Translate this page into:
Recent advances in the microwave- and ultrasound-assisted green synthesis of coumarin-heterocycles
⁎Corresponding author. rchowhan@cug.ac.in (L. Raju Chowhan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Given the importance of the highly reactive nature and attractiveness of coumarins as a precursor of natural products and pharmacological agents, tremendous efforts have been dedicated to their synthesis in the last decades. Starting from the Knoevenagel, Perkin, Kostanecki-Robinson, Pechmann, Reformansky, Baylis Hillman reactions to several new one-pot multicomponent, sequential tandem reactions have been devised. After the emergence of non-conventional energy sources like microwaves (MW) and ultrasound irradiation, the field of organic synthesis has reached an outstanding level in this century in terms of synthetic efficiency as well as green chemistry viewpoint. Enlightened by this, a great deal of attention has been paid to the synthesis of bioactive coumarin-heterocycles by employing the non-conventional approach and a vast array of synthetic procedures has been established. Therefore, a time to time investigation is required for the synthesis and biological aspects of these hybrid molecules. The present review aims to highlight the current progress achieved in the synthesis of coumarins either linked or fused with diverse bioactive five- and six-membered heterocycles by making the utilization of microwaves and ultrasound-assisted strategies from 2014 to date. Besides highlighting the development achieved in this field, we have attempted to point out the drawbacks and challenges associated with the reaction discovery, which would hopefully provide the impetus for future exploration.
Keywords
Coumarin
Microwave
Ultrasound irradiation
Green chemistry
Multicomponent reaction
1 Introduction
2H-chromen-2-one ring, popularly known as coumarin, constitutes an important class of heterocyclic compounds with the characteristics benzo-α-pyrone moiety in its structure. The existence of coumarin in nature was first interpreted in 1822, by isolating it from Coumarouna odorata Aube (Dipteryx odorata) (Gleye et al., 2003). The coumarin structural units are commonly encountered in the architecture of several natural and unnatural biologically active compounds and have been implanted into numerous pharmaceutically valuable ingredients and drug candidates (Barot et al., 2015). Examples of several marine natural products bearing coumarin core such as ningalin B (A), and lamellarins D (B) have been found to possess HIV-1 integrase inhibition, immunomodulatory activity (Fan et al., 2008; Ridley et al., 2002). Similarly, non-marine natural products such as (+)-calanolide A (C), isolated from Calophyllum lanigerum exhibited remarkable activity against HIV-1 (McKee et al., 1996; Flavin et al., 1996; Ma et al., 2008), whereas (+)-inophyllum B (D) (Patil et al., 1993) and (+)-cordatolide A (E) (Ranjith et al., 1985) are the prominent components for inhibition against HIV-reverse transcriptase (Fig. 1). The naturally occurring molecule, Warfarin (F) bearing 4-hydroxycoumarin as the core structure has been isolated from woodruff as well as from lavender and it is used to prevent clotting of blood in the veins, lungs, or heart (Thaisrivongs et al., 1994; Bell and Caldwell, 1973; Madhavan et al., 2003). Umbelliferone (G), also known as 7-hydroxycoumarin, is another well-established natural compound found in a variety of plants, such as carrots, coriander, and garden angelica, and has been employed as a sunscreen, fluorescence indicator, and dye indicator (Lima and Polonsky, 1973; Mazimba, 2017). In addition, dicoumarol (H), isolated from sweet clover hay has been used as an anticoagulant agent (Sun et al., 2020a), whereas osthole (I) was found in Cnidium monnieri and are widely present in many bioactive compounds (Shi et al., 2013; Hung et al., 2011). Furthermore, xenthyletin (J) isolated from a marine organism, Echinogorgia sp. has been established as an antitumor and anti-bacterial agent (Tillekeratne et al., 1989; Magiatis et al., 1998).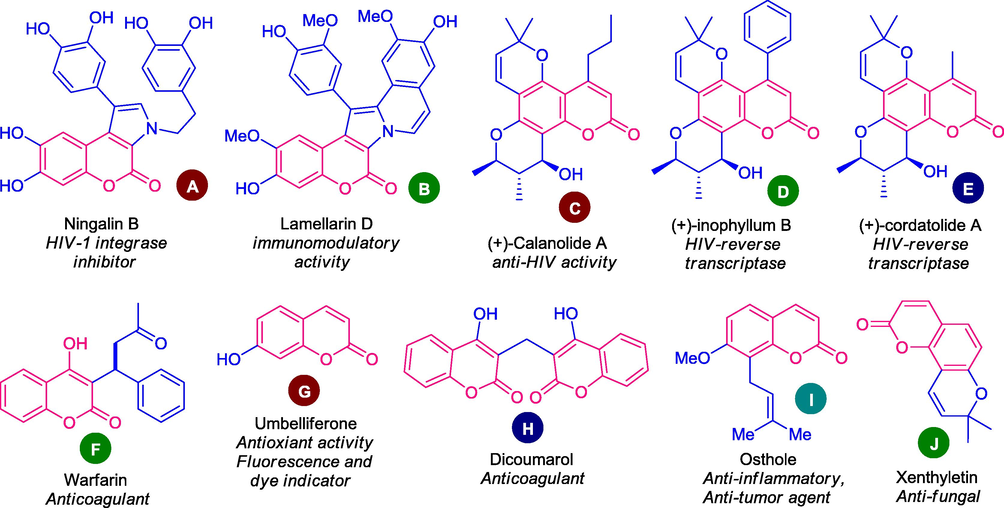
Biologically active molecules bearing coumarin moiety isolated from nature.
Besides the natural products and their analogs, the attractiveness of synthetic coumarin derivatives has drastically increased in the past decades due to their remarkable biological and pharmacological activities such as antimicrobial (Zaha and Hazem, 2002), antioxidant (Kontogiorgis and Hadjipavlou-Litina, 2003; Borges Bubols et al., 2013), analgesic (Khode et al., 2009), anticancer (Wang et al., 2002; Thakur et al., 2015; Kaur et al., 2015), antituberculosis (Keri et al., 2015), etc. Coumarin and its derivatives have also received considerable interest in material science due to their exploitation in laser dyes, fluorescent chemosensors, optical brighteners, fluorescent transthyretin folding sensors, oxygen sensors, molecular photonic devices, fluorescent whiteners, nonlinear optical chromophores, fluorescent probes, optical recording, polymers, and solar energy, etc (Trenor, et al., 2004; Traven et al., 2012; Cao et al., 2019; Sun et al., 2020b). They are generally used as dyes due to their light-emitting properties (Reynolds and Drexhage, 1975; Jones et al., 1980; Traven et al., 1997; Liu et al., 2012). Coumarin molecule is the fluorophore (fluorescent chemical compound reemit the light upon excitation) which is used in laser dyes (Nizamov et al., 2012) having brightness, photostability, and a greater stock difference that have significant commercial applications in the field of electronics. Similarly, coumarin-based fluorescent probes have attracted application in bio-imaging studies (Zhao et al., 2004; Maiti et al., 2020; Xie et al., 2021), oxygen sensors (Hara et al., 2015; Yoshihara et al., 2015), and DNA intercalators (the material used to insert the nitrogen bases at a required place in living organisms) (Al-Sehemi and El-Gogary, 2012; Li et al., 2015). Different types of fused coumarin hybrid molecules are used as photochrom (Traven et al., 2008) and also used in OLED lights (Kotchapadist et al., 2015; Puthumana and Damodaran, 2018) due to their optical and electroluminescence properties. Furthermore, coumarin and its derivatives have been utilized in the synthesis of fluorescent nanoparticles such as carbon quantum dots and graphene quantum dots that have attracted enormous application in biological imaging studies (Janus et al., 2020; Yu et al., 2020).
Considering the marvellous biological activities and wide-ranging chemical landscape of coumarins, enormous efforts have been dedicated not only just for their synthesis but also to utilizing them as a testing ground for new reaction discoveries (Lakshmi et al., 2020) that have an influential impact on the many branches of chemistry. Recently, the combination of two or more pharmacological agents into a single molecule, a hybrid molecule for providing dual activity, reactivity, and even more flexibility of the target compounds has been emerged as a key fascinating area and plays a significant role in natural and pharmaceutical chemistry (Mishra and Singh, 2016). The incorporation of another heterocycle, either as a substituent group or as a fused component into coumarin moiety, either alters or enhances the properties of the parent material, and the resulting compounds may generally display promising or even unprecedented properties.
A vast array of synthetic procedures starting from the Knoevenagel condensation (Vekariya and Patel, 2014), Perkin reaction (Vilar et al., 2006), von Pechmann (Khaligh, 2012); (Alheety et al., 2019), Kostanecki-Robinson, Reformansky (Wu et al., 2007), Baylis Hillman reactions (Kaye and Musa, 2002) to several new one-pot multicomponent, sequential tandem reactions (Chandrakar et al., 2020) has been devised. However, chemist’s ever-increasing imagination of new transformations of these products and people’s ongoing concern about their living environment as well as human health, the design and developments of new synthetic methodology to provide the efficient, atom-economical, highly reactive, and broad functionality tolerable practical route for the synthesis of new coumarin derivatives have still been desired.
Since their emergence as an alternative energy source for organic synthesis is only about a decade ago, microwaves (MW) and ultrasound irradiation have made rapid strides to be considered as the method of choice for making most of the chemical reactions sustainable for the betterment of our environment. Both microwaves and ultrasonic irradiated methods are much advantageous than conventional methods. The traditional thermal method generally involves a series of synthetic operations that required a higher amount of energy and long reaction times as well as utilize expensive catalysts and toxic volatile organic solvents that violate the green chemistry principles. Also, products could be purified by complex purification techniques which necessitate large amounts of solvents and are associated with the disadvantageous effects on the atom economy and environmental friendliness nature of the chemical process (Sheldon, 2012). To circumstance, this problem, Microwave (MW) irradiation has recently been considered as a powerful approach in organic synthesis, and medicinal chemistry for the design and construction of pharmaceutically relevant compounds as established by more than 20 years of success stories (Kaur and Kishore, 2014). After the pioneering works of Gedye and Giguere in 1986, a vast array of chemical reactions has been carried out under microwave irradiation conditions and more than 6000 articles have been subjected to microwave conditions rather than the traditional one (Polshettiwar and Varma, 2008). Microwave irradiation involves the quick heating of the reaction mixtures to high pressure- and temperatures greater than the boiling point of the solvent (Mohammadkhani and Heravi, 2020; Akondi et al., 2017). The utilization of mild reaction conditions to carry out the reactions, operational simplicity, low energy requirements, and avoidance of organic solvent or exploit less amount of solvent associated with the enhancement of the chemical transformation in terms of yields, shortened reaction times, and purity of the products. The absence of hazardous by-products also contributes to the giant popularity of microwave irradiation in organic chemistry by making them environmentally friendly and sustainable (Moseley and Kappe, 2011; Sharma et al., 2018). Because of these significant achievements, microwave technology has recently come to receive widespread global acceptance in industry and academia. However, limitations existed while using a commercial microwave due to its non-homogeneous distribution of energy and also the lack of temperature and pressure control restricted the utilization of flammable organic solvents which contribute a formidable challenge (Giguere et al., 1986; Nain et al., 2019). Similarly, ultrasound irradiation has been extensively employed as an alternative energy source in organic chemistry for the synthesis of diverse molecular scaffolds in the last decades that fulfil the goal of green and more sustainable chemistry (Banerjee, 2017a; Ziarani and Gholamzadeh, 2020). Under the influence of ultrasonic waves, molecules can oscillate around their mean position due to which the average distances of the molecules increases. Subsequently, a huge number of cavitation bubbles are formed in between the reaction mixtures, and these cavitation bubbles after growing up collapse within the reaction media to produce the fine emulsion and required amount of energy for completion of the reaction (Banerjee, 2017b). The exploitation of ultrasound in organic synthesis sometimes leads to such an environment where a catalyst is not required for promoting the reaction which contributes eco-friendly nature of the reaction. Unlike microwaves, the low energy requirements, fast reaction rate, shorter reaction time, higher reactivity and selectivity, high purity of products also make ultrasonic irradiation a very attractive and beneficial strategy for the synthesis of bioactive heterocycles (Banerjee, 2017c; Banerjee 2019) and many more review articles has covered their application in organic synthesis over the last decades (Baig, and Varma, 2012); (Kaur, 2018; Dallinger and Kappe, 2007). Consequently, in the last few years, there were immense applications of this non-conventional strategy for the synthesis of diverse coumarin heterocycles. Although several review articles covering the synthesis of coumarins (Medina et al., 2015) (Gaudino et al., 2016; Kahveci and Menteşe, 2018; Ibrar et al., 2018; Molnar et al., 2020, Zeydi et al., 2020), as well as 4-hydroxycoumarin (Jung and Park, 2009; Borah et al., 2021d) and their application in the synthesis of various heterocyclic compounds (Abdou et al., 2019a; Abdou et al., 2019b; Ziarani et al., 2019). However, none of them has especially focused on the non-conventional strategy for particularly the synthesis of coumarins containing heterocycles. In this review article, we will mainly focus on the recent signs of progress achieved in the synthesis of coumarins either linked or fused with pharmaceutically relevant five-membered heterocycles such as pyrroles, furan, thiophenes, pyrazoles/pyrazolones, imidazoles, thiazoles, triazoles, oxadiazoles as well as six-membered heterocycles like pyridines, quinolines, pyrans, and pyrimidines by making the utilization of non-conventional strategies like microwaves (MW) and ultrasound irradiation and covering the literature from 2014 to date.
2 Synthesis of coumarin-linked with five-membered heterocycles
2.1 Linked with one heteroatom bearing five-membered ring
2.1.1 Pyrroles, furans, and thiophenes
The incorporation of a biologically active compound to another biologically active compound for providing enhanced activities and reactivities of the target compounds has emerged as a rapidly growing area of research for synthetic chemists in industry and academia. The most well-known five-membered pyrroles (Borah et al., 2021b; Borah et al., 2021c), furans (Duc, 2019), and thiophenes (Gramec et al., 2014) have drawn increased attention in medicinal and synthetic organic chemistry due to their tremendous application. Consequently, their combination with bioactive coumarins for the synthesis of value-added products has received considerable attention.
On the other hand, multicomponent reactions (MCRs) has recently attracted enormous attention in both organic and medicinal chemistry for the construction of diverse molecular complexity due to their propensity of forming multiple bonds in a single operation from readily available starting materials, step- and atom-economy nature, operational simplicity, eco-friendliness, and environmentally benign nature (Jiang et al., 2010; Brauch et al., 2013; Zhi et al., 2019).
Considering the importance of both these topics, Das and co-workers in 2018 developed a highly efficient one-pot microwave-assisted strategy for the construction of coumarins-linked pyrrole derivatives 4. Using 30 mol% of InCl3 as the catalyst, the three-component reaction of coumarin 1, β-nitrostyrene 2, and dialkyl acetylene dicarboxylate 3 deliver the desired products 4 in moderate yield. The methodology was found to be limited to only 6-amino coumarin (R1 = R3 = H; R2 = NH2) and no reaction was observed with substituted coumarin 1 (R1 = NH2; R3 = Me) which marks the limitations of this protocol (Scheme 1) (Das et al., 2018). The proposed mechanism for this reaction starts with the initial formation of enamine Int-1 via the nucleophilic addition of coumarin 1a to dialkyl acetylene dicarboxylate 3. The intermediate Int-1 then underwent Michael addition with β-nitrostyrene 2 in presence of InCl3 to furnish the adduct Int-2 which on cyclization furnished intermediate Int-3 and its final aromatization afforded the desired products 4. The utilization of microwave techniques provides a high yield of the products that could be simply isolated in pure form without using any tedious process within very short reaction times.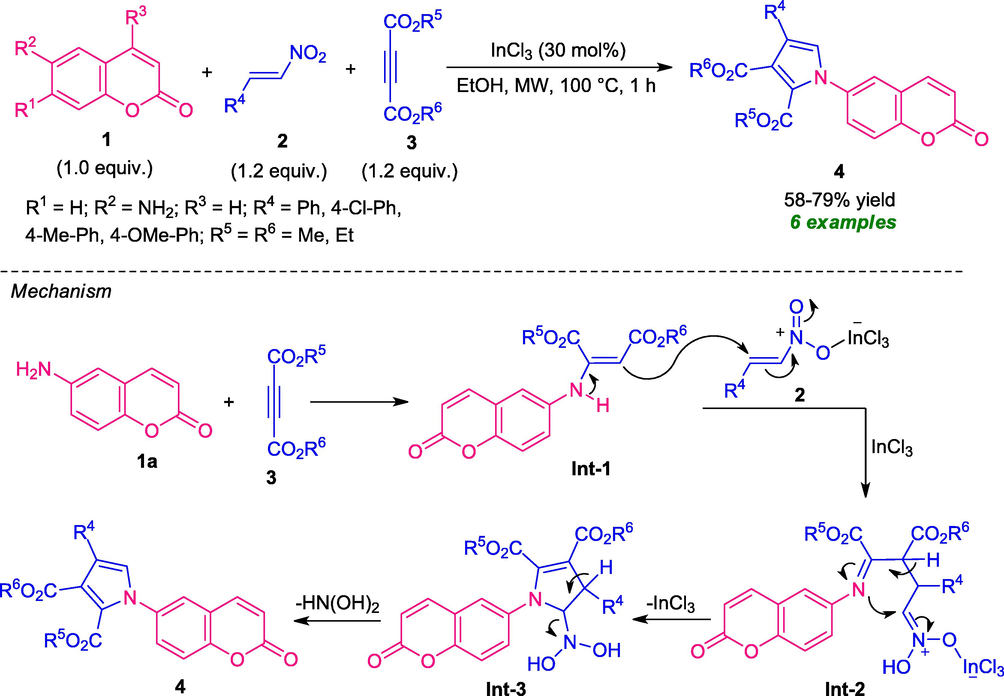
Three-component synthesis of coumarins-containing pyrrole core under MW.
Mishra et al., in 2018, reported a metal-free approach for the synthesis of diverse coumarin-linked pyrrole derivatives from the multicomponent reaction of 4-hydroxycoumarin 5, aryl glyoxals 6, and various amine source 7, 9, 11, or 13. This reaction under microwave heating at 130 °C was found to proceed smoothly to deliver the desired products 8, 10, 12, and 14 in good yields within 30 min (Scheme 2) (Mishra et al., 2018). The products were isolated by a simple workup procedure without using any chromatographic techniques. Although different sets of coumarin-linked pyrroles were synthesized, the narrow substrates scopes constitute a limitation of this approach. Therefore, broad substrate scopes for this reaction are highly demanded. Utilization of microwaves also offers additional advantages including mild reaction condition, clean reaction profile, avoidance of toxic metal and solvents, high purity of products, making the protocol useful for constructing the target compounds of pharmaceutical interest.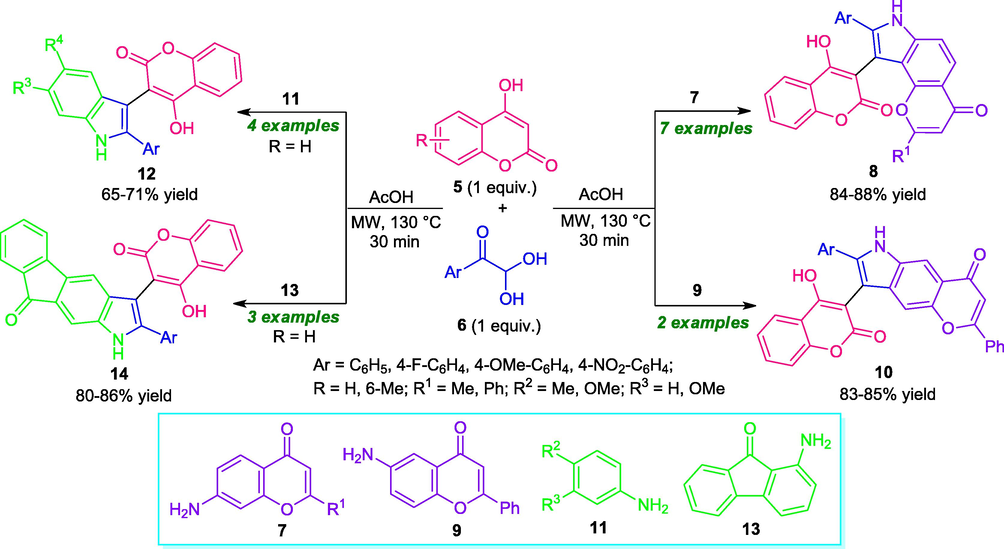
Microwave-assisted one-pot multicomponent synthesis of diverse pyrrole-substituted coumarin hybrid.
