Translate this page into:
Moisture sorption isotherms of whole and fractionated date-pits: Measurement and theoretical modelling
⁎Corresponding author. habsin@squ.edu.om (Nasser Al-Habsi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Moisture sorption properties of whole date-pits (WDP) and three fractionated date-pits; defatted date-pits (DDP), residue (REP) and supernatant (SUP) fractions were studied. Sorption isotherms were measured using Differential Thermal and Humidity chamber at different temperatures (10, 30, 50, 70 and 90 °C) and relative humidity ranged from 0.05 to 0.9. Crossovers of isotherm curves were observed for all fractions between different temperatures. At 30 °C, BET-monolayers of WDP, DDP, REP and SUP were 4.9, 4.2, 3.8 and 3.2 g/100 g ds, respectively. The complete isotherms (i.e. 0.05–0.90 water activity) were modelled by GAB with high coefficient of determination (i.e. 0.944 to 0.999). In the cases of WDP and DDP, the isosteric heat plots showed increasing trends with the decreased moisture and peaks at moisture 0.025 and 0.040 g/100 g ds. Unlike all fractions, DDP isokinetic temperatures Tis (i.e. 97.4 °C) was lower than isobound temperatures Tib (i.e. 145.0 °C) indicating complete removal of bound water occurred at higher temperature as compared to the temperature when all reactions reached at the same. The moisture sorption isotherm characteristics could be used in determining the end point of drying and storage stability as well as packaging design of the date-pits and their fractions.
Keywords
Date-pits
Water activity
BET model
GAB model
Isosteric heat
Isokinetic temperature
1 Introduction
Date-pits are waste from date fruit processing factory, and its utilization or valorization could bring economic gain, reduce environmental waste, and enhance food security, circular economy and sustainability. Date-pits are generally used as animal feeds for livestock and poultry (Vandepopuliere et al., 1995). It is a lignocellulose material mainly composed of cellulose, hemicellulose and lignin. The chemical composition of these seeds determines their proper utilization (Shi et al., 2014). Date-pits are a valuable source of fibers (60 to 80 g/100 g), oil (3.9 to 13.8 g/100 g), protein (4.8 and 12.5 g/100 g) and contain a significant antioxidant potency due to their high compositions of flavonoids and other active components (Metoui et al., 2019; Habib et al., 2017; Hamada et al., 2002; Habib and Ibrahim, 2009; Alyileili et al., 2020; Al-Khalili et al., 2021a; Nehdi et al., 2018; Besbes et al., 2009). Their components, such as polysaccharides, and oil (i.e. biofuel and cooking oil) could also be developed.
Date-pits have been used as an additive for different types of food products (Alamri et al., 2014, Almana and Mahmoud, 1994, Bouaziz et al., 2020, Ahmed et al., 2017, Anusree and Kousalya, 1998). Al-Khalili et al. (2023) reviewed the application of date-pits in coffee and other beverages, bakery goods, meat products, dairy products, desserts, spreads, and condiments. They indicated that desired functionality (i.e. sensory acceptability) could not be achieved with a high level of date-pits in food products. Therefore, it is important to measure the physico-chemical and sorption characteristics of date-pits. Other applications are to produce activated carbon for purifying water by removing different types of pollutants, such as heavy metals, boron, and pesticides. It can also be used as an ingredients for bio composed, biomass and fermentation processes (Hossain et al., 2014).
It was observed that bread containing 10 % coarse WDP powder showed a higher dietary fibre and produced similar characteristics as compared with wheat bran. However, fine WDP powder showed uniformity with overall sensory acceptability (Habibi Najafi et al., 2016). It was documented that acid and alkaline treatment of defatted date-pits increased body weight, feed intake and in-vitro digestibility when used as a chicken feed (Hussein et al., 1998). This was due to the increased solubilisation or swelling of fibres. Hydrochloric acid treatment at 100 °C for 12 h was used for DDP before its use as an ingredient for biomass fermentation (Nancib et al., 1997). Three types of date-pits fractions were developed by treating DDP with alkali at 30 °C (Al-Mawali et al., 2021, Al-Khalili et al., 2021a). The first fraction was the residue fibres after treatment with pH 13, second one was the precipitated fraction from supernatant at pH 5.5, and third one was the precipitated fraction at pH 1.5. They measured proton mobility, glass transition and solids melting-decomposition and they pointed that type 1 could be used in crackers, and type 2 could be used in bread, noodles and cookies, while type 3 could be used in edible coating and food emulsion formulation. Al-Khalili et al. (2021a) studied the hygroscopicity, solubility, morphology, structural characteristics and crystallinity of these fractions. They found that the fractionated date-pits could be used as a reinforcing bioplastic and bio-concrete (i.e. filler matrix), toxicant adsorption in the human digestive track, and as a food ingredient for breads, biscuits and rusks. In addition, they showed that there is a potential to use the alkaline digested date-pits as bioplastics or composites. Date-pits was used in other applications in different domains as well, such as biomass production (Elnajjar et al., 2021, Nancib et al., 1997, Nouri, 2021, El Hanandeh et al., 2021, El-Azazy et al., 2021), and bio-composite (Ruggiero et al., 2016, Alsewailem and Binkhder, 2010, Ejiogu et al., 2020). All of these findings supported that date-pits could be used more efficiently when treated to produce fractions with specific desired properties. Therefore, it is important to determine the properties of treated date-pits before identifying their proper applications.
Over 200 different equations were used in literature to predict the sorption isotherms at different temperatures (Van den Berg and Bruin, 1978; Al-Khalili et al., 2021b). These models are either theoretical based or rely on empirical basis (Weatherwax, 1974; Goula et al., 2008; Peng et al., 2007). The most used prediction models for determining food sample sorption isotherms are BET (Brunauer, Emett and Teller) and GAB (Guggenheim–Anderson–de Boer). This was due to their simplicity and theoretical basis (Alamri et al., 2018; Timmermann et al., 2001; Al-Khalili et al. 2021b). GAB model can be used to predict the isotherms over the complete range of water activity (i.e. 0.05 to 0.9), while the BET model is unable to predict sorption isotherms beyond 0.45 water activity (Labuza et al., 1985). Bell and Labuza (2000) indicated that an increase of 0.1 in water activity above the monolayer value could consequently decrease the materials shelf life by two to three times.
Al-Khalili et al. (2022), measured the physico-chemical, thermal, structural, and morphological characteristics of pressure cooked date-pits while soaked in 50 % water-alcohol solvent. However, it is also important to determine the water sorption properties of these treated date-pits when used in foods, biomass production and bio-composite. Moisture sorption isotherm could be used to determine date-pits shelf life, packaging and stability during storage (Fabra et al., 2009). In addition, the end point of drying could be determined from its moisture sorption isotherm. The binding nature (i.e. energy) of water could give an indication on its behaviour when date-pits are used in multicomponent formulations.
Belarbi et al. (2000) studied the moisture sorption isotherms of date-pits (Deglet-Nour variety) at 25 °C. Similarly, Suresh et al. (2017) studied the isotherm of freeze dried date-pits at room temperature (20 °C). Alyousef et al. (2020b) examined the isotherms of date-pits at three temperatures (30, 40 and 50 °C) and determined the binding nature of water. On the other hand, Ajibola et al. (2005) studied the isotherm of date-pits at temperature ranged from 40 to 60 °C within the relative humidity from 13.0 to 53.5 %. In the literature, limited research was presented on the moisture sorption isotherm of date-pits at a wider range of temperature and relative humidity. In addition, moisture sorption isotherms of fractionated date-pits were not reported earlier. Therefore, the objective of this work was to measure the moisture sorption isotherm of whole date-pits (WDP) and their pressure cooked fractions at a wider range of temperature (i.e. 10–90 °C) and relative humidity or water activity range (i.e. 0.05–0.9). The defatted date-pits (DDP) powder was fractionated by pressure cooking in a solvent of 50 % (v/v) water and alcohol mixture. The treatment provided two fractions, one as residue (REP) and another one as supernatant (SUP). Dynamic temperature and humidity (DTH) controlled chamber was used to measure their isotherms, which showed its capability to measure isotherm within a wide range of temperature at any range of relative humidity value (Al-Khalili et al., 2021b).
