Translate this page into:
Ziziphora clinopodioides Lam leaf aqueous extract mediated novel green synthesis of iron nanoparticles and its anti-hemolytic anemia potential: A chemobiological study
⁎Corresponding author. tangjin20210326@sina.com (Jin Tang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
In this study, the aqueous extract Ziziphora clinopodioides was used to biosynthesis of iron nanoparticles. A green, productive, and environmentally method was developed for the valuable study and the effective preparation of the green-synthesis of iron nanoparticles using aqueous extracts from the leaf of Ziziphora clinopodioides as a result of reducing and stabilizing factor. The simplicity of the synthesis procedures and easy work up are the benefits of the present study. The structural and morphological characterization of green‐synthesized FeNPs was performed by Uv–Vis. and FT-IR spectroscopy, XRD, SEM, and EDX techniques. The SEM images have exhibited an equal and uniform spherical morphology in size of 30.04. We also investigated the anti-hemolytic anemia property of FeNPs in an animal model of hemolytic anemia. In vivo assay, induction of hemolytic anemia was done by phenylhydrazine in mice. FeNPs significantly reduced the weight and volume of liver and spleen and the concentration of pro-inflammatory cytokines and increased the body weight, the anti-inflammatory cytokines concentration, and the total platelet, WBC, neutrophil, lymphocyte, eosinophil, monocyte, and basophil counts, and RBC parameters as compared to the untreated mice. About the biochemical parameters, FeNPs significantly increased GPx, CAT, and SOD in serum, liver, and spleen, and also HDL, total protein, and albumin in serum, and decreased GR in serum, liver, and spleen, and also erythropoietin, ferritin, ferrous, creatinine, urea, LDL, triglyceride, cholesterol, GGT, ALT, AST, and ALP in serum as compared to the anemic mice. DPPH test revealed similar antioxidant potentials for FeNPs and Butylated hydroxytoluene. FeNPs had low cell viability dose-dependently against HUVEC cell line. It appears that FeNPs can be administrated as a hematoprotective and anti-hemolytic anemia drug or supplement for the treatment of hemolytic anemia in the clinical trial.
Keywords
Ziziphora clinopodioides Lam leaf
Iron nanoparticles
Hemolytic anemia
Mouse model of hemolytic anemia
1 Introduction
The field of synthetic nanotechnology has developed the self-sufficiency of materials with specific sizes and shapes at the nanometer scale environmentally friendly. Nanomaterials can be created from the engineering of multiple or single molecules which are engineered and have several functional groups. Biocompatible nanocarriers include peptides, engineered antibodies, micelles, polymers, and liposomes (Ahmed et al., 2016; Sintubin et al., 2009; Ball, 2018; Sharma et al., 2009; Nadagouda and Varma, 2006; Rajeshkumar, 2016). Nanomaterials can be designed to gain the desired chemical and physical characteristics and purposefully direct drugs to dynamic tumor environment with high therapeutic effects and low toxicity. Features to be controlled when designing nanoparticles are surface to volume ratio, shape, size, molecular charge, and drug release. For example, nanoparticles, after binding to functional chemical components such as antibodies and ligands, bind to the surface receptors of the abnormal cells and prevent its clearance by the blood (Nadagouda and Varma, 2006; Rajeshkumar, 2016; Raut et al., 2010; Leili Shaker Ardakani, 2021; Sivaraj et al., 2014). Drug delivery by nanoparticles using biodegradable polymers solves many problems. In 1975, a drug-conjugated model of the polymer was proposed by Ringdorf, which increased drug delivery to the abnormal cell site (Zangeneh, 2019). She suggested that the conjugated pharmacological properties of drugs and polymers could be manipulated by altering the chemical and physical properties of the polymer. For example, a water-insoluble drug can be soluble by adding solvent components to a polymer. Therefore, it increases both bioavailability and biodegradability. For cytotoxic drugs to be efficiently delivered to abnormal cell tissue, first, cytotoxic agents must be removed from the bloodstream and after leaving the extracellular space, the abnormal tissue must pass through the membrane surface and be targeted inside the abnomrla cells (Abdel-Fattah and Ali, 2018; Klein et al., 2014; Zangeneh et al., 2019201820192019). The ideal properties of nano foundations that should be considered when designing are: 1- Continuous drug release 2- Inactive accumulation of the drug in abnormal tissue 3- Targeting antigens or surface receptors of abnormal cells with a controlling effect on endosomal uptake and membrane destruction. 4- Drug release into the cytoplasm and protection from enzymatic degradation (Zangeneh, 2019; Abdel-Fattah and Ali, 2018; Klein et al., 2014).
Nanoparticles are generally effective in a wide variety of sectors that if their production is based on green chemistry, they have great applications in the fields of food, medicine, cosmetics and health. Nanoparticles centered on inorganic materials such as magnetic metals, their oxides and alloys, and semiconductors have the most studies and potential in biomedicine from diagnosis to treatment of diseases (Raut et al., 2010; Leili Shaker Ardakani, 2021; Sivaraj et al., 2014). The effects of nanoparticles should be predictable, controllable and get the desired results with minimal toxicity. Metallic nanoparticles used in treatment and diagnosis, in addition to being non-toxic, must be biocompatible and stable in vivo. Also, by making appropriate changes in the surface of metallic nanoparticles, they will have a wide range of applications by binding to biomolecules and various carriers to cross the cell membrane and target the desired part in the body. One of the important points in the production of nanoparticles is the use of cost-effective and efficient precursors (Nadagouda and Varma, 2006; Rajeshkumar, 2016; Raut et al., 2010; Leili Shaker Ardakani, 2021; Sivaraj et al., 2014). There are three biological, chemical, and physical methods to synthesize the nanoparticles. Chemical and physical methods are time-consuming and costly. In addition, these methods use some toxic additive chemicals that cause adverse effects on medical applications by adsorption on the surface. Applying the principles of green chemistry has decreased the use of toxic compounds or hazardous solvents, provided optimal regeneration conditions and ameliorated materials for the chemical processes, and raised new sources for green synthesis (Rajeshkumar, 2016; Raut et al., 2010; Leili Shaker Ardakani, 2021; Sivaraj et al., 2014; Zangeneh, 2019). Therefore, one of the primary goals of green nanotechnology is to produce nanomaterials without harm to human health or environment, and to develop and design nanomaterials and products that are suitable solutions to environmental problems. The synthesis of nanoparticles by similar biological methods results in greater catalytic activity and limits the use of toxic and expensive chemicals. In biological methods, plant extracts, enzymes or proteins carrying natural resources are used to produce or stabilize nanoparticles. The nature of the materials used to make nanoparticles influences the shape, structure and morphology of these nanoparticles (Nadagouda and Varma, 2006; Rajeshkumar, 2016; Raut et al., 2010; Leili Shaker Ardakani, 2021; Sivaraj et al., 2014). Biological systems involved in the green synthesis of nanoparticles, plants and their derivatives, as well as microorganisms such as algae, fungi, and bacteria. Plant parts such as roots, leaves, stems, fruits, and tiny parts such as the kernel and skin of the fruit are suitable to synthesize the nanoparticles because their extracts are rich in phytochemicals that act as stabilizing and reducing substances (Rajeshkumar, 2016; Leili Shaker Ardakani, 2021). The use of natural plant extracts is a cheap and environmentally friendly process and does not require intermediate groups. Short time, no need for expensive equipment, precursors, high purity product and excellent quality without impurities are the features of this method. This is possible very quickly, at room temperature and pressure as well as easily on a large scale (Nadagouda and Varma, 2006; Rajeshkumar, 2016; Raut et al., 2010; Leili Shaker Ardakani, 2021; Sivaraj et al., 2014; Zangeneh, 2019).
The last decade has seen the promising emergence of nanoparticles in cancer treatment systems such as drug delivery and recombinant proteins with anti-tumor properties. Special features of the microenvironment around the tumor allow nanoscale systems to accumulate at the tumor site (Leili Shaker Ardakani, 2021; Sivaraj et al., 2014; Zangeneh, 2019; Abdel-Fattah and Ali, 2018). Therefore, some nanoparticles with adjuvant properties, when they carry peptides or proteins, can increase the activity of cells in the reticuloendocardial system and activate macrophages and dendritic cells. Activated macrophages and dendritic cells swallow and process the complex, and immune responses are formed more efficiently (Sivaraj et al., 2014; Zangeneh, 2019; Abdel-Fattah and Ali, 2018; Klein et al., 2014; Zangeneh et al., 2019201820192019; Zangeneh et al., 20192019202120202019201920192020; Naghibi et al., 2005; Behravan et al., 2007; Sonboli et al., 2006; Salehi et al., 2005; Ghafari et al., 2006; Meral et al., 2002; Mahdavi et al., 2019; Belyaev and Demeubaeva, 1999; Wei et al., 2016; Hosseinimehr et al., 2011; Arulmozhi et al., 2013; Zangeneh et al., 2019; Harshiny et al., 2015; Katata-Seru et al., 2018; Sangami and Manu, 2017; Beheshtkhoo et al., 2018; Radini et al., 2018; Oganesvan et al., 1991; Tian et al., 2010; Ghidan et al., 2016; Patra and Baek, 2017; Kuang et al., 2013; Yew et al., 2016; Nurbas et al., 2017; Bishnoi et al., 2018; Devatha et al., 2016; Rao and Pennathur, 2017; Baghayeri et al., 2018; Seydi et al., 2019; Gautam et al., 2018; Zhang et al., 2017; Devatha et al., 2018; Wu et al., 2015; Huang et al., 2014; Zangeneh et al., 2019; Shukla et al., 2012; Zangeneh et al., 2017; Saravanan and Ponmurugan, 2013; Hoseinpouran et al., 2015; Ogunlade et al., 2012; Dkhi et al., 2015; Goodarzi et al., 2017; Goodarzi et al., 2018). These nanoparticles can increase the response of the immune system to the target antigen, as well as to direct and direct this system to create a specific type of response. By using these nanoparticles as antigen carriers, the amount of recombinant protein used as well as antigen toxicity is reduced and the destructive effects of proteases on protein antigen are reduced. This strategy enhances the efficiency of the target protein in inducing immune responses against the tumor, which is important in advancing functional goals such as protein and effective drug delivery (Leili Shaker Ardakani, 2021; Sivaraj et al., 2014; Zangeneh, 2019; Abdel-Fattah and Ali, 2018; Klein et al., 2014; Zangeneh et al., 2019201820192019).
