Translate this page into:
Synthesis and biological evaluation of some 4-(6-substituted-1,3-benzothiazol-2-yl)amino-1,3-thiazole-2-amines and their Schiff bases
*Corresponding author. Tel.: +91 7104 236352; fax: +91 7104 235084 nikhilamnerkar@gmail.com (Nikhil D. Amnerkar),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 4 December 2014
Peer review under responsibility of King Saud University.

Abstract
With the aim of obtaining newer biologically active compounds, a series of 4-(6-substituted-1,3-benzothiazol-2-yl)amino-1,3-thiazole-2-amines (9–12) and 4-(6-substituted-1,3-benzothiazol-2-yl)amino-2-(4-substitutedphenyl-methylidene)amino-1,3-thiazole (13–28) were synthesized. The structures of all the synthesized compounds were confirmed by spectral (FTIR, 1H NMR and EI-MS) data and elemental (C, H, N) analysis. Furthermore, compounds (9–12) and (13–28) were screened for antibacterial, antifungal and anthelmintic activities. Almost all of these compounds showed moderate to excellent antimicrobial activity against two gram negative bacteria (Escherichia coli, Pseudomonas aeruginosa), two gram positive bacteria (Staphylococcus aureus, Bacillus subtilis), pathogenic fungal strains (Candida albicans, Aspergillus niger) and good anthelmintic activity against earthworm species (Pontoscotex corethruses). Among the compounds tested, compounds 23 and 24 showed maximum activity against Gram negative and Gram positive bacteria, respectively. Compound 22 exhibited good antifungal activity while compound 26 displayed maximum anthelmintic activity comparable to the standard drugs.
Keywords
Aminobenzothiazole
Thiazole
Schiff’s base
Antibacterial activity
Antifungal activity
Anthelmintic activity
1 Introduction
We are witnessing today a dramatic world-wide increase of serious infections by microbes. Infectious diseases are nowadays the second major cause of death worldwide and the third leading cause of death in developed countries (Rerambiah et al., 2014; Ryan-Payseur et al., 2011; Hoettecke et al., 2008). Physicians have revealed that patients with immunosuppressant are more prone toward such infections. Also, microorganism resistance to multiple antimicrobial agents has become a serious problem (Varshney et al., 2009; Aragade et al., 2009). Helminthiasis is a major medical problem and a large number of people suffered from this serious infection. Sometimes, it became more serious when multiple infections like helminth and microbe occur at the same time. Despite the availability of adequate drugs for these infections, there is a gradual rise in the number of patients suffering from these infectious diseases. This leads to increased morbidity and mortality with an overall increase in healthcare costs (Ananda Kumar et al., 2008). Due to such problems arises the need and creates considerable interest in medicinal chemists in the discovery and development of new lead structures and new chemical entities which will act both as antimicrobials and anthelmintic. Noteworthy, new and structurally unusual lead structures for the development of antimicrobial and anthelmintic agents are often found based on the screening of compounds having natural and marine origin (Sangshetti et al., 2009).
The benzothiazole scaffold and its analog are important analogs that are found in many marine compounds or natural plants (Zitouni et al., 2003; Gunwardana et al., 1988). In the past decades, 2-aminobenzothiazoles and its derivatives have received much attention due to their chemotherapeutic value in the discovery and development of newer agents effective against microbes (Bhusari et al., 2008), helminthes (Mahran et al., 2007), cancer (Caleta et al., 2004), convulsion (Amnerkar and Bhusari, 2010), inflammation (Khedekar et al., 2003), diuresis (Russo et al., 1994), etc. The Schiff base —N⚌CH— is well recognized as the pharmacophoric group in anthelmintic agents (Reddy and Mahendra, 2008; Lee et al., 1999; Mathew et al., 2010) and many clinically available antimicrobial drugs (such as nitrofurantoin, furazolidone, nifuroxazide, furoxone). Furthermore, different congeners of thiazole have also proved to exhibit good antimicrobial and anthelmintic potential (Mahler et al., 2006; Pawar et al., 2004; Coleman et al., 1994; Omar et al., 1981; Karali et al., 1998; Damico and Harman, 1955). Prompted from the chemotherapeutic importance of 2-aminobenzothiazole and thiazole derivatives and as a part of our ongoing research studies in the area of antimicrobial and anthelmintic agents (Bhusari et al., 2008; Amnerkar and Bhusari, 2011), it was thought of interest to combine group (—N⚌CH—) with these two vital moieties together into a single molecular framework. We have synthesized a series of 4-(6-substituted-1,3-benzothiazol-2-yl)amino-1,3-thiazole-2-amines 9–12 and 4-(6-substituted-1,3-benzothiazol-2-yl)amino-2-(4-substituted-phenylmethylidene)amino-1,3-thiazoles 13–28 and evaluated them for their potential as antibacterial, antifungal and anthelmintic agents.
