Translate this page into:
Enhanced Fischer-Tropsch synthesis performance using graphene Nanosheets-Supported Cobalt-Ruthenium Nanocatalysts: Comparative study with γ-Alumina and carbon nanotubes supports
⁎Corresponding author. m_masoumi@iau-tnb.ac.ir (Miresmaeil Masoumi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
In this research, graphene Nanosheets (GNS) as Cobalt-Ruthenium Nanocatalysts support have been used in Fischer-Tropsch synthesis. The characterizations of catalysts were evaluated by Fourier-transform infrared spectroscopy (FTIR), Inductively Coupled Plasma (ICP), Brunauer-Emmett-Teller (BET), X-ray diffraction (XRD), Energy-dispersive X-ray spectroscopy (EDX), Temperature Programmed Reduction (TPR), and Transmission Electron Microscope (TEM). Afterward, the catalysts' activity, selectivity, and performance were assessed in a fixed-bed reactor at 25 bar. The results were compared by changing the catalyst base to (γ-Al2O3) and carbon nanotubes (CNTs). Measurements were performed in the temperature range of 320––340 °C, the gas ratio ( ) of 2, and the gas hourly space velocity of 3 . Using GNS as cobalt catalyst support in Fischer-Tropsch synthesis leads to the 64.5 % and 25.9 % increase in the percentage of CO conversion compared to γ-Al2O3 and CNTs, respectively. Additionally, the utilization of GNS support has resulted in an 11.1 % increase in heavy hydrocarbons selectivity of , in comparison to γ-Al2O3, and a 9.5 % increase compared to CNTs. GNS-based synthesized catalyst with 10 wt% of cobalt and 0.1 wt% of ruthenium (catalyst #6) indicates the highest activity of catalyst at 320 °C with 72.3 % selectivity than heavy hydrocarbons .
Keywords
Fischer-Tropsch
Catalyst
GNS
Cobalt
Nanosheets
1 Introduction
The transformation of natural gas into a liquid state is accomplished through a catalytic procedure referred to as the Fischer-Tropsch synthesis (Piazzi et al., 2022). This method entails the conversion of natural gas, which is primarily composed of methane, into liquid hydrocarbons like diesel, gasoline, and jet fuel (Wang and Astruc, 2018). The primary objective of this process is to enhance the portability and versatility of natural gas for a broader array of applications. In this context, the catalyst employed is typically cobalt, iron, or ruthenium, which is supported on a catalyst base made of alumina, silica, or zeolite (Sapountzi et al., 2017). The catalyst facilitates the reaction between hydrogen and carbon monoxide, ultimately culminating in the production of liquid hydrocarbons (Taghavi et al., 2017). The selection of the supporting material in catalysts holds significant importance in relation to their efficacy and endurance (Abbas et al., 2020). Recent advancements in the realm of nanotechnology have yielded novel support materials, including metal oxides, carbon-based substances, and zeolites, which possess notable surface area and controlled porosity (Hodala et al., 2021). This attribute allows for the optimization of catalytic efficiency and selectivity, rendering them well-suited for employment in the catalytic alteration of natural gas into a liquid state (Santos and Alencar, 2020). Additionally, the utilization of nanoscale support materials facilitates the reclamation of metal pollutants via leaching, an environmentally sustainable approach that fosters the recycling of materials in the production of fresh catalysts (Julkapli and Bagheri, 2015).
GNS represent a remarkable class of two-dimensional substances constructed from a solitary layer of carbon atoms meticulously organized in a hexagonal configuration. This arrangement imparts upon GNS a host of extraordinary characteristics, notably encompassing an expansive surface area, elevated thermal and electrical conductivity, exceptional mechanical robustness, and commendable resistance to chemical alterations. These characteristics have made GNS an attractive material for various applications, catalysis bases (Detsios et al., 2022). In the process of converting natural gas into liquid, GNS has been used as a base material in the manufacture of catalysts due to its high surface area. GNS-based catalysts have exhibited remarkable promise in augmenting the efficiency and discriminating prowess of the Fischer-Tropsch synthesis. An illustrative instance involving the integration of GNS, manifesting as nanosheets, within the domain of catalyst creation for the conversion of natural gas into a liquid state is exemplified by the innovation of GNS-supported cobalt nanoparticles (Liu et al., 2020). This methodology encompasses the reduction of GNS oxide to generate GNSs, which then serve as a foundational substrate for cobalt nanoparticles. The resultant architecture, denoted as GNS-supported cobalt nanoparticles, has demonstrated exceptional catalytic efficiency and resilience, consequently fostering elevated yields of liquid hydrocarbons during the Fischer-Tropsch synthesis. Another exemplar lies in the application of GNS oxide as a scaffold for iron nanoparticles within the Fischer-Tropsch synthesis (Okoye-Chine et al., 2019). In this context, GNSs are functionally endowed with amine groups that serve as anchoring sites for the iron nanoparticles. This functionalization procedure engenders an amplified dispersion of the iron nanoparticles upon the GNS matrix, thereby engendering heightened catalytic efficacy and specificity (Li et al., 2016).
Despite the widespread use of as a catalyst support in Fischer-Tropsch synthesis, several challenges remain unaddressed. One of the primary issues is the limited surface area and porosity of , which can hinder the dispersion of active metals and lead to suboptimal catalytic performance. Additionally, the potential for catalytic deactivation and sintering of metal particles on the γ-Al2O3 support requires further investigation and mitigation strategies. These challenges highlight the need for alternative catalyst support materials that can overcome these limitations and enhance the performance and efficiency of Fischer-Tropsch catalysts. This study seeks to unveil fresh perspectives on the utilization of cobalt and cobalt-ruthenium catalysts, coupled with GNS and CNTs supports, within the Fischer-Tropsch process framework. By delving into uncharted territories, this research endeavors to pinpoint unique catalytic behaviors and performance-boosting factors that distinguish these catalysts. In doing so, it aims to significantly advance our comprehension of their potential within Fischer-Tropsch synthesis. Employing the incipient wetness impregnation technique, the catalysts are synthesized with varying weight ratios. Employing an array of analytical methods, the physicochemical attributes of the catalysts are scrutinized. Moreover, the catalysts' efficacy is gauged based on their carbon monoxide conversion rates and the selectivity of lighter and heavier products. Anticipated results from this investigation are poised to shed light on the creation of more effective and selective catalysts tailored for the Fischer-Tropsch process.
2 Experimental
2.1 Materials
The production of cobalt and cobalt-ruthenium catalysts involved the usage of exceptionally pure starting materials and enhancers. Cobalt nitrate and ruthenium (III) nitrosyl nitrate, acquired from Sigma-Aldrich, served as the primary precursors. These compounds exhibited an exceedingly high level of purity, surpassing 99.9 %. For the creation of catalyst substrates, modified Graphene Nanosheets (GNS) and multi-walled Carbon Nanotubes (CNTs) were procured from Changzhou Sixth Element Materials Technology Co., Ltd.
2.2 Catalyst preparation
The pretreatment process of GNS involved combining 10 g of the GNS with a solution of nitric acid (40 %
) within a round-bottom flask. The mixture was then subjected to reflux at 120 ℃ for a duration of 8 h. Following cooling to room temperature, the resultant mixture was filtered and rinsed several times with deionized water until a neutral pH was achieved. The resulting pretreated GNS material was subsequently dried at 120 ℃ for a span of 24 h. For the creation of catalysts featuring supported cobalt-ruthenium on GNS, predetermined quantities of cobalt and ruthenium were dissolved in deionized water according to the ratios outlined in Table 1. Based on our experiences in previous research works (Mosayebi and Haghtalab, 2015; Shariati et al., 2019), the selection of 10 % (wt.) for cobalt and 0.1 % (wt.) for ruthenium has been made due to the effective performance of these values in the Fischer-Tropsch reaction. These choices have been made based on empirical evidence and theoretical considerations that have been conducted thus far to achieve the desired catalytic efficiency while adhering to economic viability. The mixture of cobalt and ruthenium was then applied to the GNS using the incipient wetness method. The catalyst sample was allowed to settle over a 24-hour period before being dried for an additional 24 h at 120 ℃. This sample was further subjected to calcination under a nitrogen atmosphere at 380 ℃ for a duration of 6 h.
Catalyst
Name Catalyst
Amount of Co (wt %)
Amount of Ru (wt %)
Ru:Co Ratio
Catalyst #1
10
–
–
Catalyst #2
10
0.1
0.01
Catalyst #3
10
–
–
Catalyst #4
10
0.1
0.01
Catalyst #5
10
–
–
Catalyst #6
10
0.1
0.01
The catalysts that were produced utilizing the pretreated GNS, employing varying concentrations of nitric acid, were denoted as Co-Ru/GNS. The same procedure was replicated for the CNTs base materials.