About the hematoprotective and anti-hemolytic anemia properties of iron nanoparticles against hematotoxines, probably these effects are related with their antioxidant potentials. Studies have shown that antioxidant compounds destroy the free radicals of hematotoxic substances and prevent their toxic effects (Zangeneh et al., 2019201820192019). It seems that the synthesis of iron nanoparticles using medicinal plants as a suitable and safe method for yielding of iron nanoparticles, increase the antioxidant, hematoprotective, and anti-hemolytic anemia potentials of them (Zangeneh, 2019; Abdel-Fattah and Ali, 2018).
Plants, especially medicinal species, are the main elements of natural resources that knowledge of these species and related issues is one of the major needs of human societies. Topics related to these plants have been one of the most controversial and fascinating topics in medical science in recent decades (Zangeneh et al., 2019201820192019; Zangeneh et al., 20192019202120202019201920192020). Due to the many side effects of chemical drugs used in the treatment of diabetes, there is a need for drugs with minimal side effects and a high degree of confidence that can be used for a long time (Zangeneh et al., 20192019202120202019201920192020). Epidemiological observations show that with the change of diet culture and lifestyle from traditional to industrial, the prevalence of non-communicable diseases, especially diabetes, has increased (Zangeneh et al., 2019201820192019). However, due to the lack of complete cure of this disease with the use of various drugs, the tendency to use alternative therapies and traditional medicine is increasing, in which the role of medicinal plants cannot be ignored. Herbs and their derivatives that have long been used in the treatment of diabetes have fewer clinical side effects (Zangeneh et al., 2019201820192019; Zangeneh et al., 20192019202120202019201920192020). Ziziphora clinopodioides Lam is used in Iranian traditional medicine for the treatment of common cold, gastrointestinal and blood disorders, and inflammations (Naghibi et al., 2005; Behravan et al., 2007). Various properties such as antifungal (Sonboli et al., 2006), antibacterial (Behravan et al., 2007; Salehi et al., 2005), anti-inflammatory (Ghafari et al., 2006), and antioxidant (Salehi et al., 2005; Meral et al., 2002) have been revealed as the effects of this plant. It has chemical components including cineol, sis-isopulegone, thymol, terpenoides, α and β pinen, flavonoids, piperitenone, and pulegone (Behravan et al., 2007; Salehi et al., 2005; Mahdavi et al., 2019; Belyaev and Demeubaeva, 1999; Wei et al., 2016; Hosseinimehr et al., 2011; Arulmozhi et al., 2013; Zangeneh et al., 2019). Probably, the remedial potentials of this species are related to the above compounds.
Accordingly, the current study was conducted to assess the possible protective activity of synthesized iron nanoparticles using Z. clinopodioides against phenylhydrazine-induced blood impairments as a hemolytic compound in mice, which, following more extensive studies and ensuring the unwanted effects, can play a pivotal role in ameliorating the blood disorders especially hemolytic anemia caused by the chemical compounds in human.
2 Experimental
2.1 Preparation and extraction of Z. Clinopodioides
Five hundred grams of the powdered Z. clinopodioides leaf was soaked in 5 L of distilled water and swirled regularly for 24 hrs. The extract was decanted, filtered and concentrated under reduced pressure using a rotary evaporator at 40 °C to obtain a semi-solid residue.
2.2 Preparation and synthesis of FeNPs
The green synthesis of FeNPs was carried out according to the previous study with some modification (Wei et al., 2016). A 40 ml of aqueous extract solution (100 µg/mL) was added to 10 ml of FeCl3·6H2O in the concentration of 0.05 M (deionized water was used for the all steps of this section). The mixture was refluxed for 1 h at 50 °C. The color-changing from yellow to black indicated the formation of iron nanoparticles. The precipitate was triplet washed with water and centrifuged at 10000 rpm for 15 min subsequently. The obtained black powder was kept in a vial for the chemical characterization and evaluation of its biological activity.
2.3 Analysis of antioxidant properties of FeNPs by DPPH
Free radicals are unstable atoms that have one or more unpaired electrons. These active species are very harmful due to their high reactivity. They are most often formed when oxygen molecules in the body split into separate unstable atoms. This process can turn into a chain reaction. Free radicals excessive production in the body causes cell damage and oxidative stress. Genetics and the environment affect the extent of free radical damage in individuals. These active molecules are produced as part of the body's natural biological processes. One of the most important free radicals is DPPH. DPPH is widely used to study the antioxidant activities of natural compounds and nanoparticles (Hosseinimehr et al., 2011).
In this method, the antioxidant activity of nanoparticles is measured for DPPH radical scavenging. The basis of the action is the reduction of the alcoholic solution of DPPH in the presence of hydrogen-giving antioxidants, especially phenolic compounds. To achieve the IC50 of the samples, 11 different concentrations of nanoparticles were prepared and the percentage of inhibitory versus concentration was used to plot. In practice, 300 μl of 1 M DPPH was combined with 100 μl of diluted sample to a final volume of 2 ml using methanol. After half an hour in the dark, the absorbance was read at 517 nm and the inhibitory percentage was obtained using the following formula:
In this formula, “Control A” shows the negative control of light absorption that lacks nanoparticles, and “Sample A” expresses the amount of light absorption of different concentrations of nanoparticles (Hosseinimehr et al., 2011).
2.4 Analysis of cell toxicity of FeNPs
Investigation of cell proliferation and survival is one of the most important and basic techniques in cell laboratories. This study requires accurate quantification of the number of living cells in the cell culture medium. Therefore, cell survival calculation methods are necessary to optimize cell culture conditions, evaluate cell growth factors, detect antibiotics and anticancer drugs, evaluate the toxic effects of environmental pollutants, and study apoptosis. Many methods can be used for such purposes, but indirect methods using fluorescent or dye (chromogenic) markers provide very fast large-scale methods. Among these methods, measurement of cell survival by MTT method is the most widely used method. This method is a colorimetric method to study cell proliferation and survival, introduced in 1983 by Mossman. The method basis is based on mitochondrial activity. Mitochondrial activity in living cells is stable and therefore a raise or reduce in the living cells number is linearly related to mitochondrial activity (Arulmozhi et al., 2013). Mitochondrial dehydrogenases in living cells break the tetrazolium ring and yield NADPH and NADH, leading to forming a purple insoluble deposit called formazan. This precipitate can be dissolved by dimethyl sulfoxide or isopropanol. Dye formation is used as a marker of living cells. The color intensity produced is measured at a 540 to 630 nm wavelength and is directly proportional to the living cells number. High safety and providing a colorimetric and non-radioactive system are important advantages of this method. This kit is very easy to use, has high sensitivity and accuracy and can detect less than 950 cells. On the other hand, it has high efficiency for measuring cell proliferation, survival and mortality, and its implementation method does not require time-consuming washing steps and transfer from one plate to another. Examples studied in this method are adhesive or suspended cells and proliferating or non-proliferating cells (Arulmozhi et al., 2013).
In this research, we used the following cell line to evaluating cytotoxicity effects of FeCl3, Z. clinopodioides, and FeNPs using an MTT method.
Because nanoparticles is not soluble directly in 1640-RPMI medium and also the solvent of dimethyl sulfoxide nanoparticles (DMSO) itself has cytotoxic effects, to eliminate the effect of this substance on treated cells (Normal cell line (HUVEC)), its amount in the final solution is considered less than 1%. Dimethyl sulfoxide is not toxic to concentrations less than 1% and the concentration of this solvent is important in this regard. For this purpose, 1000 µg of nanoparticle was dissolved in 100 μl of dimethyl sulfoxide solvent after weighing. Then 1 ml of culture medium was added for better dissolution and finally the volume of solution was increased to 24 ml using culture medium: Then, successive dilutions of this stock were used in the proportions of 1–1000 μg/ml. Eleven concentrations were used for the cell lines (Arulmozhi et al., 2013).
In this study, 100 µl of culture medium containing 104 cells per plate 96 were placed. After 24 h, incubation of concentrations of 1–1000 μg/ml of nanoparticles was added to the cells, and incubated for 24, 48, and 72 h, respectively. After these times, 20 μl of MTT plate with a concentration of 5 mg/ml was added to each cell and incubated in the dark for another 4 h. After some time, the MTT medium was carefully removed, and 200 µl of acidified isopropanol were added to each plate to remove the purple formanes. After 15 min of incubation at room temperature, the light absorption of each well was read using an ELISA at 570 nm against a reference wavelength of 690 nm. The findings were reported as cell survival and IC50 (concentration that inhibits cell growth up to 50%) based on the concentration curve (μg/ml) (Arulmozhi et al., 2013).
