Translate this page into:
Phenylpropenol ester and sesquiterpenoids with antimetastatic activities from the whole plants of Chloranthus japonicus
⁎Corresponding authors. 251809@163.com (Fubo Zhang), xiechunfeng@nankai.edu.cn (Chunfeng Xie), cheng.yang@nankai.edu.cn (Cheng Yang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
One new phenylpropenol ester (1) and three new lindenane sesquiterpenes (2–4) were isolated from the whole plant of Chloranthus japonicus together with three known lindenane derivatives (5–7). Their structures were determined based on detailed spectroscopic and electronic circular dichroism computational analyses. Compound 4 showed moderate efficacy in inhibiting cell migration, invasion, and vasculogenic mimicry (VM) in human of hepatocarcinoma (HCC) HepG2 cells. Furthermore, compound 4 could alter the expression of the proteins Vimentin, N-cadherin, Snail1 and E-cadherin by down-regulating the expression of Twist1, which indicated that its antimetastatic effect was associated with restraining the epithelial-mesenchymal transition (EMT) in HepG2 cells.
Keywords
Chloranthus japonicus
Chloranthaceae
Lindenane sesquiterpenes
Antimetastatic effect
Human hepatocarcinoma
Epithelial-mesenchymal transition
1 Introduction
Primary liver cancer ranks sixth in morbidity and second in mortality in the world. Hepatocellular carcinoma is the most common primary liver cancer, accounting for more than 85% of liver cancer cases (Lee and Cheung, 2019). Patients at the early stage of HCC often need liver transplantation and surgical resection. After surgery, the 5-year recurrence rate is still higher than 70% (Tella et al., 2022). Sorafenib and lovatinib are commonly used as targeted drugs for advanced HCC, but their clinical efficacy is not perfect; they can prolong the survival time by only approximately three months (Kudo et al., 2018). Although there are many other drugs for HCC on the market, the treatment results of HCC are unsatisfactory, and the 5-year survival rate of HCC is no more than 18% (Bruix et al., 2017, Abou-Alfa et al., 2018). More effective treatment strategies and drugs with higher efficacy remain urgent.
HCC has a strong vascular invasion and metastasis ability and is one of the most common invasive malignant tumors. Its high recurrence rate is mainly due to the spread of intrahepatic metastasis (Braun et al., 2022). There is increasing evidence that epithelial-mesenchymal transformation (EMT) is a complex process that is closely related to the metastasis of HCC cells. EMT is the process that epithelial cells lose the apical-basal polarity and cell–cell adhesion, and transit to invasive mesenchymal cells. Usually, E-cadherin acts as the marker protein of epithelial phenotype, while Vimentin, Snail1 and N-cadherin are the marker proteins of mesenchymal phenotype. The changes in gene expression during EMT lead to numerous phenotypic changes, such as cell morphological changes, loss of adhesion and gain of stem cell-like features (Huang et al., 2022). The existence of VM in HCC is considered to be closely related to poor prognosis, high invasion and metastasis and high recurrence rate of HCC. Previous studies have shown that EMT is a necessary factor in the formation of VM. From this point of view, EMT is a key factor in HCC invasion and metastasis, and the inhibition of the EMT process will effectively prevent the invasion and metastasis of tumor cells (Zheng et al., 2021).
Chloranthus japonicus Sieb. is a plant species of Chloranthaceae and is distributed mainly in China. Its whole plants, namely, Yin-xian-cao, have been used to treat various diseases, such as cold cough, carbuncles and furuncles, in traditional Chinese medicine (Nanjing University of Chinese Medicine, 2006). Sesquiterpenoid derivatives, especially lindenane sesquiterpenes, are rich in C. japonicus with multiple bioactivities, such as anti-HIV, cytotoxic, and anti-inflammatory activities (Kim et al., 2016, Yan et al., 2016, Zhao et al., 2016, Zhuo et al., 2017).
In our ongoing studies on bioactive ingredients from Chloranthus plants (Xie et al., 2015), the chemical constituents of C. japonicus have been studied. Seven compounds, including one new phenylpropenol ester and three new sesquiterpenes, have been isolated from C. japonicus. The separation, structural elucidation, and evaluation of their antimetastatic effects against human hepatocarcinoma HepG2 cells for all isolates from C. japonicus are presented in this paper.
2 Materials and methods
2.1 General experimental procedures
The instruments and materials for the isolation and structural identification were the same as those in our previous report (Ding et al., 2020).
2.2 Plant material, extraction and isolation, and ECD calculations
The details on the plant material, extraction and isolation, and the calculations for the ECD spectra are provided in the Supporting Information.
2.2.1 2,3,4-Trimethoxycinnamyl senecioate (1)
Yellow oil; IR (KBr) νmax 2930, 1717, 1650, 1583, 1506, 1457, 1419, 1380, 1332, 1228, 1184, 1144, 1008, 968, 849 cm−1; 1H NMR (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3) data, see Tables 1 and 2; HR-ESIMS m/z 307.1540 [M + H]+ (calcd for C17H23O5 307.1545).
