Translate this page into:
Passivity and passivity breakdown of Al and Al–Zn alloys in SCN− solutions and the effect of some inorganic inhibitors – Galvanostatic, potentiostatic and ICP-AES studies
*Corresponding author at: Chemistry Department, Faculty of Science, Ain Shams University, 11566 Abbassia, Cairo, Egypt. Tel.: +0020 222509331 maaismail@yahoo.com (Mohammed A. Amin)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.

Available online 25 June 2010
Abstract
The susceptibility of Al and Al–Zn alloys, containing 6% and 12% (in weight) Zn, towards pitting in KSCN solutions was studied using glavanostatic and potentiostatic techniques. Potentiostatic results showed that the pitting process was categorized into the three stages, i.e., the first passivation stage, the second pit formation and growth stage and the final steady-state stage. Galvanostatic measurements allowed the pitting and repassivation potentials to be determined. The rate of pit initiation and growth was found to increase with increase in SCN− concentration, applied anodic current, applied anodic potential and solution temperature. Alloyed Zn was found to enhance pitting attack. ICP-AES method of chemical analysis confirmed results obtained from electrochemical measurements. The inhibiting effects of , , or anions were also investigated based on polarization and potentiostatic measurements, complemented with SEM and EDX examinations of the electrode surface. The pitting potential (Epit) and the incubation time (ti) were increased, while the pit growth current density (jpit) was decreased with increase in inhibitor concentration. These inorganic anions therefore inhibited pit nucleation and growth of Al in these solutions. and anions inhibited pitting corrosion more effectively than and anions.
Keywords
Pitting corrosion
Al and Al–Zn alloys
KSCN solutions
Inorganic inhibitors
1 Introduction
Al and Al alloys have many industrial applications due to their light weight, high strength and good corrosion resistance (Komisarov et al., 1996; Chen et al., 1999; Guillaumin and Mankowski, 1999; Dymek and Dollar, 2003). Al owes its excellent corrosion resistance and its usage as one of the primary metals of commerce to the barrier oxide film that is bonded strongly to its surface and, that if damaged, re-forms immediately in most environments (Despic and Parkhutik, 1989). On a surface freshly abraded and then exposed to air, the barrier oxide film is only 1 nm thick but is highly effective in protecting the Al from corrosion.
Corrosion of Al in the passive range is localized, usually manifested by random formation of pits. The pitting potential principle establishes the conditions under which metals in the passive state are subject to corrosion by pitting. For Al, pitting corrosion is most commonly produced by halide ions, of which chloride (Cl−) is the most frequently encountered in service (Polmear, 1969; Elboujdaini et al., 1988; Wolka and Virtanen, 2007; Genel, 2007; Na and Pyun, 2008; Cavanaugh et al., 2007; Ezuber et al., 2008). Pitting of Al in halide solutions open to the air occurs because, in the presence of oxygen, the metal is readily polarized to its pitting potential. Generally, Al does not develop pitting in aerated solutions of most nonhalide salts because its pitting potential in these solutions is considerably more noble (cathodic) than in halide solutions and it is not polarized to these potentials in normal service.
Our previous study (Amin et al., 2009) revealed that Al and Al–Zn alloys, when polarized to high anodic potentials, suffer from intense pitting attack in KSCN, a nonhalide salt, solutions. Indeed, we were the first who presented detailed study regarding corrosion, passivation and breakdown of passivity of Al and Al–Zn alloys in KSCN solutions. The purpose of this work, and in a continuation of our previous study (Amin et al., 2009), is to gain more information about the kinetics of passivity and passivity breakdown of Al and Al–Zn alloys in KSCN solutions. To achieve this goal, glavanostatic and potentiostatic measurements were employed under various experimental conditions. ICP-AES method of chemical analysis was also applied to confirm results obtained from electrochemical measurements that anodic potential and alloyed Zn activate pitting attack. It was also the purpose of the present work to study the effect of some inorganic inhibitors to control pitting corrosion of Al in KSCN solutions. Some SEM and EDX examinations of the electrode surface were also performed to confirm adsorption of the anions of the inorganic inhibitors.
2 Experimental
The working electrodes employed here were made of pure Al, (Al–6% Zn) and (Al–12% Zn) alloys. The composition of these materials was presented elsewhere (Amin et al., 2009). The investigated electrodes were cut as cylindrical rods, welded with Cu-wire for electrical connection and mounted into glass tubes of appropriate diameter using Araldite to offer an active flat disc shaped surface of (0.50 cm2) geometric area for the working electrode, to contact the test solution. Prior to each experiment, the surface pretreatment of the working electrode was performed by mechanical polishing (using a polishing machine model POLIMENT I, BUEHLER POLISHER) of the electrode surface with successive grades of emery papers down to 1200 grit up to a mirror finish. The electrode was then, rinsed with acetone, distilled water, and finally dipped in the electrolytic cell.
The experiments were performed in a 100 ml volume Pyrex glass cell using Pt wire and a saturated calomel electrode (SCE) as auxiliary and reference electrodes, respectively. The SCE was connected via a Luggin capillary, the tip of which was very close to the surface of the working electrode to minimize the IR drop. All potentials given in this paper are referred to this reference electrode. The experiments were carried out in 0.04 M KSCN solutions devoid of and containing various concentrations (10−4–2 × 10−3 M) of Na2MoO4, Na2WO4, Na2SiO3 or K2CrO4, as inorganic inhibitors. All solutions were freshly prepared from analytical grade chemical reagents using doubly distilled water and were used without further purification. For each run, a freshly prepared solution as well as a cleaned set of electrodes was used. Each run was carried out in aerated stagnant solutions at the required temperature (±1 °C), using water thermostat.