It should be noted that the effect of each concentration of the nanoparticles on cell lines was investigated in five independent experiments. According to the values of light absorption obtained by the ELISA reader, the percentage of growth inhibition related to each concentration was calculated using the following formula:
Finally, linear regression was done to gain IC50, which indicates the nanoparticles concentration, which causes 50% cancer cell growth inhibition. Using the curve, the line equation for cancer cells was obtained, respectively, then by replacing 50% inhibition in the equation, the IC50 value for cancer cells was obtained (Arulmozhi et al., 2013).
2.5 In vivo design
In this study, fifty male, twelve-week-old mice weighing 39–40 g were obtained. At the beginning of the study, hemolytic anemia induction was done with three doses of 20 mg/kg body weight intravenous injection of phenylhydrazine in 40 animals on days 1, 3, and 5. One group was considered as a healthy control and injected with normal saline. On day 5, the 40 phenylhydrazine-treated mice were randomly divided into four subgroups, including untreated receiving distilled water, and three groups receiving 2 mg/kg body weight intravenous injection of FeCl3, Z. clinopodioides, and FeNPs. During this period, the healthy control group received normal saline. The mice underwent injection treatment for 15 days. The mice were weighed nine times during the study. At the end of day 16 of treatment after anesthetizing the animals, the blood samples were drawn immediately from the animals’ hearts. Also, the mice livers and spleens were harvested to analyze the biochemical parameters (Zangeneh et al., 2019).
Immunological (Pro-inflammatory (IL1, IL6, IL12, IL18, IFNY, and TNFα) and anti-inflammatory (IL4, IL5, IL10, IL13, and IFNα) cytokines) and biochemical (ALP, AST, ALT, GGT, cholesterol, LDL, HDL, triglyceride, total protein, albumin, total and conjugated bilirubin, urea, creatinine, ferrous, ferritin, and erythropoietin) parameters concentration were measured in the serum by ELISA procedure and determined using kits (Ziest Chem Diagnostics) according to the manufacturers’ instructions.
The capacity of antioxidant enzymes in serum, spleens, and livers of each group was evaluated by determining the activity of SOD, CAT, GR, and GPx by ELISA procedure and determined using kits (Ziest Chem Diagnostics) according to the manufacturers’ instructions.
The number of total WBC, lymphocyte, monocyte, neutrophil, eosinophil, basophil, platelet, RBC, and nRBC, and the level of HB, PCV, MCV, MCH, and MCHC were analyzed in the plasma by automatic hematology analyzer (Sysmex XS 800i).
All hematological, immunological, and biochemical analyses were done in duplicate (Zangeneh et al., 2019).
2.6 Statistical analysis
SPSS statistical software version 22 was used for data analysis and the findings were determined as the mean standard deviation of 5 replications. Data were analyzed using one-way analysis of variance and Duncan post hoc test and the significance level in the test was considered 0.01.
3 Results and discussion
Nanotechnology is a new branch of science with a wide range of applications and nanoparticles with different compositions and sizes, shapes and surface chemical properties can have different biological and biomedical applications. Reducing the size of materials at the nanoscale can often cause electrical, magnetic, structural, morphological, and chemical changes. Nanoparticles typically have a higher percentage of atoms on their surface, which increases surface reactions (Sharma et al., 2009; Nadagouda and Varma, 2006; Rajeshkumar, 2016; Raut et al., 2010). Nanoparticles have antibacterial and magnetic properties by penetrating microorganisms due to their high surface-to-volume ratio and small size. Also, due to their photocatalytic, catalytic and ionic properties, they are widely used in the fight against human pathogenic microbes, bacteria, fungi and viruses (Leili Shaker Ardakani, 2021; Sivaraj et al., 2014; Zangeneh, 2019). By producing active bases such as oxygen ions and hydroxides, metallic nanoparticles disrupt the metabolism, proliferation and respiration of microorganisms by destroying organic structures and strongly interacting with enzymes and proteins in the electron transfer system, and it can kill more than 650 types of gram-negative and gram-positive bacteria resistant to common antibiotics in vitro up to 99.9% (Abdel-Fattah and Ali, 2018; Klein et al., 2014; Zangeneh et al., 2019201820192019; Zangeneh et al., 20192019202120202019201920192020). Metallic nanoparticles in cell cultures and human tissues yield toxins that raise inflammatory products such as cytokines and oxidative stress, ultimately leading to cell death. Larger nanoparticles are seen by the nuclei and mitochondria, causing mutations in DNA, destruction of the mitochondrial structure, and even cell death (Rajeshkumar, 2016; Raut et al., 2010; Leili Shaker Ardakani, 2021; Sivaraj et al., 2014). Solubility and density, surface baroelectricity, surface structure, shape, chemical composition, and size and dimensions are the key factors in determining the toxicity of nanoparticles. The exact effect of metallic nanoparticles on cancer cells is not fully understood, but increasing ROS production is one of the possible mechanisms (Leili Shaker Ardakani, 2021; Sivaraj et al., 2014; Zangeneh, 2019; Abdel-Fattah and Ali, 2018). When nanoparticles are in contact with cancer cells, the cellular defense mechanism is activated to minimize damage. But, if the ROS production stimulation inside the cell by nanoparticles exceeds the cell antioxidant defense capacity, the cells are destroyed during the process of apoptotic cell death (Leili Shaker Ardakani, 2021; Sivaraj et al., 2014; Zangeneh, 2019). The electrostatic interaction of nanoparticles causes them to be absorbed into target cells. Positively charged nanoparticles are attracted to cancer cells with a high percentage of anionic phospholipids and certain groups of charged proteins and carbohydrates on their outer surface (Raut et al., 2010; Leili Shaker Ardakani, 2021; Sivaraj et al., 2014). It seems the mechanism of hemolytic effects of the metallic nanoparticles is similar to their anticancer effects (Sivaraj et al., 2014; Zangeneh, 2019; Abdel-Fattah and Ali, 2018).
3.1 Characterization of FeNPs
3.1.1 UV–Visible spectroscopy analysis
The UV–Vis. spectrum of the biosynthetic nanoparticles of iron is presented in Fig. 1. The surface plasmon resonance (SPR) of FeNPs was completed using UV–Vis. spectroscopy. The peaks at 221 and 298 nm approved the creation of FeNPs. In differentiation with former reports, the presence of the mentioned band proves the creation of iron nanoparticles (Harshiny et al., 2015; Katata-Seru et al., 2018).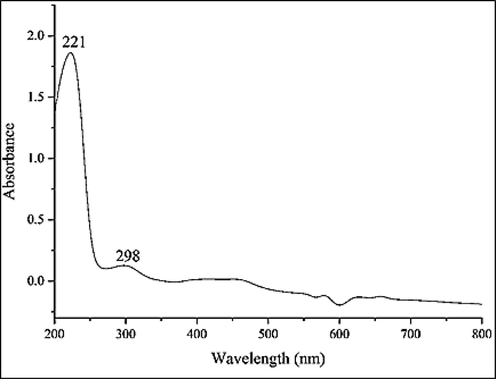
UV–Vis. The spectrum of biosynthesized FeNPs.
3.1.2 FT-IR analysis
The FT-IR spectra of the green synthetic of FeNPs and Z. clinopodioides are shown in Fig. 2. For the FT-IR analysis of metallic nanoparticles, the peaks between 400 and 700 cm−1 usually M—O vibration. The FeNPs formation was confirmed by the the peaks at 457, 524, and 638 cm−1 that is in relation with vibration of Fe—O. However, it is obvious that many organic molecules of the extract strongly protected the surface of iron nanoparticles in within the synthesis step.The similarity has been observed for the FT-IR spectrums of the Z. clinopodioides extract and FeNPs, that could be proved the biosynthetic nanoparticles of Iron . Existences of different functional groups in Z. clinopodioides extract reveal different-IR bands. Such as the peaks in 3371 and 2939 cm−1 are contributed to O—H and C—H stretching; the bands from 1421 to 1749 cm−1 belong to C⚌C and C⚌O, and the peaks at 1261 and 1081 cm−1 could be considered for —C—O and —C—O—C bonds. These peaks confirmed the presence of various organic in the plant extract which have been reported previously (Oganesvan et al., 1991; Tian et al., 2010). According to a previous study, the hydroxyl and carbonyl group of the organic compounds in the herbal extracts linked to the metallic nanoparticles (Ghidan et al., 2016).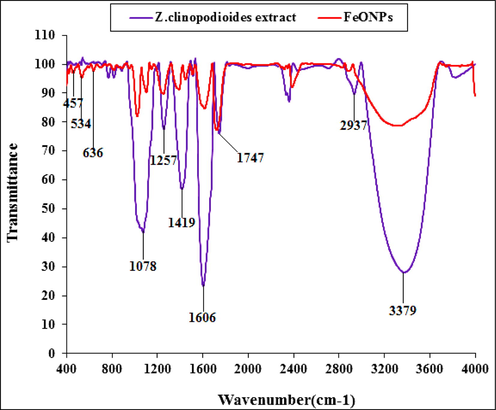
FT-IR spectra of biosynthesized FeNPs and aqueous extract of Z. clinopodioides leaf.