Microwave technology was introduced as a facile, mild and environmentally benign approach for the regioselective synthesis of a vast array of dihydrofuran substituted coumarin derivatives 19 (Tangeti et al., 2016). Under the solvent-free condition, the desired products 19 derived from readily available salicylaldehyde 15, 6-methyl, 4-hydroxy pyranone 16, aryl aldehydes 17, and pyridinium ylide 18, were obtained in 71–89% yield. Compared to conventional heating conditions, the employment of microwaves shortened the reaction times as well as increases the yield of the products. Also, broad substrates scopes, solvent-free conditions, are some of the other salient features of this approach. The reaction can proceed through the initial Knoevenagel condensation of 15 and 16 to produce the adduct Int-5 which further underwent reaction with aldehydes 17 to form the intermediate Int-6. In the meantime, the reaction of 18 with Et3N afforded the pyridinium ylide Int-7 that can then undergoes Michael addition with intermediate Int-6 and releases the enolate Int-8. The final elimination of pyridines from enolate Int-8 and its subsequent cyclization yield the target compound 19 (Scheme 3).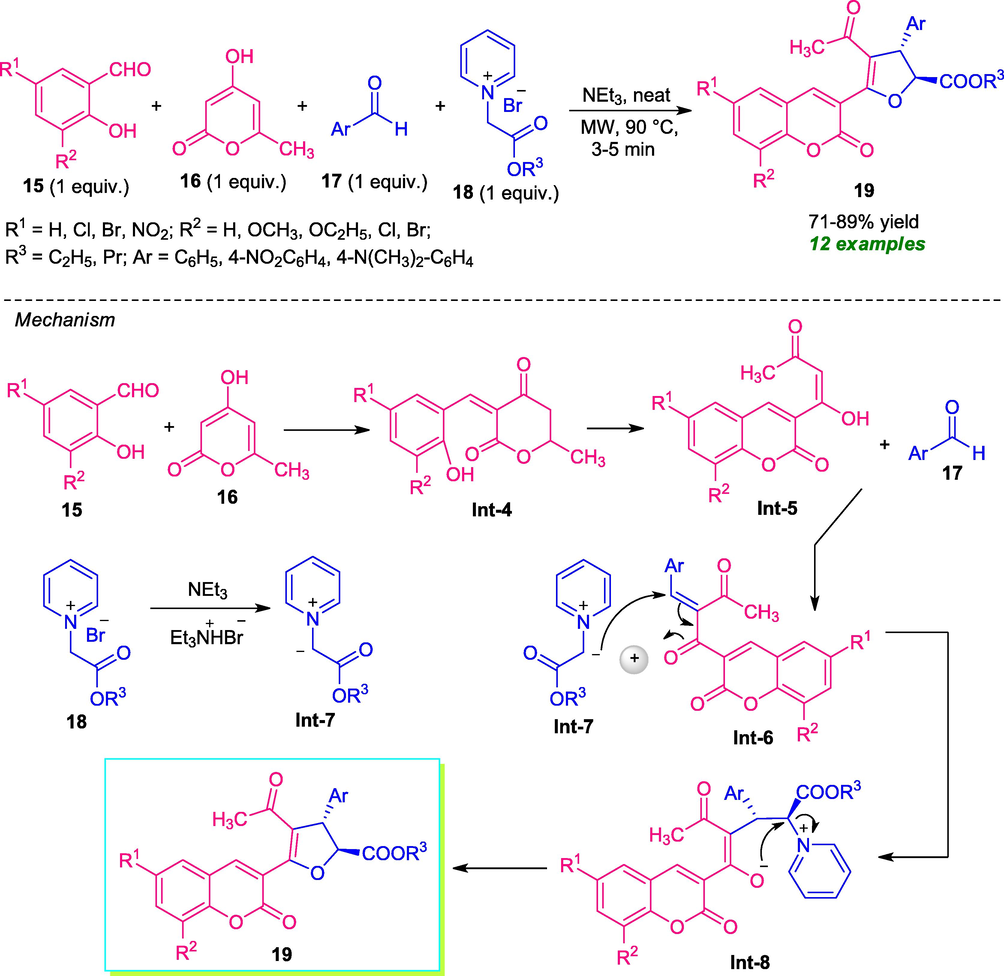
One-pot multi-component approach to C-3 functionalized dihydrofuran substituted coumarin derivatives under microwave irradiation.
A highly efficient, and robust one-pot strategy for the synthesis of coumarins-furan derivatives 22 has been developed by Gao and co-workers in 2018. Under the catalyst-free and microwave condition, the three-component reactions of 4-hydroxycoumarin 5, phenylglyoxal 20, and 4-arylaminofuran-2(5H)-one 21, afforded a variety of coumarins-furan products 22 in moderate to high yield (Scheme 4) (Gao et al., 2018). Various electron-withdrawing and electron-donating groups present on the aryl ring of glyoxals as well as amino-furan-ring smoothly participated in the reaction under microwave irradiation. Interestingly, this microwave-assisted method was established as a powerful strategy over the traditional thermal method for the construction of coumarin-linked furan hybrid owing to its mild reaction condition, short reaction time, environmentally benign reaction media, and column-free nature.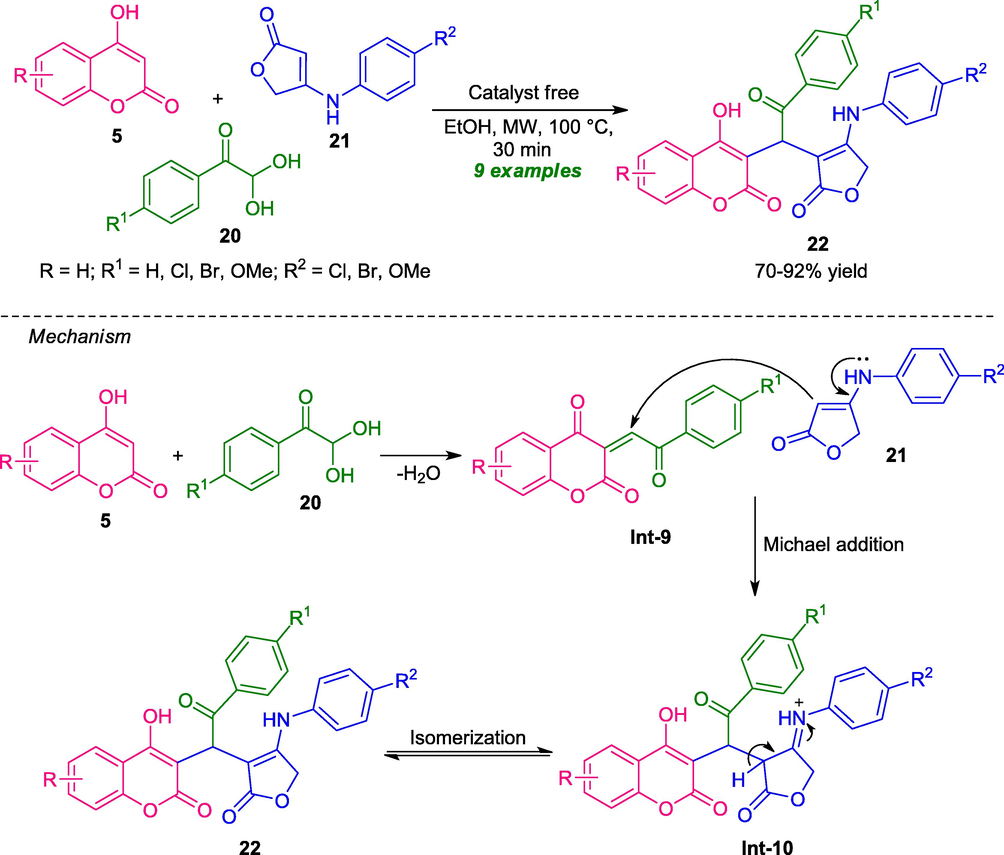
Catalyst-free microwave-assisted one-pot synthesis of coumarin-furan derivatives.
A mechanism was proposed by the authors for this transformation which involves the initial formation of intermediate Int-9 from the Knoevenagel condensation of 4-hydroxycoumarin 5 and aryl glyoxals 20. The subsequent Michael addition of Knoevenagel adduct Int-9 to amino-furan 21 then occurs to provide intermediate Int-10, which on isomerization afford the final products 22.
Around the same time, Yahaya and his group synthesized a novel coumarin-thiophene hybrid 29 by employing step-wise (Scheme 5A) and one-pot synthetic routes under both conventional and microwave heating conditions (Scheme 5B) (Yahaya et al., 2019). The conventional step-wise method afforded the products in 80–90% yield within 2–3 h; whereas the microwave-assisted step-wise method provides 90–96% yield of the products within 2–3 min. Although the step-wise method delivers the desired products 29 in high yield, however, the requirements of longer reaction conditions and large amounts of solvent constitute a shortcoming of this method. Consequently, a one-pot strategy was developed for the synthesis of 29 from a three-component reaction of coumarin 23, malononitrile 26, and sulfur 28 in presence of Et2NH as the catalyst. This one-pot method was found to be the best choice over the step-wise method in terms of the high yield of the products and fast completion rate. A comparison study of both conventional and microwave irradiation condition in one-pot approach revealed that the reaction carried out in presence of microwave heating condition were found to be better in terms of purity of the products, better yields (82–90% in MW; 80–85% in CM), and short reaction time (3–5 min for MW; 14–20 h for CM). Also, the microwave technique provides mild, efficient, environmentally as well as eco-friendly nature of the protocol.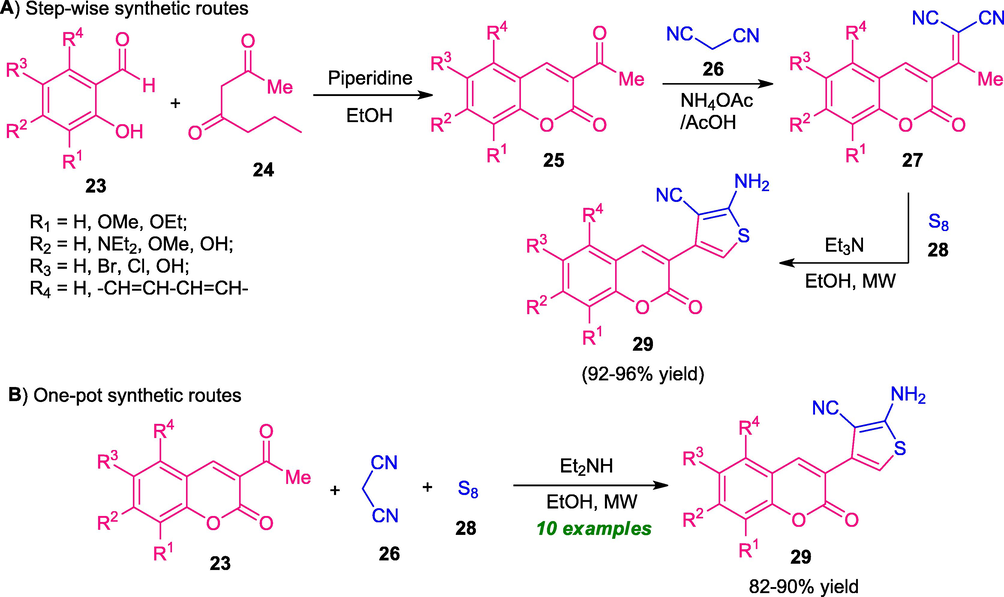
MW-assisted step-wise and one-pot construction of coumarins-bearing a thiophene core.
2.2 Linked with two heteroatoms bearing five-membered ring
2.2.1 Imidazoles, thiazoles, and pyrazoles
A simple straightforward route for the synthesis of a variety of imidazole-coumarin structures 36 was developed by Ashok et al. in 2021. Under the influence of microwave irradiation at 180 W, the step-wise reaction of resorcinol 30, ethyl acetoacetate 31, dibromo alkane 33, and chromene-imidazoles 35 afforded the desired products 22 in good yield (Scheme 6) (Ashok et al., 2021). The utilization of traditional heating condition (80 °C) instead of microwave irradiation realizes a long reaction time of up to 12 h for the completion of the reaction and provide 61–68% yield of the products. Therefore, the microwave irradiated method was established as the best method for this reaction which shortened the reaction time to 8–12 min from 10 to 12 h and increases the yield of the products (78–83% yield). Furthermore, the synthesized compounds were found to have antimicrobial activity against Bacillus subtilis, Staphylococcus aureus, Escherichia coli, and Klebsiella pneumonia as well as fungi Aspergillus Niger, Aspergillus flavus, and Fusarium oxysporum which constitutes the major significance of this approach. However, limited substrate scopes represent a limitation of this approach.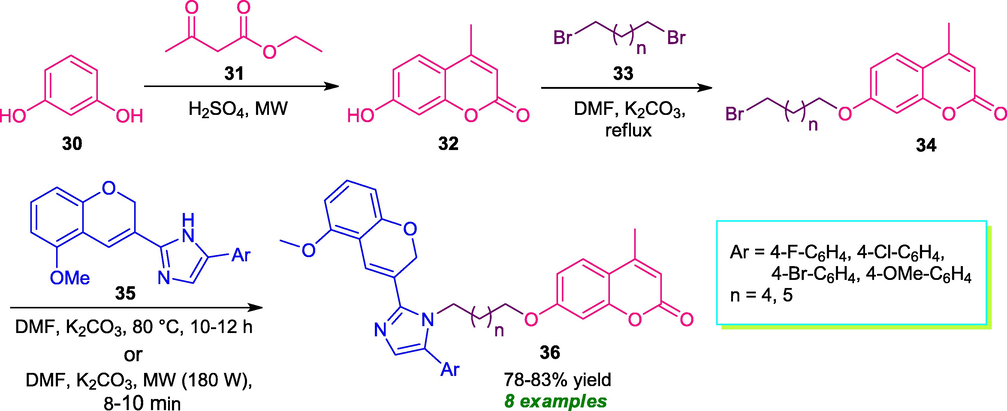
Synthesis of coumarin-linked to imidazole under microwave-irradiation condition.
In 2017, Gabr et al. reported a step-wise strategy for the synthesis of novel coumarin-thiazole derivatives 42 under microwave irradiation. Initially, the reaction of aryl aldehydes 17 with thiosemicarbazide 37 in ethanol under microwave irradiation (50 W) at 80 °C provides 2-arylidene-hydrazinocarbothioamides 38 in good yield within 10 min. On the other hand, treatment of salicylaldehyde 39 with ethyl acetoacetate 31 in presence of piperidine as the base catalyst was found to proceed under microwave irradiation (50 W) at 45 °C to form 3-acetylcoumarin 40 which on reacting with bromine furnishes 3-bromoacetyl coumarin 41. Consequently, the reaction of an equimolar amount of 38 and 41 in presence of ammonium hydroxide as the catalyst under the influences of microwave irradiation (60 W) at 100 °C afforded the corresponding products 42 in 62–89% yield (Scheme 7) (Gabr et al., 2017). The reaction condition tolerates a wide variety of substitutions at the thioamide ring 38 and delivers the highly functionalized desired product 42 in high yield. Although, exploitation of microwaves as a green strategy shortened the reaction times in every step of the reaction and improved the yield of the products; the limitations associated with the step-wise method resulted in the consumption of a large amount of solvent and involved waste formations. Therefore, a simple and one-pot strategy for the synthesis of this compound is highly desired.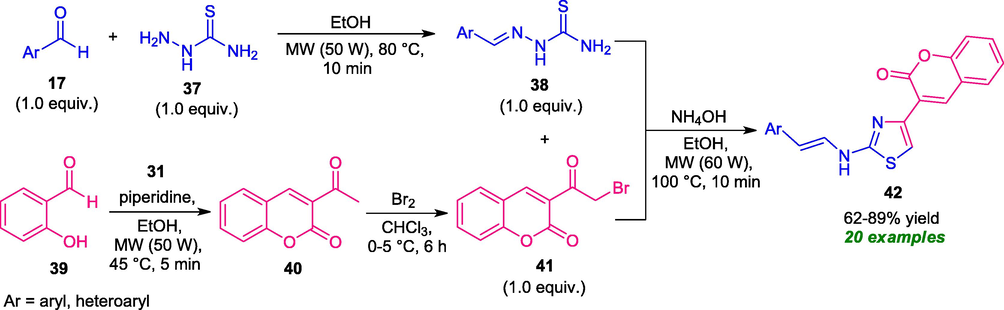
Microwave-assisted construction of coumarin-thiazole derivatives 42.
The microwave-assisted methodology was again proved to constitute an efficient green alternative for the synthesis of coumarin-thiazolidine-2,4-dione 45 by Mangasuli in 2018. With the help of anhydrous K2CO3 as the catalyst, the reaction of various coumarins 43 and thiazolidinedione 44 carried out in a microwave pressure vial and irradiated in a microwave oven with power 100 W at 50 °C, delivered the desired products 45 in good to high yield (85–94%). However, when the same reaction was carried out by the conventional method, a decrease in the product yield (75–84%) with a long reaction time was observed. From this observation, it was concluded that the microwave techniques are much more superior to traditional ones in terms of product yield and reaction time (5–9 min). Other salient features of this protocol include broad functional group tolerances, mild reaction conditions, a clean reaction profile, and prominent pharmacological activity of the synthesized compounds (Scheme 8) (Mangasuli et al., 2018).
Microwave-assisted synthesis of coumarin-thiazolidine hybrids 45.
In 2021, Mamidala and co-workers introduced microwave technology to develop a practical and efficient method for the construction of several coumarin-thiazole derivatives 49 (Mamidala et al., 2021). The synthesis was achieved via a one-pot three-component reaction of coumarins 46, aldehydes 47, and thiocarbohydrazide 48 using 20 mol% of acetic acid as the catalyst under microwave irradiation with 210 W power at a temperature of 70 °C (Scheme 9). Under this standard condition, the corresponding products 49 were obtained in good to high yield. Initial optimization for the reaction revealed that the reaction performed in microwave irradiation afforded the desired product 49 with excellent purity within a short reaction time as compared to the conventional method which required a higher amount of energy and delivers the products after a very long reaction time. However, the narrow substrate scopes mark a limitation of this strategy and calls for further developments. The plausible mechanism for this reaction begins with the initial treatment of 2 equivalents of aldehydes 47 with one equivalent of thiocarbohydrazide 48, thereby providing bis-thiocarbohydrazone Int-11. Subsequent SN2-type reaction of intermediate Int-11 to 46 deliver the intermediate Int-12, which on cyclization followed by dehydration yield the final products 49 via intermediate Int-13.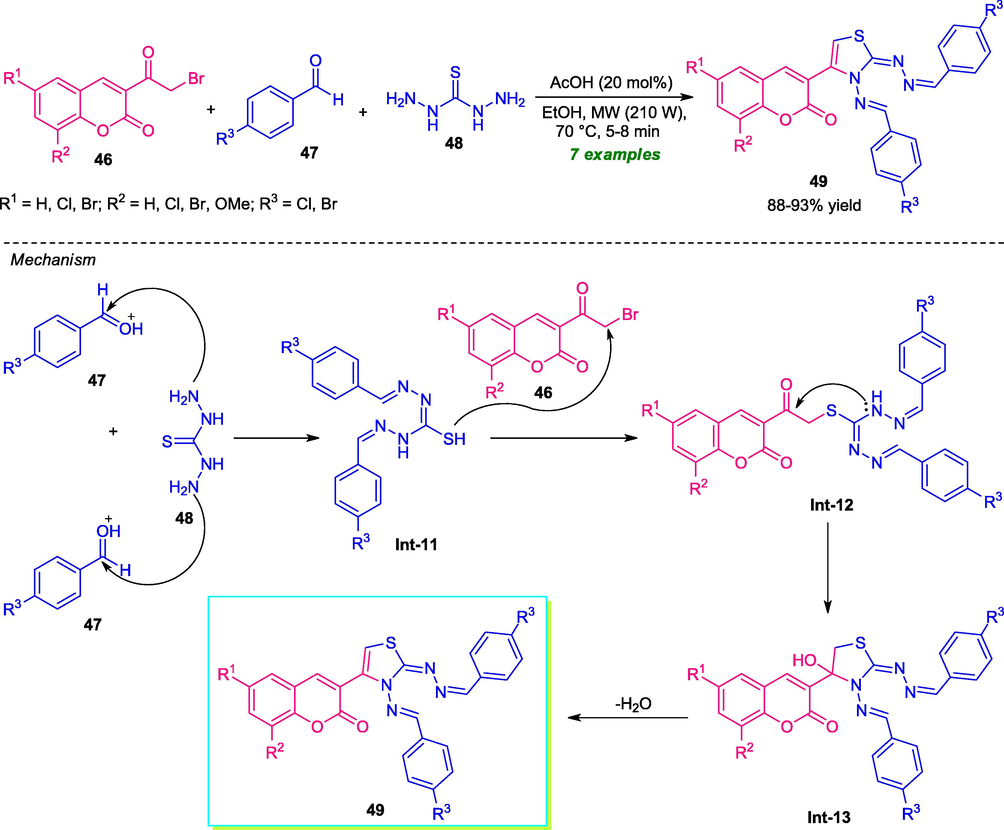
Three-component microwave-irradiated synthesis of coumarin-thiazole.
