Translate this page into:
Ethanol-free extraction of curcumin and antioxidant activity of components from wet Curcuma longa L. by liquefied dimethyl ether
⁎Corresponding author. kanda.hideki@material.nagoya-u.ac.jp (Hideki Kanda)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
As a solvent, ethanol can extract a wide range of polar substances, but its consumption is sometimes avoided for religious and cultural reasons. In this study, liquefied dimethyl ether (DME) was used to extract curcumin and antioxidants from highly moist, untreated turmeric. Higher amounts of curcumin (7.94 mg/g dry weight (DW)) were extracted using liquified DME compared with ethanol (6.77 mg/g DW). Almost all the water and 5.10 mg/g DW of lipids were extracted from raw turmeric using liquefied DME, corresponding to 56 % the amount extracted using ethanol. In addition, microscopic and spectroscopic analyses revealed that liquefied DME neither destroyed cell walls nor extracted cellulose. However, liquefied DME had a slightly lower extraction capacity for total phenolic compounds than ethanol and slightly lower antioxidant effect. DME extracts an equivalent amount of curcumin as ethanol and only slightly fewer antioxidants while simultaneously avoiding sun-drying degradation and prolonged freeze-drying.
Keywords
Extraction
Antioxidants
Polyphenols
Pigments
Subcritical fluids
Green solvents
1 Introduction
Curcumin, a low-molecular-weight polyphenolic antioxidant, is found within Curcuma longa L. (turmeric) rhizome (Machmudah, 2020a), a plant native to tropical South Asia. It is also responsible for the bright yellow color of C. longa L. Curcumin has anti-acanthamoebic (Saeed et al., 2022), anti-inflammatory (Mahjoob & Stochaj, 2021), immunomodulatory (Chamani et al., 2022), and anti-ulcer effects, as well as antioxidant (Fallahi et al., 2021) and other pharmacological activities, especially anti-tumor activity (Zhang et al., 2022). Furthermore, 1,5-bis(4-hydroxy-3-methoxyphenyl)-1,4-pentadien-3-one, which is derived from curcumin and also found in curry paste, has a better inhibitory effect on gastric cancer cell growth than curcumin (Yoshida et al., 2018). Curcumin is a fat-soluble drug that has been employed as a model for assessing drug loading and release characteristics owing to its efficacy (Zhang et al., 2021).
Here, the separation of curcumin by extraction is important for the following reasons. The possibility is that patients with current or pre-existing liver disease may overdose on another species of turmeric, which closely resembles C. longa L., causing fatty degeneration of the liver. A part of turmeric also contains high levels of iron, which is hepatotoxic to patients with chronic hepatitis C. Since turmeric supplements may contain iron, they are not suitable for patients with chronic hepatitis C, as they require an iron-restricted diet (Koike et al., 2012). Furthermore, the Spanish Agency for Food Safety and Nutrition published a report on the risks associated with the intake of turmeric-derived curcumin supplements, after which the European Food Safety Authority reassessed curcumin as a food additive and set a tolerable daily intake of 210 mg/day for an adult weighing 70 kg. In addition, the Authority advises that persons under 18 years of age and pregnant or lactating women should avoid food supplements containing curcumin. It is recommended that the labels of food supplements accurately state the amount of curcumin contained within the product (Martínez et al., 2020). In short, preclinical and clinical studies have shown that the therapeutic effect of curcumin is highly dose-dependent. Here, as is widely known, the content of curcumin in turmeric varies from one individual to another depending on the region of origin and other factors, so the importance of extracting curcumin to make the optimum amount of curcumin available, rather than directly eating turmeric. Moreover, the high hydrophobicity of curcumin results in its low water solubility and poor absorption in the intestinal tract, limiting its application in the development of functional foods and drugs. Improving the poor bioavailability of curcumin also requires innovations, such as encapsulating curcumin in micelles (Saeed et al., 2022), liposomes (Wahyudiono et al., 2022), edible polymers (Machmudah et al., 2020b), and nonionic surfactants (Mahmoud et al., 2021), which require the extraction of curcumin from turmeric as a pretreatment of these hydrophilic processes. Thus, it is important to extract curcumin from C. longa L. and to regulate its intake.
Curcumin is decomposed, and its content is reduced by sun-drying C. longa L. (Llano et al., 2022). In aqueous conditions, curcumin is photodegradable (light-sensitive) and self-degradable, even in the dark (Mondal et al., 2016). Since the rate constants of curcumin degradation are miniscule in ethanol (Mondal et al., 2016) and curcumin is hydrophobic (Machmudah et al., 2020b), ethanol is often used for extraction (Braga et al., 2003). However, some regions and cultures prohibit the use of ethanol in food processing, necessitating the use of alternative solvents. Another approach that can be used to inhibit the decomposition of curcumin is supercritical CO2 extraction; the critical point of CO2 is 31.1 °C, and thus CO2 can be made supercritical at a mild temperature. Another advantage is that no CO2 remains in the extracted product. However, since supercritical CO2 is practically immiscible with water, C. longa L. must be dried in advance, and the decomposition of curcumin during this process is a concern. Since the amount of curcumin extracted using pure supercritical CO2 is low, ethanol or isopropyl alcohol can be mixed with supercritical CO2 as a co-solvent to enhance the extraction of curcumin. However, the amount of curcumin extracted is lower than that obtained using ethanol (Braga et al., 2003). In addition, the high supercritical CO2 extraction pressure (300 bar) increases equipment costs.
