Translate this page into:
Physico-chemical and sensory acceptance of Carica papaya leaves extract edible O/W emulsion as prospective natural remedies
⁎Corresponding author. saiful-z@ukm.edu.my (Saiful Irwan Zubairi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Carica papaya Linnaeus commonly known as papaya is widely grown in Malaysia as a herbaceous plant with phytochemicals for a variety of use, particularly in the medical field. The therapeutic medicinal way of treating dengue fever using papaya leave extract mainly involves consumption a raw concoction and is very distasteful. Therefore, a study was carried out to develop stable emulsion with an acceptable taste through a ternary phase diagram system (TPDS), which comprised virgin coconut oil (VCO), isolated whey protein (WPI) and Carica papaya leaves extract (CPLE). The TPDS was developed using Chemix Software version 3.6 to identify the existence of homogenous phase region. In the first phase, a total of 11 selected samples (named as A to K) with concentrations ranging from 20% to 30% (w/w) of WPI from the homogenous phase region were used to select the best emulsion selection. Sample I with a composition of 25, 30 and 45% (w/w) comprising VCO, WPI and CPLE was selected and considered as the best and stable emulsion. In the second phase, sample I (renamed as sample M) underwent an addition of + 2% (sample L) and reduction of −2% (sample N) VCO. Analysis was carried out such as emulsion stability test (creaming index), pH value, viscosity, color and storage stability (4, 28 and 45 °C). The control sample was CPLE without any VCO or WPI. Sensory evaluation was also conducted to handpick the best formulation favored by 30 panellists. The sensory evaluation was conducted on samples L, M, N and CPLE using 7-point hedonic scale for preference on color, viscosity, odor, bitterness and overall acceptance attributes. The results showed that centrifugation test exhibited a stable emulsion for all the three samples (p > 0.05) L, M and N. In fact, there were differences between all the samples (p < 0.05) for pH, viscosity, and coloration of L* and b* values. For the storage stability test, all formulations were stable at 4 °C and there were no creaming layer and color changes developed except for sample L which was considered unstable at 28 °C and 45 °C. As for sensory preference, the color and bitterness was considered similar as compared to control (p < 0.05) except viscosity, odour and overall acceptance. In conclusion, sample N with compositional concentrations of 23% (w/w) VCO, 32% (w/w) WPI and 45% (w/w) CPLE was considered the most acceptable emulsion as it did not develop immiscible creaming layer and color changes at 4 °C and 28 °C. Based on the selected formulation, at least 270 ml emulsion by taking 3 tablespoons daily for 3 days in a row is needed as recommended by the Ministry of Health (MOH) in helping to increase the chances of curing dengue fever.
Keywords
Carica papaya leaves extract
Virgin coconut oil
Whey protein
Edible emulsion
Sensory acceptability
Alternative natural remedies
1 Introduction
Papaya (Carica papaya Linn.) belongs to the Caricaceae family and is one of the most important fruit crops in helping the economic development of countries in the tropics and subtropics. Carica papaya is also known as paw paw (Jayavalli et al., 2011; Ayoola et al., 2008; Mendoza et al., 2008 originated from Southern Mexico and Costa Rica before being planted elsewhere in the tropics region (Jari, 2009; Mendoza et al., 2008). Papaya production in Malaysia is so extensive that Malaysia has become the second largest supplier of papaya in the international market, after Mexico (Ministry of Agriculture; MOA, 2016). The demand for papaya keeps increasing because of its high nutritional and medicinal values, which can increase vitamin A, B, and C in the body (Azad et al., 2012; Imaga et al., 2010).
Papaya leaves contain mineral elements such as Ca, Mg, Na, K, Fe and Mn (Ayoola et al. 2008). Phytochemical screening of Carica papaya leaves extract (CPLE) also showed the presence of bioactive compounds such as saponins, cardiac glycosides alkaloids, tannins, phenolic, steroids and flavonoids (Abidin et al., 2016, 2015). The presence of alkaloids in the leaves indicates that this plant can be effective agents for anti-malaria (Ayoola et al. 2008). Furthermore, flavonoids (e.g. quercetin, hesperitin and naringenin) and alkaloids have been attributed to anti-inflammatory/wound-healing agents due to the availability of antioxidant (high in phenolic compound) and anti-bleeding properties (Saliasi et al. 2018). In addition, the presence of saponins supports the fact that papaya leaves have cytotoxic effect (e.g. cyanogenic glycosides) which also attributed to the bitter taste (Okwu & Okwu, 2004). Even though CPLE possess severed bitterness and potential health hazard, it can potentially be able to help treat dengue fever by increasing blood platelet counts within 40 hrs of treatment (Ismail et al., 2014; Subenthiran et al., 2013) and has been shown to decrease the bleeding and clotting time in thrombocytosis effect (Mohd Abd Razak et al. 2018). The safety and toxicity test (acute toxicity) have been conducted on mice species Sprague Dawley for 28 days found that CPLE gives no toxic effects on internal organs of rats (heart, kidney and liver) (Ismail et al., 2014; Abdullah et al., 2009).
Apart from the efficacy of papaya leaves as potential natural remedies, virgin coconut oil (VCO) also has its own properties that reduce the atherosclerosis-related inflammation, aid digestion and wound healing (Marina et al. 2009; Shahidi, 2006). The VCO has become increasingly popular as functional food oil. The increasing demand of VCO in the market, particularly in Southeast Asia including the Philippines, Thailand, Indonesia and Malaysia (Marina et al. 2009) is attributed to the ever increasing public awareness on the benefits of functional food. The VCO has many bio-active components from medium chain fatty acids (MCFA) comprising of lauric acid, capric and mysristic and is also high in phenolic content (Marina et al. 2009a,b). This makes VCO capable of reducing total cholesterol, triglycerides, phospholipids, low-density lipoprotein (LDL), very-low density lipoprotein (VLDL) and high-density lipoprotein (HDL) in serum and tissues in the body compared to coconut oil such as copra oil (Nevin & Rajamohan, 2004). In addition, the VCO can increase antioxidant enzymes (Dia et al., 2005; Nevin & Rajamohan, 2004) thus making VCO an anti-cancer agent, antimicrobial and antiviral activity from lauric acid components and through its easy digestibility from MCFA (Kamalaldin et al., 2015; Mansor et al., 2012). Hence, VCO is essentially selected as an added value substance due to its antiviral activity apart from the above said CPLE.
In developing an applicable edible O/W emulsion, an emulsifying agent is used to bridge between the oil and water by combining two parts of the molecule (hydrophilic and hydrophobic) as CPLE and VCO cannot mix spontaneously due to the different polar groups. The purpose of emulsifier addition is to enhance or improve the stability of the emulsion in order to maintain the quality of the formulation developed (Brown 2008). Whey protein isolate (WPI) has been chosen to be an emulsifying agent because WPI is widely used in the food industry. Furthermore, WPI is able to act as an emulsifier, with advantages such as in assisting in the formation and stabilization of the emulsion, particularly in the oil-in-water emulsion, reducing oxidative rancidity due to its oxygen-barrier properties (Lin and Krochta, 2005) and large amount of non-polar groups and surface-active proteins that can act as good emulsifiers (Hu et al., 2003; Girard et al., 2002). The properties of WPI depend on many factors such as the structure of an emulsion, the total charge, pH, ionic strength and the nature of the interface film produced (thickness, elasticity and viscosity) (Sun & Gunasekaran, 2009). During the formation of adsorption protein with oil droplets, the changes in the original protein conformation is dependent on the interaction between the interface of carboxylic and amino acids chains in the protein. Hydrophobic chains will bind to the surface of the oil while the hydrophilic chain was bound to the aqueous phase (Hu et al. 2003).
The development of products that combine CPLE and VCO has not yet been made commercially available in the form of edible emulsion. The existing products are in the form of capsule-like containing dried extract which may suffer from losses of essential phytochemicals during drying process apart from the unknown sources of the capsule material used which could leads to severe allergic. Therefore, it was necessary to produce potential functional foods by combining the CPLE and VCO to help cure dengue fever.
