Phenolic composition, antioxidant and enzyme inhibitory activities of Parkia biglobosa (Jacq.) Benth., Tithonia diversifolia (Hemsl) A. Gray, and Crossopteryx febrifuga (Afzel.) Benth
⁎Corresponding authors. macntamfu@yahoo.co.uk (Alfred Ngenge Tamfu), rodica.dinica@ugal.ro (Rodica Mihaela Dinica)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Medicinal plants from Chad grow under special climatic conditions in between the equatorial forest of Central Africa and the desert of North Africa and are understudied. Three medicinal plants from Chad (T. diversifolia, P. Biglobosa and C. Febrifuga) were evaluated for their phenolic composition, antioxidant and enzyme inhibition activities. The total phenolic composition varied from 203.19 ± 0.58 mg GAE/g DW in the ethyl acetate extract of P. biglobosa, to 56.41 ± 0.89 mg GAE/g DW in the methanol extract of C. febrifuga while the total flavonoid content varied from 51.85 ± 0.91 mg QE/g DW in the methanol extract of P. biglobosa to 08.56 ± 0.25 mg QE/g DW in the methanol extract of C. febrifuga. HPLC-DAD revealed that rutin, gallic acid and protocatechuic acid were the most abundant phenolics in T. diversifolia, P. Biglobosa and C. Febrifuga respectively. The antioxidant activity assayed by five different methods revealed very good activity especially in the DPPH•, ABTS•+ and CUPRAC assays where the extracts were more active than the standard compounds used. Good inhibition was exhibited against acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) with methanol (IC50: 15.63 ± 0.72 µg/mL), ethyl acetate (IC50: 16.20 ± 0.67 µg/mL) extracts of P. biglobosa, and methanol (IC50: 21.53 ± 0.65 µg/mL) and ethyl acetate (IC50: 30.81 ± 0.48 µg/mL) extracts of T. diversifolia showing higher inhibition than galantamine (IC50: 42.20 ± 0.44 µg/mL) against BChE. Equally, good inhibition was shown on α-amylase and α-glucosidase. On the α-glucosidase, the ethyl acetate (IC50 = 12.47 ± 0.61 µg/mL) and methanol extracts (IC50 = 16.51 ± 0.18 µg/mL) of P. biglobosa showed higher activity compared to the standard acarbose (IC50 = 17.35 ± 0.71 µg/mL) and on α-amylase, the ethyl acetate (IC50 = 13.50 ± 0.90 µg/mL) and methanol (IC50 = 18.12 ± 0.33 µg/mL) extracts of P. biglobosa showed higher activity compared to acarbose (IC50 = 23.84 ± 0.25 µg/mL). The results indicate that these plants are good sources of antioxidant phenolics and can be used to manage oxidative stress linked illnesses such as Alzheimer’s disease and diabetes.
Keywords
Tithonia diversifolia
Parkia Biglobosa
Crossopteryx febrifuga
Antioxidant
Anticholinesterase
Alzheimer’s disease
α-Amylase inhibition
α-Glucosidase inhibition
Antidiabetic activity
1 Introduction
Reactive oxygen species (ROS) are at the origin of many human diseases and health complications and this occurs as a result of an imbalance between ROS generation and antioxidants (disequilibrium between generation of oxidants and antioxidants) in the body that spreads over most cell targets (Talla et al., 2014; Talla et al., 2017; Forman and Zhang, 2021). This unfavorable imbalance in pro-oxidant and antioxidant creates dangerous reactive nitrogen and oxygen species such as nitric oxide, hydroxyl radicals, superoxide anion, organic and hydrogen peroxides which are notorious for cellular damage with undesirable effects on metabolic enzymes and lipids, proteins and DNA (Hassan et al., 2017). This effect of overproduction of free radicals can cause disorders such as diabetes, atherosclerosis, myocardial infarction, cardiovascular diseases, arthritis, post-ischemic injury, aging, inflammatory disorders, neurodegenerative diseases (Parkinson's disease, Alzheimer's disease, Huntington's disorder), pancreatic and liver diseases in humans due to the oxidative damage on biomolecules by the radicals and oxidative species (Fang et al., 2002; Uttara et al., 2009; Hassan et al., 2017). However, ROS also play important role as cell signaling molecules for usual physiological processes but only in situations of disproportion between generation of ROS and body’s ability of detoxification that they are harmful and this leads to oxidative stress (Asraoui et al., 2021). Some free radicals are useful, and the body has several enzymatic mechanisms of antioxidant action which helps in maintaining a delicate balance and equilibrium between antioxidants and oxidants such as removal, suppression or inactivation of ROS and up-regulation of antioxidant defenses (Talla et al., 2014; Sharifi-Rad et al., 2020). Antioxidants are exogenous or endogenous compounds from natural or synthetic origin, capable of removing free radicals, scavenging and inhibiting formation of ROS and their precursors and chelating metals which intervene in the catalysis of ROS generation (Gilgun-Sherki et al., 2001). As mentioned earlier, oxidative stress involves several pathological conditions such as obesity, hypertension and atherosclerosis besides enzymatic disorders notably neurodegenerative diseases like Alzheimer’s disease (AD) and diabetes (Liguori et al., 2018; Forman and Zhang, 2021). One of the most common types of dementia is the Alzheimer’s disease (AD) characterized by oxidative stress, progressive degeneration of neurons and is commonly accompanied by observable deposits of amyloid-β in the brain and low levels of acetylcholine (Rahman and Choudhary, 2015; Tamfu et al., 2019). The unusual deposits within the central nervous system of β-sheets of amyloid plaques has a strong correlation with memory loss and dementia, and contributes to decrease in the uptake or release of choline thereby affecting cholinergic neurotransmission (Breijyeh and Karaman, 2020). Acetylcholine and butyrylcholine are usually hydrolyzed by acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) respectively, and this leads to the decrease in the neurotransmitter levels in cholinergic synapses within certain regions of the brain and therefore the inhibition of both AChE and BChE is a beneficial therapeutic strategy to remedy AD (Uddin et al., 2021; Birsan et al., 2021). AD, dementia, cognitive impairment resulting from increasing amyloid-beta accumulation and neuroinflammation can also be induced by oxidative stress and type 2 diabetes (impaired glucose tolerance) and hyperglycemia (Breijyeh and Karaman, 2020). Since acute hyperglycemia occurs in all diabetic patients, a major strategy to remedy diabetic conditions and achieve normal and effective blood glucose levels is to control postprandial hyperglycemia by slowing down the absorption of carbohydrate hydrolysis and uptake using voglibose, miglitol and acarbose which work by inhibiting digestive enzymes (Nguelefack et al., 2020). The most probable therapeutic strategy to reduce post-prandial glucose levels is by delaying glucose absorption through the inhibition of α-amylase and α-glucosidase which are major enzymes responsible for the hydrolysis of oligosaccharides and disaccharides into monosaccharides (Salar et al., 2017).
Polyphenols and flavonoids function as chemical entities that prevents ROS induced oxidative damage and equally capable of reducing postprandial hyperglycemia by inhibiting the enzymes involved in carbohydrate hydrolysis (Ibrahima et al., 2017). Medicinal plants extracts have been a valuable source of natural antioxidants and have demonstrated their usefulness as medicinal supplements and alternative drugs that can prevent or protect the body against oxidative stress diseases and also as food additives by preventing lipid peroxidation and most recently, in the green synthetic process of metallic nanomaterials (Flieger et al., 2021). Plants provide sustainable, safer and more effective antioxidant alternatives mostly attributed to flavonoids, phenolic acids, tannins, stilbenes, and anthocyanins and these phenolic compounds are employed as a remarkable strategy to reduce pathological conditions such as diabetes, cardiovascular diseases, aging, cancer, and neurodegenerative disorders (Yu et al., 2021).
Tithonia diversifolia (Hemsl) A. Gray, also known as Mexican sunflower or tree marigold, is distributed in Africa, Asia, Central and South America (Hiransai et al., 2016). Tithonia diversifolia has been used for treatment of malaria, parasitic infections, fever, diarrhea, dermatitis, hepatitis in traditional medicine (Maregesi et al., 2007; Hiransai et al., 2016). Previous reports showed that Tithonia diversifolia contains alkaloids, tannins, flavonoids and saponins (Olayinka et al., 2015; Obayomi et al., 2021). In previous reports, antidiabetic, antiplasmodial, cytotoxic and antioxidant activities of T. diversifolia have been reported (Goffin et al., 2002; Miura et al., 2005; Hiransai et al., 2016). Parkia biglobosa (Jacq.) Benth., is commonly found in Western African Region and known as ‘African locust bean’. P. biglobosa has been used traditionally for various medicinal purposes such as, antidiabetic, anti-hypertensive, anti-inflammatory, wound healing, antihelmintic, etc. (Musara et al., 2020). Previous reports indicated that P. biglobosa is rich in phytochemicals including saponins, tannins, flavonoids, terpenoids, alkaloids, cardiac glycosides and coumarins (Yakubu et al., 2021). Antidiabetic, antimalarial, antioxidant, antibacterial, antihypertensive, antiinflammatory, anti-carcinogenic, and analgesic activities of P. biglobosa have been reported in earlier studies (Kouadio et al., 2000; Lamien-Meda et al., 2008; Builders et al., 2011; Musara et al., 2020; Oyedemi et al., 2021). Crossopteryx febrifuga (Afzel.) Benth. is largely distributed in West to Central and East Africa. It is used in traditional and indigenous medicine in African in treating inflammatory diseases, fever, malaria, diarrhoea and dysentery (Chouna et al., 2014). Reports of phytochemical studies show that C. febrifuga contains saponins and flavonoids (Kayangar et al., 2019). Also, analgesic, anti-inflammatory, antipyretic, hypoglycemic, hypolipidemic, antimicrobial, antimalarial and antiplasmodial potential of C. febrifuga have been reported (Elufioye and Agbedahunsi (2004); Salawu et al., 2008; Halilu et al., 2012; Ojewale et al., 2013).
