Translate this page into:
Synthesis, antimicrobial and anticancer activities of amido sulfonamido methane linked bis heterocycles
2nd Cancer Update
*Corresponding author. Tel.: +91 877 2249666x303; fax: +91 877 2248499 vkpuram2001@yahoo.com (Venkatapuram Padmavathi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.

Available online 28 October 2013
Abstract
A new class of amido sulfonamido methane linked bis heterocycles- bis-oxazoles, thiazoles and imidazoles were prepared and screened for antimicrobial and anticancer activities. The chloro substituted amido sulfonamido bisimidazole exhibited excellent antimicrobial activity and also it was the most potent compound on lung, colon and prostate cancer cell lines.
Keywords
Oxazoles
Thiazoles
Imidazoles
Antimicrobial activity
Anticancer activity
1 Introduction
Sulfonamide drugs are associated with a wide range of biological activities and in fact brought an antibiotic revolution in medicine (Ali et al., 2006; McCarroll et al., 2007; Wilkinson et al., 2007). Many oxazole and/or thiazole containing macrocycles are naturally occurring molecules, viz., Bistratamides (You and Kelly, 2005), Didmolamides A and B (You and Kelly, 2005), Lyngbyabellin A (Yokokawa et al., 2001), and Calyculins (Yokokawa et al., 2001; Degnan et al., 1989; Pihko and Koskinen, 1998; Perez and Faulkner, 2003; Rudi et al., 2003; Tan et al., 2003), which show cytotoxic, antimicrobial and multiple drug resistance activities. Several classes of drugs based on imidazole viz., 2-nitroimidazole commonly called Azomycin are a natural antibiotic. Some synthetic nitroimidazoles are active against intestinal infections (Breccia et al., 1986). In fact metronidazole is used for intestinal infections and also as a radiosensitizer in X-ray therapy (Middlemiss and Watson, 1994). The incorporation of sulfonamide moiety into heterocyclic rings can produce pharmacologically potent compounds. The present work comprises design and synthesis of new molecules having two pharmacophoric heterocyclic units linked by bis methane amido sulfonamido moiety which are expected to have pharmacological activity. Although chemically unrelated to these compounds, other classes of antibiotics such as the anthracyclines (Miller and Stoodley, 2011) which were originally isolated from strains of Streptomyces peucetius, show antibacterial activity (mostly against Gram positive bacteria, for example Staphylococcus aureus) and have been in clinical use for the treatment of various forms of cancer for several decades. Triazolopyrazole thiones also exhibit antibacterial, antifungal and promising anticancer activities; the latter compared with the anthracycline doxorubicin.
2 Experimental
2.1 General
Melting points were determined in open capillaries on a Mel-Temp apparatus and are uncorrected. The purity of the compounds was checked by TLC (silica gel H, BDH, ethyl acetate/hexane, 1:3). The IR spectra were recorded on a Thermo Nicolet IR 200 FT-IR spectrometer as KBr pellets and the wavenumbers were given in cm−1. The 1H NMR spectra were recorded in DMSO-d6 on a Bruker spectrospin operating at 400 MHz. The 13C NMR spectra were recorded in DMSO-d6 on Bruker spectrospin operating at 100 MHz. All chemical shifts are reported in δ (ppm) using TMS as an internal standard. The microanalyses were performed on a Perkin-Elmer 240C elemental analyzer. For anticancer activity the optical density was determined at 450 nm using a microplate reader (BioTek Instruments Inc., Winooski, VT, USA).
2.2 Synthesis of bis(carbethoxymethylsulfonyl)amine (2)
To a solution of ethyl sulfamylacetate (1) (0.003 mol) in dichloromethane (10 ml), triethylamine (0.0031 mol), and 4-dimethylaminopyridine (DMAP) (0.0001 mol) were added and stirred at room temperature for 15 min. Then, a solution of ethyl 2-chlorosulfonylacetate (0.0033 mol) in dichloromethane (5 ml) was added dropwise and the reaction mixture was stirred at 40 °C under nitrogen atmosphere for 6–10 h. After completion of reaction the solvent was removed in vacuo. The resultant residue was neutralized with saturated NaHCO3 solution and the aqueous layer was extracted with ethyl acetate (3 × 15 ml), washed with water (3 × 20 ml), and dried over anhydrous Na2SO4. The solvent was removed under vacuum and the residual solid was purified by column chromatography (silica gel, 60–120 mesh) using hexane-ethyl acetate (3:1) as eluent.
Yield 72%, m.p. 132–134 °C. IR (KBr): t = 3370 (NH), 1730 (C⚌O), 1320, 1136 (SO2) cm−1. 1H NMR (400 MHz, DMSO-d6, 25 °C, TMS): δ = 1.36 (t, 6H, CH3), 4.20 (q, 4H, OCH2), 4.42 (s, 4H, CH2), 8.01 (bs, 1H, NH) ppm; 13C NMR (100 MHz, DMSO-d6, 25 °C, TMS) δ = 14.2 (CH3), 55.8 (CH2), 61.4 (OCH2), 161.5 (C⚌O) ppm. Anal. For C8H15NO8S2 (317.35) cacld. C30.27, H4.76, N 4.41. Found C30.32, H4.74, N4.47.
2.3 Synthesis of bis(carboxymethylsulfonyl)amine (3)
A mixture of bis(carbethoxymethylsulfonyl)amine (2) (0.0025 mol), KOH (0.01 mol) in methanol (10 ml) and water (25 ml) was refluxed for 2–3 h. To this charcoal was added, boiled for 5 min. and filtered through celite. The filtrate was acidified with dil. HCl and extracted with ether. Removal of the solvent on a rotary evaporator resulted in compound 3.
