Translate this page into:
Synthesis characterization and evaluation of novel triazole based analogs as a acetylcholinesterase and α-glucosidase inhibitors
⁎Corresponding authors. shagufta_kamal@yahoo.com (Shagufta Kamal), drismat@iub.edu.pk (Ismat Bibi), bosalvee@yahoo.com (Munawar Iqbal)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
A series of novel triazole analogs (10a-k) bearing piperidine were synthesized in an aprotic solvent on the most effective pharmacophore with acetylcholinesterase (AChE) and α-glucosidase inhibitory activity. Triazole analogs (10a-k) were obtained in excellent yields (75–90 %) and characterized by EI-MS, IR, 13C NMR and 1H NMR. The newly synthesized triazole analogs (10a-k) showed potent AChE inhibitory activity in the range of Ki = 0.0155 ± 1.25 µM to 0.557 ± 0.50 µM, IC50 = 0.031 ± 0.85 to 0.537 ± 0.76 µM than Eserine (0.04 ± 0.001 µM) having strong electron-withdrawing fluorine group on the pyridine ring was recorded as a most potent inhibitor of AChE while (%) inhibition against α-glucosidase was ranging between 52.36 ± 1.67 to 85.35 ± 1.39. The kinetic study predicted that triazole analogs (10a-k) followed the un-competitive and mixed type of inhibition against AChE. In silico molecular docking was performed at the active site of the AChE co-crystal structure (PDB ID:1NEN). The results of molecular docking corelate will with the experimental findings.
Keywords
1,2,4-triazole
Piperidine
Acetylcholinesterase inhibition
α-glucosidase inhibition
1 Introduction
Alzheimerʹs disease (AD) is characterized by the presence of neurofibrillary tangles, and neuritic plaques, associated with neuronal loss leading to term memories disturbances and confusion (Türkeş et al., 2021; Li et al., 2023; Hao et al., 2023; Taslimi et al., 2020). Following the cholinergic hypothesis, a deficit in the level of acetylcholine (ACh) subsequently affecting intervening memory function results in memory reduction. AChE hydrolyzes the ACh released at cholinergic synapses into acetate and choline. This amelioration results in the depletion of Ach causing a diminution in the cognitive deficit (Güleç et al., 2017; Güleç et al., 2022). However, inhibition of AChE performs an essential role in cholinergic communication and takes part in different functions appertaining to adhesion, differentiation, and neuronal development (Avula et al., 2018). To date, there is no cure for AD (Bechara et al., 2015). However, AChE inhibitors were envisaged as remedies for AD treatment because they can reduce toxic amyloid β-peptide formation (Bechara et al., 2015). In short, AChE inhibitors (AChEIs) are responsible for improving the patient’s quality of life thus leading to improved cognitive capability yet hampering the AD progression (Bousada et al., 2020). Literature reported that neurodegenerative AD and type-2 diabetes are interlinked (Türkeş et al., 2021). Interestingly, it is observed that AChE is associated with different complications and oxidative stress leading to the pathogenesis and progression of central neural disorders such as type-2 diabetes and AD (Taslimi et al., 2021).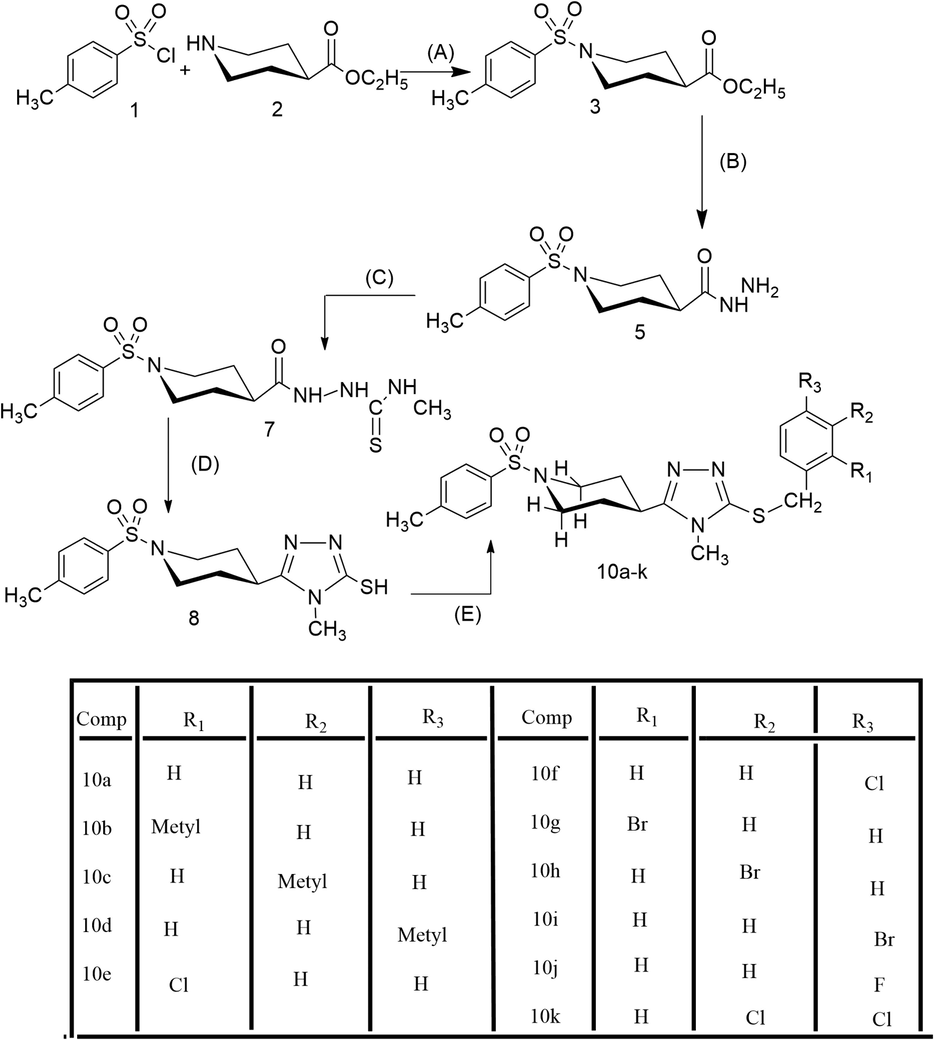
Synthesis of 1-(4-toluenesulfonyl)-4-(3-aralkylthio-4-methyl-4H-1,2,4-triazol-5-yl) piperidine (10a-k). Reagents and conditions: (A) Stirring at RT with 20 % Na2CO3(aq) and pH = 10 (B) Refluxing with NH2NH2·H2O (4) in MeOH (C) Refluxing with CH3NCS (6) in EtOH (D) Refluxing with 15 % KOH(aq) (E) Stirring at RT with LiH in DMF.
Clinically, it is proven that diabetic patients are at higher risk of cognitive impairments and dementia (Türkeş et al., 2021). Therefore, inhibition of α-glycosidase would reduce the process of carbohydrate digestion and would preclude postprandial hyperglycemia which is a major consequence of diabetes complications (Akocak et al., 2021). In light of these pieces of information, it is understood that AChE and α-glucosidase inhibitors can treat both AD and DM. Therefore, the synthesis or the development of novel compounds having appropriate bioactive properties and chemical structures to interact with these enzymes is an urgent need (Gümüş et al., 2022) Hence, because of our interest to develop potent anti-AD and anti-MD agents, we presented a series of novel triazole analogs (10a-k) by combining two essential rings of piperidine with triazole.
The heterocyclic triazole analogs have been proven to be important drug candidates owing to their activity in different biological fields including material science, nano-chemistry, biology, medicine, and agriculture (Sacchetti et al., 2022; Guan et al., 2022; Gaspar et al., 2022; Dubovis et al., 2018). In the field of medicine, analogs of triazole have acted as active antiviral (Gülçin et al., 2016), antifungal (Haddad et al., 2015), anti-inflammatory, antibacterial, anticonvulsant and anticancer agents (Honka et al., 2018; Jha et al., 2010; Kousar et al., 2020; Laube, 2002).neurodegenerative disorders require a thought-provoking resolution because of the alarming situation being faced owing to the ineffectiveness of reported drugs. Previous studies have emphasized the bioactivity of heterocyclic analogs against a variety of enzymes and pathogens which have been addressed through new active multifunctional drug candidates to deal with the ineffectiveness of drugs (Li and Zhang, 2021). Piperidine being an active synthetic pharmaceutical drug contained alkaloids such as (S)-coniine, (S)-anabasine, (–)-swainsonine, and quinine (Sacchetti et al., 2022; Guan et al., 2022; Gaspar et al., 2022; Dubovis et al., 2018; Honka et al., 2018; Jha et al., 2010; Kousar et al., 2020; Laube, 2002).
Herein, complete synthesis, structural and molecular characterization along with AChE and α-glucosidase inhibitory effects are reported and tried to discover the most potent and favorable α-glucosidase and AChE inhibition properties of novel triazole analogs (10a-k) to stretch directions to further studies.