As an environmentally benign solvent, liquefied dimethyl ether (DME) has attracted attention; previously, the extractions of lipids from algae with high water content using liquefied DME were reported (Kanda et al., 2020; Babadi et al., 2020). It was also recently reported that liquefied DME is a superior solvent that extracts more lipids than ethanol (Subratti et al., 2019). DME (CH3OCH3) is the smallest and simplest ether with the lowest molecular weight. Owing to its molecular structure, it has a boiling point of − 24.8 °C in the standard state (Wu et al., 2011); therefore, DME must be pressurized to 0.51 MPa to be used as a liquid solvent at 20 °C (Wu et al., 2011). A low boiling point ensures that no residues remain in the extracts (Kanda et al., 2020). Bioassays have also confirmed that aqueous solutions of DME gas are non-toxic to microorganisms at ambient pressure (Kanda et al., 2021a). The European Union permits its use as an extraction medium in a number of cases (Commission E, 2016). DME is also recognized as “Generally Recognized as Safe” (GRAS) by the Food and Drug Administration (Food and Drug Administration, 2017). DME is much safer than other alkyl ethers because auto-oxidation is mild, and the small amounts of oxide generated do not polymerize because DME does not contain carbon–carbon bonds within its molecular structure (Naito et al., 2005). DME is also polar owing to its bent, water-molecule-like molecular structure and weak hydrogen bonds (Tatamitani et al., 2002), making it partially miscible with water in its liquefied state (Chai et al., 2022). Therefore, when used as an extraction solvent, liquefied DME can diffuse through the surrounding water of the high-water-content target substance and come into contact with the target substance, allowing extraction of the functional substances in plants without a drying step (Kanda et al., 2021b). For example, in the case of microalgae, the water content can reach 80 %. Evaporating water is a problem in bioenergy production because the energy of drying exceeds the calorific value of the lipids obtained by microalgal photosynthesis. Liquefied DME can extract lipids from wet microalgae (Kanda et al., 2015). Although microalgae are an extreme example, other plants also have high water contents. In addition, many of the functional substances to be extracted are poorly soluble in water. Therefore, residual water owing to insufficient drying of the plants may hinder the extraction of these substances if hydrophobic solvents are used. In the case of amphiphilic solvents, the extracted solution is a mixture of water, solvent, and functional substances. Thus, distillation with water is required to recycle the solvent, which consumes a large amount of energy and may lead to oxidation of the functional substances owing to heating during the distillation process. Ethanol falls under the category of solvents with this problem, as it is amphiphilic and azeotropic with water. On the other hand, in order to boil DME, which has a low boiling point, solar hot water (<60 °C) can be used as a heat source, which is excellent in environmental friendliness. Water is also extracted by liquefied DME, but it is easy to separate water and DME because of the large difference in boiling point with water. In previous studies of water and combustible content extraction from subbituminous coal (Kanda and Makino, 2010), water, caffeine and catechin extraction from tea leaves (Kanda et al., 2013), and water and PCB extraction from PCB-polluted river sediments (Oshita et al., 2010), the recycling and reuse of liquefied DME after evaporation and condensation from the extracts has been implemented. Results indicate that function of liquefied DME as the extraction solvent is not decreased.
Curcumin has a structure in which the aromatic groups of the polyphenols are linked by unsaturated carbonyl groups. However, there are no examples of studies in which curcumin was extracted with liquefied DME, and in order to consider this possibility, examples of polyphenols and antioxidants extracted with liquefied DME in previous studies will be presented. Previous studies have shown that resveratrol and its glycosides (Kanda et al., 2021b) and catechins (Kanda et al., 2013) can be extracted as lutein (Kanda et al., 2020) and as the xanthophylls fucoxanthin (Billakanti et al., 2013) and astaxanthin (Catchpole et al., 2010) in liquid DME. Other compounds that can be extracted using liquefied DME include coenzyme Q-10 (Catchpole et al., 2010), a derivative of benzoquinone with a relatively long isoprene side chain; and xanthohumol, a bitter component of beer and polyphenol found in hops (Bizaj et al., 2022). Polychlorinated biphenyls (Oshita et al., 2010), caffeine (Kanda et al., 2013), γ-oryzanol (Wongwaiwech et al., 2020), phytosterol (Wongwaiwech et al., 2020), capsaicin and piperine, and the pungent alkaloids of chili peppers and black pepper (Catchpole et al., 2003) can also be extracted using liquefied DME. Thus, based on the findings of these previous studies, a common feature of the substances that can be extracted using liquefied DME is their lipophilic nature. Conversely, proteins (Catchpole et al., 2008) and cellulose (Machmudah et al., 2020a) are not extracted into liquefied DME, precipitating instead. Similarly to the substances that can be extracted using liquefied DME, curcumin is lipophilic. However, unlike xanthophylls, curcumin does not consist of a continuous isoprene unit, although it contains a C⚌C bond. It also differs from xanthophylls in that both ends of the carbon chain contain aromatic rings. The molecular structure of curcumin bears some resemblance to those of the substances extracted in the aforementioned studies; however, there are also significant differences. To date, no researchers have investigated whether curcumin, a polyphenol and natural pigment, can be extracted using liquefied DME.
In this study, the amount of curcumin extracted using liquefied DME was quantified, and the antioxidant capacity of the extract was determined. To understand the extraction mechanism, the residue was analyzed using scanning electron microscopy (SEM) as well as Fourier-transform infrared (FTIR) spectroscopy to determine its surface functional groups.
2 Material and methods
2.1 Materials
The C. longa L. used in this study was collected from Ishigaki Island, Okinawa, Japan, and purchased from the Ishigaki Island Healthy Bank. The water content of the C. longa L. was measured using a moisture meter (Frontlab, AS ONE Corporation, Tokyo, Japan). The water content was determined from the difference between the initial weight and the weight of the dried product when it was heated to 107 °C and remained constant. Drying at 107 °C is a condition that can define total water content in coal, including both free and hydrogen bonded water, in the ISO 13909. The moisture content of lignite and peat close to plants is also specified in this method, so we followed these conditions. The C. longa L. samples had high water contents of 80.0 ± 1.7 wt% and were not dried. Liquefied DME was purchased from Tamiya, Inc. (Shizuoka, Japan) and used without further purification. Special-grade curcumin, HPLC-grade acetonitrile, HPLC-grade water, gallic acid (98.0–103.0 %), sodium carbonate, and Folin–Ciocalteu reagent were purchased from FujiFilm Wako Pure Chemical Corporation, Osaka, Japan. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) was purchased from Sigma-Aldrich, Tokyo, Japan.
