Translate this page into:
Facile synthesis of ultrathin carbon nanosheets from waste cellulose
⁎Corresponding authors. zharbi@taibahu.edu.sa (Thaar M.D. Alharbi), zhaojun@hkbu.edu.hk (Jun Zhao), he0091@gmail.com (Shan He)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Ultrathin carbon nanosheets were fabricated using renewable carbon sources. Cellulose, an important component in the food industry, was processed to form a food byproduct and used to synthesize carbon nanosheets. Both bacterial and nonbacterial cellulose from kombucha byproducts and apple pomace, respectively, were processed via purification and pyrolysis. An inert argon atmosphere and elevated temperatures of 600 °C–800 °C for 20 min were maintained during pyrolysis. Under these conditions, the apple pomace produced a higher yield of nanosheets than the kombucha byproduct. The nanosheets with the thickness of 4 nm were characterized using different spectroscopic techniques such as Fourier transform infrared spectroscopy and Raman spectroscopy as well as microscopic techniques such as scanning electron microscopy and atomic force microscopy. This sustainable, simple, and green method of carbon nanosheet production is a promising alternative to conventional methods of production.
Keywords
Carbon
Nanosheets
Pyrolysis
Cellulose
Food processing by-products
1 Introduction
Two-dimensional (2D) carbon nanostructures have substantial potential in various fields owing to their unique electronic properties in the confined dimension of nanophase sp2-hybridized carbon, which are beneficial for certain applications (Georgakilas, Perman et al., 2015, Ashok Kumar, Bashir et al., 2022). Some of these applications include the formation of electrodes for metal-ion batteries, supercapacitors, electrocatalysts for energy conversion nanocomposites, and solar photovoltaic systems (Liu, Zhou et al., 2019, Muschi and Serre, 2019, Tian, Lu et al., 2020, Mohseni, Dehghanipour et al., 2021). Two-dimensional (2D) porous carbon nanosheets are porous carbon materials with nanometer thicknesses; some modified composites are based on these carbon materials, including heteroatom-doped porous carbon nanosheets, carbon sheet–embedded transition metal nanoparticles, and sandwich-structured carbon nanosheet composites (Brisebois and Siaj, 2020, Santamaría-Juárez, Gómez-Barojas et al., 2020). The unique 2D porous structures of carbon nanosheets endow them with many excellent physical and chemical properties. First, a porous structure effectively prevents sheet stacking, resulting in a large specific surface area rich in electrochemical active sites, which is conducive to mass and charge transfer, and thus improves the electrocatalytic activity. Second, an interconnected porous network structure reduces the ion transport distance, facilitates electrolyte penetration, and forms a good electrode–electrolyte interface. Third, a larger internal free space favors volume changes during the charging and discharging cycle, ensuring the long-term stability of a device (Sun, 2019, Brisebois and Siaj, 2020, Santamaría-Juárez, Gómez-Barojas et al., 2020).
Biomass materials, which include celluloses, hemicelluloses, lignin, biopolymers, and proteins, are naturally abundant and readily available. Different biomass materials result in different nanostructures, including hierarchically porous, multilayered, and monolayered structures (Abd El-Aziz, Kamal et al., 2018, Abou Hammad, Abd El-Aziz et al., 2019, Kucera, 2019, Pichardo-Romero, Garcia-Arce et al., 2020, Abou-Zeid, Kamal et al., 2021, BUCKLEY, 2021, Zhang, Ma et al., 2021, Malik, Kishore et al., 2022).
Among these, cellulose-based renewable resources are scalable and ecofriendly, in which bacterial and nonbacterial cellulose are minor and major byproducts.(Azeredo, Barud et al., 2019) Large amounts of nonbacterial cellulose are generated as coproducts while producing fruit beverages such as apple juice and wine (Joseph, Sathian et al., 2022, Mishra, Singh et al., 2022). With the substantial global production of apple juice and wine, in 2021, the generation of the nonbacterial cellulose-based coproducts, i.e., apple pomace and grape pomace, was estimated as approximately 4 million and 9 million tons annually, respectively. This number is expected to keep growing in the near future (Liang, Mondal et al., 2019, Vastolo, Calabrò et al., 2022).
