Translate this page into:
Evaluation of poly(L-lactic acid) (PLLA) rapid indicator film on deterioration degree of Refined, Bleached and Deodorised Malaysian Tenera palm olein oil (RBDPO) during long-term repetitive deep-fat frying
⁎Corresponding author at: Department of Food Sciences, Faculty of Science and Technology, Universiti Kebangsaan Malaysia, 43600 UKM Bangi, Selangor, Malaysia. saiful-z@ukm.edu.my (Saiful Irwan Zubairi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Palm olein oil is widely used as a medium for deep-frying purposes in the domestic and commercial sectors. However, repetitive usage beyond the recommended cycle leads to significant deterioration and spoilage of the oil. Therefore, this research investigated the effect of long-term repetitive deep-fat frying on the physicochemical properties of Refined, Bleached and Deodorised Malaysian Tenera Palm Olein oil (RBDPO) as well as the suitability of poly(L-lactic acid) (PLLA) film as a potential rapid indicator to determine the spoilage level of recycled palm olein oil. The study was carried out using three different RBDPO conditions; RBDPO without potatoes (RBDPO-1), RBDPO with potatoes (RBDPO-2), and RBDPO with Afdhal Oil (AO, an oil additive chemical) and potatoes (RBDPO-3). The deep-frying process was performed for 15 mins at 180 ± 5 °C for 15 cycles. Based on the results, the oil temperature fluctuation during the frying process was insignificant for every cycle (p > 0.05). After 15 frying cycles, all oil samples demonstrated a considerably altered and deteriorated composition, both physically and chemically. The comparison between the 1st and 15th frying cycles showed a significant difference (p < 0.05) in the decrease of moisture content and contact angle. Furthermore, the redness colour and viscosity for RBDPO-1, RBDPO-2 and RBDPO-3 increased significantly. The RBDPO-2 recorded the highest value for moisture content, contact angle, colour and viscosity after 15 frying cycles with 30.40 ± 0.2%, 26.01 ± 0.02°, 10.77 ± 0.15 Lovibond, and 133.5 ± 0.2 cP, respectively. However, the density value was insignificant (p > 0.05) between the 1st and 15th frying cycles for all types of oil. Besides, the peroxides value (PV) was steadily increased within the safety limit of Food Act (1983) (Laws of Malaysia: Act 281), with the aldehyde (C⚌O) functional group was detected in all oil samples throughout the 15 frying cycles. Hence, the high correlation between the contact angle and all parameters except density showed that contact angle analysis using PLLA film was a suitable technique for rapid oil spoilage indicator model.
Keywords
Palm olein
Deep-fat frying
Physicochemical properties
Poly(L-lactic acid)
Contact angle
Oleophilicity
Spoilage indicator model
1 Introduction
Poly(L-Lactic Acid) (PLLA) is an aliphatic polyester compound, one of the most promising, commercialized and widely used bio-based polymers. It is produced through sugars fermentation which the sugars are extracted mainly from renewable sources of starch-rich products such as sugar beet, wheat, corn and tapioca (Connolly et al., 2019; John et al., 2007; Auras et al., 2004). Besides, it can also be produced from chemical synthesis of the lactic acid monomer (Jamshidian et al., 2010; Averous, 2008). An asymmetric carbon atom of lactic acid structure creating 2 optically active configurations of L- and D-isomers. Some of the key macromolecular properties such as melting temperature, processing easiness and degree of crystallinity is affected by the ratio of L- to D-monomer units (Lim et al., 2011; Gupta et al, 2007).
As early as 1970, the U.S. Food and Drug Administration (FDA) had approved its use with biological fluids. The beneficial properties of PLA include renewable, ability to be easily composted through hydrolysis by microorganisms (Rasal et al, 2010), biocompatibility, easy to process, high mechanical strength, relatively high melting and glass transition temperatures (Albertsson et al., 2011). Besides, it also has 15% to 60% lower carbon emissions and use 50% less energy consumption (Connolly et al., 2019; Rasal et al., 2010) than petroleum-based polymers (Dorgan et al., 2006; Vink et al., 2003;). Thus, it has better advantages over other polymers. In some studies, PLLA film also has broad application for the development of food packaging material, textiles and recently as engineering plastics (Hagen, 2012). For that reason, PLLA film was selected as an indicator film for spoilage and deterioration model system of repetitive frying cooking oil through oleophilicity profiles.
The contact angle profiles (e.g., oleophilicity/hydrophilicity) is the analysis of an angle between the liquid surface and the contact surface line that shows a wetness or spreaders of a liquid on solid surface. The chemical properties of liquids and surfaces including the roughness of the surface affect the spreaders of a liquid (Zubairi et al., 2015; Rossi et al., 2009). In the case of oleophilicity fluid behaviour, contact angle is inversely proportional to the peroxide value, the amount of carbonyl compounds and the viscosity of the oil (Yerima et al., 2015). Theoretically, the level of oil droplets spread on material surface is highly dependent on the amount of carbon on the surface of the material, as well as in the palm oil used. The higher the carbon content in the palm oil used, the higher the peroxide value (PV), iodine value (IV) and carbonyl value (CV). Thus, the spread of a palm oil (either through capillary rise surface tension or drop sessile measurement) will give an indication of the level of palm oil has been damaged or spoiled due to physicochemical changes during frying (Jurid et al., 2020; Yerima et al., 2015).
The Refined, Bleached and Deodorised Malaysian Tenera Palm Olein oil (RBDPO) is widely used in various industries and culinary, especially for deep-frying purposes due to its excellent heat transfer characteristics (Choe and Min, 2007). The fresh palm olein oil contains 0.05–0.08% of free fatty acid content, 0.1% of moisture content, 1.0 meq O2/kg peroxide value, and exhibit a high boiling point of 200 °C (Lin, 2002). Since the deep-frying process involves the continuous or repeated heating of oil at high temperatures, it is suitable for a complete immersion of food products during deep-frying to increase its savoury taste and textures (Choe and Min, 2007). The deep-frying process is a highly complex procedure, which involves a series of simultaneous phenomena, such as heat, moisture and oil transfer, that takes place throughout the entire process between the product and the heating medium. The composition of the oil is progressively altered throughout the process (Oke et al., 2018). The sequence of chemical reactions during the deep-frying process, including oil hydrolysis, oxidation and polymerisation, results in the production of harmful chemical compounds that are toxic to human health, such as hydroperoxide, aldehyde and ketone (Kalogianni et al., 2011).
The oil hydrolysis process occurs when the moisture from the deep-frying of the food in the heated oil forms steam and evaporates. The water molecules react with oxygen to initiate the chemical reactions by breaking the ester linkage of triacylglycerols and produce mono- and diacyl glycerols, glycerol, and free fatty acids (Oke et al., 2018). High temperature used to heat the oil during the deep-frying can causes oil oxidation rapidly. The process begins with the reaction between polyunsaturated fatty acids and reactive oxygen species that produce primary products, such as peroxides and hydroperoxides. The reaction is followed by the formation of secondary products of aldehydes and alcohols, including volatile and non-volatile compounds. The reaction then leads to the degradation of lipids and the production of oxidative rancidity (Wójciak and Dolatowski, 2012). The influence of oxidation on the colour, nutritional value, aroma, texture, and taste induces rancidity, which results in off-flavours and unpleasant taste of the food. These are the important core factors that affect the acceptance and preference of consumers (de Lima Júnior et al., 2013).
Meanwhile, the major decomposition products of frying oil are non-volatile polar compounds, triacylglycerol dimers, and polymers that directly affect the oil quality (Rogers, 2020). The chemical reaction during deep-frying not only caused changes in the chemical composition but also alters the physical properties of the cooking oil, such as the colour quality, taste oil viscosity, and density, consequently degrading the oil quality (Lawson, 2013; Bazlul et al., 2010). Moreover, fried foods contain primary and secondary products from the chemical reaction during the deep-frying process that can increase the risk of cardiovascular disease. The altered chemical compositions enter the bloodstream and contribute to the formation of arteriosclerosis, increase the Low-Density Lipoprotein (LDL-cholesterol) levels, and increase the blood pressure (Ng et al., 2012).
With the abovementioned side effects that related to frying oil, adding antioxidants in the frying oil cocktails might perhaps aid in reducing of such problems. Antioxidant’s role is to preserve the quality and enhance shelf life of edible oils and fats. It defeats oxidation reactions by interfering or participating in with the lipid autoxidation reaction cascade through various mechanisms (Jurid et al., 2020). An extreme condition of temperature, prolonged exposure and poor thermal stability of some antioxidants of frying oil might be the biggest challenges to date due to the unpredictable oxidation rate that can cause premature decomposition. Thus, most antioxidants tend to evaporate and volatile during frying. However, adding Afdhal oil (AO) (a natural antioxidant which was extracted from Rutaceae herbs) in the cooking oil may perhaps help in slowing down the deterioration process. It is enriched with high antioxidants, antihistamines, antibacterial, improves fats, taste and quality of fried foods, preventing oil damage during frying or storage and making fried foods less hazardous. The AO usage as per manufacturer recommendation is by adding 10–15 mL (1 tablespoon) of AO into 100–150 mL (half of a cup) of frying oil (Suhaila, 2018).