Dengue fever is a viral disease spread by the bite of infected mosquito species (Aedes aegypti mosquito). Dengue fever may occur from three to fourteen days after the bite of an infected mosquito (MOH, 2015; Forattini, 2003). In 2015, the Ministry of Health (MOH) Malaysia found that the dengue virus had spread more widely than anticipated. Currently, since January to July 2020, there were a total of 2,138 dengue cases reported, bringing the cumulative number of reported cases to 58,645 as of 11th July 2020. It is a decrease compared to 68,950 cases for the same period last year with a cumulative figure of 96 deaths in 2020, compared to 102 deaths during the same period in 2019 (WHO, 2020). In the event of these alarming number of cases, the main objective of this study was to formulate and to optimize an emulsion containing VCO as the oil phase and WPI as the surfactant with appropriate ratio of oil: CPLE: surfactant. This study further aimed to analyse the physico-chemical properties and evaluate the level of consumer acceptability towards the optimized formulae through food sensory evaluation.
2 Materials and methods
2.1 Materials
The commercially available virgin coconut oil (VCO) was purchased from Bio-Asli (M) Sdn. Bhd. Carica papaya leaves used were acquired from a local private farm at Dengkil, Selangor, Malaysia (certified by the Ministry of Agriculture (MOA), Malaysia) and were free of herbicides, pesticides, and insecticides. The leaves selection were based on its maturity (stage 4) and dark green colority for consistency. The isolated whey protein powder form was purchased from Lush Protein Pte. Ltd. (purity of 99%) with no added preservative.
2.2 Preparation of Carica papaya leaves aqueous extract
The standard preparation of Carica papaya leaves extract (CPLE) were based on Ministry of Health Malaysia protocols (MOH, 2018). The leaves were removed from the branch and washed under running tap water and immersed in a container filled with reverse osmosis water for 15 mins at 25 °C and repeated 3 times. The leaves were blanched using 1L of hot reverse osmosis water (50 °C) for 15 mins. Filter papers were used to remove any excess water and later the leaves were left to dry overnight at ambient temperature (30 ± 1 °C). The dry leaves were shredded to thin slices using kitchen knives and smashed using mortar. Then, juice was extracted from 80 g of treated fresh leaves using a juice extractor without any addition of water, under sterile conditions. The characteristics of the aqueous extract (e.g. phytochemical profiling) were identified elsewhere based on prior work (Subenthiran et al., 2013; Otsuki et al., 2010).
2.3 Ternary phase diagram Experimental designs
The phase diagram preparation was carried out and adapted based on elsewhere protocols (Omar et al., 2016; Gani & Adisah, 2015). Table 1 shows the 10 different concentrations between VCO and WPI with the ratio of 0:100 to 100:0 (w/w) as a base emulsion were prepared prior to adding CPLE (Equation (1)) ranging from 0% to 100% (w/w). The theoretical weight calculation for sum of CPLE (g) that was added in each of test tubes is as follows:
aTest tube
bVCO (%, w/w)
cWPI (%, w/w)
Theoretical weight of VCO (g)
Theoretical weight of WPI (g)
Actual weight of VCO (Ce) (g)
Actual weight of WPI (Cf) (g)
*C = Ce + Cf (g)
1
100
0
0.50
0.00
Ce1
Cf1
C1
2
90
10
0.45
0.05
Ce2
Cf2
C2
3
80
20
0.40
0.10
Ce3
Cf3
C3
4
70
30
0.35
0.15
Ce4
Cf4
C4
5
60
40
0.30
0.20
Ce5
Cf5
C5
6
50
50
0.25
0.25
Ce6
Cf6
C6
7
40
60
0.20
0.30
Ce7
Cf7
C7
8
30
70
0.15
0.35
Ce8
Cf8
C8
9
20
80
0.10
0.40
Ce9
Cf9
C9
10
10
90
0.05
0.45
Ce10
Cf10
C10
a = Weight of CPLE (g)
c = Weight of VCO (Ce) (g) + weight of WPI (Cf) (g)
d = Weight of CPLE added for each circulation/weight of total sample mixture (% w/w)
The mixture with a total weight of 0.5 g was placed in a 10 ml screwcap glass tube. Then, samples were vortexed using a mixture vortex (LBS-WSS-178, India) for 15 mins and centrifuged using centrifuge (GZ-406, Maryland, U.S.) for 15 mins at 4000 rpm (operational temperature: 25 ± 2 °C). Visual observation were carried out to determine the phase behaviours. The instable emulsions were separated to two or three layer after centrifuging. The stable emulsions maintained their one phase homogenous color solution and without any immiscible phases after centrifuging. Ternary Phase Diagram System were drawn and developed accordingly using Chemix Software version 3.6 (Arne Standnes, Norway) to detect the existence of phase region.
2.4 Ternary phase diagram compositional ratios selection
Eleven points were randomly selected from the well-developed homogenous phase ternary phase diagram system (Sanjeewani & Sakeena, 2013). Table 2 shows the best and randomly selected 11 points of formulation based on the WPI concentration in the range of 20% to 30% (w/w) with a medium type of viscosity. A total of 11 g emulsion were prepared (varied ratio of VCO, WPI and CPLE) and CPLE only (labelled as samples A, B, C, D, E, F, G, H, I, J and K). All formulation were prepared in triplicates (n = 3). The stable emulsion produced were only selected (after completion of visual observations) post-centrifuged at 4000 rpm for 15 mins prior to physico-chemical analysis. Hence, sample I was considered the best formulation among the 11 formulations as it did not produce any unstable conditions such as separation phase, creaming and coagulation. Due to the high aqueous nature of the selected stable formulation, 3 new formulations were prepared for further studies. Sample I underwent an addition of + 2% (Sample L) and reduction of −2% (sample N) VCO content to determine their viscosity, storage stability and sensory accpetance. Table 3 shows the compositional ratios of VCO, WPI and papaya leaves extract for three new formulations. Sample I was considered as a new baseline (control) and labelled as M while sample L and N as mentioned above. Three formulation were prepared as minimum requirement for hedonic test (Rao et al. 2013). *Weight of each formulation = 10 g. *Addition of + 2% (L) and reduction of −2% (N) of VCO from the baseline Sample I (M).
Sample Formulation*
VCO (g)
WPI (g)
CPLE (g)
VCO: WPI: CPLE (%, w/w)
A
0.50
3.00
6.50
5 : 30 : 65
B
1.00
2.50
6.50
10 : 25 : 65
C
1.00
3.00
6.00
10 : 30 : 60
D
1.50
2.50
6.00
15 : 25 : 60
E
1.50
3.00
5.50
15 : 30 : 55
F
2.00
2.50
5.50
20 : 25 : 55
G
2.00
2.00
6.00
20 : 20 : 60
H
2.40
2.70
4.90
24 : 27 : 49
I
2.50
3.00
4.50
25 : 30 : 45
J
3.50
3.00
3.50
35 : 30 : 35
K
3.50
2.00
4.50
35 : 20 : 45
Formulation Sample*
VCO (g)
WPI (g)
CPLE (g)
VCO:WPI:CPLE (%, w/w)
L
2.70
2.80
4.50
27 : 28 : 45*
M (control)
2.50
3.00
4.50
25 : 30 : 45
N
2.30
3.20
4.50
23 : 32 : 45*
2.5 Physico-Chemical analysis of the formulated emulsion
2.5.1 Emulsions stability (Centrifugation Test)
All emulsions were transferred into glass vials containing approximately 3 ml of the formulated sample filled into 5 ml screw tube and centrifugation test at 4000 rpm for 15 mins at 25 ± 2 °C (Saharudin et al., 2016; Huang et al., 2001). After the test, the emulsions separated into optically opaque “cream layer” at the top and a transparent (or turbid) “serum layer” at the bottom. Equation (2) shows the creaming index measured based on sum of height emulsion (HE) and height of lower depleted layer (HL). Measurements were conducted in triplicates (n = 3).
HE: Sum of height emulsion (cm)
HL: Height of lower depleted layer (cm)
2.5.2 The pH profiles
Approximately 10 ml of the formulated samples were poured into 20 ml glass beaker. The pH values were measured by pH meter (PHM 210, radiometer analytical SAS, France) at 28 ± 2 °C. Three measurements were taken for each sample (n = 3). Prior to reading, the pH meter was calibrated by using pH 7.01, 4.01 and 10.01 buffer solutions respectively (Sanjeewani & Sakeena, 2013).