2 Materials and methods
2.1 Sample preparation and extraction method
Whole date-pits from khalas variety were purchased from a local market in Oman. These were cleaned by washing with hot water to remove any adhered date flesh. Whole date-pits were then dried in the oven (Gallenkamp, UK; model: 300 plus series) at 60 °C for 72 h. The dried whole date-pits were ground by a hammer mill (Model NO Cemotec 1090, Foss, Hoganas, Sweden). The whole date-pits powder was kept in a container, labelled as WDP and stored at ambient temperature for further treatment. WDP powder was defatted by petroleum ether at a ratio 1:50 (w:v) in the Soxhlet apparatus for 8 h. The defatted date-pits powder was dried in an oven for 18 h at 60 °C and considered as DDP. The DDP powder was soaked in 50 % ethanol at a ratio 1:50 (date-pits powder: solvent, w:v) with steering for 48 h. The mixture was further treated by pressure-cooking (model GPC307-6L, Geepas, Romania) at 100 °C until three blows were achieved (i.e.15 min). The mixture was left to cool for about 15 min at 20 °C. The precipitated residue was isolated manually by draining the supernatant. The residue was dried in the oven at 60 °C for 18 h and was considered as REP. The left supernatant was kept at 4 °C for 48 h and the precipitate was collected by isolating the supernatant manually. The sediment was dried in oven at 60 °C for 18 h and was considered as SUP.
2.2 Moisture content
Moisture content was measured according to the (AOAC, 2000) method. In this method, date-pits and their fractions (i.e. in triplicates) were placed in a vacuum oven (VD 23, Binder, Germany), at 70 °C for 18 h at a pressure of 10 kPa.
2.3 Sorption isotherm measurement
Moisture sorption isotherm was measured by Dynamic Thermal Humidity (DTH) controlled chamber method as recently developed by Al-Khalili et al. (2021b). This chamber utilized Advanced Preheating Technology-line™ technology, which maintains homogeneous climate conditions inside the chamber and ensured fast recovery of the humidity and temperature after opening and closing while measuring samples weight. Temperature and humidity ranged could be maintained at any incremental level between −15 to 100° C and 0 to 98 %, respectively (Al-Khalili et al. 2021b). The accuracy of the maintaining temperature (5 and 90 °C) and relative humidity (0.10–0.90) were checked by measuring temperature by a digital temperature and relative humidity meter.
Considering 0.2 g sample, initial conditioning and equilibrium time for each measurement (i.e. at specific relative humidity) was 30 min as recommended earlier by Al-Khalili et al. (2021b) considering no significant change in weight of the sample. In this procedure, date-pits and their fractions (0.2 g) were placed uniformly in a glass dish in triplicates. Dishes containing samp. les were incubated in the DTH Chamber. The chamber was set at 10 °C and 5 % relative humidity and sample weight was recorded after 30 min. The relative humidity was then changed to 10 % from 5 % and continued to increase up to 90 % with a 10 % step change. At each humidity, equilibrium time was considered as 30 min (Al-Khalili et al. (2021b). The same procedure was repeated for other temperatures (i.e. 30, 50, 70 and 90 °C) with relative humidity ranged between 5 and 90 % for each temperature.
2.4 Isotherm modelling
Moisture adsorption isotherms were commonly modelled by BET and GAB equations and it can be expressed as the following (Brunauer et al., 1938):
where Me is the equilibrium moisture and Mb is the BET monolayer moisture contents (i.e. dry basis: g water/100 g dry solids), aw is the water activity, and B is a constant that is related to the net heat of sorption (J/g-mole). BET isotherm is considered valid up to aw = 0.45, and this can predict and B (Labuza, 1968). BET parameters were predicted graphically by plotting versus aw up to 0.45. B value and Mb were estimated from the slope and the intercept of the best fitted line. The BET-monolayer (Mb) value indicated the strongly bound water with the solids matrix and these are the most inert (i.e. not reactive). In addition, the energy state of this fraction of water is low. The value of B indicates the energy required to desorop the strongly bound water (i.e. bound water below water activity 0.45). The model parameters can be plotted as a function of temperature by using regression equation, thus the effect of temperature on the isotherm could be predicted.
GAB model (Anderson, 1946, DeBoer, 1953, Guggenheim, 1966) as shown in Equation (2) was used to fit the isotherm data within 0.05 to 0.95 water activity.
where, is the GAB monolayer moisture content (i.e. dry basis, g/100 g dry solids) and C is a constant correlated to the monolayer heat sorption (J/g-mole), and K is a factor related to the multilayer heat of sorption. The GAB-monolayer is the strongly bound water considering the isotherm up to water activity 0.9 and adjusting the water sorption isotherm by adding the heat of sorption by multi-layer water. In the GAB model adsopted energies of monolayer and multilayer and separated, while BET model only considered the monolayer. The value of K is incorporated to include the effects of multilayer heat of sorption.
2.5 Isosteric heat
The isosteric heat was calculated using Clausius-Clapeyron equation and it could be expressed as:
where Qs is the net isosteric heat of sorption (kJ/kg), and can be defined as the energy needed to remove a unit mass of adsorbed water at a specific temperature (Aviara et al., 2002) and T is the temperature (K). The net isosteric heat of sorption (Qs) was determined from the slope of ln ( ) versus 1/T plots at a specific moisture content (Arslan-Tontul, 2021). The net isosteric heat indicated that how much energy is involved at a specific moisture over the temperature of the measured isotherm.
2.6 Isokinetic temperature
The isokinetic temperature (i.e. Tis), which is the temperature when all the reactions occur at a constant rate, and it was determined from the linear plot of enthalpies (ΔH) and entropies (ΔS) (Exner, 1970). The enthalpies (ΔH/R) and entropies (ΔS/R) were estimated from the slopes and intercepts of the linear plots between aw and 1/T, and it is demonstrated by Equation (4) (Telis-Romero et al., 2005):
The isokinetic temperature was then determined from the slope of the plot between the enthalpy (ΔH/R) and entropy (ΔS/R) (Exner, 1970, Telis-Romero et al., 2005). At the isokinetic temperature, the rates of all reactions or changes occur at a same rate (i.e. reactions are independent of temperature).
2.7 Isobound temperature
The isobound temperature (Tib) was determined by plotting temperature as a function of BET-monolayer solids at equilibrium temperature and it was considered as the intercept when water reached to zero (i.e. dry solids)(Rahman and Al-Saidi, 2017). This is the temperature when all water (i.e. bound and free) is desorbed.
2.8 Statistical analysis
Average and standard deviations were determined for each humidity equilibration. Standard experimental uncertainties were determined for each sample considering 0.95 level of confidence. The BET model parameters were estimated by regression of linearized isotherm Equation (1). Non-linear regression was implemented to predict the parameters of GAB model using the NLREG software (Sherrod, 1991). Coefficient of determination (R2) were determined to validate the accuracy of the models.