2.2 Liquefied DME extraction
The design of the DME extraction apparatus used in this study as well as the operating procedure were adopted from previous reports as shown in Fig. 1. (Kanda et al., 2020; Kanda et al., 2021b). The apparatus consisted of a metal tank (TVS-1: Taiatsu Techno, Saitama, Japan) containing the supplied liquefied DME, an extraction column (HPG-10–5: Taiatsu Techno, capacity 10 mL, inner diameter 11.6 mm × length 190 mm), and a tank (HPG-96–3: Taiatsu Techno, capacity 96 mL) collecting the extracted liquid, connected in series with valves in the middle and at the end of the DME flow. The extraction column and container for collecting the extract were composed of pressure-resistant glass with a volume scale printed on it and were additionally covered by a transparent polycarbonate cylinder for safety. C. longa L. was disrupted using a food mill (IFM-800DG, Iwatani Corporation, Osaka, Japan) before being placed in the extraction column. 4.96 ± 0.08 g of undried, crushed C. longa L. was loaded into the lower half of the extraction column (5 mL volume), and colorless glass beads were loaded into the cavity to prevent the sample from moving. The colorless glass beads also aided the determination of extraction completion, as the initially colorless liquefied DME gradually turned yellow as curcumin was extracted. A cellulose filter with a pore size of 0.65 µm (Advantech Toyo Kaisha, ltd., Tokyo, Japan) was installed at the outlet of the extraction column to prevent the crushed turmeric from flowing out. The tubing connecting the individual vessels was composed of SUS 316 and had an inner diameter of 1/16 in.. By heating the DME supply tank to 35 ± 1 °C in a water bath, the saturated vapor pressure of the liquid DME inside rose to 0.79 ± 0.02 MPa. As the liquefied DME supplied from this tank passed through the fine SUS316 tubes, the large specific surface area of the tubes caused the temperature to drop rapidly to 25 °C, as confirmed by a noncontact infrared thermometer. The flow rate of liquefied DME was adjusted to 10 ± 1 mL min−1 (=6.61 g min−1) (Wu et al., 2011) by manually adjusting the valve according to the volume scale of the container in which the extract was collected and the increasing amount of liquefied DME in this container. Each time the volume of the extract in the collection vessel increased, it was promptly replaced with a new, empty collection vessel. After the extract-filled recovery vessel was removed from the apparatus, the terminal valve was opened to depressurize the vessel to atmospheric pressure, which allowed the DME to evaporate. This resulted in an incremental increase in the amount of extractant corresponding to the respective incremental amount of liquefied DME. After extraction, the residue was removed from the column. Water and organic extracts, such as curcumin, remained in the collection vessel after the DME evaporated. The weight of the extracted water was determined as the difference in weight after air-drying at 60 °C. The organic extracts were recovered in a 90:10 (v/v) acetonitrile–water mixture and used for subsequent HPLC analysis. To shield the extracts from light and thus prevent curcumin degradation, the surfaces of the extraction and recovery tanks were covered with aluminum foil. To determine the reproducibility of the experiment, it was performed from extraction to analysis three times.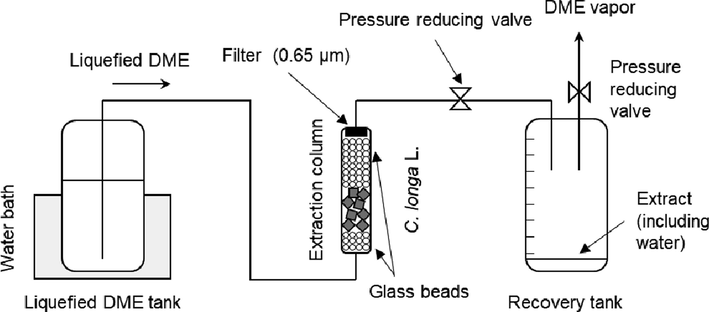
Schematic of liquefied DME extraction.
2.3 Ethanol extraction
Curcumin extraction by conventional ethanol Soxhlet was also performed to understand the effects of liquefied DME extraction. Generally, C. longa L is dried in the sun (Llano et al., 2022), degrading curcumin in the process, as mentioned previously. However, in this study, freeze-drying was used to prevent curcumin degradation, which is very time consuming and impractical on an industrial scale owing to cost constraints. First, C. longa L. was freeze-dried for 24 h and then disrupted using a food mill (IFM-800DG, Iwatani Corporation). The amounts of dried C. longa L. and ethanol used for extraction were 3.00 g and 300 mL, respectively. Ethanol Soxhlet extraction was performed for 2.5 h by following an established procedure (Braga et al., 2003). Afterward, ethanol was evaporated using a rotary evaporator (SB-1200, Eyela Co., ltd., Tokyo, Japan). To eliminate the effects of light on curcumin, the surfaces of the freeze dryer, grinder, and extraction equipment were covered with aluminum foil.
2.4 Quantitative analysis of curcumin
Liquid chromatography coupled with nuclear magnetic resonance (LC NMR) is one possible method to identify curcumin and other substances contained in the extracts, however since LC NMR requires the use of large amounts of expensive deuterated solvents to remove interfering signals due to the mobile phase, UV–vis detection was used in this study. Reversed-phase high-performance liquid chromatography (HPLC) was used to quantify the amount of extracted curcumin. The reversed-phase HPLC system consisted of a degasser (DG–980–50, Jasco Co., Inc., Tokyo, Japan), pump (PU-980, Jasco Co., Inc.), column heater (U–620, Sugai Chemie, Inc., Wakayama, Japan), and UV–Vis detector (UV-970, Jasco Co., Inc.), which was controlled using Jasco-Borwin (Ver. 1.5) software via an LC-Net II/ADC controller (Jasco Co., Inc.). To isolate curcumin, an acetonitrile/water (90/10 v/v) eluent was flushed through an Inertsil ODS-3 column (250 mm × 4.6 mm × 5 μm, GL Science, Tokyo, Japan) at a rate of 1.0 mL/min. The temperature inside the column heater was maintained at 40 °C. The UV–vis detection wavelength was 420 nm (Wahyudiono et al., 2022).
2.5 Residue characterization
Changes in the surface functional groups of C. longa L. after liquefied DME extraction were analyzed using FTIR spectroscopy (PerkinElmer Spectrum Two, PerkinElmer Japan Co, ltd, Yokohama, Japan) (Braga et al., 2003). Since liquefied DME simultaneously extracts water from C. longa L., the IR spectrum of the extracted residue was compared with that of freeze-dried C. longa L. Reference to a previous study (Kodama et al., 2015) guided the assignment of the peaks, which were compared with those of curcumin (Chen et al., 2015), C. longa L. (Rohaeti et al. 2015), and ethanol extract (Wulandari et al., 2018) in order to identify the substances extracted from the IR signal changes.
