Translate this page into:
Industrial development and applications of plant oils and their biobased oleochemicals
*Corresponding author. Tel.: +60 3 8921 5412; fax: +60 3 8921 5410 jumat@ukm.my (Jumat Salimon)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 11 August 2010
Abstract
In the concepts for new products, performance, product safety, and product economy criteria are equally important. They are taken into account already when the raw materials base for a new industrial product development is defined. Here, renewable resources gain-again after the earlier “green trend” in the 1980s—increasing attention as an alternative raw materials source compared to fossil feedstock. The industrial use of carbohydrates, proteins, and plant oils aligns perfectly with the principles of Responsible Care and is an important part of green chemistry and sustainability in general. Since the 1950s, oleochemistry has grown to a major research and technology area in several institutions and industries. A large variety of products based on fats and oils have been developed since then for different uses, such as specialties for polymer applications, biodiesel, surfactants, emollients for home and personal-care industries, pesticides and biodegradable mineral oil replacements for lubricants. However, at present it seems that the use of renewable resources, especially plant oils, has to compete more and more with the increasing demand for bioenergy, which could cause an unbalanced supply and demand in the future or even a threat for the increasing demand for food in certain areas of the world.
Keywords
Plant oils
Biolubricants
Pesticides
Surfactants
Biodiesel
1 Introduction
The industrial use of agricultural commodities has been an issue generating a significant amount of interest. As the cost of petroleum-derived products increases, the need to change to a more biobased economy can be clearly seen. In addition to food uses, plant oils have found their way into industrial products in the plastics, pharmaceutical, inks, adhesives, coatings, and many other industries. The advantages of plant oil-derived industrial products can be illustrated by several of the 12 principles of green chemistry (Schwartza et al., 2008) including the call for the use of renewable feedstock, the minimization of hazards, and the generation of substances with as little toxicity as possible. Because of their biobased nature, products formed from plant oil are often biodegradable, and because the CO2 generated from their degradation can be incorporated into the next year’s crop, they can be nearly CO2 neutral.
Development of markets for biobased products also exists in the 2002 Farm Bill (Farm Security and Rural Investment Act, published January 11, Federal Register). Section 9002 includes language directing all Federal Government Agencies to give preference to “Biobased” products, unless it is unreasonable to do so, based on price, availability or performance. Lubricants are specified in the federal guidelines. The emphasis on environmentally friendly products is largely due to the rapid depletion of world fossil fuel reserves and increasing concern for environmental pollution from excessive mineral oil use. In other words, as petrochemical sources become more expensive, the ability of plant-based products to be cost competitive, or even cost advantageous, will be realized.
2 Raw materials situation
Carbohydrates—in particular, starch, cellulose, and sucrose—proteins and natural fats and oils are key raw materials for the chemical industry using renewable resources. Although biomass in general is available in large amounts (e.g., cellulose), the annual production volumes of selected commodities are still small compared to coal or crude oil. So far, the volumes were adjusted according to the different demands. In particular, in the case of natural oils and fats, the production volume steadily increased from 30 million tons in 1960 to 131 million tons in 2004 (Petran et al., 2008). Most of it is used for food, a minor amount for animal feed and chemistry (Burt, 2004). Until now, availabilities and the different applications were quite balanced.
However, for some time we have observed a shift toward an increasing use of natural plant oils for bioenergy and biofuels, with an expected share of 15% in 2012. This increase originally started in Europe years ago with the development of biodiesel from rapeseed, to be followed later on with further development based on other plant oils, such as palm and soybean. This is one consequence of political measures such as the European Biofuel Directive 2003/30/EC, which requires a minimum proportion of biofuel in the market to comply with the Kyoto Protocol. Biodiesel production volumes were expanded significantly in the recent past, and this trend is expected to continue in Europe and other regions such as South East Asia, South America, and India, with a further increase in production capacities forecasted at least for the next 10 years (Sauthoff, 2005). After that, it might slow down again, when the so-called second-generation products, such as sun diesel or biomass-to-liquid fuels, will be ready to be launched to the market (Lichtenthaler and Peters, 2004). Those technologies are definitely needed as it can be assumed that even with increasing production volumes for fats and oils, the future bioenergy and biofuel demand cannot be satisfied by this source by far (Sauthoff, 2005). In the meantime, however, the high demands for biodiesel, further stimulated by subsidies, could create not only an over-supply of glycerol (Atadashi et al., 2010) but also a strong competition with the established uses for plant oils such as nutrition and chemical industry (oleochemistry).
3 Ecological compatibility
Many efforts to define “green” and “sustainable” have been undertaken, and principles for green chemistry have been defined (Anastas and Warner, 1998). Metrics have been developed and also applied, for example, assessing environmental, health, and safety hazards, including life-cycle inventories (“cradle to factory”) and life-cycle assessments (“cradle to grave”) (Warner et al., 2004). Based on the results from life-cycle assessments, ecological and toxicological studies for selected cases, one can assume that products based on renewable resources usually are more ecologically compatible when compared with petrochemical-based substances—an important criterion in the development of a new product, just as price and performance are (Sumathi et al., 2008). However, this general assumption has to be proven for each new product again. Therefore, ecological compatibility plays a decisive role in all research and development projects. Basically, it covers two different aspects: remaining in the environment and the effects on the environment.
