Translate this page into:
Linked drug-drug conjugates based on triterpene and phenol structures. Rational synthesis, molecular properties, toxicity and bioactivity prediction
⁎Corresponding author. apaw@ump.edu.pl (Anna Pawełczyk)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
One of the tasks of modern medicinal chemistry is to produce new molecules that have interesting and desired biological effects. In addition, the synthetic procedure for obtaining these compounds should be at least partially smart and rational e.g. “Lego” and green approaches. The study focuses on the synthesis of several hybrid type compounds that are expected to be characterized by beneficial bioactivities. In order to hybridize natural triterpene oleanolic acid and phenol structures, the linker-mode concept was selected. The synthetic goal was achieved in two stages. The first concerns the rapid introduction of the halogenoacidic linker to active phenols selected as a result of microwave-ultrasonic (MW-US) assisted O-alkylation with the use of 2-halogenoacetic acid. The next stage of the synthetic studies involves the reaction of phenoxyacetic acid derivatives obtained containing an active carboxylic group with oleanolic acid/oxime units by the methods typical of triterpene chemistry. Novel linked ester- and iminoester-type triterpene derivatives with phenols (thymol, eugenol, paracetamol, nipagins, naphthols, curcumin and genistein) were obtained and characterized. Additionally, based on the analysis of numerous references and selected methods of computational chemistry (Molinspiration Cheminformatics, Osiris Property Explorer and PASS method) the molecular parameters and the preliminary anti-inflammatory and antinociceptive activity characterising these molecules as potential drugs were calculated and predicted.
Keywords
Oleanolic acid
Phenol
Drug-drug conjugates (DDCs)
Microwave and ultrasounds assisted synthesis
Molecular parameters
Bioactivity prediction
1 Introduction
The design and discovery of novel drug candidates are the initial and most probably the crucial steps in the drug development process. Many trends have been proposed for the design of new drugs containing different structures (dimers, heterodimers, heteromers, adducts, associates, complexes, bivalent-, dual-, multifunction drugs and codrugs, identical or non-identical twin drugs, mixed or combo drugs, etc.) (Decker, 2017). The most frequently reported framework combinations are dual connections, which are completely non-specific structural entities that open up completely new structural and therapeutic perspectives (Decker, 2017; Pawełczyk et al., 2018; Tsogoeva, 2010). Fig. 1 shows the possibilities of molecular chemical structures combinations based on the “Lego” chemical concept in which molecular synthesis was as simple as building a Lego construction from single blocks and readily available structure units could be easily linked together to final form made of final molecules, in just a few steps.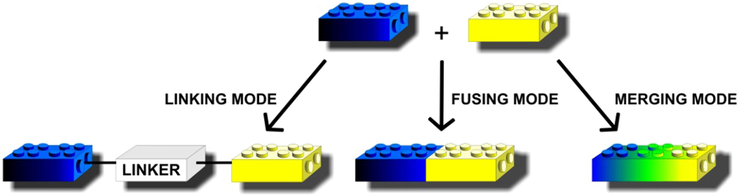
Design strategy for hybrid compounds.
One of the most popular, convenient and relatively easy to implement is the linker concept (Morphy and Rankovic, 2008). Linkers are usually small, at least bifunctional reactive molecules, necessary for chemical integration of different active substances. They can be hydrophilic or hydrophobic, di-, trifunctional and crosslinkers, linear or nonlinear, homofunctional or heterofunctional. The most common functional groups are primary amines, thiols, acids, alcohols, or halogens (Decker, 2017; Pillow, 2017). Selected components can be integrated by a properly designed linker. The choice of the right linker and the right method of attachment is a crucial part of new structure designing. Linkers are classified into different categories, e.g. according to the mechanism of drug release and drug stability in the blood circulation system (cleavable and non-cleavable). Cleavable linkers, in the physiological environment of the body metabolism, disintegrate into two molecules which could then operate independently at separate biological targets. Degradable linkers are often based on the ester linkage, which is easily hydrolysable by esterase present in the plasma. The linker chemistry determines the release profile of active ingredients, and this is crucial to the safety and efficacy of conjugates obtained.
Particularly important is the fact that new therapeutics can be obtained in fast, efficient, and selective methods using current trends in chemical and medicinal chemistry such as the “Lego” chemistry concept (Pawełczyk et al., 2018; Lohmeijer and Schubert, 2003; Maraval et al., 2003) and rational green approach (Cravotto and Cintas, 2007; Peng and Song, 2002; Martina et al., 2016; Pawełczyk et al., 2018a) (Fig. 2). The “Lego” chemistry constitutes an interesting approach to the synthesis of drug-like molecules that can accelerate the drug discovery process by utilizing a few practical and reliable reactions. This chemistry term describes reactions that are based on readily available starting materials and reagents, need no solvent or soft solvent, are high yielding, wide in scope, often are stereospecific, simple to perform and give simple product easy to isolate.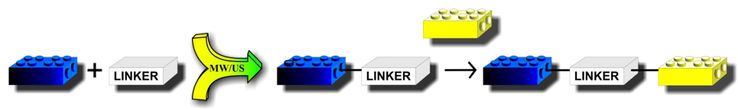
General scheme of linked hybrid derivatives synthesis.
Recently, the use of alternative activation factors such as: microwaves (MW), ultrasound (US) and their cross-combination (MW-US) also has become very promising and desirable synthetic methodologies. The favorable results are due to the combination of the phenomena of both factors - unique microwave heating and the phenomenon of ultrasound cavitation. Sonication in conjunction with microwave or catalyst-free synthesis has proven to be a highly challenging and reliable strategy in varied chemical transformations. Besides saving energy, these green techniques promote faster and more selective transformations (Martina et al., 2016; Pawełczyk et al., 2018a).
Generally, the compounds containing a phenolic group are commonly known and used. They include, among others, salicylic acid, paracetamol, levodopa, dopamine or fenoterol. Phenols exhibit the ability to denature bacterial proteins and inhibit the activity of essential enzymes. In addition, they are widespread among plants, and their antioxidant effect is used in cosmetics (Mishra and Tiwari, 2011). The second hybrid component, oleanolic acid, as many other triterpenes, is characterized by a wide range of pharmacological activities along with low toxicity and very good tolerance, such as hepatoprotective and anti-inflammatory, antiviral, antibacterial, fungicidal, antioxidant or anticancer (Pollier and Goossens, 2012; Ayeleso et al., 2017). Because natural compounds exhibit often relatively low levels of biological activity, numerous experiments have been performed in order to obtain its derivatives with higher therapeutic potential and lower toxicity. Many hybrid derivatives of triterpene compounds have been described, for example oleanolic acid-NSAID (Pawełczyk et al., 2016) or glycyrrhetinic acid-NSAID (Zhang et al., 2020) derivatives. For instance, new derivatives of aspirin (ASA), the most popular pharmaceutical phenol, mostly in order to eliminate NSAIDs-induced gastrointestinal effects and increasing the pharmacological activity have been reported (Bednarczyk-Cwynar et al., 2016; Lu et al., 2018). It has been found that the connection of oleanolic acid oxime and ASA (Bednarczyk-Cwynar et al., 2016) (Fig. 3) exhibited additional synergy antinociceptive effect and low toxicity. Studies of biological activity processes have shown that the new profile of this type conjugate activity is the expression of its analgesic activity achieved rather not via opioid mechanisms, but it is probably a component of its anti-inflammatory effect.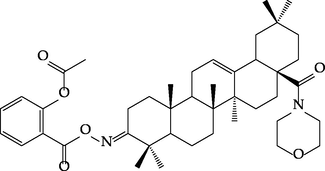
Formula of active oleanolic acid oxime and aspirin conjugate (ASAOxOAM) (Bednarczyk-Cwynar et al., 2016).
The anti-inflammatory properties of the analyzed compound are not clear but it is highly probable that the new structure shows enhanced biological effect according to a new mechanism different from the mechanisms of individual components. The obtained results regarding the analgesic and anti-inflammatory activity of new conjugate of oleanolic acid derivative and acetylsalicylic acid are interesting, but for the explanation of its mechanism of action, more detailed studies are necessary (Bednarczyk-Cwynar et al., 2016). Moreover, many other synthetic ASA derivatives (including NO-, HS-releasing, cinnamaldehyde-, gingerol- and resveratrol-based ASA derivatives, etc.) have been prepared and shown to have potent and selective anti-proliferative activity (Lu et al., 2018). Compounds of the above-mentioned type are completely new, unpredictable chemical and pharmaceutical entities. According to many literature reports (Decker, 2017; Pawełczyk et al., 2018; Tsogoeva, 2010; Morphy and Rankovic, 2008), they are supposed to show intensified and synergistic activity towards selected targets, probably according to a different, unknown mechanism involving potential new receptors. In the case of combinations of phenolic and terpene structures, the synergism of anti-inflammatory, analgesic and possibly hepatoprotective effects is expected. That is why it was decided to hybridize the structures of the triterpene unit and selected phenols.
2 Results and discussion
2.1 Synthesis of phenoxyacid derivatives by MW-US method
Proposed conjugate compounds contain elements (oleanolic acid and phenol structures) differing both in chemical and biological properties. For this synthetic task, the linker-mode method was selected. The synthetic goal was achieved in two stages. The first involves the rapid introduction of a small difunctional linker to phenolic molecules. In our work (Pawełczyk et al., 2018a), the fast functionalization of phenols was realized using a selective and efficient microwave-ultrasonic method. As a result of this stage, ether derivatives of phenols selected and chloroacetic acid (linker fragmet) were obtained (Scheme 1).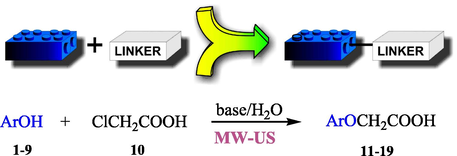
General reaction of linker introduction to the phenol molecule; Ar: selected phenolic rest.
The monophenols proposed for conjugation with triterpene included: 2-isopropyl-5-methylphenol (thymol) (1), 4-allyl-2-methoxyphenol (eugenol) (2), methyl ester of 4-hydroxybenzoic acid (nipagin M) (3), propyl ester of 4-hydroxybenzoic acid (nipagin P) (4), N-(4-hydroxyphenyl) acetamide (paracetamol) (5), 1-naphtol (6) and 2-naphtol (7). Besides the above-mentioned monophenols, two interesting and pharmacologically valuable di- and triphenolic compounds were proposed for modification according to the hybrid strategy. They are curcumin (1,7-bis(4-hydroxy-3-methoxy phenyl)hepta-1,6-dien-3,5-dione) (8) and genistein (4′,5,7-trihydroxyisoflavone) (9). The selected phenols permitted obtaining the corresponding linker-containing phenoxyacetic acid derivatives, which are non-symmetrical ethers with active carboxyl groups (Pawełczyk et al., 2018a) (Table 1). Etherification reaction was performed under different reaction conditions (US, MW and MW-US) in most cases in water solution or in a solvent-free system. The examples presented clearly show that the use of combined ultrasound and microwave irradiation, which is practically hazard-free technological innovation, deserves widespread attention in fine-pharmaceutical research. phenol structure – colored in blue; linker structure – colored in black.