The surface morphologies of the C. longa L. residue and the original sample were observed using SEM (S-4300, Hitachi High-Tech Corporation, Tokyo, Japan). To prevent drying-induced changes in the surface of the original C. longa L., it was coated in gold using a sputtering apparatus (RMC-Eiko RE vacuum coater, Eiko Engineering Co., ltd, Tokyo, Japan) at 0.1 Torr under 7 mA for 420 s. The accelerating voltage was 5.0 kV to 15.0 kV, and the maximum magnification was × 1.0 k to 40.0 k.
2.6 Total polyphenolic content assay
Since there are so many different types of polyphenolic substances and fractionating and quantifying each of them would require a great deal of effort, a standardized method was employed to define the total amount of polyphenolic content. As antioxidants besides curcumin can be extracted by liquefied DME, the total polyphenolic content (TPC) was determined using the Folin–Ciocalteu method (Blainski et al., 2013). The sample (1 mL) was mixed with 5 mL of deionized water and 6 mL of 7.5 % (w/w) sodium carbonate. After 10 min, Folin–Ciocalteu reagent (0.5 mL) was added to the mixture and stirred for 5 min. After incubation for 2 h in the dark at 20 °C, the TPC was estimated from the absorbance at 750 nm using a UV–Vis spectrophotometer (V-550, Jasco) as the equivalent amount of gallic acid (20–100 mg/L range).
2.7 DPPH radical scavenging activity assay
Since there are so many different types of antioxidants and fractionating and quantifying each of them would require a great deal of effort, a standardized method was employed to define the overall antioxidant capacity. The DPPH radical scavenging activity was measured using a UV–Vis spectrophotometer (V-550, Jasco). The samples were prepared according to the following procedure (Chhouk et al., 2017). First, the extracts were diluted with methanol to a concentration range of 0.01–2.00 mg/mL. The samples were then thoroughly stirred with 2.7 mL of DPPH solution (6 × 10−5 M in methanol) and allowed to stand for 1 h at room temperature in the dark. The DPPH radical scavenging activity was determined using Equation (1):
where A517 of the control is the absorbance of the control and reagent at a wavelength of 517 nm, and A517 of the sample is the absorbance of the sample that reacted with the reagent at this wavelength. By plotting the ratio of the DPPH radical scavenging activity against concentration, the IC50 was determined, which is the concentration of the sample required for 50 % DPPH radical scavenging activity.
3 Results and discussion
The extraction process was terminated when the color of the extract exiting the extraction column changed from yellow to colorless and transparent, i.e., the color of the original liquefied DME. The total extraction time was 48 min, and the total volume of DME that flowed through the column was 317.3 g (480 mL). Fig. 2 shows the appearance of the original wet, freeze-dried C. longa L., the residue after liquefied DME extraction, and the residue after ethanol extraction.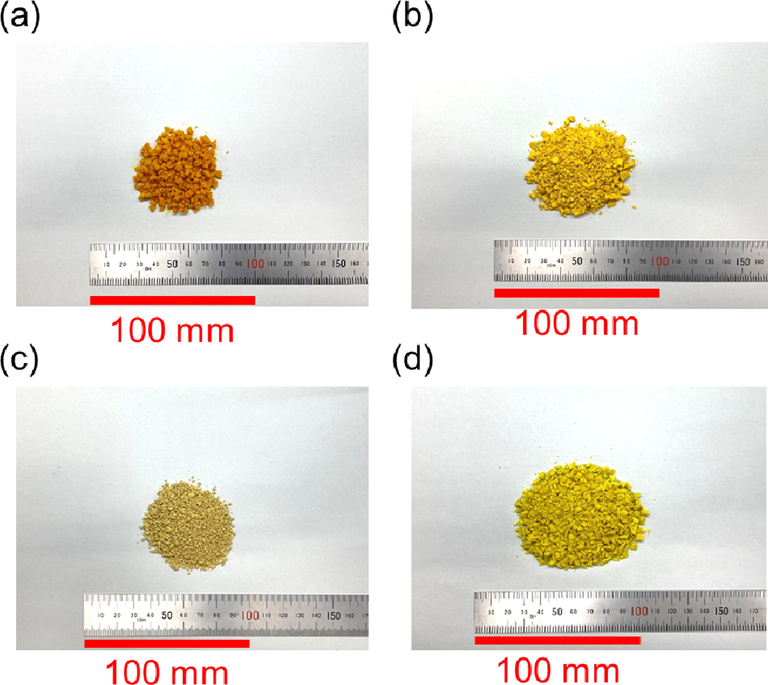
Photographic images of (a) original C. longa L., (b) freeze-dried C. longa L., (c) residue obtained after DME extraction, and (d) residue obtained after ethanol extraction.
Note that the residue after extraction with liquefied DME and ethanol is dry and should be compared with the freeze-dried sample, considering the effect of moisture on the color tone. The color tone of the residue after extraction with liquefied DME and ethanol was whiter than that of the freeze-dried samples, indicating that the extraction process removed pigments such as curcumin.
Fig. 3 shows an example of a chromatograph of the curcumin content in the extract corresponding to the eighth plot in Fig. 4(c), quantified by HPLC UV–vis. Comparison with the retention time of pure curcumin at this detection wavelength shows that curcumin was well fractionated from the extract and detected. Note that the peak of the extract with liquefied DME is small because this sample corresponds only to the increment of the extraction amount between the 7th and 8th in Fig. 4(c), while the extract with ethanol corresponds to the total curcumin amount by the Soxhlet method.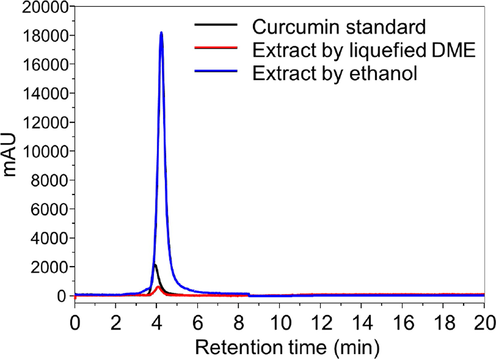
Chromatograph of curcumin fractionated from the DME-extract by HPLC detected at 420 nm.