4 Examples of products
Oils and fats are triglycerides with different composition of the alkyl chains depending on their origin. In industrial processing, they are transferred into fatty acid methyl esters, fatty acids, glycerol, and, as hydrogenation products of the fatty acid methyl esters, fatty alcohols by applying standard manufacturing technologies (Metzger, 2009). Further chemical processes lead to the desired specialty chemicals (Fig. 1).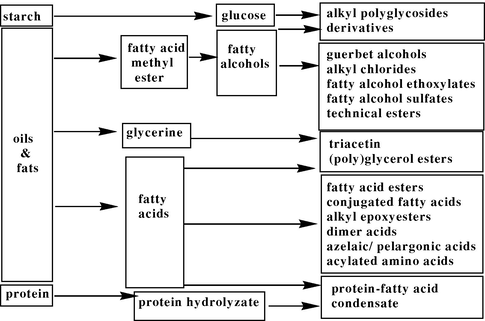
Industrial processing of natural oils and fats and selected product derivatives.
4.1 Oleochemicals for polymer applications
Oleochemicals as polymer materials represent a relatively small market, but are well established. We have to keep in mind that before crude oil was explored the only possibility to do chemistry was by using renewable resources. One example is linseed oil, which is used to produce linoleum. Here, the demand has increased from 10 000 tons in 1975 to 50 000 tons in 1998 (coming from 120 000 tons in 1960!) (Yap et al., 2010). Another example: epoxidized soybean oil (ESO) as a plastic and coating additive has a relatively stable market of approximately 100 000 tons/year (Sharma et al., 2006a). It is worth mentioning that the dicarboxylic acids are industrially produced either via ozonolysis of oleic acid to produce azelaic acid (one of the few examples of large-scale industrial ozonolysis) or by dimerization of linoleic acid and oleic acid to obtain complex mixtures of high molecular weight diacids (e.g., EMPOL® types), originally introduced in the 1950s by General Mills Chemicals and Emery (both now Cognis Corp.) (Höfer, 1996).
In a variety of polymer applications, such as coatings, large amount of solvents has to be used, including hydrocarbon and chlorinated solvents. Here, a clear trend is seen toward the so-called “green solvents”, such as ester solvents, typically produced from naturally based fatty acids and/or fatty alcohols, representing the largest group (Folić et al., 2008).
4.2 Plant oils and their oleochemicals as alternative diesel fuels
The use of plant oils in diesel engines is nearly as old as the diesel engine itself. The inventor of the diesel engine, Rudolf Diesel, reportedly used groundnut (peanut) oil as a fuel for demonstration purposes in 1900 (Kirakosyan and Kaufman, 2009). Some other work was carried out on the use of plant oils in diesel engines in the 1930’s and 1940’s. The fuel and energy crises of the late 1970’s and early 1980’s as well as the accompanying concerns about the depletion of the world’s non-renewable resources provided the incentives to seek alternatives to conventional, petroleum-based fuels. In this context, plant oils as fuel for diesel engines were remembered. They now occupy a prominent position in the development of alternative fuels. Hundreds of scientific articles and various other reports from around the world dealing with plant oil-based alternative diesel fuels (“biodiesel”) have appeared in print. They have advanced from being purely experimental fuels to initial stages of commercialization. Nevertheless, various technical and economic aspects require further improvement of these fuels.
Numerous different plant oils have been tested as biodiesel. Often the plant oils investigated for their suitability as biodiesel are those which occur abundantly in the country of testing. Therefore, soybean oil is of primary interest as biodiesel source in the United States while many European countries are concerned with rapeseed oil, and countries with tropical climate prefer to utilize coconut oil or palm oil. Other plant oils, including sunflower, and safflower, have also been investigated. Furthermore, other sources of biodiesel studied include animal fats and used or waste cooking oils. Sources of biodiesel with some emphasis on developing countries have been discussed (Shay, 1993).
However, the direct use of plant oils in fuel engines is problematic. Due to their high viscosity (about 11–17 times higher than diesel fuel) and low volatility, they do not burn completely and form deposits in the fuel injector of diesel engines (Demirbas, 2003). Different ways have been considered to reduce the high viscosity of plant oils:
-
Dilution of 25 parts of plant oil with 75 parts of diesel fuel,
-
Microemulsions with short chain alcohols such as ethanol or methanol,
-
Thermal decomposition, which produces alkanes, alkenes, carboxylic acids and aromatic compounds,
-
Catalytic cracking, which produces alkanes, cycloalkanes and alkylbenzenes, and
-
Transesterification with ethanol or methanol.
Dilution of oils with solvents and microemulsions of plant oils lowers the viscosity; some engine performance problems, such as injector coking and more carbon deposits, still exist. Among all these alternatives, the transesterification seems to be the best choice, as the physical characteristics of fatty acid esters (biodiesel) are very close to those of diesel fuel and the process is relatively simple. Furthermore, the methyl or ethyl esters of fatty acids can be burned directly in unmodified diesel engines, with very low deposit formation.
4.3 Surfactants derived from plant oil-based fatty alcohols and fatty acids
Surfactants are molecules which affect the surface or interfacial energy of surfaces. Their fundamental properties make their applications almost limitless. They are used in soaps, detergents, foaming agents, herbicides, de-foaming agents, wetting agents, emulsifiers, cosmetics, fabric softeners, and virtually any other application where two dissimilar types of compounds are brought together. Surfactants are generally made up of a hydrophilic head group and a hydrophobic tail.