As a contribution to the search of natural and alternative medicines to cure oxidative damage related ailments, precisely Alzheimer’s disease and diabetes, three medicinal plants from Chad were investigated for their potential activity using bioassays. Phenolic extracts were prepared from Parkia biglobosa (Jacq.) Benth., Tithonia diversifolia (Hemsl) A. Gray, and Crossopteryx febrifuga (Afzel.) Benth and evaluated for their antioxidant, acetylcholinesterase, butyrylcholinesterase, tyrosinase, α-amylase and α-glucosidase inhibitory activites.
2 Experimental
2.1 Plant material and extraction
The plants were collected during the month of October 2020 from Moundou locality, Lac Wey Division, Logone Occidental Region of Chad. The plants T. diversifolia, P. biglobosa and C. febrifuga were identified and voucher specimens prepared by the botanist Mr. Baroua Abouna of the Farcha Laboratory where they were deposited under the specimen numbers 1762, 1386 and 010 for T. diversifolia, P. biglobosa, and C. febrifuga respectively. The plant parts, stems for T. diversifolia, and stem barks for P. biglobosa and C. febrifuga were collected, cut into pieces, dried and then ground into powder. 200 g of each powdered plant material was extracted with 70% ethanol/water (v/v) solution. The supernatant was filtered and the solvent evaporated on a rotavapor. This was performed repeatedly three times to obtain a crude paste of extract for each plant. The crude extract was dissolved in water and re-extracted using liquid–liquid extraction with ethyl acetate and then methanol in order of increasing polarity to yield the ethyl acetate extract and the methanol extract of each plant. These extracts were freeze dried and stored at 4 °C prior to analyses.
2.2 Determination of total phenolic and total flavonoid contents
Folin-Ciocalteu reagent was employed in the determination of the total phenolic content (TPC) of the extracts according to the method described previously (Chandra et al., 2014) with little modifications. 0.2 mL of test sample was added to 0.6 mL of distilled water and 0.2 mL of Folin-Ciocalteu’s reagent (1:1). The mixture was stirred for 5 min after which 1 mL of saturated Na2CO3 solution (8% w/v in H2O) was added and the final volume made up to 3 mL using distilled water. The reaction mixture from each sample was incubated in the absence of light for 30 min giving rise to a blue colored mixture which was subjected to centrifuging and its absorbance of was measured at 765 nm. Gallic acid was used to establish a standard curve from which the phenolic content was deduced as gallic acid equivalents GAE/g of dry plant material. Each experiment was done in triplicate.
The AlCl3 colorimetric method was used in determining the total flavonoids content (TFC) of the extracts as described elsewhere (Chandra et al., 2014) with minor modifications. Quercetin standard was used in establishing the calibration curve. 5.0 mg of quercetin was dissolved in 1.0 mL methanol as a stock solution from which pure methanol was added in different amounts to give serial dilutions (5–200 μg/mL). 0.6 mL of 2% AlCl3 was added separately to 0.6 mL diluted standard quercetin solutions or extracts and mixed and the resulting solution incubated at room temperature for 1 h after which the absorbance of each mixture was read against a blank at a wavelength of 420 nm. The calibration curve was used to deduce the total flavonoid content in each test sample expressed as mg quercetin equivalent (QE)/g of dried plant material. Each experiment was done in triplicate.
2.3 Determination of HPLC–DAD phenolic profiles
The phenolic compounds in the plant extracts were detected and quantified using reversed-phase high-performance liquid chromatography (RP-HPLC) coupled with diode array detector (DAD) as described previously (Çayan et al., 2020; Tamfu et al., 2021a). Briefly, known weights of each extract were dissolved in water:methanol (80:20) then filtered on sterile 0.20 μm disposable filter disk for liquid chromatography disk and an Intertsil ODS-3 reverse phase C18 column was used for the separation employing a 1.0 mL/min solvent flow rate and 20 μL injection volume. Two mobile phases A (0.5% acetic acid H2O) and B (0.5% acetic acid in CH3OH). A gradient elution was applied as follows: 0–10% B (0–0.01 min); 10–20% B (0.01–5 min); 20–30% B (5–15 min); 30–50% B (15–25 min); 50–65% B (25–30 min); 65–75% B (30–40 min); 75–90% B (40–50 min) 90–10% B (50–55 min). A photodiode array detector set at 280 nm wavelength was employed in the detection and the UV data together with retention times were compared with authentic standards. Each analysis was performed three times. A calibration plot established through the elution of known concentrations (0.0, 0.00782, 0.01563, 0.03125, 0.0625, 0.125, 0.25, 0.5 and 1.0 ppm) of authentic compounds was used in the identification and quantification of the constituent phenolic compounds. 26 phenolic standards (gallic, p-hydroxy benzoic, protocatechuic, ellagic, chlorogenic, trans-cinnamic, 3-hydroxy benzoic, vanillic, syringic, p-coumaric, rosmarinic and ferulic acids; catechin, kaempferol, hesperetin, pyrocatechol vanillin, 6,7-dihydroxy coumarin, coumarin, rutin, myricetin, chrysin, luteolin, apigenin taxifolin and quercetin) were used. The results were expressed as μg per g dry weight of extract.
2.4 Antioxidant activity
Five different methods namely: DPPH• (2,2-diphenyl-1-picrylhydrazylhydrazyl) radical scavenging assay, ABTS•+ (2,20-azino-bis-(3-ethylbenzothiazoline-6-sulfonic) acid) radical cation, CUPRAC (cupric reducing antioxidant capacity), metal chelation and β-carotene-linoleic acid assays were used to measure the antioxidant potential of the plant extracts. Inhibition of lipid peroxidation was evaluated using the β-carotene-linoleic acid assay as decribed in a previous study (Tel-Çayan and Duru, 2019). Radical scavenging potentials on DPPH• and ABTS•+ were measure by spectrophotometric means as previously described (Tel et al., 2012; Tamfu et al., 2020b). CUPRAC was determined in accordance to a method described elswhere (Apak et al., 2004). In the above assays, α-tocopherol and BHA (Butylated Hydroxyanisole) were employed as antioxidant standards against which the activities of the extracts were compared. EDTA was used as standard in the metal chelation assay performed on Fe2+ (Decker and Welch, 1990).
2.5 Anticholinesterase activity
Cholinesterases (AChE and BChE) inhibitory activity was measured using the Ellman's method with minor changes (Ellman et al., 1961; Tamfu et al., 2020a). Two cholinesterases, AChE and BChE from electric eel and horse serum respectively were used, and acetyl- and butyryl thiocholine iodides were employed as substrates. Cholinesterase inhibitory activity was assayed using DTNB (5,5′-Dithio-bis(2-nitrobenzoic)acid) in which the reference compound used was galantamine. 96 well plates were used for the tests and the absorbances were measured on a microplate reader (SpectraMax, Molecular Devices, California, USA). The results were given as 50% inhibitory concentration (IC50).
2.6 Anti-tyrosinase activity
The inhibition of tyrosinase enzyme (mushroom source) was determined through the method published elsewhere (Masuda et al., 2005) using L-DOPA as the substrate. In a 96-well microplate, 10 μL solution of sample or kojic acid (reference) were added to 150 μL of sodium phosphate buffer (pH 6.8, 100 mM) followed by 20 μL of tyrosinase enzyme and the resulting mixture incubated at 37 °C for 10 min. 20 μL of L-DOPA were added after incubation and the absorbances of the resulting solutions were taken at 475 nm and the results expressed as concentration at which 50% inhibition was observed (IC50).
2.7 In vitro α-amylase inhibition assay
The 3,5-dinitrosalicylic acid (DNSA) method was used to evaluate α-amylase inhibition (Wickramaratne et al., 2016) with little modifications. Each sample was dissolved in minimal amounts of 10% DMSO and diluted in buffer ((Na2HPO4/NaH2PO4 (0.02 M), NaCl (0.006 M) at pH 6.9) to obtain 6.25, 12.5, 25, 50 and 100 µg/mL. 200 μL of α-amylase (2 units/mL) were added to 200 μL of extract or acarbose and the mixture incubated 30 °C for10 minutes after which 200 μL of starch (1% in water (w/v)) were added and further incubated for 3 min. 200 μL DNSA reagent (12 g of sodium potassium tartratetetrahydrate in 8.0 mL of 2 M NaOH and 20 mL of 96 Mm of 3.5-dinitrosalicylic acid solution) were added and boiled about 85–90 °C for 10 min in a water bath after which the resulting mixture was cooled and diluted using 5 mL of distilled water. The absorbance was read at 540 nm. The control experiment was that in which acarbose was used. The percentage inhibition of α-amylase inhibition was calculated according to the equation given below and IC50 values were also determined.
2.8 In vitro α-Glucosidase inhibition assay
The inhibition of α-Glucosidase was assayed using a previously described procedure (Chokki et al., 2020) with minor changes. To 20 μL sodium phosphate buffer (pH 5.0) in a 96-well plate was added 20 μL of p-nitrophenyl-β-D-glucopyranoside (1 mg/mL) and 10 μL of extract or acarbose at different concentrations (6.25, 12.5, 25, 50 and 100 µg/mL in DMSO) and the mixture incubated for 10 min at 37 °C after which 10 μL of α-Glucosidase (from almonds) solution (5 mg/mL) were introduced into each well and further incubated for 30 min at 37 °C. 140 μL of sodium carbonate buffer pH = 10 was added to quench the reaction and absorbance was read at 410 nm using a microplate reader (iTecan Microplate reader). The system without α-glucosidase was used as blank. The percentage of inhibition of α-glucosidase was deduced according to the equation below as well as the IC50.
