Translate this page into:
Novel nanofiltration membrane with low concentration of polyvinylchloride: Investigation of solvents’ mixing ratio effect (Dimethyl acetamide/Tetrahydrofuran)
⁎Corresponding author. Tel.: +98 86 32625422; fax: +98 86 32625423. A.Moghadassi@Gmail.com (A.R. Moghadassi) A.Moghadassi@Araku.ac.ir (A.R. Moghadassi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.

Abstract
In the current research polyvinylchloride (PVC) nanofiltration membrane was prepared using Dimethyl acetamide (DMAC)/Tetrahydrofuran (THF) as solvents via the phase inversion method. The effect of solvents’ mixing ratio (DMAC to THF) in the casting solution and also phase separation time in coagulation bath on membrane flux and selectivity were studied. The membrane tensile strength measurement and scanning electron microscope (SEM) analysis were also carried out in membrane characterization. The highest membrane selectivity and flux were found at (85:15) solvents’ mixing ratio (DMAC to THF).
Keywords
Nanofiltration
Polyvinylchloride
Solvent ratio effect
Dimethyl acetamide/Tetrahydrofuran
Selectivity/flux
1 Introduction
During the past years a high level of total hardness, sulfates, chlorides and bacteria in ground waters has been observed in the world (Butarewicz and Szczykowska, 2004; Jeó-Walkowiak and Sozaski, 2004; Puszkarewicz and Kaleta, 2004). Excessive increases in calcium, magnesium and sulfates derive from gypsum dissolution and ionic fertilizers (Regulations of the Polish Minister of Health, 2002). For removal of pollutants from groundwater nanofiltration (NF) seems to be a suitable method as a process capable of removing hardness, sulfates, chlorides and bacteria in one step (Mulder, 1996; Rautenbach, 1996; Van der Bruggen and Vandecasteele, 2003). For preparation of polymeric nanofiltration membranes, the exchanging of solvent and non-solvent in coagulation bath plays an important role (cheng et al., 1995). Many researchers have investigated the effect of addition of various components on membrane performance and membrane structure (King and Cantor, 1972; Hou et al., 1991; Bokhorst et al., 1981; Friedrich et al., 1981; Jianj et al., 1992; Vasarhelyi et al., 1987) Madaeni and Rahimpour studied the effect of type of solvent (DMAC, NMP and DMF) and non-solvents on morphology and performance of polysulfone and polyethersulfone ultrafiltration membranes for milk concentration (Madaeni and Rahimpour, 2005).
One of the most important parameters in membrane morphology and performance is polymer concentration. Preparation of nanofiltration membrane with low concentration of polymer (especially PVC) and effect of different concentrations of THF in casting solution via the phase inversion method have not been yet investigated in application of water treatment technology. Then the effect of solvents’ mixing ratio (THF to DMAC) in casting solution was studied. The membranes were evaluated in terms of permeate flux and Na2SO4 rejection. In addition the pure water flux (PWF), phase separation time in coagulation bath and tensile resistance of prepared membranes were measured. The scanning electron microscopy (SEM) analysis was also carried out for membrane morphological studies.
2 Experimental
2.1 Materials
Polyvinyl chloride (PVC) grade 7054 supplied by BIPC, Iran, was used as polymer binder. Dimethyl acetamide (DMAC) and Tetrahydrofuran (THF) were purchased from Merck and Dae-Jung Co. (DMAC Mw = 87.12 and THF Mw = 72.11, purity = above 99%). Distilled water was used as non-solvent (coagulation bath). Sodium sulfate (Na2SO4) (Mw = 142.04 g mol−1) from Merck was used for removal by prepared NF membranes.
2.2 Membrane preparation
Different concentrations of PVC/DMAC: THF solutions with constant concentration of PVC (12% wt) were prepared. The compositions of all prepared NF membranes have been presented in Table 1. All the membranes were prepared by the casting solution technique and the phase inversion method. For preparation of membranes, at first, homogenous solutions were prepared in DMAC: THF with different ratios (100:0, 85:15, 70:30 and 55:45) by stirrer at 7000 rpm (Multi magnetic stirrer, velp scientific model, S/N 172203, made in Europe) for 3 h. After that, they were transferred to ultrasonic cleaner for 45 min at 20 °C (Parsonic11Smodel, S/N PN-88159, made in Iran) for removing dissolved air in solutions. The obtained homogeneous solutions were casted onto glass plates by using casting knife with constant thickness of 150 μm. Then, prepared casting films were dipped into deionized water as non-solvent (after 60 s as evaporation time in air). In this step exchanging of solvent and non-solvent leads to membrane formation. After completing phase separation step and finishing membrane formation, the membranes were kept in a new container with fresh deionized water for one day to remove remaining solvent in membrane structure. Then the membranes were dried between two paper sheets at room temperature (25 ± 2 °C) for one day before testing.