3.1.3 XRD analysis
The XRD diffraction patterns of FeNPs evaluated its crystallinity. The pattern of diffractogram is showed in Fig. 3. The creation of iron nanoparticles were approved to this result. Despite the small size of FeNPs, the pattern of XRD indicated well crystallizing. The achieved data were compared with the usual database ICDD PDF card no. 96-900-5813. The peaks at positions of 35.50, 37.27, 53.95, 62.28, and 75.36 are indexed to (3 1 1), (2 2 2), (4 2 2), (4 0 4), and (5 3 3) planes which are in good agreement with those of magnetite (Fe3O4). These peaks are also reported previously (Patra and Baek, 2017; Kuang et al., 2013; Yew et al., 2016). The average crystal size of FeNPs was calculated using the Scherrer equation. The FeNPs had an average crystal size of 29.91 nm. In our literature review, A few studies have reported various crystalline size for the green-synthesized FeNPs by plants extracts. For example, 14 nm has been reported for the estimated crystallite size of the biosynthesized magnetite iron oxide for Trigonella foenum-graecum (Radini et al., 2018); 48.91 nm for Brassica rapa (Patra and Baek, 2017); 2.3 nm for Platanus orientalis (Nurbas et al., 2017); and 68.17 nm for Cynometra ramiflora fruit extract (Bishnoi et al., 2018).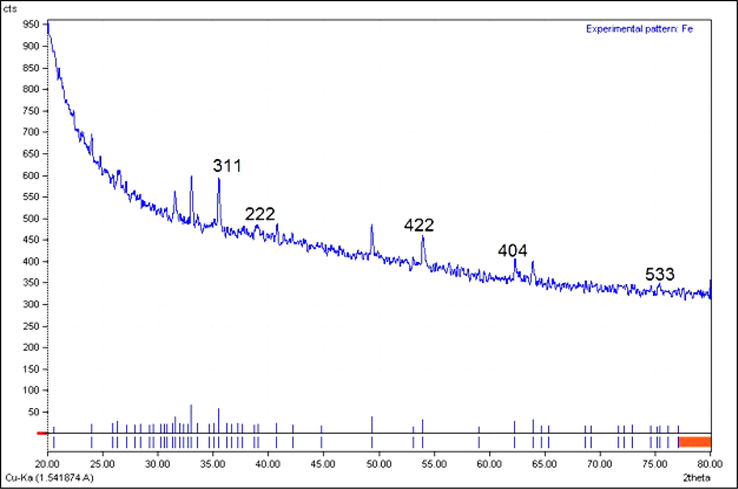
XRD pattern of FeNPs.
3.1.4 FE-SEM analysis
FE-SEM imaging investigated the morphology of surface and size of FeNPs. Fig. 4 (a-d) presents the FE-SEM of the green synthetic of iron nanoparticles. According to the result the FeNPs are in spherical morphology. The displaying of the images of synthesis of FeNPs with spherical morphology have been reported previously (Devatha et al., 2016). The images show a tendency for accumulation of the green-synthetic FeNPs and changeable size, which was reported for the other green synthetic metallic nanoparticles (Harshiny et al., 2015; Ghidan et al., 2016; Rao and Pennathur, 2017; Baghayeri et al., 2018; Seydi et al., 2019). As shown in Fig. 5d and according to the findings, the range of 13.27–43.10 nm was indicated for the diameter size of FeNPs. A 30.04 nm can be considered for the FeNPs of this study. In the literature, different sizes have been reported for the green synthesized FeNPs. The size of the iron nanoparticles was from 7.69 to 110 nm (Yew et al., 2016; Gautam et al., 2018; Zhang et al., 2017; Devatha et al., 2018; Wu et al., 2015; Huang et al., 2014).
FE-SEM image of FeNPs.
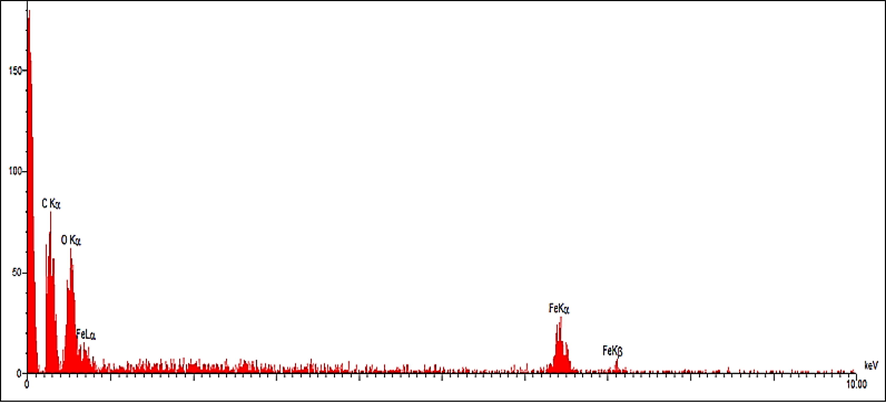
EDS analysis of FeNPs.
3.1.5 EDX analysis
The result of EDX analysis for FeNPs, as a semi-quantitative technique, is shown in Fig. 5. The existence of iron in the FeNPs was approved by the signals around 0.7 Kev for FeLα; around 6.4 Kev for FeKα; and at 7.1 Kev for FeKβ. These signals are as well as match to iron signals from a previous study (Kuang et al., 2013). On the other hand, the presences of oxygen by a single at 0.5 Kev and carbon by he peak at 0.3 Kev is approved.
3.2 Antioxidant and cytotoxicity properties of FeNPs
In this study, we assessed the antioxidant properties of Z. clinopodioides leaf aqueous extract green-synthesized FeNPs by using the DPPH test as a common free radical. Oxidation is the electrons transfer from an atom and is the aerobic life and metabolism part of living organisms. Oxygen is the receptor for electrons in the electron transport system, which yields energy from ATP (Adenosine triphosphate) in the body. Under certain conditions, oxygen may become a single electron and release free radicals. When oxygen becomes a single electron, it is called reactive oxygen species (ROS). Oxidative loss to proteins, DNA, and other macromolecules is one of the internal causes of degenerative diseases such as aging, cardiovascular disease, cancer, immune system deficiency, cataracts, and abnormal brain function. Single oxygen, high-energy, mutagenic oxygen, can be produced by lipid peroxidation by the transmission of energy from light or the respiratory tract of neutrophils (Zangeneh et al., 2019201820192019). Some free radicals have positive roles such as regulating cell growth, phagocytosis, energy production, intracellular signals, or the important biological compounds synthesis. Antioxidants produced in the body fight free radicals with two systems: enzymatic defense and non-enzymatic defense. Superoxide dismutase, catalase, and glutathione peroxidase metabolize lipid peroxide, hydrogen peroxide, and superoxide and prevent the production of toxic hydroxyl radicals (Zangeneh et al., 20192019202120202019201920192020). In non-enzymatic defense, there are two classes of fat-soluble antioxidants (such as carotenoids and vitamin E) and water-soluble (glutathione and vitamin C) that trap free radicals. These two systems help neutralize oxidants. However, oxidants can escape from antioxidants and damage tissues. In this case, the activated antioxidant repair system (which is the enzymes lipase, protease, transferase and DNA repair enzymes), counteract the oxidant effects. However, due to deficiencies in the production of antioxidants in the body or due to physiopathological factors and situations (such as smoking, air pollution, UV radiation, diets containing high unsaturated fatty acids, inflammation, ischemia, bleeding, etc) that ROS are yielded in large quantities at the wrong place and time, oral antioxidants are needed to counteract the oxidative damage cumulative effects (Zangeneh et al., 2019201820192019; Zangeneh et al., 20192019202120202019201920192020; Zangeneh et al., 2019).
In the present survey, the best result for the antioxidant activity was observed at dose 1000 µg/mL. The IC50 of BHT, Z. clinopodioides, and FeNPs were 394, 309, and 203 µg/mL, respectively (Fig. 6). About the antioxidant properties of BHT, in the previous study has been indicated that the inhibition percentage of BHT against DPPH is about 404 µg/mL (Zangeneh et al., 2019). Also, in the other studies presented that the inhibition percentage of BHT against DPPH was about 300–500 µg/mL (Zangeneh et al., 20192019202120202019201920192020).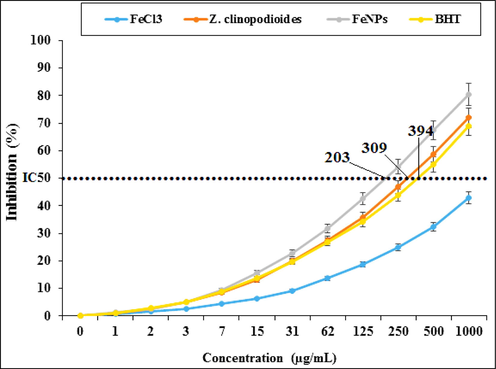
Antioxidant potential of FeCl3, Z. clinopodioides, FeNPs, and BHT. BHT: Butylated hydroxyl toluene.