2 Experimental
2.1 Chemicals and instruments
All the experiments were carried out under aerobic conditions unless stated otherwise. Alfa Aesar, Sigma Aldrich and Merck were contacted for analytical grade chemicals and solvents through local suppliers and forwarded to reaction as such. Melting points of the synthesized compounds (Acetylcholinesterase and α-Glucosidase inhibitors) were computed through Griffin and George apparatus. IR spectral patterns were recorded by Jasco-320A spectrometer using the KBr disc method. FT NMR (1H and 13C) spectra of the synthesized compounds (10a-k) were collected by Bruker 600 MHz in deuterated dimethyl sulfoxide (DMSO‑d6). JMS-HX-110 spectrometer was a tool for recording EI-MS spectra. The TLC plates were observed in UV light at 254 nm or iodine vapors and were made of aluminum plates pre-coated by silica gel.
2.2 Synthesis of 1-(4-toluenesulfonyl)-4-(ethoxycarbonyl) piperidine (3)
1,2,4 Trizole was synthesized by following the scheme-1 described by previously reported methods of (Bitla et al., 2021) 4-Ethoxycarbonylpiperidine (2; 0.05 mol) was mixed with 20 % Na2CO3(aq) in 500 mL round bottom (RB) flask. 4-Toluenesulfonyl chloride (1; 0.05 mol) was added by parts on stirring Na2CO3(aq) was used to maintain PH = 10 TLC was monitored to confirm completion. The pH of the medium was moved to 6 by dilute HCl(aq). The title compound was collected as precipitates, washed, and dried.
2.3 Synthesis of 1-(4-toluenesulfonyl)-4-(2-hydrazinocarbonyl)piperidine (5)
Hydrazine monohydrate (4; 0.045 mol) and equimolar compound 3 were shaken together in methanol (150 mL) in RB flask (500 mL). The contents of the flask were refluxed for 4 h and monitored through TLC. The precipitates were collected after distillation of methanol, washed with n-hexane and dried in air.
2.4 Synthesis of 1-(4-toluenesulfonyl)-4-[1-(methylamino thiocarbonyl)-2 hydrazinocarbonylpiperidine(7)
Equimolar compound 5 and methyl isothiocyanate (6) were shaken together in ethanol (150 mL) in RB flask (500 mL). The contents of the flask were refluxed for 2 h and monitored through TLC. The precipitates were collected after the distillation of the ethanol. Formed precipitates were washed through distilled water and dried in air.
2.5 Procedure for 1-(4-toluenesulfonyl)-4-(3-mercapto-4-methyl-4H-1,2,4-triazol-5-yl)piperidine (8)
Compound 7 (0.04 mol) was refluxed with 15 % KOH(aq) for 2 h and monitored through TLC. The pH of the medium was moved to 6 by dilute HCl(aq). The title compound was collected as precipitates, washed and dried.
2.6 Procedure for 1-(4-toluenesulfonyl)-4-(3-aralkylthio-4-methyl-4H-1,2,4-triazol-5-yl)piperidine (10a-k)
Compound 8 (2 mmol) and LiH (2 mmol) were shaken together in DMF (15 mL) in RB flask (100 mL) and stirred for 0.5 h. Equimolar aralkyl halides (9a-k) were added and the contents of the flask were stirred for 3–5 h. TLC was performed and precipitates were collected after the addition of ice-cold distilled water. The title compounds were collected as precipitates, washed and dried.
2.7 1-(4-Toluenesulfonyl)-4-(3-benzylthio-4-methyl-4H-1,2,4-triazol-5-yl)piperidine (10a)
Amorphous white solid;; M.P.: 186–188 °C; Yield: 86 %; IR (υmax, cm−1): 3061, 1670, 1548, 1367, 694;1H NMR (600 MHz, DMSO‑d6, δ ppm): 7.66 (2H, J = 7.6 Hz, d,2′' & 6′'), 7.48 (2H, J = 7.6 Hz, d,3′' & 5′'), 7.27–7.25 (3H, m, 3′'' to 5′''), 7.22 (2H, J = 6.8 Hz, d,2′'' & 6′''), 4.24 (2H, s, 7′''), 3.64–3.62 (2He, m, 2′ & 6′), 3.19 (3H, s, 6), 2.81–2.77 (1H, m, 4′), 2.42 (3H, s, 7′'), 2.42–2.39 (2Ha, m, 2′ & 6′), 1.89–1.87 (2He, m, 3′ & 5′), 1.74–1.68 (2Ha, m, 3′ & 5′);13C NMR (125 MHz, DMSO‑d6, δ ppm): 157.7 (5), 148.4 (3), 143.4 (1′'), 137.2 (1′''), 132.3 (4′'), 129.8 (3′' & 5′'), 128.8 (2′'' & 6′''), 128.3 (3′'' & 5′''), 127.5 (2′' & 6′'), 127.3 (4′''), 45.4 (2′ & 6′), 37.5 (4′), 30.4 (7′''), 29.6 (6), 28.8 (3′ & 5′), 20.9 (7′'); M.F.: C22H26N4O2S2; M.W.: 442 gmol−1; EI-MS (m/z): 442 [M]+, 319 [C15H19N4O2S]+, 293 [C14H19N3O2S].+, 287 [C15H19N4S]+, 264 [C13H16N2O2S]+, 204 [C10H10N3S]+, 155 [C7H7O2S]+, 149 [C8H7NS]+, 91 [C7H7]+, 65 [C5H5]+.
2.8 1-(4-Toluenesulfonyl)-4-[3-(2-methylbenzyl) thio-4-methyl-4H-1,2,4-triazol-5-yl]piperidine (10b
Amorphous white solid;; M.P.: 178–180 °C; Yield: 88 %;; IR (υmax, cm−1): 3069, 1656, 1577, 1374, 689;1H NMR (600 MHz, DMSO‑d6, δ ppm): 7.65 (2H, J = 7.6 Hz, d, 2′' & 6′'), 7.46 (2H, J = 7.5 Hz, d, 3′' & 5′'), 7.02–6.95 (4H, m,3′'' to 6′''), 4.19 (2H, s, 7′''), 3.63–3.61 (2He, m, 2′ & 6′), 3.20 (3H, s, 6), 2.80 (1H, br.s, 4′), 2.42 (3H, s, 7′'), 2.42–2.40 (2Ha,m, 2′ & 6′), 2.24 (3H, s, 8′''), 1.89–1.85 (2He, m, 3′ & 5′), 1.74–1.69 (2Ha, m, 3′ & 5′);13C NMR (125 MHz, DMSO‑d6, δ ppm): 157.6 (5), 148.6 (3), 143.4 (1′'), 137.8 (1′''), 132.3 (4′'), 131.4 (2′''), 130.2 (3′''), 129.8 (3′' & 5′'), 128.3 (4′''), 127.9 (6′''),127.5 (2′' & 6′'), 127.3 (5′''), 45.4 (2′ & 6′), 37.4 (4′), 30.4 (7′''), 29.5 (6), 28.8 (3′ & 5′), 20.9 (7′'), 20.6 (8′''); M.F.: C23H28N4O2S2; M.W.: 456 gmol−1. EI-MS (m/z): 456 [M]+, 319 [C15H19N4O2S]+, 301 [C16H21N4S]+, 293 [C14H19N3O2S].+, 264 [C13H16N2O2S]+, 218 [C11H12N3S]+, 163 [C9H9NS]+, 155 [C7H7O2S]+, 105 [C8H9]+, 65 [C5H5]+.
2.9 1-(4-Toluenesulfonyl)-4-[3-(3-methylbenzyl)thio-4-methyl-4H-1,2,4-triazol-5-yl]piperidine (10c)
Amorphous white solid; M.P.: 170–172 °C; Yield: 79 %;;IR (υmax, cm−1):3063, 1659, 1570, 1371, 682;1H NMR (600 MHz, DMSO‑d6, δ ppm): 7.65 (2H, J = 7.3 Hz, d, 2′' & 6′'), 7.47 (2H, J = 7.5 Hz, d, 3′' & 5′'), 7.15 (1H, J = 7.4 Hz, t, 5′''), 7.06 (1H, J = 7.4 Hz, d, 6′''), 7.01 (1H, s, 2′''), 7.01–6.99 (1H, m, 4′''), 4.18 (2H, s, 7′''), 3.63–3.62 (2He, m, 2′ & 6′), 3.19 (3H, s, 6), 2.79 (1H, br.s, 4′), 2.41 (3H, s, 7′'), 2.41–2.39 (2Ha, m, 2′ & 6′), 2.23 (3H, s, 8′''), 1.89–1.86 (2He, m, 3′ & 5′), 1.73–1.69 (2Ha, m, 3′ & 5′);13C NMR (125 MHz, DMSO‑d6, δ ppm): 157.7 (5), 148.5 (3), 143.4 (1′'), 137.5 (1′''), 137.0 (3′''), 132.3 (4′'), 129.8 (3′' & 5′'), 129.3 (5′''), 128.3 (4′''), 128.0 (2′''), 127.5 (2′' & 6′'), 125.9 (6′''), 45.4 (2′ & 6′), 37.6 (4′), 30.4 (7′''), 29.5 (6), 28.8 (3′ & 5′), 20.9 (7′'), 20.8 (8′''); M.F.: C23H28N4O2S2; M.W.: 456 gmol−1. EI-MS (m/z): 456 [M]+, 319 [C15H19N4O2S]+, 301 [C16H21N4S]+, 293 [C14H19N3O2S].+, 264 [C13H16N2O2S]+, 218 [C11H12N3S]+, 163 [C9H9NS]+, 155 [C7H7O2S]+, 105 [C8H9]+, 65 [C5H5]+.