Phenol No
Product No
Product structure/Name

Yield [%]
1
11

2-(5-Methyl-2-propan-2-ylphenoxy) acetic acid
89
2
12
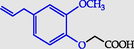
2-(2-Methoxy-4-prop-2-enylphenoxy) acetic acid
86
3
13
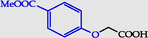
4-(Carboxymethoxy)benzoic acid methyl ester
75
4
14
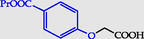
4-(Carboxymethoxy)benzoic acid propyl ester
68
5
15
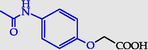
2-(4-Acetamidophenoxy) acetic acid
86
6
16
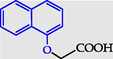
2-(Naphtalen-1-yloxy)acetic acid
88
7
17
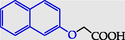
2-(Naphtalen-2-yloxy)acetic acid
91
8
18
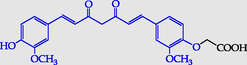
2-{4-[7-(4-Hydroxy-3-metoxy- phenyl)-3,5-dioxohepta-1,6-dienyl]-2-methoxyphenoxy} acetic acid
36
9
19
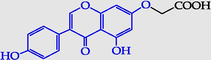
2-[5-Hydroxy-3-(4-hydroxy- phenyl)-4-oxo-4H-chromen-7-yloxy)]acetic acid
41
2.2 The reaction of phenoxyacetic acid derivatives with triterpenes
In the next stage of the hybrid compounds investigation, selected monocarboxylic derivatives of phenols, obtained in the direct rational reaction of phenols and chloroacetic acid, were used in the reactions with pentacyclic triterpene derivatives. As a backbone skeleton with the main activity, we decided to use the well-known naturally occurring 3β-hydroxyolean-12-en-28-oic acid, known as oleanolic acid (20), showing a number of beneficial properties and also its oxime derivative (22) (Scheme 2).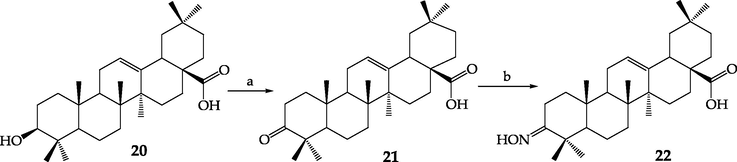
The main oleanolic acid transformations. Reagents and condition: a) Jones reagent, acetone, rt; b) NH2OH × HCl, CH3COONa, EtOH, reflux.
Structures of this type can act as bioactive molecules and as carriers of other external activities (Pollier and Goossens, 2012; Ayeleso et al., 2017). Terpenes with hydroxyl or hydroxyimino reactive groups can be used to prepare linked derivatives with selected phenoxyacetic acid derivatives. Recently, semi-synthetic acyloxyimino derivatives of oleanolic acid and acetylsalicylic acid showing analgesic and anti-inflammatory properties have been reported (Bednarczyk-Cwynar et al., 2012). In addition, the 3-hydroxyimino group is particularly valuable for the appearance of the cytotoxic activity of the derivatives obtained (Lisiak et al., 2017).
Oleanolic acid (20) and its derivative 22 are starting reagents in the synthesis of triterpene and phenoxyacetic acid derivatives. The triterpene oxime (22) (Ma et al., 2000) was obtained from 3-oxooleanolic acid (21) in the reaction with hydroxylamine hydrochloride, in the presence of sodium acetate. Corresponding carbonyl derivative (21) was obtained in the oxidation process of the starting oleanolic acid (20) with chromic anhydride in sulfuric acid (Jones reagent) (Chen et al., 2006) (Scheme 2). The main transformation to afford linked derivatives is the reaction of the secondary hydroxyl group, or hydroxyimino group resulting from it, which is present in the terpene molecule at C-3 position with a free carboxylic moiety of phenoxyacetic agents (11–19) (Scheme 3). Oleanolic acid (20) reacted by the esterification method (Pawełczyk et al., 2016) leads to the formation of linked ester-type hybrid derivatives (23–29, 37, 39), while the corresponding triterpene oxime (22) gives an ester of oximes called iminoester-type hybrid derivatives (30–36, 38, 40).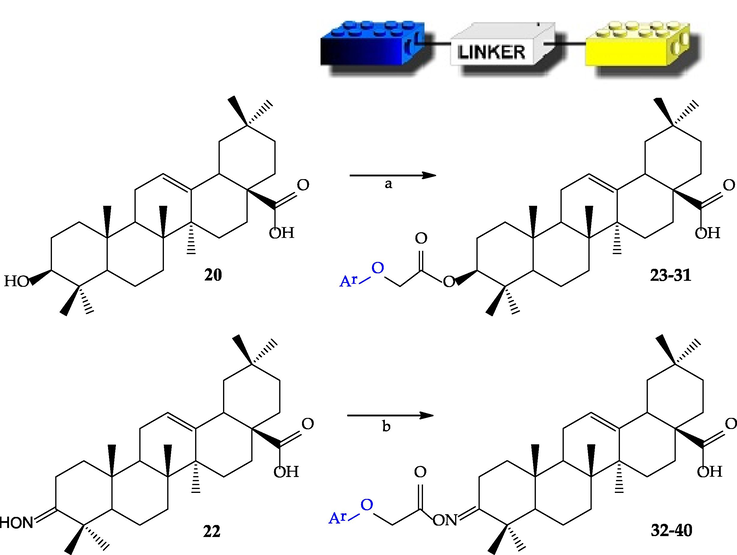
The main chemical pathways of linked hybrids synthesis: a) ester-type hybrids: selected phenoxyacetic acid derivatives (11–19), DCC, DMAP, anhyd. CH2Cl2, rt; b) iminoester-type hybrid: selected phenoxyacetic acid derivatives (11–19), DCC, anhyd. CH2Cl2, rt.
Typical methods for triterpene chemistry (Pollier and Goossens, 2012; Pawełczyk et al., 2016) were used to prepare these linked hybrid derivatives. Oleanolic acid (20) was reacted with appropriate bioactive phenols functionalized with acetate linker (11–17) in the presence of DCC (dicyclohexylcarbodiimide) and DMAP (4-(dimethylamino)piridine). DCC is a carboxylic acid activating and coupling agent, while the nucleophilic 4-(dimethylamino)pyridine (DMAP) acts as an acyl transfer reagent in the Steglich esterification reaction (Pawełczyk et al., 2016). The molar ratio of oleanolic acid, functionalized phenol, DCC and DMAP reagents used in the reaction process was 1:1.5:5:3. In the reaction of the corresponding oxime (22) towards linked iminoesters, a similar procedure was applied (Chen et al., 2006), but the presence of DMAP was not crucial. The molar ratio of reagents used was 1:1.5:5 (oleanolic acid oxime, functionalized phenol, DCC). All reactions, both towards ester (23–31) and iminoester-type compounds (32–40), were carried out at room temperature and in mild conditions. A suitable solvent is anhydrous dichloromethane or DMF solution. The reaction conditions for the formation of the corresponding esters and iminoesters were optimized and described (Pawełczyk et al., 2016) (Scheme 3). Despite the presence of more than one phenolic group in curcumin (8) and genistein (9) molecules, only monophenoxyacetic derivatives were obtained in the reaction with chloroacetic acid under the conditions used. Then they were reacted with terpenes to appropriate linked derivatives (30, 31, 39, 40).
As a result of the synthesis nine monofunctionalized phenols and eighteen linked structures of the formulas shown in Fig. 4 were prepared. The obtained hybrid derivatives of the type of esters 23–31 and iminoesters 32–40 were isolated and purified by crystallization or column chromatography with the yields ranging from 30% to 92%.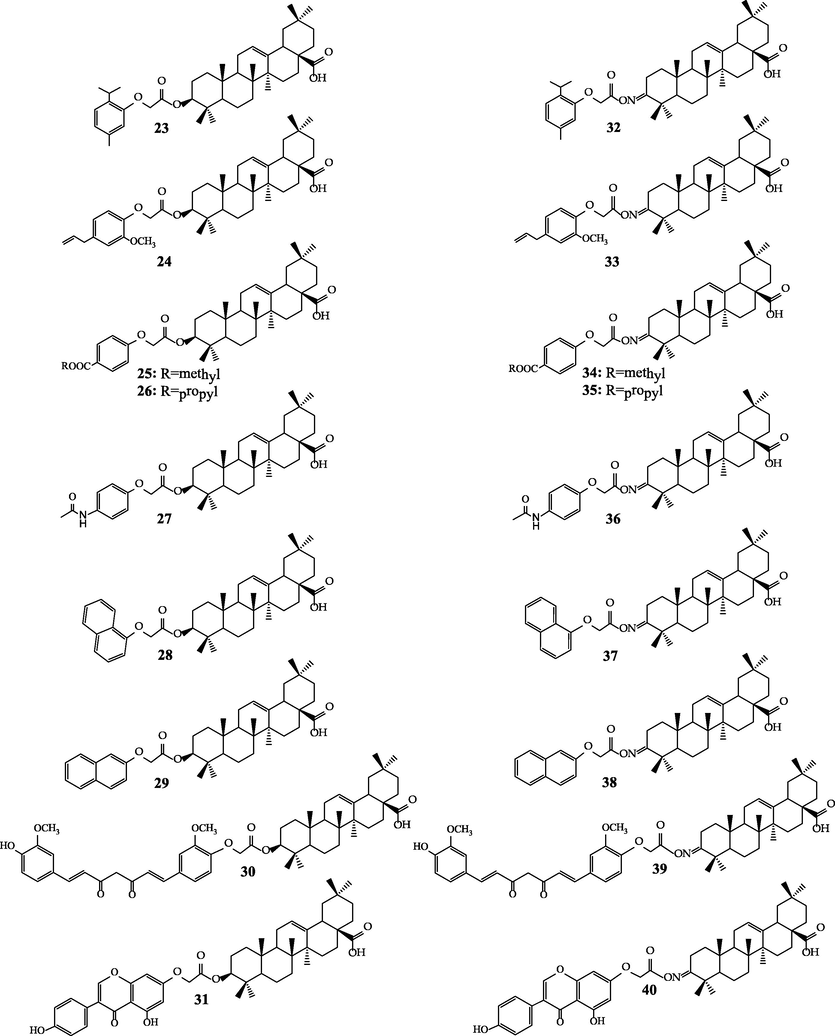
Final formulas of the linked triterpene-monophenol hybrids.