Membrane
Polymer binder (PVC, %wt)
Solvent (DMAC:THF)
M1
12
100:0
M2
12
85:15
M3
12
70:30
M4
12
55:45
2.3 Characterization of the membranes
2.3.1 Flux and rejection
For measuring permeation flux and rejection of the prepared nanofiltration membranes, the filtration stirred dead end cell with specification of outer diameter 5 cm, inner diameter 4.5 and effective filtration area about 11.94 cm2 was used. The schematic diagram of the dead end filtration system is shown in Fig. 1. Circular membrane samples were cut with a surgery knife and placed into the cell with membrane top layer in contact feed. Then prepared membranes were initially pressurized with deionized water at operating pressure of 5 bars for about 15 min to compaction. After compaction, the operating pressure was reduced to 4 bars. Permeability flux and pure water flux (PWF) were measured by passed distillated water collection from membranes at operating pressure 4 bars and determined from the below expression (Han et al., 2009):
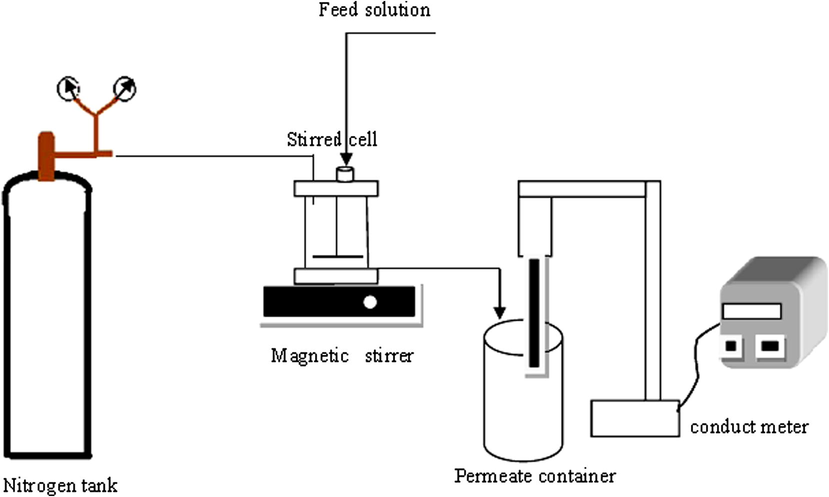
The Nanofiltration experimental setup.
The salt rejection was calculated from the following equation (Lee et al., 2008):
Where Cp and Cf are the Na2SO4 concentration in permeate and feed, respectively that were analyzed by conductivity meter (Ohaus Corporation, S/N B143385306, USA). The feed concentration was 1000 mg/l.
2.3.2 Scanning electron microscopy (SEM)
The SEM cross section of the prepared nanofiltration membrane with the optimum ratio of DMAC: THF and pure DMAC as solvents was examined at 25KV (KYKY-EM3200, SN:0198). For providing of membranes to examine with SEM, the membranes were broken in N2 liquid after 5 min and then they were sputtered with gold.
2.3.3 Tensile strength measurement
The tensile strength as a mechanical property of the prepared membranes was tested according to ASTM1922-03. All samples were cut into the standard shape in the ambient conditions before testing. For each test, three samples were used and their average values were reported.
3 Results and discussion
3.1 The effect of solvents’ mixing ratio on membrane flux and selectivity
Miscibility or solubility parameter between solvent and non-solvent is one of the most important parameters in membrane formation. If miscibility between solvent and non-solvent is high (difference of solubility parameter between solvent and non-solvent is low), the non-solvent is more able to diffuse in casting film and in this case, solvent leaves casting solution faster. Therefore exchanging of solvent and non-solvent in coagulation bath happens faster and consequently, instantaneous demixing happens. This leads to formation of porous top layer and finger like pores in support. Also formation of dense top layer is due to low miscibility of solvent and non-solvent (Madaeni and Rahimpour, 2005). The values of solubility parameters of DMAC, THF and different ratio of DMAC: THF as mixing solvents and water as non-solvent have been shown in Table 2. It should be said, solubility parameters for mixing solvents were calculated from average of solubility parameters between DMAC and THF with their composition. It can be seen from Table 2, difference between solubility parameter of DMAC and water is the lowest and opposite it, difference between solubility parameter of THF and water is the highest. In fact, in the current work THF was used for increasing phase separation time in coagulation bath and preventing macro voids and porous top layer formation and making dense top layer for preventing salt entrance.