The frying time and high temperature during deep-frying can cause thermal degradation of palm oil. Based on a pilot study by Azman et al. (2012) conducted in Kuala Lumpur, it was found that the majority of traders at the night market used the same oil repeatedly for deep-frying to save costs. The result showed that the oil used for deep-frying has a high peroxide content and has a dark colour appearance due to repeated exposure to oxygen, heat, and water during the deep-frying process. It was also reported that repeated heating of oil affected the health of the community (Kamsiah, 2013). However, recent studies carried out by Jurid et al. (2020) and Jurid (2017) showed insignificant changes in the physicochemical properties of palm olein oil after five deep-frying cycles.
Therefore, the aim of this study was to investigate the suitability of poly(L-lactic acid) (PLLA) rapid indicator film on deterioration degree of long-term repetitive deep-frying on the physicochemical properties of RBDPO. The analysis of the physicochemical properties of RBDPO includes moisture, colour, density, viscosity, Peroxide Value (PV) and aldehyde compounds in the frying oil. A total of 15 frying cycles were selected in this research as an extension to the previous study (Jurid, 2017). This study also presented an update of the literature on the development of polymeric benchmarks of poly(L-lactic acid) (PLLA) film for recycled palm olein oil. The physicochemical properties were correlated with the contact angle reading of the PLLA film coating as an alternative method to detect the spoilage of palm olein oil during the long-term repetitive frying process.
2 Materials and methods
2.1 Materials
RBDPO (Buruh, Lam Soon Edible Oils (M) Sdn. Bhd.) was used as the frying oil in this study. Russet potatoes were bought from the same source to represent each frying batch. Besides, Afdhal oil (AO) (produced by Agro-Science Resources (M) Sdn. Bhd. as a frying oil additive) was added to the frying oil and labelled as RBDPO-3 (positive control). The AO is made from 100% natural ingredients, including locally grown Rutaceae (citrus family) plants, and is therefore used as the positive control throughout the study. A deep-fat fryer (FABER, FDS-1035SS, Power: 2000 W, 220–240 V, ∼50/60 Hz, Malaysia) with an oil capacity of 3.5 L was used for the frying process. The deep-fat fryer was equipped with a thermostat to ensure a constant temperature during the heating and frying process. The PLLA film was prepared using commercial grade PLLA pellets purchased from Shenzen E-Sun Industrial Co., Ltd (Guangdong, China). The glass slide (borosilicate microslide: 76 mm × 26 mm × 1 mm) was obtained from Quasi-S Technology (M) Sdn. Bhd. prior to be attached with PLLA thin film (rapid spoilage indicator) for contact angle analysis.
2.2 Preparation of repetitive palm oil via deep-fat frying process
Prior to the frying process, the potatoes were cut to a standard size of 1 cm × 1 cm × 8 cm and approximately 300 g of cut potatoes were fried for each frying cycle (Kamisah et al., 2012; Kalogianni et al., 2011). For the frying process, 2 kg of fresh RBDPO oil was initially heated in the fryer with a frying temperature of 180 ± 5 °C for 30 mins (Fan et al., 2013). The preheating was carried out for the first 30 mins to stabilise the frying temperature. Each frying cycle took 15 mins in which the potatoes were fried in the hot oil for 8 mins, followed by a break time between the two consecutive cycles for 7 mins before the next frying process was continued (Fan et al., 2013). A total of 100 mL of oil was taken for sampling during each cycle.
The potatoes were fried using the same oil for 15 cycles (a total of 255 mins). Due to sampling of oil and oil absorption by the potatoes, the total volume of the oil decreased after every frying cycle. Nevertheless, the volume of oil was not replenished between the frying cycles since regular refuelling tends to reduce the formation of polar compounds and free fatty acids (Kamisah et al., 2012; Choe and Min, 2007). Thus, the mass of the potatoes in the fryer was reduced accordingly (frying load: 3/20 g of potatoes/mL of oil) to maintain the frying load throughout the 15 cycles. The temperature profile data were obtained by recording the refined palm oil temperature every minute during the frying process using Fluke 561 IR non-contact thermometer (Malaysia). The frying oil used for this study was as follows: (a) RBDPO-1 (Negative control: Without potatoes), (b) RBDPO-2 (RBDPO with potatoes) and (c) RBDPO-3 (Positive control: RBDPO with AO and potatoes).
2.3 Preparation of PLLA film
The fabrication of the PLLA film followed the method used by Freier et al. (2001) with several modifications. Approximately 1.0 g of PLLA polymer (powder form) was weighed and mixed with 100 mL of chloroform (Sigma-Adrich (M) Sdn. Bhd with 99.5% in purity). The solution was then heated at 60 °C and stirred at 100 rpm/min on the hot plate stirrer (HPS-280SS: Tech-Lab (M) Sdn. Bhd.) until the PLLA polymer was completely dissolved. After the polymer solution was cooled for 10 mins, the polymer solution was poured into two petri plates, with a volume of 50 mL each. Finally, the petri plates were dried overnight in a desiccator to form the thin PLLA film.
2.4 Thermal stability analysis of PLLA film
The suitability of PLLA film (modified PLA) used for the contact angle analysis in this research was determined according to its thermal properties. The analyses involved were the Thermogravimetric analysis (TGA) and Differential Scanning Calorimetry (DSC). The FTIR spectrum analysis was also performed.
2.4.1 Thermogravimetric analysis (TGA)
The PLLA film thermal stability assessment was performed using a Shimadzu TGA50 Thermogravimetric Analyser. A total of 20 ± 0.2 mg of PLLA was added to the alumina incubation bowl. The PLLA film was then heated to an ambient temperature of 25–600 °C at a scanning rate of 20 °C/min with nitrogen gas flow. The temperature, weight, and heat flow of the sample were recorded. The empty alumina incandescent bowl was used as a control.
2.4.2 Differential scanning calorimetry (DSC) analysis
The DSC analysis was performed using a Shimadzu DSC50 analyser. A total of 20 ± 0.2 mg of PLLA was added to a standard aluminium plate with a perforated cover. The initial and upper temperature was set at −20 °C and 250 °C respectively (below the PLLA decomposition temperature) with a scanning rate of 20 °C/min to eliminate any thermal and mechanical influences. The sample was then cooled to 30 °C with the same scanning rate. The glass transition, crystallisation temperature, and melting temperature were automatically determined and recorded. The empty alumina incandescent bowl was used as a control.
2.4.3 Fourier transform infrared spectroscopy (FTIR) analysis
The Perkin Elmer Spectrum™ 3 FT-IR spectrometer was used to determine the structure of the organic, inorganic materials, and functional groups, such as OH, CO, NH, and CH, in the recycled oil sample. Prior to the analysis, 0.5 g of PLLA samples was mixed with potassium bromide (KBr) to prepare the compressed pellet. The organic matter spectrum profiles were determined using the Infrared (IR) spectra at a range of 3000 to 660 cm−1.
2.5 Physical properties analysis
The physical properties analyse of the oil samples collected from the frying process include the moisture content, colour, density, viscosity and contact angle. All the analysis of the recycled oil was performed in triplicates (n = 3).
2.5.1 Moisture content
The moisture content of the recycled oil sample was determined using a pocket moisture refractometer (ATA0090, ATAGO, Tokyo). Approximately 0.2 mL of recycled oil was dropped onto the prism pocket moisture refractometer and the moisture percentage of the sample was automatically displayed on the screen (Kadir et al., 2017).
2.5.2 Colour
Approximately 60 mL of recycled oil sample was preheated in a microwave until it reached 60 °C before it was immediately poured into a 5.25″ glass cell. The oil colour was measured using a Lovibond® Tintometer Colourimeter Model F (Razali and Badri, 2003).
2.5.3 Density
The density of the recycled oil sample was heated and measured at 40 °C using the gravimetric analysis based on the mass per volume of oil (g/mL) (analytical scale ME204TE/00: METTLER TOLEDO, Malaysia).
2.5.4 Viscosity
The recycled oil sample was heated to 40 °C to dilute any form of crystalline or soluble solid in the sample before the analysis was performed. The oil viscosity was measured using a Rotational Viscometer (RV) spindle with a shear rate of 100 s−1 using Brookfield Viscometer DV-II+ (Rossi et al., 2009).
2.5.5 Contact angle
The static contact angle method was carried out using the Simplified Experimental Setup (SES) on to the borosilicate microslide using Automated Contact Angle Goniometer Model 100 (Ramé-Hart Instruments; USA) based on Ramlan et al. (2017). Prior to the contact angle analysis, the fabricated PLLA thin film was attached using the UHU™ all-purpose adhesive glue onto the microslide, rolled over using a cylinder metal roller to remove any air bubbles and air-dried for 10 mins. Then, the recycled oil sample was heated at 80 °C for 45 mins in a water bath to dilute any crystalline or solid formation in the oil (Rossi et al., 2009). Once the sample was cooled to 40 °C, approximately 0.5 μL of the recycled oil was gradually dropped onto a borosilicate-coated PLLA film microslide using a micropipette. The droplet images were captured using a 7.1 Megapixel Olympus™ digital camera with an enabled continuous burst mode of image profiles. The recycled frying oil droplet profile was then measured using MB Ruler software to detects the droplet contact angle and adjusts its profile to a perfect semi-circle curvature. The contact angle readings were automatically taken and recorded in triplicates (n = 3).