2.3 The molecular properties, toxicity risk and bioactivity prediction
Currently, several approaches have been developed to assess the drug-likeness of bioactive compounds based on topological descriptors, fingerprints of molecular structure or properties. Lipinski’s rule of five (Lipinski et al., 1997) is commonly used by pharmaceutical chemists in drug design and development to predict the bioavailability of potential drug molecules. In this work, the molecular parameters described by this rule like logP, logS, TPSA and molecular weight were calculated. Open-source program Osiris Property Explorer (Organic Chemistry Portal, 2010) and Molinspiration software (Molinspiration Cheminformatics, 1986) was used to predict the fragment-based drug-likeness of title linked-hybrid compounds. The Osiris program estimated the risks of side effects, such as mutagenic, tumorigenic, irritating and reproductive effects, as well as drug-relevant properties including partition coefficient (logP), solubility (logS), molweight (MolW), topological polar surface area (TPSA) and drug-likeness (DL). Moreover, the overall drug-score (DS) was estimated by combining the outcome of logP, logS, MolW, toxicity risks and drug-likeness. Drug-score is a measure of compound's potential to meet the criteria of a possible drug candidate. LogP, a very valuable parameter since it provides an estimate of the ability of a compound to move/cross from aqueous phase to lipid phase, hence providing a measure of the ability of compounds to cross the cell membranes. One of the parameters for the success of drug molecules is their water solubility. LogS is a measure of water solubility of compounds in water in mol/L at 25 °C, and at pH of 7.5. The total number of hydrogen bond donors (HD) and hydrogen bond acceptors (HA) should not be greater than 5 and 10, respectively. Topological polar surface area (TPSA) is the sum of all polar atoms in a molecule. It provides an estimate of the ability of a compound to cross blood brain barrier (BBB). The molecular properties of triterpene-phenol conjugates (23–40) calculated by using Molinspiration Cheminformatics and Osiris Property Explorer software are presented in Table 2. Moreover, the obtained values were compared with the results obtained for aspirin ASA (standard drug) and its corresponding triterpene derivative ASAOxOAM (Fig. 3). This connection of triterpene with acetylsalicylic acid is characterized by a strong and long-lasting analgesic and anti-inflammatory effect (about the unexplained mechanism so far), which was found in studies using animals (Bednarczyk-Cwynar et al., 2016). Moreover, years of research on the effect of oleanolic acid and its different synthetic derivatives have confirmed the antitumor activity of these compounds (Paszel-Jaworska et al., 2014; Liu et al., 2013; Shanmugam et al., 2014). Besides displaying antitumor effects, oleanolic acid such as other natural triterpenes has low toxicity, which distinguishes it from the chemotherapeutic agents currently used (Wei et al., 2013). Other studies (Tang et al., 2016) report that conjugates of selected phenols (aspirin) and triterpenes (ursolic acid) are great for targeting breast cancer metastasis. Structural modification of natural triterpene by the other molecules e.g. phenols raises the possibility of increasing the potential bioactivity of novel substances. That’s why studied compounds could be more promising drug candidates for cancer prevention or treatment. OA - oleanolic acid; OxOA - oleanolic acid oxime; ASA – aspirin; ASAOxOAM - morfolide of oleanolic acid oxime and aspirin conjugate (Fig. 3); logP - log of partition coefficient; logS - log of the solubility measured in mol/l; TPSA - topological polar surface area; HA - hydrogen bond acceptors; HD - hydrogen bond donors; MolW - molecular weight; DL - drug-likeness; DS - drug-score.
Compounds
logPMI
logPOPE
logSOPE
TPSAMI
TPSAOPE
HAMI
HDMI
MolW
DLOPE
DSOPE
OA
6.73
6.06
−6.13
57.53
57.53
3
2
456
−1.78
0.18
23
9.19
9.10
−8.70
72.84
72.83
5
1
646
−4.37
0.08
24
8.84
8.53
−8.22
82.07
82.06
6
1
660
−2.14
0.03
25
8.73
7.48
−7.63
99.14
99.13
7
1
648
−5.05
0.08
26
9.08
8.34
−8.20
99.14
99.13
7
1
676
0.03
0.11
27
8.11
7.27
−7.83
101.94
101.90
7
2
647
1.87
0.15
28
9.12
8.76
−9.09
72.84
72.83
5
1
640
−1.85
0.09
29
9.13
8.76
−9.09
72.84
72.83
5
1
640
−3.09
0.08
30
8.92
9.20
−9.79
145.68
145.60
10
2
864
−3.96
0.07
31
8.99
7.88
−8.90
143. 50
139.50
9
3
766
−0.80
0.05
OxOA
6.99
6.83
−6.55
69.89
69.89
4
2
469
−2.57
0.14
32
9.18
9.64
−9.01
85.20
85.19
6
1
659
−6.49
0.03
33
8.80
9.07
−8.52
94.44
94.42
7
1
673
−4.40
0.02
34
8.69
8.02
−7.93
111.51
111.40
8
1
661
−7.27
0.04
35
9.06
8.88
−8.50
111.51
111.40
8
1
689
−2.08
0.04
36
8.04
7.81
−8.13
114.30
114.20
8
2
660
−0.54
0.05
37
9.09
9.30
−9.40
85.20
85.19
6
1
653
−3.98
0.04
38
9.10
9.30
−9.40
85.20
85.19
6
1
653
−5.23
0.04
39
8.89
9.74
−10.09
158.04
158.00
11
2
877
−6.26
0.03
40
8.97
8.42
−9.20
155.87
151.90
10
3
779
−3.00
0.02
ASAOxOAM
8.48
8.71
−8.01
94.52
94.50
8
0
700
0.12
0.04
ASA
1.43
1.13
−1.93
63.60
63.60
4
1
180
−0.48
0.14
Molecular hydrophobicity or lipophilicity expressed as logP values of all the compounds except reference aspirin were found to be more than 5 and are in clear violation of Lipinski’s rule of five, suggesting poor permeability across the cell membrane. LogP value of aspirin, the standard drug, was found to be well under 5 justifying their oral use. The molecular weight of drug-drug conjugates was found to be greater than 500 and thus these molecules are anticipated to not be easily transported, diffused and absorbed as compared to small molecules. The number of hydrogen bond acceptors (O and N atoms) and the number of hydrogen bond donors (NH and OH) in the synthesized compounds were in accordance with Lipinski’s rule of five i.e. less than 10 and 5 respectively (except for 39). The topological polar surface area is very much correlated with the hydrogen bonding of a molecule and is a very good indicator of the bioavailability of drug molecules. TPSA of these drug-drug conjugate derivatives was observed in the range of 72.83–158.00 Å and is well below the limit of 160 Å.
A molecule having a bioactivity score of more than 0.00 is most likely to exhibit considerable biological activities, while values −0.50 to 0.00 are expected to be moderately active and if the score is less than −0.50 it is presumed to be inactive. The results clearly reveal that the physiological actions of obtained derivatives might involve multiple mechanisms and could be due to the interactions with GPCR ligands, nuclear receptor ligands, and inhibit protease and other enzymes. The bioactivity score of compounds is suggestive of moderate or low interaction with all drug targets (Table 3). The part of the selected compounds showed better bioactivity scores than aspirin for all drug targets. Results of toxicity risks and drug score assessment of synthesized derivatives were predicted by Osiris Property Explorer and Molinspiration Cheminformatics are also presented in Table 3. This online method predicts on the basis of functional group similarity of the investigated compound with the extensively in vitro – and in vivo studied compounds present in its database. The results clearly indicated that ester-type compounds (24–31, but not 23) would be safe and expected to show low or no toxicity. The results obtained for imino-ester type compounds (32–40) are much less affordable and suggest that the presence of iminoester moiety is responsible for adverse mutagenicity and less favorable tumorigenity. However, the predicted data obtained do not always correlate well with the biological tests performed. Bioactivity studies have clearly shown also that the presence of hydroxyimino groups affects the induction of autophagy and apoptosis; besides, the presence of the moiety increases the efficiency of antiproliferative activity in breast cancer cells (Lisiak et al., 2017). OA - oleanolic acid; OxOA - oleanolic acid oxime; ASA – aspirin; ASAOxOAM - morfolide of oleanolic acid oxime and aspirin conjugate (Fig. 3); L - low risk; M - medium risk; H -high risk.
Compound
GPC ligand MI
Ion chanel modulator MI
Kinase inhibitor MI
Nuclear receptor ligand MI
Protease inhibitor MI
Enzyme inhibitor MI
Mutagenicity OPE
Tumorigenicity OPE
Irritating Effects OPE
Reproductive Effects OPE
OA
0.28
−0.06
−0.40
0.77
0.15
0.65
L
L
L
L
23
−0.27
−1.16
−1.08
−0.10
−0.19
−0.23
L
L
L
L
24
−0.37
−1.29
−1.20
−0.30
−0.27
−0.34
L
H
H
L
25
−0.30
−1.16
−1.07
−0.14
−0.18
−0.25
L
L
L
L
26
−0.50
−1.46
−1.34
−0.44
−0.32
−0.49
L
L
L
L
27
−0.28
−1.17
−1.03
−0.23
−0.16
−0.26
L
L
L
L
28
−0.21
−0.09
−0.97
−0.09
−0.10
−0.17
L
L
L
L
29
−0.22
−1.10
−0.98
−0.10
−0.11
−0.17
L
L
L
L
30
−2.93
−3.62
−3.63
−3.12
−2.41
−2.96
L
L
L
L
31
−1.53
−2.99
−2.53
−1.77
−1.24
−1.64
L
L
L
H
OxOA
0.27
−0.13
−0.40
0.78
0.12
0.57
L
L
L
L
32
−0.27
−1.40
−1.21
−0.21
−0.33
−0.37
H
M
L
L
33
−0.39
−1.55
−1.34
−0.41
−0.41
−0.50
H
H
H
L
34
−0.31
−1.41
−1.20
−0.25
−0.32
−0.39
H
M
L
L
35
−0.53
−1.72
−1.50
−0.57
−0.48
−0.66
H
M
L
L
36
−0.29
−1.42
−1.16
−0.33
−0.30
−0.40
H
M
L
L
37
−0.21
−1.34
−1.10
−0.19
−0.24
−0.32
H
M
L
L
38
−0.23
−1.35
−1.10
−0.20
−0.25
−0.32
H
M
L
L
39
−3.01
−3.67
−3.67
−3.20
−2.69
−3.10
H
M
L
L
40
−1.64
−3.29
−2.77
−1.99
−1.46
−1.87
H
M
L
H
ASAOxOAM
−0.68
−1.98
−1.59
−0.94
−0.55
−0.90
H
H
L
L
ASA
−0.76
−0.32
−1.06
−0.44
−0.82
−0.28
H
H
L
H
In this study, for comparison, also the PASS method (Way2Drug.com, 2011) to estimate the selected biological activity of the substances was used. Because, there is literature and experimental data (Bednarczyk-Cwynar et al., 2016, 2012) that these types of triterpene derivatives are often characterized by antinociceptive (analgesic) and anti-inflammatory effects, it was decided to preliminary analyze them in this direction. The anti-inflammatory and antinociceptive activity (Pa factor as a probability “to be active”) for obtained compounds were predicted. Most of these compounds exhibit a very high probability of a selected type of biological activity, with more than 0.7 occurring (Tables 4). If the value of Pa factor is greater than 0.7, there is a high probability that the substance will be active in the conditions of in vitro experiment, whereas if the Pa factor is within the 0.5–0.7 range, there is a slightly lower probability confirming the activity of the substance in the biological studies. Ester-type connections, compared to the corresponding iminoester-type compounds, are potentially more active individuals. Related iminoesters also demonstrate potential activity, although many of them are in the Pa factor range of 0.5–0.7. Analyzing all results using this method, it was observed that one of the highest values for both ester and iminoester conjugates relates to the transcription factor NF kappa B stimulant, which is also additionally presented in Table 4. However, the results of biological studies of compounds containing a hydroxyimine or iminoester (as for bioactive ASAOxOAM derivative) moiety provide the direction for the design and synthesis of new antitumor drugs (Lisiak et al., 2017). OA - oleanolic acid; OxOA - oleanolic acid oxime; ASA – aspirin; ASAOxOAM - morfolide of oleanolic acid oxime and aspirin conjugate (Fig. 3).