2.10 1-(4-Toluenesulfonyl)-4-[3-(4-methylbenzyl) thio-4-methyl-4H-1,2,4-triazol-5-yl]piperidine (10d)
Amorphous white solid; M.P.: 186–188 °C; Yield: 87 %; IR (υmax, cm−1):3073, 1659, 1580, 1382, 687;1H NMR (600 MHz, DMSO‑d6, δ ppm): 7.65 (2H, J = 7.4 Hz, d, 2′' & 6′'), 7.47 (2H, J = 7.5 Hz, d, 3′' & 5′'), 7.10 (2H, J = 7.4 Hz, d, 2′'' & 6′''), 7.07 (2H, J = 7.4 Hz, d, 3′'' & 5′''), 4.19 (2H, s, 7′''), 3.63–3.62 (2He, m, 2′ & 6′), 3.20 (3H, s, 6), 2.79 (1H, br.s, 4′), 2.41 (3H, s, 7′'), 2.41–2.39 (2Ha, m, 2′ & 6′), 2.25 (3H, s, 8′''), 1.89–1.86 (2He, m, 3′ & 5′), 1.71–1.69 (2Ha, m, 3′ & 5′);13C NMR (125 MHz, DMSO‑d6, δ ppm): 157.7 (5), 148.5 (3), 143.4 (1′'), 136.6 (1′''), 134.0 (4′''), 132.3 (4′'), 129.8 (3′' & 5′'), 128.9 (3′'' & 5′''), 128.7 (2′'' & 6′''), 127.5 (2′' & 6′'), 45.4 (2′ & 6′), 37.2 (4′), 30.4 (7′''), 29.5 (6), 28.8 (3′ & 5′), 20.9 (7′'), 20.6 (8′''); M.F.: C23H28N4O2S2; M.W.: 456 gmol−1; EI-MS (m/z): 456 [M]+, 319 [C15H19N4O2S]+, 301 [C16H21N4S]+, 293 [C14H19N3O2S].+, 264 [C13H16N2O2S]+, 218 [C11H12N3S]+, 163 [C9H9NS]+, 155 [C7H7O2S]+, 105 [C8H9]+, 65 [C5H5]+.
2.11 1-(4-Toluenesulfonyl)-4-[3-(2-chlorobenzyl)thio-4-methyl-4H-1,2,4-triazol-5-yl]piperidine (10e)
Amorphous white solid; M.P.: 168–170 °C; Yield: 83 %;;IR (υmax, cm−1):3087, 1666, 1589, 1396, 702, 691;1H NMR (600 MHz, DMSO‑d6, δ ppm): 7.66 (2H, J = 8.1 Hz, d, 2′' & 6′'), 7.47 (2H, J = 8.0 Hz, d, 3′' & 5′'), 7.44 (1H, J = 7.9 Hz, d, 6′''), 7.30 (1H, J = 2.9, 6.2 Hz, dt, 4′''), 7.24–7.22 (2H, m, 3′'' & 5′''), 4.29 (2H, s, 7′''), 3.65–3.63 (2He, m, 2′ & 6′), 3.36 (3H, s, 6), 2.82–2.78 (1H, m, 4′), 2.42 (3H, s, 7′'), 2.42–2.40 (2Ha, m, 2′ & 6′), 1.89–1.87 (2He, m, 3′ & 5′), 1.75–1.68 (2Ha, m, 3′ & 5′);13C NMR (125 MHz, DMSO‑d6, δ ppm): 158.0 (5), 147.8 (3), 143.4 (1′'), 134.6 (1′''), 133.0 (5′''), 132.3 (4′'), 131.2 (3′''), 129.7 (3′' & 5′'), 129.5 (4′''), 129.4 (6′''), 127.5 (2′' & 6′'), 127.2 (2′''), 45.4 (2′ & 6′), 35.7 (4′), 30.5 (7′''), 29.5 (6), 28.8 (3′ & 5′), 20.9 (7′'); M.F.: C22H25ClN4O2S2; M.W.: 477 gmol−1 EI-MS (m/z): 479 [M + 2]+, 477 [M]+, 321 [C15H18ClN4S]+, 319 [C15H19N4O2S]+, 293 [C14H19N3O2S].+, 264 [C13H16N2O2S]+, 238 [C10H9ClN3S]+, 183 [C8H6ClNS]+, 155 [C7H7O2S]+, 125 [C7H6Cl]+, 65 [C5H5]+.
2.12 1-(4-Toluenesulfonyl)-4-[3-(4-chlorobenzyl) thio-4-methyl-4H-1,2,4-triazol-5-yl]piperidine (10f)
Amorphous white solid; M.P.: 174–176 °C; Yield: 90 %; IR (υmax, cm−1):3081, 1660, 1584, 1399, 705, 688;1H NMR (600 MHz, DMSO‑d6, δ ppm): 7.66 (2H, J = 7.5 Hz, d, 2′' & 6′'), 7.48 (2H, J = 7.6 Hz, d, 3′' & 5′'), 7.34 (2H, J = 7.9 Hz, d, 2′'' & 6′''), 7.27 (2H, J = 7.4 Hz, d, 3′'' & 5′''), 4.25 (2H, s, 7′''), 3.64–3.62 (2He, m, 2′ & 6′), 3.26 (3H, s, 6), 2.81 (1H, br.s, 4′), 2.42 (3H, s, 7′'), 2.42–2.39 (2Ha, m, 2′ & 6′), 1.90–1.87 (2He, m, 3′ & 5′), 1.73–1.69 (2Ha, m, 3′ & 5′);13C NMR (125 MHz, DMSO‑d6, δ ppm): 157.8 (5), 148.2 (3), 143.4 (1′'), 136.4 (4′''), 132.3 (4′'), 131.9 (1′''), 130.7 (3′'' & 5′''), 129.8 (3′' & 5′'), 128.3 (2′'' & 6′''), 127.5 (2′' & 6′'), 45.4 (2′ & 6′), 36.4 (4′), 30.4 (7′''), 29.6 (6), 28.8 (3′ & 5′), 20.9 (7′'); M.F.: C22H25ClN4O2S2; M.W.: 477 gmol−1; EI-MS (m/z): 479 [M + 2]+, 477 [M]+, 321 [C15H18ClN4S]+, 319 [C15H19N4O2S]+, 293 [C14H19N3O2S].+, 264 [C13H16N2O2S]+, 238 [C10H9ClN3S]+, 183 [C8H6ClNS]+, 155 [C7H7O2S]+, 125 [C7H6Cl]+, 65 [C5H5]+.
2.13 1-(4-Toluenesulfonyl)-4-[3-(2-bromobenzyl) thio-4-methyl-4H-1,2,4-triazol-5-yl] piperidine (10 g)
Amorphous white solid; M.P.: 178–180 °C; Yield: 75 %; IR (υmax, cm−1):3098, 1673, 1591, 1394, 653, 682;1H NMR (600 MHz, DMSO‑d6, δ ppm): 7.65 (2H, J = 8.0 Hz, d, 2′' & 6′'), 7.61 (1H, J = 7.7 Hz, d, 6′''), 7.46 (2H, J = 8.1 Hz, d, 3′' & 5′'), 7.32 (1H, J = 7.2 Hz, t, 4′''), 7.26 (1H, J = 6.9 Hz, t, 5′''), 6.91 (1H, J = 7.6 Hz, d, 3′''), 4.29 (2H, s, 7′''), 3.65–3.61 (2He, m, 2′ & 6′), 3.20 (3H, s, 6), 2.79 (1H, br.s, 4′), 2.42 (3H, s, 7′'), 2.42–2.41 (2Ha, m, 2′ & 6′), 1.90–1.87 (2He, m, 3′ & 5′), 1.74–1.71 (2Ha, m, 3′ & 5′);13C NMR (125 MHz, DMSO‑d6, δ,ppm): 158.0 (5), 147.8 (3), 143.4 (1′'), 136.2 (1′''), 132.3 (4′'), 131.2 (3′''), 129.8 (3′' & 5′'), 129.5 (4′''), 128.6 (6′''), 127.8 (5′''), 127.5 (2′' & 6′'), 121.9 (2′''), 45.4 (2′ & 6′), 38.2 (4′), 30.5 (7′''), 29.5 (6), 28.8 (3′ & 5′), 20.9 (7′'); M.F.: C22H25BrN4O2S2; M.W.: 521 gmol−1; EI-MS (m/z): 523 [M + 2]+, 521 [M]+, 366 [C15H18BrN4S]+, 319 [C15H19N4O2S]+, 293 [C14H19N3O2S].+, 283 [C10H9BrN3S]+, 264 [C13H16N2O2S]+, 228 [C8H6BrNS]+, 170 [C7H6Br]+, 155 [C7H7O2S]+, 65 [C5H5]+.
