Translate this page into:
The solvent extraction is a potential choice to recover asphalt from unconventional oil ores
⁎Corresponding author at: Department of Chemical Engineering, School of Chemistry and Chemical Engineering, Guizhou University, Guiyang, Guizhou 550025, China. jma3@gzu.edu.cn (Jun Ma)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The abundant unconventional oil ores (about 70% of total world oil) are playing an increasingly important role in global energy supply. To obtain asphalt in unconventional oil ores, different methods, including hot water-based extraction, pyrolysis and solvent extraction, were used to recover asphalt from oil ores (i.e., Canadian, Indonesian, and Iranian oil ores). It is found that asphalt recovery obtained by solvent extraction is the highest. Multi-staged single solvent extraction was used to recover asphalt from oil ores (i.e., toluene, tetrahydrofuran: THF, xylene, petroleum: PE, ethanol), resulting in a cumulative asphalt recovery over 98% at ambient conditions by using toluene. Take Iranian oil ores with the highest oil content (83.79 wt%) as an example, great asphalt recovery was obtained by using multi-staged composite solvents extraction (i.e., toluene@xylene, toluene@THF, toluene@PE, toluene@ethanol). It is also found that introduction of toluene in the composite solvents can significantly increase the ability of single solvent’s (xylene, THF, PE and ethanol) asphalt recovery. After solvent extraction, the solvent recovery was more than 95%. This finds suggest that solvent extraction method would be potential choice to recover asphalt from unconventional oil ores, and it possesses great prospect of industrial application in the future.
Keywords
Solvent extraction
Choice
Asphalt
Unconventional oil ores
1 Introduction
Energy and environment are two important aspects of social development (Ejaz and Younis, 2011). As one of the important energy resources, petroleum resources play an important role in the development of human society. With the large-scale exploitation and use of crude oil, the production of conventional crude oil resources are gradually reduced (He et al., 2016). Unconventional petroleum, including oil/tar sands, oil shale, heavy oil, etc., has been gradually attracted wide attention (He et al., 2015). Unconventional oil ores are a kind of petroleum resources composed of heavy oil, water and minerals (Meyer et al., 2007). It is enormous with proven resves of more than 6 trillion barrels of recoverable oil by current technologies, accounting for about 70% of total world oil reserves (Santos et al., 2014). As is known to all, unconventional oil ores including water wettability and oil wettability (He et al., 2015), During the past few decades, great efforts have been made in the exploitation of water-wetted oil ores, such as Canadian oil ores, resulting in a production of more than 1.5 million barrels of asphalt per day in 2014 (Masliyah et al., 2004). Another kind of oil ores were discovered in Indoesian islands, with about 3 billion tons of asphalt geological in situ reserves (Sui et al., 2016). However, this kind of oil ore is different from the Athabasca oil ores in asphalt content, asphalt composition, and ore types (Cloutis et al., 1995). The Indonesian asphalt rocks, with asphalt content over 25 wt%, are known as oil-wetted ores without the water film between asphalt and mineral solid surface, which are not being well-exploited until now (Wang et al., 2012). In the exploitation process of unconventional oil ores, asphalt is important oil resources, according to the polarity and solubility, asphalt is composed of saturates (S), aromatics (A), resins (R) and asphaltenes (A) (Yoon et al., 2009; Dubois and Murariu, 2009; Li et al., 2012), which can be pumped through pipelines to upgraders or refineries, where it is further processed to produce gasoline, jet fuel, heating oil and diesel fuel. Therefore, it possesses importantly practical application value to recover asphalt from unvonventional oil ores.
At present, to unlock asphalt in the unconventional oil ores, different technologies have been proposed to recover asphalt. The hot water-based extraction (HWBE) process has been industrialized in Albert, Canada, the technology is suitable to separate asphalt from water-wetted oil ores (Long et al., 2007), but it has been proved to perform poorly in separating oil-wetted unconventional oil ores (such as Indonesian asphalt rocks). This is because the interaction between oil and carbonate solid is strong, resulting it is quite difficult to separate oil and solid by using HWBE method (Srinivasa et al., 2012; Hupka et al., 2004). However, the HWBE process can cause oil–water emulsification problem. The oil–water emulsions formed will corrode equipment, increase cost and pollute the environment. The pyrolysis method also has been proposed to extract the asphalt from the unconventional oil ores (Li and Yue, 2004). This method works based on the energy-intensive reaction and high oil content in the oil ores. During the heating process, the relatively lighter compounds (volatile) in the asphalt were evaporated first, followed by the decomposition of heavier fractions (e.g., asphaltenes, resins, or larger aromatics and saturates) through pyrolysis. Consequently, only the extremely heavy hydrocarbons and the formed char or coke are left. Some researchers believed that pyrolysis would be a potential way for the exploitation of the Indonesian asphalt rocks (Wang et al., 2014; Jia et al., 2015). Because the asphalt could be directly cracked into light oil fractions or gases without adding external chemicals. However, there are also some challenges during pyrolysis industrialization, such as high energy consumption, high production of tars together with minerals, large production of gases, especially CO2, etc. (Nie et al., 2017; Wang et al., 2013). Therefore, it is necessary to find the better industrial separation method to separate asphalt from unconventional oil ores.