2.5.3 Viscosity profiles
Approximately 50 ml of the formulated samples (L, M and N) were prepared and filled into 50 ml glass beaker. Then, the emulsion was homogenised using Homogenizer Disperser Mixer (AD500S-P: 300–28,000 rpm, Nanjing, China) for 5 mins at 10,000 rpm. Emulsion viscosity was measured using Brookfield viscometer (LV2, Brookfield Inc., U.S.A) with a T-bar spindle, spindle number 7 (length 5 cm, diameter 3 cm). The viscosity of samples were recorded based on different spindle speed (10, 20, 50 and 100 rpm) for 3 mins (Rao et al. 2013). Measurements were conducted in triplicates (n = 3).
2.5.4 Color profiles
Color test (L*, a* and b*) for samples L, M and N were measured on the surface of a glass plate using Minolta colorimeter, chromameter model (CR 400, Japan). The equipment was calibrated using a white A4 plain paper prior to measuring the samples. Measurements were conducted in triplicates (n = 3).
2.5.5 Storage stability study
Approximately 10 g of the formulated samples (L, M & N) were transferred into universal bottles and tightly sealed with a cap. All samples were placed at three different temperatures (4, 28 and 45 °C) using a chiller and oven drying for 24 hrs. The occured separation phase and creaming index were recorded and calculated respectively (Sanjeewani & Sakeena, 2013; Lin and Krochta, 2005). Measurements were conducted in triplicates (n = 3).
2.6 Sensory evaluation
Sensory evaluation was carried out to determine the acceptance of panelists towards the sample formulation of L, M (Sample I) and N by using the 7 point hedonic scale (1 = most dislike, 7 = most liked). Five attributes (color, viscosity, odor, bitterness and overall acceptance) were determined (Aminah, 2015). This study was carried out involving 30 panelists (23 female and 7 male students) from the Faculty of Science and Technology (FST), UKM students and staffs.
2.7 Statistical analysis
Chemix software (Arne Standness, Norway) was used to develop the Ternary Phase Diagram System. Minitab version 17 (Minitab Inc., Sydney, Australia) was used data analysis (reported as average and standard deviation) and Fisher test was used to determine the significant difference between all formulated samples (p < 0.05).
3 Results and discussion
3.1 Ternary phase diagram system
Based on Fig. 1, two regions were succesfully formed which are the homogeneous (H) and a multi-phase (M) regions. Homogeneous region is a single phase system and stable while the multi-phase regions has more than one phase and are not stable as it produced a creaming layer. Therefore, ternary phase diagrams were used to reveal the abilities of VCO, WPI and CPLE to produce homogeneous and stable emulsion. In fact, it is clearly shown that WPI acts as an excellent emulsifier which produces large homogeneous region along the apex axis of 4% (w/w) to 53% (w/w). Maximum concentration of VCO that can be emulsified by WPI to produce a homogeneous emulsion was 40% (w/w) with 10% (w/w) of WPI. In addition, 30% (w/w) of CPLE was able to produce a homogeneous emulsion using 48% (w/w) of WPI and 21% (w/w) of VCO. The uncolored region was a region that produces formation of multi-phase emulsion. Multi-phase region occurs due to several factors such as the emulsifiers was insufficient to protect tiny oil droplets from flocculation (Thanasukarn et al. 2004). The size of the oil droplets that were not small enough for structural proteins adsorb on the surface of the droplet (Qian et al. 2011), the proximity of pH to the isoelectric point and the type of emulsifier was not suitable when applied to other types of emulsions such as water-in-oil.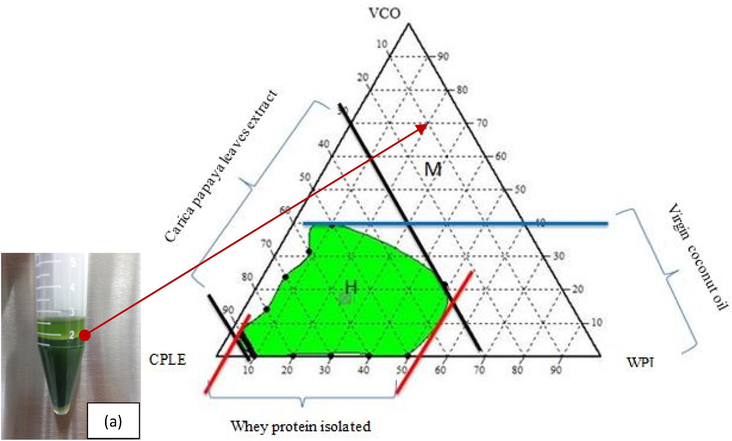
Ternary phase diagram for VCO/WPI/CPLE. The letter H indicates the homogeneous region and the letter M shows the multi-phase region. (a) The inset (bottom left) shows the pictorial view of an instable formulation at the multi-phase region M after 15 mins of vortexed and centrifuged at 4000 rpm (25 ± 2 °C).
Oil droplet size affects the formation of the emulsion as the smaller size of oil droplets is capable of forming a stable emulsion. When the size of droplets is small, WPI will easily adsorb on the surface of the droplets and protects the oil droplets from the flocculation process (Adjonu et al., 2014ab; Qian et al., 2011). In these studies, the vortex has been used to mix the VCO, WPI and CPLE preliminarily. The use of vortex was able to generate a turbulence condition by breaking down oil from large into smaller oil droplets. However, it will result in inconsistent oil droplets size as compared to using homogenize process which involves the mechanical breakdown of oil droplets which produce consistent size of droplets.
In addition, WPI was not capable to act as an emulsifier for water-in-oil because the pH of VCO was 4.47 ± 0.03, which was near the isoelectric point. When WPI is in solution with pH close to the isoelectric point, the ability of protein as amphiphilic molecule and to act as a membrane to protect the oil droplets from flocculation were affected (Lin and Krochta, 2005). An amphiphilic molecule has a hydrophobic and hydrophilic structure. Hydrophobic structures will bind with non-polar compounds such as oils, while the hydrophilic structure serves as a binder with compound polar molecules such as water (Charoen et al. 2011). This results in the formation of a creaming layer in an emulsion. Therefore, WPI is able to act as an emulsifier to produce large homogeneous region with WPI's ability to produce a homogeneous emulsion along the apex axis from 4% (w/w) to 53% (w/w).
3.2 Physical properties characteristics
3.2.1 Emulsions Stability: Creaming index
The physical stability of the formulated emulsions was investigated in this study and could be reflected by creaming index. Post stability testing under stress conditions (i.e. centrifugation test), the emulsions underwent visible phase separation, with a white cream layer on top of a clear serum layer. This test was selected specifically for this experiment because no addition of preservatives was added to the emulsion formulation samples. The creaming index stability test was performed to refrain other factors such as microorganisms or activities that promote fat oxidation which may cause destabilization of the emulsion. Based on the Fig. 2 and Table 2, a total of 11 point formulation samples were selected for further analysis to test the resulting homogeneous regions. The selected point was in the range of 20% to 30% (w/w) along the apex axis of WPI. Apart from that, the apex axis of VCO started from 5% (w/w) to 35% (w/w), while apex axis for CPLE started from 35% (w/w) to 65% (w/w). These points were selected to produce an emulsion which has a moderate viscosity (for easy mobility) prior to physico-chemical analysis (Sanjeewani & Sakeena, 2013).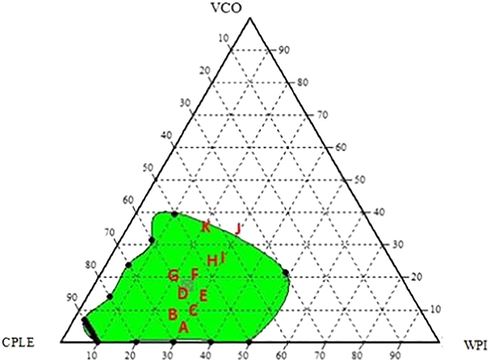
Randomly selected 11-points of emulsion formulation based on ternary phase diagram system prior to physico-chemical and stability analysis.
