Translate this page into:
Synthesis, characterization and anticancer evaluation of 2-(naphthalen-1-ylmethyl/naphthalen-2-yloxymethyl)-1-[5-(substituted phenyl)-[1,3,4]oxadiazol-2-ylmethyl]-1H-benzimidazole
2nd Cancer Update
*Corresponding author. Tel.: +91 9891872142 sallu_05@yahoo.co.in (Salahuddin)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.

Available online 11 February 2013
Abstract
In the present study o-phenylenediamine and naphtene-1-acetic acid/2-naphthoxyacetic acid were used as a starting material through a series of steps and 2-(naphthalen-1-ylmethyl/Naphthalen-2-yloxymethyl)-1H-benzimidazol-1-yl]acetohydrazide 5a, 5b were obtained. In the first series 1,3,4-oxadiazole derivatives have been synthesized from Schiff base of the corresponding hydrazide i.e. 2-[2-(naphthalen-1-ylmethyl)-1H-benzimidazol-1-yl]acetohydrazide 5a by using Chloramin-T. In the second series 1,3,4-oxadiazole has been synthesized from 2-{2-[(naphthalen-2-yloxy)methyl]-1Hbenzimidazol-1-yl}acetohydrazide 5b by using phosphorous oxychloride and aromatic acid. These compounds were evaluated by IR, NMR, Mass spectrometry, elemental analysis and finally in vitro anticancer evaluation was carried out by NCI 60 Cell screen at a single high dose (10–5 M) on various panel/cell lines. One compound 7c was found to be the most active on breast cancer cell line and compounds 4b and 7d were moderately active.
Keywords
1,3,4-Oxadiazole
Anticancer evaluation
Benzimidazole
1 Introduction
Cancer is a group of various diseases and medically known as malignant neoplasm, involving unregulated cell growth. It is a major health problem in developing as well as undeveloped countries (Abdel-Aziz, 2007; Choo et al., 2002; Al-Rasood et al., 2006). The incidence of cancer worldwide increases the search for new, safer and efficient anticancer agents, aiming the prevention or the cure of this illness. Research laboratories are still involved deeply for the research of a new anticancer drug. Product development involves application of existing products to meet the therapeutic need in addition to the discovery of new drugs. Literature review revealed that many compounds bearing a five membered heterocyclic ring containing nitrogen and oxygen like oxadiazole have been synthesized and showed a variety of biological activities like anticancer (Sengupta et al., 2008; Jin et al., 2006; Holla et al., 2005), anticonvulsant (Almasirad et al., 2004; Aziz et al., 2009), antimicrobial (Shetgiri and Nayak, 2005; Manjunatha et al., 2010; Shailaja et al., 2010; Mulwad and Chaskar, 2006; Ansari and Lal, 2009), anti-inflammatory analgesic (Bhandari et al., 2008; Dewangan et al., 2010; Amir et al., 2007; Kumar et al., 2008; Jayashankar et al., 2009), dyes and pigments (ShuiLv et al., 2010), ulcerogenic (Gilani et al., 2010), antitubercular (Ali and Shaharyar, 2007) etc.
2 Experimental
2.1 Instrumentation
The chemicals used for experimental work were commercially procured from various chemical units viz E. Merck India Ltd., CDH and S.D. Fine chem. and Qualigens. These solvents and reagents were of LR grade and purified before use. The silica gel G (160–120 mesh) used for analytical chromatography (TLC) was obtained from E. Merck India Ltd. Two solvent systems were used Benzene:Acetone (9:1) and (8:2), Toluene:Ethyl Acetate:Formic acid (5:4:1). Ashless Whattman No. 1 filter paper was used for vacuum filtration. Melting points were determined in an open glass capillary using melting point apparatus and are uncorrected. The Proton Magnetic Resonance spectra (1HNMR) were recorded on a Bruker 300 MHz instrument in DMSO-d6/CDCl3 using tetramethylsilane [(CH3)4Si] as internal standard. The Infrared spectra of compound were recorded in KBr on Perkin-Elmer FTIR Spectrometer and iodine Chamber and UV-lamp were used for visualization of TLC spots. The commercially available grades of solvents and reagents were found to be of adequate purity. However, the presence of undesirable impurities and others were likely to be used for experimental work was purified/dried.
