Translate this page into:
Exploring the antidepressant potential of Ruta chalepensis essential oil in socially isolated rats: Behavioural and neurochemical insights
* Corresponding author: E-mail address: hsalem@ksu.edu.sa (H. Attia)
-
Received: ,
Accepted: ,
Abstract
Depression is a psychiatric disorder that affects people globally, harming overall health. This study assessed the antidepressant effects of ruta oil (RO), obtained from Ruta chalepensis, using a depression model induced by social isolation (SI) for 30 days. Rats were divided into the following groups: normal control (4 rats per cage), RO control (400 mg/kg, 4 rats per cage), SI model (one rat per cage), SI+RO (200 mg/kg), SI+RO (400 mg/kg) and SI+fluoxetine (standard anti-depressant drug, 200 mg/kg). Behavioural alterations in rats were examined using the forced swim test (FST), open field test (OFT), and the sucrose preference test. Levels of lipid peroxidation, dopamine (DOP) and serotonin (SER), as well as the brain-derived neurotrophic factor (BDNF) levels in the hippocampus were quantified using enzyme-linked immunosorbent assay. Histological examination was performed using hematoxylin and eosin. SI results in the following behavior changes; suppressed locomotor activity (reflected by a decreased number of crossings in the OFT and an increase in immobility time in the FST), reduced exploratory behavior (indicated by a decreased frequency of rearing in OFT) and anhedonia (indicated by decreased sucrose preference). The hippocampal levels of DOP, SER, and BDNF levels were attenuated, while lipid peroxidation was elevated in socially isolated rats. In addition, histological examination revealed shrunken and dead pyramidal neurons and degenerated granular neurons in the granule cell layer of the hippocampus. Treatment with RO at both doses significantly attenuated the behavioural changes, biochemical alterations and neuronal damage induced by SI. In conclusion, RO effectively alleviated depression by enhancing the levels of neurotransmitters and BDNF, while reducing lipid peroxidation in the hippocampal tissues of rats. This study confirms that RO can serve as a reliable source of safe, plant-derived treatment and a powerful remedy for neurological disorders, either on its own or in combination with other medications.
Keywords
Brain derived neurotropic factor
Depression
Dopamine
Ruta oil
Social isolation
Serotonin
1. Introduction
Depression is a widespread psychiatric disorder that significantly impacts both physical and mental health, often resulting in reduced productivity. It ranks as the fifth most prevalent disorder worldwide [1]. Depression arises from multiple factors contributing to its development, including social environment, personality traits, endocrine secretions, and genetics [2]. In Saudi Arabia, anxiety, stress, and depression have been common within the general population, with levels increasing during the COVID-19 lockdown [3]. The drugs classified as selective serotonin reuptake inhibitors (SSRIs) and benzodiazepines, are frequently prescribed for the treatment of depressive disorders [4]. However, long-term benzodiazepine use leads to tolerance, and abrupt discontinuation can result in withdrawal syndrome [5]. Additionally, chronic SSRI use may cause significant adverse effects, including weight gain, sexual dysfunction, sleep disturbance, osteoporosis, bleeding disorders, hyponatremia, and diabetes mellitus [6]. Consequently, there is ongoing research to discover new compounds with strong antidepressant effects but fewer side effects. Medicinal plants are a rich source of active components that exhibit antidepressant activity, such as flavonoids, lignans, phenolic acids, stilbenes, anthraquinones, and coumarins [7-10]. These bioactive compounds exhibit anti-depressive effects by acting on neuronal transmissions, serotonergic, GABAnergic, or dopaminergic pathways, or monoamine oxidase inhibition or by enhancing nerve growth [10]. These compounds could be used in the treatment of modern diseases such as central nervous system (CNS) disorders.
In this context, Saudi Arabia is home to a diverse range of flora, including numerous medicinal herbs [11]. Ruta chalepensis, belonging to the Rutaceae family, is considered a valuable medicinal plant that is widely recognized in certain tropical countries and the Mediterranean region. Ruta has a broad range of pharmacological actions, including anthelmintic, antitumoral, emmenagogue, analgesic, spasmolytic, antipyretic, and anti-inflammatory ones [12]. An early study [13] demonstrated that the ethanolic extract of the aerial parts of R. chalepensis displayed anxiolytic, sedative, hypnotic, anticonvulsant, and antinociceptive effects, suggesting the depressant effect of R. chalepensis on the CNS. Essential oils are a type of secondary metabolite derived from a variety of plant families and show interesting biological activities always oriented by their composition. Our recent work [14] revealed that the essential oil extracted from R. chalepensis contains several monoterpenes (such as camphor and limonene) and coumarins (such as psoralene), which are known for their antidepressant activity [2,7,15-17]. In addition, Aati et al.’s study documented the antioxidant activity of the essential oil [14]. From the fact that oxidative stress significantly impacts depression and neurodegenerative diseases, and according to the chemical composition of Ruta oil (RO), we hypothesize that R. chalepensis essential oil could be a promising new antidepressant agent, which may offer fewer side effects and could be used independently or alongside other medications. The study will be the first to evaluate the antidepressant properties of Ruta chalepensis essential oil.
Social isolation (SI) or solitary housing is a type of chronic stress that is associated with the development of psychological conditions such as depression [18]. SI is known as a prominent model to induce a depression-like state in rats [19-21]. In this model, the lack of social interaction acts as a powerful stressor that leads to emotional and behavioral changes, alterations in neurochemical factors, and pathological changes [22-24]. Studies on rats have demonstrated that SI is a potent stressor that replicates human stress, contributing to the development of depression [25]. As a result, rats subjected to SI display symptoms that resemble human conditions like depression and anxiety [26,27]. Therefore, this model was employed in the current study to induce depression and assess the antidepressant effects of RO. The study focuses on investigating the modulating effect of RO on the behavioral, histochemical, and neurochemical changes induced by SI.