The potentiodynamic current–potential curves were carried out at a scan rate of 0.50 mV s−1. In galvanostatic potential/time transients, a constant anodic current density is applied on the Al samples and the variation in potential was recorded as a function of time. The potentiostatic measurements were carried out at a given step anodic potential (Es,a) at which the current transient was recorded. A Potentiostat/Galvanostat (EG&G model 273) and a personal computer were used. M352 corrosion software from EG&G Princeton Applied Research was used for the potentiodynamic polarization, galvanostatic and the potentiostatic measurements. Before each experiment, the open circuit potential of the working electrode was measured as a function of time during 60 min, the time necessary to reach a quasi-stationary value for the open circuit potential.
The acceleration influence of the applied anodic potential and alloyed Zn towards pitting corrosion of Al and Al–Zn alloys was evaluated in 0.04 M KSCN solutions at 25 °C using an independent chemical method of analysis, namely ICP-AES. The Al3+ ions concentration was determined in the aggressive solution as a function of the applied anodic potential and alloy composition after holding the sample for 5.0 min at the given potential. Measurements were carried out using Perkin–Elmer Optima 2100 Dual View inductively coupled plasma-atomic emission spectrometry (ICP-AES) instrument connected with AS 93 Plus autosampler.
The 40-MHz free-running generator was operated at a forward power of 1.3 kW; the outer, intermediate and Ar carrier gas flow rates were 15.0, 0.2 and 0.8 L/min, respectively. The carrier gas flow rate was optimized to obtain maximum signal-to-background ratios. In all experiments, the measured samples were nebulized downstream to the plasma by the autosampler and the concentrations were automatically determined using the standard calibration graph. The system adjusted to measure the samples in triplicates and the relative standard deviation was calculated. The RSD was <2% and the correlation coefficient was >0.99998.
The morphology and composition of the passive oxide films formed on the surface of Al in 0.04 M KSCN solutions without and with 0.002 M Na2MoO4, Na2WO4, Na2SiO3 or K2CrO4 was tested by SEM and EDX examinations using a JEOL JSM 6390 LA electron microscope connected with an energy dispersive spectrometer unit. In these experiments, samples of dimensions (1 cm × 1 cm × 0.10 cm) were used. These samples were first submitted to the same surface treatment used in the electrochemical techniques. For each experiment, the electrode was immersed for 5 min in electrolytic solution at 25 °C under potentiostatic regime at an anodic potential of 1300 mV (>Epit for Al in bare KSCN solution), and finally washed thoroughly and submitted to 5 min of ultrasonic cleaning in order to remove loosely adsorbed ions.
3 Results and discussion
3.1 Galvanostatic measurements
Our previous study (Amin et al., 2009), based on polarization and impedance studies, showed that Al and Al–Zn alloys pit in KSCN solutions. SCN− anions tend to rupture the passive film and initiate pitting (Amin et al., 2009). In the present work, Al and its two Al–Zn alloys were anodized galvanostatically in KSCN solutions under the effect of various experimental conditions with the aim to study the growth kinetics of the passive oxide films formed on Al and its two Al–Zn alloys, and their breakdown.
Fig. 1 presents the dependence of the anodic potential on time for anodization of the three Al samples in 0.04 M KSCN solution at a current density of 50 μA cm−2 at 25 °C. The linear increase in potential at the commencement of anodization accompanies the initial formation and growth of the oxide film. A potential maximum appears at a certain time, assigned as the incubation time, ti, which is necessary before pit growth to occur. This maximum is thought to correspond, as will be shown, to the pitting potential (Epit). The appearance of which is the correspondence of competition between two processes, namely, further oxide film growth and its breakdown.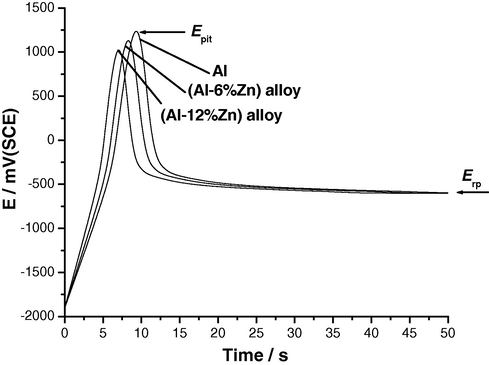
E/t curves recorded for the three Al samples in 0.04 M KSCN solution at an applied anodic current density (ja) of 50 μA cm−2 at 25 °C.
It follows from the data of Fig. 1 that the value and location of potential maximum depend on percentage of alloyed Zn in Al. The value of the potential maximum (i.e., Epit) decreases and its location shifts towards shorter times (i.e., shorter ti values) with alloyed Zn. These findings confirm results obtained in our previous study that alloyed Zn activates pitting corrosion of Al (Amin et al., 2009).
The acceleration influence of alloyed Zn towards the aggressive attack of SCN− anions was explained in our previous study (Amin et al., 2009) based on EDX examinations of the electrode surface. The obtained EDX spectra revealed increased adsorption of SCN− anions in presence of alloyed Zn. This alloyed Zn is thought to form ZnO in the Al2O3 matrix producing a more defective passive film, which enabled an easier ingress of the aggressive SCN− anions (Amin et al., 2009). These findings are in agreement with the work of Brillas et al. (1998) during studying the susceptibility of Al–Mg alloys towards localized corrosion in NaCl solutions.
After prevalence of the latter reaction at Epit, or in other words, after ti, the potential drops to a steady value, corresponding to the repassivation potential (Erp). Under galvanostatic conditions, Erp is the only potential at which the ac1tive area remains constant and independent of time (Galvele, 1978). It obvious that the values of Erp obtained from cyclic voltammetry (Amin et al., 2009) and galvanostatic measurements are in good agreements. These findings confirm that the potential maximum observed in the E/t plots corresponds to Epit.
The decline in potential following the maximum corresponds to breakdown of passive layer followed by the nucleation and formation of pits as a result of the aggressive attack of SCN− anions (Amin et al., 2009). After pit initiation, pit growth takes place immediately as a result of increasing the concentration of the aggressive anions by migration. The increased concentration of metal cations (Al3+ ions) inside the pit leads to changes in localized solution chemistry. First, following Galvele (1978), hydrolysis occurs to lower the pH. A second effect arises from the well-recognized requirement that electrical neutrality must be maintained throughout the electrolyte.
