Translate this page into:
A clean and highly efficient synthesis of 4,4′-(arylmethylene)bis(3-methyl-1-phenyl-1H-pyrazol-5-ols) using Ce(SO4)2.4H2O as heterogeneous catalyst
⁎Corresponding author. Address: Graduate University of Advanced Technology, Department of New Materials, P.O. Box 76315-117, Haft Bagh Road, Mahan, Kerman, Iran. Tel.: +98 3426226611 13; Fax: +98 3426226617. emosaddegh@gmail.com (Elaheh Mosaddegh)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.

Abstract
The synthesis of 4,4′-(arylmethylene)bis(3-methyl-1-phenyl-1H-pyrazol-5-ol) was performed effectively by the reaction of aryl aldehydes and 1-phenyl-3-methyl-5-pyrazolone in the presence of a catalytic amount of Ce(SO4)2.4H2O as reusable and environmentally friendly catalyst in water/ethanol solution within 5–25 min in 80–98% yields. All of the obtained compounds were characterized by FT IR, 1H and 13C NMR. The method has the advantages of high yields, short reaction time, simple work-up and reusability of catalyst.
Keywords
4,4′-(Arylmethylene)bis(3-methyl-1-phenyl-1H-pyrazol-5-ol)
1-Phenyl-3-methyl-5-pyrazolone
Heterogeneous catalyst
Multicomponent reaction
Ce(SO4)2.4H2O
1 Introduction
Nitrogen heterocycles such as pyrazoles and pyrazolone are of special interest because they constitute an important class of natural and unnatural products. Compounds containing these ring systems are known to display diverse pharmacological activities such as antimicrobial (Boyne et al., 2006), antifungal (Tanitame et al., 2004), anti-inflammatory, analgesic, antipyretic (Tsurumi et al., 1976) herbicidal (Vicentini et al., 2005; Waldrep et al., 1990), and also unique electrical and optical properties (Hu et al., 2007).
Nowadays, the pyrazolone derivatives were paid much attention for their various biological activities such as antitumor (Park et al., 2005; Clark et al., 2004), selective COX-2 inhibitory (Cho et al., 2004), cytokine inhibitors (Clark et al., 2005), agrochemicals, dyes and pigments. Moreover, they are capable of prototropic tautomerism (Akama et al., 1996). The compounds that contain two pyrazolone rings can be used as extractant for some metal ions (Takeishi et al., 2001) and ligands (Abdel-Latif, 1355; Pettinari et al., 2001). 2,4-Dihydro-3H-pyrazol-3-one derivatives including 4,4′-(arylmethylene)bis(3-methyl-1-phenyl-1H-pyrazol-5-ols) are being used as gastric secretion stimulatory (Rosiere and Grossman, 1951) antidepressant (Bailey et al., 1985), antibacterial (Mahajan et al., 1991) and antifilarial agents (Chauhan et al., 1993). Moreover, the corresponding 4,4′-(arylmethylene)bis(1H-pyrazol-5-ols) are applied as fungicides (Singh and Singh, 1991), pesticides (Londershausen, 1996), insecticides (Lubs, 1970) and dyestuffs (Uzoukwu, 1993; Maurya et al., 1997; Garnovskii et al., 2004). Due to the possible importance of these compounds and our interest in the development of heterocycle-based compounds (Mosaddegh and Islami, 2008), in this study, we wish to report the synthesis of some heterocycle-based chromophores based on pyrazolone.
