Translate this page into:
Study on extraction and separation of Ni and Zn using [bmim][PF6] IL as selective extractant from nitric acid solution obtained from zinc plant residue leaching
⁎Corresponding author. ghader@uk.ac.ir (Sattar Ghader) sattarghader@yahoo.com (Sattar Ghader)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
For the ever-growing demand of nickel (Ni) resources in industry, the Ni recovery from the mining residues or waste has received considerable interest. Zinc plant residue contains valuable metals it may be recovered using conventional pyrometallurgical or hydrometallurgical processes. The present communication is focused on the selective recovery of Ni from the real nitric acid leach solution of zinc plant residue by solvent extraction (i.e. 1-Butyl-3-methylimidazolium hexafluorophosphate ([bmim][PF6]) as ionic liquid, Di-(2-ethylhexyl) phosphoric acid (D2EHPA) and diphenylthiocarbazone (dithizone)). At first step, leaching of filter cake with the nitric acid solution was examined experimentally and it was observed that nitric acid as a relatively strong oxidant, can adequately dissolve Ni and Zn. After that, Ni and Zn extraction behavior in the leach solution was studied and the influence of pH and extractant concentration were investigated on the extraction of the metals. The results indicated Ni can be effectively separated by controlling the pH values. Moreover, Ni can be selectively separated using dithizone combined with [bmim][PF6] at pH = 5.5 and the separation factor βNi/Zn can reach 2.27 × 105 in one extraction stage. The extraction mechanism of Ni was investigated using slope analysis and stripping efficiencies 100% have been achieved for Zn and Ni with 2.0 M HNO3. Thus, it can be concluded that the use of [bmim][PF6] as alternatives solvents which have a less significant environmental impact than the usual solvents in terms of emission of vapors is one of the promising approaches for nickel ion extraction from the real leaching solution of zinc plant residue.
Keywords
[bmim][PF6]
D2EHPA
Dithizone
Nickel
Zinc
Nitric acid solution
Solvent extraction
1 Introduction
Development of industries and technology leads to increase in demand of primary sources. Because of limitation in primary sources, secondary sources like spent catalysts, used up electronics, construction and useable steel scrap, batteries or waste materials have recently gained a great deal of attention and began to supersede primary sources (Moradkhani et al., 2012). Several mineral processing wastes are produced during hydrometallurgical and pyro-metallurgical processing of ores materials and concentrates. The industrial residues usually have valuable minerals or metals and as well as hazardous compounds that need to be improved with an additional process. For instance, zinc plant residues that are produced through hydrometallurgical processing of zinc ores because of high concentration of heavy metals like cadmium, nickel and zinc are hazardous wastes. Consequently, metal ions recovery efficiently by low-price from natural minerals and secondary (usage) resources for high value produces is required and mainly significant to utilize the resources and harmlessly dispose (Daryabor et al., 2017; Behnajady and Moghaddam, 2017). Nickel (Ni) and zinc (Zn) are commercial metals with the wide range of applications. For example, these metals used in stainless steels, electronics, ceramics, batteries, medicines and catalysts. But because of similar chemical and physical properties, separation of them is too difficult (Ghalandari et al., 2020; Sethurajan et al., 2017).
Different hydrometallurgical techniques like ion exchange, precipitation, cementation, electrolysis and solvent extraction have been used for different matrices to recover valuable metals (Fernandes et al., 2012; Sobianowska-Turek, 2018). Between these methods, solvent extraction due to its high selectivity and simplicity is most commonly used (Shah et al., 2017). Several commercial extractants have been proposed, including acidic extractants (i.e. D2EHPA (Fatmehsari et al., 2009), PC 88A, EHEHPA, Cyanex 272 (Mantuano et al., 2006) and Cyanex 302 (Hosseini et al., 2011) as well as chelating extractants (i.e. LIX 63, LIX 984N (Kul and Cetinkaya, 2010), and LIX 84 (Jha et al., 2014) have been widely used to recover of Ni and Zn from aqueous solution. Different extractants mixtures, i.e. synergistic mixtures, have furthermore been studied to improve the selectivity or efficiency of the systems.
Although conventional solvents have some disadvantages like loss of organic diluent via volatilization, environmental toxicity and human health problem. Subsequently, application of new solvent is suggested. Supercritical fluids (SCFs) and ionic liquids (ILs) are two types of new solvents. Many advantages are reported about ILs and SCFs and they are two kinds of promising solvents to extend novel processes (Bagheri et al., 2018, 2019a, 2019b). Because of low melting point of IL, they are known as organic salts that are liquid at ambient temperature. Moreover, specific gravities of some ILs are higher than water (depends on the ILs chemical composition) and they are under the water layer in two-phase extraction. They can be formed of a huge number of anions and cations. In most instances, there is a big inconformity in size of ionic and at least anion or cation has a high asymmetry degree (Bagheri and Ghader, 2017).