Yield 80%, m.p. 145–147 °C. IR (KBr): t = 3372 (NH), 3355 (OH), 1716 (C⚌O), 1323, 1141 (SO2) cm−1. 1H NMR (400 MHz, DMSO-d6, 25 °C, TMS): δ = 4.54 (s, 4H, CH2), 7.96 (bs, 1H, NH), 10.43 (bs, 2H, OH) ppm; 13C NMR (100 MHz, DMSO-d6, 25 °C, TMS) δ = 57.6 (CH2), 174.2 (C⚌O) ppm. Anal. calcd For C4H7NO8S2 (261.24) calcd. C18.39, H2.70, N5.36. Found: C18.46, H2.71, N5.44.
2.4 General procedure for the synthesis of bis(N-(4-aryloxazol-2-yl)aminocarbonyl–methylsulfonyl)amine (7a–c)/bis(N-(4-arylthiazol-2-yl)aminocarbonyl–methylsulfonyl)amine (8a–c)/bis(N-(4-aryl-1H-imidazol-2-yl)aminocarbonyl–methylsulfonyl)amine (9a–c)
To a solution of compound 3 (0.001 mol) in dioxane (20 ml), compound 4/5/6 (0.002 mol) and 4-dimethylaminopyridine (DMAP) (0.0001 mol) were added. Then dicyclohexylcarbodiimide (DCC) (0.0014 mol) in dioxane (10 ml) was added dropwise to the contents while stirring at room temperature and continued the stirring for another 20–24 h. The separated precipitate, a dicyclohexylurea was removed by filtration. The solution was evaporated to dryness and the residual solid was purified by column chromatography (silica gel, 60–120 mesh) using hexane-ethyl acetate (3:1) as eluent.
2.4.1 Bis(N-(4-phenyloxazol-2-yl)aminocarbonylmethylsulfonyl)amine (7a)
Yield 65%, m.p. 186–187 °C. IR (KBr): t = 3344 (NH), 1690 (C⚌O), 1634 (C⚌C), 1574 (C⚌N), 1326, 1138 (SO2) cm−1. 1H NMR (400 MHz, DMSO-d6, 25 °C, TMS) δ = 4.52 (s, 4H, CH2), 7.44–7.88 (m, 12H, Ar-H and C5-H), 8.03 (bs, 1H, SO2NH), 8.46 (bs, 2H, CO-NH) ppm; 13C NMR (100 MHz, DMSO-d6, 25 °C, TMS) δ = 56.2 (CH2), 128.9, 129.1, 130.0, 132.6 (Ar-C), 138.2 (C-5), 140.3 (C-4), 149.7 (C-2), 167.2 (C⚌O) ppm. Anal calcd For C22H19N5O8S2 (545.55) calcd. C48.43, H3.51, N12.83. Found: C48.39, H3.54, N12.93.
2.4.2 Bis(N-(4-p-tolyloxazol-2-yl)aminocarbonylmethylsulfonyl)amine (7b)
Yield 67%, m.p. 172–174 °C. IR (KBr): t = 3338 (NH), 1683 (C⚌O), 1630 (C⚌C), 1580 (C⚌N), 1324, 1135 (SO2) cm−1. 1H NMR (400 MHz, DMSO-d6, 25 °C, TMS) δ = 2.40 (s, 6H, Ar-CH3), 4.48 (s, 4H, CH2), 7.15-7.48 (m, 10H, Ar-H and C5-H), 8.05 (bs, 1H, SO2NH), 8.43 (bs, 2H, CO-NH) ppm; 13C NMR (100 MHz, DMSO-d6, 25 °C, TMS) δ = 24.8 (Ar-CH3), 56.0 (CH2), 127.3, 129.4, 131.0, 137.2 (Ar-C), 137.8 (C-5), 139.5 (C-4), 148.4 (C-2), 167.2 (C⚌O) ppm. Anal. For C24H23N5O8S2 (573.60) calcd. C50.25, H, 4.04, N12.20. Found: C50.30, H4.03, N12.29.
2.4.3 Bis(N-(4-p-chlorophenyloxazol-2-yl)aminocarbonylmethylsulfonyl)amine (7c)
Yield 70%, mp 201–202 °C. IR (KBr): t = 3350 (NH), 1694 (C⚌O), 1627 (C⚌C), 1568 (C⚌N), 1332, 1145 (SO2) cm−1. 1H NMR (400 MHz, DMSO-d6, 25 °C, TMS) δ = 4.45 (s, 4H, CH2), 7.37–7.66 (m, 10H, Ar-H and C5-H), 7.98 (bs, 1H, SO2NH), 8.48 (bs, 2H, CO-NH) ppm; 13C NMR (100 MHz, DMSO-d6, 25 °C, TMS) δ = 56.5 (CH2), 127.5, 129.1, 132.4, 135.8 (Ar-C), 138.5 (C-5), 140.8 (C-4), 150.2 (C-2), 167.9 (C⚌O) ppm. Anal. For C22H17Cl2N5O8S2 (614.44) calcd. C43.00, H2.78, N11.40. Found: C43.07, H2.80, N11.48.