2.6 Chemical properties analysis
The peroxide value and aldehyde analysis were performed to study the chemical properties of the oil samples collected from the frying process. All the analysis of the recycled oil was performed in triplicates (n = 3).
2.6.1 Peroxide value (PV)
The PV of the recycled oil sample was determined according to the American Oil Chemist’s Society (AOCS) official methods Cd 8b-90 (1997). Approximately 2.0 g of recycled oil was added into a 250 mL flask containing 30 mL of acetic acid chloroform mixture (3:2) (both chemicals were 99.5% in purity: Sigma-Aldrich (M) Sdn. Bhd.). The flask was swirled before the solution was added with 0.5 mL of saturated potassium iodide (KI). The flask was swirled again for one minute and added with 30 mL of distilled water and 0.5 mL of starch solution (10%, v/v). The solution was titrated against 0.01 N sodium thiosulphate solution with constant stirring until the yellow colour has almost disappeared. The PV was expressed in milliequivalents of peroxide per kg of oil sample (meq O2 kg oil).
2.6.2 Aldehyde analysis
The aldehyde analysis was carried out to authenticate the recycled oil rapid oxidation product changes from primary (peroxides) to its secondary compounds. The Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy (ATR-FTIR) was utilised to measure the changes occurring in the total internal reflection beam when the beam is in contact with the sample. The aldehyde compounds were detected based on the presence of peak carbonyl (C⚌O) aldehydes at 1720–1740 cm−1, methyl (CH) stretches for CH2 and CH3 at 2400–2800 cm−1 and long-chain CH2 bonds at 721 cm−1 in the chromatogram generated by the infrared spectrum.
2.7 Statistical analysis
All the collected data were analysed using the Statistical Package for the Social Sciences (SPSS) 23 software. The one-way Analysis of Variance (ANOVA) and Tukey's test methods were performed to compare the mean difference between the treatments at a 95% confidence level (p < 0.05). The linear correlation between the two variables was performed using the Pearson correction analysis to study the strength of the relationship between the respective variables.
3 Results and discussion
3.1 Thermal properties of PLLA film
The PLLA is a thermoplastic polyester with good mechanical properties, biocompatibility, biodegradability, low toxicity and low cost (Raquez et al., 2013). Thus, it is widely applied in agriculture, medical devices, packaging and textiles (Mustapa and Shanks, 2013). Table 1 and Fig. 1 shows the (a) TGA, (b) DSC and (c) IR spectra analysis of the PLLA film. The decomposition temperature profile (Fig. 1a) of the PLLA film (Tonset) began at 294 °C, indicating a significant degradation stage. As the decomposition reached a maximum weight loss (Tmax) at 357 °C, the findings showed that the PLLA film exhibited strong stability and was highly resistant against degradation at high temperatures.
TGA
DSC
Tonset (°C)
Tmax (°C)
Tg (°C)
Tc (°C)
ΔHc (J/g)
Tm (°C)
ΔHm (J/g)
294.00
357.00
70.84
113.95
2.99
166.74
−31.28
FTIR
Component(s)
Infrared absorption frequency (cm−1)
O—H
3505
C—H methyl stretches of CH2 and CH3
2996 & 2946
C⚌O
1756
C—O—C ester strains
1185 & 1093
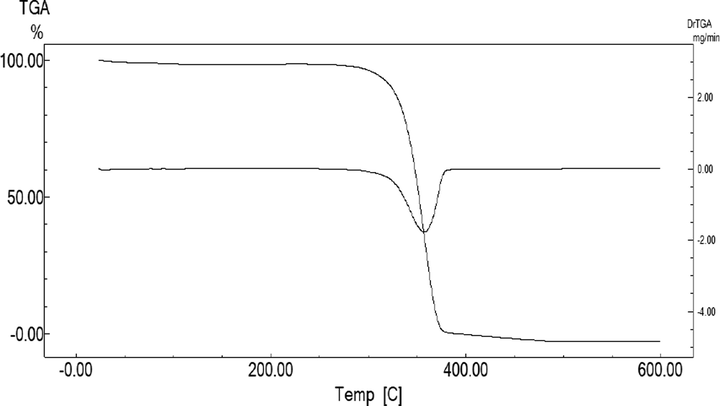
Thermogravimetric (TGA) and derivative thermogravimetry (DTG) thermogram of PLLA.
Furthermore, the DSC analysis (Fig. 1b) showed that the glass transition temperature (Tg) of the PLLA film occurred at 70.84 °C with the onset temperature at 69.04 °C. Meanwhile, the crystallisation point (Tc) was recorded at 113.95 °C with the onset, endset and heat released (ΔHc) at 102.22 °C, 12.36 °C, and 2.99 J/g, respectively. In addition, the melting point (Tm) of the PLLA film was shown at 166.74 with the onset, endset and heat absorbed (ΔHm) at 159.55 °C, 170.48 °C and −31.28 J/g, respectively. Similar findings were reported in previous studies (Neumann et al., 2017; Jamshidian et al., 2010) in which the Tg of pure PLLA was approximately 40–70 °C, while the Tc and Tm were in the range of 67.3–103.3 °C and 155.3–177.6 °C, respectively.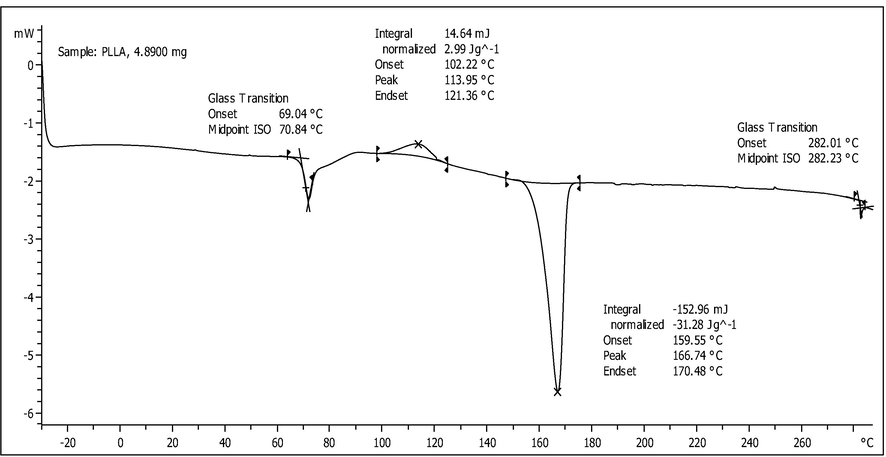
Differential scanning calorimetry (DSC) thermogram of PLLA.
The IR spectra of the PLLA film in Fig. 1c also indicated the presence of O—H, C—H methyl stretches of CH2 and CH3, C⚌O, and two C—O—C ester strains at 3505 cm−1, 2996 cm−1, 2946 cm−1, 1756 cm−1, and 1185 and 1093 cm−1, respectively. According to Neumann et al. (2017), the presence of carbon and oxygen atoms strengthen the PLLA structure, contributing to the higher thermal stability of the polymer. The PLLA film used in this study was highly stable since the frying oil temperature (40 °C) used for contact angle analysis was lower than its crystallisation, melting and decomposition temperature. Additionally, the presence of carbon and oxygen atom that produced stronger bonds in the polymer confirmed its thermal stability and the suitability of the PLLA film to be used in the rapid contact angle analysis to measure the oil degradation level in this study.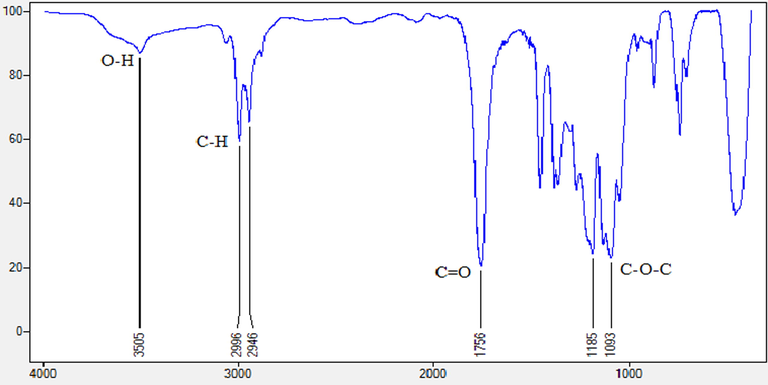
Fourier transform infrared spectroscopy (FTIR) spectrum of PLLA.