In the last two decades, tremendous efforts have been devoted to designing and synthesizing functional carbon nanosheets using cellulose-based materials. For example, a previous study reported the facile synthesis of carbon nanosheets using bacterial cellulose as the raw material (Azeredo, Barud et al., 2019, Salama, Abd El-Aziz et al., 2021); this study demonstrated a novel pyrolysis method to generate carbon nanosheets using cellulosic biofilms as the raw materials. These films are the byproducts produced at the air–water interface during kombucha fermentation from tea. The advantage of this process is the use of a sustainable feedstock–based, simple, and green route to generate carbon nanosheets. Moreover, it avoids the use of concentrated strong acids, which are normally used in the Hummers method for graphene oxide (GO) synthesis (Alkhouzaam, Qiblawey et al., 2020). Moreover, the use of bacterial cellulose improves the function of carbon nanosheets (Luo, Ao et al., 2017). The resulting carbon nanosheets not only possess large specific surface areas but also help prevent the premature release of drugs outside their target cells. Although homogenous mixing in aqueous solutions remains a critical challenge, uniformly embedding carbon nanosheets into bacterial cellulose substantially improves the degree of mixing (Li, Jellicoe et al., 2022). A previous study demonstrated the synthesis of a novel composite material with outstanding antibacterial activity from bacterial cellulose and carbon nanosheets (Li, Jellicoe et al., 2022).
Herein, we investigated the nature of ultrathin carbon nanosheets produced from kombucha cellulose and apple pomace. The ultrathin carbon nanosheets were produced at different pyrolytic temperatures in the range of 600 °C–800 °C. The highest yield was obtained at 800 °C. This study aimed to determine a method of adding value to nonbacterial cellulose-based food processing byproducts by forming ultrathin carbon nanosheets. This approach will guide future studies on the design and preparation of functional carbon nanosheets.
2 Materials and methods
2.1 Preparation of kombucha cellulose
Sucrose and green tea leaves were purchased from local market. All chemicals used in this study were purchased from Sigma-Aldrich (NSW). The first step was the preparation of kombucha using green tea. Briefly, 100 g of sucrose was dissolved in 800 mL of distilled water at 98 °C for approximately 15 min. Next, 4 g of green tea leaves were soaked in the prepared sugar solution at 98 °C for 12 min. This was followed by filtering out the tea leaves. The samples were then cooled to 25 °C and inoculated with 600 mL of a kombucha starter culture. Subsequently, fermentation was conducted at 30 °C for 15 days. Thereafter, the tops of the mixtures were collected to obtain kombucha cellulose as thin films. The films were then soaked in distilled water for 2 days. Finally, films were suspended in water evenly then filtered using a Buchner funnel to minimize the impurities and residual bacteria in the pores.
2.2 Purification of kombucha cellulose and apple pomace cellulose
Apple pomace was acquired from a local apple juice manufacturing farm. The apple pomace and kombucha cellulose byproducts were subjected to alkali treatments in 1-M NaOH at 50 °C for 12 h. Subsequently, the apple pomace and kombucha cellulose byproducts were subjected to neutralization using 1 % anhydrous CH3COOH for 1 h. Finally, they were washed repeatedly using distilled water to obtain a pH of 7. Water was then pressed out of these neutralized films.
2.3 Pyrolysis of purified kombucha cellulose and apple pomace cellulose in an inert atmosphere
Pyrolysis of purified kombucha cellulose and apple pomace cellulose samples, as shown in Scheme 1, was performed in an argon atmosphere. Three temperatures, 600, 700, and 800 °C, were selected for pyrolysis for 20 mins. The yields were calculated by heating and weighing the residues.
Description of the pyrolysis fabrication of ultrathin carbon sheets from kombucha cellulose and apple pomace.