The conventional chemical approach to 4,4′-(arylmethylene)bis(3-methyl-1-phenyl-pyrazol-5-ols) involves the successive Knoevenagel synthesis of the corresponding arylidenepyrazolones and their base promoted Michael reaction and also the one-pot tandem Knoevenagel–Michael reaction of aryl aldehydes with two equivalents of 5-methyl-2-phenyl-2,4-dihydro-3Hpyrazol-3-one performed under a variety of reaction conditions (Hamama, 2001; Li et al., 1998). The first set of procedures utilizes the catalysis of the components with piperidine in ethanolic solution (Singh and Singh, 1984; Mitra and Rout, 1961). The second set of methods involves the noncatalyzed tandem Knoevenagel–Michael reaction under neutral conditions in either ethanol (Pavlov et al., 1998) or benzene (Buzykin et al., 2224) solutions. Although it affords the corresponding 4,4′-(arylmethylene)bis(1H-pyrazol-5-ols) in reliable 70–90% yields, the reaction requires 3–12 h of initial reflux with further 24 h under ambient temperature to go to completion. Later, Li and co-worker reported the solid-state synthesis of these compounds (Li et al., 1903) and Bai et al. employed the microwave irradiation to promote the solvent-free synthesis of these compounds (Bai et al., 2004). Finally, Shi et al. (2005) and Wang et al. (2005) reported its synthesis using triethylbenzylammonium chloride (TEBA) Shi et al., 2005 and sodium dodecyl sulfate (SDS) Wang et al., 2005 as the surfactant catalyst over one hour. Further, Elinson et al. utilized the electrocatalytic procedure for its synthesis (Elinson et al., 2008). In other works these compounds were synthesized by cerium ammonium nitrate (CAN, 5 mol%) Sujatha et al., 2009, sulfuric acid ([3-(3-silicapropyl)sulfanyl]propyl)ester (0.1 g), Tayebi et al., 2011 sipmim]HSO4 (0.15 g) Baghernejad and Niknam, 2012, Silica-bonded N-propylpiperazine sulfamic acid (0.07 g) Tayebi et al., 2012, palladium nanoparticles (Saha et al., 2012), NaBr (Elinson et al., 2008) and 1,3,5-tris(hydrogensulfato) benzene (Karimi-Jaberi et al., 2012). However, there are disadvantages to these mentioned methods such as low yield, prolonged reaction time and use of toxic organic solvents, or tedious workup procedures or catalyst preparation. Thus, a search for new reagents and the development of new methods are still of practical importance. The greatly enhanced reactivity of cerium (IV) sulfate (Ce(SO4)2.4H2O) led to its emergence as a promising Lewis acid catalyst. Ce(SO4)2.4H2O is a moisture-stable compound, easy to handle, availability and with antibacterial activity in low concentration (Tayebi et al., 2011; Baghernejad and Niknam, 2012; Tayebi et al., 2012).
2 Experimental
Melting points were determined on a Gallenkamp melting point apparatus and are uncorrected. NMR spectra were recorded at 500 (1H) and 125.77 (13C) MHz on a Bruker DRX-500 Avance spectrometer. All compounds were known in the literature, the NMR and IR spectra of the products were in agreement with earlier data (Li et al., 1903; Bai et al., 2004; Shi et al., 2005; Wang et al., 2005; Elinson et al., 2008; Sujatha et al., 2009; Tayebi et al., 2011; Baghernejad and Niknam, 2012; Tayebi et al., 2012; Saha et al., 2012; Elinson et al., 2008; Karimi-Jaberi et al., 2012).
2.1 General procedure
In a typical general procedure, a mixture of aromatic aldehyde (1 mmol) and 1-phenyl-3-methyl-5-pyrazolone (2 mmol) in H2O–EtOH (1:1, 5 mL) at reflux condition, was stirred thoroughly in the presence of a catalytic amount of Ce(SO4)2.4H2O (10 mg, 2.5 mol%) to afford 4,4′-(arylmethylene)bis(3-methyl-1-phenyl-pyrazol-5-ols) in excellent yields. After completion of the reaction which is confirmed by T.L.C, the mixture was filtered. The solid product was washed with H2O and finally was recrystallized from ethanol. The structures of the products were confirmed from physical and spectroscopic data such as melting points, IR and 1H NMR spectra.
2.2 4,4′-[(2,4-Dichlorophenyl)methylene]bis(3-methyl-1-phenyl-1H-pyrazol-5-ol)
IR (KBr) νmax/cm−1 3438 (OH), 2927, 1600, 1574 (C⚌C); 1H NMR (500 MHz; DMSO; Me4Si): δ 2.30 (s, 6H, CH3), 5.10 (s, 1H, CH), 7.22–7.76 (m, 14H, Haromatic), 12.58 (s, 1H, OH), 13.81 (s, 1H, OH); 13C NMR (125.13 MHz; DMSO; Me4Si); δ: 11.73, 33.15, 120.53, 137.14, 138.86, 145.98, 145.90.
