Translate this page into:
Enhanced catalytic degradation of acetaminophen using magnesium oxide-infused clay with ultrasonic activation of hydrogen peroxide
⁎Corresponding authors. sanati@pgu.ac.ir (Ali Mohammad Sanati), b.ramavandi@bpums.ac.ir (Bahman Ramavandi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
In this study, clay modified with magnesium oxide (MgO) was used as a catalyst for the degradation of acetaminophen in wastewater, activated by hydrogen peroxide (H2O2) and ultrasonic waves. Characterization of the Clay-MgO catalyst was conducted using TGA, XRD, BET, SEM, FTIR, XRF, and EDX, revealing functional groups capable of activating H2O2. The crystalline catalyst, synthesized at 500 °C, had a surface area of 30 m2/g. Optimal conditions for acetaminophen removal, achieving 75 % efficiency, were pH 8, 3 g/L catalyst, 0.2 mL/100 mL H2O2, and 60 min of contact time. In distilled water, mineralization of acetaminophen was 42 %, while actual wastewater showed 18 %. Hydroxyl radicals played a significant role in the degradation process. The catalyst was tested for reuse up to six times and maintained a high efficiency of over 53 % in five stages. Radical scavenger studies confirmed the importance of hydroxyl radicals in the degradation kinetics, which followed pseudo-first-order (R2 > 0.96) and Langmuir-Hinshelwood (R2 = 0.95) models. The catalyst also demonstrated efficient acetaminophen removal in complex solutions, including seawater. This MgO-modified clay shows promise as an effective catalyst for the degradation of pharmaceutical pollutants through hydrogen peroxide activation, maintaining stability and reusability across multiple cycles.
Keywords
Magnesium oxide
Clay
Acetaminophen
Hydrogen peroxide
Langmuir-Hinshelwood model
1 Introduction
Pharmaceutical residues are among the most resistant pollutants to conventional treatment methods, leading to their increasing presence in water resources and subsequent adverse effects on humans and animals (Chiriac et al., 2024; Kafaei et al., 2018; Ding et al., 2024). A wide variety of drugs enter the environment through industrial and hospital/household wastewater, frequently appearing in aqueous media (Al Falahi et al., 2022). One of the most commonly prescribed drugs is acetaminophen, also known as paracetamol or acetyl-para-aminophenol (C8H9NO2) (Sybuia et al., 2024). Acetaminophen is used at an annual rate of 145,000 tons (Kumari and Kumar, 2021). It is used as a non-narcotic antipyretic and analgesic agent to alleviate headaches, colds, influenza, mild joint and muscle pains, and sometimes peripheral nerve impairments (Abdullah et al., 2016). The therapeutic dosage of acetaminophen for adults ranges from 1 to 4 g per day, with a plasma half-life of 1.5 to 2.5 h (Felisardo et al., 2024). During the therapeutic process, approximately 60 to 70 % of ingested acetaminophen remains unmetabolized and is excreted in its original form, leading to long-term environmental pollution and health risks for living organisms and humans (Srinithi et al., 2023; Villaroel et al., 2014). Acetaminophen has been found in water bodies (rivers and lakes) across several countries, including the USA, China, the United Kingdom, France, and Spain, with concentrations ranging from 0.1 to 300 mg/L (Vo et al., 2019; Pacheco-Álvarez et al., 2022). Due to its high hydrophilicity and solubility (12,900 mg/L), acetaminophen has been detected in the effluent of water and wastewater treatment plants (Kumari and Kumar, 2021; Abdullah et al., 2016) and can entry into water bodies. Acetaminophen contamination, as documented by Lari et al. (2022) (Lari et al., 2022) and Erhunmwunse et al. (2021) (Erhunmwunse et al., 2021), poses significant risks to aquatic life and human health, necessitating the development of more effective and sustainable removal technologies.
Numerous methods like coagulation and precipitation processes (Wang et al., 2023), adsorption (Yanyan et al., 2018; Gao et al., 2024; Ma et al., 2024; Wei et al., 2023), membrane and filtration processes (Shojaee Nasirabadi et al., 2016), Fenton-based processes (Ahmadzadeh and Dolatabadi, 2018), sonolysis (Sidnell et al., 2024), microwave (Wang et al., 2023), photolysis and photocatalysis (Gómez-Avilés et al., 2019; Fahoul et al., 2023; El Gaidoumi et al., 2021), heterogeneous catalysis (El Gaidoumi et al., 2019; El Gaidoumi et al., 2019), and electrochemical oxidation (Periyasamy and Muthuchamy, 2018) have been used for elimination of contaminants in aqueous media. Among these methods, physical ones such as coagulation and precipitation, membrane and filtration processes, and even adsorption only cause transition of the pollutant from the phase into another and have little impact on degradation of pollutants, thus requiring supplementary treatment stages (García-Ávila et al., 2021; Bai et al., 2023). In addition, some of these processes have downsides such as requiring greater space as well as the effects of algal blooms (García-Ávila et al., 2021; Zhen et al., 2024). The disadvantage of biological processes is that the process is time-consuming and degrades the contaminant very slowly (Akbari et al., 2021; Taoufik et al., 2021). In this regard, the low efficiency of removal of antibiotics in the treatment plants of urban wastewater can also be noted (Akbari et al., 2021).
Among the various methods, advanced oxidation processes (AOPs) have gained significant popularity among water and wastewater engineers and researchers due to their high removal efficiency (Jin et al., 2023; Ye et al., 2024; Zuo et al., 2023). AOPs are among the most efficient contemporary techniques for treating wastewater contaminated with organic pollutants. These methods rely on the generation of free radicals (HO∙, O2−∙, HO2∙) in situ, with hydroxyl radicals being the most reactive. These radicals can swiftly oxidize a wide range of organic compounds (Khankhasaeva and Badmaeva, 2020; Jamil, 2024; Kundu and Radian, 2022). Unlike other methods that merely transfer contaminants from one phase to another, AOPs degrade the contaminants (Taoufik et al., 2021; Damiri et al., 2020; Malakootian et al., 2019). These processes rapidly and non-selectively degrade pollutants through the generated hydroxyl radicals, converting them into simpler intermediates (Pan et al., 2018).
New and impactful materials are consistently a major focus in this area, playing a crucial role in catalytic techniques. Catalyst-based AOPs, such as those using clay-modified catalysts, are particularly appealing and have been extensively researched for pollutant removal from aqueous solutions (Tavasol et al., 2020; Jalali et al., 2022; Foroughi et al., 2024).
Clay offers numerous advantages, including abundance, high BET surface area, low cost, stability, sustainability, ion exchange capacity, modifiable structure, and non-toxicity (Luo et al., 2009; Mojahedimotlagh et al., 2024; Bai et al., 2022). Various clay soil-based catalysts, such as iron metal organic hydrogel/clay (Cha et al., 2023), Clay-Fe/Cu-Al (Khankhasaeva et al., 2017), Clay-covalent triazine (Yi et al., 2023), and Clay-Co/Mg/Al (Zhao et al., 2022), have been reported for the removal of antibiotics and other organic pollutants. Studies have shown that the catalytic properties of clay can be enhanced with metals like magnesium, titanium, iron, aluminum, and zinc (Boukhemkhem et al., 2023; Sangare et al., 2024; Rostamzadeh and Sadeghi, 2022).
Magnesium oxide (MgO) has been specifically chosen for this study due to its exceptional catalytic properties and stability. MgO is known for its high adsorption capacity and active surface sites, which significantly enhance its ability to catalyze oxidative reactions (Zhou et al., 2020). The inclusion of MgO in the catalyst structure improves the overall performance by increasing the generation of hydroxyl radicals when used in conjunction with H2O2 and ultrasonic activation. This enhancement is crucial for achieving efficient degradation of acetaminophen and other organic pollutants. Despite its promising properties, the potential of MgO combined with clay, H2O2, and ultrasound for acetaminophen degradation has not been extensively explored in existing research.
Using catalysts with H2O2 can enhance the oxidizing properties of the process by generating more hydroxyl radicals, thereby improving pollutant degradation performance and overcoming the limitations of H2O2 activation (Garcia-Costa and Casas, 2022; Liu et al., 2021). Ultrasound-assisted AOPs have gained attention in recent years, with studies by Sadeghi et al. (2024) (Sadeghi et al., 2024) and Bagheri et al. (2024) (Bagheri et al., 2024) demonstrating that ultrasonic cavitation can enhance mass transfer and catalyst efficiency. However, the use of Clay-MgO catalyst for the degradation of acetaminophen pollutant has yet to be thoroughly investigated.
This study presents an innovative approach by integrating MgO-infused clay, H2O2, and ultrasonic activation to achieve efficient degradation of acetaminophen. The objectives are to: (i) evaluate the impact of various operational factors (such as pH, pollutant concentration, catalyst dose, H2O2 concentration, and contact time) on acetaminophen degradation, (ii) investigate the reaction kinetics and catalyst reusability, and (iii) explore the efficacy of this process in diverse water matrices, including distilled water, tap water, hospital wastewater, and seawater. This work aims to contribute significantly to the advancement of sustainable and effective methods for the removal of pharmaceutical pollutants from aquatic environments.
2 Experimental
2.1 Materials
In this study, the utilized materials included: sodium hydroxide (NaOH, 98 %), hydrochloric acid (HCl, 37 %), magnesium chloride (MgCl2, 99.9 %), and hydrogen peroxide (H2O2, >30 %) which were purchased from Sigma-Aldrich. Acetaminophen powder (99.9 %) was prepared from Farabi Pharmaceutical Company (Iran). Materials such as silver nitrate, Tert-butanol, p-benzoquinone, and ammonium oxalate were purchased from Merck Company with purity > 99 %. Clay soil was prepared from a local soil mine in the south of Iran. The properties of the clay soil used in the present study are provided in Table 1. As observed, the utilized soil contains trivial amounts of chemical contamination while also containing useful metals which can function as catalyst.
Parameter
Value
pH
7.84 ± 0.3
EC
169.8 ± 11.1 μS/cm
TOM
0.66 ± 0.20 %
Hg
ND
Pb
ND
Cd
ND
As
ND
Cr
0.04 ± 0.01 mg/kg
Fe
232 ± 8 mg/kg
Al
346 ± 12 mg/kg
Mn
70.01 ± 3.5 mg/kg
Ti
1.52 ± 0.31 mg/kg
Mg
74.4 ± 6.5 mg/kg
EC: electrical conductivity; TOM: total organic matter
The results of the XRF analysis for the clay are presented in Table 2. Of the compounds identified by XRF, SiO2 has the highest weight percentage, followed by Al2O3, Fe2O3, K2O, and CaO.