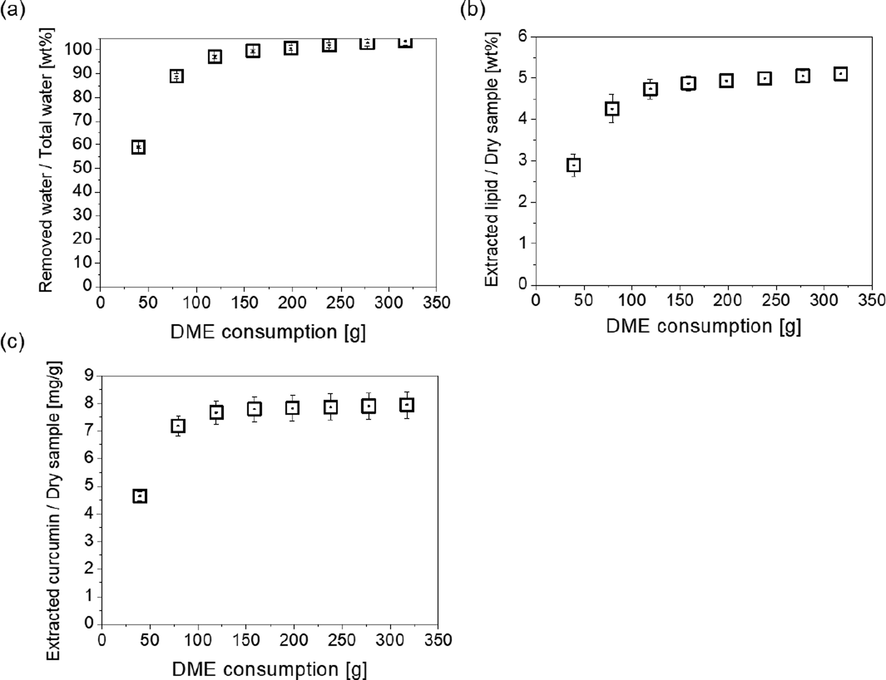
Changes over time in the amounts of materials extracted from C. longa L. by liquefied DME. (a) Water, (b) Lipid, (c) Curcumin.
The changes in the amount of water extracted from C. longa L. by liquefied DME over time are shown in Fig. 4(a). During the initial extraction stage, the amount of water extracted increased in proportion to the amount of DME, and the slope at this stage is due to the saturated solubility of water in liquefied DME at 25 °C (Chai et al., 2022). However, at the end of the extraction phase, the slope became less steep, and a phenomenon was observed in which the water inside the C. longa L. cells and hydrophilic extracellular matrix was constrained, preventing mass transfer (Dimić et al., 2021) or reducing the saturation concentration, the point at which water dissolves into liquefied DME owing to intermolecular interactional constraints (Kanda et al., 2022). This trend is similar to the results of previous studies on water extraction from various high-moisture plants and algae using liquefied DME (Kanda et al., 2021b). At the end of the extraction, 103.8 ± 1.9 % of the total water content in C. longa L. was extracted. This value exceeds 100 % of the total water content because chemically bound water may have been removed via dehydration.
The change in the amount of lipids extracted from C. longa L. by liquefied DME over time are shown in Fig. 4(b). The extraction behavior of the lipids was similar to that of water, in line with previous findings that liquefied DME has favorable extraction properties for both water and lipids (Kanda et al., 2021b). In other words, in the early stages of extraction, the amount of lipids extracted increases in proportion to the amount of DME, but at the end of the extraction phase, the slope decreases, as the C. longa L. cells and the extracellular matrix hinder mass transfer and reduce the saturation concentration. At the end of the extraction, lipids extracted from high-water-content C. longa L. by liquefied DME represented 5.10 ± 0.13 % of the dry weight of C. longa L. By contrast, the lipids extracted by ethanol constituted 9.05 ± 0.7 % of the dry weight of C. longa L. This result implies that some functional components were not extracted, or the functional components were selectively extracted at high concentrations, without being accompanied by unwanted substances.
The change in the amount of curcumin extracted from C. longa L. by liquefied DME over time is shown in Fig. 4(c). In addition, the extraction behavior of curcumin was similar to that of water and the lipids described above. The rapid increase in the amount of curcumin extracted in the early stages indicated that curcumin is highly soluble in liquefied DME. Based on the amount of extracted curcumin obtained from the first plot in Fig. 4 (4.65 ± 0.18 mg/g DW), the DW of packed wet C. longa L. (0.992 g (=4.96 g × (1 – 0.800)), and the total mass of liquid DME (39.7 g, 60 mL), the saturated solubility of curcumin in liquid DME was calculated to be 4.65 × 0.992 / 39.7 g-DME = 0.116 mg/g-DME.
At the end of the extraction process, curcumin extraction was also inhibited by the C. longa L. cells and extracellular matrix. Using liquefied DME, the lipids extracted from high-water-content C. longa L. amounted to 7.94 ± 0.47 mg/g DW on a C. longa L. DW basis, whereas those extracted using ethanol amounted to 6.77 ± 0.58 mg/g DW. In other words, even without drying C. longa L., liquefied DME had a curcumin extraction capacity comparable to that of conventional ethanol extraction. As mentioned previously, the amount of lipids extracted by liquefied DME was significantly lower than that extracted by ethanol, indicating that liquefied DME is more selective than ethanol, at least with respect to curcumin.