Some researches demonstrated that ILs are effective solvents for the removal of heavy metals from aqueous solution. Subsequently, research for new solvents based on ILs is reasonable. Larsson and Binnemans (2014) developed a recycling with new scheme of metals such as Mn, Fe, Co and Zn from metal hydride batteries using trihexyl (tetradecyl) phosphonium chloride (Cyphos IL 101), tricaprylmethylammonium chloride (Aliquat 336) and Cyanex 923 as ILs and extractant, respectively. The results indicated Cyphos IL 101 and Aliquat 336 were appropriate to remove Co, Mn, Fe and Zn. Because it can simply be scrubbed using hydrogen chloride solutions. Moreover, they demonstrated ILs direct application to recover Co, Fe, Mn and Zn because of significant properties of ILs is more effective than using a Cyanex 923. Pospiech (2015) compared the removal results of Cd from aqueous chloride solutions that is containing other metal cations, i.e., Cu, Ni and Co ions based on solvent extraction and transport across polymer inclusion membranes and Cyphos IL 104 was used as extractant and extraction carrier in both processes. Pospiech investigated the effect of various parameters as an example chloride ion concentration, hydrochloric acid concentration in aqueous phase and acid concentration. The results showed increasing of hydrogen cations concentration leads to Cd concentrations in the aqueous phases after solvent extraction and extraction efficiency reached a maximum value. Because of strong tendency of Cd to form anionic complexes with chloride ions and due to increase of the extractable anionic species concentration, the effect of chloride ion concentration was studied. The results were similar to hydrogen cations concentration. Devi (2016) investigated solvent extraction process for extraction and separation of Cu among other metal ions (Fe, Zn, Cd, Co, Ni) in kerosene using trioctylmethylammonium/2,4,4-trimethylpentyl phosphinate, ([A336/Cy272]) as bifunctional IL. Devi demonstrated [A336/Cy272] was more effective than specific extractants such as Aliquat 336 or Cyanex 272 to extract copper due to poor cation exchange property and studied on effect of shaking time, aqueous pH, IL, copper and sodium sulfate concentration and organic to aqueous volume ratio (O/A). The results showed increasing in shaking time leads to enhance Cu(II) extraction rate but after 5 min of contact time, due to reach to extraction equilibrium the extraction rate was invariant. The extraction rate changed due to IL tendency to extract H+ from the aqueous solution instead of copper ion. Referring to Fig. 2, amount of Cu(II) extraction was increased by increasing pH aqueous. In low aqueous pH, relation between the extraction rate and pH was approximately linear but near pH = 5.34, a rapid increasing in extraction rate was observed and in pH range 5.34–5.9 because of precipitation in organic phase after equilibration the decreasing trend was observed, too. Also, increasing in IL and copper concentration and O/A ratio leads to enhance Cu (II) extraction rate. But because of salting out effect, increase in concentration of sodium sulfate leads to decrease the Cu (II) extraction. Wellens et al. (2012) studied on Co separation from Ni, Mg and Ca in chloride medium based on water immiscible and non-fluorinated IL i.e. tri(hexyl) tetradecylphosphonium chloride ([P66614] [Cl]) as extractants. Co was extracted to the IL phase as the tetrachlorocobaltate (II) complex. The results indicated cobalt can selectively be separated from nickel, magnesium and calcium with solvent extraction by IL as extraction reagents. Cobalt extraction efficiency increased with increasing chloride concentration and it reached to a maximum value and after that because of formation of the hydrogen dichloride ion extraction efficiency decreased. Coll et al. (2012) investigated Ni and Co extraction from aqueous chloride solutions by bis 2,4,4-(trimethylpentyl) phosphinate ([HJMT][Cy272]) IL as extractant among continuous counter current liquid–liquid extraction process. They used Primene®JMT (JMT) to control equilibrated aqueous phase pH during the extractions of Co and Ni. The experimental results showed Co and Ni extraction increased with increasing JMT concentration because of increase in the equilibrated aqueous phase pH and its effect was more on Co and Ni. Moreover, they investigated the effects diluents on Co and Ni extraction and the results indicated Solvesso 200 (mixture of alkyl (C3-C6)‐benzenes) was more effective compared with kerosene (mixture of alkanes) and Exxon D100 (cycloparaffinic solvent).
Solvent extraction process is one of the most widespread techniques for the separation metals from solid residues (Asadi et al., 2018; Janin et al., 2009; da Silva et al., 2019). Metals contained in the zinc plant residue are leached using acid solution followed by the extraction and recovery of target metal ions from the leach solutions. However, hydrometallurgical treatment moreover generates a huge amount of acidic effluents which, if disposed into the environment causes harmful diseases in addition to ecological imbalance. Between several acids, nitric acid is the most capable leaching agent because of its high oxidation potential and less evolution of insoluble residue. Despite high cost of nitric acid, it can again be recovered using hydrometallurgical treatment. Nitric acid can be readily recycled through the external nitrous oxides oxidation. Extensive endeavors have been done for decades regarding metal extraction from zinc plant residue (e.g., metals such as cadmium (Gharabaghi et al., 2013) and zinc (Gouvea and Morais, 2007). However, numerous researchers mentioned above, could extract valuable metals from zinc plant residue, but to the best of our knowledge none of them ever concentrated on separation of nickel employing ionic liquids (ILs). Recently, ILs can be considered as alternatives solvents that have a less significant environmental influence than the conventional solvents in terms of emission of vapors.