Position
1
2
3
4
1
6.62 s
1.68 m
2.15 m
1.96 m
2α
0.74 m
0.71 m
0.98 m
2β
1.48 m
0.84 m
0.79 m
3
1.56 m
1.97 m
1.94 m
4
5
6.62 s
3.02 d (4.6)
3.52 m
3.37 d (12.4)
6
5.62 d (4.6)
5.74 d (10.7)
5.40 d (12.4)
7
6.58 d (16.1)
8
6.23 dt (6.3, 16.1)
9
4.74 d (6.3)
9.50 s
3.84 d (6.8)
4.03 s
10
11
12
13
2.18 s
2.03 s
1.92 s
14
1.22 s
0.58 s
0.96 s
15
1.58 s
4.70 br s, 5.02 br s
4.85 br s, 5.07 br s
1′
2.10 s
2.09 s
2.21 s
2′
5.73 s
3′
4′
2.19 s
5′
1.91 s
1′′
3.87 s
3.77 s
3.27 s
3.28 s
2′′
3.85 s
3′′
3.87 s
Position
1
2
3
4
1
103.7
25.1
23.1
24.1
2
153.3
5.1
15.9
16.4
3
138.1
27.3
23.5
23.3
4
153.3
91.4
149.3
147.8
5
103.7
47.4
56.5
58.9
6
132.1
66.5
63.2
69.5
7
133.8
127.4
150.9
153.5
8
123.3
165.2
105.8
109.7
9
64.1
200.8
79.2
74.7
10
58.1
43.8
42.8
11
140.9
133.9
126.3
12
169.3
170.4
171.0
13
16.6
9.4
8.5
14
15.2
20.8
17.3
15
31.9
107.5
109.1
1′
166.4
21.2
20.7
20.6
2′
115.7
169.2
169.9
170.5
3′
157.4
4′
20.3
5′
27.5
1′′
56.1
52.8
50.1
50.8
2′′
60.9
3′′
56.1
2.2.2 Chlorajapolide J (2)
Yellow oil;[α] − 21.9 (c 0.24, CH2Cl2); ECD (CH3CN) λmax (Δε) 212 (−2.43), 241 (1.55) nm; IR (KBr) νmax 2954, 2927, 1731, 1436, 1376, 1312, 1272, 1224, 1174, 1110, 1083, 1047, 1019, 962, 938, 856 cm−1; 1H NMR (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3) data, see Tables 1 and 2; HR-ESIMS m/z 351.1441 [M + H]+ (calcd for C18H23O7 351.1444).
2.2.3 Chlorajapolide K (3)
Colorless oil; [α] − 16.0 (c 0.19, CH2Cl2); ECD (CH3CN) λmax (Δε) 216 (−2.82), 234 (0.59), 255 (−2.28) nm; IR (KBr) νmax 3077, 2926, 2854, 2359, 1774, 1743, 1661, 1541, 1456, 1371, 1263, 1226, 1162, 1136, 1083, 1045, 1019, 970, 924, 896, 748 cm−1; 1H NMR (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3) data, see Tables 1 and 2; HR-ESIMS m/z 335.1490 [M + H]+ (calcd for C18H23O6 335.1495).
2.2.4 Chlorajapolide L (4)
White amorphous powder; [α] − 258.9 (c 0.14, CH2Cl2); ECD (CH3CN) λmax (Δε) 213 (12.47), 246 (4.57) nm; IR (KBr) νmax 3079, 2936, 2360, 1771, 1664, 1456, 1375, 1323, 1239, 1223, 1196, 1146, 1108, 1087, 1056, 1035, 1014, 986, 941, 921, 887, 794, 762 cm−1; 1H NMR (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3) data, see Tables 1 and 2; HR-ESIMS m/z 335.1490 [M + H]+ (calcd for C18H23O6 335.1495).
2.3 Chemicals and reagents for biological evaluation
Antibodies to GAPDH, E-cadherin, Vimentin, Snail, N-cadherin and Twist1 were purchased from Affinity (USA). Goat pAb to Rb IgG(HRP) was purchased from ImmunoWay (China). Matrigel and transwell chambers were purchased from BD Biosciences (San Jose, CA, USA). All cell culture reagents were obtained from Hyclone Co. (Suzhou, China).
2.4 Cell lines and culture
Human liver cancer cells (HepG2, ATCC) and human gastric cancer cell lines (HGC-27, ATCC) were stored in DMEM (KeyGEN BioTECH, China) with 10% fetal bovine serum (FBS, ExCell Bio, China) and 1% antibiotics (penicillin streptomycin, Gibco). Human cervical cancer cells (Hela, ATCC) and human ovarian cancer cells (SKOV3, ATCC) were stored in RPMI 1640 (KeyGEN BioTECH, China). All cells were stored in an incubator at 37 °C with 5% CO2 humidification.
2.5 MTT assay
MTT assay was used to detect the cell survival rate after different compound treatments. HepG2 cells, Hela cells and HGC-27 cells were cultured at a concentration of 5 × 104 cells/mL in a 96-well plate for 24 h, and different compounds with concentration of 50 µM were added after cell adherence. After 48 h of incubation, 20 μL MTT solution was added to each well and incubated at 37 °C for 4 h. The absorbance of the solution was quantified at 570 nm wavelength by using a microplate reader (Mul-tiskan™FC, Thermo Scientific, Waltham, MA, USA).
2.6 Wound healing assay
HepG2 cells were grown on 24-well plates to 100% confluence. The 100 µm wounds were scratched using sterile pipette tips. Then HepG2 cells were cultured with compounds 1–7 (50 µM). The scratch was observed at 0, 24, 48 h by a light microscope (Nikon, Japan). After that, the drug with the strongest inhibition of cell migration was selected and the concentration gradient was set for the wound healing assay again. Subsequently, compound 4 was selected for follow-up experiments. Compound 4 (30, 60 and 120 µM) were added to cells cultured for 0, 24, 48 h. The images of the cells were acquired with a light microscope (Nikon, Japan).
2.7 Three-dimensional culture assay
HepG2 cells were seeded in 96-well culture plates prepared with Matrigel (BD Biosciences). The cells were co-incubated with various concentrations of compound 4 (30, 60, and 120 µM). After incubation for 24 h, cell images were captured using the light microscope (Nikon, Japan).