Solvent extraction has been considered as a potential and promissing method to recover asphalt from unconventional oil ores. The method has been investigated for several decades as a result of its high extraction efficiency, low water consumption, low erergy consumption and high compatibility to all kinds of unconventional oil ores, especially the oil-wetted ores (Funk et al., 1982). He et al. (2011) researched the effects of solvent type, temperature, stirring rate, and solvent polarity on the asphalt recovery and asphalt quality from Athabasca oil ores using solvent extraction. Pal et al. (2015) and Nikakhtari et al. (2013) conducted studies on separating asphalt from Athabasca oil rocks by solvent extraction using different solvents. Their results showed that the biologically derived solvents have a lower asphalt recovery compared to those of isoprene and cyclohexane. In addition, Sui et al. (2016) investigated the feasibility of solvent extraction in recovering asphalt from Indonesian asphalt rocks. They found that a high oil recovery (up to 98%) could be achieved with the solvent of toluene or cyclohexane. However, little knowledge has been reported in extracting asphalt from different types of unconventional oil ores using various single solvent extraction or composite solvents extraction. In addition, there are few reports about the prospect of industrial application of solvent extraction in recovering asphalt from unconventional oil ores.
Accordingly, considering future industrial application, the objectives of this study are to (i) test the efficiency of HWBE, pyrolysis and solvent extraction for asphalt recovery from the different unconventional oil ores, (ii) test the efficiency of composite solvents extraction for asphalt recovery from oil-wetted oil ores (Iranian oil ores), and (iii) identify solvent extraction method is the potential choice to recover asphalt from unconvential oil ores. And discuss the great industrial application prospect of recovering asphalt by solvent extraction.
2 Experimental section
2.1 Chemicals and samples
Chemical reagents, including ethanol, toluene, tetrahydrofuran (THF), petroleum ether (PE) and xylene, were purchased at analytical grade from Tianjin Jiangtian Technology Co., Ltd, China. The asphalt rocks were obtained from Indonesia, Canada and Iran.
2.2 Composition analysis of unconventional oil ores
The composition of different unconventional oil ores was analyzed using the Dean-Stark standard method (Castro et al., 1998).The schematic diagram of experimental device is shown in Fig. 1. 10.0 g unconventional oil ores were placed in a filter paper cylinder, and then 150.0 mL toluene was added into the round bottom flask. Place the experimental device in the electric heating sleeve. Turn on the condensate and set the heating temperature to 160 °C. The oil ores was extracted by heating toluene. When the liquid dropped from the filter papercylinder became colorless and transparent, all the asphalt in the oil ores was extracted by toluene, and the heating was stopped. Remove toluene from the asphalt solution in the round bottomed flask by a rotary evaporator. The asphalt sample and the filter paper cylinder containing solid were put into the vacuum drying oven to evaporate toluene. The temperature was 80 °C and the vacuum degree was 0.1 MPa. After 4 h of drying, it were taken out and put into a dryer to cool to room temperature. Calculating the content of asphalt, solids and water in the different oil ores by Eqs. (1)–(3).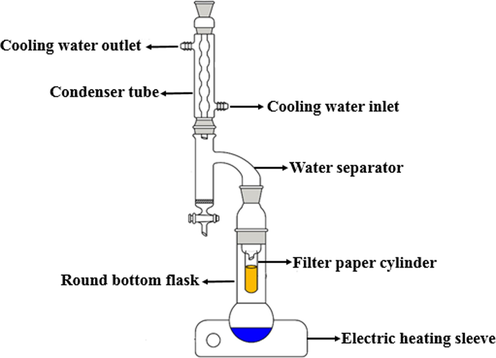
Schematic diagram of experimental device.
2.3 HWBE process of unconventional oil ores
As shown in Fig. 2, the hot water-based extraction (HWBE) process was conducted in a Denver flotation cell with a 1 L jacked stainless-steel cell connected to a thermal water bath (Sui et al., 2016). First, 900 mL hot water (50 °C and pH was adjusted to 9.03 by sodium hydroxide) and 300 g of unconventional oil ores were added to the flotation cell for conditioning for 5 min with a stirring rate at 1500 rpm. The slurry was kept at 50 °C through a thermal water bath. After the slurry conditioning, nitrogen was injected into the slurry at a flow rate of 150 mL/min for 10 min without stopping the agitation to let the heavy hydrocarbons be floated. The froth floating on the top of the water was collected for further analysis to determine its extraction rate by Eqs. (4) and (5).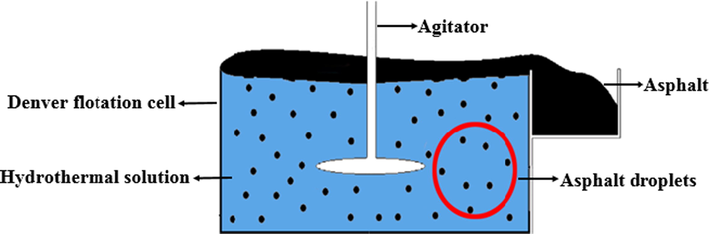
Schematic diagram of HWBE experimental device for unconventional oil ores.