2.14 1-(4-Toluenesulfonyl)-4-[3-(3-bromobenzyl)thio-4-methyl-4H-1,2,4-triazol-5-yl] piperidine (10 h)
Amorphous white solid; M.P.: 172–174 °C; Yield: 79 %;; IR (υmax, cm−1):3086, 1681, 1595, 1391, 649, 688;1H NMR (600 MHz, DMSO‑d6, δ ppm): 7.65 (2H, J = 7.6 Hz, d, 2′' & 6′'), 7.47 (2H, J = 7.5 Hz, d, 3′' & 5′'), 7.33 (1H, s, 2′''), 7.17–7.13 (2H, m, 5′'' & 6′''), 6.97 (1H, J = 7.5 Hz, d, 4′''), 4.25 (2H, s, 7′''), 3.63–3.61 (2He, m, 2′ & 6′), 3.20 (3H, s, 6), 2.79 (1H, br.s, 4′), 2.41 (3H, s, 7′'), 2.41–2.39 (2Ha, m, 2′ & 6′), 1.89–1.85 (2He, m, 3′ & 5′), 1.74–1.68 (2Ha, m, 3′ & 5′);13C NMR (125 MHz, DMSO‑d6, δ ppm): 157.9 (5), 148.2 (3), 143.4 (1′'), 140.2 (1′''), 132.3 (4′'), 131.4 (4′''), 130.4 (2′''), 130.1 (5′''), 129.8 (3′' & 5′'), 127.9 (6′''), 127.5 (2′' & 6′'), 121.3 (3′''), 45.4 (2′ & 6′), 36.5 (4′), 30.4 (7′''), 29.5 (6), 28.8 (3′ & 5′), 20.9 (7′'); M.F.: C22H25BrN4O2S2; M.W.: 521 gmol−1 EI-MS (m/z): 523 [M + 2]+, 521 [M]+, 366 [C15H18BrN4S]+, 319 [C15H19N4O2S]+, 293 [C14H19N3O2S].+, 283 [C10H9BrN3S]+, 264 [C13H16N2O2S]+, 228 [C8H6BrNS]+, 170 [C7H6Br]+, 155 [C7H7O2S]+, 65 [C5H5]+.
2.15 1-(4-Toluenesulfonyl)-4-[3-(4-bromobenzyl)thio-4-methyl-4H-1,2,4-triazol-5-yl] piperidine (10i)
Amorphous white solid; M.P.: 182–184 °C; Yield: 75 %; IR (υmax, cm−1):3076, 1679, 1597, 1388, 647, 685;1H NMR (600 MHz, DMSO‑d6, δ ppm): 7.65 (2H, J = 7.3 Hz, d, 2′' & 6′'), 7.48–7.46 (4H, m, 4H, 3′' & 5′', 2′'' & 6′''), 7.20 (2H, J = 7.2 Hz, d, 3′'' & 5′''), 4.23 (2H, s, 7′''), 3.64–3.62 (2He, m, 2′ & 6′), 3.26 (3H, s, 6), 2.82–2.79 (1H, m, 4′), 2.42 (3H, s, 7′'), 2.42–2.40 (2Ha, m, 2′ & 6′), 1.90–1.88 (2He, m, 3′ & 5′), 1.74–1.68 (2Ha, m, 3′ & 5′);13C NMR (125 MHz, DMSO‑d6, δ ppm): 157.8 (5), 148.2 (3), 143.4 (1′'), 136.8 (1′''), 132.3 (4′'), 131.2 (3′'' & 5′''), 131.0 (2′'' & 6′''), 129.8 (3′' & 5′'), 127.5 (2′' & 6′'), 120.5 (4′''), 45.4 (2′ & 6′), 36.4 (4′), 30.4 (7′''), 29.6 (6), 28.8 (3′ & 5′), 20.9 (7′'); M.F.: C22H25BrN4O2S2; M.W.: 521 gmol−1; EI-MS (m/z): 523 [M + 2]+, 521 [M]+, 366 [C15H18BrN4S]+, 319 [C15H19N4O2S]+, 293 [C14H19N3O2S].+, 283 [C10H9BrN3S]+, 264 [C13H16N2O2S]+, 228 [C8H6BrNS]+, 170 [C7H6Br]+, 155 [C7H7O2S]+, 65 [C5H5]+.
2.16 1-(4-Toluenesulfonyl)-4-[3-(4-flourobenzyl) thio-4-methyl-4H-1,2,4-triazol-5-yl]piperidine (10j)
Amorphous white solid; M.P.: 170–172 °C; Yield: 81 %; IR (υmax, cm−1):3096, 1669, 1584, 1389, 1057, 676;1H NMR (600 MHz, DMSO‑d6, δ ppm): 7.65 (2H, J = 7.6 Hz, d, 2′' & 6′'), 7.45 (2H, J = 7.6 Hz, d, 3′' & 5′'), 7.21 (2H, J = 8.1 Hz, d, 3′'' & 5′''), 7.02 (2H, J = 8.0 Hz, d, 2′'' & 6′''), 4.24 (2H, s, 7′''), 3.64–3.61 (2He, m, 2′ & 6′), 3.21 (3H, s, 6), 2.80 (1H, br.s, 4′), 2.42 (3H, s, 7′'), 2.42–2.39 (2Ha, m, 2′ & 6′), 1.90–1.88 (2He, m, 3′ & 5′), 1.72–1.69 (2Ha, m, 3′ & 5′);13C NMR (600 MHz, DMSO‑d6, δ ppm): 158.1 (5), 150.5 (4′''), 148.1 (3), 143.4 (1′'), 132.1 (4′'), 131.8 (1′''), 130.6 (2′'' & 6′''), 129.8 (3′' & 5′'), 127.5 (2′' & 6′'), 127.1 (3′'' & 5′''), 45.4 (2′ & 6′), 36.4 (4′), 30.4 (7′''), 29.6 (6), 28.8 (3′ & 5′), 20.9 (7′'); M.F.: C22H25FN4O2S2; M.W.: 460 gmol−1; EI-MS (m/z): 460 [M]+, 319 [C15H19N4O2S]+, 305 [C15H18FN4S]+, 293 [C14H19N3O2S].+, 264 [C13H16N2O2S]+, 222 [C10H9FN3S]+, 167 [C8H6FNS]+, 155 [C7H7O2S]+, 109 [C7H6F]+, 65 [C5H5]+.
2.17 1-(4-Toluenesulfonyl)-4-[3-(3,4-dichlorobenzyl)thio-4-methyl-4H-1,2,4-triazol-5-yl] piperidine (10 k)
Amorphous white solid; M.P.: 176–178 °C; Yield: 78 %; IR (υmax, cm−1):3066, 1658, 1579, 1388, 707, 686;1H NMR (600 MHz, DMSO‑d6, δ ppm): 7.66 (2H, J = 7.4 Hz, d, 2′' & 6′'), 7.59 (1H, s, 2′''), 7.47 (2H, J = 7.5 Hz, d, 3′' & 5′'), 7.33 (1H, J = 8.3 Hz, t, 5′''), 7.30 (1H, J = 8.0 Hz, d, 6′''), 4.28 (2H, s, 7′''), 3.65–3.63 (2He, m, 2′ & 6′), 3.28 (3H, s, 6), 2.84–2.80 (1H, m, 4′), 2.42 (3H, s, 7′'), 2.42–2.40 (2Ha, m, 2′ & 6′), 1.90–1.88 (2He, m, 3′ & 5′), 1.75–1.69 (2Ha, m, 3′ & 5′);13C NMR (600 MHz, DMSO‑d6, δ ppm): 158.0 (5), 147.6 (3), 143.4 (1′'), 133.99 (1′''), 133.94 (4′''), 133.0 (3′''), 132.4 (5′''), 132.3 (4′'), 129.7 (3′' & 5′'), 128.8 (2′''), 127.5 (2′' & 6′'), 127.3 (6′''), 45.4 (2′ & 6′), 34.7 (4′), 30.5 (7′''), 29.6 (6), 28.8 (3′ & 5′), 20.9 (7′'); M.F.: C22H24Cl2N4O2S2; M.W.: 511 gmol−1; EI-MS (m/z): 515 [M + 4]+, 513 [M + 2]+, 511 [M]+, 356 [C15H17Cl2N4S]+, 319 [C15H19N4O2S]+, 293 [C14H19N3O2S].+, 273 [C10H8Cl2N3S]+, 264 [C13H16N2O2S]+, 218 [C8H5Cl2NS]+, 160 [C7H5Cl2]+, 155 [C7H7O2S]+, 65 [C5H5]+.
2.18 In-vitro acetylcholinesterase (AChE) inhibition assay
Acetylcholinesterase (3.1.1.7) inhibition activity was measured by modifying the reported methods of Ellman using 96-wells plate readers (Synergy HT, BioTek, USA) (Mitsumori et al., 2003). Acetylcholine Iodide (AChEI) was used as the substrate, 5,5ʹ-dithio bis (2-nitro benzoic) acid (DTNB) to estimate the activity of AChE and Eserine as reference. The reaction mixture containing 10 μL cholinesterase enzyme (Sigma, USA), tested compounds (0.05 mM; 10 to 100 mM), 60 μL phosphate buffer (pH 7.7; 0.05 mM), and 10 μL DTNB (0.05 mM) was incubated at 37 °C for 15–30 min. The change in optical density (O.D.) at zero, 15, and 30 min was monitored at 405 nm after the initiation of the reaction by the addition of acetyl thiocholine iodide (10 μL) while Eserine (0.5 mM) was employed as a positive control. The percentage inhibition was noted as,
The Ez-Fit software (Perrella Scientific Inc. Amherst, USA) and computing data in Graph Pad Prism 5 software were used to calculate IC50 values. Inhibition parameters and Vmax were determined from Lineweaver-Burk plots (Duyckaerts et al., 2009).
