Translate this page into:
GC/MS and LC-MS/MS phytochemical evaluation of the essential oil and selected secondary metabolites of Ajuga orientalis from Jordan and its antioxidant activity
⁎Corresponding author. mahmoud.qudah@yu.edu.jo (Mahmoud A. Al-Qudah) maalqudah@imamu.edu.sa (Mahmoud A. Al-Qudah)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The current investigation aimed to shed light in the volatile and non-volatile secondary metabolites of Ajuga orientalis L. from Jordan. GC/MS and GC/FID analysis of the hydrodistilled essential oil obtained from aerial parts of the plant revealed tiglic acid (18.90 %) as main constituent. Each of the methanol and butanol fractions of A. orientalis were screened for their total phenol content (TPC), total flavonoid content (TFC), and antioxidant activity determined by DDPH and ABTS methods. The extracts were then analyzed by LC-ESI-MS/MS to unveil their chemical constituents, especially phenols and flavonoids. Results showed that the AO-B extract had the highest TPC (217.63 ± 2.65 mg gallic acid/g dry extract), TFC (944.41 ± 4.77 mg quercetin /g dry extract), highest DPPH and ABTS antioxidant activity ((4.00 ± 0.20) × 10-2; (3.00 ± 0.20) × 10-2 mg/mL, respectively) as compared to the AO-M extract. LC-ESI-MS/MS analysis of both extracts revealed the presence of several phenolics, flavonoids and nonphenolic acids.
Keywords
Ajuga orientalis
Essential oil
Antioxidant activity
LC-ESI-MS/MS
Total flavonoid content
Total phenol content
1 Introduction
Ajuga is one of the largest genera of the Lamiaceae (previously known as Labiateae) family (Amin 1991; Jalili and Jamzad 1999). Several species of this genus are well recognized as herbal remedies for the treatment of many ailments including gastrointestinal disorders, fever, dysentery, rheumatism, gout, asthma, diabetes, malaria, toothache and are reported to possess diuretic, antipyretic, tonic, diaphoretic and astringent properties (Chen et al., 1996; Ben Jannet et al., 2006; Israili and Lyoussi., 2009) in addition to their antibacterial, antitumor, antifeedant, antioxidant and neuroprotective effects (Turkoglu et al., 2010; Zerroug et al., 2011; Guo et al., 2011; Makni et al., 2013). Ajuga plants are known for their sundry of volatile and nonvolatile phyto-constituents including terpenoids, iridoids, sterols, flavonoids and many others (Teismann 2000; Küçükbay et al., 2013; Al-Qudah et al., 2014; Al-Qudah et al., 2017a,b).
Three Ajuga species were reported in the Flora of Jordan, these are Ajuga chia Schreber., Ajuga Iva L., and A. orientalis L. (Alhamad 2006., Oran 2015). Ajuga orientalis L. is a perennial herb that is 20–40 cm length characterized by its basal, erect wooly stems and blue violet colors. The plant is known to grow wild in humid places of Ajloun, Salt, Amman and Al-Karak. Flowering occurs in the spring season, during April and May (Al-Eisawi, 1998). Previous studies on phytochemical investigation of volatile constituents (Küçükbay et al., 2013; Sajjadi and Ghannadi 2004) and non-volatile secondary metabolites were limited (Oran et al., 2022), especially from Jordanian origin. Accordingly, the current study was designed to investigate the chemical composition of the hydro-distilled essential oil obtained from the aerial parts of A. orientalis (AO-HDEO) from Jordan and its antioxidant activity. Moreover, extracts of different polarities obtained from the aerial parts of the plant material were screened for their total phenols content (TPC), total flavonoids content (TFC) and antioxidant activities (by DPPH and ABTS methods). The presence of selected phenolic acids, flavonoids and other constituents in theses extracts was determined by LC-ESI-MS/MS technique.
2 Experimental
2.1 General
Gas chromatography-Mass spectrometry (GC–MS) analysis was performed using Agilent 6890 series II – 5973 mass spectrometers interfaced with HP chemstation. UV–vis spectra were recorded on Shimadzu UV-1800 UV/Visible Scanning Spectrophotometer. Detection of the selected phenolic acids, flavonoids and nonphenolic acids and compounds was done utilizing a Bruker Daltonik (Bremen, Germany) Impact II ESI-Q-TOF System equipped with Bruker Dalotonik Elute UHPLC system (Bremen, Germany). n-Hexane (GC-grade), the n-alkanes (C8-C20) standard mixture, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS, purity > 99 %), 2,2-diphenyl-2-picrylhydrazyl ((DPPH, purity > 99 %), ascorbic acid (purity > 98 %), methanol, potassium persulfate, sodium carbonate, Folin and Ciocalteu's Phenol reagent, sodium nitrite, aluminum chloride, and sodium hydroxide were all products of Sigma-Aldrich.