3 Results and discussion
3.1 Moisture sorption isotherm
The standard uncertainties of the measured equilibrium moisture for all samples were varied from ± 0.07 to ± 0.08 g water/100 g ds (considering 95 % confidence level). Fig. 1 shows sigmoid shape (i.e., type II according to the International Union of Pure and Applied Chemistry, IUPAC) isotherms of the whole and date-pits fractions as a function of temperature (Brunauer, Deming, Deming, & Troller, 1940; Alothman, 2012; Zheng et al., 2018). Similar sigmoid shape was observed for the freeze dried date-pits isotherm (Suresh et al., 2013, Belarbi et al., 2000). The sigmoidal sorption isotherm was also commonly observed for foods, plant products and different types of plant leaves and biomaterials (Mulet et al., 1999, Rahman and Al-Belushi, 2006, Siripatrawan and Jantawat, 2006, Peng et al., 2007, Staudt et al., 2013, Alamri et al., 2018; Arslan & Togrul, 2006; Sinija & Mishra, 2008; Bahloul et al., 2008; Mohamed et al., 2005; Argyropoulos et al., 2012; López‐Vidaña et al., 2021; López-Vidaña et al., 2016). Date-pits are mainly a lignocellulosic biomaterial that contain significant amount of cellulose, hemicellulose and lignin. Al-Khalili et al. (2021b) also observed similar shape of isotherm for commercial cellulose and lignin.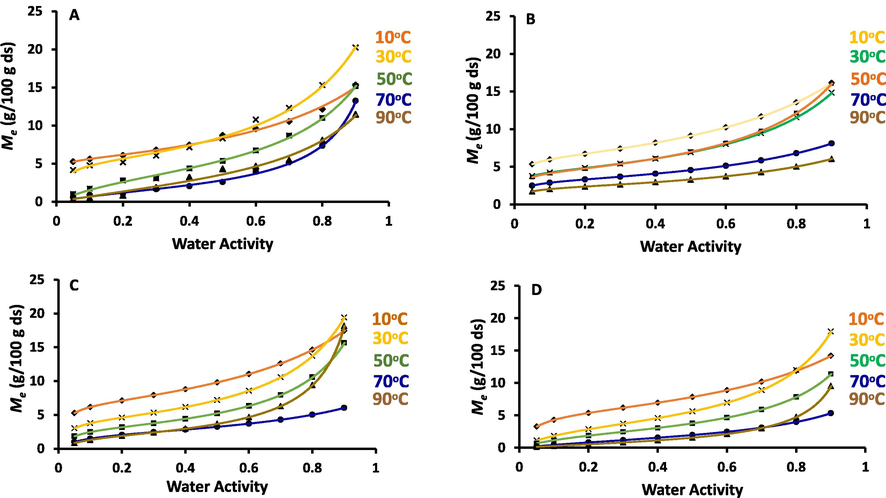
Adsorption isotherm curves of A; WDP (CV: 0.07 g/100 g ds), B; DDP (CV: 0.08 g/100 g ds), C: REP (CV: 0.07 g/100 g ds) and D; SUP (CV: 0.08 g/100 g ds) for the temperatures from 10 to 90 °C.
In general, isotherms shifted downward as temperature increased. This was due to the escaping tendency of the water at higher temperature (Kapsalis, 2017, Siripatrawan and Jantawat, 2006). The elevated temperature was found to decrease the number of molecules linked to the polar sites and cohesive pressure of the solid matrix (Alyousef et al., 2020b). Similarly, date-pits isotherms at temperatures 30, 40 and 60 °C showed similar behavior (Alyousef et al., 2020b) as well as starchy foods (Rohvein et al., 2004, Barreiro et al., 2003, Jorge, 1982) and fibers such as cellulose and lignin (Al-Khalili et al., 2021b). Kapsalis (2017) mentioned that at higher temperature, water molecules mobility enhanced the dynamic equilibrium between water vapor and adsorbed phases. The temperature effect on sorption behavior of constant moisture content of packed products is particularly important with different ambient temperature in different countries. High ambient temperature makes these products susceptible to increased water activity and thus the stability of the product will be affected negatively (Alam and Islam, 2015).
However, crossovers of the isotherms were observed at selected temperatures. The crossovers were observed at water activity of 0.50 (i.e. between 10 and 30 °C) and above 0.80 (i.e. between 70 and 90 °C) in the case of WDP. In the case of DDP, only one crossover was observed at water activity 0.50 (i.e. between 30 and 50 °C). REP showed three crossovers of the isotherm curves, first one at water activity 0.30 (i.e. between 10 and 30 °C), second ones at 0.85 (i.e. between 50 and 90 °C) and third ones at 0.50 (i.e. between 70 and 90 °C). On the other hand, SUP fraction showed two intersections, first one at 0.70 (i.e. between 70 and 90 °C), and second one at 0.80 (i.e. between 10 and 30 °C). The crossover behavior of isotherm was identified earlier for starch and sugar based foods, such as beetroot (Iglesias et al., 1975), and barley mallet (Barreiro et al., 2003). In the case of sugar or carbohydrate-based products, they suggested that crossover could be due to the increased solubility (i.e. dissolution of sugars) of low molecular weight saccharides at a critical water activity (Balderrama and Cadima, 2014, Alhamdan and Hassan, 1999, Myhara et al., 1998). In the case of sugar based foods, crossover was explained by dissolution of sugars within water activity 0.45 and 0.55 (Alhamdan and Hassan, 1999, Myhara et al., 1998). The variations of the crossovers in the cases WDP and other treated fractions could be due to the variations of the swelling ability at critical water activity. It was expected to observe swelling variations of polysaccharides in whole date-pits and their function due to the removal of oils and pressure cooking in alcohol. In addition, in the case of starch, the creation of active sites and hygroscopicity was due to the variation of swelling above critical water activity, and this could result in the inversion of isotherm (Perdomo et al., 2009). However, Brett et al. (2009) pointed that the crossover could be correlated to the glass-rubber transition at a critical water activity.
3.2 BET isotherm model
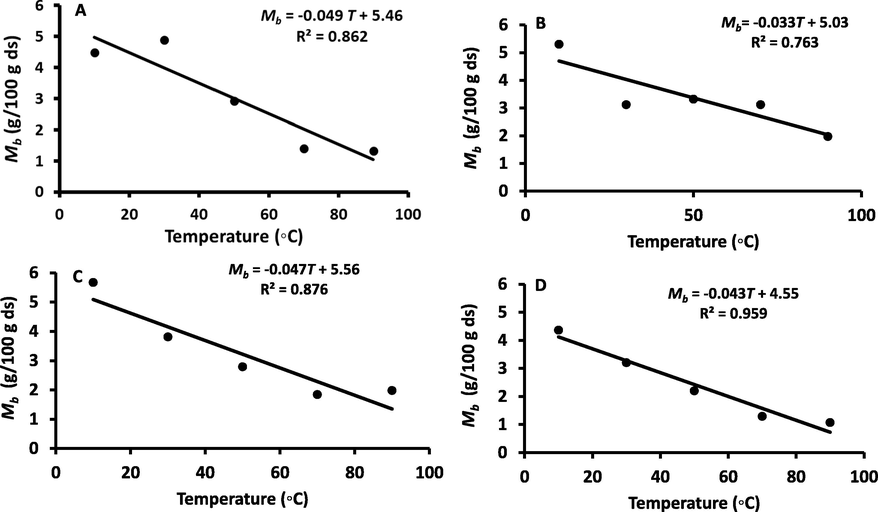
Table 1 shows the BET model parameters for the WDP, DDP, REP and SUP at temperatures 10, 30, 50, 70 and 90 °C. The BET adsorption isotherm was fitted linearly up to water activity 0.40 and the coefficient of determination (R2) varied from 0.885 to 0.999 (Table 1). At 10 °C the Mb values were 5.75 and 5.67 g/100 g for the DDP and REP, while WDP and SUP Mb values were 4.49 and 4.32, respectively. Mb value of freeze dried date-pits were estimated as 4.30 g/100 g ds at 20 °C (Suresh et al., 2013) and 4.27 g/100 g ds at 25 °C (Belarbi et al., 2000), while in this study it was observed as 4.49 g/100 g ds at 30 °C (Table 2). The BET-monolayer value indicated the active polar sites in the solid matrix. DDP and REP showed higher Mb values as compared to the WDP and SUP, this could indicate that former absorbs more water at same temperature and water activity (Alam and Islam, 2015). The BET monolayer values showed a general decreasing trend as the temperature increased for date-pits and fractionated fibers (Fig. 2). The reduction in Mb values could be due to the chemical and physical changes as affected by elevated temperature (McMinn and Magee, 2003). The decreasing trends of Mb with increasing temperature were also reported for many starchy food products (i.e. barley malt, rice, crackers and cookies) (Barreiro et al., 2003, Kim et al., 1998, Benado and Rizvi, 1985). Considering more than 40 foods, Iglesias and Chirife (1984) pointed that the effect of temperature on BET monolayer values varied differently depending on the types of foods and biomaterials. In this study, Mb values of WDP, DDP, REP and SUP decreased from 4.49 to 1.33 g/100 g ds, 5.75 to 2.40 g/100 g ds, 5.67 to 1.98 g/100 g ds, and 4.32 to 1.07 g/100 g ds, respectively when temperature increased from 10 to 90 °C. *Relative Humidity Range.
