Translate this page into:
High-efficiency absorption of low NOX concentration in metallurgical flue gas using a three dimensional printed large-flow microstructured reactor
⁎Corresponding authors at: Faculty of Metallurgical and Energy Engineering, Kunming University of Science and Technology, Kunming 650093, PR China (S. Ju); Kunming Institute of Precious Metals, Kunming 650106, China (Y. Gu). 1014108819@qq.com (Shaohua Ju), guyongwan@163.com (Yongwan Gu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
In the process of nitric acid dissolving precious metals, a large amount of NOx exhaust gas will be produced. This research aims at the development of a new method for the removal of low-concentration nitrogen oxides from metallurgical flue gas. In this process, a printed three-dimensional large-flow microstructure reactor and urea solution are used for the removal of NOx, which facilitates the greater efficiency of denitrification(≥94%). Urea plays an important role in the redox of NO2, such as NO2 is reduced to N2 in solution. Both the gas and the liquid phase simultaneously react in the microchannels of the microfluidic reactor. The channels allow the proper mixing of urea and NaClO2 during the flow which efficiently removes NOx at low concentrations. The optimum condition for high denitration efficiency is outlined: the urea solution with 3%,temperature of the mixed solution is 293.15 K, gas–liquid flow mass ratio is 1:1, pH value (8.0–10.0), CNaClO2 = 0.02 mol/L. This work successfully describes the use of a microfluidic reactor to enhance and maintain the denitration efficiency. This work describes how to successfully enhance and maintain the denitration efficiency while using a printed three-dimensional large-flow microstructure reactor.
Keywords
Microstructure reactor
Microfluidic mixing
NOX
NaClO2
Urea
1 Introduction
NOx is one of the most harmful pollutants in human life (Zhang et al., 2019). Air quality deterioration has been observed for the past few years in developing countries due to rapid industrialisation causing serious environmental threats. The emission of NOx from the metallurgical production process has become an important matter of concern for air pollution that cannot be ignored (Dao et al., 2009; Wang et al., 2021). Presently, precious metal smelting companies are using aqua regia to dissolve platinum, gold, and other precious metals (Lahtinen et al., 2017). Aqua regia is a strong oxidizing agent and composed of hydrochloric acid and nitric acid. In the process of dissolving platinum in aqua regia, a large amount of nitrogen oxide gas will be produced (Dong et al., 2015). The traditional method of platinum extraction involves direct emission of nitrogen oxides or other harmful gases to the atmosphere through the open top of the container, which is not only very harmful to the health of the operators but also causes air pollution. NOx emission into the atmosphere will cause great harm to the cardiovascular and respiratory system, lead to acid rain, photochemical smog, and other natural disasters (Ma et al., 2021; He et al., 2014; Cheng et al., 2016; Lg and Bf, 1974; Acid Rain in China, 2006). In the process of dissolving platinum in aqua regia, the traditional vacuum pump is often used to dry the gas in the reactor, and the alkali liquor is used to wash the nitrogen oxide gas in the spray tower (Chen et al., 2020). However, this method has several limitations: a large amount of nitrogen oxide waste gas is generated during the platinum dissolution process, the concentration of nitrogen oxides in discharge is high, the waste gas treatment is not easy to reach the standard, and the denitration efficiency is low (Han et al., 2017). The electron beam and EB-mixing system can remove high-concentration NOX in the flue gas, adding NaClO and NaClO2. Using the NaClO2phosphate buffer-seawater system, the total removal rate of NOX reached 95.03% (Zhao et al., 2020; Zwolińska et al., 2020). The use of heterogeneous catalysts including precious metals and metals for denitration can be effectively applied in practice (Jing et al., 2016). Using urea in the denitration process is the most promising method for the removal of nitrogen oxides from flue gas in the future (Dao et al., 2009). It can avoid that the above EB requires a lot of electric energy, catalytic wet air oxidation requires expensive catalysis, and deacidification tower requires huge investment cost. The removal of NOx can be processed through different methods but the urea process is the most selective one for the catalytic reduction of NOx from the flue gas where ammonia derived from urea is the key controlling chemical (Xie et al., 2011). Pressure driven or air assisted atomization of urea aqueous solution largely determines the decomposition of urea and the formation of mixture (Spiteri et al., 2015). The urea process is superior in economy point of view and the absorption rate is also very good (Li et al., 2019). The main obstacle of removing NOx from flue gas in a liquid phase is the low solubility of NO in water, which increases the mass transfer resistance of liquid phase. The use of NaClO and NaClO2 can oxidize NO to NO2, which is easier to react with water. Finally, the NOX in the exhaust gas becomes NO3− and NO2− in the solution (Zhiping et al., 2019; Fuel Research, 2017). The solubility of NO in water could not be improved by changing the temperature and pH value of the solution. Therefore, the development of a reactor to convert NO into easily absorbed form is the key to denitration.