2.3 4,4′-[(Phenyl methylene)bis(3-methyl-1-phenyl-1H-pyrazol-5-ol)
IR (KBr) νmax/cm−1: 3361(OH), 2927, 1625, 1574 (C⚌C); 1H NMR (500 MHz; DMSO; Me4Si): δ 2.49 (s, 6H, CH3), 4.94 (s, 1H, CH), 7.15–7.70 (m, 14H, Haromatic), 12.44 (s, 1H, OH), 13.98 (s, 1H, OH); 13C NMR (125.13 MHz; DMSO; Me4Si); δ: 11.56, 33.14, 120.46, 125.43, 125.78, 127.10, 128.03, 128.13, 142.28, 146.21.
2.4 4,4′-[(4-Chlorophenyl)methylene]bis(3-methyl-1-phenyl-1H-pyrazol-5-ol)
IR (KBr) νmax/cm−1: 3463 (OH), 2927, 1625, 1574 (C⚌C); 1H NMR (500 MHz; DMSO; Me4Si); δ 2.30 (s, 6H, CH3), 4.95 (s, 1H, CH), 7.22–7.70 (m, 14H, Haromatic), 12.50 (s, 1H, OH), 13.87 (s, 1H, OH); 13C NMR (125.13 MHz; DMSO; Me4Si); δ: 11.50, 32.56, 120.47, 125.51, 127.81, 128.81, 129.04, 130.47, 137.33, 141.11, 146.15.
2.5 4,4′-[(4-Methylphenyl)methylene]bis(3-methyl-1-phenyl-1H-pyrazol-5-ol)
IR (KBr) νmax/cm−1: 3432(OH), 2921, 1600, 1501, 1408, 1294, 1026; 1H NMR (500 MHz; DMSO; Me4Si); δ 2.21 (s, 3H, CH3), 2.28 (s, 6H, CH3), 4.87 (s, 1H), 7.03–7.67 (m, 14H, Haromatic); 13C NMR (125.13 MHz; DMSO; Me4Si); δ 12.2, 21.1, 33.3, 121.0, 122.8, 124.7, 125.1, 126.1, 127.6, 129.2, 129.5, 135.3, 139.7, 146.8.
2.6 4,4′-[(4-Methoxyphenyl)methylene]bis(3-methyl-1-phen-yl-1H-pyrazol-5-ol)
IR (KBr) νmax/cm−1: 3060(OH), 2920, 2836, 1604, 1580, 1404, 1252, 1036, 752, 692; 1H NMR (500 MHz; DMSO; Me4Si); δ 2.12 (s, 6 H, 2 CH3), 3.72 (s, 3 H, OCH3), 4.73 (s, 1 H, CH), 6.77–7.28 (m, 14H, Haromatic); 13C NMR (125.13 MHz; DMSO; Me4Si); δ 11.6, 32.4, 54.9, 105.0, 113.5, 120.4, 125.4, 128.1, 128.9, 129.2, 134.6, 137.9, 146.1, 157.5.
3 Results and discussion
In continuation of our interest on the application of heterogeneous catalysts for the development of a useful synthetic methodology (Mosaddegh and Hassankhani, 2011; Mosaddegh et al., 2010; Mosaddegh and Hassankhani, 2013), we wish to report herein a simple and highly efficient procedure for the preparation of 4,4′-(arylmethylene)bis(3-methyl-1-phenyl-pyrazol-5-ols) derivatives via a one-pot tandem Knoevonagel–Michael reaction using Ce(SO4)2.4H2O (2.5 mol%) as environmentally friendly mild Lewis acid catalyst with high catalytic activity and reusability in H2O–EtOH media (Scheme 1).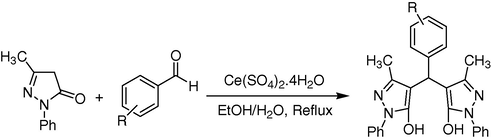
Synthesis of 4,4′-(arylmethylene)bis(3-methyl-1-phenyl-1H-pyrazol-5-ol) using cerium (IV) sulfate.