2.3 Characterization
The catalysts underwent an array of characterization methodologies to ascertain their attributes. Fourier Transform Infrared Spectroscopy (FTIR) was employed to detect functional groups. This procedure was executed using a PerkinElmer spectrometer. Powder X-ray Diffraction (XRD) analysis was conducted utilizing an X'Pert Pro X-ray diffractometer from PANalytical in the Netherlands. Cu Kα irradiation (λ = 1.5406 Å) was employed at 40 kV and 30 mA. To scrutinize the morphology of cobalt catalysts situated on the support surfaces, Transmission Electron Microscopy (TEM) was engaged. This was carried out using a Zeiss EM10C device operating at 100 kV. Quantitative determination of Cobalt (Co) and Ruthenium (Ru) loadings in the synthesized catalysts was accomplished through the utilization of the Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP-AES) methodology. Catalyst compositions were ascertained employing Energy-Dispersive X-ray Spectroscopy (EDX) utilizing a JEOL JED-2300 instrument. Nitrogen adsorption isotherms were acquired at a temperature of 77 K, employing the Brunauer-Emmett-Teller (BET) (Costech Sorptometer 1042). The catalysts were subjected to (Temperature-Programmed Reduction) profiling in order to assess the ease of reduction of the metal species present. The samples, each weighing approximately 0.05 g, underwent a purging process with helium at a temperature of 140 °C to remove any residual gases. Subsequently, the temperature was lowered to 40 °C. For the TPR analysis, a gas mixture consisting of 5 % in Ar was utilized. This analysis was performed using the Micrometrics TPD-TPR 2900 analyzer, which was equipped with a thermal conductivity detector (TCD). The gas flow rate for the analysis was maintained at 40 , and the procedure was carried out at atmospheric pressure. The samples were heated linearly at a rate of 10 °C up to 850 °C. Using the Micromeritics TPD-TPR 290 system, the quantity of chemisorbed hydrogen on the catalysts was assessed. Initially, a 0.25 g sample was reduced with hydrogen flow at 400 °C for 12 h, followed by cooling to 100 °C while under hydrogen flow. Next, the hydrogen flow was replaced with argon at the same temperature for approximately 30 min, eliminating weakly adsorbed hydrogen. The temperature-programmed desorption (TPD) was then performed by raising the temperature of the samples to 400 °C under argon flow at a ramp rate of 10 °C . The TPD profile obtained was used to determine the dispersion of cobalt and its average crystallite size on the surface. After -TPD, 10 % oxygen in helium pulses were used to reoxidize the sample at 400 °C to assess the degree of reduction.
2.4 Hydrocarbon synthesis in the reaction system
A fixed-bed reactor (Fig. 1) system employing a downward flow configuration was utilized for the Fischer-Tropsch synthesis process. The reactor itself comprised a stainless-steel tube characterized by an inner diameter of 22 mm and a length of 450 mm. Within this reactor, 1 g of catalyst was positioned. Controlled addition of
and CO was executed into the reactor using Brooks 5850 mass flow controllers, operating at the specified rates. To initiate the process, the catalyst underwent reduction via a continuous flow of hydrogen at a temperature of 380 °C for a duration of 12 h. Subsequent to reduction, a mixture of CO and
was introduced into the reactor. This mixture was maintained at a pressure of 20 bar, with a flow rate of 50 mL
, and a temperature of 320–340 °C. The ratio of
to CO in the mixture was maintained at 2. The generated products were continuously extracted from the reactor and channeled through two separate traps: a hot trap maintained at 100 °C and a cold trap held at 0 °C. The gaseous products, including
,
, and others, were subjected to periodic analysis at 2-hour intervals. The liquid products were collected and subjected to analysis using three distinct gas chromatographs. Selectivity data were collected after the first 24 h.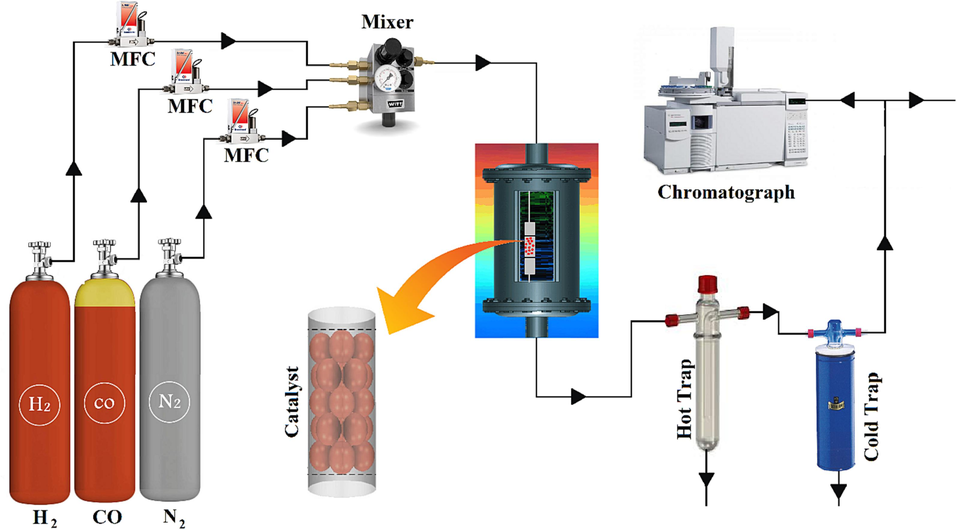
The schematic diagram of the Fischer-Tropsch synthesis setup.
An analysis system involving a Shimadzu 4C gas chromatograph was utilized. This system incorporated two interconnected packed columns, namely Porapak Q and Molecular Sieve 5 Å. The gas chromatograph was equipped with a thermal conductivity detector (TCD) that employed argon as the carrier gas for hydrogen analysis. For the comprehensive examination of liquid products and to obtain a detailed product distribution, a Varian CP 3800 instrument was employed. This setup featured a Petrocol Tm DH100 fused silica capillary column and a flame ionization detector (FID). Furthermore, an additional Varian CP 3800 instrument was employed, equipped with a chromosorb column and a thermal conductivity detector (TCD). This configuration was specifically utilized for the analysis of non-condensable gases, such as CO, , and other similar components. To ensure a uniform temperature distribution throughout the catalytic bed, the reactor was situated within a molten salt bath ( 7 % wt, 53 % wt, 40 % wt), complemented by a stirrer. The temperature of the bath was meticulously controlled through the utilization of a PID (Proportional-Integral-Derivative) temperature controller.