In present investigation has been planned to examine selective separation and extraction of Ni from zinc plant residue, which is a mixture of various metals like Ni, Zn, Sb, Pb, Fe, Al. At the first step, the zinc plant residue was leached using nitric acid solution, after that the extraction and separation of Ni and Zn from the acidic leach solution were examined with the ([bmim][PF6]) as IL extraction system compared to conventional organic solvent.
2 Experimental
2.1 Characterization of the zinc plant residue
The zinc plant residue employed in the experiments was supplied from the zinc manufacturing plant in Zanjan, Iran. The filter cake was oven-dried (at 30 °C) for 24 h and then crashed with a ball mill until achieving the desired powder size (particle sizes ranged from less than 0.1 mm) for further analysis. The chemical composition of filter cake was determined by X-Ray Fluorescence Spectroscopy (XRF) (ARL ADVANTX+, Switzerland). Results of the XRF analysis of the filter cake are given in Table 1. As can be seen, the residue is mostly composed of zinc, nickel, lead, and cadmium. Mineralogical analysis was performed using X'PertPro model (Panalytical Company, Netherland) X-ray diffractometer with Cu Kα radiation (40 kV and 30 mA) and scanning rate of 0.05° s−1. The XRD pattern of the zinc plant residue is presented in Fig. 1. Characteristic peak intensity positions show that the main phases presented in this filter cake is zinc oxide.
Elements
Zn
Ni
Si
Ca
Mn
Fe
Al
Cu
Mg
Cd
Sb
SO4
Pb
L.O.I
Wt %
50.12
4.60
0.37
1.20
0.02
0.66
0.36
0.3
0.25
14.02
0.14
14.97
1.15
11.81
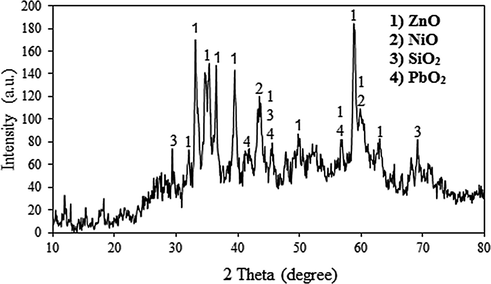
XRD pattern of the zinc plant residue.
2.2 Materials
Solvent extraction experiments were carried out using real leaching solution from filter cake. The aqueous solutions of the specified metal ions were prepared by the dissolution of various amounts of nitric acid (Merck, 65% pure) as leaching reagent in deionized water. The pH of each solution was adjusted by the addition of 10 M sodium hydroxide or 1 M nitric acid solution. Organic phases containing acidic extractant, i.e. D2EHPA, dithizone (DTZ), and ionic liquid, i.e. [bmim][PF6] were purchased from Merck, Germany. The chemical structures of the main components are shown in Fig. 2. The properties of the extractants are given in Table 2. Moreover, kerosene was purchased from Esfahan Oil Refinery in Iran and used as the diluent for the D2EHPA and dithizone. a: The properties are from reference (Jafari et al., 2018). b: The properties are from reference (Irving, 1977). c: The properties are from reference (Masilela and Ndlovu, 2019).![The chemical structures of D2EHPA, [bmim][PF6], and dithizone.](/content/184/2020/13/6/img/10.1016_j.arabjc.2020.04.019-fig2.png)
The chemical structures of D2EHPA, [bmim][PF6], and dithizone.
Characteristic
D2EHPAa
Dithizoneb
[bmim][PF6]c
Molecular formula
C16H35O4P
C13H12N4S
C8H15F6N2
Molar mass (g.mol−1)
322.43
256.3
284.186
Solubility in water (g.L-1)
<0.01
≈5 × 10−5
24.6
Density (g.ml−1)
0.9758
1.35
1.38
Appearance
Colorless or yellowish, liquid
Violet-black
Light yellow liquid
Purity/wt.%
97
98
99
2.3 Leaching experiments
Several leaching experiments were performed to study the leaching efficiency of target metals (i.e. Zn and Ni) from the filter cake. All leaching experiments were performed in a flat-bottom three-necked flask of 500 ml using from high purity acid solutions at a solid/liquid ratio (S/L) of 1:10 (20 g of filter cake was added to a 200 ml nitric acid solution with predetermined concentration) with shaking at 600 rpm. The recovery of elements was studied at reaction times range of 30–120 min and different temperatures: 25, 65 and 95 °C. A water-cooled condenser was attached to central opening of a flask in order to prevent solution loss by evaporation. The concentration of elements in the leach solution were determined using the Atomic Absorption Spectrophotometer (AAS) (SpectorAA 220, Australia). The leaching efficiency of metal ions (% LE) was calculated according to the following equation:
2.4 Solvent extraction experiments
Solvent extraction of the real leaching solution from filter cake was carried out in the following way: the equal volumes of organic phase (i.e., [bmim][PF6], D2EHPA, and dithizone) and aqueous phase containing metal ions (phase volume ratio O/A = 1:1) were mechanically shaken (600 rpm) for 30 min in the glass beaker (the experimental setup is shown in Fig. 3). All experiments were carried out at room temperature (25 ± 2 °C). In experiments, after pH measurement during the experiments by the pH meter (PTR79, Iran) and its adjustment using sodium hydroxide or nitric acid, 10 min was considered for full transfer of the metal to the organic phase.
The experimental setup.