Over the past 20 years, microfluidic technology as a new concept has attracted significant interest because of its impressive advantages characteristics in the field of chemical engineering. The advantages of microfluidic technology are: controls the fluid in the micron-scale which increases the physical quantity gradient between the fluids, greatly improves the driving force of heat and mass transfer, and has higher heat and mass transfer efficiency, unit volume production, and process safety (Jaehnisch et al., 2004; Watts and Haswell, 2005; Yuen and Ki, 2013). At present, micro-processing technologies such as heterogeneous wet chemical etching, deep plate printing, micro forming technology, and mechanical finishing technology are used to manufacture microreactors. These processes are failed to meet the need for large-scale processing of microfluid reactors due to high production cost and process time, material selection, and accuracy. In addition, microfluidic chip is often used in scientific research which is limited by the processing capacity, so it is difficult to meet the requirements of industrial production practice (Ehrfeld et al., 2014). Thus, it is necessary to scale up the microfluidic technology and enhance its industrial application for effective reduction of NOx is the key to the problem.
Presently, 3D printing technology has been developed and gained special attention for employing a novel mechanism to process microfluidic chips (Zhang and Fu, 2020). Compared with the traditional micro-processing technology, 3D printing microfluidic chip technology shows distinctive properties; fast design and processing, wide material adaptability, and low cost (Dai, 2016). 3D printing technology can significantly simplify the processing of microfluidic chips. Besides various polymer materials, biomaterials can also be printed directly (Wu et al., 2019). Compared with other microfluidic technologies, it greatly reduces the technical threshold and processing cost of the microfluidic chip, which is a very positive sign for the promotion and application of microfluidic chip technology (Bhattacharjee et al., 2016; Li et al., 2019).
Recently, the unconventional metallurgy team of Kunming University of science and technology has made some progress in the application of 3D printing technology for the fabrication of microreactors (Li et al., 2019; Zhou et al., 2019; Miniaturized application, 2022). In the present work, the authors have reported the fabrication of a microfluidic chip through 3D printing and also developed an absorption method for the removal of low concentration NOx from metallurgical flue gas using urea and additive as absorption solution. The objective of this work is to prepare a porous microreactor, maximize the NOx reduction efficacy and maintain the process stability (Zhou et al., 2019). The performance of the equipment is satisfactory. The as-prepared device has a simple structure, convenient operation and maintenance, small space occupation, and highly efficient. In addition, on the basis of the research on wet denitrification of microreactors, microchannel phase separation technology is being developed and pilot-tested in precious metal production enterprises, which is expected to achieve industrial-scale denitrification.
2 Materials and methods
2.1 Chemicals and gases
Materials and instruments used in this experiment are listed in Tables 1 and 2.