Sample
BET Model
ParametersGAB Model
ParametersThermodynamic
Parameters
T
(oC)
Mb
g/100g ds
B
R2
Mg
g/100g ds
C
K
R2
Tis
(oC)
Tib
(oC)
WDP
10
4.49
82.44
0.997
5.38
426.73
0.71
0.995
111.6
102.8
30
4.90
7.50
0.999
5.08
65.04
0.84
0.995
50
2.92
9.81
0.971
3.81
6.49
0.85
0.998
70
1.40
2.05
0.956
1.79
4.97
0.97
0.998
90
1.33
1.54
0.970
3.14
2.80
0.84
0.988
DDP
10
5.75
173.00
0.994
6.00
170.00
0.70
0.988
97.4
145.0
30
4.20
28.00
0.942
4.22
150.00
0.80
0.998
50
4.16
32.89
0.964
4.16
120.00
0.82
0.994
70
3.13
16.00
0.885
3.02
110.00
0.70
0.955
90
2.40
19.80
0.922
2.18
90.00
0.71
0.944
REP
10
5.67
47.00
0.997
6.50
100.00
0.70
0.997
97.4
110.3
30
3.81
72.86
0.985
4.16
50.00
0.87
0.999
50
2.79
34.78
0.981
3.00
30.00
0.90
0.999
70
1.84
23.81
0.968
2.30
20.00
0.70
0.997
90
1.98
13.10
0.989
2.00
13.00
0.99
0.999
SUP
10
4.36
71.69
0.989
5.34
40.00
0.70
0.995
111.6
104.8
30
3.20
9.60
0.992
3.50
9.34
0.90
0.999
50
2.20
8.30
0.988
2.45
8.00
0.88
0.999
70
1.29
4.04
0.968
1.65
3.80
0.80
0.997
90
1.07
2.69
0.999
1.09
2.68
0.99
0.999
BET Model Parameters
GAB Model Parameters
Study
Variety/ Form
Temperatue (oC)
RHR*
(%)
Mb
(g/100 g ds)B
Mg
(g/100 g ds)C
K
Net Isostatic
Heat (kJ/kg)
Current study
Khalasdate-pits powder
10
5.0–90.0
4.49
82.44
5.38
426.73
0.71
3450
30
4.90
7.50
5.08
65.04
0.84
50
2.92
9.81
3.81
6.49
0.85
70
1.40
2.05
1.79
4.97
0.97
90
1.33
1.54
3.14
2.80
0.84
Suresh et al. (2017)
Khalas
freeze dried date-pits powder20
11.5–84.3
4.30
–
4.1
–
–
–
Ajibola et al. (2005)
Whole
date-pits powder40
13.0–53.5
–
–
–
–
–
3250
50
–
–
–
–
–
60
–
–
–
–
–
70
–
–
–
–
–
80
–
–
–
–
–
Belarbi et al. (2000)
Deglet-Nour
date-pits powder25
11.0–90
4.27
52.2
4.89
3.75
0.93
–
BET (A: WDP, B: DDP, C: REP, D: SUP) monolayer values as a function of temperature.
The BET heat of sorption (i.e. B value) showed decreasing trends with the increase of temperature. At 10 °C, B values were 82.4, 173.0, 47.0 and 71.7 J/g-mol, in the cases of WDP, DDP, REP and SUP, respectively (Table 1 and Fig. 3). Belarbi et al. (2000) determined the B value of WDP (i.e. Deglet-Nour variety) as 52.2 at 25 °C, while in this study it was 65.0 at 30 °C (i.e. close to 25 °C).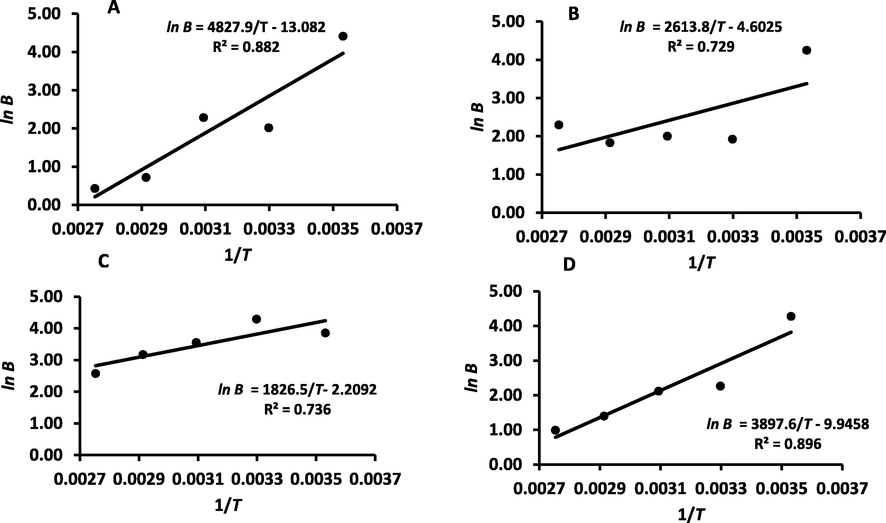
BET model B-values (A: WDP, B: DDP, C: REP, D: SUP).
3.3 GAB isotherm model
The GAB model parameters are presented in Table 1 and the coefficient of determination (R2) varied from 0.944 to 0.999 for WDP and fractionated fibers (Table 1). At 10 °C, DDP and REP showed higher Mg values (i.e. 6.00 and 6.50 g/100 g ds, respectively) as compared to WDP and SUP (i.e. 5.38 and 5.34 g/100 g ds, respectively). In both cases (i.e. BET and GAB), monolayer moisture increased with the removal of oil, as this trend was also observed by De Jong et al. (1996) for whole and defatted foods. In the case of freeze dried date-pits, Suresh et al. (2013) estimated the Mg value as 4.1 g/100 g ds, while Belarbi et al. (2000) found Mg of Deleget-Nour date-pits as 4.89 g/100 g ds. In this study, the Mg values were higher as compared to the reported values. The variation in sorption capacity between different samples particularly at higher water activity values is attributed to differences in the structural integrity (Alam and Islam, 2015). Similar to BET model, the Mg values from GAB model followed a decreasing trend with the increase in temperature (Fig. 4). However, in the case of WDP (Fig. 4A), the variability was higher at the elevated temperature and it could be due to the different types of components reacting at higher temperature. Fig. 5 shows that moisture residual plots as a function of water activity. These figures show low errors (i.e. ± 0.008 g/g-dry-solids or ± 0.8 g/100 g ds) of the model from experimental data.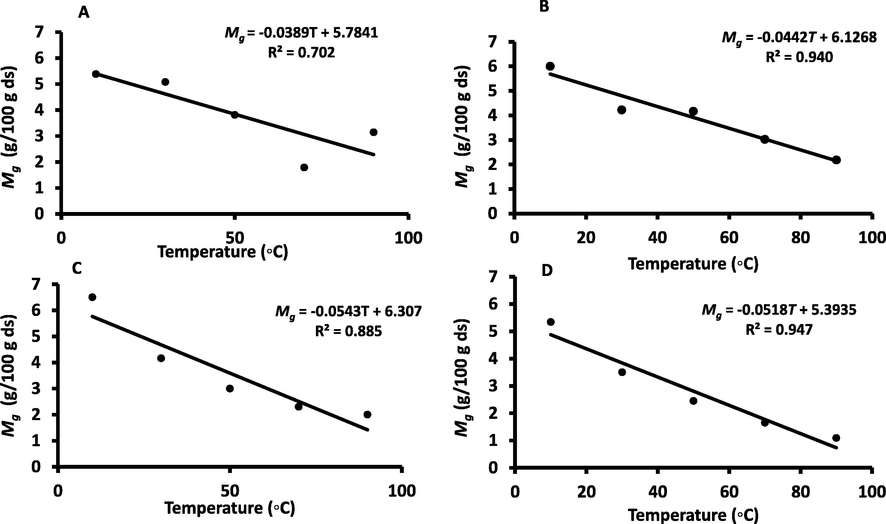
GAB (A: WDP, B: DDP, C: REP, D: SUP) monolayer values as a function of temperature.