3.2 Temperature profile of recycled RBDPO oil via deep-frying process
Fig. 2 shows the temperature profile of the recycled RBDPO oil during the heating and frying process throughout the 15 cycles. The oil temperature dropped rapidly from 184 °C to 145 °C when the raw potatoes were added to the fryer. A similar trend of decreasing temperatures was observed from the first minute to the third minute of each frying cycle. However, the temperature started to rise gradually until the eighth minute. Once the potatoes were removed from the frying pan after 8 mins of frying, the oil continued to heat for the next 7 mins to allow the oil temperature to return to its original frying temperature (temperature recovery period). There was no significant difference in terms of oil temperature throughout the heating cycle since the deep-fat fryer in this study was equipped with a thermostat to maintain the oil temperature at 180 ± 5 °C. The same oscillating trend was shown at every 5th frying cycle throughout the analysis.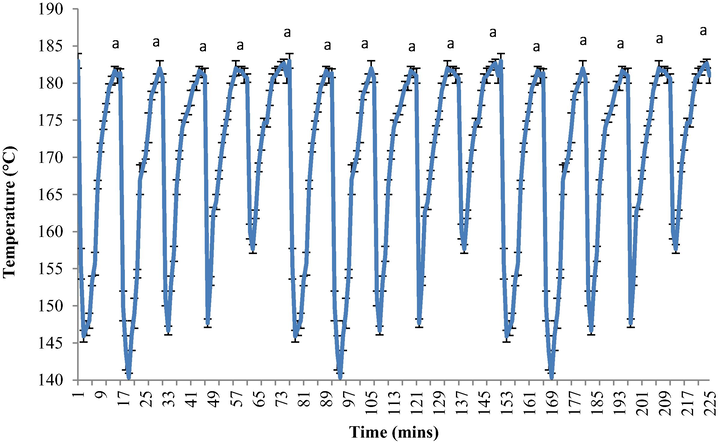
The temperature profile of RBDPO for 15 frying cycles.
During the frying process, the frying oil acts as the medium to facilitate simultaneous heat and mass transfer. While the heat transfer into the food takes place via convection, the moisture that migrates out of the food is replaced by the oil absorption into the food via conduction (Budžaki and Šeruga, 2005). Besides, two main factors that could lead to the decrease of oil temperature were determined, which were the amount of food added to the oil (food to oil ratio) and the presence of water or moisture content in the food. For each cooking cycle, the ratio of food to oil must be accurate, where the general recommended ratio of food to oil is 1:6 (Berger, 2005). The ratio of potatoes-to-oil was maintained throughout the study. Furthermore, the oil hydrolysis products during the frying process, such as water, volatilised and decreased the oil temperature (Choe and Min, 2007). The oil temperature should not fall too low beyond its setting operational temperature as the temperature will become too slow to return to the original setting frying temperature. Hence, the fluctuations in the oil temperature, moisture potatoes contents, and the potatoes-to-oil ratio were constant throughout the study.
3.3 Physical properties analysis
3.3.1 Moisture analysis
The mass transfer during the frying process involves water loss, heat transfer, and oil absorption (Vitrac et al., 2002). Fig. 3 shows the moisture content (%) of RBDPO oil during the frying cycles. The result demonstrated that the decreasing trend of moisture content of the recycled oil over successive frying cycles was significant (p < 0.05) between the 1st and 15th frying cycles for RBDPO-1, RBDPO-2 and RBDPO-3 in this study.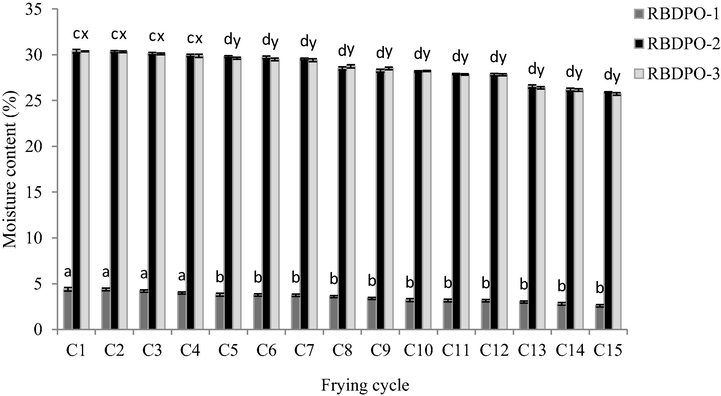
The moisture content of RBDPO-1, RBDPO-2 and RBDPO-3 for 15 frying cycles.
The significance difference (p < 0.05) was observed as early as the 5th frying cycle until the 15th cycle due to the exposure of the oil to air and moisture within the time of the frying process. The moisture evaporation during the deep-frying process was caused by the conductive heat transfer from the hot oil to the raw potatoes (ambient temperature: 25 ± 2 °C) as the inside of the potatoes were cooked (Orthoefer and List, 2006). As a result, a huge mass transfer occurs, which removed the water from the food and into oil as steam. Simultaneously, the process facilitates the transfer of chemical components from the food into the frying oil and vice versa (Lazarick, 2009). Given that the remaining non-volatile compounds from the oil hydrolysis products remained and accumulated in the frying oil, therefore, the overall chemical composition of the frying oil have been altered (Choe and Min, 2007).
3.3.2 Colour profiles
Fig. 4 shows the increasing redness of the RBDPO-1, RBDPO-2, and RBDPO-3 throughout the 15 frying cycles. The redness of RBDPO-1 significantly increased (p < 0.05) after the 2nd frying cycle (lag phase) with the subsequent increment at the 5th, 6th, 9th, 12th and 15th frying cycles remained significant (p < 0.05). In contrast, it was observed that the increment of redness (lag phase) of RBDPO-2 between the 1st until the 4th frying cycle was significant (p < 0.05). The noticeably subsequent increment (log phase) at 6th, 9th, 10th, 12th and 14th frying cycles were significant (p < 0.05). Meanwhile, for RBDPO-3, a significant increment (p < 0.05) of redness colour between the lag phase of 1st and 5th. The subsequent increment (log phase) at the 9th, 12th, and 14th frying cycles was also significant (p < 0.05).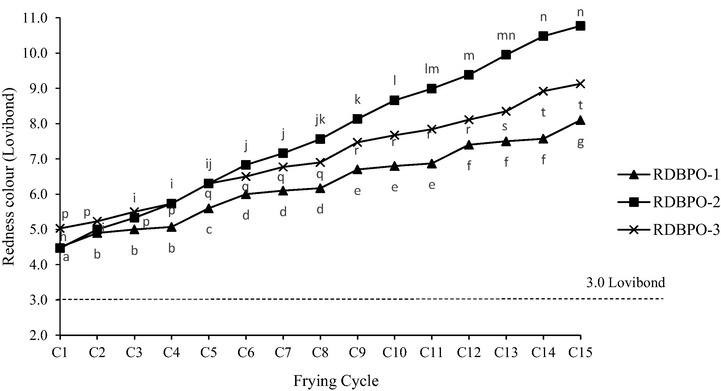
Effect of heating on the redness of RBDPO-1, RBDPO-2 and RBDPO-3 for 15 frying cycles.
Furthermore, the results showed that the RBDPO-2 has the darkest redness colour in comparison to the redness of RBDPO-3 and RBDPO-1. The colour differences could be explained based on the Maillard reaction and lipid oxidation, where these two essential reactions affect the colour of food products. The Maillard reaction and lipid oxidation in this study was a result of the decomposition of non-volatile compounds from the fried potatoes into the oil (Delgado-Andrade et al., 2010; Zamora and Hidalgo, 2005; Xu, 2003). The Maillard reaction involved the interactions between nucleophilic amino groups of amino acids, peptides, or proteins and the carbonyl groups (mainly of reducing sugars), which led to the sugar–amine condensation and rearrangement to Heyns or Amadori products (Oracz and Nebesny, 2019). On the other hand, the lipid oxidation, which was contributed by the reaction between polyunsaturated fatty acid and reactive oxygen at high temperatures, gave rise to the formation of harmful compounds, such as peroxides and aldehydes that deteriorate the oil colour (de Lima Júnior et al., 2013).
RBDPO-3 is found to be more stable compared to RBDPO-2 due to the addition of AO as an oil additive that makes the oil sample becomes more resistant to discolouration. Ahmad Suhael (2015) reported that incorporation of AO in cooking oil reduced the oil absorption into the food but increased the shelf life of the cooking oil up to 80 times. The AO was formulated from a special nutritional extract ingredient that is found in curry leaves or Rutaceae herbs, which are known as a natural antioxidant that preserved the cooking oil quality during frying. Based on the Malaysian Standard (2007), the maximum redness colour of RBD palm oil (MS 814:2007) and palm olein (MS 816: 2007) was set at 3.0 Lovibond. However, all oil used in this study was considered as unacceptable as it exceeded the standard limit.
3.3.3 Density profiles
Fig. 5 shows the density of the recycled oil samples throughout the 15 frying cycles. The result showed that the density of each sample gradually increased starting from RBDPO-1, RBDPO-2 and RBDPO-3 as compared to control (fresh oil: 0.933 g/ml). However, there was no significant difference in oil density over the 15 frying cycles (p > 0.05) for all types of oil. In addition, the Tuckey’s test with confidence intervals (CI) of 95% was only significant from the 20th frying cycle onwards. Nevertheless, the density results as shown in Fig. 4 were expected as the frying process was carried out only up to 15 frying cycles.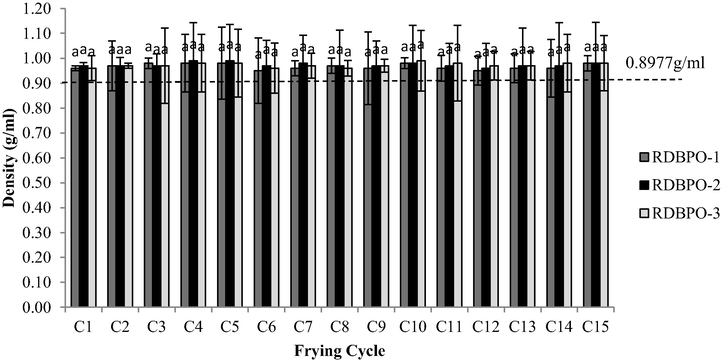
The density for RBDPO-1, RBDPO-2 and RBDPO-3 for 15 frying cycles.