3 Results and discussion
3.1 Functional groups analysis
Fig. 2 illustrates the FTIR analysis of the functional groups on the GNS (a) and CNT (b). In GNS, adsorption bands related to the oscillation of the bond of the carboxyl group with a wavelength of 1096
, and the aromatic ring bond seen in the 1469
region of the spectrum was observed. Symmetric and asymmetric C–H stretches were observed for CH2 and CH3 at 2918
and C–H at 571
. The peaks observed in 1712
and 3425
were related to the tensile vibrations of the carbonyl groups and the hydroxyl groups in carboxylic acid, respectively. These results indicate that the treatment of GNS with nitric acid caused the formation of carboxyl groups on the surface of GNS. In addition, the peak observed at wavelength 1572
is related to the carbon double bonds (C = C) in the structure of GNS (Vasseghian et al., 2022). In CNT, the peak at 3420
suggests the presence of hydroxyl groups or adsorbed water molecules on the CNT surface. The peak at 2930
corresponds to aliphatic C–H stretching vibrations, possibly indicating organic residues or impurities. Peaks at 1630
and 1500
are indicative of sp2-hybridized carbon structures, characteristic of CNTs, as they represent C = C stretching vibrations within aromatic rings. The peak at 1450
could be attributed to C–H bending vibrations or carboxylic acid functional groups. Peaks at 1384
and 1109
may relate to defects in the carbon lattice (D band) and the in-plane stretching of carbon atoms (G band), respectively, reaffirming the presence of CNTs.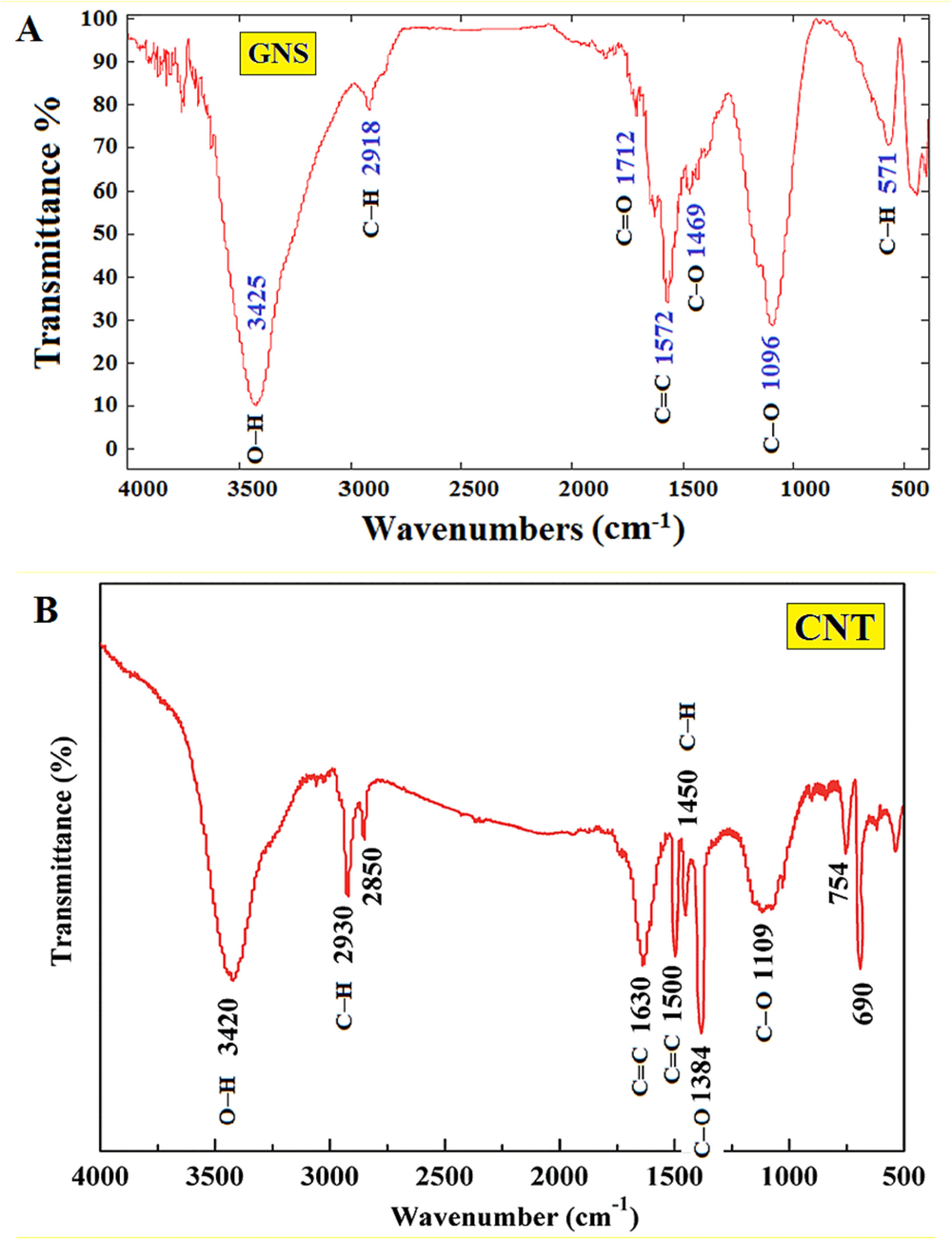
The FTIR spectrum of functionalized GNS (a) and CNT (b).
Functional groups on CNTs and GNS significantly enhance Fischer-Tropsch synthesis catalysts. These groups act as active sites, promote metal dispersion, and affect reactant/product interactions. Oxygen-containing groups facilitate reactant adsorption and dissociation, vital in Fischer-Tropsch reactions. They also disperse catalytic metals (cobalt, ruthenium), anchoring nanoparticles to ensure uniform dispersion, increasing active sites, and overall performance (Davari et al., 2014). Functional groups influence reactant/product adsorption and desorption through strength variations. This impacts Fischer-Tropsch selectivity, favoring specific hydrocarbon formation and reducing byproducts. Additionally, functional groups hinder metal sintering, preserving catalyst stability and extending its lifespan. They modify support material properties, affecting surface area and pore structure, ultimately shaping mass transport and diffusion within the catalyst (Haghtalab and Mosayebi, 2014).
3.2 Elemental composition
The composition of the catalysts using ICP analysis is shown in Table 2. The results show that the loading value of cobalt and ruthenium is close to the theoretical value (Cheng et al., 2021; Wang and Astruc, 2018). The table presents the results of ICP analysis for the elemental composition of various catalysts. These results indicate that the actual loading values of ruthenium and cobalt in the catalysts are generally close to their theoretical values. Here are the key findings: Based on these results, the loading of cobalt and ruthenium in many of the catalysts is very close to the theoretical values, which are the expected values based on theory. These results confirm that the catalysts have been produced and prepared with the specified elemental loading values. Small differences between the actual and theoretical values may be attributed to various factors related to the production or measurement process.
Catalyst
Catalyst Name
Cobalt loading (%)
Ru loading (%)
ICP
Theory
ICP
Theory
Catalyst #1
9.57
10
–
–
Catalyst #2
9.52
10
0.084
0.1
Catalyst #3
9.62
10
–
–
Catalyst #4
9.56
10
0.092
0.1
Catalyst #5
9.41
10
–
–
Catalyst #6
9.49
10
0.088
0.1
3.3 Surface area and porosity
The BET analysis was conducted to quantify the surface area (
), mean pore radius (nm), and pore volume (
) of both the support materials and the catalysts. The outcomes are outlined in Table 3. In the calcined catalysts, all three parameters exhibit reduced values compared to the supports. This is attributed to the coverage of active sites and the obstruction of pores by the active metal species (cobalt and ruthenium) that are introduced during the catalyst synthesis process (Fang et al., 2021; Li et al., 2022). Interestingly, when increasing the amount of ruthenium in the bimetallic catalyst, there are limited changes in the pore volume and average pore values. However, the surface area experiences a decline, dropping from 265.9 to 260.1
. The reduction in BET surface area and porosity in the loaded catalysts compared to the pristine support is primarily attributed to the occupation of pores by active metal components. The increase in ruthenium loading has a limited impact on pore volume and average pore size but does affect the surface area (Haghtalab and Mosayebi, 2014).
Special Surface Area (BET =
)
Support/Catalyst
Pore Volume (
)
Average pore radius (nm)
268.81
1.10
13.21
215.04
0.90
12.70
254.01
0.57
12.32
195.30
0.49
11.81
332.37
1.59
12.18
265.90
0.85
11.54
3.4 Crystallographic characterization
XRD analysis was employed to discern the crystalline phases present in the Co and Co-Ru catalysts situated on γ-Al2O3, CNTs and GNS. As depicted in Fig. 3, the peaks registered at 2θ = 25.73° and 2θ = 43.53° correspond to GNS and CNT. Notably, cobalt oxide peaks are discernible on the GNS surface, manifesting at 2θ = 32.36°, 36.9°, 54.6°, 59.35°, and 65.21°. The most pronounced peak is discerned at the 2θ angle of 36.9°.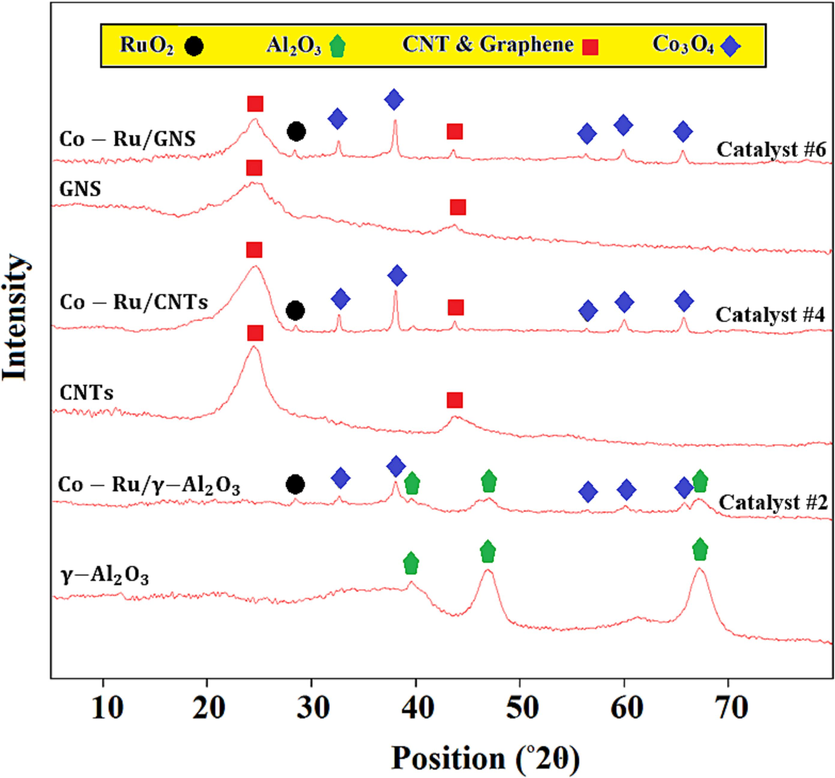
XRD patterns of Catalyst #2, Catalyst #4, Catalyst #6, and the base of the catalysts.