2.4 Characterizations
Scanning electron microscopy (SEM) images were obtained using an Inspect F50 scanning electron microscope (PS216, FEI). The prepared sample (20 L) was dropcast onto a silicon wafer and air-dried overnight. Platinum sputtering was then performed to form a 2-nm coating. Samples of the freeze-dried materials were prepared by submerging them in liquid nitrogen for 2 min and cutting them with a surgical knife into 5 mm × 5 mm × 2 mm (length × width × thickness) sections. Next, the samples were sputtered again with platinum to form another 2-nm layer. X-ray diffraction (XRD) measurements were performed using an X-ray diffractometer (PW3040/60, PANalytical). The voltage and current were fixed at 40 kV and 40 mA, respectively, and measurements were made in the 2θ range of 10°–40°, with a step speed of 10°/min. The ratios of the areas under the crystalline peaks to the total area (under the crystalline and amorphous peaks) were used to calculate the degrees of crystallinity. Interplanar distances were calculated using Bragg’s law. The Scherrer equation was used to determine the crystallite sizes (D) corresponding to the main peaks: where β denotes the full width at half maximum of the peak (in radians) and θ denotes the Bragg angle. Fourier transform infrared (FTIR) spectroscopy was performed using a spectrometer equipped with an attenuated total reflection accessory and a diamond crystal. The characterization was performed at room temperature with a spectral range of 400–4000 cm−1 and 40 scans. The resolution was fixed at 4 cm−1 and approximately 10 mg of each freeze-dried sample was deposited on the diamond crystal. Raman spectroscopy was performed using a Dilor LabRAM 1B microscope, with a 633-nm excitation wavelength and 2-mW laser power used as the input parameters. Zeta potential measurements were performed using a Nano ZS Zetasizer (Malvern Panalytical) zeta potential analyzer. The measurements were performed using 100 mg of powdered samples suspended in 100 mL of aqueous solutions. The solutions were continuously stirred, with their pH levels maintained using commercially obtained 0.1-M HNO3 and 0.1-M or 0.01-M NaOH solutions (VWR). The starting pH was adjusted to 3 and the solution was allowed to equilibrate for a few minutes before collecting and analyzing an aliquot. The aliquot was then poured back into the solution and the pH adjusted using NaOH; another aliquot was collected and analyzed after reaching a stable pH value. This process was repeated until the pH value was ∼ 10. These measurements helped determine the isoelectric point (IEP; the pH value at which the zeta potential ζ = 0 V). Atomic force microscopy (AFM) was performed using a MultiMode 8 microscope (Bruker) equipped with a Nanoscope V controller and operated in tapping mode in air. The operating parameters, including the set point, scan rate, and feedback gains were optimized to obtain high-quality AFM images. Mikromasch HQ:NSC15 silicon AFM probes with a spring constant and tip diameter of 40 N/m and 16 nm, respectively, were used for performing AFM experiments. The scanner was calibrated in the x, y, and z directions using the silicon calibration grids VGRP (Bruker; 10 μm pitch, and 180 nm depth) and PG (Bruker; 1 μm pitch, 110 nm depth). The AFM images were analyzed using the Nanoscope analysis software (version 2.0).
2.5 Statistical analyses
Each measurement was repeated three times and the data are presented as means with standard deviations (SDs). Statistical analyses included one-way analysis of variance (ANOVA) and least significant difference (LSD) calculations performed on Minitab. Statistical significance was set at p ≤ 0.05.
3 Results and discussion
3.1 Pyrolysis of cellulose samples in argon atmosphere
The fabrication process began with the pyrolysis of the purified kombucha and apple pomace cellulose samples in an argon atmosphere at 600 °C–800 °C. Table 1 shows the percentage yields of the residues formed under different pyrolytic conditions. The yield was determined based on the weight of the material after pyrolysis divided by weight of the material before pyrolysis. Pyrolysis performed at higher temperatures produced a high yield for both the kombucha and apple pomace cellulose residues. At 800 °C, the yields for the kombucha and apple pomace cellulose residues were 19.65 % and 25.12 %, respectively. These samples were used for further studies. *Probability (P) ≤ 0.05 was considered statistically significant. *± SD was obtained by taking an average of three measurements per trial. For each trial, the letters indicate differences (P < 0.05) based on the one-way ANOVA and LSD test.
