniflora Exhibits Antioxidant and Hepatoprotective Activities In Vivo. Pharmaceuticals (Basel).. 2020;13:84.
- [CrossRef] [Google Scholar]
- Protective effect of chlorogenic acid against carbon tetrachloride-induced acute liver damage in rats. Chin. Herb. Med.. 2014;6:36-41.
- [CrossRef] [Google Scholar]
- Quantitative analysis of six phenolic acids in artemisia capillaris (Yinchen) by HPLC-DAD and their transformation pathways in decoction preparation process. J. Anal. Methods Chem.. 2020;2020:8950324.
- [CrossRef] [Google Scholar]
- Research Advances on Health Effects of Edible Artemisia Species and Some Sesquiterpene Lactones Constituents. Foods.. 2020;10:65.
- [CrossRef] [Google Scholar]
- Anti-Obesity Effect of Nostoc commune Ethanol Extract In Vitro and In Vivo. Nutrients.. 2022;14:968.
- [CrossRef] [Google Scholar]
- Long term treatment with quercetin in contrast to the sulfate and glucuronide conjugates affects HIF1alpha stability and Nrf2 signaling in endothelial cells and leads to changes in glucose metabolism. Free Radic. Biol. Med.. 2019;137:158-168.
- [CrossRef] [Google Scholar]
- Extraction and analysis for insecticidal active components of six plants of artemisia. Harbin: Northeast Forestry University; 2008. https://d.wanfangdata.com.cn/thesis/Y2991399
- Anti-oxidant Chemical Constituents from Patrinia Villosa. Chin. Herb. Med.. 2019;50:5206-5211.
- [CrossRef] [Google Scholar]
- Isochlorogenic acid (ICGA): natural medicine with potentials in pharmaceutical developments. Chin. J. Nat. Med.. 2020;18:860-871.
- [CrossRef] [Google Scholar]
- The biological activities, chemical stability, metabolism and delivery systems of quercetin: A review. Trends Food Sci. Tech.. 2016;56:21-38.
- [CrossRef] [Google Scholar]
- The hepatoprotective effects of Sedum sarmentosum extract and its isolated major constituent through Nrf2 activation and NF-kappaB inhibition. Phytomedicine.. 2019;53:263-273.
- [CrossRef] [Google Scholar]
- Potential implications of polyphenols on aging considering oxidative stress, inflammation, autophagy, and gut microbiota. Crit. Rev. Food Sci. Nutr.. 2021;61:2175-2193.
- [CrossRef] [Google Scholar]
- The Composition and Anti-Aging Activities of Polyphenol Extract from Phyllanthus emblica L. Fruit. Nutrients.. 2022;14:857.
- [CrossRef] [Google Scholar]
- Protective properties of laggera alata extract and its principle components against d-Galactosamine-Injured hepatocytes. Sci. Pharm.. 2012;80:447-456.
- [CrossRef] [Google Scholar]
- Antioxidant activities of extract and fractions from receptaculum nelumbinis and related flavonol glycosides. Int. J. Mol. Sci.. 2012;13:7163-7173.
- [CrossRef] [Google Scholar]
- Determination of scopolin, chlorogenic acid, scopoletin, isochlorogenic acid A, isochlorogenic acid B and isochlorogenic acid C in plants of Erycibe. China J. Chin. Mater. Med.. 2015;40:1119-1122.
- [Google Scholar]
- Scutellarin protects against diabetic cardiomyopathy via inhibiting oxidative stress and inflammatory response in mice. Ann. Palliat. Med.. 2021;10:2481-2493.
- [Google Scholar]
- Identification of chemical components in capillary wormwood herb by UPLC-Q-TOF/MS. Cent. South Pharm.. 2019;17:656-661.
- [CrossRef] [Google Scholar]
- Study on Processing Technology for Artemisia japonica T. Tea. Food Ferment. Ind.. 2008;32:168-171.
- [Google Scholar]
- Chemical profiling of Artemisia rupestris using HPLC-IT-TOF-MS. Chin. Pharm. J.. 2020;45:4658-4666.
- [Google Scholar]
- Antioxidation and Genetic Toxicity of Artemisia japonica Extract. Nat. Prod. Res.. 2011;23:39-42.
- [Google Scholar]
- Phytochemistry and bioactivities of sesquiterpenoids from the Artemisia species. J. Chin. Pharm. Sci.. 2017;26:317-334.
- [CrossRef] [Google Scholar]
- Metabolic profiling of antioxidants constituents in Artemisia selengensis leaves. Food Chem.. 2015;186:123-132.
- [CrossRef] [Google Scholar]
- The therapeutic detoxification of chlorogenic acid against acetaminophen-induced liver injury by ameliorating hepatic inflammation. Chem-Biol. Interact.. 2015;238:93-101.
- [CrossRef] [Google Scholar]