Considering the importance of pyrazolone in organic and medicinal chemistry (Borah et al., 2021a), Jadhav and co-workers reported the combinatorial synthesis of diverse coumarin-linked pyrazolone derivatives 52 by employing a microwave-assisted one-pot multicomponent approach (Jadhav et al., 2015). With the help of alum [KAl (SO4)2·12H2O] as the catalytic system and polyethylene glycol (PEG-400) as the reaction medium, the treatment of 4-hydroxycoumarin 5, aldehydes 50, ethyl acetoacetate 31, and hydrazine hydrate 51 under microwave irradiation (400 W) at 100 °C results in the formation of the desired products 52 in moderate to excellent yield. A variety of aryl- alkyl- and heteroaryl substituted aldehydes smoothly underwent the reaction without affecting the outcome of the reaction. A mechanism was proposed to account for this reaction which proceeds either through the Michael addition of Knoevenagel adduct Int-16 (generated in situ from the alum catalyzed reaction of 5 with 50) to O-H form of pyrazolone Int-14 (produced in situ from ethyl acetoacetate and hydrazine) or alum catalyzed reaction of 5 to arylidene pyrazolone Int-15. By both of these ways, the final product 52 could be formed (Scheme 10).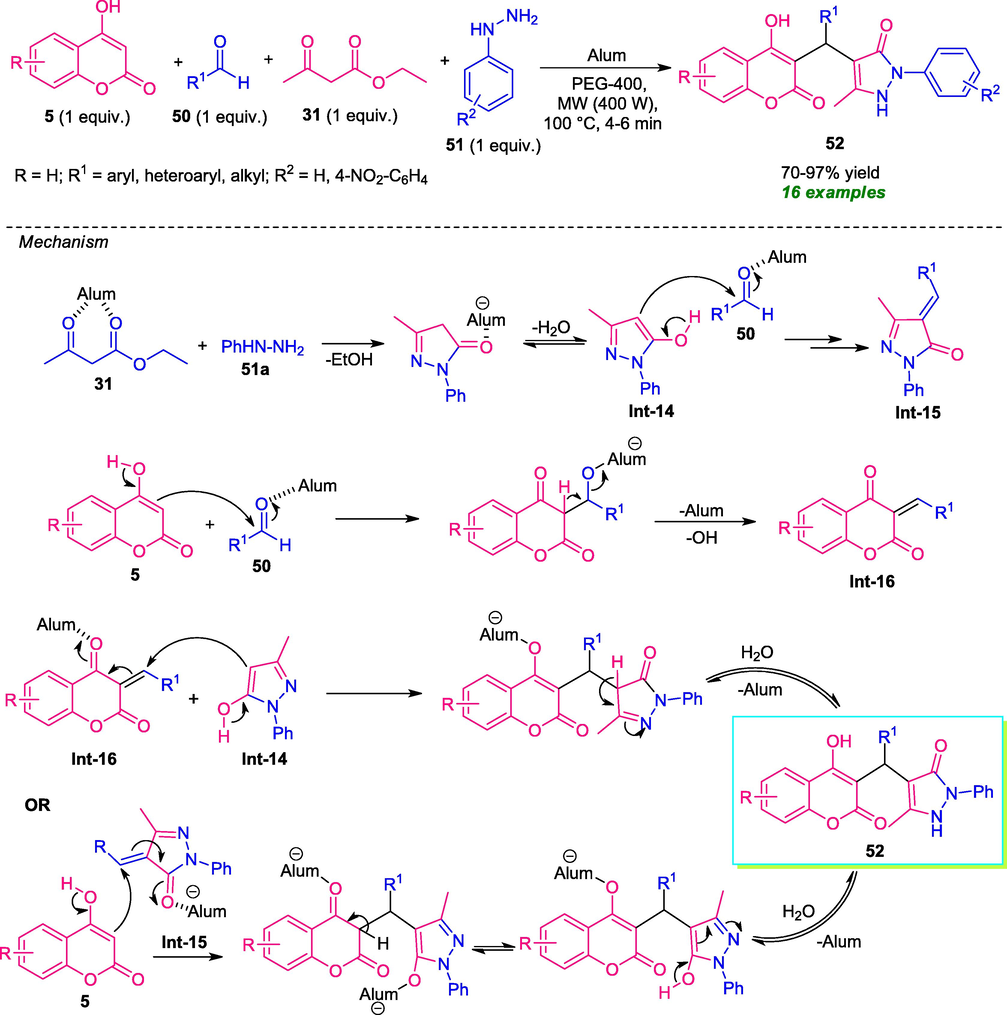
One-pot multicomponent reaction under microwave irradiation toward the synthesis of coumarin-pyrazolone hybrid.
In 2016, Seydimemet and co-workers reported a highly convenient one-pot strategy for the synthesis of a variety of coumarin-pyrazoles by introducing ultrasonication technology. With the help of L-proline as the organocatalyst, the ultrasound-irradiated four-component reaction of coumarin 53, hydrazine 51a, aldehyde 50, and malononitrile 26 afforded the desired products 54 in moderate to good yield. The wide-substrate scopes, energy-efficient, short reaction time, eco-friendly, and environmentally friendly nature are some of the salient features of this protocol (Scheme 11) (Seydimemet et al., 2016). The overall process can be initiated through the formation of coumarin-pyrazolone intermediate Int-17, which under influence of L-proline afforded the adduct Int-18. This adducts Int-18 then undergoes Michael addition with arylidene malononitrile Int-20 produced in situ from L-proline catalyzed Knoevenagel condensation of aldehydes 50 with malononitrile 26, leading to intermediate Int-22. Consequently, the 6-exo-dig cyclization of Int-22 followed by tautomerization provides the final products 54.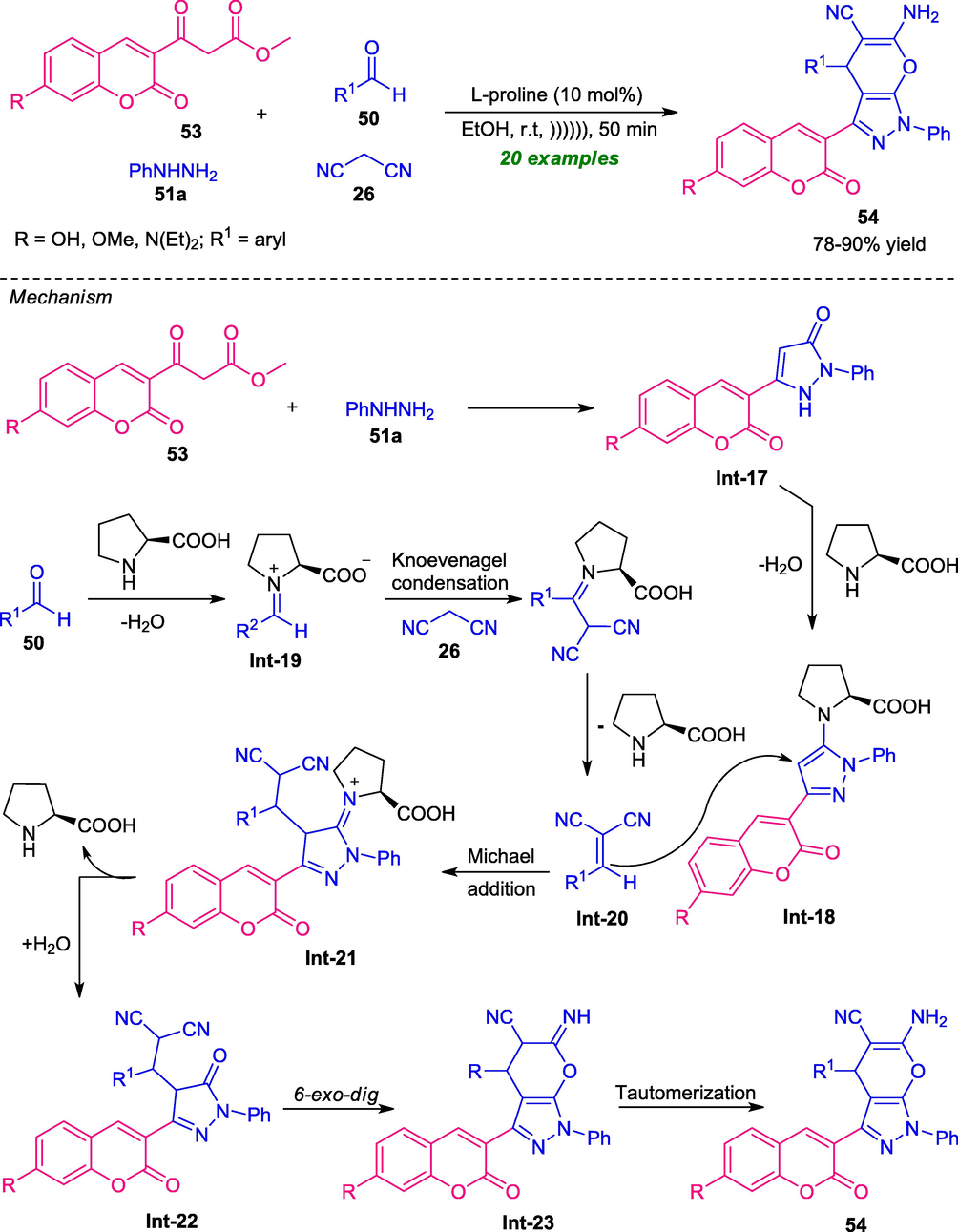
Ultrasound-assisted secondary amine catalyzed synthesis of coumarin-pyrazoles 54.
A step-wise microwave-irradiated procedure has been developed for the synthesis of several coumarin-pyrazole hybrids 57 from ethyl acetoacetate 31, salicylaldehyde 39 aldehyde 47, and hydrazine 56. The resultant products were obtained in moderate yield. The synthesized compound was found to have significant anti-malarial and anti-microbial activity (Scheme 12) (Akhtar et al., 2017). Despite, notable improvements in the reaction time, a yield of the products, as well as purity of the products, has been achieved by using microwave conditions, the step-wise method involves the waste of a large amount of solvent in the separation of the product intermediates which contributes to a major limitation of this approach. Consequently, a simple, practicable, atom-economical, and highly efficient one-pot strategy for the synthesis of these pharmaceutically relevant compounds is still desired.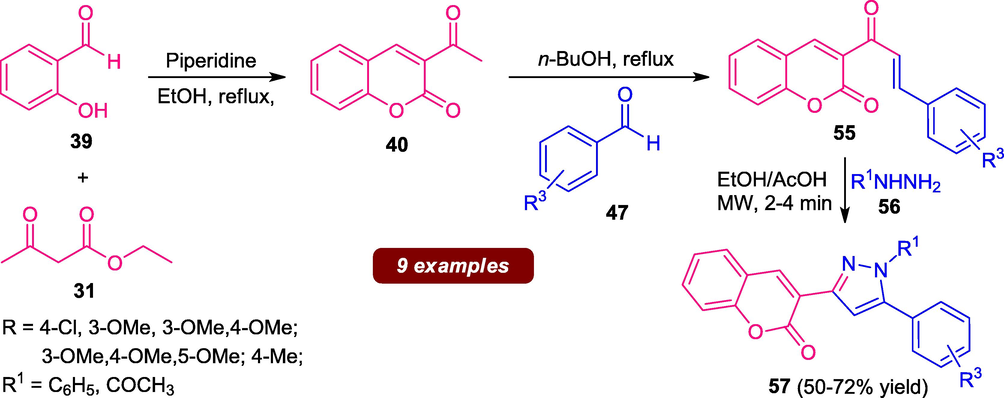
Microwave-assisted synthesis of coumarin-pyrazole hybrid 57.
The utilization of water as green solvents (Borah et al., 2021a) for the synthesis of coumarin-pyrazole structure 59 has been demonstrated by Nikpassand and co-workers in 2018. By using 10 mol% of citric acid as the catalyst, the reaction of coumarin 5 and pyrazole 58 under the influence of ultrasound irradiation deliver the desired product 59 in good to high yield (Scheme 13) (Nikpassand et al., 2018). Interestingly, a comparison of the ultrasonic method with the conventional method was carried out for this reaction. Out of these conditions optimized by the authors, it was found that the reaction carried out under ultrasonic irradiation proceeded at room temperature, and therefore making the protocol energy efficient as compared to the conventional method which required a high energy source for the completion of the reaction. Ultrasonic technology also increases the rate of the reaction and provides a high level of yield. The strategy offers other significant advantages such as environmentally and eco-friendly nature, wide-substrate scopes, short reaction time, mild conditions are advantages of this protocol.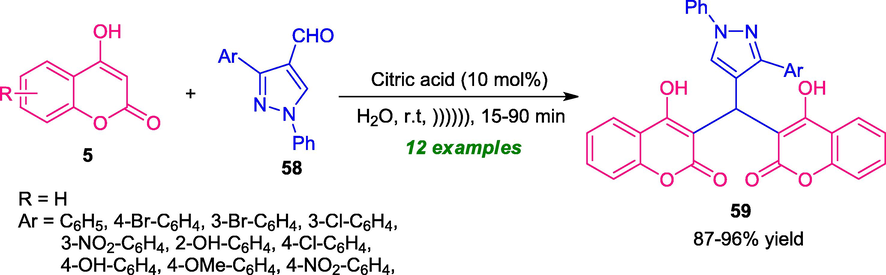
Ultrasound-irradiated synthesis of coumarin-pyrazole derivatives 59 in water.
Almost at the same time, Chavan and Hosamani realized a rapid strategy for the synthesis of value-added coumarin-pyrazole derivatives by using microwave technology. In the presence of K2CO3 as the base catalyst and acetone as the solvent system, the coumarin-pyrazole products 61 derived from various 4-bromomethyl coumarins 43 and pyrazolone 60 bearing Schiff base structure under microwave irradiation with the power of 200 W at the temperature of 35 °C, were obtained in good to high yield (Scheme 14) (Chavan and Hosamani, 2018). Interestingly, the synthesized compounds were found to have antibacterial and anti-inflammatory activity. The use of microwave condition make this strategy environmentally as well as eco-friendly benign and provides a high yield of the products in a short reaction time (8–12 min) with perfect purity over the traditional thermal method that unusually required a longer reaction time to complete the reaction. Despite this notable development, the limited substrate scopes constitute a drawback of this protocol.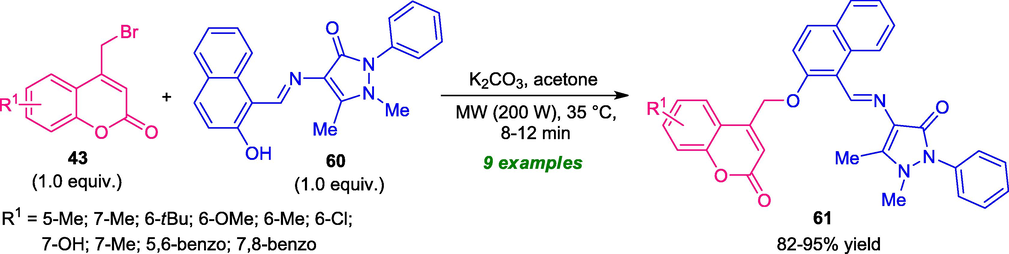
Synthesis of coumarin-pyrazole derivatives 61 under the influence of MW.
2.3 Linked with three heteroatoms bearing five-membered ring
2.3.1 Triazoles, and oxadiazoles
The term “click chemistry” introduced by Sharpless is used for describing a “spring-loaded” chemical reaction in which Cu-catalyzed azide − alkyne cycloaddition reaction has received enormous attention for the synthesis of medicinally important 1,2,3-triazoles (Medina et al., 2015).
In this regard, Li and co-workers developed an “on water” Cu(I)-catalyzed 1,3-dipolar cycloaddition of coumarin azides 62 and terminal alkynes 63 under ultrasonic irradiation to afford a series of coumarin derivatives 64 linked with 1,2,3-triazole moiety. The desired coumarin-triazole products 64 were obtained in good to high yield in a completely green and eco-friendly fashion. This reaction condition was found to be suitable for diverse substitutions on the terminal alkyne rings. The developed methodology was further applied to include coumarin alkyne of type 65 with azides 66 that delivers the coumarin-triazole product 67 in good yield (Scheme 15) (Li et al., 2016). The utilization of water and ultrasonic approach leads to the fast acceleration of the reaction as compared to other organic solvents and makes the protocol very attractive to move toward more green and sustainable chemistry. However, the requirement of a slightly higher reaction time unnecessarily points toward a limitation of this approach otherwise outstanding developments.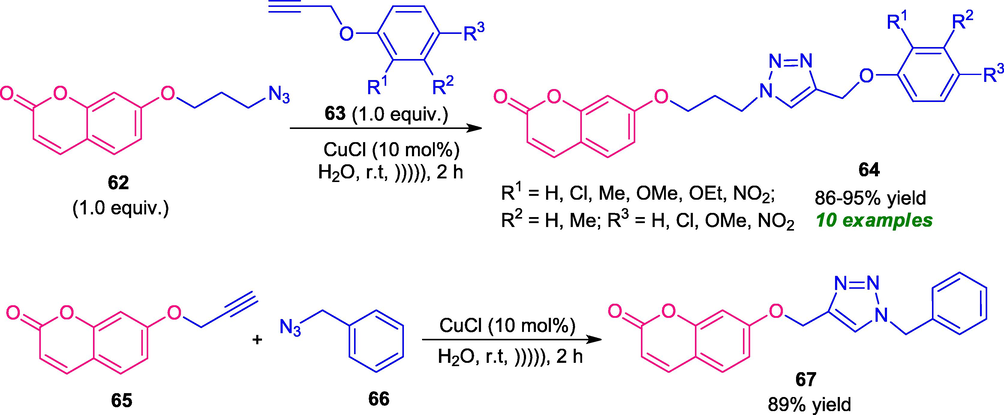
Ultrasound irradiated 1,3-dipolar mediated synthesis of coumarin-triazoles derivatives.
In 2018, Ashok et al. reported a three-step synthesis of dimmer of coumarin-triazoles 72 under microwave irradiation. The process involves the initial construction of 7-hydroxycoumarin 69 via a Pechmann condensation of resorcinols 68 and ethyl acetoacetate 31, which further underwent alkylation with propargyl bromide to form 70. The final step involves the Cu(I)-catalyzed 1,3-dipolar cycloaddition of 70 with diazidoalkanes (generated in situ from 71 and sodium azide) under microwave irradiation at 180 W, leads to the formation of final products 72 in 75–86% yield (Scheme 16) (Ashok et al., 2018). The same reaction (final step) when carried out under conventional heating at 80 °C takes 24 h to achieve completion of the reaction and provides a lower yield (43–53%) of the products (72). Pleasingly, the microwave approach served as the main reason for this reaction to improve the product yield within a very short reaction time (10 min). The use of green reaction media, the biological activity of synthesized compounds marks other advantages of this protocol.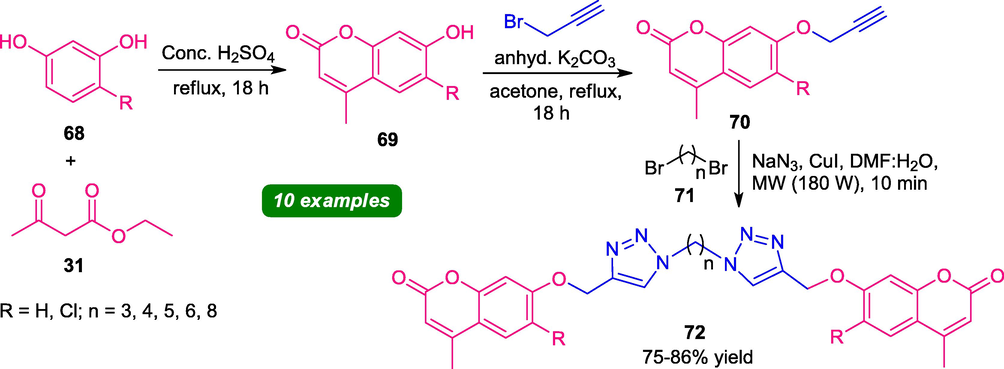
Synthesis of coumarin-triazoles under microwave irradiation.
In a different approach, Jain et al. has designed a magnetic Fe3O4 core decorated with cyclodextrin-citric acid (Fe3O4@CD-CIT) as a nano-phase transfer catalyst with low Cu-loading for the synthesis of coumarin-triazole 75 (Scheme 17) (Jain et al., 2019). By using water as the green reaction media under ultrasound irradiation, the resultant products 75 derived from various coumarin azide 73 and terminal alkyne 74, were obtained in high to excellent yield. The effectiveness of the protocol was established by its applicability in the scale-up synthesis under low catalyst loading. The mild reaction condition, energy-efficient, cost-effective, recyclability, and reusability of the catalyst, easily available starting materials make this protocol very attractive from the viewpoint of green and sustainable chemistry.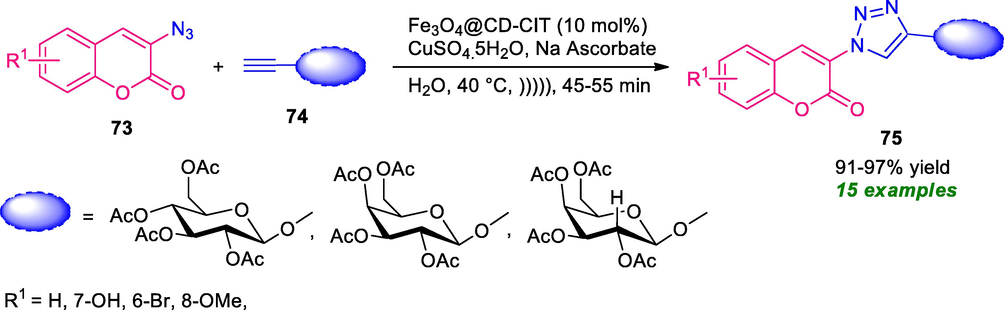
Nano PTC catalyzed synthesis of coumarin-triazoles in water under ultrasound irradiation.