2. Materials and Methods
2.1. Plant material, extraction of essential oil, and GC/MS analysis
A total 600 g of freshly collected aerial parts of R. chalepensis were harvested from the Fayfa Mountain in Jazan Province during the plant’s flowering phase in May 2021. Dr. Rajakrishnan Rajagopal, a taxonomist at the Science College Herbarium, King Saud University (KSU), identified the plant. A voucher specimen labelled NO. 20278, was stored in the herbarium of the College of Science, KSU. The freshly harvested plants underwent hydro-distillation (HD) according to the method outlined by Aati et al. [14] Furthermore, GC/MS analysis and identification were performed using the procedures specified by Aati et al. [28]
2.2. Animals
Albinos male Wistar rats (n=48), aged between 8 and 12 weeks and weighing 180-220 g, were procured from the Animal Care Center at the College of Pharmacy, KSU. The control group rats were housed in standard polypropylene cages, with four rats per cage. For the depression model and treatment groups, rats were housed individually (one rat per cage). The animals were allowed a one-week acclimatization period before any experimental procedures began and were handled with care and compassion. They were kept at a temperature of 22°C ± 2°C, relative humidity of 50% ± 5%, a 12-hr light/12-hr dark cycle, and fed freely with access to water. The study was carried out following the guidelines established by the Research Ethics Committee and the Institutional Animal Care and Use Committee, with the research protocol receiving approval from the Research Ethics Committee at KSU (No. KSU-SE-22-71).
2.3 Induction of depression
Depression was induced through SI following previously established protocols [29-31]. All rats, except those in the control group, were placed individually in opaque polypropylene glycol cages (36.5 × 20.5 × 14.0 cm) for 30 days. Rats in control groups were housed as four per cage (37.0 × 57.0 × 19.0 cm cage) for 30 days.
2.4 Experimental design
Rats were allocated to six groups, each comprising eight rats, as detailed: (i) G1 (normal control): rats were housed in pairs (four rats per cage) and administered normal saline for 30 days; (ii) G2 (oil control): rats were housed in pairs (four per cage) and given RO (400 mg/kg/day) for 30 days via oral gavage; (iii) G3 (depression model group, SI model): rats were housed individually (one per cage) for 30 days and received normal saline; (iv) G4 and G5 (SI+RO): rats were housed individually and treated with 200 mg/kg/day and 400 mg/kg/day of RO by oral gavage for 30 days, respectively; (v) G6 (SI + fluoxetine): rats were housed individually and treated with fluoxetine (20 mg/kg/day) by oral gavage for 30 days, with fluoxetine serving as the standard antidepressant [32]. The RO doses were selected based on prior research [33,34]. Three behavioral tests were performed during the last three days of the experiment; these included open field test (OFT), forced swimming test (FST), and sucrose preference test (SPT) to assess locomotor and exploratory activities as well as anhedonia (inability to feel pleasure). At the end of the experiment, histological examination was performed to assess the neuronal damage induced by SI, and enzyme-linked immunosorbent assay (ELISA) assay was conducted to determine the levels of neurochemicals, including serotonin (SER), dopamine (DOP), and brain-derived neurotropic factor (BDNF) in the hippocampus. These chemicals are known to decrease during depression. Lipid peroxidation was also determined using ELISA kits.
2.5. Behavioral tests
2.5.1 Open-field test
The OFT is widely utilized to assess exploratory behavior and locomotor activity [35]. It is a useful, non-invasive method that can be repeated multiple times during a study [36]. The OFT was performed as described previously [37,38]. The apparatus consists of a 100 × 100 cm black square wooden box with 30 cm high walls and an open top. The floor of the arena is marked with 16 equal squares. Each individual rat was placed in the center of the arena, and 5 mins were given for exploration. During this time, the following parameters were recorded: the number of squares crossed, latency time (the time taken to start moving), and frequency of rearing (number of times the animal stood on its hind legs to explore). Locomotor activity was determined by counting the squares crossed and timing the latency, while the frequency of rearing served as a measure of exploratory behavior, indicating the rat’s curiosity to explore.
2.5.2 Forced swimming test (FST)
This experiment assesses the rats’ despair behavior. During the pretest phase, rats were placed in transparent glass cylinders with a height of 40 cm and a diameter of 28 cm. The cylinder was filled with water (30 cm) at 25 ± 1°C, and then each rat was forced to swim for 15 mins, following the protocol outlined by Bian et al. [37] Subsequently, the rats were taken out, dried, and placed back in their cages. After a 24-hr rest period, the rats were placed back in the cylinders for a 6-min test session and the immobility time was recorded for the last 4 mins. Immobility time is the period during which the rats floated with minimal limb movement, just enough to keep their head above the water.
2.5.3 Sucrose preference test (SPT)
SPT was used to test the anhedonia, a primary symptom of severe depression [39,40]. The SPT was conducted on the 28th day, as described previously [41,42]. Initially, during the first 24 hrs, two bottles containing sucrose solution (1 % w/v) were given to the animals to acclimate them to the sucrose solution. Following this, the rats were housed individually, with one bottle replaced by tap water and both filled with 150 mL of each. The rats were then exposed to both the sucrose and water for another 24 hrs. To prevent positional bias, the positions of the bottles were randomly alternated during the experiment. After 24 hrs, the volume (mL) of water and sucrose consumed was measured, and then the SPT was determined as the percentage of sucrose consumption in relation to the total liquid intake:
A lower sucrose consumption indicates a lack of pleasure (interest) and is associated with depression.
2.6. Separation of hippocampus and preparation of homogenate
After 24 hrs of completing the behavioral tests, the rats were anesthetized using carbon dioxide and then sacrificed by decapitation. The brains were quickly extracted and washed with phosphate-buffered saline (PBS. pH 7.4). The hippocampus was carefully dissected, weighed, and frozen at -80°C until further processing. The hippocampal homogenate was prepared in PBS to create a 20% solution. The homogenate was then centrifuged for 15 mins at 3000 rpm using a refrigerated centrifuge. The resulting supernatant was kept at -80°C for later investigations. A sample of the hippocampal tissue was preserved in 10% formalin for tissue examination using the hematoxylin and eosin (H&E) stain.
2.7. Histological examination
Hippocampal tissue samples were fixed for 24 hrs in formalin (10%, pH 7.4). The tissue was then dehydrated with increasing concentrations of alcohol and embedded in paraffin. The paraffin blocks were sectioned into 5-μm-thick slices and then deparaffinized, rehydrated, and stained with H&E. These sections were examined under a light microscope to evaluate structural changes and neurodegeneration.
2.8. Assay of lipid peroxidation
The levels of malondialdehyde (MDA), a marker of lipid peroxidation, in the hippocampus were quantified using the Rat Malondialdehyde ELISA Kit (Catalogue # MBS738685). For this assay, 100 µL of hippocampal tissue samples, standards, and PBS (as blank) were added to the designated wells. Then 50 µL of enzyme conjugate was added to each well, followed by mixing. The plate was sealed and incubated at 37°C for 1 hour. After five washes, a mixture of substrate A (50 µL) and substrate B (50 µL) was added to each well. The plate was then incubated in the dark for 20 mins at 37°C. As the last step, 50 µL of stop solution was added to each well, including the blank and the absorbance was measured immediately at 450 nm using a microplate reader. The MDA concentration was determined from the standard curve.
