Translate this page into:
Selective liquid-phase oxidation of toluene over heterogeneous Mn@ZIF-8 derived catalyst with molecular oxygen in the solvent-free conditions
⁎Corresponding authors at: College of Chemistry and Materials Engineering, Huaihua University, Huaihua 418000, PR China. cg@hhtc.edu.cn (Gui Chen), xbl@hhtc.edu.cn (Bailin Xiang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
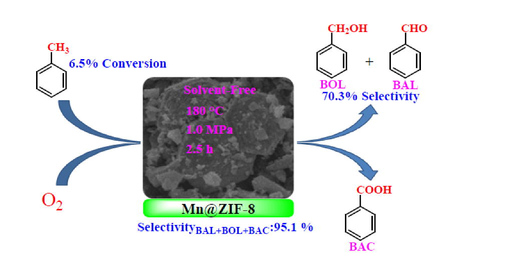
A novel and heterogeneous Mn@ZIF-8 derived catalyst was prepared by solvothermal method. Mn species were good dispersion in the catalyst and its high specific surface area were responsible for excellent catalytic performance. This work affords a new way into selective preparation of aromatic alcohols and aldehydes.
Abstract
In this work, liquid-phase catalytic oxidation of toluene was carried out under solvent-free conditions, and highly selective synthesis of benzaldehyde (BAL) and benzyl alcohol (BOL) and benzoic acid (BAC) in the presence of Mn@ZIF-8 calcined material as catalyst with oxygen molecules. As a heterogeneous catalyst, the zeolitic imidazolate framework Mn@ZIF-8 derived material exhibited reasonable substrate-product selectivity (70.3% of selectivity to BAL and BOL, 95.1 % of selectivity to BAL, BOL and BAC) and conversion (6.5%) under optimum reaction conditions. The catalysts were characterized by BET-specific surface area determination, XRD, XPS, FT-IR, TG-DTG and SEM-EDS-Mapping. The results demonstrated that the catalytic capacity of the catalysts was enhanced by the good dispersion of amorphous Mn species in ZIF-8 derivatives and high specific surface area. The possible reaction pathway for the catalytic oxidation of toluene was also suggested. Maybe this method employing Mn@ZIF-8 as efficient catalyst affords a new and environmentally friendly route for the synthesis of BOL and BAL from the selective oxidation of toluene.
Keywords
Molecular oxygen
Mn@ZIF-8
Toluene
Benzaldehyde
Benzyl alcohol
1 Introduction
As every-one knows, liquid-phase oxidation of toluene has been critical to the development and utilization of fine chemicals (aromatic alcohol and aldehydes) from organic chemical material (Gardner and Mayer, 1995; Tan et al., 2017). The partial oxidation of toluene is an important method for the preparation of benzaldehyde (BAL), benzyl alcohol (BOL) and benzoic acid (BAC) (Somma et al., 2017; Chen et al., 2022). BOL and BAL are important chemical intermediates with high added value, it is widely used in industrial chemical production as organic synthesis raw materials, solvent, stabilizer and preservation, daily chemical essence, and so on (Alabbad et al., 2014; Assal et al., 2019; Rezaei et al., 2017; Zhang et al., 2011). In addition, BAC is an important bulk raw materials for the preparation of sodium benzoate preservatives, and the additives for drugs, dyes (Afnan et al., 2020; Zhu et al., 2016). The methods of toluene as the raw material to prepare BAL and BOL, such as benzyl chloride hydrolysis, indirect electrooxidation, and gas-phase partial oxidation, are extensive limited; mostly because the product obtained by these methods contains chlorine, high environmental pollution and excessive power consumption (Ding et al., 1996; Brown and Hudson, 1951). Due to advantages of environment-friendly way, high atom utilization and suitable reaction conditions, liquid-phase oxidation of toluene is an important method for the preparation of oxygen-containing compounds (Scheme 1). In the oxidation of toluene, heterogeneous catalyst has widespread application, due to their remarkable advantages in efficient selectivity of products, catalyst recovery and utilization, post-processing.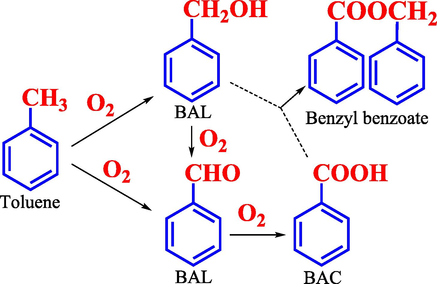
Routes of the synthesis of BOL, BAL and BAC.
However, in the early research process of catalytic oxidation of toluene used peroxide as oxidant, acetic acid as solvent, bromide salt as assistant. Now, it mostly used expensive catalyst in the case of no assistant solvent, such as metal porphyrin, doped Pd, Au and other precious metals catalysts, or that catalyst with complex preparation process. For instance, Wang et al. (Wang et al., 2009) explored bis(acetylacetonato) oxovanadium (VO(acac)2) catalysts for toluene oxidation with H2O2 as oxidant in acetic acid, giving 19.8 % conversion of toluene and 56.1 % selectivity to BAL + BOL, 22.2 % selectivity to BAC. Hu et al. (Hu et al., 2006) investigated metalloporphyrin catalyst μ-oxo-bis[tetra-phenylporphyrin (III)] to catalyze toluene oxidation, the conversion was 7.4 %, the selectivity to BAL + BOL was 59.0 %. In another study, Zhang et al. (Zhang et al., 2008) immobilized Au-Pd alloy nanoparticles on carbon or TiO2 as catalysts to oxidize toluene under solvent-free conditions, the main products are benzyl benzoate (greater than 80 % selectivity) and the by-products are BAL and BAC. Jiang et al. (Jiang et al., 2013) used Au-γMnO2 catalysts for solvent-free toluene oxidation with oxygen, giving 13.5 % conversion of toluene and 67.6 % selectivity to BAL + BOL, 16.8 % selectivity to BAC, 15.6 % selectivity to benzyl benzoate. Therefore, the development of efficient catalysts that are high toluene conversion and efficient selectivity to BAL, BOL in an O2-based system is a great challenge.
Recently, metal–organic frameworks (MOFs) were received considerable attention to catalytic reaction, oxidative degradation and electrochemical sensing because of their properties, such as large surface area, well-defined structures, structural diversity and easy processing (Taghavi et al., 2022; Dehdashtian et al., 2022; Alamgholiloo et al., 2020; Alamgholiloo et al., 2021; Zhao et al., 2020). Pliekhov et al. (Pliekhov et al., 2018) reported Co-MOF-74@NDHPI catalytic system with 16 % toluene conversion; and good selectivity to BAL + BOL (66.0 %) and relatively higher selectivity to BAC (34.0 %), resectively. However, their application is limited by their complicated preparation process and expensive raw materials (Jiang et al., 2010). To our delight, this research has led to a strong interest in catalytic oxidation of toluene using MOFs or zeolitic imidazolate frameworks (ZIFs) to find low-cost, simple and efficient mono-metal-doped metal–organic frameworks catalytic system (Hu et al., 2018; Mohtasham et al., 2022).
Carry on with our work, we successfully synthesized Mn@ZIF-8. Furthermore, based on the previous researcher of liquid-phase oxidation reactions in the solvent- and additives-free conditions (Ni et al., 2021; Jian et al., 2020; Liu et al., 2019; Chen et al., 2022); we will report a simple eco-friendly method for synthesis of BAL, BOL employing Mn@ZIF-8 derived material as an effectual catalytic system for the partial oxidation of toluene with oxygen in this work. This is the meaningful report about the oxidation of toluene to three oxidation products in industry.