2 Chemistry
The reaction sequence involved in the formation of 4-(6-substituted-1,3-benzothiazol-2-yl)amino-1,3-thiazole-2-amines 9–12 and 4-(6-substituted-1,3-benzothiazol-2-yl)amino-2-(4-substituted-phenylmethylidene)amino-1,3-thiazoles 13–28 is outlined in Scheme 1. The compounds, 6-substituted-1,3-benzothiazol-2-amines 1–4 were synthesized in an excellent yield following the methodology as described by Jimonet et al. (1999) from aryl amines. The reaction of compounds 1–4 with chloroacetylchloride afforded 2-chloro-N-(6-substituted-1,3-benzothiazol-2-yl)acetamides 5–8 (Papadopoulou et al., 2005) which on treatment with thiourea undergoes cyclization to yield 4-(6-substituted-1,3-benzothiazol-2-yl)amino-1,3-thiazole-2-amines 9–12. Compounds 9–12 were condensed with different aromatic aldehydes in absolute ethanol to give schiff base 4-(6-substituted-1,3-benzothiazol-2-yl)amino-2-(4-substitutedphenyl-methylidene)amino-1,3-thiazoles 13–28. All the reaction products were obtained in good yield. The structures of the newly synthesized compounds 9–28 were elucidated by elemental, FTIR, 1H NMR and EI-MS analyses.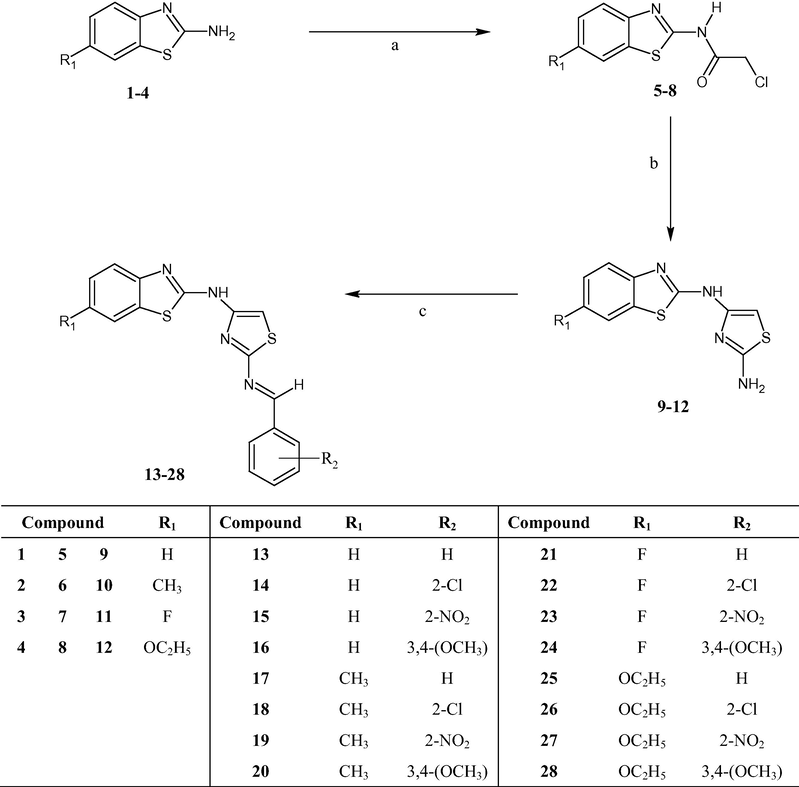
General Scheme I for the synthesis of compounds 9–28. Reagents and conditions: (a) dry benzene, ClCOCH2Cl, TEA, reflux, 3 h, 80 °C; (b) dry EtOH, H2NCSNH2, reflux, 12 h; (c) EtOH, Ar-CHO, reflux, 10–12 h.
3 Biological activity
Acute toxicity study was conducted using Organization for Economic Co-operation and Development (OECD) guidelines for nitro substituted compounds (15, 19, 23 and 27) (OECD, 2000). All the newly synthesized compounds 9–28 were evaluated for their in vitro antibacterial, antifungal and anthelmintic activities. The antibacterial activity was carried out against two Gram-negative bacteria (Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853) and two Gram-positive bacteria (Staphylococcus aureus ATCC 25923 and Bacillus subtilis ATCC 6633) by the test tube dilution technique using Mueller–Hinton nutrient broth (Jones et al., 1991). Ciprofloxacin and ampicillin were used as a reference standard. The antifungal activity was examined against Candida albicans (NCIM No. 3471) and Aspergillus niger (NCIM 1196) by the serial plate dilution method (Khan, 1997) using fluconazole as standard. The anthelmintic activity was carried out against earthworm Pontoscotex corethruses (ICARBC 408) species (Poojary and Belagali, 2005) at a concentration of 0.2% w/v. Mebendazole was used as a standard for comparison of anthelmintic results.
4 Results and discussion
In the present work, a series of 20 new aminobenzothiazole derivatives were synthesized and evaluated for antibacterial, antifungal and anthelmintic potential. The compounds 9–12 and 13–28 were synthesized in good yield (58–79%) as illustrated in Scheme 1 and their structures were characterized by spectral data. The IR spectra of the compounds 13–28 showed, in each case, stretching band of N—H, C⚌N and C—N groups in the regions of 3348–3274 cm−1, 1562–1522 cm−1 and 1144–1088 cm−1, respectively. The 1H NMR spectra showed, in each case, the signals as multiplet at δ 7.58–8.28 ppm attributed to Ar-H in addition to the singlet of the N—H group in the region 10.92–13.02 ppm. The singlet appeared for C-5 of the thiazole ring in the regions 5.24–6.21 ppm integrating for one proton. The singlet also appeared at δ 8.52–9.20 ppm attributed to one proton of N⚌CH. Thus, it confirmed the formation of Schiff bases. EI-MS of all compounds displayed the [M+H]+ confirming their molecular weight. The elemental (CHN) analyses were found within the limit of theoretical values.