2.4.4 Bis(N-(4-phenylthiazol-2-yl)aminocarbonylmethylsulfonyl)amine (8a)
Yield 72%, m.p. 190–192 °C. IR (KBr): t = 3355 (NH), 1685 (C⚌O), 1632 (C⚌C), 1570 (C⚌N), 1322, 1140 (SO2) cm−1. 1H NMR (400 MHz, DMSO-d6, 25 °C, TMS) δ = 4.45 (s, 4H, CH2), 7.20–7.56 (m, 12H, Ar-H and C5-H), 7.94 (bs, 1H, SO2NH), 8.42 (bs, 2H, CO-NH) ppm; 13C NMR (100 MHz, DMSO-d6, 25 °C, TMS) δ = 55.9 (CH2), 103.7 (C-5), 126.7, 127.6, 128.8, 132.0 (Ar-C), 148.0 (C-4), 162.8 (C-2), 167.4 (C⚌O) ppm. Anal. For C22H19N5O6S4 (577.68) calcd. C45.74, H3.31, N12.12. Found: C45.79, H3.30, N12.03.
2.4.5 Bis(N-(4-p-tolylthiazol-2-yl)aminocarbonylmethylsulfonyl)amine (8b)
Yield 69%, m.p. 183–185 °C. IR (KBr): t = 3348 (NH), 1673 (C⚌O), 1641 (C⚌C), 1565 (C⚌N), 1326, 1137 (SO2) cm−1. 1H NMR (400 MHz, DMSO-d6, 25 °C, TMS) δ = 2.38 (s, 6H, Ar-CH3), 4.43 (s, 4H, CH2), 7.10-7.43 (m, 10H, Ar-H and C5-H), 8.01 (bs, 1H, SO2NH), 8.39 (bs, 2H, CO-NH) ppm; 13C NMR (100 MHz, DMSO-d6, 25 °C, TMS) δ = 24.5 (Ar-CH3), 55.3 (CH2), 103.0 (C-5), 127.9, 129.5, 130.6, 136.8 (Ar-C), 147.7 (C-4), 162.1 (C-2), 166.6 (C⚌O) ppm. Anal. For C24H23N5O6S4 (605.73) calcd. C47.58, H3.82, N11.56. Found: C47.54, H3.85, N11.66.
2.4.6 Bis(N-(4-p-chlorophenylthiazol-2-yl)aminocarbonylmethylsulfonyl)amine (8c)
Yield 75%, m.p. 215–217 °C. IR (KBr): t = 3368 (NH), 1687 (C⚌O), 1631 (C⚌C), 1575 (C⚌N), 1328, 1143 (SO2) cm−1. 1H NMR (400 MHz, DMSO-d6, 25 °C, TMS) δ = 4.38 (s, 4H, CH2), 7.26-7.60 (m, 10H, Ar-H and C5-H), 8.02 (bs, 1H, SO2NH), 8.50 (bs, 2H, CO-NH) ppm; 13C NMR (100 MHz, DMSO-d6, 25 °C, TMS) δ = 56.2 (CH2), 104.2 (C-5), 127.8, 129.0, 131.4, 135.0 (Ar-C), 148.4 (C-4), 163.2 (C-2), 167.8 (C⚌O), Anal. For C22H17Cl2N5O6S4 (646.57) calcd. C40.86, H2.65, N10.83. Found: C40.90, H2.63, N10.90.
2.4.7 Bis(N-(4-phenyl-1H-imidazol-2-yl)aminocarbonylmethylsulfonyl)amine (9a)
Yield 77%, m.p. 224–226 °C. IR (KBr): t = 3372 (NH), 1665 (C⚌O), 1643 (C⚌C), 1560 (C⚌N), 1330, 1140 (SO2) cm−1. 1H NMR (400 MHz, DMSO-d6, 25 °C, TMS) δ = 4.40 (s, 4H, CH2), 7.15–7.50 (m, 12H, Ar-H and C5-H), 8.04 (bs, 1H, SO2NH), 8.38 (bs, 2H, CO-NH), 11.35 (bs, 2H, NH) ppm; 13C NMR (100 MHz, DMSO-d6, 25 °C, TMS) δ = 55.0 (CH2), 120.3 (C-5), 127.4, 128.2, 129.7, 132.0 (Ar-C), 137.5 (C-2), 140.0 (C-4), 166.4 (C⚌O) ppm. Anal. For C22H21N7O6S2 (543.59) calcd. C48.61, H3.89, N18.03. Found: C48.68, H3.90, N18.14.
2.4.8 Bis(N-(4-p-tolyl-1H-imidazol-2-yl)aminocarbonylmethylsulfonyl)amine (9b)
Yield 74%, m.p. 198–200 °C. IR (KBr): t = 3370 (NH), 1660 (C⚌O), 1638 (C⚌C), 1550 (C⚌N), 1325, 1130 (SO2) cm−1. 1H NMR (400 MHz, DMSO-d6, 25 °C, TMS) δ = 2.36 (s, 6H, Ar-CH3), 4.38 (s, 4H, CH2), 7.09–7.40 (m, 10H, Ar-H and C5-H), 7.96 (bs, 1H, SO2NH), 8.35 (bs, 2H, CO-NH), 11.28 (bs, 2H, NH) ppm; 13C NMR (100 MHz, DMSO-d6, 25 °C, TMS) δ = 24.7 (Ar-CH3), 54.7 (CH2), 120.1 (C-5), 127.7, 129.6, 130.8, 135.4 (Ar-C), 137.1 (C-2), 139.7 (C-4), 166.0 (C⚌O) ppm. Anal. For C24H25N7O6S2 (571.64) calcd. C50.42, H4.40, N17.15. Found: C50.49, H4.44, N17.24.