2.19 In-vitro α-Glucosidase assay
α-Glucosidase inhibition assay was performed following the reported methods of with minor modifications using acarbose as a reference standard (Tao et al., 2013). Tested compounds (10 to 100 μL; 0.05 mM), α-glucosidase enzyme (10 μL) (Sigma, USA) and phosphate buffers (70 μL; pH 6.8; 0.05 M) were mixed and pre-incubated at 37 °C for 10 min followed by pre-reading at 400 nm. Change in absorbance was monitored by adding p-nitrophenyl glucopyranoside (10 μL; 0.5 mM). The previously mentioned formula was used to assess the % inhibition and IC50 values.
2.20 Molecular docking simulations
The inhibitory potential of produced compounds was determined theoretically by virtual molecular docking software by Molegro Virtual Docker version 2010. Proteins for anti-acetylcholine esterase were saved as pdb after downloading from an online protein data bank. Chemdarw (Pro; 12.0.2; 875–317589-4732; Cambridgesoft) was used to draw the structures of produced compounds (10a-k) and saved as a MOL2 file by copying it into 3D chem draw. Files of pdb proteins were imported in the docking software to prepare a template then all produced compounds were run one by one.
3 Results and discussion
The target compounds 1,2,4-triazole analogs (10a-k) bearing piperidine were prepared under multiple-step synthesis presented having different functional groups depicted in Scheme 1. The main core of the 1,2,4-triazole nucleus (8) was achieved through the formation of different intermediates including carboxylate (3), hydrazide (5), and thiosemicarbazide (7). The last step resulted in 1,2,4-triazole analogs (10a-k). Similar to our findings, (Castaneda et al., 2010)also reported the synthesis of 1,3,5 substituted 1,2,4 triazole from carboxylic acid but with only 25–84 % yield. Furthermore, two enzymes including acetylcholinesterase (AChE) and α-glucosidase were employed to demonstrate the enzyme inhibition potential of synthesized compounds (10a-k).
3.1 Chemistry
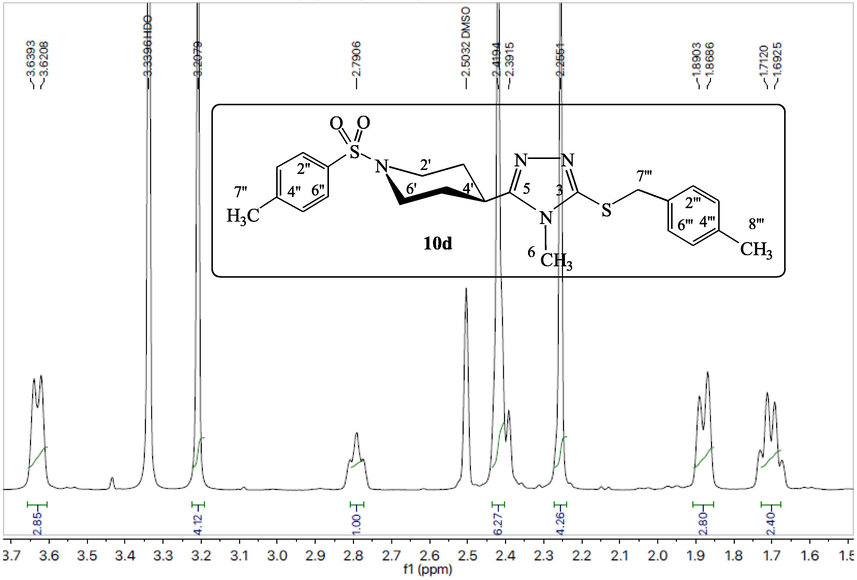
The compound 10d has been explicated as a single compound discussion from this alkyl halide series (10a-k). The compounds, 10a-c,e-k, have been structurally elucidated similarly. The white amorphous solid, 10d (C23H28N4O2S2, 456 g mol−1, justified through mass spectrum), possessed a melting point of 186–188 °C with 87 % yield. The IR data was known to possess the following stretching frequencies for prominent functionalities, 3073, 1659, 1580, 1382 and 687. 4-Methylphenylsulfonyl moiety was justified through three signals in the 1H NMR spectrum (Figs. 1, 2) at δ 7.65 (2H, J = 7.4 Hz, d, 2′' & 6′'), 7.47 (2H, J = 7.5 Hz, d, 3′' & 5′') and 2.41 (3H, s, 7′'); and five signals in the 13C NMR spectrum at δ 143.4 (1′'), 132.3 (4′'), 129.8 (3′' & 5′'), 127.5 (2′' & 6′') and 20.9 (7′'). 4-Methylbenzyl moiety was justified through four signals in 1H NMR spectrum (Figs. 1, 3) at δ 7.10 (2H, J = 7.4 Hz, d, 2′'' & 6′''), 7.07 (2H, J = 7.4 Hz, d, 3′'' & 5′''), 4.19 (2H, s, 7′'') and 2.25 (3H, s, 8′''); and six signals in 13C NMR spectrum (Figure-4, Figure-5) at δ 136.6 (1′''), 134.0 (4′''), 128.9 (3′'' & 5′''), 128.7 (2′'' & 6′''), 30.4 (7′'') and 20.6 (8′''). 3,5-Disubstituted-6-methyl-1,2,4-triazole moiety was justified through one signal in the 1H NMR spectrum (Fig. 2) at δ 3.20 (3H, s, 6); and three signals in the 13C NMR spectrum (Figs. 4, 5) at δ 157.7 (5), 148.5 (3) and 29.5 (6). Piperidine moiety was justified through five signals in the 1H NMR spectrum (Fig. 2) at δ 3.63–3.62 (2He, m, 2′ & 6′), 2.79 (1H, br.s, 4′), 2.41–2.39 (2Ha, m, 2′ & 6′), 1.89–1.86 (2He, m, 3′ & 5′) and 1.71–1.69 (2Ha, m, 3′ & 5′); and three signals in the 13C NMR spectrum (Fig. 5) at δ 45.4 (2′ & 6′), 37.2 (4′) and 28.8 (3′ & 5′). 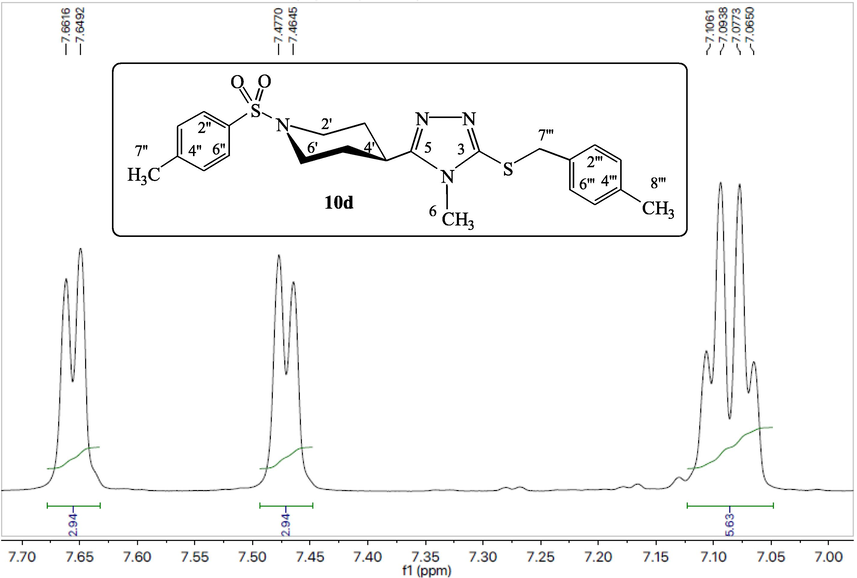
1H NMR spectrum (aromatic) of compound 10d.
1H NMR spectrum (shielded aliphatic) of compound 10d.
1H NMR spectrum (deshielded aliphatic) of compound 10d.