2 Experimental
2.1 Reagents and instrument
Mn(CH3COO)2·4H2O (AR, 99.0 %) was obtained from Tianjin Guangfu Fine Chemical Institute, China. Toluene (AR, 99.5 %), Zn(NO3)2·6H2O (AR, 99.0 %) and Ce(NO3) 3·6H2O (AR, 99.0 %) were gained from Sinopharm Chemical Reagent Co., ltd., China. 2-Methylimidazole (AR, 99.0 %) and other reagents were purchased from Shanghai Mclean Co., ltd. Miniature high pressure reactor (BE100) was obtained from Shanghai LABE Instrument Co., ltd. GC–MS qualitative analysis of all products was carried out by Japan Shimadzu GCMS-QP2010 plus. The quantitative analysis of reaction products was detected by GC Agilent 7890B with HP-5 column and hydrogen flame ion detector.
2.2 Catalyst preparation
Mn@ZIF-8 was synthesized by solvothermal method (Zheng et al., 2020). Typically, Mn@ZIF-8 with the 10:1 Zn/Mn molar ratio was prepared by the following steps: firstly, 0.2 g Mn(CH3COO)2·4H2O, 0.6 g 2-methylimidazole and 2.1 g Zn(NO3)2·6H2O were added into 126.5 mL N,N-dimethylformamide under stirring, and then set in a 200 mL autoclave. After autoclaving at 140 °C overnight, the resulting powder was rinsed repeatedly with anhydrous ethanol, dried at low temperature, then calcined at 300 °C for 2.0 h under air atmosphere (static in muffle) to acquire the fresh Mn@ZIF-8 derived catalyst, and the catalyst after reuse was calcined and defined as regenerated catalyst. Other MOF series catalysts (MOF-5, MOF-74) were prepared according to reference (Pliekhov et al., 2018; Xiang et al., 2019).
2.3 Catalyst characterization
The nitrogen adsorption–desorption isotherms were collected by a Quantachrome NOVA-2200e (Degassing was performed at high vacuum for 12 h, followed by N2 adsorption tests at 77 K). XRD analysis were tested by a Rigaku D/Max-2550 V+ diffractometer (Single source CuKα target diffraction source, λ = 1.5418 Å, working voltage 30 kV, working current 40 mA, 2θ = 5-90°, scanning speed 10°/min). XPS analysis were recorded by a ESCALAB 250Xi analyser (Al Ka (1486.6e V) with monochromator as the X-ray excitation source, 150 W power, C 1 s, 284.8 eV). FT-IR spectra were measured on a Nicolet-380 instrument in the range of 4000–400 cm−1 (High purity KBr tablets were pressed and then tested). TG/DTG curves were tested by a Mettler thermogravimetric analyser (Vacuum dried at 105 °C for 12 h, the air flow rate was 40 mL/min, and the room temperature was heated to 800 °C at a rate of 10 °C/min). SEM images were obtained with Sigma HD, Carl Zeiss (FE-SEM, 20 kV acceleration voltage).
2.4 Catalytic experimental procedure
Typically, 21.0 g toluene and 0.1 g Mn@ZIF-8 derived catalyst were added into the 100 mL stainless steel reactor. When the temperature of reactor heated to a certain temperature, molecular oxygen in a high-pressure oxygen tank was continuously put into the reaction equipment after decompression. The reaction was implemented at 180 °C and 1.0 MPa for 2.5 h with 800 r/min stirring. After reaction, the resulted products were quantified by GC (the internal standard substance chlorobenzene).
The toluene conversion and the selectivity to BOL, BAL and BAC were calculated using the following formulas.
3 Results and discussion
3.1 Catalyst characterization
3.1.1 Textural analysis and X-ray diffraction (XRD)
BET-specific surface area determination of fresh and regenerative samples are shown in Fig. 1. The isotherms of two samples have a type-IV curve (type-H3 hysteresis loop), exhibiting the presence of abundant mesoporous structures (Lü et al., 2020; Movahed et al., 2018). Meanwhile, Table 1 shows the textural properties of regenerative sample have little change compare with fresh sample, attributing to great number of C species of the catalyst in the calcination process. ICP result shows low levels of Mn in the catalyst. In addition, two samples have large specific surface area (greater than650 m2/g), which is one of the reasons for their good catalytic performance. This is consistent with the experimental results.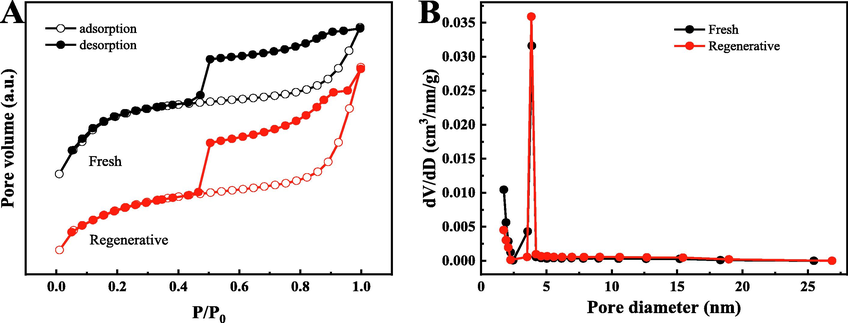
N2 adsorption–desorption isotherms(A) and pore diameter distribution curves of samples(B).
Samples
ICP
(mZn/mMn)Surface area (m2/g)
t-plot micropore area (m2/g)
Pore volume (cm3/g)
t-plot micropore volume (cm3/g)
Pore diameter (nm)
Fresh
161/1
669.7
394.9
0.44
0.21
2.62
Regenerative
157/1
654.5
348.4
0.42
0.19
3.05
Fig. 2 exhibits the XRD curves of four samples. Firstly, the pattern of ZIF-8 was consistent with that reported previously (Abdollahi et al., 2021), confirming the formation of pure crystalline ZIF-8 phase (Jing et al., 2014).The four diffraction peaks appear at 31.8°, 34.4°, 36.3° and 47.5°, belonging to ZnO (JCPDS 36–1451). Compared with ZnO, the patterns of ZIF-8 and Mn@ZIF-8 show specific diffraction peaks. As expected, the characteristic peak (1 1 0) of ZIF-8 at 7.3° was found in four samples. However, the introduction of manganese species in this catalyst leaded to its weakening at 7.3°. Even more, XRD pattern of Mn@ZIF-8 didn’t have a diffraction peak assigned to Mn species, indicating that the Mn species may be present in the framework of the molecule, or the Mn content was below the detection limit (Liu et al., 2016; Deng et al., 2017).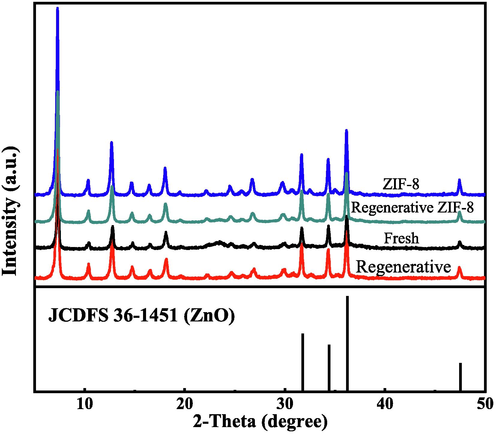
XRD curves of samples.