2.4.9 Bis(N-(4-p-chlorophenyl-1H-imidazol-2-yl)aminocarbonylmethylsulfonyl)amine (9c)
Yield 78%, m.p. 243–245 °C. IR (KBr): t = 3375 (NH), 1673 (C⚌O), 1640 (C⚌C), 1562 (C⚌N), 1335, 1144 (SO2). 1H NMR (400 MHz, DMSO-d6, 25 °C, TMS) δ = 4.42 (s, 4H, CH2), 7.20-7.55 (m, 10H, Ar-H and C5-H), 7.99 (bs, 1H, SO2NH), 8.40 (bs, 2H, CO-NH), 11.40 (bs, 2H, NH) ppm; 13C NMR (100 MHz, DMSO-d6, 25 °C, TMS) δ = 55.8 (CH2), 120.7 (C-5), 128.9, 130.1, 130.9, 135.5 (Ar-C), 137.9 (C-2), 140.4 (C-4), 166.8 (C⚌O) ppm. Anal. For C22H19Cl2N7O6S2 (612.48) calcd. C43.14, H3.12, N16.00. Found: C43.20, H3.11, N16.08.
2.5 Antimicrobial activity
The compounds 3–9 were evaluated for antimicrobial activity by the agar well diffusion method and broth dilution methods.
2.5.1 Microbial cultures
Bacterial strains S. aureus, Bacillus subtilis, Pseudomonas aeruginosa, Klebsiella pneumoniae and fungi Aspergillus niger and Penicillium chrysogenum were obtained from the Department of Microbiology, S.V University, Tirupati, India.
2.5.2 Antibacterial and antifungal assays
The in vitro antimicrobial studies were carried out by the agar well diffusion method against test organisms (Chung et al., 1990; Azoro, 2002). Nutrient broth (NB) plates were swabbed with 24 h old broth culture (100 μl) of test bacteria. Using the sterile cork borer, wells (6 mm) were made into each petriplate. The compounds were dissolved in DMSO of 5 mg/ml and from this 10 and 20 μL (50, 100 μg/well) were added into the wells by using sterile pipettes. The standard antibiotics, Chloramphenicol, for antibacterial activity and Ketoconazole, for antifungal activity (as positive control) were simultaneously tested against the pathogens. The samples were dissolved in DMSO which showed no zone of inhibition acts as a negative control. The plates were incubated at 37 °C for 24 h for bacteria and at 28 °C for 48 h for fungi. After appropriate incubation, the diameter of zone of inhibition of each well was measured. Duplicates were maintained and the average values were calculated for eventual antibacterial activity.
2.5.3 Minimum inhibitory concentration assay
Broth dilution test was used to determine Minimum Inhibitory Concentration (MIC) of the above mentioned samples (Janovska et al., 2003; Bishnu et al., 2009). Freshly prepared nutrient broth was used as diluents. The 24 h old culture of the test bacteria S. aureus, B. subtilis, P. aeruginosa, K. pneumoniae and the test fungi A. niger and P. chrysogenum were diluted 100 fold in nutrient broth (100 μl bacterial cultures in 10 ml NB). The stock solution of the synthesized compounds was prepared in dimethyl sulfoxide (DMSO) by dissolving 5 mg of the compound in 1 ml of DMSO. Increasing concentrations of the test samples (1.25, 2.5, 5, 10, 20, and 40 μl of stock solution contains 6.25, 12.5, 25, 50, 100, and 200 μg of the compounds) were added to the test tubes containing the bacterial and fungal cultures. All the tubes were incubated at 37 °C for 24 h for bacteria and at 28 °C for 48 h for fungi. The tubes were examined for visible turbidity and using NB as control. Control without test samples and with solvent was assayed simultaneously. The lowest concentration that inhibited visible growth of the tested organisms was recorded as MIC.
2.5.4 Minimum bactericidal/fungicidal concentration
To determine the minimum bactericidal concentration (MBC) (NCCLS publication M7-A3; Villanova, PA, 1993) and Minimum Fungicidal Concentration (MFC) (NCCLS Document M27-P; Villanova, PA, 1992) for each set of test tubes in the MIC determination, a loopful of broth was collected from those tubes which did not show any growth and inoculated on sterile nutrient broth (for bacteria) and PDA (for fungi) by streaking. Plates inoculated with bacteria and fungi were incubated at 37 °C for 24 h and at 28 °C for 48 h, respectively. After incubation, the lowest concentration was noted as MBC (for bacteria) or MFC (for fungi) at which no visible growth was observed.
2.6 Anticancer assays
2.6.1 Compounds
The compounds 7c, 8c, 9a, 9b, and 9c were screened for anticancer activity against NCI-H1299 (Human non-small lung cancer cells; ATCC, Manassas, VA, USA), HCT-166 p53 (Human colorectal adenocarcinoma; ATCC, Manassas, VA, USA), and PC-3 (Human prostate cancer cells; ATCC, Manassas, VA, USA) cells by EZ-cytox cell viability assay kit.
2.6.2 Cell cultures
NCI-H1299 (Human non-small lung cancer cells; ATCC, Manassas, VA, USA), and HCT-166 p53 (Human colorectal adenocarcinoma; ATCC, Manassas, VA, USA), cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) (Sigma-Aldrich, St. Louis, MO, USA) and PC-3 (Human prostate cancer cells; ATCC, Manassas, VA, USA) cells were cultured in Roswell Park Memorial Institute Medium-1640 (RPMI-1640) (Sigma–Aldrich, St. Louis, MO, USA) supplemented with 10% fetal bovine serum (FBS), penicillin 100 U/ml, streptomycin 100 μg/ml, N-(2-hydroxyethyl)-piperazine-N′-2-ethanesulfonic acid (HEPES) 8 mM, and l-glutamine 2 mM. Cells were maintained at 37 °C in a humidified 5% CO2 incubator.