Compound
Value (W%)
SiO2
53
Al2O3
14.5
Fe2O3
7.4
CaO
2.7
MgO
0.8
Na2O
0.82
K2O
3.4
TiO2
1.3
MnO
0.6
SO3
0.24
P2O5
0.32
Cl2
0.2
BaO
0.7
ZnO
0.3
CuO
0.3
LOI
13.02
LOI: Loss on ignition
2.2 Production of Clay-MgO catalyst
To prepare the Clay-MgO catalyst, a flask containing 3 g clay was mixed in 10, 35, 70, and 100 mL of MgCl2 solution (at 1,000 mg/L) plus 5 mL of 1 M NaOH solution, and then diluted with distilled water to 250 mL. The names of the Clay-MgO catalysts that were obtained with these magnesium chloride solutions were named 10-Clay-MgO, 35-Clay-MgO, 70-Clay-MgO, and 100-Clay-MgO, respectively. The prepared mixture was placed inside shaker incubator device (300 rpm) for 5 h. Next, the suspension was placed still for a specific period of time (2 h) to precipitate. After this stage, its top water was discharged and the remaining solution was filtered through Whatman filter paper 0.42 µm. The remaining materials were placed inside oven for 990 min at 98 °C in order to dry off. The obtained materials were put inside electrical furnace (without oxygen and nitrogen) at 500 °C for 2 h. Ultimately, a Clay-MgO catalyst was produced and utilized to study the removal of acetaminophen from wastewater and other aqueous solutions, using hydrogen peroxide and ultrasonic waves.
2.3 Method of optimizing the influential parameters
To evaluate the effect of pH, a range of 5–9 was tested with an acetaminophen concentration of 100 mg/L. The pH levels (5, 6, 7, 8, 9) were adjusted using HCl and NaOH. Then, 3 g/L of catalyst and 0.2 mL of H2O2 were added. After 1 h of contact time, the acetaminophen content was measured.
To assess the effect of the Clay-MgO catalyst on acetaminophen removal, six flasks containing acetaminophen at pH 8 were prepared. Each flask received 0.2 mL of H2O2 and specific amounts of catalyst (0.1, 0.5, 1, 2, 3, and 4 g) and was placed in an ultrasonic device for 1 h. The solution was subsequently filtered using 0.42 μm filter paper, and the remaining acetaminophen was measured.
To evaluate the effect of H2O2 on acetaminophen removal, a solution with a specific amount of acetaminophen at the optimal pH was prepared, and 3 g of catalyst was added. Various amounts of H2O2 (0, 0.1, 0.2, 0.3, 0.5, and 1 mg/L) were then introduced. After a 1 h reaction time, the pollutant content was measured.
To investigate acetaminophen removal using the Clay-MgO catalyst with hydrogen peroxide and ultrasonic waves, optimal pH, catalyst dose, and H2O2 concentration were first determined. Acetaminophen concentrations of 10, 50, 100, 200, and 300 mg/L were then subjected to various reaction times (5, 10, 20, 30, 40, 60, and 100 min) in a shaker. After each reaction, the solution was filtered using 0.42 μm filter paper, and the remaining drug was measured.
To recover the catalyst, it was first isolated after use and subjected to a washing process. The catalyst was initially immersed in 250 mL of distilled water for 1 h (1 g of the recycled catalyst), followed by immersion in 250 mL of 0.1 M ethanol for 1 h (1 g of the water-washed catalyst). Finally, 1 g of the recycled catalyst was immersed in 250 mL of 0.1 M acetonitrile for 1 h. After washing, the catalyst was dried at 70 °C for one day and then used in subsequent cycles.
All experiments were conducted with stirring at 200 rpm, and the temperature was kept at 24 ± 1 °C. Each series of experiments was performed in triplicate, with the mean and standard deviation reported.
2.4 Characterization of the synthesized catalyst and measurement of acetaminophen
To characterize the physicochemical properties of the synthesized Clay-MgO catalyst, a specific amount of the prepared catalyst was tested with acetaminophen under optimal operational circumstances. Then, fresh catalysts and those exposed to the drug were evaluated using different tests including BET (Asap2020 − Micromeritics), FT/IR 4600-JASCO, FE-SEM (ZEISS Sigma), TGA (STA6000- Perkin Elmer), and XRD (D8 Advance-BRUKER). X-ray fluorescence (XRF) analysis was applied to quantify the chemical compositions of Clay (Rigaku Primus IV) (Zhan et al., 2024). The particle size distribution of Clay-MgO particles was determined using Dynamic Light Scattering (DLS) on a Nano Plus DLS/ELS instrument, which utilizes a 5 mW He-Ne laser with a 632.8 nm wavelength as its light source. DLS measurements were performed at a scattering angle (θ) of 173° and a temperature of 25 °C. The samples were diluted in ethanol and sonicated for 10 min prior to analysis (del Álamo et al., 2020).
The acetaminophen concentration was determined using a high-performance liquid chromatography (HPLC) device (Shimadzu LC, HPLC 20A) with a UV detector (UV/VIS, SPD-20A). An InertSustain C18 column was used for the analysis. The mobile phase was a 25:75 volumetric mixture of methanol and deionized water, flowing at 1 mL/min. The C18 column was kept at 30 °C, and measurements were taken at a wavelength of 245 nm. This method has been previously employed in other studies for the measurement of acetaminophen (Sanjeev and Valsan, 2024; Sun et al., 2024). The LOD and LOQ of this method was 0.016 µg/L and 0.05 µg/L, respectively. The extent of acetaminophen mineralization in the studied system was measured using TOC device (Shimadzu, VCSH model, Japan). The ICP-OES instrument (730-ES, Varian) was used to analyze the metals present in the clay and the treated solution following each cycle of the catalyst reuse. The extent of acetaminophen mineralization was calculated by Formula (1):
3 Results and discussion
3.1 Properties of fresh and used Clay-MgO catalyst
FTIR analysis is used to examine and identify functional groups and to show the interactions between the different components of the materials used. Fig. 1a presents the FTIR spectra for clay, MgO, and Clay-MgO both before and after the degradation of the target pollutant. The results show vibrations within the range of 3500–3600 cm−1, attributed to the –OH vibrations within the structure of the samples (Foroughi et al., 2024). Additionally, vibrations are observed at 1448, 1214, 1014, and 460–570 cm−1 in the clay structure, corresponding to the asymmetric vibrations of CO3 (Najafi et al., 2024; Largo et al., 2020), Si-O (Bougdour et al., 2024), Si-O-Si (Barhdadi et al., 2024), and Al-O-Al/Si-O-Si (Mbognou et al., 2024; Bai et al., 2024), respectively. In the MgO spectrum, vibrations appear at 1430 and below 1000 cm−1, attributed to Mg-O-Mg and Mg-O (Jin et al., 2023; Foroutan et al., 2021). After modifying clay with MgO, the absorption peaks of both components are clearly visible in the Clay-MgO composite, indicating good interaction between the materials and successful integration of MgO into the clay structure. Post-acetaminophen degradation, no significant changes are observed in their functional groups and ranges, suggesting that the catalyst is stable and does not decompose during the degradation process.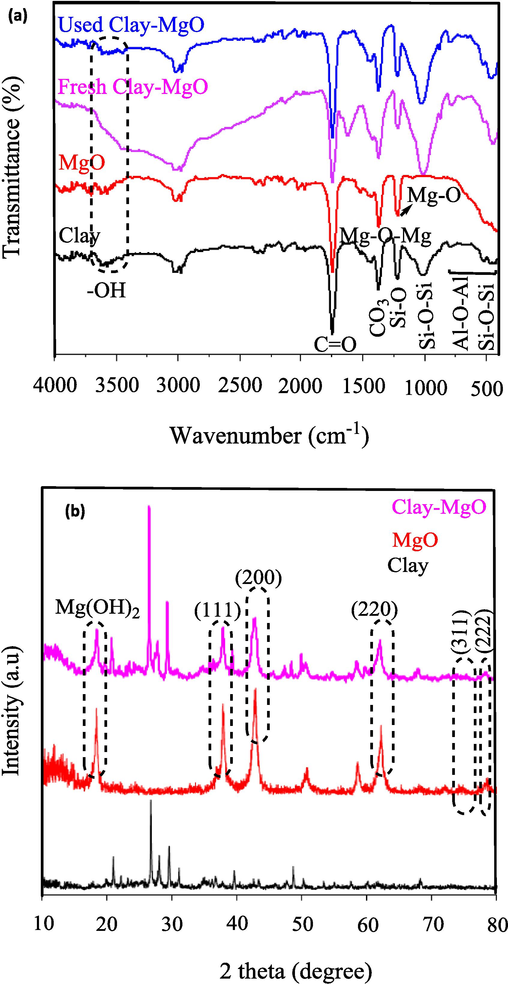
(a) FTIR and analysis for fresh and used catalyst and its components and (B) XRD analysis for fresh catalyst and its components.
Fig. 1b shows the XRD spectra for clay, MgO, and Clay-MgO samples, confirming the crystalline and amorphous structures of the samples. The spectra reveal peaks of varying intensities, indicating the crystalline nature of the samples. Specific peaks are observed in the clay structure at 2θ values of 20.92, 26.68, 29.5, 30.74, 34.94, 36.64, 39.52, 40.36, 42.28, 50.16, 60.18, and 68.2. These peaks correspond to the presence of illite, kaolinite, quartz, calcite, and dolomite within the clay structure (Bellaj et al., 2024; Bellaj et al., 2024; Bai et al., 2024). In the presented MgO structure, XRD spectrum confirms existence of peaks at 2θ degrees of 37.84, 43.15, 62.26, 74.84, and 78.66; based on the JCPDS card number 04–0829 standard, they are attributed to the crystalline planes of (111), (200), (220), (311), and (222) (Shkir et al., 2024; He et al., 2023; Chen et al., 2024). It should also be noted that in the XRD spectrum related to MgO, a very high intensity peak has emerged at 18.52° which is attributed to the Mg(OH)2 state (Foroutan et al., 2021). After modifying the clay with MgO, the peaks associated with both materials successfully appear together. This indicates successful integration and good interaction between MgO and the clay.