After centrifuged at 4000 rpm for 15 mins, the results on eleven formulations found that only sample I had good stability and produced a homogeneous emulsion. Fig. 3 shows the creaming indexes that have been measured for all samples which aims to determine the stability of the samples and were found to be significantly different between samples (p < 0.05). The creaming index (%) shows formulation sample K has the highest creaming index 47.78 ± 3.85% as compared to other formulations of formulation samples (p < 0.05). In contrast, formulation sample I showed the absence of the creaming index (%).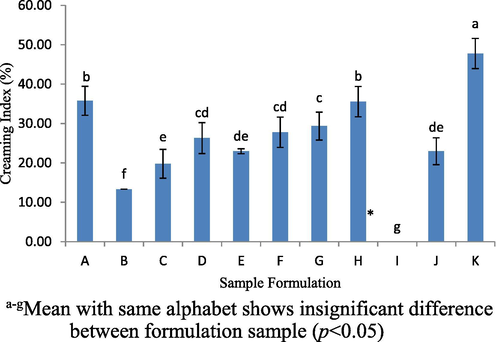
Creaming index (%) for 11-formulated samples determined by centrifugation test at 4000 rpm for 15 mins. *creaming index = 0, indicating stable emulsions after stability testing.
The resulting of creaming index (%) shows the emulsion was quite unstable condition. This was because, all sample formulations were produced by using a vortex (homogenizer is considered the best option). Although, the use of a vortex was not sufficient enough to breakdown the oil droplets and protein to a consistent size but compared to the use of low molecular weight surfactants (e.g. Tween 20, Tween 80 and Tween 85), they easily adsorb the oil droplets even just using a vortex alone (Gani et al. 2015). However, this experiment clearly demonstrates the formulation of sample I create a stable emulsion system using centrifuge at 4000 rpm. Therefore, sample I was selected for further studies by adding (+2%) and subtracting (-2%) of VCO composition of the sample I (labelled as M and N respectively) prior to the hedonic test which requires minimum of 3 different concentrations.
When the emulsion formulation samples were centrifuged at 4000 rpm, oil droplets do not have strong protein membranes to protect the oil droplets from easily and quickly being attracted to each other to form a creaming layer in the emulsion. For this reason, emulsions tend to create different layers commonly consisting of an oil layer. The oil layer will be at the water surface as oil compounds have lower density than water (McClements & Decker, 2000). Furthermore, a stable emulsion has sufficient charge to prevent oil droplets from being attracted to one other in emulsion thus does not produce a link between oil droplets (Thanasukarn et al. 2004). Multi-phase emulsions were formed due to clustering of oil droplets. This happens because of the possibility of destabilizing the droplets resulting from the formation of the weak film interface around the droplets that promote the formation of larger droplets thus creating a layer of cream (Van Aken et al., 2003; McClements & Decker, 2000). Overall, sample L, M and N produce no creaming layer/coagulation and were stable (do not produce any multi-phase emulsion) after centrifuged at 4000 rpm for 15 mins.
3.2.2 The pH profiles of formulated emulsion
In the emulsion formulations, pH test should be conducted to investigate the effect of pH in the solution. This is because protein is susceptible to the pH in the solution, especially when the pH of the emulsion is near or at the isoelectric point thus quickly generates agglutination of oil droplets in the formulation (Adjonu et al., 2014ab; Lin and Krochta, 2005). The pH of the prepared CPLE was neutral in nature (7.02 ± 0.02) and this is in accordance to the MOH guidelines in preparing the remedies. Besides, no additional water and preservatives in the CPLE was added and was stored at a temperature of −18 °C to avoid any spoilage due to the growth of microorganisms or fungi. However, pH of VCO was slightly acidic (4.47 ± 0.03). This was due to the added preservatives in product to prevent the growth of microorganisms and impair product characteristics such as taste and odor VCO.
Fig. 4 shows that the average pH of all samples was ranging from 6.0 to 6.4 (p < 0.05). In addition, the pH of all samples was also approaching 7. Increasing the composition (%) of the VCO, WPI and CPLE exhibited different changes to the sample pH values. Samples A and K have higher pH than the other samples (p < 0.05). For sample A, the pH was nearly neutral because lower concentration of VCO which was 5% (w/w) and 35% (w/w) may not influence the pH formulation to become acidic and tends to be neutral (Kulmyrzaey et al. 2000). Besides, the pH of the sample B and sample D was the lowest as compared to the other samples (p < 0.05) due to the fact that the amount of WPI and CPLE was more than VCO thus affecting their acidity.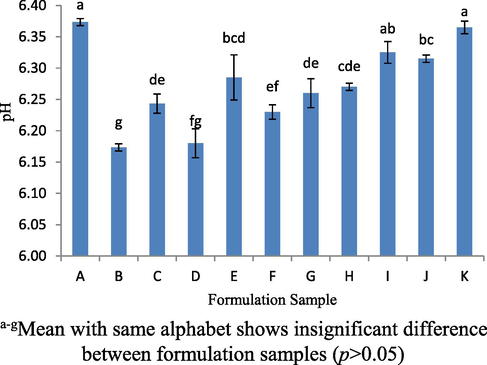
The pH values for 11 formulation samples.
Next, WPI was found to be easily affected by changes in pH due to isoelectric point (charge of the protein was neutral or zero) (Chanamai & McClements, 2002). When the protein is in neutral charge, this means that the structure of the protein lacks the ability to act as a good emulsifier and will result in quick flocculation of oil droplets. Therefore, there was no interaction formed between protein structures with oil droplets. The isoelectric point of the WPI was about pH 4.8 (Lin and Krochta, 2005). Thus, the pH factor was not the cause of the samples having different emulsion layers as a sample I was able to produce a homogeneous phase. Other factors such as the size of the oil droplets that was not small enough to disable the ability of protein to adsorb oil droplets that form protective membranes to prevent flocculation of oil droplets. Therefore, it would lead to an increase in electrostatic repulsion between oil droplets (Chanamai & McClements, 2002).
Fig. 5 shows the pH value of the formulation samples L, M and N which were the extension of formulation sample I. All samples had pH values around pH 6.09 to 6.56 (p < 0.05). Sample M has a lower pH than the samples L and N (p < 0.05). This was because when the amount of both VCO and WPI was almost high, the protein could easily influence the carboxyl group by becoming more acidic due to obtaining more negative charge (Kulmyrzaey et al. 2000). However, the pH of the emulsion of all samples did not approach the isoelectric point but approaching pH 7. Proteins are made up of two groups, namely β-lactoglobulin and α-lactalbumin. At pH 7 (room temperature: 28 ± 2 °C), WPI comprising the β-lactoglobulin and α-lactalbumin will adsorb oil droplets with the same affinity, but at a lower pH or higher temperature, α-lactalbumin adsorb more readily than β- lactoglobulin (Thanasukarn et al. 2004).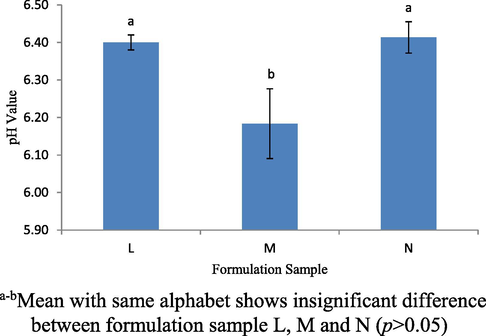
The pH values for formulation samples L, M and N.
When WPI is used as an emulsifier, the pH near the isoelectric point causes low charges on the oil droplets and the electrostatic repulsion. The increase in ionic strength causes electrostatic repulsion between the droplets to decrease as a counter ion in the aqueous phase to protect the charge on the surface of the oil droplets (Kulmyrzaey et al. 2000). Therefore, flocculation of droplets easily occurred and leads to unstable emulsions. In terms of the charge on hydrophobic and hydrophilic groups, when the pH is higher than the isoelectric point, the magnitude of the positive charge of the oil droplets will decrease because some carboxyl groups (–COO-) become negatively charged and the amino groups become neutral (NH2). On the other hand, a further increase in pH results in oil droplets having an increased net negative charge as the increasing number of negatively charged groups and positively charged groups (Kulmyrzaey et al. 2000). Overall, all of the formulations produced a pH value, which was not close to the isoelectric point.