In the current study, the cytotoxicity properties of FeCl3, Z. clinopodioides, and FeNPs were measured against HUVEC cells (Fig. 7). HUVECs are the human normal cells that are separated from umbilical vein endothelial cells. These cells are used for investigating the cytotoxicity effects of metallic nanoparticles such as iron nanoparticles (Zangeneh et al., 20192019202120202019201920192020).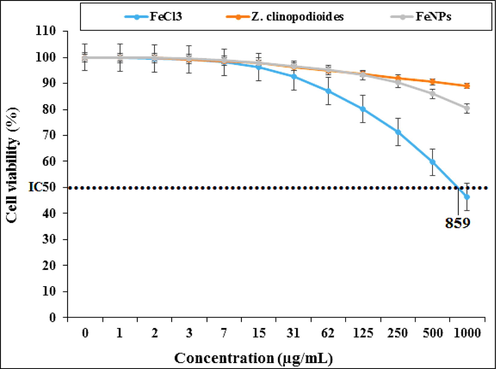
Percent viability measured on HUVEC after treatment with present FeCl3, Z. clinopodioides, and FeNPs.
The absorbance rate was determined at 570 nm, which indicated extraordinary viability on normal cell line (HUVEC) even up to 1000 μg/mL for Z. clinopodioides and FeNPs. The IC50 of FeCl3 was 859 μg/mL. The absence of any significant toxicity of Z. clinopodioides and FeNPs has numerous safe applications in pharmaceutical domains. In the previous study has been indicated when FeCl3 is combined with plant extracts, its cytotoxicity capacity significantly reduces against HUVECs (Zangeneh et al., 2019).
3.3 Anti-hemolytic anemia potential of FeNPs
Many factors such as industrial chemicals, some commercial pesticides or improper use of some drugs or some natural factors such as animal toxins or some parasites lead to red blood cell lysis and hemolytic anemia. One of the most obvious side effects of this type of anemia is an increase in the amount of tissue iron due to the lysis of red blood cells and a decrease in oxygen (hypoxia) caused by anemia (Zangeneh et al., 2019; Shukla et al., 2012). The amount of intracellular iron is precisely regulated and it is believed that increasing the amount of this substance outside the cell causes oxidative stress conditions and damage to the fat of cell membranes and organs (Mahdavi et al., 2019; Belyaev and Demeubaeva, 1999; Shukla et al., 2012). It can also be stated that about 20 million people live at altitudes of more than 3,000 m above sea level and under hypoxic conditions. Recently, the population of people working in the highlands has also increased. In the last decade, a number of studies on growth, development and production have been performed under hypoxic conditions (Mahdavi et al., 2019; Belyaev and Demeubaeva, 1999; Zangeneh et al., 2019; Shukla et al., 2012).
Hydrazines are colorless liquid and oxidant compounds with hemolytic properties that cause toxicity in various tissues and at different levels - which are widely used in industry, industrial pesticides, pesticides, explosives, chemical, pharmaceutical, agricultural and laboratory industries (Mahdavi et al., 2019; Belyaev and Demeubaeva, 1999). These compounds are recognized by the World Health Organization as carcinogenic pollutants that can cause many public health problems and cause irreversible cellular damage (Belyaev and Demeubaeva, 1999; Shukla et al., 2012). Phenylhydrazine was first developed from hydrazine by Fischer Emil in 1875. Phenylhydrazine is used to prepare indoles, which is an intermediate substance for the production of various dyes and drugs. Phenylhydrazine is also used to produce phenylhydrazones from natural compounds that are used to distinguish simple sugars from each other (Mahdavi et al., 2019; Belyaev and Demeubaeva, 1999; Zangeneh et al., 2019; Shukla et al., 2012). According to studies, phenylhydrazine exerts its effects through direct damage to the erythrocyte membrane. Phenylhydrazine, which is also used clinically in the treatment of polycythemia due to its hemolytic effects, it causes a wide range of side effects such as hemolytic anemia, hypoxia, vascular disorders, liver, kidney, respiratory, central and autoimmune nervous system, malignancies, genetic abnormalities and inflammatory reactions (Shukla et al., 2012). Phenylhydrazine auto-oxidation results in the production of reactive oxygen species and phenylhydrazine-derived radicals such as phenylhydrazyl radicals. In addition to reactive oxygen species, phenylhydrazine metabolites can also react with cell membranes and cause red blood cell destruction and hemolytic anemia due to lipid peroxidation and protein oxidation (Mahdavi et al., 2019; Shukla et al., 2012). On the other hand, increased hematopoietic activity and iron absorption following severe phenylhydrazine-induced severe hemolytic anemia increase iron storage in tissues, that high levels of tissue iron also increase the production of reactive oxygen species and cause several pathophysiological changes (Mahdavi et al., 2019; Belyaev and Demeubaeva, 1999; Zangeneh et al., 2019; Shukla et al., 2012). In this regard, several experimental studies have confirmed the role of hemolytic anemia and phenylhydrazine-induced vascular disorders in the development of systemic inflammatory reactions and histopathological changes in various organs such as the heart (Shukla et al., 2012).
In our experiment, the number of total WBC, lymphocyte, monocyte, neutrophil, eosinophil, basophil, RBC, platelet counts and the other RBC parameters including Hb, PCV, MCV, MCH, and MCHC decreased significantly (p ≤ 0.01) and the number of nRBC counts raised significantly (p ≤ 0.01) in the phenylhydrazine-treated group (Fig. 8,9). Above results show that phenylhydrazine successfully has induced hemolytic anemia in mice. Phenylthydrazine is known to induce hemolytic anemia mixed from insufficiency of many organs such as heart, testis, spleen, stomach, kidney, and liver (Shukla et al., 2012). According to our study, in similar studies indicated that phenylhydrazine with decreasing the number of RBC to 4 × 1012/L induced severe hemolytic anemia. In the previous studies, the levels of Hb, PCV, MCV, MCH, MCHC reduced to 8 g/dL, 29%, 49fL, 18 pg, and 20 g/dL, respectively. About the number of total WBC and platelet, these parameters also reduced in the phenylhydrazine-treated group (Zangeneh et al., 2019; Zangeneh et al., 2017). Z. clinopodioides and FeNPs improved significantly (p ≤ 0.01) the hematological parameters, so that they could significantly (p ≤ 0.01) enhance the number of total WBC, lymphocyte, monocyte, neutrophil, eosinophil, basophil, RBC, platelet counts and the other RBC parameters including Hb, PCV, MCV, MCH, and MCHC and reduce the number of nRBC in comparison to untreated group. There isn’t any significant difference (p ≤ 0.01) between Z. clinopodioides, FeNPs, and control groups in the number of nRBC. In the previous studies, the hematoprotective and anti-hemolytic anemia potentials of aqueous extract of medicinal plants have been related to their antioxidant compounds. (Zangeneh et al., 2019; Zangeneh et al., 2017) Due to Z. clinopodioides is rich of the antioxidant compounds including cineol, sis-isopulegone, thymol, terpenoides, α and β pinen, flavonoids, piperitenone, and pulegone, so its hematoprotective and anti-hemolytic anemia potentials are normal [Behravan et al., 2007; Salehi et al., 2005; Mahdavi et al., 2019; Belyaev and Demeubaeva, 1999; Zangeneh et al., 2019, Zangeneh et al., 2017; Saravanan and Ponmurugan, 2013; Hoseinpouran et al., 2015; Ogunlade et al., 2012]. Fig. 9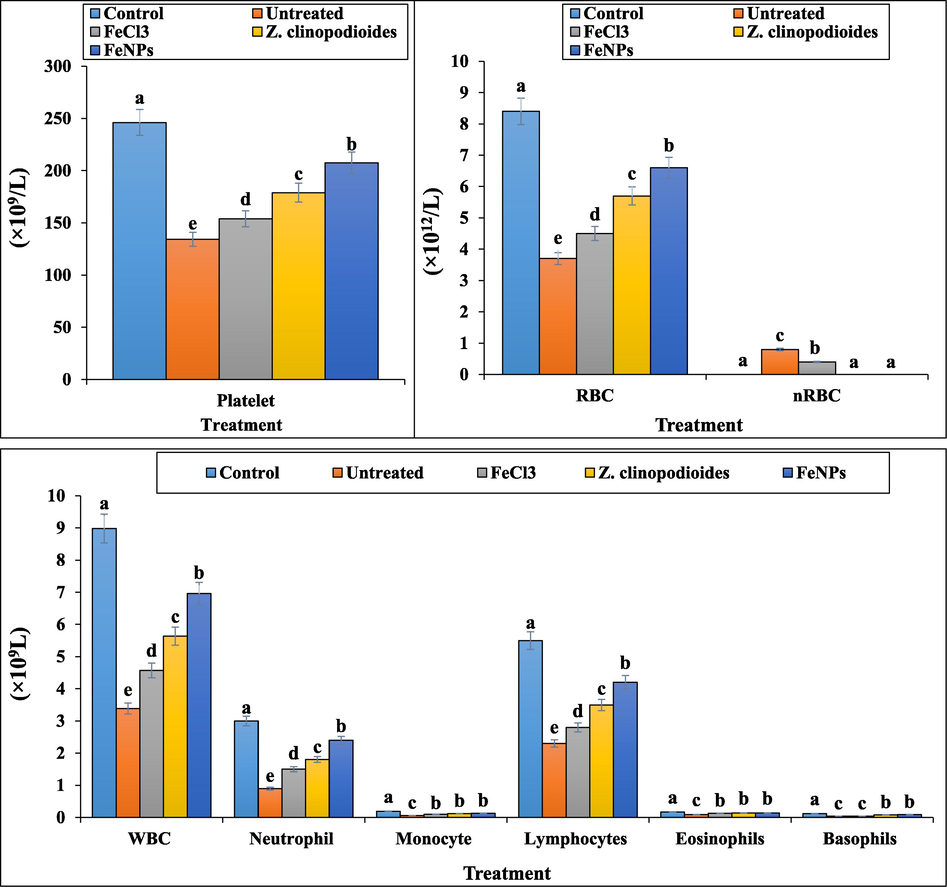
The number of platelet, RBC, nRBC, and WBC in the experimental groups.