2.9 Statistical analysis
Each measurement and absorbance for bioassays was done in triplicate. The results were recorded as the means ± standard error of the mean for three parallel measurements. Statistical analysis was performed with MINITAB 16 and ANOVA (analysis of variance) procedure was employed to determine the significant differences between means and the level of p < 0.05 were regarded as significant.
3 Results
3.1 Total phenolic and flavonoid contents
Phenolic and flavonoids compounds are very important classes of bioactive constituents that occur in plants and are famous for their antioxidant activity. Phenolic content measured with the use of Folin-Ciocalteu reagent and gallic acid (0–250 µg/mL) and expressed in terms milligrams of gallic acid equivalent per gram dry weight of extract (GAE/g DW) are shown on Table 1. The total phenolic composition varied from 203.19 ± 0.58 mg GAE/g DW in the ethyl acetate extract of P. biglobosa, to 56.41 ± 0.89 mg GAE/g DW in the methanol extract of C. febrifuga. For T. diversifolia, TPC were 112.16 ± 0.33 and 105.74 ± 0.25 mg GAE/g DW respectively for ethylacetate extract and MeOH extracts. In P. biglobosa, 203.19 ± 0.58 and 158.07 ± 1.34 mg GAE/g DW in the ethyl acetate and MeOH extracts respectively and in the C. febrifuga TPC was 69.82 ± 0.65 and 56.41 ± 0.89 mg GAE/g DW respectively in the ethyl acetate and MeOH extracts. Colorimetric method was used with quercetin as standard to evaluate the total flavonoid content (TFC) of the plant extracts and the results reported in milligrams of quercetin equivalents per gram dry extract weight (mg QE/g DW) on Table 1. The TFC varied from 51.85 ± 0.91 mg QE/g DW in the methanol extract of P. biglobosa to 08.56 ± 0.25 mg QE/g DW in the methanol extract of C. febrifuga. Significant variation (p < 0.05) in the total content of phenolic and flavonoids was observed between the different plants extracts.
| Plant | Extract | TPC (mg GAE/g dry extract weight) |
TFC (mg QE/g dry extract weight) |
|---|---|---|---|
| T. diversifolia | AcOEt | 112.16 ± 0.33 | 36.10 ± 0.58 |
| MeOH | 105.74 ± 0.25 | 25.49 ± 0.31 | |
| P. biglobosa | AcOEt | 203.19 ± 0.58 | 51.85 ± 0.91 |
| MeOH | 158.07 ± 1.34 | 44.27 ± 1.03 | |
| C. febrifuga | AcOET | 69.82 ± 0.65 | 19.08 ± 0.71 |
| MeOH | 56.41 ± 0.89 | 08.56 ± 0.25 |
Values expressed are means ± S.E.M. of three parallel measurements (p < 0.05).
3.2 HPLC-DAD phenolic composition
Target phenolic constituents of extracts were evaluated and reported as μg/g extract using HPLC‐DAD (Table 2). Out of the twenty-six standard and authentic phenolic compounds used in the analyses, ten phenolic compounds were detected in ethyl acetate extract of T. diversifolia, seven in methanol extract of T. diversifolia, ten in ethyl acetate and butanol extracts of P. biglobosa, eight in extracts of C. febrifuga. The chemical structures of the component compounds detected and quantified by HPLC-DAD are presented on Fig. 1. Amongst the detected compounds, rutin (113.2 ± 1.25 µg/g) was the most abundant compound in the ethyl acetate extract of the T. diversifolia, followed by ferulic acid (35.84 ± 0.29 µg/g) and caffeic acid (32.51 ± 0.40 µg/g). Rutin (13.84 ± 0.63 µg/g), coumarin (11.24 ± 0.45 µg/g) and apigenin (10.61 ± 0.52) were found as the most dominant phenolic compounds in the methanol extract of T. diversifolia. Gallic acid was revealed as the major phenolic constituent compound of ethyl acetate (65.10 ± 0.61 µg/g) and methanol (23.85 ± 0.49 µg/g) extracts of P. biglobosa. The other major compounds were found as p-coumaric acid (16.83 ± 0.33 µg/g) in ethyl acetate extract of P. biglobosa and ferulic acid (15.28 ± 0.37 µg/g) in the methanol extract of P. biglobosa. Protocatechuic acid was the most abundant compound in ethyl acetate (36.78 ± 0.24 µg/g) and butanol (9.84 ± 0.31 µg/g) extracts of C. febrifuga. Gallic acid, caffeic acid, ferulic acid and rutin were identified in all studied extracts with different amounts.
| No | Phenolic compounds | RT (min) |
T. diversifolia AcOEt extract |
T. diversifolia MeOH extract |
P. biglobosa AcOEt extract |
P. biglobosa MeOH extract |
C. febrifuga AcOEt extract |
C. febrifuga MeOH extract |
|---|---|---|---|---|---|---|---|---|
| 1 | Gallic acid | 5.70 | 20.44 ± 0.48 | 6.58 ± 0.21 | 65.10 ± 0.61 | 23.85 ± 0.49 | 9.67 ± 0.35 | 6.33 ± 0.20 |
| 2 | Protocatechuic acid | 8.85 | 7.15 ± 0.27 | – | 8.58 ± 0.32 | 5.51 ± 0.27 | 36.78 ± 0.24 | 9.84 ± 0.31 |
| 3 | Catechin | 10.68 | 6.79 ± 0.31 | – | 4.42 ± 0.24 | 8.68 ± 0.41 | 1.78 ± 0.17 | – |
| 4 | Pyrocatechol | 11.04 | 6.92 ± 0.25 | – | – | – | – | – |
| 5 | Chlorogenic acid | 12.35 | 6.57 ± 0.42 | – | 3.43 ± 0.36 | 5.78 ± 0.20 | 4.65 ± 0.49 | – |
| 6 | p-hydroxy benzoic acid | 12.77 | -b | – | – | – | – | – |
| 7 | 6.7-Dihydroxy coumarin | 14.10 | – | – | – | – | – | – |
| 8 | Caffeic acid | 15.09 | 32.51 ± 0.40 | 4.24 ± 0.15 | 5.86 ± 0.29 | 5.52 ± 0.33 | 15.38 ± 0.41 | 5.88 ± 0.36 |
| 9 | 3- hydroxy benzoic acid | 15.98 | – | – | – | – | – | – |
| 10 | Syringic acid | 16.56 | – | – | – | – | – | – |
| 11 | Vanillin | 17.78 | – | – | – | – | – | – |
| 12 | p-Coumaric acid | 20.56 | 7.35 ± 0.18 | – | 16.83 ± 0.33 | 9.31 ± 0.52 | – | – |
| 13 | Taxifolin | 21.26 | – | – | 8.73 ± 0.21 | 6.41 ± 0.45 | – | – |
| 14 | Ferulic acid | 22.14 | 35.84 ± 0.29 | 3.49 ± 0.22 | 8.53 ± 0.16 | 15.28 ± 0.37 | 5.36 ± 0.14 | 2.58 ± 0.24 |
| 15 | Coumarin | 24.49 | – | 11.24 ± 0.45 | – | – | – | – |
| 16 | Rutin | 25.30 | 113.2 ± 1.25 | 13.84 ± 0.63 | 7.82 ± 0.25 | 4.95 ± 0.42 | 8.66 ± 0.37 | 2.04 ± 0.18 |
| 17 | Ellagic acid | 26.11 | – | – | 5.45 ± 0.31 | 6.33 ± 0.38 | – | – |
| 18 | Rosmarinic acid | 26.77 | – | – | – | – | 6.75 ± 0.25 | 3.74 ± 0.42 |
| 19 | Myricetin | 27.35 | – | – | – | – | – | – |
| 20 | Quercetin | 30.83 | – | – | – | – | – | – |
| 21 | trans-cinnamic acid | 31.34 | – | – | – | – | – | – |
| 22 | Luteolin | 31.70 | – | – | – | – | – | – |
| 23 | Hesperetin | 32.14 | – | 7.86 ± 0.41 | – | – | – | – |
| 24 | Kaempferol | 33.21 | – | – | – | – | – | – |
| 25 | Apigenin | 33.57 | 12.49 ± 0.35 | 10.61 ± 0.52 | – | – | – | – |
| 26 | Chrysin | 38.43 | – | – | – | – | – | – |
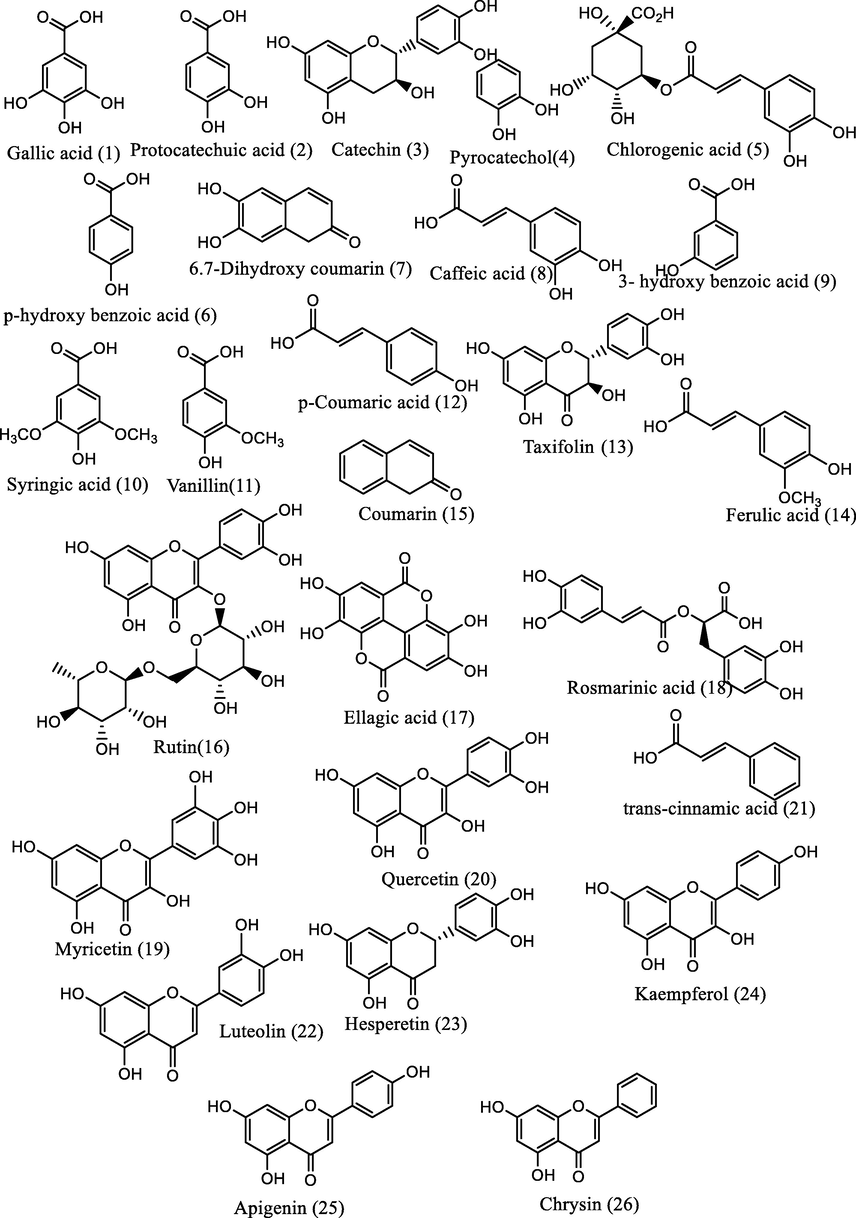
- Structures of identified phenolic compounds in the plant extracts.