2.2 Plant material
Fresh aerial parts of A. orientalis were collected at full flowering stage in April/2018 from Ajloun city, north of Jordan (N 32.363932; E 35.775043). The plant identity was confirmed by Prof. Dr. Jamil Laham, Yarmouk University, Irbid, Jordan. A voucher specimen (AO/L/2018) was deposited in Prof. Mahmoud A. Al-Qudah Laboratory, Department of Chemistry, Faculty of Science, Yarmouk University, Irbid, Jordan.
2.3 Hydro-distillation of essential oil and extracts preparation
Fresh aerial parts of A. orientalis (200 g) were minced, suspended in 250 mL distilled water and the mixture was subjected to hydro-distillation in a Clevenger type apparatus for 4 h. The obtained yellow oil was dissolved in n-hexane (GC-grade), dried over anhydrous sodium sulfate, and then stored in amber glass vial at 4–6 °C until analysis.
Extraction and fractionation of the aerial parts of A. orientalis was performed according to the procedure listed in the literature (Al-Jaber et al., 2012). Each of the aqueous methanol (AO-A) and the butanol (AO-B) fractions were then assayed for their TPC, TFC, antioxidant activity (by the DPPH and ABTS assay methods) and then were subjected to LC-ESI-MS/MS analysis for the detection of selected phenolic acids, flavonoids and nonphenolic compounds.
2.4 Determination of essential oil constituents and their % concentration
The chemical constituents of A. orientalis hydro-distilled essential oil (AO-HDEO) and their relative percentage composition were determined according to the procedure listed in the literature in the literature using the same instruments and under identical chromatographic conditions (Abu-Orabi et al., 2020).
Identification of chemical constituents was achieved by comparing their calculated Kovats retention index (KI) values relative to (C8–C20) n-alkanes literature values measured with columns of identical polarity, or by matching their recorded mass spectra with the built-in mass spectral libraries (NIST, Gaithhersburg, MD, USA, and Wiley Co., Hoboken, NJ, USA) in addition to mass spectrum matching to the available authentic standards.
2.5 Total phenols and total flavonoids contents
The total phenols and total flavonoids contents of the AO-A and AO-B extracts were determined by Folin-Ciocalteu method and aluminum chloride assay, respectively as previously described (Al-Humaidi., 2017).
2.6 Antioxidant activity
The antioxidant activity of the AO-HDEO and each AO-A and AO-B fractions was determined by the DPPH and ABTS methods according to the procedure listed in the literature (Al-Qudah 2016; Al-Qudah et al., 2015; Al-Qudah et al., 2014; Teismann and Ferger., 2000). The ability of the AO-HDEO/fractions to scavenge radicals was calculated using the following equation:
Where Ac is the absorbance of the blank and As is the absorbance in the presence of essential oil/extract.
2.7 LC-MS analysis of secondary metabolites
Analysis of selected secondary metabolites was performed on Bruker Daltonik (Bremen, Germany) Impact II ESI-Q-TOF System equipped with Bruker Daltonik Elute UHPLC system (Bremen, Germany) in both positive (M + H) and negative (M−H) electrospray ionization modes. Chromatographic separation was performed on a C-18 reversed phase column (100 × 2.1 mm, 2.0 μm) from Bruker Daltonik (Germany) at 40 °C, with an autosampler temperature of 8 °C. The elution gradient consisted of mobile phase A: water with 0.05 % formic acid and mobile phase B: acetonitrile. The gradient elution program was: linear gradient 5–80 % B (0 – 27 min); 95 % B (27 – 29 min); 5 % B (29.1–35.0 min). The flow rate of the solvent was 0.51 mL/min and the injection volume of the sample was 3.0 μL. Mass spectrum was operating at the following conditions: the capillary voltage was 2500 V, the nebulizer gas was 2.0 bar, dry gas (N2) gas flow was 8.0 L/min and the dry temperature was 200 °C. The mass accuracy was < 1 ppm; the mass resolution was 50,000 FSR (Full Sensitivity Resolution) and the TOF repetition rate was up to 20 kHz.
A stock solution containing standard compounds (0.5 mg/mL) was prepared in HPLC-grade. Plant samples were dissolved with 2.0 mL DMSO, the volume was completed to 50 mL by acetonitrile, then each sample was centrifuged at 4000 rpm for 2 min and 3.0 µL was injected. The composition of the samples was identified based on the identification of m/z ratio with reference to the retention time of the used standards.