2.4 Pyrolysis process of unconventional oil ores
Unconventional oil ores pyrolysis was carried out in a Vacuum tube furnace from 25 °C to 400 °C at 10 °C/min heating rate, and maintain 30 min at 400 °C. The schematic diagram of the experimental device is shown in Fig. 3. Pyrolysis experiments were performed in the nitrogen atmosphere, the average initial oil ores sample mass was 50.0 g. According to Eqs. (6) and (7), the relative recovery of asphalt was calculated after pyrolysis (6) and (7).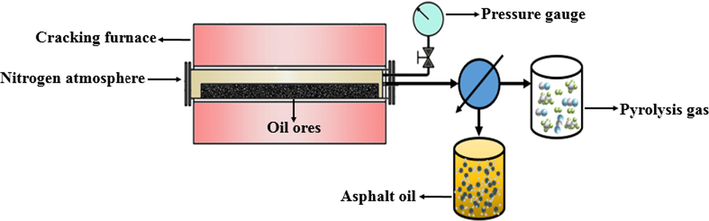
Schematic diagram of pyrolysis experimental device for unconventional oil ores.
2.5 Solvent extraction process of unconventional oil ores
A multi-staged solvent extraction for the asphalt recovery process has been applied in this study (Sui et al., 2016), shown in Fig. 4. A volume of weighted solvent (150 mL) and 50 g unconventional ores were first added to a jar for agitation at 450 rpm using a paddle mixer for 25 min. After the agitation, the mixture was sent for settling in a glass cylinder to recover asphalt solution. The settled solids were separated for the second and third extraction using 100 and 50 mL of solvent, respectively. The asphalt recovery has been measured three times by centirfugation, distillation, and vacuum drying, accordingly (Qiu, 2010). The solvents were recycled. The residual solids from extraction step were analyzed to obtain how much of solvent was left together with the waste solids. The application of the second and third extractions is to increase the asphalt recovery, which may facilitate the removal and recovery of residual solvent from the waste solids. The final residual solvents and asphalt from the third extraction were recovered by gas bubbling method (Wang et al., 2020). According to the above experimental process, the effect of different composite solvents on the extraction rate of asphalt was studied. The relative recovery of asphalt by single solvent or composite solvents was calculated by Eqs. (8) and (9).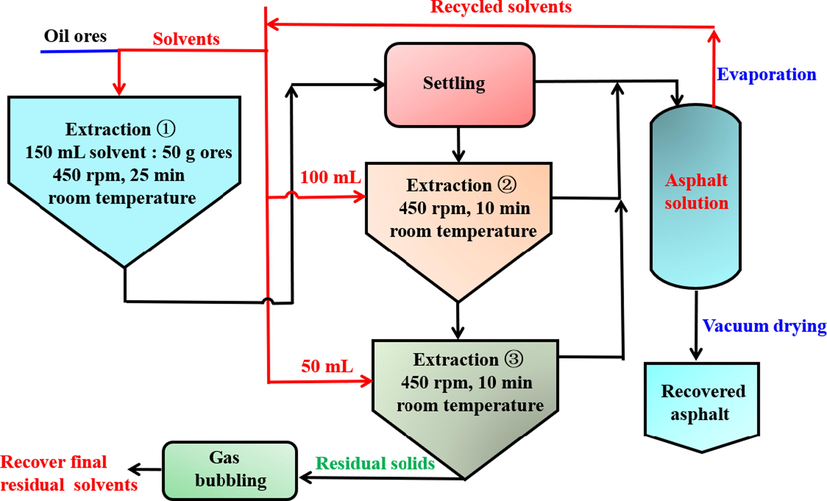
Schematic of the solvent extraction process for recovering asphalt.
2.6 Hansen solubility parameters (HSP) calculation of solvents
Teas converted HSP to fractions fd, fp, and fh, which were defined as in equations 10–12 (Hansen, 2007; Barton, 1991). Petroleum ether are mainly composed of pentane and hexane, so, the HSP of pentane and hexane explained that petroleum ether can be used as solvent to extract asphalt from unconventional oil ores. According to the fraction of the solubility parameter, every solvent can be positioned in a Teas triangle. HSP and the fractional parameters of solvents are given in Table 1.