2.9. ELISA assay of monoamines and brain derived neurotrophic factor
Rat ELISA kits for measuring DOP in the hippocampus (catalog No. SEKSM-0021), SER (catalog No. SEKSM-0016), and BDNF (catalog No. SEKR-0076) were obtained from Solarbio Life Sciences (Beijing, China). The quantification of these biomarkers was carried out following the manufacturer’s instructions. Briefly, samples and standards were pipetted into wells and mixed with 50 µL of biotinylated antibody, and then the plate was incubated at 37°C for 45 mins. After four washes, 100 µL of horseradish peroxidase conjugate was added, and the plate was incubated again for 30 mins at 37°C. For color development, substrate solution (90 µL) was added, and the plate was incubated for 15 mins at 37°C. Stop solution (50 µL) was added, and the absorbance was measured at 450 nm using a microplate reader. The concentration of each sample was determined from the standard curve.
2.10. Statistical analysis
The statistical analyses were conducted using GraphPad Prism software (version 9.0) employing one-way analysis of variance (ANOVA) followed by the Tukey-Kramer test. The statistically significant result will be achieved when a p-value < 0.05.
3. Results and Discussion
3.1 GC/MS analysis results
The chemical composition of the essential oil extracted from the aerial parts of R. chalepensis was evaluated using gas chromatography/mass spectrometry (GC-MS) (Figure 1) and has been presented in Table 1. The compounds are arranged based on their elution order on the column. Hydrodistillation (HD) of the aerial parts of R. chalepensis produced a yellow essential oil with a 0.92% (v/w) yield, calculated from the fresh weight of the plant. The relative percentages of individual components were calculated based on the gas chromatography (GC) peak areas. The analysis identified 29 aromatic compounds, which together made up 95.90% of the total oil. The primary constituents included 2-Nonanone (43.00%), 2-Undecanone (30.20%), 2-Nonyl acetate (7.40%), Geijerene, 2-Undecyl acetate (1.80%), 2-Decanone (1.60%), psoralen and bergaptene (1.50%). The essential oil is predominantly composed of aliphatic ketones, with minor amounts of aliphatic alcohols and a small terpene fraction. Our analysis showed that long-chain aliphatic ketones are the dominant components, making up approximately 70.20% of the total oil. Key compounds include 2-Undecanone, which makes up 30.20% of the oil, and 2-Nonanone, accounting for 43.00%. Monoterpenes, such as terpinen-4-ol and limonene, along with small amounts of sesquiterpenes (0.40%) and diterpenes (0.30%) were also detected in the oil extracted from R. chalepensis grown in Saudi Arabia.
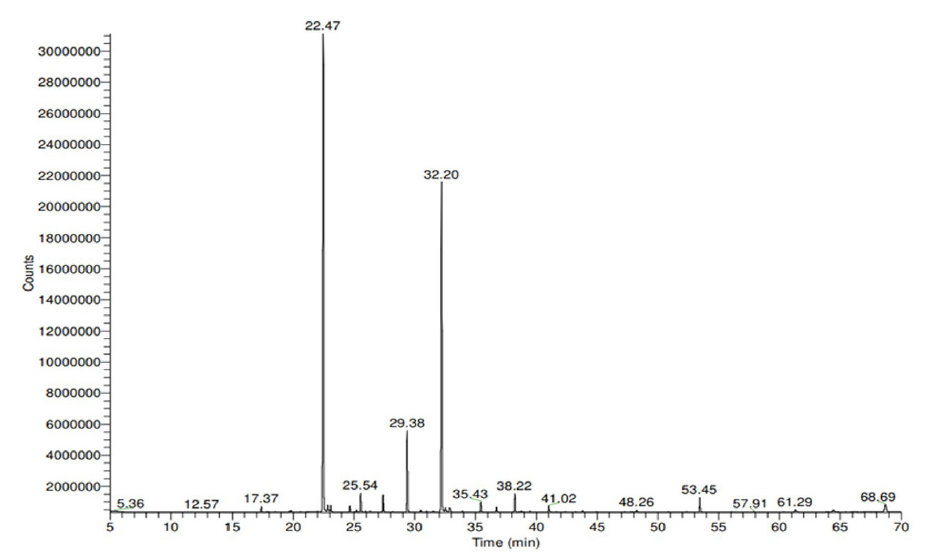
- The GC-MS chromatogram of R. chalepensis essential oil showed the main components at retention times (Rts) of 22.74, 29.38, and 32.20, which were identified as 2-Nonanone, 2-Nonyl acetate, and 2-Undecanone, respectively. GC-MS: Gas chromatography/mass spectrometry.
| # | Compound | Rt (minutes) | % Conc. |
|---|---|---|---|
| 1 | 2-Octanone | 17.37 | 0.50 |
| 2 | α-Phellandrene | 18.48 | 0.10 |
| 3 | Limonene | 19.70 | 0.10 |
| 4 | 2-Heptyl acetate | 19.82 | 0.10 |
| 5 | 2-Nonanone | 22.47 | 43.00 |
| 6 | 2-Nonanol | 22.83 | 0.70 |
| 7 | Linalool | 22.93 | 0.10 |
| 8 | Nonanal | 23.07 | 0.60 |
| 9 | 2-Octyl acetate | 24.66 | 0.60 |
| 10 | Geijerene | 25.54 | 1.80 |
| 11 | Terpinen-4-ol | 27.15 | 0.10 |
| 12 | 2-Decanone | 27.39 | 1.60 |
| 13 | Octyl acetate | 28.19 | 0.10 |
| 14 | 2-Nonyl acetate | 29.38 | 7.40 |
| 15 | Isogeijerene C | 30.97 | 0.10 |
| 16 | 2-Undecanone | 32.20 | 30.20 |
| 17 | 2-Undecanol | 32.51 | 0.50 |
| 18 | Nonyl acetate | 32.84 | 0.40 |
| 19 | Pregeijerene | 32.91 | 0.30 |
| 20 | 6-Dodecanone | 35.43 | 1.00 |
| 21 | 2-Dodecanone | 36.71 | 0.50 |
| 22 | 2-Undecyl acetate | 38.22 | 1.80 |
| 23 | (E)-β-caryophyllene | 38.74 | 0.10 |
| 24 | 2-Tridecanone | 41.02 | 0.60 |
| 25 | α-Elemol | 43.80 | 0.20 |
| 26 | 10-epi-γ-Eudesmol | 47.27 | 0.10 |
| 27 | Psoralen | 53.45 | 1.50 |
| 28 | Moskachane D | 61.29 | 0.30 |
| 29 | Bergaptene | 68.69 | 1.50 |
| Cyclic Monoterpenes | 0.3 | ||
| Acyclic (Hydrocarbon) Monoterpenes | 0.1 | ||
| Cyclic Sesquiterpenes | 0.4 | ||
| Cyclic Diterpenes | 0.3 | ||
| Other Chemical Classes | 94.8 | ||
| Total % | 95.9 | ||
“Rt” refers to the retention time, and “%Conc” indicates the percentage concentration of the compound, determined by its peak area.