2.6.3 Measurement of cancer cell viability
Cell viability and proliferation were determined with EZ-cytox cell viability assay kit (Daeil Labservice, Korea) based on the cleavage of the tetrazolium salt to water-soluble formazan by succinate-tetrazolium reductase system, which belongs to the respiratory chain of the mitochondria and is active only in the viable cells. Therefore the amount of formazan dye increased with an increase in cell viability (Kwon et al., 2010). Initially, the cells were seeded into 96-well culture plates at 1 × 104 cells/ml and NCI-H1299 and HCT-166 p53 cells were cultured in DMEM and PC-3 cells were cultured in RPMI-1640 media containing 10% FBS at 37 °C. When cells reached 70% confluence, the medium was replaced with DMEM or RPMI-1640 containing 10% FBS and each 100 μM of compounds for 24 h. EZ-cytox cell viability kit reagents were added to the medium, and the cells were incubated for 1 h. The index of cell viability was determined by measuring formazan production with a microplate reader at an absorbance of 450 nm. The % cell viability was calculated by the formula:
As the % cell viability decreases the % inhibition increases. The % inhibition was calculated by the formula:
More the value of % inhibition more potent the drug is. Cells in fresh medium without any test compound were used as the control.
2.6.4 Statistical analysis
Experimental results are expressed as mean ± S.E.M. One-way ANOVA followed by Dunnett’s test was used for multiple comparisons. P values of <0.01 represent statistically significant differences.
3 Results and discussion
3.1 Chemistry
The compound ethyl sulfamylacetate (1) was obtained by the reaction of ethyl 2-chlorosulfonylacetate with ammonia solution (Hinman and Locatell, 1959). The compound bis(carbethoxymethylsulfonyl)amine (2) was prepared from ethyl 2-chlorosulfonylacetate and 1 in the presence of catalytic amounts of 4-dimethylaminopyridine (DMAP) and triethylamine (Scheme 1). The 1H NMR spectrum of 2 displayed a triplet and a quartet at δ 1.36 and 4.20 for ethoxy protons, a singlet at 4.42 for methylene protons and a broad singlet at 8.01 ppm for NH. The compound 2 on hydrolysis gave bis(carboxymethylsulfonyl)amine (3). The absence of signals due to the ethoxy group in the 1H NMR spectrum of 3 indicated that hydrolysis occurred. Besides, a broad singlet was observed at δ 10.43 ppm due to hydroxy protons in addition to signals of the methylene and NH protons. The signals due to highly acidic protons in 2 and 3 disappeared on deuteration. The compounds 4-aryloxazol-2-amine (4) and 4-arylthiazol-2-amine (5) were prepared by adopting the literature precedent from phenacyl bromide and urea/thiourea (Potewar et al., 2008). The compound 4-aryl-1H-imidazole-2-amine (6) was obtained by the reaction of phenacyl bromide with acetyl guanidine followed by hydrolysis under acidic conditions (Little and Webber, 1994). The coupling reaction of 3 with 4 in the presence of DCC and DMAP resulted in bis(N-(4-aryloxazol-2-yl)aminocarbonylmethylsulfonyl)amine (7). Similarly, the compounds bis(N-(4-arylthiazol-2-yl)aminocarbonylmethylsulfonyl)amine (8) and bis(N-(4-aryl-1H-imidazol-2-yl)aminocarbonylmethylsulfonyl)amine (9) were synthesized by the reaction of 3 with 5 and 6 (Scheme 1). The 1H NMR spectra of 7a, 8a and 9a exhibited a singlet at δ 4.52, 4.45 and 4.40 for methylene protons, two broad singlets at δ 8.03, 7.94, 8.04 and 8.46, 8.42, and 8.38 for SO2NH and CONH, respectively. However, a singlet due to C5-H appeared in a more downfield region, merged with aromatic protons at 7.53, 7.50 and 7.45 ppm. The structures of all the compounds were further ascertained by IR, 13C NMR spectra and microanalyses.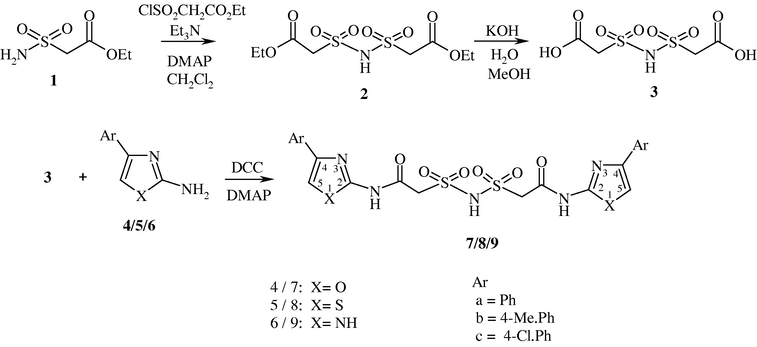
Synthesis of amido sulfonamido methane linked bis heterocycles.