Therefore, aggressive ions (SCN− in the present study) must migrate into the pit to compensate the local increase in metal cations concentration, as shown by the theoretical analysis of Ateya and Pickering (Szklarska-Smialowska, 1986). The solution inside pits in this way becomes aggressive and allows active dissolution of Al and formation of soluble corrosion products that could be removed from pits by diffusion. This reflects the auto-catalytic nature of the pitting corrosion process. Consequently, the pit once initiated continues to propagate and enlarge (i.e., enhances in size and depth). These events reflect the decline in potential after Epit till reaching Erp (Amin et al., 2009).
The effect of various experimental conditions, including SCN− concentration, anodic current density and solution temperature, on the E/t responses of Al and its two Al–Zn alloys was studied. Results obtained for Al are depicted in Figs. 2–4. Similar results were obtained from the two Al–Zn alloys. It follows from Figs. 2–4 that the values of maximum (i.e., Epit) are dependent upon the SCN− concentration, constant current density applied, ja, and solution temperature. The repassivation potentials, Erp, are, however, unaffected by these parameters, reaching almost identical values after the potential drop.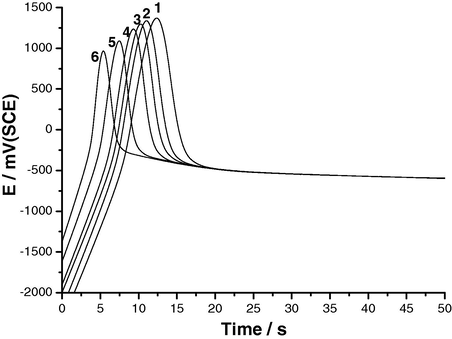
E/t curves recorded for Al in KSCN solutions of various concentrations at ja = 50 μA cm−2 at 25 °C. (1) 0.01 M KSCN; (2) 0.02 M KSCN; (3) 0.03 M KSCN; (4) 0.04 M KSCN; (5) 0.05 M KSCN; (6) 0.06 M KSCN.
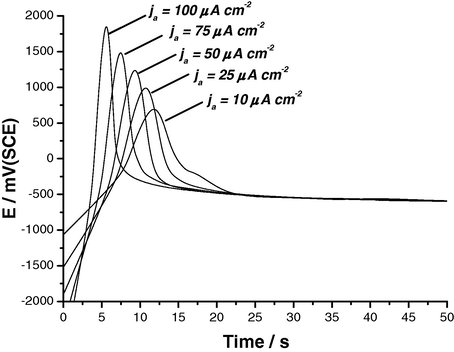
E/t curves recorded for Al in 0.04 M KSCN solutions at different applied anodic current densities at 25 °C.
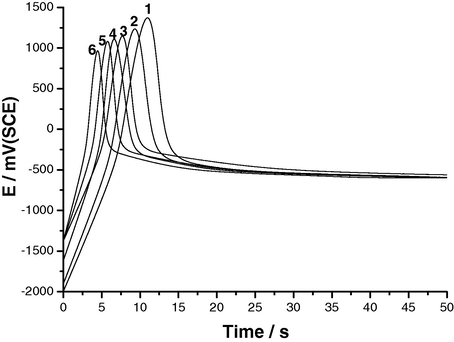
E/t curves recorded for Al in 0.04 M KSCN solutions at ja = 50 μA cm−2 at different temperatures. (1) 15 °C; (2) 25 °C; (3) 35 °C; (4) 45 °C; (5) 55 °C; (6) 65 °C.
Fig. 3 reveals that the slope of the linear part of the potential against time curves, and therefore the rate of oxide film growth, increases with increase in current density. This behaviour is due to the increase in the rate of ion transport across the pre-existing oxide film toward the metal/oxide interface, where the growth of the oxide film takes place (Albella et al., 1987). An increase in current density enhances the electric field across the oxide film, and therefore increases the rate of ion transport. Thus, the higher the current density used, the higher the rate of the oxide growth. This may explain the positive shift of the pitting potential with increasing current density (see again Fig. 3).
Referring again to Figs. 2–4 it is obvious that the incubation time, ti, decreases with increasing ja, SCN− concentration and temperature. These observations can be confirmed by plotting the rate of pit nucleation (i.e., the number of events per unit time), defined as (
), for the three samples vs. ja,[SCN−] or temperature, as shown in Figs. 5a–c, respectively. These relations denote that the rate of pit nucleation increases with these three parameters. The rate enhances with increase in Zn content in the Al samples, confirming the accelerating influence of the alloyed Zn (Amin et al., 2009).![Dependence of the rate of pit nucleation, t i − 1 , for the three samples in KSCN solutions, and (a) ja, (b)[SCN−] or (c) temperature.](/content/184/2011/4/2/img/10.1016_j.arabjc.2010.06.028-fig5.png)
Dependence of the rate of pit nucleation,
, for the three samples in KSCN solutions, and (a) ja, (b)[SCN−] or (c) temperature.