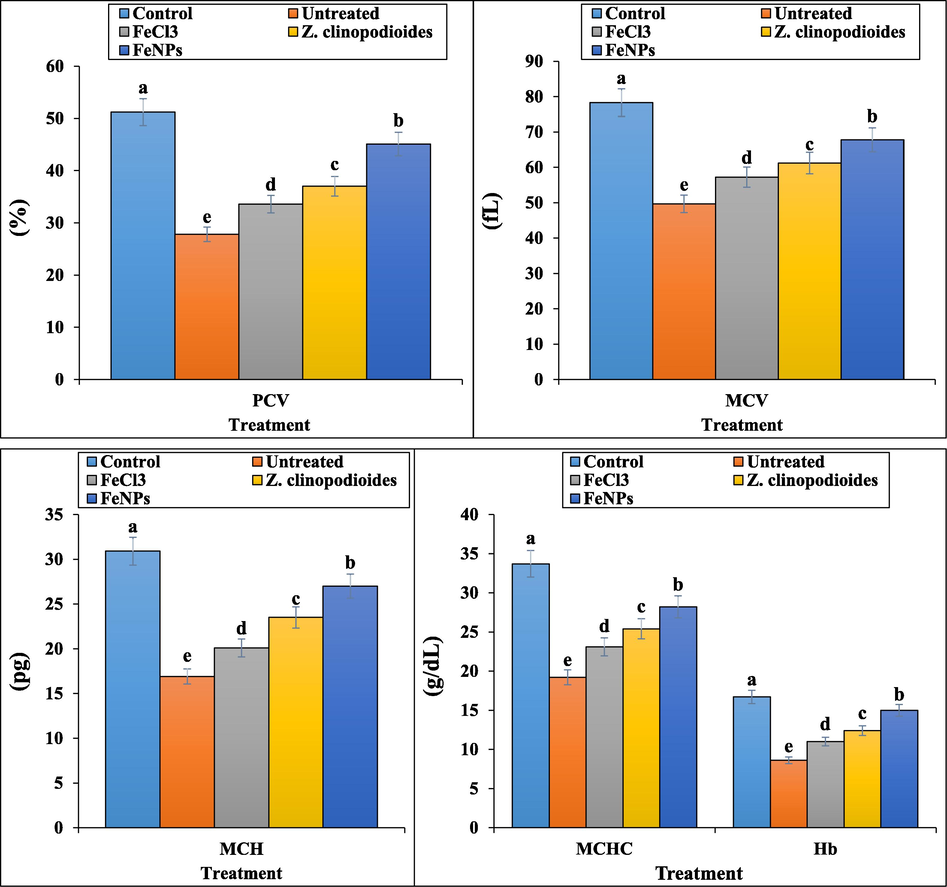
The levels of RBC parameters in tested groups.
About immunosuppressive property of phenylhydrazine, the anti-inflammatory cytokines including IL4, IL5, IL10, IL13, and IFNα reduced significantly (p ≤ 0.01) and the pro-inflammatory cytokines including IL1, IL6, IL12, IL18, IFNY, and TNFα increased significantly (p ≤ 0.01) in the phenylhydrazine-treated group (Fig. 10). Agreement with our study, in a similar study has been indicated that phenylhydrazine as inflammation inducing agent, increases the concentration of pro-inflammatory cytokines and decreases the concentration of anti-inflammatory cytokines in serum (Zangeneh et al., 2017). Both groups of Z. clinopodioides leaf aqueous extract and FeNPs significantly (p ≤ 0.01) ameliorated the levels of anti-inflammatory and pro-inflammatory cytokines near to normal. The best results have been observed in FeNPs-treated group. No significant difference (p ≤ 0.01) was seen between FeNPs and control groups in the concentrations of IL6, IL13, IL18, and IFNα. In this study, FeNPs were introduced as immunoprotective nanoparticles.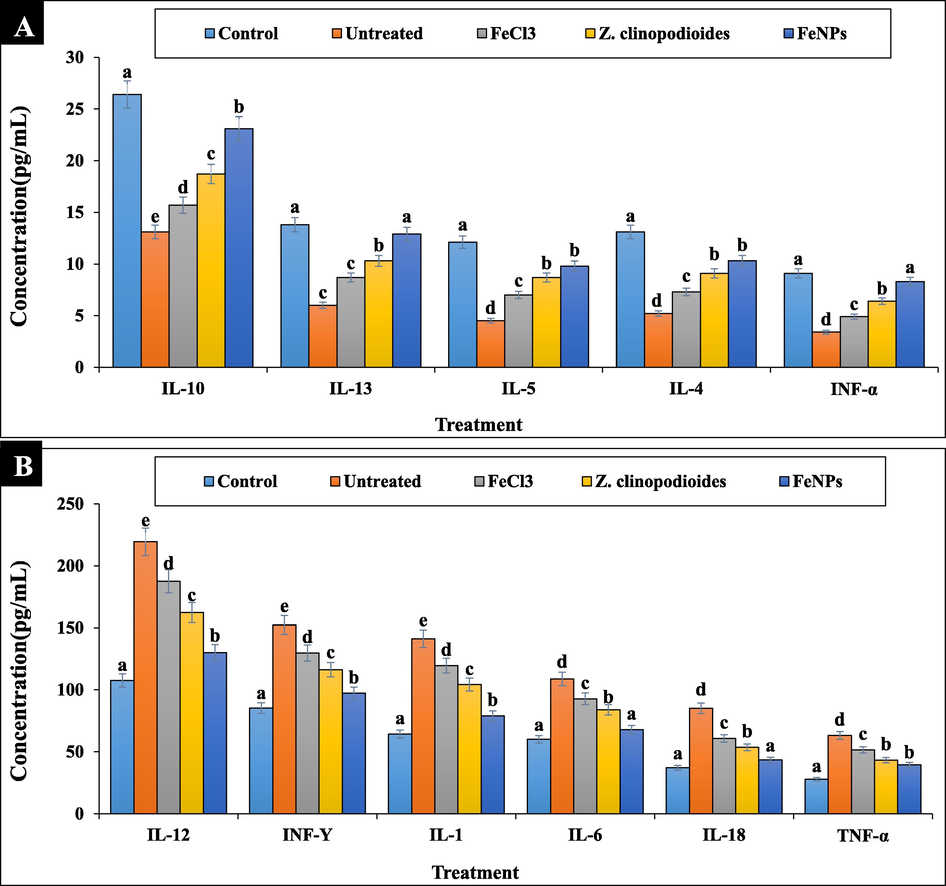
The levels of immunological parameters in tested groups.
In the current research, the levels of serum, liver, and spleen SOD, CAT, and GPx increased and GR reduced significantly (p ≤ 0.01) at Z. clinopodioides leaf aqueous extract and FeNPs-treated mice in comparison to the untreated group. No significant differences (p ≤ 0.01) were found between Z. clinopodioides, FeNPs and control groups in the concentration of serum GR and liver GPx (Fig. 11). In the study of Saravanan and Ponmurugan (Saravanan and Ponmurugan, 2013), it revealed the strong antioxidant effect of medicinal plants with ameliorating the concentrations of SOD, CAT, and GPx (Saravanan and Ponmurugan, 2013).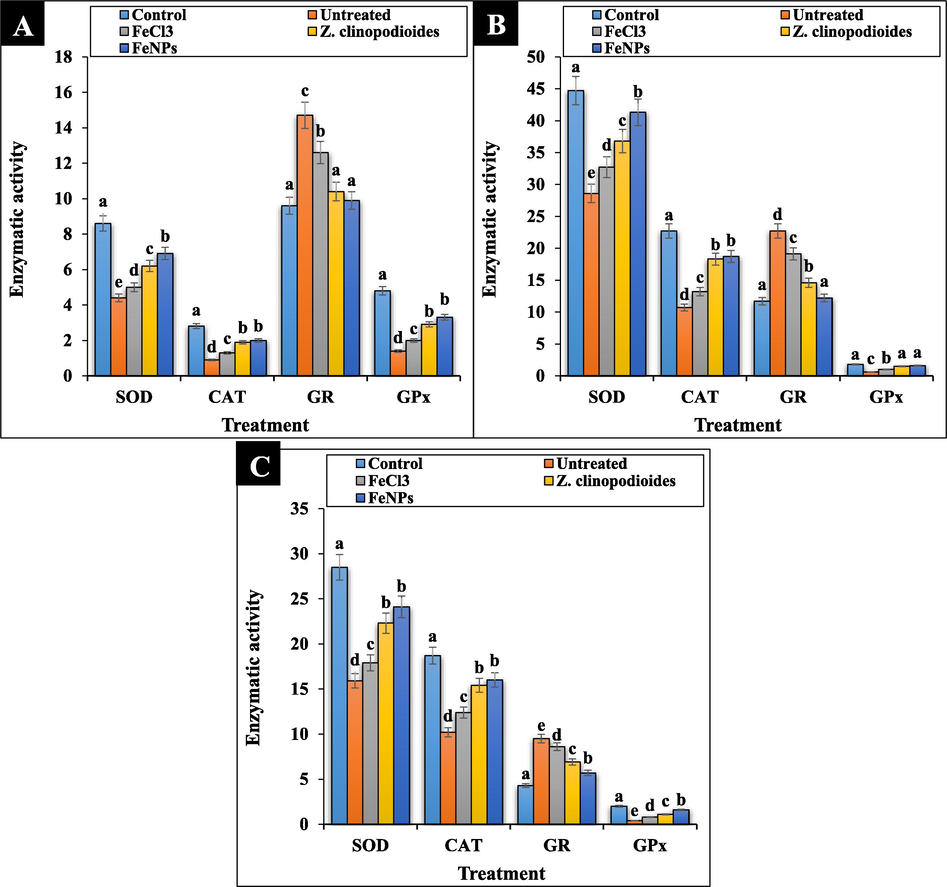
The levels of antioxidant enzymes of serum (A), liver (B), and spleen (C) in tested groups.