SEM, EDX-map analysis has been presented for investigating the superficial properties and changes made in the morphology of clay and Clay-MgO samples before and after acetaminophen degradation process (Fig. 2a–i). As observed from the results, the surface of the clay exhibits various roughnesses, peaks, and troughs, with different elements dispersed across its surface, which can influence the acetaminophen degradation process. After modification with MgO, particles are visible within the clay structure, likely due to the integration of MgO particles. EDX-Map analysis showed that the MgO content in the clay structure increased from 2.3 W% to 7 W% after modification, confirming the successful integration of MgO via the chemical precipitation method. Post-oxidation process, SEM results indicated that the Clay-MgO structure remained largely unchanged, demonstrating its structural stability. Additionally, EDX-Map analysis revealed the presence of similar elements to those found in Clay-MgO before the oxidation process, though the percentages of some elements varied. These changes in element percentages may result from leaching or different points of analysis during imaging, as EDX-Map analysis is a point-based method where elemental composition can vary with the location of analysis.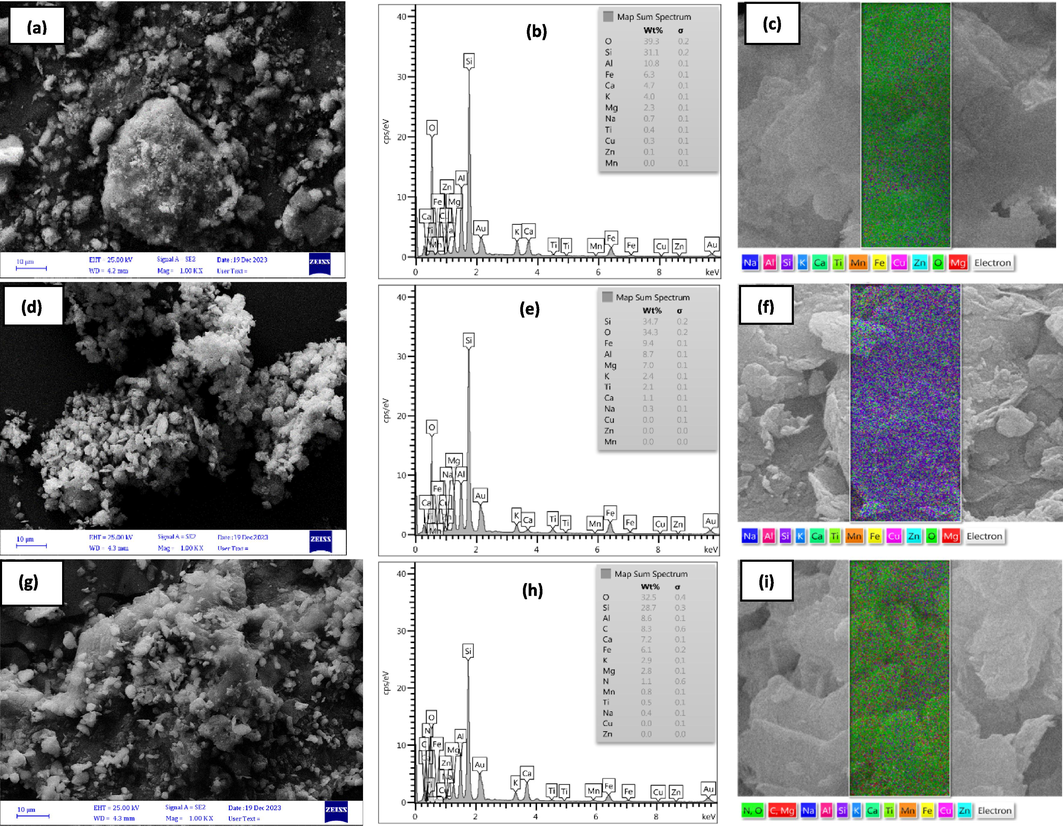
SEM, EDX, and mapping results for (a-c) Clay, (d-f) fresh Clay-MgO, and (g-i) used Clay-MgO.
Fig. 3 represents a DLS analysis of magnesium-modified clay, showing particle size distribution from 100 to 500 nm. The peak intensity occurs between 200–300 nm, indicating dominant particle sizes within this range. Studies on clay modification with magnesium typically show similar particle sizes, as reported in other works, such as those focusing on modified kaolinite or bentonite clays (El Bouraie and Masoud, 2017; Lin et al., 2020). These studies often report size ranges between 50–500 nm, with magnesium enhancing stability and dispersion, aligning well with the presented data.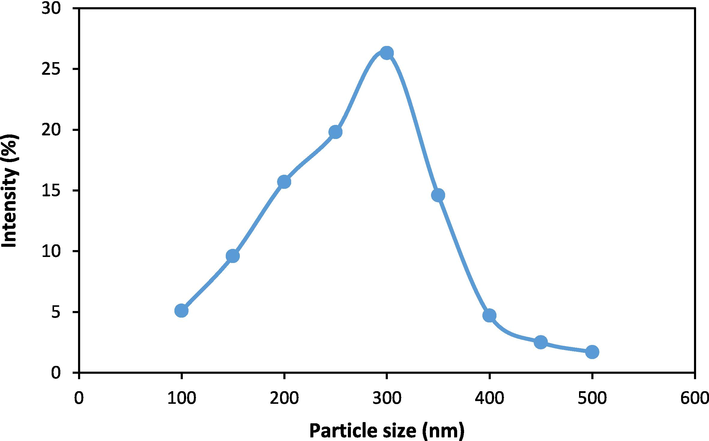
DLS analysis of Clay-MgO sample.
Thermal gravimetric analysis (TGA) was conducted to examine the thermal behavior properties of both the clay and Clay-MgO samples over the temperature range of 25–950 °C, with the results depicted in Fig. 4. The initial stage of weight loss, occurring between 25–150 °C, is attributed to the evaporation of surface moisture from the samples. The subsequent significant weight loss, spanning from 150-750 °C, is primarily due to the release of moisture from internal layers (Bée et al., 2017), the removal of water species associated with interlayer cations, and the hydroxylation of both clay and MgO (Kaya et al., 2015). No substantial change in weight loss is observed beyond 750 to 950 °C, indicating the complete removal of organic compounds and moisture from the sample structures (Wu et al., 2024). However, it is noteworthy that the percentage of weight loss for Clay-MgO is lower compared to that of clay, likely due to the incorporation of MgO within the clay structure. Therefore, it can be inferred that MgO has been effectively integrated into the Clay structure, constituting approximately 2.5 % of the Clay structure.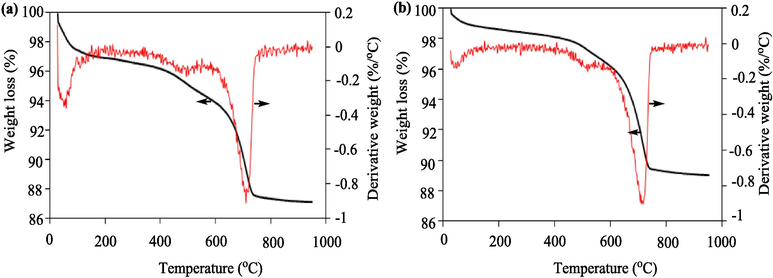
TGA-DTG analysis for (a) Clay and (b) Clay-MgO.
The structural properties of the Clay-MgO catalyst, evaluated before and after the acetaminophen degradation process, provide valuable insights into its catalytic performance. The BET surface area, pore volume, and pore diameter are crucial factors influencing the effectiveness of heterogeneous catalysts. In this study, BET analysis was conducted on MgO, Clay, and Clay-MgO samples both before and after acetaminophen removal, with the results summarized in Table 3 and Fig. 5.
Parameter
MgO
Clay
Fresh Clay-MgO
Used Clay-MgO
BET area (m2/g)
41.63
38.42
30.11
22.12
Pore volume (m3/g)
0.122
0.053
0.048
0.035
Average pore width (nm)
11.738
5.52
6.40
6.46
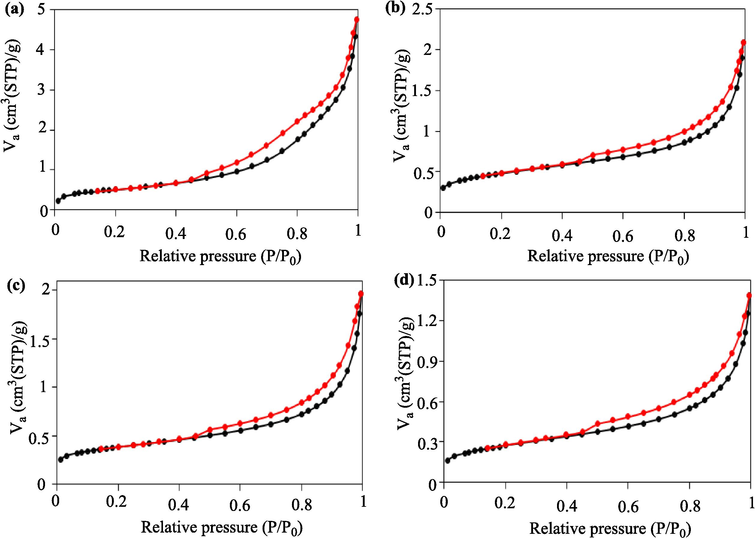
BET analysis for (a) MgO, (b) Clay, (c) fresh Clay-MgO, and (d) used Clay-MgO.
The specific surface areas of MgO, Clay, fresh Clay-MgO, and used Clay-MgO were measured as 41.63, 38.42, 30.11, and 22.12 m2/g, respectively. This decline in surface area is a clear indication of catalyst fouling during the degradation process, likely due to the accumulation of reaction byproducts within the pores. This behavior is common in oxidation processes, where byproducts can block active sites and reduce the overall surface area available for further reactions (Bai et al., 2024).
In addition, the reduction in pore volume from 0.048 m3/g in fresh Clay-MgO to 0.035 m3/g in the used sample confirms the partial clogging of the pores. This decrease correlates with the reduction in specific surface area and suggests that the catalyst's effectiveness diminishes over time due to physical obstruction of the pores by residual organics and other byproducts of the degradation process (Vaschetto et al., 2023).
Despite this, mesoporous nature of the Clay-MgO catalyst is maintained, as demonstrated by the N2 adsorption–desorption isotherms. The type IV curves observed for all samples, according to the IUPAC classification (Zhang et al., 2024; Gao et al., 2024), confirm that the material remains mesoporous, with pore diameters ranging from 2 to 50 nm (Foroutan et al., 2024; Foroughi et al., 2024). This characteristic is beneficial for drug degradation processes, as mesopores allow better diffusion of reactants and products, ensuring high catalytic activity (Lin et al., 2021; Yu et al., 2024).
Comparing the results with other studies, we observe similar trends in surface area reduction post-reaction. For example, TiO2-based catalysts often exhibit a surface area between 40–60 m2/g but suffer from similar deactivation due to pore blockage during degradation (Gaya and Abdullah, 2008). Zeolite-based catalysts, known for their high surface areas (100–300 m2/g), also experience reductions in catalytic efficiency due to agglomeration (Cundy and Cox, 2003). However, the moderate surface area of Clay-MgO coupled with its stability and cost-effectiveness makes it a competitive candidate for environmental applications.
3.2 Effect of MgO concentrations in the catalyst on the acetaminophen removal
Four Clay-MgO catalysts (10-Clay-MgO, 35-Clay-MgO, 70-Clay-MgO, and 100-Clay-MgO) was tested for acetaminophen removal based on the content of their Mg. The Mg content of these catalysts were obtained 3 %, 7 %, 9.7 %, and 11 %, respectively. As can be seen in Fig. 6, increasing the amount of magnesium in the catalyst increased the efficiency from 3 % to 7 %. For magnesium percentages above 7 %, the efficiency has not changed significantly. Therefore, the catalyst containing 7 % magnesium was selected to continue the removal of the target drug. Based on past research, increasing the amount of magnesium can increase the active area of the catalyst, and a higher range can also block the pores and lead to a decrease in surface area (Jalali et al., 2021). In two separate researches, Jalali et al. used zeolite modified with sea water ions and magnesium oxide to remove amoxicillin and found a similar process in which the increase of magnesium was useful up to a certain range and then it was useless (Jalali et al., 2022; Jalali et al., 2021).![Effect of MgO concentrations in the catalyst on the acetaminophen removal (pH: 6.8, [Acet.]: 100 mg/L, [catalyst]: 3 g/L, [H2O2]: 0.2 mL/100 mL, time: 60 min).](/content/184/2024/17/12/img/10.1016_j.arabjc.2024.106047-fig6.png)
Effect of MgO concentrations in the catalyst on the acetaminophen removal (pH: 6.8, [Acet.]: 100 mg/L, [catalyst]: 3 g/L, [H2O2]: 0.2 mL/100 mL, time: 60 min).