3.2.3 Emulsion viscosity
Based on Fig. 6, there was a protuberant differences of fluid behaviours between all formulated samples L, M and N (p < 0.05). The profiles were considered as dilatant non-Newtonian fluid. Sample N has the lowest viscosity as compared to other samples (p < 0.05) despite the fact all samples had the same concentration of CPLE (45% (w/w)). It seems that the amount of VCO affects the viscosity of all samples where the higher the oil content, the lower the viscosity attained. The findings were consistent with the oil content in sample N, M and L of 22, 25 and 28% (w/w) respectively.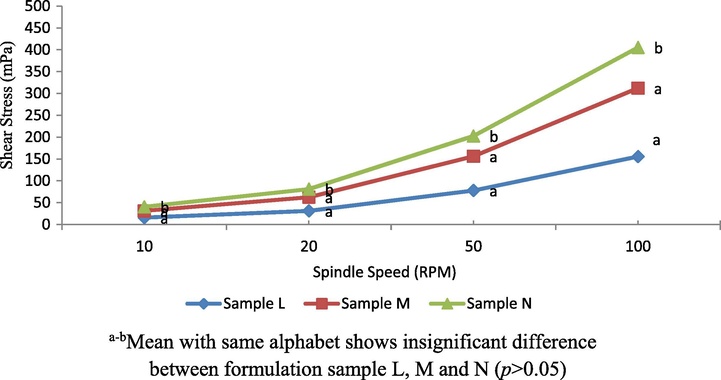
Dilatant non-Newtonian fluid profiles of sample L, M and N.
Previous studies stated that the higher the oil content, the higher the viscosity that is produced (Qian & McClements, 2011; Girard et al., 2002). This clearly shows that the addition of oil in emulsion will affect the viscosity of the resulting emulsion. In this study, the samples L and M produce higher viscosity as compared to sample N. This is because when oil content was high and oil droplet size was small, this caused oil droplets to be very close to each other and tend to create clustering due to colloidal interactions thereby increasing the viscosity of the emulsion (Van Aken et al. 2003). Shear rate depends on the force of attraction between the oil droplets. The stronger attractive forces, the higher the shear rate (Hayati et al. 2007). In addition, the emulsion that has a lower droplet size will have a strong attraction between the droplets. This is because a smaller distance between the droplets is produced when the droplet size is small compared to the large droplet size (Qian & McClements, 2011; Hayati et al., 2007).
The increase of WPI (%) also affects the viscosity. The presence of protein in the continuous phase of the emulsion can increase the viscosity, reduce movement and absorb the oil droplets in the emulsion (Lam & Nickerson, 2013). Concentration of WPI in the emulsion can also be the cause of the initial increase in viscosity. This however improves the stability of the emulsions as the spindle movement was limited by the formation of protein adsorption of the oil droplets (Kulmyrzaey et al. 2000).
3.2.4 Emulsion color profiles
The color profiles of samples L, M, N and control (CPLE) was measured to observe of any changes that was attributed to the addition of CPLE into VCO and whey protein base mixture. The formulation color profiles will indeed affect the acceptance of the consumers and this will be an essential quality indicator prior to final product appearances (Jha, 2010). Three indicators were used to determine the color of the L*, a* and b*, where the higher L* value the brighter the sample. While the + a value was a reddish color and -a value was a greenish color. + b value also was a yellowish color while -b was a bluish color. Comparisons were also made between samples L, M, N and CPLE to find the resulting differences between samples. Fig. 7 shows a significant difference (p < 0.05) of L* values between samples CPLE and L. CPLE showed lower L* value compared to other samples. This was because WPI was white color while VCO was lacquer color. When they are mixed into the samples L, M and N, formulation samples will be brighter than the CPLE. However, the L* values of all samples indicated no significant difference (p > 0.05). Fig. 8 shows the a* value of among samples. No significant difference (p > 0.05) was observed between samples CPLE, L and M. as well as between samples L, M and N. From the results obtained, significant difference (p < 0.05) was only observed between CPLE and M only. All samples had negative values of a* which was showing green color hue. The green color comes from the chlorophyll contained in CPLE. This study showed the addition of WPI and VCO does not affect the green color of CPLE. The highest b* values showed in CPLE sample compared to other samples (p < 0.05). Referring to Fig. 9, b* values for all samples were positive which indicate yellowish color. Samples L, M and N contain lower yellowish color as compared to CPLE sample. This was because the addition of the VCO and WPI had reduced the yellowness color in samples L, M and N. Overall, the addition of VCO and WPI changed the L* and b* values in the sample L, M and N except the a* value. Those profiles were quite anticipated especially on the (-ve) a* value as it would succumb into greenish-like emulsion due to its prominently strong chlorophylls color concentration.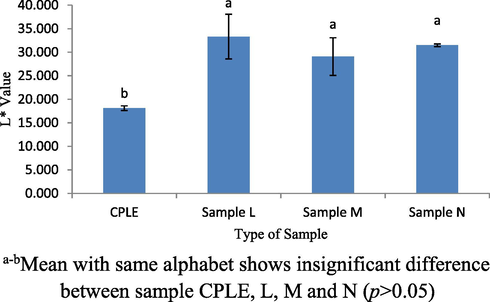
L* value for CPLE, L, M and N sample.
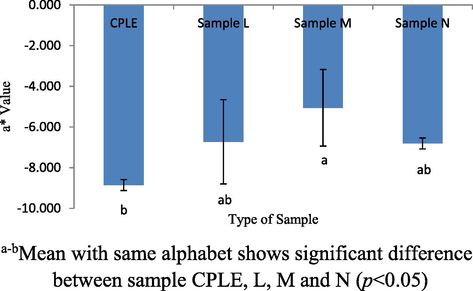
a* value for CPLE, L, M and N sample.
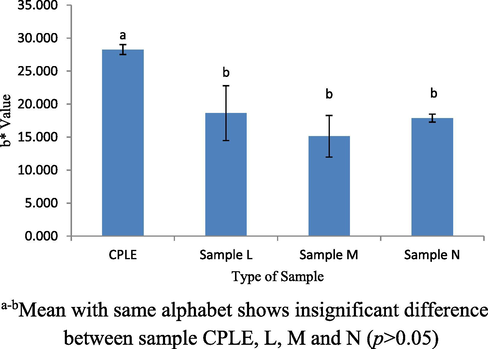
b* value for CPLE, L, M and N samples.
3.2.5 Storage stability
Storage stability test was conducted to study the stability of the emulsion at different temperatures within a certain duration. In this study formulation samples L, M and N were placed in three different temperatures to evaluate the changes that occur. Based on the studies, all samples were kept at different temperatures, which were at 4, 28 and 4 °C/45 °C for 24 hrs. At 4 °C, all formulation for samples L, M and N were in stable condition, do not produce creaming layer and generate multi-emulsion (immiscible layers) but not at 28 °C and 4 °C/45 °C. Based on the Fig. 10, sample L shows separation layer of emulsion exists at a temperature of 28 °C. The creaming index for the sample L was 56.67 ± 5.77% which by far distinguish with the other 2 samples (p < 0.05). Meanwhile when samples were stored at 4 °C/45 °C, all samples produced a creaming layer. Fig. 11 shows the creaming index profiles for the samples L, M and N was 61.67 ± 2.89%, 15.00 ± 5% and 5.00 ± 0.00%, respectively, which was stored at 4 °C/45 °C (p < 0.05). In this study, it clearly shows sample L produce a larger creaming layer as compared to sample M and N at 28 °C and 45 °C. This was because, the VCO concentration was higher in sample L and may easily be influenced by lipid oxidation activity of VCO and WPI. Thus, it tends to produce a separation layer.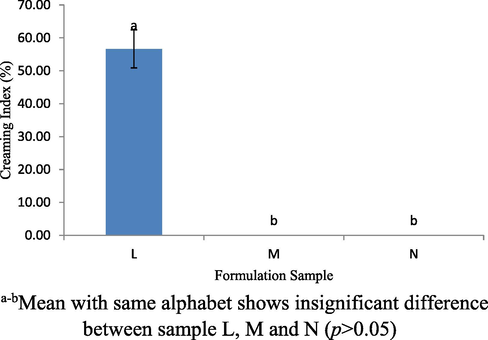
Creaming index (%) for L, M and N sample stored at 28 °C for 24 hrs.