After the extraction was completed, organic and aqueous phases were separated in a separator funnel. After separation of the aqueous and organic phases, metal concentrations in the aqueous phase were measured by Atomic Absorption Spectrophotometer (AAS) (SpectorAA 220, Australia). The concentrations of the metal ions in the organic phase was calculated by mass balance. The percentage of extraction of each metal (%ME) was calculated as:
The separation factor of each metal was calculated as:
2.5 Stripping and recycling process
The stripping behavior of Ni and Zn from the extracting phase was examined by nitric solutions at A/O ratio of 1:1. The concentration of metal ions in the aqueous phase was measured using the Atomic Absorption Spectrophotometer (AAS). The stripping efficiency (R %) of metal ion was defined as:
For the recycling experiments, the resulting extracting phase from the leach solution was mixed by mechanical shaker (600 rpm) with an equal volume of the nitric acid stripping solution at O/A ratio of 1/1, 25 °C for 30 min. The extracting phase separated from the aqueous stripping phase was reused as extracting phase for the next fresh aqueous solution. The extraction and stripping processes were repeated five recycles.
3 Results and discussion
To recover metals from the filter cake at the first step, metals must be extracted from the solid matrix by leaching process. The leaching process involves dissolving the required metal in the waste using an appropriate reagent. The leaching solution contains various co-extracted metal ions besides the targeted metal ion (i.e. Zn and Ni) at the same time. The initial part of experiments is leaching of filter cake by the nitric acid solution to find conditions of rather a complete dissolution of the pointed out metals by examining the effect of concentration, temperature, and time.
3.1 Effect of nitric acid concentration
In general, nitric acid concentration has pronounced effect on metal extraction and it is one of the important factors for the metal ions dissolution condition optimizing. Several leaching agents like sulfuric, nitric, and hydrochloric acid were used to leach of Zn and Ni (Sethurajan et al., 2017; Khalid et al., 2019; Li et al., 2011; Safarzadeh et al., 2008). However, nitric acid is more effective than sulfuric and hydrochloric acid to recovery of nickel. Similar results have been also reported by Kim et al. (2007) that experimentally observed that nickel leaching from multi-layer ceramic capacitors in HNO3 solution. In their study, HNO3 was more effective than HCl and H2SO4 solutions. Fig. 4 showed the leaching efficiency of Zn and Ni from filter cake using altering nitric acid concentration in the range of 1–6 M, keeping solid/liquid ratio of 1:10 and with the contact time (120 min) and temperature (95 °C).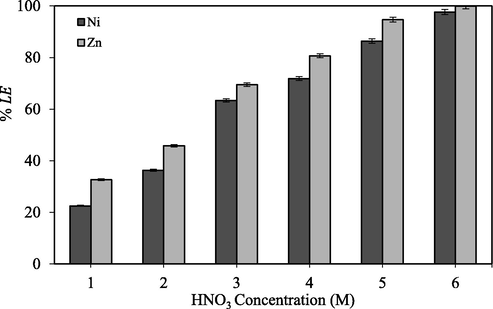
Effect of nitric acid concentration on the leaching of nickel and zinc (leaching temperature: 95 °C; leaching time: 120 min; agitation speed: 600 rpm; L/S ratio 1:10).
As observed from Fig. 4, by the increasing of nitric acid concentration, Ni and Zn leaching efficiency was increased. The highest leaching efficiency for both Ni and Zn was reached when leaching process was performed in 6 M HNO3 (97.65% and 99.91%, respectively), while lower values were observed using 1 M HNO3 (22.52% and 32.71%, respectively). The reactions of the main species (Ni, Zn) that exist in the filter cake with nitric acid are presented in following equations (Mecucci and Scott, 2002):
The presence of H+ ions encourages the dissolution of Ni and Zn subsequently; nitric acid was selected as solution media. Nitric acid is the primary choice on several grounds, e.g., it is an oxidizing acid that can dissolve most metals to form soluble metal nitrates. It has poor oxidizing strength below 2 M but is a powerful oxidizing acid in concentrated form. Its oxidizing power can be enhanced by increasing its temperature. Referring to Eqs. (6) and (7), nitric acid has strong activators, NO3− which can dissolve both Zn and Ni. The increase in nitric acid concentration leads to increase of available hydrogen ions that interact with solid matrix and increases the rate of dissolution of Zn and Ni from it. Consequently, increasing nitric acid concentration had a positive effect on both Ni and Zn leaching efficiency. A similar trend was also observed by increasing nitric acid concentration to 5 M at 80 °C and 120 min leaching efficiency of Ni increasing to 97% (Lasheen et al., 2009).
3.2 Effect of temperature and time
To investigate the effect of temperature and time on Zn and Ni leaching efficiency, the leaching process was performed in the wide range of temperature (25–95 °C) and time (30–120 min) with contact nitric acid concentration (6 M). The results were shown in Fig. 5.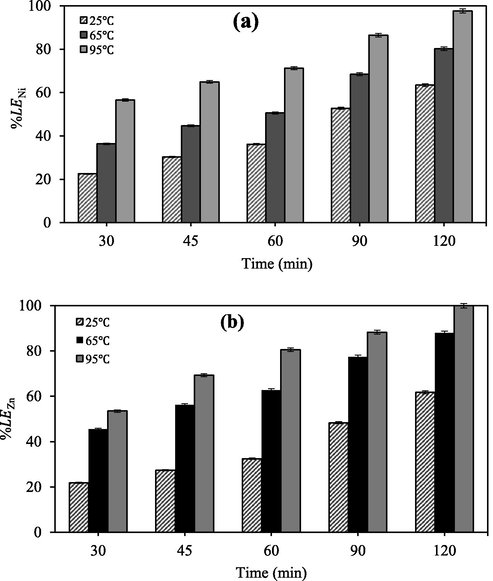
Effect of time and temperature on the leaching of (a) nickel and (b) zinc (nitric acid concentration: 6 M; agitation speed: 600 rpm; L/S ratio 1:10).