The average crystallite sizes of four samples are calculated with the Scherrer equation (Ho et al., 2020), the crystallite size of fresh, regenerative samples, ZIF-8 and regenerative ZIF-8 were 38.5, 43.0, 55.5 and 59.2 nm, respectively. It indicated that manganese was successfully incorporated into the catalyst, resulting in a smaller grain size. Combined with the experimental results, its catalytic performance was enhanced.
3.1.2 X-ray photoelectron spectroscopy (XPS)
XPS measurement was conducted to probe the electrons of C 1 s, N 1 s, O 1 s, Mn 2p3/2 and Zn 2p centered at 284.8, 398.9, 531.4, 641.5 and 1021.7 eV, respectively (Fig. 3A). Fig. 3B displays C 1 s spectra, it is fitted into three peaks centered at 284.8 eV (C—C), 286.3 (C—N), and 288.1 (N-C⚌N), respectively (Zhao et al., 2020). However, due to the effects of oxidation reaction, the N-C⚌N is a little bit of a shift towards higher electron volts (An et al., 2022). Fig. 3C shows the N 1 s spectra, it can be fitted into two peaks at 399.9 eV (N—H) and 398.8 eV (Zn-N), this is in good agreement with previously reported (Ran et al., 2020). The O 1 s spectra are decomposed into two peaks containing different oxygen chemical bonds (Fig. 3D). The first peak at the lower bond energies (531.1 eV) is attributed to lattice oxygen (Olatt). Another peak (531.8 eV) is ascribed to chemisorption oxygen or weakly bonded oxygen (Oads) (Du et al., 2018). Fig. 3E shows the Zn 2p spectra of the catalysts, which are decomposed into two peaks (1044.9 and 1021.8 eV) (Zhao et al., 2020; Ran et al., 2020; Li et al., 2022). The Mn 2p3/2 peak (640.5 eV) correspond to superficial Mn2+ species (Fig. 3F), the chemical state of the superficial Mn species on the catalysts is Mn2+ (Musić et al., 2009). Combined XRD analysis, it indicated that the Mn species may be present in the framework of the molecule.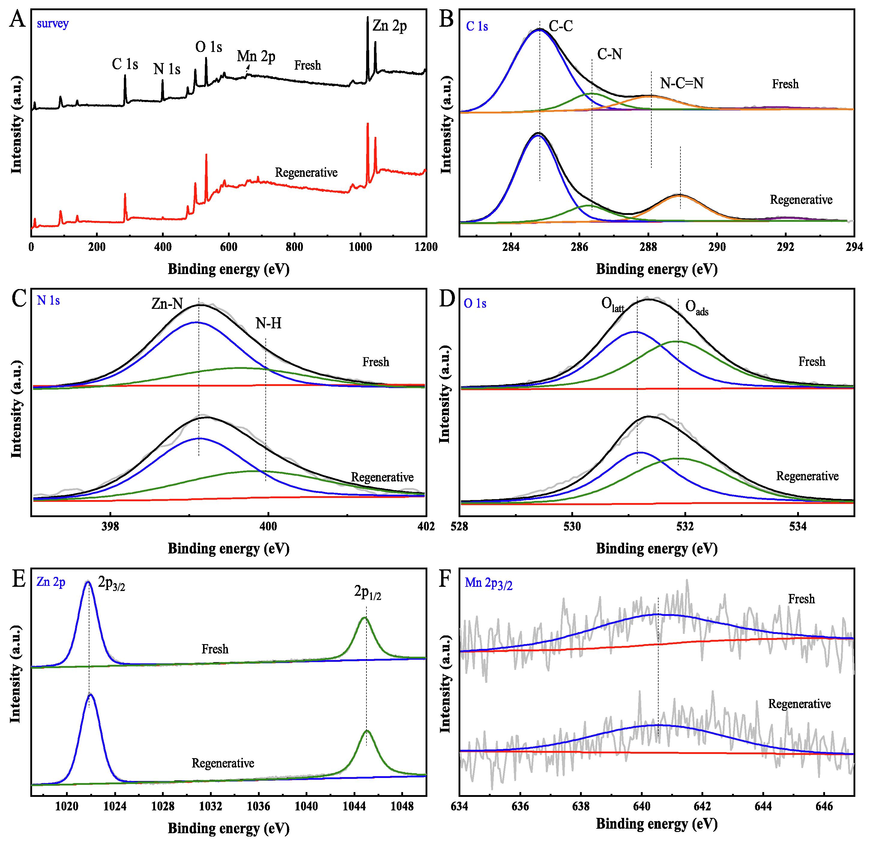
XPS spectra of samples.
3.1.3 Fourier transform infrared spectrogra (FT-IR) and thermal stability characterization
FT-IR (Fig. 4) exhibits many characteristic peaks of three samples. A sharp peak (421 cm−1) is attributed to a typical Zn-N stretching vibration, it indicates that Zn-N form a porous ZIF-8 coordination structure (Jing et al., 2014; Nagarjun and Dhakshinamoorthy, 2019). The peaks at 680 (C—H bending), 750 (C—H bending), 1323 (CH2 wagging) and 2922 cm−1 (C—H symmetric stretch) correspond to the bending signals of the imidazole ring (Movahed et al., 2018; Yang et al., 2018; Mohammed et al., 2021). Furthermore, the peaks at 995 (=C—H in-plane bend), 1146 (=C—H in-plane deformation vibration), 1425 cm−1 (CH2 asymmetric bend) are the stretching vibration of the imidazole ring (Zhao et al., 2020; Yang et al., 2018). At 490 cm−1 for fresh and regenerative samples, it can be ascribed to Mn-O stretching vibration (Musić et al., 2009; Wang et al., 2020). The broad peak (3420 cm−1) was assigned to –OH deformation vibration and N—H stretching mode (Zhang et al., 2020). The peak (1620 cm−1) can be ascribed to C⚌N stretch/ C⚌C stretching vibration (Asadzadeh-Khaneghah et al., 2021; Awadallah-F et al., 2019; Chen et al., 2019). However, compared with ZIF-8, fresh and regenerative samples have two new peaks at 1375 (CH3 asymmetric bend) and 818 cm−1, which may be caused by the product left over from the reaction process. The results demonstrated that the chemical structure of the Mn@ZIF-8 derived samples did not change significantly after regeneration, so that its catalytic performance had a little change in the oxidation of toluene.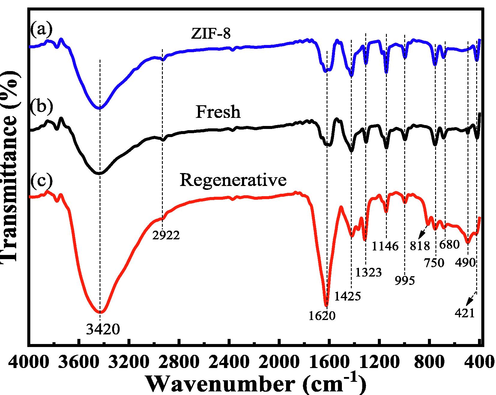
FT-IR spectra of samples.