3.2. Effect of RO on the depressive behavior tests
3.2.1 Effect on the immobility time determined in FST in socially isolated rats
As illustrated in Figure (2a), and according to FST, a significant increase in immobility time (P < 0.001) was shown by rats exposed to SI compared to the normal control and RO control groups. Treatment with fluoxetine and RO at 200 and 400 mg/kg significantly reduced immobility time (P < 0.001) compared to the SI model. No significant differences were detected between the two RO doses, nor between the RO and fluoxetine doses.
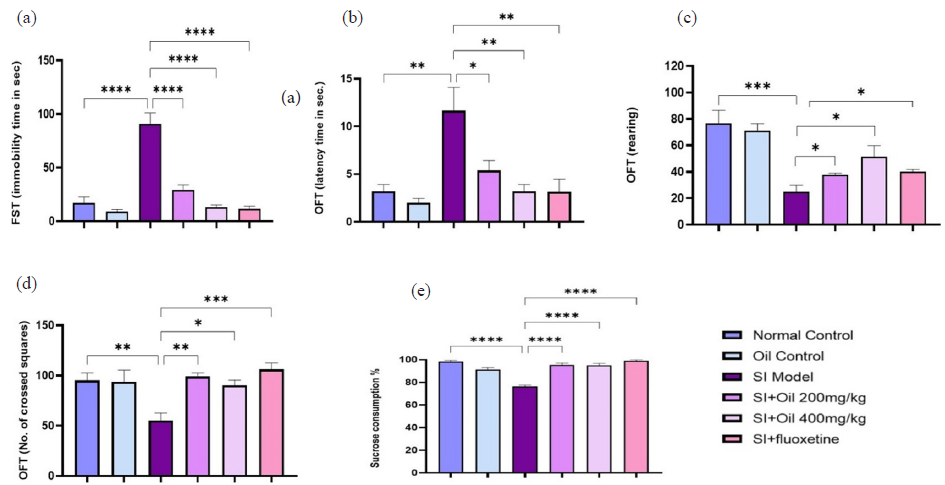
- (a-e) Effect of Ruta oil (RO) on depressive behavior tests. Treatment with RO at 200 and 400mg/kg alleviates the depressive behavior as indicated by the improvement in locomotor activity (reflected by the significant reduction in the latency time (b) and the increased number of crossed squares (d) in open field test (OFT) and the decreased immobility time (a) in forced swimming test (FST) compared to the SI model. RO also improved the exploratory behavior as indicated by the significant increased number of rearing* (c) in OFT. Treatment with RO also ameliorated the anhedonia (indicated by increased consumption of sucrose (e) with RO treatment) compared to SI group. ****P<0.0001, ***P<0.001, **P<0.01, *P<0.05. * Rearing means that an animal stands on its hind legs to explore.
3.2.2. Effect on latency time, frequency of rearing, and number of crossed squares in OFT in SI rats
SI resulted in a significant increase in latency time (P < 0.01, Figure 2b), along with a notable decrease in the frequency of rearing (P < 0.001, Figure 2c) and the number of crossed squares (P < 0.01, Figure 2d) in the OFT group when compared with the normal control group. Treatment with fluoxetine (P < 0.01) and RO at 400 mg/kg (P < 0.01) significantly reduced the latency time by 5 mins. RO at 200 mg/kg (P < 0.05) showed a lesser effect compared to the SI model. Fluoxetine treatment significantly increased the frequency of rearing, as did RO at 200 and 400 mg/kg, when compared to the SI model (P < 0.05). The number of crossed squares was significantly increased by fluoxetine (P < 0.001), RO at 200 mg/kg (P < 0.01), and RO at 400 mg/kg (P < 0.05) in comparison to the SI group.
3.2.3. Effect on anhedonia in SPT in socially isolated rats
In this study, an SPT was done for the rats. Rats in the SI model group showed a significantly lower sucrose preference compared to the control rats (Figure 2e). However, rats treated with fluoxetine, as well as RO at 200 and 400 mg/kg, displayed a significant increase in sucrose intake compared to the SI group (P < 0.001), indicating a significant reversal of the SI-induced loss of pleasure. No significant differences were observed between the different treatment groups.
3.3 Biochemical measurements
3.3.1 Effect of RO treatment on lipid peroxidation in socially isolated rats
As shown in Figure 3, SI resulted in a significant increase in the MDA levels (P < 0.0001), reflecting lipoid peroxidation in the hippocampal tissue. However, RO treatment at both doses (200 and 400 mg/kg) resulted in a significant decrease (P < 0.01) in the level of MDA in the hippocampus compared to SI rats. A greater extent of improvement in MDA levels was shown by treatment with fluoxetine (P < 0.0001).
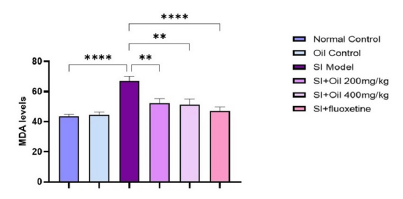
- Effects of Ruta oil (RO, 200 and 400 mg/kg) on hippocampal levels of malondialdehyde (MDA, marker of lipid peroxidation) in depressive rats. RO significantly ameliorated the increased levels of MDA mediated by social isolation (SI). ****p<0.0001, **P<0.01.
3.3.2 Effect of RO on hippocampal levels of serotonin (SER) and dopamine (DOP) and BDNF in socially isolated rats
We also examined the impact of RO on neurotransmitter levels in the hippocampus of socially isolated rats. The results indicated that the levels of SER and DOP were significantly reduced in the hippocampus of socially isolated rats compared to the control groups (P < 0.0001) (Figure 4a and 4b). Treatment with fluoxetine (P < 0.001) and RO at 400 mg/kg (P < 0.001) significantly reversed these reductions in SER and DOP levels, with a lesser effect observed with RO at 200 mg/kg (P < 0.01). Additionally, the hippocampal levels of BDNF were significantly increased in the socially isolated rats (P < 0.0001) (Figure 4c), and this increase was significantly reduced by treatment with fluoxetine (P < 0.01), RO at 200 mg/kg (P < 0.05), and RO at 400 mg/kg (P < 0.05).
