Translate this page into:
Chemical speciation distribution, desorption characteristics, and quantitative adsorption mechanisms of cadmium/lead ions adsorbed on biochars
⁎Corresponding author. fanshisuo@ahau.edu.cn (Shisuo Fan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
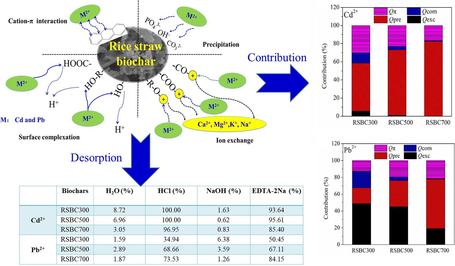
The adsorption characteristics of Cd2+ and Pb2+ with biochars have been widely reported. However, the desorption characteristics, chemical speciation, and stability of Cd2+ and Pb2+ adsorbed on biochars remain unclear. In this work, rice straw biochars were obtained at different pyrolysis temperatures (RSBC300, RSBC500, RSBC700). The chemical speciation and desorption characteristics of Cd- and Pb-loaded on biochars were investigated through Tessier sequential extraction and different desorption agents, and possible quantitative mechanisms of metal-laden biochars were revealed. Pyrolysis temperature influenced the properties of biochars obviously, including surface morphology, element and mineral composition, and carbon structure defects. RSBC700 and RSBC500 exhibited the highest adsorption capacity toward Cd2+ and Pb2+, respectively. The majority of adsorbed metal ions on rice straw biochars are distributed in the carbonate fraction and the exchangeable fraction. Cd and Pb in all metal-loaded biochars showed a high risk with risk assessment code values larger than 50 %. HCl and EDTA-2Na could desorb most of the Cd2+ from the Cd-laden biochars, and desorb most of Pb2+ from high-temperature Pb-laden biochars. Precipitation with minerals (Qpre, 52.77 %–82.37 %) and interaction with π-electrons (Qπ, 16.57 %–30.26 %) were the main Cd2+ adsorption mechanisms for all biochars, whereas precipitation with minerals (Qpre, 18.82 %–58.71 %) and ion exchange (Qexc, 19.18 %–48.81 %) were the main removal mechanisms for Pb2+. Therefore, Cd2+ and Pb2+ adsorbed on biochars were not stable and easily released to the environment, which showed a high potential environmental risk.
Keywords
Rice straw biochars
Cd and Pb
Tessier’s sequential extraction
Desorption behavior
Adsorption mechanisms
1 Introduction
Cadmium (Cd) and lead (Pb) belong to the typical toxic heavy metals in wastewater, which can pose a potential threat to human health through the food chain (Lian et al., 2020). Among the different methods for metal elimination in wastewater, adsorption technology is widely applied because of its simple, cheap, and effective (Liu et al., 2022b). It is crucial to select a suitable cleanup, eco-friendly, and inexpensive adsorbent for heavy metal ions purification from wastewater efficiently.
Nowdays, biochar has caused great attention as a promising adsorbent because of its well-developed porous structure, abundant functional groups, good aromatic structure, and endogenous minerals (Qin et al., 2020; Pang et al., 2023; Vuppaladadiyam et al., 2023). The adsorption performance of biochar is highly relied on its physicochemical properties (Luo et al., 2022; Cheng et al., 2023; Li et al., 2023; Pinky et al., 2023). The pyrolysis temperature strongly determined the basic properties of biochars, which influenced their adsorption performance toward metal ions (Li et al., 2022; Zhang et al., 2023).
To date, adsorption characteristics of Cd2+ and Pb2+ on various biochars have been widely investigated (Qiu et al., 2021). In most cases, typical kinetic equations, and the isotherm equations were the most frequently used models to describe the adsorption characteristics of Cd2+ and Pb2+ on biochars (Liu and Zhang, 2022; Pathy et al., 2023). In addition, adsorption mechanisms of Cd2+ and Pb2+ by biochars were also fully explored, including mineral ion participation, surface functional groups action, and aromatic structure (Li et al., 2017). The dominant adsorption mechanisms depended considerably on the feedstocks and pyrolysis conditions (Lakshmi et al., 2021). Furthermore, the quantification of multiple adsorption mechanisms of Cd2+ and Pb2+ on biochars was also studied. For instance, some studies had quantified the different Cd2+ adsorption mechanisms, referring to cation exchange and precipitation: 59.55 %–76.05 %: 59.55–76.05 % (Huang et al., 2020); Cd2+–π interaction: 61.83–82.17 % (Wang et al., 2018); complexation and coordination: 22.4–62.0 % (Huang et al., 2018). Meanwhile, previous studies also quantified the contribution of different Pb2+ adsorption mechanisms, including precipitation and cation exchange: 85.6–92.6 % (Wang et al., 2015b); complexation and coordination: 7.6–24.9 %, 30.3–35.5 %, and 4.9–15.6 % (Lu et al., 2012); Pb2+–π interaction: 19.5–39.1 % (Gong and Chi, 2022), respectively. These abovementioned studies focused on exploration of the adsorption mechanisms and quantification of Cd2+ and Pb2+ on biochars. However, the speciation distribution of heavy metals adsorbed on biochars lacks attention, and their desorption characteristics have a shortage of studies.
Adsorption stability is involved in the release behavior, environmental risk, bioavailability, and final disposal of heavy-metal-laden biochars. Desorption studies and sequential extraction procedures were widely used to evaluate the stability of heavy metals in solid waste. In general, a desorption experiment is conducted to regenerate the adsorbent and assess its recycling potential. Among the desorption reagents, HCl and EDTA-2Na are the most widely used agents, which can desorb most metal ions from biochars. Many studies found that HCl and EDTA could desorb most of the Cd/Pb from the Cd/Pb-laden biochars, and were used as eluents for the regeneration of biochars (Wang et al., 2015a; Huang et al., 2019; Zhang et al., 2019). However, the comparison of different reagents and the desorption kinetics of Cd2+ and Pb2+ on biochars were rarely investigated. Meanwhile, speciation distribution can reflect the leaching assessment and ecological risk of heavy metals in biochars (Shen et al., 2020). The sequential extraction procedure was more adopted to evaluate the remediation effect of biochars on heavy-metal-contaminated soil. The majority of adsorbed Cd2+ and Pb2+ onto the biochars was located in the acid-soluble fraction (Shen et al., 2017; Wang et al., 2021; Yang et al., 2022). Cd2+ was mainly distributed in the carbonate fraction (61.35–92.96 %) and the exchangeable fraction (5.59–29.78 %) in biochars after adsorption, and Pb2+ was mainly located in carbonate fraction (79.30–98.06 %) (Tian et al., 2021). But little information has been reported on the speciation of Cd2+ and Pb2+ adsorbed on biochars using the Tessier sequential extraction procedure. Furthermore, the potential ecological risk of Cd/Pb adsorbed on biochars has rarely been assessed.
In this work, rice straw biochars were obtained at 300, 500, and 700 °C. Then, the properties of biochars were elucidated using SEM–EDS, BET, XRD, Raman, FTIR, XPS, and element analysis, and used to adsorb Cd2+/Pb2+. A desorption study and Tessier sequential extraction procedure was conducted to assess the stability of Cd2+ and Pb2+ in Cd/Pb-loaded biochars. The detailed purposes of this work were as follows: (1) to investigate the influence of pyrolysis temperature on the physicochemical properties of biochars, (2) to study the desorption behavior of Cd2+/Pb2+ from Cd/Pb-loaded biochars, (3) to assess the speciation distribution of Cd/Pb in Cd/Pb-loaded biochars, and (4) to quantify the main adsorption mechanisms of Cd2+/Pb2+ on biochars. Therefore, this study could provide insight into the quantitative contribution of Cd2+/Pb2+ adsorption mechanisms by rice straw biochars, and the stability of Cd2+ Pb2+ on biochars after adsorption.
2 Materials and methods
2.1 Biochar preparation
The procedure of pristine and demineralized biochars preparation could be seen in our previous study (Liu and Fan, 2018), which was shown in the Supplementary Information (SI).
