Translate this page into:
Quality differentiation method of similar phytomedicines with high sugar content based on the sugar-marker: Taking Schisandrae Chinensis Fructus and Schisandrae Sphenantherae Fructus as an example
⁎Corresponding authors at: College of Pharmaceutical Engineering of Traditional Chinese Medicine, Tianjin University of Traditional Chinese Medicine, Tianjin 301617, China. suotc@tjutcm.edu.cn (Tongchuan Suo), lizheng@tjutcm.edu.cn (Zheng Li), pharmwch@126.com (Chunhua Wang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Sugar was used as quality marker to distinguish similar phytomedicines. Taking SCF and SSF as an example, a set of quality control methods was established. SCF and SSF from ten different areas were classified into three categories.
Abstract
Quality evaluation of phytomedicines is limited to small molecules as quality markers, even for herbs with high sugar contents. We established a high-resolution method for distinguishing similar medicinal materials using sugar as quality marker, taking Schisandrae Chinensis Fructus (SCF) and Schisandrae Sphenantherae Fructus (SSF) as an example. High performance liquid chromatography with an evaporative light-scattering detector (HPLC-ELSD), high performance size exclusion chromatography coupled with multi-angle laser light scattering detector and refractive index detector (HPSEC-MALLS-RID) and high performance liquid chromatography with photo-diode array (HPLC-PDA) after 1-phenyl-3-methyl-5-pyrazolone (PMP) pre-column derivatization were used respectively to determine monosaccharide contents, molecular weights and monosaccharide compositions of polysaccharides in this study. The differences of SCF and SSF from ten producing areas were compared by principal component analysis (PCA) and hierarchical cluster analysis (HCA). Results showed that contents of fructose and glucose were similar between SCF and SSF. Molecular weight (Mw) of SCF polysaccharides was ranging from 1.561 × 102 to 6.599 × 102 kDa, and that of SSF polysaccharides was ranging from 8.524 × 102 to 1.7416 × 103 kDa. Schisandra polysaccharides were mainly composed of mannose, galacturonic acid, glucose, galactose and arabinose. Based on PCA and HCA, SCF and SSF from ten different areas were classified into three categories. With great accuracy, sensitivity and stability, the methods established in this study had important reference value for quality evaluation, development and utilization of saccharide components in medicinal materials.
Keywords
Schisandrae Chinensis Fructus
Schisandrae Sphenantherae Fructus
Contents determination
Molecular weight
Monosaccharide composition
Quality evaluation
- SCF
-
Schisandrae Chinensis Fructus
- SSF
-
Schisandrae Sphenantherae Fructus
- Mw
-
molecular weight
- HPLC-ELSD
-
high performance liquid chromatography with an evaporative light-scattering detector
- HPSEC-MALLS-RID
-
high performance size exclusion chromatography coupled with multi-angle laser light scattering detector and refractive index detector
- HPLC-PDA
-
high performance liquid chromatography with photo-diode array
- PMP
-
1-phenyl-3-methyl-5-pyrazolone
- PCA
-
principal component analysis
- HCA
-
hierarchical cluster analysis
- Na2SO4
-
anhydrous sodium sulfate
- RSD
-
relative standard deviation
- TFA
-
trifluoroacetic acid
Abbreviations
1 Introduction
The selection of quality control markers of plant drugs has always been a difficulty due to their complex compositions. Quality control of phytomedicines is almost based on small molecules (Fan et al., 2012; Zhao et al., 2015; Cai et al., 2021; Kumar et al., 2021). However, for phytomedicines which have high saccharide contents, small molecules are also chosen for quality control markers (Tan et al., 2015; Peng et al., 2016; Mohammat et al., 2017; Luo et al., 2020; Yue et al., 2021). With the development of molecular biology, scientists have recognized the importance of saccharides. In recent years, researches have shown that polysaccharides have immunomodulatory, anti-tumor, anti-oxidation, anti-diabetic, hypoglycemic and other pharmacological activities (Zhao et al., 2014; Yu et al., 2018; Zhang et al., 2018; Chen et al., 2019; Du et al., 2019; Shan et al., 2019). More importantly, molecular weight and monosaccharide composition of polysaccharides can affect the biological activity of herbs (Sun et al., 2009; Lo et al., 2011; Zeng et al., 2015; Chen et al., 2017; Fang et al., 2020; Lv et al., 2021). Therefore, it might be not comprehensive and objective to evaluate the quality of herbs with high sugar contents by small molecular phytochemicals. A simple, efficient and reliable method for identifying and quantifying saccharides in herbs is needed. We have established quality control methods of phytomedicines with high sugar contents using sugar as quality marker (Cheng et al., 2021).