The hydrophobic end group is usually a saturated hydrocarbon chain, which can be either petrochemically based such as a long chain alcohol or from an oleochemical, such as a fatty acid chain. The head group can also be from either source. Some commonly used biobased hydrophiles include amino acids (Reznik et al., 2010; Infante et al., 2004), polyglycerides, polysaccharides and the carboxylate groups on fatty acid chains. For a surfactant to be biobased, either the hydrophobe or the hydrophile should be made from natural sources. A class of useful surfactants has been made using oleochemically based fatty esters as the hydrophobe and combining them with ethylene oxide as the hydrophile (Abd Maurad et al., 2006; Deleu and Paquot, 2004; Maneerat, 2005; Warwel et al., 2001). They have even (Johansson and Svensson, 2001) been able to create a mild surfactant by using their catalyst on a plant oil.
The use of carbohydrates as the hydrophilic part of a surfactant has been known for half of a century (Bazin et al., 1998; Gouéth et al., 1995). The carbohydrates range from lignin and lecithin to sugars (Bazito and El Seoud, 2001), such as sorbitol. Sorbitol-based surfactants have enjoyed great success and are currently commercially available from multiple sources, most commonly as the Span™ series (Fig. 2). These surfactants are considered to have excellent properties for emollients, or emulsifiers in the food and personal-care industries. An additional benefit is that in some of these surfactants, such as Span™ 60, both the hydrophilic part and the hydrophobic part of the surfactant are biobased, a sugar and fatty acid, respectively.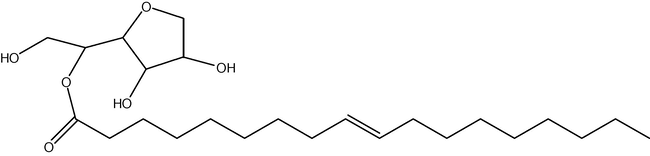
The structure of a sugar surfactant (sorbitan mono-oleate). The most common isomer at the five sugar position is shown, but substitution at the two and six positions is also common.
Biobased glycerol, produced in excess from the biodiesel process, is another low cost source for a biobased surfactant precursor. Polymers of glycerol (polyglycerides) have been shown to be good hydrophobes. Polyglyceryl laurates have been shown to have similar or even superior detergency to alcohol ethoxylates commonly used in laundry detergents (Yangxin et al., 2008). Polyglycerol surfactants can be made from epoxide-containing compounds like glycidol (Istratov et al., 2003), or from glycerol polymerization (Mendrek et al., 2010). Recently, another class of polyglyceride surfactants with potential for introduction of branching points was made from glycerol and methyl oleate (Doll and Erhan, 2006) using only sodium hydroxide as a catalyst (Fig. 3). This surfactant also demonstrated similar surfactant properties to those of alcohol ethoxylates. Additionally, the potential for introducing branches to the surfactant makes it of even more interest.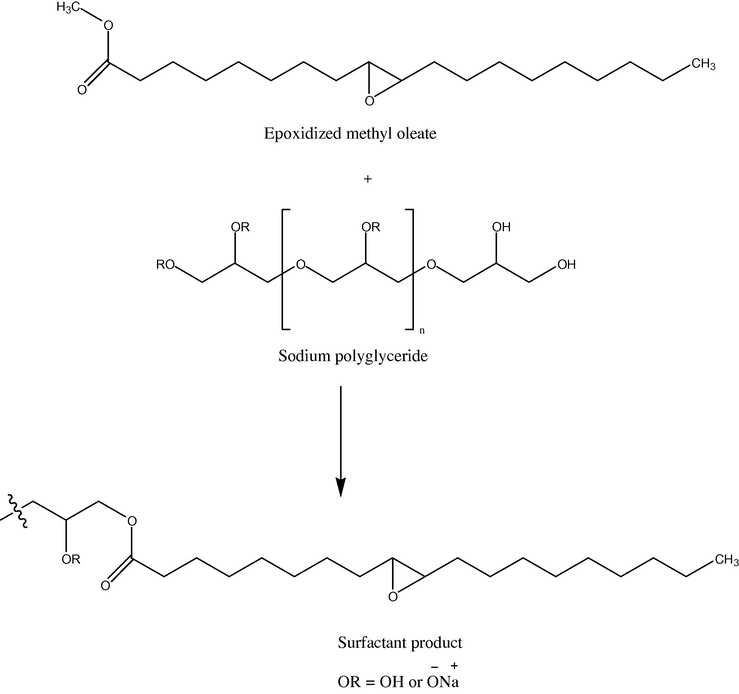
The synthesis of oleochemical- and glyceride-based surfactants. Only the major product is shown.