Compouds/Activity
Pa Factor
Transcription factor NF kappa B stimulant
Anti-inflammatory
Antinociceptive
OA
0.831
0.895
0.954
23
0.761
0.788
0.892
24
0.738
0.789
0.886
25
0.752
0.792
0.896
26
0.752
0.763
0.891
27
0.704
0.715
0.878
28
0.755
0.828
0.907
29
0.758
0.813
0.909
30
0.788
0.743
0.867
31
0.766
0.655
0.860
OxOA
0.693
0.710
0.929
32
0.579
0.613
0.861
33
0.559
–
0.856
34
0.558
0.620
0.866
35
0.564
0.574
0.861
36
0.508
0.521
0.850
37
0.551
0.686
0.880
38
0.558
0.658
0.882
39
0.655
0.548
0.822
40
0.614
0.453
0.824
ASAOxOAM
0.636
0.543
0.751
ASA
0.765
0.530
–
3 Materials and methods
The melting points of all compounds used in this study were determined on a Boetius apparatus and were uncorrected. The 1H- and 13C NMR spectra were recorded using a Varian Gemini 300 VT spectrometer, 300 and 75 MHz respectively (Agilent Technologies, Santa Clara, CA, USA). Chemical shifts were expressed in parts per million (ppm), relative to tetramethylsilane (TMS) as an internal standard, using CDCl3 as a solvent. Coupling constants (J) are expressed in Hertz (Hz). Signals are labeled as follows: s, singlet; d, doublet; dd, double doublet; t, triplet; and m, multiplet. MS spectra were recorded on a 402 AMD INTECTRA apparatus (AMD Intectra GmbH, Harpstedt, Germany) by the electron impact technique (EI), operating at 75 eV and ESI-MS (QTOF mass spectrometer - Impact HD, Bruker, positive ion mode). The progress of reactions and purity of products was checked using the TLC method on silica gel plates (25DC-Alufolien Kieselgel 60 F254 from Merck, Darmstadt, Germany). The following eluents were used: mixture of chloroform/ methanol (9:1 and 5:1, v/v) for functionalized phenols and hexane/ethyl acetate (2:1 and 4:1, v/v) for triterpene products. The TLC spots on the plates were observed in UV light (λ = 254 nm) for functionalized phenols and were visualized by spraying the chromatograms with 10% H2SO4 in ethanol and heating the plates at 110 °C for approximately 2–3 min. for final triterpene products. Silica gel 60 (63–200 μm particle size, Merck) was used for column chromatography. Oleanolic acid (Bio-Tech, Beijing, China, plant material: Olea Europea), DCC, DMAP, phenolic compounds (paracetamol, eugenol, thymol, nipagin M and P, 1- and 2-naphtol, curcumin, genistein), and solvents (methylene chloride, chloroform, methanol, ethyl acetate, hexane) from Aldrich®, Fluka®, Chempur® and POCh® were used. MW-US reactions were carried out in a UWave-1000 reactor (Sineo Microwave Chemistry Technology, 2014), equipped with a microwave generator (0–1000 W), an ultrasonic probe (26–28 kHz, 0–800 W), a UV lamp (λ = 365 nm, 300 W), contact and non-contact thermometer, magnetic stirrer, cooler and reaction visualization system. The crude reaction products were purified by means of crystallization process or flash column chromatography. The data on compounds obtained (11–19) confirmed the literature information (Pawełczyk et al., 2018a). The structures of all linked derivatives obtained were confirmed by 1H, 13C NMR spectra and MS data.
Calculation of molecular parameters such as logP, logS, TPSA, HD, HA, MolW, toxicity potential and initial biological activity has been determined by online cheminformatics tools: Osiris Property Explorer and Molinspiration Chemoinformatics. Osiris Property Explorer (Organic Chemistry Portal) was used to determine pharmacokinetic parameters such as toxicity potential, solubility and overall drug-likeness of synthesized conjugates and Molinspiration Chemoinformatics software was used to calculate various molecular properties and to predict bioactivity score for drug targets including enzymes and nuclear receptors, kinase inhibitors, GPCR ligands, and ion channel modulators. Molecular properties were calculated to evaluate the drug-likeness (DL) of the synthesized compounds. The PASS method (Prediction of Activity Spectra for Substance) was used to estimate the selected biological activity of the obtained triterpene conjugates.
3.1 Synthetic procedures
3.1.1 General procedure for the synthesis of phenoxyacetic acid derivatives (11–19)
In a 50 mL flask, 1 mmol of phenol (1–9), 1.2 mmol (0.11 g) chloroacetic acid (10), the appropriate amount of sodium hydroxide (respectively 2.3 mmol for monophenols, 3.3 mmol for curcumin, 4.3 mmol for genistein) and 10 mL of water were mixed. A flask with carefully mixed reagents was placed in the MW-US reactor and the contents reacted with the following parameters: MW = 200 W, US = 100 W at 85–90 °C for 5 min. After the process was complete, concentrated hydrochloric acid was added to the reaction mixture until an acidic pH ∼ 3 was obtained to isolate the free products. The separated precipitate was filtered off and purified by crystallization or column chromatography on silica gel using a mixture of chloroform and methanol (9:1 or 5:1, v:v) as an eluent.
2-(5-Methyl-2-propan-2-ylphenoxy)acetic acid (11) (Pawełczyk et al., 2018a): in the reaction of 1 and 10 the product 11, after crystallization process, was obtained as a light-yellow solid (W = 89%); m.p. 140–145 °C; MS (m/z): 208.2 [M]+.
2-(2-Methoxy-4-prop-2-enylphenoxy)acetic acid (12) (Pawełczyk et al., 2018a): in the reaction of 2 and 10 the product 12, after crystallization process, was obtained as a cream solid (W = 86%); m.p. 88–92 °C; MS (m/z): 222.1 [M]+.
4-(Carboxymethoxy)benzoic acid methyl ester (13) (Pawełczyk et al., 2018a): in the reaction of 3 and 10 the product 13, after crystallization process, was obtained as a white solid (W = 75%); m.p. 143–145 °C; MS (m/z): 211.2 [M]+.
4-(Carboxymethoxy)benzoic acid propyl ester (14) (Pawełczyk et al., 2018a): in the reaction of 4 and 10 the product 14 was obtained as a white solid (W = 68%); m.p. 86–89 °C; MS (m/z): 238.0 [M]+.
2-(4-Acetamidophenoxy)acetic acid (15) (Pawełczyk et al., 2018a): in the reaction of 5 and 10 the product 15, after crystallization process, was obtained as a beige solid (W = 86%); m.p. 85–90 °C; MS (m/z): 209.1 [M]+.
2-(Naphthalen-1-yloxy)acetic acid (16) (Pawełczyk et al., 2018a): in the reaction of 6 and 10 the product 16, after crystallization process, was obtained as a blue-grey solid (W = 88%); m.p. 173–177 °C; MS (m/z): 201.9 [M]+.
2-(Naphtalen-2-yloxy)acetic acid (17) (Pawełczyk et al., 2018a): in the reaction of 7 and 10 the product 17, after crystallization process, was obtained as a grey solid (W = 91%); m.p. 85–87 °C; MS, m/z (%): 201.9 [M]+.
2-{4-[7-(4-Hydroxy-3-metoxyphenyl)-3,5-dioxohepta-1,6-dienyl]-2-metoxyphenoxy}acetic acid (18) (Pawełczyk et al., 2018a): in the reaction of 8 and 10 the product 18, after column chromatography, was obtained as a brick-red solid (W = 36%); m.p. 150–155 °C; MS (m/z): 426.3 [M]+.
2-[5-Hydroxy-3-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-yloxy)]acetic acid (19) (Pawełczyk et al., 2018a): in the reaction of 9 and 10 the product 19, after column chromatography, was obtained as a light brown solid (W = 41%); m.p. 255–260 °C; MS (m/z): 328.0 [M]+.
3.2 Procedure for the oleanolic acid oxime (22) synthesis
At first, oleanolic acid (20) was oxidized with Jones reagent in acetone at room temperature (Ma et al., 2000). The 3-oxoderivative obtained (21) was subjected to a reaction with hydroxylamine hydrochloride in ethanol, in accordance with the procedure known for many different oxotriterpenoids (Chen et al., 2006). Heating oleanolic acid 3-ketone (21) with hydroxylamine hydrochloride produced the corresponding oleanolic oxime (22). The spectral data of this compound were in agreement with the literature information (Ma et al., 2000; Chen et al., 2006).
3.2.1 General procedure for the synthesis of ester-type triterpene-phenol conjugates (23–31)
Oleanolic acid (20) (0.46 g, 1 mmol), DCC (1.03 g, 5 mmol), DMAP (0.37 g, 3 mmol) and selected functionalized phenols (1.5 mmol) were mixed at 0 °C and then stirred at room temperature in dry CH2Cl2 (12 mL) for 2 h. Hexane (8 mL) was added to the reaction mixture and cooled. Next, the resulting precipitate of dicyclohexylurea was filtered. The organic phase was washed with 5% aqueous hydrochloric acid, 5% aqueous sodium bicarbonate and water and dried over anhydrous sodium sulfate. After removal of the drying agent and solvent evaporation, the crude new esters (23–31) were purified with column chromatography on silica gel using a hexane/ethyl acetate (2:1 or 4:1, v:v) mixture as eluent.
3-[2-(5-Methyl-2-propan-2-ylphenoxy)acetoxy]olean-12-en-28-oic acid (23): in the reaction of 11 and 20, after chromatographic separation, the product 23 was obtained as a white fine crystals (W = 62%); m.p. 145–146 °C; Rf = 0.70 (hexane/ethyl acetate 4:1); 1H NMR (CDCl3): 10.31 (s, 1H, COOH), 7.14–7.12 (m, 1H, Ar), 6.80–6.78 (m, 1H, Ar), 6.55 (s, 1H, Ar), 5.33 (t, 1H, J = 3,6Hz, -CH-12terp), 4.66 (s, 2H, —OCH2—), 4.33 (1H, m, CH-3terp), 3.42–3.37 (m, 1H, —CH—), 2.84 (dd, 1H, J = 13.6, 3.6 Hz, CH-18terp), 2.30 (s, 3H, —CH3), 1.25 (s, 3H, —CH3), 1.23 (s, 3H, —CH3), 1.16–0.80 (s, 21H, 7 × CH3); 13C NMR (CDCl3): 172.91 (COOH), 169.20 (-C(O)Ophenol-terp), 154.93 (C-1Ar), 143.22 (C-13terp), 136.18 (C-5Ar), 134.32 (C-2Ar), 126.20 (C-3Ar), 122.92 (C-4Ar), 122.01 (C-12terp), 111.97 (C-6Ar), 82,23 (C-3terp), 73.2 (-OCH2-), 55.30 (C-5), 47.60 (C-9), 46.72 (C-17), 45.85 (C-19), 41.72 (C-14), 41.30 (C-18), 39.50 (C-8), 38.10 (C-1), 37.90 (C-4), 36.91 (C-10), 33.88 (C-21), 33.15 (C-29), 32.70 (C-7), 32.52 (C-22), 30.70 (C-20), 29.81 (C-15), 27.96 (C-23), 27.80 (C-2), 26.59 (CH3 tym), 25.98 (C-27), 25.85 (CH3tym), 23.66 (C-30), 23.55 (C-16), 23.45 (C-11), 18.20 (C-6), 17.88 (C-26), 16.85 (C-24), 15.30 (C-25), 22.79 (CHtym), 21.28 (CH3tym); MS (m/z) 646.9 [M]+ (C42H62O5).