Kalogianni et al. (2011) suggested that frying cycle number was a significant variable (p < 0.05) that affects the density of oil. Thus, the significant change in oil density indicates a formation of polymeric compounds (e.g., polymerization of unsaturated fatty acid) and other chemical reactions occur including oxidation, hydrolysis and cyclization that may lead to additional chemical formation in palm oil. Based on the findings in the present study, the minimum and maximum density values of RBDPO-1, RBDPO-2 and RBDPO-3 were considered non-quality palm olein oil throughout the frying cycle as the maximum density of palm olein exceeded standard limit (MS 816: 2007) of 0.8977 g/mL, as stated in the Malaysian Standard (2007). In fact, the results were reliably anticipated since the changes are heavily depending on the temperature, frying cycles, type of samples used and storage method.
3.3.4 Viscosity profiles
Fig. 6 showed the measured viscosity of RBDPO-1, RBDPO-2, and RBDPO-3 at 40 °C. The findings showed a similar trend of shear thickening viscosity increment for all three oils. RBDPO-2 has higher viscosity compared to RBDPO-1, while the viscosity of RBDPO-3 oil was almost constant over each frying cycle. There was a significant increase (p < 0.05) in the viscosity of the RBDPO-1 between the 1st and 3rd, 10th, and 12th frying cycles. The RBDPO-2 also recorded a significant difference (p < 0.05) between the 1st and 3rd until 15th frying cycles, with an increasing viscosity value. However, the difference in viscosity value for RBDPO-3 was insignificant. It was also observed that RBDPO-3 recorded the lowest viscosity value, indicating that RBDPO-3 was more stable during long repetitive deep-frying cycles due to the presence of AO that maintains the oil viscosity, therefore, preserving its quality.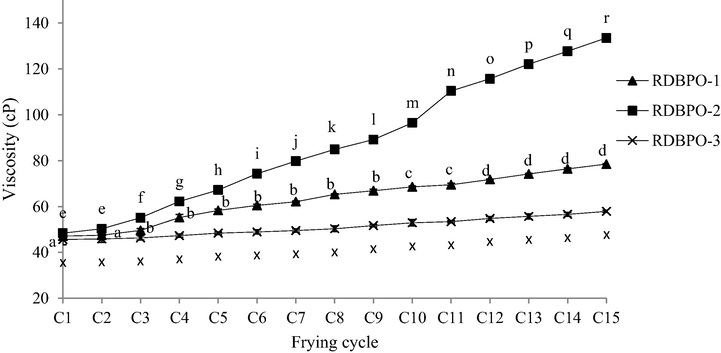
The viscosity of RBDPO-1, RBDPO-2 and RBDPO-3 for 15 frying cycles.
All three types of oil were found to deteriorate due to the exceeding viscosity beyond 38.6 cP. The viscosity of the recycled oil increased with increasing polymerisation levels and fatty acid length (Lioumbas et al., 2012). In fact, the viscosity of the recycled oil affected the rate of heat transfer, causing a longer time taken to fry the food and a higher amount of oil absorption into the food. The results were in agreement with prior studies (Kalogianni et al., 2011; Lazarick, 2009), where the measured oil viscosity increased with the increasing number of frying cycles as a result of the chemical breakdown during the frying process. According to Lawson (2013), the frying oil becomes more viscous after the oil heating takes place several times. The long heating periods (increasing number of cycles) and the exposure to high temperatures and air during the heating process were identified as the main factors that caused the increase in oil viscosity (Saguy and Dana, 2003).
3.3.5 Contact angle profiles
Fig. 7 shows the contact angle profiles of RBDPO-1, RBDPO-2, and RBDPO-3 on the PLLA thin film at 40 °C throughout the 15 frying cycles. The contact angle measurements provide information on the hydrophobicity of the PLLA and the oil blends. The results demonstrated a significant decrease in the contact angle between the 1st frying cycle and the 2nd frying cycle for RBDPO-2 and the 3rd frying cycle for RBDPO-1 and RBDPO-3 (p < 0.05). The decreasing values were significant until the 15th frying cycle. The range of contact angle between the 1st and 15th frying cycle for RBDPO-1, RBDPO-2, and RBDPO-3 was 25.07 ± 0.30° to 20.14 ± 0.16°, 34.80 ± 0.15° to 26.01 ± 0.02°, and 30.49 ± 0.17° to 20.76 ± 0.07°, respectively. The RBDPO-3 was more stable and resistant to major changes compared to the RBDPO-2 due to the addition of AO as the additive in the oil sample.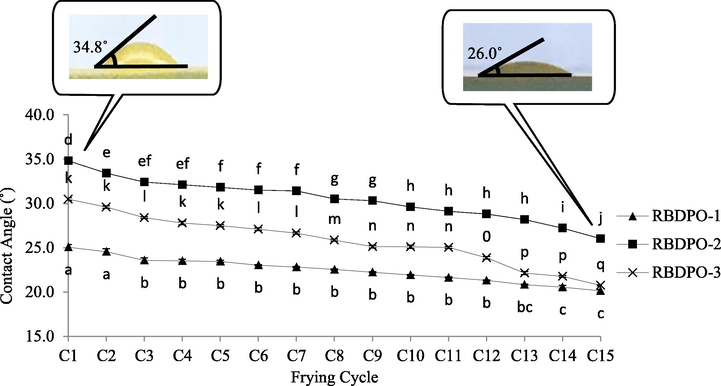
Contact angle profiles (°) of RBDPO-1, RBDPO-2 and RBDPO-3 on the PLLA thin film at 40 °C for 15 frying cycles.
The results were similar to prior reports (Aydar, 2020; Sahasrabudhe et al., 2017) in which the contact angle decreased as the frying time increased. The findings could be due to the oxidation and oil hydrolysis process that occurred during the frying process, which led to the formation of by-products, such as aldehydes and ketones. Consequently, the surface tension between the food-oils decreased, thus, reducing the contact angle values (https://www.researchgate.net/publication/282666366_Determination_of_Contact_Angle_of_Olive_oil_and_Canola_Oil_on_a_PTFE_surface_at_Elevated_Temperatures). Besides, the results could be contributed by the reaction between the hydroxyl groups in the PLLA structure and the carbonyl groups of fatty acids in the palm olein oil (Xu and Qu, 2009). A contact angle below 45° indicates that the surface exhibits high wetting, where water droplet spreads out further on the surface (Aydar, 2020). A small contact angle value is vital for the oil industry to ensure that the oil possesses a higher wetting ability on the surface based on the wetting phenomena. Furthermore, smaller contact angle represents a smaller amount of build-up carbonized material from the frying process. Hence, the higher the wetting value (via smaller contact angle), the fresher the oil is. Fresher oil contained fewer toxic compounds (e.g., aldehyde) and low in rancid scent due to its rancidity oxidation mechanism.
3.4 Chemical properties analysis
3.4.1 Peroxide value (PV)
Fig. 8 shows the PV results for RBDPO-1, RBDPO-2, and RBDPO-3 throughout the 15 frying cycles. The RBDPO-1, RBDPO-2, and RBDPO-3 oil demonstrated a significant increase (p < 0.05) of the PV between the 1st and 15th frying cycles. It was observed that PV for all oil samples throughout the 15 frying cycles was within the acceptable range based on the Food Act (1983) (Laws of Malaysia: Act 281), which was between 1.0 and 5.0 meq O2 kg oil and below the maximum value as stated in Food Regulations (1985), which was 10 meq O2/kg of oil.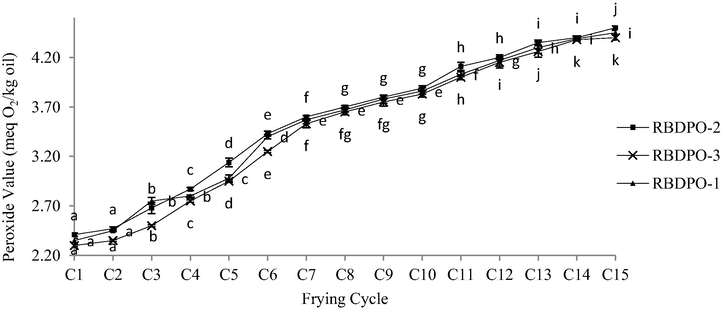
Peroxide value (PV) for RBDPO-1, RBDPO-2 and RBDPO-3 (n = 3) for 15 frying cycles.