3.3 Antioxidant activity
The results of the antioxidant effects of the ethyl acetate and MeOH extracts of T. diversifolia, P. biglobosa and C. Febrifuga, measured according to the β-carotene-linoleic acid, DPPH•, ABTS+•, CUPRAC and metal chelation models are presented on Table 3. Inhibition of lipid peroxidation capacity of the extracts was determined by β-carotene-linoleic acid assay. The ethyl acetate and methanol extracts of P. biglobosa exhibited the strongest inhibition activity of lipid peroxidation with IC50 values of 4.05 ± 0.15 and 4.46 ± 0.18 µg/mL, respectively. Also, ethyl acetate and MeOH extracts of T. diversifolia exhibited good activities with IC50 values of 9.05 ± 0.34 and 12.42 ± 0.25 µg/mL, respectively in β-carotene-linoleic acid assay while extracts of C. febrifuga were shown to possess low activity when compared to the other tested extracts.
| Antioxidant activity | ||||||
|---|---|---|---|---|---|---|
| Plants | Extracts | β-carotene-linoleic acid assay IC50 (µg/mL)a |
DPPH• assay IC50 (µg/mL) |
ABTS•+ assay IC50 (µg/mL) |
CUPRAC assay A0.5 (µg/mL)b |
Metal chelating assay IC50 (µg/mL) |
| T. diversifolia | AcOEt | 9.05 ± 0.34 | 16.51 ± 0.15 | 9.74 ± 0.21 | 13.37 ± 0.16 | 45.61 ± 1.05 |
| MeOH | 12.42 ± 0.25 | 25.80 ± 0.21 | 15.40 ± 0.28 | 19.69 ± 0.35 | 68.21 ± 1.36 | |
| P. biglobosa | AcOEt | 4.05 ± 0.15 | 5.15 ± 0.20 | 3.45 ± 0.19 | 5.80 ± 0.30 | 41.70 ± 1.22 |
| MeOH | 4.46 ± 0.18 | 5.83 ± 0.14 | 4.18 ± 0.27 | 6.21 ± 0.42 | 53.25 ± 1.48 | |
| C. febrifuga | AcOEt | 43.57 ± 0.35 | 37.52 ± 0.51 | 28.47 ± 0.74 | 35.41 ± 0.88 | >100 |
| MeOH | 70.25 ± 0.49 | 96.44 ± 0.89 | 65.61 ± 1.32 | 79.33 ± 1.15 | >100 | |
| Standards | BHAc | 1.45 ± 0.02 | 19.70 ± 0.20 | 12.80 ± 0.50 | 25.17 ± 0.01 | NT |
| α-tocopherolc | 2.23 ± 0.03 | 38.70 ± 0.25 | 34.50 ± 0.47 | 85.41 ± 0.01 | NT | |
| EDTAc | NT | NT | NT | NT | 5.45 ± 0.65 | |
The ability of the extracts to scavenge radical species was performed using a radical cation ABTS•+ and radical DPPH•. In scavenging assay on DPPH•, the ethyl acetate and MeOH extracts of P. biglobosa revealed the best scavenging capacity with IC50 values of 5.15 ± 0.20 and 5.83 ± 0.14 µg/mL, respectively when the compared to the standards α-tocopherol (IC50: 38.70 ± 0.25 µg/mL) and BHA (IC50: 19.70 ± 0.20 µg/mL). Also, Ethyl acetate extract of T. diversifolia possessed better activity than reference compounds with IC50 value of 16.51 ± 0.15 µg/mL. Similar results were observed in ABTS•+ assay where the ethyl acetate, MeOH extracts of P. biglobosa and ethyl acetate extract of T. diversifolia exhibited better activity than reference compounds with IC50 values of 3.45 ± 0.19, 4.18 ± 0.27 and 9.74 ± 0.21 µg/mL, respectively.
The Cu2+ reduction ability of the plant extracts was evaluated via the CUPRAC assay. Ethyl acetate, MeOH extracts of P. biglobosa and T. diversifolia were found to possess higher potent activity when the compared BHA (IC50: 25.17 ± 0.01) and α-tocopherol (IC50: 85.41 ± 0.01) with IC50 values of 5.80 ± 0.30, 6.21 ± 0.42, 13.37 ± 0.16 and 19.69 ± 0.35 µg/mL, respectively
In metal chelating assay, ethyl acetate extract of P. biglobosa and T. diversifolia displayed highest activity amongst the tested extracts with IC50 values of 41.70 ± 1.22 and 45.61 ± 1.05 µg/mL, respectively
3.4 Anticholinesterase activity
Cholinesterases (AChE and BChE) are the major enzymes responsible for the hydrolysis of acetylcholine (ACh), thereby reducing the levels of Ach which is necessary for neurotransmission and causing neurodegenerative disorders notably AD. Therefore inhibition of these enzymes is beneficial as remedy to AD. The AChE and BChE inhibitory activity of T. diversifolia, P. biglobosa and C. febrifuga ethyl acetate extracts and MeOH extracts were evaluated and reported on Table 4. The MeOH and ethyl acetate extracts of P. biglobosa exhibited the strongest AChE and BChE activities amongst all the tested extracts. The methanol (IC50: 15.63 ± 0.72 µg/mL), ethyl acetate (IC50: 16.20 ± 0.67 µg/mL) extracts of P. biglobosa, methanol (IC50: 21.53 ± 0.65 µg/mL) and ethyl acetate (IC50: 30.81 ± 0.48 µg/mL) extracts of T. diversifolia showed higher activity than galantamine (IC50: 42.20 ± 0.44 µg/mL) against BChE. Similarly, MeOH and ethyl acetate extracts of P. biglobosa and methanol extract of T. diversifolia displayed highest activities against AChE with IC50 values of 20.43 ± 0.81, 23.85 ± 0.46 and 28.75 ± 0.92 µg/mL, respectively.
| Cholinesterase inhibition | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AChE | BChE | Tyrosinase inhibition | α-amylase inhibition | α-glucosidase inhibition | |||||||
| Plants | Extracts | Inhibition (%) (at 100 µg/mL) |
IC50 (µg/mL)a | Inhibition (%) (at 100 µg/mL) |
IC50 (µg/mL) | Inhibition (%) (at 100 µg/mL) |
IC50 (µg/mL) | Inhibition (%) (at 100 µg/mL) |
IC50 (µg/mL) | Inhibition (%) (at 100 µg/mL) |
IC50 (µg/mL) |
| T. diversifolia | AcOEt | 70.48 ± 0.87 | 46.43 ± 0.77 | 78.91 ± 0.68 | 30.81 ± 0.48 | 22.46 ± 0.31 | >100 | 70.90 ± 0.84 | 31.22 ± 0.41 | 77.52 ± 1.80 | 26.30 ± 1.21 |
| MeOH | 80.21 ± 1.46 | 28.75 ± 0.92 | 82.07 ± 1.55 | 21.53 ± 0.65 | 15.62 ± 0.79 | >100 | 76.63 ± 1.10 | 28.35 ± 0.25 | 83.65 ± 1.25 | 24.59 ± 0.73 | |
| P. biglobosa | AcOEt | 81.35 ± 1.15 | 23.85 ± 0.46 | 84.11 ± 1.44 | 16.20 ± 0.67 | 65.39 ± 0.97 | 76.34 ± 1.24 | 87.73 ± 1.52 | 13.50 ± 0.90 | 91.31 ± 0.79 | 12.47 ± 0.61 |
| MeOH | 82.58 ± 0.89 | 20.43 ± 0.81 | 84.63 ± 1.02 | 15.63 ± 0.72 | 70.22 ± 1.30 | 51.28 ± 0.77 | 85.91 ± 0.26 | 18.12 ± 0.33 | 88.74 ± 0.16 | 16.51 ± 0.18 | |
| C. febrifuga | AcOEt | 20.72 ± 0.60 | >100 | 58.81 ± 0.75 | 82.31 ± 0.94 | 14.74 ± 0.87 | >100 | 69.44 ± 1.73 | 91.58 ± 2.10 | 72.38 ± 0.42 | 84.21 ± 1.75 |
| MeOH | 17.63 ± 1.34 | >100 | 32.17 ± 0.43 | >100 | 8.51 ± 0.74 | >100 | 45.31 ± 0.11 | >100 | 55.64 ± 1.11 | 93.10 ± 0.29 | |
| Standards | Galantamine | 85.40 ± 0.52 | 5.50 ± 0.20 | 74.63 ± 0.25 | 42.20 ± 0.44 | NT | NT | NT | NT | NT | NT |
| Kojic acid | NT | NT | NT | NT | 79.48 ± 0.32 | 23.75 ± 0.24 | NT | NT | NT | NT | |
| Acarbose | NT | NT | NT | NT | NT | NT | 82.59 ± 1.10 | 23.84 ± 0.25 | 88.14 ± 0.43 | 17.35 ± 0.71 | |
Values (inhibition % and IC50) represent the means ± SEM of three parallel sample measurements (p < 0.05).