3 Results and discussion
3.1 Essential oil
Hydro-distillation of the fresh aerial parts of A. orentalis afforded a yellow oil (yield 0.05 %, w/w). GC–MS analysis of the obtained HD-AOEO (Fig. 1) resulted in the identification of a total of 92 compounds amounting to 90.49 % of the total oil content (Table 1). The HD-AOEO was dominated by different classes of terpenoids, aliphatic hydrocarbons and their derivatives (Table 1), mainly oxygenated sesquiterpenoids (27.29 %). Individual main components included tiglic acid (18.90 %), ageratochromene (8.09 %), α-thujene (6.20 %), and 5-cedranone (5.82 %). Moreover, the obtained HDEO was assayed for its antioxidant activity using the DPPH and ABTS methods, results (Table 2) indicated a relatively high activity as compared to the employed positive controls (DPPH: (6.92 ± 0.22) × 10-3 mg/mL; ABTS: 6.44 ± 0.18) × 10-3 mg/mL). * a(Lit.):Literature Kovats index; b(Exp.): Experimentally calculated Kovats index using C8 – C20 n-alkanes on HP-5MS capillary column. cMS: Identification by mass spectrum (NIST and our local generated libraries were used for all MS comparisons). dCol: Co-Injection with an authentic compound, **: no of compounds detected in each class.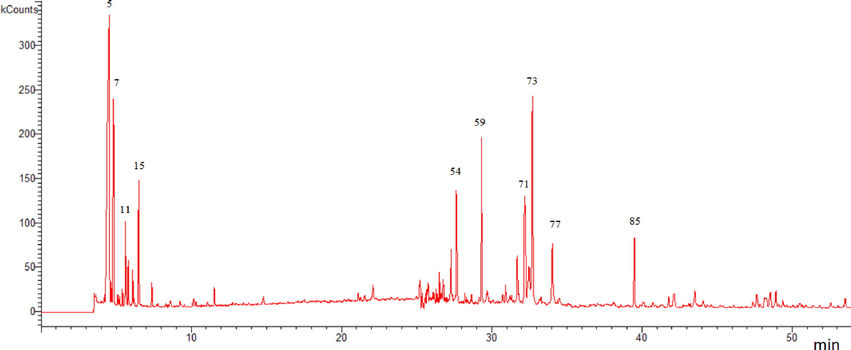
GC–MS, peaks were numbered as reported in Table 1.
No
KI
Compound
% Composition
Identification mode KI b, MS c, Col d
Lit. a
exp.b
1
862
861
2E-Hexenol
0.10
MS, RI
2
867
866
2Z-Hexenol
0.13
MS, RI
3
870
869
n-Hexanol
0.09
MS, RI
4
900
902
n-Nonane
0.23
MS, RI
5
912
914
Tiglic acid
18.90
MS, RI, Col
6
923
919
2-Methyl-4-heptanone
0.43
MS, RI
7
930
926
α-Thujene
6.20
MS, RI, Col
8
937
937
Tetrahydro citronellene
0.34
MS, RI
9
938
941
Allyl isovalerate
0.20
MS, RI
10
930
948
Cumene
0.31
MS, RI
11
960
956
Thuja-2,4(10)-diene
2.31
MS, RI
12
965
964
2-Methyl-(3E)-octen-5-yne
0.90
MS, RI
13
967
975
Verbenene
0.82
MS, RI
14
978
979
Hexanal, dimethyl acetal
0.16
MS, RI
15
995
991
Mesitylene
2.61
MS, RI, Col
16
1025
1019
psi-Cumene
0.58
MS, RI
17
1037
1046
E-β-Ocimene
0.10
MS, RI
18
1069
1053
m-Tolualdehyde
0.28
MS, RI, Col
19
1081
1072
p-Tolualdehyde
0.24
MS, RI
20
1090
1080
Dehydro linalool
0.07
MS, RI
21
1096
1098
Linalool
0.31
MS, RI, Col
22
1104
1102
2-Isopropyl-5-methyl-(2E)-hexenal
0.14
MS, RI
23
1121
1121
exo-Fenchol
0.14
MS, RI
24
1138
1132
Benzeneacetonitrile
0.53
MS, RI
25
1213
1212
Octanol acetate
0.31
MS, RI
26
1361
1361
γ-Nonalactone
0.26
MS, RI
27
1362
1364
Hydroxy citronellol
0.11
MS, RI
28
1375
1370
α-Copaene
0.17
MS, RI
29
1386
1384
δ-Nonalactone
0.56
MS, RI
30
1460
1459