3-[2-(2-Methoxy-4-prop-2-enylphenoxy)acetoxy]olean-12-en-28-oic acid (24): in the reaction of 12 and 20, after chromatographic separation, the product 24 was obtained as a white fine crystals (W = 68%); m.p. 77–79 °C; Rf = 0.41 (hexane/ethyl acetate 4:1); 1H NMR (CDCl3): 9.96 (s, 1H, COOH), 6.92 (d, 1H, J = 8.6 Hz, H-6Ar), 6.76 (d, 1H, J = 8.6 Hz, H-5Ar), 6.74 (s, 1H, H-3Ar), 5.96 (m, 1H, ⚌CH—), 5.32 (t, 1H, J = 3,6Hz, -CH-12terp), 5.12 and 5.19 (each d, 1H, J = 19.7, CH2⚌C—), 4.60 (s, 2H, —OCH2—), 4.33 (m, 1H, CH-3terp), 3.86 (s, 3H, —OCH3), 3.38 (d, 2H, J = 6.6 Hz, —CH2—), 2.81 (dd, 1H, J = 13.6, 3.6 Hz, CH-18terp), 1.10–0.72 (s, 21H, 7 × CH3); 13C NMR (CDCl3): 172.91 (COOH), 169.20 (-C(O)Ophenol-terp), 146.91(C-2Ar), 144.34 (C-1Ar), 143.22 (C-13terp), 138.15 (C-4Ar), 132.11 (⚌CH—), 122.01 (C-12terp), 121.73 (C-5Ar), 115.62 (C-3Ar), 114.76 (C-6Ar), 111.73 (CH2⚌), 82,23 (C-3terp), 73.20 (—OCH2—), 56.35 (OCH3), 55.30 (C-5), 47.60 (C-9), 46.72 (C-17), 45.85 (C-19), 41.72 (C-14), 41.30 (C-18), 40.36 (CH2), 39.50 (C-8), 38.10 (C-1), 37.90 (C-4), 36.91 (C-10), 33.88 (C-21), 33.15 (C-29), 32.70 (C-7), 32.52 (C-22), 30.70 (C-20), 29.81 (C-15), 27.96 (C-23), 27.80 (C-2), 25.98 (C-27), 23.66 (C-30), 23.55 (C-16), 23.45 (C-11), 18.20 (C-6), 17.88 (C-26), 16.85 (C-24), 15.30 (C-25); MS (m/z) 659.9 [M]+ (C42H60O6).
3-[2-(4-Methoxycarbonylphenoxy)acetoxy]olean-12-en-28-oic acid (25): in the reaction of 13 and 20, after chromatographic separation, the product 25 was obtained as a white fine crystals (W = 82%); m.p. 250–255 °C; Rf = 0.29 (hexane/ethyl acetate 4:1); 1H NMR (δ[ppm]): 10.34 (s, 1H, COOH), 8.07 (m, 2H, 3,5-HAr), 7.02 (m, 2H, 2,6-HAr), 5.34 (t, 1H, J = 3,6 Hz, -CH-12terp,), 4.74 (s, 2H, —OCH2—), 4.35 (m, 1H, CH-3terp), 3.96 (s, 3H, —OCH3), 2.82 (dd, 1H, J = 13.6, 3.6 Hz, CH-18terp), 1.15–0.76 (s, 21H, 7 × CH3); 13C NMR (δ[ppm]): 183.64 (COOH), 168.52 (-C(O)Ophenol-terp), 166.41 (-C(O)O-CH3), 161.42 (C-1Ar), 143.55 (C-13terp), 131.46 (C-3,5Ar), 123.86 (C-4Ar), 122.36 (C-12terp), 115.02 (C-2,6Ar), 82.59 (C-3terp), 70.10 (—OCH2—), 57.40 (—OCH3), 55.19 (C-5), 47.38 (C-9), 46.50 (C-17), 45.74 (C-19), 41.58 (C-14), 41.21 (C-18), 39.42 (C-8), 38.00 (C-1), 37.48 (C-4), 36.76 (C-10), 33.51 (C-21), 33.01 (C-29), 32.59 (C-7), 32.46 (C-22), 30.69 (C-20), 29.68 (C-15), 27.78 (C-23), 27.67 (C-2), 25.84 (C-27), 23.57 (C-30), 23.43 (C-16), 23.38 (C-11), 22.04 (—CH2—); 18.11 (C-6), 17.49 (C-26), 16.79 (C-24), 15.30 (C-25); MS (m/z) 648.9 [M]+ (C40H56O7).
3-[2-(4-Propoxycarbonylphenoxy)acetoxy]olean-12-en-28-oic acid (26): in the reaction of 14 and 20, after chromatographic separation, the product 26 was obtained as a white fine crystals (W = 79%); m.p. 233–237 °C; Rf = 0.34 (hexane/ethyl acetate 4:1); 1H NMR (δ[ppm]): 10.30 (s, 1H, COOH), 8.02 (m, 2H, 3,5-HAr), 6.95 (m, 2H, 2,6-HAr), 5.30 (t, 1H, J = 3,6 Hz, -CH-12terp,), 4.71 (s, 2H, —OCH2—), 4.32 (m, 1H, CH-3terp), 4.27 (m, 2H, —OCH2—C2H5), 2.84 (dd, 1H, J = 13.6, 3.6 Hz, CH-18terp,), 1.80 (m, 2H, —CH2—), 1.15–0.76 (s, 21H, 7 × CH3), 1.04 (m, 3H, CH3); 13C NMR (δ[ppm]): 183.57 (COOH), 168.29 (-C(O)Ophenol-terp), 166.32 (-C(O)O-C3H7), 161.43 (C-1Ar), 143.60 (C-13terp), 131.62 (C-3,5Ar), 123.90 (C-4Ar), 122.47 (C-12terp), 115.15 (C-2,6Ar), 82.67 (C-3terp), 70.16 (—OCH2—), 66.40 (—CH2—C2H5), 55.23 (C-5), 47.52 (C-9), 46.53 (C-17), 45.85 (C-19), 41.72 (C-14), 41.30 (C-18), 39.50 (C-8), 38.10 (C-1), 37.90 (C-4), 36.91 (C-10), 33.88 (C-21), 33.15 (C-29), 32.70 (C-7), 32.52 (C-22), 30.70 (C-20), 29.81 (C-15), 27.96 (C-23), 27.80 (C-2), 25.98 (C-27), 23.66 (C-30), 23.55 (C-16), 23.45 (C-11), 22.15 (–CH2-); 18.20 (C-6), 17.88 (C-26), 16.85 (C-24), 15.30 (C-25), 10.54 (CH3); MS (m/z) 675.9 [M]+ (C42H60O7).
3-[2-(4-Acetamidophenoxy)acetoxy]olean-12-en-28-oic acid (27): in the reaction of 15 and 20, after chromatographic separation, the product 27 was obtained as a white fine crystals (W = 92%); m.p. 234–240 °C; Rf = 0.30 (hexane/ethyl acetate 4:1); 1H NMR (CDCl3): 10.51 (s, 1H, COOH), 8.31 (br s, 1H, NH), 7.38 (m, 2HAr), 6.61 (m, 2HAr), 5.21 (t, 1H, J = 3.4, CH-12terp), 4.74 (s, 2H, —OCH2—), 4.46 (m, 1H, CH-3terp), 2.80 (dd, 1H, J = 14.1, 3.4, CH-18terp), 2.03 (s, 3H, CH3), 1.09–0.61 (s, 21H, 7 × CH3); 13C NMR (CDCl3): 184.52 (COOH), 175.53 (C(O)Ophenol-terp), 167.44 (C(O)NH), 153.15 (C-1Ar), 143.33 (C-13terp), 130.99 (C-4Ar), 122.53 (C-12terp),120.99 (C-3.5Ar), 114.97 (C-2.6Ar), 80.79 (C-3terp), 70.43 (—OCH2—), 55.33 (C-5), 47.59 (C-9), 46.69 (C-17), 45.88 (C-19), 41.79 (C-14), 41.54 (C-18), 39.60 (C-8), 38.16 (C-1), 37.95 (C-4), 37.01 (C-10), 33.98 (C-21), 33.23 (C-29), 32.81 (C-7), 32.65 (C-22), 30.78 (C-20), 29.86 (C-15), 28.02 (C-23), 27.91 (C-2), 26.00 (C-27), 23.67 (C-30), 23.68 (CH3), 23.64 (C-16), 23.51 (C-11), 18.43 (C-6), 17.96 (C-26), 16.89 (C-24), 15.42 (C-25); MS (m/z) 646.9 [M]+ (C40H57NO6).
3-[2-(Naphtalen-1-yloxy)acetoxy]olean-12-en-28-oic acid (28): in the reaction of 16 and 20, after chromatographic separation, the product 28 was obtained as a colorless resin (with tendency to crystallization) (W = 54%); Rf = 0.68 (hexane/ethyl acetate 2:1); 1H NMR (CDCl3): 10.60 (s, 1H, COOH), 8.23–8.19 (m, 1HAr), 7.81–7.76 (m, 1HAr), 7.52–7.37 (m, 3HAr), 7.28–7.20 (m, 1HAr), 6.80–6.71 (m, 1HAr), 5.25 (t, 1H, J = 3.4, CH-12terp), 4.77 (s, 2H, —OCH2—), 4.51 (m, 1H, CH-3terp), 2.83 (dd, 1H, J = 14.1, 3.4, CH-18terp), 1.12–0.69 (s, 21H, 7 × CH3); 13C NMR (CDCl3): 185.25 (COOH), 176.33 (C(O)Ophenol-terp), 156.20 (CAr), 143.33 (C-13terp), 134.91 (CAr), 128.02 (CAr), 127.64 (CAr), 126.42 (CAr), 126. 23 (CAr), 124.92 (CAr), 122.53 (C-12terp), 122. 11 (CAr), 80.82 (C-3terp), 70.46 (—OCH2—), 55.34 (C-5), 47.60 (C-9), 46.72 (C-17), 45.92 (C-19), 41.81 (C-14), 41.58 (C-18), 39.63 (C-8), 38.16 (C-1), 37.99 (C-4), 37.08 (C-10), 34.02 (C-21), 33.32 (C-29), 32.87 (C-7), 32.67 (C-22), 30.82 (C-20), 29.92 (C-15), 28.08 (C-23), 27.99 (C-2), 26.06 (C-27), 23.69 (C-30), 23.75 (C-16), 23.62 (C-11), 18.46 (C-6), 17.98 (C-26), 16.92 (C-24), 15.50 (C-25); MS (m/z) 639.6 [M]+ (C42H56O5).