Surfactants are used in a wide range of fields. By far, the most important field of application is the washing and cleansing sector as well as textile treatment and cosmetics; these use more than 50% of the total amount of surfactants. Surfactants are also used in food sector, crop protection, mining, and production of paints, coatings, inks, and adhesives. The basic manufacturing routes to important surfactants are laid out in Fig. 4. It is true that the most important surfactant from the amount produced apart from soap is still the petrochemical-based alkyl benzene sulfonate; however, a continuous trend toward surfactants based on renewable resources has become apparent (Falbe, 1987).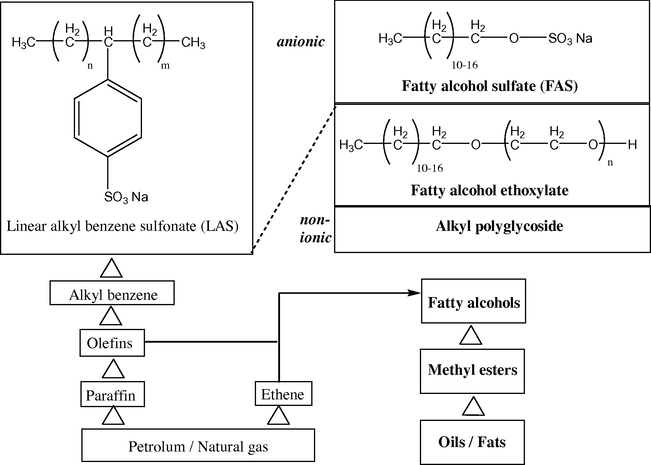
Production of surfactants and examples of products.
4.3.1 Determining the environmental acceptability of surfactants
The term “environmentally acceptable” is not likely to have the same meaning to all persons. In the regulatory context, only those surfactants that pass some environmental muster can be designated environmentally acceptable. Any process that evaluates the environmental properties will result in some sort of score and hence lead to debates on the meaning of such scores. Despite the pitfalls of interpretation and value judgments, a process for scientific evaluation has emerged.
The process begins with a consideration of the production of surfactants. The environmental impact of a surfactant can be examined in a Life Cycle Inventory (LCI), which is the well-defined part of LCA (interchangeably called Life Cycle Analysis or Assessment). The European surfactant industry undertook an LCI in 1992 for the major classes of surfactants used in Europe. An LCI quantifies the energy and raw materials consumed to produce a surfactant and its feedstocks as well as the amounts of atmospheric, waterborne, and solid waste emissions intrinsic to these processes. It is also a balance sheet that allows manufacturers to assess opportunities for improving the environmental profile of their surfactants and intermediates. Although an LCI is not intended to compare products, it can be used to highlight excessive energy and materials consumption and/or emissions associated with a particular feedstock or process.
The results of the European LCI (Stalmans et al., 1995) are summarized in Table 1. The data show that no technical or scientific basis exists to support a general environmental superiority claim, either for an individual surfactant or for various options for sourcing from petrochemical, oleochemical, or agricultural feedstocks and minerals. Therefore, environmental acceptability is not associated with raw material source, at least in the LCI context.
Surfactant
Requirements
Releases
Raw materials (kg/1000 kg)
Energy consumption (GJ/1000 kg)
Atmospheric (kg/1000 kg)
Waterborne (kg/1000 kg)
Solid waste (kg/1000 kg)
LAS (Pc)
1040
61
1661
5
65
AS
Pc
1091
73
2604
7
81
Oc
2077
57
1684
26
75
AE3S
Pc
1310
73
2335
5
68
Oc
2167
67
2054
24
96
Soap (Oc)
2167
50
4451
45
144
SAS (Pc)
1013
52
1261
2
64
AE3
Pc
1448
83
2366
6
67
Oc, Pc
2401
73
2024
28
66
AE7
Pc
1570
79
2281
6
64
Oc, Pc
2264
72
2062
21
64
AE11
(Oc, Pc)
2064
82
2299
11
63
APG (Oc)
2060
63
2015
35
142
Most of the issues on environmental acceptability focus on the effects on the environment associated with the use and disposal of these surfactants. These effects are taken into account by a risk assessment. The first step in a risk assessment is an estimate of the concentrations of surfactants in the environmental compartments of interest, such as wastewater treatment plant effluents, surface waters, sediments, and soils (Mulligan, 2005; Lehmler, 2005). This estimate is generated either by actual measurement or by prediction via modeling. The measured or predicted concentrations are then compared to the concentrations of surfactant known to be toxic to organisms living in these environmental compartments. If the measured or predicted concentration is less than the no-toxic-effect level, then a margin of safety exists. Just to be sure, a safety factor of 10, 100, or 1000 is often applied. That is, the margin of safety between the actual, measured and no-effect concentrations should differ by one, two, or three orders of magnitude.
4.4 Emollients
The physicochemical nature of the oil-phase components in a cosmetic emulsion, the emollients, determines their hair- and skin-care effects, such as smoothing, spreading, and sensorial appearance (Keng et al., 2009). Test methods have been developed to characterize and classify the numerous emollients available on the market, such as silicones, paraffin, and oleochemical-based products. The latter include glycerides, esters, alcohols, ethers, and carbonates with tailor-made structures depending on the performance needed (Table 2). However, especially with regard to additional effects, there is still a demand for new products and product combinations with unique performance properties.
Structure
INCI name
Ester
Hexyl laurate, coco-caprylate, hexyldecyl stearate Decyl oleate, oleyl erucate, caprylic/capric triglyceride
Guerbet alcohols
Hexyl decanol, octyl dodecanol
Hydrocarbons
Diethylhexyl cyclohexane
Ethers
Dicaprylyl ether
Carbonates
Dicaprylyl carbonate
For example, a compound based on dioctyl ether (INCI: dicaprylyl ether) from coconut- or palm kernel-oil-based octanol (e.g., Cetiol® LDO) allows the formulation of silicon oil-free hair-care products, particularly for the use as hair cleansing preparations in order to improve the tactile hair feeling and hair gloss. In combination with wax esters and cationic polymers, additional benefits like improved sensorial feeling are achieved (Lodén and Sc, 2003).