Samples
Pyrolysis conditions
Yield*
Kombucha cellulose
at 600 °C, in Argon
15.36c ± 0.54 %
at 700 °C, in Argon
18.24d ± 0.75 %
at 800 °C, in Argon
19.65d ± 0.56 %
Apple pomace cellulose
at 600 °C, in Argon
22.80f ± 0.56 %
at 700 °C, in Argon
23.28 g ± 0.42 %
at 800 °C, in Argon
25.12 h ± 0.32 %
The morphologies of the fabricated ultrathin carbon sheets were further studied using AFM in the tapping mode in air. Fig. 1a and 1b show that the developed ultrathin carbon sheets exhibited good smooth surface morphologies with thicknesses of ∼ 4 nm. Fig. 1c and 1d show the SEM images of the kombucha and apple pomace cellulose pyrolysis products in argon at 800 °C. The carbon sheets were exfoliated to form thin flakes, which largely included single carbon sheet in a semitransparent form. Upon irradiation with a 20-kV high-energy electron beam, the ultrathin carbon sheets were observed beneath the thin semitransparent single flakes due to the penetration of the electron beam. These images validated the formation of considerably thin-layered GO structures via pyrolysis, consistent with previous studies. (Amarasekara and Wang, 2020).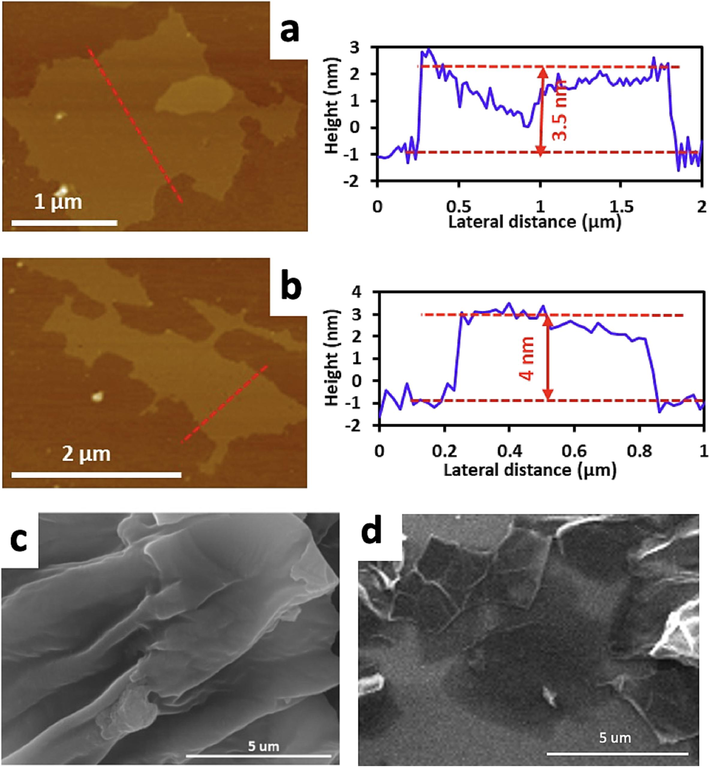
Atomic force microscopy (AFM) images of fabricated ultrathin carbon sheets processed (at 800 °C in argon) of the pyrolysis products formed from kombucha cellulose (a) and apple pomace (b); red lines indicate a cross section of the carbon nanosheets. Scanning electron microscopy (SEM) images of fabricated ultrathin carbon sheets processed (at 800 °C in argon) of the pyrolysis products formed from kombucha cellulose (c) and apple pomace (d).
FTIR spectroscopy was used to study the sample morphologies (Fig. 2a). The Characteristic absorption peaks for the samples were obtained at 1650, 1210, 998, and 2420 cm−1 for C = C, C–O–C, C–H, and C = O = C, respectively. These peak values were consistent with those of carbon nanosheets synthesized using Hummers method in previous studies (Ebajo Jr, Santos et al., 2019) (Loudiki, Matrouf et al., 2022). Fig. 2b represents the XRD patterns of carbon nanosheets obtained from kombucha and apple pomace cellulose products after pyrolysis. As seen in the XRD patterns, two distinct peaks were obtained at 2θ = 22° and 43°. These patterns were similar to those of ultrathin carbon sheets developed using the modified Hummers method (Sujiono, Zabrian et al., 2020). Thus, the proposed method was viable for producing ultrathin carbon sheets compared with other conventional techniques owing to its additional advantages of value-adding sustainable feedstock, green route to generate carbon nanosheets, and avoiding use of concentrated strong acids, which are commonly used in Hummer’s method for graphene oxide synthesis.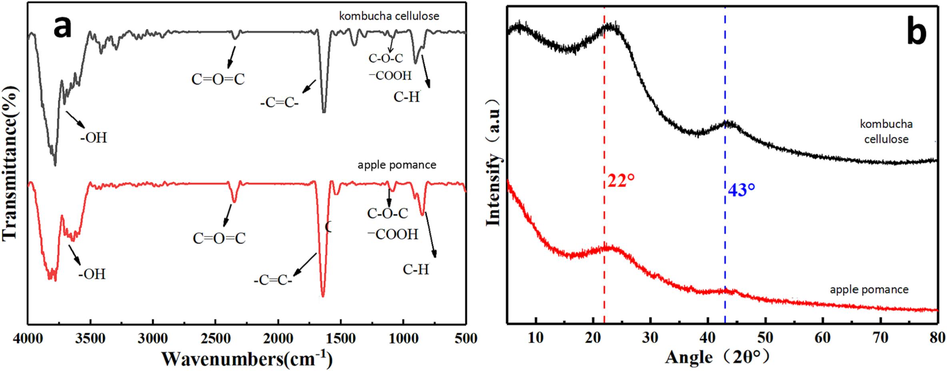
Fourier transform infrared (FTIR) spectra (at 800 °C in argon) of pyrolyzed products formed from kombucha and apple pomace cellulose. X-ray diffraction (XRD) (at 800 °C in argon) of pyrolyzed products produced from kombucha and apple pomace cellulose.