2.8 Transwell assays
HepG2 cells were seeded onto a chamber coated with matrigel (BD, USA) and inserted into the wells of a 24-well plate. The 24-well plate were filled with FBS-containing complete medium. Different concentrations of compound 4 were added into the chamber, after 24 h HepG2 cells migrating to the lower surface were fixed and stained.
2.9 Western blot analysis
Proteins were extracted from the HepG2 cells treated with different concentrations of compound 4 and analyzed through western blot analysis. Whole-cell lysates were analyzed with sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE), transferred to polyvinylidene fluoride (PVDF) membrane (Milan, Italy), then the membranes were blocked with 5% fat free milk. The membrane incubated at 4 ℃ overnight with monoclonal antibodies against GAPDH, E-cadherin, Vimentin, N- cadherin, Snail1 and Twist1. The membrane was further incubated with secondary antibodies (Affinity, 1:10000). Finally, the membrane was subjected to an electrophoresis gel imaging system (ChemiScope 6000, CLIX, Shanghai, China).
2.10 Statistical analysis
Statistical analyses were performed with GraphPad Prism 7.0. The significance differences between groups were calculated by student t test. Significant differences among multiple groups were detected by one-way ANOVA. P < 0.05 was considered as statistically significance.
3 Results and discussion
3.1 Structural elucidation
Chemical research on C. japonicus resulted in one new phenylpropenol ester (1), three new sesquiterpenes (2–4) and three known compounds (5–7) (Fig. 1). The known compounds were identified as 8-epimer-chlorajapolide F (5) (Zhang et al., 2012), chlorajapolide F (6) (Zhang et al., 2012), and shizukanolide (7) (Kawabata et al., 2014), respectively.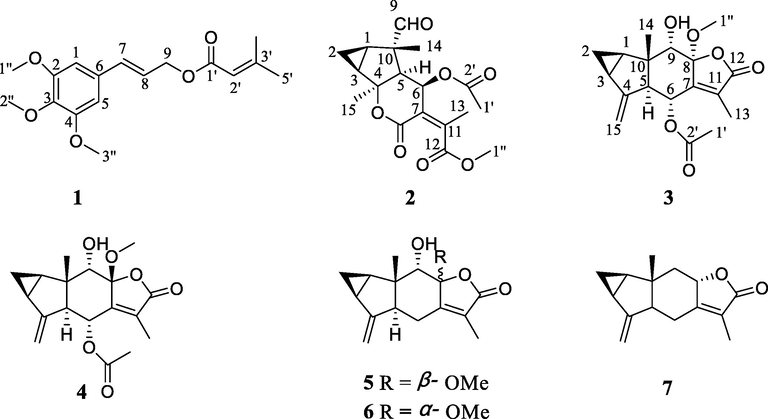
Chemical structures of compounds 1–7.
Compound 1 was obtained as a yellow oil. Its HR-ESIMS showed a [M + H]+ peak at m/z 307.1540 and determined the molecular formula of C17H22O5 (calcd for C17H23O5 307.1545). The carbonyl (1717 cm−1) and double bond (1650 cm−1) functional groups were found in the IR spectrum. The 1H NMR spectrum (Table 1) showed the presence of three methoxy groups at δH 3.87 (s, 6H) and 3.85 (s, 3H), two methyl groups at δH 2.19 (s, 3H) and 1.91 (s, 3H), five olefinic methines at δH 6.62 (s, 1H*2), 6.58 (d, J = 16.1 Hz, 1H), 6.23 (dt, J = 6.3, 16.1 Hz, 1H) and 5.73 (s, 1H), and an oxygenated methylene at δH 4.74 (d, J = 6.3 Hz, 2H). The 13C NMR data (Table 2) of 1 included 14 carbon signals. It was apparent that one senecioyl group (δC 166.4, 157.4, 115.7, 27.5, and 20.3) appeared in the NMR spectra (D'Ambrosio, et al., 2015). The remaining resonances comprised a symmetrical 1, 3, 4, 5-tetrasubstitution benzene at δC 153.3 (×2), 138.1, 132.1, and 103.7 (×2), one oxygenated methylene at δC 64.1, two olefinic methines at δC 123.3 and 133.8, and three methoxy groups at δC 56.1 (×2) and 60.9. According to the spectroscopic data, compound 1 might be a phenylpropenol ester derivative (Fan et al., 2009). Detailed comparison with the known compound 3′,4′,5′-trimethoxy cinnamyl caproate revealed that the NMR data of 1 (Tables 1 and 2) resembled those of this compound (Sun, et al., 2016), except for a senecioyl group in 1 instead of a hexanoyl group attached to C-9 in 3′,4′,5′-trimethoxycinnamyl caproate, which was confirmed by the 1H–1H COSY correlations of H-7/H-8/H2-9 and HMBC correlation from H-9 (δH 4.74) to C-1′ (δC 166.4) (Fig. 2). The configuration of the double bond Δ7, 8 was established as E according to the coupling constant (J = 16.1 Hz). The structure of 1 was therefore elucidated as 2, 3, 4-trimethoxycinnamyl senecioate.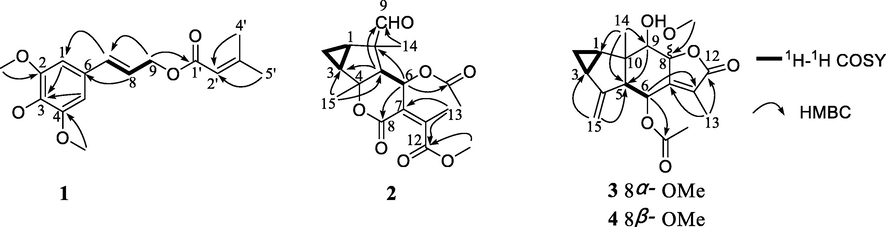
Selected 1H–1H COSY and HMBC correlations of compounds 1–4.