2.1.1 Procedure for the synthesis of 2-naphthalen-1-yl/naphthoxy-methyl-1H-benzimidazole (3a, 3b)
A mixture of o-phenylenediamine 1 (0.05 mol; 5.40 g) and naphthylacetic acid/naphthoxyacetic acid 2 (0.05 mol) was refluxed in 4N HCl for 4 h on a heating mantle. After completion of reaction, the solution was poured onto crushed ice, ammonia solution was added drop wise to neutralize and the resulting solid was filtered, washed with cold water, dried and recrystallized with ethanol (see Scheme 1).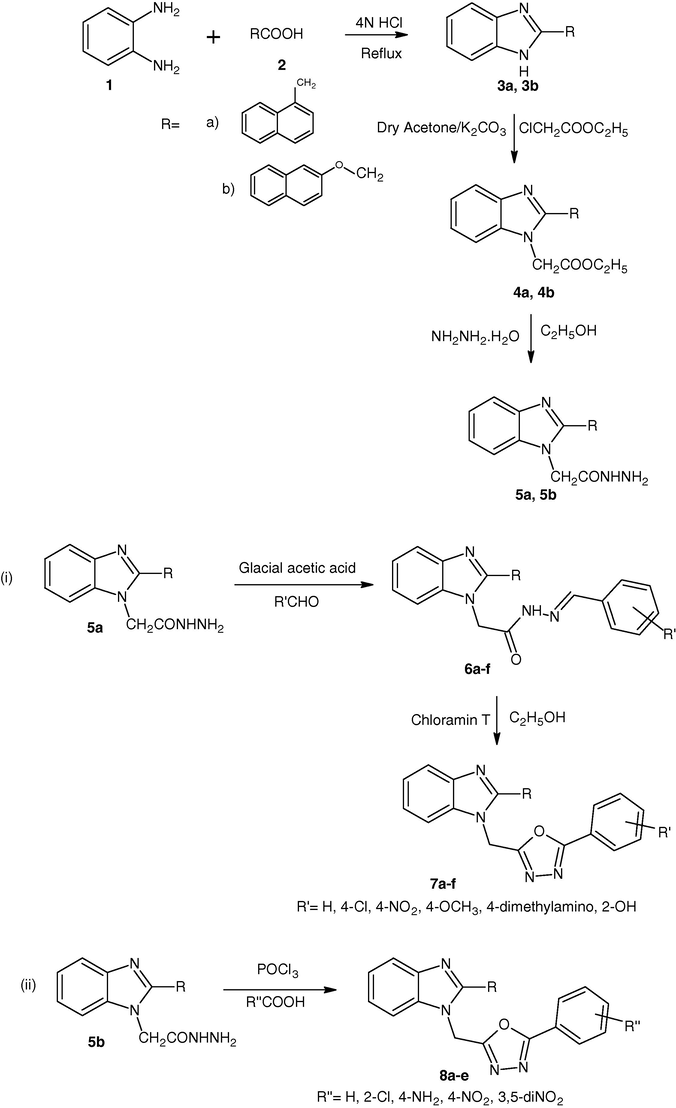
2.1.1.1 Synthesis of 2-(naphthalen-1-ylmethyl)-1H-benzimidazole (3a)
Yield 85%, m.p. 125–126 °C, IR (KBr) cm−1: 1528 (C⚌N), 3302 (N-H): 1H-NMR (DMSO-d6) δ ppm: 4.62 (s, 2H, CH2), 7.06–8.19 (m, 11H, aromatic), 12.37 (s, 1H, NH). EI-MS 274 (M+); Anal. Calcd. for C18H14N2C, 83.69; H, 5.46; N, 10.84. Found: C, 83.67; H, 5.49; N, 10.82.
2.1.1.2 Synthesis of 2-[(naphthalen-2-yloxy)methyl]-1H-benzimidazole (3b)
Yield 84%, m.p. 205–207 °C, IR (KBr) cm−1: 1226 (C–O), 1464 (C⚌N), 3297 (N–H), 3010 (CH, aromatic): 1H-NMR (DMSO-d6) δ ppm: 5.34 (s, 2H, CH2O), 7.26–7.70 (m, 11H, aromatic); EI-MS 258 (M+); Anal. Calcd. for C18H14N2O C, 78.81; H, 5.14; N, 10.21; O, 5.83 Found: C, 78.84; H, 5.15; N, 10.25; O, 5.80.
2.1.2 Synthesis of ethyl [2-(naphthalen-1-yl/naphthalen-2-yloxymethyl)-1H-benzimidazol-1-yl]acetate (4a, 4b)
To a suspension of 2-(naphthylmethyl)-1H-benzimidazole 3 (0.01 mol), anhydrous potassium carbonate (2 g) in dry acetone, ethyl chloroacetate (0.01 mol; 1.2 ml) was added drop wise at room temperature for a period of 20–30 min. The reaction mixture was stirred at room temperature for 10–12 h. The inorganic solid was filtered off and the filtrate was concentrated under reduced pressure.
2.1.2.1 Synthesis of ethyl [2-(naphthalen-1-ylmethyl)-1H-benzimidazol-1-yl]acetate (4a)
Yield 72%, m.p. 108–112 °C, IR (KBr) cm−1: 1229 (C–O), 1436 (C⚌N), 3206 (N–H), 3010 (CH, aromatic); 1H-NMR (DMSO-d6) δ ppm: 4.14 (s, 2H, CH2 naphthyl), 5.06 (s, 2H, CH2) 7.46–7.79 (m, 11H, aromatic); EI-MS 360 (M+); Anal. Calcd. for C22H20N2O2, C, 76.72; H, 5.85; N, 8.13; O, 9.29. Found: C, 76.70; H, 5.88; N, 8.12; O, 9.29.
2.1.2.2 Synthesis of ethyl {2-[(naphthalen-2-yloxy)methyl]-1H-benzimidazol-1-yl}acetate (4b)
Yield 69%, m.p. 105–109 °C, IR (KBr) cm−1: 1226 (C–O), 1464 (C⚌N), 1736 (C⚌O), 2950 (CH, aromatic); 1H-NMR (DMSO-d6) δ ppm: 4.72 (s, 2H, CH2), 5.21 (s, 2H, CH2O) 6.90–7.61 (m, 11H, aromatic); EI-MS 344 (M+); Anal. Calcd. for C22H20N2O3C, 73.32; H, 5.59; N, 7.77; O, 13.32. Found: C, 73.29; H, 5.60; N, 7.80; O, 13.35.