Fig. 5 shows the FTIR spectra of C. longa L. and its residue after liquefied DME extraction. The freeze-dried C. longa L. generally matched the FTIR spectra of C. longa L. in the previous study (Rohaeti et al. 2015). First, the hydrogen-bonded O—H stretch (3600–3000 cm−1) weakened after DME extraction. In a previous study, the absorption peak at 3508 cm−1 was attributed to the phenolic OH stretching vibration of curcumin (Chen et al., 2015). Cellulose, one of the main components of C. longa L., has also a broad OH absorption peak spanning the range of 3600–3000 cm−1 (Kassanov et al., 2017, Kodama et al., 2015, Wulandari et al., 2018). Since cellulose is not extracted into liquefied DME (Machmudah et al., 2020a), its presence in the IR spectrum is considered to be due to the extraction of various phenolic compounds, including curcumin. The peak at 2927 cm−1 did not change significantly after DME extraction. In fact, curcumin does not give rise to any distinguishable peaks (Chen et al., 2015), while the spectrum of cellulose contains peaks in the range of 3000–2800 cm−1 owing to the methyl and methylene symmetric and asymmetric stretching vibrations (Kassanov et al., 2017, Rohaeti et al. 2015). In other words, this peak corresponds to the cellulose that was not extracted by DME. However, as with previous ethanol extraction results (Wulandari et al., 2018), this means that these corresponding substances, namely curcumin and polyphenols, could have been extracted. The peak around 1740–1680 cm−1 in the freeze-dried sample is due to ketone and carbonyl C⚌O stretching (Kodama et al., 2015, Rohaeti et al. 2015), and its contraction in the residue after extraction suggests curcumin extraction. The other major change in the spectrum was the lower intensities of the peaks at 1634 cm−1 and 1538–1517 cm−1 detected in the spectrum of the original C. longa L. In a previous study (Kodama et al., 2015), the absorption owing to the stretching of the benzene ring appeared at 1632 cm−1, and ketone and carbonyl C⚌O stretching was observed at 1560–1510 cm−1. Curcumin also gives rise to absorption peaks at similar wavenumbers, namely, the benzene stretching vibration at 1628 cm−1 and ketone C⚌O stretching vibration at 1509 cm−1 (Chen et al., 2015). There is also an automatic skeletal stretching vibration at 1613 and 1450 cm−1 (Kodama et al., 2015), and the lower intensities around it are consistent with the extraction of curcumin. This suggests that the diminishing of the peaks could be due to the extraction of curcumin and similar aromatic compounds with ketone or carbonyl groups. The absorption peak at 1402 cm−1 corresponds to C—H bending (Kodama et al., 2015), but the spectrum of curcumin does not contain a peak at this wavenumber (Chen et al., 2015). The surrounding peaks in the range of 1450–1350 cm−1 also decreased in intensity and correspond to O–CH3 (1450–1350 cm−1) and O—H bending of ether and carboxylic acid groups, respectively (Kodama et al., 2015), implying that the components containing these functional groups were extracted. In addition, the automatic skeletal stretching vibration around 1510 cm−1 (Rohaeti et al. 2015) corresponds to curcumin and polyphenols and could be considered as their extraction. Otherwise, the broad peak centered at ≈1235 cm−1 disappeared. In this range, C—O—C and C—O stretching of phenol occurred at 1232 cm−1 and 1215 cm−1, respectively (Kodama et al., 2015). In the spectrum of curcumin, aromatic C—O stretching vibrations appeared at 1278 cm−1 (Chen et al., 2015). A peak at 997 cm−1 was also apparent, which was not associated with curcumin (Chen et al., 2015); this peak was considered to originate from cellulose or other extraneous sources (Kassanov et al., 2017) such as phenols and compounds with ether bonds. A peak corresponding to the C-OH stretching vibration characteristic of curcumin and polyphenols has been detected in ethanol extracts at 1030 cm−1 in the previous study (Wulandari et al., 2018), but this peak is buried by a larger peak at 997 cm−1.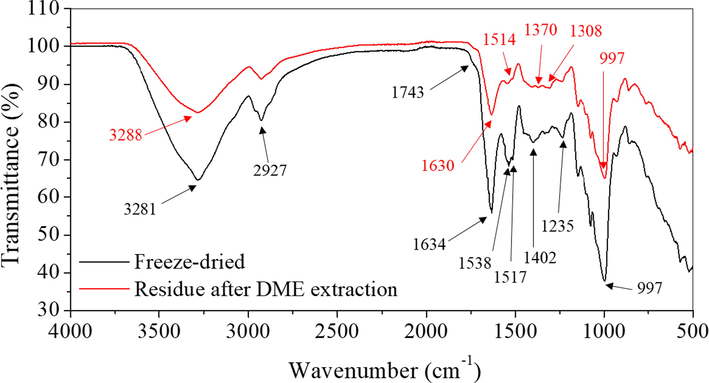
FTIR spectra of freeze-dried C. longa L. and its residue after liquefied DME extraction.
In other words, the FTIR results supported the extraction of curcumin However, it cannot be determined whether other phenolic compounds were extracted.
Fig. 6 shows the SEM morphologies of C. longa L. before extraction along with its residue after extraction. The surface of the original C. longa L. appears very smooth under all magnifications. By contrast, the residue obtained after liquefied DME extraction contains holes on the order of submicrons. However, previous studies, for example in the case of the macroalgae Monostroma nitidum, have shown that the surface of the residue obtained from liquefied DME extraction was not cracked or roughened (Kanda et al., 2020). In other words, it is envisioned that DME moves in and out of the cell through very narrow spaces in the cell walls. Therefore, it is logically consistent to assume that a certain component is ubiquitous in C. longa L., and this particular component was extracted.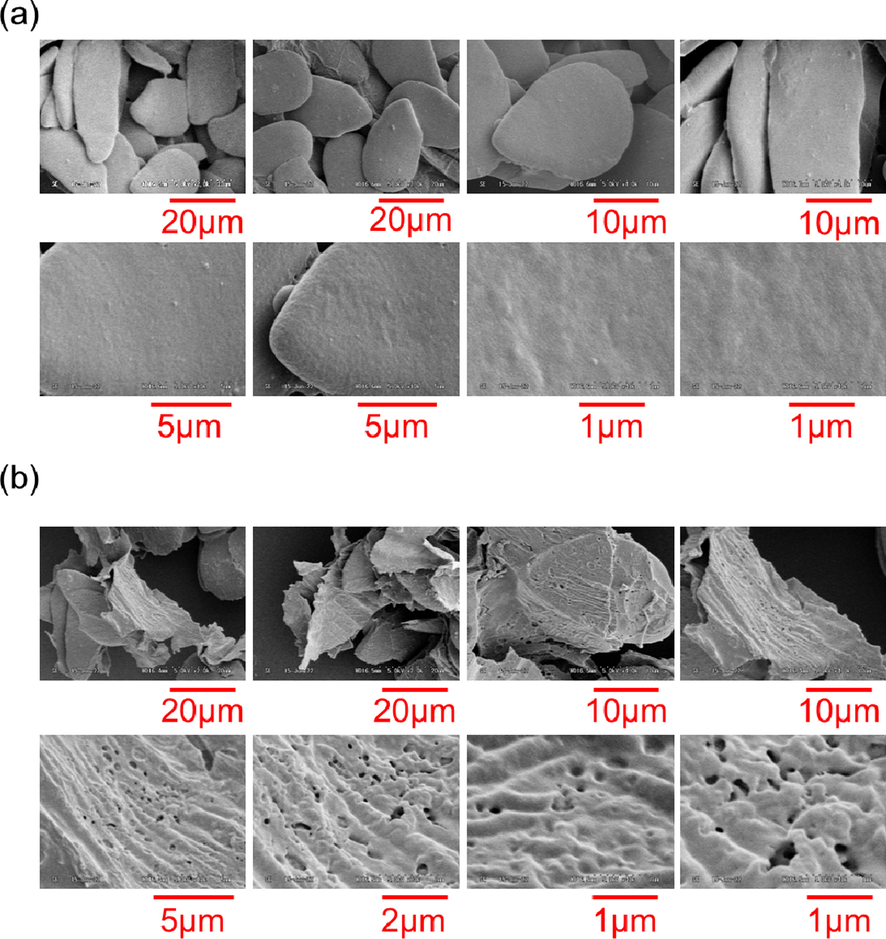
SEM images: (a) original C. longa L., and (b) its residue after liquefied DME extraction.