The results of antibacterial activity are expressed as MIC (μg/mL) and summarized in Table 1. Compared to standard drugs, compounds 9–12 exhibited insignificant antibacterial activity against both Gram-negative and Gram-positive bacteria. Compound 23 (MIC of 3 μg/mL) with the electron withdrawing (nitro) group on 2-position of the phenyl ring showed excellent activity against Gram-negative (E. coli and P. aeruginosa) bacteria. While, compound 24 (MIC of 4–8 μg/mL) with the electron donating (methoxy) group exhibited very good antibacterial activity against Gram-positive (S. aureus and B. subtilis) bacteria. Investigation of the structure activity relationship study revealed that compounds (21–24) with electron withdrawing (fluoro) substitution on 6-position of the benzothiazole ring favors antibacterial activity against both Gram-negative and Gram-positive bacteria, whereas compounds with no substitution (R1⚌H) (13–16) showed moderate antibacterial activity. The good activity may be due to the presence of pharmacologically active fluorine (highly electronegative) in the molecule. All other compounds (17–20 and 25–28) in the series were found to have comparatively less or poor activity against both bacterial strains.
Compound
Gram negative bacteria*
Gram positive bacteria*
Fungi*
E. coli
P. aeruginosa
S. aureus
B. subtilis
C. albicans
A. niger
9
54
66
47
59
92
>100
10
82
>100
57
86
74
88
11
36
44
38
46
22
52
12
>100
>100
64
88
57
79
13
18
19
32
28
56
48
14
09
15
27
24
23
29
15
06
11
18
14
34
31
16
22
27
16
12
41
37
17
27
31
42
39
27
20
18
14
21
35
32
11
28
19
12
18
29
23
14
37
20
34
36
24
21
22
47
21
05
07
13
05
09
10
22
04
05
11
04
02
04
23
03
03
10
08
03
05
24
08
10
08
04
05
09
25
44
33
69
59
21
27
26
38
36
56
39
06
08
27
29
24
38
24
12
11
28
51
58
33
22
17
24
Ciprofloxacin
03
04
06
02
−
−
Ampicillin
02
02
01
01
−
−
Fluconazole
−
−
−
−
01
02
From the antifungal activity data (Table 1), it was observed that compound 22 (MIC of 2–4 μg/mL) exhibited maximum activity among all tested compounds against both fungal strains. However, all other compounds in the series were found to have mild to moderate antifungal activity compared to reference standard. Compounds 21, 23, 24 and 26 showed less antifungal activity (compared to most active compound 22) while compounds 9–12 were found 50-fold less active than standard drug fluconazole. The compounds with no substitution (R1⚌H) showed very less activity against both the fungal strains. The investigation of structure activity relationship revealed that the compounds with electron withdrawing (fluoro) substituents at 6-position of the benzothiazole ring and even on the 2-position of the phenyl ring (chloro) encourage the antifungal activity.
The result of anthelmintic activity is depicted in Table 2 and presented as mean ± S.E.M. The majority of the compounds showed statistically significant anthelmintic activity. Compound 26 was found to possess more potent anthelmintic activity compared to standard drug mebendazole. Compound 25–28 containing the electron donating (ethoxy) group on 6-position of the benzothiazole ring displayed excellent anthelmintic activity. However, compounds 17–24 and 13–16 showed moderate to mild activity. On the other hand, compounds 13–28 showed good potency due to the presence of (—N⚌CH—) group with phenyl substitution as a characteristic feature as compared to compounds 9–12. Among the series, it is significant to note that compounds 14, 18, 22 and 26 having electron withdrawing (chloro) substituent on the phenyl ring showed better anthelmintic activity, whereas substitutions with the electron donating (3,4-dimethoxy) group as seen in compounds 16, 20, 24 and 28 exhibited least activity.
Compound
Mean paralyzing time (min)a
Mean death time (min)a
9
85.46 ± 0.35
97.51 ± 0.36
10
82.84 ± 0.28
89.20 ± 0.10
11
81.58 ± 0.41
87.38 ± 0.14
12
78.26 ± 0.08
84.58 ± 0.44
13
56.55 ± 0.36
68.17 ± 0.35
14
53.87 ± 0.58
64.32 ± 0.11
15
60.14 ± 0.59
71.27 ± 0.14
16
63.92 ± 0.63
73.73 ± 0.43
17
29.84 ± 0.35
49.98 ± 0.60
18
29.79 ± 1.45
47.13 ± 0.54
19
35.24 ± 0.53
52.00 ± 0.72
20
35.55 ± 0.28
54.89 ± 0.29
21
44.54 ± 0.29
59.38 ± 0.13
22
42.67 ± 0.32
58.73 ± 0.43
23
46.90 ± 0.41
60.36 ± 0.54
24
52.98 ± 0.41
63.05 ± 0.43
25
21.16 ± 0.22
38.23 ± 0.55
26
14.48 ± 0.89
28.94 ± 0.61
27
21.95 ± 0.44
37.57 ± 0.74
28
34.51 ± 0.88
44.52 ± 0.32
Mebendazole
19.84 ± 1.24
31.39 ± 0.34
The statistical analyses were carried out using one way ANOVA (Dunnet’s test) at a 95% confidence interval and all the activity data reach statistical significance with p < 0.05.