The results depicted increasing temperature and time is in favor of Zn and Ni leaching effectively. For instance, at 25 °C only 63.4% Ni and 61.7% Zn were recovered and the recovery process was completed at 95 °C. In general, both reaction time and temperature had an important effect on the leaching of Ni and Zn. Obviously, Ni and Zn are covered by other constituents in the filter cake matrix which recovery of these metals from nitric acid solution is too hard at ambient temperature. Therefore, leaching of these components which is inherently slow due to such barriers becomes reasonable as temperature is raised (Sheik et al., 2013; Oza et al., 2011). Similar results were also obtained by MacCarthy et al. (2016) that investigated the effects of agitation rate and temperature on leaching of Ni from the saprolitic nickel laterite ore and they indicated temperature parameter has a significant impact on the leaching behavior.
3.3 Extraction of Ni and Zn from nitric acid solution
After the leaching process to evaluate the extraction of Ni and Zn using various extractants, the extraction percentages (% ME) of these metal ions in 6 M nitric acid at 25 °C were determined. Due to the nitric acid leaching solution of the filter cake is a complex system that includes of multi-metal existence, thus the selective extraction of Ni is a critical subject. Therefore, Ni and Zn extraction were evaluated for a series of different extractants. In this study, dithizone, D2EHPA, and [bmim][PF6] which are chelating, phosphinic, and IL, respectively, were investigated for the Ni and Zn extraction. The concentration of each extractant is as follows: 0.014 M dithizone in different solvents (kerosene and IL), 0.14 M D2EHPA in kerosene, and the mixture of D2EHPA and dithizone (0.14 M: 0.014 M) in kerosene at pH = 6.5, temperature 25 °C, and O/A = 1/1. As can be observed from Fig. 6, D2EHPA and dithizone are also the most efficient compounds in nickel extraction, with all achieving near 100% extraction. However, dithizone in [bmim][PF6] is more efficient in the separation of Zn and Ni due to the lower % ME Zn. The results indicated the extraction efficiency of extractants, are dependent on the pH of the system. Consequently, the concentration of extractants and pH influences on the separation of Ni and Zn are investigated.![Effect of the extracting agent on the extraction of nickel and zinc from the 6 M nitric acid solution ([Ni] = 4.36 g/L and [Zn] = 48.67 g/L) at pH = 6.5, O/A phase ratio of 1:1, and temperature 25 °C.](/content/184/2020/13/6/img/10.1016_j.arabjc.2020.04.019-fig6.png)
Effect of the extracting agent on the extraction of nickel and zinc from the 6 M nitric acid solution ([Ni] = 4.36 g/L and [Zn] = 48.67 g/L) at pH = 6.5, O/A phase ratio of 1:1, and temperature 25 °C.
3.3.1 Effect of pH
In order to investigate the influence of pH, on the extraction of Ni and Zn with 0.14 M D2EHPA in kerosene and 0.014 M dithizone in different solvents (kerosene and IL), experiments were conducted at the pH range of 2–7.5, the organic/aqueous (O/A) ratio at 1:1, and temperature of 25 °C. The percentage extraction results of Ni and Zn vs. pH are shown in Figs. 7 and 8.![Effect of pH on the extraction of nickel and zinc from the 6 M nitric acid solution ([Ni] = 4.36 g/L and [Zn] = 48.67 g/L) at the O/A phase ratio of 1:1, D2EHPA concentration of 0.14 M in kerosene and the temperature of 25 °C.](/content/184/2020/13/6/img/10.1016_j.arabjc.2020.04.019-fig7.png)
Effect of pH on the extraction of nickel and zinc from the 6 M nitric acid solution ([Ni] = 4.36 g/L and [Zn] = 48.67 g/L) at the O/A phase ratio of 1:1, D2EHPA concentration of 0.14 M in kerosene and the temperature of 25 °C.
![Effect of pH on the extraction of nickel and zinc from the 6 M nitric acid solution ([Ni] = 4.36 g/L and [Zn] = 48.67 g/L) at the O/A phase ratio of 1:1, dithizone concentration of 0.014 M in (a) kerosene and (b) IL ([bmim][PF6]) and the temperature of 25 °C.](/content/184/2020/13/6/img/10.1016_j.arabjc.2020.04.019-fig8.png)
Effect of pH on the extraction of nickel and zinc from the 6 M nitric acid solution ([Ni] = 4.36 g/L and [Zn] = 48.67 g/L) at the O/A phase ratio of 1:1, dithizone concentration of 0.014 M in (a) kerosene and (b) IL ([bmim][PF6]) and the temperature of 25 °C.