It was unclear from the FTIR results whether other phenols were extracted, so the TPC assay was performed. The IC50, defined as the DPPH radical scavenging activity, was also measured. The TPC was 68.45 ± 3.65 mg GAE/g DW for the liquefied DME extraction and 80.73 ± 2.20 mg GAE/g DW for the ethanol extraction. The IC50 values were 67.21 ± 4.79 µg/mL and 53.43 ± 3.08 µg/mL for the liquid DME extract and ethanol extract, respectively, indicating that the latter had a slightly higher TPC and higher antioxidant activity.
4 Conclusion
This study was carried out to address the fact that many people cannot consume ethanol for cultural or religious reasons and to circumvent the need for drying pretreatment, which is necessary in ethanol extraction. Our results demonstrated that curcumin and antioxidants can be extracted from raw C. longa L. by liquefied DME without the pre-drying step, even in the case of high water contents. DME can extract the same high yield of curcumin as ethanol without having the same drawbacks, eliminating the need for the cumbersome and risky process of drying pretreatment, which can degrade curcumin. DME is an effective alternative to ethanol in food processing; however, liquefied DME had a slightly lower extraction capacity for phenolic compounds than ethanol and a slightly lower antioxidant effect.
CRediT authorship contribution statement
Hideki Kanda: Conceptualization, Investigation, Methodology, Writing – original draft, Writing – review & editing, Project administration, Resources, Funding acquisition, Supervision. Li Zhu: Investigation, Data curation, Visualization. Wanying Zhu: Investigation, Data curation, Visualization. Tao Wang: Investigation, Data curation, Visualization.
Acknowledgments
Part of this study was supported by JSPS KAKENHI (grant number JP20H02515).
Declaration of Competing Interest
All authors declare that (i) no financial support or other support was received from any organization that may have an interest in the submitted research, and (ii) no other relationships or activities influenced the submitted research.
References
- Identification of carotenoids and chlorophylls from green algae Chlorococcum humicola and extraction by liquefied dimethyl ether. Food Bioprod. Process.. 2020;23:296-303.
- [CrossRef] [Google Scholar]
- Enzyme-assisted extraction of fucoxanthin and lipids containing polyunsaturated fatty acids from Undaria pinnatifida using dimethyl ether and ethanol. Process Biochem.. 2013;48:1999-2008.
- [CrossRef] [Google Scholar]
- Hop (Humulus lupulus L.) essential oils and xanthohumol derived from extraction process using solvents of different polarity. Horticulturae. 2022;8:368.
- [CrossRef] [Google Scholar]
- Application and analysis of the Folin Ciocalteu method for the determination of the total phenolic content from Limonium brasiliense L. Molecules. 2013;18:6852-6865.
- [CrossRef] [Google Scholar]
- Comparison of yield, composition, and antioxidant activity of turmeric (Curcuma longa L.) extracts obtained using various techniques. J. Agric. Food Chem.. 2003;51:6604-6611.
- [CrossRef] [Google Scholar]
- Extraction of chili, black pepper, and ginger with near-critical CO2, propane, and dimethyl ether: analysis of the extracts by quantitative nuclear magnetic resonance. J. Agric. Food Chem.. 2003;51:4853-4860.
- [CrossRef] [Google Scholar]
- Extraction of lipids from fermentation biomass using near-critical dimethyl ether. J. Supercrit. Fluids. 2010;53:34-41.
- [CrossRef] [Google Scholar]
- Extraction of lipids from a specialist dairy stream. J. Supercrit. Fluids. 2008;45:314-321.
- [CrossRef] [Google Scholar]
- Vapor–liquid equilibrium (VLE) prediction for dimethyl ether (DME) and water system in DME injection process with Peng-Robinson equation of state and composition dependent binary interaction coefficient. J. Pet. Sci. Eng.. 2022;211:110172
- [CrossRef] [Google Scholar]
- Immunomodulatory effects of curcumin in systemic autoimmune diseases. Phytother. Res.. 2022;36:1616-1632.
- [CrossRef] [Google Scholar]
- The stability, sustained release and cellular antioxidant activity of curcumin nanoliposomes. Molecules. 2015;20:14293-14311.
- [CrossRef] [Google Scholar]
- Extraction of phenolic compounds and antioxidant activity from garlic husk using carbon dioxide expanded ethanol. Chem. Eng. Process.. 2017;117:113-119.
- [CrossRef] [Google Scholar]
- Commission E. 2016. Commission Directive (EU) 2016/1855 of 19 October 2016 amending Directive 2009/32/EC of the European Parliament and of the Council on the approximation of the laws of the Member States on extraction solvents used in the production of foodstuffs and food i. Official Journal of the European Union, 284, 19–20.
- Supercritical fluid extraction kinetics of cherry seed oil: kinetics modeling and ANN optimization. Foods. 2021;10:1513.
- [CrossRef] [Google Scholar]
- Curcumin and inflammatory bowel diseases: from in vitro studies to clinical trials. Mol. Immunol.. 2021;130:20-30.
- [CrossRef] [Google Scholar]
- Food and Drug Administration 2017. GRAS Notice for the use of dimethyl ether as an extraction solvent. GRAS Notice No. GRN 000741.
- Energy-efficient coal dewatering using liquefied dimethyl ether. Fuel. 2010;89:2104-2109.