Almost at the same time, Dharavath and co-workers demonstrated a Cu(I)-catalyzed 1,3-dipolar cycloaddition of coumarin alkyne of type 81 and aryl azides 82 for the synthesis of coumarin-triazoles 83 by introducing microwave conditions (Dharavath et al., 2020). The coumarin alkynes 81 were prepared in a step-wise manner from 76 and 79. Under microwave irradiation (200 W) at a fixed temperature of 40–100 °C, a variety of coumarin-triazole derivatives 83 were synthesized within a very short reaction time in good yield (Scheme 18). To broaden the scope of this reaction, various substituted alkynes 81 and azides 82 were employed which efficiently provided the corresponding products with sufficient reactivity and selectivity. Although, the reaction was carried out under both conventional as well as microwaves condition; however, the microwave condition was found to be very efficient over conventional one in terms of products yield, and reaction time.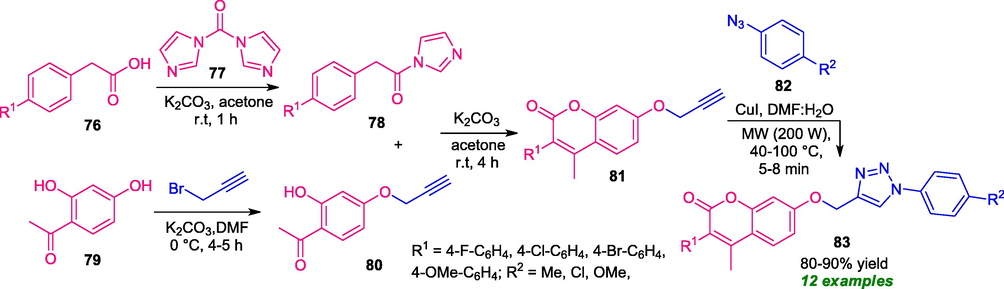
MW-assisted Cu-catalyzed 1,3-cycloaddition to access coumarin-triazoles 83.
Unlike triazoles, incorporation of oxadiazole into coumarin moiety for providing enhanced reactivity was recently established by Kapadiya and Dholaria. With the help of microwave irradiation with the power of 180 W, various coumarin-oxadiazole derivatives 90 were synthesized from oxadiazole-2-thiol 87 and coumarin 89 in poor to high yield. The intermediate 87 was synthesized in a two-step reaction from methyl benzoate 84 and coumarin intermediate 89 was synthesized in a one-step reaction from 4-hydroxycoumarin 5 (Scheme 19) (Kapadiya and Dholaria, 2021). The reaction condition tolerates a vast array of substitution on the oxadiazole-2-thiol ring 87. However, no reaction was observed with chloro-substituents present on the oxadiazole ring 87 (R = 3-Cl, 4-Cl), which marks the limitation of this protocol. In this case, a high yield of the products was achieved by the conventional method as compared to the microwave method. However, it takes much more time generally 4–6 h as compared to the microwave technique which required only 15–25 min for completing the reaction.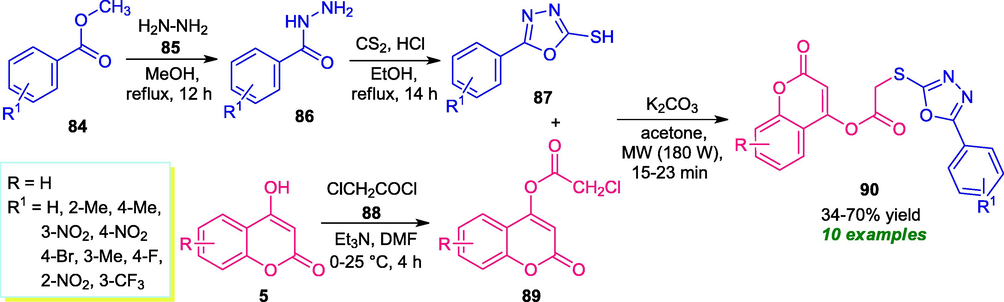
Synthesis of coumarin-oxadiazoles derivatives under microwave-irradiation.
3 Synthesis of coumarin-linked with six-membered heterocycles
3.1 Linked with one heteroatom bearing six-membered ring
3.1.1 Pyridine, quinoline, and pyrans
As a result of their tremendous application, the nitrogen-containing pyridine, quinoline, and oxygen-containing pyran molecule (Dwivedi et al., 2020a; Borah and Chowhan, 2021e; Borah et al., 2021f) have attracted considerable attention in synthetic as well as medicinal chemistry.
Desai et al. has demonstrated that the reaction of various 3-acetyl coumarin 40, aldehydes 47, and malononitrile 26 when irradiated by microwaves (350 W) for 6–10 min at 100 °C in the presence of ammonium acetates, coumarin-pyridine structure 91 was formed in good yield (Desai et al., 2017). Further treatment of 91 with furfural 92 catalyzed by ZnCl2 under microwave irradiation (300 W) at 100 °C, delivered furan substituted coumarin-pyridine hybrid 93 in 65–82% yield (Scheme 20). Aldehydes possessing various electron-withdrawing as well as electron-donating substituents were found to smoothly participate in the reaction under the microwave condition. The utilization of microwave reduces the reaction time in both cases and proved to be an efficient, powerful environmentally benign technique for the clean synthesis of the target compound.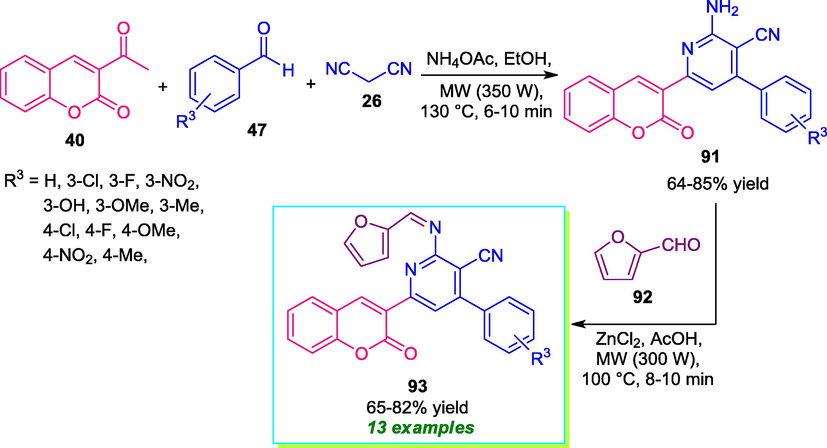
Microwave-assisted synthesis of coumarin-pyridine derivatives 93.
El-Naggar and co-workers disclosed microwave techniques to develop a convenient approach for the synthesis of several coumarin-pyridine derivatives (El-Naggar et al., 2017). The process involves the reaction of coumarin chalcone 95 (generated from the piperidine catalyzed Claisen-Schmidt condensation of 3-acetyl coumarin 40 and aldehydes 94 in microwave condition) with different active methylene compounds such as malononitrile 26 and ethyl acetoacetate 31 by using ammonium acetate as the catalyst and polyethylene glycol as the solvent under microwave irradiation of power 200–400 W and at a temperature of 120 °C. The resultant products 96 and 97 were obtained within a very short time in high yield (Scheme 21). Microwave condition was again established as the best condition to synthesize both the derivatives over the traditional thermal method. Nevertheless, the narrow substrate scopes point toward disadvantages of this approach and substantial developments have been required in this perspective.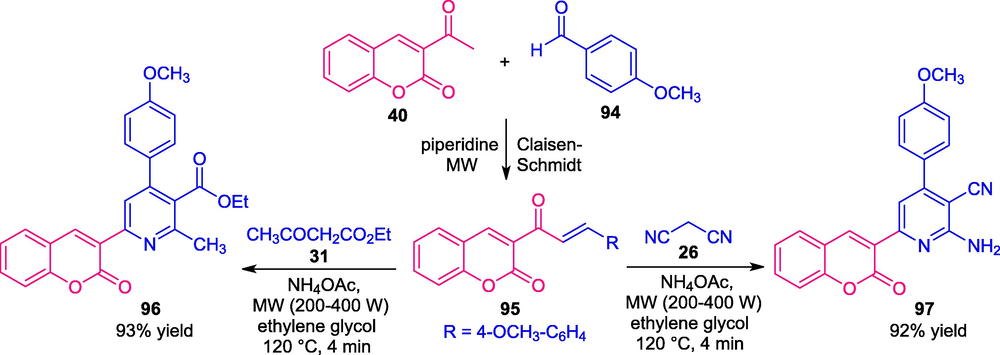
Synthesis of different coumarin-pyridine derivatives under microwave irradiation.
Very recently, a series of coumarin-quinoline derivatives were synthesized by Teja et al., based on a Michael addition/annulation sequence. Using deep eutectic solvent (DES) as the catalytic system as well as reaction media, the reaction of 4-hydroxy coumarin 5 with chalcone 98 under microwave irradiation with power 350 W at 90 °C for 15 min, delivered the desired products 99 good to high yield. The mild reaction condition, metal-free nature, low cost, makes this protocol environmentally as well as eco-friendly benign (Scheme 22) (Teja et al., 2021). However, narrow substrate scopes mark a limitation of this approach, and therefore, the development of a methodology that broadens the substrate scopes of this reaction is in high demand.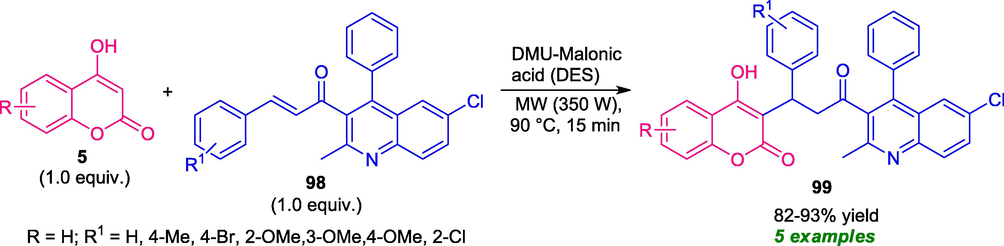
Deep Eutectic Solvent catalyzed synthesis of coumarin-quinolines under MW-irradiation.
As a continuation of El-Naggar’s work, it is important to note that they also synthesized a variety of coumarin-pyran hybrids, unlike coumarin-pyridines. Under microwave irradiation (200–400 W power, 120 °C), the reaction of coumarin chalcone 95 with malononitrile 26 in presence of piperidines as catalyst afforded the coumarin-pyran derivatives 103 in good to high yield. While, the reaction of 95 with ethyl cyanoacetate 101 formed the coumarin-pyran 102 in 86% yield (Scheme 23) (El-Naggar et al., 2017). A comparison of two reaction conditions emphasizes the microwave techniques as the key factor of this reaction to achieve the reaction in a short reaction time with pure products obtained in good yield. Heating the reaction under the thermal method does not increase the yield of the products. This method also needs ample attention to broaden the possible scope of the product substrates.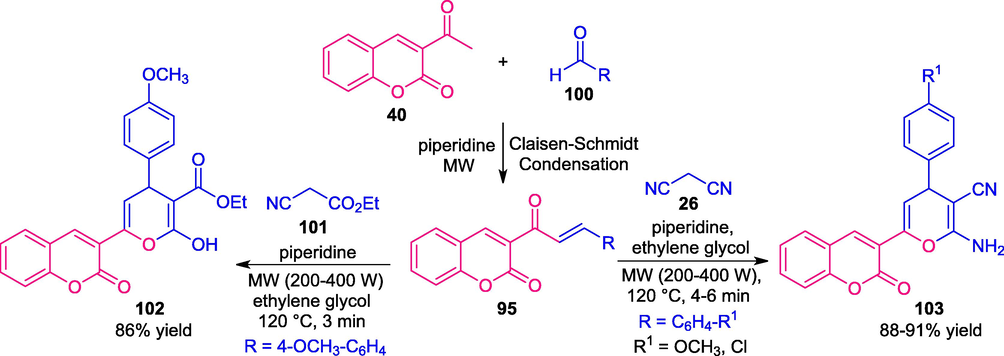
Microwaves driven synthesis of different coumarin-pyran derivatives.
Koparde et al. (2018) reported a two-component microwave-assisted reaction of previously prepared 4-bromomethylcoumarins 43 and maltol 104 for the construction of various coumarin-pyran derivatives 105 (Koparde et al., 2018). With the help of K2CO3 as the base catalyst, the desired products 105 were obtained in good to high yield within 5–10 min under microwave irradiation of power 100–200 W and at a temperature of 60 °C. On the other hand, when the same reaction was carried out in thermal condition, the corresponding products were obtained only in 57–72% yield after 3–6 h (Scheme 24). The results revealed that the optimized reaction condition not only improved the yield and reduces the reaction time, but also improve the purity of the products compared to the conventional method. Another salient feature of this approach included short reaction time, wide-substrate scopes, mild reaction conditions, and the biological activity of the synthesized compounds.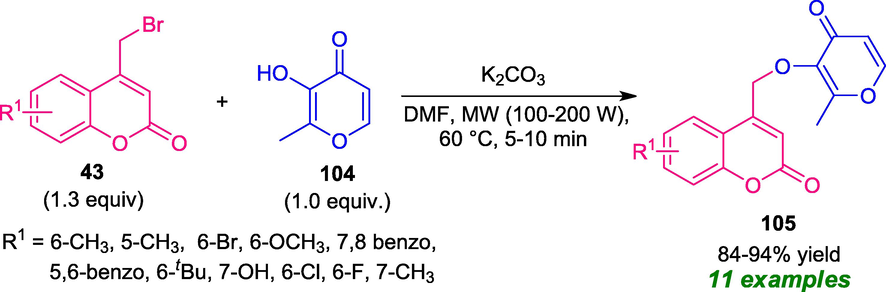
Construction of coumarin-pyran hybrids 105 under microwave irradiation.
A one-pot five-component reaction of substituted salicylaldehyde 89, with 6-methyl-4-hydroxy-pyranone 90, aldehydes 83, ethyl acetoacetate 17, and hydrazines 91 were established for the construction of coumarin-pyran derivatives 92 under microwave irradiation at power level 540 W with temperature 90 °C. By using Et3N as the catalyst in solvent-free conditions, various substituted coumarin-pyran products 92 were achieved in moderate to high yield within a very short reaction time (Scheme 25) (Tangeti et al., 2018). A variety of substituents present on the aryl ring of aldehydes as well as salicylaldehyde were well tolerated by this methodology. Initially, the reaction was carried out under the influence of different base catalysts as well as catalyst-free conditions and in different solvent systems; among them, the utilization of Et3N in solvent-free conditions was established as the best condition for this reaction. The combination of microwave and solvent-free condition not only enhances the products yield and clean reaction profile but also make this strategy environmentally as well as eco-friendly benign.![One-pot multicomponent synthesis of coumarin-pyrano[2,3-c]pyrazoles.](/content/184/2022/15/3/img/10.1016_j.arabjc.2021.103654-fig26.png)
One-pot multicomponent synthesis of coumarin-pyrano[2,3-c]pyrazoles.
A mechanism was proposed for this transformation which is depicted in Scheme 25. Initially, the reaction involves the formation of pyrazolone Int-24 from nucleophilic addition of ethyl acetoacetate 31 and hydrazine 106, which upon condensed with aryl aldehydes afforded intermediate Int-25. The intermediate Int-25 then experiences nucleophilic attack from coumarin intermediate Int-26 generated in situ from 15 and 16 deliver the adduct Int-27. The intramolecular cyclization of the resulting intermediate Int-27 followed by tautomerization yields the final product 107 via intermediate Int-28.
3.2 Linked with two heteroatoms bearing six-membered ring
3.2.1 Pyrimidines
A highly efficient and rapid strategy for the synthesis of coumarin-pyrimidine derivatives was developed by Helmy and his group. Under the influences of microwave irradiation, the treatment of acetyl coumarin 40 with aldehydes 100 delivers the coumarin chalcone 95 which upon subsequent condensation with guanidine hydrochloride 108 afford the desired coumarin-pyrimidines 109 in 80–91% yield (Scheme 26) (Helmy et al., 2015). Subsequent optimization of the reaction under both conventional and microwave conditions showed the effectiveness of the microwave technique over the conventional method in terms of fast completion of the reactions, clean reaction profile, mild conditions, high yield of the products, operational simplicity, and cost-effective manner. Despite these achievements, the scope of the reaction was found to be very limited and required further developments.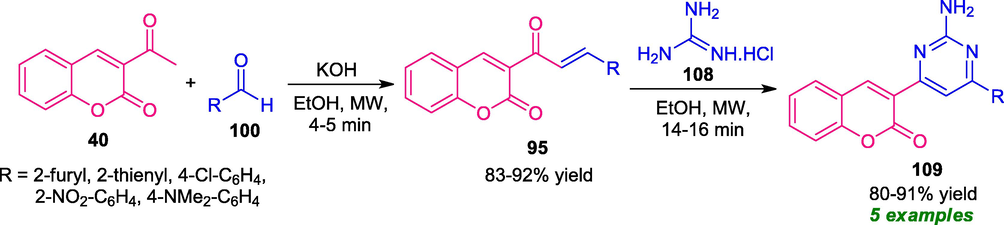
Microwave-assisted synthesis of coumarin-pyrimidine derivatives 109.
In 2019, Brahmachari et al. developed a one-pot multicomponent method for the synthesis of different coumarin-pyrimidine derivatives 111 and 113 by introducing ultrasonic techniques as an alternative to the thermal method. With the help of 30 mol% of sulfamic acid as the catalyst and aqueous ethanol as the solvent system, the reaction of 4-hydroxy coumarin 5, aldehydes 39, with different amine source 6-aminouracil 110 or 112 was found to proceed smoothly under the ultrasonic condition to provide the corresponding products 111 and 113 in 66–97% yield and 80–98% yield respectively. Not only the aryl and heteroaryl-substituted aldehydes but also alkyl-substituted aldehydes were well worked under this condition when 112 were employed as the amine source. However, in the case with 110, the scope of the reaction was limited only to an aryl as well as heteroaryl substituted aldehydes, and no reaction was observed with aliphatic aldehydes which represents the failure of this reaction condition. The utilization of easily accessible starting material, metal-free nature, high atom economy, short reaction time, moderate to excellent yield, gram-scale synthesis, and green reaction media are advantages of this protocol (Scheme 27) (Brahmachari et al., 2019). Although ultrasonic conditions allowed the best yield of the products and green reaction profile, the requirement of a large amount of catalyst also marks a shortcoming of this approach.
Ultrasound-assisted one-pot multicomponent synthesis of diverse coumarin-pyrimidine derivatives 111 and 113.
The plausible mechanism for the formation of product 113 is depicted in Scheme 27. The first step involves the sulfamic acid-catalyzed Claisen-Schmidt condensation of 47 to 112, thereby providing intermediate Int-29 which after removal of water furnish adduct Int-30. Subsequent nucleophilic attack of 4-hydroxycoumarin 5 to intermediate Int-30 afford the intermediate Int-31, which undergo tautomerization to yield the final product 113.
4 Synthesis of coumarin-fused with five-membered heterocycles
4.1 Fused with one heteroatom bearing five-membered heterocycles
4.1.1 Pyrroles, furans, and indoles
Due to the frequent occurrence of nitrogen-containing heterocycles, pyrroles, and indoles in natural products and pharmaceuticals as a result of which intensive interest has been made for their synthesis and functionalization. In this perspective, the derivatization of complex molecules from pyrroles and indoles by incorporating these rings into coumarins has been devised.