Allo-aromadendrene
0.71
MS, RI
31
1463
1463
cis-Cadina-1(6),4-diene
0.34
MS, RI
32
1465
1469
cis-Muurola-4(14),5-diene
0.50
MS, RI
33
1472
1471
Dauca-5,8-diene
0.55
MS, RI
34
1476
1473
trans-Cadina-1(6),4-diene
0.76
MS, RI
35
1477
1476
γ-Gurjunene
0.43
MS, RI
36
1479
1477
γ-Muurolene
0.17
MS, RI
37
1481
1479
Amorpha-4,7(11)-diene
0.25
MS, RI
38
1481
1481
Germacrene D
0.48
MS, RI
39
1482
1483
Widdra-2,4(14)-diene
0.42
MS, RI
40
1484
1486
α-Amorphene
0.36
MS, RI
41
1488
1488
Aristolochene
0.37
MS, RI
42
1494
1491
epi-Cubebol
0.78
MS, RI
43
1495
1494
γ-Amorphene
0.31
MS, RI
44
1499
1495
4-epi-cis-Dihydroagarofuran
0.22
MS, RI
45
1500
1497
α-Muurolene
1.03
MS, RI
46
1500
1500
β-Himachalene
0.14
MS, RI
47
1502
1502
trans-β-Guaiene
0.24
MS, RI
48
1505
1504
α-Cuprenene
0.19
MS, RI
49
1505
1506
β-Bisabolene
0.22
MS, RI
50
1512
1510
δ-Amorphene
1.76
MS, RI
51
1513
1513
γ-Cadinene
0.35
MS, RI
52
1513
1516
trans-Cycloisolongifol-5-ol
0.12
MS, RI
53
1522
1518
trans-Calamenene
0.27
MS, RI
54
1523
1520
δ-Cadinene
3.96
MS, RI, Col
55
1538
1534
α-Cadinene
0.14
MS, RI
56
1545
1537
α-Calacorene
0.22
MS, RI
57
1548
1546
Italicene epoxide
0.24
MS, RI
58
1565
1558
β-Calacorene
0.19
MS, RI
59
1567
1563
Maaliol
4.67
MS, RI
60
1575
1572
Germacrene D-4-ol
0.67
MS, RI
61
1583
1582
Caryophyllene oxide
0.1
MS, RI
62
1590
1588
Globulol
0.09
MS, RI
63
1592
1593
Viridiflorol
0.09
MS, RI
64
1594
1599
Carotol
0.44
MS, RI
65
1607
1604
β-Oplopenone
0.76
MS, RI
66
1619
1611
1,10-di-epi-Cubenol
0.43
MS, RI
67
1623
1615
10-epi-γ-Eudesmol
0.32
MS, RI
68
1623
1618
α-Corocalene
0.14
MS, RI
69
1628
1624
1-epi-Cubenol
1.94
MS, RI
70
1631
1627
Eremoligenol
0.29
MS, RI
71
1628
1638
5-Cedranone
5.82
MS, RI
72
1650
1645
β-Eudesmol
2.52
MS, RI
73
1660
1652
Ageratochromene
8.09
MS, RI
74
1661
1664
cis-Calamenen-10-ol
0.19
MS, RI
75
1665
1667
Junicedranone
0.27
MS, RI
76
1676
1671
Cadalene
0.16
MS, RI
77
1685
1687
5-neoCedranol
3.11
MS, RI
78
1700
1696
Amorpha-4,9-dien-2-ol
0.11
MS, RI
79
1702
1699
10-nor-Calamenen-10-one
0.57
MS, RI
80
1760
1757
Benzyl benzoate
0.08
MS, RI
81
1763
1763
Aristolone
0.26
MS, RI
82
1807
1800
2-Ethyl hexyl salicylate
0.12
MS, RI
83
1805
1802
2-α-Acetoxy-amorpha-4,7(11)-diene
0.10
MS, RI
84
1811
1815
β-Chenopodiol
0.09
MS, RI
85
1864
1842
cis-Thujopsenic acid
2.45
MS, RI
86
1865
1859
Benzyl salicylate
0.25
MS, RI
87
1881
1878
Cyclohexyl anthranilate
0.16
MS, RI
88
1912
1909
Kudtdiol
0.46
MS, RI
89
1921
1920
Methyl hexadecanoate
1.02
MS, RI
90
1939
1951
11-Acetoxyeudesman-4-α-ol
0.18
MS, RI
91
1960
1964
Hexadecanoic acid
0.92