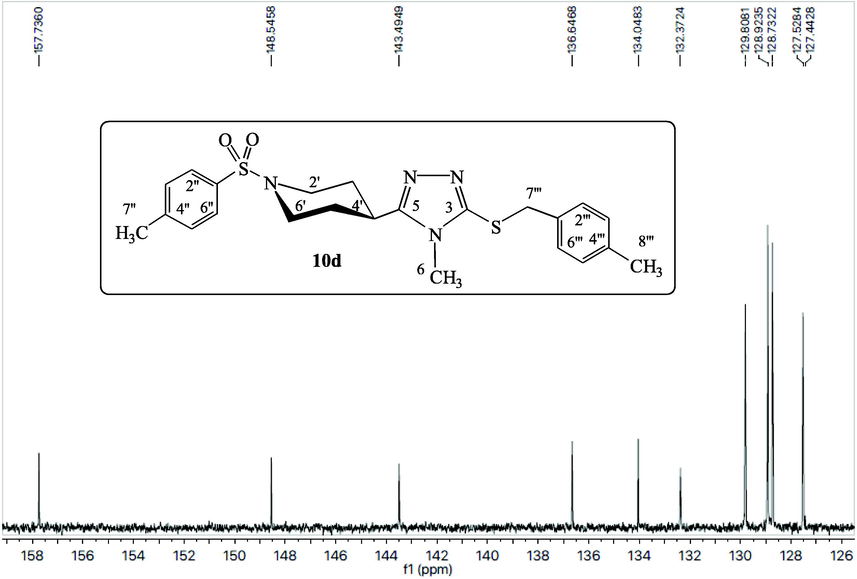
13C NMR spectrum (aromatic) of compound 10d.
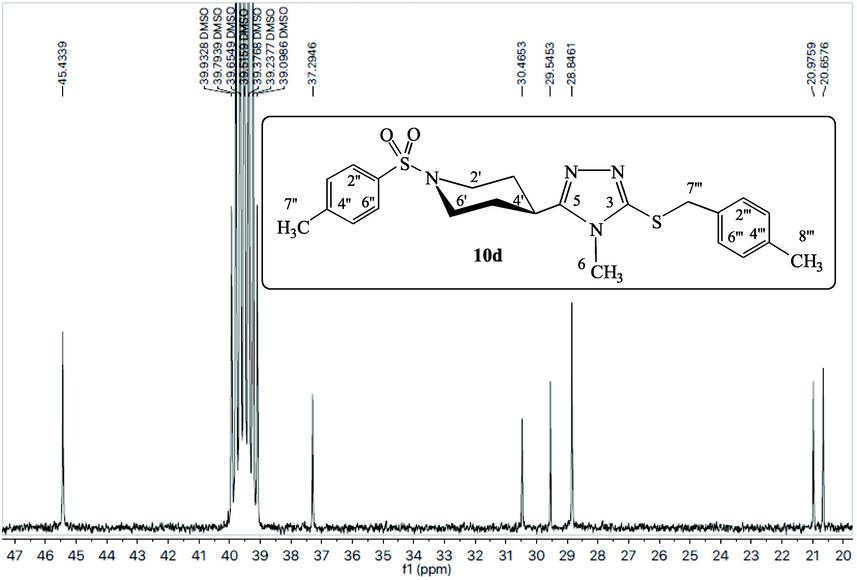
13C NMR spectrum (aliphatic) of compound 10d.
3.2 Biological activities
The biological activities of the prepared compounds were evaluated, which are important for practical applications (Muhammad et al., 2020; Suhail and Ali, 2020; Asif and Abida, 2019; Deeba et al., 2018; Pathak et al., 2021). The 1,2,4-triazole analogs (10a-k) were further evaluated for their inhibitory activities against AChE and α-glucosidase enzymes (Table 1, 2). It is investigated that AD and DM have an elevated activity of AChE (E.C. 3.1.1.7) which leads to alteration in cholinergic neurotransmission (Gulçin et al., 2018). Any alteration in the expression and activity of AChE that’s a prime cause of neurodegenerative disorders may act as a biomarker for neurotoxicity determination (Isik et al., 2015) Inhibitors of AChE are widely recommended to eliminate neurotoxic effects produced by the change of the cholinergic hypothesis. Inhibitors of AChE may reduce the multiple β-amyloid neurotoxic products and protects the cell from oxidative damage (Taslimi et al., 2021). AChE inhibitors commonly used in treatment slow down excessive ACh hydrolysis and avert AD progression which leads to relaxation and improves cognitive functions (Isik et al., 2019)The structure–activity relationship (SAR) analysis revealed that the presence of electron-donating or electron-withdrawing groups at meta, ortho, or para positions of the whole series of derivatives (1,2,4-triazole analogs 10a-k) presented a vital role in enhancing their anti-enzymatic role against AChE. The whole series of derivatives (1,2,4-triazole analogs 10a-k) were active against the AChE enzyme (Table 1, 2, Figs. 6a & 6b). The excellent activity was possessed by four compounds, 10b (bearing 2-methyl benzyl moiety), 10c (bearing 3-methylbenzyl moiety), 10j (bearing 4-fluorobenzyl moiety), and 10 k (bearing 3,4-dichlorobenzyl moiety). among methyl-benzyl substituents, the most active ones were ortho and meta-substituted molecules, ortho substituted in the chlorobenzyl substituents, meta substituted in the bromobenzyl substituents, while ortho and meta substituted in the mono-halo-benzyl substituents whereas 4-fluorobenzyl bearing molecules were the exception. The only molecule bearing dichloro benzyl moiety remained the most efficient compound against AChE. Note: a = Eserine, b = Acarbose.
Compound
AChE Inhibition
α-Glucosidase Inhibition
Inhibition (%) at 0.5 mM
IC50 (µM)
Inhibition (%) at 0.5 mM
IC50 (µM)
10a
63.24 ± 1.46
0.251 ± 1.24
52.36 ± 1.67
452.75 ± 1.27
10b
87.23 ± 1.12
0.528 ± 0.92
85.35 ± 1.39
56.43 ± 1.13
10c
87.95 ± 1.24
0.537 ± 0.76
82.16 ± 1.74
82.53 ± 1.43
10d
76.52 ± 1.67
0.158 ± 1.22
62.81 ± 1.45
213.62 ± 1.24
10e
64.82 ± 1.46
0.250 ± 1.15
65.34 ± 1.73
325.14 ± 1.36
10f
58.93 ± 1.58
0.339 ± 1.21
62.57 ± 1.72
379.62 ± 1.45
10 g
65.42 ± 1.35
0.262 ± 1.13
62.37 ± 1.57
397.26 ± 1.32
10 h
79.75 ± 1.49
0.115 ± 1.14
76.74 ± 1.81
154.26 ± 1.26
10i
77.42 ± 1.56
0.135 ± 1.31
71.54 ± 1.57
192.43 ± 1.24
10j
87.18 ± 1.23
0.031 ± 0.85
68.87 ± 1.62
317.84 ± 1.32
10 k
90.46 ± 1.21
0.169 ± 0.97
75.21 ± 1.53
413.61 ± 1.29
Standard
91.27 ± 1.17a
0.04 ± 0.001a
65.73 ± 1.93b
375.82 ± 1.76b
Sr. No.
Triazole derivative
Type of inhibition
Ki (μM)
Std drug
Un-competitive
0.018 ± 1.00
10a
Mixed type
0.211 ± 0.85
10b
Mixed type
0.492 ± 0.75
10c
Mixed type
0.548 ± 0.50
10d
Mixed type
0.198 ± 1.50
10e
Mixed type
0.248 ± 1.80
10f
Competitive
0.156 ± 1.75
10 g
Mixed type
0.251 ± 0.25
10 h
Un-competitive
0.557 ± 0.50
10i
Mixed type
0.124 ± 2.5
10j
Un-competitive
0.015 ± 1.25
10 k
Un-competitive
0.084 ± 0.05
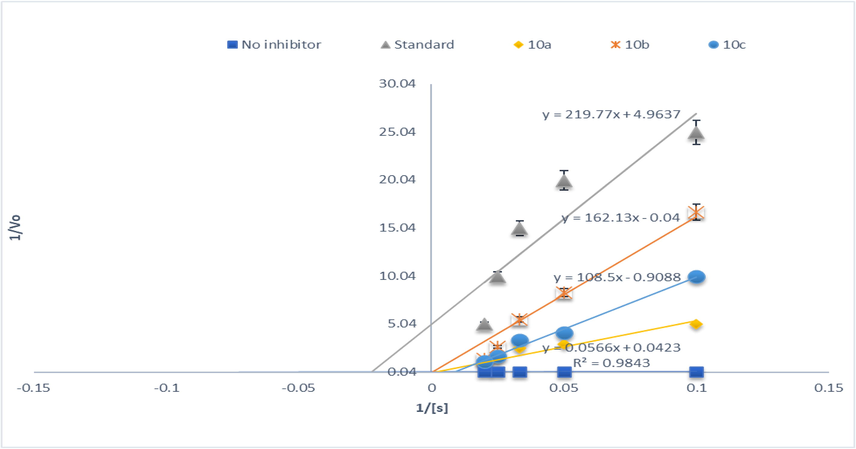
AChE inhibition of synthesized compounds (10a-10c).
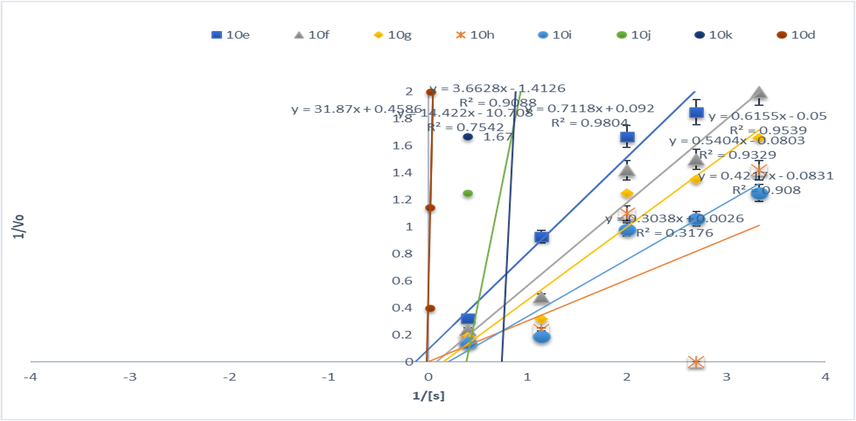
AChE inhibition of synthesized compounds (10d-10 k).