Fig. 5 shows the TG/DTG curves of two samples. Main weight loss of two samples are concentrated between 350 and 800 °C, meaning to the collapse of the frameworks of ZIF-8 (Fu and Ren, 2020). Notably, the regenerative catalyst exhibits a higher percentage of weight loss, implying that organic matters of the toluene reaction process are primarily embedded in the interior of the Mn@ZIF-8 derived catalyst. These results showed that the catalyst had good thermo-stability in the oxidation of toluene (reaction temperature is lower than 200 °C) (Yang et al., 2018; Wen et al., 2021; Abdelmigeed et al., 2021).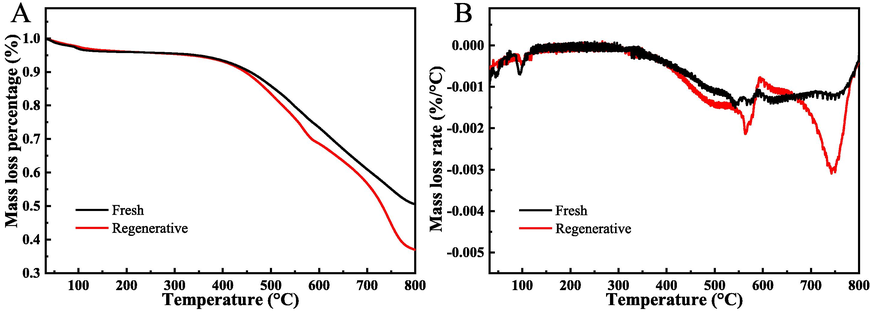
TG (A) and DTG (B) curves of samples.
3.1.4 Morphology and element distribution characterization
SEM characterization is shown in Fig. 6. The shape of samples exhibits polyhedron morphology with size of 6 μm (Guo et al., 2018). Fresh sample is small and irregular pieces (Son et al., 2019). However, regenerative sample is a little aggregated particle. In the process of toluene oxidation reaction, combined with repeated experiments, carbon deposition had a little impact on the morphology and catalytic activity of the catalyst.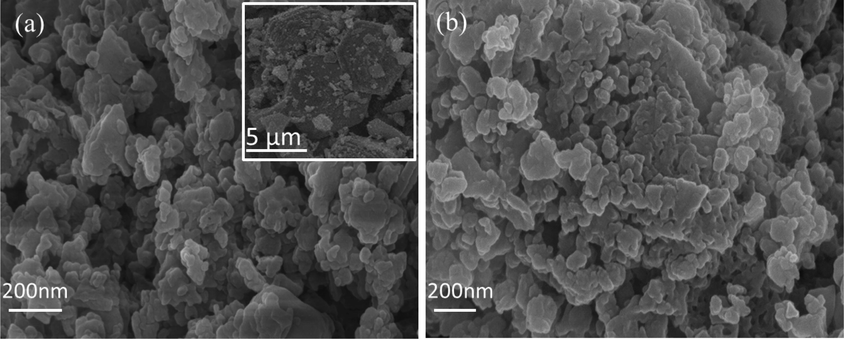
SEM images of fresh(a) and regenerative (b) samples.
The elemental mappings and EDS were done to identify the presence and distribution of four elements (O, N, Mn and Zn) in the two samples (Fig. 7). It indicates that O, N, Mn and Zn are distributed relatively uniformly within two samples. It is noteworthy to mention that the homogeneity of four elements is one reason for its excellent performance in the reaction process (Hao et al., 2018). However, the element ratio of the regenerative sample is different with the fresh sample, which may be the reason for a little decrease of catalytic activity.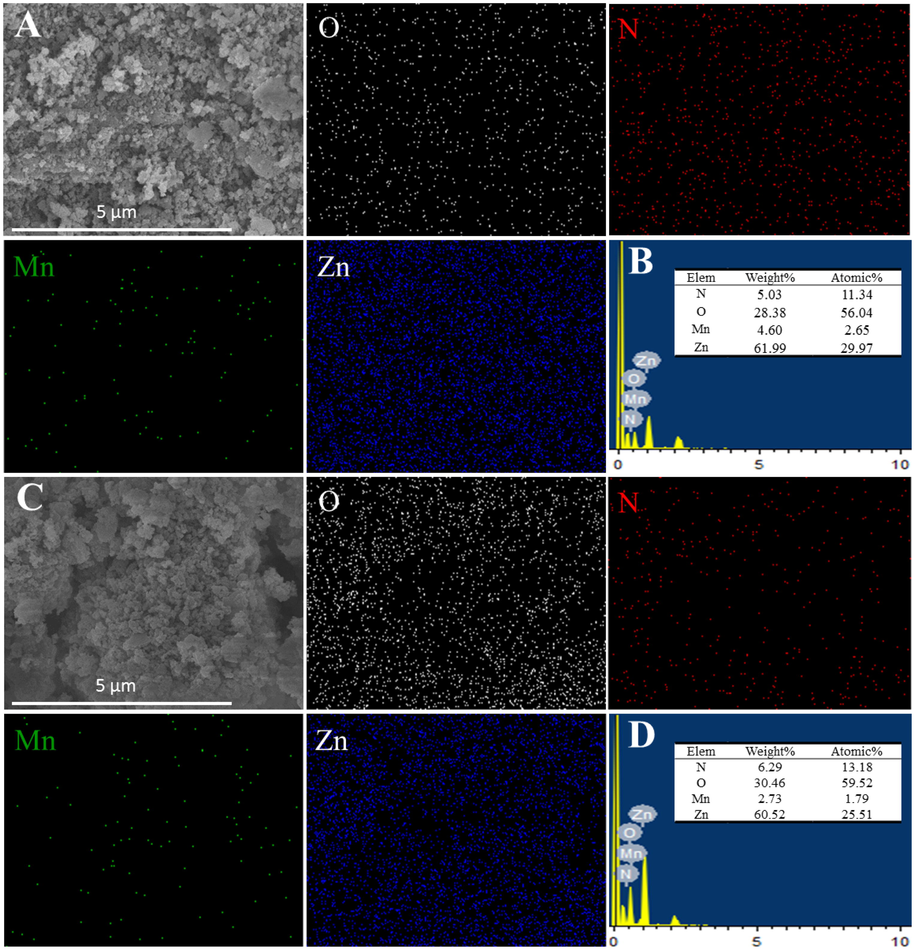
SEM-Mapping images and EDS analyzer of fresh (A, B) and regenerative samples (C, D).
3.2 Catalytic performance
To find an effective catalyst, we examined some MOFs catalysts in the oxidation of toluene (Table 2). Actually, BAL and BOL were obtained in the oxidation of toluene without any catalysts, only 2.5 % of toluene conversion (entry 1). To our delight, it was showed good catalytic activity of ZIF-8 with 5.1 % toluene conversion, 73.6 % selectivity to BAL + BOL (entry 2). However, other catalysts were weak catalysts (entries 3–6).
Entry
Catalyst
Conversion (%)
Selectivity (%)
BAL
BOL
BAC
BAL + BOL
Total b
Others c
1
None
2.5
41.2
30.8
23.7
72.0
95.7
4.3
2
ZIF-8
5.1
38.0
35.5
22.0
73.5
95.5
4.5
3
MOF-74
2.6
56.4
27.4
13.1
83.8
96.9
3.1
4
MOF-5
2.9
55.4
22.0
21.9
77.4
99.3
0.7
5
ZnO
3.9
40.4
31.9
23.3
72.3
95.6
4.4
6
MnO
4.2
44.6
30.2
19.0
74.8
93.8
6.2
7
Mn@MOF-74
5.8
34.5
33.6
26.6
68.1
94.7
5.3
8
Mn@MOF-5
7.0
27.2
21.2
47.8
48.4
96.2
3.8
9
Mn@ZIF-8
6.5
31.6
38.7
24.8
70.3
95.1
4.9
Next, we attempted to incorporate Mn during the in-situ synthesis catalyst to enhance the catalytic activity of MOFs (entries 7–9). Compared with other catalysts, the addition of Mn into ZIF-8 ungently improve the catalytic activity. These results indicates that Mn and ZIF-8 of this catalyst have an interaction and improve the oxidation activity.