MS, RI
92
1993
1981
Ethyl hexadecanoate
0.37
MS, RI
Classes detected (no. of compounds/class)
Oxygenated hemiterpenoids
19.1 (2)**
Monoterpene hydrocarbons
9.77 (5)
Oxygenated monoterpenes
0.77 (5)
Sesquiterpene hydrocarbons
14.83 (28)
Oxygenated sesquiterpenes
27.29 (28)
Esters
3.13 (9)
Phenolic compounds
12.64 (7)
Non-phenolic compounds
2.96 (8)
Total identified
90.49 %
Extracts
TPC
TFC
IC50 (mg/mL)
DPPH
ABTS
HDEO
–
–
(6.92 ± 0.22) × 10-3
(6.44 ± 0.18) × 10-3
AO-M
52.35 ± 1.35
281.24 ± 1.50
(14.00 ± 0.60) × 10-2
(7.00 ± 0.10) × 10-2
AO-B
217.63 ± 2.65
944.41 ± 4.77
(4.00 ± 0.20) × 10-2
(3.00 ± 0.20) × 10-2
Ascorbic acid
–
–
1.58 × 10-3 ± 3.0 × 10-5
1.78 × 10-3 ± 6.0 × 10-5
α-tocopherol
–
–
1.79 × 10-3 ± 1.0 × 10-5
2.33 × 10-3 ± 4.0 × 10-5
The chemical composition of the essential oils of A. orientals from Turkey (Küçükbay et al., 2013) and from Iran (Sajjadi and Ghannadi, 2004); was quite different when compared to current results. The essential oil obtained from Turkish A. orientalis was dominated by phytol (36.7 %) while Iranian A. oreintalis EO was dominated by germacrene (24.2 %). Fig. 2 shows the main variations among the different classes of constituents detected in the essential oils A. orientalis from Jordan (current study), Iran, and Turkey. This variation in composition could be attributed to the different climatic conditions, different soil properties in addition to other factors like time of collection and different extraction procedures (Mercy and David Udo., 2018).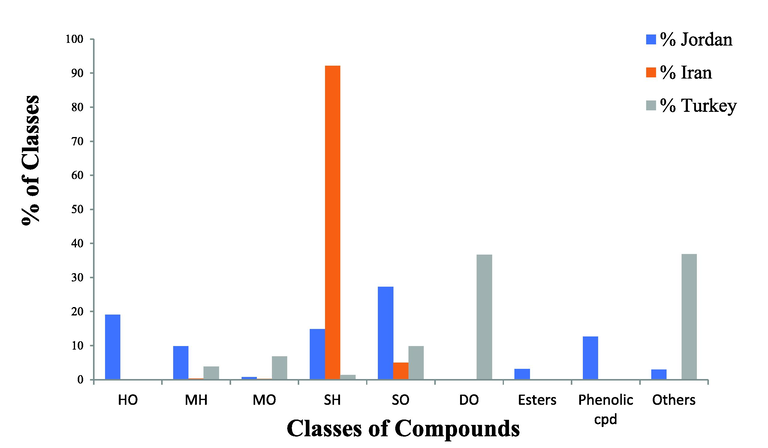
A classification of the constituents of the A. orientalis L. and their % composition from Jordan, Iran, and Turkey. Hemiterpenoids oxygenated (HO), monoterpene hydrocarbons (MH), monoterpenes oxygenated (OM), sesquiterpene hydrocarbons (SH), sesquiterpenes oxygenated (SO), diterpene oxygenated (DO).
3.2 TPC, TFC, antioxidant activity
In the current study, each of the aqueous methanol (AO-A) and butanol (AO-B) fractions were investigated for their TPC, TFC and antioxidant activity using two assay methods and according to the procedures listed in the literature ((Al-Qudah, 2016; Al-Qudah et al., 2014, 2015; Govindan et al., 2016; Sanchez-Moreno, 2002). As could be deduced from the results shown in Table 2, AO-B fraction had the highest TPC and TFC (217.63 ± 2.65 mg gallic acid/g extract; 944.41 ± 4.77 mg quercetin/g extract, respectively). This extract had also the highest antioxidant activity as measured by the DPPH ((4.00 ± 0.20) × 10-2 mg/mL) and ABTS ((3.00 ± 0.20) × 10-2 mg/mL) assay methods.