3.2 In-vitro antimicrobial activity
The compounds 3–9 were tested for antimicrobial activity by agar well diffusion and broth dilution methods. The results of antibacterial activity shown in Table 1 revealed that Gram-negative bacteria were more susceptible toward the tested compounds than Gram-positive bacteria (Fig. 1). The compound 3 exhibited moderate antibacterial activity. Among 4, 5 and 6, compound 6c showed good antibacterial activity against Gram-negative bacteria. However, the compounds having amido-sulfonamido linkage 7–9 displayed greater activity when compared with the synthetic intermediates 3–6. The compound 9c exhibited pronounced activity particularly against B. subtilis and P. aeruginosa at 50 and 100 μg/mL when compared with the standard drug Chloramphenicol. The bis heterocycles having thiazole moiety (8) showed moderate activity against the tested bacteria. On the other hand, compounds with bisoxazole unit (7) exhibited least activity. The presence of the chloro substituent on the aromatic ring enhanced the activity. All the tested compounds inhibited the spore germination against tested fungi except the compounds 4 and 7. It was observed that the amido-sulfonamido linked bis-heterocycles 7–9 showed greater antifungal activity than the compounds 3–6. In fact, compound 9c displayed higher activity than the standard drug Ketoconazole against both fungi (Table 2). All the compounds displayed slightly higher antifungal activity toward P. chrysogenum than A. niger (Fig. 2). (–) No activity. (–) No activity.
Compound
Concentration (μg/Well)
Zone of inhibition (mm)
Gram-positive bacteria
Gram-negative bacteria
S. aureus
B. subtilis
P. aeruginosa
K. pneumoniae
3
50
18
20
17
16
100
21
22
21
19
4a
50
–
–
–
–
100
–
–
10
–
4b
50
–
–
–
–
100
–
–
–
–
4c
50
–
–
9
–
100
–
–
12
10
5a
50
8
–
9
9
100
10
9
12
–
5b
50
–
–
8
–
100
–
–
10
5c
50
10
8
12
10
100
13
11
16
12
6a
50
14
13
12
10
100
17
15
16
13
6b
50
9
8
9
8
100
11
12
11
10
6c
50
19
20
17
15
100
21
23
19
18
7a
50
–
–
12
10
100
–
–
15
12
7b
50
–
–
–
–
100
–
–
10
10
7c
50
10
12
14
13
100
13
14
15
15
8a
50
16
15
15
12
100
18
18
19
16
8b
50
13
12
14
13
100
15
15
17
16
8c
50
17
16
17
15
100
19
17
20
18
9a
50
22
23
20
20
100
24
28
24
22
9b
50
20
19
18
17
100
23
20
22
20
9c
50
25
34
28
36
100
30
37
32
38
Chloramphenicol
50
33
34
27
40
100
35
38
30
42
Control (DMSO)
–
–
–
–
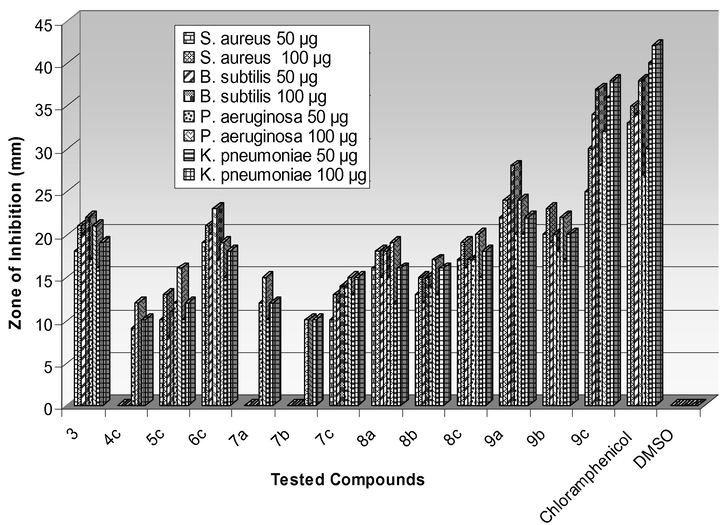
Antibacterial activity of 7–9.
Compound
Concentration (μg/Well)
Zone of inhibition (mm)
A. niger
P. chrysogenum
3
50
25
24
100
28
28
4a
50
–
–
100
–
–
4b
50
–
–
100
–
12
4c
50
10
13
100
13
15
5a
50
14
16
100
17
18
5b
50
12
13
100
16
16
5c
50
18
20
100
22
23
6a
50
22
23
100
25
27
6b
50
18
20
100
21
24
6c
50
25
27
100
29
31
7a
50
–
–
100
–
–
7b
50
–
–
100
–
–
7c
50
10
14
100
12
17
8a
50
20
23
100
24
26
8b
50
19
21
100
22
24
8c
50
24
24
100
26
28
9a
50
27
29
100
30
34
9b
50
22
24
100
25
26
9c
50
32
36
100
36
39
Ketoconazole
50
33
36
100
36
38
Control (DMSO)
–
–
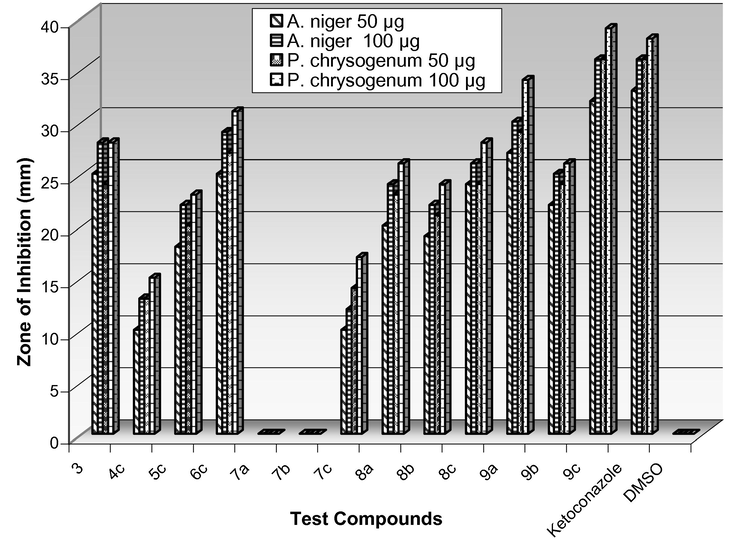
Antifungal activity of 7–9.