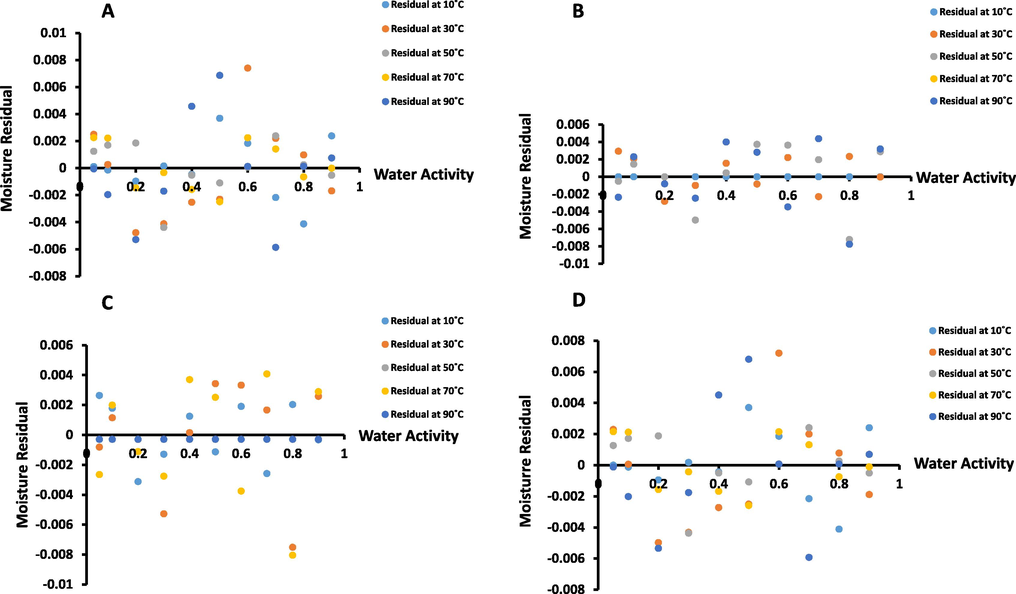
Moisture residual plots of (A: WDP, B: DDP, C: REP, D: SUP) as water activity.
The C values (i.e., constant related to sorption energy difference between upper layers and monolayer) shows a decreasing trend with the increase of temperature for all samples (R2 ≥ 0.931) (Fig. 6). The constant C of WDP dropped from 426.7 (i.e. at 10 °C) to 65.04, 6.49, 4.97 and 2.80, as the temperature increased to 30, 50, 70 and 90 °C, respectively (Table 1). At 25 °C, Belarbi et al. (2000) observed much lower value of C (i.e. 3.75) in the case of date-pits, while in this study it was observed as 65.04. The constant K was a positive value less than unity for WDP powder and the fractionated fibers in all isotherms (Table 1 and Fig. 7). The K values were similar to the values observed for date-pits (Belarbi et al., 2000), cellulose and lignin (Al-Khalili et al., 2021b). Theoretically, K values should be less than unity as observed in this study (Chirife et al., 1992, Rahman, 2009, Belarbi et al., 2000). In the case of SUP, the multilayer sorption energy (i.e. K-value) was highly affected by temperature, however WDP and RES showed lower effects. The DDP fraction showed the minimal effect by temperature. This could be due to the varitions of the compsotions in defferent fractions and their surface energy.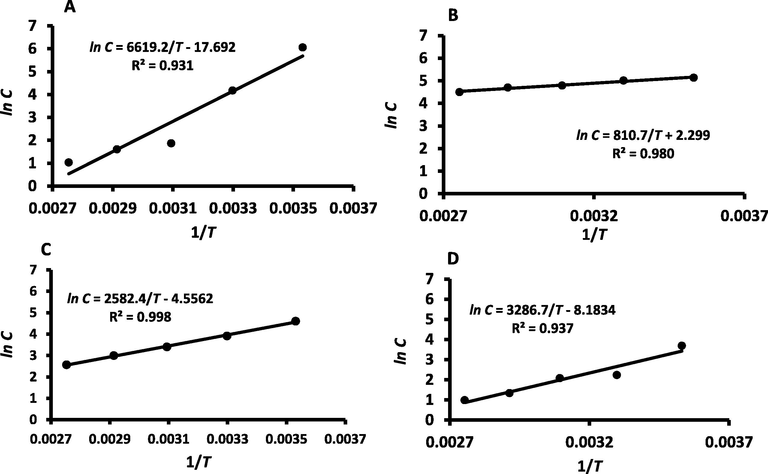
GAB model C-values (A: WDP, B: DDP, C: REP, D: SUP) as a function of temperature.
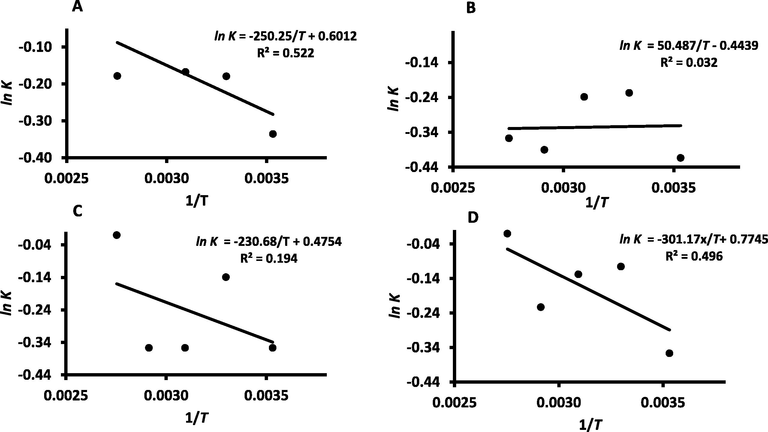
GAB model K-values (A: WDP, B: DDP, C: REP, D: SUP) as a function of temperature.
3.4 Isosteric heat of adsorption
Fig. 8 showed the isosteric heat of sorption in relation to the moisture content. In the case of WDP and DDP, the isosteric heat curves showed peaks at moisture 4.0 and 6.0 g/100 g ds, respectively. A sharp decrease was observed below the critical moisture. Similarly, Viollaz and Rovedo (1999) observed a sharp decrease at low moisture in the case of starch and gluten. The net isosteric heat of adsorption at 4.0 g/100 g ds (i.e. peak) was 4000 kJ/kg in the case of WDP, while DDP showed 2000 kJ/kg at critical moisture of 6.0 g/100 g ds. This indicated that the water binding energy was much higher in the case of WDP as compared to DDP (i.e. removal of oil reduced the water binding energy). Considering statistical models, Alyousef et al. (2020a) determined that water in date-pits was physically bound to the solids matrix. Therefore, removal of oil may change the surface characteristics of the matrix and allowed to spread the adsorbed water and caused lower energy of water binding. The WDP and DDP fractions possessed complex structural matrix with varied polar sites and these could cause variations of water binding energy. In the case of whole date-pits, Ajibola et al. (2005) determined the net isostatic heat as 3250 kJ/kg at moisture content 5.0 g/100 g ds, while in this study it was 3450 kJ/kg (Table 2). However, they did not measure the isostatic heat below moisture 5.0 g/100 g ds. In the case of serum albumin, isosteric heat showed a sharp increase up to moisture 5.0 g/100 g ds and below 5.0 g/100 g ds it remained nearly constant. They identified this as step function with rounded corners and low moisture (e.g. monolayer or below) possessed lower energy similar to multi-layer water. These variations depended on the type of foods or biomaterials. They also mentioned that isotherm models (e.g. BET or GAB) did not provide nature of adsorbed water (i.e. binding nature), and real representation can be obtained from the variation of water activity with temperature at constant moisture using Eq. (3). In the case of cooked wheat, Turhan et al. (2003) also observed rounded corner below moisture 5.0 g/100 g ds. There was a sharp increase from 15.0 to 5.0 g/100 g ds, moisture content. They mentioned that below BET-monolayer the binding energy did not change.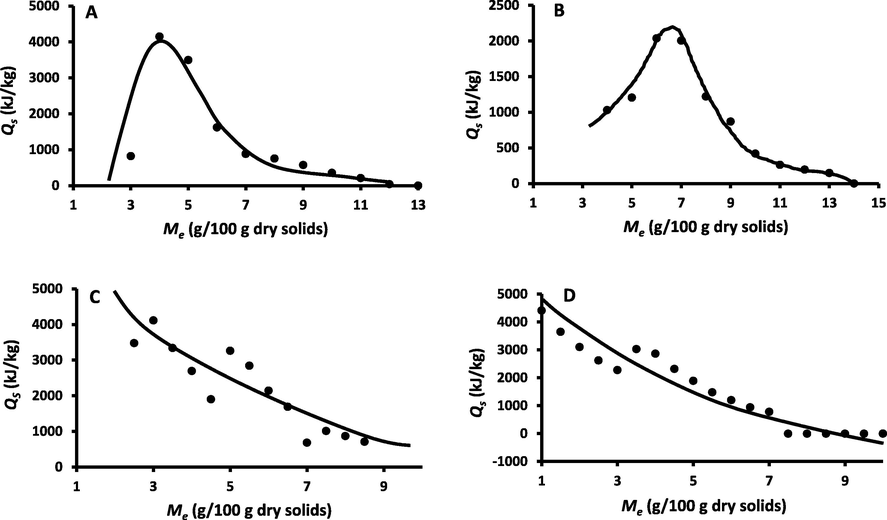
Isosteric heat of adsorption as a function of moisture content (A: WDP, B: DDP, C: REP, D: SUP) (Line shows the trend).