Cobalt oxide peaks were observed on the GNS surface, appearing at 2θ = 32.36° (JCPDS No. 78–2043), 36.9° (JCPDS No. 42–1467), 54.6° (JCPDS No. 71–2174), 59.35° (JCPDS No. 78–2044), and 65.21° (JCPDS No. 78–2045), with the most pronounced peak at 2θ = 36.9°. A specific peak corresponding to ruthenium oxide ( ) was observed at 2θ = 29.1° (JCPDS No. 76–1383).
In accordance with prior research (Sasson Bitters et al., 2022), the cobalt oxide present in the XRD pattern typically conforms to a specific structure. Cobalt oxidation proceeds through two distinct steps, shown in Eq. (1) and (2). These findings are consistent with the results obtained from the TPR analysis.
Catalyst
diameter (nm)
Reference
12.7
In this research
13.2
In this research
11.6
In this research
12.1
In this research
11.4
In this research
11.9
In this research
Co-Ru/MWCNTs
14.9
(Trépanier et al., 2009)
Co/CNTs
15.4
(Davari et al., 2014)
Co/rGO
19.5
(Jiang et al., 2021)
Co/GNS
21.6
(Karimi et al., 2015a)
Co/NGA
23.2
(Wang et al., 2020)
Notably, within the context of the bimetallic Catalyst #6, the dimensions of cobalt oxide nanocrystals register an increment of 0.5 nm when contrasted with the single-metal Catalyst #5. This alteration is attributed to the reduction in the count of cobalt oxide crystal sites engendered by the inclusion of ruthenium, thereby culminating in the formation of a bimetallic catalyst.
3.5 EDX and TPR results
In order to confirm the presence of all constituent elements in the
and
configurations, EDX spectra were acquired. As depicted in Fig. 4a, Catalyst #5 displayed discernible peaks corresponding to C, O, and Co, while according to Fig. 4b Catalyst #6 exhibited peaks indicative of C, O, Co, and Ru. The detection of oxygen within the samples is indicative of the oxidation of metal atoms situated on the surface of the catalyst. TPR test is used to evaluate the rate of reduction of oxide phases. Using the catalyst reduction peaks, information about the interaction between the active metal and the supports is obtained. Fig. 5 shows the TPR patterns of the Catalyst #1- Catalyst #6.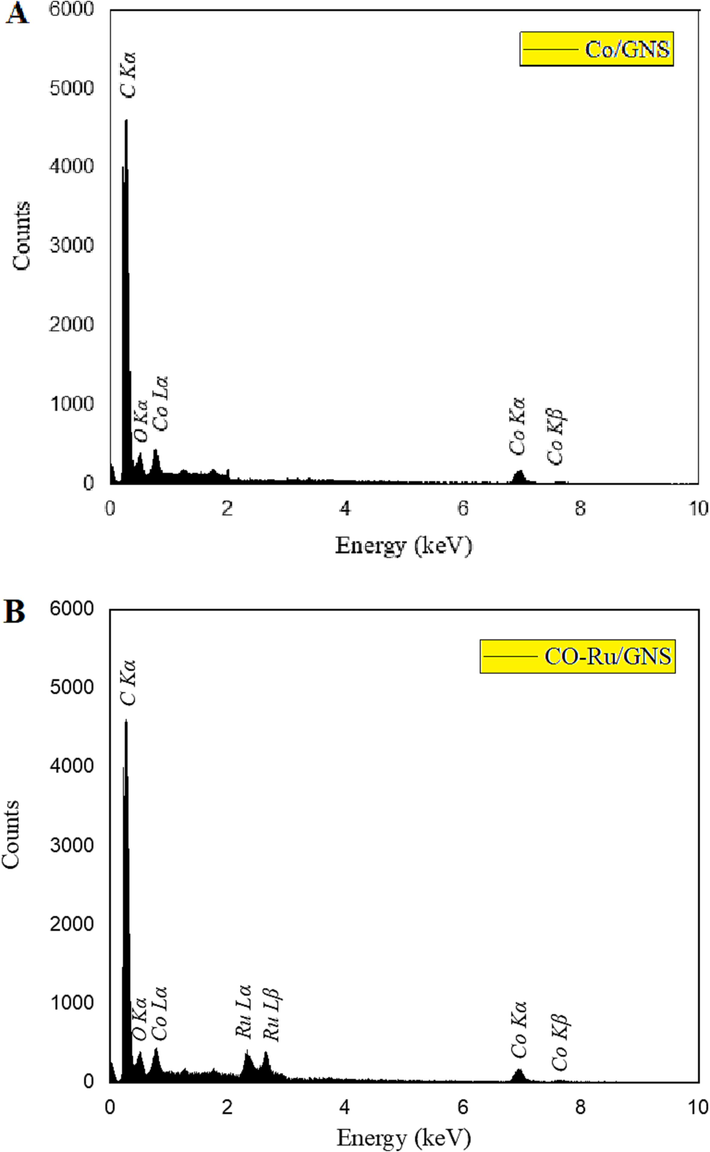
The EDX test for Catalyst #5 (a) and Catalyst #6 (b).
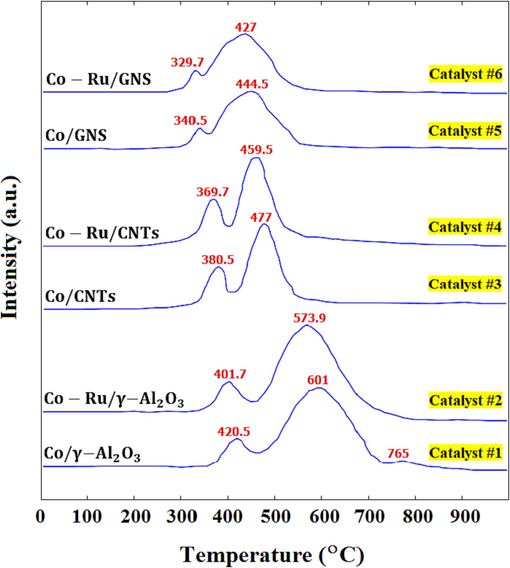
TPR patterns of the Catalyst #1 - Catalyst #6.
In the TPR spectrum of Catalyst #1, distinct reduction peaks are evident at 420.5 °C and 601 °C. Similarly, Catalyst #3 showcases these same peaks at lower temperatures of 380.5 °C and 477 °C, while for Catalyst #5, they appear at even lower temperatures of 340.5 °C and 444.5 °C. The initial peak corresponds to the reduction of to while the subsequent peak corresponds to the reduction of CoO into metallic cobalt. Furthermore, a minor peak is discernible at 765 °C, indicative of the reduction of cobalt aluminate, a compound formed through the reaction between cobalt oxide and γ-Al2O3. This interaction prompts a shift in the TPR peak towards higher temperatures, thereby elevating the reduction temperature. This complexity in cobalt oxide reduction leads to a more intricate reduction process and subsequently diminishes catalyst activity (Vasseghian et al., 2022). In alignment with Fig. 5, the cobalt aluminate peak is notably absent in both the CNT and GNS support contexts. This absence suggests a more straightforward reduction mechanism for cobalt oxide within catalysts founded on these support bases.
For Catalyst #3, the surface area beneath the second peak is roughly threefold greater than the area beneath the first peak. This observation implies that a majority of the crystals situated on the CNTs support exist in the
form (Mu et al., 2021). Conversely, the surface area beneath the peak for Catalyst #5 surpasses that of the reference catalyst. This discrepancy signifies a more pronounced and comprehensive reduction process facilitated by the GNS-based catalyst. Consequently, this enhancement leads to a heightened consumption of hydrogen and a more thorough reduction, thereby making a larger number of cobalt atoms accessible for engagement in the Fischer-Tropsch reaction. Table 5 compiles data on the temperatures of the first and second peaks for various catalysts, along with the levels of hydrogen consumption and the degree of reduction as assessed through the TPR analysis. Notably, the reduction temperatures of the first and second peaks for catalysts founded on CNTs are significantly lower than those observed in cobalt-based alumina catalysts. Furthermore, in GNS-based catalysts, these reduction temperatures are even lower in comparison to the other two support materials.
