Translate this page into:
Evaluation of chromium(VI) sorption efficiency of modified Amberlite XAD-4 resin
⁎Corresponding author. Tel.: +92 (22) 9213430; fax: +92 (22) 9213431. shahabuddinmemon@yahoo.com (Shahabuddin Memon)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.

Abstract
The present work deals with the removal of Cr(VI) from aqueous media by modified Amberlite XAD-4 (MAX-4) resin through the solid phase extraction method. Different parameters such as pH, dosage and temperature were optimized during the batch experiment. The experimental data were analyzed by Freundlich, Langmuir, Dubinin-Radushkevich (D-R) and Temkin equilibrium isotherms. Each characteristic parameter of isotherms was determined. The kinetic sorption experiments show that the sorption process follows pseudo second order kinetics. The sorption mechanism was investigated by Reichenberg (R-B) and Morris–Weber equations. From the thermodynamic parameters, it could be concluded that the sorption process is endothermic and spontaneous in nature. The interference and desorption studies were also performed. The results show that MAX-4 resin has the capability to remove Cr(VI) significantly from aqueous media even in the presence of interfering ions.
Keywords
Chromium
Sorption
Amberlite XAD
Solid phase extraction
Isotherms
1 Introduction
Many industries such as leather tanning, dye and electroplating extensively use chromium as alloy or trivalent or hexavalent salt. As a result, these industries release a large quantity of chromium without treatment to our environment, which causes great hazard to humans and aquatic life (Goyal et al., 2003; Yusof and Malek, 2009; Raji and Anirudhan, 1998). In aqueous systems, chromium mainly exists in two forms, i.e. Cr(III) and Cr(VI) subsequently; toxicity and reactivity mainly depend on the chemical form or oxidation state of the chromium. In trace amounts, Cr(III) is an essential nutrient for humans and to mammals for their maintenance of normal glucose tolerance factor, lipid and protein metabolism (Kocaoba and Akcin, 2002). Cr(VI) is a well known carcinogen and due to its high solubility in water, high oxidation potential and relatively small size enable it to penetrate through biological cell membrane and being mutagenic and genotoxic causes different types of DNA damages (Ertul et al., 2010). Cr(VI), i.e. and mainly exist as divalent anions with oxide functionalities at the periphery, which are potential sites for hydrogen bonding (Yilmaz et al., 2007). As it has been mentioned that Cr(VI) is highly soluble in water as compared to Cr(III) and the maximum permissible level for Cr(VI) in potable and industrial wastewater is 0.05 and 0.25 mg/L, respectively (Sayin et al., 2010; Deligöz et al., 2008). Therefore, there is an essential need of treatment of wastewater containing Cr(VI) as compared to Cr(III).
Solid phase extraction (SPE) has been favored by many researchers and preferred over liquid–liquid extraction (Qureshi et al., 2009; Ozcan et al., 2009). In SPEs, chelating resins have often been used because they provide good thermal stability and high sorption capacity toward ions with better flexibility in working conditions however; insignificance in selectivity and regenerability makes them unsuitable. Among them 5-palmitoyl oxine-functionalized XAD-2 resin (Filik et al., 2003), Amberlite XAD-16 (Elci et al., 2000), Amberlite XAD-2000 (Narin et al., 2001), Amberlite IRC-718, 4-vinyl pyridine-divinylbenzene/acrylonitrile-divinylbenzene copolymer, Amberlite IRA-400, silica based C-18 Dowex A-26 have been employed for sorption of Cr(VI) (Mondal, 2003). Besides these resins, Amberlite XAD-4 has been known as non-ionic polymeric sorbent material with superior physical properties, economical, easily available, thermally stable and can be easily modified (Uzun et al., 2001; Soylak et al., 2001). Previously, we have modified Amberlite XAD-4 by introducing amino groups into the aromatic ring for the removal of fluoride ion from aqueous environment (Solangi et al., 2010). Therefore, keeping in view the above observations and results, in this study we have used the same resin, i.e. modified Amberlite XAD-4 for the removal of Cr(VI) ion from aqueous media using the solid phase extraction method by optimizing various parameters.