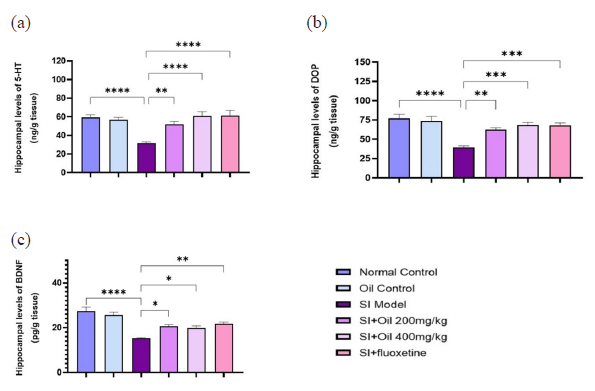
- Effects of Ruta oil (RO, 200 and 400 mg/kg) on hippocampal levels of (a) serotonin (SER, 5-HT), (b) dopamine (DOP) and (c) brain derived neurotropic factor (BDNF) in depressive rats. RO significantly increased the hippocampal levels of SER, DOP and BDNF compared to the socially isolated (SI) group. ****P<0.0001, ***P<0.001, **P<0.01, *P<0.05.
3.4 Histological examination
H&E stained sections of the rat hippocampus revealed the normal control and RO control groups (Figure 5, panels A and B) show a normal view of compact aggregations of granule neurons called granule cell layer (GCL) in between the polymorphic layer (POL) and molecular layer (ML). On the other hand, the hippocampus of the socially isolated group (Figure 5, Panel C) exhibits shrunken and dead pyramidal neurons and degenerated and faded granular neurons in the GCL. Hippocampus from socially isolated rats treated with fluoxetine (Figure 5, Panel D) displayed marked improvement represented by aggregations of abundant good-looking granular neurons in GCL. In addition, the hippocampus of socially isolated rats treated with RO (200 mg/kg) showed improvement except for some shrunken and degenerated granular neurons in the GCL (Figure 5, Panel E). The hippocampus of socially isolated rats treated with RO (400 mg/kg) exhibited obvious improvement represented by healthy granular neurons in GCL (Figure 5, Panel F).
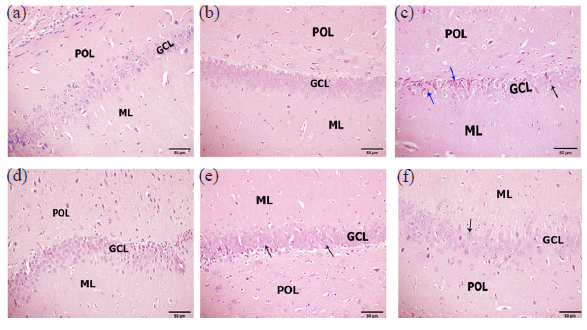
- Photomicrograph of hippocampus morphology; (a) Control hippocampus showing a normal view of compact aggregations of granule neurons called granule cell layer (GCL) in between polymorphic layer (POL) and molecular layer (ML). (b) Control hippocampus treated with Ruta oil (RO) revealing normal view of compact aggregations of granule neurons in GCL. (c) The hippocampus of the socially isolated group exhibits shrunken and dead pyramidal neurons (blue arrows) and degenerated and faded granular neurons (black arrow) in the GCL. (d) Hippocampus of socially isolated rats treated with fluoxetine displaying marked improvement represented by aggregations of abundant good-looking granular neurons in GCL. (e) The hippocampus of socially isolated rats treated with RO (200 mg/kg) showed some improvement except for some shrunken and degenerated granular neurons in the GCL. (f) The hippocampus of socially isolated rats treated with RO (400 mg/kg) posting obvious improvement represented by healthy granular neurons in GCL. (Hematoxylin and eosin, 200x).
3.5 Discussion
The identification of highly effective and low-toxicity drugs is crucial for depression treatment. Ruta chalepensis essential oil has many pharmacological effects, but its potential to alleviate depression has not been explored. This research highlights the potential of RO as a natural, plant-derived alternative to conventional antidepressants, such as SSRIs and benzodiazepines, which have been associated with significant side effects. It positions the oil as a safer and potentially more sustainable therapeutic option. For this purpose, we used a multifaceted approach to assess the antidepressant effects of the oil including behavioral changes, neurochemical markers, and histological evidence. Results of the current study revealed, for the first time, that RO has promising antidepressant activity, suggesting that it could be used as a standalone therapy or in combination with existing antidepressant medications, potentially enhancing treatment efficacy while minimizing side effects.
First, we studied the effect of RO on behavioral changes. OFT, FST, and SPT were often performed to evaluate depressive-like behaviors. In the current study, SI for 30 days led to anxiety and depressive-like behavior as indicated by impaired locomotor activity, decreased exploratory desires, and loss of pleasant desire. The impaired locomotor activity was indicated by higher immobility time in FST, decreased number of crossed squares, and higher latency time in OFT compared to the normal group. Decreased exploratory desire was reflected by reduced rearing activity in OFT, and the loss of pleasant desire was indicated by decreased sucrose consumption in SPT. These findings align with those of Grigoryan et al. [43]; Rai et al. [44], and Yang et al. [45] who observed behavioral changes in socially isolated rats and mice. In the current study, and compared to the model group, treatment with RO at 200 and 400mg/kg alleviates depressive behavior as indicated by the improvement in locomotor activity. This improvement was reflected by the significant reduction in the latency time and the increased number of crossed squares in OFT, in addition to the decreased immobility time in FST compared to the SI model. RO also improved the exploratory behavior as indicated by the significantly increased number of rearing in OFT. Rearing refers to the animal standing on its hind legs to explore. Treatment with RO also ameliorated the anhedonia, as indicated by increased consumption of sucrose with RO treatment compared to the SI group. These results indicate that RO has a role in alleviating depression and could be used successfully to manage the depressive-like behavior and anxiety resulting from stressful conditions such as SI.