Solvent/non-solvent
δ (solubility parameter)
Refs.
DMAC
11.13
Madaeni and Rahimpour, 2005
THF
11.44
Belmares et al., 2004
DMAC:THF (85:15)
11.17
Cal
DMAC:THF (70:30)
11.223
Cal
DMAC:THF (55:45)
11.2695
Cal
Water
9.4
Madaeni and Rahimpour, 2005
The results for permeability flux of prepared membranes with PVC and constant concentration of 12% wt versus different ratios of DMAC: THF as mixing solvents are shown in Fig. 2. As shown in Fig. 2, membrane containing pure DMAC as solvent has more flux than other prepared membranes. Results indicate that the permeability flux of prepared membranes is decreased by employing THF. It is seen that increasing of THF concentration in casting solution leads to decreasing of flux. Also at (55:45) solvent ratio (DMAC:THF) flux declines sharply (near zero) which is due to increasing of phase separation time in coagulation bath (delay demixing). There are the same results for PWF for prepared NF membranes (Fig. 3). The lower flux for the membrane at 1000 mg salt concentration compared to PWF is related to formation of cake layer in membrane surface that is described elsewhere (Nghiem and Schäfer, 2006).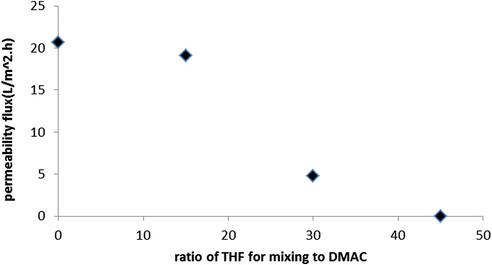
Permeability flux versus different ratios of solvents (DMAC:THF).
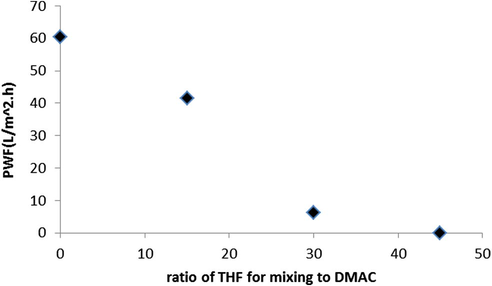
PWF versus different ratios of solvents (DMAC:THF).
The experimental results for the salt rejection of NF membranes versus different ratios of THF in casting solution are given in Fig. 4. As shown in this figure, salt rejection of membranes was increased by increase of THF ratio in casting solution because of phase separation time increasing in coagulation bath which makes dense structure with thick top layer for the membranes (Van den Boomgaard, 1998).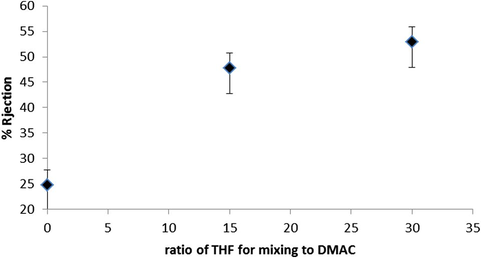
Salt rejection versus different ratios of used solvents.
The phase separation time of casting film (in deionized water as coagulation bath) versus different ratios of DMAC:THF is shown in Fig. 5. As shown in this figure, the phase separation time increased by an increase of THF ratio in casting solution which leads to formation of dense structure for the membrane with a denser and thicker top layer. The results confirm that increasing of THF with different concentration leads to reducing permeability flux and PWF and increasing % Na2SO4 rejection.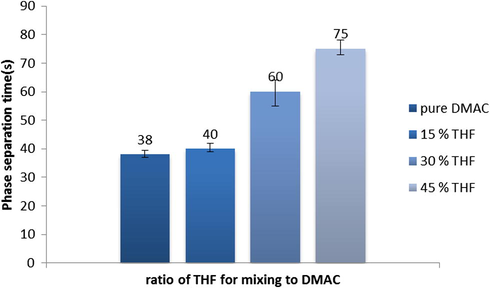
Effect of solvents’ mixing ratio on phase separation time in coagulation bath.