3.3 Effect of the preparation factors of catalyst
The performance of Mn@ZIF-8 derived catalyst with the different molar ratio of Zn/Mn and calcination temperature were also explored. As shown in Table 3 and Table 4, With the decrease of Zn/Mn molar ratio, the toluene conversion revealed an upward trend. As the calcination temperature is too high, ZIF-8 skeleton is collapses, which leads to the degradation of catalytic performance (Li et al., 2006; Zhang et al., 2018). when Zn/Mn molar ratio and calcination temperature were 10:1 and 300 °C, respectively, BOL + BAL were obtained the highest selectivity (70.3 %) under the same conditions.
Zn/Mn
(molar ratio)Conversion (%)
Selectivity (%)
BAL
BOL
BAC
BAL + BOL
Total
Others
20/1
5.5
33.2
37.2
24.9
70.4
95.3
4.7
10/1
6.5
31.6
38.7
24.8
70.3
95.1
4.9
5/1
6.9
25.6
30.1
37.1
55.7
92.8
7.3
Calcination
temperature (°C)Conversion (%)
Selectivity (%)
BAL
BOL
BAC
BAL + BOL
Total
Others
300
6.5
31.6
38.7
24.8
70.3
95.1
4.9
500
3.4
46.0
35.5
13.8
81.5
95.3
4.7
700
3.4
46.0
34.4
15.4
80.4
95.8
4.2
900
3.2
39.9
35.9
19.7
75.8
95.5
4.5
3.4 Reaction conditions optimization
This work optimized the toluene oxidation reaction parameters (in Fig. 8). Fig. 8A and 8B showed that the conversion of toluene was gently increased, while BOL + BAL selectivity displayed trend downward with raised the amount of catalyst and oxygen pressure, and the BAC selectivity was increased, other by-products were both increased gradually in a small range. It was that aromatic alcohols and aldehydes could be increase acceleratory oxidized to other productes in higher reaction parameters.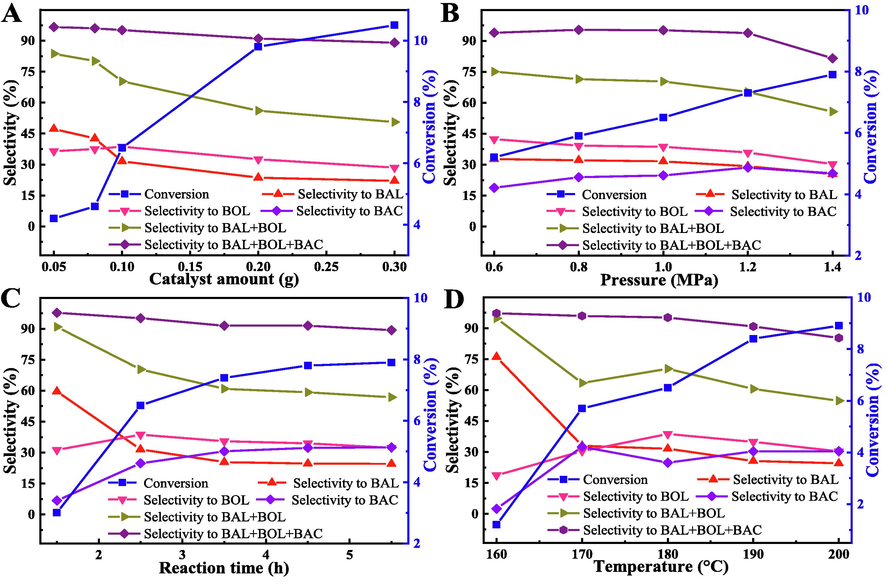
Optimization of reaction parameters. Influences factors: (A) 1.0 MPa oxygen pressure, 180 °C reaction temperature, 2.5 h reaction time; (B) 0.1 g catalyst, 180 °C reaction temperature, 2.5 h reaction time; (C) 0.1 g catalyst, 1.0 MPa oxygen pressure, 180 °C reaction temperature; (D) 0.1 g catalyst, 1.0 MPa oxygen pressure, 2.5 h reaction time.
However, with the increase of reaction time and reaction temperature (Fig. 8C and 8D), the conversion of toluene was an increasing trend firstly, and then decreasing trend, BOL + BAL selectivity showed a falling trend firstly, and then increasing trend, while the selectivity to other by-products were opposite to the trend of BOL + BAL. For reaction time, the performance of catalyst could not be fully exerted when reaction was in the induction phase (Fan et al., 2017) (1.5 h), so the BOL + BAL selectivity was very high. Finally, the pressure of the gas phase toluene also increased as the temperature rising gradually, and the molecular oxygen was actually decreases in the reactor, so it was leaded to the above results (Fu et al., 2022).
Therefore, under the oxidation of toluene reaction conditions: 21.0 g toluene, 180 °C, reaction time of 2.5 h, 0.1 g Mn@ZIF-8 derived catalyst, oxygen pressure of 1.0 MPa, a good substrate-product selectivity (70.3 % of BOL + BAL, 95.1 % of total selectivity to three products) and conversion (6.5 %) were obtained.
3.5 The recycle of Mn@ZIF-8 derived catalyst in the toluene oxidation
The recycle of heterogeneous Mn@ZIF-8 derived catalyst was used in the reaction under the above optimal conditions. Firstly, the catalyst separated by the centrifugation after reaction, washed with ethanol, dried at 105 °C. After calcining at 300 °C for 2.0 h, it was used the following reaction.
As shown in Fig. 9, it was clear that the catalytic activity of recovered catalyst was stable in five runs, the selectivity to BAL + BOL maintained 69.4 % with 5.8 % of toluene conversion. The results indicated that the stability and catalytic activity of Mn@ZIF-8 was a litter changed in the recycle oxidation reaction.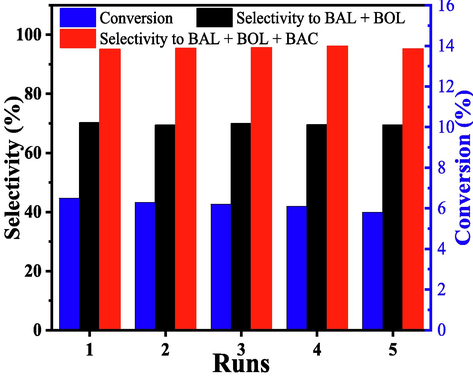
Results of recycle of Mn@ZIF8 catalyst. Reaction conditions: 0.1 g Mn@ZIF-8, 1.0 MPa, 180 °C, 2.5 h, 21.0 g toluene.