Experimental models of SI rats demonstrated that behavioral alterations were associated with oxidative stress in the rat brain [43,44]. This is because SI is associated with impaired mitochondrial function, which results in increased production of reactive oxygen species (ROS) [46]. ROS could attack the cellular macromolecules, such as cell membrane phospholipids, leading to lipid peroxidation and cell injury. Consistent with this fact, SI in our study led to elevated levels of MDA (the marker of lipid peroxidation). However, this elevation was corrected by treatment with RO at both doses. This improvement could be related to the antioxidant activity of the oil, which has been detected in our recent work [14], in which the antioxidant activity of RO was determined using three assays. Upon chemical analysis of the extracted oil, 68 and 29 components were detected by headspace solid-phase microextraction [14] and HD [Table 1, current study], respectively. Among these compounds, six were identified to be responsible for the antioxidant activity of the oil, as shown through docking modeling. The modeling revealed that germacrene D, psoralen, d-cadinene, (E)-α-bisabolene, β-eudesmol, bergapten, and α-selinene are components of RO that may act as inhibitors of NADPH oxidase, a main source of ROS. These compounds may individually or synergistically contribute to the antioxidant properties of RO and, therefore, participate in modulating the behavioral changes. As a conclusion, this research establishes a connection between the antioxidant properties of the oil and its antidepressant efficacy.
Neurotrophic factors play a critical role in the development of depression in mammals. Among these factors is BDNF, which has been reported as an important factor in the pathogenesis of depression [47]. Normally, BDNF plays an important role in neuronal proliferation, axonal growth, and maintaining normal brain function. However, the hippocampal levels of BDNF are found to be reduced in depression triggering depressive-like behavior and anxiety [48,49]. These studies confirm our data, where BDNF levels were significantly reduced in the socially isolated rats. These reduced levels of BDNF were associated with pathological changes including shrunken and dead pyramidal neurons and degenerated and faded granular neurons in the GCL (Figure 5, Panel C). In the current study, the reduced expression of BDNF was attenuated by the treatment with RO at both doses, which was accompanied by amelioration of histological changes in the form of improvement of granular neurons in the GCL particularly with the dose 400 mg/kg. This effect on BDNF is comparable to fluoxetine. These results suggest that the downregulation of BDNF contributes to the beneficial effects of RO in improving depressive-like behavior, anxiety, and histological changes.
Disturbance in brain neurochemicals was previously documented in the pathogenesis of depression. The main neurotransmitter players in the pathogenesis of depression are DOP and SER, which show a reduction in their levels with the progression of depression [50,51]. Consistent with this data, the hippocampal levels of SER and DOP in our study were significantly suppressed by SI confirming their implication in the pathogenesis of SI-induced depression. On the other hand, the present results revealed that RO at both doses restored SER and DOP levels in the socially isolated rats, the effect which could be related to the bioactive components of RO. It has been reported that psoralen, a component of RO isolated from Saudi species [14], exhibits potent antidepressant-like properties, which are relatively mediated by improving serotonergic activity [51]. In the CNS, DOP is involved in the regulation of various functions, including locomotor activity [52]; therefore, the impaired locomotor activity induced by SI and the improvement by RO are strongly linked to the DOP concentrations in the brain. Fluoxetine, as one of the SSRIs, is widely used as a standard treatment for depression and various anxiety disorders by increasing SER and DOP [32]. In the present study, the results of RO were comparable to the results of fluoxetine, suggesting its powerful antidepressant effect as SSRIs. Collectively, this study revealed a new anti-depressant biological function of RO, which expanded its clinical use. Summarizing, RO can significantly increase the levels of DOP, SER, and BDNF in the brain to achieve the effect of treating depression.
The antidepressant effects of RO could be credited to its components such as psoralen, camphor, limonene, psoralen, and α-phellandrene (Table 1) [14], which are previously tested for their neuroprotective effects in some models of depression. For example, in the spared nerve injury model of neuropathic pain in rats, limonene and α-phellandrene exhibited anti-depressive effects and inhibited increased immobility in the FST [53]. Treatment with limonene in mice showed anxiolytic activity, increased locomotor activity, increased expression of tyrosine hydroxylase (key enzyme in dopamine biosynthesis), and significantly increased levels of tissue DOP [54]. Recently, limonene alleviated the depression-like behavior in rats subjected to chronic restraint stress [15]. Camphor, that represents about 2.7 % of the oil contents [14], demonstrated protective effects against depression induced by ciprofloxacin in rats [16]. In this study, treatment with camphor for 21 days reduced the serum levels of MDA and increased the levels of SER and DOP in brain tissue. Psoralen (1.5 % of the oil content) is one of the most studied coumarins with antidepressant effects [7]. Validation of the antidepressant activity of these related active compounds is necessary for future direction. 2-Nonanone and 2-Undecanone are the major components that were identified in the oil at 43.0% and 30.2 %, respectively. However, there is no previous work on their antidepressant or anti-anxiety effects. Therefore, future studies are needed to investigate their anti-depressant potential and their role in the beneficial effects of RO on socially isolated rats.
4. Conclusions
This study highlights the significant antidepressant potential of RO in mitigating the effects of SI-induced depression in rats. RO effectively alleviated depressive behavior, restored neurotransmitter levels (dopamine and serotonin), increased BDNF expression, and reduced lipid peroxidation in hippocampal tissues. These findings demonstrate RO’s ability to improve neurochemical balance and protect against neuronal damage, suggesting its potential as a natural, plant-derived remedy with a favorable safety profile. The broader significance of this work lies in advancing the use of natural bioactive compounds as alternatives to conventional antidepressants, particularly in addressing the need for therapies with fewer side effects. Future research should focus on investigating the antidepressant activity and the specific mechanisms of the major bioactive components of RO, such as 2-nonanone and 2-undecanone. Additionally, exploring the synergistic effects of combining RO with conventional antidepressants could uncover complementary treatment strategies. Further studies could assess the potential applications of RO in managing other neuropsychiatric or oxidative stress-related disorders, expanding its therapeutic scope. By addressing these areas, this research paves the way for innovative, natural solutions to mental health challenges.
CRediT authorship contribution statement
Conceptualization and study design: HAA and HYA. Investigations: HAA, RMB and NMA. Data collection: HAA, HYA, RMB and NMA. All authors contribute to methodology, resources, formal analysis and visualization. Supervision: HAA and HYA. Funding acquisition: HYA. Project administration: HAA and HYA. Writing-Original Draft: HAA. All authors read and approved the final manuscript.
Declaration of competing interest
The authors declare that there is no competing of interest.
Declaration of Generative AI and AI-assisted technologies in the writing process
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Funding
The authors would like to thank the Researchers Supporting Project number (RSP2025R504), King Saud University, Riyadh, Saudi Arabia.