The obtained results revealed that modified membrane with (85:15) solvent ratio (DMAC:THF) showed good performance (flux and salt rejection) compared to others.
Figs. 6 and 7 show relationship between phase separation time in coagulation bath and flux and salt rejection of prepared membranes respectively. As seen in these figures by an increase of phase separation time of casting film in coagulation bath, the flux decreases and salt rejection increases.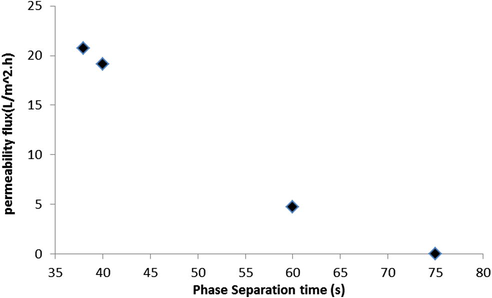
Relationship between phase separation time in coagulation bath and flux.
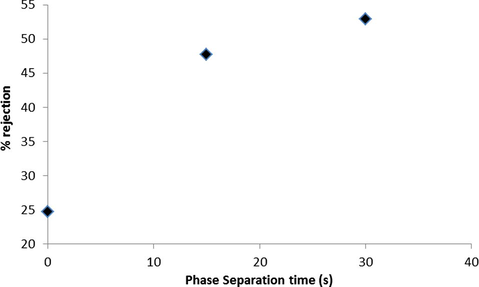
Relationship between phase separation time in coagulation bath and salt rejection.
3.2 Scanning electron microscopy
To obtain a better effect of THF addition to casting solution on membrane structure, SEM cross section (Fig. 8) of the membranes was prepared from pristine membrane and modified type (optimum ratio of mixing DMAC:THF (85:15)). As seen in the SEM images, both membranes have assymetric structures with dense top layer and finger like porous sublayer. Also membrane with pure DMAC has porous and thin top layer and its support has high porosity (Fig. 8a). Addition of THF to casting solution with a ratio of 85:15 (DMAC:THF) results in formation of denser and thicker top layer in comparison of alone DMAC as solvent (Fig. 8b). As shown in Table 2 (solubility parameter), difference of solubility parameter between THF and water is significant. Then the presence of THF in casting solution decreases micibility of solvent (mixing DMAC:THF) and non solvent (dionised water) and leads to an increase in phase separation time in coagulation bath and consequently formation of membrane with denser toplayer. In this situation, permeability and PWF decrease and salt rejection increases.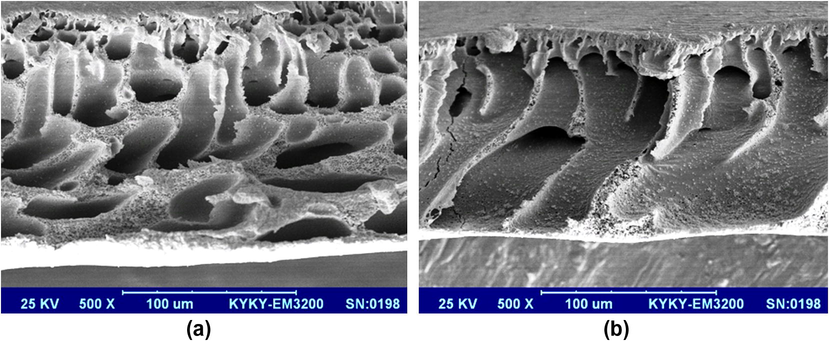
SEM Cross sectional images of (a) PVC/DMAC pure, (b) PVC/DMAC:THF (85:15).
3.3 Effect of solvents’ mixing ratios on membrane tensile resistance
The effect of solvents’ mixing ratios in the casting solution on membrane tensile resistance is shown in Fig. 9. The results incicate that addition of solvents with low miscibility to non-solvent in casting solution (with increasing of phase separation time in coagulation bath and happening of delay demixing) increases membrane mechanical resistance due to formation of denser structure. As shown in this figure, the lowest resistance versuse tensile is related to prepared membrane with pure DMAC which has the lowest phase separation time in coagulation bath and consequently the most porosity.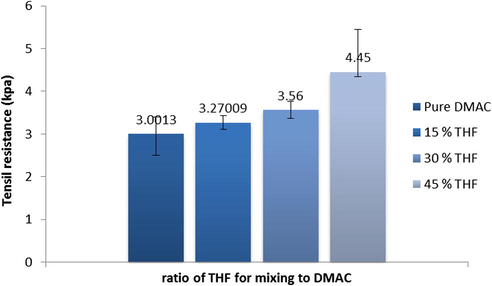
Effect of different ratios of solvents (DMAC:THF) on membrane tensile resistance.