Solvent
δt
HSP
HSP fractional parameter
δd
δp
δh
fd
fp
fh
Pentane
14.500
14.500
0.000
0.000
1.000
0.000
0.000
Hexane
14.900
14.900
0.000
0.000
1.000
0.000
0.000
Xylene
18.000
17.800
1.000
3.100
0.813
0.046
0.142
Toluene
18.200
18.000
1.400
2.000
0.841
0.065
0.093
THF
19.400
16.800
5.700
8.000
0.551
0.187
0.262
Ethanol
26.500
15.800
8.800
19.400
0.359
0.200
0.441
3 Results and discussion
3.1 Comparison of different extraction methods
Fig. 5 shows the results of asphalt recovery from three unconventional oil ores by HWBE and pyrolysis methods. As shown in Fig. 5a, the recovery rates of asphalt from oil ores are different by HWBE method (Canadian: 21.3%, Indonesian: 7.8%, Iranian: 1.7%). The results indicated the HWBE method is more suitable for separating Canadian oil ores. Because the Canadian oil ores belong to the water-wetted type, while Indonesian and Iranian oil ores belong to the oil-wetted type (Fig. 6) (Takamura, 1982). In Canadian oil ores, there is a water film between solid and asphalt, which can increase the affinity between oil and water, while Indonesian and Iranian oil ores possess weak affinity for water. Therefore, the amount of asphalt recovered from Indonesian and Iranian oil ores by HWBE method is lower than Canadian. In addition, the solid content in Indonesian oil ores is higher than Iran (Table 2). It is beneficial to increase hydrophilicity (Li et al., 2018), so the asphalt recovery of Indonesian oil ores is higher than Iran. Fig. 5b shows more asphalts can be recovered from three oil ores by pyrolysis method, and the effect of separating asphalt is better than HWBE method. It is because of the acceleration of movement rate of asphalt molecules at high temperature, accelerating the separation of asphalts and solids. Table 2 shows the content of Iranian oil ores is the highest (83.79%), so the asphalt recovery by pyrolysis is the highest (62.2%).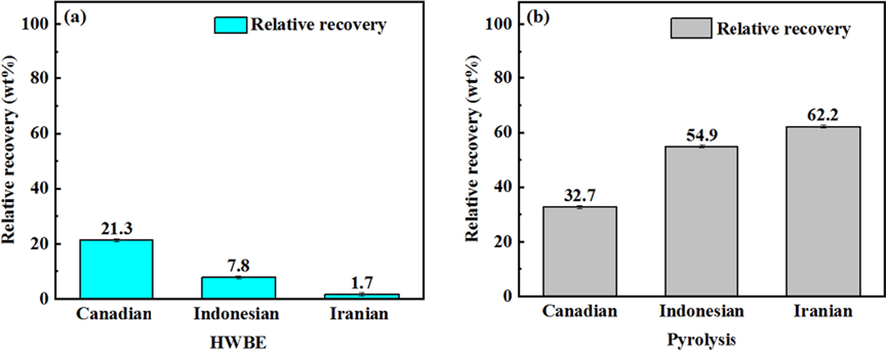
Asphalt recovery from three unconventional oil ores by different methods (a: HWBE, b: Pyrolysis).

Structure of unconventional oil ores.
Oil ores type
Water (wt%)
Asphalt (wt%)
Solids (wt%)
Canadian oil ores
2.02
10.46
87.52
Indonesian oil ores
2.00
27.10
70.90
Iranian oil ores
2.57
83.79
13.64
Single solvent multistage extraction was applied to enhance the recovery of asphalt from various oil ores, as shown in Fig. 7. Combined with Fig. 5, it was observed that, the effect of solvent extraction method was better than HWBE and pyrolysis method. On the one hand, because asphalt in oil ores is organic matter, according to the principle of similar compatibility, organic solvent can better dissolve asphalt and promote the stripping of asphalt and solid. On the other hand, organic solvents can effectively reduce the interaction between asphalt and solid, and promote the separation of asphalt in oil ores (Zhang et al., 2019). However, the various organic solvents were used to extract asphalt at one time, the asphalt recovery was different. As shown in Fig. 7a–c, distinct organic solvents were used to extract asphalt from Canadian, Indonesian and Iranian oil ores, respectively. The order of asphalt recovery of each solvent are toluene (76.8%, 78.8%, 85.6%) > THF (74.6%, 75.8%, 78.3%) > xylene (72.3%, 73.4%, 75.8%) > petroleum ether (68.6%, 69.8%, 69.7%) > ethanol (43.3%, 44.7%, 45.3%). These results indicate toluene possess the best extraction effect, and ethanol’s extraction effect is the worst. According to the HSP, this is due to the high fd and fh and low fp values of the good extraction solvents (Table 1 and Fig. 8). To be mentioned, it is also found that increasing the extraction times (2–3 times) significantly facilitates the recovery, exceeding 98% recovery, shown in Fig. 7. This is because multiple extraction can further reduce the interaction between oil components and solid particles (Natarajan et al., 2011), so more asphalts can be stripped from oil ores.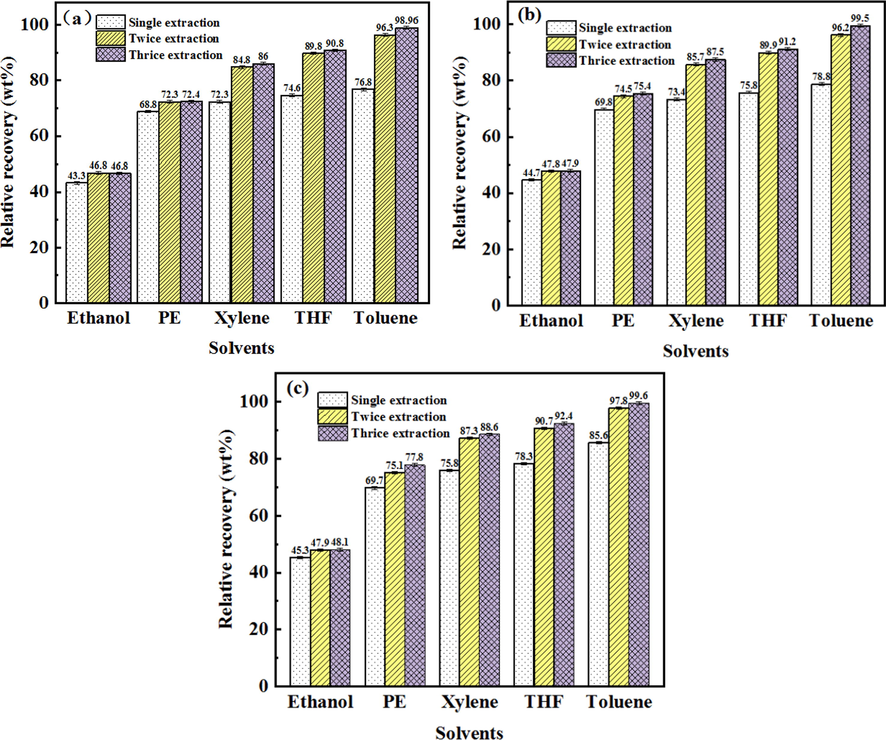
Asphalt recovery from differernt oil ores by solvent extraction with different times of the extraction by solvents (a: Canadian oil ores, b: Indonesian oil ores, c: Iranian oil ores).
