Translate this page into:
Specific interactions within micelle microenvironment in different charged dye/surfactant systems
⁎Corresponding author at: University Politehnica of Bucharest, Research Centre for Environmental Protection and Eco-friendly Technologies, Polizu 1, RO-011061 Bucharest, Romania. Tel./fax: +40 213154193. maria.mihaly@upb.ro (Maria Mihaly)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The interactions of two ionic dyes, Crystal Violet and Methyl Orange, with different charged surfactants and also with a nonionic surfactant were investigated using surface tension measurements and visible spectroscopy in pre-micellar and post-micellar regions. It was found that for the water dominant phase systems the dye was localized between the polar heads, at the exterior of the direct micelle shells for all the systems. For the oil dominant phase systems, in case of the same charged dye/surfactant couples, the dye was localized in the micelle shell between the hydrocarbon chain of the surfactant nearby the hydrophilic head groups while for nonionic surfactant and oppositely charged dye/surfactant, localization of dye was between the oxyethylenic head groups towards the interior of the micelle core. Mixed aggregates of the dye and surfactant (below the critical micellar concentration of cationic surfactant), dye-surfactant ion pair and surfactant-micelles were present. The values of equilibrium constants (for TX-114/MO and TX-114/CV systems were 0.97 and 0.98, respectively), partition coefficients between the micellar and bulk water phases and standard free energy (for the nonionic systems were −12.59 kJ/mol for MO and −10.97 kJ/mol for CV) were calculated for all the studied systems. The partition processes were exothermic and occurred spontaneously.
Keywords
Dye-surfactant interactions
Partition coefficient
Spectroscopy
Micellar systems
1 Introduction
Studies of dye-surfactant interactions provide useful information for industrial applications, chemical research and dye separation processes (Bilski et al., 1997; Purkait et al., 2004). Dye-surfactant associations depend principally on the chemical structure of the compounds (Kartal and Akbas, 2005; Tehrani Bagha et al., 2007). Hydrophobic interactions, electrostatic interactions, hydrogen bonds, π-stacking and Van der Waals forces are typical examples of the intermolecular forces that dominate the interactions of dye molecules with surfactant aggregates (Naeem et al., 2000; Dezhampanah and Firouzi, 2012; Karukstis et al., 2010). In pre-micellar region, monomers of surfactant interact with the dye molecules to form ion association complexes, while, in post-micellar region, the dye molecules are likely to be localized at micelle surface. These interactions are responsible for changes in the position and intensity of absorption band in electronic spectra (Ghosh et al., 2012; Haq et al., 2014).
Despite the occurrence of many investigations of dye-surfactant systems and the behaviour of surfactant and dye mixtures, the mechanism underlying dye-surfactant interactions in surface films and micelles is not fully understood yet (Ghosh, 2001; Chakraborty et al., 2011; Ghosh et al., 2011; Muntaha and Khan, 2014; Kert and Simoncic, 2008). These studies may be applied in: (i) solubilization (Goddard and Ananthapadmanabhan, 1998) and photographic industries (Simoncic and Kert, 2002) where the dye should be localized inside the direct micelles; and (ii) dyes sensitized cells (Guo et al., 2013), nonlinear optical materials (Rao et al., 2002; Cyprych et al., 2015) and photosensitizer (Prakash, 2002) when the chromophore is intended to be placed in the hydrophilic shell of the surfactant micelles. Moreover, these dye-surfactant complexes find wide applications in extraction of dyes from wastewater using water-in-oil systems (Fleancu et al., 2013).
In the present investigation, the interactions of three different types of surfactants with cationic and anionic dyes have been thoroughly studied, given their intrinsic environmental and economic importance for dye removal from wastewater. Anionic dye Methyl Orange (MO) and cationic dye Crystal Violet (CV), respectively, being largely present in the dyeing process and rinsing effluents of the textile industries were selected as being representative for this study (Shah et al., 2013; Rahimi et al., 2010; Alqaragully, 2014).
The surfactants were chosen from three classes: cationic, anionic and nonionic surfactants in order to get complete information on the dye-surfactant complex formation between opposite- or the same-charged dye and surfactant molecules.
The surfactant and dye-surfactant aggregations were investigated by Ostwald stalagmometric method, while the dye-surfactant interactions were monitored by UV–VIS spectroscopy. The equilibrium constant, surfactant/water partition coefficient and standard free energy change of dye transfer from bulk water to micellar phase were quantified as well.