In our initial endeavor, we carried out the reaction of 1-phenyl-3-methyl-5-pyrazolone (2 eq) with aromatic aldehyde (1 eq) using 2.5 mol% of cerium (IV) sulfate in a mixture of water–ethanol (1:1) as solvent at reflux condition. The reaction proceeded to completion within 5–25 min. With these optimistic results in hand, further investigation was carried out for the catalytic evaluation of cerium (IV) sulfate for the optimum reaction conditions. The increase in the amount of cerium (IV) sulfate up to 10 mol% did not show much difference in terms of yield or reaction time. However in the absence of cerium (IV) sulfate, only 70% of the product was obtained even after stirring for 24 h. We found decreasing of temperature to 50 °C leads to yield of 77%. In order to optimize the conditions, we refluxed this reaction. An increase in temperature leads to increasing product yields and rate of the reaction about 93% at 10 min. To show that Ce(SO4)2.4H2O is an efficient catalyst, we accomplished the reaction at 50 °C in the absence of catalyst for 3 h. The reaction just produced the product in 75% yield (Table 1). This proves the essential effect of Ce(SO4)2.4H2O as a mild Lewis acid catalyst on the progress of the reaction.
Entry
Catalyst
Condition
Time
Yield (%)
1
–
r.t
24 h
70
2
–
50 °C
3 h
75
3
0.1 g
50 °C
1 h
77
4
0.1 g
Reflux
10 min
93
A range of aromatic aldehydes was subjected to react with 3-methyl-5-pyrazolones in the presence of 2.5 mol% of Ce(SO4)2.4H2O and H2O–EtOH as solvent (Table 1). 4,4′-(arylmethylene)bis(3-methyl-1-phenyl-pyrazol-5-ols) derivatives containing electron-withdrawing groups such as nitro and halide groups or electron-donating groups such as hydroxyl and alkoxy groups (Table 2) were formed in a short experimental time (5–25 min) with high yields (80%–98%).
Entry
Ar
Time (min)
Yields (%)a
mp (°C)
1
C6H5
25
81
170–172
2
4-ClC6H4
10
93
213–215
3
2,4-Cl2C6H3
7
96
229–231
4
3-NO2-C6H4
5
98
150–152
5
4-BrC6H4
5
92
177–180
6
4-CH3C6H4
20
80
203–205
7
3-CH3C6H5
12
97
196–197
8
4-CH3OC6H5
17
95
142–145
9
3-OHC6H4
8
91
166–168
10
2,4-(OCH3)2-C6H4
15
94
164–167
A reasonable mechanism for the formation of 1–10 is proposed in Scheme 2. The first step involves the formation of benzylidene III by the nucleophilic addition of 1-phenyl-3-methyl-5-pyrazolone II to aromatic aldehyde I followed by dehydration. Then, the second molecule of 1-phenyl-3-methyl-5-pyrazolone II is added to intermediate III by the Michael addition fashion to give 4,4′-(arylmethylene)bis(3-methyl-1-phenyl-pyrazol-5-ols) 1–10 (Scheme 2).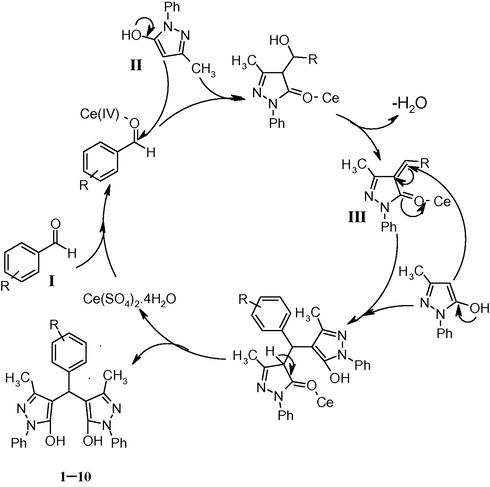
Proposed mechanism for the formation of 4,4′-(arylmethylene)bis(3-methyl-1-phenyl-pyrazol-5-ols).
The reusability of the catalysts is one of the most important benefits and makes them useful for commercial applications. Thus the recovery and reusability of Ce(SO4)2.4H2O were investigated. In these experiments, the reaction mixture was filtered and washed with H2O. The soluble catalyst was easily reused after distillation of solvent, washing with CHCl3 and drying at 60 °C. The recycled catalyst has been examined in the next run in the reaction between 2,4-dichlorobenzaldehyde and 1-phenyl-3-methyl-5-pyrazolone. The Ce(SO4)2.4H2O catalyst could be reused three times without any loss of its activity.