2.2 Biochar characterization
The biochars was fully analyzed and the specific information was shown in SI, including scanning electron microscope and energy dispersive spectroscopy (SEM–EDS), N2 adsorption–desorption isotherms (BET), X-ray diffraction (XRD), Raman, Fourier transform infrared spectroscopy (FTIR), X-ray photoelectron spectroscopy (XPS), and element analysis.
2.3 Adsorption experiments
The adsorption experiments were also showed in the SI.
2.4 Tessier sequential extraction and risk assessment code
To assess the adsorption stability of Cd2+/Pb2+ on biochar and the potential release risk of Cd2+/Pb2+ from biochar, Tessier sequential extraction procedure (Tessier et al., 1979) and the risk assessment code (RAC) method (Liu et al., 2016) was performed. The detailed experimental processes and calculation procedure was shown in the SI.
2.5 Desorption study
The detailed desorption study was shown in the SI.
2.6 Exploration of adsorption mechanisms
Many characterizations and measurements were performed to elucidate the adsorption mechanisms. In particular, Cd/Pb-loaded biochars were characterized using SEM–EDS, XRD, XPS, FTIR, and Raman analysis. In addition, the pH values of supernatants after Cd2+/Pb2+ adsorption onto biochars and acid-washed biochars were also measured. The concentration of PO43– in the supernatant after Cd2+/Pb2+ adsorption was determined using ion chromatography (ICS-3000, Dionex Inc., USA). The concentrations of CO32– and HCO3– in the supernatant were obtained through the titration method, whereas the concentrations of Ca2+, Mg2+, Na+, and K+ in the supernatant were measured using ICP–OES.
2.7 Quantification of different adsorption mechanisms
Based on the previous literatures (Wang et al., 2015b; Cui et al., 2016), the main adsorption mechanisms of Cd2+/Pb2+ onto biochars are ion exchange, precipitation with minerals, complexation with organic functional groups (OFGs), and Cd/Pb–π interaction. Quantification of different adsorption mechanisms was performed and shown in Supplementary Information (SI).
2.8 Practical application of biochars
The detailed practical application of biochars was presented in the SI.
3 Results and discussion
3.1 Characterization of biochars
The SEM-EDS of biochars are displayed in Fig. S1. As the increase in pyrolysis temperature, the surface of the biochar became more irregular, coarse, and porous. More particles could be observed on the surface of the biochars. Moreover, more minerals could be condensed in high-temperature biochars.
As shown in Fig. S2a, the biochars exhibit typical I/IV-type isothermal curves, indicating the presence of micropores structure (Ye et al., 2019). Moreover, the small hysteresis loop of the desorption branch extending from P/P0 at 0.5 to 0.9 demonstrates the existence of abundant mesopores structure (Cheng et al., 2020). Thus, the pore structure of biochars showed multilayer and hierarchical. According to Fig. S2b, adsorption pores of biochars are mainly distributed in micropores and mesopores with the pore size ranging from 2 to 50 nm. The pore size distribution of biochar at three temperatures was different, and RSBC700 presented a high mesoporous volume, followed by RSBC500 and RSBC300.
The textural parameters of biochars are listed in Table 1. With the pyrolysis temperature increasing from 300 to 700 °C, the surface area of biochars increased from 70 to 155 m2·g−1, and the pore volume of biochars increased from 0.045659 to 0.100370 cm3·g−1. The micropore surface area and micropore volume also increased with the increase in pyrolysis temperature, suggesting that they were predominant in the biochars. The average pore diameter of RSBC700 was less than that of RSBC300 and RSBC500, which was beneficial for the diffusion of metal ions (Yuan et al., 2024).
Biochars
RSBC300
RSBC500
RSBC700
STotal/(m2·g−1)
70
98
155
SMicro/(m2·g−1)
61.0056
83.5069
134.0205
SExternal/(m2·g−1)
9.3974
14.9701
20.9314
VTotal/(cm3·g−1)
0.045659
0.071233
0.100370
VMicro/(cm3·g−1)
0.031606
0.043078
0.071451
average pore diameter (nm)
16.4860
22.0595
11.3716
C (%)
52.31
54.50
60.27
H (%)
3.435
1.857
0.82
O (%)
18.761
13.389
6.377
N (%)
1.09
0.93
0.61
S (%)
0.184
0.244
0.293
Ash (%)
24.22
29.08
31.63
H/C
0.0657
0.0341
0.0136
O/C
0.3587
0.2457
0.1058
(N + O)/C
0.3795
0.2627
0.1159
pH
8.54
9.67
9.73
The contents of elements, ash, and elemental ratio are presented in Table 1. The contents of C, S, and ash in the biochars increased, whereas the contents of H, O, and N decreased with increasing pyrolysis temperature. These changes were attributed to several reactions such as decarboxylation, dehydrogenation, decarbonylation, and deoxygenation that occurred during the pyrolysis process (Chen et al., 2020; Gupta et al., 2022). The H/C, O/C, and (N + O)/C ratios are generally used to evaluate the aromaticity, polarity, and hydrophilicity of biochars (Wang et al., 2016). As the pyrolysis temperature increased, the atomic ratios of H/C, O/C, and (N + O)/C decreased, suggesting that higher aromaticity, lower polarity, and hydrophilicity were observed in RSBC700. The pH value of the biochars increased with increasing pyrolysis temperature, which was ascribed to the condensation of mineral components in biochars (Yuan et al., 2011).
Fig. 1a shows the main minerals in biochars, namely, SiO2, CaCO3, and KCl. Two broad and weak peaks at 2θ = 24° ((0 0 2)) and 42° (1 0 0) were observed in the XRD pattern of the biochars (Hu et al., 2020; Tang et al., 2020). The appearance of the (0 0 2) peak implies the formation of amorphous carbon in the biochars. The intensity of peak (1 0 0) became more obvious in RSBC700, suggesting that more graphitic carbon structure was formed in biochar prepared at higher temperatures.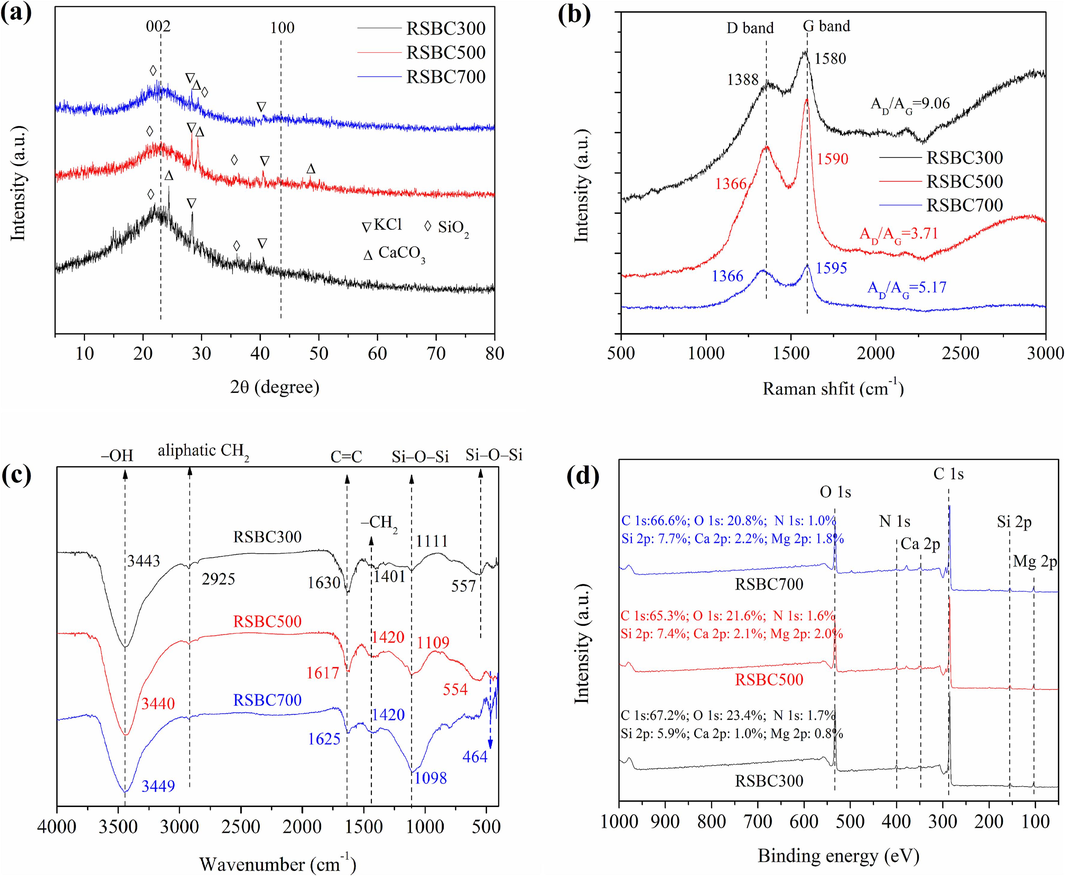
XRD (a), Raman (b), FTIR (c), and XPS survey (d) of biochars.