In 2019, Yadav and co-workers developed a solvent-free domino strategy for the synthesis of a variety of coumarin-fused pyrroles under microwave irradiation. By using In(OTf)3 or Gd(OTf)3 as the catalytic system, the reaction of amino coumarin 114 with malonates 115 or 3-ylidene-2-oxindoles 117 as 1,2-bielectrophiles under microwave irradiation with power 150 W at 90 °C or 100 °C, delivered the desired coumarin-fused pyrroles 116 in 0.5–1.5 h and 118 in 1.5–2.0 h respectively. In both cases, sufficient yields were observed (Scheme 28) (Yadav et al., 2019). To broaden the scope of the reaction, several substitutions on the amino coumarin ring as well as α-malonates were examined. The incorporation of electron-withdrawing groups into the amino coumarin rings leads to a decrease in the yield of the products as compared to amino coumarin-bearing electron-donating groups. Similarly, aryl-, heteroaryl- and alkyl-substituted α-malonates efficiently worked under this condition. α-acetylidene malonates, which are often less explored as a coupling partner, were also found to be very active in this reaction. Broad functionality, solvent-free condition, short reaction times are advantages of this method. Based on the experimental results, a mechanism was suggested for this reaction which starts with the coordination of In(OTf)3 with 115 to form a more reactive intermediate 115´ by lowering the LUMO energy. This reactive intermediate then underwent Michael reaction with 114, leading to intermediate Int-32. Subsequent intramolecular cyclization of Int-32 followed by [1,3]-H shift delivered the final products 116 via intermediate Int-33.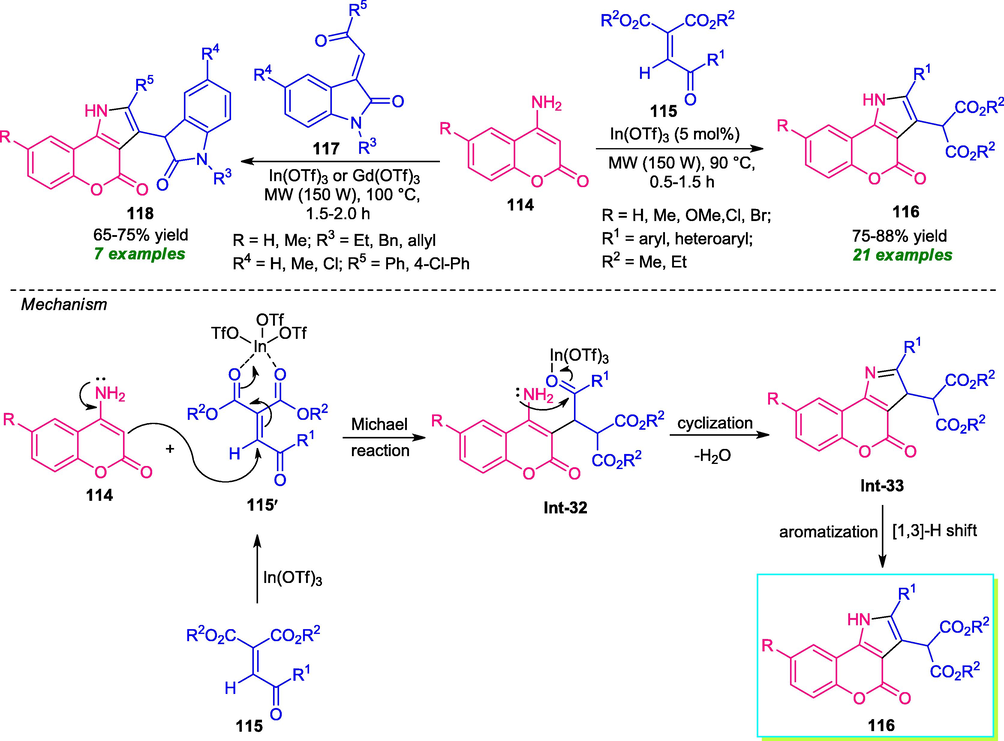
Microwave-assisted domino synthesis of coumarin-fused pyrroles.
A highly efficient and diastereoselective one-pot ultrasound irradiated approach for the synthesis of coumarin-fused furans was developed by Safaei-Ghomi et al. In the presence of MgO-nanoparticle as a highly reactive and recyclable catalyst, the desired products 121 synthesized from a four-component reaction of 4-hydroxycoumarin 5, aldehydes 47, 2,4-dibromoacetophenone 119, and pyridine 120 has been achieved in good to high yield (Safaei-Ghomi et al., 2017). Several electron-withdrawing, as well as electron-donating substituents on the aryl ring of aldehydes smoothly, underwent the reaction under this ultrasonic approach. The exploitation of ultrasonic conditions allows the fast completion of the reaction within only 10–15 min and provides a clean synthesis of the target complex in a high yield. The effectiveness of the protocol was also demonstrated by successfully carried out gram-scale synthesis in ultra-low catalyst loading. Other advantages of this approach include easy separation of the products; reusability of the catalyst up to five consecutive cycles without change in catalytic activity (Scheme 29). However, the catalyst requires multistep synthesis at higher temperature conditions, which represents a shortcoming of this approach. The reaction proceeded through the Knoevenagel condensation of 4-hydroxycoumarin 5 with aldehydes 47 to form intermediate Int-34 on the active sites of nano-MgO. The Michael addition of pyridinium ylide Int-35 generated from 119 and 120 to intermediate Int-34 then took place. Consequently, the intramolecular cyclization of intermediate Int-36, followed by removal of pyridines afforded the final product 121.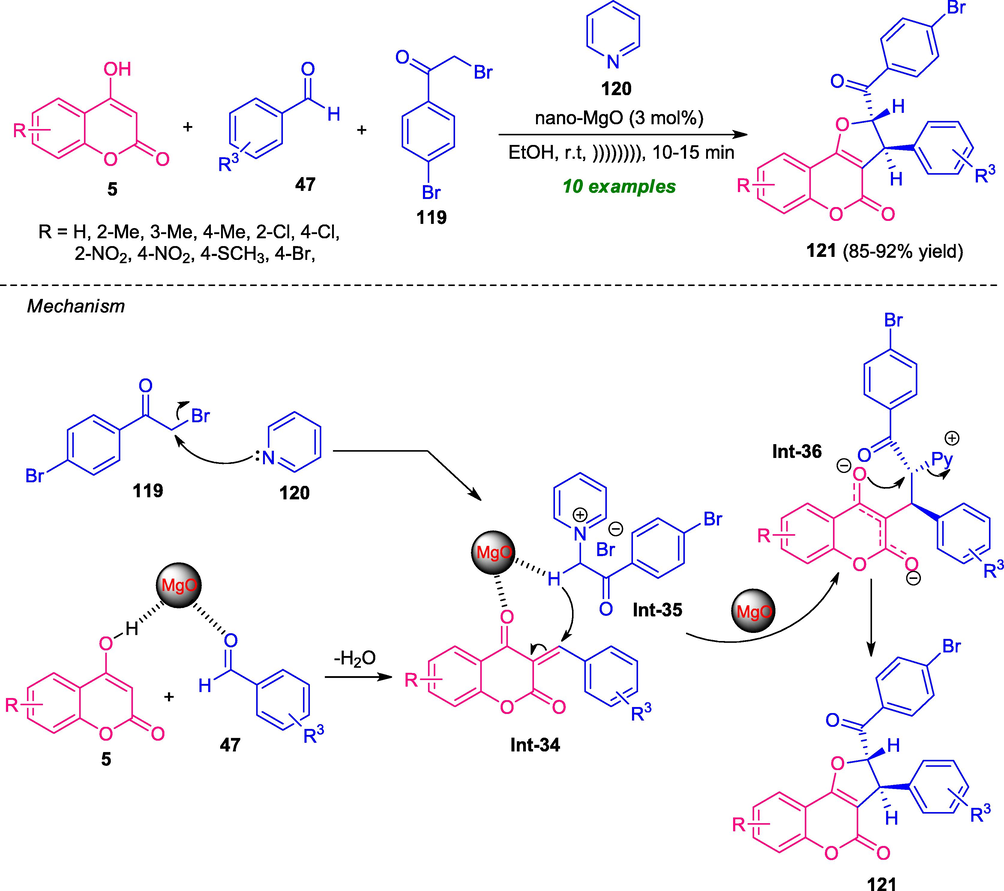
Ultrasound-assisted one-pot multicomponent synthesis of coumarin-fused furan.
Another example for the synthesis of coumarin-fused furan was established by the research group of Rani under microwave irradiation (Rani et al., 2020). Treatment of bromoacetophenone 123 with substituted acetyl 4-hydroxycoumarin 122 or coumarin chalcone 125 in presence of K2CO3 as the catalyst in microwave condition at 80 °C with stirring at 8 bar pressure leads to a variety of coumarin-fused furans 124 and 126 respectively (Scheme 30). In both cases, products were formed in good to excellent yield. The strategy offers several advantages like a metal-free catalyst, mild reaction conditions. Also, the utilization of microwaves shortened the reaction time and increases the product yield. Furthermore, most of the synthesized compounds were found to have an anti-microbial activity which represents a benefit of this approach. Besides these achievements, the limited substrate scope represents a drawback of this approach, and therefore, the development of a highly efficient protocol to extend the substrate scopes of this reaction is still desired.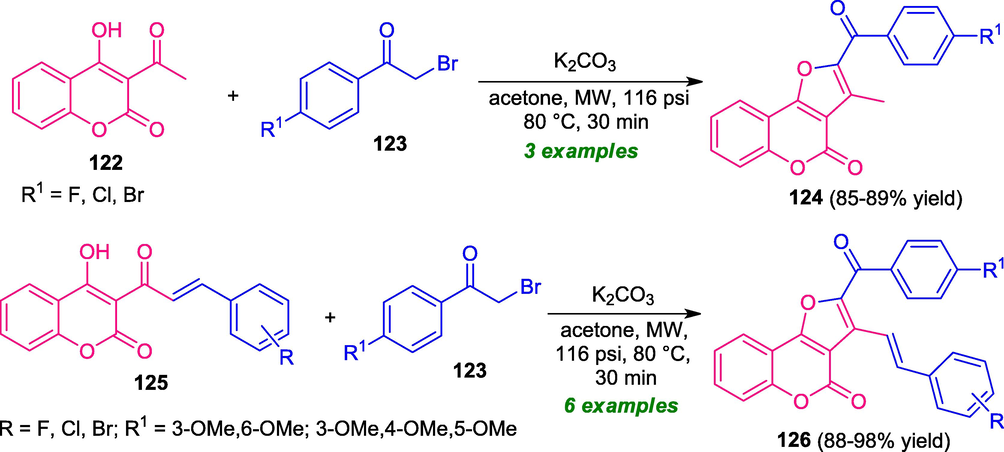
Microwave-assisted synthesis of different coumarin-fused furan derivatives.
A straightforward palladium-catalyzed intramolecular cross dehydrogenative coupling strategy has been realized to access a series of coumarin-fused indoles 128 from easily available 3-arylamino coumarins 127 (Gu et al., 2019). Under the influence of microwave (150 W) heating conditions at 140 °C, the desired products 128 were synthesized in moderate to high yield by employing Cu(OAc)2 as the oxidant in base-free conditions. Despite, remarkable functional group tolerance, green reaction condition, high atom economy makes this suitable among others, the requirement of a large amount of catalyst constitutes a major drawback of this approach (Scheme 31) Gratifyingly, the electronic influences of various substituents on the aryl ring of the amine as well as coumarin ring were tested. Noteworthy, all the substituents smoothly underwent the reaction under microwave conditions. Although exploitation of microwave techniques provides a high atom economy of the reaction, however, the unusual requirement of long reaction time, as well as high heating condition (140 °C), represents the limitation of this approach. The suggested mechanistic pathway starts with the initial palladation of coumarin preferentially occurred at the C-4 position to form Pd(II) intermediate Int-37. The tentative metalation-deprotonation of Int-37 furnished intermediate Int-39 which on reductive elimination provides the final product 128. Finally, the Pd(II) catalyst was regenerated for the next cycles by oxidant Cu(II) salt.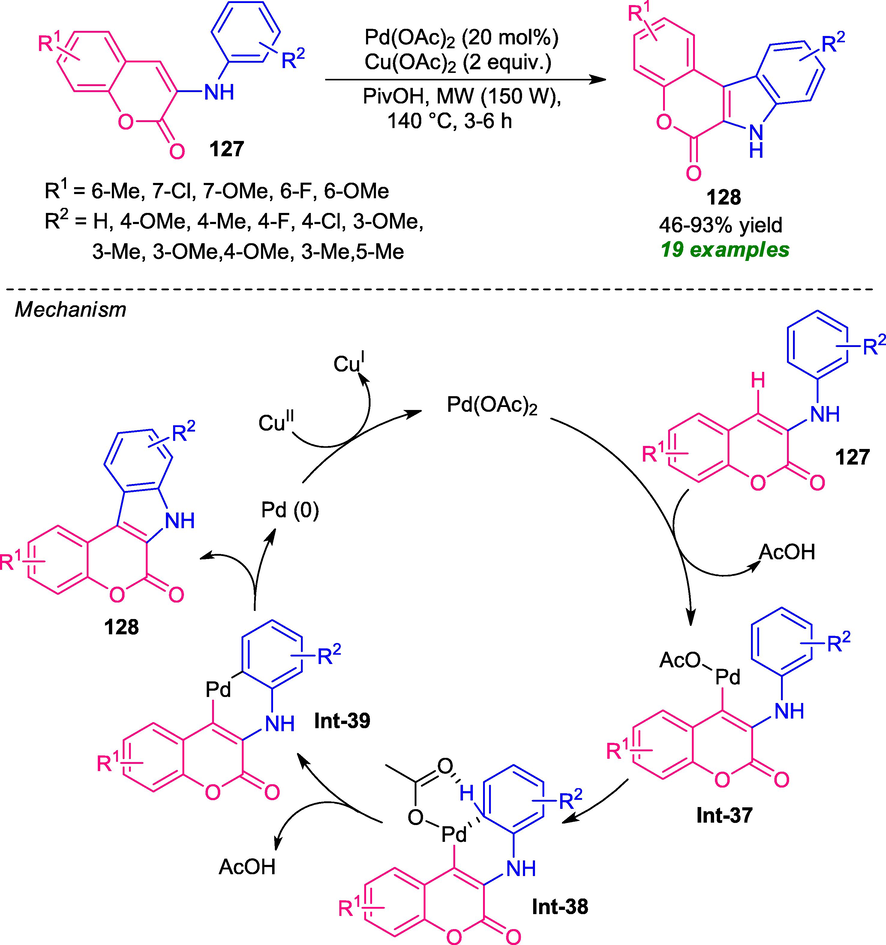
Base-free microwave-assisted synthesis of coumarin-fused indoles.
4.2 Fused with two/three heteroatom bearing five-membered heterocycles
4.2.1 Thiazoles, and triazoles
The sequential multicomponent 1,3-dipolar cycloaddition of azomethine ylide to activated has been comprehensively explored in recent times for the synthesis of five-membered nitrogen heterocycles (Reddy et al., 2018; Dwivedi et al., 2020b).
In this regard, a sequential multicomponent reaction of salicylaldehyde 15, dimedone 129, isatin 131, and thiazolidine-4-carboxylic acid 132 under ultrasonic irradiation has been developed for the synthesis of coumarin-fused thiazoline hybrids. The reaction can proceed through the formation of coumarin-carboxylic acid 130 from 15 and 129 which undergo 1,3-dipolar cycloaddition with azomethine ylide generated in situ from 131 and 132 to afford the desired product 133 in good to high yield (Scheme 32) (Kanchithalaivan et al., 2014). To explore the generality of the reaction, several substituted salicylaldehyde and isatins were employed under ultrasonic conditions. Almost all electron-rich and electron-poor substituents gave sufficient yield of the products within a very short reaction time. Furthermore, the effectiveness of this ultrasonic strategy was established by comparing the same reaction in the traditional thermal method. The ultrasonic method provides a clean and column-free synthesis of the products in high yield in short reaction time, whereas the conventional method required much more time to complete the reaction, and products were isolated by using column chromatographic techniques in 50–61% yield. The utilization of chromatography techniques for purification purposes also wastes a large amount of solvent due to which the conventional method is not considered as an environmentally benign approach. Therefore, the ultrasonic method was much more advantageous than the conventional method for the synthesis of the target compounds.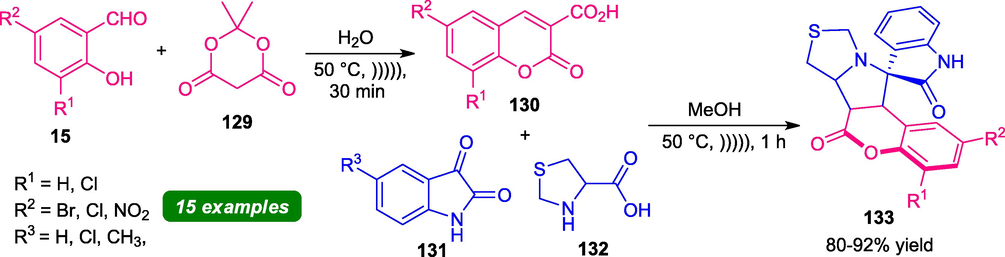
Multicomponent synthesis of coumarin-fused thiazole under ultrasonication.
A metal-free 1,3-dipolar cycloaddition of cyclic nitroalkenes 135 (prepared from salicylaldehyde 15 and ethyl 2-nitroacetate 134) to sodium azide under microwave-irradiation was designed (Schwendt and Glasnov, 2017). The reaction afforded a variety of coumarin-fused triazole derivatives 136 in 69–94% yield. Although short reaction time, metal-free nature, a high level of yield are major advantages, the substrate scopes of the reaction were found to be limited and demand further development of this approach (Scheme 33). Furthermore, the reaction was performed at a higher temperature condition (150–160 °C) due to which it was also not considered as an energy-efficient method.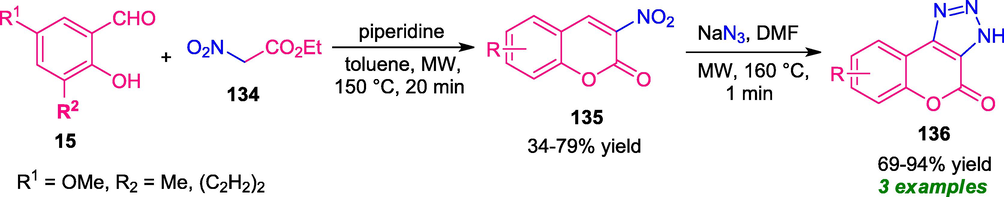
Microwave-assisted rapid access to coumarin-fused triazoles 136.
5 Synthesis of coumarin-fused with six-membered heterocycles
5.1 Fused with one heteroatom bearing six-membered heterocycles
5.1.1 Pyridine, quinoline, and pyrans
An environmentally and eco-friendly benign approach towards the synthesis of a series of coumarin-fused pyridine derivatives 138 by employing Ultrasonic technology was developed by Kumari and co-workers in 2016 (Kumari et al., 2016). In this regard, a three-component reaction of 4-hydroxycoumarin 5 with aldehydes 50 and amino-isoxazole 137 was carried out in presence of ionic liquid [C4mim][HSO4] under both conventional as well as ultrasound irradiation. By using conventional heating at 80 °C, the desired product 138 was obtained in 90–95% yield within 20–45 min, whereas the reaction under ultrasonic irradiation takes place at room temperature to afford the desired product 138 in almost the same quantity. Although both of these two conditions were found to be efficient, the ultrasonic technique was much more superior to the conventional one in terms of reaction time and consumed less amount of energy. Not only aryl aldehydes but also heteroaryl aldehydes were well tolerated by this condition. The overall process can involve the initial condensation of 50 with 137 to form intermediate Int-40 that can then experiences the nucleophilic attack from 5 to provide intermediate Int-41. Cleavage of resulting intermediate Int-41 form alkene Int-42 which undergo 1,2-addition with 137 to produce Int-43, which on intramolecular cyclization followed by [1,3]-H shift afforded the final product 138 (Scheme 34).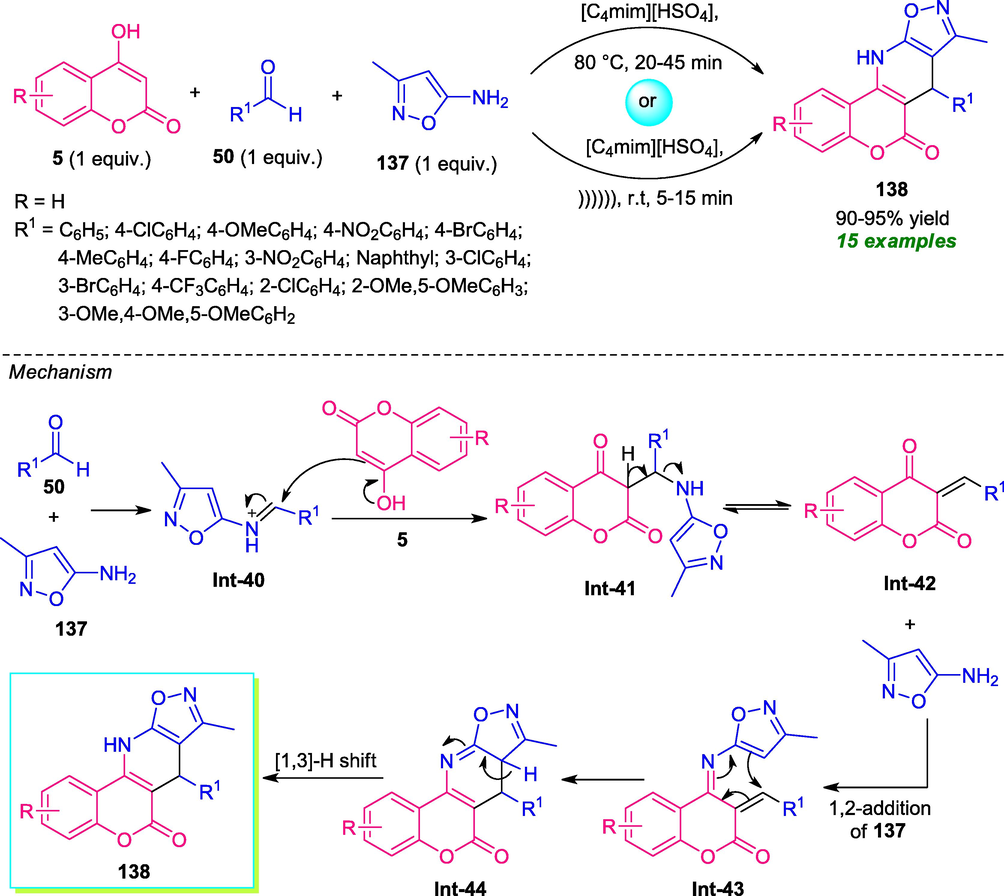
Multicomponent synthesis of coumarin-fused pyridines 138 under ultrasound irradiation.