3.3 Effect of operational parameters
3.3.1 Solution pH
The pH level significantly impacts pollutant removal by affecting catalyst surface properties, the chemical nature of the pollutant, and reaction kinetics (Zhu et al., 2024). To investigate the density of surface charges and the effect of pH on the degradation efficiency of acetaminophen, pH was varied within the range of 5–9, and the results are depicted in Fig. 7.![(a) pHzpc for Clay-MgO catalyst and (b) Effect of pH ([Acet.]: 100 mg/L, [catalyst]: 3 g/L, [H2O2]: 0.2 mL/100 mL, time: 60 min).](/content/184/2024/17/12/img/10.1016_j.arabjc.2024.106047-fig7.png)
(a) pHzpc for Clay-MgO catalyst and (b) Effect of pH ([Acet.]: 100 mg/L, [catalyst]: 3 g/L, [H2O2]: 0.2 mL/100 mL, time: 60 min).
Fig. 7a illustrates that the point of zero charge (pHzpc) for the catalyst of interest was determined to be 7.65. This indicates that the catalyst exhibits positive superficial charges at pH values below pHzpc and negative superficial charges at pH values above pHzpc. Additionally, as shown in Fig. 7b, an increase in the initial pH of the aqueous solution from 3 to 9 resulted in a rise in the efficiency of acetaminophen degradation from 48.25 % to 75.08 %. This increase in degradation efficiency can be attributed to the pKa value of acetaminophen, which is 9.3 (Zyoud et al., 2020). At an initial pH of 9, the proportion of protonated or dissociative species is expected to be maximal (Wang et al., 2016). Since the catalyst possesses a negative superficial charge at pH values greater than 7.65, an electrostatic attraction occurs between the catalyst surface and the protonated species of acetaminophen, leading to the absorption and degradation of the drug.
Given that no significant difference was observed between the efficiencies achieved at pH 8 and 9, pH 8 was selected for subsequent experiments to mitigate the potential financial burden associated with further alkaline elevation if this method were to be implemented. Similarly, in a study by Zyoud et al. (2020) (Zyoud et al., 2020), the effectiveness of acetaminophen removal was examined using a ZnO and TiO2 catalyst. The authors reported that the efficiency of acetaminophen degradation was significantly influenced by pH, with higher pH values leading to improved removal rates. Their findings indicated that an optimal pH range, which exceeded the pHzpc of the catalyst, facilitated better pollutant adsorption and degradation. This supports our choice of pH 8 for subsequent experiments, balancing high degradation efficiency with cost considerations.
3.3.2 Quantity of Clay-MgO catalyst
The influence of Clay-MgO catalyst concentration, ranging from 0 to 4 g/L, on acetaminophen removal from aqueous solutions was examined, and the outcomes are depicted in Fig. 8a. It is evident that the efficiency of acetaminophen degradation increased from 11 % to around 74 % with an escalation in catalyst dosage from 0 to 3 g/L. However, at higher catalyst concentrations, the efficiency reached a plateau. Consequently, the optimal catalyst mass for acetaminophen oxidation was determined to be 3 g/L. The improvement in acetaminophen removal with the rise in Clay-MgO concentration can be attributed to the larger surface area, providing more active sites for interactions between hydrogen peroxide and the catalyst. This interaction facilitates the initiation and propagation of radical generation reactions, leading to improved acetaminophen degradation efficiency (Jalali et al., 2022; Kasprzyk-Hordern et al., 2003; Yaghmaeian et al., 2017). As a result, the probability of interaction between the contaminant and the release of radicals into the solution increases, leading to a higher removal efficiency. Furthermore, with the augmentation in the number of active sites on the catalyst, the degradation process becomes more prominent (Jalali et al., 2022). The efficiency of catalytic processes in eliminating pollutants depends on the quantity of catalyst available for radical formation. In this study, augmenting the catalyst dosage leads to the formation of a greater number of active sites and the production of additional hydroxyl radicals, consequently amplifying the degree of pollutant removal (Yang et al., 2019). Another point is that the extent of removal when the catalyst concentration in the solution is zero is around 11 %, which can be due to oxidation of acetaminophen with hydrogen peroxide.![Effect of (a) mass of Clay-MgO catalyst (pH: 8, [Acet.]: 100 mg/L, [H2O2]: 0.2 mL/100 mL, time: 60 min) and (b) H2O2 content (pH: 8, [Acet.]: 100 mg/L, [catalyst]: 3 g/L, [time: 60 min).](/content/184/2024/17/12/img/10.1016_j.arabjc.2024.106047-fig8.png)
Effect of (a) mass of Clay-MgO catalyst (pH: 8, [Acet.]: 100 mg/L, [H2O2]: 0.2 mL/100 mL, time: 60 min) and (b) H2O2 content (pH: 8, [Acet.]: 100 mg/L, [catalyst]: 3 g/L, [time: 60 min).
3.3.3 Quantity of H2O2
Various amounts of H2O2 (ranging from 0 to 1 mL/100 mL) were investigated for their impact on the removal of acetaminophen from aqueous solution in the presence of Clay-MgO catalyst, and the findings are illustrated in Fig. 8b. It is evident that the presence of H2O2 has a dual effect: as the concentration of this oxidant increases from 0 to 0.2 mL/100 mL, the extent of acetaminophen removal rises from 18 % to 74 %. However, beyond this concentration, the efficiency decreases with further elevation of H2O2. The enhancement in removal efficiency with an increase in H2O2 concentration can be attributed to the greater generation of oxidizing radicals, which are crucial components of the reaction. As larger amounts of H2O2 are present, the production of these radicals increases, consequently leading to higher removal efficiency (Moussavi et al., 2009). Increasing the concentration of H2O2 beyond 0.2 mL/100 mL has led to a decrease in the removal rate. This decline could be attributed to the inhibitory effect of H2O2, acting as an inhibitor and/or radical inhibitor (Suh and Mohseni, 2004). A high content of H2O2 can be absorbed onto the catalyst, and reduce the removal of pollutants, since H2O2 reacts executively with the radicals (Wang et al., 2021). Decline of removal efficiency with H2O2 elevation can also be due to the fact that it causes generation of radicals with less reactivity than hydroxyl. Indeed, excess H2O2 can contribute to removal of OH• (OH + H2O2 → HO2 + H2O•) from the solution, causing generation of HO2•, which has less oxidation potential compared to hydroxyl radicals (Deng et al., 2018). Hence, a small quantity of H2O2 contributes positively to degradation efficiency, whereas larger amounts have a detrimental effect (Bansal and Verma, 2017). Increasing the H2O2 concentration doesn't significantly contribute to system enhancement. Another noteworthy observation is that when the H2O2 concentration in the reaction solution is zero, approximately 18 % removal still occurs, possibly due to adsorption onto the catalyst.
Mojahedimotlagh et al. (2024) (Mojahedimotlagh et al., 2024) investigated the decomposition of azithromycin using H2O2 activation over a clay catalyst modified with nanofiltration brine. They observed that increasing the concentration of the clay catalyst led to higher degradation efficiencies, similar to the improvement observed in this study. However, like our results, they also noted that beyond a certain concentration, the efficiency did not increase significantly and plateaued.
3.3.4 Concentration and time
The starting concentration of the pollutant is pivotal in shaping the degradation process. In this investigation, we examined how the initial concentration of acetaminophen affects removal efficiency within the 10–300 mg/L range under optimal conditions. The removal of different drug concentrations was evaluated across contact times spanning from 5 to 100 min using the Clay-MgO/H2O2/Ultrasonic process, with the findings presented in Fig. 9.![Effect of acetaminophen concentration and time (pH: 8, [Acet.]: 100 mg/L, [catalyst]: 3 g/L, [H2O2]: 0.2 mL/100 mL).](/content/184/2024/17/12/img/10.1016_j.arabjc.2024.106047-fig9.png)
Effect of acetaminophen concentration and time (pH: 8, [Acet.]: 100 mg/L, [catalyst]: 3 g/L, [H2O2]: 0.2 mL/100 mL).
As depicted in the figure, prolonging the contact time and reducing the initial acetaminophen concentration lead to a higher level of drug degradation in the aqueous solution. Initially, when the acetaminophen concentration is high, there is a higher likelihood of contact between pollutant molecules and hydroxyl free radicals. Over time, these hydroxyl free radicals oxidize the acetaminophen metabolites (Jalali et al., 2022; Damiri et al., 2020). In catalytic oxidation processes, several factors can contribute to a decrease in pollutant removal efficiency at higher initial concentrations:
Higher pollutant concentrations may occupy a larger number of active sites on the catalyst surface, thereby inhibiting the production of oxidizing agents, primarily hydroxyl radicals.
An increase in pollutant content leads to more oxidizing agents reacting, resulting in a reduction in the amount of these oxidizing radicals and consequently lowering the removal efficiency.
With a consistent catalyst quantity employed in catalytic processes, and hence a fixed volume of OH radicals produced in the reactor, the proportion of radicals to pollutant molecules declines as the initial concentration rises. This decline results in a decrease in degradation percentage (Yaghmaeian et al., 2017).
The findings suggest that lower concentrations and longer contact times yield higher efficiency and benefits. This may also stem from an inadequate amount of radicals generated by H2O2 for pollutant removal as the concentration increases (Jalali et al., 2022; Su et al., 2012). Similar findings have also been reported for degradation of acetaminophen using TiO2 photocatalytic process (Yang et al., 2008). Chen et al. (2024) (Chen et al., 2024) investigated trimethoprim degradation using a palladium-based catalyst and self-generated hydrogen peroxide. They observed that as trimethoprim concentration increased, the pollutant removal efficiency dropped, particularly with shorter contact times. This aligns with our findings, where acetaminophen degradation also decreased at higher concentrations due to competition for catalyst active sites and limited OH radicals. However, extending the contact time improved degradation efficiency, highlighting the importance of balancing radicals with pollutant levels for optimal removal.
3.4 Kinetic study
The reaction kinetics play a pivotal role in comprehending the rate and mechanism of the conversion of reactants into products (Moradi et al., 2021). In this study, the pseudo first-order and Langmuir-Hinshelwood models (Eqs. (2), 3) was employed to assess the kinetic behavior of acetaminophen catalytic decontamination. Langmuir-Hinshelwood is the commonplace kinetic model to explain heterogeneous catalytic processes. In this model, the pollutant adsorption on the catalyst active sites is evaluated (Rajabi et al., 2022).
The amount of kobs was achieved by scheming
versus t in the different concentrations that are shown in Table 4.
[Acet.]0 (mg/L)
R2
kobs (1/min)
Line equation
10
0.9738
0.0906
y = -0.1176x + 0.135
50
0.9673
0.0253
y = -0.0144x − 0.3596
100
0.9861
0.0184
y = -0.0203x − 0.4133
200
0.9897
0.0118
y = -0.0118x − 0.2433
300
0.9724
0.0096
y = -0.0096x − 0.199
After that, a linear equation was obtained by plotting the curve 1/kobs against the acetaminophen initial concentration and by using it, kc and kL-H values were calculated (Fig. 10). Based on the achieved results the amounts of kL-H and kc were 0.52 L/mg and 1.79 mg/L.min, respectively, and it was shown that degradation of the acetaminophen follows Langmuir-Hinshelwood and pseudo-first-order kinetics. In a study, metronidazole removal using CuCoFe2O4@MC/AC catalyst follows pseudo-first-order and Langmuir-Hinshelwood kinetics (Rajabi et al., 2022). In a study by Jalali et al. (Jalali et al., 2022), degradation of amoxicillin from wastewater using Zeolite Y-MgO catalyst in the presence of hydrogen peroxide had followed pseudo-first-order kinetics.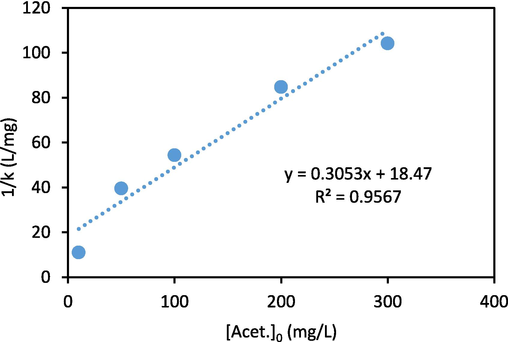
Langmuir-Hinshelwood kinetic curve.