Gas and absorbents
Details
Companies
N2 cylinder
greater than 99.999% purity
Meiser gas Co., Ltd, Sichuan, China
O2 cylinder
greater than 99.999% purity
NO cylinder
0.05% purity and balance N2
(NH2)2CO
99.0%,AR
Zhiyuan, Tianjin, China
NaClO2
80%
Macklin Biochemical Co.,Ltd, Shanghai, China
NaClO
AR
Zhiyuan, Tianjin, China
H2O2
AR
Chuangdao, Chongqing, China
NaOH
96%,AR
Zhiyuan, Tianjin, China
Instrument
Specifications
Gas mixing device
Dahua, Najing, China
Mass flowmeter controller
FLOWS instruments Co., Ltd., Beijing, China
Microreactor
Curable Resin, custom instrument
Path controller
PTFE, Alibaba
Gas analyzer
OS60, Skyeaglee, China
pH meter
pHS-3C, Shanghai sincere dedication of science and technology innovation CO., LTD, China
Constant temperature heating magnetic stirrer
DF-101S, Yuhua Instrument Co.,Ltd, China
Advection pump
2PB-1000III, Beijing Xingda Technology Development Co., Ltd, China
Current regulator
007, Beijing Qixing Huachuang flowmeter Co., Ltd, China
2.2 Gas absorption process system
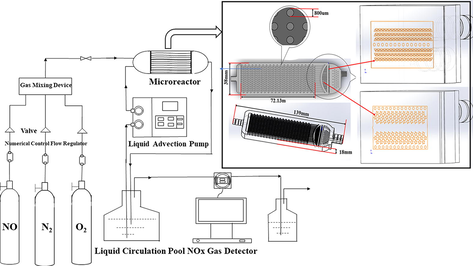
Urea and oxidant solution is used in the gas absorption system as the circulating liquid for gas absorption and treatment. The flow chart of gas absorption and treatment in the system is shown in Fig. 1. The device of gas absorption and treatment system includes 1. Prepared gas; 2. Cylinder pressure reducing valve; 3. Numerical control flow stabilizer; 4. Valve; 5. Gas mixing device; 6. Gas mixing valve; 7. Microreactor; 8. Liquid advection pump; 9. Liquid circulation pool; 10. NOx gas detector; 11. Tail gas absorption.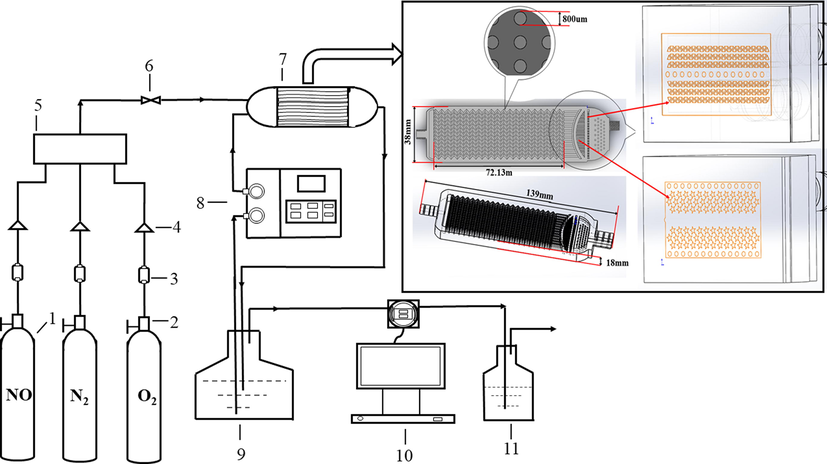
Flow chart of the gas absorption system.
The model diagram of the microreactor is shown in Fig. 2, and its main features consist of the following components: liquid phase inlet 2, gas phase inlet 1, mixed liquid collection chamber (3,6,8), droplet cutting sieve plate 4, mixing chamber separator 5, mixing reaction microchannel 7, and mixed liquid outlet 9. Taking the microreactor involved in this experiment as an example, the characteristic sizes of the two-phase inlet and outlet are 3 mm, the characteristic sizes of the sieve plate are 800 μm, the number of Z-type mixing channels is 91, the characteristic sizes are 800 um, the saw tooth angle is 90° and the length of each microchannel is 72.13 mm. The structure of the sieve plate, the number of the micromixing channels, and the characteristic sizes of the basic structure of the microreactor were optimized. For the appropriate adjustment, the two-phase fluid was allowed to pass through the sieve plate to form enough small droplets and collide with each other, to increase the contact area of the two-phase. During the experiment, the two-phase fluid respectively enters the reactor through the inlet 1 and 2, and the liquid phase is cut into small droplets through a layer of droplet cutting sieve plate 4, and then enters the mixed liquid collecting cavity 3 for the first contact and mixing with the gas phase, and then the mixed fluid will successively pass through the separator 5, the mixed liquid collecting cavity 6, the mixed reaction microchannel 7 (the main area of gas–liquid reaction), and the mixed liquid collecting cavity 8, and finally flows out of the reactor through the mixed liquid outlet 9. Each condition of the experiment was repeated at least third, average value was reported.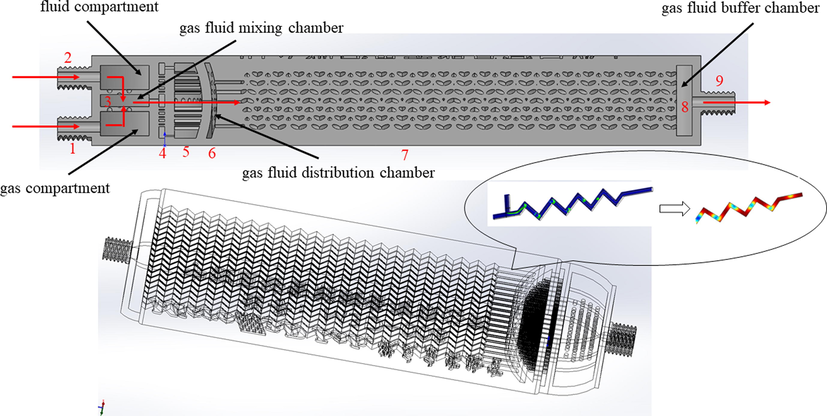
The enlarged schematic diagram of the microreactor.