Catalyst
Catalyst Name
TPR peaks
H2 consumption
(mmol)
Reduction (%)
1st (°C)
2nd (°C)
Catalyst #1
420.5
601.0
0.1480
47.3
Catalyst #2
401.7
573.9
0.1662
71.1
Catalyst #3
380.5
477.0
0.1367
56.4
Catalyst #4
369.7
459.5
0.1701
69.6
Catalyst #5
340.5
444.5
0.1535
64.2
Catalyst #6
329.7
427.0
0.1818
80.3
In the realm of catalysis research, a thorough examination of the reduction behavior of catalyst systems supported on diverse substrates has unveiled valuable insights into the intricate interplay between metal species and their underlying support materials. A comparative review of several pertinent studies sheds light on the main reduction peaks of various catalysts, along with additional information elucidating the underlying dynamics. In a study by Gonzalo-Chacón et al. (Gonzalo-Chacón et al., 2014), the reduction peak of 480 °C (RuO) for Ru/Graphite catalysts was observed. The investigation highlighted the pivotal role of the interaction between metal species and the GNS support in dictating the reducibility of the catalyst, thereby modulating its catalytic efficacy. In a separate line of inquiry, Karimi et al. (Karimi et al., 2015a) delved into Co/GNS catalysts and uncovered a reduction peak at 350 °C (CoO). Hydrogen temperature-programmed reduction (H2-TPR) analyses in this context provided invaluable insights into the complex interaction dynamics between Co species and the GNS support, thereby offering a deeper understanding of the catalyst's behavior. Building upon the exploration of support interactions, Taghavi et al. (Taghavi et al., 2019) scrutinized Ru/N-GNS catalysts, revealing dual reduction peaks at 225 °C and 525 °C (RuO). Employing H2-TPR, the study not only elucidated the reducibility of metal species but also emphasized the influence of the GNS support on the electronic properties of the catalyst system. Intriguingly, Taghavi et al. (Taghavi et al., 2017) revisited the Co/GNS catalyst system, identifying a distinct reduction peak at 400 °C (CoO). The authors attributed the shift of this peak to a lower temperature to a stronger interaction between Co species and the GNS support. This interaction, in turn, impacted the overall characteristics of the catalyst. The study conducted by Hemmati et al. (Hemmati et al., 2012) investigated Ru/GNS catalysts, revealing a broader reduction peak within the range of 380–560 °C (RuO). This distinctive observation was ascribed to the presence of multiple RuOx species with varying reducibility. The diversity in reducibility was attributed to the intricate interaction between Ru species and the GNS support. A different avenue of exploration led Shariati et al. (Shariati et al., 2019) to explore Co-Ru/CNT catalysts, uncovering reduction peaks at 300 °C (CoO) and 450 °C (RuO). Notably, these catalysts displayed a robust interaction with the CNT support, contributing to enhanced reducibility of cobalt and ruthenium species, thereby underscoring the critical role of support interactions in catalytic performance. Turning attention to Co/CNT catalysts, Karimi et al. (Karimi et al., 2015b) reported a reduction peak at 200 °C (CoO). The weak interaction observed between cobalt species and the CNT support played a role in shaping the catalyst's reducibility, subsequently influencing its overall effectiveness. In a final investigation, Bahome et al. (Bahome et al., 2007) studied Ru/CNT catalysts, revealing a reduction peak at 320 °C (RuO). The findings indicated a weak interaction between ruthenium species and the CNT support, thereby imparting distinctive characteristics to the catalyst system.
Table 5 elucidates that augmenting the concentration of the ruthenium promoter induces a downward shift of the high-temperature and low-temperature peaks in the respective catalysts (#2, #4, and #6). This shift results in the transfer of these peaks to lower temperatures. Notably, the reduction of bimetallic Co-Ru catalysts generally exhibits a higher ease of reduction compared to the single-metal Co catalysts. By incorporating a 1 wt% content of ruthenium into the cobalt catalyst anchored on GNS the temperature of the first peak decreased from 340.5 °C to 329.7 °C, and the temperature of the second peak reduced from 444.5 °C to 427 °C. In the context of Catalyst #2, the inclusion of a ruthenium layer atop cobalt atoms prevented the interaction between cobalt and aluminum atoms. This prevention averted the formation of cobalt aluminate, which in turn resulted in the absence of the third reduction peak in the catalyst (Li et al., 2015).
The reduction extent exhibited by bimetallic catalysts (#2, #4, and #6) markedly surpassed that of their monometallic counterparts (#1, #3, and #5). The inclusion of ruthenium within the bimetallic catalysts induced a decrease in the temperature requisite for cobalt oxide reduction. As a consequence, the reduction process exhibited enhanced ease, leading to a heightened presence of active sites on the catalyst surface, which was notably conducive for the Fischer-Tropsch reaction (Sasson Bitters et al., 2022). While alterations in hydrogen consumption might not precisely mirror the extent of reduction, the quantity of hydrogen consumed exhibits behavior akin to the degree of reduction (Wang et al., 2021).
3.6 TPD results
The findings derived from TPD analysis of cobalt catalyst samples (#1, #3, and #5) utilizing oxygen are presented in Table 6. Notably, in the case of Catalysts founded on CNTs and GNS as catalyst bases, there is an observed increase in the cobalt crystal dispersion, concomitant with a decrease in the size of cobalt particles. This phenomenon stands in contrast to catalysts synthesized using the same methodology but based on γ-Al2O3. The elevated surface area characteristic of GNS and CNTs exerts an augmenting influence on the parameters aforementioned. The size reduction of cobalt particles, stemming from their diminished dimensions, culminates in an amplified surface area. This size reduction, in turn, contributes to a heightened catalyst activity. Furthermore, the reduction extent of Catalyst #1 surpasses that of Catalyst #3 by approximately 18 %, while the reduction extent of Catalyst #5 exceeds that of Catalyst #3 by roughly 15 %. This elevation in catalyst reduction can be attributed to the interaction between the supporting material and the active metal cobalt, as well as the reduction of both constituents. The combination of heightened dispersion and smaller particle size yields a greater count of active sites suitable for the conversion of carbon monoxide, thereby fostering an augmentation in the rate of hydrocarbon production (Chernyak et al., 2020; Wolf et al., 2021; Zhang et al., 2021).
Catalyst
Name Catalyst
H2 desorption
Dispersion (%)
Reduction (%)
dparticles (nm)
Catalyst #1
0.910
7.8
40.8
12.4
Catalyst #3
0.942
9.2
48.1
11.1
Catalyst #5
0.990
12.3
55.3
10.6
3.7 Visualization of particle morphology
Fig. 6a and 6c presents the TEM and SEM outcomes for Catalyst #6. The presence of dark spots corresponds to the spherical configuration of cobalt oxide nanoparticles situated on the surface of GNS. The TEM imagery distinctly reveals that the cobalt nanoparticles, with sizes ranging from 5 to 15 nm, are effectively distributed across the support surface. The mean size of the cobalt oxide particles within the catalyst approximates 12.04 nm, a measurement that aligns with the findings derived from XRD analysis. Additionally, an evaluation of the cobalt particle size distribution has been performed based on the cobalt particle population. This distribution is depicted in Fig. 6b and 6d. However, it's important to note that the dimensions obtained through TEM testing are relatively larger than those yielded by XRD analysis. This divergence is attributed to particle agglomeration during the synthesis process (Ghogia et al., 2021). The analysis by Dey and Dhal (Dey et al., 2019; Dey and Dhal, 2020) demonstrated that the average particle sizes for the CuMnOx catalysts were 1.45–2.35
and,
catalyst was 20–95 nm (Dey and Chandra Dhal, 2020).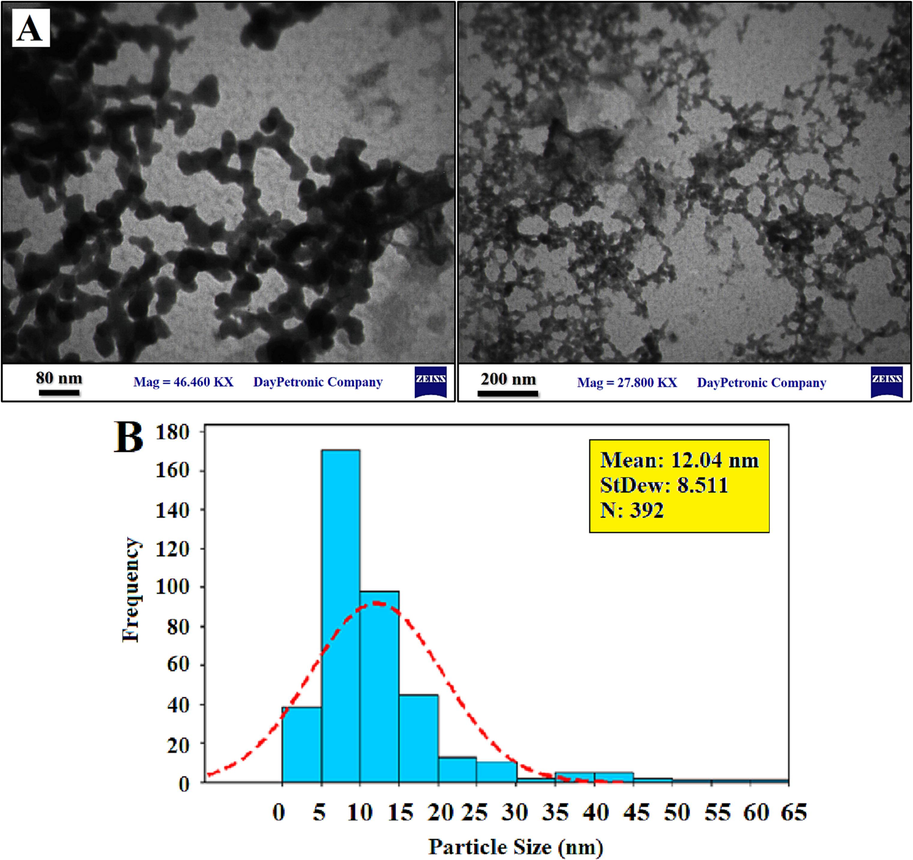
TEM images of the Catalyst #6 (a), and cobalt oxide particle size histogram (b) SEM images of the Catalyst #6 (c), and cobalt oxide particle size histogram (d).