It can be seen that by increasing pH almost complete extraction was achieved for Ni by all extractants. However, the extraction efficiency of Zn was low in the case of dithizone in kerosene and no extraction of Zn was observed in IL. It needs to be noted that in the presence of acidic extractants like D2EHPA, due to release hydrogen ions into the solution during the extraction (Eq. (8)) (Bidari et al., 2013):
Acidic extractant follow a cation exchange extraction mechanism which involves the displacement of hydrogen ion from the extractant by the extracted metal, forming a neutral organic soluble complex. Based on Eq. (8), D2EHPA release ion during the solvent extraction process. Consequently, increasing the pH of solution increases the metal ion extraction from aqueous solution. D2EHPA is an ideal extractant for the Ni and Zn extraction owing to its chemical stability, low aqueous solubility and high loading characteristics thus the proton in the D2EHPA is easily substituted by the nickel and zinc ions (Narita et al., 2006; Zhang et al., 2016). Besides, since the Ni and Zn extraction is highly pH dependent, an acidic extractant like D2EHPA is more preferable. Also, Fig. 8 showed the comparison of extraction of Ni and Zn from the aqueous phase to ionic liquid and to kerosene. The higher percentage extraction of Ni in [bmim][PF6] than in kerosene indicates that ionic liquid is a better receptor phase for metal removal and the use of [bmim][PF6] as the solvent for liquid/liquid extraction of metal ions allows the extraction to be performed at lower pH.
The selectivity of extractants for desired Ni and Zn separation can be quantified using separation factor (see Eq. (3)). The highest value of the separation factor corresponds to the highest selectivity in metals separation. Separation factor of Ni from Zn (βNi/Zn) at various pH are presented in Table 3. According to the data shown in Table 3, dithizone in IL is the most selective extractant of the three studied ones as it extracts only Ni (βNi/Zn = ∞). The pH value higher than 6.5 seems to be the most adequate for selective extraction of Ni from Zn using dithizone in kerosene and separation factor of Ni extraction over Zn for dithizone in kerosene is equal to 4.433. However, D2EHPA does not separate Ni from Zn. Thus this value of separation factor is not acceptable for separation of the two ions in these conditions.
βNi/Zn
pH
2
4
5
5.5
6
6.5
D2EHPA
0.006
0.058
0.118
0.111
1.84
0.820
DTZ in kerosene
0.198
0.297
0.228
0.2
1.823
4.433
DTZ in IL
40.06
697
1458.25
2.27 × 105
–
–
3.3.2 Effect of concentration
Since the amount of extractant in liquid–liquid extraction has a significant effect on the metal ion extraction, the effect of the different extractant concentrations on the selective extraction was investigated and the results are shown in Fig. 9. As can be seen from Fig. 9a, the extraction percentage of Ni and Zn increased with increasing concentration of D2EHPA. As expected, the increase in the concentration of D2EHPA resulted in higher metal extractions (Pereira et al., 2007). The results demonstrate that upon increasing D2EHPA concentration from 0.14 to 0.56 M, the extraction of Ni and Zn was increased from 0.41% to 93.40% and 35.30 to 98.59%, respectively. This is consistent with observations of Vahidi et al. (Innocenzi and Veglio, 2012). Also, Fig. 9b shows that extraction percentages of Ni and Zn increased with increasing dithizone concentration from 0.014 to 0.044 M. In particular, extraction percentage of Ni increased from 7.34% to 99.5%. Further increase in the [bmim][PF6] concentrations from 0.2 to 1.0 M had no effect on the extraction of Ni, because Ni extraction had already reached nearly maximum (∼100%), whereas Zn slightly increased in the ranges of 2.09–3.21% (Fig. 9c). On the basis of the results nickel content of the real leaching solution was recovered through a single-stage extraction process. In some of the previous works, high nickel recoveries from nitric acid solution have been obtained by multi-stage solvent extraction approach and also a modifier or another extractant should be used, however recoveries as high as the present 100% have not been generally achieved using the previous single-stage extraction processes (Hutton-Ashkenny et al., 2015; Mubarok and Hanif, 2016).![Effect of concentration (a) D2EHPA in kerosene, (b) dithizone in kerosene, (c) dithizone in IL ([bmim][PF6]) on the extraction of nickel and zinc from 6 M nitric acid solution ([Ni] = 4.36 g/L and [Zn] = 48.67 g/L) at the O/A phase ratio of 1:1 and the temperature of 25 °C.](/content/184/2020/13/6/img/10.1016_j.arabjc.2020.04.019-fig9.png)
Effect of concentration (a) D2EHPA in kerosene, (b) dithizone in kerosene, (c) dithizone in IL ([bmim][PF6]) on the extraction of nickel and zinc from 6 M nitric acid solution ([Ni] = 4.36 g/L and [Zn] = 48.67 g/L) at the O/A phase ratio of 1:1 and the temperature of 25 °C.
Moreover, the changes in selectivity factors with varying extractant concentrations are presented in Table 4. Increasing D2EHPA concentration cannot improve selectivity separation Ni from Zn. This is due to the fact that selectivity separation of Ni from Zn using D2EHPA is very low and separation factor fluctuate is around 1.0. The difference between the separation factors characterizing extraction with various dithizone concentrations is much more visible. For the lowest dithizone concentration (i.e. 0.014 M) separation factor is about 4.5, while for the higher concentration (i.e. 0.028) is equal to 19.