2.3 Nox absorption in the presence of urea and oxidant
Fig. 1 shows the absorption process of NOx gas. All experiments were carried out in the temperature range of 293.15 K-323.15 K. 500 ppm of standard gas (filling gas is N2) was produced by Kunming gas plant and then the mixture of oxygen and nitrogen was used to simulate industrial NOx exhaust gas. The desired concentration of NOx gas was prepared by mixing the appropriate amount of N2 and O2. About 3 h after starting up, a certain concentration of urea and oxidant solution was used to absorb NOx, and the NOx concentration of tail gas passing through the gas absorption system was measured by the NOx detector to determine the efficiency of the system in treating and absorbing NOx.
2.4 Calculation of gas–liquid flow volume ratio
2.5 Calculation of gas–liquid contact time (T)
The contact time of gas–water two-phase in microchannel reactor is calculated as follows:
3 Results and discussion
Gas phase and liquid phases are cut into micron-scale fluids in microchannels to realize micron scale mixing, mass transfer, and the reaction of multiphase fluids. The advantages lie in the sharp increase of the gradient of temperature, pressure, concentration, and density between fluids, the reduction of transfer distance to the micro nanoscale, the enhancement of heat and mass transfer driving force, and the interface volume ratio reached 104 times of that of conventional kettle reaction. Therefore, mass transfer and reaction may be greatly enhanced (Ehrfeld et al., 2014; Yang et al., 2015; Pohorecki et al., 2008).
3.1 Selection of oxidant in urea solution
The reaction conditions of gas–liquid two-phase in the absorption treatment gas system are: temperature 293.15 K; reaction time 30 min; gas flow rate 150 mL / min; liquid flow rate 150 mL / min; O2 21%; NO content 400 ppm. Fig. 3 shows that with the increase of urea concentration from 3% to 15%, the denitration efficiency increases from 29.85% to 41.29%. The denitration efficiency is increased by 11.44% when the concentration of absorption solution is increased by two times, and the improvement of denitration efficiency is not obvious. Considering the economy of flue gas desulfurization and denitrification, the use of a higher concentration of absorption liquid leads to higher operation costs, therefore, the concentration of absorption liquid cannot be increased without limit. However, the influence of other process conditions on the denitration effect of urea depicts that microfluid characteristics are responsible for the temperature, pressure, concentration, density, and other physical properties of fluids. the urea solution with 3% can be used as the absorption liquid.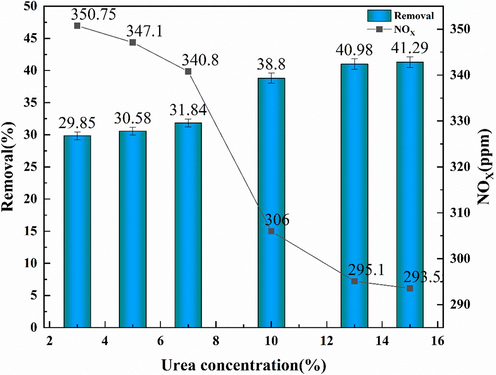
NOx absorption effect of urea with different concentrations.