5 Conclusion
Herein, we have described an efficient and convenient synthesis of a new series of 4-(6-substituted-1,3-benzothiazol-2-yl)amino-1,3-thiazole-2-amine 9–12 and 4-(6-substituted-1,3-benzothiazol-2-yl)amino-2-(4-substitutedphenylmethylidene)amino-1,3-thiazole 13–28. The structures of these new heterocyclic compounds containing both aminobenzothiazole and thiazole ring systems were confirmed by spectral (IR, 1H NMR, Mass) and elemental (C, H, N) analysis and were evaluated for their antibacterial, antifungal and anthelmintic activities. The results showed that several of the synthesized aminobenzothiazole derivatives exhibited significant antibacterial, antifungal and anthelmintic activities. The compounds with the electron withdrawing (fluoro) group on 6-position of the benzothiazole ring supports the antibacterial and antifungal activities while anthelmintic activity is favored by the electron donating (ethoxy, methyl) group. The substitutions with the electron withdrawing (nitro) group on the phenyl ring favors antibacterial activity against Gram-negative bacterial strain while the electron donating (methoxy) group supports for Gram-positive bacterial strain. Compounds with electron withdrawing substituents (fluoro, chloro) on both aminobenzothiazole and phenyl ring encourage the antifungal activity. The results of anthelmintic studies revealed that substitutions with the electron withdrawing (chloro) group on the phenyl ring exhibited potential activity. Thus, the significant antibacterial, antifungal and anthelmintic profiles of some 2-aminobenzothiazole derivatives offer them as promising lead molecules for further optimization using molecular modeling techniques.
6 Experimental protocols
6.1 Instrumentation and chemicals
All the chemicals and solvents employed in the synthesis were supplied by Merck (Germany), Fluka (Germany) and SD Fine chemicals (India) and used without purification. Melting points were determined on a digital melting point apparatus Electrothermal 1A 9200 (U.K.) and are uncorrected. All the reactions were monitored by TLC performed on 2.0–6.0 cm aluminum sheets precoated with silica gel 60 (HF-254, E. Merck, India). The IR spectra were recorded on a Shimadzu FTIR 8400S spectrophotometer (Kyto, Japan) using KBr optics. 1H NMR spectra were recorded in CDCl3 on a Varian Mercury YH-300 MHz spectrophotometer (Palo Alto, CA, USA) and chemical shifts (δ) are given in ppm relative to TMS. Mass spectra were recorded at 70 eV on a Jeol D-300 spectrometer (Tokyo, Japan). Elemental analyses were carried out using FLASH EA 1112 CHN analyzer (Thermo Finnigan, Italy) and found within ± 0.4% of theoretical values.
6.2 Synthesis
6.2.1 General synthesis of 4-(6-substituted-1,3-benzothiazol-2-yl)amino-1,3-thiazole-2-amine (9–12)
A mixture of 2-chloro-N-(6-substituted-1,3-benzothizol-2-yl)acetamides (5–8), thiourea (0.01 mol), and anhydrous potassium carbonate (0.01 mol) in absolute ethanol (15 mL) was heated under reflux on water bath for 12 h. The excess of ethanol was removed by distillation and the residue was treated with 5% sodium carbonate solution to remove acid impurities, filtered, washed with water and dried. The crude product was crystallized from ethanol.
6.2.1.1 4-(1,3-Benzothiazol-2-yl)amino-1,3-thiazole-2-amine (9)
Light yellow crystals; yield: 79%; mp 191–193 °C; Rf 0.61 (ethyl acetate:ammonia, 9:0.01); IR (KBr, cm−1): 3344 (NH), 1544 (C⚌N), 1077 (C—N); 1H NMR (CDCl3, ppm): δ 4.45 (s, 2H, NH2), 5.24 (s, 1H, C-5 of thiazole), 7.72–8.02 (m, 4H, Ar-H), 12.42 (s, 1H, NH); EI-MS: m/z [M+H]+ 249; Anal. Calcd for C10H8N4S2: C, 48.37; H, 3.25; N, 22.56. Found: C, 48.22; H, 3.21; N, 22.49.
6.2.1.2 4-(6-Methyl-1,3-benzothiazol-2-yl)amino-1,3-thiazole-2-amine (10)
Yellow solid; yield: 66%; mp 203–205 °C; Rf 0.56 (ethyl acetate:ammonia, 9:0.01); IR (KBr, cm−1): 3298 (NH), 1564 (C⚌N), 1105 (C—N); 1H NMR (CDCl3, ppm): δ 2.31 (s, 3H, CH3), 4.72 (s, 2H, NH2), 5.53 (s, 1H, C-5 of thiazole), 7.66–8.16 (m, 3H, Ar-H), 11.23 (s, 1H, NH); EI-MS: m/z [M+H]+ 263; Anal. Calcd for C11H10N4S2: C, 50.36; H, 3.84; N, 21.36. Found: C, 50.21; H, 3.76; N, 21.28.
6.2.1.3 4-(6-Fluoro-1,3-benzothiazol-2-yl)amino-1,3-thiazole-2-amine (11)
Brown solid; yield: 64%; mp 213–215 °C; Rf 0.48 (ethyl acetate:ammonia, 9:0.01); IR (KBr, cm−1): 3314 (NH), 1562 (C⚌N), 1117 (C—N); 1H NMR (CDCl3, ppm): δ 4.50 (s, 2H, NH2), 5.67 (s, 1H, C-5 of thiazole), 7.78–8.12 (m, 3H, Ar-H), 11.82 (s, 1H, NH); EI-MS: m/z [M+H]+ 267; Anal. Calcd for C10H7FN4S2: C, 45.10; H, 2.65; N, 21.04. Found: C, 45.21; H, 2.72; N, 21.09.