Since the ternary water/surfactant/oil system is reported as a more efficient removal agent for different types of pollutants (Fleancu et al., 2013) and knowing that the dye microenvironment into the micelles is influenced by the oil addition (Mihaly et al., 2014), the influence of ethyl acetate (EtAc) on the preferential location of dye into the micelles was checked.
2 Experimental
2.1 Materials
Methyl Orange (MO), Crystal Violet (CV) dyes, nonionic surfactant, (1,1,3,3-tetramethylbutyl)phenyl-polyethylene glycol (Triton X-114, hereafter termed TX-114) and ethyl acetate (EtAc) were purchased from Sigma–Aldrich. Anionic surfactant sodiumdodecyl sulphate (SDS) was provided by Merck and cationic surfactant dodecylthrimethylammonium bromide (DTAB) was obtained from Alfa Aesar. All chemicals were used as received. Solutions were prepared in distilled water. The chemical structures of dyes and surfactants used in this work are presented in Fig. 1.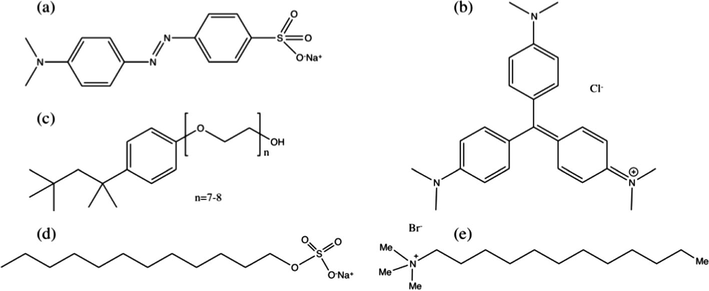
Chemical structure of (a) Methyl Orange (MO), (b) Crystal Violet (CV), (c) (1,1,3,3-tetramethylbutyl)phenyl-polyethylene glycol (TX-114), (d) sodiumdodecyl sulphate (SDS) and (e) dodecylthrimethylammonium bromide (DTAB).
2.2 Methods
Surface tension measurements were carried out at 298.15 K by Ostwald stalagmometric method (method of counting drops) (Mihaly et al., 2007), which is often used to measure the relative surface tension
, i.e. to compare the surface tension of the tested liquid σ to surface tension of the standard liquid σ0. The formula for the relative surface tension is as follows:
The critical micellar concentration (CMC) values were obtained from the surface tension dependence against the surfactant concentrations. Also, the binding equilibrium constants were calculated from the surface tension data in the pre-micellar region.
Absorbance measurements have been performed with UV–VIS spectrophotometer type V-670, Jasco. The micelle/water partition coefficients for dye-surfactant system were determined from the absorbance changes occurred as a result of the interaction between dye and surfactant molecules (Duman et al., 2012). The concentration of each dye was kept constant (CDye = 0.01 mM) and the concentration of surfactants was increasing during the experiments.
For surface tension analysis and spectroscopic study, different solutions have been used as presented in Table 1.
System
Concentration range, mM
Dye/Water
0.008–0.164
DTAB
2–60
SDS
1–40
TX-114
0.032–3.00
Dye/Surfactant/Water
DTAB
8; 30
SDS
5; 12
TX-114
0.07; 0.35
Dye
0.005–0.500
The micellar systems investigated were DTAB/MO/Water, DTAB/CV/Water, SDS/MO/Water, SDS/CV/Water, TX-114/MO/Water and TX-114/CV/Water.
For the investigation of the charge effect on spectral behaviour of the dyes in Water/Surfactant/Oil media, EtAc/Dye/Water systems have been prepared and surfactant was added until a clear, transparent, single phase system has been obtained. Then using the titration method, the organic phase (EtAc) was added until two different clear, transparent phases (one containing water/surfactant/oil and the other with excess oil phase) and the electronic spectra for every system were recorded. The same procedure was followed for the addition of water as well, but in this case a biphasic system was obtained, one having water/surfactant/oil and the other excess water. The corresponding compositions for every system are collected in Table 2, where ∗R is the volumetric ratio between water and EtAc.