Based on the results (Fig. 3), the effects of different reagents on the removal of NOx in urea solution were further investigated. As shown in the Fig. 4, adding NaClO to urea solution can effectively improve the efficiency of NOx removal by urea when the concentration of NaClO is 0.1 mol/L, and the removal rate of NOx is increased from 29.85% in Fig. 3 to 51.25%. When 0.4 mol/L NaClO was added to the urea solution, the highest NOx removal rate was 73.02%, and the NOx concentration of tail gas was 108 ppm. The NO oxidation ability of NaClO decayed rapidly during the solution treatment, and the absorption rate decreased to 35.5%. When the concentration of NaClO was increased, the removal efficiency of NOx was found to be highest, only increased by 21.77%, After 50 min of reaction, the removal rate of 0.4 mol / L solution with the highest concentration of NaClO also decreased rapidly to 39.14%. The reaction mechanism of NOx removal by NaClO in urea solution follows as below:
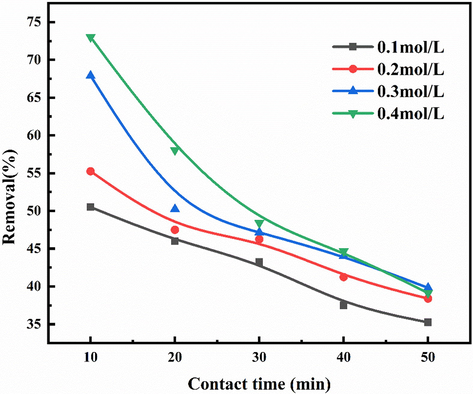
Efficiency diagram of NOx treatment with urea 3% + NaClO solution.
As shown in Fig. 5, when the concentration of H2O2 is 0.1 mol/L, the NOx removal efficiency of urea can be effectively improved by adding H2O2 to the urea solution. The highest NOx removal rate can reach 61%, and the NOx concentration of tail gas is 156 ppm. When the concentration of H2O2 was increased from 0.1 mol / L to 0.4 mol / L, the removal efficiency of NOx increased from 61% to 82.94%. However, it can be seen from the figure that after adding H2O2 to the urea solution for 20 min, the reaction tends to be balanced, and the denitration of the solution is significantly lower than that of the first 10 min.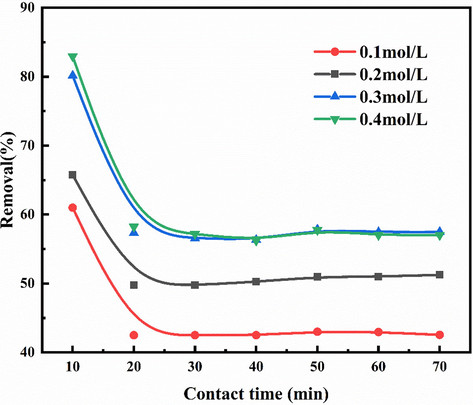
The efficiency of NOx treatment with urea 3% + H2O2 solution.
According to the results in Fig. 3, the effects of different reagents on the removal of NOx in urea solution were further investigated. As shown in Fig. 6, adding NaClO2 to the urea solution can greatly improve the NOx removal efficiency. When the concentration of NaClO2 is 0.005 mol/L,the NOx removal rate increases from 29.85% in to 83% (Fig. 3). NO can be oxidized in a solution (Chien et al., 2001):
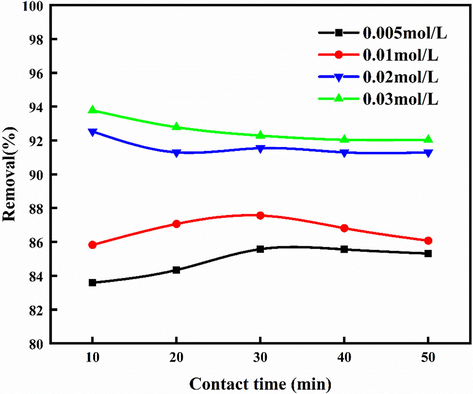
Effects of different concentrations of NaClO2 and 3% urea on NOx removal.
The solubility of NO in water is very low, which increases the mass transfer resistance of the liquid phase. The key role of the process is that NO is converted into an easily absorbed form NO2 by NaClO. When NO is oxidized to NO2, the increase of NOx solubility is mainly due to the formation of N2O3 and N2O4. In the liquid phase, NOX is oxidized to NO3– or NO2–. Nitrite can be reduced to nontoxic N2 by urea. The reaction between NO and (NH2)2CO in NaClO2 aqueous solution is as follows:
The role of the concentration of NaClO2 in the absorption solution was investigated. With the increase of NaClO2 concentration from 0.005 mol/L to 0.03 mol/L, the efficiency of NOx absorption by urea increased from 83% to 93.78%, among which the concentration of NO and NO2 decreased from 264 ppm and 138 ppm to 0 ppm and 20 ppm from the beginning. Compared with other reactors, micro-reactors can take advantage of its own process enhancement characteristics to realize the oxidation of NO with a lower concentration of NaClO2 (the NO concentration in the tail gas is 0 ppm). However, considering the cost and removal efficiency of NaClO2, the optimum concentration was 0.02 mol/L.