The molecular formula of compound 2 was deduced to be C18H22O7 according to HR-ESIMS (m/z 351.1441 [M + H]+, calcd for C18H23O7 351.1444). In the 1H NMR spectrum, five methyl signals at δH 2.18 (s, 3H), 1.22 (s, 3H), 1.58 (s, 3H), 2.10 (s, 3H), and 3.77 (s, 3H), one oxygenated methine at δH 5.62 (d, J = 4.6 Hz, 1H), and one formyl proton at δH 9.50 (s, 1H) were observed (Table 1). The 13C NMR spectrum exhibited eighteen carbon resonances (Table 2). It was obvious that an acetoxy group (δH 2.10 and δC 169.2, 21.2) and a methoxy (δH 3.77 and δC 52.8) group were present, which suggested 2 as a sesquiterpene with one acetoxy and one methoxy groups. The 13C and 1H NMR data of 2 demonstrated a resemblance with those of the known compound chloranerectuslactone V isolated from C. erectus (Thu Huong et al., 2014) except for an additional C-6 acetoxy group. This deduction was further verified by the HMBC correlation from H-6 (δH 5.62) to C-2′ (δC 169.2) as shown in Fig. 2. The relative configuration of 2 was established from the NOESY spectrum (Fig. 3). The cross-peaks of H3-15/H-6, H3-15/H-5, H-9/H-5, H-9/H-1, H-3/H-2α, and H-1/H-2α indicated that they adopted an α-orientation, while H3-14 was assigned as β-oriented. Additionally, the NOESY correlation of H-6/H3-13 suggested a Z-configuration of the Δ7, 11 olefinic bond. The absolute configurations were assigned as 1R, 3S, 4S, 5S, 6R, and 10S using the calculated and experimental ECD comparison (Fig. 4). The structure of 2 was thus finally elucidated and named chlorajapolide J. Compound 2 represented a rare example of the rearranged 8,9-seco-lindenanes previously found in Sarcandra glabra (Chi et al., 2019), Chloranthus erectus (Thu Huong et al., 2014), Lindera strychnifolia (Kouno et al., 2001; Liu et al., 2013; Sumioka et al., 2011), L. chunii (Zhang et al., 2002), and L. aggregata (Gan et al., 2009).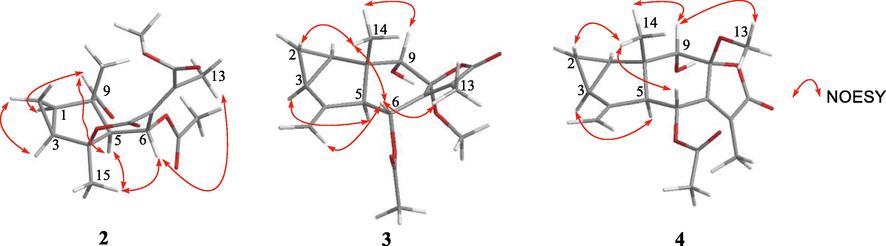
Selected NOESY correlations of compounds 2–4.
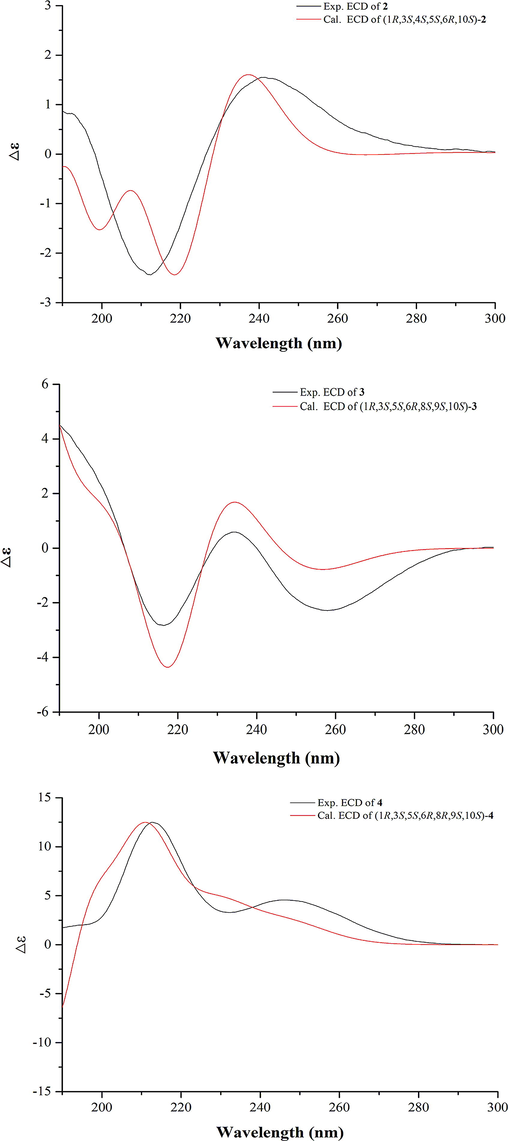
Calculated and experimental ECD spectra of compounds 2–4.