3.5 Comparison of the performance process with other processes
The results of applying the developed method in this paper for the removal of pharmaceutical contaminants are compared with other published works in Table 5. Comparing different studies with varying laboratory conditions is very challenging. Factors such as the type of contaminant, catalyst dosage, reaction time, reactor conditions, radical scavengers, catalyst levels, and the presence of enhancers like light or ultrasonic waves all affect the removal efficiency. Although the efficiency of this study is lower than that of many other works, the working conditions such as reaction time and catalyst dosage are relatively lower, which can be an advantage.
Catalyst
Pollutant
Initial Conc. (mg/L)
Catalyst quantity (g/L)
Time (min)
H2O2
Efficiency (%)
Kinetic model
Ref.
Ultrasonic wave/Clay-MgO
Acetaminophen
50
3
60
2 mL/L
81.8
Pseudo first-order and Langmuir-Hinshelwood
This work
Sea sediment-titanate/H2O2/US/UV
Cephalexin
100
1.5
100
1.63 mg/L
87.01
first-order
(Tavasol et al., 2021)
Sonication/ultraviolet/ H2O2/zeolite-titanate photocatalyst system
Ibuprofen
100
1
100
0.05 mM
99
first-order
(Jalali et al., 2022)
Catalyst/H2O2/ultrasound
Amoxicillin
5
4.5
60
50 mM
98
first-order
(Damiri et al., 2020)
Fly ash/UV/H2O2
Amoxicillin
50
−
240
10 mM
36.1
−
(Ramírez-Franco et al., 2019)
Magnesium oxide nanocatalyst/ultrasound
Amoxicillin
11
1.8
80
−
84.1
−
(Soltani et al., 2018)
AGCu-hsCN
Acetaminophen
20
0.2
180
1000 ppm
94.8
first-order
(Tian et al., 2023)
3.6 Removal of acetaminophen and TOC from different environments
Fig. 11 illustrates the removal percentage of acetaminophen and TOC in various solutions, including distilled water, wastewater, urban piping systems, and seawater. It is evident that the extent of acetaminophen removal in distilled water, urban piping systems, wastewater, and seawater is approximately 75 %, 53 %, 44 %, and 35 %, respectively. The corresponding percentages for TOC removal (mineralization) in these waters are approximately 42 %, 30 %, 20 %, and 13 %. Distilled water and seawater exhibit the highest and lowest efficiencies in acetaminophen and TOC removal, respectively. The decreased efficiency in TOC and drug removal from seawater and wastewater compared to other environments may be attributed to the presence of interfering factors and radical scavengers (Stucchi et al., 2018). The findings of this study have been confirmed with the study done for removal of amoxicillin from wastewater with activation of H2O2 through zeolite catalyst (Jalali et al., 2022). As depicted in this figure, the percentage of TOC removal has exceeded that of acetaminophen removal in distilled water and tap water. This variance in the removal efficiency of TOC and acetaminophen suggests the presence of intermediate compounds generated during the degradation process of the pollutant. Conversely, in wastewater and seawater, the efficiency of TOC removal has been lower than that of acetaminophen removal. This reduced value can be attributed to the presence of additional organic agents, aside from acetaminophen, in wastewater and seawater (Wang et al., 2018; Moradi et al., 2020).![The acetaminophen and TOC removal by Ultrasonic wave/Clay-MgO/H2O2 system in different aqueous medium (pH: 8, [Acet.]: 100 mg/L, [catalyst]: 3 g/L, [H2O2]: 0.2 mL/100 mL, time: 60 min).](/content/184/2024/17/12/img/10.1016_j.arabjc.2024.106047-fig11.png)
The acetaminophen and TOC removal by Ultrasonic wave/Clay-MgO/H2O2 system in different aqueous medium (pH: 8, [Acet.]: 100 mg/L, [catalyst]: 3 g/L, [H2O2]: 0.2 mL/100 mL, time: 60 min).
3.7 Reusability and stability
Reusability and stability are important parameters for use of catalysts under real conditions, and provide the possibility of using catalysts in the long term (Tang et al., 2018). To assess catalyst reusability, it was subjected to six consecutive cycles under optimal conditions, and the results are depicted in Fig. 12. It can be observed that the efficiency of acetaminophen degradation decreased as the catalyst was used in more cycles. After six cycles of catalyst recovery, the efficiency reached approximately 50 %. The outcomes of multiple catalyst uses suggest its stability and potential applicability in the water and wastewater industry. The reduction in efficiency could be attributed to several factors, including deactivation of some active sites of the nanocomposite due to reactions with the pollutant or by-products, loss of some nanocomposites during the collection and drying process, and release of certain ions (Ghosh et al., 2017). In other words, the decline of the catalyst efficiency with increase in the consumption cycle can be due to leakage of its vital components into the treated solution and damage to the active sites (Jalali et al., 2022). Empirical observations of Dutta et al. (Dutta and Ray, 2004) indicated that intermediate products which are absorbed strongly on the catalyst, occupy the active sites on the catalyst surface, leading to diminished catalytic degradation.![The reusability of the Clay-MgO catalyst for acetaminophen removal (pH: 8, [Acet.]: 100 mg/L, [catalyst]: 3 g/L, [H2O2]: 0.2 mL/100 mL, time: 60 min).](/content/184/2024/17/12/img/10.1016_j.arabjc.2024.106047-fig12.png)
The reusability of the Clay-MgO catalyst for acetaminophen removal (pH: 8, [Acet.]: 100 mg/L, [catalyst]: 3 g/L, [H2O2]: 0.2 mL/100 mL, time: 60 min).
An essential factor in the application of heterogeneous catalysts is the stability of their structural integrity during repeated cycles. In this study, we evaluated the durability of the magnesium-clay catalyst, specifically focusing on the retention of magnesium as the active component (Fig. 12). Our experiments revealed that with increasing catalytic cycles, a progressive leaching of magnesium into the treated solution occurred. After five cycles, the concentration of leached magnesium reached a critical threshold, indicating substantial degradation of catalytic efficiency. Beyond five cycles, further use of the catalyst resulted in a significant decline in performance, attributed to the depletion of this essential component. These findings suggest that while the magnesium-clay catalyst demonstrates initial effectiveness in conjunction with H2O2 and ultrasonic waves for acetaminophen degradation, its practical application is limited by the gradual loss of active material, necessitating regeneration or replacement after five cycles to maintain optimal performance.
To investigate the structure of Clay/MgO after fifth cycle of the degradation process, SEM analysis with 1 µm and 200 nm magnification was used so that the layers and the empty space between the particles can be clearly seen (Fig. 13). As the results show, after the degradation and regeneration process, the desired catalyst has a layered structure with pores that can be suitable for absorbing and destroying the desired pollutant.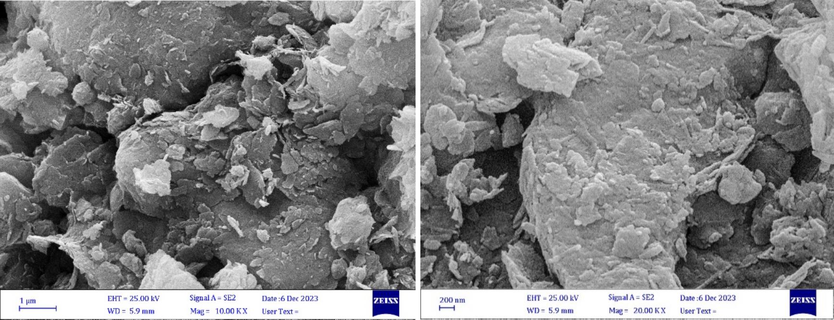
SEM images with magnifications of (a) 1 µm and (b) 200 nm for the desired catalyst after five times reuse.
3.8 Effect of system components on acetaminophen elimination
The efficiency of acetaminophen elimination using various components of the treatment process employed in this study (including Clay-MgO alone, H2O2 alone, ultrasonic waves alone, ultrasonic/H2O2/MgO, ultrasonic/H2O2/Clay, ultrasonic/H2O2/Clay-MgO) was examined, and the results are illustrated in Fig. 14a. It can be observed that the adsorption of acetaminophen onto Clay-MgO particles had minimal impact on its removal from aqueous media, with the maximum removal percentage being around 10–12 %.![(a) The effect of system components on acetaminophen removal (pH: 8, [Acet.]: 100 mg/L, [catalyst]: 3 g/L, [H2O2]: 0.2 mL/100 mL, time: 60 min) and (b) The effect scavengers on acetaminophen removal by the developed system (pH: 8, Acet. concentration: 100 mg/L, catalyst dose: 3 g/L, [H2O2]: 2 mL/100 mL, time: 60 min).](/content/184/2024/17/12/img/10.1016_j.arabjc.2024.106047-fig14.png)
(a) The effect of system components on acetaminophen removal (pH: 8, [Acet.]: 100 mg/L, [catalyst]: 3 g/L, [H2O2]: 0.2 mL/100 mL, time: 60 min) and (b) The effect scavengers on acetaminophen removal by the developed system (pH: 8, Acet. concentration: 100 mg/L, catalyst dose: 3 g/L, [H2O2]: 2 mL/100 mL, time: 60 min).
Furthermore, the maximum removal of acetaminophen for H2O2 alone and ultrasonic waves alone was less than 10 %. These findings suggest that Clay-MgO is not an effective adsorbent for acetaminophen and direct oxidation with H2O2 and ultrasonic waves alone is also ineffective. Hydrogen peroxide (H2O2) serves as a source of hydroxyl radicals under the right conditions. In the presence of ultrasonic waves and/or catalysts, H2O2 undergoes homolytic cleavage to generate •OH radicals: H2O2 → 2 •OH(4)
However, as observed in the experiments, using H2O2 alone was not effective because the activation energy required for the efficient generation of hydroxyl radicals was not met without the aid of ultrasound or a catalyst.
Ultrasound generates cavitation bubbles in the aqueous solution, which collapse and produce localized high temperatures and pressures (Yuan et al., 2024; Xie et al., 2024). This intense energy is sufficient to break chemical bonds in water and hydrogen peroxide, leading to the formation of hydroxyl radicals (Nasiri et al., 2021): H2O → •OH + H•(5)
The radicals generated via sonolysis enhance the degradation of acetaminophen, though ultrasound alone is not highly efficient due to the limited concentration of •OH radicals (Malakootian et al., 2020).