Surfactant
MO
CV
∗R = VWater/VEtAc
CSf, % (w/w)
CEtAc, % (w/w)
CWater, % (w/w)
∗R = VWater/VEtAc
CSf, % (w/w)
CEtAc, % (w/w)
CWater, % (w/w)
DTAB
0.8
18.74
42.93
38.33
0.8
18.75
42.00
39.25
1.0
20.49
37.66
41.85
1.0
20.10
37.85
42.05
9.7
4.41
8.12
87.47
8.5
4.83
9.09
86.08
SDS
0.7
10.14
49.74
40.12
0.7
12.05
48.69
39.26
1.0
11.75
41.8
46.45
1.0
13.92
40.78
45.30
6.6
3.26
11.6
85.14
8.8
3.06
8.98
87.96
TX-114
0.4
16.24
58.53
25.23
0.2
15.37
68.87
15.76
1.0
32.18
32.13
35.69
1.0
33.93
31.30
34.77
2.6
15.70
21.96
62.34
13.7
6.27
5.78
87.95
3 Results and discussion
3.1 Tensioactive properties of surfactant in aqueous media in absence/presence of different dyes
The experimental data revealed that the surfactant aqueous systems exhibit the same value of CMC (in presence/absence of both dyes) (Table 3), which shows that the surface-active properties of the surfactants were not influenced by the addition of the dye in the system. This behaviour suggests that the dye-surfactant interactions do not affect the surface tension and the dyes are surface-inactive, in both molecular and associated form.
System
CMC (mM)
RSD (%)
System
CMC (mM)
RSD (%)
System
CMC (mM)
RSD (%)
DTAB/Water
14.43
0.78
SDS/Water
8.00
0.98
TX-114/Water
0.24
1.34
DTAB/CV/Water
14.48
0.86
SDS/CV/Water
8.11
0.92
TX-114/CV/Water
0.25
1.08
DTAB/MO/Water
14.49
0.54
SDS/MO/Water
8.14
1.01
TX-114/MO/Water
0.25
0.97
To establish whether the dyes are surface-inactive, in both molecular and associated form, the behaviour of different concentrations of MO and CV in aqueous surfactant media was investigated. The surface tension values for Surfactant/Dye/Water systems in pre-micellar and post-micellar regions are presented in Table 4. One may observe that in both the pre- and post-micellar regions the surface tension was not strongly dependent upon either the dye used or the dye concentration. The calculated relative standard deviations had values smaller than 5% which confirm that the dye concentration does not influence the tensioactive properties of the surfactant.
Dye
[Dye], mM
Pre-micellar region
Post-micellar region
Surface tension, dyne/cm
RSD %
Surface tension, dyne/cm
RSD %
DTAB/Dye/Water
MO
0.005
53.50
3.79
42.60
0.46
0.05
55.19
42.47
0.5
57.68
42.22
CV
0.005
55.20
3.49
42.47
0.61
0.05
57.68
43.00
0.5
59.16
42.73
SDS/Dye/Water
MO
0.005
53.70
0.23
39.48
0.16
0.05
53.91
39.59
0.5
53.91
39.59
CV
0.005
53.07
2.78
40.16
0.29
0.05
50.23
39.93
0.5
51.34
40.05
TX-114/Dye/Water
MO
0.005
68.71
2.8
35.08
2.02
0.05
69.39
34.87
0.5
65.80
33.77
CV
0.005
70.44
2.53
34.35
0.85
0.05
68.05
34.87
0.5
67.07
34.87
3.2 Investigation of the local microenvironment in micelles
3.2.1 Interactions in dye/cationic surfactant systems
Variation in the absorption maximum, λmax, for 0.01 mM MO dye as function of DTAB concentrations is presented in Fig. 2a. In the pre-micellar region of the surfactant, DTAB molecules flocculate in the presence of —SO3− anions of MO dye, creating an aggregate with pollutant-removing properties (Talens et al., 1998; Aboulhassan et al., 2006). Therefore, in the pre-micellar region, the electronic spectra could not be registered. However, in the post-micellar region a hypsochromic shift, from 463 nm to 433 nm, was observed. This blue shift can be assigned to the incorporation of the dye molecules into the shell of the DTAB direct micelles. Although these measurements provided no direct information about the structure of the surfactant-dye aggregates, the cause of the blue shift was assigned to the formation of an ion pair accompanied by surfactant-dye aggregation. The formation of dye-surfactant ion pair is a consequence of mutual influences of long range electrostatic forces and short range hydrophobic interactions (Reeves and Harkaway, 1977; Engberts et al., 1999; Engberts and Buwalda, 2001).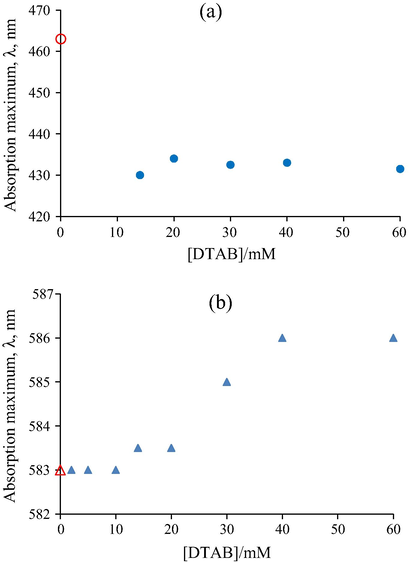
Variation of the absorption maxima of (a) Methyl Orange (0.01 mM) and (b) Crystal Violet (0.01 mM) as function of the DTAB concentration; symbols: (
 ) λmax of MO in aqueous solution and (
) λmax of MO in aqueous solution and (
 ) λmax of CV in aqueous solution.