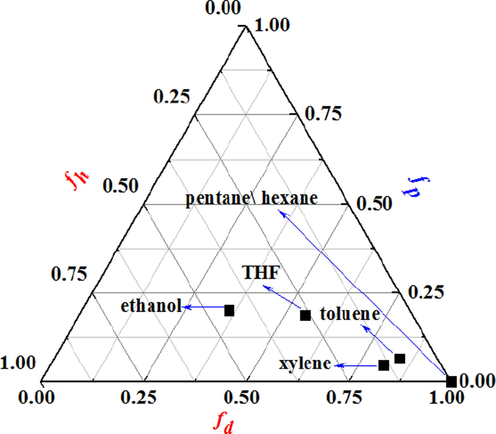
Solvent Teas triangle.
3.2 Composite solvents extraction
The different methods were used to recover asphalt from unconventional oil ores, indicating the effect of solvent extraction was better than HWBE and pyrolysis. In addition, no matter which kind of oil ores, the toluene’s extraction rate was the highest in selected solvents. In this section, take Iranian oil ores as the research object, investigate the extraction effect of various proportions (volume ration) of composite solvents (toluene@xylene, toluene@THF, toluene@PE, toluene@ethanol) recover asphalt from Iranian oil ores.
As shown in Fig. 9, the different composite solvents was used to extract asphalt from Iranian oil ores. The order of asphalt recovery of each composite solvent are toluene@xylene > toluene@THF > toluene@PE > toluene@ethanol, indicating the toluene@xylene composite solvents possess the strongest asphalt recovery capacity. When toluene@xylene (1:1) were used to recover asphalt, the volume fraction of toluene in the composite solvents was 50%, the asphalt recovery was the highest, and the asphalt recovery was 99.7% with secondary extraction, which is higher than single solvent extraction (toluene: 97.8%). Although the extraction effect of toluene@ethanol was the worst in composite solvents, its extraction effect was better than that of single ethanol (Fig. 9d and Fig. 7c). Fig. 9 also shows the asphalt recovery decreases with the decrease of toluene content in the composite solvents. Indicating toluene plays an important role in the process of asphalt extraction. It also shows among these organic solvents, toluene possess the best extraction effect, which is consistent with the result in Fig. 7c. Furthermore, the introduction of toluene in the composite solvents can significantly increase the ability of single solvent’s (xylene, THF, PE and ethanol) asphalt recovery. No matter single solvent extraction or composite solvents extraction, the solvent recovery was more than 95% (Fig. 10).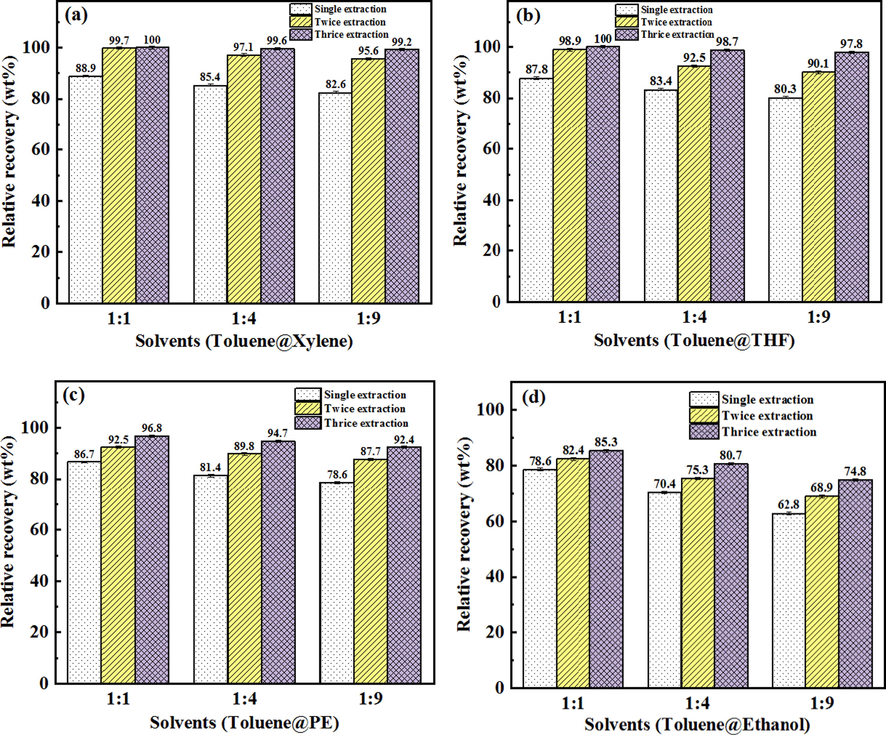
Asphalt recovery from Iranian oil ores by composite solvent extraction with different times of the extraction by solvents (a: Toluene@Xylene, b: Toluene@THF, c: Toluene@PE, d: Toluene@Ethanol).