3.2 Potentiostatic measurements
Potentiostatic measurements were also performed alongside galvanostatic ones to gain more information regarding the kinetics of passivity and passivity breakdown of the three tested Al in SCN− solutions. Fig. 6 shows j/t transients recorded for Al in 0.04 M KSCN solution under the influence of the applied step anodic potential, Es,a, (before and after Epit, see polarization measurements in Amin et al., 2009) at 25 °C. Similar results were obtained for the two Al–Zn alloys (data not included here). When Es,a < Epit, the anodic current density initially decreases and then tends to attain steady-state current density, jss. The decrease in the current density is due to the formation and growth of Al2O3 on the anode surface. The steady-state current density corresponds to the current density passing through the passive layer (i.e., the passive current density, jpass) that is previously discussed in the polarization measurements of Al/SCN− system (Amin et al., 2009).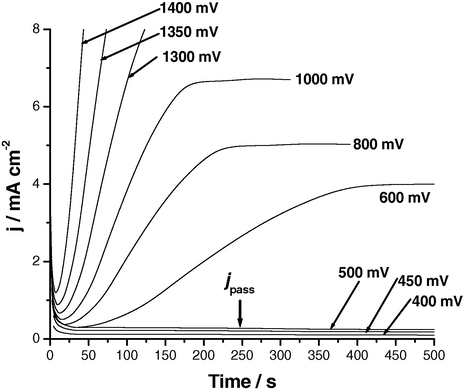
Potentiostatic j/t transients recorded for Al in 0.04 M KSCN solution under the influence of the applied step anodic potential, Es,a, at 25 °C.
The constancy of the steady-state current may be explained on the basis that the rate of oxide formation equals the rate of its dissolution so that the oxide film hardly grows. It is clear that this steady-state current density increases with increase in Es,a, reflecting thinning of the passive layer and formation of a less protective (thin and/or porous) passive film. The value of jss, at any given time, is found to be greater in presence of alloyed Zn, corresponding to the formation of a less protective passive film susceptible to the aggressive attack of SCN− anions. These data agree well with the potentiodynamic polarization results (Amin et al., 2009).
On the other hand, when Es,a > Epit, the anodic current density decreases initially to a minimum value determining the characteristic pitting parameter, namely the incubation time (ti). The magnitude of ti reflects, as previously shown, the susceptibility of sample to pitting corrosion. Beyond ti (i.e., after pit initiation), the current density increases rapidly due to propagation and growth of pits. It is possible that pitting corrosion products precipitate inside the pits. The corrosion products block up the pits, and therefore hinder the current flow through the pits. Thus, a steady-state was then attained between the metal dissolution and oxide film formation including a blockade by pitting corrosion product. Within this steady sate, the increment in current density caused due to the metal dissolution just equals the sum of the decrement in current density due to oxide film formation and the decrement of current density due to the blockage by pitting corrosion product, leading to nearly constant current density.
The effect of various experimental parameters including, applied anodic potential, SCN− concentration and solution temperature on the j/t transients of Al and its two Al–Zn alloys has been studied. Figs. 6–8 depict results obtained for Al, as a representative example. Similar results were obtained for the two Al–Zn alloys. The data reveal that jpit (the rising part of the transient) increases and ti decreases with increase in Es,a, SCN− concentration or solution temperature corresponding to increased susceptibility towards pitting. The relationships between the rate of pitting initiation,
, and these three parameters for the three Al samples in 0.04 M KSCN solutions have been constructed (not included here). The obtained plots showed that, for the three samples, the rate of pit initiation increases with increase in Es,a, SCN− concentration or temperature. The rate of pitting is always greater in presence of alloyed Zn. These findings confirm polarization and impedance results previously obtained (Amin et al., 2009).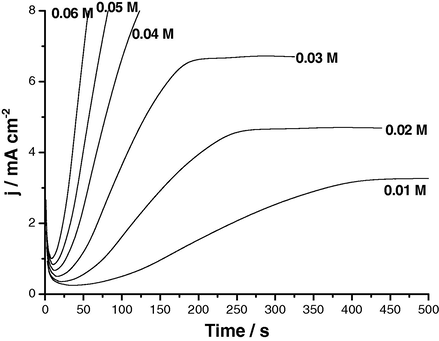
Potentiostatic j/t transients recorded for Al in KSCN solutions of various concentrations at Es,a = 1300 mV at 25 °C.
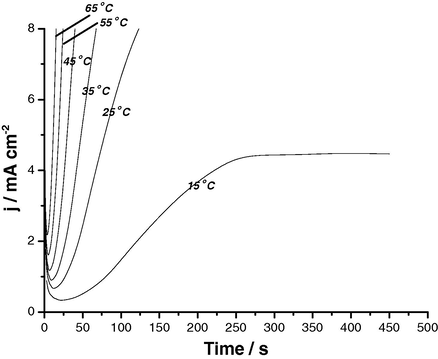
Potentiostatic j/t transients recorded for Al in 0.04 M KSCN solutions at Es,a = 1300 mV at different temperatures.
The increase in the rate of pitting initiation with SCN− concentration may be due to the adsorption of more aggressive SCN− anions on the available active sites. On the other hand, the increase of this rate with temperature could be explained on the basis that the increase in temperature accelerates the rates of migration and diffusion of the reactant and product species into and from pits. In addition, an increase in temperature enhances the solubility of the passive oxide film (Amin et al., 2006). All these events facilitate the mission of the aggressive SCN− anion to destruct passivity of the oxide film, resulting in the occurrence of intense pitting corrosion (Amin et al., 2009). EDX spectra presented in our previous study (Amin et al., 2009) revealed increased adsorption of SCN− at high temperatures probably as a result of increased porosity of the passive film. These findings are in accordance with Augustynski et al. (1978), who used the XPS method to confirm the increased adsorption of Cl− at high temperatures.
Finally, the dependence of the rate of pit nucleation on the applied potential suggests that there is a distribution of nucleation sites of different energies which nucleate at distinct potential (Metikos-Hukovic, 1992). In other words, the more positive is the applied potential, the more will be the active sites available for pit nucleation. This in turn enhances the adsorption of the aggressive SCN− anions on the passive electrode surface. It has been shown in our previous studies, based on XPS and EDX examinations, that the adsorption of SCN− (Amin et al., 2009) and and anions (Amin, 2009) on aluminium oxide surface increases with increasing anodic polarization even above the pitting potential. Kolics et al. (1998) came to the same conclusion regarding the adsorption of Cl− on passivated Al.