) λmax of CV in aqueous solution.
The effects of different DTAB concentrations on the absorption spectrum of CV dye are shown in Fig. 2b where the maximum absorption peak of the dye in aqueous solution is centred initially at 583 nm. As the surfactant concentration increases, a slight bathochromic shift to 586 nm takes place due to the electrostatic repulsion and hydrophobic interaction of alkyl chain (dodecylthrimethyl ammonium ion) of DTAB and phenyl rings of the cationic dye, resulting in the solubilization of CV in surfactant micelles (Ghosh et al., 2012).
3.2.2 Interactions in dye/anionic surfactant systems
The effect of anionic surfactant SDS on the absorption spectra of CV and MO was also studied. One can notice that the CMC estimated from stalagmometric measurements is supported, in all cases, by the spectral behaviour of these surfactant-dyes aqueous systems.
The observed hypsochromic shift, in the case of anionic dye, MO, from 463 nm to 460 nm (Fig. 3a), may result from the interaction between aromatic rings present in the structure of the dye (π–π stacking) (Abbot et al., 2004) and formation of dye J-aggregates (head-to-tail).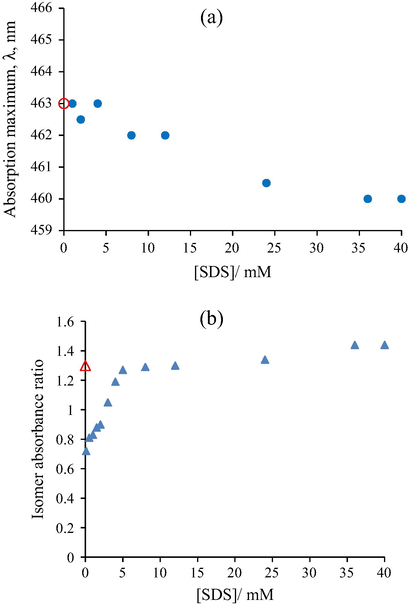
(a) Variation of the absorption maxima of Methyl Orange (0.01 mM) and (b) variation of the isomer absorbance ratio of Crystal Violet (0.01 mM) as function of the SDS concentration; Symbols: (
 ) λmax of MO in aqueous solution and (
) λmax of MO in aqueous solution and (
 ) isomer absorbance ratio of CV in aqueous solution.
) isomer absorbance ratio of CV in aqueous solution.
In the case of cationic triphenylmethane dye CV, in aqueous solution, there is a maximum absorption peak at 583 nm and a shoulder at 532 nm due to different isomers in equilibrium. The origin of the shoulder may be interpreted by assuming the existence of two fundamental isomers, one with planar structure (583 nm) for the ground state and the other one with a pyramidal structure (532 nm) in which three bonds of the central atom are bent (Ghosh et al., 2012). A maximum absorbance ratio between the planar and pyramidal structures was calculated. From Fig. 3b it can be seen that at small concentrations of surfactant (bellow 8 mM), the value of the isomers absorbance ratio is smaller than 1, which indicates that association complexes between the surfactant and the pyramidal isomer are formed. For higher surfactant concentrations (8–40 mM), the isomers absorbance ratio is higher than 1 and is similar to that in water (1.3). This situation contributes to the solubilization of dye molecules into the micelles. The reason for this behaviour may be explained by the electrostatic interactions of CV with SDS micelles. In the presence of the anionic surfactant, dye molecules do not act simply as counter ions, but are incorporated into the water-rich Stern layer of the micelle in a sandwich arrangement. This permits not only the hydration of hydrophilic (⚌N+ (CH3)2) group, but also the solubilization of the aromatic ring of the dye by the —SO3− group with participation of Van der Waals interactions between adjacent surfactant chains and the dye organic moiety (hydrophobic forces). In this situation, the microenvironment of the chromophore has changed and this behaviour is the main cause of the spectral change observed (Garcia and Sanzmedel, 1986).