6.2.1.4 4-(6-Ethoxy-1,3-benzothiazol-2-yl)amino-1,3-thiazole-2-amine (12)
Dark grayish green; yield: 72%; mp 221–223 °C; Rf 0.58 (ethyl acetate:ammonia, 9:0.01); IR (KBr, cm−1): 3324 (NH), 1548 (C⚌N), 1106 (C—N); 1H NMR (CDCl3, ppm): δ 1.47 (t, 3H, OCH2—CH3), 4.1 (q, 2H, OCH2), 4.9 (s, 2H, NH2), 5.82 (s, 1H, C-5 of thiazole), 7.66–7.92 (m, 3H, Ar-H), 10.92 (s, 1H, NH); EI-MS: m/z [M+H]+ 293; Anal. Calcd for C12H12N4OS2: C, 49.29; H, 4.14; N, 19.16. Found: C, 49.37; H, 4.09; N, 19.12.
6.2.2 General synthesis of 4-(6-substituted-1,3-benzothiazol-2-yl)amino-2-(4-substitutedphenyl-methylidene)amino-1,3-thiazole (13–28)
To a solution of compound 4-(6-substituted-1,3-benzothiazol-2-yl)amino-1,3-thiazole-2-amine (9–12) (0.01 mol) in ethanol (60 mL), substituted aromatic aldehydes (0.01 mol) along with few drops of glacial acetic acid were added. The resulting mixture was refluxed for 10–12 h. The excess of the ethanol was distilled off and the remaining mixture was cooled, poured onto crushed ice and filtered. The crude product was crystallized from ethanol.
6.2.2.1 4-(1,3-Benzothiazol-2-yl)amino-2-(phenylmethylidene)amino-1,3-thiazole (13)
Orange brown solid; yield: 63%; mp 168–170 °C; Rf 0.42 (ethyl acetate); IR (KBr, cm−1): 3295 (NH), 1562 (C⚌N), 1096 (C—N); 1H NMR (CDCl3, ppm): δ 5.92 (s, 1H, C-5 of thiazole), 7.72–8.14 (m, 9H, Ar-H), 8.52 (s, 1H, N⚌CH), 12.64 (s, 1H, NH); EI-MS: m/z [M+H]+ 337; Anal. Calcd for C17H12N4S2: C, 60.69; H, 3.60; N, 16.65. Found: C, 60.52; H, 3.58; N, 16.68.
6.2.2.2 4-(1,3-Benzothiazol-2-yl)amino-2-(2-chlorophenyl-methylidene)amino-1,3-thiazole (14)
Dark yellow solid; yield: 58%; mp 160–162 °C; Rf 0.49 (ethyl acetate); IR (KBr, cm−1): 3321 (NH), 1522 (C⚌N), 1092 (C—N), 720 (C—Cl); 1H NMR (CDCl3, ppm): δ 5.78 (s, 1H, C-5 of thiazole), 7.62–7.96 (m, 8H, Ar-H), 8.74 (s, 1H, N⚌CH), 11.92 (s, 1H, NH); EI-MS: m/z [M+H]+ 371; Anal. Calcd for C17H11ClN4S2: C, 55.05; H, 2.99; N, 15.11. Found: C, 55.14; H, 2.96; N, 15.09.
6.2.2.3 4-(1,3-Benzothiazol-2-yl)amino-2-(2-nitrophenyl-methylidene)amino-1,3-thiazole (15)
Dark yellow solid; yield: 63%; mp 178–180 °C; Rf 0.56 (ethyl acetate); IR (KBr, cm−1): 3276 (NH), 1547 (C⚌N), 1420 (O⚌N⚌O), 1133 (C—N); 1H NMR (CDCl3, ppm): δ 5.86 (s, 1H, C-5 of thiazole), 7.58–7.81 (m, 8H, Ar-H), 8.86 (s, 1H, N⚌CH), 12.37 (s, 1H, NH); EI-MS: m/z [M+H]+ 382; Anal. Calcd for C17H11N5O2S2: C, 53.53; H, 2.91; N, 18.36. Found: C, 53.56; H, 2.87; N, 18.31.
6.2.2.4 4-(1,3-Benzothiazol-2-yl)amino-2-(3,4-dimethoxyphenyl-methylidene)amino-1,3-thiazole (16)
Dark brown crystals; yield: 68%; mp 173–175 °C; Rf 0.73 (ethyl acetate); IR (KBr, cm−1): 3313 (NH), 1562 (C⚌N), 1094 (C—N); 1H NMR (CDCl3, ppm): δ 3.82 (s, 6H, 2xOCH3), 6.14 (s, 1H, C-5 of thiazole), 7.89–8.14 (m, 7H, Ar-H), 8.65 (s, 1H, N⚌CH), 12.46 (s, 1H, NH); EI-MS: m/z [M+H]+ 397; Anal. Calcd for C19H16N4O2S2: C, 57.56; H, 4.07; N, 14.13. Found: C, 57.51; H, 4.09; N, 14.17.
6.2.2.5 4-(6-Methyl-1,3-benzothiazol-2-yl)amino-2-(phenylmethylidene)amino-1,3-thiazole (17)
White crystals; yield: 72%; mp 198–200 °C; Rf 0.41 (ethyl acetate); IR (KBr, cm−1): 3342 (NH), 1545 (C⚌N), 1088 (C—N); 1H NMR (CDCl3, ppm): δ 2.45 (s, 3H, CH3), 5.75 (s, 1H, C-5 of thiazole), 7.58–7.84 (m, 8H, Ar-H), 8.55 (s, 1H, N⚌CH), 13.02 (s, 1H, NH); EI-MS: m/z [M+H]+ 351; Anal. Calcd for C18H14N4S2: C, 61.69; H, 4.03; N, 15.99. Found: C, 61.61; H, 4.05; N, 15.95.