2 Experimental
pH meter (781-pH/Ion meter, Metrohm, Herisau Switzerland) with glass electrode and internal reference electrode was used for pH measurements. The pH of the solution was adjusted by mixing an appropriate amount of 0.1 M (HCl/KOH). Janke and Kunikel automatic shaker model KS 501 D Singapore was used at ambient temperature (25 ± 2 °C). All chemicals used were of analytical or equivalent grade. Stock standard solution (1000 mg/L) was prepared using K2Cr2O7 purchased from Sigma (St. Loius, MO, USA). Calibration standards were prepared by diluting stock solution. Amberlite XAD-4™ (surface area of 825 m2/g, pore diameter 14.4 nm and bead size 20.50 mesh) was procured from Fluka, Germany. The surface area, pore diameter and mesh size were quoted by the supplier. All glassware were thoroughly washed and soaked overnight in 5 M HNO3, and rinsed with de-ionized water before use. All aqueous solutions were prepared with deionized water that had been passed through a Millipore milli-Q Plus water purification system (ELGA Model CLASSIC UVF, UK).
2.1 Synthesis
Amberlite XAD-4™ has been modified (Scheme 1) by using previously developed method (Solangi et al., 2010). The modification of the resin was confirmed by FT-IR spectroscopy.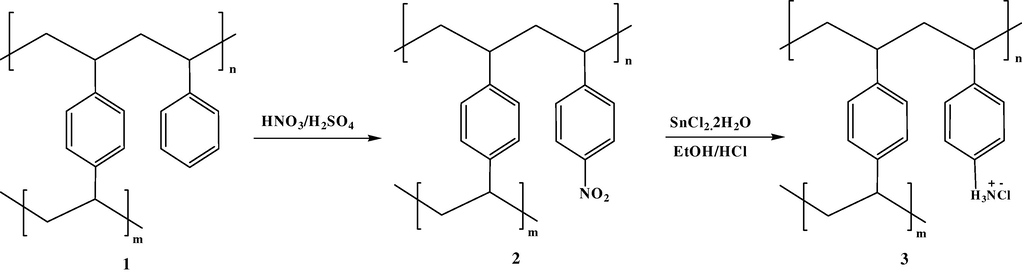
Modification of Amberlite XAD-4 resin.
2.2 Sorption procedure
Batch experiment was carried out for the sorption process of Cr(VI) on MAX-4 resin at ambient temperature. A 10 ml sample solution containing Cr(VI) (5 mg/L) was taken in a 25 mL Erlenmeyer flask and MAX-4 resin (0.1 g) was added. The mixture was equilibrated for a fixed period of time (1 h). The mixture was filtered and the concentration of Cr(VI) was analyzed by UV–Visible spectrophotometer. The percent sorption of Cr(VI) ion was calculated as follows: where Ci and Cf (mol/L) are the initial and final concentrations of solution before and after the sorption of the Cr(VI), respectively.
3 Results and discussion
3.1 Effect of sorbent dosage
Sorbent dosage is an important parameter because this concludes the ability of a sorbent for given initial concentration of the sorbate at the operating conditions (Solangi et al., 2009). The effect of sorbent dosage on the sorption of Cr(VI) is represented in Fig. 1. It was observed that as the amount of MAX-4 resin increases, the sorption of Cr(VI) increases due to more surface area available for sorption. Maximum sorption (98.7 %) was achieved at 0.1 g of the MAX-4 resin and sorption remained almost constant up to 0.2 g. There is no significant change in removal efficiency of sorbent after 0.1 g due to the availability of large number of sorption sites. Keeping in view the above results further study was processed at 0.1 g of sorbent.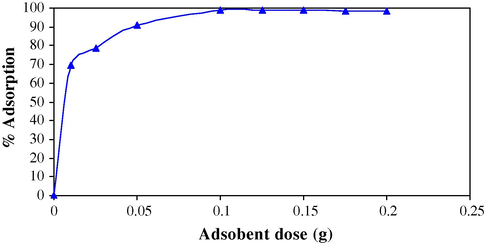
Effect of sorbent dosage on the percent sorption (10 ml of Cr(VI) with concentration 1 × 10−4 mol/L, 60 min contact time).