3.2.3 Interactions in dye/nonionic surfactant systems
In the case of TX-114, the maximum absorption peak of MO dye shows a hypsochromic shift from 463 nm to 433 nm (Fig. 4a). For the CV dye in the pre-micellar region (bellow 0.3 mM) also a hypsochromic shift is obtained from 583 nm to 579 nm, and returns to the maximum wavelength in water in post-micellar region (Fig. 4b). These shifts in the absorption spectra of both dyes correspond to the changes of the microstructure around the chromophore molecules, which can be attributed to the interactions between the phenyl ring of the dyes and polyoxyethylene phenyl ether moiety of nonionic micelles. The non-polar part of the dye is localized in the hydrophobic core of the micelle, whereas the polar part resides in the head group region. A greater hypsochromic shift was observed in the case of the anionic dye, MO, due to the fact that the ether linkages in nonionic surfactants are weakly cationic (http://www4.mpbio.com/ecom/docs/proddata.nsf/%28webtds2%29/807426). Therefore, in this case, the anionic dye charge decides the microenvironment structure.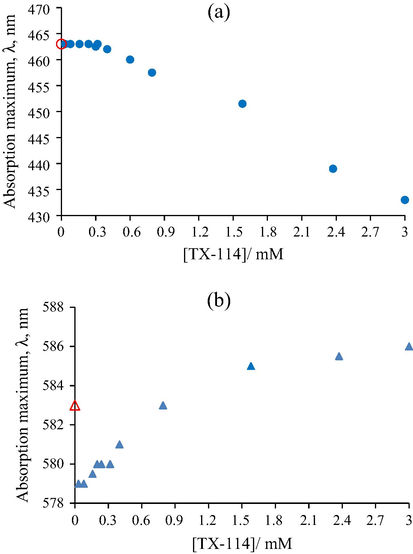
Variation of the absorption maximum of (a) Methyl Orange (0.01 mM) and (b) Crystal Violet (0.01 mM) as a function of the TX-114 concentration; symbols: (
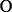 ) λmax of MO in aqueous solution and (
) λmax of MO in aqueous solution and (
 ) λmax of CV in aqueous solution.
) λmax of CV in aqueous solution.
3.2.4 Charge effect on spectral behaviour of the dyes in Water/Surfactant/Oil media
The preferential location of the dyes as well as the reasons for this preference in colloidal systems, among oil–water interface, bulk oil and bulk water, can be properly analysed by monitoring the spectral behaviour. The aim of this study was to deeper understand the possible interactions within micelle aggregation in various charged types of dye/surfactant couples in water/oil systems. By increasing the environment polarity from oil to water, two types of spectral changes are expected: one in band intensity (hyper/hypochromic effects) as a result of increasing/decreasing in the transition probability, and the other one in the position of maximum of absorbance band (hipso/bathochromic shifts) due to solvation of either fundamental or excited state. Fig. 5 illustrates the electronic spectra of colloidal systems of the anionic dye (MO) in ternary systems where oil phase is represented by ethyl acetate (EtAc) and the surfactants are cationic (DTAB – Fig. 5a), anionic (SDS – Fig. 5b) and nonionic (TX-114 – Fig. 5c). Each figure represents the evolution of spectral pattern while passing from major oil phase (R < 1) towards major aqueous phase (R > 1) by stepwise addition of water. One may notice that for all the three surfactants in couple with anionic dye, MO, a significant hyperchromic effect is observed, which can be interpreted by the confinement quantum effect produced at nanoscale while the dye is incorporated within the micelle.
Electronic spectra of Methyl Orange in (a) Water/DTAB/EtAc system, (b) Water/SDS/EtAc system and (c) Water/TX-114/EtAc system, where MO_AD and MO_ETAC are the electronic spectra of MO in distillate water and ethyl acetate, respectively.