Raman spectroscopy was used to further analyze and confirm our final products are carbon nanosheets. In general, carbon nanomaterials, in Raman spectrum, have two main peaks in the D-band region (1321–1337 cm−1) and G-band region (1584–1592 cm−1). which are associated with disorders in the sp2 carbon network and C–C inside the graphite network, respectively (Yang, Hu et al., 2016), (Wang, Li et al., 2015). Fig. 3a and 3b show Raman spectrum for both ultrathin carbon sheets produced from kombucha cellulose and apple pomace. Both materials of carbon sheets show shows the presence of D and G bands at 1337 and 1584 cm−1 respectively. Which confirmed that our products are carbon nanosheets (Lopez-Diaz, Delgado-Notario et al., 2020). Analysis of the Raman spectra usually involves a comparison between the ratio of intensity of the G band and D bands. The intensity ratio of the D- and G-bands, a significant characterization parameter, was defined as ID/IG. The kombucha and apple pomace cellulose products had ID/IG values of 1.061 and 1.184, respectively. The difference, in the ratio, is not dramatic. The results suggests that both carbon sheets produced via pyrolyzed from kombucha cellulose and apple pomace have some oxygen functional groups and defects on the surface of these carbon sheets, this is consisting with SEM observations. Also, this was comparable with the values of GO produced using the conventional Hummers method (Alkhouzaam, Qiblawey et al., 2020).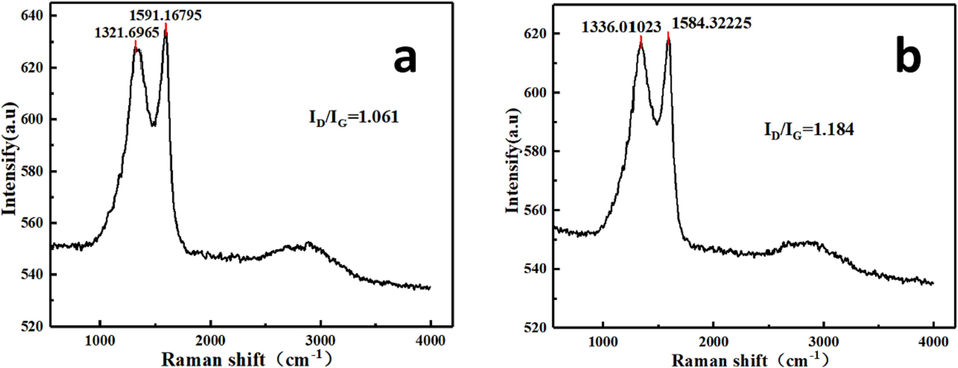
Raman spectrum (at 800 °C in argon) of the pyrolyzed products formed from kombucha cellulose (a) and apple pomace (b).
Table 2 shows the zeta potentials of the pyrolyzed products of obtained in an argon atmosphere at 800 °C. The zeta potential values for both processed products were within the range of − 51 to − 34 mV, which was consistent with that of ultrathin carbon sheets synthesized in a previous study (Shi, Han et al., 2022). *Probability (P) ≤ 0.05 was used statistically to determine the significance. *± SD is the mean of three measurements per trial. Within each trial, different letters indicate significant differences (P < 0.05), according to one-way ANOVA and LSD test.