In the present work, the increase in the rate of pitting corrosion of Al with the increase of applied anodic potential and alloyed Zn is further confirmed chemically using inductively coupled plasma-atomic emission spectroscopy (ICP-AES). This method of chemical analysis involved the determination of the dissolved Al3+ ions as a function of applied anodic potential (>Epit) and alloy composition. Obtained data are collected in Table 1. It is obvious that the concentration of Al3+ in solution, due to pitting corrosion, increases as the potential is made more positive. The Al3+ concentrations are always greater in presence of increasing amounts of alloyed Zn, reflecting the ability of the alloying element (Zn) to activate pitting corrosion process.
Es,a/mV (SCE)
[Al3+]/ppm
Al
Al–6% Zn alloy
Al–12% Zn alloy
1300
0.065
0.15
0.22
1350
0.092
0.27
0.47
1400
0.22
0.38
0.65
1450
0.35
0.66
1.18
1500
0.98
1.85
3.55
3.3 Role of some inorganic inhibitors
The polarization responses of Al have been studied in 0.04 M KSCN solutions in the absence and presence of various concentrations of Na2MoO4, Na2WO4, Na2SiO3 or K2CrO4 as inorganic inhibitors, see Figs. 9a–d.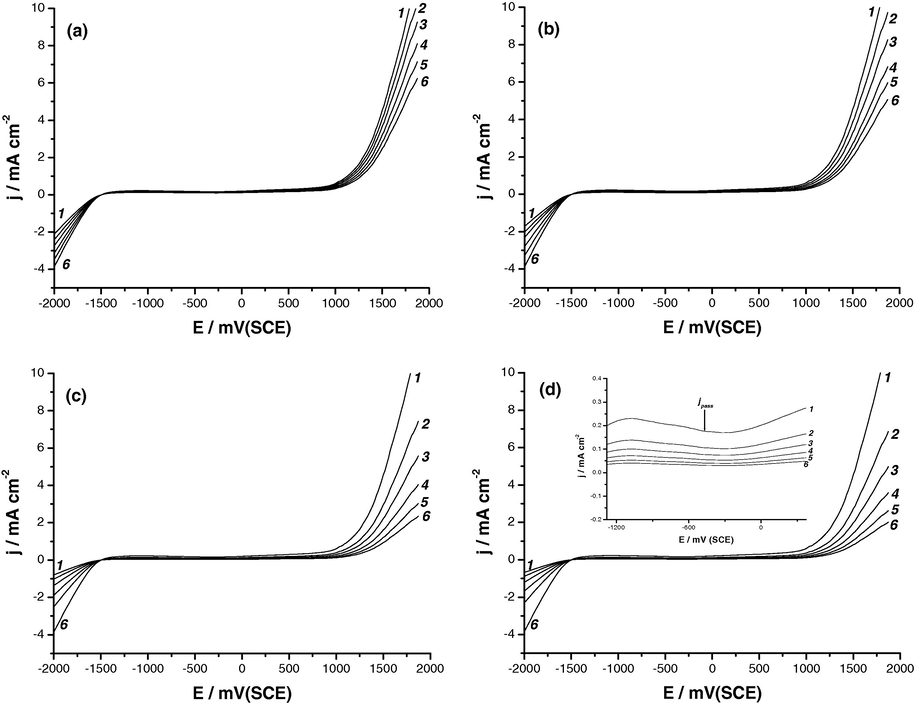
Potentiodynamic anodic polarization curves recorded for Al in 0.04 M KSCN solutions in the absence and presence of various concentrations of (a) Na2MoO4, (b) Na2WO4, (c) Na2SiO3 or (d) K2CrO4 at a scan rate of 0.5 mV s−1 at 25 °C. (1) Blank; (2) 10−4 M Inhib.; (3) 2 × 10−4 M Inhib.; (4) 5 × 10−4 M Inhib.; (5) 10−3 M Inhib.; (6) 2 × 10−3 M Inhib.
In all cases, the polarization curves show a passive region. This passive region is followed by pitting potential, Epit, indicated by a permanent rise in current in the passive region. Fig. 10 depicts the relationship between Epit and the logarithmic concentration of the inhibitors[log (Cinhib/M)]. It follows that Epit values are shifted in the noble direction and the values of jpass are decreased (see, for example, the insert of Fig. 9d) to an extent depending on the type and concentration of the introduced inhibitor. A retardation in the pitting events therefore results. At a fixed concentration of additive, Epit was found to increase in the order:
<
≈
. These results indicate that
and
inhibit the aggressive attack caused by SCN− anions more effectively than
and
.![Relationship between Epit and the logarithmic concentration of the inhibitors[log (Cinhib/M)] for Al in 0.04 M KSCN solutions at 25 °C.](/content/184/2011/4/2/img/10.1016_j.arabjc.2010.06.028-fig10.png)
Relationship between Epit and the logarithmic concentration of the inhibitors[log (Cinhib/M)] for Al in 0.04 M KSCN solutions at 25 °C.