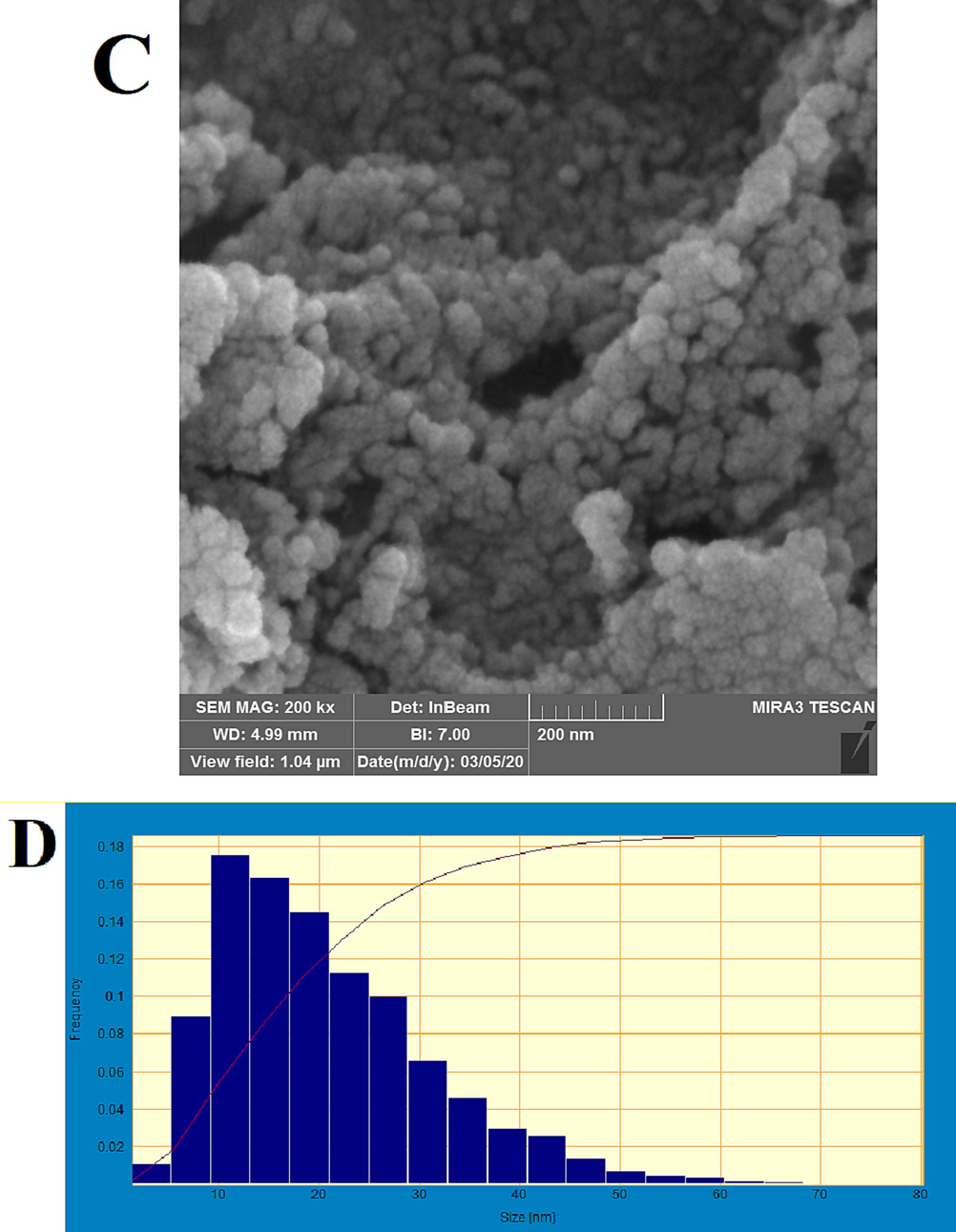
TEM images of the Catalyst #6 (a), and cobalt oxide particle size histogram (b) SEM images of the Catalyst #6 (c), and cobalt oxide particle size histogram (d).
3.8 Catalyst results
Fischer-Tropsch synthesis was conducted employing various catalysts, and the outcomes of these experiments are presented in terms of carbon monoxide conversion percentage and product selectivity, as detailed in Table 7. The diminished extent of reduction exhibited by Catalyst #1 results in a reduction of available active sites for carbon monoxide conversion compared to Catalysts #3 and #5. Consequently, the carbon monoxide conversion rate for the γ-Al2O3-based Catalyst #1 is lower in comparison to the catalysts founded on CNTs and GNS. The lower percentage of
with Catalyst #1 can be attributed to its monometallic cobalt composition, which may favor the production of hydrocarbons over
. The presence of promoter metals and unique support materials in other catalysts can lead to different selectivity patterns, resulting in varying percentages of
and other reaction products.
Catalyst
Catalyst Name
conversion
Selectivity
Catalyst #1
31.4
23.3
13.2
62.5
Catalyst #2
36.6
19.5
10.3
67.2
Catalyst #3
41.1
22.6
12.9
63.4
Catalyst #4
47.3
19.6
9.9
68.5
Catalyst #5
51.7
20.4
10.5
69.4
Catalyst #6
58.2
16.3
8.4
72.3
The findings presented by Kazemnejad et al. (2019) underscore the significant impact of catalyst composition and preparation methods on conversion efficiency and selectivity in catalytic processes. Specifically, the study highlights notable improvements in CO conversion rates with catalysts prepared through chemical vapor deposition, especially the catalyst, which demonstrated a 5.3 % increase compared to conventional impregnation-prepared catalysts. Additionally, the Co/GNS catalyst, supported on graphene nanosheets, exhibited the highest CO conversion rate at 78.4 %, attributed to the abundant active sites and unique properties of graphene nanosheets.
The observed differences in conversion efficiency between Catalyst #3 and Catalyst #5 in the present work align with the findings of Kazemnejad et al. (2019). The superior performance of GNS-based catalysts over CNTs-based counterparts is evident, and this enhanced performance can be attributed to the distinctive properties of GNS, including its elevated specific surface area and two-dimensional sheet-like structure. These attributes contribute to a heightened dispersion of particles on the support surface, corroborating the importance of catalyst composition and support structure in catalytic reactions. Authors should consider incorporating this contextual information to elucidate the relevance and significance of their own work in relation to the established findings in the field.
The results obtained with Catalyst #6 in Table 7 indicate its performance in Fischer-Tropsch synthesis. Catalyst #6 is a bimetallic catalyst, composed of cobalt and ruthenium, and it is supported on graphene nanosheets (GNS). Catalyst #6 exhibits a relatively high carbon monoxide conversion rate of 58.2 %. This means that a significant portion of the CO feedstock is being converted into hydrocarbons, which is a key indicator of the catalyst's activity.
The selectivity of Catalyst #6 is as follows:
16.3 % for : This indicates that a relatively small fraction of the converted CO is being transformed into .
8.4 % for hydrocarbons: This represents the selectivity for ethylene, propylene, and butylene, which are valuable in the petrochemical industry.
72.3 % for hydrocarbons: The majority of the converted CO is directed toward the production of heavier hydrocarbons, which have higher commercial value.