MgO is a metal oxide catalyst that plays a crucial role in enhancing •OH radical production by acting as an electron donor or acceptor in heterogeneous catalysis. MgO particles catalyze the decomposition of H2O2 to produce more hydroxyl radicals through the following reaction: H2O2 + MgO → 2 •OH(6)
Additionally, MgO may provide surface active sites that stabilize intermediates during acetaminophen oxidation, which facilitates pollutant degradation.
In addition, the removal efficiency of acetaminophen using the Ultrasonic/H2O2/Clay process increased to approximately 40 %. This indicates the positive contribution of Clay to the removal of acetaminophen from aqueous environments, possibly due to its catalytic and adsorption properties. However, a removal efficiency of 40 % is not considered highly effective for pollutant removal from aqueous media, and this low efficiency is likely attributed to the low concentration of •OH radicals formed under the experimental conditions.
By employing Ultrasonic/H2O2/MgO and Ultrasonic/H2O2/Clay-MgO, the efficiency of acetaminophen removal significantly increased, reaching 70 % and 75 %, respectively. This can be attributed to the presence of MgO particles, which induce and accelerate the formation of oxidizing radicals in the process, likely due to the catalytic potential of MgO particles (Yaghmaeian et al., 2017; Du et al., 2016). These observations also suggest that MgO coating aspects on clay and its usage in pharmaceutical pollutant degradation such as acetaminophen have been well effective. In addition, by applying Clay-supported MgO catalyst plus hydrogen peroxide (H2O2), the oxidizing properties of the process have also improved (Bilal et al., 2022; Chen et al., 2011).
The combination of clay and MgO has a synergistic effect on acetaminophen removal. The clay acts as an adsorbent, providing a surface where acetaminophen can accumulate, which increases its local concentration near active MgO catalytic sites. The MgO coating enhances the clay's catalytic properties, increasing the generation of •OH radicals and accelerating oxidation. The combined processes result in more efficient pollutant breakdown: Acetaminophen + •OH → Degradation Products(7)
This synergistic effect explains the higher removal efficiency (∼75 %) observed when Clay-MgO, H2O2, and ultrasonic waves are used together.
3.9 Radical scavenger study
Fig. 14b illustrates the influence of various radical scavengers, including tert-butanol (OH radical scavenger), p-benzoquinone (O2– radical scavenger), silver nitrate (e- scavenger), and ammonium oxalate (h+ radical scavenger), on the acetaminophen degradation process. Specifically, 5 mM of each scavenger was added to the reaction medium under optimal conditions, and the efficiency of acetaminophen removal was measured.
It is evident that the acetaminophen removal efficiency under optimal conditions, which was approximately 74 % without adding radical scavengers, decreased to 57 %, 14 %, 11.6 %, and 7 % in the presence of tert-butanol, p-benzoquinone, silver nitrate, and ammonium oxalate, respectively. These results highlight the significant inhibitory effect of p-benzoquinone, silver nitrate, and ammonium oxalate. It suggests that superoxide and hydroxyl radicals play a crucial role in the decomposition process of pollutants in the studied process (Maktedar et al., 2017). The great impact on superoxide radical reveals that dissolved oxygen plays a central role in acetaminophen degradation process (Zeng et al., 2023). Chen et al. (Chen et al., 2022) reported that alcohols like isopropyl (IPA) and methanol can cause absorption of hydroxyl radicals. Also, Huang et al. (Hwang et al., 2010) stated that isopropyl alcohol can absorb OH radicals.
3.10 Acetaminophen degradation pathway
In this investigation, after 60 min, approximately 74 % of acetaminophen was eliminated through the Clay-MgO/US/H2O2 process, indicating its efficacy in removing pharmaceutical contaminants. In this phase of the research, the potential pathway of acetaminophen degradation and its resulting byproducts was elucidated using a GC-Mass device by analyzing the sample treated under optimal conditions (see Fig. 15).
A possible degradation pathway of Acetaminophen and its intermediates in H2O2/Ultrasonic/Clay-MgO.
As depicted, upon the attack of oxidizing agents and free radicals (such as hydroxyl) at one of the carbon positions within the acetaminophen structure, byproducts such as 2-hydroxy-4-(N-acetyl)aminophenol, 1,4-benzoquinone, and acetamide are generated. The formation of these intermediate products in response to acetaminophen degradation in aqueous solutions has also been documented in previous studies (Yang et al., 2008; Le et al., 2017). Among these three intermediate products, including 2-hydroxy-4-(N-acetyl)aminophenol and 1,4-benzoquinone, they still retain a benzene ring in their structure. Under the influence of degrading agents, they transform into products such as acetamide, benzaldehyde, and benzoic acid. Benzaldehyde and benzoic acid are also products containing a benzene ring. Subsequently, the benzene structure of these by-products undergoes further breakdown, leading to the formation of a series of aliphatic organic acids (Fig. 15), while acetamide is converted to oxalic acid. Ultimately, with the progression of the oxidation process, these organic acids with a linear structure are transformed into mineral ions such as nitrate and ammonium, along with the final by-products of oxidation, namely water and carbon dioxide.
4 Conclusions
In this research, clay soil underwent modification with magnesium oxide to serve as a catalyst alongside hydrogen peroxide and ultrasonic waves for removing acetaminophen from wastewater. Key parameters affecting the catalyst's efficacy, such as optimal pH, catalyst dosage, H2O2 concentration, and acetaminophen concentration, were initially explored. The physiochemical properties of the catalyst were characterized using TGA, XRD, SEM, FTIR, and EDX. The crystalline catalyst exhibited a BET surface area of 30 m2/g and featured functional groups suitable for activating hydrogen peroxide. Under optimal conditions, the removal efficiency reached approximately 75 %. Notably, the catalyst maintained its effectiveness over six cycles of reuse. Radical scavenger studies highlighted the pivotal role of hydroxyl radicals in acetaminophen degradation. The catalyst demonstrated efficient removal of acetaminophen from complex solutions, including hospital wastewater, tap water, and seawater. The degradation process followed which followed pseudo-first-order and Langmuir-Hinshelwood models. Hence, MgO-modified clay proves to be a promising candidate for hydrogen peroxide activation and removal of pharmaceutical pollutants.
CRediT authorship contribution statement
Roshana Rashidi: Writing – original draft, Methodology, Investigation, Formal analysis. Seyed Hamed Meraji: Supervision, Investigation, Conceptualization. Amin Mahmoudi: Methodology, Investigation, Conceptualization. Ali Mohammad Sanati: Supervision, Methodology, Investigation, Formal analysis, Conceptualization. Bahman Ramavandi: Writing – review & editing, Methodology, Investigation, Conceptualization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Assessing the treatment of acetaminophen-contaminated brewery wastewater by an anaerobic packed-bed reactor. J. Environ. Manage.. 2016;168:273-279.
- [Google Scholar]
- Removal of acetaminophen from hospital wastewater using electro-Fenton process. Environ. Earth Sci.. 2018;77:53.
- [Google Scholar]
- Review of antibiotics treatment by advance oxidation processes. Environ. Adv.. 2021;5:100111
- [Google Scholar]
- Occurrence of pharmaceuticals and personal care products in domestic wastewater, available treatment technologies, and potential treatment using constructed wetland: A review. Process Saf. Environ. Prot.. 2022;168:1067-1088.
- [Google Scholar]
- Investigating COVID-19 active pharmaceutical ingredients (APIs) degradation using Peroxydisulfate/FeMnOx binary metal oxide/Ultrasound System. Water Resour. Ind.. 2024;31:100232
- [Google Scholar]
- The remediation efficiency of heavy metal pollutants in water by industrial red mud particle waste. Environ. Technol. Innov.. 2022;28:102944
- [Google Scholar]
- A high-strength red mud–fly ash geopolymer and the implications of curing temperature. Powder Technol.. 2023;416:118242
- [Google Scholar]
- The migration process and temperature effect of aqueous solutions contaminated by heavy metal ions in unsaturated silty soils. Heliyon. 2024;10
- [Google Scholar]
- Migration trajectories and blocking effect of the fine particles in porous media based on particle flow simulation. AIP Adv.. 2024;14
- [Google Scholar]
- Corrosion effect of acid/alkali on cementitious red mud-fly ash materials containing heavy metal residues. Environ. Technol. Innov.. 2024;33:103485
- [Google Scholar]
- Synergistic effect of dual process (photocatalysis and photo-Fenton) for the degradation of Cephalexin using TiO2 immobilized novel clay beads with waste fly ash/foundry sand, Journal of Photochemistry Photobiology A. Chemistry. 2017;342:131-142.
- [Google Scholar]
- Detailed study of Safranin-O adsorption on sepiolite clay: Kinetics, thermodynamics, isotherms and theoretical calculations for optimal water treatment efficiency. J. Mol. Struct.. 2024;1308:138130
- [Google Scholar]
- Magnetic chitosan/clay beads: A magsorbent for the removal of cationic dye from water. J. Magn. Magn. Mater.. 2017;421:59-64.
- [Google Scholar]
- Eco-friendly synthesis of clay-chitosan composite for efficient removal of alizarin red S dye from wastewater: A comprehensive experimental and theoretical investigation. Environ. Res.. 2024;247:118352
- [Google Scholar]
- Cationic and anionic dyes adsorption from wastewater by clay-chitosan composite: An integrated experimental and modeling study. Chem. Eng. Sci.. 2024;285:119615
- [Google Scholar]
- Hydrogen-based catalyst-assisted advanced oxidation processes to mitigate emerging pharmaceutical contaminants. Int. J. Hydrogen Energy. 2022;47:19555-19569.
- [Google Scholar]
- Applicability and characterization of the natural Moroccan clay for cationic dye removal: kinetic, Isotherms studies. In: And Application on Effluent Treatmentf for Textile Dyeing. Progress in Engineering Science; 2024. p. :100001.
- [Google Scholar]
- Degradation of pesticides by heterogeneous Fenton using iron-exchanged clays. Catal. Commun.. 2023;183:106771
- [Google Scholar]
- Comprehensive evaluation of antibiotic tetracycline and oxytetracycline removal by Fe-metal organic framework/biopolymer-clay hydrogel. Ceram. Int.. 2023;49:12201-12213.
- [Google Scholar]
- Durability evaluation of a high-performance red mud-based composite material. Mater. Today Commun.. 2024;39:108684
- [Google Scholar]
- Heterogeneous activation of self-generated H2O2 by Pd@UiO-66(Zr) for trimethoprim degradation: Efficiency and mechanism. J. Environ. Manage.. 2024;366:121868
- [Google Scholar]
- Removal of tetracycline from aqueous solutions using polyvinylpyrrolidone (PVP-K30) modified nanoscale zero valent iron. J. Hazard. Mater.. 2011;192:44-53.
- [Google Scholar]
- Enhanced UV photoreductive destruction of perfluorooctanoic acid in the presence of alcohols: Synergistic mechanism of hydroxyl radical quenching and solvent effect. Appl Catal B. 2022;316:121652
- [Google Scholar]
- Fate of pharmaceutical residue in two Romanian rivers receiving treated water: Occurrence, distribution and risk assessment. Sci. Total Environ.. 2024;923:171359
- [Google Scholar]
- The hydrothermal synthesis of zeolites: history and development from the earliest days to the present time. Chem Rev. 2003;103:663-702.
