Translate this page into:
Synthesis and application of 1,4-bis(triphenyl phosphonium)butane peroxodisulfate for conversion of alkyl benzenes to their corresponding acylbenzenes
*Corresponding author. Tel.: +98 6113348345 Mohammadi@iauahvaz.ac.ir (Mohammad Kazem Mohammadi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.

Available online 11 January 2011
Abstract
1,4-Bis(triphenyl phosphonium)butane peroxodisulfate (BTPPBPDS) was synthesized by treating 1,4-bis(triphenyl phosphonium)butane dibromide and potassium peroxydisulfate in aqueous solution. This was used as a mild and efficient reagent for the oxidation of alkyl benzenes to their corresponding acylbenzenes in very good to high yields.
Keywords
1,4-Bis(triphenyl phosphonium)butane peroxodisulfate (BTPPBPDS)
Alkyl benzenes
Potassium peroxydisulfate
Acylbenzenes
1 Introduction
The oxidation of organic substrates in aprotic solvents, under mild and neutral conditions, was important in modern organic synthesis. Therefore, the search for new oxidizing agents is of interest to synthetic organic chemists. Many such reagents have been developed in recent years with some success (Fieser et al., 1967–1984).
The oxidation of alkyl arenes and alcohols to their corresponding carbonyl compounds is of significant importance in organic chemistry, both for fundamental research and industrial manufacturing. The worldwide annual production of carbonyl compounds is over 1 × 107 and most of these are produced from the aerobic oxidation of alkyl arenes and alcohols (Zhan and Thompson, 2004; Ishii et al., 2001; Schultz and Sigman, 2006).
The oxidation of alkyl benzenes to the corresponding carbonyl compounds is one of the most important and frequently used synthetic procedures, and the development of such a procedure is still desirable in academic as well as in industrial research. The readily available peroxydisulfate ion,
, is an excellent and versatile oxidant for a variety of organic and inorganic compounds (Fieser et al., 1967–1984; House, 1962; Behrman et al., 1968; Wilmarth and Haim, 1962; Bacon et al., 1966). Peroxodisulfate radical anion
, is one of the most important and strong one-electron oxidants in organic synthesis (Mohammadpoor-Baltork et al., 1992, 1998; Hashemi and Ahmadi Beni, 1999; Varma and Meshran, 1997). Despite the relatively high oxidation potential for the half-reaction (Eq. (1)), many oxidations by peroxydisulfate do not proceed at a convenient rate at ambient temperatures (Didier and Philipe, 1996; Chen et al., 1996; Dave et al., 1986):
These reagents suffer from either one or more of the following disadvantages such as, availability of the reagent, cumbersome work-up procedure, high cost of the reagent, over oxidation or oxidation of other functional groups. As a result, there is still a need for the development of general, efficient, and new reagents to synthesize carbonyl compounds from the corresponding arenes under mild reaction conditions.
Herein, we have reported the synthesis and application of 1,4-bis(triphenyl phosphonium)butane peroxodisulfate (BTPPBPDS) as an efficient and selective oxidant in non aqueous medium that use of a mild, efficient, inexpensive, simple, convenient and selective method for the oxidation of alkylbenzenes to their corresponding carbonyl compounds with (BTPPBPDS) under neutral conditions and in the absence of metal catalysts.
2 Experimental
2.1 Materials and methods
Arenes, dialkyl halid, PPh3, K2S2O8 (Merck, P.A.) were used without further purification. Solvents were purified by standard methods. Infrared spectra were recorded as KBr disks on a Shimadzu model 420 spectrophotometer. The UV/Vis measurements were made on an Uvicon model 922 spectrometer. 1H and 13C (for product) were carried out on a Bruker AVANCE DRX 500 spectrometer at 500, 125 and 470.66 MHz. All the chemical shifts are quoted in ppm using the high-frequency positive convention; 1H and 13C NMR spectra were referenced to external SiMe4 and 19F NMR spectra to external CFCl3. The relative concentrations of carbon, hydrogen and nitrogen were obtained from the microanalytical laboratories, Department of Chemistry, OIRC, Tehran. Melting points were measured on an Electrothermal 9100 melting point apparatus.
2.2 Synthesis of 1,4-bis(triphenyl phosphonium)butane peroxodisulfate (BTPPBPDS)
1,4-Bis(triphenyl phosphonium)butane dibromide (3.4 g, 4 mmol) was mixed with H2O (60 ml), the mixture was completely stirred to obtain a clear solution. Then, chloroform (2–3 ml) was added and the organic and aqueous phases were separated. The aqueous phase was filtered. In other sections K2SO4 (1.081 g, 4 mmol) and H2O (20.79 ml) were added and completely stirred to obtain a clear solution. This solution was added to the aqueous phase (obtained in previous section) with stirring for 3 h to obtain a white powder, this powder was washed with H2O (6 × 10 ml) and then was dried in a desiccator. 1,4-Bis(triphenyl phosphonium)butane peroxodisulfate (BTPPBPDS) was obtained in 81% yield. m.p. (Scheme 1).