Two prominent peaks, the D band (≈1360 cm−1) and G band (≈1580 cm−1) in biochars, were found in the Raman spectra (Fig. 1b). The D-band intensity originated from sp3 hybridization, and the G band was induced by crystalline graphitic/sp2 carbon atoms (Ma et al., 2020). The area ratio of the D band to the G band (AD/AG) of RSBC300 was larger than that of RSBC500 and RSBC700, suggesting the existence of structural defects and disorder in RSBC300. As the pyrolysis temperature increased from 500 to 700 °C, the AD/AG ratio increased from 3.71 to 5.17, indicating the increase in the proportion of condensed aromatic ring structures with defects (Mei et al., 2021; Xu et al., 2022; Huang et al., 2023).
The surface functional groups of the biochars are presented in Fig. 1c; the pyrolysis temperature also influenced the surface functional groups in biochars. In the FTIR spectra, the position of the C⚌C (1630 cm−1), −CH2 (1401 cm−1), Si—O—Si (1111 and 557 cm−1) significantly changed with the increasing pyrolysis temperature (Fan et al., 2018). The change of surface functional groups may influence the binding ability toward Cd2+ and Pb2+ ions.
The valence states of the surface elements and composition of the biochars were measured using XPS (Fig. 1d). The peaks of C 1s, O 1s, N 1s, Si 2p, Ca 2p, and Mg 2p were observed on the surface of the biochars. The relative content of various elements also changed with the increase in pyrolysis temperature. Obviously, minerals also could be found on the surface of the biochars.
According to the XPS peak fitting results (Fig. S2), the high-resolution spectra of the C 1s peak of RSBC300 show that the C 1s peaks located at 284.73, 285.66, and 288.35 eV are ascribed to C–C/C⚌C/C–H, C–O, and C⚌O, respectively (Yang et al., 2016). The O 1s spectrum of RSBC300 could be decomposed into two peaks at 533.08 and 531.42 eV, which are ascribed to the C⚌O and C–O, respectively (Yao et al., 2022), respectively. In addition, pyrolysis temperature affected the types and relative percentage of functional groups (C⚌O and C—O).
3.2 Adsorption capacity of Cd2+ and Pb2+ by biochars and acid washed biochars
The adsorption capacity of Cd2+ and Pb2+ by biochars and acid-washed biochars are presented in Fig. 2a and 2b. Specifically, RSBC700 exhibited the highest adsorption capacity for Cd2+ and were 62.45 mg∙g−1, whereas RSBC500 showed the greatest adsorption capacity for Pb2+ and reached 167.49 mg∙g−1. However, the adsorption capacity of Cd2+ and Pb2+ by biochars extremely decreased after acid demineralization treatment, implying that soluble minerals played a crucial role in the adsorption process of Cd2+ and Pb2+, which contributed 50 %–80 % for Cd2+ adsorption, 61 %–75 % for Pb2+ adsorption. In addition, RSBC300-A showed the highest adsorption capacity toward Cd2+ and Pb2+ among the acid-washed biochars, which could be ascribed to the presence of functional groups in low-temperature biochars.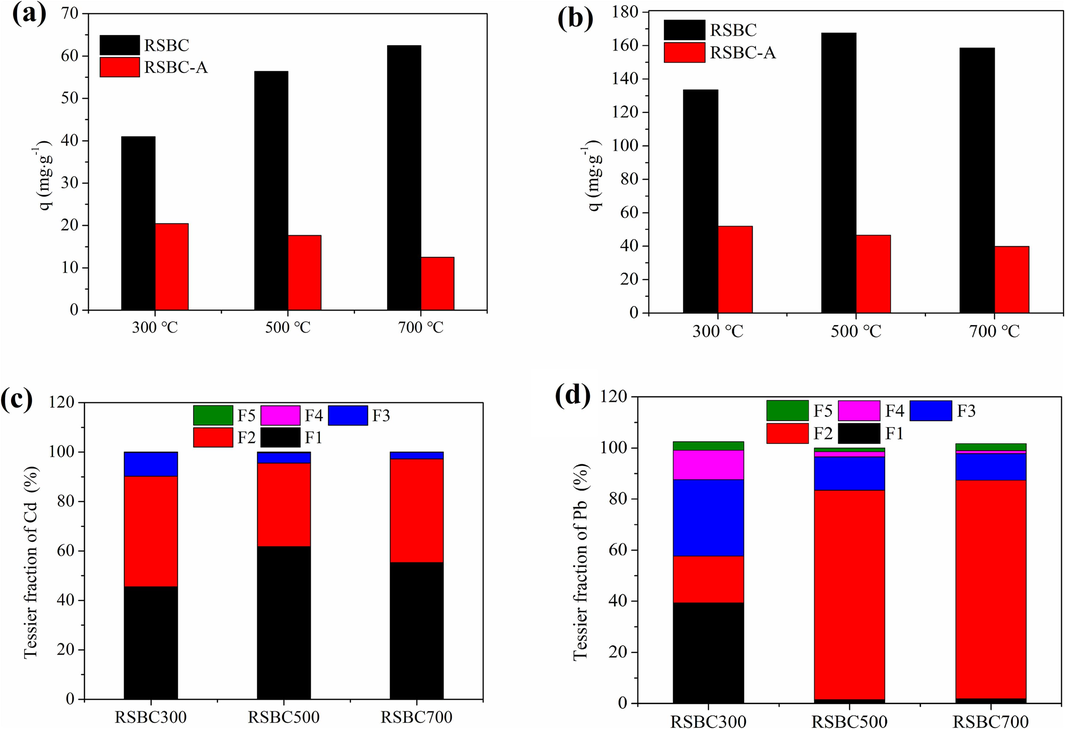
Adsorption capacity of Cd2+ (a) and Pb2+ (b) by biochars and acid washed biochars, and the chemical speciation distribution of Cd2+ (c) and Pb2+ (d) on biochars.
Other studies have also confirmed that minerals could play an crucial role and take part in the adsorption process through ion exchange and precipitation mechanisms, especially for Cd2+, Pb2+, Cu2+, etc (Xu et al., 2017; Gholizadeh and Hu, 2021; Wu et al., 2021). Meanwhile, surface functional groups and the aromatic graphite structure of biochars could bind with heavy-metal ions via complexation and cation–π interaction. (Berslin et al., 2022).
3.3 Speciation distribution and RAC of Cd2+ and Pb2+ on biochars
Fig. 2c and 2d shows the chemical distribution of Cd and Pb adsorbed on the Tessier fractions of biochars. The majority of adsorbed Cd2+ on rice straw biochars was in the F1 and F2 fractions. Cd2+ was bound to the carbonate fraction (45.52 %–61.77 %) and the exchangeable fraction (33.79 %–44.70 %). For Pb2+, the majority of adsorbed Pb2+ on RSBC300 was in the F1 (39.9 %) and F3 (29.96 %) fractions, followed by the F2 fraction (18.30 %). However, most of the adsorbed Pb2+ on RSBC500 and RSBC700 was distributed in the F2 fraction (82.00 %−85.56 %). The speciation of heavy metals adsorbed on biochars also could reflect the adsorption mechanisms. In this work, the majority of adsorbed Cd2+ and Pb2+ on rice straw biochars were in the F1 and F2 fractions, suggesting that occurrence of precipitation and ion exchange (Frišták et al., 2014; Ke et al., 2023).