3.2 pH effect on sorption of Cr(VI)
Effect of pH on sorption of Cr(VI) on MAX-4 resin was also checked because in the sorption experiment pH plays a vital role (Memon et al., 2008). Surface binding sites of sorbent and aqueous chemistry are widely affected with change in pH. At a fixed concentration of Cr(VI), sorption behavior was observed at various pH values as shown in Fig. 2 and it has been found that the maximum sorption (qmax = 1.08 mmol/g) was achieved at pH 6.9.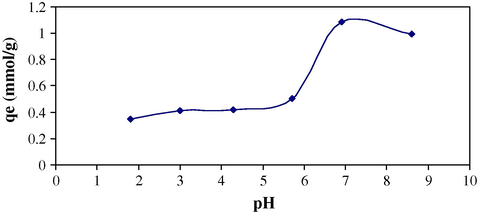
Effect of pH on sorption of Cr(VI) on MAX-4 resin (10 ml of Cr(VI) with concentration 1 × 10−4 mol/L, 60 min contact time).
3.3 Sorption isotherms
Optimizing the design of a sorption system to evaluate the most appropriate correlations for equilibrium curves is important. The sorption isotherms may be applied to predict the sorbate behavior that has not been experimentally investigated. Langmuir, Freundlich, D-R and Temkin isotherm models were applied to experimental data (Langmuir, 1918; Clayton, 1926; Kamboh et al., 2011). These nonlinear isotherm models can be recast as linear equations (Ruthven, 1984; McKay et al., 1982; Abdelwahab, 2007).
The Langmuir isotherm (Eq. (1)) is based on the assumption of monolayer surface coverage, equivalent sorption sites, and independent sorption sites.
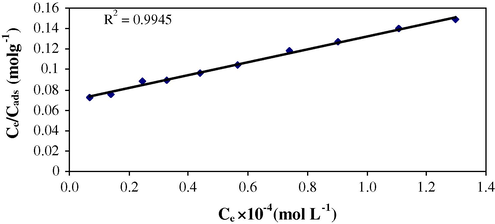
Langmuir isotherm (Conc. 1.0 × 10−4–1.0 × 10−3 mol/L, 0.1 g sorbent per 10 mL of sorbate with 60 min shaking time at 25 °C).
The Freundlich isotherm (Eq. (2)) is useful to identify sorption phenomena with the heterogeneous sorbent media. This isotherm is derived from the assumption that the sorption sites are distributed exponentially with respect to the heat of sorption (Helfferich, 1995).
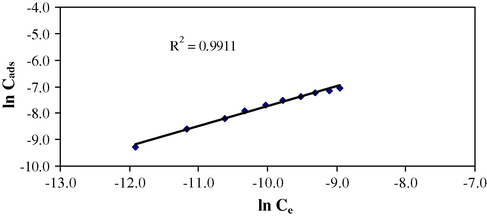
Freundlich isotherm (Conc. 1.0 × 10−4–1.0 × 10−3 mol/L, 0.1 g sorbent per 10 mL of sorbate with 60 min shaking time at 25 °C).
Parameters
Langmuir
Freundlich
D-R
Temkin
Q mmol/g
1.581 ± 0.054
–
–
–
A mmol/g
–
0.526 ± 0.094
–
–
Xm mmol/g
–
–
13.72 ± 0.034
–
A mol/g
–
–
–
1.08 ± 0.000034
b × 102 L/mol
91.32
–
–
–
1/n
–
0.746
–
–
E kJ/mol
–
–
9.449
–
b J/mol
–
–
–
680.1
RL
0.522–0.108
–
–
–
n
–
1.338
–
–
R2
0.994
0.991
0.990
0.980
The Dubinin-Radushkevich, (D-R) isotherm (Eq. (3)) model is more general than the Langmuir isotherm as its deviations are not based on ideal assumptions such as equipotential of sorption sites, absence of steric hindrances between adsorbed and incoming particles and surface homogeneity on microscopic level (Itodo and Itodo, 2010).