The increment of PV was due to the lipid oxidation during the repetitive deep-frying process. This was in line with previous reports in which the high PV indicates the oxidation level of an oil sample (Jurid et al., 2020). However, the prior work PV was critically high on the 1st cycle of frying (8–9 meq O2/kg of oil) as compared to the current values (15 frying cycles) that was steadily increased as the cycle increased. The anomaly might be due to the usage of an older batch oil product that might have started to deteriorate during long storage. During the deep-frying process, the polyunsaturated fatty acid reacts with oxygen to produce peroxides and hydroperoxides, leading to the formation of secondary products, such as ketones and aldehydes. As a result, these by-products contribute to the off-flavour and unpleasant taste of the food, which highly affects the preference of consumers (de Lima Júnior et al., 2013; Allendorf, 2010). The constituent of fatty acids and the storage period of oil could also influence the PV (Lawson, 2013).
Additionally, the PV showed a fluctuating trend throughout the 15 frying cycles (Fig. 8). According to Marmesat et al. (2008), the unstable PV was caused by the degradation of peroxide compounds at high frying temperatures, leading to the formation of dimers and evaporated products. Since the rate of peroxide decomposition was higher than the rate of peroxide formation, thus, the PV results in this study may not accurately indicate the real extent of oil spoilage and degradation. Therefore, further analysis was carried out to detect the presence of aldehyde (secondary product oxidation) that may occurred on the peroxide compound due to its prolonged high temperature exposure and repetitive usage.
3.4.2 The presence of aldehyde
Fig. 9 shows the IR spectra for RBDPO-1 (Fig. 9a), RBDPO-2 (Fig. 9b), and RBDPO-3 (Fig. 9c) at the 5th, 10th, and 15th frying cycles, respectively. The chemical structure of aldehydes comprising functional groups of carbonyl, hydroxyl, carbon, and hydrogen molecules are circled and highlighted on each of the IR spectra to indicate the occurrence of the lipid oxidation process in the sample. The IR spectra as shown in Table 2 also indicate the presence of (i) methyl chain, (ii) methyl chain for CH2 and CH3, (iii) aldehyde carbonyl chain (C⚌O), (iv) alcohol, ester, ether, carboxylic acid, and anhydride carbonyl chain, and (v) CH2 long chain group that represent the occurrence of lipid hydrolysis, oil oxidation, and polymerisation throughout the 15 frying cycles.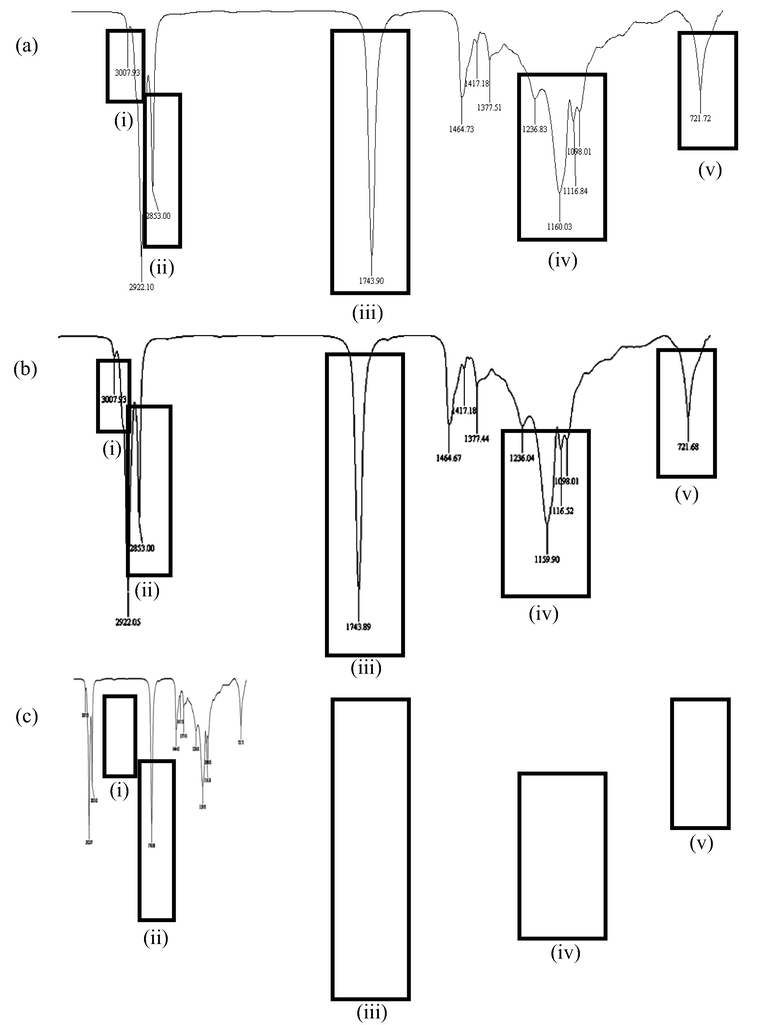
Chromatogram for a) RBDPO-1b) RBDPO-2 and c) RBDPO-3 at 5th frying cycle.
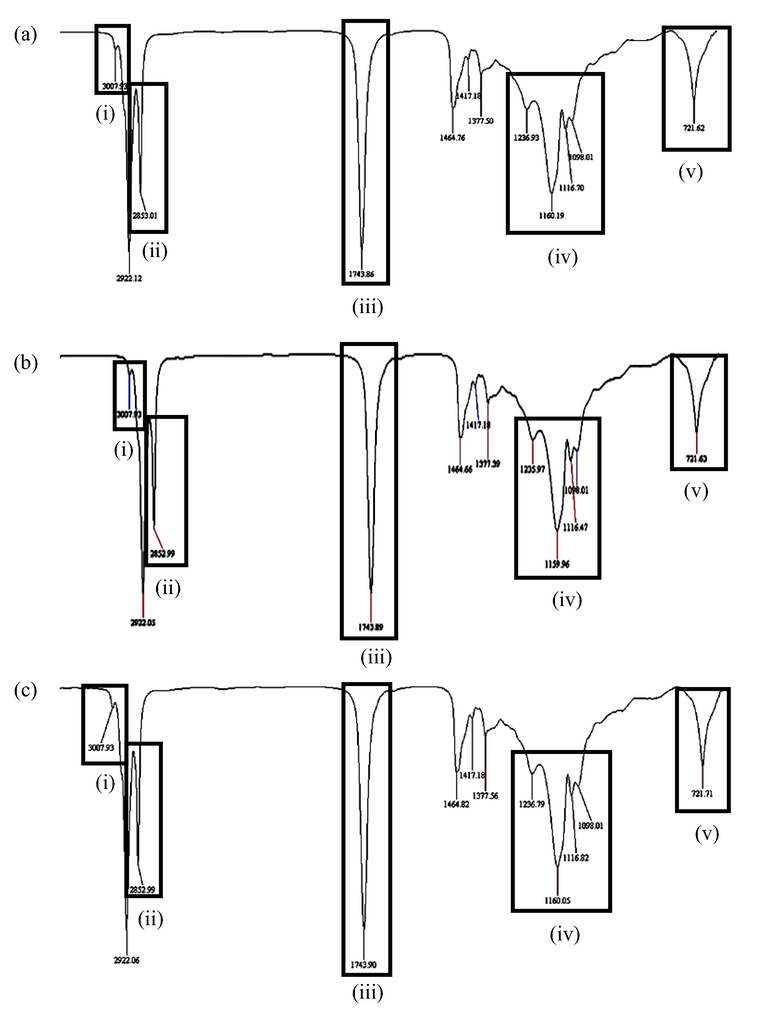
Chromatogram for a) RBDPO-1b) RBDPO-2 and c) RBDPO-3 at 10th frying cycle.
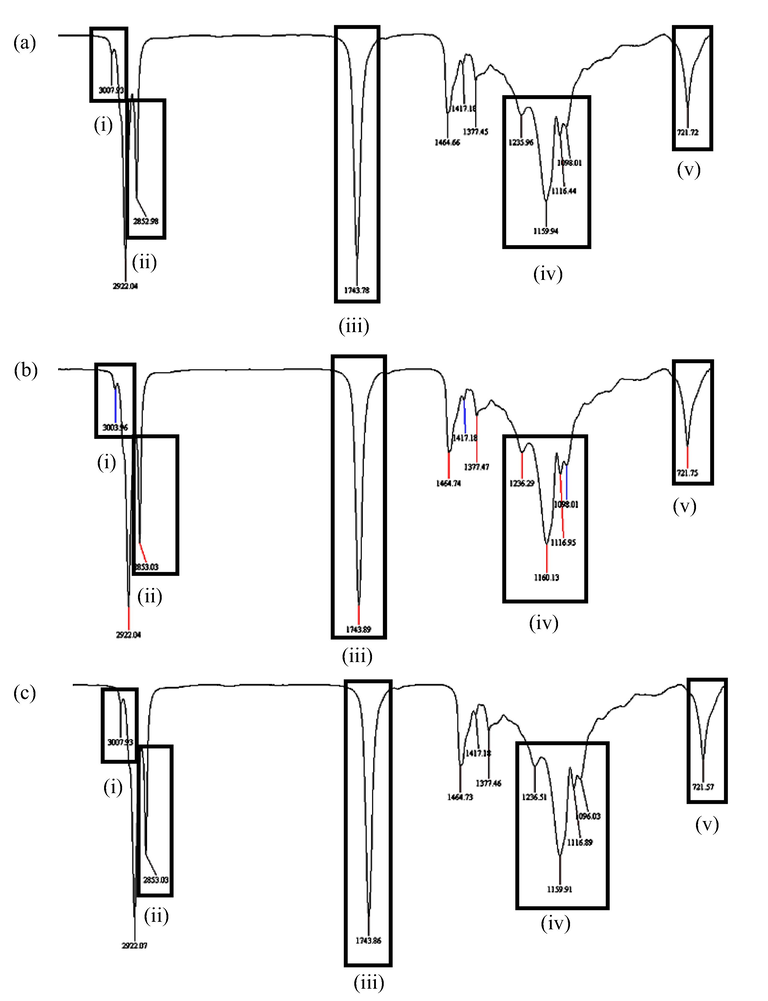
Chromatogram for a) RBDPO-1b) RBDPO-2 and c) RBDPO-3 at 15th frying cycle.