In our study, phenylhydrazine-induced hemolytic anemia significantly (p ≤ 0.01) decreased the concentrations of HDL, total protein, and albumin and raised ALP, AST, ALT, GGT, cholesterol, LDL, triglyceride, total and conjugated bilirubin, urea, creatinine, ferrous, ferritin, and erythropoietin in serum. Z. clinopodioides leaf aqueous extract and FeNPs significantly (p ≤ 0.01) ameliorated the above parameters. There were no significant changes (p ≤ 0.01) between Z. clinopodioides, FeNPs, and control groups in conjugated bilirubin, triglyceride, and GGT levels. The best results in improving the biochemical parameters were seen in FeNPs-treated group (Fig. 12, Fig. 13.). In this regards, in a study of Dkhil et al. (2015) has been indicated that metallic nanoparticles such as iron nanoparticles have an excellent protective role against free radicals, so that they could regulate the concentrations of biochemical and immunological parameters of serum near to normal (Dkhi et al., 2015).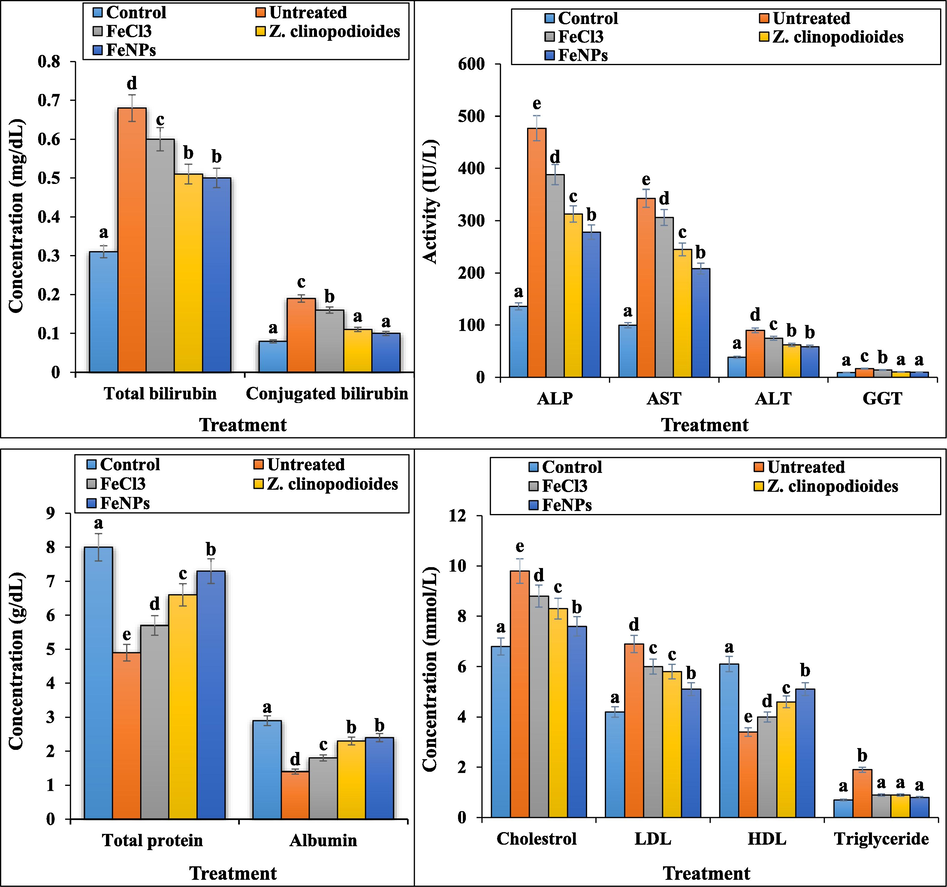
The levels of hepatic biochemical parameters in tested groups.
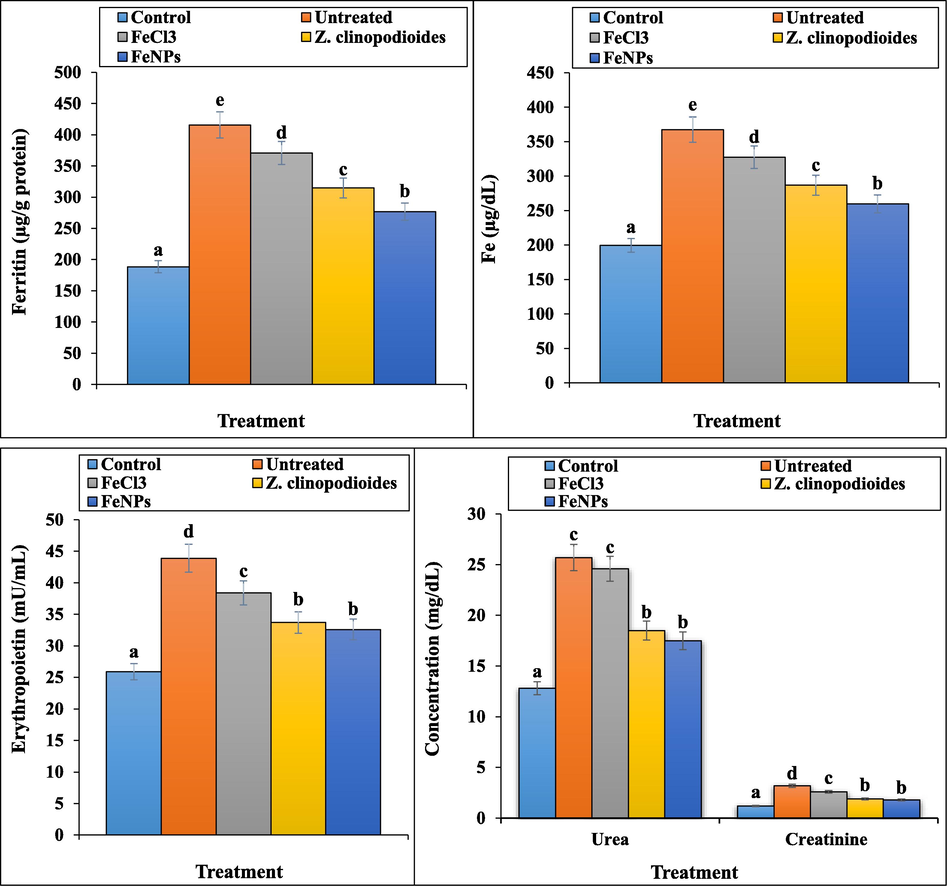
The levels of Fe, ferritin, erythropoietin, urea, and creatinine in tested groups.
In Figs. 14 and 15, phenylhydrazine increased significantly (p ≤ 0.01) the weights and volumes of spleen and liver and reduced the weight of the body. Treatment with Z. clinopodioides leaf aqueous extract and FeNPs reduced significantly (p ≤ 0.01) the above parameters in liver and spleen and increased significantly (p ≤ 0.01) the weight of the body. The best results were seen in FeNPs-treated group.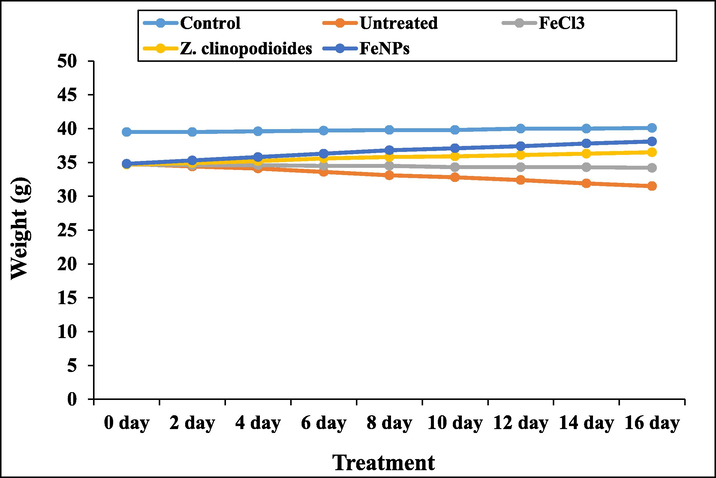
The weight of the body in tested groups.