4 Conclusions
In this paper, PVC nanofiltration membrane with low concentration of polymer (12% wt) was prepared with pure DMAC and different ratios of DMAC:THF as mixing solvents (85:15, 70:30 and 55:45) to investigate the effect of addition of THF with low micibility to non-solvent (deionized water as coagulation bath). The results of experiments confirm that addition of THF to casting solution leads to decreasing of permeability and PWF and increasing of % salt rejection. In addition it leads to increasing of phase separation time of casting film in coagulation bath and membrane tensile resistance too. The optimum ratio of DMAC:THF was found to be 85:15 in terms of permeability flux, PWF, % salt rejection and phase separation time. The SEM cross sectional of PVC membrane prepared from mixing of DMAC:THF with optimum ratio (85:15), indicated the denser and thicker top layer in comparison if pure DMAC.
Acknowledgment
The authors gratefully acknowledge Arak University for the financial support during this research.
References
- Hildebrand and hansen solubility parameters from molecular dynamics with applications to electronic nose polymer sensors. J. Comput. Chem.. 2004;25(15)
- [Google Scholar]
- Formation of asymmetric cellulose acetate membranes. Desalination. 1981;38:349-360.
- [Google Scholar]
- A. Butarewicz, J. Szczykowska, 6th International Scientific and Technical Conference, Water Supply and Water Quality, Pozna, 2004, p. 257.
- Membrane formation by isothermal precipitation in polyamide-formic acid-water systems. J. Polym. Sci.. 1995;33:323-325.
- [Google Scholar]
- Asymmetric reverse osmosis and ultrafiltration membranes prepared from sulfonated polysulfone. Desalination. 1981;36:39.
- [Google Scholar]
- Effect of NaA zeolite particle addition on poly(phthalazinone ether sulfone ketone) composite ultrafiltration (UF) membrane performance. J. Membr. Sci.. 2009;345:5-12.
- [Google Scholar]
- The study of mechanism of organic additives action in the polysulfone membrane casting solution. Desalination. 1991;83:343.
- [Google Scholar]
- J. Jeó-Walkowiak, M. Sozaski, 6th International Scientific and Technical Conference, Water Supply and Water Quality, Pozna, 2004, p. 107.
- The nucleation of organic additives in membrane casting (I) (The theory and direct evidence) Desalination. 1992;85:297-320.
- [Google Scholar]
- King WM, Cantor PA. Reverse osmosis and process and composition for manufacturing cellulose acetate membranes wherein the swelling agent is a di or tri basic aliphatic acid. US Patent, 3,673,084, 1972.
- Polyamide thin-film nanofiltration membranes containing TiO2 nanoparticles. Desalination. 2008;219:48-56.
- [Google Scholar]
- Effect of type of solvent and non-solvents on morphology and performance of polysulfone and polyethersulfone ultrafiltration membranes for milk concentration. Polym. Adv. Technol.. 2005;16:717-724.
- [Google Scholar]
- Basic Principles of Membrane Technology (2nd ed.). Dordrecht, The Netherlands: Kluwer Academic; 1996.
- Critical risk points of nano filtration and reverse osmosis processes in water recycling applications. Desalination. 2006;187:303-312.
- [Google Scholar]
- A. Puszkarewicz, J. Kaleta, 6th International Scientific and Technical Conference, Water Supply and Water Quality, Pozna, 2004, p. 747.
- R. Rautenbach, Membrane Processes, WNT, 1996.
- Regulations of the Polish Minister of Health, Dz.U.No. 203, 19.11.2002, Point 1718, App. 1. Microbiological requirements, App. 2, Physicochemical requirements.
- Van den Boomgaard A. 1998. Membrane formation by phase inversion in multi component polymer system. Ph.D. Thesis, University of Twente, Enschede.
- Environ. Poll.. 2003;122:435.
- Development of wet–dry reversible reverse osmosis membranes with high performance from cellulose acetate and cellulose triacetate blends. Desalination. 1987;61:211.
- [Google Scholar]