3.6 Performance comparison of catalysts reported in the literatures
Table 5 shows the catalytic results of different catalysts in the literatures. It can be seen that different catalytic systems require different reaction conditions, and the toluene conversion is higher in the presence of solvents or additives, but these bring inconvenience to industrial production. If hydrogen peroxide is used as an oxidant, there are also safety problems in industry. Although noble metals or metalloporphyrins have high catalytic activity, their preparation process is complex and their stability is poor, which limits their industrial application. We reported a simple eco-friendly method for synthesis of BAL, BOL employing Mn@ZIF-8 as an effectual catalytic system for the partial oxidation of toluene with oxygen in this work.
Catalyst
Oxidizer
Solvent/ (Initiator)
Temperature (°C)
Time (h)
Conversion (%)
Selectivity (%)
Ref.
BAL
BOL
BAC
VO(acac)2
H2O2
Glacial acid
90
4.0
19.8
50
6.1
22.2
(Wang et al., 2009)
Co-MOF-74@NDHPI
O2
(NDHPI)
100
2.0
16.0
18.0
48.0
34.0
(Alamgholiloo et al., 2020)
Mn3O4/CNTs
O2
(TBHP)
90
12.0
24.63
43.5
47.0
-
(Feng and Zeng, 2020)
Au-γMnO2
O2
–
160
8.0
13.5
3.5
64.1
16.8
(Jiang et al., 2013)
Pt/ZrO2
O2
–
90
3.0
37.2
19.6
6.5
70.4
(Ilyas and Sadiq, 2009)
[TPPFeIII]2O
O2
–
165
3.8
7.4
59.0
-
(Hu et al., 2006)
Co(II)TPP
air
–
160
3.5
8.9
33.0
27.0
39.0
(Guo et al., 2005)
Cu-Mn oxides
O2
–
190
2.0
17.2
11.7
18.7
63.1
(Li et al., 2006)
NHPI/Cu-BTC
O2
–
110
2.0
7.6
44.1
11.0
44.9
(Bao et al., 2016)
SO42-/TiO2-CeO2
O3-O2
–
30
3.0
9.7
77.0
(Wang et al., 2009)
mpg-C3N4
O2
–
160
16.0
3.1
99.0
(Li et al., 2012)
Mn@ZIF-8
O2
–
180
2.5
6.5
31.6
38.7
24.8
[this work]
3.7 Possible catalytic oxidation reaction pathway of toluene over Mn@ZIF-8 derived catalysts
Fig. 10 shows a possible catalytic oxidation reaction pathway of toluene to BAL and BOL over heterogeneous Mn@ZIF-8 derived catalyst. The catalyst has been critical effect at the beginning of the oxidation reaction through the activation of reactant toluene, and then formation of the benzyl radical (C7H7•) and the intermediate benzyl peroxyradical (ArOO•) (Partenheimer, 2003). The reactive oxygen species can react with absorbed toluene to form the radical intermediate (ArOOH) (Biswas et al., 2020; Laribi et al., 2016). To prove the existence of the radical, tert-butylalcohol (hydroxyl radical scavenger) was add to the reaction system (Biswas et al., 2020). Just as we expected, a significant decrease of toluene conversion (1.2 %) was observed, which confirmed that the reactive radical intermediates reacted with tert-butylalcohol, resulting in a relative decrease of the toluene conversion. Finally, the resulting ArOOH intermediate were decomposed to BAL, and some of them were simultaneously reduced to BOL. The resulting BOL can be to BAL, even to BAC (Xu et al., 2021).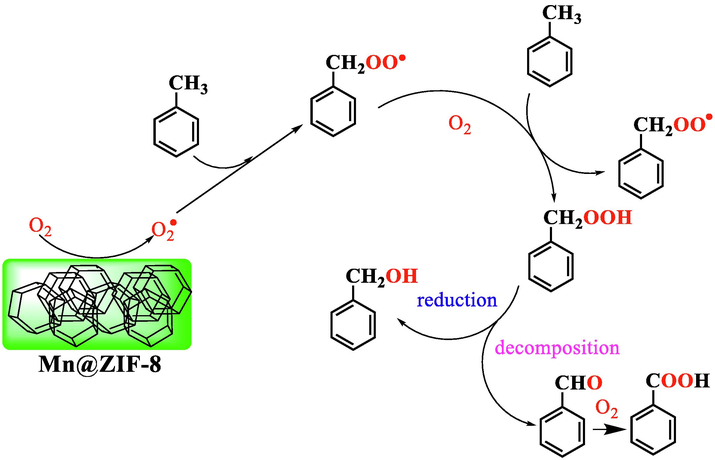
Possible reaction pathway of toluene oxidation to BAL and BOL.
4 Conclusions
In summary, a novel Mn@ZIF-8 derived catalyst with low-cost, high catalytic activity, good heat resistance and stability was constructed, and used for the selective partial oxidation of toluene to BOL, BAL and BOL. 70.3 % selectivity to BAL + BOL with 6.5 % toluene conversion was acquired under optimum reaction conditions (21.0 g toluene, 180 °C, reaction time of 2.5 h, 0.1 g Mn@ZIF-8 catalyst, oxygen pressure of 1.0 MPa). Besides, the possible reaction pathway was suggested, and the results of characterization showed that the catalytic capacity of the catalyst was enhanced by the good dispersion of amorphous Mn species in ZIF-8 and high specific surface area (669.7 m2/g). This way using Mn@ZIF-8 calcined material as catalyst, molecular oxygen as green oxidant is a favorable industrial applications routine for efficient production of BOL and BAL.
Acknowledgments
The authors are grateful for the financial support of Key Research and Development Program of Hunan Province (2016NK2134), Project of Hunan Engineering Laboratory for Preparation Technology of Polyvinyl Alcohol (PVA) Fiber Material (HGY201810), Innovation Training Program for College Students of China (202110548015), Innovation Training Program for College Students of Hunan Province (S202110548055), Research Project of Huaihua University (HHUY2019-17).
References
- Magnetized ZIF-8 impregnated with sodium hydroxide as a heterogeneous catalyst for high-quality biodiesel production. Renew Energ.. 2021;165:405-419.
- [CrossRef] [Google Scholar]
- Fabrication of ZiF-8 metal organic framework (MOFs)-based CuO-ZnO photocatalyst with enhanced solar-light-driven property for degradation of organic dyes. Arab. J. Chem.. 2021;14:103444
- [CrossRef] [Google Scholar]
- Synthesis of magnetic CuFe2O4 nanoparticles as green catalyst for toluene oxidation under solvent-free conditions. Arab. J. Chem.. 2020;13:4945-4953.
- [CrossRef] [Google Scholar]
- Gold & silver nanoparticles supported on manganese oxide: Synthesis, characterization and catalytic studies for selective oxidation of benzyl alcohol. Arab. J. Chem.. 2014;7:1192-1198.
- [CrossRef] [Google Scholar]
- Formation and stabilization of colloidal ultra-small palladium nanoparticles on diamine-modified Cr-MIL-101: synergic boost to hydrogen production from formic acid. J. Colloid Interf. Sci.. 2020;567:126-135.
- [CrossRef] [Google Scholar]
- Synergistic advanced oxidation process for the fast degradation of ciprofloxacin antibiotics using a GO/CuMOF-magnetic ternary nanocomposite. J. Environ. Chem. Eng.. 2021;9:105486
- [CrossRef] [Google Scholar]
- Sacrificial template induced Fe-, N-, and S-tridoped hollow carbon sphere as a highly efficient electrocatalyst for oxygen reduction reaction. Mol. Catal.. 2022;530:112589
- [CrossRef] [Google Scholar]
- Fabrication of novel g-C3N4 nanosheet/carbon dots/Ag6Si2O7 nanocomposites with high stability and enhanced visible-light photocatalytic activity. J. Taiwan Inst. Chem. Eng.. 2021;122:320-321.