3-[2-(Naphtalen-2-yloxy)acetoxy]olean-12-en-28-oic acid (29): in the reaction of 17 and 20, after chromatographic separation, the product 29 was obtained as a white fine crystals (W = 58%); m.p. 178–183 °C; Rf = 0.43 (hexane/ethyl acetate 2:1); 1H NMR (CDCl3): 10.62 (s, 1H, COOH), 7.98–7.90 (m, 1HAr), 7.88–7.80 (m, 1HAr), 7.60–7.52 (m, 2HAr), 7.50–7.42 (m, 1HAr), 7.39–7.35 (m, 1HAr), 7.20–7.15 (m, 1HAr), 5.28 (t, 1H, J = 3.4, CH-12terp), 4.79 (s, 2H, —OCH2—), 4.56 (m, 1H, CH-3terp), 2.81 (dd, 1H, J = 14.1, 3.4, CH-18terp), 1.11–0.71 (s, 21H, 7 × CH3); 13C NMR (CDCl3): 185.43 (COOH), 176.32 (C(O)Ophenol-terp), 156.30 (CAr), 143.37 (C-13terp), 134.94 (CAr), 128.12 (CAr), 127.76 (CAr), 126.53 (CAr), 126.32 (CAr), 124.98 (CAr), 122.56 (C-12terp), 122.12 (CAr), 80.86 (C-3terp), 70.44 (—OCH2—), 55.42 (C-5), 47.67 (C-9), 46.78 (C-17), 45.95 (C-19), 41.87 (C-14), 41.63 (C-18), 39.72 (C-8), 38.14 (C-1), 38.03 (C-4), 37.15 (C-10), 34.24 (C-21), 33.51 (C-29), 32.97 (C-7), 32.76 (C-22), 30.86 (C-20), 29.94 (C-15), 28.18 (C-23), 28.00 (C-2), 26.13 (C-27), 23.74 (C-30), 23.80 (C-16), 23.65 (C-11), 18.48 (C-6), 18.00 (C-26), 16.97 (C-24), 15.55 (C-25); MS (m/z) 639.6 [M]+ (C42H56O5).
3-[2-{4-[7-(4-hydroxy-3-metoxyphenyl)-3,5-dioxohepta-1,6-dienyl]-2-metoxyphenoxy)acetoxy} olean-12-en-28-oic acid (30): in the reaction of 18 and 20, after chromatographic separation, the product 30 was obtained as a pale yellow resin (with tendency to crystallization) (W = 38%); Rf = 0.28 (hexane/ethyl acetate 2:1); 1H NMR: 16.03 (br s, 1H, OHenol), 10.89 (s, 1H, COOH), 7.54 (d, J = 16 Hz, 2H, HC = C, 1.7-H); 7.11 (m, 2H, 6-Ar); 6.87 (m, 4H, 2.5-Ar); 6.49 (d, J = 16 Hz, 2H, ⚌CH—CO—, 2.6-H); 5.84 (broad s, 1H, OHphenol); 5.79 (s, 1H, —CH⚌C(OH)—), 5.26 (t, 1H, J = 3.4, CH-12terp), 4.64 (s, 2H, OCH2); 4.44 (m, 1H, CH-3), 3.88 (s, 6H, 3-Ar-OCH3), 2.82 (dd, 1H, J = 14.1, 3.4, CH-18terp), 1.13–0.68 (s, 21H, 7CH3); 13C NMR: 183.50 (COOH), 183.16 (C3), 174.35, (C(O)Ophenol-terp), 147.75 (C4-Ar), 146,64 (C3-Ar), 143.58 (C-13), 140.32 (C1), 127.58 (C1-Ar), 122.72 (C6-Ar), 122.53 (C-12), 121.65 (C2), 114.57 (C5-Ar), 109.45 (C2-Ar), 101.12 (C4), 80.85 (C-3), 70.37 (OCH2), 55.88 (OCH3), 55.30 (C-5), 47.60 (C-9), 46.72 (C-17), 45.85 (C-19), 41.72 (C-14), 41.30 (C-18), 39.50 (C-8), 38.10 (C-1), 37.90 (C-4), 36.91 (C-10), 33.88 (C-21), 33.15 (C-29), 32.70 (C-7), 32.52 (C-22), 30.70 (C-20), 29.81 (C-15), 27.96 (C-23), 27.80 (C-2), 25.98 (C-27), 23.66 (C-30), 23.55 (C-16), 23.45 (C-11), 18.20 (C-6), 17.88 (C-26), 16.85 (C-24), 15.30 (C-25); MS (m/z) 864.0 [M]+ (C53H68O10).
3-[2-{5-Hydroxy-3-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-yloxy)acetoxy}olean-12-en-28-oic acid (31): in the reaction of 19 and 20, after chromatographic separation, the product 31 was obtained as a light beige resin (with tendency to crystallization) (W = 30%); Rf = 0.16 (hexane/ethyl acetate 2:1); 1H NMR (CDCl3): 12.98 (s, 1H, 5-OH), 10.53 (s, 1H, COOH), 9.72 (s, 1H, 4′-OH), 8.39 (s, 1H, 2-H), 7.44–7.36 (m, 2H, 2′,6′-HAr), 6.87–6.79 (m, 2H, 3′,5′-HAr), 6.43 (s, 1H, 8-H), 6.27 (s, 1H, 6-H), 5.21 (t, 1H, J = 3.4, CH-12terp), 4.68 (s, 2H, —OCH2—), 4.46 (m, 1H, CH-3terp), 2.80 (dd, 1H, J = 14.1, 3.4, CH-18terp), 1.11–0.66 (s, 21H, 7 × CH3); 13C NMR (CDCl3): 184.59 (COOH), 182.24 (C⚌O), 175.57 (C(O)Ophenol-terp), 165.93 (Cg-7), 163.85 (Cg-5), 159.69 (Cg-9), 158.81 (Cg-4′), 154.76 (Cg-2), 143.38 (C-13terp), 131.38 (Cg-2′,6′), 124.73 (Cg-3), 123.29 (Cg-1′), 122.58 (C-12terp), 116.25 (Cg-3′,5′), 106.28 (Cg-10), 100,12 (Cg-6), 94.27 (Cg-8), 80.82 (C-3terp), 70.47 (—OCH2—), 55.35 (C-5), 47.61 (C-9), 46.72 (C-17), 45.93 (C-19), 41.82 (C-14), 41.57 (C-18), 39.61 (C-8), 38.21 (C-1), 37.97 (C-4), 37.06 (C-10), 34.04 (C-21), 33.29 (C-29), 32.86 (C-7), 32.68 (C-22), 30.82 (C-20), 29.91 (C-15), 28.08 (C-23), 27.98 (C-2), 26.03 (C-27), 23.70 (C-30), 23.72 (CH3), 23.69 (C-16), 23.55 (C-11), 18.47 (C-6), 17.99 (C-26), 16.94 (C-24), 15.47 (C-25); MS (m/z) 765.7 [M]+ (C47H58O9).
3.2.2 General procedure for the synthesis of iminoester-type triterpene-phenol conjugates (32–40)
Oleanolic acid oxime (22) (0.46 g, 1 mmol), DCC (1.03 g, 5 mmol) and functionalized phenols (1.5 mmol) were mixed at 0 °C and then stirred at room temperature in dry CH2Cl2 (12 mL) for 2 h. Hexane (8 mL) was added to the reaction mixture and cooled. Next, the resulting precipitate of dicyclohexylurea was filtered. The organic phase was washed with 5% aqueous hydrochloric acid, 5% aqueous sodium bicarbonate and water and dried over anhydrous sodium sulfate. After removal of the drying agent and solvent evaporation, the crude new iminoesters (32–40) were purified with column chromatography on silica gel using hexane/ethyl acetate (2:1 or 4:1, v:v) mixture as eluent.
3-[2-(5-Methyl-2-propan-2-ylphenoxy)acetoxy]iminoolean-12-en-28-oic acid (32): in the reaction of 11 and 22, after chromatographic separation, the product 32 was obtained as a colorless resin (W = 56%); Rf = 0.37 (hexane/ethyl acetate 4:1); 1H NMR (CDCl3): 9.92 (s, 1H, COOH), 7.12–7.10 (m, 1H, Ar), 6.79–6.74 (m, 1H, Ar), 6.49 (s, 1H, Ar), 5.30 (t, 1H, J = 3,6Hz, -CH12-terp), 4.63 (s, 2H, —OCH2—), 3.39–3.33 (m, 1H, —CH—), 2.80 (dd, 1H, J = 13.6, 3.6 Hz, CH-18terp), 2.28 (s, 3H, —CH3), 1.23 (s, 3H, —CH3), 1.20 (s, 3H, —CH3), 1.13–0.76 (s, 21H, 7 × CH3); 13C NMR (CDCl3): 178.91 (COOH), 173.11 (C-3terp), 168.19 (—C(O)ONphenol-terp), 154.16 (C-1Ar), 142.23 (C-13terp), 135.56 (C-5Ar), 133.59 (C-2Ar), 125.69 (C-3Ar), 122.46 (C-4Ar), 122.06 (C-12terp), 111.76 (C-6Ar), 73.03 (—OCH2—), 55.10 (C-5), 47.30 (C-9), 46.32 (C-17), 45.64 (C-19), 41.53 (C-14), 41.20 (C-18), 39.25 (C-8), 37.89 (C-1), 37.69 (C-4), 36.76 (C-10), 33.54 (C-21), 33.05 (C-29), 32.48 (C-7), 32.39 (C-22), 30.56 (C-20), 29.31 (C-15), 27.66 (C-23), 27.43 (C-2), 26.37 (CH3 tym), 25.68 (C-27), 25.55 (CH3 tym), 23.35 (C-30), 23.22 (C-16), 23.17 (C-11), 18.02 (C-6), 17.59 (C-26), 16.63 (C-24), 15.22 (C-25), 22.61 (CHtym), 21.06 (CH3 tym); MS (m/z) 659.9 [M]+ (C42H61NO5).
3-[2-(2-Methoxy-4-prop-2-enylphenoxy]iminoolean-12-en-28-oic acid (33): in the reaction of 12 and 22, after chromatographic separation, the product 33 was obtained as a white fine crystals (W = 55%); m.p. 77–80 °C; Rf = 0.41 (hexane/ethyl acetate 2:1); 1H NMR (CDCl3): 9.96 (s, 1H, COOH), 6.92 (d, 1H, J = 8.6 Hz, H-6Ar), 6.76 (d, 1H, J = 8.6 Hz, H-5Ar), 6.74 (s, 1H, H-3Ar), 5.96 (m, 1H, ⚌CH—), 5.32 (t, 1H, J = 3,6Hz, -CH12-terp), 5.12 and 5.19 (each d, 1H, J = 19.7, CH2⚌C—), 4.60 (s, 2H, —OCH2—), 3.86 (s, 3H, —OCH3), 3.38 (d, 2H, J = 6.6 Hz, —CH2—), 2.81 (dd, 1H, J = 13.6, 3.6 Hz, CH-18terp), 1.10–0.72 (s, 21H, 7 × CH3); 13C NMR (CDCl3): 176.81 (COOH), 172.97 (C-3terp), 169.03 (—C(O)ONphenol-terp), 146.57 (C-2Ar), 144.71 (C-1Ar), 143.34 (C-13terp), 138.03 (C-4Ar), 131.89 (⚌CH—), 122.00 (C-12terp), 121.52 (C-5Ar), 115.48 (C-3Ar), 114.51 (C-6Ar), 111.42 (CH2⚌), 73.04 (—OCH2—), 56.16 (OCH3), 55.19 (C-5), 47.60 (C-9), 46.62 (C-17), 45.47 (C-19), 41.58 (C-14), 41.23 (C-18), 40.12 (CH2), 39.41 (C-8), 38.00 (C-1), 37.78 (C-4), 36.82 (C-10), 33.79 (C-21), 33.02 (C-29), 32.53 (C-7), 32.39 (C-22), 30.56 (C-20), 29.79 (C-15), 27.88 (C-23), 27.65 (C-2), 25.76 (C-27), 23.43 (C-30), 23.42 (C-16), 23.38 (C-11), 18.04 (C-6), 17.61 (C-26), 16.72 (C-24), 15.08 (C-25); MS (m/z) 672.8 [M]+ (C42H59NO6).