: IR (KBr) cm−1: 3050–3007 C–H (arom., strech), 2980–2900 C–H (aliph., strech), 1700–1450 (C⚌C), 1100 C–P (strech). 1H NMR (300 MHz, CDCl3) δ 7.21–7.68 (m, 15H), 2.04 (t, 4H), 1.5 (m, 4H). 13C NMR (300 MHz, CDCl3) δ 141, 132, 115, 28, 24.
2.3 General procedure for oxidation of alkyl benzenes with (BTPPBPDS)
To a solution of arenes (1 mmol) were dissolved in CH3CN/H2O (5:1, 10 ml) in a 50 ml round-bottomed flask equipped with a condenser and a magnetic stirrer. Oxidant (2 mmol) in small portions over (7–60) min was added under reflux condition. The progress of the reaction was monitored by TLC (eluent; CCl4/Et2O (6:1)). After completion of the reaction, the mixture was filtered and the solvent was evaporated. The resulted crude product was purified on a silica-gel (20 × 20 cm) plate with appropriate solvent (hexane/EtOAc, 4:1). Pure carbonyl compounds were obtained in 57–96% yields which characterized with compared to similar compounds (Shaabani et al., 2008; Rajabi et al., 2011) (Scheme 2, Table 1).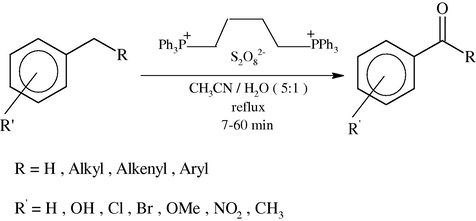
Entry
Substrate
Product
Time (min)
Yield (%)
1
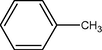
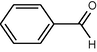
8
95
2
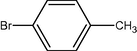
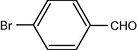
12
93
3
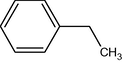
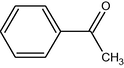
9
94
4
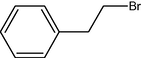
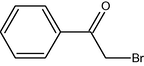
10
85
5

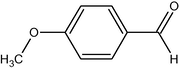
7
96
6
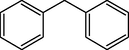
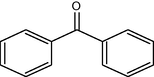
10
87
7
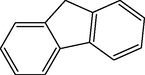
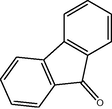
10
92
8
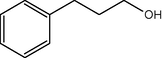
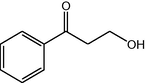
10
70
9
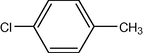
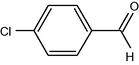
10
88
10
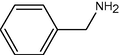
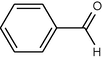
15
90
11
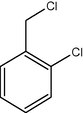
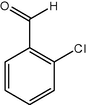
12
91
12
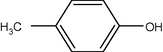
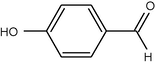
10
90
13
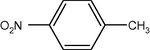
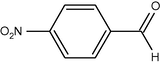
35
57
14
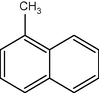
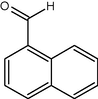
14
86
15
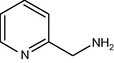
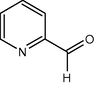
60
75
16
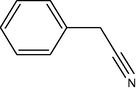
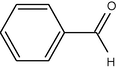
10
90
17
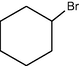
–
120
–
18
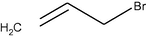
–
120
–
19
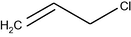
–
120
–
20
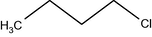
–
120
–
Data characteristic of compounds 4, 7, 8, and 14:
2.3.1 Entry 4: 2-bromo-1-phenylethanone (C8H7BrO)
IR (KBr) cm−1: 3045–3012 C–H (arom., strech), 2967–2935 C–H (aliph., strech), 1725 (C⚌O, strech), 1630–1460 (C⚌C), 1050 C–Br (strech). 1H NMR (300 MHz, CDCl3) δ 4.73 (s, 2H), 7.32–7.78 (m, 5H). 13C NMR (300 MHz, CDCl3) δ 40, 118, 129, 135, 195.
2.3.2 Entry 7: 9H-fluoren-9-one (C13H7BrO)
IR (KBr) cm−1: 3080–3025 C–H (arom., strech), 1684 (C⚌O, strech), 1642–1490 (C⚌C). 1H NMR (300 MHz, CDCl3) δ 7.27–7.86 (m, 8H). 13C NMR (300 MHz, CDCl3) δ 123, 130, 134, 137, 148, 201.