3.3 LC-MS/MS profiling of selected secondary metabolites
In the current investigation, AO-M and AO-B fractions were screened for the presence of a selected set of secondary metabolites by LC-ESI-MS/MS using both, the positive and negative ionization modes. The list of the 33 different phenolic and nonphenolic compounds detected in both extracts are shown in Table 3, chromatograms are shown in Fig. 3. Both extracts were found to contain acteoside as a major phenolic acid derivative. It was noticed that each of 8-Prenylnaringenin, 3-gal(1–2)gluA soyasapogenol B, hederagenin, myristic acid and (Z)-3-hydroxyoctadec-7-enoic acid were detected in the AO-A fraction only. The phenolic and flavonoids profiles detected in the extracts of A. orientalis from Jordan in our current study were completely different from those reported for the plant from Turkish origin (Göger et al., 2015; Zengin et al., 2018).
No.
Rt
Name
Structure
Molecular Formula
m/z meas.
Mwt
Compounds*
AO-M
AO-B
1
0.99
Succinic acid
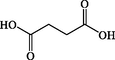
C4H6O4
117.0193
118.0266
+
+
2
1.8
2,5-Dihydroxybenzoic acid
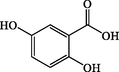
C7H6O4
153.0192
154.0265
+
+
3
2.81
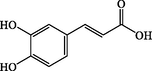
Caffeic Acid
C9H8O4
179.0343
180.0415
+
+
4
3.16
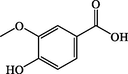
Vanillic acid
C8H8O4
167.0351
168.0423
+
+
5
4.4
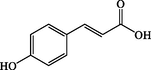
p-Coumaric acid
C9H8O3
163.0399
164.0471
+
+
6
4.44
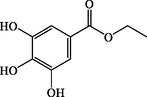
Ethyl gallate
C9H10O5
197.0457
198.053
+
+
7
4.82
3,5-Dimethoxy-4-hydroxyacetophenone
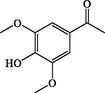
C10H12O4
195.0644
196.0716
+
+
8
5.45
Vitexin
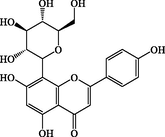
C21H20O10
431.0976
432.1049
–
+
9
5.51
Eriodictyol-7-neohesperidoside
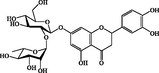
C27H32O15
595.167
596.1743
+
+
10
5.89
Salicylic acid
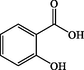
C7H6O3
137.0243
138.0315
+
+
11
5.89
Luteolin 7-O-glucoside (Cynaroside)
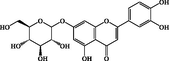
C21H20O11
447.0931
448.1004
+
+
12
5.95
Acteoside = Verbascoside
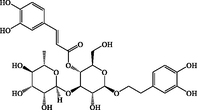
C29H36O15
623.1981
624.2056
+
+
13
6.05
3-O-Neohesperidoside Kaempferol
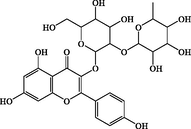
C27H30O15
593.1511
594.1583
+
+
14
6.17
Rutin
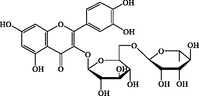
C27H30O16
609.145
610.1523
+
+
15
6.77
Kaempferol-3-O-glucoside
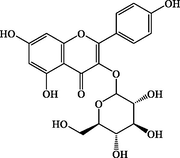
C21H20O11
447.0931
448.1004
+
+
16
6.78
3,6,2′,4′-Tetrahydroxyflavone
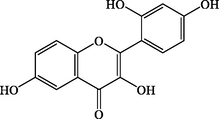
C15H10O6
285.0399
286.0472
+
+
17
7.00
Diosmin
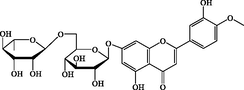
C28H32O15
607.166
608.1733
+
+
18
7.09
7-Glu-Chrysoeriol
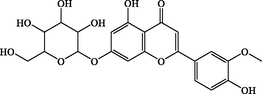
C22H22O11
461.1085
462.1157
+
+
19
7.23
Kaempferol-7-O-glucoside
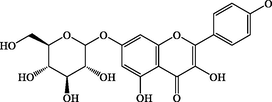
C21H20O11
447.0928
448.1
+
+
20
8.55
Luteolin
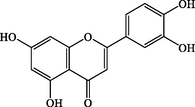
C15H10O6