Lineweaver-Burk and Michaelis Menten equation was used to determine the value of Ki and type of inhibition at eight different concentrations both in the presence and in absence of triazole derivatives (Table 2 and Figs. 6a & 6b). It can be suggested that derivative 10 k inhibited the AChE un-competitively by binding AChE and AChEI complex reversibly with weak interactions. Furthermore, its Ki value was determined as 0.084 ± 0.05 µM. Literature reported that many neurodegenerative disorders such as Parkinson's disease, Lewy body dementia, myasthenia gravis, and glaucoma especially AD could be treated by cholinesterase inhibitors In another study, (Shelke et al., 2015) investigated water-soluble triazole substituted metal-free and metallo-phthalocyanines for AChE inhibition potential. In line with our findings, 4a phthalocyanine having 0.040 µM IC50 was active against AChE which was 1.5-fold active as compared to standard.
The whole series of derivatives were active against the α-glucosidase enzyme (Table 2, Fig. 7). Most of the compounds among the series were active and were better inhibitors than that of the reference standard. The excellent activity was possessed by two compounds, 10b (bearing 2-methylbenzyl moiety) and 10c (bearing 3-methylbenzyl moiety). Among the methyl benzyl substituents, the most active ones were ortho and meta-substituted molecules while ortho-substituted in chlorobenzyl substituents, meta-substituted in bromobenzyl substituents, ortho and meta in the mono-halo benzyl substituents whereas derivatives bearing unsubstituted benzyl moiety and di chlorobenzyl moiety were found to be the least active ones. Triazole due to its poor basicity as compared to other azaheterocycles is not protonated at physiological pH. The better inhibition potential of derivative compounds might be due to excellent mimic partial positive charge at the anomeric carbon in the transition state of glucosidase catalyzed reaction in non-protonated sp2-hybridized nitrogen atoms of triazole derivative than corresponding basic nitrogen of iminosugars. Similar to our findings, it has been reported that several triazole derivatives showed an inhibitory effect against α-glucosidase among which 10b showed promising results and was considered as the most active analog with IC50 value of 14.2 µM (Taslimi et al., 2017). Whereas compound 6 showed contrary results due to a low IC50 value of 218.1 µM. Similarly, (Ye et al., 2019) determined the antidiabetic potential of xanthone triazole derivatives by inhibiting the α-glucosidase and glucose uptake in HepG2 cells. They reported that compound 5e was found to be most potent having 2.06 µM IC50 than parental (1,3-dihydroxyxanthone IC50 = 160.8 µM) and 1-deoxynojirimycin (Std. IC50 = 59.5 µM). The position of different substituents in 1,2,4-triazole analogs (10a-k) helped to establish SAR. In-vitro studies have indicated that chloro and fluoro groups present at R2 and R3 positions in derivatives best inhibit the activities of both AChE and α-glucosidase. The reason might be due to the presence of chloro and fluoro groups as a substituent on triazole ring have a negative inductive effect causing destabilization of the triazole ring resulting in enhanced activity of 1,2,4-triazole analogs. The compounds 10 k, 10j, 10b, and 10c are the most active and presented the most potent inhibitory potential as compared to others. When all 1,2,4-triazole analogs (10a-k) were examined, it was observed that chloro, fluoro and methyl groups containing 1,2,4-triazole analogs (10a-k) are the most active compounds. The findings revealed that the triazole analogs prepared in the present investigation showed promising biological activity (Hassan and Arshad, 2022; Tegegne et al., 2020; Shadrach et al., 2020), which have potential for partical application.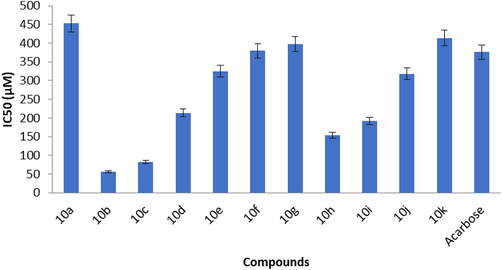
α-Glucosidase inhibition of synthesized compounds (10a-k).
3.3 Molecular docking simulation
Docking imitations of applicant ligands with AChE co-crystal structure (PDB ID:1NEN) using Auto dock tools predicted that the compound has a value of binding affinity of −9.9 kcal/mol which is the best binding score (Taslimi et al., 2017; Pradhan and Vishwakarma, 2020). The detailed analysis along with types of bonds with their distances formed between the amino acids was provided in Table 3. Drug-target interactions were assumed in the provision of interfacing amino acids fragments, hydrogen bonding, docking energy investigation, presumed binding sites and peculiarities of active site amino acid residues (Fig. 8). Total binding strength is a consequence of different kinds of bonds inclusive of ionic, hydrophobic interactions, Vander Waals forces and hydrogen bonds which are the promoter. Compound (10j) showed conventional hydrogen bonding interactions with amino acid HIS 45, THR 46, ASN 218, ALA 201 and electrostatic interaction with HIS 354, ARG 399, GLU 388 (Virk et al., 2019). It has convenient van der Waals interactions with HIS 354, ALA 49, LEU 252, LYS 38, HIS 354, ALA49, LYS 38 (Ye et al., 2016). In the form of numerous types such as Pi-Pi T-shaped, Alkyl and Pi-Alkyl (Yin et al., 2009). All these interactions with certain amino acids occurred at different distances are shown in Table 3.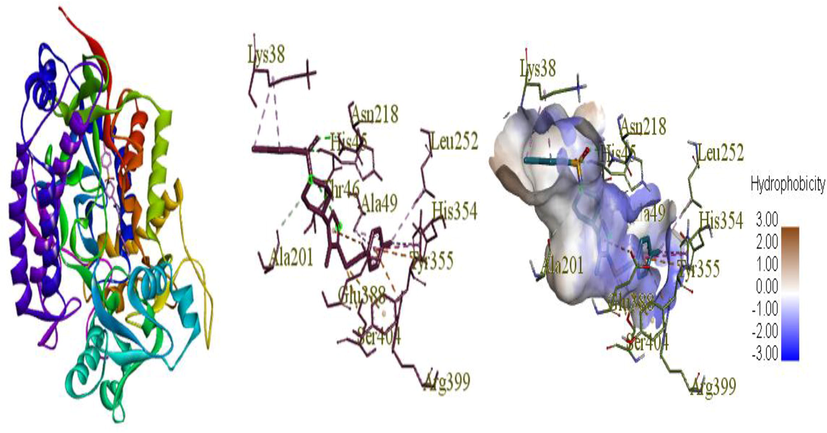
Binding modes of compound 10j in acetyl cholinesterase (PDB ID:1NEN). Dotted lines presented the hydrogen bonds.
Ligand
Binding affinity, ΔG (Kcal/mol)
Hydrogen Bonding
Types of Interactions Hydrophobic
Electrostatic
Amino acids
Type
Distance
Amino acids
Distance
Type
Amino acids
Distance
Type
Ligand 10j
−9.9
HIS 45
THR 46
ASN 218
ALA 201Conventional
Conventional
Conventional
C—H bond2.183
2.647
2.171
3.795HIS 354
ALA 49
LEU 252
LYS 38
HIS 354
ALA49
LYS 385.053
4.292
5.115
4.502
4.934
4.143
4.518Pi-Pi T-shaped
Alkyl
Alkyl
Alkyl
Pi-Alkyl
Pi-Alkyl
Pi-AlkylHIS 354
ARG 399
GLU 3884.4154
4.4118
4.5183Pi-Cation
Pi-Cation
Pi-Anion
4 Conclusion
As a result, 1,2,4 triazole derivatives (10a-k) are stronger inhibitors in very low concentrations by the attachment of functional electronegative groups such as -F and -Cl to a triazole ring. As these compounds have an electron density, can inhibit AChE and α-glucosidase by getting them simpler to attach to the functional groups of amino acids. Different inhibitors of these enzyme classes are in clinical practice but are riddled with efficacy, potency and safety challenges. It is concluded from the results of the present study that novel triazole derivatives (10b, 10j and 10 k) are the candidate drugs with anticholinergic and antidiabetic potential for the treatment of some diseases like type 2 DM, dementia, altitude (mountain) sickness and AD. The identified derivatives may be subjected to further analysis by pharmacological industries to check out their use as new drug candidates.
Funding
This research was funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R18), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Acknowledgments
The authors express their gratitude to Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R18), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. Research services and analytical facilities provided by Central Hi-Tech Laboratory, Govt. College University, Faisalabad is thankfully acknowledged.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Synthesis, characterization, and inhibition study of novel substituted phenylureido sulfaguanidine derivatives as α-glycosidase and cholinesterase inhibitors. Chem. Biodivers.. 2021;18(4):e2000958.