- [CrossRef] [Google Scholar]
- Production of decaffeinated green tea leaves using liquefied dimethyl ether. Food Bioprod. Process.. 2013;91:376-380.
- [CrossRef] [Google Scholar]
- Energy-saving lipid extraction from wet Euglena gracilis by the low-boiling-point solvent dimethyl ether. Energies. 2015;8:610-620.
- [CrossRef] [Google Scholar]
- Direct Extraction of Lutein from Wet Macroalgae by Liquefied Dimethyl Ether without Any Pretreatment. ACS Omega. 2020;5:24005-24010.
- [CrossRef] [Google Scholar]
- Surfactant-free decellularization of porcine aortic tissue by subcritical dimethyl ether. ACS Omega. 2021;6:13417-13425.
- [CrossRef] [Google Scholar]
- Ethanol-free extraction of resveratrol and its glycoside from Japanese knotweed rhizome by liquefied dimethyl ether without pretreatments. Asia Pac. J. Chem. Eng.. 2021;16:e2600.
- [Google Scholar]
- Thermodynamic model of extraction equilibrium in cylindrical nanopores validated with molecular dynamics simulation. Chem. Eng. Sci.. 2022;248:117115
- [CrossRef] [Google Scholar]
- Cellulose enzymatic saccharification and preparation of 5-hydroxymethylfurfural based on bamboo hydrolysis residue separation in ionic liquids. RSC Adv.. 2017;7:30755-30762.
- [CrossRef] [Google Scholar]
- Enhancing pressurized water extraction of β-glucan from barley grain by adding CO2 under hydrothermal conditions. Chem. Eng. Process.. 2015;97:45-54.
- [CrossRef] [Google Scholar]
- The current status of health food- or supplement-related adverse health effects. Iyakuhin Johogaku. 2012;14:134-143.
- [Google Scholar]
- Effect of drying methods and processing conditions on the quality of curcuma longa powder. Processes. 2022;10:702.
- [CrossRef] [Google Scholar]
- Water removal from wood biomass by liquefied dimethyl ether for enhancing heating value. Energy Rep.. 2020;6:824-831.
- [CrossRef] [Google Scholar]
- Formation of Fine Particles from Curcumin/PVP by the Supercritical Antisolvent Process with a Coaxial Nozzle. ACS Omega. 2020;5:6705-6714.
- [CrossRef] [Google Scholar]
- Curcumin nanoformulations to combat aging-related diseases. Ageing Res. Rev.. 2021;69:101364
- [CrossRef] [Google Scholar]
- Scrutinizing the Feasibility of Nonionic Surfactants to Form Isotropic Bicelles of Curcumin: a Potential Antiviral Candidate Against COVID-19. AAPS PharmSciTech. 2021;23:44.
- [CrossRef] [Google Scholar]
- Martínez, M. R., Hurtado, M. C., Daschner, Á., Pons, R. M. G., Navas, F. J. M., Baquedano, M. del P. P. et al. 2020. Informe del Comité Científico de La Agencia Española de Seguridad Alimentariay Nutrición (AESAN) sobre el riesgo asociado al consume de complementos alimenticios que contienen curcumina como ingredient. Revista del Comité Científico de la AESAN, 32, 85–112. https://www.aesan.gob.es/AECOSAN/docs/documentos/seguridad_alimentaria/evaluacion_riesgos/informes_comite/CURCUMINA.pdf.
- Stability of curcumin in different solvent and solution media: UV–visible and steady-state fluorescence spectral study. J. Photochem. Photobiol. B Biol.. 2016;158:212-218.
- [CrossRef] [Google Scholar]
- A comparative study on the autoxidation of dimethyl ether (DME) comparison with diethyl ether (DEE) and diisopropyl ether (DIPE) J. Loss Prev. Process Ind.. 2005;18:469-473.
- [CrossRef] [Google Scholar]
- Extraction of PCBs and water from river sediment using liquefied dimethyl ether as an extractant. Chemosphere. 2010;78:1148-1154.
- [CrossRef] [Google Scholar]
- Fourier transform infrared spectroscopy combined with chemometrics for discrimination of Curcuma longa, Curcuma xanthorrhiza and Zingiber cassumunar. Spectrochim. Acta A Mol. Biomol. Spectrosc.. 2015;137:1244-1249.
- [CrossRef] [Google Scholar]
- Nanovesicles containing curcumin hold promise in the development of new formulations of anti-acanthamoebic agents. Mol. Biochem. Parasitol.. 2022;247:111430
- [CrossRef] [Google Scholar]
- Efficient extraction of black cumin (Nigella sativa L.) seed oil containing thymol, using liquefied dimethyl ether (DME) J. Food Process. Preserv.. 2019;43:e13913.
- [Google Scholar]
- Weak, improper, C−O...H−C hydrogen bonds in the dimethyl ether dimer. JACS. 2002;124:2739-2743.
- [CrossRef] [Google Scholar]
- Curcumin-loaded liposome preparation in ultrasound environment under pressurized carbon dioxide. Foods. 2022;11:1469.
- [CrossRef] [Google Scholar]
- Subcritical dimethyl ether extraction as a simple method to extract nutraceuticals from byproducts from rice bran oil manufacture. Sci. Rep.. 2020;10:21007.
- [CrossRef] [Google Scholar]
- An equation of state for the thermodynamic properties of dimethyl ether. J. Phys. Chem. Ref. Data. 2011;40:023104
- [CrossRef] [Google Scholar]
- Liquid chromatography and fourier transform infrared spectroscopy for quantitative analysis of individual and total curcuminoid in Curcuma longa extract. Journal of Applied Pharmaceutical Science. 2018;8:107-113.
- [CrossRef] [Google Scholar]
- Dietary intake of pyrolyzed diketene curcumin inhibits gastric carcinogenesis. J. Funct. Foods. 2018;50:192-200.
- [CrossRef] [Google Scholar]
- Enhancing the stability of zein/fucoidan composite nanoparticles with calcium ions for quercetin delivery. Int. J. Biol. Macromol.. 2021;193:2070-2078.
- [CrossRef] [Google Scholar]
- Curcumin induces autophagic cell death in human thyroid cancer cells. Toxicol. In Vitro. 2022;78:105254
- [CrossRef] [Google Scholar]