REP and SUP fractions showed an increasing trend with the decreasing moisture content without any peak at low moisture. Similar, trends were observed in the case of caseins (27–80 °C) (Diamante, 1991), dried fruits (Lim et al., 1995), macadamia nuts (Palipane and Driscoll, 1993), fruits (Tsami, 1991, Ayranci et al., 1990, Hossain et al., 2001, Myhara et al., 1998), vegetables (Kiranoudis et al., 1993, Kaymak-Ertekin and Sultanoğlu, 2001), alfalfa (Fasina and Sokhansanj, 1993), and corn chips (Achoba et al., 1991). It is important to point that most of the cases the isosteric heat was reported up to moisture 5.0 g/100 g ds. This was due to the missing of isotherm at high temperature range (i.e. mainly above 60 °C). In the current study, water binding in whole date-pits and their fractions could be assessed at low moisture since the isotherm measurement was up to 90 °C.
3.5 Isokinetic temperature
Fig. 9 demonstrates the plotting of the differential enthalpy (ΔH/R) as a function of differential entropy (ΔS/R) for WDP and the fractionated date-pits fibres. A linear trend satisfied the enthalpy–entropy compensation theory. The linear correlations (R2) were 0.990, 0.925, 0.993 and 0.983, respectively for WDP, DDP, REP and SUP. The isokinetic temperatures (Tis) were 111.6, 111.6 and 97.4 °C for WDP, REP and SUP, respectively (Table 1). These were close to the isobound temperature (Tib) for WDP, REP and SUP (i.e. 102.8, 110.3 and 104.7 °C, respectively) (Table 1). Similar findings were also established for banana (Rahman and Al-Saidi, 2017) and mango (Telis-Romero et al., 2005). However, in the case of DDP, Tis (i.e. 97.39 °C) showed to be lower as compared to Tib (i.e. 145.01 °C) (Table 1), which indicated that complete removal of bound water occurred after the temperature when all reactions were at the same rate.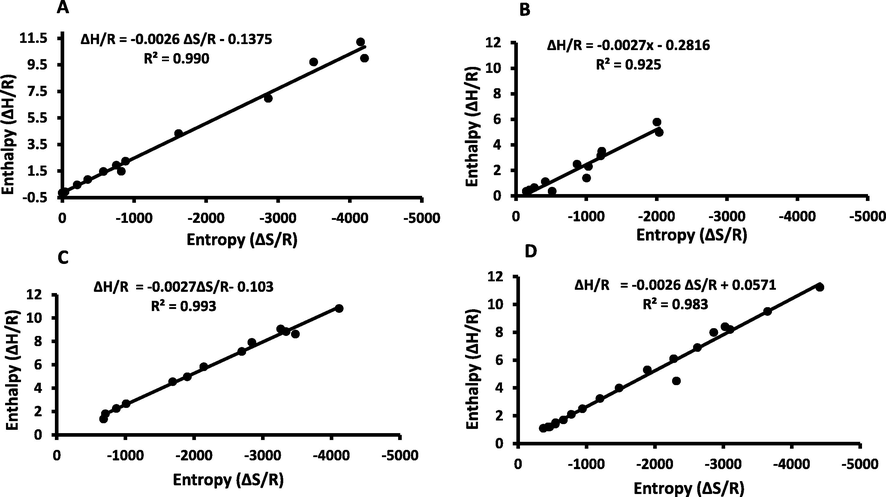
Differential enthalpy (ΔH/R) as a function of differential entropy (ΔS/R) for A: WDP, B: DDP, C: REP and D: SUP.
Greater Tis values compared to the Tib values for WDP and REP could corroborates to the enthalpy–entropy compensation theory, which indicated that their sorption processes are enthalpy-driven mechanisms. Similarly, the compensation theory was used to assess moisture sorption processes of different fruit seeds such as prickly pear seeds (Hassini et al., 2015) and jambolana seeds (De Araújo and Da Silva Pena, 2022). It was observed that these processes were also controlled by enthalpy. On the other hand, higher Tib values of DDP and SUP fractions compared to their Tis values implied entropy-driven processes, which indicated greater freedom of the molecules within the matrix of these fibres (Spada et al., 2013).
4 Conclusion
Sorption isotherms of the WDP, and their fractions (i.e. DDP, REP and SUP) were measured and modeled by BET and GAB equations. At temperature 10 °C, BET-Mb and GAB-Mg were observed higher for DDP and REP as compared to WDP and SUP, which indicated higher level in the strongly bound water in the case of DDP and REP at the same conditions. WDP in this study showed higher values of BET monolayer at ambient temperature (20–30 °C) compared to whole date-pits presented in the literature. This could be due to the variety and maturity level as well as processing conditions. Isosteric heats of sorption was decreasing trends in the case of fractions REP and SUP, however WDP and DDP fractions showed peaks followed by a sharp decrease of the isosteric heat. This could be an indication of the variable binding nature (i.e. energy) with the types of treatments (i.e. petroleum ether and alcohol). Isokinetic temperature of WDP and SUP were observed higher as compared to the DDP and REP fractions. This indicated that all reactions reached at the same rate at much lower temperature (i.e. variability of the reactions in the WDP and SUP was higher). In the cases of WDP and their fractions, isokinetic temperatures were close to the isobound temperatures in all cases, except for DDP fraction. Over all, the treatments and composition of the extracted fractions affected the sorption characteristics of the fibers.
Acknowledgement
This project was supported by the His Majesty Trust Funds (SR/AGR/FOOD/2019/1) on the valorisation of under-utilized food waste.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Moisture isotherms of cornchips at three temperatures. Int. J. Food Sci. Technol.. 1991;26(5):547-552.
- [Google Scholar]
- Evaluation and Sensorial Assesment of Date Pit-Based Functional Drink. J. Food Process. Preserv.. 2017;41(3):e12890.
- [Google Scholar]
- Moisture sorption equilibrium and thermodynamic properties of palm kernel. Int. Agrophys.. 2005;19(4):273-283.
- [Google Scholar]
- Study on water sorption isotherm of summer onion. Bangladesh J. Agric. Res.. 2015;40:35-51.
- [Google Scholar]
- Alamri, M., Mohamed, A., Hussain, S., Ibraheem, M., Qasem, A., & Akram, A. 2018. Determination of Moisture Sorption Isotherm of Crosslinked Millet Flour and Oxirane Using GAB and BET. Journal of Chemistry, 2018.