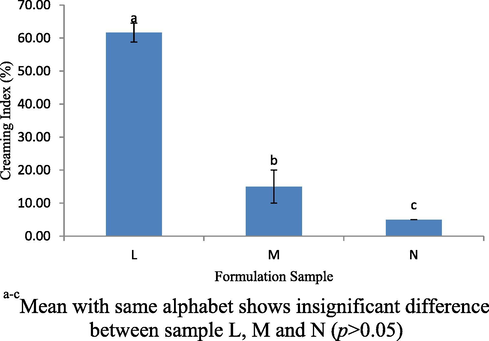
Creaming index (%) for L, M and N sample stored at 4 °C/45 °C for 24 hrs.
In the storage stability test, flocculation of droplets was slower at 4 °C compared to at temperatures of 28 °C and 45 °C because the rate and frequency of collisions between droplets of oil was lower at storage temperature and reduce the increasing size of the oil droplets in the emulsion (Mcclements, 2004). Previous study has shown that nanoparticles of solid fat storage at 50 °C makes the oil droplet size increase rapidly due to the increase in the kinetic energy and accelerate the collision between the oil droplets (Mcclements, 2004). This corresponds to the conditions in this study where all samples produce a creaming layer at 45 °C. Increased collision between droplets will influence the oil droplets with the tendency to produce a creaming layer. In addition, the droplet size distribution in emulsion that is kept at 45 °C and 28 °C shifts toward larger droplet size than the emulsion stored at 4 °C.
Generally, the formulation of samples L, M and N that was stored at 28 °C and 4 °C/45 °C show a dry creaming layer/dry in the upper part of the storage bottle. This may be due to protein molecules adsorbed at the interface of the oil droplets consisting of large and small proteins (Van Aken et al. 2003) resulting in an uneven adsorption, discontinuous and weak mechanical stability of the adsorbed film interface. Therefore, the oil drip rate has increased. Oil droplets were then combined and produced a creaming layer in emulsion. Furthermore, dry creaming layer that exists at storage temperature 28 °C and 45 °C may be associated with the evaporation of water from the emulsion droplet interface and resulting in production of dry films (Van Aken et al. 2003). Next, flocculation in the depletion emulsion occurs when the attractive force between the oil droplets rose against repulsive forces between the oil droplets (Tesch & Schubert, 2002; Thanasukam et al., 2004). When Van der Waals bonding dominate the oil droplets, it will cause the attraction between the droplets but not when the electrostatic repulsion dominates the oil droplets (Lin and Krochta, 2005). In addition, the Fig. 12(a) shows that all samples which were stored at 4 °C exhibited no changes in coloration. However, based on Fig. 12(b), sample L changed from green to brown color when stored at 28 °C for 24 hrs. Based on the Fig. 12(c), all samples changed to brown color when stored at 45 °C for 24 hrs. This was because the VCO was a functional food which has medium chain fatty acids such as lauric, capric and myristic acid (Marina et al. 2009a,b). Therefore, VCO can easily be exposed to the fatty acid oxidation activity with the presence of oxygen (McClements & Decker, 2000). Fatty acids oxidation activities produced by fat in the WPI and VCO also affect the color, texture and smell (Hu et al. 2003). In addition, the change in the color of samples may be due to the occurrence of browning reaction because the CPLE does not contain any preservatives and was exposed to high temperatures. Thus, this leads to instability of the emulsion due to increased clustering of oil droplets in the samples. However, samples L, M and N did not show any changeof color from green to brown at low temperature which was at 4 °C. This was because the storage of perishable materials such as WPI, VCO and CPLE at low temperature can protect and maintain the quality of products from microbial growth and depletion of chemical reactions such as oxidation of fats (Charoen et al. 2011).
The pictorial representatives of creamy formulations of sample L, M and N stored for 24 hrs at (a) 4 °C; (b) 28 °C; (c) 4 °C/45 °C.
3.3 Sensory acceptance profiles
Sensory evaluation should be performed to test whether the sample is acceptable to consumer in terms of taste. If it does not meet consumers’ preference, the product will suffer a loss in the market. Sensory evaluation tests were carried out using 7 point hedonic scale. The hedonic test can be defined as a relationship to or associated with satisfaction and to measure directly the degree of preference or acceptance of the product by panellists. Hedonic scale test was selected for this study because information on preference can be obtained compared with other tests (Aminah, 2015). To the best of our knowledge, there is no existing edible emulsion product containing VCO and CPLE that uses WPI as a food grade emulsifier in the market. By referring to Fig. 13, in terms of the color attribute, sample L, M, N and CPLE did not show any significant difference (p > 0.05). This was due to the clear color from VCO and white color from WPI. Thus, both compounds did not give any effect on the color of CPLE since it was added in larger quantity than VCO and WPI. Meanwhile, the highest (p < 0.05) viscosity was found for CPLE. It is because the viscosity of CPLE was not similar to the sample L, M and N samples with added VCO and WPI. The panellists preferred formulation which is highly liquid. There were significant differences (p < 0.05) towards odor attributes between sample L, M, N if compared to CPLE. The higher preference score for odor in samples L, M and N were attributed from VCO. Most of the panellists preferred the odor of samples L, M, and N which has mild coconut smell compared to strong leaf odor in CPLE. For the bitterness attribute, significant difference (p < 0.05) can be observed between samples N and CPLE (p < 0.05). As overall acceptance, samples L, M, N were significantly different (p < 0.05) compared to CPLE. However, no significant differences were observed between samples L, M and N. This is because CPLE containing slightly higher bitter taste than the samples L, M and N. Therefore, the addition of WPI and VCO in samples L, M and N was accepted by panellists in the study as compared to CPLE (without additional VCO and WPI).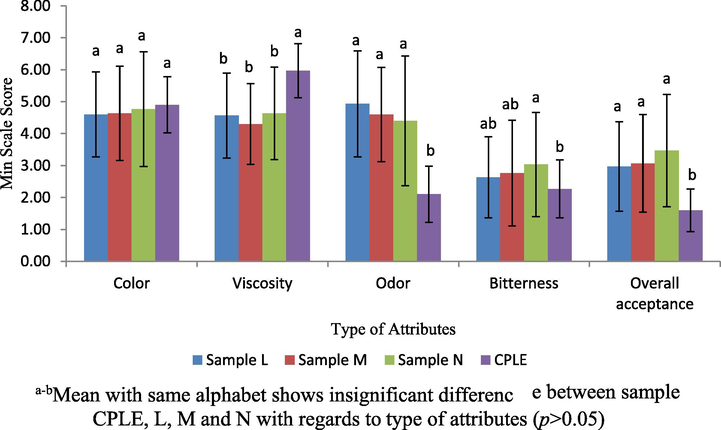
Scoring scale sensory evaluation based on color, viscosity, odor, bitterness and overall acceptance attributes towards sample L, M, N and CPLE.
Most of the panellists were attracted to the odor of all sample formulations compared to the odor of CPLE. But when panellists tasted all the samples, the perspective of odor pleasantness towards sample L, M and N changed because of the bitterness. Bitterness comes from CPLE which is rich in saponins (Syed Amran et al. 2018) and essentially flavonoids, which has its own particular properties as an agent to increase blood platelets in dengue fever patients (Subenthiran et al. 2013). Although sample L has VCO as high as 27% (w/w) compared to the sample M and N which was 25% (w/w) and 23% (w/w) respectively, panellists were not able to identify the differences in terms of the color, viscosity, odor, bitterness and overall acceptance attributes. This was because VCO and WPI has no prominent taste when consumed (Villarino et al. 2007). Therefore, they are not able to mask the bitter taste from CPLE. Overall, sample N was better prefered than samples L, M and CPLE because it has a low concentration of VCO with acceptable viscosity, bitterness, odor and overall acceptance attributes. Medicinal products from leave extracts which have a bitter taste, tend to get a lower rating for preference score from the panellists. This is because not many people in general would like to consume bitter medicine and will eventually opt for sweet/savory taste medicines. It is note worthy for future analysis, some flavoring can be added to the product to reduce the bitter attribute.