Samples
zeta potential* (mV)
pyrolysis products produced from kombucha cellulose
−43a + 2.56 %
pyrolysis products produced from apple pomace
−40a + 2.72 %
4 Conclusion
We used a simple, noninvasive, and green route to generate ultrathin carbon sheets. Certain materials, such as sustainable organic cellulose–based byproducts, eliminate the need for concentrated strong acids and oxidizing agents in the fabrication process. Different atmospheric conditions and temperatures were tested, and the highest yield was obtained in an argon atmosphere at 800 °C for 20 min. Further analysis was performed using characterization techniques such as FTIR, Raman spectroscopy, XRD, SEM, and AFM. We successfully developed a novel and industrially feasible green process, where renewable waste products from food industries can be used to develop inexpensive ultrathin carbon nanosheets, which has huge potential applications in many value-adding products, such as battery.
CRediT authorship contribution statement
Thaar M.D. Alharbi: Investigation. Mohammed J.K. Bashir: Methodology. Anindya Nag: Validation. Wael H. Alsaedi: . Matt Jellicoe: Investigation. Jonathan Woon Chung Wong: Investigation. Liwen Luo: Formal analysis. Xin Xiong: Investigation. Zihan Feng: Investigation. Jiayue Fang: Investigation. Jun Zhao: . Shan He: .
Acknowledgement
The authors are thankful for the support provided by the German Research Foundation (DFG, Deutsche Forschungsgemeinschaft) as part of Germany’s Excellence Strategy—EXC 2050/1—Project ID 390696704—Cluster of Excellence, “Centre for Tactile Internet with Human-in-the Loop” (CeTI) of Technische University Dresden. The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for support.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Biodegradable grafting cellulose/clay composites for metal ions removal. Int. J. Biol. Macromol.. 2018;118:2256-2264.
- [Google Scholar]
- A novel electromagnetic biodegradable nanocomposite based on cellulose, polyaniline, and cobalt ferrite nanoparticles. Carbohydr. Polym.. 2019;216:54-62.
- [Google Scholar]
- Grafted TEMPO-oxidized cellulose nanofiber embedded with modified magnetite for effective adsorption of lead ions. Int. J. Biol. Macromol.. 2021;167:1091-1101.
- [Google Scholar]
- Synthesis of graphene oxides particle of high oxidation degree using a modified Hummers method. Ceram. Int.. 2020;46(15):23997-24007.
- [Google Scholar]
- Pyrolysis route for the conversion of bacterial cellulose to graphene oxide. ACS Sustain. Chem. Eng.. 2020;9(1):113-119.
- [Google Scholar]
- A review on graphene and its derivatives as the forerunner of the two-dimensional material family for the future. J. Mater. Sci.. 2022;57(26):12236-12278.
- [Google Scholar]
- Bacterial cellulose as a raw material for food and food packaging applications. Front. Sustain. Food Syst.. 2019;3:7.
- [Google Scholar]
- Harvesting graphene oxide–years 1859 to 2019: a review of its structure, synthesis, properties and exfoliation. J. Mater. Chem. C. 2020;8(5):1517-1547.
- [Google Scholar]
- BUCKLEY, P. (2021). Low temperature synthesis of nanographite and nanoquartz using surfactant phases, Durham University.
- Regenerable acidity of graphene oxide in promoting multicomponent organic synthesis. Sci. Rep.. 2019;9(1):15579.
- [Google Scholar]
- Georgakilas, V., J. A. Perman, J. Tucek and R. J. C. r. Zboril (2015). “Broad family of carbon nanoallotropes: classification, chemistry, and applications of fullerenes, carbon dots, nanotubes, graphene, nanodiamonds, and combined superstructures.” 115(11): 4744-4822.
- Joseph, T. M., A. Sathian, A. B. Unni, D. K. Mahapatra, A. Kallingal, J. K. S, J. Hapniuk and S. Thomas (2022). Applications of Flavors and Fragrances in Meat Products. Flavors and Fragrances in Food Processing: Preparation and Characterization Methods, ACS Publications: 405-435.