The effect of these inhibitors on the incubation time of the pitting corrosion was also studied, see for example Figs. 11a and b. The samples were first passivated at −600 mV (SCE) in 1.0 M Na2SO4 solution for 15 min, in order to get a stable Al2O3 film on the electrode surface (Amin et al., 2008), and then SCN− was added to the sulphate solution to establish a 0.04 M thiocyanate concentration. Exactly the same procedure was followed for Al in 1.0 M Na2SO4 solution containing 0.04 M SCN− + 0.002 M of the inorganic inhibitor. Similar results were obtained for the other tested inhibitors. Within this study, the incubation time was defined as the time period form thiocyanate addition until significant rise in the current transient results, denoting the occurrence of pitting corrosion.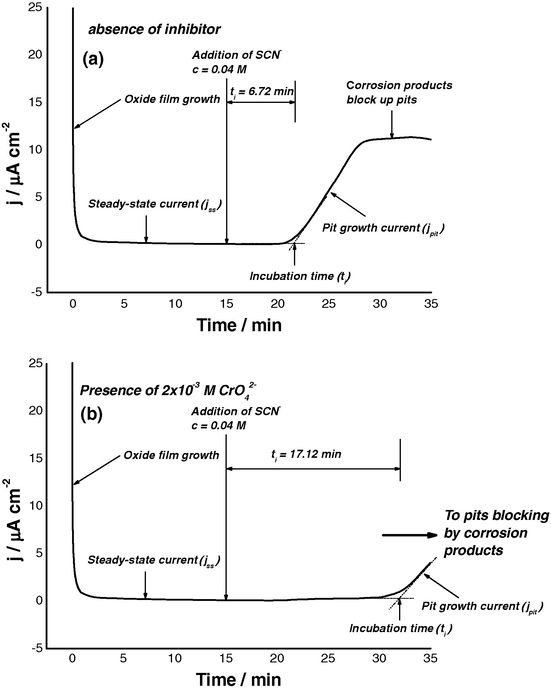
Potentiostatic current/time transients recorded for Al in 1.0 M Na2SO4 solution at 25 °C and at a step anodic potential (Es,a) of −600 mV(SCE), followed by addition (after 15.0 min) of KSCN to establish a concentration of 0.04 M SCN− in the
solution (a) without and (b) with 0.002 M
.
Addition of
anions to thiocyanate-sulphate containing solutions significantly decreases jpit and extends ti. These events were found to be significant in the presence of
and
(see for example Fig. 11b), indicating a significant increase in resistance of the passive film towards pitting attack in presence of these two anions. These findings can be confirmed by plotting the relation between the rate of pitting initiation,
, for Al and log Cinhib (Fig. 12). It follows from the data of Fig. 12 that, for all inhibitors, the rate of pitting initiation decreases with increasing their concentrations, and this decrease in the rate of pit initiation enhances following the order:
<
≈
. It seems that pits, as will be discussed, find it more difficult to develop in the presence of the inhibitors. The transition from passive state to pitting has therefore been made more difficult by the inhibitors. The inhibition mechanism of these oxyanions will be discussed later, after surface analysis has been presented.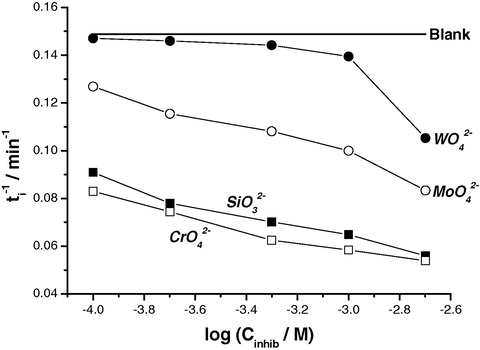
Relation between the rate of pitting initiation,
, and log Cinhib for Al in 0.04 M KSCN solutions at 25 °C.
3.4 Surface analysis
A further insight into the processes occurring at Al in 0.04 M KSCN solutions without and with 0.002 M
,
,
or
anions was enabled by EDX data (Fig. 13). EDX was first performed in water (Fig. 13a), only Al and O were detected, which indicated that the passive film contained only alumina, Al2O3. However, in KSCN solution (Fig. 13b), the EDX spectra showed additional lines characteristic for the existence of S, C and N. This means that the oxide film formed in KSCN solution contains a certain amount of incorporated S, C and N, presumably as SCN− anions. This indicates a deep penetration of this anion into the bulk of the passive oxide film. These results confirm that SCN− anions generate passivity breakdown by their adsorption and incorporation into the passive film (Amin et al., 2009).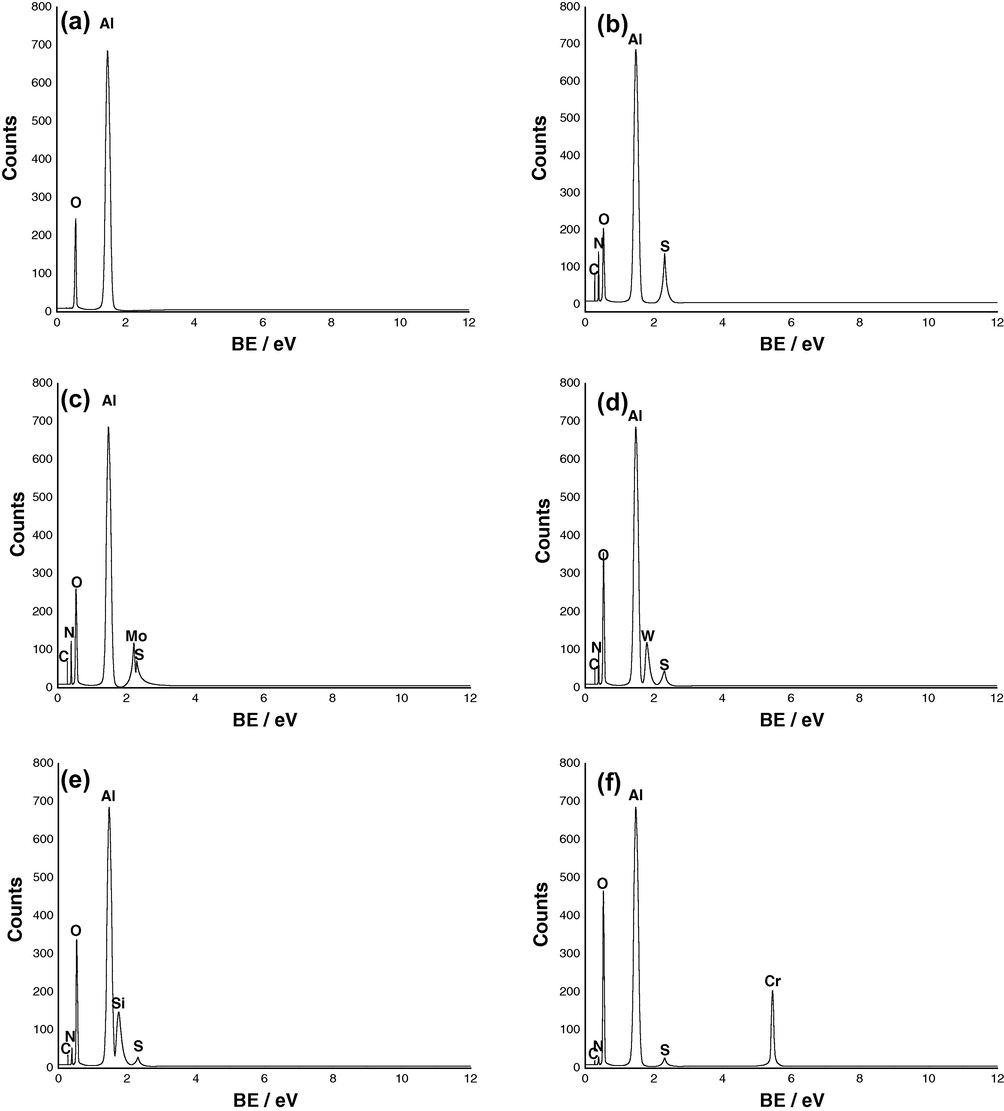
EDX spectra recorded for Al in (a) water and in 0.04 M KSCN solution in the absence (b) and presence of 0.002 M (c)
, (d)
(e)
, or (f)
anions at 25 °C.