3.4 Product formulation in accordance to MOH intake recommendation for therapeutic application (Dengue fever Treatment)
To the best of our knowledge, there are no specific alternative natural therapeutic products to treat dengue fever commercially available in Malaysia. The traditional way of treating dengue fever is by consuming directly of the CPLE. With it undesirable bitter taste, the patient would become very unpleasant throughout the whole treatment period. For that reason, this section represents the effective dosage edible emulsion preparation (based on the best formulation attained) in accordance to the Ministry of Health (MOH) recommendation.
3.4.1 Calculation of effective dose of raw CPLE (Based on the MOH Recommendation)
Table 4 shows the specification of the best formulation (sample I) which has been obtained from the developed ternary phase diagram (Fig. 1). The composition of CPLE in the formulation should bare the minimum effective dose to cure dengue fever by increasing the platelet count in patients (Subenthiran et al. 2013). According to the Ministry of Health, Malaysia (MOH, 2018), patients are recommended to be given two tablespoons of CPLE (34 g) for three consecutive days based on the phase 1 clinical trial beforehand (40 patients involved). Therefore, an approximately 90 ml of CPLE (equally to 102 g = 34 g × 3 days) is considered as a minimum effective dosage that should be taken by the patient (based on the CPLE density). *Density of emulsion Sample I = 5 g/4.22 ml = 1.18 g/ml.
Ingredient
Concentration(%, w/w)
Weight (g)
Density (g/ml)*
VCO
25
1.25
1.24
WPI
30
1.50
Powder form
CPLE
45
2.25
1.13
3.4.2 Satisfactory effective dosage should be taken based on the formulated emulsion
-
Approximate volume of 1 standard tablespoon: 30 ml of formulated emulsion.
-
Weight of 1 tablespoon of formulated emulsion:
30 ml/1.18 g/ml (emulsion density) = 25 g.
Compositional weight distribution of the formulated emulsion (based on Sample I)
VCO = 25% x 25 g = 6.25 g.
WPI = 30% x 25 g = 7.50 g.
CPLE = 45% x 25 g = 11.25 g.
Therefore, approximately 3 tablespoons/day (twice daily) of the formulated emulsion are needed for 3 consecutive days ( tablespoons in the morning and tablespoons in the evening) with a total volume of 270 ml (320 g emulsion weight) for the treatment to be equally potent as consuming raw CPLE. In fact, any excess intake of CPLE has no adverse effects on patients. Previous study to determine the effect of CPLE on rats for 28 days has showed oral doses given to mice (Sprague Dawley) at 0.01, 0.14 and 2 g/kg did not give any toxic effect to the rodent’s internal organs such as heart, liver and kidney (Ismail et al. 2014, Abdullah et al. 2009). Furthermore, the emulsified product appeals should perhaps be taken seriously as consumer nowadays are looking for a good looking coloration with the addition of ‘superfood-based’ ingredient which could provide great health benefits (e,g. antiviral and antioxidant properties).
4 Conclusion
The three-phase diagram was constructed consisting of three components, namely, VCO,WPI and CPLE. Further research has been conducted by taking eleven points in homogenous regions by labeling the sample A, B, C, D, E, F, G, H, I, J and K. It was found that only sample I produced a homogeneous phase stable and the best emulsion. Physico-chemical characteristics of the sample L, M and N were determined by emulsion stability test (creaming index), pH test, viscosity test, color test and storage stability test. The centrifugation test found that sample L, M and N did not produce creaming layer on the sample. For pH test, the sample L, M and N did not approach isoelectric point of WPI. Tests also showed that the viscosity of sample N was lower than samples L and M (p < 0.05). The L* and b* value indicate significant differences between the control (CPLE), L, M and N sample except for the a* value. For storage stability tests, samples did not produce any creaming layer and discoloration at 4 °C. Sensory evaluation using hedonic scale test was conducted and samples were evaluated for viscosity, odor, bitterness and overall acceptance attributes which had significant difference with the control sample (CPLE) (p < 0.05) except for the color attributes. This shows the addition of VCO and WPI affect the assessment of the viscosity, odor, bitterness and overall acceptability as compared with CPLE sample. Overall, sample N was the best formulation causing with lower viscosity than sample L and M. In fact, sample N was stable during storage treatment at a temperature of 4 °C and 28 °C for 24 hrs. Based on calculations of the minimum effective dose formulation, at least 320 g emulsion (270 ml) by taking 3 tablespoons daily for 3 days in a row is needed for the effectiveness of this emulsion to work in helping to cure dengue fever.
Acknowledgment
The authors would like to thank Universiti Kebangsaan Malaysia (UKM) for providing financial support to this project (GUP-2018-057).
Data Availability Declaration
No data were used elsewhere to support this study and it was entirely a new set of data.
Conflict of Interest Declaration
The authors declare that they have no conflicts of interest. The publication of this work in your journal is approved by all authors and we responsible that work was carried out, and, if accepted, it will not be published elsewhere including in English or in any other language, without the written consent of the copyright-holder.
References
- Acute Toxicity of Orthosiphon stamineus Benth Standardized Extract in Sprague Dawley Rats. Phytomedicine. 2009;16(2):222-226.
- [Google Scholar]
- Proliferative Activity of Saponin-Reducing and Carica Papaya Leaves Extracts on Human Lung Fibroblast Cell (IMR90) Jurnal Teknologi (Sciences & Engineering). 2015;75(1):1-6.
- [Google Scholar]
- Whey Protein Peptides as Components of Nanoemulsions: A Review of Emulsifying and Biological Functionalities. Journal of Food Engineering. 2014;122:15-27.
- [Google Scholar]
- Formation of Whey Protein Isolate Hydrolysate Stabilised Nanoemulsion. Food Hydrocolloids. 2014;41:169-177.
- [Google Scholar]
- Panduan Makmal Penilaian Sensori. Bangi: Penerbit Universiti Kebangsaan Malaysia; 2015.
- Adsorption of Saponin Compound in Carica papaya Leaves Extract Using Weakly Basic Ion Exchanger Resin. AIP Conference Proceeding. 2016;1784:030039
- [Google Scholar]
- Phytochemical Screening and Antioxidant Activities of Some Selected Medicinal Plants Used for Malaria Therapy in Southwestern Nigeria. Tropical Journal of Pharmaceutical Research. 2008;7(3):1019-1024.
- [Google Scholar]
- Plant Regeneration and Somatic Embryogenesis from Immature Embryos Derived through Interspecific Hybridization among Different Carica Species. International Journal of Molecular Sciences. 2012;13(12):17065-17076.
- [Google Scholar]
- Understanding Food: Principles and Preparation (3 Ed). Hawaii, Manoa: Thomson Learning Inc.; 2008.
- Comparison of Gum Arabic, Modified Starch, and Whey Protein Isolate as Emulsifiers: Influence of pH, CaCl2 and Temperature. Journal of Food Science. 2002;67(1):120-125.
- [Google Scholar]
- Influence of Biopolymer Emulsifier Type on Formation and Stability of Rice Bran Oil-in-Water Emulsions: Whey Protein, Gum Arabic, and Modified Starch. Journal of Food Science. 2011;76(1):165-172.
- [Google Scholar]
- Comparative Physicochemical Characteristics of Virgin Coconut Oil Produced by Different Methods. Philippine Agricultural Scientist. 2005;88(4):462-468.
- [Google Scholar]
- Epidemiology and Phylogenetic Relationships of Dengue Viruses. Dengue Bulletin. 2003;27:91-94.
- [Google Scholar]
- Phase Behaviour Study of Swiftlet Nest Using Virgin Coconut Oil with Non-Ionic Surfactants. Malaysian Journal of Analytical Sciences. 2015;19(1):184-193.
- [Google Scholar]
- Emulsifying Properties of Whey Protein-Carboxymethylcellulose Complexes. Journal of Food Science. 2002;67(1):113-119.
- [Google Scholar]
- Stability and Rheology of Concentrated O/W Emulsions Based on Soybean Oil/Palm Kernel Olein Blends. Food Research International. 2007;40(8):1051-1061.