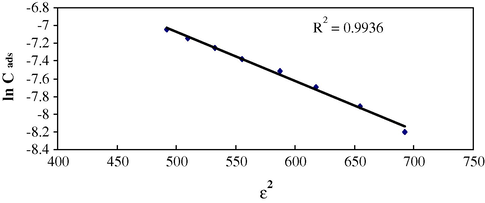
D-R isotherm (Conc. 1.0 × 10−4–1.0 × 10−3 mol/L, 0.1 g sorbent per 10 mL of sorbate with 60 min shaking time at 25 °C).
Temkin isotherm, assumes that the heat of sorption decreases linearly with the coverage due to sorbent-sorbate interaction.
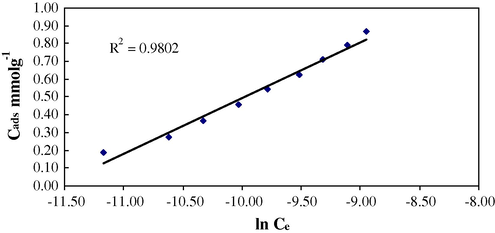
Temkin isotherm (Conc. 1.0 × 10−4–1.0 × 10−3 mol/L, 0.1 g sorbent per 10 mL of sorbate with 60 min shaking time at 25 °C).
3.4 Sorption kinetics
The batch experiment plays a key role in designing and evolution for the presentation of sorption kinetics. According to the literature (Dabrowski, 2001) sorption kinetics can be determined by the following main steps:
Internal diffusion: Diffusion of molecules inside the pores.
External diffusion: Diffusion of molecules from the bulk phase toward the interface space.
Surface diffusion: Diffusion of molecules in the surface phase.
Adsorption/desorption elementary processes.
Lagergren/pseudo first order and pseudo second order kinetic equation are as follows:
Temperature
Pseudo-first order kinetic model
Pseudo-second order kinetic model
K1 min−1
qe mol/g
R2
K2 g mol/min
qe mol/g
R2
303
0.1602
2.300
0.951
162.08
0.00772
0.999
308
0.1183
1.568
0.963
43.971
0.00946
0.996
313
0.1325
2.244
0.987
47.529
0.00911
0.997
Reichenberg equation (Eq. (9)) was used to test the sorption process, whether sorption is film diffusion or intra-particle.
It is clear from the linear plot of Bt versus time t (Fig. 7) that intra-particle diffusion is a rate controlling step with a small friction of sorption that occurs through film diffusion because the plot does not pass through the origin.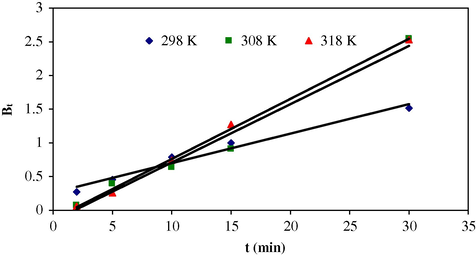
Reichenberg (R-B) plot at different temperatures for the sorption of Cr(VI) on MAX-4 resin.
Morris–Weber equation was also used to explore the kinetic sorption process.
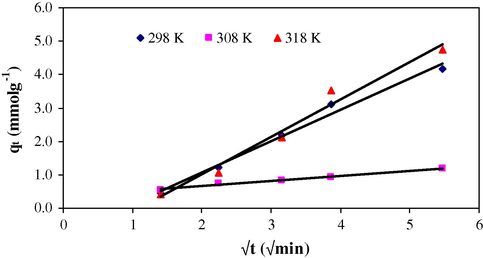
Morris–Weber plot at different temperatures for the sorption of Cr(VI) on MAX-4 resin.
3.5 Thermodynamics of sorption
Sorption of Cr(VI) onto MAX-4 Resin was analyzed at different temperatures. It has been observed that as temperature increases the sorption capacity value increases (Fig. 9). The obtained values from the plot are 0.085 (298 K), 0.1017 (308 K) and 0.1188 mmol/g (318 K), respectively.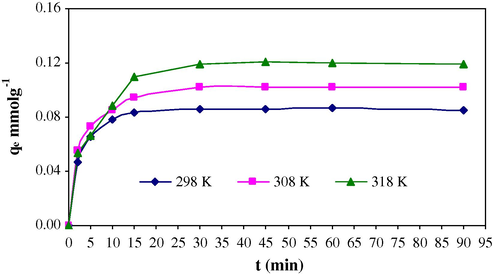
Sorption of Cr(VI) onto MAX-4 resin as a function of time at different temperatures.