NT: not tested.
3.5 Anti-tyrosinase activity
The antityrosinase activity of T. diversifolia, P. biglobosa and C. febrifuga ethyl acetate extracts and MeOH extracts are shown on Table 4. The MeOH and ethyl acetate extracts of P. biglobosa showed significant tyrosinase inhibitory activities with IC50 values of 51.28 ± 0.77 and 76.34 ± 1.24 µg/mL respectively while kojic acid had IC50 value of 23.75 ± 0.24 µg/mL. Extracts of T. diversifolia and C. febrifuga exhibited lower inhibitions against tyrosinase when the compared the extracts of P. biglobosa and the reference compound kojic acid used in the study.
3.6 α-amylase and α-glucosidase inhibitory activities
The α-amylase as well as α-glucosidase are key enzymes found in the digestive system which intervene in the hydrolysis of carbohydrate to glucose and inhibiting these enzymes will reduce the amounts of glucose that enters the body. The ethyl acetate and MeOH extracts of T. diversifolia, P. biglobosa and C. febrifuga were evaluated for α-amylase as well as α-glucosidase inhibitory ability and the results are presented on Table 4. On the α-glucosidase inhibition, the ethyl acetate and MeOH extracts of P. biglobosa exhibited greater activity with IC50 values of 12.47 ± 0.61 µg/mL and 16.51 ± 0.18 µg/mL when compared to the standard acarbose whose IC50 value was 17.35 ± 0.71 µg/mL. For T. diversifolia, the ethyl acetate extract (IC50 = 26.30 ± 1.21 µg/mL) and the MeOH extract (IC50 = 24.59 ± 0.73 µg/mL) equally showed good activity while the extracts of C. febrifuga had moderate activity. On the α-amylase, the ethyl acetate and MeOH extracts of P. biglobosa exhibited better activity with IC50 values of 13.50 ± 0.90 µg/mL and 18.12 ± 0.33 µg/mL with respect to the standard acarbose whose IC50 value was 23.84 ± 0.25 µg/mL. For T. diversifolia, the the MeOH extract (IC50 = 28.35 ± 0.25 µg/mL) and the ethyl acetate extract (IC50 = 31.22 ± 0.41 µg/mL) equally showed good activity and the ethyl extract of C. febrifuga had moderate inhibitory potential with IC50 of 91.58 ± 2.10 µg/mL.
4 Discussion
Phenolic compounds are ubiquitous in plant kingdom and chemically have a single or multiple aromatic rings with at least a hydroxyl group directly bonded to the aromatic ring. Phenolic compounds possess interesting bioactivities, according to many scientific studies and more research is still needed on them so as to understand their main actions in organisms and improve their bioavailability, sustainable extraction methods, refine modification, and procedures of stability to increase the fields of application (Albuquerque et al., 2021). Flavonoids constitute one of the largest groups of phenolic metabolites that has attracted great attention for research (Su et al., 2021). Ethyl acetate and methanol were used in the extraction because it is generally believed that solvents of high polarity easily dissolve and remove phenolic compounds from plant material and hence phenolic constituents are easily extractable in these solvents (Alara et al., 2021). TPC and TFC from three medicinal plants T. diversifolia, P. biglobosa and C. febrifuga showed variation in composition between the different species which might be explained from a genetic perspective. Within the same plant species, the amounts varied between the ethyl acetate and methanol solvents indicating that phenolic compounds and flavonoids in the plants have different affinities for methanol and ethyl acetate. In P. biglobosa the ethyl acetate extract had higher TPC than the methanol extract meanwhile the methanol extract had higher TFC than the ethyl acetate extract. The TPC and TFC in this study are higher those reported for P. biglobosa collected from Burkina Faso (Windmi et al., 2021). In another study, TPC and TFC were found to be 225.2 ± 18.25 mg GAE/g and 99.28 ± 12.3 mg QE/g respectively (Oyedemi et al., 2021) and these values confirm the high contents determined in this study. As reported in this study, for P. biglobosa the ethyl acetate extract had lesser phenolic content than the methanol extract and a similar trend was reported in another study in which ethanol extract had higher TPC than the ethyl acetate extract (Ibrahim et al., 2013). For T. diversifolia and C. febrifuga the ethyl acetate extracts had more TPC and TFC than their corresponding methanol extracts. Similar trend was also observed for T. diversifolia collected from Phillipines where TPC was found to be 15.20 mg GAE/g dry sample and the TFC where 12.50 mg QE/g dry sample (Aileen et al., 2019). Our contents were higher than these but lower than those detected in T. diversifolia from Nigeria which showed TPC of 251.63 mg. g−1 GAE and TFC of 98.21 mg. g−1 QE (Ojo et al., 2020). The TPC and TFC were low in C. febrifuga and probably because this plant is usually rich in triterpene and saponins instead (Kayangar et al., 2019). The low amounts of phenolic compounds and flavonoids detected in this work for C. febrifuga is in agreement with the works of some researchers who determined low contents of phenolics and flavonoids in this plant (Ouedraogo, et al., 2019).
The detection and quantification of specific phenolic compounds were carried out using HPLC-DAD against 26 standard phenolic compounds. Since HPLC-DAD analysis can provide precisely useful information about the nature of individual compounds, it is more advantageous over total phenolics content as determined by the Folin-Ciocalteu method (Komolafe et al., 2014). In the T. diversifolia, ten compounds were detected in the ethyl acetate extract while seven compounds were identified and subsequently quantified in the methanol extracts. Our results corroborates with some studies in which RP-HPLC in which this method was used to identify phenolic compounds in T. diversifolia previously (da Costa Inácio et al., 2020) and most especially, some identified major compounds were detected in T. diversifolia collected from Nigeria notably phenolic acids and flavonoids like p-coumaric, gallic, caffeic and chlorogenic acids and apigenin (Ojo et al., 2020) some of which are also identified and quantified in our extracts, though in different amounts. Phenolic compounds and flavonoids have also been reported in T. diversifolia using UPLC/MS (Omokhua et al., 2018). Flavonoids and phenolic compounds are known to be contained in this plant as one of the major bioactive constituents (Bouberte et al., 2006; Ambrósio et al., 2008; Pantoja Pulido et al., 2017; Chagas-Paula et al., 2011). In P. biglobosa, ten compounds were identified and quantified in both ethyl acetate and methanol extracts. The phenolic compounds identified in our study corroborates well with one previous study in which gallic acid, quercetin, catechin, rutin, chlorogenic acid, epigallocatechin, epigallocatechin gallate, caffeic acid and kaempferol were identified and quantified in P. biglobosa (Komolafe et al., 2017). Previously, phenolic compounds such as quercetin, caffeic acid, gallic acid, rutin, catechin and epigallocatechin have been equally identified in the extract from P. biglobosa stem bark using an HPLC analysis (Oyedemi et al., 2021). Bioactive compounds including phenolic compounds such as trans-ferulic acid and 4-O-methyl-epi-gallocatechin have been isolated from the stem bark (Tringali et al., 2000). The identified phenolic compounds in this study therefore are in agreement with the reports in literature. In C. febrifuga, a total of eight and six compounds were identified and quantified in the ethyl acetate and methanol extracts respectively. Previously, some phenolic compounds were reported in C. febrifuga using GC-MS (Nma et al., 2018). This supports the identification of phenolics in this study. However, the class of phenolic compounds that have been isolated from C. febrifuga are mostly flavonoids (Tomás-Barberán and Hostettmann, 1988).