The high selectivity for hydrocarbons is a significant feature of Catalyst #6. This indicates that the catalyst is effective at producing long-chain hydrocarbons, which are valuable for applications such as diesel fuel and lubricants. The relatively low selectivity for methane suggests that Catalyst #6 is capable of minimizing the production of lighter and less valuable hydrocarbons.
The enhanced performance of Catalyst #6 can be attributed to the presence of ruthenium as a promoter metal. Ruthenium plays a crucial role in enhancing the activity of the cobalt catalyst, increasing the extent of reduction and, consequently, the number of active sites available for the Fischer-Tropsch synthesis.
The use of graphene nanosheets as a support material also contributes to the catalyst's performance. The high specific surface area and unique structure of graphene nanosheets facilitate better dispersion of metal particles, leading to smaller crystal sizes, improved catalytic activity, and selectivity for hydrocarbons.
Notably, the carbon monoxide conversion percentage for Catalyst #2 exceeds that of Catalyst #1, an effect attributed to the formation of a bimetallic structure. Upon introducing ruthenium into the catalyst composition and generating the bimetallic configuration, the carbon monoxide conversion rate escalates from 31.4 % to 36.6 %. This substantial increase in conversion rate can be directly linked to a significant rise in the extent of reduction, surging from 47.3 % to 71.7 %. Analogous findings can be observed for Catalysts #4 and #6 when compared to their corresponding Catalysts #3 and #5. In summation, the cobalt-ruthenium bimetallic catalyst showcases a pivotal role for ruthenium in catalytic activity, with cobalt as the primary enhancing metal (Navalon et al., 2016).
Ruthenium serves as a promoter for cobalt metal, exerting a positive influence on its performance. The saturation of ruthenium with hydrogen on the cobalt surface enhances the reduction process of cobalt, subsequently augmenting the pool of active sites available for the Fischer-Tropsch synthesis. This enhancement in active sites leads to an escalation in the percentage of carbon monoxide conversion. As a result, Catalysts #2, #4, and #6 exhibit heightened activity in Fischer-Tropsch synthesis when compared to Catalysts #1, #3, and #5 (Pereira Lopes and Astruc, 2021).
The selectivity of methane for bimetallic catalysts #2, #4, and #6 is notably lower in comparison to that of monometallic catalysts #1, #3, and #5. Conversely, the selectivity for heavy hydrocarbons follows an opposing trend. Notably, the introduction of enhanced ruthenium content in Catalyst #6 leads to an increment in the selectivity of
hydrocarbons, increasing from 69.4 % to 72.3 % in contrast to Catalyst #5. These observed outcomes can be directly attributed to the specific configuration of the bimetallic catalyst. Within this architecture, ruthenium emerges as a pivotal contributor to the catalytic traits of the Fischer-Tropsch reaction. Additionally, it's worth noting that ruthenium displays a heightened propensity for methane production compared to cobalt. The results of calculation of
hydrocarbon yield are presented in Fig. 7. This yield of heavy hydrocarbons (
) holds significance in the Fischer-Tropsch process due to its amalgamation of carbon monoxide conversion percentage and
selectivity. Remarkably, the highest yield is attributed to Catalyst #6 (the cobalt-ruthenium bimetallic catalyst based on GNS, characterized by the highest ruthenium percentage) operating at a temperature of 320–340 °C. The effect of temperature on Fischer-Tropsch synthesis results in a complex interplay of factors, which vary depending on specific catalyst compositions and conditions. Generally, higher temperatures accelerate the reaction kinetics, increasing the conversion of CO and
to hydrocarbons. However, this doesn't always lead to higher yields of desired products. Elevated temperatures tend to favor the formation of unwanted
, which competes with the production of valuable
. Catalyst deactivation can occur more rapidly at higher temperatures due to issues like carbon deposition (coking), reducing overall hydrocarbon production and affecting selectivity. Catalysts with different compositions and support materials respond differently to temperature changes. Additionally, thermodynamics play a role, with higher temperatures shifting the equilibrium toward lighter hydrocarbons. These factors contribute to the observed temperature-dependent effects on Fischer-Tropsch synthesis.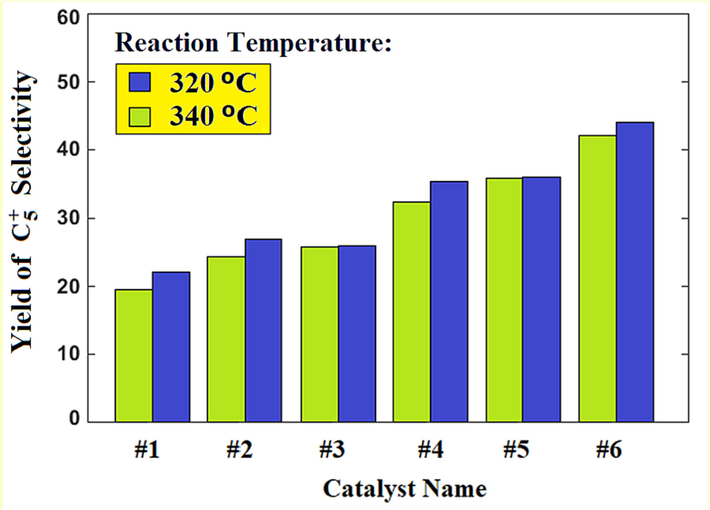
The yield of
for catalyst #1 – catalyst #6.
Similar to the results of this research, A. Chernyak et al. (Chernyak et al., 2022) developed catalysts containing iron and potassium within a three-dimensional structure, supported by graphene nanoflakes and carbon nanotubes (These catalysts were designed for the conversion of syngas into hydrocarbons); The results of their study showed that the selectivity for reached 70 %. Also, Karimi et al. (Karimi et al., 2015b) reported that the conversion efficiency of CO increased from 61 % to 74 % when using the graphene-supported cobalt catalyst, leading to a notable 22 % enhancement in the Fischer–Tropsch synthesis rate.
In terms of product selection, the study demonstrated a reduction in selectivity for light hydrocarbons, including a 34 % decrease in methane selectivity and a 25 % decrease in selectivity for gaseous hydrocarbons, when employing the graphene-supported catalyst. Conversely, the selectivity to liquid products increased by approximately 5 %. The results also indicated a higher selectivity for the 15.0 wt% Co/graphene catalyst compared to the 15.0 wt% Co/CNTs catalyst, attributed to the influence of increased CO conversion and water partial pressure on the water–gas shift reaction. Furthermore, the Anderson–Schultz–Floury distribution analysis revealed a chain growth probability (α) of 0.85 for Co/graphene and 0.80 for Co/CNTs catalysts, affirming the propensity of the graphene-supported catalyst to produce higher molecular weight hydrocarbons.
The comparative analysis of various catalysts underscores the pivotal role of factors such as bimetallic configurations, support materials like graphene nanosheets, and promoter effects in shaping the carbon monoxide conversion rates and product selectivity during Fischer-Tropsch synthesis.
4 Conclusions
In this study, the effect of support on the activity of cobalt and cobalt-ruthenium catalysts in Fisher-Tropsch synthesis has been reviewed. 10 wt% cobalt catalysts with 0.1 % wt% loads of ruthenium were made by incipient wetness impregnation on , CNTs and GNS support, respectively. Characterization tests have also reported desirable chemical-physical properties, such as high surface area and better reduction, for catalysts on GNS. The surface properties of functionalized GNS lead to better distribution and a higher reduction rate of cobalt particles. Also, the high surface area of the GNS support causes better dispersion of metal nanoparticles on the surface, smaller metal particle size, increasing the number of active metal sites, and increasing catalyst activity. The results show that GNS support Fisher-Tropsch synthesis catalysts more than γ-Al2O3 and CNTs supports. Using of GNS has increased the selectivity of , compared to γ-Al2O3 and CNTs. Catalyst.
showed the highest catalyst activity at 320 °C with 72.3 % selectivity .
Statement:
The authors of this article declare that they have not published this article anywhere before. The authors declare no conflict of interest/competing interest. The authors declare that they have not received funding from anywhere in this research.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Hierarchical porous spinel MFe2O4 (M=Fe, Zn, Ni and Co) nanoparticles: Facile synthesis approach and their superb stability and catalytic performance in Fischer-Tropsch synthesis. Int. J. Hydrogen Energy. 2020;45:10754-10763.
- [CrossRef] [Google Scholar]
- Fe-Ru small particle bimetallic catalysts supported on carbon nanotubes for use in Fischer-Tröpsch synthesis. Appl. Catal. A Gen.. 2007;328:243-251.