2.1.3 Synthesis of 2-[2-(naphthalen-1-yl/naphthalen-2-yloxy methyl)-1H-benzimidazol-1-yl]acetohydrazide (5a, 5b)
To an ethanolic solution of ethyl [2-(naphthalen-1-ylmethyl)/(naphthalen-2-yloxy)methyl]-1H-benzimidazol-1-yl]acetate 4a, 4b (0.01 mol), hydrazine hydrate (98%) (0.01 mol; 0.49 ml) was added and the mixture was refluxed for 3 h. After completion of the reaction, the mixture was cooled and the solid so obtained was filtered, washed with cold water and recrystallized from methanol.
2.1.3.1 Synthesis of 2-[2-(naphthalen-1-ylmethyl)-1H-benzimidazol-1-yl]acetohydrazide (5a)
Yield 82% mp. 147–150 °C, IR (KBr) cm−1: 1233 (N–N), 1528 (C⚌N); 1643 (C⚌O), 3043 (CH–Ar), 3302 (N–H); 1H-NMR (DMSO-d6) δ ppm: 2.50 (s, 1H, NH2), 4.90 (s, 2H, CH2), 7.13–8.15 (m, 11H, aromatic), 9.25 (s, 1H, CONH); EI-MS 346 (M+); Anal. Calcd. for: C22H18N4OC, 72.71; H, 5.49, N, 16.96; O, 4.84 Found: C, 72.71; H, 5.50, N, 16.94; O, 4.82.
2.1.3.2 Synthesis of 2-{2-[(naphthalen-2-yloxy)methyl]-1H-benzimidazol-1-yl}acetohydrazide (5b)
Yield 82% mp. 208–210 °C, IR (KBr) cm−1: 1254 (N–O), 1466 (C⚌N); 1668 (C⚌O), 3056 (CH–Ar), 3292 (N–H); 1H-NMR (DMSO-d6) δ ppm: 2.53 (s, 1H, NH2), 4.89 (s, 2H, CH2), 5.35 (s, 1H, CH2O), 6.92–7.70 (m, 11H, aroamtic), 9.05 (s, 1H, CONH); EI-MS 330 (M+); Anal. Calcd. for: C22H18N4O2C, 69.35; H, 5.24; N, 16.17; O, 9.24 Found: C, 69.30; H, 5.21; N, 16.21; O, 9.27.
2.1.4 Synthesis of 2-[2-(naphthylmethyl)-1H-benzimidazol-1-yl]-N’-[substituted phenylmethylidene] acetohydrazide (6a–f)
A mixture of 2-[2-(naphthylmethyl)-1H-benzimidazol-1-yl]acetohydrazide 5 (0.0025 mol) and substituted benzaldehyde (0.0025 mol) in ethyl alcohol (10 ml) and few drops of glacial acetic acid were refluxed for 5 h. After completion of reaction, the reaction mixture was concentrated, cooled, poured in ice cold water, the precipitate so formed was filtered, dried and recrystallized with ethanol to give desired compound.
2.1.4.1 (2-Naphthalen-1-ylmethyl-benzimidazol-1-yl)-acetic acid benzylidene-hydrazide (6a)
Yield 76%; m.p. 208–209 °C, IR (KBr) cm−1: 1134 (N–N), 1594 (C⚌N); 1669 (C⚌O), 3045 (CH–Ar), 3193 (N–H); 1H-NMR (DMSO-d6) δ ppm: 4.08 (s, 2H, CH2 naphthyl), 4.46 (s, 2H, CH2), 7.43–8.66 (m, 16H, aroamtic), 11.81 (s, 1H, CONH); EI-MS 435 (M + 1)+; Anal. Calcd. for C27H22N4O: C, 77.49; H, 5.30; N, 13.39; O, 3.82 Found: C, 77.46; H, 5.33; N, 13.41; O, 3.80.
2.1.4.2 (2-Naphthalen-1-ylmethyl-benzimidazol-1-yl)-acetic acid (4-chloro-benzylidene)-hydrazide (6b)
Yield 80%; m.p. 200–202 °C, IR (KBr) cm−1: 1252 (N–N), 1489 (C⚌N); 1667 (C⚌O), 2962 (CH–Ar), 3181 (N–H); 1H-NMR (DMSO-d6) δ ppm: 4.05 (s, 2H, CH2 naphthyl), 5.47 (s, 2H, CH2), 7.42–8.26 (m, 15H, aroamtic), 11.51 (s, 1H, CONH); EI-MS 469 (M + 1)+; Anal. Calcd. for C27H19ClN4O: C, 71.60; H, 4.67; Cl, 7.83; N, 12.37; O, 3.53 Found: C, 71.60; H, 4.65; Cl, 7.82; N, 12.38; O, 3.52.