Among these five fractions, the exchangeable fraction is the most threatening fraction, and the sum of the exchangeable and carbonate fractions can be regarded as the bioavailable fraction (Sun et al., 2022). In this work, Cd2+ and Pb2+ adsorbed on rice straw biochars were not stable and easily released to the environment, which poses a potential environmental threat. Compared with Pb2+, Cd2+ displayed a high environmental risk due to its high F1 fraction.
Furthermore, the values of RAC for the Cd and Pb in metal-loaded biochars are displayed in Table 2. Cd and Pb in all metal-loaded biochars showed a high risk with RAC values greater than 50 %. In particular, Cd2+ and Pb2+ loaded on RSBC700 presented a higher risk. Furthermore, the environmental risk of Cd was greater than that of Pb. Therefore, the biochars after Cd2+ and Pb2+ adsorption should be disposed of suitably to avoid secondary pollution or environmental risk.
Biochars
Cd (%)
Pb (%)
RSBC300
90.22
57.70
RSBC500
95.56
83.49
RSBC700
97.24
87.40
3.4 Desorption characteristics of Cd2+ and Pb2+ from biochars after adsorption
The desorption amount of Cd2+ from Cd-laden biochars with different desorption agents is shown in Fig. 3a-3d. With the increase in desorption time, more Cd2+ ions were released into the solution. The desorption capacity was larger at the initial stage, and gradually tended to be stable at the later stage. The desorption deficiency of different desorption agents showed the order of HCl > EDTA-2Na > H2O > NaOH. When H2O acted as the desorption reagent, RSBC500 presents a high desorption capacity. In contrast, when HCl, NaOH, and EDTA-2Na were used as the desorption reagents, RSBC700 showed a high desorption capacity due to its high adsorption capacity toward Cd2+.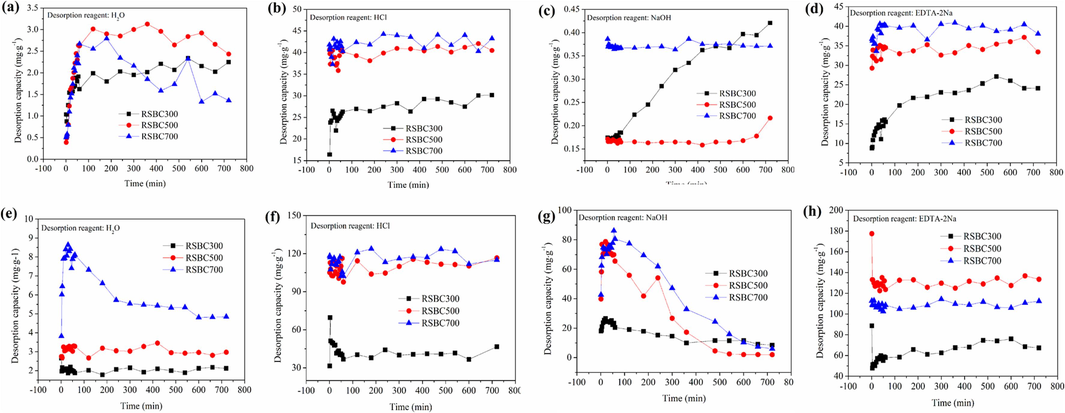
Desorption kinetics of Cd2+ from Cd-laden biochars (a-d), and Pb2+ from Pb-laden biochars (e-h).
For Pb2+, the desorption capacity from Pb-laden biochars with different desorption agents is shown in Fig. 3e-3 h. As desorption time increased, the desorption amount was larger at the initial stage, and gradually decreased at a later stage. The order of different desorption agents toward Pb desorption from biochar was EDTA-2Na > HCl > NaOH > H2O. When H2O was acting as the desorption reagent, RSBC700 showed a high desorption capacity, followed by RSBC500 and RSBC300. RSBC500, RSBC700, and RSBC300 exhibited a high desorption amount when HCl was used as the desorption agent. The desorption capacity from RSBC500 and RSBC700 was greater than that from RSBC300. When NaOH acted as the desorption agent, the desorption amount first increased but then decreased with increasing desorption time. The desorption capacity of Pb from biochars became low when the desorption time was 12 h because most Pb had been desorbed from the metal-laden biochars or the desorbed Pb2+ occurred secondary adsorption on the surface of biochars. When EDTA-2Na was applied as the desorption agent, the desorption amount of Pb from RSBC300 and RSBC700 first increased, then tended to become stable with the increase in desorption time. The desorption amount of Pb from RSBC500 using EDTA-2Na was stable.
The desorption extent of Cd/Pb from the Cd/Pb-laden biochars is listed in Table 3. Herein, HCl and EDTA-2Na exhibited the highest desorption extent, indicating that most of the Cd/Pb can be desorbed from biochars. HCl can destroy the mineral composition and the structure of the biochar, resulting in the release of Cd/Pb into the solution (Zhang et al., 2019). EDTA-2Na has a strong chelating ability and realizes desorption by chelating with Cd/Pb on the surface of the biochar (Pal and Maiti, 2019).
Biochars
H2O (%)
HCl (%)
NaOH (%)
EDTA-2Na (%)
Cd2+
RSBC300
8.72
100.00
1.63
93.64
RSBC500
6.96
100.00
0.62
95.61
RSBC700
3.05
96.95
0.83
85.40
Pb2+
RSBC300
1.59
34.94
6.38
50.45
RSBC500
2.89
68.66
3.59
67.11
RSBC700
1.87
73.53
1.26
84.15
For RSBC300 and RSBC700, the desorption extent of Pb2+ by EDTA-2Na was larger than that of HCl. For RSBC500, the desorption extent of Pb2+ by HCl was slightly greater than that of EDTA-2Na. The results of the desorption experiment stayed the same with the analysis of the Tessier sequential extraction procedure. Exchangeable and carbonate fractions of Cd/Pb on biochars could be extracted when HCl and EDTA-2Na were used as desorption reagents.
In this study, the desorption kinetic experiment lasted 720 min due to the stable leaching of metal ions. However, future study was required to be performed in a long-term time to validate the results of desorption kinetics.
3.5 Adsorption mechanisms of Cd2+ and Pb2+
Precipitation with minerals
Based on the SEM–EDS of biochars after Cd2+ and Pb2+ adsorption (Fig. S4 and Fig. S5), many particles and crystal minerals were found on the surface of the biochars. The Cd and Pb could be directly found on the surface of the biochars, which confirmed that precipitation with minerals occurred during the adsorption process.
The typical anion concentrations in the supernatant after Cd2+ and Pb2+ adsorption are showed in Table S1. Compared with the control group, the concentrations of CO32–, HCO3–, and PO43– decreased after Cd2+ adsorption, indicating that potential precipitation occurred. Furthermore, the decrease in concentration of HCO3– and PO43– after Pb adsorption directly confirmed the presence of precipitation.