One example of a new product type for skin care is dioctyl carbonate (INCI: dicaprylyl carbonate, e.g., Cetiol® CC). The product is synthesized by the trans-esterification reaction of octanol and dimethyl carbonate in the presence of alkali catalyst. Dioctyl carbonate is a dry emollient with excellent dermatological compatibility and a comprehensive performance profile, such as solubilizing and dispersing ability for sun-care filters, and a unique sensorial feeling.
R. C. Guerbet in 1899 discovered the self-condensation reaction of alcohols, which, via the aldehyde as an intermediate, leads to branched structures (2-alkyl alcohols)–the Guerbet alcohols. Starting from fatty alcohols from plant sources, such as octanol and decanol, the corresponding C16 and C20 alcohols, 2-hexyl decanol and 2-octyl decanol, are produced. The reaction is carried out under alkali catalysis and high temperatures (>200 °C). Over the years, both products have proven to be efficient emollients in skin-care product formulations, but are also used for other applications, such as plasticizers.
4.5 Plant oils and their oleochemicals in pesticide formulations
Oils and fats, particularly plant based, are important raw materials for the chemical industry worldwide. Plant oil and their oleochemicals have major advantages over mineral oils in that they are renewable and readily available all around the world, often as a result of by-products from other industrial activities. Also, they are generally biodegradable, non-flammable and cause fewer medical problems and allergies to the end users (Fukuyama et al., 2009). Baumann and Biermann (1994) showed that oils and fats are biological raw materials which are constantly renewed. This will be reflected, at least in the long term as an economic advantage for chemical products from oils and fats, compared with those from mineral oil. Favorable ecological characteristics can be expected from linear fatty acid molecules which have no branches or ring structures.
Plant oils such as coconut, soybean, corn, palm, palm kernel oils and their oleochemicals are used to produce inert ingredients such as surfactants, wetting agents, dispersing agents, emulsifying agents, adjuvants, solvents and carrier/diluents. for pesticide formulations. In general, preparation of pesticide formulations will include two main components, i.e., the active ingredients and inert ingredients. Active ingredients mean the chemical or chemicals in a product responsible for the desired effect, which are capable of preventing, destroying, repelling or mitigating insects, fungi, weeds, rodents, or other pests. Meanwhile, inert ingredients also called supplements are inactive ingredients which have no pesticidal action (Abhilash and Singh, 2009). There are various types of inert ingredients normally found in pesticide formulation, for example, emulsifiers, organic solvents, dispersing agents, and wetting agents.
4.5.1 Spray adjuvants and solvents
The structure and general properties of mineral and plant oils used as spray adjuvants had been studied (Hamilton, 1993). Table 3 shows the fatty acid compositions of some plant oils. HEAR = High erucic acid rape oil. LEAR = Low erucic acid rape oil.
Oil
Fatty acid
14:0
16:0
16:1
18:0
18:1
18:2
18:3
20:1
22:1
Soybean
–
12.0
–
3.6
23.7
51.4
8.8
–
–
Cottonseed
1.1
27.3
1.4
3.1
16.7
50.4
–
–
–
Linseed
–
6.5
tra
4.5
19.5
16.5
53.0
–
–
Palm
1.0
43.1
0.2
4.0
39.0
10.0
0.2
–
–
Palm Olein
1.0
39.8
tr
4.0
42.8
11.0
0.3
–
–
Peanut
–
9.8
0.4
3.7
60.9
18.1
–
–
–
Sunflower
–
6.0
–
3.0
27.0
64.0
–
–
–
HEAR
–
3.0
–
2.0
22.6
15.0
14.0
15.0
28.0
LEAR
–
4.0
–
2.0
56.0
26.0
10.0
2.0
tr
Palm oil, which is semi solid in temperate climates, is not suitable to be used as spray adjuvants. However, other plant oils including palm olein which is a liquid fraction from palm oil products would be satisfactory. Researchers indicated that plant oils such as soybean, cottonseed, linseed, peanut, rape and sunflower adhere better to leaves of plants or insects even after heavy rainfall because of their polyunsaturated nature (Knowles, 2008). This increases the persistency of the active ingredients, making further treatment of the crop unnecessary and hence allowing considerable cost reactions and the quantities of active ingredients lost to the environment are reduced. Flury (1996) indicated that methyl esters and hydrocarbons (mineral oils) which have comparable viscosities and surface tensions are suitable vehicles for the transport of pesticides onto the surface of plants or insects.
Kapusta (1985) evaluated the concentrates of soybean oil (SOC) and petroleum oil (POC) as enhancing agents with post emergence herbicides, such as sethoxydim, fluazifop-butyl and HOE 581 (code name) for Jahnson grass control in soybeans. Results from this study and Henkel research (Henkel, 1995) indicated that SOC is equal to POC in enhancing the activity of essentially all post emergence herbicides.
Many researchers produced various types of methyl esters from plant oils, for example, methyl laurate, methyl oleate, methyl soyte, methyl sunflowerate, and methyl coconate. (Chi, 1999; Prateepchaikul et al., 2007; Petchmala et al., 2008; Rashid et al., 2008). They are found to be biodegradable, have low toxicity with LD50 > 20 g/kg, low viscosity mobile fluids (<7 cps at 25 °C), with good solvency and adjuvant functions. Table 4 shows the solubility of various insecticides in methyl esters derived from plant oils. The results indicated that methyl esters from plant oils have good solvency property to pesticide. The pesticide solubility varied from 3.0% (w/w) solubility to miscible in all portions. E-2209 = Methyl caprylate/caprate. E-2270 = Methyl laurate. E-2301 = Methyl canolate. M = Miscible in all proportion.