2.1.4.3 (2-Naphthalen-1-ylmethyl-benzimidazol-1-yl)-acetic acid (4-nitro-benzylidene)-hydrazide (6c)
Yield 78%; m.p. 226–227 °C, IR (KBr) cm−1: 1520 (N⚌C); 1662 (C⚌O); 3042 (CH–Ar); 3176 (N–H); 1H-NMR (DMSO-d6) δ ppm: 3.99 (s, 2H, CH2 naphthyl), 4.41 (s, 2H, CH2), 6.95–8.15 (m, 15H, aroamtic), 11.35 (s, 1H, CONH); EI-MS 478 (M+); Anal. Calcd. for C27H21N5O3: C, 69.97; H, 4.57; N, 15.11; O, 10.36. Found: C, 69.95; H, 4.60; N, 15.10; O, 10.39.
2.1.4.4 (2-Naphthalen-1-ylmethyl-benzimidazol-1-yl)-acetic acid (4-methoxy-benzylidene)-hydrazide (6d)
Yield 75%; m.p. 186–187 °C, IR (KBr) cm−1: 1512 (N⚌C); 1661 (C⚌O); 3051 (CH–Ar); 3202 (N–H); 1H-NMR (DMSO-d6) δ ppm: 3.98 (s, 2H, CH2 naphthyl), 4.40 (s, 2H, CH2), 6.69–8.12 (m, 15H, aroamtic), 11.18 (s, 1H, CONH); EI-MS 465 (M + 1)+; Anal. Calcd. for C28H24N4O2: C, 74.98; H, 5.39; N, 12.49; O, 7.13. Found: C, 74.98; H, 5.39; N, 12.49; O, 7.13.
2.1.4.5 (2-Naphthalen-1-ylmethyl-benzimidazol-1-yl)-acetic acid (4-dimethylamino-benzylidene)-hydrazide (6e)
Yield 82%; m.p. 289–290 °C, IR (KBr) cm−1: 1496 (N⚌C); 1654 (C⚌O); 3041 (CH–Ar); 3194 (N–H); 1H-NMR (DMSO-d6) δ ppm: 3.30 (s, 3H, CH3), 4.05 (s, 2H, CH2 naphthyl), 4.47 (s, 2H, CH2), 7.43–8.25 (m, 15H, aroamtic), 11.58 (s, 1H, CONH); EI-MS 478 (M + 1); Anal. Calcd. for C29H27N5O: C, 75.47; H, 5.91; N, 15.19; O, 3.50. Found: C, 75.43; H, 5.89; N, 15.14; O, 3.49.
2.1.4.6 (2-Naphthalen-1-ylmethyl-benzimidazol-1-yl)-acetic acid (2-hydroxy-benzylidene)-hydrazide (6f)
Yield 82%; m.p. 289–290 °C, IR (KBr) cm−1: 1466 (N⚌C); 1739 (C⚌O); 3048 (CH–Ar); 3294 (N–H); 1H-NMR (DMSO-d6) δ ppm: 4.04 (s, 2H, CH2 naphthyl), 4.46 (s, 2H, CH2), 6.98–8.21 (m, 15H, aroamtic), 10.18 (s, 1H, CONH), 11.39 (s, 1H, OH); EI-MS 451 (M + 1)+; Anal. Calcd. for C27H22N4O2: C, 74.64; H, 5.10; N, 12.89; O, 7.36. Found: C, 74.62; H, 5.13; N, 12.90; O, 7.39.
2.1.5 General procedure for the synthesis of 2-Naphthalen-1-ylmethyl-1-(5-substituted phenyl-[1,3,4]oxadiazol-2-ylmethyl)-1H-benzimidazole (7a–f)
To an ethanolic solution of 2-[2-(Naphthylmethyl)-1H-benzimidazol-1-yl]-N’-[phenylmethylidene] acetohydrazide (0.0025 mol), chloramin T (0.0125 mol) was added. The solution was refluxed for 4 h, sodium chloride which separated out during the course of reaction was filtered off. Excess ethanol was completely removed from the filtrate by distillation under reduced pressure, leaving behind a solid mass which was crystallized from ethanol to give desired compound.
2.1.5.1 2-Naphthalen-1-ylmethyl-1-(5-phenyl-[1,3,4]oxadiazol-2-ylmethyl)-1H-benzimidazole (7a)
Yield 65%; m.p. 172–174 °C, IR (KBr) cm−1: 1034 (N–N); 1231 (C–O); 1598 (C⚌N); 2963 (CH–Ar); 1H-NMR (DMSO-d6) δ ppm: 5.33 (s, 2H, CH2 naphthyl), 5.55 (s, 2H, CH2), 7.16–7.86 (m, 16H, aromatic); EI-MS 431 (M+); Anal. Calcd. for C27H20N4O: C, 77.87; H, 4.84; N, 13.45; O, 3.84. Found: C, 77.85; H, 4.85; N, 13.43; O, 3.80.
2.1.5.2 1-[5-(4-Chloro-phenyl)-[1,3,4]oxadiazol-2-ylmethyl]-2-naphthalen-1-ylmethyl-1H-benzimidazole (7b)
Yield 71%; m.p. 221–223 °C, IR (KBr) cm−1: 773 (C–Cl); 1085 (N–N); 1228 (C–O); 1489 (C⚌N); 2962 (CH–Ar); 1H-NMR (DMSO-d6) δ ppm: 4.70 (s, 2H, CH2 naphthyl), 5.24 (s, 2H, CH2), 7.13–8.21 (m, 15H, aromatic); EI-MS 467 (M + 1)+; Anal. Calcd. for C27H19ClN4O: C, 71.92; H, 4.25; Cl, 7.86; N, 12.43; O, 3.55. Found: C, 71.92; H, 4.25; Cl, 7.83; N, 12.45; O, 3.51.