Compound 3 was isolated as a colorless oil and assigned the molecular formula of C18H22O6 deduced from the HR-ESIMS data. The 1H NMR spectrum of 3 (Table 1) exhibited signals typical of the cyclopropane ring of lindenane sesquiterpenoids (Yan et al., 2013), including four upfield protons at δH 0.71 (1H, m, H-2b), 0.84 (1H, m, H-2a), 1.97 (1H, m, H-3), and 2.15 (1H, m, H-1). Additionally, two terminal olefinic protons at δH 5.02 (1H, br s, H-15a) and 4.70 (1H, br s, H-15b) implied that 3 bore a 4(15)-en-lindane skeleton. This induction was supported by the occurence of the 1H–1H COSY correlations of H-1, H2-2 and H-3 and the correlations of H2-15 to C-5 (δC 56.5) and C-3 (δC 23.5) in the HMBC spectrum (Fig. 2). The carbon signals at δC 169.9, 20.7, and 50.1 in the 13C NMR spectrum (Table 2) together with the corresponding 1H NMR data suggested the existence of an acetoxy and a methoxy groups. The 13C and 1H NMR spectroscopic data of 3 were comparable to those of 8-epimer-9-hydroxy-heterogorgiolide isolated from the same plant species (Zhang et al., 2012), apart from an additional acetoxy group. The significant down-field shift of C-6 revealed that the acetoxy group was attached at C-6 (δC 63.2, Δδ + 40.4), which was sustained through the correlation of δH 5.74 with δC 169.9 in the HMBC spectrum (Fig. 2). The relative configuration of 3 was elucidated through a NOESY experiment (Fig. 3). The correlations of H-6/H-15a, H3-13, and H3-14, H3-14/H-9 and H-2β, H-2α/H-1 and H-3, H-5/8-OMe indicated that the cyclopropane ring and H3-14 were β-oriented, and that H-5, 6-OAc, 8-OMe and 9-OH were α-oriented. The absolute configurations of (1R, 3S, 5S, 6R, 8S, 9S, 10S) were determined for 3 by ECD analysis (Fig. 4). The structure of 3 was thus elucidated and named chlorajapolide K.
Compound 4 was isolated as a white amorphous powder. Its molecular formula was determined to be C18H22O6 according to the protonated molecular ion peak at m/z 335.1490 [M + H]+ (calcd for C18H23O6 335.1495) and 1D NMR data. The 1H and 13C NMR spectroscopic data of 4 closely resembled those of 3 with the exception of the down-field shifted C-6 (δC 69.5) and C-7 (δC 153.5) as well as the up-field shifted C-9 (δC 74.7) and C-11 (δC 126.3) (Table 2). This indicated that compound 4 bore a similar structure as 3 with a different stereochemistry. In the NOESY spectrum (Fig. 3), the correlations of 8-OMe/H-9, H3-14, H3-14/H-2β, H-6, H-9, H-2α/H-3, and H-1 suggested that the cyclopropane ring, 8-OMe and H3-14 were β-oriented, and that H-5, 6-OAc, and 9-OH were α-oriented. The absolute configuration was assigned as 1R, 3S, 5S, 6R, 8R, 9S, and 10S because of the similarity between the calculated and experimental ECD data (Fig. 4). Consequently, the structure of compound 4 was determined to be a C-8 epimeric isomer of 3 and named chlorajapolide L.
3.2 Compound 4 can significantly inhibits the migration and invasion but not the cell viability of HepG2 cells in vitro