285.0403
286.0475
+
+
21
10.24
Hispidulin
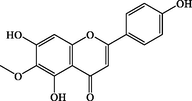
C16H12O6
299.0557
300.063
+
+
22
13.73
Caffeic acid phenethyl ester
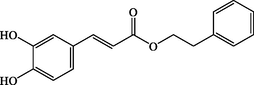
C17H16O4
283.1011
284.1084
+
+
23
14.65
8-Prenylnaringenin
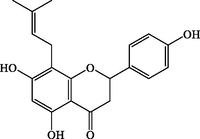
C20H20O5
339.1235
340.1308
+
–
24
16.46
3-Gal(1–2)GluA Soyasapogenol B
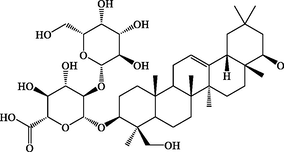
C42H68O14
795.4529
796.4602
+
–
25
21.63
Hederagenin
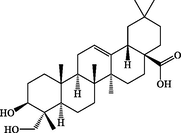
C30H48O4
471.3472
472.3545
+
–
26
22.58
Glc-octadecatrienoyl-sn-glycerol
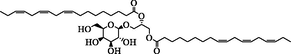
C27H46O9
513.3099
514.3172
+
+
27
26.4
(Z)-3-Hydroxyoctadec-7-enoic acid
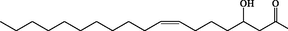
C18H34O3
297.2435
298.2508
+
+
28
26.7
Myristic acid
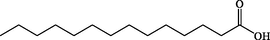
C14H28O2
227.2012
228.2085
+
–
29
28.78
Pentadecanoic acid
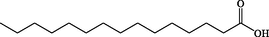
C15H30O2
241.2176
242.2249
+
+
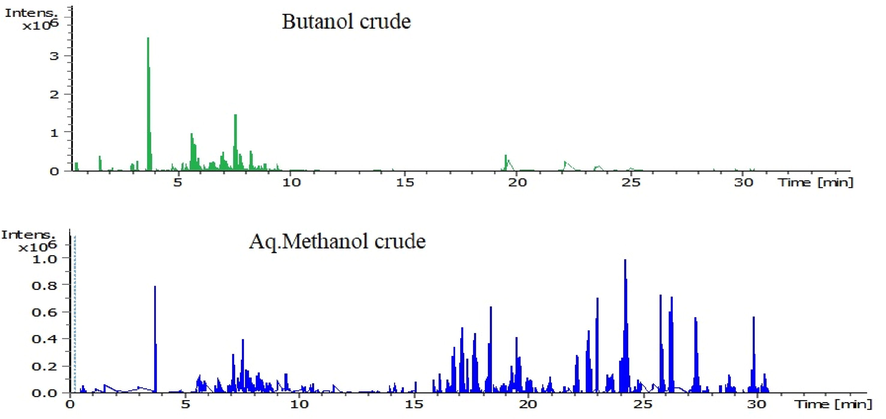
LC-MS/MS chromatograms of AO-B and AO-M fractions of A. orientalis from Jordan.
4 Conclusions
Different extracts of A. orientalis from Jordan were investigated for their TPC, TFC and antioxidant activities using the DPPH and ABTS assay methods, Results of the current study revealed that A.orientalis fractions had a relatively high TPC, TFC and good antioxidant activity as determined by the two assay methods (DDPH and ABTS), especially the butanol (AO-B) fraction. The detection of several phenolic and flavonoids compounds could justify the observed activity.
Acknowledgments
We would like to thank the Deanship of Scientific Research and Graduate Studies at Yarmouk University for funding this research project (Grant no. 29/2020). And also thank to Imam Mohammad Ibn Saud Islamic University (IMSIU), Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abu-Orabi, S.T., Al-Qudah, M.A.,Saleh, N.R., Bataineh, T.T., , Obeidat, S.M., Al-Sheraideh, M.S., .Al-Jaber, H.I., Tashtoush, H.I., Lahham, J.M., 2020. Antioxidant activity of crude extracts and essential oils from flower buds and leaves of Cistus creticus and Cistus salviifolius. AJC. 13(7), 6256-6266.
- Field Guide to Wild Flowers of Jordan and Neighboring Countries. Amman, Jordan: The National Library; 1998.
- Ecological and species diversity of arid Mediterranean grazing land vegetation. J. Arid Environ.. 2006;66(4):698-715.
- [Google Scholar]
- Phytochemical investigation and in vitro antioxidant activities of Cleome amblyocarpa Barratte & Murb and Cleome ramosissima Parl. growing in Saudi Arabia. Jordan J. Chem.. 2017;12(4):241-254.
- [Google Scholar]
- New terpenes from Salvia palaestina Benth. and Salvia syriaca L. growing wild in Jordan. J. Asian Nat. Prod. Res.. 2012;14(7):618-625.
- [Google Scholar]
- Antioxidant activity and chemical composition of essential oils of fresh and air-dried Jordanian Nepeta curviflora Boiss. J. Biol. Act. Prod. Nat.. 2016;6(2):101-111.