3-[2-(4-Methoxycarbonylphenoxy)acetoxy]iminoolean-12-en-28-oic acid (34): in the reaction of 13 and 22, after chromatographic separation, the product 34 was obtained as white fine crystals (W = 68%); m.p. 190–196 °C; Rf = 0.36 (hexane/ethyl acetate 4:1); 1H NMR (δ[ppm]): 10.38 (s, 1H, COOH), 8.10 (m, 2H, 3,5-HAr), 7.11 (m, 2H, 2,6-HAr), 5.35 (t, 1H, J = 3,6 Hz, -CH-12terp), 4.69 (s, 2H, —OCH2—), 3.97 (m, 2H, —OCH3), 2.97 (dd, 1H, J = 13.6, 3.6 Hz, CH-18terp), 1.20–0.82 (s, 21H, 7 × CH3); 13C NMR (δ[ppm]): 184.07 (COOH), 175.20 (—C(O)ONphenol-terp), 174.07 (C-3terp), 164.33 (—C(O)O—CH3), 161.48 (C-1Ar), 143.78 (C-13terp), 131.76 (C-3,5Ar), 126.73 (C-12), 123.95 (C-4Ar), 122.87 (C-12terp), 115.45 (C-2,6Ar), 70.22 (—OCH2—), 58.40 (—OCH3), 55.26 (C-5), 47.58 (C-9), 46.62 (C-17), 45.94 (C-19), 41.83 (C-14), 41.36 (C-18), 39.64 (C-8), 38.21 (C-1), 37.98 (C-4), 36.99 (C-10), 33.30 (C-29), 32.87 (C-22), 33.98 (C-21), 32.81 (C-7), 30.83 (C-20), 29.91 (C-15), 27.98 (C-23), 27.88 (C-2), 25.97 (C-27), 23.70 (C-30), 23.64 (C-16), 23.52 (C-11), 18.23 (C-6), 17.84 (C-26), 16.87 (C-24), 15.34 (C-25); MS (m/z) 661.9 [M]+ (C40H55NO7).
3-[2-(4-Propoxycarbonylphenoxy)acetoxy]iminoolean-12-en-28-oic acid (35): in the reaction of 14 and 22, after chromatographic separation, the product 35 was obtained as a white fine crystals (W = 81%); m.p. 178–183 °C; Rf = 0.42 (hexane/ethyl acetate 4:1); 1H NMR (δ[ppm]): 10.31 (s, 1H, COOH), 8.05 (m, 2H, 3,5-HAr), 7.08 (m, 2H, 2,6-HAr), 5.37 (t, 1H, J = 3,6 Hz, -CH-12terp,), 4.68 (s, 2H, —OCH2—), 4.27 (m, 2H, —OCH2-C2H5), 2.96 (dd, 1H, J = 13.6, 3.6 Hz, CH-18terp,), 1.74 (m, 2H, —CH2—), 1.20–0.82 (s, 21H, 7 × CH3), 1.22 (m, 3H, CH3); 13C NMR (δ[ppm]): 183.57 (COOH), 174.29 (—C(O)ONphenol-terp), 173.77 (C-3terp), 166.32 (—C(O)O—C3H7), 161.43 (C-1Ar), 143.60 (C-13terp), 131.62 (C-3,5Ar), 126.60 (C-12), 123.90 (C-4Ar), 122.47 (C-12terp), 115.15 (C-2,6Ar), 70.16 (—OCH2—), 66.40 (—OCH2—C2H5), 55.23 (C-5), 47.52 (C-9), 46.53 (C-17), 45.89 (C-19), 41.74 (C-14), 41.30 (C-18), 39.58 (C-8), 38.10 (C-1), 37.92 (C-4), 36.92 (C-10), 33.15 (C-29), 32.54 (C-22), 33.86 (C-21), 32.72 (C-7), 30.73 (C-20), 29.82 (C-15), 27.95 (C-23), 27.80 (C-2), 25.95 (C-27), 23.65 (C-30), 23.58 (C-16), 23.43 (C-11), 22.15 (—CH2—), 18.20 (C-6), 17.80 (C-26), 16.86 (C-24), 15.30 (C-25), 10.54 (CH3); MS (m/z) 688.7 [M]+ (C42H59NO7).
3-[2-(4-Acetamidophenoxy)acetoxy]iminoolean-12-en-28-oic acid (36): in the reaction of 15 and 22, after chromatographic separation, the product 36 was obtained as a white fine crystals (W = 82%); m.p. 197–205 °C; Rf = 0.53 (hexane/ethyl acetate 2:1); 1H NMR (CDCl3): 10.51 (s, 1H, COOH), 8.36 (br s, 1H, NH), 7.28 (m, 2HAr), 6.34 (m, 2HAr), 5.23 (t, 1H, J = 3.4, CH-12terp), 4.72 (s, 2H, —OCH2—), 2.81 (dd, 1H, J = 14.1, 3.4, CH-18terp), 2.02 (s, 3H, CH3), 1.11–0.73 (s, 21H, 7 × CH3); 13C NMR (CDCl3): 184.52 (COOH), 175.42 (C(O)ONphenol-terp), 172.65 (C-3terp), 167.56 (C(O)NH), 153.34 (C-1Ar), 143.53 (C-13terp), 130.87 (C-4Ar), 122.49 (C-12terp),120.86 (C-3.5Ar), 114.84 (C-2.6Ar), 70.24 (—OCH2—), 55.21 (C-5), 47.45 (C-9), 46.57 (C-17), 45.76 (C-19), 41.51 (C-14), 41.44 (C-18), 39.51 (C-8), 38.04 (C-1), 37.85 (C-4), 36.97 (C-10), 33.82 (C-21), 33.19 (C-29), 32.74 (C-7), 32.52 (C-22), 30.62 (C-20), 29.53 (C-15), 28.34 (C-23), 28.03 (C-2), 26.14 (C-27), 23.78 (C-30), 23.92 (CH3), 23.59 (C-16), 23.75 (C-11), 18.68 (C-6), 17.69 (C-26), 16.76 (C-24), 15.71 (C-25); MS (m/z) 659.8 [M]+ (C40H56N2O6).
3-[2-(Naphtalen-1-yloxy)acetoxy]iminoolean-12-en-28-oic acid (37): in the reaction of 16 and 22, after chromatographic separation, the product 37 was obtained as a colorless resin (with tendency to crystallization) (W = 54%); Rf = 0.77 (hexane/ethyl acetate 2:1); 1H NMR (CDCl3): 10.63 (s, 1H, COOH), 8.22–8.18 (m, 1HAr), 7.79–7.75 (m, 1HAr), 7.50–7.34 (m, 3HAr), 7.25–7.18 (m, 1HAr), 6.79–6.70 (m, 1HAr), 5.26 (t, 1H, J = 3.4, CH-12terp), 4.73 (s, 2H, —OCH2—), 2.81 (dd, 1H, J = 14.1, 3.4, CH-18terp), 1.08–0.61 (s, 21H, 7 × CH3); 13C NMR (CDCl3): 184.52 (COOH), 175.64 (C(O)ONphenol-terp), 156.81 (CAr), 143.33 (C-13terp), 135.21 (CAr), 128.11 (CAr), 127.72 (CAr), 126.54 (CAr), 126.33 (CAr), 124.98 (CAr), 122.53 (C-12terp), 121.02 (CAr), 119.93 (CAr), 108.95 (CAr), 70.43 (—OCH2—), 55.33 (C-5), 47.59 (C-9), 46.69 (C-17), 45.88 (C-19), 41.79 (C-14), 41.54 (C-18), 39.60 (C-8), 38.16 (C-1), 37.95 (C-4), 37.01 (C-10), 33.98 (C-21), 33.23 (C-29), 32.81 (C-7), 32.65 (C-22), 30.78 (C-20), 29.86 (C-15), 28.02 (C-23), 27.91 (C-2), 26.00 (C-27), 23.67 (C-30), 23.68 (CH3), 23.64 (C-16), 23.51 (C-11), 18.43 (C-6), 17.96 (C-26), 16.89 (C-24), 15.42 (C-25); MS (m/z) 652.6 [M]+ (C42H55NO5).
3-[2-(Naphtalen-2-yloxy)acetoxy]iminoolean-12-en-28-oic acid (38): in the reaction of 17 and 22, after chromatographic separation, the product 38 was obtained as a white fine crystals (W = 61%); m.p. 150–154 °C; Rf = 0.39 (hexane/ethyl acetate 4:1); 1H NMR (CDCl3): 10.63 (s, 1H, COOH), 8.22–8.18 (m, 1HAr), 7.79–7.75 (m, 1HAr), 7.50–7.34 (m, 3HAr), 7.25–7.18 (m, 1HAr), 6.79–6.70 (m, 1HAr), 5.26 (t, 1H, J = 3.4, CH-12terp), 4.73 (s, 2H, —OCH2—), 2.81 (dd, 1H, J = 14.1, 3.4, CH-18terp), 1.08–0.61 (s, 21H, 7 × CH3); 13C NMR (CDCl3): 184.57 (COOH), 175.67 (C(O)ONphenol-terp), 157.22 (CAr), 143.43 (C-13terp), 134.58 (CAr), 129.87 (CAr), 128.98 (CAr), 127.77 (CAr), 126.54 (CAr), 126.39 (CAr), 123.66 (CAr), 122.56 (C-12terp), 117.72 (CAr), 109.57 (CAr), 70.47 (—OCH2—), 55.31 (C-5), 47.52 (C-9), 46.63 (C-17), 45.84 (C-19), 41.80 (C-14), 41.60 (C-18), 39.59 (C-8), 38.13 (C-1), 37.91 (C-4), 37.11 (C-10), 33.92 (C-21), 33.53 (C-29), 32.79 (C-7), 32.63 (C-22), 30.81 (C-20), 29.84 (C-15), 28.12 (C-23), 27.86 (C-2), 25.99 (C-27), 23.65 (C-30), 23.63 (CH3), 23.61 (C-16), 23.47 (C-11), 18.38 (C-6), 17.89 (C-26), 16.76 (C-24), 15.38 (C-25); MS (m/z) 652.6 [M]+ (C42H55NO5).
3-[2-{4-[7-(4-hydroxy-3-metoxyphenyl)-3,5-dioxohepta-1,6-dienyl]-2-metoxyphenoxy)acetoxy} iminoolean-12-en-28-oic acid (39): in the reaction of 18 and 22, after chromatographic separation, the product 39 was obtained as a pale yellow resin (with tendency to crystallization) (W = 39%); Rf = 0.32 (hexane/ethyl acetate 2:1); 1H NMR: 16.03 (br s, 1H, OHenol), 10.89 (s, 1H, COOH), 7.54 (d, J = 16 Hz, 2H, HC = C, 1.7-H); 7.11 (m, 2H, 6-Ar); 6.87 (m, 4H, 2.5-Ar); 6.49 (d, J = 16 Hz, 2H, ⚌CH—CO—, 2.6-H); 5.84 (broad s, 1H, OHphenol); 5.79 (s, 1H, —CH⚌C(OH)—), 5.26 (t, 1H, J = 3.4, CH-12terp), 4.64 (s, 2H, OCH2), 3.88 (s, 6H, 3-Ar-OCH3), 2.82 (dd, 1H, J = 14.1, 3.4, CH-18terp), 1.13–0.68 (s, 21H, 7CH3); 13C NMR: 183.48 (COOH), 183.14 (C3), 175.65 (C(O)ONphenol-terp), 174.33 (C-3terp), 147.72 (C4-Ar), 146,61 (C3-Ar), 143.56 (C-13), 140.30 (C1), 127.55 (C1-Ar), 122.69 (C6-Ar), 122.50 (C-12), 121.62 (C2), 114.53 (C5-Ar), 109.41 (C2-Ar), 101.09 (C4), 70.33 (OCH2), 55.84 (OCH3), 55.28 (C-5), 47.58 (C-9), 46.69 (C-17), 45.81 (C-19), 41.69 (C-14), 41.26 (C-18), 39.47 (C-8), 38.06 (C-1), 37.88 (C-4), 36.86 (C-10), 33.83 (C-21), 33.04 (C-29), 32.66 (C-7), 32.48 (C-22), 30.67 (C-20), 29.76 (C-15), 27.90 (C-23), 27.78 (C-2), 25.97 (C-27), 23.62 (C-30), 23.50 (C-16), 23.39 (C-11), 18.17 (C-6), 17.85 (C-26), 16.83 (C-24), 15.28 (C-25); MS (m/z) 877.0 [M]+ (C53H67NO10).