Through the comparison of the above experimental data, it is found that the adding effect of various oxidants is: NaClO2 > NaClO > H2O2. H2O2 as an additive can improve the denitration efficiency of the urea process in a green and clean way, however, the treatment efficiency of H2O2 is not high, and the consumption in the reaction process is too fast. NaClO produces hypochlorite under acidic conditions and NaClO can react in the presence of Cl- in the solution:
The pH of absorption solution needs to be controlled accurately. In alkaline conditions, the consumption of hypochlorite oxidant is too fast, and the removal efficiency of NOx is low; NaClO2 can maintain strong oxidation stability for a long time under normal temperature and pressure. Interestingly, NOx removal rate of NaClO2 and urea solution in NOx absorption treatment system remains above 90%, therefore it has a good application prospect as an additive.
3.2 Operating parameters factors NO removal efficiency of urea + NaClO2 solution
3.2.1 The influence of temperature on the removal efficiency of NOX
According to the results in Fig. 7, the removal of NOx (400 ppm) was carried out with 3% urea and 0.02 mol/L NaClO2. 500 mL solution was prepared and the experiment lasted for 50 min. At low temperatures (293.15 K,303.15 K), the temperature has little effect on the removal efficiency of urea and NaClO2 solution. With the increase in temperature,the effect of urea and NaClO2 solution on the removal of NOx is more and more obvious. After 10 min of reaction, the removal rate of NOx is reduced from 93.78% at 293.15 K to 91.05% at 323.15 K. In the actual precious metal production process, the temperature of the exhaust gas will not be as high as the sintering exhaust gas and blast furnace exhaust gas. On the other hand, the total heat transfer coefficient of the microreactor exceeds 20 kW/(m2•K), which is much higher than that of conventional heat exchange equipment (Yang et al., 2016). Compared with SCR technology, microfluidic technology does not require very high temperature. Compared with other reactors using NaClO2 solution for denitration, microfluidic technology does not require additional heat exchange devices (Zhiping et al., 2019).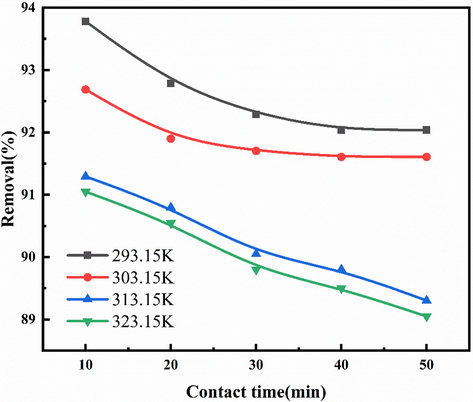
NOx removal efficiency of urea + NaClO2 solution at different temperatures.
3.2.2 The influence of calculation of gas–liquid flow mass ratio (R) on the removal efficiency of NOX
The actual contact area of gas and liquid two-phase fluid in the microchannel in the micro reactor will be affected by the change of mixed fluid flow pattern, and the contact time T of two-phase fluid in the micro reactor is determined by the flow, so the contact time T is very important for the effect of solution removal of NOX. The longer the contact time of gas–liquid two phases in the micro reaction channel, the greater the possibility of the reaction between NO in the gas phase and NaClO2, (NH2) 2CO in the solution. The contact time T mentioned above is regulated by the flow rate of two phases entering the reactor and the ratio of gas to liquid, and the flow rate is regulated by digital flow regulator and flat flow pump. Fig. 8 shows the NOx removal efficiency of urea and NaClO2 solution at different gas–liquid flow mass ratios (R), which is controlled by adjusting the circulating pump. When the gas–liquid ratio R is reduced, the residence time t of the gas phase and liquid phase in the microchannel of the microreactor becomes smaller.When the gas–liquid mass ratio R is reduced from 1 to 1 / 3, the residence time T is also reduced from 1.68 s to 0.56 s, resulting in the reduction of NOx removal rate from 93.78% to 77.31%.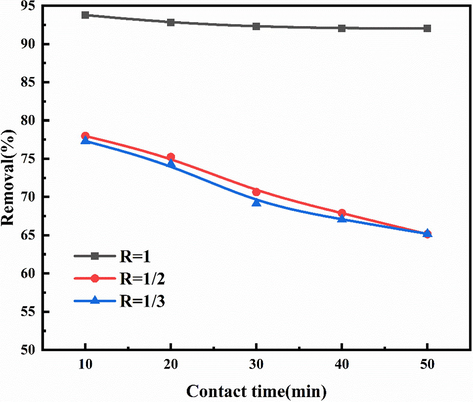
The efficiency of NOx removal from urea + NaClO2 solution at different gas–liquid flow mass ratios (R).