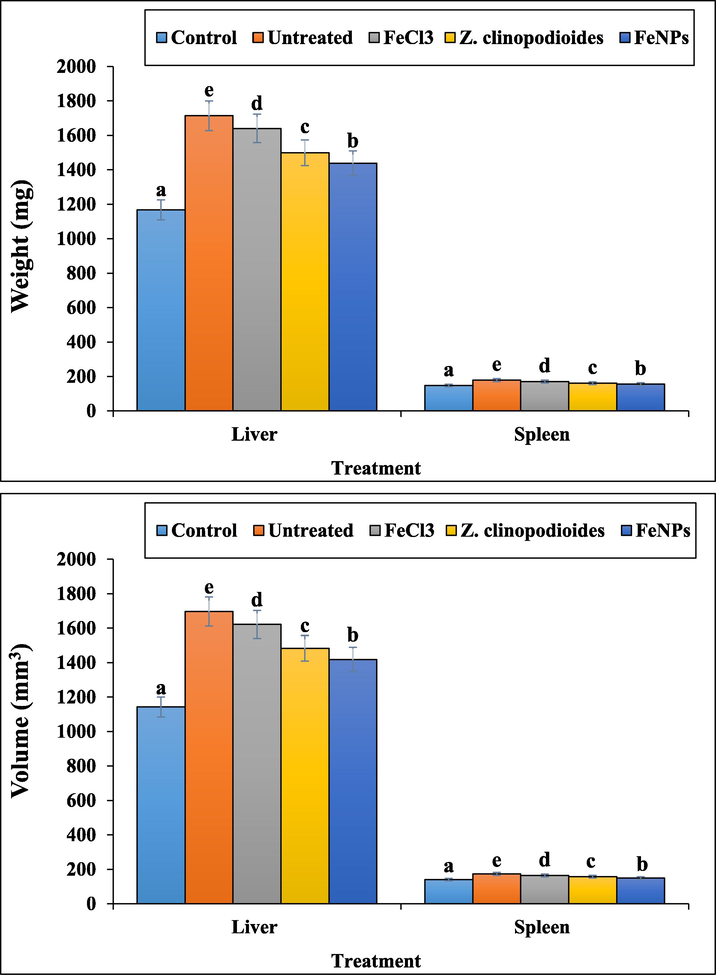
The weights and volumes of liver and spleen in tested groups.
4 Conclusion
The gradual maturation of nanotechnology has been considered not only for treating anemia but also for a wide variety of applications, especially for drug delivery and diagnostic and imaging cases. There are many types of nanoparticles available and choosing the right carriers according to demand is a key issue. Nanoparticles are very close in size to biological molecules in terms of size and can easily penetrate into the cell, for this reason, one of the goals of nanotechnology is to mount molecules and drugs on nanoparticles and transfer them to the target cell. It is also possible to create different surface properties for nanoparticles by attaching protective ligands to increase the nanoparticles' resistance to the immune system and increase their presence in the bloodstream, and even binding ligands to specifically bind the nanoparticles to the target tissue. In the present study, the green synthesis of FeNPs were successfully carried out by the bioreduction of FeCl3 solution using the extract of Z. clinopodioides leaf. FeNPs have been appropriately characterized and confirmed using UV–Vis., FT-IR, XRD, EDS and FE-SEM. In vivo field, FeNPs significantly (p ≤ 0.01) ameliorated the levels of immunological, hematological, and biochemical parameters in anemic mice. In vitro field, FeNPs indicated the excellent antioxidant property against DPPH. Also, FeNPs had high cell viability dose-dependently on HUVEC cell line. Seemingly, FeNPs can be used for the treatment of hemolytic anemia in human after confirming in the clinical trial.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- J. Adv. Res.. 2016;7:17-28.
- Appl. Microbiol. Biotechnol.. 2009;84:741-749.
- Front. Bioeng. Biotechnol.. 2018;17:109.
- Adv. Colloid Interface Sci.. 2009;145:83.
- Green Chem.. 2006;8:516.
- Resour Effic Technol.. 2016;2:230.
- Nano-Micro Lett.. 2010;2:106.
- Green synthesis of iron-based nanoparticles using Chlorophytum comosum leaf extract: methyl orange dye degradation and antimicrobial properties. Heliyon. 2021;7:e06159
- [Google Scholar]
- Acta Part A: Mol. Biomol. Spectrosc.. 2014;133:178-181.
- Appl. Organometal. Chem. 2019
- [CrossRef]
- J. Appl. Biotechnol. Bioeng.. 2018;5:00116.
- J. Phys. Chem. B. 2014;118:6159-6166.
- (a) Zangeneh, M.M., Pooyanmehr, M. Zangeneh, A. Comp. Clin. Pathol. 2019. doi: 10.1007/s00580-019-02997-w. (b) Goorani, S., Zangeneh, M.M., Koohi, M.K., Seydi, N., Zangeneh, A., Souri, N., Hosseini, M.S. Comp. Clin. Pathol. 2018. DOI: 10.1007/s00580-018-2866-3. (c) Jalalvand, A.R., Zhaleh, M., Goorani, S., Zangeneh, M.M., Seydi, N., Zangeneh, A., Moradi, R., J. Photochem. Photobiol. B. 2019, 192, 103–112. (d) Goorani, S., Shariatifar, N., Seydi, N., Zangeneh, A., Moradi, R., Tari, B., Nazari, F., Zangeneh, M.M. Orient. Pharm. Exp. Med. 2019. DOI: 10.1007/s13596-019-00361-5.
- (a) Zangeneh, M.M., Bovandi, S., Gharehyakheh, S., Zangeneh, A., Irani, P. Appl. Organometal. Chem. 2019, DOI:10.1002/aoc.4961. (b) Mohammadi, G., Zangeneh, M.M., Zangeneh, A., Siavash Haghighi, Z.M., 2019. Appl. Organometal. Chem. DOI:10.1002/aoc.5136. (c) Zareshahrabadi, Z., Karami, F., Taghizadeh, S., Iraji, A., Amani, A.M., Motamedi, M., Zomorodian, K., 2021. J. Nano Res. 67, 55-67. (d) Sadeghipour, Y., Alipour, M., Ghaderi Jafarbeigloo, H., Salahvarzi, A., Mirzaii, M., Amani, A., Mosleh-shirazi, S., Mehrabi, M., 2020. Evaluation antibacterial activity of Biosynthesized Silver Nanoparticles by using extract of Euphorbia Pseudocactus Berger (Euphorbiaceae). Nanomed. Res. J. 5(3): 265-275. doi: 10.22034/nmrj.2020.03.007 (e) Tahvilian, R., Zangeneh, M.M., Falahi, H., Sadrjavadi. K., Jalalvand, A.R., Zangeneh, A., 2019. Appl Organometal Chem. e5234. (f) Zangeneh, M.M., Joshani, Z., Zangeneh, A., Miri, E., 2019. Appl. Organometal Chem., DOI: 10.1002/aoc.5016. (g) Zhaleh, M., Zangeneh, A., Goorani, S., Seydi, N., Zangeneh, M.M., Tahvilian, R., Pirabbasi, E., 2019. Appl. Organometal Chem. DOI: 10.1002/aoc.5015. (h) Shiri, P., 2020. Appl. Organometal Chem. DOI: 10.1002/aoc.5600.
- Pharm. Res.. 2005;2:63-79.
- J. Essent Oil Bear. 2007;10:339-345.
- Naturforsch.. 2006;61:677-680.
- Biol. Pharm. Bull.. 2005;28:1892-1896.
- Hum. Exp. Toxicol.. 2006;6:325-332.
- Fitoterapia. 2002;23:716-718.
- Appl. Organometal Chem. 2019
- [CrossRef]
- Chem. Nat. Compd.. 1999;35:52-54.
- Mater. Lett.. 2016;185:384-386.
- Cancer Biother. Radiopharm.. 2011;26:325-329.
- Colloids Surf. B Biointerfaces.. 2013;110:313-320.
- Com. Clin. Pathol.. 2019;28:427-434.
- Powder Technol.. 2015;286:744-749.
- J. Mol. Liq.. 2018;256:296-304.
- Environ. Technol. Innov.. 2017;8:150-163.
- Appl. Phys. A. 2018;124:363-369.
- J. Photochem. Photobiol. B. 2018;183:154-163.
- Chem Nat.. 1991;27:247.
- Pharmacog. Mag.. 2010;6:116-119.
- Environ. Nanotechnol. Monitor Manag.. 2016;6:95-98.
- J. Photochem. Photobiol. B. 2017;173:291-300.
- J. Colloid Interface Sci.. 2013;410:67-73.
- Nanoscale Res. Lett.. 2016;11:276-282.
- Dig J. Nanomater. Bios.. 2017;12:993-1000.
- Mater. Res. Bull.. 2018;97:121-127.
- J. Clean Prod.. 2016;139:1425-1435.
- Mater. Res. Bull.. 2017;85:64-73.
- Appl. Organometal Chem.. 2018;32:e4057
- Appl. Organometal Chem. 2019:5009.
- Environ. Nanotechnol. Monitor Manage.. 2018;10:377-387.
- Food Func.. 2017;8:3219-3227.
- Environ. Nanotechnol. Monitor Manage.. 2018;9:85-94.
- Sep. Purif. Technol.. 2015;154:161-167.
- Spectrochim Acta A Mol. Biomol. Spectrosc.. 2014;117:801-804.
- Appl. Organometal Chem. 2019e5246
- IJBAS. 2012;2:86-91.
- Iranian J. Pharmacol. Ther.. 2017;15:1-9.
- Int. J. Endocrinol. Metab. Disord.. 2013;11:e10927
- Crescent J. Med. Biol. Sci.. 2015;2:125-129.
- Am. J. Biochem. Mol. Biol.. 2012;2:67-81.
- Biomed. Res.. 2015;27:214-219.
- J. Rafsanjan Univ. Med. Sci.. 2017;16:227-238.
- Sci. Res. J. Shahed Univ.. 2018;25:21-30.