Another successful application of microwave technology for the rapid and concise access to various coumarin-fused pyridine rings 141 bearing pyrazole moiety has been accomplished via the reaction of acetyl coumarin 139 and amino-pyrazoles 140 by using silica sulfuric acid (SSA) as the catalyst and DDQ as the oxidant (Lin et al., 2017). Interestingly, this reaction under the influence of microwave irradiation at 140 °C, delivers the desired products 141 in 37–51% yield (Scheme 35). The reaction condition was found to be suitable for amino-pyrazole bearing both aliphatic and aromatic rings. Despite, these great achievements, the low yield of the products, limited substrate scopes, high reaction temperature are major disadvantages of this approach otherwise groundbreaking development. Therefore, the development of a novel methodology consistent with the green chemistry principle for the facile access to these heterocycles in broader scopes with excellent yield is in high demand.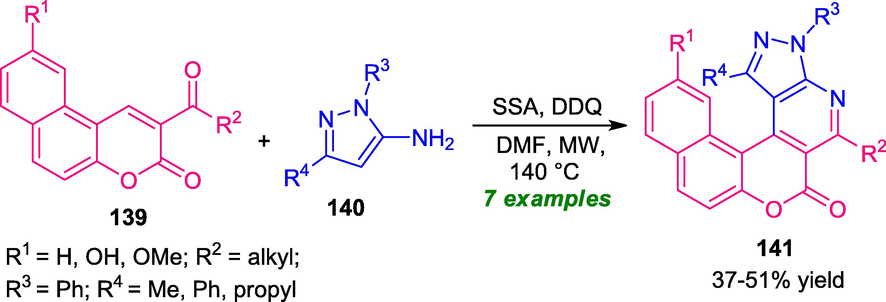
One-pot MW-irradiated synthesis of coumarin-fused pyridine derivatives.
After this report, another non-conventional strategy for the synthesis of coumarin-fused pyridines was developed by Kolita and Bhuyan. The reaction of coumarin 142 with aryl ketones 143 or ethyl acetoacetate 31 in presence of ammonium acetate was found to proceed smoothly under microwave irradiation (35 W) at 130 °C to afford the corresponding products 143 and 145 respectively in moderate to good yield within a very short reaction time. The wide substrate scopes, solvent-free conditions make this approach very attractive in terms of synthetic efficiency as well as green chemistry points of view (Scheme 36) (Kolita and Bhuyan, 2018).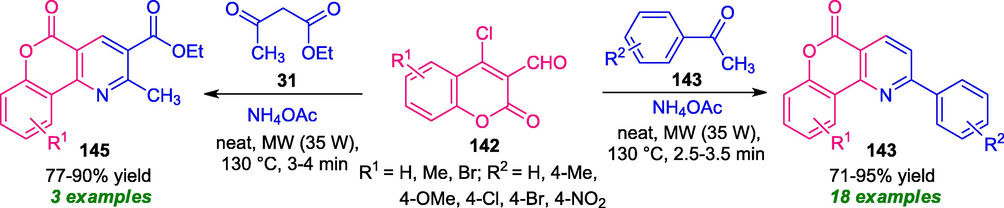
Solvent-free synthesis of coumarin-fused pyridines under MW-irradiation.
In 2014, Khan and co-workers disclosed a one-pot multicomponent approach for the synthesis of diverse coumarin-fused dihydroquinoline 146 by introducing microwave technology as a powerful energy source (Khan et al., 2014). Initially, the reaction of 4-hydroxycoumarin 5 with aldehydes 50 and substituted anilines 11 was performed in presence of different catalysts including Bi(OTf)3, Sc(OTf)3, L-proline, imidazole, InCl3, and a different solvent system like H2O, EtOH, CH3CN, THF, toluene. Among these different reaction conditions, the exploitation of 10 mol% of Bi(OTf)3 in H2O medium under microwave heating at 130 °C was chosen as the best reaction condition for the formation of desired products 146 (Scheme 37). Various aryl and heteroaryl substituted aldehydes efficiently worked under this condition. Similarly, electron-rich, as well as electron-poor substituents on the aniline ring, had no significant effect on the yield of the products. However, when para-substituted nitro aniline was employed as the substrate; no reaction was observed which constitutes a limitation of this approach. The synthesized product 146 upon subsequent reaction with NBS in THF at room temperature provided the coumarin-fused quinoline products 147. The mechanistic pathway for this reaction begins with the condensation of 11 with 50 to produce intermediate Int-45, which can then experiences the nucleophilic attack from 5 to deliver adduct Int-47. The resulting intermediate Int-47 undergoes 1,2-addition with 11 to form adduct Int-48, which subsequently cyclized to afford 146 and aromatized to yield the product 147.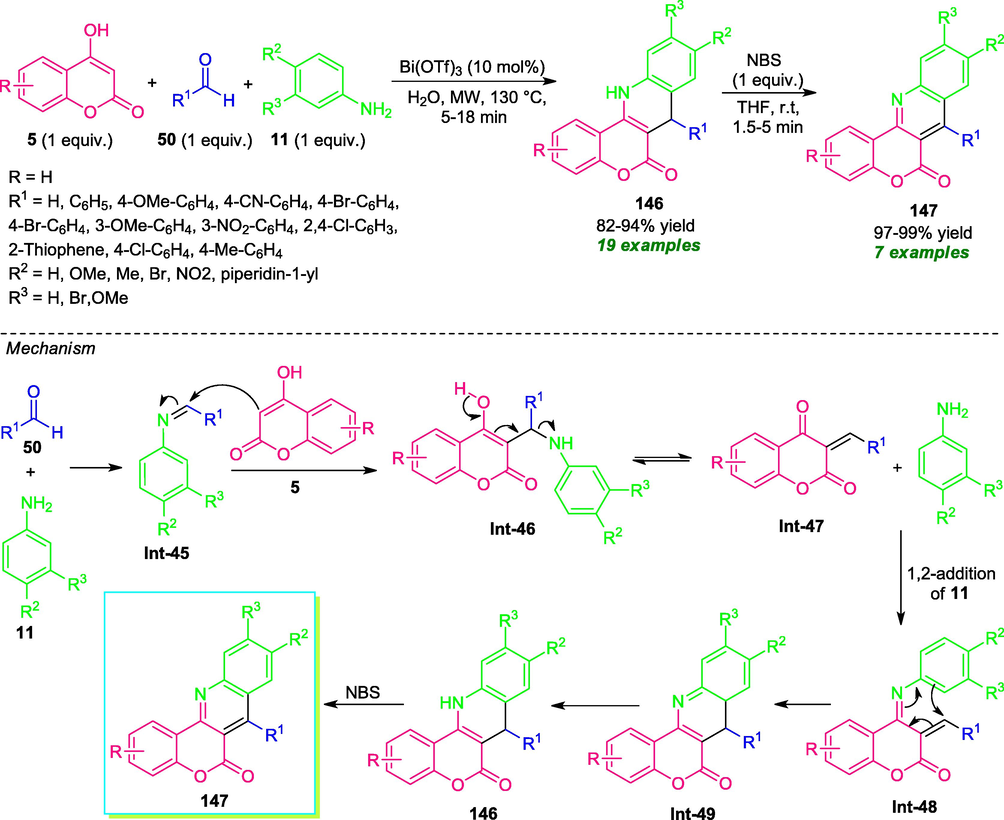
Microwave irradiated rapid construction of coumarin-fused quinolines in aqueous medium.
Molecular iodine catalyzed one-pot multicomponent tandem reactions of 4-hydroxycoumarin 5, aldehydes 50, and arylamines 148 have been realized for the synthesis of a series of coumarin-fused quinoline derivatives 149. Using microwave-irradiation with 400 W powers at the temperature of 165 °C, a total of 26 compounds were synthesized regioselectively by this Povarov type reaction in 80–94% yield in 30 min. Not only the aryl aldehyde but also heteroaryl aldehydes were well tolerated by this method (Scheme 38) (Sashidhara et al., 2015). Despite this tremendous advancement, the reaction required high-temperature conditions (165 °C), due to which the developed approach was not considered as an energy-efficient method. Here, the microwave was used a powerful green approach that shortened the reaction time and provides a clear pathway for the reaction to complete. A mechanism was proposed which proceeded through the initial condensation of aldehyde 50 and amine 148 which form intermediate Int-50. The nucleophilic attack of 5 to intermediate Int-50, leading to adduct Int-51 which underwent rearrangement to produce the intermediate Int-55 via transition states Int-52, Int-53, and Int-54. The subsequent [1,3]-H shift of Int-55 produces thermodynamically stable adduct Int-56, which on oxidation in presence of iodine deliver the final product 149.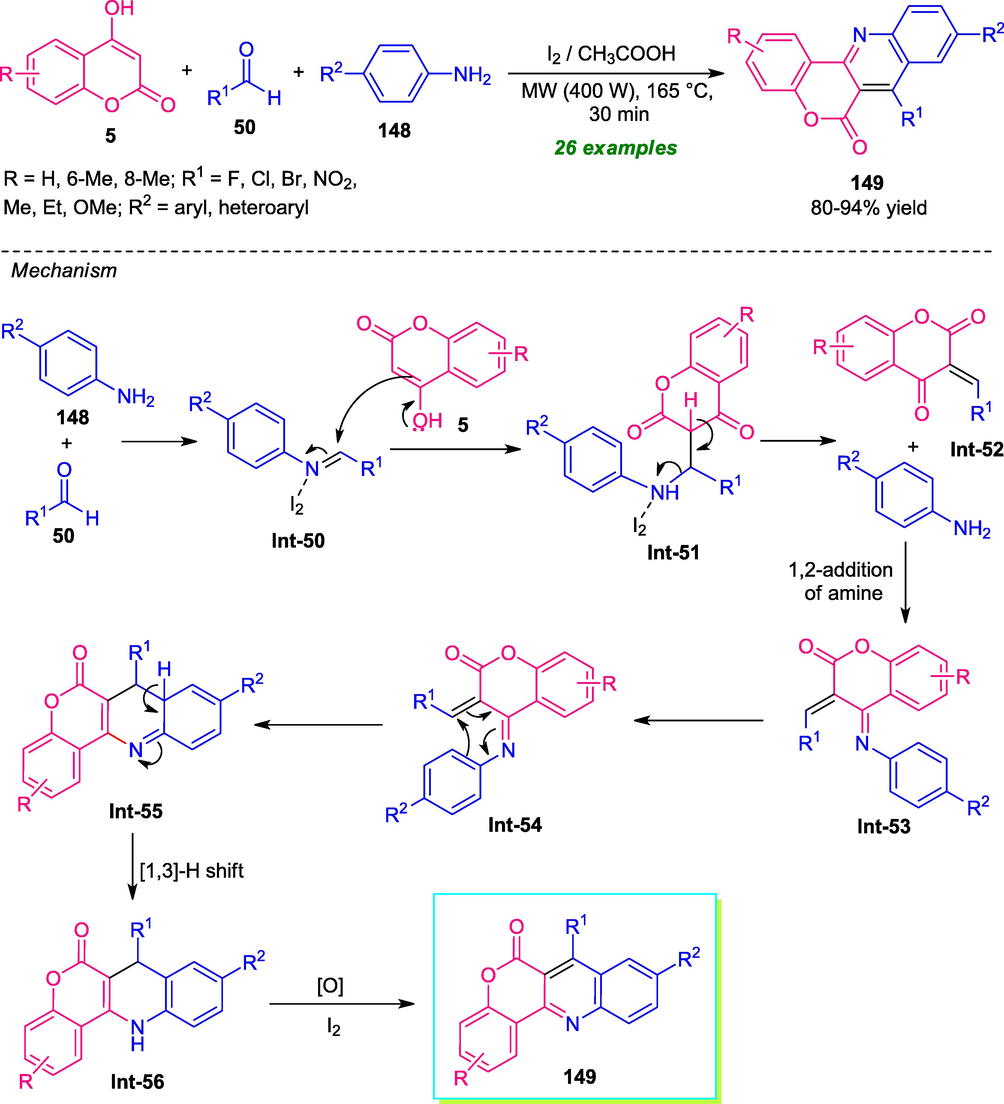
MW-assisted one-pot multicomponent synthesis of coumarin-fused quinolines.
As discussed previously, oxygen-containing heterocycle mainly pyrans being received immense importance for their synthesis due to their pharmacological profile as well as material science applications (Borah et al., 2021a; Borah et al., 2021f; Dwivedi et al., 2020a). In this perspective, the incorporation of pyrans into the coumarin ring for their pharmaceutical application has been extensively examined by Al-bogami in 2015 (Scheme 39). With the help of ultrasonic technique, a three-component reaction of 4-hydroxycoumarin 5 with active methylene compound 150 and aldehyde 47 was carried out by using piperidine as the catalyst that afforded the corresponding pyrano[3,2-c]coumarin derivatives 151 in 86–94% yield (Al-bogami, 2015). The reaction condition was found to be suitable for aryl aldehydes possessing different electron-rich and electron-poor substituents as well as for substituted active methylene compounds. A comparative study between both conventional and ultrasound irradiation methods realize the preference of ultrasonic technique for this reaction over conventional one in terms of synthetic efficiency as well as green chemistry point of view. The ultrasonic technique improved the rate of the reaction as well as product yield and the reaction required only a few minutes to complete. A tentative mechanism was proposed to account for this reaction. The first step of reaction involves the Knoevenagel condensation of aldehydes 47a with active methylene compounds 151a to form the adduct Int-57. In the next step, Michael addition of adduct Int-57 to 4-hydroxycoumarin 5 takes place to produce the intermediate Int-58, which underwent intramolecular cyclization and subsequent tautomerization via intermediate Int-59, leading to final product 151a.![Ultrasound-assisted one-pot three-component synthesis of diverse pyrano[3,2-c]coumarin hybrid.](/content/184/2022/15/3/img/10.1016_j.arabjc.2021.103654-fig40.png)
Ultrasound-assisted one-pot three-component synthesis of diverse pyrano[3,2-c]coumarin hybrid.
Almost at the same time, Esmaeilpour and his group demonstrated a practical one-pot procedure by employing ultrasonic technique as an environmentally and eco-friendly benign approach for the synthesis of a series of coumarin fused pyran hybrids (Esmaeilpour, et al., 2015). By using Fe3O4@SiO2-imid-PMAn nanoparticles as the catalyst, a three-component reaction of readily available 4-hydroxycoumarin 5, aldehydes 50, and malononitrile 26 in an aqueous medium under ultrasound irradiation at room temperature was performed. From this reaction, the desired coumarin-fused pyran derivatives 152 were obtained in 88–97% yield. The reaction condition tolerates a wide variety of aryl, heteroaryl, and alkyl-substituted aldehydes (Scheme 40). The practicality of this approach was established by demonstrating gram scale synthesis with low catalyst loading and recyclability of the catalyst up to eight consecutive cycles with negligible loss in catalytic properties. This reaction under conventional heating conditions (reflux) also provides a high yield of the products unlike the ultrasonic approach, however, they required a slightly higher time in comparison to the ultrasound irradiation method due to which the ultrasonic approach was chosen as the best condition for this reaction. Another salient feature of this approach included mild reaction condition, operational simplicity, broad functional group tolerance, green reaction medium, short reaction time, etc.
Magnetic nanoparticles catalyzed efficient synthesis of coumarin fused pyrans under ultrasound irradiation.
In the past decades, the construction of spiro-heterocycles especially spirooxindole framework has drawn much more attention due to their wide prevalence in natural products and pharmaceutical candidates (Singh and Desta, 2012).
In view of these importances, Karimi and co-workers developed two novel protocols for the efficient synthesis of spirooxindole derivatives 154 bearing coumarin and pyran unit via a multicomponent reaction of 4-hydroxycoumarin 5, malononitrile 26, and substituted isatin 153 under ultrasound irradiation (Karimi et al., 2015a; Karimi et al., 2015b). They prepared two novel magnetic nanoparticles as the catalytic system for this reaction including S-alkyl O-hydrogen sulfothioate functionalized silica-coated magnetic nanoparticles (AHST-MNPs) and silica sulfuric acid magnetic nanoparticles (SSA-MNPs) (Scheme 41). Both of these two nanoparticles efficiently catalyzed the reaction in ultrasonic conditions in presence of either water or aqueous ethanol as the solvent system and the corresponding products 154 were obtained in high yield. The utilization of ultrasound in this reaction alongside water as reaction media makes this protocol environmentally and eco-friendly benign. In addition, the substrate scope of the reaction was found to be limited in both the cases which mark a major shortcoming of both these two approach that needs ample attention to broaden the scope of this reaction.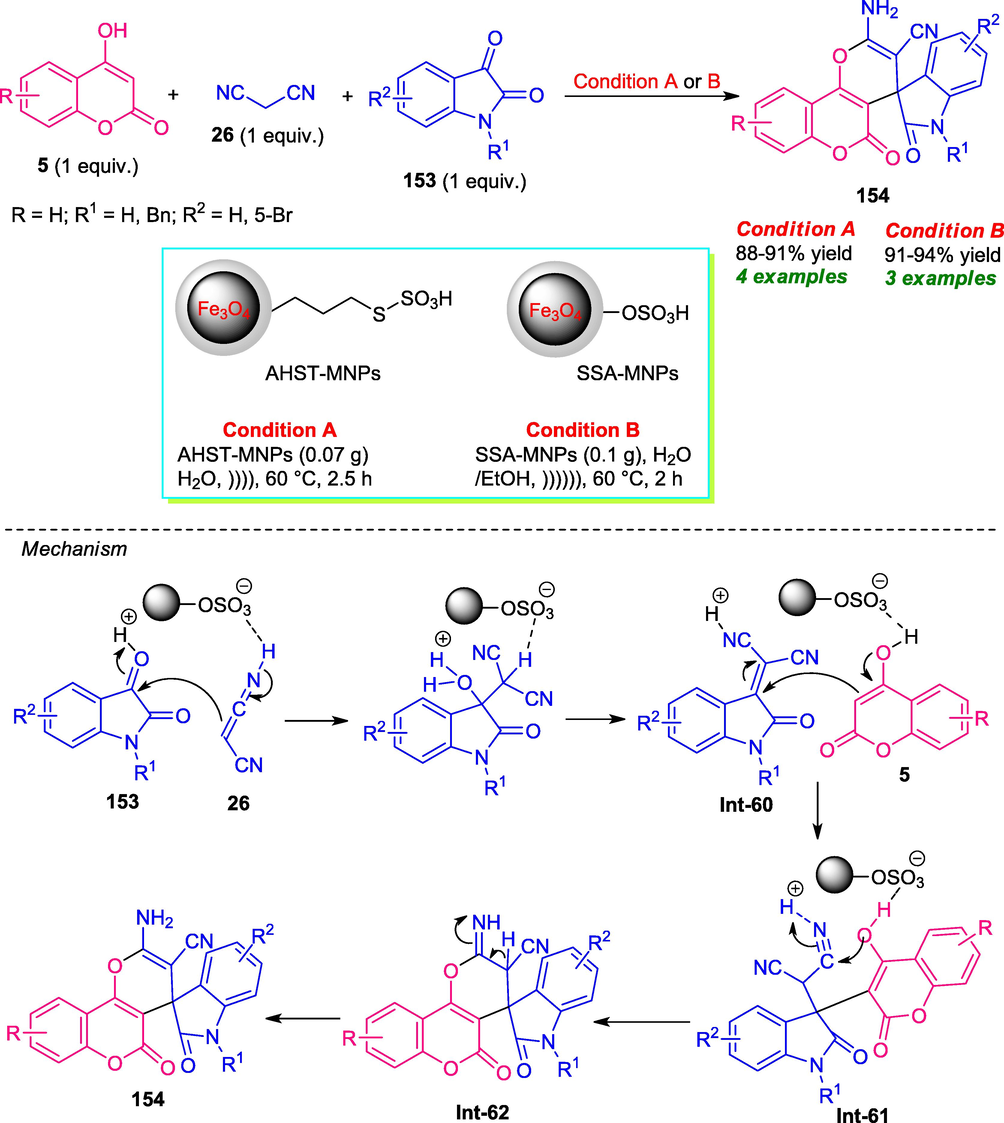
Ultrasound-assisted multicomponent approach towards the synthesis of coumarin fused spiro-pyrans under two different conditions.
A plausible mechanistic pathway for SSA-MNP catalyzed formation of 154 was postulated in Scheme 41 where an initial Knoevenagel condensation of 153 to 26 was presumed to occur under influence of SSA-MNPs. The resulting adduct Int-60 then underwent Michael addition with 5 to form intermediate Int-61, which on intramolecular cyclization followed by subsequent tautomerization afforded final product 154 via transition structure Int-62.