6.2.2.6 4-(6-Methyl-1,3-benzothiazol-2-yl)amino-2-(2-chlorophenyl-methylidene)amino-1,3-thiazole (18)
Light yellow crystals; yield: 63%; mp 221–223 °C; Rf 0.39 (ethyl acetate); IR (KBr, cm−1): 3335 (NH), 1526 (C⚌N), 1112 (C—N), 690 (C-Cl); 1H NMR (CDCl3, ppm): δ 2.52 (s, 3H, CH3), 6.21 (s, 1H, C-5 of thiazole), 7.66–8.16 (m, 7H, Ar-H), 8.78 (s, 1H, N⚌CH), 12.24 (s, 1H, NH); EI-MS: m/z [M+H]+ 385; Anal. Calcd for C18H13ClN4S2: C, 56.17; H, 3.40; N, 14.56. Found: C, 56.24; H, 3.41; N, 14.52.
6.2.2.7 4-(6-Methyl-1,3-benzothiazol-2-yl)amino-2-(2-nitrophenyl-methylidene)amino-1,3-thiazole (19)
Dark yellow solid; yield: 58%; mp 204–206 °C; Rf 0.43 (ethyl acetate); IR (KBr, cm−1): 3295 (NH), 1543 (C⚌N), 1425 (O⚌N⚌O), 1123 (C—N); 1H NMR (CDCl3, ppm): δ 2.45 (s, 3H, CH3), 5.9 (s, 1H, C-5 of thiazole), 7.78–8.09 (m, 7H, Ar-H), 8.9 (s, 1H, N⚌CH), 11.97 (s, 1H, NH); EI-MS: m/z [M+H]+ 396; Anal. Calcd for C18H13N5O2S2: C, 54.67; H, 3.31; N, 17.71. Found: C, 54.59; H, 3.28; N, 17.73.
6.2.2.8 4-(6-Methyl-1,3-benzothiazol-2-yl)amino-2-(3,4-dimethoxyphenyl-methylidene)-amino-1,3-thiazole (20)
Light yellow crystals; yield: 69%; mp 212–214 °C; Rf 0.49 (ethyl acetate); IR (KBr, cm−1): 3321 (NH), 1556 (C⚌N), 1092 (C—N); 1H NMR (CDCl3, ppm): δ 2.61 (s, 3H, CH3), 3.9 (s, 6H, 2xOCH3), 6.2 (s, 1H, C-5 of thiazole), 7.64–8.12 (m, 6H, Ar-H), 8.75 (s, 1H, N⚌CH), 12.43 (s, 1H, NH); EI-MS: m/z [M+H]+ 411; Anal. Calcd for C20H18N4O2S2: C, 58.52; H, 4.42; N, 13.65. Found: C, 58.43; H, 4.39; N, 13.69.
6.2.2.9 4-(6-Fluoro-1,3-benzothiazol-2-yl)amino-2-(phenylmethylidene)amino-1,3-thiazole (21)
Light brown crystals; yield: 59%; mp 243–245 °C; Rf 0.61 (ethyl acetate); IR (KBr, cm−1): 3354 (NH), 1527 (C⚌N), 1131 (C—N), 560 (C−F); 1H NMR (CDCl3, ppm): δ 5.9 (s, 1H, C-5 of thiazole), 7.79–8.28 (m, 8H, Ar-H), 9.2 (s, 1H, N⚌CH), 12.86 (s, 1H, NH); EI-MS: m/z [M+H]+ 355; Anal. Calcd for C17H11FN4S2: C, 57.61; H, 3.13; N, 15.81. Found: C, 57.72; H, 3.17; N, 15.76.
6.2.2.10 4-(6-Fluoro-1,3-benzothiazol-2-yl)amino-2-(2-chlorophenyl-methylidene)amino-1,3-thiazole (22)
Orange yellow solid; yield: 71%; mp 224–226 °C; Rf 0.41 (ethyl acetate); IR (KBr, cm−1): 3286 (NH), 1547 (C⚌N), 1097 (C—N), 710 (C−Cl), 590 (C−F); 1H NMR (CDCl3, ppm): δ 5.85 (s, 1H, C-5 of thiazole), 7.61–8.06 (m, 7H, Ar-H), 8.75 (s, 1H, N⚌CH), 12.24 (s, 1H, NH); EI-MS: m/z [M+H]+ 389; Anal. Calcd for C17H10ClFN4S2: C, 52.51; H, 2.59; N, 14.41. Found: C, 52.51; H, 2.59; N, 14.41.
6.2.2.11 4-(6-Fluoro-1,3-benzothiazol-2-yl)amino-2-(2-nitrophenyl-methylidene)amino-1,3-thiazole (23)
Yellow–brown solid; yield: 63%; mp 250–252 °C; Rf 0.65 (ethyl acetate); IR (KBr, cm−1): 3305 (NH), 1556 (C⚌N), 1410 (O⚌N⚌O), 1124 (C—N), 610 (C−F); 1H NMR (CDCl3, ppm): δ 6.15 (s, 1H, C-5 of thiazole), 7.74–8.17 (m, 7H, Ar-H), 8.9 (s, 1H, N⚌CH), 11.96 (s, 1H, NH); EI-MS: m/z [M+H]+ 400; Anal. Calcd for C17H10FN5O2S2: C, 51.12; H, 2.52; N, 17.53. Found: C, 51.18; H, 2.57; N, 17.51.