The temperature effect was analyzed by using Arrhenius equation (Solangi et al., 2011) at optimized conditions,
where Ao (1/min) is the “frequency factor” and independent of temperature, Ea (kj/mol) is the activation energy, R (8.314 J/mol/K) is the gas law constant and T is the absolute temperature. The value of Ea (94.32 kJ/mol) and Ao (0.757/min) is calculated from the plot of lnk1 versus 1/T (Fig. 10). The value of activation energy is in the range of 40–800 kJ/mol that suggests the sorption is chemical in nature (Nollet et al., 2003; Singh and Rawat, 1994).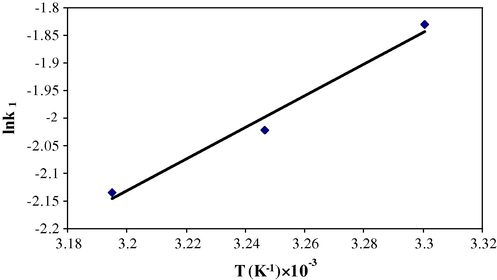
Arrhenius plot of the sorption of Cr(VI) on MAX-4 resin.
From the plot of ln kc versus 1/T (Fig. 11), numerical values of ΔH (kJ/mol), ΔS (kJ/mol /K) and ΔG (kJ/mol) were calculated using following equations.
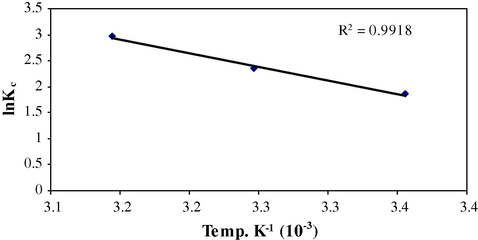
Effect of temperature on sorption of Cr(VI) on MAX-4 resin.
ΔH (kJ/mol)
ΔS (kJ/mol/K)
ΔG (kJ mol)
298 K
308 K
318 K
43.655
0.161731
−4.608
ln Kc = 1.861−6.006
ln Kc = 2.346−7.851
ln Kc = 2.971
3.6 Interference study
Most industrial effluents are source of many contaminations. They may interfere with uptake of target species, for example existing of other anions such as F−, Cl−, Br−,
, etc. The study was carried out with different concentration ratios of target and interfering anion, i.e. 1:1, 1:5 and 1:10. From the results it is concluded that there is no significant change in uptake of target species in the presence of other co-existing anions at optimized conditions (Fig. 12).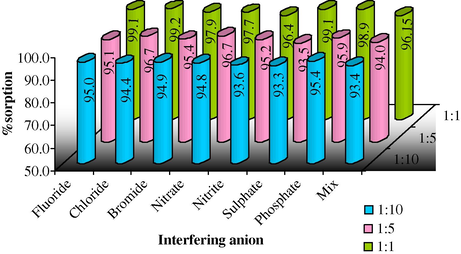
Sorption of Cr(VI) on MAX-4 resin in the presence of other anions.
3.7 Desorption
Efficiency of resin can be justified with its reusability and comparative efficiency (Table 4). The used resin can be regenerated by washing with 5 M HCl (Fig. 13).
Resin
pH
Capacity mmol/g
Reference
Azophenolcarboxylic acid resin
2.0
0.69
Pramanik, et al. (2007)
6-Mercapto Purinylazo Resin
1.0.
1.06
Mondal (2003)
Amberlite XAD-7 impregnated with Aliquat 336
6.0
0.97
Saha, et al. (2004)
Amberlite XAD-16
1.0
0.40
Tunceli and Turker (2002)
Lignocellulosic substrate
2.1
0.72
Dupont and Guillon (2003)
Modified Amberlite XAD-4 (MAX-4)
6.9
1.08
Present study
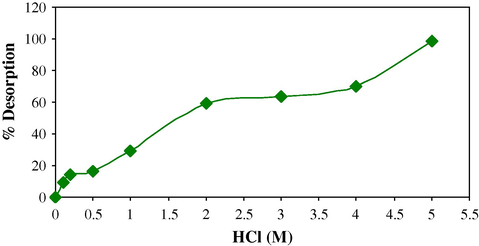
Regeneration profile of resin with HCl.