Concentration of:
D2EHPA, M
DTZ, M
0.14
0.28
0.56
0.014
0.029
0.044
βNi/Zn
0.82
1.01
0.20
4.43
19.01
17.09
3.4 Extraction mechanism and extraction stoichiometry
The presence of two opposite charge species of the IL makes the metal ion separation in aqueous/IL biphasic system a much more complex process than in traditional solvents. Several studies have illustrated the difference of metal extraction mechanism between ionic liquid and convenient organic solvents (Wei et al., 2003; Prabhu et al., 2017). The metal extraction mechanism changed from ion-pair mechanism in organic solvent (denoted by org, Eq. (9).) to cation exchange in ionic liquid (Eq. (10)) (Shimojo and Goto, 2004).
The separation efficiency of metal ions in solvent extraction is reported in terms of percentage extraction or distribution ratio which can be predicted from a set of equilibrium constants. Combining all the results obtained in this study, Confirmation of the metal ions extraction dependence on pH was done by considering the extraction of Eq. (11) which leads to the relationship between logD and proton concentration in Eq. (14). Also, the possible assumption of extraction equilibria of metal ions studied with dithizone in ionic liquid ([bmim][PF6]) could be proposed as in the following Fig. 10 (H2L denoting dithizone).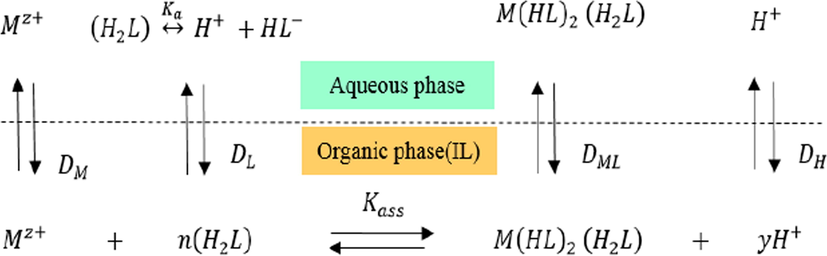
Chemical equilibriums in the extraction of metal ions using dithizone (H2L) with IL.
The extraction mechanism of the metal ion (in this case nickel) with dithizone in IL may be expressed as follows:
The equilibrium constant of this extraction reaction (Kex) can be written as
The distribution ratio, D, can be substituted into the above equation, which gives:
By taking the logarithm of Eq. (13)
The slope analysis was carried out for determining the stoichiometry of extracted metal complexes in solvent extraction. The plots of logDNi against equilibrium pH and log (H2L2), are shown in Fig. 11a and b. From Fig. 11(a), the slope of logDNi-pH curve is 1.701, so the value of y is close to 2. From Fig. 11(b), the slope of logDNi-log(H2L2) curve is 2.87, and the value of n is close to 3. Therefore, from the Eq. (14), the extraction reaction equation can be expressed as Eq. (15).
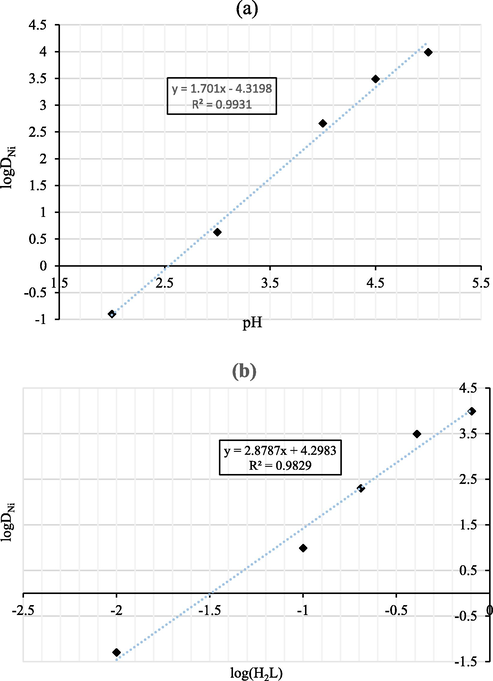
Plots of logDNi versus equilibrium (a) pH and (b) log(H2L).
3.5 Stripping of Ni and recycling of the IL
After the extraction of Ni and Zn, it is significant to recover Ni and Zn from the IL phase and recycle the IL in a sustainable way. Since the previous results demonstrated that the extraction of metal ions depended on the pH of solution, the stripping experiment was carried out using varying concentrations of nitric acid solution (0.5–3 M). The results are shown in Table 5. The stripping efficiency of Ni and Zn increased gradually along with acid concentration. The maximum stripping efficiency 100% could be obtained with 2 M HNO3. After the stripping process, the IL could be reused in the next extraction process. The recycling efficiency of IL has been tested (Fig. 12). The extraction and stripping processes were repeated five cycles. The results indicate that IL still retains its extraction ability toward Ni after five cycles and the extraction percentage of Ni is around 94.5%. Therefore, the IL had a good reuse property for the selective extraction of Ni.