4 Conclusion
In conclusion, the present method is an operationally simple, cleaner and highly efficient procedure for the synthesis of compound III using a catalytic amount of Ce(SO4)2.4H2O. In addition low cost, easy availability, recyclability, moderate Lewis acidity and moisture compatibility of the catalyst, excellent yields of products, short reaction time, simple experimental and isolation procedures make this methodology a valid contribution to the existing processes in the field of 4,4′-(arylmethylene)bis(3-methyl-1-phenyl-pyrazol-5-ol) derivatives synthesis.
References
- Abdel-Latif, S. A., Synth. React. Inorg. Met.-Org. Chem. 8, 1355 (2001).
- Akama, Y., and Tong, A., Microchem.l J. 53, 34, (1996).
- Int. J. Chem.. 2012;4:52.
- Chin. J. Org. Chem.. 2004;6:616. (in Chinese)
- J. Med. Chem.. 1985;28:256.
- ACS Chem. Biol.. 2006;1:43.
- Buzykin, B. I., and Lonshchakova, T. I., Bull. Acad. Sci., USSR Div. Chem. Sci. (Engl. Transl.) 2224 (1971).
- Indian J. Chem. Sect. B.. 1993;32:858.
- Cho, I. H., Noh, J. Y., Park, S. W., Ryu, H. C., Lim, J. W., Kim, J. H., Chae, M. Y., Kim, D. H., Jung, S. H., Park, H. J., Kim, Y. H., and Min, I. K., US Patent. 2, 004, 002, 532 (2004).
- Med. Chem.. 2004;11:2724.
- Clark, M. P., Laughlin, S. K., Golebiowski, A., Brugel, T. A., and Sabat, M., WO Patent. 2, 005, 047, 287 (2005).
- Synthesis. 2008;12:1933.
- Synthesis. 2008;12:1933.
- Garnovskii, A. D., Uraev, A. I., Minkin, V. I., 2004. Arkivoc, (iii), 29, 23.
- Synth. Commun.. 2001;31:1335.
- J. Mol. Struct.. 2007;839:50.
- Chin. J. Catal.. 2012;33:1945.
- Li, X. -L., Ma, H., Wang, Y. -M., Meng, J. -B., Chem. J. Chin. Univ. 12, 1903 (1995) (in Chinese).
- J. Heterocycl. Chem.. 1998;35:129.
- Pestic. Sci.. 1996;48:269.
- The Chemistry of Synthetic Dyes and Pigments. Washington DC: American Chemical Society; 1970.
- J. Indian Chem. Soc.. 1991;68:245.
- Indian J. Chem. Sect. A. 1997;36:596.
- J. Indian Chem. Soc.. 1961;38:893.
- Tetrahedron Lett.. 2011;52:488.
- Catal. Commun.. 2013;33:70-75.
- Org. Prep. Proc. Int.. 2008;40:586.
- Mosaddegh, E., Hassankhani, A., and Baghizadeh, A., J. Chil. Chem. Soc. 55, N 4 (2010).
- Bioorg. Med. Chem. Lett.. 2005;15:3307.
- Pavlov, P. T., Goleneva, A. F., Lesnov, A. E., Prokhorova, T. S., Pharm. Chem. J. (Engl. Transl.) 32, 370 (1998).
- J. Chem. Soc. Dalton Trans.. 2001;11:1790.
- Science. 1951;113:651.
- RSC Adv.. 2012;2:6397.
- Chin. J. Org. Chem.. 2005;4:405.
- J. Chem. Eng. Data. 1984;29:355.
- J. Indian Chem. Soc.. 1991;68:165.
- Bioorg. Med. Chem. Lett.. 2009;19:3611.
- Anal. Chim. Acta. 2001;1:69.
- J. Med. Chem.. 2004;47:3693.
- Chin. J. Catal.. 2011;32:1477.
- Iran J. Catal. 2012;2:69.
- Folia Pharmacologica Japonica. 1976;72:41.
- Polyhedron. 1993;12:2719.
- Agric. Food Chem.. 2005;53:3848.
- Waldrep, T. W., Beck, J. R., Lynch, M. P., Wright, F. L. J., 1990. Agric. Food Chem. 38, 541, 47, 3367 (2004).
- Synth. Commun.. 2005;9:1263.