- Behri dates pits-enriched bread: effect on dough rheology, bread quality, and shelf life. Ital. J. Food Sci.. 2014;26:1-11.
- [Google Scholar]
- Water sorption isotherms of date pastes as influenced by date cultivar and storage temperature. J. Food Eng.. 1999;39(3):301-306.
- [Google Scholar]
- Structural characteristics of alkaline treated fibers from date-pits: Residual and precipitated fibers at different pH. Bioact. Carbohydr. Diet. Fibre. 2021;25:100251
- [Google Scholar]
- Al-Khalili, M., Al-Habsi, N., & Rahman, M. S. (2021). Application of Dynamic Temperature-Humidity Chamber for Measuring Moisture Sorption Isotherms of Biomaterials as Compared to the Conventional Isopiestic Method. Adsorption Science & Technology, 2021.
- Al-Khalili, M., Al-Habsi, N., Al-Kindi, M. & Rahman, M. S. 2022. Characteristics of crystalline and amorphous fractions of date-pits as treated by alcohol-water pressure cooking. Bioactive Carbohydrates and Dietary Fibre, 28, 100331.Al-Mawali, M., Al-Habsi, N., & Rahman, M. S., 2020.
- Al-Khalili, M., Al-Habsi, N., & Rahman, M. S. (2023). Applications of Date-Pits in Foods for Enhancing their Functionality and Quality: A Review. Foranteirs in sustainaable food system (acceped).
- Palm date seeds as an alternative source of dietary fiber in Saudi bread. Ecol. Food Nutr.. 1994;32(3–4):261-270.
- [Google Scholar]
- Al-Mawali, M., Al-Habsi, N. and Rahman, M.S., 2021. Thermal characteristics and proton mobility of date-pits and their alkaline treated fibers. Food Engineering Reviews, 13, pp.236-246.. Food Engineering Reviews, 1-11.
- A review: fundamental aspects of silicate mesoporous materials. Materials. 2012;5:2874-2902.
- [Google Scholar]
- Preparation and characterization of polymer/date pits composites. J. Reinf. Plast. Compos.. 2010;29(11):1743-1749.
- [Google Scholar]
- Effect of inclusion of degraded and non-degraded date pits in broilers’ diet on their intestinal microbiota and growth performance. Animals. 2020;10:2041.
- [Google Scholar]
- New insights on physico-chemical investigation of water adsorption isotherm into seed of dates using statistical physics treatment: Pore size and energy distributions. J. Mol. Liq.. 2020;298:112041
- [Google Scholar]
- Statistical physics modeling of water vapor adsorption isotherm into kernels of dates: Experiments, microscopic interpretation and thermodynamic functions evaluation. Arab. J. Chem.. 2020;13(3):4691-4702.
- [Google Scholar]
- Modifications of the Brunauer, Emmett and Teller equation1. J. Am. Chem. Soc.. 1946;68(4):686-691.
- [Google Scholar]
- Anusree, K., & Kousalya, L. 1998. Evaluation of caffeine content in date seed-an alternative source for caffeinated coffee.
- Moisture sorption isotherms and isosteric heat of sorption of leaves and stems of lemon balm (Melissa officinalis L.) established by dynamic vapor sorption. LWT. 2012;47:324-331.
- [Google Scholar]
- The fitting of various models to water sorption isotherms of tea stored in a chamber under controlled temperature and humidity. J. Stored Prod. Res.. 2006;42:112-135.
- [Google Scholar]
- Moisture sorption isotherm and thermodynamic analysis of quinoa grains. Heat Mass Transf.. 2021;57(3):543-550.
- [Google Scholar]
- PH—Postharvest technology: Thermodynamics of moisture sorption in sesame seed. Biosyst. Eng.. 2002;83(4):423-431.
- [Google Scholar]
- Moisture sorption isotherms of dried apricot, fig and raisin at 20 C and 36 C. J. Food Sci.. 1990;55(6):1591-1593.
- [Google Scholar]
- Moisture desorption–adsorption isotherms and isosteric heats of sorption of Tunisian olive leaves (Olea europaea L.) Ind. Crop. Prod.. 2008;28:162-176.
- [Google Scholar]
- Balderrama, J. G. P., & Cadima, S. C. C. 2014. Water adsorption isotherms of amaranth (Amaranthus caudatus) flour. Food and Nutrition Sciences, 2014.
- Water sorption characteristics of six row barley malt (Hordeum vulgare) LWT-Food Science and Technology. 2003;36(1):37-42.
- [Google Scholar]
- Water desorption isotherms for eleven varieties of dates. J. Food Eng.. 2000;43(2):103-107.
- [Google Scholar]
- Bell, L. N. & Labuza, T. P. 2000. Moisture sorption: practical aspects of isotherm measurement and use.
- Thermodynamic properties of water on rice as calculated from reversible and irreversible isotherms. J. Food Sci.. 1985;50(1):101-105.
- [Google Scholar]
- Adding value to hard date (Phoenix dactylifera L.): Compositional, functional and sensory characteristics of date jam. Food Chem.. 2009;112:406-411.
- [Google Scholar]
- Date Seeds as a Natural Source of Dietary Fibers to Improve Texture and Sensory Properties of Wheat Bread. Foods (Basel, Switzerland). 2020;9(6):737.
- [Google Scholar]
- Moisture sorption characteristics of starchy products: oat flour and rice flour. Food Biophys.. 2009;4(3):151-157.
- [Google Scholar]
- Adsorption of gases in multimolecular layers. J. Am. Chem. Soc.. 1938;60(2):309-319.
- [Google Scholar]
- On a theory of the van der Waals adsorption of gases. Journal of the American Chemical society. 1940;62:1723-1732.
- [Google Scholar]
- Some features of the parameter k of the GAB equation as applied to sorption isotherms of selected food materials. J. Food Eng.. 1992;15(1):75-82.
- [Google Scholar]
- Moisture desorption behavior and thermodynamic properties of pulp and seed of jambolan (Syzygium cumini) Heliyon. 2022;8:e09443.
- [Google Scholar]
- Water vapour sorption behaviour of original and defatted wheat gluten. Int. J. Food Sci. Technol.. 1996;31(6):519-526.
- [Google Scholar]
- DeBoer, J. H. 1953. The dynamical character of adsorption (Vol. 76): LWW.
- Diamante, L. M. 1991. Casein drying: a thesis presented in partial fulfilment of the requirements for the degree of Doctor of Philosophy in Food Technology at Massey University. Massey University.
- Biodegradable hybrid polymer composite reinforced with coconut shell and sweet date seed (Phoenix dactylifera) powder: a physico-mechanical study; part A. Multiscale and Multidisciplinary Modeling, Experiments and Design. 2020;3(1):41-51.
- [Google Scholar]
- Modelling of the adsorption of Pb, Cu and Ni ions from single and multi-component aqueous solutions by date seed derived biochar: Comparison of six machine learning approaches. Environ. Res.. 2021;192:110338
- [Google Scholar]
- Eco-Structured adsorptive removal of tigecycline from wastewater: Date pits’ biochar versus the magnetic biochar. Nanomaterials. 2021;11(1):30.
- [Google Scholar]
- Experimental investigations of bio-syngas production using microwave pyrolysis of UAE’S palm date seed pits. Fuel. 2021;303:121348
- [Google Scholar]
- Sorption isotherm and state diagram of grapefruit as a tool to improve product processing and stability. J. Food Eng.. 2009;93(1):52-58.
- [Google Scholar]
- Equilibrium moisture relations and heat of sorption of alfalfa pellets. J. Agric. Eng. Res.. 1993;56(1):51-63.
- [Google Scholar]
- Water sorption isotherms and glass transition temperature of spray dried tomato pulp. J. Food Eng.. 2008;85(1):73-83.
- [Google Scholar]
- Guggenheim, E. A. (1966). Applications of statistical mechanics.
- Habib, H., Othman, A., AL-Marzooqi, S., AL-Bawardi, A., Pathan, J. Y., Hilary, S., Souka, U., AL-Hammadi, S., Ibrahim, W. & Platat, C. 2017. The antioxidant activity of date seed: preliminary results of a preclinical in vivo study. Emirates Journal of Food and Agriculture, 822-832.