2.3.3 Entry 8: 3-hydroxy-1-phenylpropan-1-one (C9H10O2)
IR (KBr) cm−1: 3430 O–H (OH), 3063–3020 C–H (arom., strech), 1710 (C⚌O, strech), 1600–1470 (C⚌C), 800 C–C (strech). 1H NMR (300 MHz, CDCl3) δ 2.77 (t, 2H), 3.81 (t, 2H), 7.31–7.73 (m, 5H). 13C NMR (300 MHz, CDCl3) δ 43, 62, 125, 136, 139,196.
2.3.4 Entry 11: 2-chlorobenzaldehyde (C7H5ClO)
IR (KBr) cm−1: 3080–3000 C–H (arom., strech), 2855, 2770 C–H (aldeh.), 1660–1490 (C⚌C), 850 (C–Cl). 1H NMR (300 MHz, CDCl3) δ 7.38–7.89 (m, 3H), 9.81 (s, 1H). 13C NMR (300 MHz, CDCl3) δ 130, 133, 135, 139, 143, 188.
3 Results and discussion
BTPPBPDS was an easily prepared reagent, which was used recently for the oxidation of alcohols. The oxidative formation of carbonyl compounds with this reagent was investigated in CH3CN/H2O. As shown in Table 1, a series of appropriate arenes were reacted with 2 M equivalents of the reagent to afford the corresponding carbonyl group in excellent yields Use of a mild, efficient, inexpensive, simple, convenient and selective method for the oxidation of alkylbenzenes to their corresponding carbonyl compounds with (BTPPBPDS) under neutral conditions and in the absence of metal catalysts. This reagent was easily prepared in a two step reaction. Thus treatment of two moles of triphenylphosphine with 1 mol of 1,4-dibromo butane at 200–250 °C afforded, after filtration and purification and ion exchanges 1,4-bis(triphenyl phosphonium)butane peroxodisulfate in (57–96%) yield (Schemes 1 and 2).
The procedure developed for oxidation of alkylbenzenes consists of simply addition of BTPPBPDS in small portions to a refluxing solution of alkylbenzenes in aqueous acetonitrile. The results obtained are summarized in Table 1.
Because of the stability and solubility of BTPPBPDS, reactions could be performed at reflux condition and the separation of the products was facile. The mechanism for the present oxidation is still unclear. However we assumed that the mechanism of oxidation was similar to that of other similar oxidants. In addition, this oxidant and the oxidation conditions could be used for the synthesis of highly functionalized molecules.
It seems from Table 1, that functional groups such as, amino and nitrile (entry 15 and 16) were easily converted to carbonyl compounds. This was probably because the removal of these functional groups in the presence of peroxide reagent in N2 or other forms. In other compounds with OH, NO2 or other functional groups, no interaction between these groups and reagent was found.
It has been found also that this reagent has certain advantages over similar oxidizing agents in terms of amounts of oxidant and solvent required, and especially in the short reaction times required and in higher product yields (Schultz and Sigman, 2006; House, 1962; Behrman et al., 1968; Wilmarth and Haim, 1962; Bacon et al., 1966; Menghani and Bakore, 1968; Mohammadpoor-Baltork et al., 1992, 1998; Hashemi and Ahmadi Beni, 1999; Varma and Meshran, 1997).
Acknowledgment
We gratefully acknowledge the support of this work by Islamic Azad University, Science and Research Branch, Ahvaz, Iran.
References
- J. Chem. Soc.. 1966;1384:1388.
- Annual Reports on Mechanisms of Reactions of Sulfur Compounds. Vol vol. 2. Santa Monica, CA: Intra-Science Research Foundation; 1968. p. 235
- Synth. Commun. 1996:253.
- Synth. Commun.. 1986;16:1343.
- Tetrahedron Lett.. 1996;43:7725.
- Reagents for Organic Synthesis. Vol vols. 1–11. New York: Wiley; 1967–1984.
- Chem. Res. 1999:434.
- Chem. Rev.. 1962;62:185.
- Adv. Synth. Catal.. 2001;343:393.
- Bull. Chem. Soc. Jpn.. 1968;41:2547.
- Chem. Res. 1992:102.
- Bull. Chem. Soc. Jpn.. 1998;71:1649.
- Catal. Commun.. 2011;12:510.
- Tetrahedron. 2006;62:8227.
- Appl. Catal. A: Gen.. 2008;338:14.
- Tetrahedron Lett. 1997:5427.
- Edwards J.O., ed. Peroxide Reaction Mechanisms. New York, NY: John Wiley and Sons Inc.; 1962. p. 175 ff
- Tetrahedron. 2004;60:2917.