Pesticide
Solvent
E-2209
E-2270
E-2301
Malathion
M
M
M
Permethrin
78
67
60
Chlorpyrifons
74
66
60
2,4-d-Isooctyl
M
M
M
Esters dimethoate
12
4.2
2.7
4.6 Biolubricants from chemically modified plant oils
There has been a constant demand for environmentally friendly or “green” lubricants. A significant lubricant market of some nine million metric tons per year of industrial and automotive lubricants exists. Currently, plant oils (VO) provide only a fraction of the lubricant market. VOs are already in use as lubricants due to their superior lubricity, good anticorrosion, better viscosity-temperature characteristics and low evaporation loss in industrial applications such as rolling, cutting, drawing, quenching operations, and greases either alone or in combination with mineral oils (Campanella et al., 2010; Erhan and Asadauskas, 2000). Their volatility is low due to the high molecular weight of the triglyceride molecule and they have a narrow range of viscosity changes with temperature. Polar ester groups are able to adhere to metal surfaces and, therefore, possess good boundary lubrication properties. In addition, VOs have high solubilizing power for polar contaminants and additive molecules. From the environmental point of view their importance is evident especially in areas of total loss lubrication, military applications, and in outdoor activities such as forestry, mining, railroads, dredging, fishing and agriculture hydraulic systems. However, their use is still restricted due to low thermo-oxidative stability and poor cold flow behavior. There are various ways to improve the oxidation and the cold flow behavior of VOs. These include use of heat-, chemically, and genetically modified plant oils and additives.
Because the most active site for various oxidation reactions of a triacylglycerol is at allylic site of the carbon chain, elimination of the double bonds in the triacylglycerol is an important step in increasing the oxidative stability of the oil. The double bonds in triacylglycerol can be eliminated by polymerization using heat modification method (Fox and Stachowiak, 2007; Erhan et al., 2006), resulting in lubricating base oils with different viscosities. Heat modification is usually carried out at a temperature range of 232–330 °C in an inert atmosphere. At this high temperature the double bond migrates and conjugated dienes are formed, which readily add to active double bonds to form a six-membered ring containing one double bond. This polymerization process often leads to an increase in oil viscosity. Microwave-irradiation also provides higher viscosity oils like heat-modified oils (Biswas et al., 2007). These heat-modified VOs, which have improved oxidation properties, are successfully used in a formulation of hydraulic elevator fluid for the Statue of Liberty elevator and for the metalworking fluid industry.
Chemical modifications such as epoxidation, estolides formation, and tranesterification of plant oils with polyols have been shown to improve the oxidative stability of plant oil-based lubricants and to achieve optimal characteristics for extreme applications (Wagner et al., 2001). In the field of lubricants, environmental and economic reasons lead to the utilization of plant oils and animal fats, or used oils and fats after appropriate chemical modifications. The temperature flow property of pant oils is extremely poor, and this limits their use at low operating temperatures, especially in automotive and industrial fluids. Plant oils have a tendency to form macrocrystalline structures at low temperatures through uniform stacking of the ‘‘bend’’ in the triacylglycerol backbone. Such macrocrystals restrict flow due to the loss of kinetic energy of individual molecules during self-stacking. Several diester compounds have been synthesized from commercially available oleic acid and common fatty acids (Salimon and Salih, 2009a,b). The key steps in the three-step synthesis of oleochemical diesters include epoxidation and ring opening of epoxidized oleic acid with different fatty acids (octanoic, nonanoic, lauric, myristic, palmitic, stearic, and behenic acids) using p-toluenesulfonic acid (PTSA) as a catalyst to yield mono-ester compounds. The esterification reaction of these compounds with butanol, isobutanol, octanol, and 2-ethylhexanol was further carried out in the presence of 10 mol% H2SO4, producing the desired diester compounds (Fig. 5) (Salimon and Salih, 2009c,d).
Epoxidation of oleic acid, followed by ring-opening acylation of EOA using PTSA as a catalyst. RCOOH is octanoic, nonanoic, lauric, myristic, palmitic, stearic and behenic acid. The esterification reaction was carried out using alcohol (butanol, isobutanol, 2-ethylhexanol, octanol) and H2SO4 as a catalyst.
Not surprisingly, as the length of the mid-chain increases, a corresponding improvement in low temperature behavior is observed. This phenomenon is due to the increased ability of the long chain esters to disrupt macrocrystalline formation at low temperatures. Another observation is the positive effect of branching at the chain end on the low temperature performance of the resultant products, which leads to the formation of microcrystalline structures rather than macrocrystalline structures.
Oleic acids are more thermally stable than polyunsaturated fats, and therefore are highly desired components in VOs for lubricant applications. Successes in genetic modification have helped to develop high oleic VOs, which are now available for commercial uses. The combination of high-oleic VOs and chemical additives offers the best option for achieving the ultimate goal. The lubricants formulated using this approach exhibit similar or better oxidative stability and low temperature properties compared to petroleum-based lubricants (Sharma et al., 2006b). Greases are solid or semi-solid products obtained by the dispersion of a thickening agent in a liquid lubricant. Development of VO-based greases has been an area of active research for several decades (Awawdeha et al., 2009). It has been found that the thermo-oxidative and tribochemical behavior of soybean oil-based greases is affected by the composition of the metal soap thickener (Sharma et al., 2005). Biobased grease with high oxidative stability can be prepared using epoxy VOs for industrial, agriculture/ farming equipment, and forestry applications (Sharma et al., 2006b). These greases deliver at par or better performance properties (effective lubrication, wear protection, corrosion resistance, friction reduction, heat removal, etc.) than existing mineral oil-based grease currently used in similar trade.