Initials
Reference wavelength (cm−1)
Component(s)
i
3007
Methyl chain (CH3)
ii
2400–2800
Methyl chain for CH2 and CH3
iii
1720–1740
Aldehyde carbonyl chain (C⚌O)
iv
1000–1200
Alcohol, ester, ether, carboxylic acid, and anhydride carbonyl chain
v
721
CH2 long-chain group
As mentioned earlier, the aldehyde is a secondary lipid oxidation product from the degradation of fatty acids in oil via hydroperoxide. Guillen and Uriarte (2012) reported that the non-volatile aldehydes remained in the oil during the deep-frying process, hence, they can be found in fried foods. Aldehyde is a toxic compound as it can cause cancer and neurodegenerative disorders in humans, such as Alzheimer and Parkinson diseases. Given their very reactive nature, aldehydes can react with hormones, enzymes, and proteins in the organism and inhibit the proper functioning of these biochemical compounds. Moreover, aldehydes also have an unpleasant flavour that affects overall consumer acceptance.
Thus, to reduce of such toxic secondary oxidation product (aldehydes) includes fresh oil replenishment, reducing frying time and temperature, well-maintained fryer, low unsaturated fatty acids/free fatty acids oil and adding antioxidants additives (e.g., Afdhal oil) to increase the oxidative stability may affect the quality and flavour of oil during deep-fat frying (Wang et al., 2017; Choe and Min, 2007). In fact, intermittent frying with a higher turnover rate and lower temperature diminishes the oxidation and polymerization of oil during deep-fat frying. Moreover, frying using small surface-to-volume ratio fryer is desirable to slow down the frying oil oxidation (http://dx.doi.org/10.21037/hbsn.2018.04.013; Negishi et al., 2003). Further lab analysis regarding the aldehyde concentration should be carried out in the future to determine the level of toxicity of aldehyde in recycled oil.
3.5 The correlation of contact angle to physicochemical characteristics of palm olein oil
In this study, the correlation test was conducted to evaluate the suitability of contact angle using PLLA film as a fast-indicating method and to provide a general statistical overview of the dataset to measure the spoilage of recycled oil. The correlation analysis, r describes the “degree of relationship” between two variables and ranges from −1 to +1. A negative correlation indicates that one variable increases as the other variable decreases. In contrast, a positive correlation indicates that both variables increase simultaneously. A correlation value closer to 0 suggests a weak relationship between the two variables (Ching, 2018).
Based on the Pearson correlation test results shown in Table 3, the contact angle was negatively correlated (p < 0.01) for almost all the physicochemical properties studied during the 15 frying cycles, including colour, density, viscosity and PV. As the contact angle value decreased, the recycled oil samples became darker, denser, more viscous, and possessed higher PV as the repetitive frying process reduced the unsaturation degree of the oil, making the oil more prone to oxidative rancidity. This condition represents the spoilage level of recycled oil. Nevertheless, the contact angle only recorded a positive correlation with the moisture content. The moisture content was reduced as the contact angle decreased throughout the 15 frying cycles, which was due to the evaporation and conductive heat transfer that occurred between the potatoes and the oil during the frying process.
Interestingly, a weak correlation between the contact angle and density indicated that the density may not be a suitable parameter to assess the quality of recycled oil, which corresponded with the insignificant density change throughout the frying process. On the contrary, the coefficient values showed a high relationship between the contact angle and colour, viscosity, moisture and PV of the oil. Therefore, they are well-fitted to be utilised as oil degradation parameters.
Therefore, based on the aforementioned well-fitted correlation values, the index guidelines (Table 4) were created to produce a quick/rapid novel oil spoilage indicator and to distinguish basic safety level of the unknown oil used. The attached PLLA film microslide will certainly be a rapid spoilage model system (Yusof et al., 2018) as the unknown recycled oil contact angle will spread and fall over on either of these three different circular indicators (oil droplet diameter) that represent its respective physicochemical properties within 15 frying cycles. Table 4 shows a suggestion of the index guidelines for the degradation of RBDPO during the deep-frying process at 180 °C using the contact angle method (PLLA film). It was reported that the contact angle modification of the liquid oils was significantly correlated with the common indices of hydrolytic, oxidative, and thermal phenomena and proven to be a sound index of the overall oil degradation in the actual frying experiments (Jurid et al., 2020; Rossi et al., 2009).
Frying cycle
Output
Oil droplet diameter of PLLA film (cm)
Contact angle (°)
Peroxide Value (PV) (meq O2/kg oil)
Redness colour (Lovibond)
Moisture (%)
1–5
32.65–29.65
2.30–3.14
4.75–6.30
25.82–27.88
0.6 ± 0.2a
6–10
29.03–27.36
3.15–3.70
6.67–8.17
28.21–29.62
0.8 ± 0.3b
11–15
27.07–23.39
3.71–4.52
8.42–9.95
29.72–30.39
1.0 ± 0.1c
4 Conclusion
This study successfully investigated the effect of long-term repetitive frying on the physicochemical properties of RBDPO, in particular, the moisture, colour and aldehyde detection, as well as to evaluate the potential usage of PLLA thin film as a reliable rapid indicator technique via contact angle analysis, to determine the spoilage of the recycled oil. The significant changes of FOUR selected physicochemical properties are summarized as follows: (1) The oil moisture content was significantly decreased between the 1st and 15th frying cycles for all oil samples due to the volatilisation process of the hydrolysis products during the frying process. (2) The red colour and viscosity of the recycled oil were significantly increased after 15 frying cycles. (3) Furthermore, it was observed that PV for all oil samples throughout the 15 frying cycles (carbonyl (C⚌O) aldehyde was detected in all oil samples throughout the whole cycles) was steadily increased and within the acceptable range based on the Food Act (1983). (4) Finally, the contact angle showed a significant decrease (p < 0.05) between the 1st and 15th frying cycles for all oil samples. The strong positive correlation (ranging from 0.7 − 0.95) between the contact angle (oleophilicity) and various parameters, including colour, viscosity, moisture and PV, indicated that the contact angle method using PLLA film was considered a primary physical property for a rapid and thermally stable technique in determination of recycled oil spoilage. The innovative index guidelines of RBDPO deep-frying degradation profiles (Table 3) were generated successfully as it will serves as a potential guideline for the food industry and local health authorities in detecting of any oil quality deterioration.
Acknowledgements
The authors are grateful to Universiti Kebangsaan Malaysia (UKM) for the financial support (GUP-2018-057, GUP-2018-080) and the Department of Food Sciences, Faculty of Science and Technology, UKM Bangi for allowing this study to be carried out.
Data Availability Statement
No data were used elsewhere to support this study and it was entirely a new set of data.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: [Saiful Irwan Zubairi reports financial support, administrative support, article publishing charges, and statistical analysis were provided by National University of Malaysia.]
References
- Ahmad Suhael, A., 2015. Minyak masak Afdhal boleh goreng 80 kali. Retrieved from (https://upm.edu.my/berita/penyelidik_cipta_minyak_masak_afdhal_boleh_goreng_80_kali-25139). Accessed July 11th, 2020.
- Design and synthesis of different types of poly(lactic acid). Poly(lactic acid): synthesis, structures, properties, processing and applications. Chichester: Wiley; 2011. p. :43-55.
- Application of a handheld portable infrared sensor to monitor oil quality. Ohio State University; 2010. Doctoral Thesis
- American Oil Chemist’s Society, AOCS, 1998. Method Cd 8b-90, 4th ed., AOCS Press: Champaign, IL.
- An overview of polylactides as packaging materials. Macromoacular Biosci.. 2004;4:835-864.
- [Google Scholar]
- The relationship between the contact angle and some quality parameters of frying oils. La Rivista Italiana Delle Sostanze Grasse Journal. 2020;97(2):17-23.
- [Google Scholar]
- Polylactic acid: synthesis, properties and applications. Monomers, polymers and composites from renewable resources. Kidlington: Elsevier Ltd; 2008. p. :433-450.
- Level of knowledge, attitude and practice of night market food outlet operators in Kuala Lumpur regarding the usage of repeatedly heated cooking oil. Med. J. Malaysia. 2012;67(1):91-101.