6.2.2.12 4-(6-Fluoro-1,3-benzothiazol-2-yl)amino-2-(3,4-dimethoxyphenyl-methylidene)-amino-1,3-thiazole (24)
Yellow solid; yield: 71%; mp 217–219 °C; Rf 0.52 (ethyl acetate); IR (KBr, cm−1): 3323 (NH), 1546 (C⚌N), 1117 (C—N), 590 (C−F); 1H NMR (CDCl3, ppm): δ 3.85 (s, 6H, 2xOCH3), 5.95 (s, 1H, C-5 of thiazole), 7.60–8.09 (m, 6H, Ar-H), 8.7 (s, 1H, N⚌CH), 12.31 (s, 1H, NH); EI-MS: m/z [M+H]+ 415; Anal. Calcd for C19H15FN4O2S2: C, 55.06; H, 3.65; N, 13.52. Found: C, 55.18; H, 3.61; N, 13.54.
6.2.2.13 4-(6-Ethoxy-1,3-benzothiazol-2-yl)amino-2-(phenylmethylidene)amino-1,3-thiazole (25)
Brown solid; yield: 64%; mp 224–226 °C; Rf 0.65 (ethyl acetate); IR (KBr, cm−1): 3286 (NH), 1527 (C⚌N), 1131 (C—N); 1H NMR (CDCl3, ppm): δ 1.46 (t, J⚌8.85 Hz, 3H, OCH2—CH3), 4.32 (q, 2H, OCH2), 6.05 (s, 1H, C-5 of thiazole), 7.76–8.15 (m, 8H, Ar-H), 9.1 (s, 1H, N⚌CH), 12.09 (s, 1H, NH); EI-MS: m/z [M+H]+ 381; Anal. Calcd for C19H16N4OS2: C, 59.98; H, 4.24; N, 14.73. Found: C, 59.86; H, 4.21; N, 14.68.
6.2.2.14 4-(6-Ethoxy-1,3-benzothiazol-2-yl)amino-2-(2-chlorophenyl-methylidene)amino-1,3-thiazole (26)
Yellow solid; yield: 58%; mp 233–235 °C; Rf 0.41 (ethyl acetate); IR (KBr, cm−1): 3274 (NH), 1558 (C⚌N), 1108 (C—N), 710 (C—Cl); 1H NMR (CDCl3, ppm): δ 1.39 (t, J = 8.76 Hz, 3H, OCH2—CH3), 4.42 (q, 2H, OCH2), 5.95 (s, 1H, C-5 of thiazole), 7.69–7.93 (m, 7H, Ar-H), 8.85 (s, 1H, N⚌CH), 12.46 (s, 1H, NH); EI-MS: m/z [M+H]+ 415; Anal. Calcd for C19H15ClN4OS2: C, 55.00; H, 3.64; N, 13.50. Found: C, 55.12; H, 3.67; N, 13.46.
6.2.2.15 4-(6-Ethoxy-1,3-benzothiazol-2-yl)amino-2-(2-nitrophenyl-methylidene)amino-1,3-thiazole (27)
Yellow solid; yield: 67%; mp 228–230 °C; Rf 0.53 (ethyl acetate); IR (KBr, cm−1): 3319 (NH), 1545 (C⚌N), 1410 (O⚌N⚌O),1137 (C—N); 1H NMR (CDCl3, ppm): δ 1.52 (t, J = 8.11 Hz, 3H, OCH2—CH3), 4.19 (q, 2H, OCH2), 6.10 (s, 1H, C-5 of thiazole), 7.76–8.17 (m, 7H, Ar-H), 8.9 (s, 1H, N⚌CH), 12.77 (s, 1H, NH); EI-MS: m/z [M+H]+ 426; Anal. Calcd for C19H15N5O3S2: C, 53.63; H, 3.55; N, 16.46. Found: C, 53.69; H, 3.58; N, 16.41.
6.2.2.16 4-(6-Ethoxy-1,3-benzothiazol-2-yl)amino-2-(3,4-dimethoxyphenyl-methylidene)amino-1,3-thiazole (28)
White solid; yield: 61%; mp 231–233 °C; Rf 0.47 (ethyl acetate); IR (KBr, cm−1): 3294 (NH), 1537 (C⚌N), 1115 (C—N); 1H NMR (CDCl3, ppm): δ 1.41 (t, J = 8.57 Hz, 3H, OCH2—CH3), 3.9 (s, 6H, 2xOCH3), 4.28 (q, 2H, OCH2), 6.1 (s, 1H, C-5 of thiazole), 7.84–8.23 (m, 6H, Ar-H), 9.2 (s, 1H, N⚌CH), 12.32 (s, 1H, NH); EI-MS: m/z [M+H]+ 441; Anal. Calcd for C21H20N4O3S2: C, 57.25; H, 4.58; N, 12.72. Found: C, 57.32; H, 4.53; N, 12.78.
6.3 Acute toxicity studies
Albino mice of either sex were used for the study and randomly group housed three per cage. The suspensions of test compounds were prepared in methyl cellulose in water (0.5%) and administered orally at a dose level 2 g/kg. This is the limit test at one dose level. The mortality is unlikely at the highest starting dose level. The initial dose of 2 g/kg was administered orally. The food was withheld for further 6 h. The treated mice were observed for mortality once during 30 min, periodically during the first 24 h, with a special attention given during the first 4 h and daily thereafter for a total of 14 days. None of the test compounds at a limit one dose level for a dose 2 g/kg showed any mortality. No mortality, no body weight changes, no toxic signs were noticed during the 14 day period of observation. Thus, the cut off LD50 was >2 g/kg for each test compound when given orally.