On the other hand, the EDX spectra recorded for Al in 0.04 M KSCN solution containing 0.002 M , , or anions (Fig. 13c–f) showed additional lines characteristic for the existence of Mo, W, Si or Cr, respectively. In addition, the O signal is enhanced due to oxygen atoms present in these oxy-anions. Moreover, the spectra of Fig. 13c–f show that the S, C and N signals are considerably suppressed relative to the samples prepared in bare KSCN solution (Fig. 13b). These findings confirm that these anions compete with SCN− anions for surface adsorption sites, and consequently suppress the adsorption and incorporation of the aggressive SCN− anions into the passive film, thereby reducing the pitting susceptibility. It seems that these elements or their reduced forms, as will be seen, become part of Al2O3 passive film and tend to plug its pores and flaws, thereby imparting to it better protective properties.
Further inspection of Fig. 13 reveals that signals due to SCN− are significantly suppressed in presence of and (Fig. 13e and f) as compared with and anions (Fig. 13c and d). These results may confirm the results obtained from electrochemical measurements that and anions are more effective than and anions in the inhibition of pitting corrosion of Al.
The adsorption and inhibition action of these oxyanions was further confirmed by SEM examinations of the electrode surface before and after exposure to the inhibitor solution (Fig. 14). The morphology of specimen surface presented in Fig. 14a reveals that, in the absence of inhibitor, a well-defined irregular shaped pit is developed on the electrode surface. However, in presence of the four tested inorganic inhibitors (images b–e), the rate of corrosion is suppressed, as can be seen from the observed improvement in surface morphology.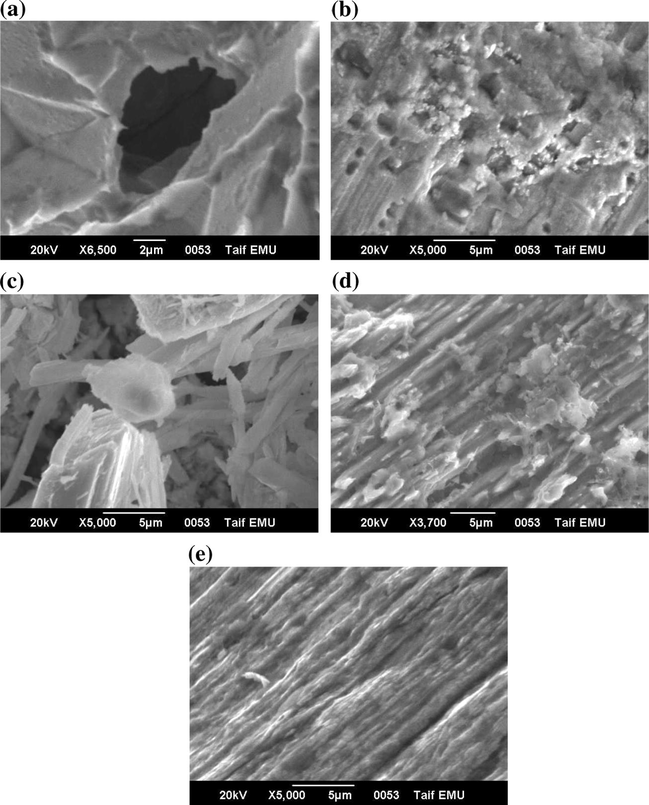
SEM examinations of Al surface in 0.04 M KSCN solution (a) without and with 0.002 M (b)
, (c)
(d)
, or (e)
anions at 25 °C. In each solution, the Al specimen was potentiostatically held for 5.0 min at Es,a = 1300 mV.
3.5 Inhibition mechanism
The decrease of the rate of pitting initiation and growth of Al in SCN− solutions due to the presence of these oxyanions may be explained on the basis that the inhibitors block the active sites available for a pit initiation. The inhibitor species penetrate the oxide film in parallel with SCN− anions under the influence of the electric field (Ilevbare and Burstein, 2001). Penetration of aggressive (and inhibiting) anions is thus expected to occur preferentially at active sites. The inhibitors would have either to hinder or to prevent completely the migration of the aggressive SCN− anions through the oxide film. This could be achieved by improving the oxide film. These inhibitors may improve the oxide film by incorporation into the defective film sites, and subsequent reduction into stable oxides, following Eqs. (1)–(3):
The reduced forms of the inhibitors, namely Cr2O3, WO2 and MoO2 may be incorporated in the passive film and tend to repair any flawed regions on the surface. This may either change the electronic properties for instance by reversing the anion selectivity (Baszkiewicz et al., 1992; Sakashita and Sato, 1979; Craig, 1991) of the film making it more difficult for SCN− anions to migrate through it, or take part in enhancing the oxide film by increasing its thickness (Sugimoto and Sawada, 1977).