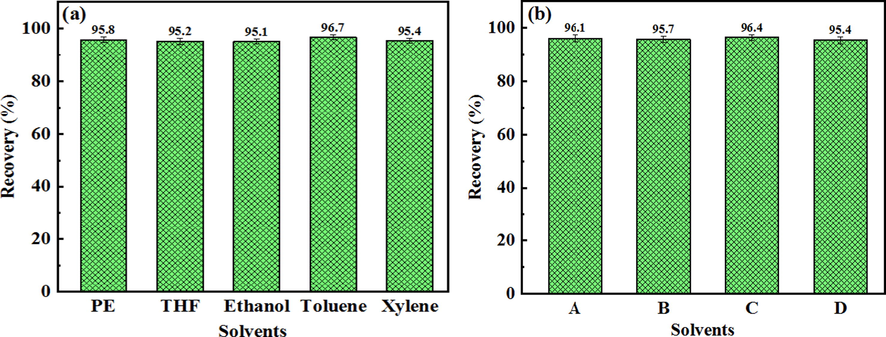
Solvent recovery of single solvent (a) and composite solvents (b). Notes: A is Toluene@Xylene, B is Toluene@THF, C is Toluene@PE, D is Toluene@Ethanol.
3.3 Industrial application prospect of solvent extraction
The above results show the ability of solvent extraction method to separate asphalt from unconventional oil ores is better than HWBE and pyrolysis method. Although the HWBE has been applied in industry, there are some shortcomings (Vedoy and Soares, 2015; Alamgir et al., 2012; Lin et al., 2017). (1) The operation temperature of process water is high, which needs to consume lots of heat energy and emit a lot of greenhouse gas. (2) The HWBE needs a lot of fresh surface water. On average 3–4 barrels of water are consumed for each barrel of asphalt production. Only 75–87% of the process water can be recycled. (3) The HWBE will produce a large number of tailings when recycling asphalt. The tailings are the mixture of water, sands, clay and residual asphalt. The existence of tailings will cause serious environmental harm. The chemical substances such as ammonia, mercury and naphthenic acid in tailings will have toxic effects on animals, especially aquatic organisms. At the same time, the water and pollutants in the tailings will penetrate into the surrounding soil, contaminate the groundwater and damage the surrounding wildlife. (4) Heavy oil–water emulsions are easy to be formed in the process of HWBE. The emulsions will not only corrode the equipments, but also seriously reduce the oil quality and increase the production cost. In addition, the asphalt recovery of oil-wet oil ores is low by HWBE. The pyrolysis method can be used to recover asphalt from different unconventional ores, and it does not need to consume a lot of water in the pyrolysis process. But the pyrolysis process need high operating temperature, large energy consumption, large equipment investment (Zhang et al., 2017). In addition, coke, CO2 and CO will be produced after pyrolysis. These are harmful to the environment. Therefore, pyrolysis method is not suitable for large-scale industrial application in recovering asphalt from unconventional oil ores.
Compared with HWBE and pyrolysis methods, solvent extraction method possess many advantages, including normal temperature operation, no water participation, solvent can be recovered in large quantities, high extraction rate and wide application range. However, in order to make the solvent extraction process better for industrial application, the following three key problems need to be solved in the process of solvent extraction (process flow chart). However, in order to make the solvent extraction process better for industrial application in recovering asphalt from unconventional oil ores, the following three key problems need to be addressed in the process of solvent extraction (Fig. 11).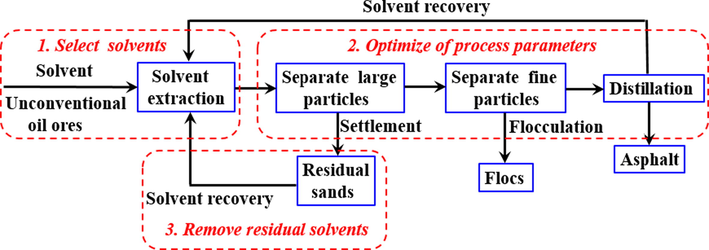
Schematic diagram of solvent extraction process.
(1) The appropriate solvent should be selected, and the effect, cost toxicity of solvent extraction should be considered. Based on this, our group developed green environment-friendly amino acid ionic liquid and switchable solvent, which can be used to recover asphalt from unconventional oil ores, and the asphalt recovery can reach 95.33% and 96.00% respectively (Li et al., 2018; Zhang et al., 2018). The results show the green and environment-friendly solvents will be a better choice for the recovery of asphalt from unconventional oil ores by solvent extraction.