2.1.5.3 2-Naphthalen-1-ylmethyl-1-[5-(4-nitro-phenyl)-[1,3,4]oxadiazol-2-ylmethyl]-1H-benzimidazole (7c)
Yield 71%; m.p. 245–246 °C, IR (KBr) cm−1: 1070 (N–N); 1232 (C–O); 1519 (C⚌N); 3041 (CH–Ar); 1H-NMR (DMSO-d6) δ ppm: 5.41 (s, 2H, CH2 naphthyl), 5.65 (s, 2H, CH2), 7.09–7.85 (m, 15H, aromatic); EI-MS 477 (M+); Anal. Calcd. for C27H20N5O3: C, 70.27; H, 4.15; N, 15.18; O, 10.40. Found: C, 70.25; H, 4.17; N, 15.17; O, 10.40.
2.1.5.4 1-[5-(4-Methoxy-phenyl)-[1,3,4]oxadiazol-2-ylmethyl]-2-naphthalen-1-ylmethyl-1H-benzimidazole (7d)
Yield 82%; m.p. 170–172 °C, IR (KBr) cm−1: 1168 (N–N); 1257 (C–O); 1508 (C⚌N); 3041 (CH–Ar); 1H-NMR (DMSO-d6) δ ppm: 2.46 (s, 3H, CH3), 4.05 (s, 2H, CH2 naphthyl), 4.42 (s, 2H, CH2), 7.35–8.93 (m, 15H, aromatic); EI-MS 463 (M + 1)+; Anal. Calcd. for C28H22N4O2: C, 75.32; H, 4.97; N, 12.55; O, 7.17. Found: C, 75.31; H, 4.97; N, 12.54; O, 7.18.
2.1.5.5 Dimethyl-{4-[5-(2-naphthalen-1-ylmethyl-benzimidazol-1-ylmethyl)-[1,3,4]oxadiazol-2-yl]-phenyl}-amine (7e)
Yield 71%; m.p. 187–190 °C, IR (KBr) cm−1: 1139 (N–N); 1234 (C–O); 1531 (C⚌N); 2857 (CH–Ar); 1H-NMR (DMSO-d6) δ ppm: 2.50 (s, 6H, CH3), 3.97 (s, 2H, CH2 naphthyl), 4.48 (s, 2H, CH2), 7.16–8.27 (m, 15H, aromatic); EI-MS 344 (M + 1)+; Anal. Calcd. for C29H25N5O: C, 75.80; H, 5.48; N, 15.24, O, 3.48. Found: C, 75.82; H, 5.48; N, 15.21, O, 3.45.
2.1.5.6 2-[5-(2-Naphthalen-1-ylmethyl-benzimidazol-1-ylmethyl)-[1,3,4]oxadiazol-2-yl]-phenol (7f)
Yield 74%; m.p. 102–104 °C, IR (KBr) cm−1: 1027 (N–N); 1216 (C–O); 1503 (C⚌N); 2979 (CH–Ar); 1H-NMR (DMSO-d6) δ ppm: 4.01 (s, 2H, CH2 naphthyl), 4.40 (s, 2H, CH2), 7.30–8.11 (m, 15H, aromatic), 9.82 (s, 1H, OH); EI-MS 449 (M + 1)+; Anal. Calcd. for C27H20N4O2: C, 74.98; H, 4.66; N, 12.95; O, 7.40. Found: C, 74.98; H, 4.66; N, 12.95; O, 7.40.
2.1.6 Synthesis of 2-(Naphthalen-2-yloxymethyl)-1-(5-substituted phenyl-[1,3,4]oxadiazol-2-ylmethyl)-1H-benzimidazole (8a–e)
A mixture of 2-{2-[(naphthalen-2-yloxy)methyl]-1H-benzimidazol-1-yl}acetohydrazide 5b (0.0025 mol) and suitable aromatic acid (0.0025 mol) was refluxed in the presence of POCl3 (5 ml) for 5 h at a temperature of 110–120 °C. After completion of reaction, the mixture was cooled at room temperature and poured onto crushed ice. On basification with sodium bicarbonate (5%), a solid mass separated out was filtered to get crude product. Finally the product was heated with charcoal in hydrated ethanol and then re-crystallized from ethanol to obtain 8a–e.
2.1.6.1 Synthesis of 2-(Naphthalen-2-yloxymethyl)-1-(5-phenyl-[1,3,4]oxadiazol-2-ylmethyl)-1H-benzimidazole (8a)
Yield 67% m.p. 204–208 °C IR (KBr) cm−1: 1031 (N–N), 1250 (C–O), 1605 (C⚌N); 1H-NMR (DMSO-d6) δ ppm: 4.67 (s, 2H, CH2), 5.64 (s, 2H, OCH2), 7.09–7.76 (m, 16H, aromatic); EI-MS 416 (M+); Anal. Calcd. for C27H20N4O2: C, 74.98; H, 4.66; N, 12.95; O, 7.40 Found: C, 74.95; H, 4.69; N, 12.93; O, 7.45.