Zhang and co-workers in 2016, employed microwave technology for the efficient synthesis of diverse coumarin-fused pyran derivatives 156 and 158 based on a metal-free approach (Zhang et al., 2016). By using DMAP as the catalyst, the two-component reaction of 4-hydroxycoumarin 5 with substituted acetoacetate 155 was found to proceed in microwave condition (640 W) at room temperature to afford the desired coumarin-fused pyran products 156 in poor to good yield (Scheme 42). Although diverse substitutions on the 4-hydroxycoumarin ring could be tolerated by microwave irradiated method within a very short reaction time, the same reaction when carried out under conventional heating provides an only poor yield of the target compounds within a longer reaction time. On the other hand, the reaction of 4-hydroxycoumarin 5 with cyclic ethyl acetoacetate 157 under the same reaction condition provides functionalized coumarin-fused pyrans 158 in 65–75% yield. Broad functional group tolerances, short time, simple work-up procedure are some of the key advantages of this protocol. Despite these, the low yield of the products constitutes a major limitation of this approach and calls for further developments otherwise excellent work.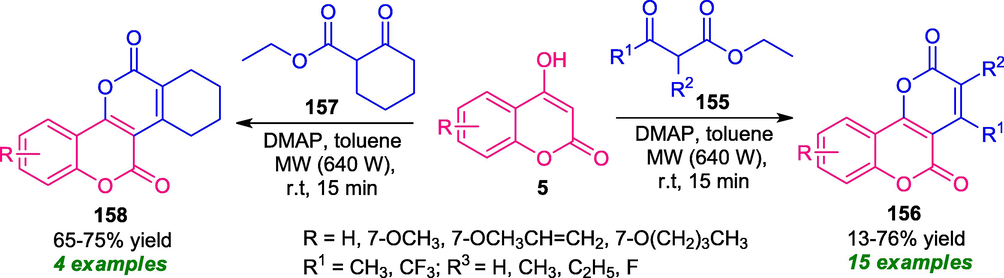
Microwave irradiated synthesis of coumarin-fused pyrans.
A tandem double condensation reaction under microwave irradiation for the construction of coumarin-fused pyrans has been realized by Parthasarathy in 2016 (Parthasarathy et al., 2016). The synthesis involves the reaction of 2 equivalent of 4-hydroxycoumarin 5 with one equivalent of isatins 153 in presence of 2 mol% of Au(III) as the catalyst under microwave irradiation with power 200 W at 40 °C for 15 min (Scheme 43). The reaction condition was found to be very suitable for N-substituted (R1 = alkyl, aryl, allyl) as well as N-unsubstituted isatins (R1 = H). Also, various electron-donating and electron-donating groups were efficiently tolerated by this method and had no detrimental effect on the reactivity of the reaction. Here, the microwave was used as a powerful green energy source that facilitates a simple and cleaner pathway to completion of the reaction and improves the yield of the products, and also reduces the reaction time. However, utilization of expensive catalysts points towards the drawback of this approach otherwise outstanding development.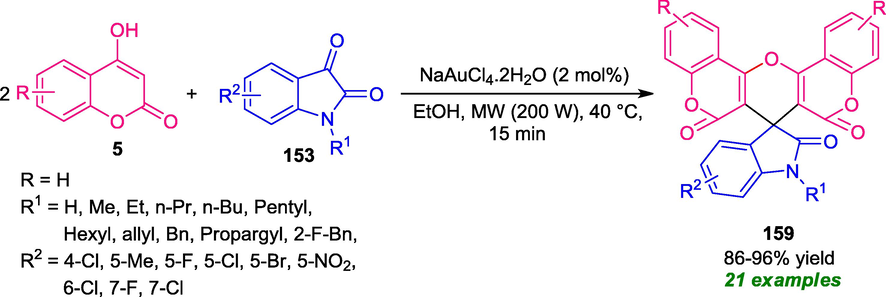
Gold catalyzed microwave-assisted double condensation reaction towards the synthesis of coumarin fused spiro-pyrans.
In 2021, Teja and his group developed a highly advantageous microwave-assisted one-pot approach for the construction of a different set of coumarin-fused pyran structures 161 and 163 (Teja et al., 2021). By using deep eutectic solvent (DES) as the catalyst system, treatment of 4-hydroxycoumarin 5 with substituted chalcones 160 and 162 in solvent-free and microwave irradiation (350 W) at 90 °C, delivered the desired product 161 and 163 in high yield within 15 min respectively. This microwave-assisted approach was carried out under metal-free conditions. Clean and mild reaction profiles, short reaction times mark the highlights of this approach. However, narrow substrate scope represents a major limitation of this approach and calls for further developments (Scheme 44).
Deep eutectic solvent catalyzed synthesis of different coumarin-fused pyrans.
5.2 Fused with two heteroatoms bearing six-membered heterocycles
5.2.1 Pyrimidines
An environmentally friendly as well as eco-friendly approach for the construction of coumarin-fused with two heteroatom-bearing heterocycles namely pyrimidine was developed by Brahmachari and his group in 2018 (Brahmachari et al., 2018). Under the influence of ultrasonic irradiation, the one-pot three-component reaction of 4-hydroxycoumarin 5, several aldehydes 50, and 2-aminopyridine 164 in presence of 20 mol% of sulphamic acid as the catalyst in aqueous ethanol afforded various substituted coumarin-fused pyrimidine derivatives 165 in moderate to excellent yield. Not only aryl aldehydes possessing different electron-rich and electron-deficient substituents, but also heteroaryl substituted aldehydes were well tolerated under this reaction condition. The sonochemistry in this reaction facilitates a fast completion rate and improves the product yield. This reaction does not require any co-catalyst as well as additives and reagents. The utilization of green reaction media, broad substrate scopes, high atom economy as well as low E-factor are some of the salient features of this approach (Scheme 45).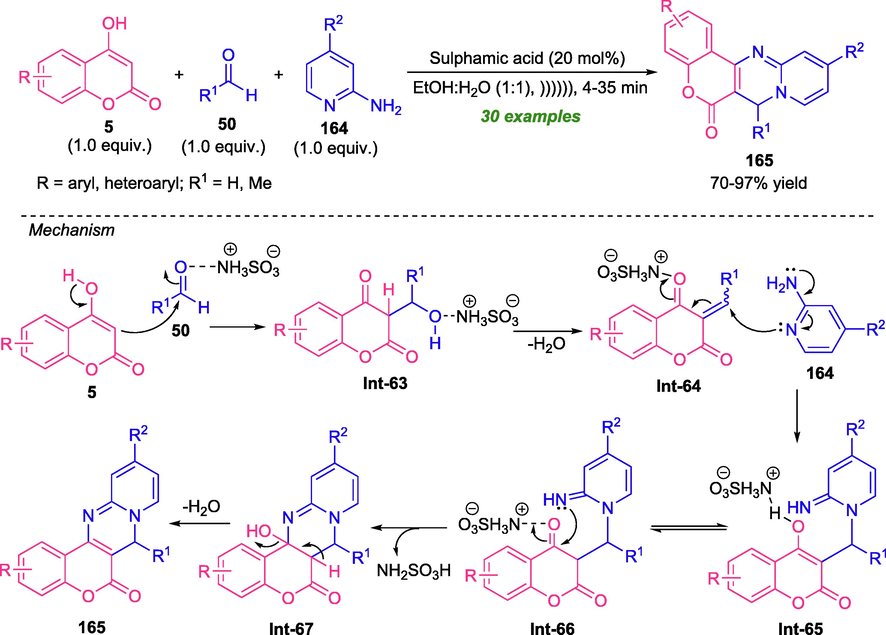
Ultrasound irradiated multicomponent synthesis of coumarin-fused pyrimidines.
In this transformation, an initial Claisen-Schmidt condensation reaction between sulfamic acid-activated aldehydes 50 and 4-hydroxycoumarin 5 takes place to furnish chalcone adduct Int-64, which can then experiences a nucleophilic attack from 2-aminopyridine 164 to afford intermediate Int-65. The tautomerization of intermediate Int-65 followed by cyclization yield the cycloadduct Int-67, which subsequently provide the final product 165 after elimination of water.
In 2019, Kaurav and co-workers developed an organocatalytic one-pot multi-component strategy for the synthesis of coumarin-fused pyrimidines 167 under microwave conditions (Kaurav et al., 2019). Treatment of readily available 4-hydroxycoumarin 5 with aldehydes 50 and urea/thiourea 166 under microwave irradiation at 300 W power in aqueous medium using 10 mol% of L-proline as the organocatalyst afforded the corresponding coumarin fused pyrimidine 167 in good to high yield with good to excellent enantioselectivity (Scheme 46). The scope of the reaction was extended to a variety of aryl and heteroaryl substituted aldehydes. Interestingly, microwave condition was found to be very suitable for over conventional method for this reaction. Microwave chemistry shortened the reaction times as well as improves the product yield and also improves the stereochemical induction of this reaction as compared to the conventional method. The overall process started with the formation of iminium ion Int-68 from L-proline and aldehydes 50, which undergoes Knoevenagel condensation with 4-hydroxycoumarin 5 to form chalcone adduct Int-70. Subsequently, the Michael addition of adduct Int-70 to 166 takes place smoothly to produce the intermediate Int-71, which on cyclization give the target compound 167.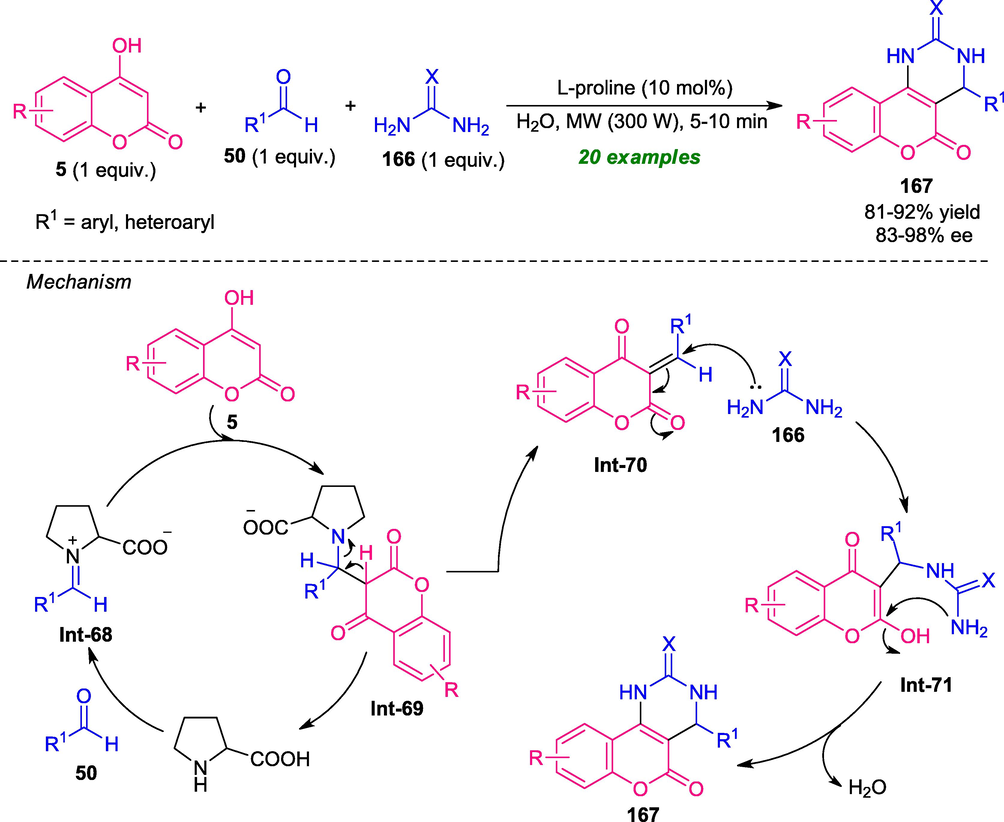
L-proline catalyzed three-component synthesis of coumarin fused pyrimidines under microwave-irradiation.
6 Conclusion
Considering the widespread prevalence of coumarins in natural products, pharmaceutical agents, and optoelectronic materials, intensive efforts have been subjected not only for their synthesis but also their utilization in selective transformation and construction of value-added compounds. Over the last decades, a vast array of synthetic procedures for the synthesis of coumarins starting from Knoevenagel, Perkin, Kostanecki-Robinson, Pechmann, Reformansky, Wittig reaction to green synthesis has been devised. In recent years, the synthesis of hybrid molecules by combining various heterocycles for providing enhanced biological activity, reactivity, and flexibility has attracted much more attention from both medicinal and synthetic chemistry perspectives. Due to this, the synthesis of coumarins directly connected or fused with different active heterocycles has been gaining importance and has become one of the most important topics of current research. Despite, remarkable developments achieved so far, most of them utilized highly toxic reagents, solvents, expensive catalysts, transition metal catalysts, ligands, and traditional energy sources which have disadvantageous effects on environments as well as human health. However, after the renaissance of alternative green energy sources like- microwave as well as ultrasound irradiation, the field of organic chemistry for the betterment of our environment as well as product development has reached an exceptional level in this century.
In this review article, we have summarized the recent progress achieved in the synthesis of coumarin derivatives either linked with or fused with different heterocyclic compounds under the influence of non-conventional energy sources like- microwave and ultrasound irradiations.
These reactions undoubtedly demonstrated the synthesis of diverse coumarin-heterocycles that may show promise as biologically active compounds or pharmaceutical targets. By using microwaves and ultrasonic technology, the products could be achieved in pure form with an excellent level of yield within a very short reaction time. One of the major risks associated with the environment is the utilization of toxic and volatile organic solvents. Hence, avoiding or minimizing the utilization of organic solvents in the chemical process is in high demand. Microwaves and ultrasonic protocol are found to be the better replacement of the traditional method which utilizes a large number of toxic solvents and turns to very attractive in terms of synthetic efficiency as well as green chemistry point of view. They also avoid expensive metal catalysts which usually contaminated the final products, and provide a high atom economy of the reaction. Most of the strategies developed for the synthesis of coumarin-heterocycles under microwaves and ultrasound irradiations have been efficiently carried out in catalyst as well as solvent-free conditions and were found to be superior to the conventional process. As a current fascinating area for the construction of several bonds in a one-pot operation, multicomponent reactions for the synthesis of coumarin-heterocycles under microwaves and ultrasound irradiation were also successfully performed.
Despite these so far developments, narrow substrate scopes, low yield of the product, high catalyst loading in some cases demands further developments.
We hope, this review article will help in identifying the current progress achieved in the synthesis of coumarin-heterocycles and stimulate further inventive developments not only for methodology but also for creative derivatization by utilizing them as a key building block.
Acknowledgments
The author thanks the Central University of Gujarat, Gandhinagar, India, and Prof. Rama Shanker Dubey, Vice-Chancellor, the Central University of Gujarat for the encouragement and continuous support. BB thanks UGC-India for the Non-NET fellowship.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Recent advances in 4-hydroxycoumarin chemistry. Part 1: Synthesis and reactions. Arab. J. Chem.. 2019;12:88-121.
- [Google Scholar]
- Recent advances in 4-hydroxycoumarin chemistry. Part 2: Scaffolds for heterocycle molecular diversity. Arab. J. Chem.. 2019;12:974-1003.
- [Google Scholar]
- An expedient microwave assisted regio-and stereoselective synthesis of spiroquinoxaline pyrrolizine derivatives and their AChE inhibitory activity. New J. Chem.. 2017;41:873-878.
- [Google Scholar]
- Coumarin-pyrazoline derivatives: their one-pot microwave assisted synthesis and antimalarial activity. J. Pharm. Med. Chem.. 2017;3:1-9.
- [Google Scholar]
- One-pot, three-component synthesis of novel pyrano [3, 2-c] coumarins containing sulfone moiety utilizing ultrasonic irradiation as eco-friendly energy source. Res. Chem. Intermed.. 2015;41:93-104.
- [Google Scholar]
- Synthesis of coumarin by Pechman reaction-A Review. J. Pharm. Sci. Res.. 2019;11:3344-3347.
- [Google Scholar]
- Synthesis and Photooxygenation of Furo [3, 2-c] coumarin Derivatives as Antibacterial and DNA Intercalating Agent. Chin. J. Chem.. 2012;30:316-320.
- [Google Scholar]
- Microwave-assisted synthesis of substituted 2-(2 H-chromen-3-yl)-5-phenyl-1 H-imidazole based coumarin derivatives and their antimicrobial activity. Russ. J. Gen. Chem.. 2021;91:711-716.
- [Google Scholar]
- Dimers of coumarin-1, 2, 3-triazole hybrids bearing alkyl spacer: Design, microwave-assisted synthesis, molecular docking and evaluation as antimycobacterial and antimicrobial agents. J. Mol. Struct.. 2018;1157:312-321.
- [Google Scholar]
- Alternative energy input: mechanochemical, microwave and ultrasound-assisted organic synthesis. Chemical Society Reviews. 2012;41:1559-1584.
- [CrossRef] [Google Scholar]
- Recent developments on ultrasound-assisted organic synthesis in aqueous medium. J. Serb. Chem. Soc.. 2017;82:755-790.
- [Google Scholar]
- Recent developments on ultrasound-assisted one-pot multicomponent synthesis of biologically relevant heterocycles. Ultrason. Sonochem.. 2017;35:15-35.
- [Google Scholar]
- Recent developments on ultrasound-assisted synthesis of bioactive N-heterocycles at ambient temperature. Aust. J. Chem.. 2017;70:872-888.
- [Google Scholar]
- Ultrasound and nano-catalysts: an ideal and sustainable combination to carry out diverse organic transformations. ChemistrySelect. 2019;4:2484-2500.
- [Google Scholar]
- Recent advances and therapeutic journey of coumarins: current status and perspectives. Med. Chem. Res.. 2015;24:2771-2798.
- [Google Scholar]
- Mechanism of warfarin resistance. Warfarin and the metabolism of vitamin K1. Biochemistry. 1973;12:1759-1762.
- [Google Scholar]
- Borah, B., Dwivedi, K. D., Chowhan, L. R., 2021a. Applications of pyrazolone in multicomponent reactions for the synthesis of dihydropyrano [2, 3-c] pyrazoles and spiro-pyrano [2, 3-c] pyrazoles in aqueous medium. Arkivoc, part i, 273-328.
- Recent approaches in the organocatalytic synthesis of pyrroles. RSC Adv.. 2021;11:13585-13601.
- [Google Scholar]
- Recent Advances in metal-and organocatalyzed asymmetric functionalization of pyrroles. Asian J. Org. Chem.. 2021;10:2709-2762.
- [Google Scholar]
- Borah, B., Dwivedi, K. D., Chowhan, L. R., 2021d. 4‐Hydroxycoumarin: a versatile substrate for the transition‐metal‐free multicomponent synthesis of bioactive heterocycles. Asian J. Org. Chem. https://doi.org/10.1002/ajoc.202100550.
- Recent advances in the transition-metal-free synthesis of quinoxalines. RSC Adv.. 2021;11:37325-37353.
- [Google Scholar]
- Review on synthesis and medicinal application of dihydropyrano [3, 2-b] pyrans and spiro-pyrano [3, 2-b] pyrans by employing the reactivity of 5-hydroxy-2-(hydroxymethyl)-4 H-pyran-4-one. Polycycl. Aromat. Compd. 2021:1-45.
- [Google Scholar]
- The antioxidant activity of coumarins and flavonoids. Mini Rev. Med. Chem.. 2013;13:318-334.
- [Google Scholar]
- Ultrasound-promoted expedient and green synthesis of diversely functionalized 6-amino-5-((4-hydroxy-2-oxo-2 H-chromen-3-yl)(aryl) methyl) pyrimidine-2, 4 (1 H, 3 H)-diones via one-pot multicomponent reaction under sulfamic acid catalysis at ambient conditions. ACS Sustain. Chem. Eng.. 2019;7:6369-6380.
- [Google Scholar]
- Ultrasound-assisted expedient and green synthesis of a new series of diversely functionalized 7-aryl/heteroarylchromeno [4, 3-d] pyrido [1, 2-a] pyrimidin-6 (7 H)-ones via one-pot multicomponent reaction under sulfamic acid catalysis at ambient conditions. ACS Sustain. Chem. Eng.. 2018;6:11018-11028.
- [Google Scholar]
- Higher-order multicomponent reactions: beyond four reactants. Chem. Soc. Rev.. 2013;42:4948-4962.
- [Google Scholar]
- Coumarin-based small-molecule fluorescent chemosensors. Chem. Rev.. 2019;119:10403-10519.
- [Google Scholar]
- Recent advances in on-water multicomponent synthesis of coumarin derivatives. Curr. Org. Chem.. 2020;24:2601-2611.
- [Google Scholar]
- Microwave-assisted synthesis, computational studies and antibacterial/anti-inflammatory activities of compounds based on coumarin-pyrazole hybrid. Royal Soc. Open Sci.. 2018;5:172435
- [Google Scholar]
- Microwave-assisted, one-pot three-component synthesis of 6-(Pyrrolyl) coumarin/quinolone derivatives catalyzed by In (III) chloride. ChemistrySelect. 2018;3:9592-9595.
- [Google Scholar]
- A microwave-assisted facile synthesis of novel coumarin derivatives containing cyanopyridine and furan as antimicrobial agents. J. Saudi Chem. Soc.. 2017;21:S153-S162.
- [Google Scholar]
- Microwave-assisted synthesis, biological evaluation and molecular docking studies of new coumarin-based 1, 2, 3-triazoles. RSC Adv.. 2020;10:11615-11623.