(2) It is necessary to optimize the process conditions of solvent extraction, and the operation parameters of solvent extraction have an important influence on the separation effect. For example, Li et al. studied the effects of various operating parameters on the separation of Athabasca oil ores by n-heptane@toluene composite solvents by multi factor orthogonal method, the results show the liquid–solid ratio and stirring rate are important parameters affecting asphalt recovery, while the stirring time and temperature have little influence (Li et al., 2012). The content of asphaltene in asphalt product is more sensitive to temperature and mixing time. Painter et al. used ionic liquid to assist organic solvent for solvent extraction. Three-phase system was formed by adding ionic liquid into the extraction system, and asphalt extraction liquid was obtained by standard solid–liquid and liquid–liquid separation (Pulati et al., 2012, 2016; Hogshead et al., 2011). The bottom were sand and clay, the middle was ionic liquid layer, and the top is asphalt-organic solvent layer. The asphalt and organic solvent layer at the top can be easily separated by decantation or other methods. The addition of ionic liquid promoted the separation of asphalt from the surface of sand and clay particles, effectively avoided the entrainment of clay particles in organic phase, and the sand in tailings was relatively clean. Sui et al. developed a separation process of hydrophilic switchable solvent assisted organic solvent extraction for separation of asphalt in unconventional oil ores (Sui et al., 2016). In order to improve the recovery rate and reduce the entrainment of residual sands, water-soluble switchable solvent (tertiary amine) was used to assist organic solvent to extract asphalt from oil ores. The improvement of separation effect is mainly due to the adsorption of water-soluble reversible tertiary amine protonated cations, adsorbed at the surface of asphalt and mineral solids and formed ion pairs, it leads to the improve wettability of mineral solids. The weakening of interaction between asphalt and mineral solids makes asphalt more effectively separated from oil ores.
(3) The residual solvents in residual sands should be removed after extraction. After extraction, there will be a certain amount of residual solvent in the residual sand, which not only causes the loss of solvent, but also brings environmental harm. Due to the existence of residual solvent, the residual sand cannot be discharged directly. The series of treatments need to be carried to reduce the residual solvent content to reach the environmental protection standard before discharge. Aiming at the problem of solvent residue, our group developed the gas bubbling method to remove residual solvents in residual sands. After using organic solvents to extract asphalt from unconventional oil ores, the 97.6% toluen in residual sands can be removed by gas bubbling method (Wang et al., 2020). It shows the gas bubbling method possess potential application prospect in the separation process of unconventional oil ores.
To sum up, solvent extraction is a better choice for recovering asphalt from unconventional oil ores, it possess potential industrial application prospect in the separation of unconventional oil ores, and it is expected to carry out large-scale industrial application in the future.
4 Conclusions
In this work, the HWBE, pyrolysis and solvent extraction method have been applied for recovering asphalt from different unconventional oil ores. It is found that the solvent extraction method performs much better than that of HWBE and pyrolysis method. The three oil ores have been extracted by solvent extraction with four different typical solvents, including toluene, THF, xylene, PE and ethanol. It is discovered that toluene possess the best extraction effect, and the asphalt recovery is more than 96% after second extraction. The various composite solvents, including toluene@xylene, toluene@THF, toluene@PE, and toluene@ethanol, are used to recover asphalt from oil-wetted Iranian oil ores. It is found that composite solvents perform much better than single solvent in separating asphalt from Iranian oil ores, and composite solvents (toluene@xylene) possess more strong ability in recovering asphalt than other three composite solvents. In addition, it is observed that the volume ratio of composite solvents is 1:1, the most asphalt is recovered from Iranian oil ores. No matter single solvent or composite solvents is used to recover asphalt from unconventional oil ores, more than 95% of the solvent can be recovered. It is also found that increasing the extraction times is proven to significantly enhance the asphalt recovery. Therefore, solvent extraction method is the potential choice to recover asphalt from unconventional oil ores. Indeed, our previous research results also showed that switchable solvent and amino acid ionic liquids have excellent performance in the separation of unconventional oil ores, and in the removal of residual solvents by gas bubbling method. As a result, in order to promote the industrialization process of solvent extraction in recovering asphalt from unconventional oil ores, we suggest that future studies modify the two kinds of green solvents, further improve the asphalt extraction rate and optimize the gas bubbling method process parameters.
Acknowledgements
This work was supported by National Natural Science Foundation of China (Grant No. 22208071). Guizhou Provincial Science and Technology Projects (Grant No. ZK[2022]088). The cultivation project of Guizhou University (Grant No. [2020]38), Natural Science Special Foundation of Guizhou University (Grant No. X2021073), Innovation Group Project of Education Department in Guizhou Province (Grant No. 2021010).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Al-PAM assisted filtration system for abatement of mature fine tailings. Chem. Eng. Sci.. 2012;80:91-99.
- [Google Scholar]
- Barton, A., 1991. Handbook of solubility and other cohesion parameters.
- Soxhlet extraction of solid materials: an outdated technique with a promising innovative future. Anal. Chim. Acta. 1998;369:1-10.
- [Google Scholar]
- Characterization of minerals in oil sands by reflectance spectroscopy. Fuel. 1995;74:874-879.
- [Google Scholar]
- New trends in pla-based (nano) composites. In: 8th World Congress of Chemical Engineering. 2009. p. :23-27.
- [Google Scholar]
- Production of biodiesel: a technical review. Renew. Sustain. Energy Rev.. 2011;15:4732-4745.
- [Google Scholar]
- Hansen, C., 2007. Solubility parameters-an introduction.
- Parameters of solvent extraction for bitumen recovery from oil sands. Adv. Mat. Res. 2011:3728-3731.