The possibility of any of these occurring is still being debated. These inhibitors as a result increase the difficulty of soluble film formation required for film rupture to occur (Foley, 1986). This difficulty in soluble film formation prevents direct contact of the bare metal surface with aggressive SCN− anions so that further metal dissolution and, therefore, pit formation is prevented or at least retarded.
A further explanation for the inhibiting ability of K2CrO4, Na2MoO4 and Na2WO4 is that they reduce the local acidity at the sites where they react. Eq. (1), as an example, shows that for 2 moles of
reduced, 5 mol of H2O are consumed and 10 mol of OH− ions are produced which increase local pH. In addition, it has been shown that the dissolution of Na2SiO3 in water generally increases the pH since silicic acid, H2SiO3, is a weak acid with pK1 = 9.5 and pK2 = 12.7 (Armstrong et al., 1994). This means that when Na2SiO3 is dissolved in water the following reaction occurs:
For example, in one of the experiments of the present Na2SiO3 is added to 0.04 M KSCN solution to establish a concentration of 0.002 M Na2SiO3. The resultant solution actually contains: 0.04 M SCN−, 0.001 M , 0.001 M and 0.001 M OH−, giving a pH of 11. This increase in pH would affect the transition from passive state to pitting since a low pH is important to maintain the aggressiveness necessary for pit development.
Another factor, which might affect the strength of an inhibitor, is the size of the inhibiting species. It is important to determine whether the inhibitor species are able to penetrate or be pulled through the oxide film under the influence of the electric field. It has been shown that the radii and volumes (assuming perfect spheres) of SCN− and polyatomic anions of the tested inhibitors are comparable (Weast, 1976). It is therefore possible for these polyanions to be pulled through the oxide film under the influence of an electric field since they are not much larger than the SCN− anion.
Once these inhibitor species migrate through the oxide film, they reduce the amount of SCN− anions, which eventually migrate to a particular site to react and cause a rupture. This might increase the strength of the passive layer, corresponding to low passive current, as observed in Fig. 9, since the large amounts of SCN− anions required for the formation of soluble species to activate pitting would not be readily accumulated.
Because these ions carry a negative charge as the aggressive SCN− anion it also suggests that SCN− anions would be repelled from these sites. Thus the rate at which SCN− anions migrate through the oxide film will be reduced by the parallel migration of other species. This would cause a reduction in the probability of generating pits, and therefore the rate of pit growth ceases. It is also possible that the presence of these inhibitor species assists in repairing defects (may be via repassivation) of the oxide film so that further metal dissolution and, therefore, pit formation is prevented. Repassivation may be enhanced because the amount of SCN− anions present at these sites in the presence of the inhibitors is reduced. This would also make the transition from passivity to its breakdown (pit initiation and growth) more difficult because the necessary amount of SCN− anions required for transition to take place is no longer available.
4 Conclusion
Potentiostatic and galvanostatic studies of the pitting corrosion of Al and Al–6% Zn and Al–12% Zn alloys in aerated neutral 0.04 M KSCN solutions showed that:
-
The three Al samples tend to pit in KSCN solutions to an extent depending on SCN− concentration, applied anodic current, applied anodic potential, solution temperature and sample composition.
-
The susceptibility of Al towards pitting attack was enhanced when the percentage of alloyed Zn in Al was increased.
-
An incubation time (ti) was necessary before pit nucleation and growth to occur.
-
The rate of pit nucleation and growth ( ) was found to enhance with the above mentioned parameters.
-
The presence of inorganic inhibitors such as , , or anions suppressed the rate of pit nucleation and growth ( ), with and anions being more effective than and .
References
- Electrochim. Acta. 1987;32:255.
- Electrochim. Acta. 2009;54:1857.
- Electrochim. Acta. 2006;51:4754.
- Electrochim. Acta. 2008;53:5644.
- Electrochim. Acta. 2009;54:4288.
- J. Appl. Electrochem.. 1994;24:1244.
- Augustynski, J., Frankenthal, R.P., Kruger, J., (Eds.), 1978. Proceedings of the Fourth International Symposium on Passivity. The Electrochemical Society, Pennington, NJ, p. 997.
- Corros. Sci.. 1992;33:815.
- Electrochim. Acta. 1998;43:799.
- Scripta Mater.. 2007;56:995.
- Electrochim. Acta. 1999;44:2751.
- Fundamental Aspects of Corrosion Films in Corrosion Science. New York, USA: Plenum Press; 1991.
- Bockris J.O’.M., White R.E., Conway B.E., eds. Modern Aspects of Electrochemistry. Vol vol. 20. New York: Plenum; 1989. (Chapter 6)
- Mater. Chem. Phys.. 2003;81:286.
- J. Appl. Electrochem.. 1988;18:257.
- Mater. Des.. 2008;29:801.
- Corrosion. 1986;42:277.
- Frankenthal R.P., Kruger J., eds. Passivity of Metals. Princeton, NJ: The Electrochemical Society, Inc.; 1978. p. :285.
- Scripta Mater.. 2007;57:297.
- Corros. Sci.. 1999;41:421.
- Corros. Sci.. 2001;43:485.
- Electrochim. Acta. 1998;43:3605.
- Mater. Sci. Eng. A. 1996;221:113.
- J. Appl. Electrochem.. 1992;22:448.
- Corros. Sci.. 2008;50:248.
- Light Alloys (second ed.). London: Edward Arnold; 1969.
- Corrosion. 1979;35:351.
- Corros. Sci.. 1977;17:3.
- Pitting Corrosion of Metals. Houston, TX: NACE; 1986.
- Weast R.C., ed. Handbook of Chemistry and Physics. Cleveland, OH: CRC Press; 1976.
- Acta Mater.. 2007;55:6666.