3-[2-{5-Hydroxy-3-(4-hydroxyphenyl)-4-oxo-4H-chromen-7-yloxy)acetoxy}iminoolean-12-en-28-oic acid (40): in the reaction of 19 and 22, after chromatographic separation, the product 40 was obtained as a light beige resin (with tendency to crystallization) (W = 37%); Rf = 0.21 (hexane/ethyl acetate 2:1); 1H NMR (CDCl3): 13.00 (s, 1H, 5-OH), 10.54 (s, 1H, COOH), 9.74 (s, 1H, 4′-OH), 8.41 (s, 1H, 2-H), 7.40–7.31 (m, 2H, 2′,6′-HAr), 6.85–6.74 (m, 2H, 3′,5′-HAr), 6.46 (s, 1H, 8-H), 6.31 (s, 1H, 6-H), 5.23 (t, 1H, J = 3.4, CH-12terp), 4.71 (s, 2H, —OCH2—), 2.82 (dd, 1H, J = 14.1, 3.4, CH-18terp), 1.13–0.70 (s, 21H, 7 × CH3); 13C NMR (CDCl3): 184.61 (COOH), 182.27 (C⚌O), 175.36 (C(O)ONphenol-terp), 172.68 (C-3terp), 165.95 (Cg-7), 163.87 (Cg-5), 159.71 (Cg-9), 158.84 (Cg-4′), 154.79 (Cg-2), 143.41 (C-13terp), 131.42 (Cg-2′,6′), 124.77 (Cg-3), 123.33 (Cg-1′), 122.62 (C-12terp), 116.29 (Cg-3′,5′), 106.32 (Cg-10), 100,15 (Cg-6), 94.29 (Cg-8), 70.51 (—OCH2—), 55.38 (C-5), 47.66 (C-9), 46.74 (C-17), 45.95 (C-19), 41.86 (C-14), 41.60 (C-18), 39.64 (C-8), 38.23 (C-1), 38.00 (C-4), 37.12 (C-10), 34.07 (C-21), 33.33 (C-29), 32.91 (C-7), 32.72 (C-22), 30.86 (C-20), 29.94 (C-15), 28.11 (C-23), 28.01 (C-2), 26.07 (C-27), 23.73 (C-30), 23.75 (CH3), 23.72 (C-16), 23.58 (C-11), 18.49 (C-6), 18.04 (C-26), 16.98 (C-24), 15.51 (C-25); MS (m/z) 778.7 [M]+ (C47H57NO9).
4 Conclusions
The linked triterpene and phenolic-type derivatives were obtained in two-stage synthesis. Rational synthetic strategy for the functionalization of selected phenols and typical mild chemistry for triterpene structures were used in order to obtain final linker-mode conjugates. Unconventional factors such as microwave irradiation and ultrasonic waves were used to support the reactions involving the introduction of bifunctional small linkers to modify the functionality of the phenolic compounds, as well as their pharmacological properties. O-Alkylation reactions with chloroacetic acid enabled rapid functionalization and change in the reactivity of selected phenolic compounds. The resulting COOH-functionalized phenols were used in subsequent hybridization reactions with selected oleanolic derivatives, that contained a secondary hydroxyl group, by use of esterification type reaction. The described experiments confirmed the beneficial effect of non-classical reaction conditions, using microwaves and ultrasounds combination (MW-US) on the etherification reaction. Microwave-ultrasonic techniques can successfully compete with classical synthesis methods and are among the simplest, cheapest and most valuable tools in applied chemistry. The processes were carried out using environmentally safe energy sources, mild solvents, mainly water, which is also consistent with the idea of green chemistry. The synthesis of conjugate type compounds with the character of the multi-target drug is a huge hope in the treatment of cancer, infection and other civilization diseases. In addition, the use of a rational, “Lego”, green and synergistic synthetic approach to obtain new individuals for applications in the pharmaceutical field is a very promising area, which perfectly fits in the current trends in medicinal chemistry. In order to determine the drug-likeness of compounds (23–40), in silico calculation and prediction of some physicochemical parameters, bioactivity score and theoretical toxicity risks were carried out. The results predicted for the derivatives obtained are not model, however, literature reports allow to conclude on the possibility of a favorable bioactivity profile for this type of compounds, despite their violation from the Lipinski’s rule, the significant hydrophobicity and not optimal molecular weight.
Funding
The task was financially supported by the NCN as a part of Miniatura 1 project - decision 2017/01/X/ST5/00341.
References
- Oleanolic acid and its derivatives: Biological activities and therapeutic potential in chronic diseases. Molecules. 2017;22:1915.
- [CrossRef] [Google Scholar]
- The analgesic and anti-inflammatory effect of new oleanolic acid acyloxyimino derivative. Eur. J. Pharm. Sci.. 2012;47:549-555.
- [CrossRef] [Google Scholar]
- Strong and long-lasting antinociceptive and anti-inflammatory conjugate of naturally occurring oleanolic acid and aspirin. Front. Pharmacol.. 2016;7:202.
- [CrossRef] [Google Scholar]
- Pentacyclic triterpenes. Part 3: Synthesis and biological evaluation of oleanolic acid derivatives as novel inhibitors of glycogen phosphorylase. Bioorg. Med. Chem. Lett.. 2006;16:2915-2919.
- [CrossRef] [Google Scholar]
- The combined use of microwaves and ultrasound: Improved tools in process chemistry and organic synthesis. Chem. Eur. J.. 2007;13:1902-1909.
- [CrossRef] [Google Scholar]
- Design of Hybrid Molecules for Drug Development. Elsevier; 2017.
- Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug. Deliver Rev.. 1997;23:4-25.
- [CrossRef] [Google Scholar]
- Semisynthetic oleanane triterpenoids inhibit migration and invasion of human breast cancer cells through downregulated expression of the ITGB1/PTK2/PXN pathway. Chem. Biol. Interact.. 2017;268:136-147.
- [CrossRef] [Google Scholar]
- Synthesis and antitumor activities of naturally occurring oleanolic acid triterpenoid saponins and their derivatives. Eur. J. Med. Chem.. 2013;64:1-15.
- [CrossRef] [Google Scholar]
- Playing LEGO with macromolecules: design, synthesis, and self-organization with metal complexes. J. Polym. Sci. Part A Polym. Chem.. 2003;41:1413-1427.
- [CrossRef] [Google Scholar]
- Novel cinnamaldehyde-based aspirin derivatives for the treatment of colorectal cancer. Bioorg. Med. Chem. Lett.. 2018;28:2869-2874.
- [CrossRef] [Google Scholar]
- Chemical modification of oleanene type triterpenes and their inhibitory activity against HIV-1 protease dimerization. Chem. Pharm. Bull.. 2000;48:1681-1688.
- [CrossRef] [Google Scholar]
- Lego chemistry for the straightforward synthesis of dendrimers. J. Org. Chem.. 2003;68:6043-6046.
- [CrossRef] [Google Scholar]
- Combined microwaves/ultrasound, a hybrid technology. Top. Curr. Chem.. 2016;374:79.
- [CrossRef] [Google Scholar]
- Natural products: an evolving role in future drug discovery. Eur. J. Med. Chem.. 2011;46:4769-4807.
- [CrossRef] [Google Scholar]
- Molinspiration Cheminformatics, 1986. https://www.molinspiration.com/ (accessed on 20 July 2020).
- Multi-target drugs: strategies and challenges for medicinal chemists. In: Wermuth C.G., ed. The Practice of Medicinal Chemistry. New York: Elsevier; 2008. p. :549-571.
- [Google Scholar]
- Organic Chemistry Portal, 2010. https://www.organic-chemistry.org/prog/peo/ (accessed on 20 July 2020).
- PASS Online-Way2Drug.com © 2011 - 2020. http://www.pharmaexpert.ru/passonline/ (accessed on 20 July 2020).
- Molecular mechanisms of biological activity of oleanolic acid - a source of inspiration for a new drugs design. Mini Rev. Org. Chem.. 2014;11:330-342.
- [CrossRef] [Google Scholar]
- Hybrid compounds strategy in the synthesis of oleanolic acid skeleton-NSAID derivatives. Molecules. 2016;21:420.
- [CrossRef] [Google Scholar]
- Molecular consortia-various structural and synthetic concepts for more effective therapeutics synthesis. Int. J. Mol. Sci.. 2018;19:1104.
- [CrossRef] [Google Scholar]
- Microwave (MW), Ultrasound (US) and Combined Synergic MW-US strategies for rapid functionalization of pharmaceutical use phenols. Molecules. 2018;23:2360.
- [Google Scholar]
- Combined microwave and ultrasound assisted Williamson ether synthesis in the absence of phase-transfer catalysts. Green Chem.. 2002;4:349-351.
- [CrossRef] [Google Scholar]
- Novel linkers and connections for antibody-drug conjugates to treat cancer and infectious disease. Pharm. Pat. Anal.. 2017;6:25-33.
- [CrossRef] [Google Scholar]
- Oleanolic acid and its synthetic derivatives for the prevention and therapy of cancer: preclinical and clinical evidence. Cancer Lett.. 2014;346:206-216.
- [CrossRef] [Google Scholar]
- Sineo Microwave Chemistry Technology, 2014. http://www.sineomicrowave.com/Upload/%E4%BA%A7%E5%93%81%E5%9B%BE%E7%89%87/hechengyi/UWave1000-20315522903.pdf (accessed on 20 July 2020).
- A novel co-drug of aspirin and ursolic acid interrupts adhesion, invasion and migration of cancer cells to vascular endothelium via regulating EMT and EGFR-mediated signaling pathways: multiple targets for cancer metastasis prevention and treatment. Oncotarget.. 2016;8:73114-73129.
- [Google Scholar]
- Recent progress in the development of synthetic hybrids of natural or unnatural bioactive compounds for medicinal chemistry. Mini. Rev. Med. Chem.. 2010;10:773-793.
- [CrossRef] [Google Scholar]
- Oleanolic acid arrests cell cycle and induces apoptosis via ROS-mediated mitochondrial depolarization and lysosomal membrane permeabilization in human pancreatic cancer cells. J. Appl. Toxicol.. 2013;33:756-765.
- [CrossRef] [Google Scholar]
- Synthesis and anti-hepaticfibrosis of glycyrrhetinic acid derivatives with inhibiting COX-2. Bioorg. Chem.. 2020;99:103804
- [CrossRef] [Google Scholar]