- [Google Scholar]
- Ligand free one-pot synthesis of pyrano [2, 3-c] pyrazoles in water extract of banana peel (WEB): a green chemistry approach. Front. Chem.. 2020;7:944.
- [Google Scholar]
- Isatin N, N′-cyclic azomethine imine 1, 3-dipole mediated regio and diastereoselective synthesis of isoxazole-containing spirooxindoles by an abnormal [3+2] cycloaddition. Tetrahedron Lett.. 2020;61:152664
- [Google Scholar]
- An efficient one-pot synthesis of new coumarin derivatives as potent anticancer agents under microwave irradiation. J. Heterocycl. Chem.. 2017;54:3519-3526.
- [Google Scholar]
- A green one-pot three-component synthesis of tetrahydrobenzo [b] pyran and 3, 4-dihydropyrano [c] chromene derivatives using a Fe 3 O 4@ SiO 2–imid–PMA n magnetic nanocatalyst under ultrasonic irradiation or reflux conditions. RSC Adv.. 2015;5:26625-26633.
- [Google Scholar]
- Lamellarins and related pyrrole-derived alkaloids from marine organisms. Chem. Rev.. 2008;108:264-287.
- [Google Scholar]
- Synthesis, chromatographic resolution, and anti-human immunodeficiency virus activity of (±)-calanolide A and its enantiomers. J. Med. Chem.. 1996;39:1303-1313.
- [Google Scholar]
- One-pot synthesis of 3-Functionalized 4-hydroxycoumarin under catalyst-free conditions. Molecules. 2018;23:235.
- [Google Scholar]
- Microwave-assisted synthesis and antitumor evaluation of a new series of thiazolylcoumarin derivatives. EXCLI J.. 2017;16:1114.
- [Google Scholar]
- Recent advances and perspectives in the synthesis of bioactive coumarins. RSC Adv.. 2016;6:46394-46405.
- [Google Scholar]
- Application of commercial microwave ovens to organic synthesis. Tetrahedron Lett.. 1986;27:4945-4948.
- [Google Scholar]
- Acaricidal activity of tonka bean extracts. synthesis and structure−activity relationships of bioactive derivatives. J. Nat. prod.. 2003;66:690-692.
- [Google Scholar]
- Bioactivation potential of thiophene-containing drugs. Chem. Res. Toxicol.. 2014;27:1344-1358.
- [Google Scholar]
- Synthesis of indolo [2, 3-c] coumarins and indolo [2, 3-c] quinolinones via microwave-assisted base-free intramolecular cross dehydrogenative coupling. Tetrahedron. 2019;75:1605-1611.
- [Google Scholar]
- Water-soluble phosphorescent ruthenium complex with a fluorescent coumarin unit for ratiometric sensing of oxygen levels in living cells. Bioconjug. Chem.. 2015;26:645-649.
- [Google Scholar]
- Microwave-assisted synthesis of new series some acetyl coumarin derivatives and studying of some their pharmacological activities. J. Pharm. Sci. Res.. 2015;7:83.
- [Google Scholar]
- Osthole suppresses hepatocyte growth factor (HGF)-induced epithelial-mesenchymal transition via repression of the c-Met/Akt/mTOR pathway in human breast cancer cells. J. Agric. Food Chem.. 2011;59:9683-9690.
- [Google Scholar]
- Developing hybrid molecule therapeutics for diverse enzyme inhibitory action: active role of coumarin-based structural leads in drug discovery. Bioorg. Med. Chem.. 2018;26:3731-3762.
- [Google Scholar]
- An alum [KAl (SO4) 2. 12H2O] catalyzed microwave assisted multicomponent synthesis of bioactive functionalized benzylpyrazolyl coumarin and quinolinone derivatives in PEG. Chem. Mat. Res. 2015;7:105-111.
- [Google Scholar]
- Robust synthesis of sugar-coumarin based fluorescent 1, 4-disubstituted-1, 2, 3-triazoles using highly efficient recyclable citrate grafted β-cyclodextrin@ magnetite nano phase transfer catalyst in aqueous media. Carbohydr. Res.. 2019;482:107736
- [Google Scholar]
- Coumarin-modified CQDs for biomedical applications—two-step synthesis and characterization. Int. J. Mol. Sci.. 2020;21:8073.
- [Google Scholar]
- Multicomponent reactions for the synthesis of heterocycles. Chem. Asian J.. 2010;5:2318-2335.
- [Google Scholar]
- Medium effects on fluorescence quantum yields and lifetimes for coumarin laser dyes. Chem. Phys. Lett.. 1980;72:391-395.
- [Google Scholar]
- Synthetic approaches and biological activities of 4-hydroxycoumarin derivatives. Molecules. 2009;14:4790-4803.
- [Google Scholar]
- Microwave assisted synthesis of coumarins: a review from 2007 to 2018. Curr. Micro. Chem.. 2018;5:162-178.
- [Google Scholar]
- Ultrasound-assisted sequential multicomponent strategy for the combinatorial synthesis of novel coumarin hybrids. ACS Comb. Sci.. 2014;16:566-572.
- [Google Scholar]
- Microwave and conventional study of coumarin-oxadiazole adducts and their anti-microbial evaluation. Folia Med.. 2021;63:105.
- [Google Scholar]
- Synthesis of mono-and bis-spiro-2-amino-4 H-pyrans catalyzed by S-alkyl O-hydrogen sulfothioate functionalized silica-coated magnetic nanoparticles under ultrasound irradiation. Res. Chem. Intermed.. 2015;41:7427-7435.
- [Google Scholar]
- Silica sulfuric acid magnetic nanoparticle: an efficient and ecofriendly catalyst for synthesis of spiro [2-amino-4 H-pyran-oxindole] s. Can. J. Chem.. 2015;93:546-549.
- [Google Scholar]
- Coumarin: a promising scaffold for anticancer agents. Anti-Cancer Agents Med. Chem.. 2015;15:1032-1048.
- [Google Scholar]
- Microwave-assisted synthesis of six-membered O-heterocycles. Synth. Commun.. 2014;44:3047-3081.
- [Google Scholar]
- Ultrasound-assisted green synthesis of five-membered O-and S-heterocycles. Synth. Commun.. 2018;48:1715-1738.
- [Google Scholar]
- An efficient, mild and metal free l-proline catalyzed construction of fused pyrimidines under microwave conditions in water. RSC Adv.. 2019;9:3755-3763.
- [Google Scholar]
- A convenient and improved Baylis-Hillman synthesis of 3-substituted 2H-1-benzopyran-2-ones. Synthesis. 2002;2002:2701-2706.
- [Google Scholar]
- Synthesis and pharmacological evaluation of a novel series of 5-(substituted) aryl-3-(3-coumarinyl)-1-phenyl-2-pyrazolines as novel anti-inflammatory and analgesic agents. Eur. J. Med. Chem.. 2009;44:1682-1688.
- [Google Scholar]
- Recent progress in the drug development of coumarin derivatives as potent antituberculosis agents. Eur. J. Med. Chem.. 2015;100:257-269.
- [Google Scholar]
- Synthesis of coumarins via Pechmann reaction catalyzed by 3-methyl-1-sulfonic acid imidazolium hydrogen sulfate as an efficient, halogen-free and reusable acidic ionic liquid. Catal. Sci. Technol.. 2012;2:1633-1636.
- [Google Scholar]
- Multicomponent reactions for facile access to coumarin-fused dihydroquinolines and quinolines: synthesis and photophysical studies. New J. Chem.. 2014;38:4722-4729.
- [Google Scholar]
- An efficient synthesis of pyrido [3, 2-c] coumarins under microwave irradiation in solvent-free conditions. ChemistrySelect. 2018;3:1411-1414.
- [Google Scholar]
- Biological evaluation of several coumarin derivatives designed as possible anti-inflammatory/antioxidant agents. J. Enzyme Inhib. Med. Chem.. 2003;18:63-69.
- [Google Scholar]
- Microwave synthesis of coumarin-maltol hybrids as potent antitumor and anti-microbial drugs: an approach to molecular docking and DNA cleavage studies. Chem. Data Collect.. 2018;15:41-53.
- [Google Scholar]
- Synthesis, characterisation, and electroluminescence properties of N-coumarin derivatives containing peripheral triphenylamine. Eur. J. Org. Chem.. 2015;2015:496-505.
- [Google Scholar]
- A green approach for the synthesis of novel 7, 11-dihydro-6H-chromeno [3, 4-e] isoxazolo [5, 4-b] pyridin-6-one derivatives using acidic ionic liquid [C4mim][HSO4] Aust. J. Chem.. 2016;69:1049-1053.
- [Google Scholar]
- Highly efficient catalyst-free domino conjugate addition, decarboxylation and esterification/amidation of coumarin carboxylic acid/esters with pyrazolones: a green chemistry approach. RSC Adv.. 2020;10:13866-13871.
- [Google Scholar]
- Highly efficient ultrasonic-assisted CuCl-catalyzed 1, 3-dipolar cycloaddition reactions in water: synthesis of coumarin derivatives linked with 1, 2, 3-triazole moiety. J. Heterocycl. Chem.. 2016;53:1402-1411.
- [Google Scholar]
- Novel coumarin-containing aminophosphonatesas antitumor agent: synthesis, cytotoxicity, DNA-binding and apoptosis evaluation. Molecules. 2015;20:14791-14809.
- [Google Scholar]
- The flavonoid constituents of cephalanthus spathelliferus. Phytochemistry. 1973;12:913-916.
- [Google Scholar]
- Molecular origins of optoelectronic properties in coumarin dyes: toward designer solar cell and laser applications. J. Phys. Chem. A. 2012;116:727-737.
- [Google Scholar]
- Chemical resolution of (±)-calanolide A,(±)-cordatolide A and their 11-demethyl analogues. Bioorg. Med. Chem. Lett.. 2008;18:1079-1083.
- [Google Scholar]
- Umbelliferone: sources, chemistry and bioactivities review. Bull. Fac. Pharm. Cairo Univ.. 2017;55:223-232.
- [Google Scholar]
- Microwave-assisted synthesis of novel hetero [5] helicene-like molecules and coumarin derivatives. Org. Biomol. Chem.. 2017;15:7909-7916.
- [Google Scholar]
- Novel coumarin derivatives of heterocyclic compounds as lipid-lowering agents. Bioorg. Med. Chem. Lett.. 2003;13:2547-2551.
- [Google Scholar]
- Synthesis and cytotoxic activity of pyranocoumarins of the seselin and xanthyletin series. J. Nat. Prod.. 1998;61:982-986.
- [Google Scholar]
- A coumarin embedded highly sensitive nitric oxide fluorescent sensor: kinetic assay and bio-imaging applications. Org. Biomol. Chem.. 2020;18:8450-8458.
- [Google Scholar]
- Microwave irradiated one pot, three component synthesis of a new series of hybrid coumarin based thiazoles: antibacterial evaluation and molecular docking studies. J. Mol. Struct.. 2021;1225:129114
- [Google Scholar]
- Synthesis, molecular docking studies and biological evaluation of potent coumarin-thiazolidinone hybrids: an approach to microwave synthesis. Chem. Data Collect.. 2018;17:327-338.
- [Google Scholar]
- New pyranocoumarins isolated from calophyllum lanigerum and calophyllum teysmannii. J. Nat. Prod.. 1996;59:754-758.
- [Google Scholar]
- Coumarin heterocyclic derivatives: chemical synthesis and biological activity. Nat. Prod. Rep.. 2015;32:1472-1507.
- [Google Scholar]
- Hybrid molecules: the privileged scaffolds for various pharmaceuticals. Eur. J. Med. Chem.. 2016;124:500-536.
- [Google Scholar]
- Synthesis of fused pyrroles containing 4-hydroxycoumarins by regioselective metal-free multicomponent reactions. Org. Biomol. Chem.. 2018;16:3289-3302.
- [Google Scholar]
- Microwave-assisted synthesis of quinazolines and quinazolinones: an overview. Front. Chem.. 2020;8:580086
- [Google Scholar]
- Green chemistry approaches to the synthesis of coumarin derivatives. Curr. Org. Chem.. 2020;24:4-43.
- [Google Scholar]
- A critical assessment of the greenness and energy efficiency of microwave-assisted organic synthesis. Green Chem.. 2011;13:794-806.
- [Google Scholar]
- Importance of microwave heating in organic synthesis. Adv. J. Chem. A.. 2019;2:94-104.
- [Google Scholar]
- Ultrasound-promoted regioselective synthesis of pyrazolyl-bis coumarinyl methanes using citric acid as a natural and efficient catalyst. Polycycl. Aromat. Compd.. 2018;40:1-16.
- [Google Scholar]
- Phosphorylated 3-heteroarylcoumarins and their use in fluorescence microscopy and nanoscopy. Chem. Eur. J.. 2012;18:16339-16348.
- [Google Scholar]
- Gold catalyzed double condensation reaction: synthesis, antimicrobial and cytotoxicity of spirooxindole derivatives. Bioorg. Med. Chem. Lett.. 2016;26:4310-4317.
- [Google Scholar]
- The inophyllums, novel inhibitors of HIV-1 reverse transcriptase isolated from the Malaysian tree, Calophyllum inophyllum Linn. J. Med. Chem.. 1993;36:4131-4138.
- [Google Scholar]
- Microwave-assisted organic synthesis and transformations using benign reaction media. Acc. Chem. Res.. 2008;41:629-639.
- [Google Scholar]
- Multicomponent-reaction-(MCR-) assisted synthesis of a coumarin-based deep blue emitter for OLEDs and related applications. ChemistrySelect. 2018;3:2951-2957.
- [Google Scholar]
- Efficient microwave-assisted synthesis, in vitro antimicrobial evaluation of furo [3, 2-c] pyrones and furo [3, 2-c] coumarins along with molecular docking studies. ChemistrySelect. 2020;5:14041-14049.
- [Google Scholar]
- Triterpenoids and coumarins from the leaves of Calophyllum Cordato-Oblongum. Phytochemistry. 1985;24:1553-1556.
- [Google Scholar]
- New coumarin dyes with rigidized structure for flashlamp-pumped dye lasers. Opt. Commun.. 1975;13:222-225.
- [Google Scholar]
- Heterogeneous graphene oxide as recyclable catalyst for azomethine ylide mediated 1, 3 dipolar cycloaddition reaction in aqueous medium. RSC Adv.. 2018;8:35587-35593.
- [Google Scholar]
- Total synthesis and evaluation of lamellarin α 20-Sulfate analogues. Bioorg. Med. Chem.. 2002;10:3285-3290.
- [Google Scholar]
- Diastereoselective synthesis of trans-2, 3-dihydrofuro [3, 2-c] coumarins by MgO nanoparticles under ultrasonic irradiation. J. Saudi Chem. Soc.. 2017;21:929-937.
- [Google Scholar]
- Molecular iodine catalysed one-pot synthesis of chromeno [4, 3-b] quinolin-6-ones under microwave irradiation. Green Chem.. 2015;17:3766-3770.
- [Google Scholar]
- Intensified synthesis of [3, 4-d] triazole-fused chromenes, coumarins, and quinolones. Monatsh. Chem.. 2017;148:69-75.
- [Google Scholar]
- L-Proline catalyzed four-component one-pot synthesis of coumarin-containing dihydropyrano [2, 3-c] pyrazoles under ultrasonic irradiation. Tetrahedron. 2016;72:7599-7605.
- [Google Scholar]
- Microwave-assisted organic synthesis: overview of recent applications. Green Tech. Org. Synth. Med. Chem. 2018:441-468.
- [Google Scholar]
- Fundamentals of green chemistry: efficiency in reaction design. Chem. Soc. Rev.. 2012;41:1437-1451.
- [Google Scholar]
- Osthole protects lipopolysaccharide-induced acute lung injury in mice by preventing down-regulation of angiotensin-converting enzyme. Eur. J. Pharm. Sci.. 2013;48:819-824.
- [Google Scholar]
- Isatins as privileged molecules in design and synthesis of spiro-fused cyclic frameworks. Chem. Rev.. 2012;112:6104-6155.
- [Google Scholar]
- Tangeti, V. S., Varma K, R., Siva Prasad, G. V., Satyanarayana, K. V. V. V., 2016. Synthesis of C3-dihydrofuran substituted coumarins via multicomponent approach. Synth. Commun. 46, 613-619.
- A pharmacological review of dicoumarol: an old natural anticoagulant agent. Pharmacol. Res.. 2020;105193
- [Google Scholar]
- Synthesis and application of coumarin fluorescence probes. RSC Adv.. 2020;10:10826-10847.
- [Google Scholar]
- Green chemical multicomponent approach for the synthesis of C3-pyranopyrazole-substituted coumarins. J. Iran. Chem. Soc.. 2018;15:823-829.
- [Google Scholar]
- Diversity oriented synthesis of oxygen-heterocycles, warfarin analogs utilizing microwave-assisted dimethyl urea-based deep eutectic solvents. Polycycl. Aromat. Compd. 2021;41:1-11.
- [Google Scholar]
- Structure-based design of HIV protease inhibitors: 4-hydroxycoumarins and 4-hydroxy-2-pyrones as non-peptidic inhibitors. J. Med. Chem.. 1994;37:3200-3204.
- [Google Scholar]
- Coumarins as anticancer agents: a review on synthetic strategies, mechanism of action and SAR studies. Eur. J. Med. Chem.. 2015;101:476-495.
- [Google Scholar]
- Xanthyletin and xanthoxyletin from a gorgonian, Echinogorgia sp. J. Nat. Prod.. 1989;52:1303-1304.
- [Google Scholar]
- Electronic absorption spectra and structure of hydroxycoumarin derivatives and their ionized forms. Can. J. Chem.. 1997;75:365-376.
- [Google Scholar]
- Coumarinyl (thienyl) thiazoles: novel photochromes with modulated fluorescence. Org. Lett.. 2008;10:1319-1322.
- [Google Scholar]
- New reactions, functional compounds, and materials in the series of coumarin and its analogs. Russ. Chem. Bull.. 2012;61:1342-1362.
- [Google Scholar]
- Coumarins in polymers: from light harvesting to photo-cross-linkable tissue scaffolds. Chem. Rev.. 2004;104:3059-3078.
- [Google Scholar]
- Recent advances in the synthesis of coumarin derivatives via Knoevenagel condensation: a review. Synth. Commun.. 2014;44:2756-2788.
- [Google Scholar]
- Design, synthesis, and vasorelaxant and platelet antiaggregatory activities of coumarin–resveratrol hybrids. Bioorg. Med. Chem. Lett.. 2006;16:257-261.
- [Google Scholar]
- Inhibition of cell cycle progression in human leukemia HL-60 cells by esculetin. Cancer Lett.. 2002;183:163-168.
- [Google Scholar]
- Antioxidant properties of Cortex Fraxini and its simple coumarins. Food Chem.. 2007;104:1464-1471.
- [Google Scholar]
- Coumarin-based fluorescent probes for bioimaging: recent applications and developments. Curr. Org. Chem.. 2021;25:2142-2154.
- [Google Scholar]
- An expedient lewis-acid-catalyzed microwave-assisted domino approach to coumarin-fused pyrroles and related heterocycles under neat conditions. ChemistrySelect. 2019;4:12768-12773.
- [Google Scholar]
- Improved one-pot synthetic conditions for synthesis of functionalized fluorescent coumarin-thiophene hybrids: Syntheses, DFT studies, photophysical and thermal properties. Tetrahedron. 2019;75:2143-2154.
- [Google Scholar]
- Ratiometric molecular probes based on dual emission of a blue fluorescent coumarin and a red phosphorescent cationic iridium (III) complex for intracellular oxygen sensing. Sensors. 2015;15:13503-13521.
- [Google Scholar]
- Coumarin-modified graphene quantum dots as a sensing platform for multicomponent detection and its applications in fruits and living cells. ACS Omega. 2020;5:7369-7378.
- [Google Scholar]
- Antimicrobial activity of two novel coumarin derivatives: 3-cyanonaphtho [1, 2-(e)] pyran-2-one and 3-cyanocoumarin. New Microbiol.. 2002;25:213-222.
- [Google Scholar]
- Overview on developed synthesis procedures of coumarin heterocycles. J. Iran. Chem. Soc. 2020:1-64.
- [Google Scholar]
- Consecutive multicomponent reactions for the synthesis of complex molecules. Org. Biomol. Chem.. 2019;17:7632-7650.
- [Google Scholar]
- The molecular diversity scope of 4-hydroxycoumarin in the synthesis of heterocyclic compounds via multicomponent reactions. Mol. Divers.. 2019;23:1029-1064.
- [Google Scholar]
- Ultrasound-assisted synthesis of heterocyclic compounds. Mol. Divers.. 2020;24:771-820.
- [Google Scholar]
- Microwave-assisted synthesis and antifungal activity of novel coumarin derivatives: Pyrano [3, 2-c] chromene-2, 5-diones. Eur. J. Med. Chem.. 2016;116:76-83.
- [Google Scholar]
- New caged coumarin fluorophores with extraordinary uncaging cross sections suitable for biological imaging applications. J. Amer. Chem. Soc.. 2004;126:4653-4663.
- [Google Scholar]