5 Conclusion
The successful development of environmentally compatible and powerful products in the sense of a sustainable development has been demonstrated by various examples of recent product innovations from plant oil. The ability to replace petrochemicals with renewable resources is becoming a worldwide issue. Deriving products that meet consumer demands and performance expectations is an ongoing challenge. Issues such as ultimate bio-degradability, co-product utilization and amount of available bio-resources will always be paramount. It can be assumed that in the future further possibilities for using renewable resources will be intensely investigated. This review has given just a few examples of what is being done in the area as we change to our industries of the future.
Acknowledgements
The authors acknowledge the Universiti Kebangsaan Malaysia for funding (“Code UKM-GUP-NBT-08-27-113” and “UKM-OUP-NBT-29-150/2010”), and the direct contributions of the support staff from the School of Chemical Sciences and Food Technology, the Faculty of Science and Technology, Universiti Kebangsaan Malaysia.
References
- Alpha-sulfonated methyl ester as an active ingredient in palm-based powder detergents. J. Surfactants Deterg.. 2006;9:161-167.
- [Google Scholar]
- Pesticide use and application: an Indian scenario. J. Hazard. Mater.. 2009;165:1-12.
- [Google Scholar]
- Green Chemistry: Theory and Practice. New York: Oxford University Press; 1998.
- High quality biodiesel and its diesel engine application: a review. Renew. Sust. Energy Rev. 2010
- [CrossRef] [Google Scholar]
- Effects of yellow grease or soybean oil on performance, nutrient digestibility and carcass characteristics of finishing Awassi lambs. Anim. Feed Sci. Technol.. 2009;153:216-227.
- [Google Scholar]
- Synthesis of sucrose-based surfactants through regioselective sulfonation of acylsucrose and the nucleophilic opening of a sucrose cyclic sulfate. Carbohydr. Res.. 1998;309:189-205.
- [Google Scholar]
- Sugar-based anionic surfactants: synthesis and micelle formation of sodium methyl-acylamido-2-deoxy-6-O-sulfo-d-glucopyranosides. Carbohydr. Res.. 2001;332:95-102.
- [Google Scholar]
- Microwave irradiation effects on the structure, viscosity, thermal properties and lubricity of soybean oil. Ind. Crops Prod.. 2007;25:1-7.
- [Google Scholar]
- Essential oils: their antibacterial properties and potential applications in foods – a review. Int. J. Food Microbiol.. 2004;94:223-253.
- [Google Scholar]
- Lubricants from chemically modified plant oils. Bioresour. Technol.. 2010;101:245-254.
- [Google Scholar]
- Chi, L., 1999. The production of methyl esters from plant oil/ fatty acid mixtures, M.Sc thesis, Graduate Department of Chemical Engineering and Applied Chemistry, University of Toronto, Canada.
- From renewable plants resources to microorganisms: new trends in surfactants. C. R. Chim.. 2004;7:641-646.
- [Google Scholar]
- Biodiesel fuels from plant oils via catalytic and non-catalytic supercritical alcohol transesterifications and other methods: a survey. Energy Convers. Manage.. 2003;44:2093-2109.
- [Google Scholar]
- Synthesis and performance of surfactants based on epoxidized methyl oleate and glycerol. J. Surfactants Deterg.. 2006;9:377-383.
- [Google Scholar]
- Oxidation and low temperature stability of plant oil-based lubricants. Ind. Crops Prod.. 2006;24:292-299.
- [Google Scholar]
- Heidelberg: Springer; 1987. Surfactants in Consumer Products: Theory, Technology Applications
- Experimental evidence of transport of pesticides through field soils – a review. J. Environ. Qual.. 1996;25:25-45.
- [Google Scholar]
- Systematic selection of green solvents for organic reacting systems. Chin. J. Chem. Eng.. 2008;16:376-383.
- [Google Scholar]
- Plant oil-based lubricants – a review of oxidation. Tribol. Int.. 2007;40:1035-1046.
- [Google Scholar]
- Allergic reaction induced by dermal and/or respiratory exposure to low-dose phenoxyacetic acid, organophosphorus, and carbamate pesticides. Toxicology. 2009;261:152-161.
- [Google Scholar]
- Garst, R., 1995. Methyl esters. Paper presented in natural oleochemicals as adjuvant in argo-chemical formulations. Organized by Henkel Oleochemical (M) Sdn. Bhd.
- Synthesis of novel bis(glycosyl) ethers as bolaamphiphilc surfactants. Carbohydr. Res.. 1995;266:171-189.
- [Google Scholar]
- Structure and general properties of mineral and plant oils used as spray adjuvants. Pestic. Sci.. 1993;37:141-146.
- [Google Scholar]
- Agriculture solutions. A newsletter for the agriculture industry. Henkel Corporation, 11501 Northlake Drive. OH: Cincinnati; 1995.