- [Google Scholar]
- M. Asif, Abida, M.T. Alam, Diverse chemical and biological potentials of various pyridazine and pyridazinone derivatives, Chemistry International 5(3) (2019) 206-231.
- Synthesis of 1H–1, 2, 3-triazole derivatives as new α-glucosidase inhibitors and their molecular docking studies. Bioorg. Chem.. 2018;81:98-106.
- [Google Scholar]
- One-pot synthesis of 3, 4, 5-trisubstituted 1, 2, 4-triazoles via the addition of hydrazides to activated secondary amides. Org. Lett.. 2015;17(5):1184-1187.
- [Google Scholar]
- Design and synthesis, biological evaluation of bis-(1, 2, 3-and 1, 2, 4)-triazole derivatives as potential antimicrobial and antifungal agents. Bioorg. Med. Chem. Lett.. 2021;41:128004
- [Google Scholar]
- G.M. Bousada, B.L. de Sousa, G. Furlani, A.P. Agrizzi, P.G. Ferreira, J.P.V. Leite, T.A.d.O. Mendes, E.V. Varejão, E.J. Pilau, M.H. Dos Santos, Tyrosol 1, 2, 3-triazole analogues as new acetylcholinesterase (AChE) inhibitors, Computational Biology and Chemistry 88 (2020) 107359.
- The phosphatidyl inositol 3-kinase/AKT signaling pathway in breast cancer. Cancer Metastasis Rev.. 2010;29(4):751-759.
- [Google Scholar]
- Synthesis, characterization and biological activities of 1, 3, 4-oxadiazole derivatives of nalidixic acid and their copper complexes. Chem. Int.. 2018;4(4):206-215.
- [Google Scholar]
- A new method of synthesis of substituted 1-(1H-imidazole-4-yl)-1H-1,2,3-triazoles and their fungicidal activity. Tetrahedron. 2018;74(6):672-683.
- [Google Scholar]
- Classification and basic pathology of Alzheimer disease. Acta Neuropathol.. 2009;118(1):5-36.
- [Google Scholar]
- 1,3-Dipolar cycloaddition reactions of enaminones and azides: synthesis of 4-acyl-1,2,3-triazoles and mechanistic studies. Tetrahedron 2022132856
- [Google Scholar]
- Facile synthesis of N2-substituted-1,2,3-triazole from aryl ethynylene and azide via a one-pot two-step strategy. Tetrahedron. 2022;108:132670
- [Google Scholar]
- Rosmarinic acid inhibits some metabolic enzymes including glutathione S-transferase, lactoperoxidase, acetylcholinesterase, butyrylcholinesterase and carbonic anhydrase isoenzymes. J. Enzyme Inhib. Med. Chem.. 2016;31(6):1698-1702.
- [Google Scholar]
- Antidiabetic and antiparasitic potentials: Inhibition effects of some natural antioxidant compounds on α-glycosidase, α-amylase and human glutathione S-transferase enzymes. Int. J. Biol. Macromol.. 2018;119:741-746.
- [Google Scholar]
- Synthesis and carbonic anhydrase inhibitory properties of new spiroindoline-substituted sulphonamide compounds. Arch. Physiol. Biochem.. 2017;123(5):306-312.
- [Google Scholar]
- Cytotoxic effect, enzyme inhibition, and in silico studies of some novel N-substituted sulfonyl amides incorporating 1, 3, 4-oxadiazol structural motif. Mol. Divers. 2022:1-21.
- [Google Scholar]
- Discovery of sulfadrug–pyrrole conjugates as carbonic anhydrase and acetylcholinesterase inhibitors. Arch. Pharm.. 2022;355(1):2100242.
- [Google Scholar]
- A strategic approach to the synthesis of functionalized spirooxindole pyrrolidine derivatives: in vitro antibacterial, antifungal, antimalarial and antitubercular studies. New J. Chem.. 2015;39(1):520-528.
- [Google Scholar]
- Integration of metabolomics and network pharmacology to validate the mechanism of Schisandra chinensis(Turcz.)Baill - Acorus tatarinowii Schott ameliorating the Alzheimer's disease by regulating the aromatase activity to affect local estrogen in brain of AD model rats. Arab. J. Chem.. 2023;16(2):104457
- [Google Scholar]
- Nutra-pharmaceutical potential evaluation of Trignollafoenum graecum seeds extracts in different solvents. Chem. Int.. 2022;8(1):23-32.
- [Google Scholar]
- Insulin-stimulated glucose uptake in skeletal muscle, adipose tissue and liver: a positron emission tomography study. Eur. J. Endocrinol.. 2018;178(5):523-531.
- [Google Scholar]
- Drug target prioritization by perturbed gene expression and network information. Sci. Rep.. 2015;5(1):1-13.
- [Google Scholar]
- Bidirectional relationship between caregiver burden and neuropsychiatric symptoms in patients with Alzheimer's disease: a narrative review. Int. J. Geriatr. Psychiatry. 2019;34(9):1326-1334.
- [Google Scholar]
- Design, synthesis and biological evaluation of 1, 3, 4-oxadiazole derivatives. Eur. J. Med. Chem.. 2010;45(11):4963-4967.
- [Google Scholar]
- Phytochemicals from selective plants have promising potential against SARS-CoV-2: investigation and corroboration through molecular docking, MD simulations, and quantum computations. Biomed Res. Int.. 2020;2020
- [Google Scholar]
- Advances in treating diabetes with aerosolized insulin. Diabetes Technol. Ther.. 2002;4(4):515-518.
- [Google Scholar]
- SAR studies of quinoline and derivatives as potential treatments for Alzheimer’s disease. Arab. J. Chem.. 2023;16(2):104502
- [Google Scholar]
- The antibacterial activity of 1, 2, 3-triazole-and 1, 2, 4-triazole-containing hybrids against Staphylococcus aureus: An updated review (2020-present) Curr. Top. Med. Chem. 2021
- [Google Scholar]
- Synthesis and properties of novel highly fluorescent pyrrolopyridazine derivatives. Chem. Mater.. 2003;15(20):3759-3768.
- [Google Scholar]
- Antibacterial and antioxidant activity of p-quinone methide derivative synthesized from 2, 6-di-tert-butylphenol. Chem. Int.. 2020;6(4):260-266.
- [Google Scholar]
- Design, synthesis, antibacterial evaluation, and computational studies of hybrid oxothiazolidin–1, 2, 4-triazole scaffolds. Arch. Pharm.. 2021;354(6):2000473.
- [Google Scholar]
- Synthesis of quinolone derivatives and their molecular docking for antiepileptic activity. Chem. Int.. 2020;6(4):224-231.
- [Google Scholar]
- Synthesis of DPA-triazole structures and their application as ligand for metal catalyzed organic reactions. Tetrahedron. 2022;104:132581
- [Google Scholar]
- Nutraceutical potential of ripe and unripe plantain peels: A comparative study. Chem. Int.. 2020;6(2):83-90.
- [Google Scholar]
- Microwave-assisted catalyst-free synthesis of substituted 1, 2, 4-triazoles. Synlett. 2015;26(03):404-407.
- [Google Scholar]
- Gas chromatography: A tool for drug analysis in biological samples. Chem. Int.. 2020;6(4):277-294.
- [Google Scholar]
- Rapid screening and identification of α-glucosidase inhibitors from mulberry leaves using enzyme-immobilized magnetic beads coupled with HPLC/MS and NMR. Biomed. Chromatogr.. 2013;27(2):148-155.
- [Google Scholar]
- Synephrine and phenylephrine act as α-amylase, α-glycosidase, acetylcholinesterase, butyrylcholinesterase, and carbonic anhydrase enzymes inhibitors. J. Biochem. Mol. Toxicol.. 2017;31(11):e21973.
- [Google Scholar]
- Anti-Alzheimer, antidiabetic and antioxidant potential of Satureja cuneifolia and analysis of its phenolic contents by LC-MS/MS. Arab. J. Chem.. 2020;13(3):4528-4537.
- [Google Scholar]
- Benzenesulfonamide derivatives as potent acetylcholinesterase, α-glycosidase, and glutathione S-transferase inhibitors: biological evaluation and molecular docking studies. J. Biomol. Struct. Dyn.. 2021;39(15):5449-5460.
- [Google Scholar]
- Nutritional potential and mineral profiling of selected rice variety available in Ethiopia. Chem. Int.. 2020;6(1):21-29.
- [Google Scholar]
- Novel inhibitors with sulfamethazine backbone: synthesis and biological study of multi-target cholinesterases and α-glucosidase inhibitors. J. Biomol. Struct. Dyn. 2021:1-13.
- [Google Scholar]
- Biological screening and docking studies of unique hybrids synthesized by conventional versus microwave assisted techniques. Trop. J. Pharm. Res.. 2019;18(5):1109-1117.
- [Google Scholar]
- Therapeutic potential of spirooxindoles as antiviral agents. ACS Infect. Dis.. 2016;2(6):382-392.
- [Google Scholar]
- Effect of organic loading rate on the recovery of nutrients and energy in a dual-chamber microbial fuel cell. Bioresour. Technol.. 2019;281:367-373.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.104626.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