Cell activity detect is a classical research method for evaluating the antitumor effect of compounds. To explore the antitumor activity of compounds 1–7, we evaluated the inhibitory effects on the activity of liver cancer cells (HepG2), cervical cancer cells (Hela), stomach cancer cells (HGC-27) and ovarian cancer cells (SKOV3), which are common and high incidence tumor types, by the MTT method. Different cancer cells were treated with all compounds for 48 h. At a concentration of 50 μM, none of the isolates showed obvious antitumor activity on four cancer cells (Fig. 5A). The high migration and invasion ability of tumor cells is an important basis of tumor metastasis (Gundamaraju et al., 2022). HCC has a strong vascular invasion and metastasis ability and is one of the most common invasive malignant tumors. In order to further explore the inhibitory activity of all compounds on tumor cell migration, we evaluated the inhibitory effect of all compounds on HepG2 cells migration. HepG2 cells were treated with seven compounds with 50 μM for 48 h. The results of migration inhibit rate showed that compound 4 exhibited the most obvious inhibitory effect on cell migration (Fig. 5B, 5C), revealing that the C-8 configuration and the substituents at C-6 might have an important effect on the antimetastatic activities of lindenrane sesquiterpenoids. Besides, we also detected the inhibitory effect of compound 4 on four cancer cells migration. The results showed that HepG2 cells are the most sensitive to compound 4 (Fig. S38, Supporting Information).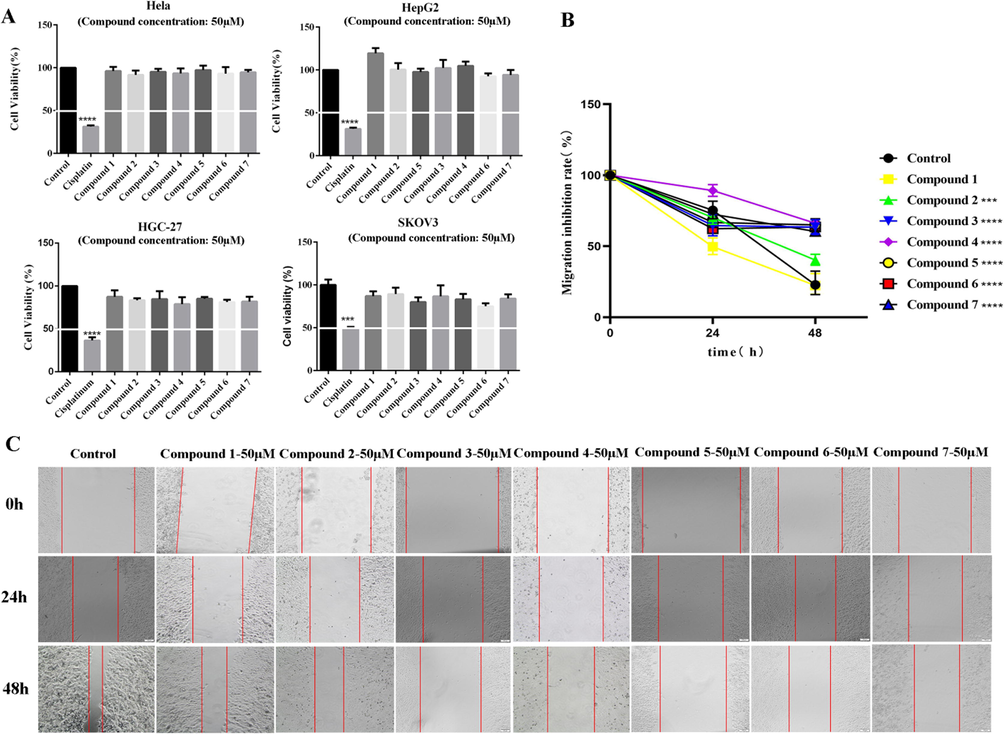
Compound 4 can significantly inhibit the migration but not the cell viability of HepG2 cells in vitro. (A) Cell viability tests on Hela, HepG2, HGC-27 and SKOV3 cells showed that compounds 1–7 did not inhibit the activity of cancer cells at the concentration of 50 μM within 48 h. (B, C) Compounds 1–7 were added to HepG2 cells for wound healing assays. Compound 4 had the strongest migration inhibitory effect on HepG2 cells at a concentration of 50 μM. (Data are represented as the means ± SDs, n = 3. * P < 0.05, ** P < 0.01, *** P < 0.001, ****P < 0.0001.).
Invasion and metastasis of cancer cells are the main factors of poor prognosis and easy recurrence and metastasis of cancer. Invasion and metastasis are multistep and complex process regulated by a series of genes, involving the adhesion of tumor cells, matrix degradation, tumor cell migration, tumor angiogenesis and so on (You et al., 2017, Prahl and Odde, 2018). To further study the effects of compound 4 on the invasion, migration and vasculogenic mimicry formation of liver cancer cells, we conducted wound healing, transwell and 3D culture assays with different concentrations of compound 4. Wound healing assay results showed that compound 4 can significantly inhibit the migration ability of HepG2 cells in a dose-dependent manner (Fig. 6A, 6B). In the transwell assay, the invasion ability of the HepG2 cells was significantly inhibited by compound 4 (Fig. 6C, 6D). The 3D culture assay showed that compound 4 inhibited VM formation of HepG2 cells (Fig. 6E, 6F). These results indicates that compound 4 will be a potential drug candidate for HCC invasion, metastasis and angiogenesis.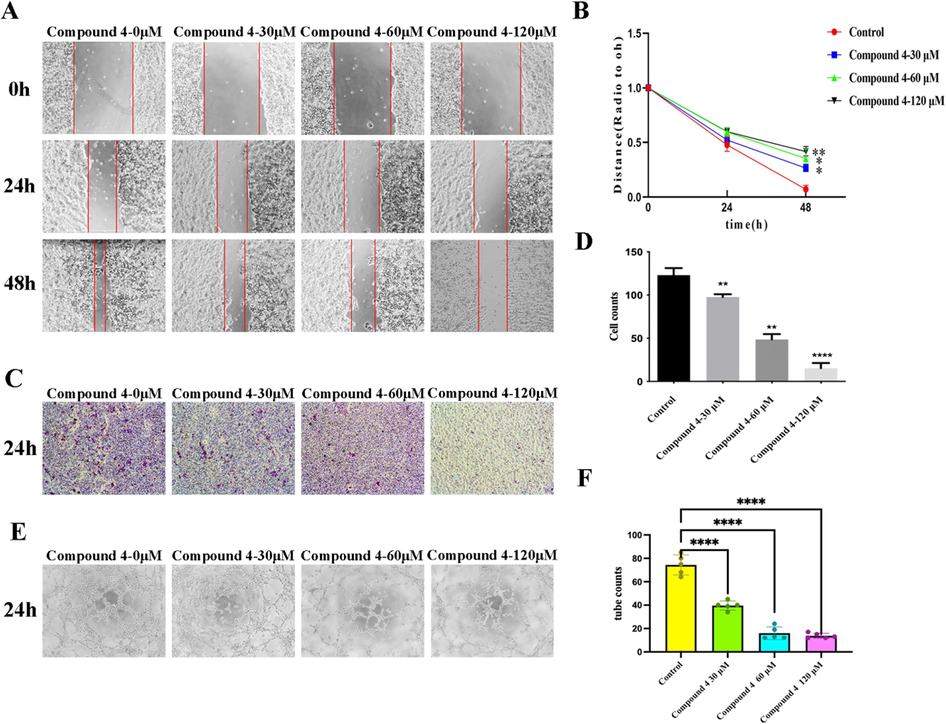
Compound 4 inhibited the migration, invasion and tube formation of HepG2 cells in vitro. (A, B) Compound 4 inhibited the migration of HepG2 cells in a dose-dependent manner. (C, D) The invasion of HepG2 cells was observed at 24 h, and compound 4 had an inhibitory effect on the invasion of HepG2 cells. (E, F) At the end of the 24th hour, compound 4 decreased the number of pipe-like structures in HepG2 cells. (Data was represented as the means ± SD, n = 3. * P < 0.05, ** P < 0.01, *** P < 0.001).