References
- Prevalence of depressive disorders and associated demographic characteristics in Shandong: An epidemiological investigation. Journal of Affective Disorders. 2022;311:198-204. https://doi.org/10.1016/j.jad.2022.05.084
- [CrossRef] [PubMed] [Google Scholar]
- Bergapten alleviates depression-like behaviour by inhibiting cyclooxygenase 2 activity and NF-κB/MAPK signalling pathway in microglia. Experimental Neurology. 2023;365:114426. https://doi.org/10.1016/j.expneurol.2023.114426.
- [CrossRef] [PubMed] [Google Scholar]
- Prevalence of depression, anxiety, and stress among the general population in Saudi Arabia during Covid-19 pandemic. International Journal of Environmental Research and Public Health. 2020;17:9183. https://doi.org/10.3390/ijerph17249183
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Molecular and cellular mechanisms of antidepressant action. Current Topics Behavioral Neuroscience. 2013;14:309-325. https://doi.org/10.1007/7854_(2012)_216
- [Google Scholar]
- Treatment of benzodiazepine dependence. New England Journal of Medicine. 2017;376:1147-1157. https://doi.org/10.1056/NEJMra1611832
- [CrossRef] [PubMed] [Google Scholar]
- Antidepressant effects of coumarins and their derivatives: A critical analysis of research advances. European Journal of Pharmacology. 2023;956:175958. https://doi.org/10.1016/j.ejphar.2023.175958
- [CrossRef] [PubMed] [Google Scholar]
- Long-term antidepressant use: Patient perspectives of benefits and adverse effects. Patient Prefer Adherence. 2016;10:1401-1407. https://doi.org/10.2147/PPA.S110632
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Phenolic profile, antidepressant-like and neuroprotective effects of maclura tinctoria leaves extract. Natural Product Research. 2022;36:4692-4695. https://doi.org/10.1080/14786419.2021.2000407
- [CrossRef] [PubMed] [Google Scholar]
- Polyphenols as novel interventions for depression: Exploring the efficacy, mechanisms of action, and implications for future research. Neuroscience and Biobehavioral Reviews. 2023;151:105225. https://doi.org/10.1016/j.neubiorev.2023.105225
- [CrossRef] [PubMed] [Google Scholar]
- Emerging role of flavonoids as the treatment of depression. Biomolecules. 2021;11:1825. https://doi.org/10.3390/biom11121825
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Traditional use of ethnomedicinal native plants in the Kingdom of Saudi Arabia. Journal of Ethnobiology and Ethnomedicine. 2019;15:2. https://doi.org/10.1186/s13002-018-0263-2
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Anti-inflammatory effect of ruta graveolens l. in murine macrophage cells. Journal of Ethnopharmacology. 2006;104:234-239. https://doi.org/10.1016/j.jep.2005.09.008
- [CrossRef] [PubMed] [Google Scholar]
- Neuropharmacological profile of an ethanol extract of ruta chalepensis l. in mice. Journal of Ethnopharmacology. 2006;106:129-135. https://doi.org/10.1016/j.jep.2005.12.014
- [CrossRef] [PubMed] [Google Scholar]
- Headspace solid phase micro-Extraction of volatile constituents produced from Saudi ruta chalepensis and molecular docking study of potential antioxidant activity. Molecules. 2023;28:1891. https://doi.org/10.3390/molecules28041891
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- D-Limonene reduces depression-like behaviour and enhances learning and memory through an anti-neuroinflammatory mechanism in male rats subjected to chronic restraint stress. European Journal of Neurosci. 2024;60:4491-4502. https://doi.org/10.1111/ejn.16455
- [Google Scholar]
- Neuroprotective role of camphor against ciprofloxacin induced depression in rats: Modulation of nrf-2 and TLR4. Immunopharmacology and Immunotoxicology. 2021;43:309-318. https://doi.org/10.1080/08923973.2021.1905658
- [CrossRef] [PubMed] [Google Scholar]
- Paeoniflorin: A neuroprotective monoterpenoid glycoside with promising anti-depressive properties. Phytomedicine. 2021;90:153669. https://doi.org/10.1016/j.phymed.2021.153669
- [CrossRef] [PubMed] [Google Scholar]
- The role of social isolation and social cognition in thought disorder. Psychiatry Research. 2018;269:56-63. https://doi.org/10.1016/j.psychres.2018.08.048
- [CrossRef] [PubMed] [Google Scholar]
- Social isolation stress induces anxious-Depressive-Like behavior and alterations of neuroplasticity-Related genes in adult male mice. Neural Plasticity. 2016;2016:6212983. https://doi.org/10.1155/(2016)/6212983
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Age-dependent effects of social isolation on mesolimbic dopamine release. Experimental Brain Research. 2022;240:2803-2815. https://doi.org/10.1007/s00221-022-06449-w
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Long-Term behavioral effects of post-weaning social isolation in males and females. Frontiers in Behavioral Neuroscience. 2019;13:66. https://doi.org/10.3389/fnbeh.2019.00066
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Social isolation and the brain in the pandemic era. Nature Human Behavior. 2022;6:1333-1343. https://doi.org/10.1038/s41562-022-01453-0
- [CrossRef] [Google Scholar]
- The growing problem of loneliness. Lancet. 2018;391:426. https://doi.org/10.1016/S0140-6736(18)30142-9
- [CrossRef] [Google Scholar]
- Neurobiology of loneliness, isolation, and loss: Integrating human and animal perspectives. Frontiers in Behavioral Neuroscience. 2022;16:846315. https://doi.org/10.3389/fnbeh.2022.846315
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Social isolation stress in adolescence, but not adulthood, produces hypersocial behavior in adult male and female C57BL/6J mice. Frontiers in Behavioral Neuroscience. 2020;14:129. https://doi.org/10.3389/fnbeh.2020.00129
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Loneliness and the onset of new mental health problems in the general population. Social Psychiatry and Psychiatric Epidemiology. 2022;57:2161-2178. https://doi.org/10.1007/s00127-022-02261-7
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Social isolation and memory decline in later-life. The Journals of Gerontology. Series B, Psychologic. 2020;75:367-376. https://doi.org/10.1093/geronb/gbz152
- [CrossRef] [Google Scholar]
- Chemical composition and antimicrobial activity of the essential oils of artemisia absinthium, artemisia scoparia, and artemisia sieberi grown in Saudi Arabia. Arabian Journal of Chemistry. 2020;13:8209-8217. https://doi.org/10.1016/j.arabjc.2020.09.055
- [CrossRef] [Google Scholar]
- HINT1 is involved in the behavioral abnormalities induced by social isolation rearing. Neuroscience letters. 2015;607:40-45. https://doi.org/10.1016/j.neulet.2015.08.026
- [CrossRef] [PubMed] [Google Scholar]
- Prolonged social isolation, started early in life, impairs cognitive abilities in rats depending on sex. Brain Sciences. 2020;10:799. https://doi.org/10.3390/brainsci10110799
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Uwhangchungsimwon, A standardized herbal drug, exerts an anti-Depressive effect in a social isolation stress-Induced mouse model. Frontiers in Pharmacology. 2020;10:1674. https://doi.org/10.3389/fphar.2019.01674
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Oleuropein reverses repeated corticosterone-Induced depressive-like behavior in mice: Evidence of modulating effect on biogenic amines. Science Reports. 2020;10:3336. https://doi.org/10.1038/s41598-020-60026-1
- [CrossRef] [Google Scholar]
- Oral acute toxicity, influence on the gastrointestinal microbiota and in vivo anti-salmonellosis effect of zizyphus lotus (L.) and ruta chalepensis (L.) essential oils. Journal of Applied Biotechnology Reports. 2021;8:13-26. https://doi.org/10.30491/jabr.2020.229267.1217.