- [Google Scholar]
- Waste sludge from shipping docks as a catalyst to remove amoxicillin in water with hydrogen peroxide and ultrasound. Ultrason. Sonochem.. 2020;68:105187
- [Google Scholar]
- Waste sludge from shipping docks as a catalyst to remove amoxicillin in water with hydrogen peroxide and ultrasound. Ultrason. Sonochem.. 2020;68:105187
- [Google Scholar]
- Fenton-like catalyst based on a reticulated porous perovskite material: Activity and stability for the on-site removal of pharmaceutical micropollutans in a hospital wastewater. Chem. Eng. J.. 2020;401:126113
- [Google Scholar]
- Nanoscale zero-valent iron/biochar composite as an activator for Fenton-like removal of sulfamethazine. Sep. Purif. Technol.. 2018;202:130-137.
- [Google Scholar]
- Water security assessment for effective water resource management based on multi-temporal blue and green water footprints. J. Hydrol.. 2024;632:130761
- [Google Scholar]
- Efficient activation of peroxymonosulfate by magnetic Mn-MGO for degradation of bisphenol A. J. Hazard. Mater.. 2016;320:150-159.
- [Google Scholar]
- Experimental investigation of Taylor vortex photocatalytic reactor for water purification. Chem. Eng. Sci.. 2004;59:5249-5259.
- [Google Scholar]
- Adsorption of phosphate ions from aqueous solution by modified bentonite with magnesium hydroxide Mg (OH) 2. Appl. Clay Sci.. 2017;140:157-164.
- [Google Scholar]
- Co(II)-pyrophyllite as Catalyst for Phenol Oxidative Degradation: Optimization Study Using Response Surface Methodology. Waste Biomass Valoriz.. 2019;10:1043-1051.
- [Google Scholar]
- Catalytic Efficiency of Cu-Supported Pyrophyllite in Heterogeneous Catalytic Oxidation of Phenol. Arab. J. Sci. Eng.. 2019;44:6313-6325.
- [Google Scholar]
- Sol–gel fluorinated TiO2–clay nanocomposite: study of fluor-titanium interaction on the photodegradation of phenol. Res. Chem. Intermed.. 2021;47:5203-5228.
- [Google Scholar]
- Acute effects of acetaminophen on the developmental, swimming performance and cardiovascular activities of the African catfish embryos/larvae (Clarias gariepinus) Ecotoxicol. Environ. Saf.. 2021;208:111482
- [Google Scholar]
- Development of a new CoS-Supported ZnAl2O4 catalyst for the visible photodegradation of a basic textile dye from water. Opt. Mater.. 2023;143:114148
- [Google Scholar]
- Electrochemical degradation of acetaminophen in urine matrices: Unraveling complexity and implications for realistic treatment strategies. Water Res.. 2024;261:122034
- [Google Scholar]
- Simultaneous degradation of methyl orange and indigo carmine dyes from an aqueous solution using nanostructured WO3 and CuO supported on Zeolite 4A. Sep. Purif. Technol.. 2024;344:127265
- [Google Scholar]
- Simultaneous anionic dyes degradation via H2O2 activation using Zeolite 4A/ZnO/Fe2(MoO4)3 nanoparticles in a sono-photocatalytic process. Adv. Powder Technol.. 2024;35:104320
- [Google Scholar]
- One-pot transesterification of non-edible Moringa oleifera oil over a MgO/K2CO3/HAp catalyst derived from poultry skeletal waste. Environ. Technol. Innov.. 2021;21:101250
- [Google Scholar]
- Evaluation of two cationic dyes removal from aqueous environments using CNT/MgO/CuFe2O4 magnetic composite powder: A comparative study. J. Environ. Chem. Eng.. 2021;9:104752
- [Google Scholar]
- Amendment of Sargassum oligocystum bio-char with MnFe2O4 and lanthanum MOF obtained from PET waste for fluoride removal: A comparative study. Environ. Res.. 2024;251:118641
- [Google Scholar]
- B. Gao, F. Bi, Z. Zhou, Y. Zhang, J. Wei, X. Lv, B. Liu, Y. Huang, X. Zhang, A bimetallic MOF-derived MnCo spinel oxide catalyst to enhance toluene catalytic degradation††Electronic supplementary information (ESI) available: Experimental section and supporting figures and tables. See DOI: https://doi.org/10.1039/d4cc01732c, Chemical Communications, 60 (2024) 7455-7458.
- Graphene-based aerogels in water and air treatment: A review. Chem. Eng. J.. 2024;484:149604
- [Google Scholar]
- The challenge of improving the efficiency of drinking water treatment systems in rural areas facing changes in the raw water quality. S. Afr. J. Chem. Eng.. 2021;37:141-149.
- [Google Scholar]
- Intensification strategies for thermal H2O2-based advanced oxidation processes: current trends and future perspectives. Chemical Engineering Journal Advances. 2022;9:100228
- [Google Scholar]
- Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J Photochem Photobiol C: Photochem Rev. 2008;9:1-12.
- [Google Scholar]
- Preparation of TiO2/cobalt ferrite/reduced graphene oxide nanocomposite based magnetically separable catalyst with improved photocatalytic activity, Journal of Nanoscience. Nanotechnology. 2017;17:4694-4703.
- [Google Scholar]
- C-modified TiO2 using lignin as carbon precursor for the solar photocatalytic degradation of acetaminophen. Chem. Eng. J.. 2019;358:1574-1582.
- [Google Scholar]
- Excellent microwave absorption performance of LaFeO3/Fe3O4/C perovskite composites with optimized structure and impedance matching. Carbon. 2023;213:118200
- [Google Scholar]
- Fenton-like degradation of MTBE: Effects of iron counter anion and radical scavengers. Chemosphere. 2010;78:563-568.
- [Google Scholar]
- Removal of amoxicillin from wastewater in the presence of H2O2 using modified zeolite Y- MgO catalyst: An optimization study. Chemosphere. 2021;274:129844
- [Google Scholar]
- Elimination of amoxicillin using zeolite Y-sea salt as a good catalyst for activation of hydrogen peroxide: Investigating degradation pathway and the effect of wastewater chemistry. J. Environ. Manage.. 2022;302:114045
- [Google Scholar]
- Application of ball Clay/MnO2 based catalytic ozonation process for textile wastewater treatment. Desalin. Water Treat.. 2024;318:100394
- [Google Scholar]
- Synergistic mechanism of Ce-Mn in ZSM-5 carrier catalysts for catalytic oxidation of toluene. Fuel. 2023;342:127921
- [Google Scholar]
- Occurrence, distribution, and potential sources of antibiotics pollution in the water-sediment of the northern coastline of the Persian Gulf, Iran. Sci. Total Environ.. 2018;627:703-712.
- [Google Scholar]
- Catalytic ozonation and methods of enhancing molecular ozone reactions in water treatment. Appl Catal B. 2003;46:639-669.
- [Google Scholar]
- Preparation and characterization of “green” hybrid clay-dye nanopigments. J. Phys. Chem. Solid. 2015;78:95-100.
- [Google Scholar]
- Removal of p-aminobenzenesulfanilamide from water solutions by catalytic photo-oxidation over Fe-pillared clay. Water Res.. 2020;185:116212
- [Google Scholar]
- Oxidative degradation of sulfanilamide catalyzed by Fe/Cu/Al-pillared clays. Appl. Clay Sci.. 2017;146:92-99.
- [Google Scholar]
- River water treatment using electrocoagulation for removal of acetaminophen and natural organic matter. Chemosphere. 2021;273:128571
- [Google Scholar]
- Surface confinement of per-fluoroalkyl substances on an iron-decorated clay-cyclodextrin composite enables rapid oxidation by hydroxyl radicals. Chem. Eng. J.. 2022;431:134187
- [Google Scholar]
- Adsorptive removal of both cationic and anionic dyes by using sepiolite clay mineral as adsorbent: Experimental and molecular dynamic simulation studies. J. Mol. Liq.. 2020;318:114247
- [Google Scholar]
- Metabolomic analysis predicted changes in growth rate in Daphnia magna exposed to acetaminophen. Aquat. Toxicol.. 2022;249:106233
- [Google Scholar]
- Correlation between degradation pathway and toxicity of acetaminophen and its by-products by using the electro-Fenton process in aqueous media. Chemosphere. 2017;172:1-9.
- [Google Scholar]
- Evaluation of phosphate adsorption on zirconium/magnesium-modified bentonite. Environ. Technol.. 2020;41:586-602.
- [Google Scholar]
- Fenton/Fenton-like processes with in-situ production of hydrogen peroxide/hydroxyl radical for degradation of emerging contaminants: Advances and prospects. J. Hazard. Mater.. 2021;404:124191
- [Google Scholar]
- Catalytic property of Fe-Al pillared clay for Fenton oxidation of phenol by H2O2. Appl Catal B. 2009;85:201-206.
- [Google Scholar]
- Review of irradiation treatments on MOFs and COFs: Synthesis, modification, and application. Sep. Purif. Technol.. 2024;339:126636
- [Google Scholar]
- Ultrasound assisted simultaneous reduction and direct functionalization of graphene oxide with thermal and cytotoxicity profile. Ultrason. Sonochem.. 2017;34:856-864.
- [Google Scholar]
- Synthesis and stabilization of ZnO nanoparticles on a glass plate to study the removal efficiency of acid red 18 by hybrid advanced oxidation process ultraviolet/ZnO/ultrasonic. Desalin. Water Treat.. 2019;170:325-336.
- [Google Scholar]
- Efficiency of novel Fe/charcoal/ultrasonic micro-electrolysis strategy in the removal of Acid Red 18 from aqueous solutions. J. Environ. Chem. Eng.. 2020;8:103553
- [Google Scholar]
- Silane modified clay for enhanced dye pollution adsorption in water. Results Surf. Interfaces. 2024;14:100183
- [Google Scholar]
- Azithromycin decomposition from simple and complex waters by H2O2 activation over a recyclable catalyst of clay modified with nanofiltration process brine. Environ. Technol. Innov.. 2024;33:103512
- [Google Scholar]
- A review on pollutants removal by Sono-photo-Fenton processes. J. Environ. Chem. Eng.. 2020;8:104330
- [Google Scholar]
- Ultrasound-assisted synthesis of FeTiO3/GO nanocomposite for photocatalytic degradation of phenol under visible light irradiation. Separation Purification Technology. 2021;261:118274
- [Google Scholar]
- The removal of formaldehyde from concentrated synthetic wastewater using O3/MgO/H2O2 process integrated with the biological treatment. J. Hazard. Mater.. 2009;171:907-913.
- [Google Scholar]
- Copper oxide-incorporated pillared clay granular nanocomposite for efficient single and binary, batch and fixed bed column adsorption of levofloxacin and crystal violet. Chem. Eng. Sci.. 2024;295:120184
- [Google Scholar]
- CoFe2O4@Methylcelloluse as a New Magnetic Nano Biocomposite for Sonocatalytic Degradation of Reactive Blue 19. J. Polym. Environ.. 2021;29:2660-2675.
- [Google Scholar]
- A critical review on paracetamol removal from different aqueous matrices by Fenton and Fenton-based processes, and their combined methods. Chemosphere. 2022;303:134883
- [Google Scholar]
- Synergistic degradation of antibiotic sulfamethazine by novel pre-magnetized Fe0/PS process enhanced by ultrasound. Chem. Eng. J.. 2018;354:777-789.