3.3 Compound 4 inhibits the invasion and metastasis of HepG2 cells by inhibiting EMT
Metastasis of hepatocellular carcinoma consists of multiple processes, including EMT, characterized by loss of epithelioid features and gain of mesenchymal properties (Babaei et al., 2021). Epithelial-mesenchymal transformation (EMT) is considered a mechanism leading to tumor metastasis, and E-cadherin, Snail1, N-cadherin and Vimentin are known as EMT markers. E-cadherin is one of the epithelial markers, while Snail1, N-cadherin and Vimentin are mesenchymal markers (Loh et al., 2019). Recently, several articles have been demonstrated the EMT involvement in cancer progression and in metastasis formation, underlining that EMT is a significant event for triggering metastatic process (Smith and Bhowmick, 2016). In tumor cells with epithelial-mesenchymal transition, E-cadherin expression was decreased and N-cadherin, Vimentin and Snail1 expression were increased. In order to further determine whether compound 4 inhibited the invasion and migration of HCC cells by inhibiting EMT, we detected the expression of EMT markers in HepG2 cells by western blotting method. Western blotting results showed that compound 4 inhibited the expression of Snail1, N-cadherin and Vimentin in a dose-dependent manner. The expression levels of E-cadherin were increased by compound 4 (Fig. 7A, 7B). The results showed that compound 4 can inhibits the EMT process in HepG2 cells. Twist1, a member of the basic helix-loop-helix transcription factor family, is one of the important transcription factors that induce EMT, cell migration and invasion during embryonic development and in cancer cells. Ectopic expression of Twist1 in Twist1-negative cancer cells is sufficient to induce EMT and cancer stem-like cell properties (Greco et al., 2021). In this study, we also evaluated the effect of compound 4 on the expression of Twist1 in HepG2 cells. The results showed that compound 4 inhibited the expression of Twist1 in a dose-dependent manner. These results indicate that compound 4 inhibits the EMT process of HCC cells by inhibiting the expression of Twist1. To our knowledge, this is the first investigation of the mechanism of the antimetastatic activity of lindenrane sesquiterpenoids. EMT plays an important role in the migration and invasion of HCC. Inhibitors targeting the EMT process will be used as adjuvant therapy for chemotherapy or targeted drugs, which can improve the clinical outcome of current cancer treatment.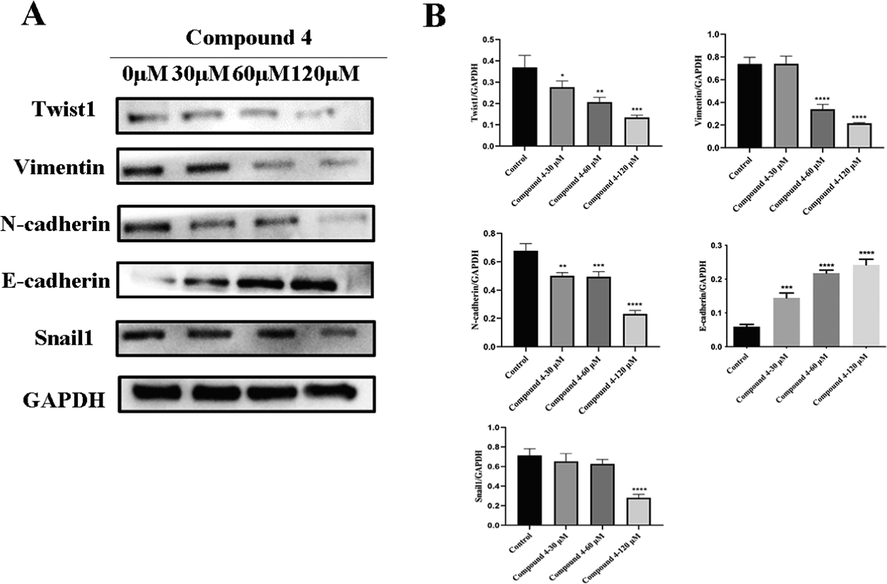
Compound 4 inhibited the invasion and metastasis of HepG2 cells by inhibiting EMT. (A, B) Western blot analysis showed that E-cadherin expression was increased and Vimentin, Snail1, N-cadherin and Twist1 expression were decreased in compound 4 treated groups compared with the negative control. (Data was represented as the means ± SD, n = 3. * P < 0.05, ** P < 0.01, *** P < 0.001).
4 Conclusions
Seven compounds, including one new phenylpropenol ester and three new lindenrane sesquiterpenes, were separated and elucidated from C. japonicus. The anti-migration potencies of all isolates were screened with HepG2 cells through the wound healing assay and compound 4 exhibited the best antimigration activity. Furthermore, compound 4 inhibited cell migration, invasion, and vasculogenic mimicry in a dose-dependent manner. The antimetastatic effect of compound 4 was associated with restraining the epithelial-mesenchymal transition in HepG2 cells by down-regulating the expression of Twist1. Compound 4 deserves further investigation as an anticancer candidate against metastatic hepatic carcinoma.
Acknowledgements
This study was supported by the National Key Research and Development Program of China (Grant No. 2018YFA0507203), the National Natural Science Foundation of China (Grant No. 81871972), the 111 Project (Grant No. B20016), and Open fund research project of State Key Laboratory of Medicinal Chemical Biology (Nankai University) (Grant No. 2018121).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N. Engl. J. Med.. 2018;379:54-63.