- [Google Scholar]
- Lipid Oxidation in Corn Oil-in-Water Emulsions Stabilized by Casein, Whey Protein Isolate, and Soy Protein Isolate. Journal of Agricultural and Food Chemistry. 2003;51(6):1696-1700.
- [Google Scholar]
- Hydrocolloids in Emulsions: Particle Size Distribution and Interfacial Activity. Food Hydrocolloids. 2001;15(4):533-542.
- [Google Scholar]
- Phytochemical and Antioxidant Nutrient Constituents of Carica papaya and Parquetina nigrescens Extracts. Scientific Research and Essays. 2010;5(16):2201-2205.
- [Google Scholar]
- Safety Evaluation of Oral Toxicity of Carica papaya Linn. Leaves: A Subchronic Toxicity Study in Sprague Dawley Rats. Evidence-Based Complementary and Alternative Medicine Volume 2014. Article ID. 2014;741470:1-10.
- [Google Scholar]
- Papayas are Yummy Easy to Grow. Hawaii: University of Hawaii-Manoa College of Tropical Agric. & Human Resources; 2009.
- Colour measurements and modeling. In: Nondestructive evaluation of food quality. Springer; 2010. p. :17-40.
- [Google Scholar]
- Breaking the Intergeneric Hybridization Barrier in Carica papaya and Vasconcellea cauliflora. Scientia Horticulturae. 2011;130(4):787-794.
- [Google Scholar]
- Apoptosis in Lung Cancer Cells Induced by Virgin Coconut Oil. Regenerative Research. 2015;4(1):30-36.
- [Google Scholar]
- Influence of pH and CaCl2 on the Stability of Dilute Whey Protein Stabilized Emulsions. Food Research International. 2000;33(1):15-20.
- [Google Scholar]
- Whey Protein Coating Efficiency on Surfactant-Modified Hydrophobic Surfaces. Journal of Agricultural and Food Chemistry. 2005;53(12):5018-5023.
- [Google Scholar]
- Food Proteins: A Review on Their Emulsifying Properties Using a Structure-Function Approach. Food Chemistry. 2013;141(2):975-984.
- [Google Scholar]
- Chemical Properties of Virgin Coconut Oil. Journal of the American Oil Chemists' Society. 2009;86(4):301-307.
- [Google Scholar]
- Virgin Coconut Oil: Emerging Functional Food Oil. Trends in Food Science & Technology. 2009;20(10):481-487.
- [Google Scholar]
- Ministry of Agriculture (MOA). Buah-Buahan Malaysia. (2011). http://www.moa.gov.my/web/guest/buah-buahan/ Accessed 20th May 2016
- Ministry of Health (MOH). Institute Medical Research (IMR), Malaysia. Carica papaya Extract for Dengue Treatment (2018). https://www.imr.gov.my/en/highlightsfeatured-articles/2245-carica-papayaextract-for-dengue-treatment.html/ Accessed 05th December 2018.
- Mohd Abd Razak, M. R., Mohmad Misnan, N., Md Jelas, N. H., Norahmad, N. A., Muhammad, A., Dee Ho, T. C., Jusoh, B., Sastu, U. R., Zainol, M., Wasiman, M. I., Muhammad, H., Thayan, R. & Syed Mohamed, A. F. 2018. The Effect of Freeze-Dried Carica papaya Leaf Juice Treatment on NS1 and Viremia Levels in Dengue Fever Mice Model. BMC Complementary and Alternative Medicine Volume 18, Article number: 320.
- Situasi Semasa Demam Denggi Di Malaysia. 2015 Accessed 24th July 2017
- Physicochemical properties of virgin coconut oil extracted from different processing methods. International Food Research Journal. 2012;19(3):837-845.
- [Google Scholar]
- Lipid Oxidation in Oil-in-Water Emulsions: Impact of Molecular Environment on Chemical Reactions in Heterogeneous Food Systems. Journal of Food Science. 2000;65(8):1270-1282.
- [Google Scholar]
- Protein-Stabilized Emulsions. Current Opinion in Colloid & Interface Science. 2004;9(5):305-313.
- [Google Scholar]
- Recent Advances in the Development of Transgenic Papaya Technology. Biotechnology Annual Review. 2008;14:423-462.
- [Google Scholar]
- Beneficial Effects of Virgin Coconut Oil on Lipid Parameters and in vitro LDL Oxidation. Clinical Biochemistry. 2004;37(9):830-835.
- [Google Scholar]
- Aqueous extract of Carica papaya leaves exhibits anti-tumor activity and immunomodulatory effects. Journal of Ethnopharmacology. 2010;127(3):760-767.
- [Google Scholar]
- Preparation and Sensory of Emulsified Virgin Coconut Oil (VCO)-Carica papaya Extract Concoction Using Alkali/Acidic Treated Cyclodextin. AIP Conference Proceeding. 2016;1784:030035
- [Google Scholar]
- Chemical Composition of Spondias mombin Linn Plant Parts. Journal Sustainable Agriculture Environment. 2004;6(2):140-147.
- [Google Scholar]
- Formation of Nanoemulsions Stabilized by Model Food-Grade Emulsifiers Using High-Pressure Homogenization: Factors Affecting Particle Size. Food Hydrocolloids. 2011;25(5):1000-1008.
- [Google Scholar]
- Optimization of Metronidazole Emulgel. Journal of Pharmaceutics Volume 2013. Article ID. 2013;501082:1-9.
- [Google Scholar]
- Role of Xanthan Gum on Physicochemical and Rheological Properties of Rice Bran Oil Emulsion. International Food Research Journal. 2016;23(4):1361-1366.
- [Google Scholar]
- Formulation and Characterization of Virgin Coconut Oil (VCO) Based Emulsion. International Journal Science Research Publication. 2013;3(12):528-536.
- [Google Scholar]
- Syed Amran, S. N., Abidin, A. Z. & Zubairi, S. I. 2018. Saponin Bitterness Reduction of Carica papaya Leaves Extract through Weakly Basic Ion Exchange Resins Adsorption. Journal of Food Quality Volume 2018, Article ID 5602729, 11 pages.
- Nutraceutical and Specialty Lipids and Their Co-Products. CRC Press; 2006.
- Saliasi, I., Llodra, J. C., Bravo, M., Tramini, P., Dussart, C., Viennot, S. & Carrouel, F. 2018. Effect of a Toothpaste/Mouthwash Containing Carica papaya Leaf Extract on Interdental Gingival Bleeding: A Randomized Controlled Trial. International Journal of Environmental Research and Public Health 27; 15(12): p. 2660.
- Carica papaya Leaves Juice Significantly Accelerates the Rate of Increase in Platelet Count among Patients with Dengue Fever and Dengue Haemorrhagic Fever. Evidence-Based Complementary and Alternative Medicine Volume 2013. Article ID. 2013;616737:1-7.
- [Google Scholar]
- Effects of Protein Concentration and Oil-Phase Volume Fraction on the Stability and Rheology of Menhaden Oil-in-Water Emulsions Stabilized by Whey Protein Isolate with Xanthan Gum. Food Hydrocolloids. 2009;23(1):165-174.
- [Google Scholar]
- Influence of Increasing Viscosity of the Aqueous Phase on the Short-Term Stability of Protein Stabilized Emulsions. Journal of Food Engineering. 2002;52(3):305-312.
- [Google Scholar]
- Impact of Fat and Water Crystallization on the Stability of Hydrogenated Palm Oil-in-Water Emulsions Stabilized by Whey Protein Isolate. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2004;246(1):49-59.
- [Google Scholar]
- Colloidal Destabilisation Mechanisms in Protein-Stabilised Emulsions. Current Opinion in Colloid & Interface Science. 2003;8(4):371-379.
- [Google Scholar]
- Descriptive Sensory Evaluation of Virgin Coconut Oil and Refined, Bleached and Deodorized Coconut Oil. LWT-Food Science and Technology. 2007;40(2):193-199.
- [Google Scholar]
- World Health Organization (WHO). Dengue Situation Update Number 599. Update on the Dengue situation in the Western Pacific Region. (2020). https://www.who.int/docs/default-source/wpro---documents/emergency/surveillance/dengue/dengue-20200716.pdf?sfvrsn=b42cfbd0_34 Accessed 02nd Sept 2020.