2.1.6.2 Synthesis of 1-[5-(2-Chloro-phenyl)-[1,3,4]oxadiazol-2-ylmethyl]-2-(naphthalen-2-yloxymethyl)-1H-benzimidazole (8b)
Yield 63%; m.p. 108–110 °C, IR (KBr) cm−1: 740 (C–Cl); 1028 (N–N), 1238 (C–O), 1583 (C⚌N), 3026 (CH–Ar); 1H-NMR (DMSO-d6) δ ppm: 4.45 (s, 2H, CH2), 5.03 (s, 2H, OCH2), 6.73–7.79 (m, 15H, aroamtic), EI-MS 451 (M + 1)+. Anal. Calcd. for C27H19CIN4O2: C, 69.45; H, 4.10;Cl, 7.59; N, 12.00; O, 6.85 Found: C, 69.41; H, 4.14; Cl, 7.61; N, 11.97; O, 6.83.
2.1.6.3 Synthesis of 2-{5-[2-(Naphthalen-2-yloxymethyl)-benzoimidazol-1-ylmethyl]-[1,3,4]oxadiazol-2-yl}-phenylamine (8c)
Yield 66%; m.p. 220–222 °C, IR (KBr) cm−1: 1037 (N–N), 1246 (C–O), 1598 (C⚌N), 3067 (CH–Ar); 1H-NMR (DMSO-d6) δ ppm: 4.0 (s, 2H, NH2), 4.91 (s, 2H, CH2), 5.31 (s, 2H, OCH2), 6.73–7.79 (m, 15H, aroamtic), EI-MS 432 (M + 1)+. Anal. Calcd. for C27H21N5O2: C, 72.47; H, 4.73; N, 15.65; O, 7.15 Found: C, 72.45; H, 4.77; N, 15.67; O, 7.13.
2.1.6.4 Synthesis of 2-(Naphthalen-2-yloxymethyl)-1-[5-(4-nitro-phenyl)-[1,3,4]oxadiazol-2-ylmethyl]-1H-benzimidazole (8d)
Yield 69%; m.p. 80-84 °C, IR (KBr) cm−1: 1035 (N–N); 1239 (C–O); 1568 (NO2); 1685 (C⚌N); 3032 (CH–Ar); 1H-NMR (DMSO-d6) δ ppm: 4.50 (s, 2H, CH2), 5.28 (s, 2H, OCH2), 6.91–7.71 (m, 15H, aromatic); EI-MS 461 (M+); Anal. Calcd. for C27H19N5O4: C, 67.92; H, 4.01; N, 14.67; O, 13.40 Found: 67.89; H, 4.05; N, 14.70; O, 13.37.
2.1.6.5 Synthesis of 12-(Naphthalen-2-yloxymethyl)-1-[5-(3,5-dinitro-phenyl)-[1,3,4]oxadiazol-2-ylmethyl]-1H-benzimidazole (8e)
Yield 74%; m.p. 155-157 °C, IR (KBr) cm−1: 1035 (N–N); 1239 (C–O); 1566 and 1350 (NO2); 1685 (C⚌N); 3063 (CH–Ar); 1H-NMR (DMSO-d6) δ ppm: 5.12 (s, 2H, CH2), 5.37 (s, 2H, OCH2), 6.91–7.78 (m, 14H, aroamtic), EI-MS 507 (M + 1)+; Anal. Calcd. for C27H18N6O6: C, 62.07; H, 3.47; N, 16.09; O, 18.37 Found: C, 62.12; H, 3.44; N, 16.11; O, 18.35.
2.2 Anticancer activity
2.2.1 Treatment of tumor cell lines
All compounds submitted to the NCI 60 Cell screen were tested initially at a single high dose (10−5 M) on leukemia, melanoma, lung, colon, CNS, ovarian, renal, prostate, and breast cancer cell lines, nearly 60 in number. The one-dose data were reported as a mean graph of the percent growth of treated cells. The number reported for the one-dose assay is growth relative to the no-drug control, and relative to the time zero number of cells. The anticancer screening was carried out as per the NCI US protocol reported elsewhere (http://dtp.nci.nih.gov; Turner, 1964; Monks et al., 1991; Boyd and Paull, 1995; Shoemaker, 2006). Using the seven absorbance measurements [time zero, (Tz), control growth, (C), and test growth in the presence of drug at the five concentration levels (Ti)], the percentage growth was calculated at each of the drug concentration levels.
Percentage growth inhibition is calculated as:
3 Result and discussion
3.1 Chemistry
2-Naphthalen-1-yl/naphthoxy-methyl-1H-benzimidazole 3a, 3b were prepared by refluxing with o-phenylenediamine 1 and naphthalene-1-acetic acid/2-naphthoxyacetic acid 2 in the presence of 4NHCl. The compounds 3a, 3b on reacting with ethylchloroacetate in the presence of potassium carbonate in dry acetone gave ethyl [2-(naphthalen-1-yl/naphthalen-2-yloxymethyl)-1H-benzimidazol-1-yl]acetate 4a, 4b which on treatment with hydrazine hydrate result in the formation of 2-[2-(naphthalen-1-ylmethyl/naphthalen-2-yloxymethyl)-1H-benzimidazol-1-yl]acetohydrazide 5a, 5b. The compound 5a on treatment with aromatic aldehyde and alcohol gave Sciff base which is (2-Naphthalen-1-ylmethyl-benzoimidazol-1-yl)-acetic acid substituted benzylidene-hydrazide 6a–f. Sciff base 6a–f on reacting with ethyl alcohol and Chloramin-T gives 2-Naphthalen-1-ylmethyl-1-(5-substitutedphenyl-[1,3,4]oxadiazol-2-ylmethyl)-1H-benzimidazole 7a–f. Finally the compound 5b on treating with various aromatic acids and phosphorous oxychloride gave 2-(Naphthalen-2-yloxymethyl)-1-(5-substituted phenyl-[1,3,4]oxadiazol-2-ylmethyl)-1H-benzimidazole 8a–d.