- [Google Scholar]
- Electrochemical oxidation of paracetamol in water by graphite anode: Effect of pH, electrolyte concentration and current density. J. Environ. Chem. Eng.. 2018;6:7358-7367.
- [Google Scholar]
- Enhanced activation of persulfate by CuCoFe2O4@MC/AC as a novel nanomagnetic heterogeneous catalyst with ultrasonic for metronidazole degradation. Chemosphere. 2022;286:131872
- [Google Scholar]
- Fly ash as photo-Fenton catalyst for the degradation of amoxicillin. J. Environ. Chem. Eng.. 2019;7:103274
- [Google Scholar]
- Ni doped zinc oxide nanoparticles supported bentonite clay for photocatalytic degradation of anionic and cationic synthetic dyes in water treatment. J. Photochem. Photobiol. A Chem.. 2022;431:113947
- [Google Scholar]
- Ultrasound-assisted removal of Imidacloprid from aqueous solutions using carboxymethyl cellulose-based bionanocomposite hydrogel beads (CMC/Fe3O4-Zeolite): Emphasis on effects Fe3O4-Zeolite nanoparticles and ultrasound, Journal of Environmental. Chem. Eng.. 2024;12:112281
- [Google Scholar]
- Iron-TiO2 pillared clay nanocomposites: Eco-friendly solutions for photocatalytic removal of organic and pathogen contaminants. Inorg. Chem. Commun.. 2024;160:111923
- [Google Scholar]
- Photocatalytic and antibacterial activity of green synthesized and immobilized zinc oxide nanoparticles for the removal of sulfadiazine and acetaminophen: Effect of operational parameters and degradation pathway. J. Environ. Chem. Eng.. 2024;12:112649
- [Google Scholar]
- Novel MgO and Ag/MgO nanoparticles green-synthesis for antibacterial and photocatalytic applications: A kinetics-mechanism & recyclability. J. Photochem. Photobiol. A Chem.. 2024;449:115398
- [Google Scholar]
- Membrane processes used for removal of pharmaceuticals, hormones, endocrine disruptors and their metabolites from wastewaters: a review. Desalin. Water Treat.. 2016;57:24146-24175.
- [Google Scholar]
- Increasing efficiency and treatment volumes for sonolysis of per- and poly-fluorinated substances, applied to aqueous film-forming foam. Ultrason. Sonochem.. 2024;105:106866
- [Google Scholar]
- Hybrid sonocatalysis/electrolysis process for intensified decomposition of amoxicillin in aqueous solution in the presence of magnesium oxide nanocatalyst. J. Ind. Eng. Chem.. 2018;64:373-382.
- [Google Scholar]
- Ultra-sonic assisted synthesis of nitrogen rich-g-C3N5 @MoO3 Z-scheme system for efficient removal of acetaminophen in aqueous environment via photo- and electro-catalytically. J. Alloy. Compd.. 2023;957:170249
- [Google Scholar]
- Water treatment: Mn-TiO2 synthesized by ultrasound with increased aromatics adsorption. Ultrason. Sonochem.. 2018;44:272-279.
- [Google Scholar]
- Degradation of amoxicillin in aqueous solution using sulphate radicals under ultrasound irradiation. Ultrason. Sonochem.. 2012;19:469-474.
- [Google Scholar]
- A study on the relationship between biodegradability enhancement and oxidation of 1, 4-dioxane using ozone and hydrogen peroxide. Water Res.. 2004;38:2596-2604.
- [Google Scholar]
- A TiO2 nanotube photoanode (blue TiO2 nanotube/RuO2/BiVO4) for efficient acetaminophen degradation and nitrogen removal. J. Water Process Eng.. 2024;60:105230
- [Google Scholar]
- Application of the white-rot fungus Trametes sp. (C3) laccase in the removal of acetaminophen from aqueous solutions. J. Water Process Eng.. 2024;57:104677
- [Google Scholar]
- Microwave-assisted preparation of ZnFe2O4@ methyl cellulose as a new nano-biomagnetic photocatalyst for photodegradation of metronidazole. Int. J. Biol. Macromol.. 2020;154:1036-1049.
- [Google Scholar]
- Construction of novel Z-scheme Ag/FeTiO3/Ag/BiFeO3 photocatalyst with enhanced visible-light-driven photocatalytic performance for degradation of norfloxacin. Chem. Eng. J.. 2018;351:1056-1066.
- [Google Scholar]
- Comparative overview of advanced oxidation processes and biological approaches for the removal pharmaceuticals. J. Environ. Manage.. 2021;288:112404
- [Google Scholar]
- Design a new photocatalyst of sea sediment/titanate to remove cephalexin antibiotic from aqueous media in the presence of sonication/ultraviolet/hydrogen peroxide: Pathway and mechanism for degradation. Ultrason. Sonochem.. 2020;65:105062
- [Google Scholar]
- Photocatalyst production from wasted sediment and quality improvement with titanium dioxide to remove cephalexin in the presence of hydrogen peroxide and ultrasonic waves: A cost-effective technique. Chemosphere. 2021;284:131337
- [Google Scholar]
- Atomically dispersed Cu-N3 on hollow spherical carbon nitride for acetaminophen degradation: Generation of 1O2 from H2O2. Sep. Purif. Technol.. 2023;318:124016
- [Google Scholar]
- Engineering more sustainable catalysts based in ecological and economic synthesis routes from renewable raw material: Novel mesoporous silicas for remediation technologies. Microporous Mesoporous Mater.. 2023;360:112719
- [Google Scholar]
- Ultrasonic degradation of acetaminophen in water: Effect of sonochemical parameters and water matrix. Ultrason. Sonochem.. 2014;21:1763-1769.
- [Google Scholar]
- Acetaminophen micropollutant: Historical and current occurrences, toxicity, removal strategies and transformation pathways in different environments. Chemosphere. 2019;236:124391
- [Google Scholar]
- Visible-LED-light-driven photocatalytic degradation of ofloxacin and ciprofloxacin by magnetic biochar modified flower-like Bi2WO6: The synergistic effects, mechanism insights and degradation pathways. Sci. Total Environ.. 2021;764:142879
- [Google Scholar]
- Construction of microwave/PMS combined dual responsive perovskite-MXene system for antibiotic degradation: Synergistic effects of thermal and non-thermal. Appl. Surf. Sci.. 2023;639:158263
- [Google Scholar]
- Reaction kinetics and oxidation product formation in the degradation of acetaminophen by ferrate VI. Chemosphere. 2016;155:583-590.
- [Google Scholar]
- Synthesis of magnetically recoverable Fe0/graphene-TiO2 nanowires composite for both reduction and photocatalytic oxidation of metronidazole. Chem. Eng. J.. 2018;337:372-384.
- [Google Scholar]
- A versatile nanocomposite Fe0@SiO2@CaO2 for reductive, oxidative, and coagulation removal of organic and inorganic contaminants from water. Chem. Eng. J.. 2023;454:140048
- [Google Scholar]
- PVP-modified spindle-shaped MIL-88B(Fe) to enhance the degradation of tetracycline by activated peroxodisulfate: A comparative study and mechanistic investigation. Prog. Nat. Sci.: Mater. Int.. 2023;33:872-880.
- [Google Scholar]
- Temperature-driven coupled transport of pollutants and suspended particles established by granular thermodynamics. Int. J. Heat Mass Transf.. 2024;228:125645
- [Google Scholar]
- Plasma-catalyzed combined dynamic wave scrubbing: A novel method for highly efficient removal of multiple pollutants from flue gas at low temperatures. J. Hazard. Mater.. 2024;461:132518
- [Google Scholar]
- Oxidation of acetaminophen in the ozonation process catalyzed with modified MgO nanoparticles: effect of operational variables and cytotoxicity assessment, Process Safety. Environ. Prot.. 2017;109:520-528.
- [Google Scholar]
- Degradation of paracetamol in aqueous solutions by TiO2 photocatalysis. Water Res.. 2008;42:3480-3488.
- [Google Scholar]
- Recent advances in photo-activated sulfate radical-advanced oxidation process (SR-AOP) for refractory organic pollutants removal in water. Chem. Eng. J.. 2019;378:122149
- [Google Scholar]
- Enhanced removal of acetaminophen from synthetic wastewater using multi-walled carbon nanotubes (MWCNTs) chemically modified with NaOH, HNO3/H2SO4, ozone, and/or chitosan. J. Mol. Liq.. 2018;251:369-377.
- [Google Scholar]
- Denitrifying communities enriched with mixed nitrogen oxides preferentially reduce N2O under conditions of electron competition in wastewater. Chem. Eng. J.. 2024;155292
- [Google Scholar]
- Enhanced hydrophilicity and promoted charge transfer in covalent triazine frameworks/sepiolite complexed via hydrogen bonding for visible-light driven degradation of antibiotics. Appl. Clay Sci.. 2023;238:106921
- [Google Scholar]
- The activation of PMS for PFOA degradation by adjustable metal stripping Fe/Co/N@BC catalysts: Degradation performance with single-line oxygen and high-valent metal oxides as the main active substances. Sep. Purif. Technol.. 2024;351:127589
- [Google Scholar]
- Ultrasound: A new strategy for artificial synapses modulation. InfoMat 2024:e12528.
- [Google Scholar]
- Role of superoxide radical and singlet oxygen in peroxymonosulfate activation by iron-doped bone char for efficient acetaminophen degradation. Chem. Eng. J.. 2023;459:141642
- [Google Scholar]
- Dynamic hysteresis compensation and iterative learning control for underwater flexible structures actuated by macro fiber composites. Ocean Eng.. 2024;298:117242
- [Google Scholar]
- Defects materials of Institut Lavoisier-125(Ti) materials enhanced photocatalytic activity for toluene and chlorobenzene mixtures degradation: Mechanism study. J. Colloid Interface Sci.. 2024;660:423-439.
- [Google Scholar]
- Activation of peroxymonosulfate by a stable Co-Mg-Al LDO heterogeneous catalyst for the efficient degradation of ofloxacin. Sep. Purif. Technol.. 2022;294:121231
- [Google Scholar]
- Synergistic Integration of Anammox and Endogenous Denitrification Processes for the Simultaneous Carbon, Nitrogen, and Phosphorus Removal. Environ. Sci. Tech. 2024
- [Google Scholar]
- Efficient degradation of phenol in aqueous solution by catalytic ozonation over MgO/AC. J. Water Process Eng.. 2020;36:101168
- [Google Scholar]
- Activating peroxymonosulfate by high nitrogen-doped biochar from lotus pollen for efficient degradation of organic pollutants from water: Performance, kinetics and mechanism investigation. Sep. Purif. Technol.. 2024;346:127456
- [Google Scholar]
- Photoinduced C-H heteroarylation of enamines via quadruple cleavage of CF2Br 2. Org. Chem. Front.. 2023;10:6112-6116.
- [Google Scholar]
- Removal of acetaminophen from water by simulated solar light photodegradation with ZnO and TiO2 nanoparticles: Catalytic efficiency assessment for future prospects. J. Environ. Chem. Eng.. 2020;8:104038
- [Google Scholar]