- [Google Scholar]
- Antioxidant activity and chemical composition of essential oils from Jordanian Ononis natrix L. and Ononis sicula Guss. J. Biol. Act. Prod. Nat.. 2014;4(1):52-61.
- [Google Scholar]
- New isoflavones from Gynandriris sisyrinchium Parl. growing wild in Jordan and their antioxidant and cytotoxic activities. Fitoterapia. 2015;107:15-21.
- [Google Scholar]
- New flavonol glycoside from Scabiosa prolifera L. aerial parts with in vitro antioxidant and cytotoxic activities. Nat. Prod. Res.. 2017;31(24):2865-2874.
- [Google Scholar]
- Composition, antioxidant and anticancer activities of the essential oil from fresh and air-dried aerial parts of Pallenis spinosa. Chem. Biodivers.. 2017;14:e1700146.
- [Google Scholar]
- Amin, G. 1991. Popular medicinal plants of Iran, Vol. 1. Ministry of Health Publications, Tehran, 40, 41-55
- Structure–antibacterial activity relationship of secondary metabolites from Ajuga pseudoiva Rob. leaves. Nat. Prod. Res.. 2006;20(3):299-304.
- [Google Scholar]
- Antibacterial neoclerodane diterpenoids from Ajuga lupulina. J. Nat. Prod.. 1996;59(7):668-670.
- [Google Scholar]
- Phytochemical characterization of phenolics by LC-MS/MS and biological evaluation of Ajuga orientalis from Turkey. Bangladesh J. Pharmacol.. 2015;10(3):639-644.
- [Google Scholar]
- Comparative study on the antioxidant activity of methanolic and aqueous extracts from the fruiting bodies of an edible mushroom Pleurotus djamor. Food Sci. Biotechnol.. 2016;25(2):371-377.
- [Google Scholar]
- Guo, P., Li, Y., Xu, J., Liu m C., Ma., Y., Guo, Y., 2011. Bioactive neo-clerodane diterpenoids from the whole plants of Ajuga ciliata Bunge. J. Nat. Prod. 74(7), 1575-1583.
- Ethnopharmacology of the plants of genus Ajuga. Pak. J. Pharm. Sci.. 2009;22(4):425-462.
- [Google Scholar]
- Red data book of Iran, a preliminary survey of endemic. In: Rare & Endangered Plant Species in Iran. Tehran, Iran: Research Institute of Forest and Rangelands; 1999. p. :1-4.
- [Google Scholar]
- Essential oil composition from the aerial parts of Ajuga orientalis L. growing in Turkey. Asian J. Chem.. 2013;25(16):9126-9128.
- [Google Scholar]
- Antioxidant, free radical scavenging, and antimicrobial activities of Ajuga iva leaf extracts. Int. J. Food Prop.. 2013;16(4):756-765.
- [Google Scholar]
- Natural products as lead bases for drug discovery and development. Res. Rep. Med. Sci.. 2018;2(1):1-2.
- [Google Scholar]
- Chemical composition, in vitro assessment of antioxidant properties and cytotoxicity activity of ethanolic and aqueous extracts of Ajuga orientalis L. (Lamiaceae) J. Pharm. Pharmacogn. Res.. 2022;10(3):486-495.
- [Google Scholar]
- Oran, S.A.S., 2015. Flora of Bader Al-Jadida County, western high mountains of Amman city/Jordan. Int. J. Herb. Med. 3(4 Part A), 49-59.
- Volatile oil composition of the aerial parts of Ajuga orientalis L. from Iran. Z. Naturforsch. C. 2004;59(3–4):166-168.
- [Google Scholar]
- Methods used to evaluate the free radical scavenging activity in foods and biological systems. Food Sci. Technol. Int.. 2002;8:121-137.
- [Google Scholar]
- The salicylate hydroxylate ion assay to measure hydroxyl free radicals induced by local application of glutamate in vivo or induced by the Fenton reaction in vitro. Brain Res. Prot.. 2000;5:204-210.
- [Google Scholar]
- Determination of antimicrobial and antioxidant activities of Turkish endemic Ajuga chamaepitys (L.) Schreber subsp. euphratica PH Davis (Lamiaceae) J. Med. Plant Res.. 2010;4(13):1260-1268.
- [Google Scholar]
- Exploring the therapeutic potential and phenolic composition of two Turkish ethnomedicinal plants–Ajuga orientalis L. and Arnebia densiflora (Nordm.) Ledeb. Ind. Crops Prod.. 2018;116:240-248.
- [Google Scholar]
- Antibacterial activity of extracts of Ajuga Iva, and Teucrium Polium. Adv. Environ. Biol.. 2011;5(2):491-495.
- [Google Scholar]