The MIC, MBC and MFC values of the compounds tested are listed in Table 3. The compound 9c exhibited low MIC values when compared with 9a. The MBC value of compound 9c is 2× MIC in case of B. subtilis and P. aeruginosa and MFC value is 2× MIC in case of A. niger and P. chrysogenum. However, 9a showed bactericidal and fungicidal effects greater than 2× MIC. The structure-antimicrobial activity relationship of the tested compounds indicated that the amido-sulfonamido methane linked bisimidazoles are more active than bisthiazoles and bisoxazoles. The chloro substituted bisimidazole showed strong antibacterial activity against B. subtilis and P. aeruginosa and also strong antifungal activity against A. niger and P. chrysogenum. S, Staphylococcus; B, Bacillus; P, Pseudomonas; K, Klebsiella; A, Aspergillus; P, Penicillium.
Compound
Minimum inhibitory concentration MIC (MBC/MFC) μg
S. aureus
B. subtilis
P. aeruginosa
K. pneumoniae
A. niger
P. chrysogenum
9a
50 (200)
25 (100)
25 (100)
100 (>200)
50 (200)
100 (>200)
9c
25 (100)
6.25 (12.5)
6.25 (12.5)
50 (200)
6.25 (12.5)
12.5 (25.0)
Chloramphenicol
6.25
6.25
6.25
12.5
–
–
Ketoconazole
–
–
–
–
6.25
12.5
3.3 In-vitro anticancer activity
The amido-sulfonamido methane linked bis heterocycles 7, 8 and 9 were screened against lung (NCI-H1299), colon (HCT-166 p53) and prostate (PC-3) cancer cell lines by EZ-cytox cell viability assay kit. To determine the anticancer activity of bis heterocycles 7–9, the cancer cells were treated at concentrations from 100 μM for 24 h and measured the cell viability using the EZ-cytox cell viability kit. As shown in Fig. 3, the evidence of the anticancer effect of compound 9c on NCI-H1299, HCT-166 p53 and PC-3 cancer cells can be seen. The inhibition percentage of compound 9c was 78.60 (NCI-H1299), 58.62 (HCT-166 p53) and 67.91 (PC-3) at 100 μM, respectively (Table 4 and Fig. 3). With compound 9c (0–100 μM) stimulation for 24 h, cancer cells also decreased in a dose-dependent manner (Table 5 and Fig. 4). This result suggests that the compound 9c pre-treatment was clearly shown to modulate the anticancer activity.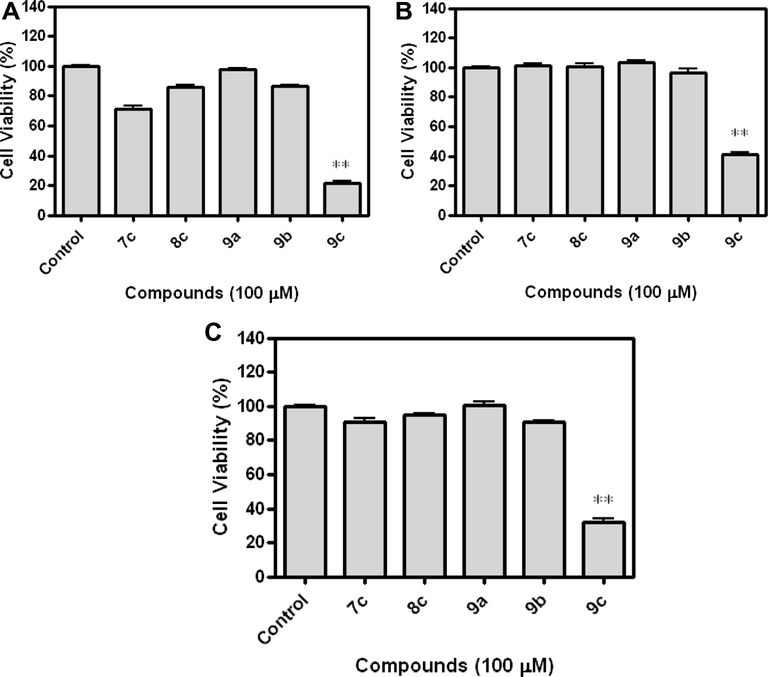
Effects of amido sulfonamido methane linked bis heterocycles on cancer cell lines. Cells were seeded in 96-well culture plates at 1 × 104 cells/ml NCI-H1299 and HCT-166 p53 cells were cultured in DMEM and PC-3 cells were cultured in RPMI-1640 media containing 10% FBS at 37 °C. When cells reached 70% confluence, the medium was replaced with DMEM or RPMI-1640 containing 10% FBS and 100 μM of amido sulfonamido methane linked bis heterocycles (7c, 8c, 9a, 9b and 9c) for 23 h. Values represent means ± S.E.M. from three different assays. (A) NCI-H1299, (B) HCT-166 p53, and (C) PC-3 cancer cell line. ∗∗p < 0.01 compared with control.