As referring to the position of the wavelength of absorption maximum, a bathochromic shift is observed only in the case of the anionic surfactant (SDS). This phenomenon can be assigned to the dominance of the ionic form against molecular form of MO in polar environment, accompanied by decreasing of transition energy as a result of hydration of the excited state.
Similar spectra have been registered for the cationic dye (CV) presented in Fig. 6a–c. In this case no wavelength shift is evidenced for all three surfactants, while no significant change in intensity is observed for the cationic surfactant, hyperchromic effect for nonionic surfactant and hypochromic effect for anionic surfactant. This different behaviour of CV dye as compared to the MO dye may be explained by the more voluminous highly conjugated structure of the three phenylmethanic dye which will determine in couple with the anionic surfactant bigger size micelles than with the nonionic (partially aromatic) surfactant, whence hypochromic instead of hyperchromic effect appears in micellar confinement.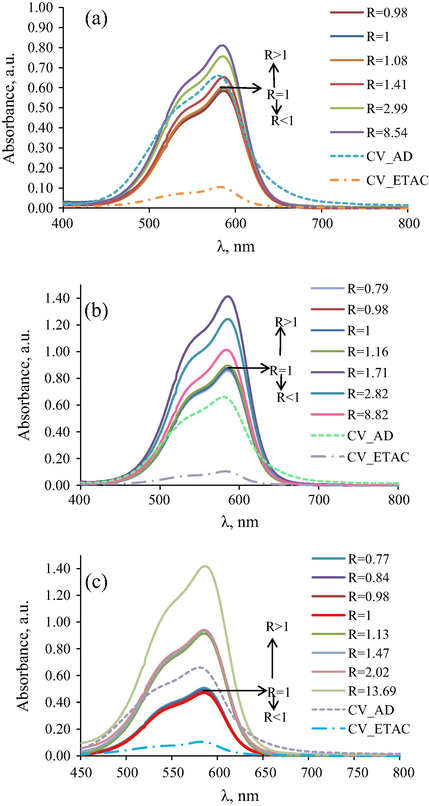
Electronic spectra of Crystal Violet in (a) Water/DTAB/EtAc system, (b) Water/SDS/EtAc system and (c) Water/TX-114/EtAc system, where CV_AD and CV_ETAC are the electronic spectra of CV in distillate water and ethyl acetate, respectively.
One may conclude that for spectral behaviour much more important is the molecular structure of the dyes and not their charge in couple with various surfactants, particularly the existence of diverse equilibriums between molecular and ionic forms, or between dye isomers.
3.2.5 Localization of the dye molecules in Water/Surfactant/EtAc systems
Based on the electronic spectra shown before, different variants for possible localization of the dye molecules in the ternary system are illustrated in Scheme 1.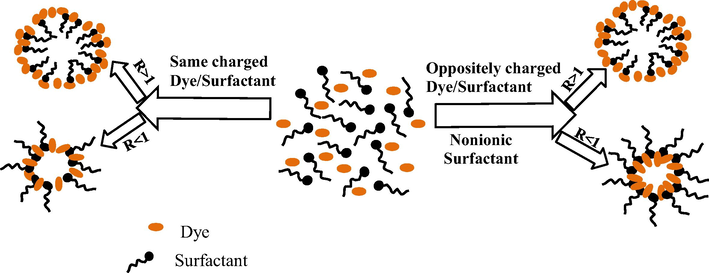
Representation of micelle structure in various ternary systems.
On the left side of Scheme 1 the same charged dye/surfactant couples are represented while on the right side both oppositely charged dye/surfactant and dye/nonionic surfactant are illustrated.
In water dominant phase systems (R > 1) direct micelles (O/W) are obtained (upper side) and in oil dominant phase systems (R < 1) reverse micelles (W/O) are formed (down side).
In case of the same charged dye/surfactant systems, for R < 1, the molecular form of dyes and surfactants are present, and the dye is localized in the micelle shell between the hydrocarbon chain of the surfactant nearby the hydrophilic head groups.
For nonionic surfactant and oppositely charged dye/surfactant, for R < 1, localization of dye is between the oxyethylenic head groups towards the interior of the micelle core.
For the water dominant phase systems (R > 1) the dye is localized between the polar heads but at the exterior of the direct micelle shells for all the systems does not matter the type of dye/surfactant couples.
3.3 Thermodynamic parameters
3.3.1 Determination of association equilibrium constant
Using the surface tension measurements and according to the methods previously described (Mihaly et al., 2007) the values of the equilibrium constant K were subsequently estimated. The changes of surface tension values measured in the pre-micellar region, both in the absence and presence of dyes, where there is not self-aggregation of surfactant or dye molecules association, are related to the binding of dyes to surfactant molecules.