6.4 Antibacterial activity
The stock solution (1000 μg/mL) of the test compounds was prepared by dissolving test compounds (10 mg) in dimethylsulfoxide (DMSO) (10 mL). The stock solution was sterilized by passing through a 0.2 mm polycarbonate sterile membrane (Nucleopore) filter. Further, serial dilutions of the test compounds were carried out and the following concentrations were used 1000, 500, 250, 125, 62, 32, 16, 8, 4 and 1 μg/mL. Test compounds at various concentrations were added to culture medium in a sterilized borosilicate test tube and different bacterial strains were inoculated at 106 bacilli/mL concentration. A control was also prepared for the plates in the same way using solvent DMSO. The tubes were incubated at 37 °C for 24 h and then examined for the presence or absence of growth of the test organisms. All experiments were performed in triplicate. The MIC values were obtained from the lowest concentration of the test compounds where the tubes remained clear (i.e. no turbidity), indicating that the bacterial growth was completely inhibited at this concentration. The MIC values were also determined for the well-known antibiotics (ciprofloxacin and ampicillin) to compare the antibacterial activity of our test compounds with the antibiotics, which are currently in therapy. The MIC values are expressed in μg/mL and summarized in Table 1.
6.5 Antifungal activity
Sabouraud Dextrose Agar (Merck) media were used for the cultivation of fungi. Normal saline was used to make a suspension of spore of fungal strain. A loopful of particular fungal strain was transferred to 3 mL saline to get a suspension of corresponding species. A solution of agar media (20 mL) was poured into each petri dish. Excess of suspension was decanted and the plates were dried. After drying, wells were made using an agar punch and test samples, reference standard and negative control (DMSO) were placed in labeled wells in each petri plate. The petri plates were incubated at 37 °C for 48 h. The MIC values were noted and the activity of each compound was compared with fluconazole as standard drug. The results of antifungal activity are given in MIC values as μg/mL and are illustrated in Table 1.
6.6 Anthelmintic activity
Suspensions of test compounds were prepared by triturating the newly synthesized compounds with 0.5% Tween 80 and distilled water. The resulting mixture was stirred for 30 min using mechanical stirrer and the suspensions were diluted to contain 0.2% w/v of test samples. A suspension of the standard drug, mebendazole, was prepared in a similar way to get final concentration of 0.2% w/v. Three sets of five earthworms of almost similar sizes were placed in Petri plates of 4 inch diameter containing 50 mL of suspension of the test sample and standard drug at room temperature. Another set of five earthworms was kept as control in 50 mL suspension of distilled water and 0.5% Tween 80. The compounds were evaluated by the time taken for complete paralysis and death of earthworms and their mean was calculated for triplicate sets. The time taken by the worm to become motionless was noted as paralyzing time. To ascertain the death motionless worms were placed in warm water at 50 °C, which stimulate and induce the movement in the worms, if alive. The results are shown in Table 2.
Acknowledgements
We are thankful to SAIL, School of Pharmaceutical Sciences, RGPV, Bhopal for mass spectra and CHN analysis. Also, great thanks to Punjab University, Chandigarh and N.C.L., Pune for providing NMR spectra.
References
- Eur. J. Med. Chem.. 2010;45(1):149-159.
- J. Enz. Inhib. Med. Chem.. 2011;26:22-28.
- J. Enz. Inhib. Med. Chem.. 2008;23:462-469.
- Arch. Pharm. Chem. Life Sci.. 2009;342:361-366.
- Asian J. Res. Chem.. 2008;1:53-57.
- II Farmaco. 2004;59:297-305.
- J. Antimicrob. Chemother.. 1994;35:704-708.
- J. Am. Chem. Soc.. 1955;77:476-481.
- J. Am. Chem. Soc.. 1988;110:4856-4858.
- Bioorg. Med. Chem.. 2008;16:10319-10325.
- J. Med. Chem.. 1999;42:2828-2843.
- Lennette E.H., Balows A., Hausler W.J., Shadomy H.J., eds. A Manual of Clinical Microbiology. Washington, DC, USA: American Society of Microbiology; 1991.
- Arzneim-Forsch./Drug Res.. 1998;48:758-761.
- Khan, Z.K., 1997. In: Proceeding International Workshop. UNIDO-CDRI, Lucknow, India.
- Arzneim-Forsch/Drug Res.. 2003;9:640-647.
- Bioorg. Med. Chem. Lett.. 1999;9:1727-1732.
- Bioorg. Med. Chem. Lett.. 2006;16:1309-1311.
- Molecules. 2007;12:622-633.
- Der Pharma Chem. 2010;2:337-343.
- OECD, Guidance Documents on Acute Oral Toxicity, Environmental Health and Safety Monograph Series on Testing and Assessment, 2000, 24.
- Eur. J. Med. Chem.. 1981;16:77-80.
- II Farmaco. 2005;60:969-973.
- Eur. J. Pharm. Sci.. 2004;21:115-118.
- Eur. J. Med. Chem.. 2005;40:407-412.
- Int. J. Infect. Dis.. 2014;29:48-53.
- Eur. J. Med. Chem.. 1994;29:569-578.
- J. Infect. Dis.. 2011;204(9):1450-1462.
- Bioorg. Med. Chem. Lett.. 2009;19:3564-3567.
- Bioorg. Med. Chem. Lett.. 2009;19:3573-3576.
- Eur. J. Med. Chem.. 2003;39:267-272.