Compound
NCI-H1299
HCT-166 p53
PC-3
% Viability (% inhibition)
SD
% Viability (% inhibition)
SD
% Viability (% inhibition)
SD
Control
100.00 (0)
±1.76
100.00 (0)
±2.01
100.00 (0)
±1.34
7c
70.93 (29.07)
±4.08
101.50 (−1.5)
±2.43
90.68 (9.32)
±4.76
8c
85.68 (14.32)
±3.40
100.80 (−0.8)
±3.10
95.21 (4.79)
±1.39
9a
97.45 (2.55)
±2.67
102.52 (−2.52)
±2.07
100.53 (−0.53)
±4.29
9b
86.24 (13.76)
±2.47
96.69 (3.31)
±5.64
90.95 (9.05)
±1.15
9c
21.40 (78.60)
±2.25
41.38 (58.62)
±1.78
32.09 (67.91)
±4.23
Compound 9c (μM)
NCI-H1299
HCT-166 p53
PC-3
% Viability (% inhibition)
SD
% Viability (% inhibition)
SD
% Viability (% inhibition)
SD
0
100.00 (0)
±3.10
100.00 (0)
±3.10
100.00 (0)
±3.10
10
98.26 (1.74)
±3.08
102.67 (−2.67)
±8.11
54.90 (45.10)
±3.00
50
19.99 (80.01)
±8.33
89.12 (10.88)
±1.43
39.38 (60.62)
±1.28
100
19.28 (80.72)
±3.60
44.00 (56.00)
±0.34
36.61 (63.39)
±3.40
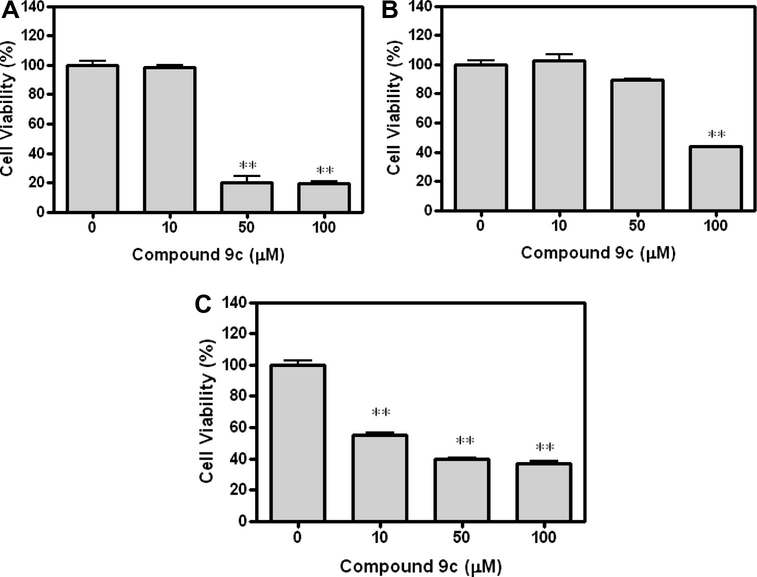
The effect of compound 9c on NCI-H1299, HCT-166 p53 and PC-3 cancer cell lines: precultured, treated with compound 9c (0–100 μM) for 23 h, EZ-cytox cell viability kit reagents were added to the medium and the cells were incubated for 1 h. The optical density was determined at 450 nm using a microplate reader. Values represent means ± S.E.M. from three different assays. (A) NCI-H1299, (B) HCT166 p53, and (C) PC-3 cancer cell line. ∗∗p < 0.01 compared with control.
4 Conclusion
-
A new class of amido-sulfonamido methane linked bis heterocycles-bisoxazoles (7), bisthiazoles (8) and bisimidazoles (9) were prepared from bis(carboxymethyl–sulfonyl)amine and amino-oxazoles (4), thiazoles (5) and imidazoles (6).
-
Bis heterocycles (7–9) were found more active than the respective mono heterocycles (4–6).
-
The compounds having oxazole unit 4 and 7 were found inactive against both bacteria and fungi.
-
Compounds having chloro substituent showed good antimicrobial activity.
-
The chloro substituted bisimidazole 9c exhibited excellent antibacterial activity against B. subtilis, P. aeruginosa and antifungal activity against A. niger, and P. chrysogenum.
-
The compound 9c was the most potent compound on cancer cells which may potentiate cancer therapy regimens now in the development of lung, colon and prostate cancer cell lines.
Acknowledgements
One of the authors V. Padmavathi is grateful to DST, New Delhi, for financial assistance under a major research project.
References
- J. Med. Chem.. 2006;49:7342-7356.
- World J. Biotechnol.. 2002;3:347-357.
- J. Sci. Eng. Technol.. 2009;5:143-150.
- Chemistry, Pharmacology and Clinical Applications. VCH, Florida: Plenum Press; 1986.
- J. Appl. Bacteriol.. 1990;69:498-503.
- J. Med. Chem.. 1989;32:1354-1359.
- J. Am. Chem. Soc.. 1959;5:5655-5658.
- J. Food Sci.. 2003;21:107-110.
- BMC Cancer. 2010;10:392.
- J. Org. Chem.. 1994;59:7299-7305.
- J. Med. Chem.. 2007;50:1707-1710.
- Tetrahedron. 1994;50:13049-13080.
- J. Saudi Chem. Soc.. 2011;15:275-281.
- NCCLS Document M27-P. PA: Villanova; 1992.
- National committee for clinical laboratory standards methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 3d edition; approved standard, NCCLS publication M7-A3; Villanova, PA, 1993.
- J. Nat. Prod.. 2003;66:247-250.
- J. Org. Chem.. 1998;63:92-98.
- Tetrahedron. 2008;64:5019-5022.
- J. Nat. Prod.. 2003;66:575-577.
- J. Nat. Prod.. 2003;66:764-771.
- J. Med. Chem.. 2007;50:1651-1657.
- Tetrahedron Lett.. 2001;42:4171-4174.
- Tetrahedron. 2005;61:241-249.
- Tetrahedron Lett.. 2005;46:2567-2570.