As shown in Fig. 4a, CdCO3 in RSBC300, and CdCO3 and Cd(OH)2 in RSBC500 and RSBC700 were formed after Cd adsorption. Therefore, precipitation with minerals was key for the high removal of Cd2+ by rice straw biochars (Yang et al., 2021). As shown in Fig. 5a, much Pb-containing precipitation was observed in biochars after Pb2+ adsorption. To be specific, PbC2O4, Pb3(PO4)2, Pb2(SO4)O, and 3PbCO3⋅2Pb(OH)2⋅H2O were detected on the surface of RSBC300. For RSBC500 and RSBC700, Pb5(PO4)3Cl, Pb3(CO3)2(OH)2, PbCO3, Pb4O(PO4)2, Pb3C2O7, Pb4(SO4)(CO3)2(OH)2, and Pb5(PO4)3OH were found on the surface of the biochars. Thus, precipitation with minerals was responsible for the Pb2+ adsorption (Chang et al., 2020).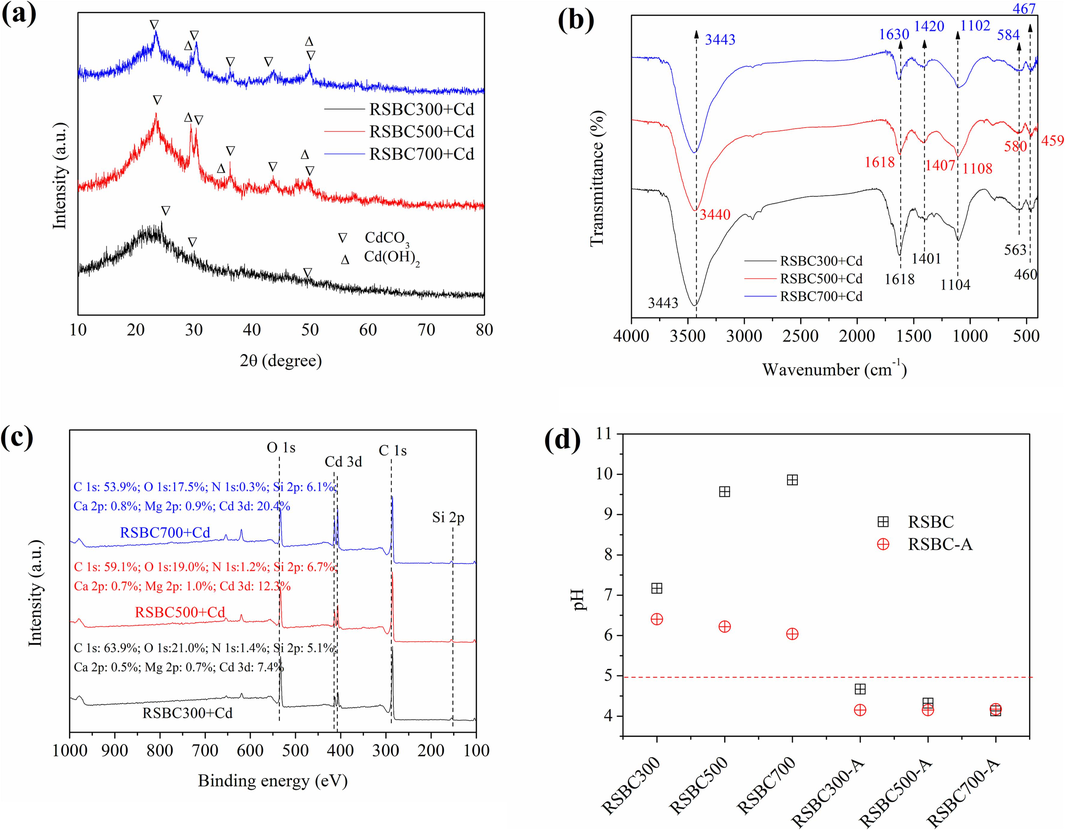
XRD (a), FTIR (b), XPS (c), and pH change on biochars after Cd2+ adsorption.
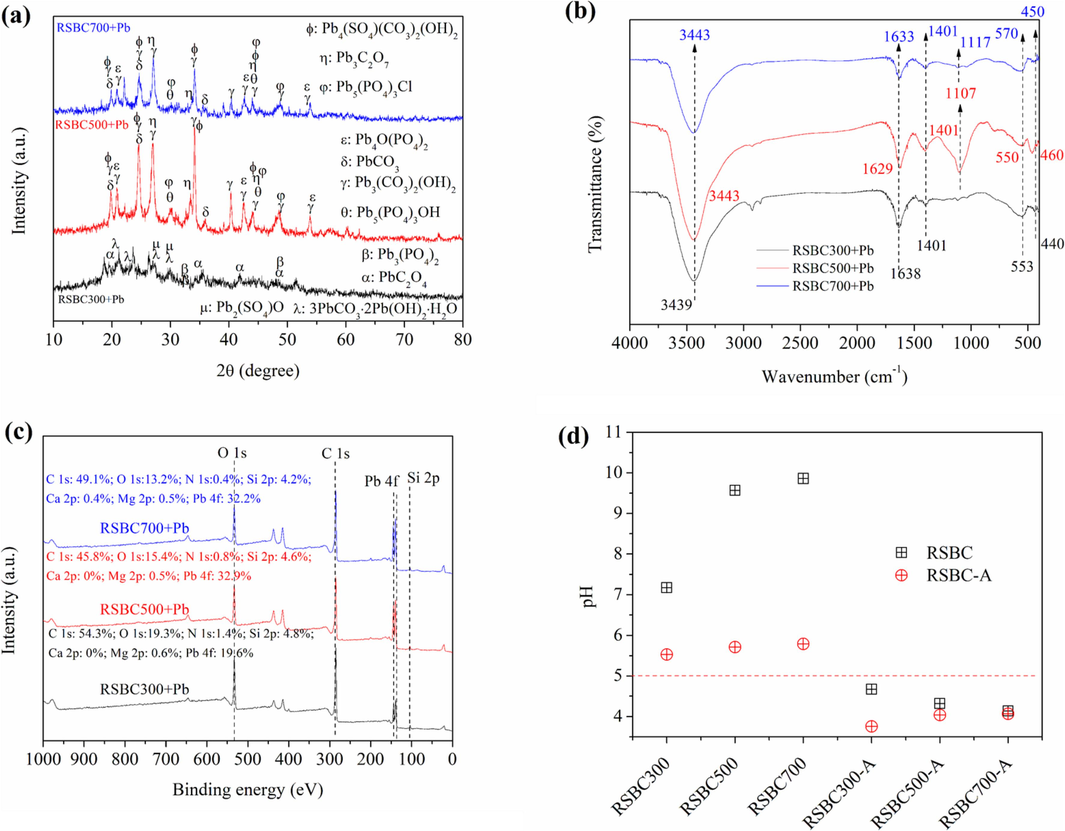
XRD (a), FTIR (b), XPS (c), and pH change on biochars after Pb2+ adsorption.
Overall, the above characterization and analysis confirmed that the occurrence of precipitation during the adsorption process was caused by the anions in the biochars.
Ion exchange
Comparing the results of Fig. S1 with Fig. S4 and Fig. S5, typical minerals cation ions on the surface of biochar disappeared after Cd2+ and Pb2+ adsorption, suggesting the occurrence of ion exchange.
Comparing the XPS analysis of biochar in Fig. 1d with Fig. 4c and Fig. 5c, the relative content of Ca 2p and Mg 2p of the biochars decreased after Cd and Pb adsorption, which suggested that ion exchange took place during the Cd2+ and Pb2+ adsorption process.
The release of minerals cation ions by all biochars was measured after Cd2+ and Pb2+ adsorption and listed in Table S1. More Ca2+, Mg2+, and K+ were released to the solution after Cd2+ and Pb2+ adsorption on biochars, implying the occurrence of an ion exchange mechanism.
In short, the above results also confirmed ion exchange happened during the adsorption process.
Complexation interaction
FTIR and XPS of biochars after heavy metal ions adsorption were characterized to elucidate the complexation interaction.
Compared with the FTIR of biochars in Fig. 1c, some peaks shifted after Cd adsorption on biochars (Fig. 4b). For RSBC300, the peaks at 1630, 1111, and 557 cm−1 shifted to 1618, 1104, and 563 cm−1, respectively. For RSBC500, 1420 and 554 cm−1 shifted to 1407 and 580 cm−1, respectively. For RSBC700, 3449, 1625, 1098, and 464 cm−1 shifted to 3442, 1630, 110, and 467 cm−1, respectively. Compared with the FTIR of biochars in Fig. 1c, some peaks also shifted after Pb adsorption on biochar (Fig. 5b). For RSBC300, the peaks at 3443, 1630, and 557 cm−1 shifted to 3439, 1639, and 553 cm−1, respectively. For RSBC500, 3440, 1617, 1420, and 554 cm−1 shifted to 3443, 1629, 1401, and 550 cm−1, respectively. For RSBC700, 3449, 1625, 1098, and 464 cm−1 shifted to 3443, 1633, 1117, and 450 cm−1, respectively. These changes suggested that OFGs in biochar toke prat in the binding of Cd2+/Pb2+ through complexation.