- Nutritional quality evaluation of eighteen date pit varieties. International journal of food sciences and nutrition. 2009;60:99-111.
- [Google Scholar]
- Development of sourdough fermented date seed for improving the quality and shelf life of flat bread: study with univariate and multivariate analyses. J. Food Sci. Technol.. 2016;53(1):209-220.
- [Google Scholar]
- Preliminary analysis and potential uses of date pits in foods. Food Chem.. 2002;76:135-137.
- [Google Scholar]
- Desorption isotherms and thermodynamic properties of prickly pear seeds. Ind. Crop. Prod.. 2015;67:457-465.
- [Google Scholar]
- Sorption isotherms and heat of sorption of pineapple. J. Food Eng.. 2001;48(2):103-107.
- [Google Scholar]
- Chemcial compistion of date-pits and its potential for developing value-added product- a review. Polish Journal of Food and Nutrition Sciences. 2014;64(4):215-226.
- [Google Scholar]
- The use of dates and date pits in broiler starter and finisher diets. Bioresour. Technol.. 1998;66(3):219-223.
- [Google Scholar]
- Water sorption isotherms in sugar beet root. Int. J. Food Sci. Technol.. 1975;10(3):299-308.
- [Google Scholar]
- Jorge, C. (1982). Handbook of food isotherms: water sorption parameters for food and food components. Food science and technology (USA).
- Influences of hysteresis and temperature on moisture sorption isotherms. In: Water Activity: Theory and Applications to Food. Routledge; 2017. p. :173-213.
- [Google Scholar]
- Moisture sorption isotherm characteristics of peppers. J. Food Eng.. 2001;47(3):225-231.
- [Google Scholar]
- Moisture sorption characteristics of composite foods filled with strawberry jam. LWT-Food Science and Technology. 1998;31(4):397-401.
- [Google Scholar]
- Equilibrium moisture content and heat of desorption of some vegetables. J. Food Eng.. 1993;20(1):55-74.
- [Google Scholar]
- Effect of temperature on the moisture sorption isotherms and water activity shift of two dehydrated foods. J. Food Sci.. 1985;50:385-392.
- [Google Scholar]
- Moisture sorption characteristics of freeze dried blueberries. J. Food Sci.. 1995;60(4):810-814.
- [Google Scholar]
- Evaluation of the Sorption Equilibrium and Effect of Drying Temperature on the Antioxidant Capacity of the Jaboticaba (Myrciaria cauliflora) Chem. Eng. Commun.. 2016;203:809-821.
- [Google Scholar]
- Moisture sorption isotherms, isosteric heat, and Gibbs free energy of stevia leaves. J. Food Process. Preserv.. 2021;45:e15016.
- [Google Scholar]
- Thermodynamic properties of moisture sorption of potato. J. Food Eng.. 2003;60(2):157-165.
- [Google Scholar]
- Chemical Composition, Antioxidant and Antibacterial Activity of Tunisian Date Palm Seed. Pol. J. Environ. Stud.. 2019;28
- [Google Scholar]
- Moisture sorption isotherms and heat of sorption of bitter orange leaves (Citrus aurantium) J. Food Eng.. 2005;67:491-498.
- [Google Scholar]
- Sorption isosteric heat determination by thermal analysis and sorption isotherms. J. Food Sci.. 1999;64(1):64-68.
- [Google Scholar]
- Water sorption isotherms of dates: modeling using GAB equation and artificial neural network approaches. LWT-Food Science and Technology. 1998;31(7–8):699-706.
- [Google Scholar]
- Use of waste date products in the fermentative formation of baker's yeast biomass by Saccharomyces cerevisiae. Bioresour. Technol.. 1997;60(1):67-71.
- [Google Scholar]
- Chemical composition of date palm (Phoenix dactylifera L.) seed oil from six Saudi Arabian cultivars. J. Food Sci.. 2018;83:624-630.
- [Google Scholar]
- Potentials and challenges of date pits as alternative environmental clean-up ingredients. Biomass Convers. Biorefin. 2021:1-28.
- [Google Scholar]
- Moisture sorption characteristics of in-shell macadamia nuts. J. Food Eng.. 1993;18(1):63-76.
- [Google Scholar]
- Modeling of water sorption isotherm for corn starch. J. Food Eng.. 2007;80(2):562-567.
- [Google Scholar]
- Glass transition temperatures and water sorption isotherms of cassava starch. Carbohydr. Polym.. 2009;76(2):305-313.
- [Google Scholar]
- Food properties handbook. CRC Press; 2009.
- Dynamic Isopiestic Method (DIM): Measuring Moisture Sorption Isotherm of Freeze-Dried Garlic Powder and Other Potential Uses of DIM. Int. J. Food Prop.. 2006;9(3):421-437.
- [Google Scholar]
- Exploring validity of the macro-micro region concept in the state diagram: Browning of raw and freeze-dried banana slices as a function of moisture content and storage temperature. J. Food Eng.. 2017;203:32-40.
- [Google Scholar]
- Note: Estimation of sorption isotherm and the heat of sorption of quinoa (Chenopodium quinoaWilld.) seeds. Food Sci. Technol. Int.. 2004;10(6):409-413.
- [Google Scholar]
- Exploitation of waste date seeds of Phoenix dactylifera in form of polymeric particle biocomposite: Investigation on adhesion, cohesion and wear. Compos. B Eng.. 2016;104:9-16.
- [Google Scholar]
- Nonlinear regression analysis program (NLREG). TN: Nashville; 1991.
- Date Pits: Chemical Composition, Nutritional and Medicinal Values, Utilization. Crop Sci.. 2014;54:1322-1330.
- [Google Scholar]
- Moisture sorption isotherms and heat of sorption of instant (soluble) green tea powder and green tea granules. J. Food Eng.. 2008;86:494-500.
- [Google Scholar]
- Determination of Moisture Sorption Isotherms of Jasmine Rice Crackers Using BET and GAB Models. Food Sci. Technol. Int.. 2006;12(6):459-465.
- [Google Scholar]
- Water adsorption isotherms of microcapsules with hydrolyzed pinhão (Araucaria angustifolia seeds) starch as wall material. J. Food Eng.. 2013;114:64-69.
- [Google Scholar]
- A new method for predicting sorption isotherms at different temperatures using the BET model. J. Food Eng.. 2013;114(1):139-145.
- [Google Scholar]
- Thermal characteristics, chemical composition and polyphenol contents of date-pits powder. J. Food Eng.. 2013;119(3):668-679.
- [Google Scholar]
- Thermal characteristics and state diagram of freeze-dried broccoli: freezing curve, maximal-freeze-concentration condition, glass line and solids-melting. Thermochim Acta. 2017;655:129-136.
- [Google Scholar]
- Enthalpy-entropy compensation based on isotherms of mango. Food Sci. Technol. (. 2005;25(2):297-303.
- [Google Scholar]
- Water sorption isotherms of foods and foodstuffs: BET or GAB parameters? J. Food Eng.. 2001;48:19-31.
- [Google Scholar]
- C. Van den Berg and S. Bruin, “Water activity and its estimation in food systems,” in: Proceedings Int. Symp. Properties of Water in Relation to Food Quality and Stability, Osaka, 1978.
- Dates and date pits as ingredients in broiler starting and Coturnix quail breeder diets. Poult. Sci.. 1995;74(7):1134-1142.
- [Google Scholar]
- Equilibrium sorption isotherms and thermodynamic properties of starch and gluten. J. Food Eng.. 1999;40(4):287-292.
- [Google Scholar]
- A modified isopiestic method for adsorp tion of water at high relative vapor pressure. J. Colloid Interface Sci.. 1974;48(3):518-519.
- [Google Scholar]
- Theoretical Insight into Gate-Opening Adsorption Mechanism and Sigmoidal Adsorption Isotherm into Porous Coordination Polymer. J. Am. Chem. Soc.. 2018;140:13958-13969.
- [Google Scholar]