3.2.3 The effect of solution pH on the removal efficiency of NOX
The main reason is that the temperature changes affect the pH of the solution. Fig. 9 shows the relationship between NOx removal rate and pH of urea and NaClO2 solution that the removal efficiency of NOx is greatly affected by pH. when the pH of the solution is 8.0–10.0, the removal efficiency of NOx of urea and NaClO2 solution is stable between 92% and 95%. When the temperature of the mixed solution rises to 323.15 K, the pH also changes to 11.02, and the nitrogen removal efficiency decreases.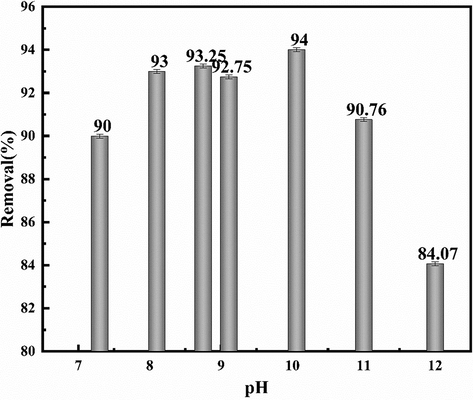
Relationship between NOx removal rate of urea + NaClO2 solution and pH.
4 Conclusions
In this paper, microfluidic technology was used to screen the different effect of low concentration NOx removal by mixing oxidants such as NaClO、H2O2 and NaClO2 with urea solution. Comparing the experimental data, it was found that the adding effect of various oxidants follows as NaClO2 > NaClO > H2O2. The influencing factors of NOx removal in urea and NaClO2 solutions were studied.
Objective to explore the influencing factors on NOx removal in urea and NaClO2 solutions.
The results show that the temperature, the gas–liquid mass ratio (R) and solution pH have a great influence on NOx removal. The main conclusions of this study are as follows:
a. The mixed solution of urea and NaClO2 has the high NOx removal efficiency, good stability and oxidation controllability.
b. The gas–liquid mass ratio (R) and pH of the mixed solution are the main factors that affecting the denitration performance of the solution. The higher the gas–liquid mass ratio (R), the higher the denitration efficiency and the more stable reaction; The pH value of the mixed solution is between 8.0 and 10.0, which can keep high denitration efficiency.
The following further studies have been proposed: (1) Understanding the effects of channel length and residence time on the denitration efficiency; (2) Demonstrate the effects of increasing number of microchannels and longer reacting time at a larger gas–liquid flow rate; (3) Multi-stage denitration experiments will be carried out through multiple microreactors that connected in series and parallel; (4) Improvising the total efficiency of denitrification of urea solution by using green and environmental friendly additives.
Acknowledgements
This work was supported by the National Natural Science of China (Nos. 51964032).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Drivers of improved PM2.5 air quality in China. In: Proceedings of the National Academy of Sciences 116. 2019. from 2013 to 2017
- [Google Scholar]
- No reduction by selective noncatalytic reduction using ammonia-effects of additives. Int. J. Energy Clean Environ.. 2009;10
- [Google Scholar]
- Recent discoveries in the reaction mechanism of heterogeneous electrocatalytic nitrate reduction. Catal. Sci. Technol. 2021
- [Google Scholar]
- Selective recovery of gold from electronic waste using 3D-printed scavenger. ACS Omega. 2017;2:7299-7304.
- [Google Scholar]
- Recovery of platinum group metals from spent catalysts: a review. Int. J. Miner. Process.. 2015;145
- [Google Scholar]
- Comprehensive study about the photolysis of nitrates on mineral oxides. Environ. Sci. Technol. 2021
- [Google Scholar]
- Correction: corrigendum: mineral dust and NOx promote the conversion of SO2to sulfate in heavy pollution days. Sci. Rep.. 2014;4
- [Google Scholar]
- Reactive nitrogen chemistry in aerosol water as a source of sulfate during haze events in China. Sci. Adv.. 2016;2
- [Google Scholar]
- E. LG, H BF, Acid rain: a serious regional environmental problem, Science, New York, NY 184 (1974).