Compared with the XPS of biochars in Fig. S3, the binding energy and distribution of carbon- and oxygen-related groups changed after Cd2+ adsorption on biochars (Fig. S6). In addition, the OFGs also changed after Cd2+ adsorption. Moreover, the Cd 3d3/2 and Cd 3d5/2 peaks were observed on the surface of biochars after Cd2+ adsorption (Liu et al., 2022a; Tan et al., 2022). Compared with the XPS of biochars in Fig. S3, the binding energy and distribution of carbon- and oxygen-containing groups changed after Pb2+ adsorption on biochars (Fig. S7). Furthermore, the Pb 4f5/2 and Pb 4f7/2 peaks were found on the surface of biochars after Pb2+ adsorption (Wu et al., 2021).
As shown in Fig. 4d and Fig. 5d, the pH of RSBC-A after Cd2+ and Pb2+ adsorption was less than the control groups without Cd2+ and Pb2+ adsorption, illustrating the complexation interaction between Cd2+/Pb2+ and OFGs. The complexation reactions can be seen in the following reactions.
Cd-π interaction
Based on the FTIR and XPS, the aromatic carbon structure (1630 cm−1 in FTIR) and graphene-like structure (C–C/C—H/C⚌C in XPS) in biochars changed after Cd2+ and Pb2+ adsorption, suggesting that Cd/Pb–π interaction took place during the adsorption process. In addition, the Cd/Pb–π interaction could be observed directly in the XPS analysis of Cd 3p and Pb 4f.
Raman spectroscopy analysis also could be used to revela the adsorption mechanism (Fan and Zhang, 2021). According to the Raman spectra (Fig. S8) of biochar after Cd2+ and Pb2+ adsorption, the decrease in AD/AG values indicated the presence of Cd/Pb–π interaction when compared with the results in Fig. 1b.
3.6 Quantitative contributions of different sorption mechanisms
The pyrolysis temperature significantly influenced the heavy metal adsorption mechanisms on biochars. The Qexc, Qpre, Qcom, and Qπ values of biochars were 0.02–2.36, 21.63–51.44, 0.64–4.60, and 10.35–12.93 mg∙g−1, respectively (Fig. 6a.). As the pyrolysis temperature increases, the Qexc values of biochars decreased due to the minerals becoming stable at high temperatures, and Qcom values of biochars decreased due to the lower abundance of functional groups. The Qpre values of biochars also greatly increased with the increasing pyrolysis temperature. Precipitation with minerals played a key role in the adsorption of Cd2+, especially for the high-temperature biochar. As shown in Fig. 6b, the relative contribution percentage of Qexc, Qpre, Qcom, and Qπ values of biochars were 0.03 %–5.75 %, 52.77 %–82.37 %, 1.03 %–11.22 %, and 16.57 %–30.26 %, respectively. The contributions of Qexc, Qcom, and Qπ decreased with increasing temperature, whereas the contribution of Qpre was gradually predominant at high pyrolysis temperatures.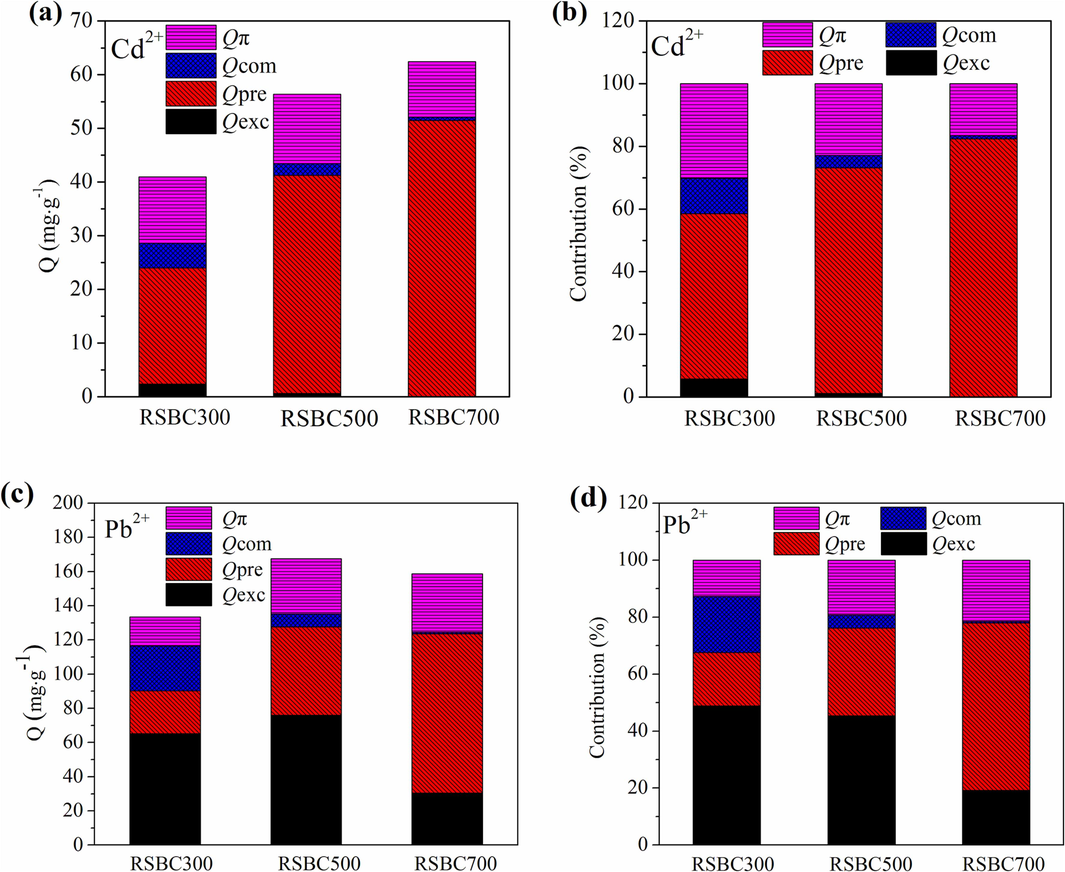
Adsorption capacity and quantitative contributions of different sorption mechanisms, (a) and (b) for Cd2+, (c) and (d) for Pb2+.
For Pb2+ (Fig. 6c), the Qexc, Qpre, Qcom, and Qπ values of biochars were 30.42–75.93, 25.14–93.17, 0.88–26.22, and 17.00–34.14 mg∙g−1, respectively. As the increasing of pyrolysis temperature, the Qpre and Qπ values of biochars increased, whereas the Qcom values of biochars decreased. The Qexc values of biochars first increased and then decreased with the increasing pyrolysis temperature. Thus, minerals in biochar could participate in the interaction of precipitation and exchange, which became the dominant mechanism during the adsorption process. As shown in Fig. 6d, the relative contribution percentages of Qexc, Qpre, Qcom, and Qπ values of biochars were 19.18 %–48.81 %, 18.82 %–58.71 %, 0.55 %–19.63 %, and 12.73 %–21.52 %, respectively. As the pyrolysis temperature increased, the relative contributions of Qpre and Qπ increased, whereas the relative contributions of Qexc and Qcom decreased. The increased contributions of Qpre and Qπ were attributed to the soluble minerals in biochar and graphitic structure, but the increased contributions of Qexc and Qcom were ascribed to the passivation of minerals in biochar, and the disappearance of OFGs.
Therefore, quantitative contributions of different sorption mechanisms could be used to explain the speciation distribution and environmental risk of Cd2+ and Pb2+ adsorbed on biochars, and the desorption characteristics of Cd2+ and Pb2+ from biochars after adsorption.