- Anwendungstechnische aspekte der verwendung natürlicher le und ihrer derivate in der polymer-synthese und -verarbeitung. In: Eierdanz H., ed. Perspektiven Nachwachsender Rohstoffe in der Chemie. Weinheim: VCH; 1996. p. :91-106.
- [Google Scholar]
- Linear-dendritic nonionic poly(propylene oxide)-polyglycerol surfactants. Tetrahedron. 2003;5:4017-4024.
- [Google Scholar]
- Surfactants based on fatty acids and other natural hydrophobes. Curr. Opin. Colloid Interface Sci.. 2001;6:178-188.
- [Google Scholar]
- Uses of soybean oil in the application of herbicides. J. Am. Oil Chem. Soc.. 1985;62:923-926.
- [Google Scholar]
- Newly synthesized palm esters for cosmetics industry. Ind. Crops Prod.. 2009;29:37-44.
- [Google Scholar]
- Kirakosyan, A., Kaufman, P.B., 2009. Recent Advances in Plant Biotechnology, Springer US. doi:10.1007/978-1-4419-0194-1_9.
- Recent developments of safer formulations of agrochemicals. Environmentalis.. 2008;28:35-44.
- [Google Scholar]
- Synthesis of environmentally relevant fluorinated surfactants – a review. Chemosphere. 2005;58:1471-1496.
- [Google Scholar]
- Carbohydrates as green raw materials for the chemical industry. C. R. Chim.. 2004;7:65-90.
- [Google Scholar]
- The skin barrier and use of moisturizers in atopic dermatitis. Clin. Dermatol.. 2003;21:145-157.
- [Google Scholar]
- Production of biosurfactants using substrates from renewable-resources. Songklanakarin J. Sci. Technol.. 2005;27:676-683.
- [Google Scholar]
- Amphiphilic behaviour of poly(glycidol)-based macromonomers and its influence on homo-polymerisation in water and in water/benzene mixture. Polymer. 2010;51:342-354.
- [Google Scholar]
- Fats and oils as renewable feedstock for chemistry. Eur. J. Lipid Sci. Technol.. 2009;111:865-876.
- [Google Scholar]
- Production methyl esters from palm fatty acids in supercritical methanol. Chiang. Mai. J. Sci.. 2008;35:23-28.
- [Google Scholar]
- Methyl ester production from high free fatty acid mixed crude palm oil. Songklanakarin J. Sci. Technol.. 2007;29:1552-1561.
- [Google Scholar]
- Production of sunflower oil methyl esters by optimized alkali-catalyzed methanolysis. Biomass Bioenergy. 2008;32:1202-1205.
- [Google Scholar]
- Use of sustainable chemistry to produce an acyl amino acid surfactant. Appl. Microbiol. Biotechnol 2010
- [CrossRef] [Google Scholar]
- A. Preparation and characterization of 9, 10-epoxyoleic acid α-hydroxy ester derivatives as biolubricant base oil. Eur. J. Sci. Res.. 2009;31:265-272.
- [Google Scholar]
- Substituted esters of octadecanoic acid as potential biolubricants. Eur. J. Sci. Res.. 2009;31:273-279.
- [Google Scholar]
- C. Improved low temperature properties of 2-ethylhexyl 9(10)-hydroxy-10(9)-acyloxystreate derivatives. Eur. J. Sci. Res.. 2009;31:583-591.
- [Google Scholar]
- Oleic acid diestrs: synthesis, characterization and low temperature properties. Eur. J. Sci. Res.. 2009;32:216-222.
- [Google Scholar]
- Sauthoff, H., 2005. Opportunity or threat for oils and fats trading, Handout FOSFA “Contact Day”, Bioenergy and Biofuels, London.
- Tocopherol, tocotrienol and plant sterol contents of plant oils and industrial fats. J. Food Comp. Anal.. 2008;21:152-161.
- [Google Scholar]
- Soybean oil based greases: influence of composition on thermo-oxidative and tribochemical behavior. J. Agric. Food Chem.. 2005;53:2961-2968.
- [Google Scholar]
- Synthesis of hydroxy thio-ether derivatives of plant oil. J. Agric. Food Chem.. 2006;54:9866-9872.
- [Google Scholar]
- Biobased grease with improved oxidation performance for industrial application. J. Agric. Food Chem.. 2006;54:7594-7599.
- [Google Scholar]
- Diesel fuel from plant oils: status and opportunities. Biomass Bioenergy. 1993;4:227-242.
- [Google Scholar]
- European life-cycle inventory for detergent surfactant production. Tenside, Surfactants, Deterg.. 1995;32:84-109.
- [Google Scholar]
- Utilization of oil palm as a source of renewable energy in Malaysia. Renew. Sust. Eng. Rev.. 2008;12:2404-2421.
- [Google Scholar]
- Lubricant base fluids based on renewable raw materials: Their catalytic manufacture and modification. Appl. Catal. A Gen.. 2001;221:429-442.
- [Google Scholar]
- Polymers and surfactants on the basis of renewable resources. Chemosphere. 2001;43:39-48.
- [Google Scholar]
- Development of surfactants and builders in detergent formulations. Chin. J. Chem. Eng.. 2008;16:517-527.
- [Google Scholar]
- Application of plant oils in the treatment of polycyclic aromatic hydrocarbons-contaminated soils. J. Hazard. Mater.. 2010;177:28-41.
- [Google Scholar]