There is an increasing interest and attention towards medicinal plant and their phenolic content and those rich in phenolic compounds because of their beneficial therapeutic applications. The most famous bioactivity of phenolic compounds is antioxidant activity and capacity to prevent or reduce oxidative stress associated diseases. After determining the TPC and TFC of the different plant extracts, their antioxidant capacities were determined using five. In the β-carotene-linoleic acid assay, no extract was more active than the standards BHA and α-tocopherol but the activities of T. diversifolia and P. biglobosa were good as their IC50 values remained close to those of standards while the activity of C. febrifuga was low. For the radical scavenging of DPPH•, the ethyl acetate and MeOH extracts of P. biglobosa and equally the ethyl acetate extract of T. diversifolia were more active than the standard compounds α-tocopherol and BHA used. In the CUPRAC and ABTS•+ assays, all extracts of T. diversifolia and P. biglobosa were more active than the standards α-tocopherol and BHA as their IC50 values were lower than those of the standards used. For C. febrifuga only the ethyl acetate extract was more active than α-tocopherol which was used as one of the standards but BHA was more active than this extract. In the metal chelating assay, the activity of the extracts were low to moderate and the standard used was EDTA and it was more active than all the extracts. The high antioxidant activity observed in this study can confirm the one reported in another study in which extracts of T. diversifolia exhibited competitive activity with the standard compounds in the capacity to scavenge free radicals and this was attributed to the phenolic compounds present in the extracts since the phenolic compounds possess hydrogen donating ability which helps to reduce the radicals (Ojo et al., 2010; Hiransai et al., 2016). In another study, n-butanol and ethyl acetate extracts of T. diversifolia were shown to possess good antioxidant activity in DPPH and metal chelating assays (Pulido et al., 2017). It was shown through DPPH scavenging mechanism of free radicals that antioxidant activity of T. diversifolia could reduce oxidative stress and improve cytoprotection (Juang et al., 2014). T. diversifolia has exhibited properties that are important in health and which are attributable to both its free radical scavenging capacity and ability to induce cellular protective systems involved in stress defences in the cells (Di Giacomo et al., 2015). The antioxidant activity is in agreement with the findings reported elsewhere in which the extracts of P. biglobosa showed better antioxidant activities that the standards trolox and quercetin used in the assay (Windmi et al., 2021). This high antioxidant activity of P. biglobosa is attributable to its phenolic and flavonoid contents since these classes of compounds are known to confer antioxidant activity on plant extracts. The results in this study agrees with some work in which P. biglobosa showed significant inhibition of lipid peroxidation, reactive oxygen species and scavenging capacities on ABTS•+ and DPPH• (Komolafe et al., 2014; Oyedemi et al., 2021). Various extracts of P. biglobosa possess promising antioxidative properties in several experiments demonstrated as free radical scavengers, electron donors and nitric oxide inhibitors and the stem bark extract as well as the ethanol extracts of leaves and roots exhibited the most powerful activity (Ibrahim et al., 2013). P. biglobosa can therefore be used as antioxidant substance in living systems to overcome free radical species and consequently reducing the risk of ROS related ailments such as cancer, arthritis and diabetes (Nwahujor et al., 2011). Antioxidant activity was also exhibited by C. febrifuga although its activity was moderate with respect to other tested plant extracts and higher than that of α-tocopherol in the CUPRAC, ABTS•+ and DPPH•. In some study, the extract of C. febrifuga showed higher antioxidant activity than quercetin though with relatively low phenolic and flavonoid contents for the extracts (Ouedraogo et al., 2019).
The plant extracts showed good inhibition on AChE and BChE except the methanol extract of C. febrifuga. In both AChE and BChE inhibitory assays, the activity of each extract was higher in the BChE than in the AChE inhibition. No extract was more active than galantamine in the AChE assay but the ethyl acetate and methanol extracts of T. diversifolia and P. biglobosa were more active than galantamine in the BChE assay. The anticholinesterase activity observed here is in accordance with previous experimental report in which extracts of T. diversifolia showed higher BChE and AChE inhibitory activities than the drug reference prostigmine used in the assay and the activity was attributed to high amounts of caffeic and chlorogenic acids and this was considered a promising mechanism for the treatment of AD (Ojo et al., 2018). In another study, BChE and AChE inhibition of T. diversifolia were good but lower than our results and AChE inhibition had IC50 of 73.9 ± 11.06 μg/mL attributable to terpenes and sesquiterpene lactones found in T. diversifolia (Pantoja-Pulido et al., 2020). Though reports on the anticholinesterase activities of this plant are scarce, it has been shown to possess oxidative stress and to have effect on some illnesses related to the central nervous system and many enzymes that intervene in neuro-metabolic processes (Saleh et al., 2021; Oyedemi et al., 2021). P. biglobosa extracts exhibited good AChE and BChE inhibitions. However, in one study, the inhibition of cerebral acetylcholinesterases by phenolic extracts of P. biglobosa were found to be low compared to the standard (Ramipril) used in the assay (Komolafe et al., 2017). C. febrifuga extracts showed no activity on AChE as the IC50 was not found within tested concentration range and only its ethyl acetate extract exhibited very weak inhibition on BChE. Although no previous works described anticholinesterase activity of C. febrifuga, it is one of the plants used traditionally as memory enhancer to manage neurodegenerative disorders like Alzheimer’s disease and Parkinson disease (Babawale et al., 2016). Plant extracts particularly those rich in phenolic compounds and flavonoids possess good inhibition of angiotensin-converting enzymes including AChE and BChE and the plausible mechanism is the generation of chelating substrates capable of forming complexes within the active sites of the angiotensin-converting enzymes thereby inactivating them (Guerrero et al., 2012; Oboh et al., 2014; Komolafe et al., 2017). Acetylcholine is known to be involved in many physiological processes in the brain and is necessary in neurotransmission but the enzymes AChE and BChE reduce its levels in the brain leading to neurological problems such as loss of memory. Therefore, simultaneous inhibition of both BChE and AChE will reduce the rate of the hydrolysis of acetylcholine and therefore is an effective strategy for the improvement of the pathological conditions in Alzheimer’s disease patients (Khoobi et al., 2013). Amongst the plant extracts tested for anti-tyrosinase activity, only the extracts of P. biglobosa showed activity, though weak when compared to the standard thiourea and the methanol extract was more potent than the ethyl acetate extract. Phenolic compounds are amongst the good cholinesterase inhibitors and therefore estracts which are rich in phenolic compounds can possess justified AChE and BChE inhibitory activities (Tamfu et al., 2021b). Inhibitors of tyrosinase constitute an attraction to the cosmetic industry because they can find applications as agents of depigmentation and also in the food industry where they are used for enzymatic browning and phenolic compounds are considered as being capable of mimicking the tyrosinase substrate and competitively inhibiting the enzyme as well (Zolghadri et al., 2019).
Inhibition of α-glucosidase and α-amylase is a widely accepted approach for controlling levels of glucose found in the blood of diabetes mellitus patients (Quan et al., 2019). The plant extracts showed significant inhibitory activities against the enzymes α-amylase and α-glucosidase. Particularly, extracts of P. biglobosa exhibited higher activities than acarbose which used as reference compound against both enzymes. P. biglobosa is used in the management and treatment of diabetes in folk medicine by local people in African countries (Ekperikpe et al., 2019). According to Sunmonu and Lewu (2019), extract of P. biglobosa demonstrated strong α-amylase inhibitory activity. In another study, phenolic rich extract of P. biglobosa showed significant inhibitory potential against both enzymes (Ademiluyi and Oboh, 2012). The present study is in agreement previous reports and showed that great potential of extracts from P. biglobosa as antidiabetic agents. Also, on α-glucosidase and α-amylase, extracts of T. diversifolia possessed good inhibitory activity. Although, there is no study about α-glucosidase and α-amylase inhibition activities of T. diversifolia, antidiabetic potentials have been investigated in animal studies (Miura et al., 2005; Kadima et al., 2016; Fauziyah et al., 2018; Yazid et al., 2021). Extracts of C. febrifuga demonstrated relatively lower activities when the compared acarbose and other tested plant extracts. To the best of our knowledge, α-glucosidase and α-amylase potential inhibitory activities of extracts from C. febrifuga were investigated first time in this study. Plants extracts and compounds are possible alternatives for α-glucosidase and α-amylase inhibition and hence can find applications in combatting diabetes through their capacity of reducing blood glucose levels (Feunaing et al., 2021; Tchuente Djoko et al., 2021).
5 Conclusion
Though reactive oxygen species can have some physiological relevance in the body, their over-production can lead to tissue damage and oxidative stress whose ultimate effect can lead to some neurodegenerative diseases and diabetes amongst others. The body has mechanisms of maintaining a healthy balance between oxidants and antioxidants but oxidative stress occurs as a result of an imbalance between the pro-oxidants and antioxidants. Several antioxidants from sources external to the organism can restore the balance and they are believed to produce beneficial health effects in terms of disease prevention especially diseases that result from or are linked to oxidative stress. Phenolic compounds are one of the most powerful and commonly used antioxidants and they are widely distributed in plants. Medicinal plants provide a cheap, safe and effective source of bioactive phenolic compounds whose extraction, characterization and application have become a subject of intense research. In this study, ethylacetate and methanol extracts of three plants T. diversifolia, P. biglobosa and C. febrifuga collected from Chad showed high contents of phenolic and flavonoids and certain target phenolic compounds were identified and quantified by HPLC-DAD method. The plants displayed good antioxidant activity assayed through five different methods with the extracts of P. biglobosa showing good activity. The anticholinesterase activity results revealed good activity of T. diversifolia and P. biglobosa extracts indicating that these plants could be used to remedy Alzheimer’s disease. The capacity of these plant extracts to inhibit the key enzymes, α-amylase and α-glucosidase, which are responsible for hydrolyses of starch to glucose shows that the plants can be used to reduce postprandial blood glucose levels and manage diabetic conditions. This study ascertains the usage of these medicinal plants popularly used in African traditional medicine in the treatment of various diseases and also in other cultures in developing countries where modern medicine is inaccessible to a large population. Proper and documented knowledge on chemical composition and bioactivities of medicinal plants is very crucial for the lives of the 80% of the world’s population who rely on them for their health care.
Acknowledgement
The authors are grateful to Dunarea de Jos University, Galati Romania, Mugla Sitki Kocman University, Mugla Turkey and the University of Ngaoundere, Ngaoundere Cameroon for material support. We are equally grateful to Mr. Baroua Abouna, botanist at the Farcha Laboratory, Chad for plant identification.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Phenolic-rich extracts from selected tropical underutilized legumes inhibit α-amylase, α-glucosidase, and angiotensin I converting enzyme in vitro. J. Basic Clin. Physiol. Pharmacol.. 2012;23(1):17-25.