- [CrossRef] [Google Scholar]
- Manipulating metal-support interactions of metal catalysts for Fischer-Tropsch synthesis. Chinese J. Chem. Eng.. 2021;35:220-230.
- [CrossRef] [Google Scholar]
- Effect of type and localization of nitrogen in graphene nanoflake support on structure and catalytic performance of Co-based Fischer-Tropsch catalysts. Catal. Today. 2020;357:193-202.
- [CrossRef] [Google Scholar]
- Graphene Nanoflake- and Carbon Nanotube-Supported Iron-Potassium 3D-Catalysts for Hydrocarbon Synthesis from Syngas. Nanomaterials. 2022;12
- [CrossRef] [Google Scholar]
- Enhancement of activity, selectivity and stability of CNTs-supported cobalt catalyst in Fischer-Tropsch via CNTs functionalization. Appl. Catal. A Gen.. 2014;485:133-142.
- [CrossRef] [Google Scholar]
- Detsios, N., Zisopoulos, G., Atsonios, K., Nikolopoulos, N., Grammelis, P., Kaltenmorgen, J., 2022. Dynamic process simulations and control of a lignite / waste to Fischer Tropsch liquids plant 236. https://doi.org/10.1016/j.fuproc.2022.107395.
- Controlling carbon monoxide emissions from automobile vehicle exhaust using copper oxide catalysts in a catalytic converter. Mater. Today Chem.. 2020;17:100282
- [CrossRef] [Google Scholar]
- Synthesis of silver promoted CuMnOx catalyst for ambient temperature oxidation of carbon monoxide. J. Sci. Adv. Mater. Devices. 2019;4:47-56.
- [CrossRef] [Google Scholar]
- Synthesis of CuMnOx catalysts by novel routes for selective catalytic oxidation of carbon monoxide. Comput. Toxicol.. 2020;16:100132
- [CrossRef] [Google Scholar]
- Low-temperature conversion of methane to oxygenates by supported metal catalysts: From nanoparticles to single atoms. Chinese J. Chem. Eng.. 2021;38:18-29.
- [CrossRef] [Google Scholar]
- Cobalt catalysts on carbon-based materials for Fischer-Tropsch synthesis: a review. Appl. Catal. A Gen.. 2021;609:117906
- [CrossRef] [Google Scholar]
- Effects of the reduction temperature over ex-chloride Ru Fischer-Tropsch catalysts supported on high surface area graphite and promoted by potassium. Appl. Catal. A Gen.. 2014;480:86-92.
- [CrossRef] [Google Scholar]
- Co@Ru nanoparticle with core-shell structure supported over γ-Al2O3 for Fischer-Tropsch synthesis. Int. J. Hydrogen Energy. 2014;39:18882-18893.
- [CrossRef] [Google Scholar]
- Cobalt supported on CNTs-covered γ- And nano-structured alumina catalysts utilized for wax selective Fischer-Tropsch synthesis. J. Nat. Gas Chem.. 2012;21:713-721.
- [CrossRef] [Google Scholar]
- Catalyst design for maximizing C5+ yields during Fischer-Tropsch synthesis. Int. J. Hydrogen Energy. 2021;46:3289-3301.
- [CrossRef] [Google Scholar]
- Van der waals heterostructures by single cobalt sites-anchored graphene and g-C3N4 nanosheets for photocatalytic syngas production with tunable CO/H2 ratio. Appl. Catal. B Environ.. 2021;295:120261
- [CrossRef] [Google Scholar]
- Graphene supported heterogeneous catalysts: An overview. Int. J. Hydrogen Energy. 2015;40:948-979.
- [CrossRef] [Google Scholar]
- Enhancement of cobalt catalyst stability in Fischer-Tropsch synthesis using graphene nanosheets as catalyst support. Chem. Eng. Res. Des.. 2015;104:713-722.
- [CrossRef] [Google Scholar]
- Cobalt supported on Graphene - A promising novel Fischer-Tropsch synthesis catalyst. Appl. Catal. A Gen.. 2015;499:188-196.
- [CrossRef] [Google Scholar]
- Highly dispersed cobalt Fischer-Tropsch synthesis catalysts supported on γ-Al2O3, CNTs, and graphene nanosheet using chemical vapor deposition. Int. J. Ind. Chem.. 2019;10:321-333.
- [CrossRef] [Google Scholar]
- Graphene oxide: A promising nanomaterial for energy and environmental applications. Nano Energy. 2015;16:488-515.
- [CrossRef] [Google Scholar]
- Design of highly stable and selective core/yolk-shell nanocatalysts-review. Appl. Catal. B Environ.. 2016;188:324-341.
- [CrossRef] [Google Scholar]
- Synthesis strategies of carbon nanotube supported and confined catalysts for thermal catalysis. Chem. Eng. J.. 2022;431:133970
- [CrossRef] [Google Scholar]
- Effects of initial crystal structure of Fe2O3 and Mn promoter on effective active phase for syngas to light olefins. Appl. Catal. B Environ.. 2020;261
- [CrossRef] [Google Scholar]
- The comprehensive kinetic modeling of the Fischer-Tropsch synthesis over Co at Ru/γ-Al2O3 core-shell structure catalyst. Chem. Eng. J.. 2015;259:191-204.
- [CrossRef] [Google Scholar]
- Pretreating Co/SiO2 to generate highly active Fischer-Tropsch synthesis catalyst with low CH4 selectivity. Ranliao Huaxue Xuebao/journal Fuel Chem. Technol.. 2021;49:1592-1598.
- [CrossRef] [Google Scholar]
- Metal nanoparticles supported on two-dimensional graphenes as heterogeneous catalysts. Coord. Chem. Rev.. 2016;312:99-148.
- [CrossRef] [Google Scholar]
- A critical review of the impact of water on cobalt-based catalysts in Fischer-Tropsch synthesis. Fuel Process. Technol.. 2019;192:105-129.
- [CrossRef] [Google Scholar]
- Biochar as a support for nanocatalysts and other reagents: Recent advances and applications. Coordination Chemistry Reviews. Elsevier b.v. 2021
- [CrossRef] [Google Scholar]
- Energy and exergy analysis of different biomass gasification coupled to Fischer-Tropsch synthesis configurations. Energy. 2022;249:123642
- [CrossRef] [Google Scholar]
- Electrocatalysts for the generation of hydrogen, oxygen and synthesis gas. Prog. Energy Combust. Sci.. 2017;58:1-35.
- [CrossRef] [Google Scholar]
- Utilizing bimetallic catalysts to mitigate coke formation in dry reforming of methane. J. Energy Chem.. 2022;68:124-142.
- [CrossRef] [Google Scholar]
- Fischer-Tropsch synthesis using Co and Co-Ru bifunctional nanocatalyst supported on carbon nanotube prepared via chemical reduction method. J. Energy Chem.. 2019;28:9-22.
- [CrossRef] [Google Scholar]
- Enhancement of performance and stability of Graphene nano sheets supported cobalt catalyst in Fischer-Tropsch synthesis using Graphene functionalization. Chem. Eng. Res. Des.. 2017;119:198-208.
- [CrossRef] [Google Scholar]
- Loading and promoter effects on the performance of nitrogen functionalized graphene nanosheets supported cobalt Fischer-Tropsch synthesis catalysts. Int. J. Hydrogen Energy. 2019;44:10604-10615.
- [CrossRef] [Google Scholar]
- Co, Ru and K loadings effects on the activity and selectivity of carbon nanotubes supported cobalt catalyst in Fischer-Tropsch synthesis. Appl. Catal. A Gen.. 2009;353:193-202.
- [CrossRef] [Google Scholar]
- Spotlighting graphene-based catalysts for the mitigation of environmentally hazardous pollutants to cleaner production: A review. J. Clean. Prod.. 2022;365
- [CrossRef] [Google Scholar]
- Recent developments of metallic nanoparticle-graphene nanocatalysts. Prog. Mater. Sci.. 2018;94:306-383.
- [CrossRef] [Google Scholar]
- Cobalt-based Fischer-Tropsch synthesis: Effect of the catalyst granule thermal conductivity on the catalytic performance. Mol. Catal.. 2021;502:111395
- [CrossRef] [Google Scholar]
- Construction of three-dimensional nitrogen-doped graphene aerogel (NGA) supported cobalt catalysts for Fischer-Tropsch synthesis. Catal. Today. 2020;355:10-16.
- [CrossRef] [Google Scholar]
- Formation of metal-support compounds in cobalt-based Fischer-Tropsch synthesis: A review. Chem Catal.. 2021;1:1014-1041.
- [CrossRef] [Google Scholar]
- Photothermal catalytic CO2 reduction over nanomaterials. Chem Catal.. 2021;1:272-297.
- [CrossRef] [Google Scholar]