- [Google Scholar]
- EMT, cancer stem cells and autophagy; The three main axes of metastasis. Biomed. Pharmacother.. 2021;133:110909
- [Google Scholar]
- Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56-66.
- [Google Scholar]
- Diverse chemosensitizing 8, 9-secolindenane-type sesquiterpenoid oligomers and monomers from Sarcandra glabra. J. Org. Chem.. 2019;84:9117-9126.
- [Google Scholar]
- Structure and cytotoxic activity of sesquiterpene glycoside esters from Calendula officinalis L.: studies on the conformation of viridiflorol. Phytochemistry. 2015;117:1-9.
- [Google Scholar]
- Sesquiterpenoids isolated from the flower of Inula japonica as potential antitumor leads for intervention of paclitaxel-resistant non-small-cell lung cancer. Bioorg. Chem.. 2020;101:103973
- [Google Scholar]
- Chemical constituents of Heteroplexis micocephala. J. Nat. Prod.. 2009;72:1184-1190.
- [Google Scholar]
- Sesquiterpene lactones from the root tubers of Lindera aggregata. J. Nat. Prod.. 2009;72:1497-1501.
- [Google Scholar]
- Epithelial to mesenchymal transition: a challenging playground for translational research. Current models and focus on TWIST1 relevance and gastrointestinal cancers. Int. J. Mol. Sci.. 2021;22:11469.
- [Google Scholar]
- Autophagy and EMT in cancer and metastasis: Who controls whom? Biochim. Biophys. Acta, Mol. Basis Dis.. 2022;1868:166431
- [Google Scholar]
- Epithelial-mesenchymal transition: the history, regulatory mechanism, and cancer therapeutic opportunities. MedComm.. 2022;3:e144.
- [Google Scholar]
- Isolation and structural elucidation of four sesquiterpenes from Chloranthus japonicus (Chloranthaceae) Agri. Biol. Chem.. 2014;45:1447-1453.
- [Google Scholar]
- Hitorins A and B, hexacyclic C(25) terpenoids from Chloranthus japonicus. Org. Lett.. 2016;18:5420-5423.
- [Google Scholar]
- New eudesmane sesquiterpenes from the root of Lindera strychnifolia. J. Nat. Prod.. 2001;64:286-288.
- [Google Scholar]
- Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173.
- [Google Scholar]
- STAT3: An emerging therapeutic target for hepatocellular carcinoma. Cancers (Basel). 2019;11:1646.
- [Google Scholar]
- Sesquiterpene lactones from the roots of Lindera strychnifolia. Phytochemistry. 2013;87:112-118.
- [Google Scholar]
- The E-cadherin and N-cadherin switch in epithelial-to-mesenchymal transition: signaling, therapeutic implications, and challenges. Cells. 2019;8:1118.
- [Google Scholar]
- Dictionary of Chinese Traditional Medicine (Zhong Yao Da Ci Dian) (second ed.). Shanghai: Shanghai Science and Technology Press; 2006.
- Linderolides A-F, eudesmane-type sesquiterpene lactones and linderoline, a germacrane-type sesquiterpene from the roots of Lindera strychnifolia and their inhibitory activity on NO production in RAW 264.7 cells in vitro. Phytochemistry. 2011;72:2165-2171.
- [Google Scholar]
- Two new cinnamyl isovalerate derivatives from Sabina gaussenii. Molecules. 2016;21:571.
- [Google Scholar]
- First-line targeted therapy for hepatocellular carcinoma: role of atezolizumab/bevacizumab combination. Biomedicines. 2022;10:1304.
- [Google Scholar]
- Chloranerectuslactone V, a new sesquiterpene from Chloranthus erectus verdc. Lett. Org. Chem.. 2014;11:639-642.
- [Google Scholar]
- Bioactive ent-pimarane and ent-abietane diterpenoids from the whole plants of Chloranthus henryi. J. Nat. Prod.. 2015;78:2800-2807.
- [Google Scholar]
- Lindenane sesquiterpenoid dimers from Chloranthus japonicus inhibit HIV-1 and HCV replication. Fitoterapia. 2016;115:64-68.
- [Google Scholar]
- Chlojaponilactones B-E, four new lindenane sesquiterpenoid lactones from Chloranthus japonicus. Helv. Chim. Acta. 2013;96:1386-1391.
- [Google Scholar]
- NOR1 promotes hepatocellular carcinoma cell proliferation and migration through modulating the Notch signaling pathway. Exp. Cell Res.. 2017;352:375-381.
- [Google Scholar]
- Chlojaponilactone B from Chloranthus japonicus: suppression of inflammatory responses via inhibition of the NF-kappa B signaling pathway. J. Nat. Prod.. 2016;79:2257-2263.
- [Google Scholar]
- Sesquiterpenes and alkaloids from Lindera chunii and their inhibitory activities against HIV-1 integrase. Chem Pharm Bull (Tokyo). 2002;50:1195-1200.
- [Google Scholar]
- Sesquiterpenes from the aerial part of Chloranthus japonicus and their cytotoxicities. Fitoterapia. 2012;83:1604-1609.
- [Google Scholar]
- Regulatory mechanisms and therapeutic targeting of vasculogenic mimicry in hepatocellular carcinoma. Pharmacol. Res.. 2021;166:105507
- [Google Scholar]
- Chlorajaponols A-F, sesquiterpenoids from Chloranthus japonicus and their in vitro anti-inflammatory and anti-tumor activities. Fitoterapia. 2017;119:90-99.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.104100.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