- [CrossRef] [Google Scholar]
- Antioxidant and cytotoxic activity of the leaf ethanolic extracts of Tithonia diversofilia and Gliricidia sepium from Bukidnon, Philippines. Asian J. Biol. Sci.. 2019;8(1):8-15.
- [CrossRef] [Google Scholar]
- Extraction of phenolic compounds: a review. Curr. Res. Food Sci.. 2021;4:200-214.
- [CrossRef] [Google Scholar]
- Phenolic compounds: current industrial applications, limitations and future challenges. Food Funct.. 2021;12(1):14-29.
- [CrossRef] [Google Scholar]
- Constituents of glandular trichomes of Tithonia diversifolia: relationships to herbivory and antifeedant activity. Phytochemistry. 2008;69:2052-2060.
- [CrossRef] [Google Scholar]
- Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem.. 2004;52(26):7970-7981.
- [CrossRef] [Google Scholar]
- Phytochemical investigation and antioxidant activity of Globularia alypum L. Molecules. 2021;26(3):759.
- [CrossRef] [Google Scholar]
- Ethnobotanical survey of plants used as memory enhancer in three states of Southwestern Nigeria. J. Appl. Pharm. Sci.. 2016;6(9):209-214.
- [CrossRef] [Google Scholar]
- Anticholinesterase activities of different solvent extracts of brewer’s spent grain. Foods. 2021;10:930.
- [CrossRef] [Google Scholar]
- Comprehensive review on alzheimer's disease: causes and treatment. Molecules. 2020;25(24):5789.
- [CrossRef] [Google Scholar]
- Antiplasmodial activities of Parkia biglobosa leaves: in vivo and in vitro studies. Ann. Biol. Res.. 2011;2:8-20.
- [Google Scholar]
- Identification and quantification of phenolic acid compounds of twenty-six mushrooms by HPLC–DAD. J. Food Meas. Charact.. 2020;14:1690-1698.
- [CrossRef] [Google Scholar]
- Chlorogenic acids from Tithonia diversifolia demonstrate better anti-inflammatory effect than indomethacin and its sesquiterpene lactones. J. Ethnopharmacol.. 2011;136:355-362.
- [CrossRef] [Google Scholar]
- Assessment of total phenolic and flavonoid content, antioxidant properties, and yield of aeroponically and conventionally grown leafy vegetables and fruit crops: a comparative study. Evid Based Complement Alternat Med.. 2014;253875
- [CrossRef] [Google Scholar]
- New caffeic acid derivative from Tithonia diversifolia (Hemsl.) A. Gray butanolic extract and its antioxidant activity. Food Chem. Toxicol.. 2017;109:1079-1085.
- [CrossRef] [Google Scholar]
- Exploring antioxidant and enzymes (α-amylase and β-glucosidase) inhibitory activity of Morinda lucida and Momordica charantia leaves from Benin. Foods. 2020;9:434.
- [CrossRef] [Google Scholar]
- Feeding deterrence towards Helicoverpa armigera by Tithonia diversifolia tagitinin C-enriched extract. Arab. J. Chem.. 2020;13:5292-5298.
- [CrossRef] [Google Scholar]
- Role of ferritin as a lipid oxidation catalyst in muscle food. J. Agric. Food Chem.. 1990;38(3):674-677.
- [CrossRef] [Google Scholar]
- Effects of Tithonia diversifolia (Hemsl.) A. gray extract on adipocyte differentiation of human mesenchymal stem cells. PLoS ONE. 2015;10(4):e0122320
- [CrossRef] [Google Scholar]
- Effects of Parkia biglobosa aqueous seed extract on some biochemical, haematological and histopathological parameters in streptozotocin induced diabetic rats. J Ethnopharmacol.. 2019;228:1-10.
- [CrossRef] [Google Scholar]
- A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol.. 1961;7:88-95.
- [CrossRef] [Google Scholar]
- Antimalarial activities of Tithonia diversifolia (Asteraceae) and Crossopteryx febrifuga (Rubiaceae) on mice in vivo. J. Ethnopharmacol.. 2004;93:167-171.
- [CrossRef] [Google Scholar]
- Free radicals, antioxidants, and nutrition. Nutrition.. 2002;18(10):872-879.
- [CrossRef] [Google Scholar]
- Fauziyah, Y., Sunarti, S., Hanoum, I.F., Wahyuningsih, M.S.H., 2018. Ethanol Extract of Tithonia diversifolia (Hemsley) A Gray Standardized Ameliorates Hyperglycemia, Polyphagia, and Weight Loss in Diabetic Rats. Molekul, 13 (1), 72-79. https://doi.org/10.20884/1.jm.2018.13.1.417.
- A new abietane-type diterpenoid from roots of Burkea africana Hook (Fabaceae) with α-amylase inhibitory potential. Nat. Prod. Res.. 2021;20:1-8.
- [CrossRef] [Google Scholar]
- Antioxidants: classification, natural sources, activity/capacity measurements, and usefulness for the synthesis of nanoparticles. Materials (Basel).. 2021;14(15):4135.
- [CrossRef] [Google Scholar]
- Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov.. 2021;20:689-709.
- [CrossRef] [Google Scholar]
- Oxidative stress induced-neurodegenerative diseases: the need for antioxidants that penetrate the blood brain barrier. Neuropharmacology. 2001;40(8):959-975.
- [CrossRef] [Google Scholar]
- In vitro antiplasmodial activity of Tithonia diversifolia and identification of its main active constituent: tagitinin C. Planta Med.. 2002;68:543-545.
- [CrossRef] [Google Scholar]
- Inhibition of angiotensin-converting enzyme activity by flavonoids: structure activity relationship studies. PLoS ONE. 2012;7:e49493
- [CrossRef] [Google Scholar]
- Antimicrobial and preliminary phytochemical studies of methanol extract of root bark of Crossopteryx febrifuga (Rubiaceae) J. Appl. Pharm. Sci.. 2012;2:66-70.
- [CrossRef] [Google Scholar]
- Oxidative stress and antioxidant potential of one hundred medicinal plants. Curr. Top. Med. Chem.. 2017;17(12):1336-1370.
- [CrossRef] [Google Scholar]
- Anti-nitric oxide production, anti-proliferation and antioxidant effects of the aqueous extract from Tithonia diversifolia. Asian Pac. J. Trop. Biomed.. 2016;6:950-956.
- [CrossRef] [Google Scholar]
- In vitro anti-oxidative activities of the various parts of Parkia biglobosa and GC-MS analysis of extracts with high activity. Afr. J. Trad. Complement. Altern. Med.. 2013;10(5):283-291.
- [CrossRef] [Google Scholar]
- Computational, chemical profiling and biochemical evaluation of antidiabetic potential of Parkia biglobosa stem bark extract in type 2 model of rats. J. Biomol. Struct. Dyn.. 2021;1–14
- [CrossRef] [Google Scholar]
- Antioxidant -amylase inhibitors flavonoids from Iris germanica rhizomes. Revista Brasileira de Farmacognosia. 2017;27:170-174.
- [CrossRef] [Google Scholar]
- Investigation of antioxidative stress in vitro and water apparent diffusion coefficient in MRI on rat after spinal cord injury in vivo with Tithonia diversifolia ethanolic extracts treatment. BMC Complement. Altern. Med.. 2014;14:447.
- [CrossRef] [Google Scholar]
- Comparative antidiabetic potential and survival function of Harungana madagascariensis, Physalis peruviana, Solanum americanum and Tithonia diversifolia extracts on alloxan-induced diabetes in guinea pigs. Int. J. Pharm. Pharm. Sci.. 2016;5(3):196-206.
- [Google Scholar]
- Design, synthesis, biological evaluation and docking study of 5-oxo-4,5-dihydropyrano[3,2-c]chromene derivatives as acetylcholinesterase and butyrylcholinesterase inhibitors. Eur. J. Med. Chem.. 2013;68:260-269.
- [CrossRef] [Google Scholar]
- Angiotensin-1-converting enzyme inhibition, antioxidant activity, and modulation of cerebral Na+/K+ ATPase by free phenolics of African locust bean (Parkia biglobosa) Health Sci. Rep.. 2017;1(1):e17
- [CrossRef] [Google Scholar]
- In vitro antioxidant activity and effect of Parkia biglobosa bark extract on mitochondrial redox status. J. Acupunct. Meridian. Stud.. 2014;7(4):202-210.
- [CrossRef] [Google Scholar]
- Kouadio, F., Kanko, C., Juge, M., Grimaud, N., Jean, A., N.'Guessan, Y.T., Petit, J.Y., 2000. Analgesic and antiinflammatory activities of an extract from Parkia biglobosa used in traditional medicine in the Ivory Coast. Phytother. Res. 14, 635-637. https://doi.org/10.1002/1099-1573(200012)14:8<635::aid-ptr427>3.0.co;2-t.
- Polyphenol content and antioxidant activity of fourteen wild edible fruits from Burkina Faso. Molecules. 2008;13:581-594.
- [CrossRef] [Google Scholar]
- Oxidative stress, aging, and diseases. Clin. Interv. Aging. 2018;13:757-772.
- [CrossRef] [Google Scholar]
- Ethnopharmacological survey of the Bunda district, Tanzania: plants used to treat infectious diseases. J. Ethnopharmacol.. 2007;113:457-470.
- [CrossRef] [Google Scholar]
- Screening for tyrosinase inhibitors among extracts of seashore plants and identification of potent inhibitors from Garcinia subelliptica. Biosci. Biotechnol. Biochem.. 2005;69:197-201.
- [CrossRef] [Google Scholar]
- Antidiabetic effect of Nitobegiku, the herb Tithonia diversifolia, in KK-Ay diabetic mice. Biol. Pharm. Bull.. 2005;28:2152-2154.