3.7 Practical application of biochars
The regeneration performance of biochars for metal ions is shown in Fig. 7a and Fig. 7b. The adsorption performance of biochars toward metal ions decreased significantly due to the loss of minerals since the precipitation with minerals and ion exchange were the dominant mechanisms for metal ions adsorption, especially Cd2+. Thus, the recycle potential of biochars was limited due to the decrease of binding sites.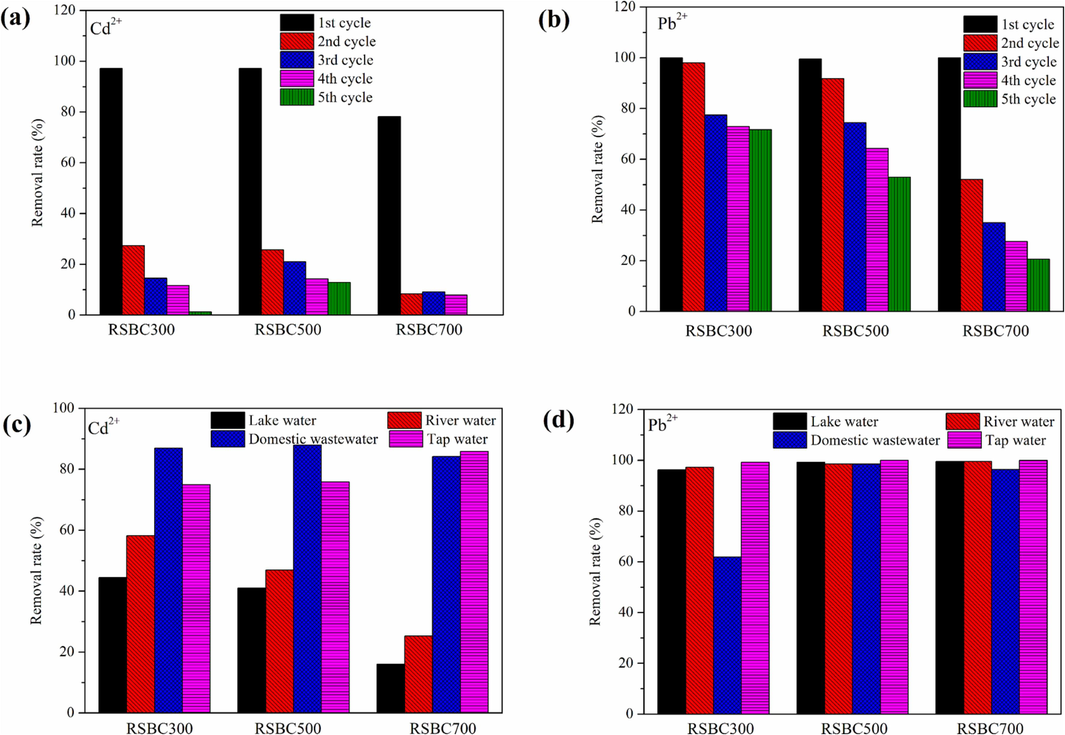
Practical application of biochars, (a) and (b) for recycle, (c) and (d) for real waterbodies.
The real application of biochars toward metal ions removal in various waterbodies is presented in Fig. 7c and Fig. 7d. As seen in the results, biochars exhibited a good adsorption performance toward Cd2+ and Pb2+ in domestic wastewater and tap water. Furthermore, biochars displayed a high removal rate toward Pb2+ in lake water and river water. Further study should be conducted to modify the biochars to improve their adsorption performance.
4 Conclusions
The majority of adsorbed Cd2+ on rice straw biochars was in the carbonate fraction and the exchangeable fraction. Cd and Pb in all metal-loaded biochars presented high risk, with RAC values greater than 50 %. HCl and EDTA-2Na could desorb most of the Cd2+ and Pb2+ from metal-laden biochars. Precipitation with minerals, ion exchange, and interaction with π-electrons were the main Cd2+/Pb2+ adsorption mechanisms, which was controlled by pyrolysis temperature. Therefore, Cd2+ and Pb2+ adsorbed on biochar were not stable and easily released to the environment, which shows a potential environmental risk. Biochars after Cd2+ and Pb2+ adsorption should be treated suitably to avoid causing secondary pollution.
CRediT authorship contribution statement
Li Liu: Methodology, Writing – original draft, Software, Formal analysis, Investigation. Shisuo Fan: Investigation, Conceptualization, Supervision, Project administration. Zixin Wang: Methodology, Formal analysis, Investigation, Data curation. Jingjing Hu: Methodology, Software, Investigation, Data curation.
Acknowledgements
This study was supported by the Natural Science Foundation of Anhui Province (2108085QD175) and Natural Science Foundation of the Education Department of Anhui Province (KJ2021A0675).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Remediation of emerging metal pollutants using environment friendly biochar - review on applications and mechanism. Chemosphere. 2022;290:133384
- [Google Scholar]
- Spent Ganoderma lucidum substrate derived biochar as a new bio-adsorbent for Pb2+/Cd2+ removal in water. Chemosphere. 2020;241:125121
- [Google Scholar]
- Insight into KOH activation mechanism during biomass pyrolysis: chemical reactions between O-containing groups and KOH. Appl. Energy. 2020;278:115730
- [Google Scholar]
- From macroalgae to porous graphitized nitrogen-doped biochars – Using aquatic biota to treat polycyclic aromatic hydrocarbons-contaminated water. Bioresour. Technol.. 2020;303:122947
- [Google Scholar]
- Optimized Ca-Al-La modified biochar with rapid and efficient phosphate removal performance and excellent pH stability. Arabian J. Chem.. 2023;16(8):104880
- [Google Scholar]
- Potential mechanisms of cadmium removal from aqueous solution by Canna indica derived biochar. Sci. Total Environ.. 2016;562:517-525.
- [Google Scholar]
- Removal of tetracycline from aqueous solution by biochar derived from rice straw. Environ. Sci. Pollut. Res.. 2018;25(29):29529-29540.
- [Google Scholar]
- Production and characterization of tea waste–based biochar and its application in treatment of Cd-containing wastewater. Biomass Convers. Biorefin.. 2021;11(5):1719-1732.
- [Google Scholar]
- Utilization of biochar sorbents for Cd2+, Zn2+, and Cu2+ ions separation from aqueous solutions: comparative study. Environ. Monit. Assess.. 2014;187(1):4093.
- [Google Scholar]
- Removal of heavy metals from soil with biochar composite: a critical review of the mechanism. J. Environ. Chem. Eng.. 2021;9(5):105830
- [Google Scholar]
- Contribution of different mechanisms to Pb2+ and Cd2+ sorption on magnetic wheat straw biochars: impact of pyrolysis temperature and DOM in biochar. J. Environ. Chem. Eng.. 2022;10(3):107851
- [Google Scholar]
- Catalytic pyrolysis using a nickel-functionalized chemically activated biochar catalyst: insight into process kinetics, products, and mechanism. ACS Sustainable Chem. Eng.. 2022;10(18):5770-5780.
- [Google Scholar]
- Synthesis of ZnO nanoparticle-anchored biochar composites for the selective removal of perrhenate, a surrogate for pertechnetate, from radioactive effluents. J. Hazard. Mater.. 2020;387:121670
- [Google Scholar]
- Effects of KMnO4 pre- and post-treatments on biochar properties and its adsorption of tetracycline. J. Mol. Liq.. 2023;373:121257
- [Google Scholar]
- Quantitative contribution of Cd2+ adsorption mechanisms by chicken-manure-derived biochars. Environ. Sci. Pollut. Res.. 2018;25(28):28322-28334.
- [Google Scholar]
- Qualitative and quantitative characterization of adsorption mechanisms for Cd2+ by silicon-rich biochar. Sci. Total Environ.. 2020;731:139163
- [Google Scholar]
- Synthesis of amino-functionalized magnetic aerobic granular sludge-biochar for Pb(II) removal: adsorption performance and mechanism studies. Sci. Total Environ.. 2019;685:681-689.