The equilibrium reaction for the binding of the dye to the surfactant micelles can be written as
Based on stalagmometric measurements (Eq. (5)), the parameters n and K can be determined:
The ln γC was plotted as function of ln γSf/Dye/Water (Fig. 7) according to Eq. (6) using the surface tension values, in order to find the n and K values (Mihaly et al., 2007) from the slopes and intercepts of the plots and the corresponding results are given in Table 5.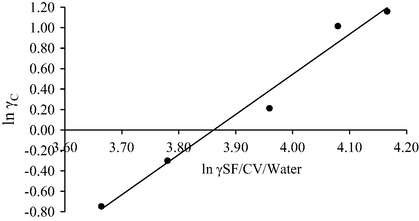
Plot of ln γC versus ln γSF/CV/Water for the interaction of CV with SDS.
Surfactant/Dye
K (×10)
n = mole Sf/mole Dye
Kc (×10 M−1)
Kx (×10−1)
(kJ/mol)
DTAB/MO
8.9
2
0.9
0.53
−4.15
DTAB/CV
8.5
3
–
–
–
SDS/MO
6.8
4
–
–
–
SDS/CV
8.1
7
111.6
61.98
−15.93
TX-114/MO
9.7
0.9
29.01
16.10
−12.59
TX-114/CV
9.8
0.6
15.12
8.39
−10.97
3.3.2 Determination of partition coefficient
Partition coefficient, Kx, is an important parameter to determine the partition of dye between the micellar and the bulk water phases. Kx is defined according to the pseudo-phase model as follows (Rohatgi-Mukerjee et al., 1985; Dutta and Bihat, 1996):
Replacing
and
in Eq. (8) by Eq. (10) and
and
in Eq. (9) by Eq. (11) can be deduced as follows:
The fraction f of the associated dye may be defined as
At a certain dye concentration, CDye, the fraction f is equal to zero in the non-micellar region up to CMC and increases with increasing the concentration of surfactant above CMC. When surfactant concentration, CSf, tends to infinity, f approaches unity since all added dye should be solubilized in micelles.
The fraction f can be directly calculated from the experimental data using Eq. (16):
By using Eqs. (15) and (16), (14) can be written in linear form as follows:
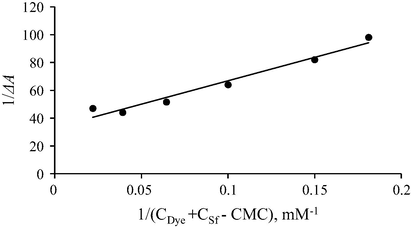
Plot of 1/ΔA versus 1/(CDye + CSf − CMC) for the interaction of MO with DTAB.
The Kc and Kx calculated values for the partition of the studied dyes between the micellar and the bulk water phases are shown in Table 5. These data are comparable with the literature values reported previously for some cationic dye molecules bound to non-ionic surfactant micelles and for the interactions of some anionic dye with cationic and anionic surfactants (Sarkar and Poddar, 1999; Göktürk and Talaman, 2008; Duman et al., 2012)
From Eq. (18), the standard free energy change of the transfer of dye from bulk water phase to micellar phase can be obtained as follows:
The values of are summarized in Table 5.
As seen from Table 5, the ΔGo values are negative which indicates that the partition process of both dyes between the micellar and the bulk water phases occurs spontaneously.
For the same charged dyes and surfactants the partition coefficient and the standard free energy could not be calculated. The equilibrium constant values for Nonionic surfactant/Dye systems are slightly higher than those for Ionic surfactant/Dye systems as mostly nonionic micelles are much stronger than ionic ones for incorporation of dye molecules into micelles in terms of binding tendency (Table 5). From Table 5 it can be also seen that in the case of MO, the partition coefficient is higher for non-ionic surfactant than for ionic one. In the case of CV interaction with SDS, Kx has higher values compared to non-ionic surfactant due to a combined effect provided by the complex association between the pyramidal isomer of the dye and surfactant and the electrostatic interactions between the planar isomer and the surfactant molecules.