4 Conclusion
It has been concluded that modified Amberlite XAD-4 (MAX-4) resin is an efficient ion exchange material for the removal of Cr(VI) from aqueous media. The maximum sorption of Cr(VI) achieved at pH 6.9 means that the MAX-4 resin can work at neutral pH. The isotherm models, kinetics and thermodynamic study further confirm the experimental results. The interference study shows selectivity of resin for Cr(VI) at optimized conditions. Hopefully, this study will find applicability in analytical, environmental and industrial fields.
Acknowledgement
We are highly thankful to the National Centre of Excellence in Analytical Chemistry, University of Sindh, Jamshoro (Project No. NCEAC/246/2010) for the financial support.
References
- Kinetic and isotherm studies of copper (II) Removal from wastewater using various adsorbents. Egyptian Journal of Aquatic Research. 2007;33:125-143.
- [Google Scholar]
- Removal of copper(II) ions from aqueous solution by biosorption onto agricultural waste sugar beet pulp. Process Biochemistry. 2005;40:3031-3044.
- [Google Scholar]
- Andal, N., Sakthia, M. V., 2010. A Comparative Study on the Sorption Characteristics of Pb(II) and Hg(II) onto Activated Carbon. E-Journal of Chemistry 7, 967-74.
- Capillary and colloid chemistry. By Prof. H. Freundlich. Translated by H. Stafford Hatfield, B.Sc., Ph.D. pp. xv+883. London, Methuen and Co., Ltd., 1926. Price, 50s. Journal of the Society of Chemical Industry. 1926;45:797-798.
- [Google Scholar]
- Adsorption – from theory to practice. Advances in Colloid and Interface Science. 2001;93:135-224.
- [Google Scholar]
- Azocalixarene. 5: p-Substituted azocalix[4]arenes as extractants for dichromate anions. Pakistan Journal of Analytical & Environmental Chemistry. 2008;9:1-5.
- [Google Scholar]
- Removal of hexavalent chromium with a lignocellulosic substrate extracted from wheat bran. Environmental Science & Technology. 2003;37:4235-4241.
- [Google Scholar]
- Pore and solid diffusion kinetics in fixed adsorption constant pattern conditions. Indian Engineering Chemistry Research. 1966;5:212-223.
- [Google Scholar]
- Determination of trace impurities in some nickel compounds by flame atomic absorption spectrometry after solid phase extraction using Amberlite XAD-16 resin. Fresenius Journal of Analytical Chemistry. 2000;368:358-361.
- [Google Scholar]
- Removal of chromate and phosphate anion from aqueous solutions using calix[4]aren receptors containing proton switchable units. Journal of Hazardous Materials. 2010;181:1059-1065.
- [Google Scholar]
- Speciation analysis of chromium by separation on a 5-palmitoyl oxine-functionalized XAD-2 resin and spectrophotometric determination with diphenylcarbazide. Analytical and Bioanalytical Chemistry. 2003;376:928-933.
- [Google Scholar]
- Comparative studies on the microbial adsorption of heavy metals. Advances in Environmental Research. 2003;7:311-319.
- [Google Scholar]
- Ion exchange. Dover Publications; 1995. New York
- Utilizing D-R and temkin isotherms with gcms external standard technique in forecasting liquid Phase herbicide sorption energies. Electronic Journal of Environmental, Agriculture and Food Chemistry. 2010;9:1792-17802.
- [Google Scholar]
- A highly efficient calix[4]arene based resin for the removal of azo dyes. Desalination. 2011;268:83-89.
- [Google Scholar]
- Removal and recovery of chromium and chromium speciation with MINTEQA2. Talanta. 2002;57:23-30.
- [Google Scholar]
- About the theory of so-called adsorption of soluble substances. Kung Svenska Vetenskapsakad Handlingar. 1898;24:1-39.
- [Google Scholar]
- The adsorption of gases on plane surfaces of glass, mica and platinum. Journal of the American Chemical Society. 1918;40:1361-1403.