- [CrossRef] [Google Scholar]
- Ag2O nanoparticles/MnCO3, -MnO2 or -Mn2O3/highly reduced graphene oxide composites as an efficient and recyclable oxidation catalyst. Arab. J. Chem.. 2019;12:54-68.
- [CrossRef] [Google Scholar]
- On the nanogate-opening pressures of copper-doped zeolitic imidazolate framework ZIF-8 for the adsorption of propane, propylene, isobutane, and n-butane. J. Mater. Sci.. 2019;54:5513-5527.
- [CrossRef] [Google Scholar]
- N-hydroxyphthalimide incorporated onto Cu-BTC metal organic frameworks: an novel catalyst for aerobic oxidation of toluene. Res. Chem. Intermed.. 2016;42:5527-5539.
- [CrossRef] [Google Scholar]
- AgNPs immobilized over functionalized 2D hexagonal SBA-15 for catalytic C-H oxidation of hydrocarbons with molecular oxygen under solvent-free conditions, ACS Sustainable Chem. Eng.. 2020;8:5856-5867.
- [CrossRef] [Google Scholar]
- Controllable preparation of porous hollow carbon sphere@ZIF-8: novel core-shell nanomaterial for Pb2+ adsorption. Colloid Sur. A.. 2019;568:461-469.
- [CrossRef] [Google Scholar]
- Solvent–free catalytic oxidation of toluene over heterogeneous CeMnOx composite oxides. Res. Chem. Intermed.. 2022;48:2593-2606.
- [CrossRef] [Google Scholar]
- Solvent-free liquid-phase selective catalytic oxidation of toluene to benzyl alcohol and benzaldehyde over CeO2-MnOx composite oxides. React. Chem. Eng.. 2022;7:898-907.
- [CrossRef] [Google Scholar]
- Electrochemical sensing of indole in plasma using Pd nanoparticles modified metal-organic framework Cr-MIL-101/ionic liquid sensor. Microchem. J.. 2022;181:107839
- [CrossRef] [Google Scholar]
- Well-defined ZIF-derived Fe-N codoped carbon nanoframes as efficient oxygen reduction catalysts. ACS Appl. Mater. Inter.. 2017;9:9699-9709.
- [CrossRef] [Google Scholar]
- A study on wastewater minimization in indirect electrochemical synthesis of benzaldehyde. Water. Sci. Technol.. 1996;34:113-120.
- [CrossRef] [Google Scholar]
- Capacity fading of nanoporous carbon electrode derived from ZIF-8 during insertion-desertion of lithium ions. Chem. Phys. Lett.. 2018;712:7-12.
- [CrossRef] [Google Scholar]
- Effect of acidic and red-ox sites over modified ZSM-5 surface on selectivity in oxidation of toluene. Mol. Catal.. 2017;442:20-26.
- [CrossRef] [Google Scholar]
- Selective liquid-phase oxidation of toluene with molecular oxygen catalyzed by Mn3O4 nanoparticles immobilized on CNTs under solvent-free conditions. Catalysts.. 2020;10:623-635.
- [CrossRef] [Google Scholar]
- Synthesis of Double-shell hollow TiO2@ZIF-8 nanoparticles with enhanced photocatalytic activities. Front. Chem.. 2020;8:578847
- [CrossRef] [Google Scholar]
- Ultrasound-assisted co-precipitation synthesis of mesoporous Co3O4-CeO2 composite oxides for highly selective catalytic oxidation of cyclohexane. Front. Chem. Sci. Eng.. 2022;16:1211-1223.
- [CrossRef] [Google Scholar]
- Understanding C-H bond oxidations: H· and H- transfer in the oxidation of toluene by permanganate. Science. 1995;269:1849-1851.
- [CrossRef] [Google Scholar]
- Comparative studies on ZIF-8 and SiO₂ nanoparticles as carrier for immobilized β-glucosidase. Mol. Catal.. 2018;459:1-7.
- [CrossRef] [Google Scholar]
- Selective liquid phase oxidation of toluene with air. Appl. Catal. A.. 2005;282:55-59.
- [CrossRef] [Google Scholar]
- Direct imaging for single molecular chain of surfactant on CeO2 nanocrystals. Small. 2018;14:1093-1100.
- [CrossRef] [Google Scholar]
- One-pot synthesis of 5-FU@ZIF-8 and ibuprofen@ZIF-8 nanoparticles. Inorg. Chim. Acta.. 2020;500:119229
- [CrossRef] [Google Scholar]
- Catalysis of μ-oxo-bis[porphyriniron(III)] for toluene oxidation with molecular oxygen. J. Porphyrins. Phthalocyanines.. 2006;10:948-952.
- [CrossRef] [Google Scholar]
- Taking organic reactions over metal-organic frameworks as heterogeneous catalysis. Micropor. Mesopor. Mat.. 2018;256:111-127.
- [CrossRef] [Google Scholar]
- Oxidation of toluene to benzoic acid catalyzed by platinum supported on zirconia in the liquid phase-solvent free conditions. Catal. Lett.. 2009;128:337-342.
- [CrossRef] [Google Scholar]
- Highly dispersed Co/SBA-15 mesoporous materials as efficient and stable catalyst for partial oxidation of cyclohexane with molecular oxygen. Mater. Chem. Phys.. 2020;246:122814
- [CrossRef] [Google Scholar]
- Copper metal-organic framework: Structure and activity in the allylic oxidation of cyclohexene with molecular oxygen. J. Catal.. 2010;270:26-33.
- [CrossRef] [Google Scholar]
- Au/γ-MnO2 catalyst for solvent-free toluene oxidation with oxygen. Chin. J. Catal.. 2013;34:1683-1689.
- [CrossRef] [Google Scholar]
- Photocatalytic degradation of methylene blue in ZIF-8. RSC Adv.. 2014;4:54454-54462.
- [CrossRef] [Google Scholar]
- Elaboration of nickel-impregnated over hexagonal mesoporous materials and their catalytic application. Arab. J. Chem.. 2016;9:1388-1393.
- [CrossRef] [Google Scholar]
- Magnetic Fe3O4/ZIF-8 optimization by Box-Behnken design and its Cd(II)-adsorption properties and mechanism. Arab. J. Chem.. 2022;15:104119
- [CrossRef] [Google Scholar]
- Liquid-phase oxidation of toluene by molecular oxygen over copper manganese oxides. Catal. Lett.. 2006;110:149-154.
- [CrossRef] [Google Scholar]
- Liquid-phase oxidation of toluene by molecular oxygen over copper manganese oxides. Catal. Lett.. 2006;110:255-260.
- [CrossRef] [Google Scholar]
- Solvent-free and metal-free oxidation of toluene using O2 and g-C3N4 with nanopores: Nanostructure boosts the catalytic selectivity. ACS Catal.. 2012;2:2082-2086.
- [CrossRef] [Google Scholar]
- NiO-PTA supported on ZIF-8 as a highly effective catalyst for hydrocracking of Jatropha oil. Sci. Rep-UK. 2016;6:23667.
- [CrossRef] [Google Scholar]
- Hydrotalcite-derived Co-MgAlO mixed metal oxides as efficient and stable catalyst for the solvent-free selective oxidation of cyclohexane with molecular oxygen. Mol. Catal.. 2019;466:130-137.