4 Conclusions
Surface properties and spectroscopic characterization of the studied systems revealed formation of three species: mixed aggregates of the dye and surfactant (below the critical micellar concentration of cationic surfactant), dye-surfactant ion pair and surfactant micelles. The critical micellar concentration value of the surfactant was not influenced by the addition of Methyl Orange and Crystal Violet. The preferential location of the dye molecules among oil–water interface was determined by the spectral behaviour. For the water dominant phase systems (R > 1) the dye is localized between the polar heads but at the exterior of the direct micelle shells for all the systems. For the oil dominant phase systems (R < 1), in case of the same charged dye/surfactant couples, the dye is localized in the micelle shell between the hydrocarbon chain of the surfactant nearby the hydrophilic head groups while for nonionic surfactant and oppositely charged dye/surfactant, localization of dye is between the oxyethylenic head groups towards the interior of the micelle core.
The negative ΔGo values indicate that the partition process of Methyl Orange and Crystal Violet between the micellar and the bulk water phase occurs spontaneously and is exothermic. The equilibrium constant values for Nonionic surfactant/Dye systems are higher than those for Ionic surfactant/Dye systems as mostly nonionic micelles are much stronger than ionic ones for incorporation of dye molecules into micelles in terms of binding tendency. Therefore the adequate microenvironment for dyes extraction from wastewater is Nonionic Surfactant/Dye/Water system as the binding of the dye to the surfactant micelles is faster (ΔGo < 0) and easier (K ≈ 1).
Acknowledgements
The work was funded by the Sectorial Operational Programme Human Resources Development 2007-2013 of the Ministry of European Funds through the Financial Agreement POSDRU/159/1.5/S/134398.
Also we want to thank for the financial support provided by the Project CB-PhotoDeg, PN-II-PT-PCCA-2013-4-0747, contract no. 282/2014.
References
- J. Phys. Chem. B. 2004;108:13726-13735.
- Int. J. Environ. Sci. Technol.. 2006;3(4):327-332.
- Int. J. Adv. Res. Chem. Sci.. 2014;1:48-59.
- J. Photochem. Photobiol. A. 1997;110:67-74.
- Arab. J. Chem.. 2011;4:265.
- J. Phys. D: Appl. Phys.. 2015;48:1-8.
- Int. Res. Phys. Chem.. 2012;2:45-48.
- Dyes Pigm.. 2012;94:233-238.
- Colloid Surf. A. 1996;106:127-134.
- Langmuir. 1999;15:1083-1089.
- Langmuir. 2001;17:1054-1059.
- Fluid Phase Equilib.. 2013;337:18-25.
- Talanta. 1986;33:255-264.
- J. Colloid Interface Sci.. 2001;244:128.
- J. Phys. Chem. B. 2011;115:11098.
- Fluid Phase Equilib.. 2012;332:1-6.
- Goddard, E.D., Ananthapadmanabhan, K.P., 1998. Polymer-Surfactant Systems. In: Kwak, J.C.T. (Ed.), New York.
- J. Solution. Chem.. 2008;37:1709-1723.
- Electrochim. Acta. 2013;90:24-29.
- J. Mol. Liq.. 2014;196:264-269.
- Dyes Pigm.. 2005;65:191-195.
- Spectrochim. Acta A. 2010;75:1354-1361.
- Dyes Pigm.. 2008;79:59-68.
- Rev. Chim.. 2007;58:929-932.
- Comptes Rendus Chim.. 2014;17:342-351.
- J. Mol. Liq.. 2014;197:191-196.
- Monatsh. Chem.. 2000;131:761-767.
- J. Mater. Sci. Mater. Electron.. 2002;13:735.
- Sep. Purif. Technol.. 2004;37:81-92.
- Rahimi, R., Kerdari, H., Rabbania, M., 2010. 14th International Electronic Conference on Organic Synthetic Chemistry (ECSOC-14).
- Chem. Phys. Lett.. 2002;361:439.
- Micellization Solubilization and Microemulsions. New York: Plenum Press; 1977. ed
- J. Colloid Interface Sci.. 1985;106:45-50.
- Spectrochim. Acta A. 1999;55:1737-1742.
- OA Biotechnology 01. 2013;2(2):11.
- Dyes Pigm.. 2002;54:221-237.
- Langmuir. 1998;14(18):5046-5050.
- Dyes Pigm.. 2007;72:331-338. <http://www4.mpbio.com/ecom/docs/proddata.nsf/%28webtds2%29/807426> (accessed 18.05.15)
Appendix A
Supplementary material
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.arabjc.2015.09.009.
Appendix A
Supplementary material