- [Google Scholar]
- Adsorption of dyes on chitin. I. Equilibrium studies. Journal of Applied Polymer Science. 1982;27:3043-3057.
- [Google Scholar]
- Adsorption of methyl parathion pesticide from water using watermelon peels as a low cost adsorbent. Chemical Engineering Journal. 2008;138:616-621.
- [Google Scholar]
- Use of 6-mercapto purinylazo resin in chromium speciation. Bulletin of the Chemical Society of Japan. 2003;76:111-114.
- [Google Scholar]
- Separation and enrichment of chromium, copper, nickel and lead in surface seawater samples on a column filled with Amberlite XAD-2000. Analytical Letters. 2001;34:1935-1947.
- [Google Scholar]
- Preparation and application of calix[4]arene-grafted magnetite nanoparticles for removal of dichromate anions. Materials Science and Engineering C. 2009;29:2378-2383.
- [Google Scholar]
- A new chelating resin containing azophenolcarboxylate functionality: synthesis, characterization and application to chromium speciation in wastewater. Analytica Chemica Acta. 2007;584:469-476.
- [Google Scholar]
- Estimation of chromium(VI) sorption efficiency of novel regenerable p-tert-butylcalix[8]areneoctamide impregnated Amberlite resin. Journal of Hazardous Materials. 2009;164:675-682.
- [Google Scholar]
- Batch Cr(VI) removal by polyacrylamide-grafted sawdust: Kinetics and thermodynamics. Water Research. 1998;32:3772-3780.
- [Google Scholar]
- Properties of ion-exchange resins in relation to their structure. III. Kinetics of exchange. Journal of the American Chemical Society. 1953;75:589-597.
- [Google Scholar]
- Principles of adsorption and adsorption processes. Wiley; 1984. New York
- Sorption of Cr(VI) from aqueous solution by Amberlite XAD-7 resin impregnated with Aliquat 336. Reactive & Functional Polymers. 2004;60:223-244.
- [Google Scholar]
- Synthesis and oxoanions (dichromate/arsenate) sorption study of n-methylglucamine derivative of calix[4]arene immobilized onto poly[(phenyl glycidyl ether)-co-formaldehyde] Journal of Inclusion Phenomena and Macrocyclic Chemistry. 2010;67:385-391.
- [Google Scholar]
- Comparative sorption equilibrium studies of toxic phenols on flyash and impregnated flyash. Journal of Chemical Technology & Biotechnology. 1994;61:307-317.
- [Google Scholar]
- Synthesis and application of a highly efficient tetraester calix[4]arene based resin for the removal of Pb2+ from aqueous environment. Analytica Chimica Acta. 2009;638:146-153.
- [Google Scholar]
- An excellent fluoride sorption behavior of modified Amberlite resin. Journal of Hazardous Materials. 2010;176:186-192.
- [Google Scholar]
- Comparative fluoride sorption study of new calix[4]arene-based resins. Desalination. 2011;272:98-106.
- [Google Scholar]
- Solid phase extraction of trace metal ions with Amberlite XAD resins prior to atomic absorption spectrometric analysis. Journal of Trace and Microprobe Techniques. 2001;19:329-344.
- [Google Scholar]
- Speciation of Cr(III) and Cr(VI) in water after preconcentration of its 1,5-diphenylcarbazone complex on amberlite XAD-16 resin and determination by FAAS. Talanta. 2002;57:1199-1204.
- [Google Scholar]
- Preconcentration and separation with Amberlite XAD-4 resin; Determination of Cu, Fe, Pb, Ni, Cd and Bi at trace levels in waste water samples by flame atomic absorption spectrometry. Talanta. 2001;54:197-202.
- [Google Scholar]
- Kinetics of adsorption on carbon from solution. Journal of Sanitary Engineering Division American Society of Chemical Engineering. 1963;89:31-59.
- [Google Scholar]
- Synthesis and dichromate anion extraction ability of p-tert-butylcalix[4]arene diamide derivatives with different binding sites. Tetrahedron. 2007;63:5000-5005.
- [Google Scholar]
- Removal of Cr(VI) and As(V) from aqueous solutions by HDTMA-modified zeolite Y. Journal of Hazardous Materials. 2009;162:1019-1024.
- [Google Scholar]