3.2 Anticancer activity
The synthesized title compounds displayed moderate to low activity in the in vitro screen on all tested cancer cell lines and are given in Table 1. The compound 7c was found to be the most active compound of the series that showed 72.85 growth percent (GP) and highly active on MDA-MB-468 (Breast cancer) and SK-MEL-28 (Melanoma) (GP = 36.23 and 47.56, respectively). The compounds 7d and 4b showing moderate activity were highly active on NCI-H522 (Non-small cell lung cancer) with GP of 32.73 and 47.08, respectively and on UO-31 (Renal cancer) with 40.53 and 57.33, respectively, while rest of the compounds showed less activity with an average growth percent of 84.91–96.59.
Compound
60 cell lines assay in 1 dose 10–5 M conc.
NSC code
Mean growth %
Range of growth %
The most sensitive cell line
Growth % of the most sensitive cell line
3b
764,708
89.44
61.59–123.30
A-498 (renal cancer)
61.59
HOP-92 (non-small cell lung cancer)
63.30
4b
764,709
79.28
47.08–97.79
NCI-H522 (Non-small Cell Lung Cancer)
47.08
UO-31 (renal cancer)
57.33
6d
764,703
95.73
69.53–109.35
MOLT-4 (leukemia)
69.53
UO-31 (renal cancer)
72.14
6f
764,706
94.99
63.19–108.33
UO-31 (renal cancer)
63.19
UACC-62 (melanoma)
74.16
7a
764,700
84.91
25.17–110.90
HOP-92 (non-small cell lung cancer)
25.17
UO-31 (renal cancer)
63.88
7b
764,701
91.53
51.36–107.82
SNB-75 (CNS cancer)
51.36
NCI-H522 (non-small cell lung cancer)
72.21
7c
764,702
72.85
36.23–101.81
MDA-MB-468 (Breast Cancer)
36.23
SK-MEL-28 (melanoma)
47.56
7d
764,704
74.09
32.73–100.64
NCI-H522 (non-small cell lung cancer)
32.73
UO-31 (renal cancer)
40.53
7e
764,705
95.10
64.10–109.28
UO-31 (renal cancer)
64.10
HOP-92 (non-small cell lung cancer)
70.12
7f
764,707
96.59
62.97–111.45
UO-31 (renal cancer)
62.97
HOP-92 (non-small cell lung cancer)
78.32
8a
764,710
92.20
56.74–111.52
UO-31 (renal cancer)
56.74
MOLT-4 (leukemia)
72.02
8b
764,711
80.54
40.96–111.19
UO-31 (renal cancer)
40.96
MOLT-4 (leukemia)
41.37
8c
764,712
101.87
71.70–118.83
UO-31 (renal cancer)
71.70
HOP-92 (non-small cell lung cancer)
74.53
8d
764,713
96.10
54.01–110.84
UO-31 (renal cancer)
54.01
HOP-92 (non-small cell lung cancer)
76.98
8e
764,714
92.62
47.35–108.14
UO-31 (renal cancer)
47.35
PC-3 (prostate cancer)
69.80
4 Conclusion
A novel 2-Naphthalen-1-ylmethyl-1-(5-substituted phenyl-[1,3,4]oxadiazol-2-ylmethyl)-1H-benzimidazole (7a–f) and 2-(Naphthalen-2-yloxymethyl)-1-(5-phenyl-[1,3,4]oxadiazol-2-ylmethyl)-1H-benzimidazole (8a–e) have been synthesized by using chloramin-T from Schiff base and phosphrous oxychloride from hydrazides. The in vitro anticancer studies reveal that the compound with para substituent like p-NO2 (7c) showed a prominent activity against MDA-MB-468 (Breast cancer) and SK-MEL-28 (Melanoma) (GP = 36.23 and 47.56, respectively) probably because of more electron withdrawing power of the other substituents. While the other compounds 7d and 4b showed moderate activity against selected cancer cell line. Thus the study revealed that these compounds have potential anticancer activity and structural modification may lead to the synthesis of more 1,3,4-oxadiazole derivatives and can be evaluated for their anticancer activities in vitro as well as in vivo.
Acknowledgement
The author are hihly thankful to Managing Director,Noida Institute of Pharmaceutical Technology for providing the research facilities. The author also wish to express their thanks to all the staffs ofNational Cancer Institute,Betehesda,MD,USA for in vitro evaluation of anticancer activity.
References
- Novel and versatile methodology for synthesis of cyclic imides and evaluation of their cytotoxic, DNA binding, apoptotic inducing activities and molecular modeling study. Eur. J. Med. Chem.. 2007;42:614.