- [CrossRef] [Google Scholar]
- Parkia biglobosa (Mimosaceae): botany, uses, phytochemical properties and pharmacological potential. J. Pharm. Nutr. Sci.. 2020;10:101-115.
- [Google Scholar]
- Multimodal α-glucosidase and α-amylase inhibition and antioxidant effect of the aqueous and methanol extracts from the trunk bark of Ceiba pentandra. Biomed. Res. Int.. 2020;2020:3063674.
- [CrossRef] [Google Scholar]
- GC-MS analysis of bioactive compounds in the ethyl acetate fraction of Crossopteryx febrifuga leaves. J. Chem. Pharm. Res.. 2018;10(3):75-79.
- [Google Scholar]
- A new ursane-type triterpene oxoglucopyranoside from Crossopteryx febrifuga. Z. Naturforsch. C J. Biosci.. 2019;74:289-293.
- [CrossRef] [Google Scholar]
- Anti-inflammatory and anti-oxidant activities of the methanolic extracts of the stalk of Parkia biglobosa (jacq.) Benth. Hygeia. J. D. Med.. 2011;3(1):34-40.
- [Google Scholar]
- Biosynthesis of Tithonia diversifolia leaf mediated Zinc Oxide Nanoparticles loaded with flamboyant pods (Delonix regia) for the treatment of Methylene Blue Wastewater. Arab. J. Chem.. 2021;14:103363
- [CrossRef] [Google Scholar]
- Phenolic compounds from sandpaper (ficus exasperata) leaf inhibits angiotensin 1 converting enzyme in high cholesterol diet fed rats. J Ethnopharmacol.. 2014;157:119-125.
- [CrossRef] [Google Scholar]
- Hypoglycemic and hypolipidemic activities of ethanolic roots extract of Crossopteryx febrifuga in alloxan-induced diabetic rats. Mintage J. Pharm. Med. Sci.. 2013;2:1-4.
- [Google Scholar]
- Phytochemical and proximate composition of Tithonia diversifolia (Hemsl.) A. Gray. Annals Food Sci. Technol.. 2015;16:195-200.
- [Google Scholar]
- A comprehensive study of the potential phytomedicinal use and toxicity of invasive Tithonia species in South Africa. BMC Complement Altern Med. 2018;18:272.
- [CrossRef] [Google Scholar]
- Ouedraogo, V., Compaoré, E., Rouamba, A., Compaoré, M., Kiendrebeogo, M. 2019. Anti-virulence Activity of Three Medicinal Plants: Cassia occidentalis L., Crossopteryx febrifuga (Afzel ex G. Don) Benth. and Zanthoxylum zanthoxyloides (Lam) Zep. and Timl. Microbiol. Res. J. Int. 29 (1), 1-7. https://doi.org/10.9734/mrji/2019/v29i130152.
- New caffeic acid derivative from Tithonia diversifolia (Hemsl.) A. Gray butanolic extract and its antioxidant activity. Food Chem. Toxicol. 2017
- [CrossRef] [Google Scholar]
- Antioxidant, α-amylase and α-glucosidase inhibitory activities and potential constituents of Canarium tramdenum bark. Molecules. 2019;24(3):605.
- [CrossRef] [Google Scholar]
- Rahman, A.U., Choudhary, M.I., (Eds.), 2015. Drug Design and Discovery in Alzheimer’s Disease; Elsevier: Amsterdam, The Netherlands, p. 784.
- Salar, U., Khan, K.M., Chigurupati, S. Taha M., Wadood A., Vijayabalan S., Ghufran M., Perveen S., 2017. New Hybrid Hydrazinyl Thiazole Substituted Chromones: As Potential α-Amylase Inhibitors and Radical (DPPH & ABTS) Scavengers. Sci Rep 7, 16980. https://doi.org/10.1038/s41598-017-17261-w,
- Analgesic, antiinflammatory, anti-pyretic and antiplasmodial effects of the methanolic extract of Crossopteryx febrifuga. J. Med. Plant Res.. 2008;2:213-218.
- [Google Scholar]
- Genus Parkia: phytochemical, medicinal uses, and pharmacological properties. Int. J. Mol. Sci.. 2021;22:618.
- [CrossRef] [Google Scholar]
- Lifestyle, oxidative stress, and antioxidants: back and forth in the pathophysiology of chronic diseases. Front. Physiol.. 2020;11:694.
- [CrossRef] [Google Scholar]
- The comparison of total phenolics, total antioxidant, and anti-tyrosinase activities of korean sargassum species. J. Food Qual.. 2021;2021:6640789.
- [CrossRef] [Google Scholar]
- Phytochemical analysis, in vitro antioxidant activity and inhibition of key diabetic enzymes by selected nigerian medicinal plants with antidiabetic potential. Indian J. Pharm. Educ. Res.. 2019;53(2):250-260.
- [Google Scholar]
- Phytochemical screening, antioxidant activity, total polyphenols and flavonoids content of different extracts of propolis from Tekel (Ngaoundal, Adamawa region, Cameroon) J. Phytopharmacol.. 2014;3:321-329.
- [Google Scholar]
- New mono-ether of glycerol and triterpenes with DPPH radical scavenging activity from Cameroonian propolis. Nat. Prod. Res.. 2017;31:1379-1389.
- [CrossRef] [Google Scholar]
- HPLC-DAD phenolic profiles, antibiofilm, anti-quorum sensing and enzyme inhibitory potentials of Camellia sinensis (L.) O. Kuntze and Curcuma longa L. LWT- Food. Sci Technol.. 2020;133:110150
- [CrossRef] [Google Scholar]
- Tamfu, A.N., Kucukaydin, S., Ceylan, O., Sarac, N., Duru, M.E., 2021a. Phenolic composition, enzyme inhibitory and anti-quorum sensing activities of cinnamon (Cinnamomum zeylanicum Blume) and Basil (Ocimum basilicum Linn). Chem. Africa. https://doi.org/10.1007/s42250-021-00265-5.
- Non-alkaloid cholinesterase inhibitory compounds from natural sources. Molecules. 2021;26(18):5582.
- [CrossRef] [Google Scholar]
- Tamfu, A.N., Tagatsing, F.M., Talla, E., Ozturk, M., Mbafor, J.T., Duru, M.E., Farzana, S., 2019. Chemical composition and evaluation of anticholinesterase activity of essential oil from Cameroonian propolis. Issues Biol. Sci. Pharm. Res. 7 (3), 58–63. https://doi.org/10.15739/ibspr.19.007.
- Antibiofilm, antiquorum sensing and antioxidant activity of secondary metabolites from seeds of Annona senegalensis. Persoon. Microb. Pathog.. 2020;144:104191
- [CrossRef] [Google Scholar]
- An α-Sophoradiol glycoside from the root wood of Erythrina senegalensis DC. (Fabaceae) with α-amylase and α-glucosidase inhibitory potential. Nat. Prod. Com.. 2021;16(9):1-6.
- [CrossRef] [Google Scholar]
- Tel-Çayan, G., Duru, M.E., 2019. Chemical characterization and antioxidant activity of Eryngium pseudothoriifolium and E. thorifolium essential oils. J. Res. Pharm. 23 (6), 1106-1114. http://dx.doi.org/10.35333/jrp.2019.75.
- Cytotoxic triterpenoid and flavonoids from Crossopteryx febrifuga. Planta Med.. 1988;54(3):266-267.
- [CrossRef] [Google Scholar]
- Bioactive constituents of the bark of Parkia biglobosa. Fitoterapia. 2000;71(2):118-125.
- [CrossRef] [Google Scholar]
- Uddin, M.J., Russo, D., Rahman, M.M., Uddin, S.B., Halim, M.A., Zidorn, C., Milella, L., 2021. Anticholinesterase Activity of Eight Medicinal Plant Species: In Vitro and In Silico Studies in the Search for Therapeutic Agents against Alzheimer's Disease. Evid. Based Complement. Alternat. Med. 2021, 9995614. https://doi.org/10.1155/2021/9995614.
- Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol.. 2009;7(1):65-74.
- [CrossRef] [Google Scholar]
- In-vitro alpha amylase inhibitory activity of the leaf extracts of Adenanthera pavonina. BMC Complement. Altern. Med.. 2016;16(1):466.
- [CrossRef] [Google Scholar]
- Polyphenols quantification and antioxidant activity of methanolic and aqueous extracts from eight medicinal plants used to manage avian diseases in Burkina Faso. J. Med. Plants Res.. 2021;15(5):226-231.
- [CrossRef] [Google Scholar]
- HPLC-DAD Fingerprinting analysis, antioxidant activities of Tithonia diversifolia (Hemsl.) A. Gray Leaves and its inhibition of Key enzymes linked to Alzheimer’s disease. Toxicol. Rep. 2010
- [CrossRef] [Google Scholar]
- Fermentation of locust bean (Parkia biglobosa): modulation in the anti-nutrient composition, bioactive profile, in vitro nutrient digestibility, functional and morphological characteristics. Int. J. Food Sci. Technol. 2021
- [CrossRef] [Google Scholar]
- Yazid, F., Salim, S.O., Rahmadika, F.D., Rosmalena, R., Artanti, N., Sundowo, A., Prasasty, V.D., 2021. Antidiabetic Effects of Tithonia diversifolia and Malus domestica Leaf Extracts in Alloxan-Induced Sprague Dawley Rats. Sys. Rev. Pharm. 12 (1), 1630-1638. https://doi.org/10.31838/srp.2021.1.232.
- Phytochemical and antioxidant analysis of medicinal and food plants towards bioactive food and pharmaceutical resources. Sci. Rep.. 2021;11(1):10041.
- [CrossRef] [Google Scholar]
- A comprehensive review on tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem.. 2019;34(1):279-309.
- [CrossRef] [Google Scholar]