- [CrossRef] [Google Scholar]
- Fischer-tropsch synthesis: ZIF-8@ ZIF-67-derived cobalt nanoparticle-embedded nanocage catalysts. Ind. Eng. Chem. Res.. 2020;59:12352-12359.
- [CrossRef] [Google Scholar]
- Insights into methyl orange adsorption behavior on a cadmium zeolitic-imidazolate framework Cd-ZIF-8: A joint experimental and theoretical study. Arab. J. Chem.. 2021;14:102897
- [CrossRef] [Google Scholar]
- Nano-architecture of MOF (ZIF-67)-based Co3O4 NPs@N-doped porous carbon polyhedral nanocomposites for oxidative degradation of antibiotic sulfamethoxazole from wastewater. Chemosphere.. 2022;310:136625
- [CrossRef] [Google Scholar]
- A nitrogen-doped porous carbon derived from copper phthalocyanines on/in ZIF-8 as an efficient photocatalyst for the degradation of dyes and the Csingle bondH activation of formamides. J. Photoch. Photobio. A.. 2018;351:208-224.
- [CrossRef] [Google Scholar]
- Synthesis and microstructure of porous Mn-oxides. J. Mol. Struct.. 2009;924-926:243-247.
- [CrossRef] [Google Scholar]
- A Cu-Doped ZIF-8 metal organic framework as a heterogeneous solid catalyst for aerobic oxidation of benzylic hydrocarbons. New. J. Chem.. 2019;43:18702.
- [CrossRef] [Google Scholar]
- Highly selective preparation of cyclohexanone oxime from liquid-phase catalytic oxidation of cyclohexylamine with molecular oxygen over glucose-modified TiO2 under solvent-free conditions. Ind. Eng. Chem. Res.. 2021;60:3907-3913.
- [CrossRef] [Google Scholar]
- The effect of zirconium in metal/bromide catalysts during the autoxidation of p-xylene: Part i. Activation and changes in benzaldehyde intermediate formation. J. Mol. Catal. A: Chem.. 2003;206:105-119.
- [CrossRef] [Google Scholar]
- The Co-MOF-74 modified with N, N′-dihydroxypyromellitimide for selective, solvent free aerobic oxidation of toluene. Catal. Commun.. 2018;110:88-92.
- [CrossRef] [Google Scholar]
- One-step in-situ growth of zeolitic imidazole frameworks-8 on cotton fabrics for photocatalysis and antimicrobial activity. Cellulose. 2020;27:10447-10459.
- [CrossRef] [Google Scholar]
- Selective oxidation of toluene to benzaldehyde by H2O2 with mesoporous silica KIT-6 supported VOHPO40.5H2O catalyst. J. Environ Chem. Eng.. 2017;5:3529-3539.
- [CrossRef] [Google Scholar]
- Kinetic modelling of benzyl alcohol selective oxidation in aqueous mixtures of nitric and sulfuric acids. Chem. Eng. J.. 2017;308:738-744.
- [CrossRef] [Google Scholar]
- N, S-doped nanocarbon derived from ZIF-8 as a highly efficient and durable electro-catalyst for oxygen reduction reaction. J. Solid State. Chem.. 2019;274:237-242.
- [CrossRef] [Google Scholar]
- Magnetite metal-organic frameworks: applications in environmental remediation of heavy metals, organic contaminants, and other pollutants. Inorg. Chem.. 2022;61:15747-15783.
- [CrossRef] [Google Scholar]
- Enhancing the oxidation of toluene with external electric fields: a reactive molecular dynamics study. Sci. Rep.. 2017;7:1-11.
- [CrossRef] [Google Scholar]
- ZIF-8-modified MnFe2O4 with high crystallinity and superior photo-Fenton catalytic activity by Zn-O-Fe structure for TC degradation. Chem. Eng. J.. 2020;392:124851
- [CrossRef] [Google Scholar]
- A mild simple method for liquid-phase selective catalytic oxidation of toluene with ozone over CeO2 promoted sulfated TiO2. Ind. Eng. Chem. Res.. 2009;48:440-445.
- [CrossRef] [Google Scholar]
- Partial oxidation of toluene in CH3COOH by H2O2 in the presence of VO(acac)2 catalyst. J. Phys. Chem. C. 2009;113:14270-14278.
- [CrossRef] [Google Scholar]
- Mesoporous silicon sulfonic acid as a highly efficient and stable catalyst for the selective hydroamination of cyclohexene with cyclohexylamine to dicyclohexylamine in the vapor phase. Front. Chem. Sci. Eng.. 2021;15:654-665.
- [CrossRef] [Google Scholar]
- Two synthesis methods for Fe(III)@MOF-5-derived porous carbon composites for enhanced phenol hydroxylation. ChemistrySelect.. 2019;12:13638-13645.
- [CrossRef] [Google Scholar]
- Selective aerobic oxidation of toluene to benzaldehyde catalyzed by covalently anchored N-hydroxyphthalimide and cobaltous ions. Mol. Catal.. 2021;503:111440
- [CrossRef] [Google Scholar]
- Synthesis of ZnO/ZIF-8 hybrid photocatalysts derived from ZIF-8 with enhanced photocatalytic activity. Inorg. Chem. Front.. 2018;5:687-693.
- [CrossRef] [Google Scholar]
- Synthesis of mesoporous ZIF-8 nanoribbons and their conversion into carbon nanoribbons for high-performance supercapacitors. Chem. Eur. J.. 2018;24:11185-11192.
- [CrossRef] [Google Scholar]
- Simulation and optimization in the process of toluene liquid-phase catalytic oxidation. Chin. J. Chem. Eng.. 2008;16:36-38.
- [CrossRef] [Google Scholar]
- Preparation of novel bimetallic CuZn-BTC coordination polymer nanorod for methanol synthesis from CO2 hydrogenation. Mater. Chem. Phys.. 2018;215:11-20.
- [CrossRef] [Google Scholar]
- Solvent-free liquid phase oxidation of benzyl alcohol to benzaldehyde over superfine MgAl2O4 supported Co-based catalysts: effects of support MgAl2O4. Adv. Mater. Res.. 2011;233–235:1100-1107.
- [CrossRef] [Google Scholar]
- Preparation of magnetic CuFe2O4@Ag@ZIF-8 nanocomposites with highly catalytic activity based on cellulose nanocrystals. Molecules. 2020;25:124-139.
- [CrossRef] [Google Scholar]
- In situ growth of Co3O4 nano-dodecahedeons on In2O3 hexagonal prisms for toluene catalytic combustion. Arab. J. Chem.. 2020;13:4857-4867.
- [CrossRef] [Google Scholar]
- Efficient production of H2O2 via two-channel pathway over ZIF-8/C3N4 composite photocatalyst without any sacrificial agent. Appl. Catal. B: Environ.. 2020;278:119289
- [CrossRef] [Google Scholar]
- One-step synthesis of ZIF-8/ZnO composites based on coordination defect strategy and its derivatives for photocatalysis. J. Alloys Compd.. 2020;838:155219
- [CrossRef] [Google Scholar]
- Solar thermoelectric field plus photocatalysis for efficient organic synthesis exemplified by toluene to benzoic acid. Appl. Catal. B: Environ.. 2016;193:151-159.
- [CrossRef] [Google Scholar]