- [Google Scholar]
- Effect of combined aging treatment on biochar adsorption and speciation distribution for Cd(II) Sci. Total Environ.. 2023;867:161593
- [Google Scholar]
- Artificial intelligence (AI) applications in adsorption of heavy metals using modified biochar. Sci. Total Environ.. 2021;801:149623
- [Google Scholar]
- Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere. 2017;178:466-478.
- [Google Scholar]
- Facile preparation of magnetic porous biochars from tea waste for the removal of tetracycline from aqueous solutions: effect of pyrolysis temperature. Chemosphere. 2022;291:132713
- [Google Scholar]
- Engineered biochar derived from lemon peel waste for highly efficient removal of organic pollutants from water. Arabian J. Chem.. 2023;16(10):105158
- [Google Scholar]
- Utilization of biochar produced from invasive plant species to efficiently adsorb Cd (II) and Pb (II) Bioresour. Technol.. 2020;317:124011
- [Google Scholar]
- Enhanced removal of Cd2+ from water by AHP-pretreated biochar: adsorption performance and mechanism. J. Hazard. Mater.. 2022;438:129467
- [Google Scholar]
- Removal of cadmium in aqueous solution using wheat straw biochar: effect of minerals and mechanism. Environ. Sci. Pollut. Res.. 2018;25(9):8688-8700.
- [Google Scholar]
- Adsorption of cadmium and lead from aqueous solution using modified biochar: a review. J. Environ. Chem. Eng.. 2022;10(1):106502
- [Google Scholar]
- Chemical forms and risk assessment of heavy metals in sludge-biochar produced by microwave-induced low temperature pyrolysis. RSC Adv.. 2016;6(104):101960-101967.
- [Google Scholar]
- Modified-biochar adsorbents (MBAs) for heavy-metal ions adsorption: a critical review. J. Environ. Chem. Eng.. 2022;10(2):107393
- [Google Scholar]
- Relative distribution of Pb2+ sorption mechanisms by sludge-derived biochar. Water Res.. 2012;46(3):854-862.
- [Google Scholar]
- Novel insights into the adsorption of organic contaminants by biochar: a review. Chemosphere. 2022;287:132113
- [Google Scholar]
- Ionothermal carbonization of biomass to construct sp2/sp3 carbon interface in N-doped biochar as efficient oxygen reduction electrocatalysts. Chem. Eng. J.. 2020;400:125969
- [Google Scholar]
- Effect of Fe–N modification on the properties of biochars and their adsorption behavior on tetracycline removal from aqueous solution. Bioresour. Technol.. 2021;325:124732
- [Google Scholar]
- Abatement of cadmium (Cd) contamination in sediment using tea waste biochar through meso-microcosm study. J. Cleaner Prod.. 2019;212:986-996.
- [Google Scholar]
- Hierarchical porous biochar for persulfate activation: non-radical pathway for rapid degradation of organic pollutants. Arabian J. Chem.. 2023;16(11):105242
- [Google Scholar]
- Activation methods increase biochar's potential for heavy-metal adsorption and environmental remediation: a global meta-analysis. Sci. Total Environ.. 2023;865:161252
- [Google Scholar]
- Facile preparation of micro-porous biochar from Bangladeshi sprouted agricultural waste (corncob) via in-house built heating chamber for cationic dye removal. Arabian J. Chem.. 2023;16(9):105080
- [Google Scholar]
- Understanding structure-performance correlation of biochar materials in environmental remediation and electrochemical devices. Chem. Eng. J.. 2020;382:122977
- [Google Scholar]
- Biochar as a low-cost adsorbent for aqueous heavy metal removal: a review. J. Anal. Appl. Pyrolysis. 2021;155:105081
- [Google Scholar]
- Effect of pyrolysis temperature on characteristics, chemical speciation and environmental risk of Cr, Mn, Cu, and Zn in biochars derived from pig manure. Sci. Total Environ.. 2020;704:135283
- [Google Scholar]
- Qualitative and quantitative characterisation of adsorption mechanisms of lead on four biochars. Sci. Total Environ.. 2017;609:1401-1410.
- [Google Scholar]
- Leaching of heavy metals from lead-zinc mine tailings and the subsequent migration and transformation characteristics in paddy soil. Chemosphere. 2022;291:132792
- [Google Scholar]
- Enhancing Cd(II) adsorption on rice straw biochar by modification of iron and manganese oxides. Environ. Pollut.. 2022;300:118899
- [Google Scholar]
- Microwave-assisted production of CO2-activated biochar from sugarcane bagasse for electrochemical desalination. J. Hazard. Mater.. 2020;383:121192
- [Google Scholar]
- Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem.. 1979;51(7):844-851.
- [Google Scholar]
- Impact of pyrolysis temperature and activation on oily sludge-derived char for Pb(II) and Cd(II) removal from aqueous solution. Environ. Sci. Pollut. Res.. 2021;28(5):5532-5547.
- [Google Scholar]
- Bio-oil and biochar from the pyrolytic conversion of biomass: a current and future perspective on the trade-off between economic, environmental, and technical indicators. Sci. Total Environ.. 2023;857:159155
- [Google Scholar]
- Sorption of four hydrophobic organic contaminants by biochars derived from maize straw, wood dust and swine manure at different pyrolytic temperatures. Chemosphere. 2016;144:285-291.
- [Google Scholar]
- Investigating the adsorption behavior and the relative distribution of Cd2+ sorption mechanisms on biochars by different feedstock. Bioresour. Technol.. 2018;261:265-271.
- [Google Scholar]
- High-efficiency removal capacities and quantitative adsorption mechanisms of Cd2+ by thermally modified biochars derived from different feedstocks. Chemosphere. 2021;272:129594
- [Google Scholar]
- Investigating the mechanisms of biochar’s removal of lead from solution. Bioresour. Technol.. 2015;177:308-317.
- [Google Scholar]
- Regeneration of magnetic biochar derived from eucalyptus leaf residue for lead(II) removal. Bioresour. Technol.. 2015;186:360-364.
- [Google Scholar]
- A novel modified method for the efficient removal of Pb and Cd from wastewater by biochar: enhanced the ion exchange and precipitation capacity. Sci. Total Environ.. 2021;754:142150
- [Google Scholar]
- Improved adsorption properties of tetracycline on KOH/KMnO4 modified biochar derived from wheat straw. Chemosphere. 2022;296:133981
- [Google Scholar]
- Indispensable role of biochar-inherent mineral constituents in its environmental applications: a review. Bioresour. Technol.. 2017;241:887-899.
- [Google Scholar]
- Enhanced immobilization of cadmium and lead adsorbed on crop straw biochars by simulated aging processes. Environ. Pollut.. 2022;302:119064
- [Google Scholar]
- Adsorption characteristics and the removal mechanism of two novel Fe-Zn composite modified biochar for Cd(II) in water. Bioresour. Technol.. 2021;333:125078
- [Google Scholar]
- The interfacial behavior between biochar and soil minerals and its effect on biochar stability. Environ. Sci. Technol.. 2016;50(5):2264-2271.
- [Google Scholar]
- Magnetic MgFe2O4/biochar derived from pomelo peel as a persulfate activator for levofloxacin degradation: effects and mechanistic consideration. Bioresour. Technol.. 2022;346:126547
- [Google Scholar]
- Facile assembled biochar-based nanocomposite with improved graphitization for efficient photocatalytic activity driven by visible light. Appl. Catal. B. 2019;250:78-88.
- [Google Scholar]
- Biochar derived from traditional Chinese medicine residues: an efficient adsorbent for heavy metal Pb(II) Arabian J. Chem.. 2024;17(3):105606
- [Google Scholar]
- The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour. Technol.. 2011;102(3):3488-3497.
- [Google Scholar]
- Desorption of calcium-rich crayfish shell biochar for the removal of lead from aqueous solutions. J. Colloid Interface Sci.. 2019;554:417-423.
- [Google Scholar]
- Molecular-level investigation on removal mechanisms of aqueous hexavalent chromium by pine needle biochar. Arabian J. Chem.. 2023;16(8):104966
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2024.105669.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