- [Google Scholar]
- Interfacial sciences in unconventional petroleum production: from fundamentals to applications. Chem. Soc. Rev.. 2015;44:5446-5494.
- [Google Scholar]
- A regulatory policy to promote renewable energy consumption in China: Review and future evolutionary path. Renew. Energy. 2016;89:695-705.
- [Google Scholar]
- Studies of bitumen silica and oil silica interactions in ionic liquids. Energy Fuel. 2011;25:293-299.
- [Google Scholar]
- Water-based bitumen recovery from diluent-conditioned oil sands. Can. J. Chem. Eng.. 2004;82:978-985.
- [Google Scholar]
- Pyrolysis behavior of Indonesia oil sand by TG-FTIR and in a fixed bed reactor. J. Anal. Appl. Pyrol.. 2015;114:250-255.
- [Google Scholar]
- Operational parameters, evaluation methods, and fundamental mechanisms: aspects of nonaqueous extraction of bitumen from oil sands. Energy Fuel. 2012;26:3553-3563.
- [Google Scholar]
- A hybrid process for oil-solid separation by a novel multifunctional switchable solvent. Fuel. 2018;221:303-310.
- [Google Scholar]
- Understanding desorption of oil fractions from mineral surfaces. Fuel. 2018;232:257-266.
- [Google Scholar]
- Study of different kinetic models for oil shale pyrolysis. Fuel Process. Technol.. 2004;45:140-144.
- [Google Scholar]
- Recent advances in nonaqueous extraction of bitumen from mineable oil sands: a review. Org. Process Res. Dev.. 2017;21:492-510.
- [Google Scholar]
- Effect of operating temperature on water-based oil sands processing. Can. J. Chem. Eng.. 2007;85:726-738.
- [Google Scholar]
- Understanding water-based bitumen extraction from Athabasca oil sands. Can. J. Chem. Eng.. 2004;82:628-654.
- [Google Scholar]
- Heavy oil and natural bitumen resources in geologic basins of the world. Open-File Report 2007
- [Google Scholar]
- Understanding molecular interactions of asphaltenes in organic solvents using a surface force apparatus. J. Phys. Chem. C. 2011;115:16043-16051.
- [Google Scholar]
- Influence of temperature on the product distribution during the fast pyrolysis of Indonesian oil sands and the relationships of the products to the oil sand organic structure. Energy Fuel. 2017;31:1318-1328.
- [Google Scholar]
- Solvent screening for non-aqueous extraction of Alberta oil sands, The. Can. J. Chem. Eng.. 2013;91:1153-1160.
- [Google Scholar]
- Performance of solvent mixtures for non-aqueous extraction of Alberta oil sands. Energy Fuel. 2015;29:2261-2267.
- [Google Scholar]
- Low-temperature treatment of illinois no. 6 coal in ionic liquids. Energy Fuels. 2012;26:3548-3552.
- [Google Scholar]
- Bitumen-silica interactions in a deep eutectic ionic liquid analogue. Energy Fuel. 2016;30:249-255.
- [Google Scholar]
- Effect of oil sands slurry conditioning on bitumen recovery from oil sands ores. Canada: University of Alberta; 2010.
- An overview of heavy oil properties and its recovery and transportation methods. Braz. J. Chem. Eng.. 2014;31:571-590.
- [Google Scholar]
- Study of bitumen liberation from oil sands ores by online visualization. Energy Fuels. 2012;26:2883-2890.
- [Google Scholar]
- Recovery of heavy hydrocarbons from Indonesian carbonate asphalt rocks. Part 1: solvent extraction, particle sedimentation, and solvent recycling. Energy Fuel. 2016;30:9242-9249.
- [Google Scholar]
- Understanding the roles of switchable-hydrophilicity tertiary amines in recovering heavy hydrocarbons from oil sands. Chem. Eng. J.. 2016;290:312-318.
- [Google Scholar]
- Water-soluble polymers for oil sands tailing treatment: a review. Can. J. Chem. Eng.. 2015;93:888-904.
- [Google Scholar]
- Inhibitory action of flame retardant on the dynamic evolution of asphalt pyrolysis volatiles. Fuel. 2013;105:757-763.
- [Google Scholar]
- Combustion characteristics of Indonesian oil sands. Fuel Process. Technol.. 2012;99:110-114.
- [Google Scholar]
- Pyrolysis model of oil sand using thermogravimetric analysis. J. Therm. Anal. Calorim.. 2014;116:499-509.
- [Google Scholar]
- Removal of residual solvent from solvent-extracted unconventional oil ores gangue by gas bubbling. Sep. Purif. Technol.. 2020;254:117551
- [Google Scholar]
- Separation and characterization of bitumen from Athabasca oil sand. Korean J. Chem. Eng.. 2009;26:64-71.
- [Google Scholar]
- Understanding the co-pyrolysis behavior of Indonesian oil sands and corn straw. Energy Fuel. 2017;31:2538-2547.
- [Google Scholar]
- Synthesis and application of amino acid ionic liquid-based deep eutectic solvents for oil-carbonate mineral separation. Chem. Eng. Sci.. 2018;181:264-271.
- [Google Scholar]
- Novel synthesis of choline-based amino acid ionic liquids and their applications for separating asphalt from carbonate rocks. Nanomaterials. 2019;9:504.
- [Google Scholar]