- Biofouling of polyamide membranes: Fouling mechanisms, current mitigation and cleaning strategies, and future prospects. Membranes. 2019;9(9):111.
- [Google Scholar]
- Continuous flow vortex fluidic transformation of kombucha cellulose into more compact and crystalline fibers. ACS Sustain. Chem. Eng.. 2022;10(13):4279-4288.
- [Google Scholar]
- Graphene-based planar microsupercapacitors: recent advances and future challenges. Adv. Mater. Technol.. 2019;4(1):1800200.
- [Google Scholar]
- Recent advances in friction and lubrication of graphene and other 2D materials: Mechanisms and applications. Friction. 2019;7:199-216.
- [Google Scholar]
- Towards understanding the Raman spectrum of graphene oxide: the effect of the chemical composition. Coatings. 2020;10(6):524.
- [Google Scholar]
- Graphene oxide synthesized from zinc-carbon battery waste using a new oxidation process assisted sonication: Electrochemical properties. Mater. Chem. Phys.. 2022;275:125308
- [Google Scholar]
- Bacterial cellulose/graphene oxide nanocomposite as a novel drug delivery system. Curr. Appl. Phys.. 2017;17(2):249-254.
- [Google Scholar]
- A comprehensive review on nanobiotechnology for bioremediation of heavy metals from wastewater. J. Basic Microbiol.. 2022;62(3–4):361-375.
- [Google Scholar]
- Mishra, S., P. K. Singh, R. Pattnaik, S. Kumar, S. K. Ojha, H. Srichandan, P. K. Parhi, R. K. Jyothi and P. K. Sarangi (2022). “Biochemistry, synthesis, and applications of bacterial cellulose: A review.” Frontiers in bioengineering and biotechnology 10.
- Enhancement of the photovoltaic performance and the stability of perovskite solar cells via the modification of electron transport layers with reduced graphene oxide/polyaniline composite. Sol. Energy. 2021;213:59-66.
- [Google Scholar]
- Progress and challenges of graphene oxide/metal-organic composites. Coord. Chem. Rev.. 2019;387:262-272.
- [Google Scholar]
- Current advances in biofouling mitigation in membranes for water treatment: An overview. Processes. 2020;8(2):182.
- [Google Scholar]
- Salama, D. M., M. E. Abd El-Aziz, M. E. El-Naggar, E. A. Shaaban and M. S. Abd EL-Wahed (2021). “Synthesis of an eco-friendly nanocomposite fertilizer for common bean based on carbon nanoparticles from agricultural waste biochar.” Pedosphere 31(6): 923-933.
- Safer modified Hummers’ method for the synthesis of graphene oxide with high quality and high yield. Mater. Res. Express. 2020;6(12):125631
- [Google Scholar]
- Synthesis mechanisms, structural models, and photothermal therapy applications of top-down carbon dots from carbon powder, graphite, graphene, and carbon nanotubes. Int. J. Mol. Sci.. 2022;23(3):1456.
- [Google Scholar]
- Graphene oxide based coconut shell waste: synthesis by modified Hummers method and characterization. Heliyon. 2020;6(8):e04568.
- [Google Scholar]
- Structure and synthesis of graphene oxide. Chin. J. Chem. Eng.. 2019;27(10):2251-2260.
- [Google Scholar]
- Advanced electrocatalysts for the oxygen reduction reaction in energy conversion technologies. Joule. 2020;4(1):45-68.
- [Google Scholar]
- A review on the use of agro-industrial CO-products in animals’ diets. Ital. J. Anim. Sci.. 2022;21(1):577-594.
- [Google Scholar]
- Controllable tailoring graphene nanoribbons with tunable surface functionalities: an effective strategy toward high-performance lithium-ion batteries. ACS Appl. Mater. Interfaces. 2015;7(31):17441-17449.
- [Google Scholar]
- Longitudinal splitting versus sequential unzipping of thick-walled carbon nanotubes: Towards controllable synthesis of high-quality graphitic nanoribbons. Carbon. 2016;110:480-489.
- [Google Scholar]
- Flow electrode capacitive deionization (FCDI): recent developments, environmental applications, and future perspectives. Environ. Sci. Tech.. 2021;55(8):4243-4267.
- [Google Scholar]