- Acid Rain in China, 2006. Environmental Science & Technology 40: 418-425 (2006).
- FeII(EDTA)–NO reduction by mn powder in wet flue gas denitrification technology coupled with Mn2+ recycling: performance, kinetics, and mechanism. Energy Fuels. 2020;34:2590-2598.
- [Google Scholar]
- New experimental results of NO removal from simulated flue gas by wet scrubbing using NaClO solution. Energy Fuels. 2017;31:3047-3054.
- [Google Scholar]
- NO oxidation with NaClO, NaClO2, and NaClO3 solution using electron beam and a one stage absorption system. Plasma Chem. Plasma Process.. 2020;433–447
- [Google Scholar]
- Removal of high concentrations of NOx and SO2 from diesel off-gases using a hybrid electron beam technology. Energy Rep.. 2020;952–964
- [Google Scholar]
- No reduction by selective noncatalytic reduction using ammonia-effects of additives. Clean Air. 2009;10:121-133.
- [Google Scholar]
- Experimental research of affects of the additives on the removal of NO_x from flue gas by aqueous urea solution. Proc. CSEE. 2011;31:41-46.
- [Google Scholar]
- Comparative analysis on the performance of pressure and air-assisted urea injection for selective catalytic reduction of NOx. Fuel. 2015;161
- [Google Scholar]
- Simultaneous removal of SO2 and NOx from flue gas by wet scrubbing using a urea solution. Environ. Technol.. 2019;40
- [Google Scholar]
- Simultaneous wet desulfurization and denitration by an oxidant absorbent of NaClO2/CaO2. Environ. Sci. Pollut. Res. Int.. 2019;26
- [Google Scholar]
- Fuel Research, 2017. Researchers from Dalian Maritime University Report Details of New Studies and Findings in the Area of Fuel Research (New Experimental Results of NO Removal from Simulated Flue Gas by Wet Scrubbing Using NaClO Solution). Energy Weekly News (2017).
- The application of microreactors for small scale organic synthesis. ChemInform. 2005;36
- [Google Scholar]
- Yuen and Ki P, Fluid control in microfluidic devices using a fluid conveyance extension and an absorbent microfluidic flow modulator. Lab on A Chip 13 (2013) 1737-1742.
- Microreactors: New Technology For Modern Chemistry. Microreactors: New Technology For Modern Chemistry; 2014.
- Research Progress of 3D Printing Microfluidic Chip. J. Phys. Conf. Ser.. 2020;1549:052055
- [Google Scholar]
- Zhong-Bin XU and Tie-Feng LI, progress in 3D micro-nano printing technology and its application. China Plastics Ind. 2016
- [Google Scholar]
- Research progress of organoids-on-chips in biomedical application. Chin. Sci. Bull. 2019
- [Google Scholar]
- Mixing processes in a 3D printed large-flow microstructured reactor: finite element simulations and experimental study. Chem. Eng. J.. 2019;370
- [Google Scholar]
- Extraction and separation of In∼(3+) and Fe∼(3+) using a 3D printing multichannel microreactor. Chem. Ind. Eng. Progr. 2019
- [Google Scholar]
- Miniaturized application of 3D rinted large‐flow microreactor in extraction and separation of platinum, palladium, and rhodium. Journal of Chemical Technology & Biotechnology (2020).
- Visualization of Gas-Liquid Mass Transfer Around a Taylor Bubble during the Forming-Stage and the Flowing-Stage in Microreactors. In: 12th international conference on gas-liquid and gas-liquid-solid reactir engineering. 2015.
- [Google Scholar]
- Hydrodynamic regimes of gas–liquid flow in a microreactor channel. Chem. Eng. J.. 2008;135:S185-S190.
- [Google Scholar]
- Spray scrubbing of the nitrogen oxides into NaClO2 solution under acidic conditions. J. Environ. Sci. Health. Part A Toxic/Hazard. Subst. Environ. Eng.. 2001;36:403-414.
- [Google Scholar]
- Visualization and characterization of gas–liquid mass transfer around a Taylor bubble right after the formation stage in microreactors. Chem. Eng. Sci.. 2016;143
- [Google Scholar]