- [Google Scholar]
- Oxadiazole mannich bases: synthesis and antimycobacterial activity. Bioorg. Med. Chem. Lett.. 2007;17:3314.
- [Google Scholar]
- Synthesis and anticonvulsant activity of new 2-substituted-5-[2-(2-fluorophenoxy)phenyl]-1,3,4-oxadiazoles and 1,2,4-triazoles. Bioorg. Med. Chem. Lett.. 2004;14:6057.
- [Google Scholar]
- Synthesis, dihydrofolate reductase inhibition, antitumor testing, and molecular modeling study of some new 4(3H)-quinazolinone analogs. Bioorg. Med. Chem.. 2006;14:860.
- [Google Scholar]
- Synthesis of some 1,3,4-oxadiazole derivatives as potential anti-inflammatory agents. Ind. J. Chem.. 2007;46B:1014.
- [Google Scholar]
- Synthesis and evaluation of some new benzimidazole derivatives as potential antimicrobial agents. Eur. J. Med. Chem.. 2009;44:2294.
- [Google Scholar]
- Synthesis of novel pyrazole derivatives and evaluation of their antidepressant and anticonvulsant activities. Eur. J. Med. Chem.. 2009;44:3480.
- [Google Scholar]
- Design, synthesis and evaluation of antiinflammatory, analgesic and ulcerogenicity studies of novel S-substituted phenacyl-1,3,4-oxadiazole-2-thiol and Schiff bases of diclofenac acid as nonulcerogenic derivatives. Bioorg. Med. Chem.. 2008;16:1822.
- [Google Scholar]
- Some practical considerations and applications of the National Cancer Institute in vitro anticancer drug discovery screen. Drug Dev. Res.. 1995;34:91.
- [Google Scholar]
- Solid-phase combinatorial synthesis and cytotoxicity of 3-aryl-2,4-quinazolindiones. Bioorg. Med. Chem.. 2002;10:517.
- [Google Scholar]
- Synthesis of some novel 2,5-disubstituted 1,3,4-oxadiazole and its analgesic, anti-inflammatory, anti-bacterial and anti-tubercular activity. Int. J. Chemtech Res.. 2010;3:1397.
- [Google Scholar]
- Synthesis and pharmacological evaluation of condensed heterocyclic 6-substituted 1,2,4-triazolo-[3,4-b]-1,3,4-thiadiazole and 1,3,4-oxadiazole derivatives of isoniazid. Bioorg. Med. Chem. Lett.. 2010;16:4762.
- [Google Scholar]
- Synthesis of some novel 2,5-disubstituted 1,3,4-oxadiazole and its analgesic, anti-inflammatory, anti-bacterial and anti-tubercular activity. Ind. J. Chem.. 2005;44B:1669.
- [Google Scholar]
- Synthesis and pharmacological evaluation of 1,3,4-oxadiazole bearing bis(heterocycle) derivatives as anti-inflammatory and analgesic agents. Eur. J. Med. Chem.. 2009;44:3898.
- [Google Scholar]
- Synthesis, structure, and bioactivity of N′-substituted benzylidene-3,4,5-trimethoxybenzohydrazide and 3-acetyl-2-substituted phenyl-5-(3,4,5-trimethoxyphenyl)-2,3-dihydro-1,3,4-oxadiazole derivatives. Bioorg. Med. Chem. Lett.. 2006;16:5036.
- [Google Scholar]
- 1,3,4-Oxadiazole/thiadiazole and 1,2,4-triazole derivatives of biphenyl-4-yloxy acetic acid: synthesis and preliminary evaluation of biological properties. Eur. J. Med. Chem.. 2008;43:2688.
- [Google Scholar]
- Synthesis and biological evaluation of some 1,3,4-oxadiazole derivatives. Eur. J. Med. Chem.. 2010;45:5225.
- [Google Scholar]
- Feasibility of a high-flux anticancer drug screen utilizing a diverse panel of human tumor cell lines in culture. J. Natl. Cancer Inst.. 1991;83:757.
- [Google Scholar]
- Synthesis and antibacterial activity of new oxadiazolo[1,3,5]triazine, 1,2,4-triazolo and thiadiazolo[1,3,4]oxadiazole derivatives. Ind. J. Chem.. 2006;45B:1710.
- [Google Scholar]
- Evaluation of anticancer activity of some 1,3,4-oxadiazole derivatives. Ind. J. Chem.. 2008;47B:460.
- [Google Scholar]
- Synthesis and biological activity of novel 2,5-disubstituted-1,3,4-oxadiazoles. Ind. J. Chem.. 2010;49B:1088.
- [Google Scholar]
- Synthesis and antimicrobial activities of oxadiazoles, phthalazines and indolinones. Ind. J. Chem.. 2005;44B:1267.
- [Google Scholar]
- The NCI60 human tumour cell line anticancer drug screen. Nat. Rev. Cancer. 2006;6:813.
- [Google Scholar]
- The synthesis, characterization and optical properties of novel, substituted, pyrazoly 1,3,4-oxadiazole derivatives. Dyes Pigm.. 2010;86:25.
- [Google Scholar]
- Screening Methods in Pharmacology. New York, London: Academic Press; 1964. p. 165. http://dtp.nci.nih.gov, (accessed on 22/10/2011)