HNO3 (M)
Stripping efficiency (R%)
Ni
Zn
0.5
49.83
51.29
1
71.59
76.95
1.5
91.07
94.77
2
100
100
3
100
100
![Reuse ability of [bmim][PF6] for selective extraction of nickel (extraction and stripping conditions: [bmim][PF6] = 0.4 M; pH = 5; extraction time = 30 min; [Ni] = 4.36 g/L, [Zn] = 48.67 g/L; stripping solution of 2 M HNO3; temperature: 25 °C).](/content/184/2020/13/6/img/10.1016_j.arabjc.2020.04.019-fig12.png)
Reuse ability of [bmim][PF6] for selective extraction of nickel (extraction and stripping conditions: [bmim][PF6] = 0.4 M; pH = 5; extraction time = 30 min; [Ni] = 4.36 g/L, [Zn] = 48.67 g/L; stripping solution of 2 M HNO3; temperature: 25 °C).
4 Conclusions
In this study, separation of Ni and Zn from zinc plant residue in nitrate medium was investigated through the solvent extraction process using D2EHPA, dithizone, and ionic liquid (1-Butyl-3-methylimidazolium hexafluorophosphate, [bmim][PF6]). At first, leaching of filter cake with the nitric acid (HNO3) solution was examined experimentally and it was observed that nitric acid as a relatively strong oxidant can adequately dissolve Ni and Zn. The effect of nitric acid concentration, temperature and time on leaching was also studied. The experiments indicated that approximately 100% of Ni and Zn are leached in 6 M HNO3 at 95 °C for 120 min. After that, extraction behavior of Ni and Zn in the real leaching solution was studied at different pH and extractant concentration. Also, the results suggest that the pH have great effect on the selective extraction of Ni. Increase in concentration of extractant would most likely show a positive effect on the amount of Ni extracted however, is not necessarily required. Furthermore, the results show that application of hydrophobic [bmim][PF6] combined with dithizone allowed the selective separation of Ni from Zn. The separation factor βNi/Zn can reach to 2.27 × 105 in one extraction stage. The extraction mechanism of Ni was investigated using slope analysis and stripping efficiencies 100% have been achieved for Zn and Ni with 2.0 M HNO3. This work supplies a promising approach for the selective separation of Ni from zinc plant residue, which making IL a potential replacement for volatile organic solvents.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- J. Dispers. Sci. Technol.. 2018;39:1328-1334.
- J. Mol. Liq.. 2017;236:172-183.
- J. Mol. Liq.. 2018;261:174-188.
- Comput. Particle Mech.. 2019;6:721-737.
- J. Mol. Liq.. 2019;281:490-505.
- Chem. Eng. Res. Des.. 2017;117:564-574.
- J. Environ. Chem. Eng.. 2013;1(4):1269-1274.
- Hydrometallurgy. 2012;125–126:24-28.
- J. Clean Prod.. 2019;210:786-794.
- Ultrason. Sonochem.. 2017;34:931-937.
- Trans. Nonferr. Metal Soc.. 2016;26:874-881.
- Hydrometallurgy. 2009;98:143-147.
- J. Power Sources.. 2012;220:286-291.
- J. Fluid Phase Equilib.. 2020;508:112433
- Chem. Eng. Res. Des.. 2013;91(2):325-331.
- Miner. Eng.. 2007;20(9):956-958.
- Hydrometallurgy. 2011;105:277-283.
- Miner. Eng.. 2015;77:42-51.
- Hydrometallurgy. 2012;129–130:50-58.
- Dithizone. Burlington House, London: The Chemical Society; 1977.
- Sep. Purif. Technol.. 2018;197:210-219.
- Hydrometallurgy. 2009;96(4):318-326.
- Sep. Purif. Technol.. 2014;122:119-127.
- J. Cleaner Prod.. 2019;215:1005-1013.
- Hydrometallurgy. 2007;86:89-95.
- Solvent Extr. Ion Exch.. 2010;28:225-243.
- Green Chem.. 2014;16:4595-4603.
- Int. J. Miner. Process.. 2009;92:109-114.
- Miner. Eng.. 2011;24:859-863.
- Hydrometallurgy. 2016;160:26-37.
- J. Power Sources. 2006;159:1510-1518.
- J. Environ. Chem. Eng.. 2019;7:102810
- J. Chem. Technol. Biotechnol.. 2002;77(4):449-457.
- Hydrometallurgy. 2012;84–92:115-116.
- Procedia Chem.. 2016;19:743-750.
- Solvent. Extr. Ion. Exch.. 2006;24:693-702.
- J. Chem. Technol. Biotechnol.. 2011;86:1276-1281.
- Sep. Purif. Technol.. 2007;53(1):89-96.
- Hydrometallurgy. 2015;154:88-94.
- Solvent Extr. Ion Exch.. 2017;35(6):423-438.
- Sep. Purif. Technol.. 2008;58:367-376.
- J. Hazard. Mater.. 2017;324:71-82.
- J. Environ. Chem. Eng.. 2017;5:5260-5269.
- J. Taiwan Inst. Chem. Eng.. 2013;44:34-39.
- Anal. Chem.. 2004;76(17):5039-5044.
- Waste Manag.. 2018;77:213-219.
- Anal Chim Acta.. 2003;488:183-192.
- Green Chem.. 2012;14:1657-1665.
- Chem. Eng. J... 2016;3002:233-238.