- [Google Scholar]
- Physico-chemical properties of blends of palm olein with other vegetable oils. Grasas y Aceites (Sevilla). 2010;61(4):423-429.
- [Google Scholar]
- Moisture loss and oil uptake during deep fat frying of “Kroštula” dough. Eur. Food Res. Technol.. 2005;220(1):90-95.
- [Google Scholar]
- Ching, T.L., 2018. Correlation and regression with R. Retrieved from https://sphweb.bumc.bu.edu/otlt/MPH-Modules/BS/R/R5_Correlation-Regression/R5_Correlation-Regression_print.html. Accessed February 17th, 2021.
- Novel polylactic acid (PLA)-organoclay nanocomposite bio-packaging for the cosmetic industry; migration studies and in vitro assessment of the dermal toxicity of migration extracts. Polym. Degrad. Stab.. 2019;168
- [Google Scholar]
- Development of the Maillard reaction in foods cooked by different techniques. Intake of Maillard-derived compounds. Food Chem.. 2010;122(1):145-153.
- [Google Scholar]
- Oxidação lipídica e qualidade da carne ovina. Acta Veterinaria Brasilica. 2013;7(1):14-28.
- [Google Scholar]
- Poly (lactic acids): a brief review. In: Degradable polymers and materials. ACS Symposium Series. 2006. p. :102-125.
- [Google Scholar]
- Frying stability of rice bran oil and palm olein. Int. Food Res. J.. 2013;20(1):403-407.
- [Google Scholar]
- Food Act, 1983. Food (amendment) (no. 3) regulations 2014. Retrieved from http://www.federalgazette.agc.gov.my/index.php. Accessed February 15th, 2020.
- Solvent removal from solution-cast films of biodegradable polymers. J. Mater. Sci. Lett.. 2001;20(21):1929-1931.
- [Google Scholar]
- Aldehydes contained in edible oils of a very different nature after prolonged heating at frying temperature: Presence of toxic oxygenated α, β unsaturated aldehydes. Food Chem.. 2012;131(3):915-926.
- [Google Scholar]
- Polylactic Acid: Polymers for a Sustainable Environment and Green Energy. Polymer Sci.: A Comprehensive Ref.. 2012;10:231-236.
- [Google Scholar]
- Poly-Lactic Acid: Production, applications, nanocomposites, and release studies. Compr. Rev. Food Sci. Food Saf.. 2010;9(5):552-571.
- [Google Scholar]
- Fermentative production of lactic acid from biomass: An overview on process developments and future perspectives. Appl. Microbiol. Biotechnol.. 2007;74:524-534.
- [Google Scholar]
- Kesan-kesan penggorengan terhadap sifat fizikokimia minyak masak olein sawit. Universiti Kebangsaan Malaysia; 2017. Master Thesis
- The effect of repetitive frying on physicochemical properties of refined, bleached and deodorized Malaysian tenera palm olein during deep-fat frying. Arabian J. Chem.. 2020;13(7):6149-6160.
- [Google Scholar]
- Physicochemical properties of recycled refined, bleached and deodorized (RBDPO) palm olein: Which cycle should the olein considered spoilage? Jurnal Teknologi. 2017;79(4):17-25.
- [Google Scholar]
- Effect of repeated frying on the viscosity, density and dynamic interfacial tension of palm and olive oil. J. Food Eng.. 2011;105(1):169-179.
- [Google Scholar]
- Minyak masak terpakai ancaman kepada kesihatan. Bangi: Penerbit Universiti Kebangsaan Malaysia; 2013.
- Deep-fried keropok lekors increase oxidative instability in cooking oils. Malaysian J. Medical Sci.. 2012;19(4):57-62.
- [Google Scholar]
- Food oils and fats: Technology, utilization and nutrition. Springer Science & Business Media; 2013.
- Lazarick, K., 2009. Cause of colour component formation oils during frying. Master Thesis. University of Lethbridge.
- Processing of poly(lactic acid). Poly(lactic acid): synthesis, structures, properties, processing, and applications. Chichester: Wiley; 2011. p. :191-251.
- Vegetable oils in food technology: Composition, properties and uses. John Wiley & Sons; 2002.
- Effect of potato deep-fat frying conditions on temperature dependence of olive oil and palm oil viscosity. J. Food Eng.. 2012;113(2):217-225.
- [Google Scholar]
- Malaysian Standard, 2007. Department of standards Malaysia. Cyberjaya, Selangor.
- Quantitative determination of epoxy acids, keto acids and hydroxy acids formed in fats and oils at frying temperatures. J. Chromatogr. A. 2008;1211(1):129-134.
- [Google Scholar]
- Poly (lactic acid)-hemp-nanosilica hybrid composites: Thermomechanical, thermal behavior and morphological properties. Int. J. Adv. Sci. Eng. Technol.. 2013;3(1):192-199.
- [Google Scholar]
- Effect of a modified deep-fat fryer on chemical and physical characteristics of frying oil. J. Am. Oil. Chem. Soc.. 2003;80:163-166.
- [Google Scholar]
- Biodegradable poly (L-lactic acid) (PLLA) and PLLA-3-arm blend membranes: The use of PLLA-3-arm as a plasticizer. Polym. Test.. 2017;60:84-93.
- [Google Scholar]
- Involvement of inflammation and adverse vascular remodelling in the blood pressure raising effect of repeatedly heated palm oil in rats. Int. J. Vasc. Med.. 2012;2012 Article ID 404025
- [Google Scholar]
- Effect of roasting parameters on the physicochemical characteristics of high-molecular-weight Maillard reaction products isolated from cocoa beans of different Theobroma cacao L. groups. Eur. Food Res. Technol.. 2019;245(1):111-128.
- [Google Scholar]
- Dynamics of frying. In: Erickson M.D., ed. Deep frying: Chemistry, Nutrition, and Practical Applications. Champaign, IL: AOCS Press; 2006. p. :253-275.
- [Google Scholar]
- Effects of chemical surface coating (rain-ZTM) on the powder yield of spray-drying: A preliminary approach. Acta Horticulture. 2017;1152(23):165-174.
- [Google Scholar]
- Polylactide (PLA)-based nanocomposites. Prog. Polym. Sci.. 2013;38(10–11):1504-1542.
- [Google Scholar]
- Oil absorption, polymer and polar components formation during deep-fat frying of French fries in vegetable oils. Palm Oil Develop.. 2003;38:11-15.
- [Google Scholar]
- Rogers. K., 2020. Polymerization, Encyclopedia Britannica. Retrieved from https://www.britannica.com/science/polymerization. Accessed May 17, 2021.
- Suitability of contact angle measurement as an index of overall oil degradation and oil uptake during frying. Food Chem.. 2009;112(2):448-453.
- [Google Scholar]
- Integrated approach to deep fat frying: engineering, nutrition, health and consumer aspects. J. Food Eng.. 2003;56(2):143-152.
- [Google Scholar]
- Density, viscosity, and surface tension of five vegetable oils at elevated temperatures: Measurement and modeling. Int. J. Food Prop.. 2017;20(2):1965-1981.
- [Google Scholar]
- Suhaila, M., 2018. Afdhal Oil. https://wazan.upm.edu.my/kandungan/minyak_afdhal-35243?L=en. Accessed December 24th, 2021.
- Applications of life cycle assessment to NatureWorks™ polylactide (PLA) production. Polym. Degrad. Stab.. 2003;80(3):403-419.
- [Google Scholar]
- Characterization of heat and mass transfer during deep-fat frying and its effect on cassava chip quality. J. Food Eng.. 2002;53(2):161-176.
- [Google Scholar]
- Role of Chinese cooking emissions on ambient air quality and human health. Sci. Total Environ.. 2017;589:173-181.
- [Google Scholar]
- Oxidative stability of fermented meat products. Acta Scientiarum Polonorum Technologia Alimentaria. 2012;11(2):99-109.
- [Google Scholar]
- A chromametric method for the rapid assessment of deep-frying oil quality. J. Sci. Food Agric.. 2003;83(13):1293-1296.
- [Google Scholar]
- Mechanical and rheological properties of epoxidized soybean oil plasticized poly (lactic acid) J. Appl. Polym. Sci.. 2009;112(6):3185-3191.
- [Google Scholar]
- Temperature Dependence of Density and Dynamic Surface Tension of Groundnut Oil and Palm Oil. Int. J. Eng. Sci.. 2015;4(6):49-55.
- [Google Scholar]
- Rapid Microbial Detection Model System in UHT Milk Products Using Poly-L-Lactide (PLLA) Thin Film. Sains Malaysiana. 2018;47(11):2677-2683.
- [Google Scholar]
- Coordinate contribution of lipid oxidation and Maillard reaction to the nonenzymatic food browning. Crit. Rev. Food Sci. Nutr.. 2005;45(1):49-59.
- [Google Scholar]
- The Effect of Surface Heterogeneity on Wettability of Porous Three Dimensional (3-D) Scaffolds of Poly(3-Hydroxybutyric Acid) (PHB) and Poly(3-Hydroxybutyric-co-3-Hydroxyvaleric Acid) (PHBV) J. Teknologi (Sci. Eng.). 2015;75(1):305-312.
- [Google Scholar]