- [Google Scholar]
- In Vivo Hepato-Protective Properties of the Essential Oils of Boswellia papyrifera (Del.) Hochst (Burseraceae) and Ruta chalepensis L. (Rutaceae) Journal of Biomedical Science. 2020;8:117-131. https://doi.org/10.4236/jbm.2020.810011
- [Google Scholar]
- Techniques and Basic Experiments for the Study of Brain and Behavior. Netherlands: Elsevier Science Publishers; 2016.
- Behavioral and locomotor measurements using an open field activity monitoring system for skeletal muscle diseases. Journal of Visualized Experiments. 2014:51785. https://doi.org/10.3791/51785
- [CrossRef] [Google Scholar]
- Dihydrolipoic acid protects against lipopolysaccharide-induced behavioral deficits and neuroinflammation via regulation of Nrf2/HO-1/NLRP3 signaling in rat. Journal of Neuroinflammation. 2020;17:166. https://doi.org/10.1186/s12974-020-01836-y
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Sex-dependent impact of different degrees of maternal separation experience on OFT behavioral performances after adult chronic unpredictable mild stress exposure in rats. Physiology and Behavior. 2018;194:153-161. https://doi.org/10.1016/j.physbeh.2018.04.034
- [CrossRef] [PubMed] [Google Scholar]
- Making sense of rodent models of anhedonia. The International Journal of Neuropsychopharmacolo. 2018;21:1049-1065. https://doi.org/10.1093/ijnp/pyy083
- [CrossRef] [Google Scholar]
- Exploratory rearing: A context- and stress-sensitive behavior recorded in the open-field test. Stress. 2018;21:443-452. https://doi.org/10.1080/10253890.2018.1438405
- [CrossRef] [PubMed] [Google Scholar]
- Sucrose preference test for measurement of stress-induced anhedonia in mice. Nature protocols. 2018;13:1686-1698. https://doi.org/10.1038/s41596-018-0011-z
- [CrossRef] [PubMed] [Google Scholar]
- Social isolation after chronic unpredictable mild stress perpetuates depressive-like behaviors, memory deficits and social withdrawal via inhibiting ERK/KEAP1/NRF2 signaling. Journal of affective disorders. 2023;324:576-588. https://doi.org/10.1016/j.jad.2022.12.092
- [CrossRef] [PubMed] [Google Scholar]
- Effects of social isolation on the development of anxiety and depression-Like behavior in model experiments in animals. Neuroscience and Behavioral Physiology. 2022;52:722-738. https://doi.org/10.1007/s11055-022-01297-1
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Role of acorus calamus in preventing depression, anxiety, and oxidative stress in long-term socially isolated rats. Veterinary World. 2023;16:1755-1764. https://doi.org/10.14202/vetworld.2023.1755-1764
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Ginkgolide-platinum (II) complex GPt(II) exhibits therapeutic effect on depression in mice via upregulation of DA and 5-HT neurotransmitters. Medical Science Monitor. 2020;26:e922052. https://doi.org/10.12659/MSM.922052.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Lifestyle, oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Frontiers Physiology. 2020;11:694. https://doi.org/10.3389/fphys.2020.00694
- [CrossRef] [Google Scholar]
- Hippocampal BDNF in physiological conditions and social isolation. Reviews Neuroscience. 2017;28:675-692. https://doi.org/10.1515/revneuro-2016-0072
- [Google Scholar]
- Homocysteine modulates social isolation-Induced depressive-Like behaviors through BDNF in aged mice. Molecular Neurobiology. 2023;60:4924-4934. https://doi.org/10.1007/s12035-023-03377-w
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Reduction of BDNF results in GABAergic neuroplasticity dysfunction and contributes to late-life anxiety disorder. Behavioral Neuroscience. 2019;133:212-224. https://doi.org/10.1037/bne0000301
- [CrossRef] [PubMed] [Google Scholar]
- Therapeutic effect of resveratrol on mice with depression. Exp Ther Med. 2019;17:3061-3064. https://doi.org/10.3892/etm.2019.7311
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Antidepressant-like effects of psoralen isolated from the seeds of psoralea corylifolia in the mouse forced swimming test. Biological & Pharmaceutical Bulletin. 2008;31:1109-1114. https://doi.org/10.1248/bpb.31.1109
- [CrossRef] [PubMed] [Google Scholar]
- Dopamine receptors: From structure to function. Physiological Reviews. 1998;78:189-225. https://doi.org/10.1152/physrev.1998.78.1.189
- [CrossRef] [PubMed] [Google Scholar]
- Antihyperalgesic and antidepressive actions of (R)-(+)-limonene, α-phellandrene, and essential oil from schinus terebinthifolius fruits in a neuropathic pain model. Nutritional Neuroscience. 2015;18:217-224. https://doi.org/10.1179/1476830514Y.0000000119
- [CrossRef] [PubMed] [Google Scholar]
- Limonene has anti-anxiety activity via adenosine A2A receptor-mediated regulation of dopaminergic and GABAergic neuronal function in the striatum. Phytomedicine. 2021;83:153474. https://doi.org/10.1016/j.phymed.2021.153474
- [CrossRef] [PubMed] [Google Scholar]