Schisandra (Wuweizi) is a kind of functional dietary supplement with very high medicinal and nutritional value, which has been used for more than 2000 years. It is divided into Schisandrae Sphenantherae Fructus (Nan Wuweizi, SSF) and Schisandrae Chinensis Fructus (Bei Wuweizi, SCF) in the Pharmacopoeia of People's Republic of China (2000), as shown in Fig. 1. SSF, the dried ripe fruit of Schisandra sphenanthera Rehd. et Wils, is mainly produced in the south of the Yellow River Basin in China (Zhou, 2002) and also called “Huazhongwuweizi” in Chinese. Similarly, SCF is the dried fruit of S. chinensis (Turcz.) Baill. and mainly grown in North China (Gao, 2003). Schisandra can be made into tea, which can relieve fatigue and improve human functions (Chi et al., 2016). In addition, as one of the most commonly used phytomedicines, it can astringe lung for relieving cough, arrest sweating and prevent diarrhea, etc. Main active ingredients in Schisandra include lignans (Onay et al., 2020), polysaccharides, volatile oil and so on (Bai et al., 2019; Liu et al., 2019; Yang et al., 2021).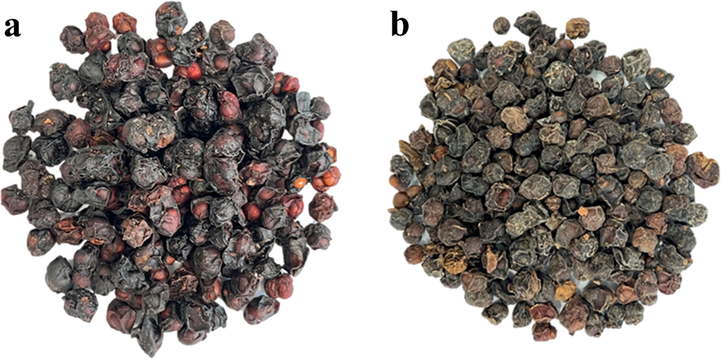
SCF (a) and SSF (b).
Nowadays, the research on polysaccharides of SCF and SSF mainly focuses on the content of total polysaccharides, separation, structure characterization and pharmacological effects of polysaccharides (Li et al., 2018). Qualitative and quantitative characterization of carbohydrates concerning SCF and SSF are comparatively few. At the same time, due to the different geographical distributions (Sun et al., 2010), SCF and SSF also have some differences in chemical compositions and pharmacological activities (Li et al., 2018; Wang et al., 2018).
Therefore, taking SCF and SSF as an example, we aimed to establish qualitative and quantitative methods to distinguish similar phytomedicines with high sugar contents using sugar as quality marker. High performance liquid chromatography with an evaporative light-scattering detector (HPLC-ELSD) was used to determine monosaccharide contents. Molecular weight (Mw) and distribution of polysaccharides were detected by high performance size exclusion chromatography coupled with multi-angle laser light scattering detector and refractive index detector (HPSEC-MALLS-RID). High performance liquid chromatography with photo-diode array (HPLC-PDA) after 1-phenyl-3-methyl-5-pyrazolone (PMP) pre-column derivatization was used to measure monosaccharide compositions. The differences of saccharide components in Schisandra from different regions were compared by principal component analysis (PCA) and hierarchical cluster analysis (HCA). The aim was to provide a basis for content, compositions of saccharides and quality evaluation of Schisandra. The results showed that the methods established in this paper were accurate, reliable and stable. It is indicated that sugar could be quality marker of phytomedicines with high sugar content.
2 Materials and methods
2.1 Materials and reagents
D-mannose, rhamnose, D-galactose, D-anhydrous glucose, D-fructose, sucrose, L-arabinose, D-xylose, D-glucuronic acid, D-galacturonic acid, maltose and 1-phenyl-3-methyl-5-pyrazolone reagent were all purchased from Yuanye Biological Technology Co., Ltd (Shanghai, China). Anhydrous sodium sulfate (Na2SO4) was purchased from Tianjin North Tianyi Chemical Reagent Factory (Tianjin, China). Biological preservative (Proclin 300) and ammonium acetate were purchased from Beijing Solaibo Technology Co., Ltd (Beijing, China). Chromatography-grade methanol and acetonitrile were purchased from Fisher (Hampton, NH, USA). All other reagents were of analytical grade. Pure water was produced by Milli-Q Ultrapure Water System (Q-POD, Millipore, Illkirch-Graffenstaden, France).
Five batches of SSF and five batches of SCF were purchased from Anhui Yishengyuan Chinese Medicine Decoction Pipe Technology Co., Ltd (Anhui, China). They were authenticated by Dr. Chunhua Wang from Tianjin University of Traditional Chinese Medicine. Voucher specimens have been deposited in the College of Pharmaceutical Engineering of Traditional Chinese Medicine, Tianjin University of Traditional Chinese Medicine (Tianjin, China). The details of samples are listed in Table 1.
Herbs
Regions
Batch number
SCF
Qingyuan, Liaoning
181201
Fushun, Liaoning
190301
Xiuyan, Liaoning
181201
Changbaishan, Jilin
181201
Benxi, Liaoning
181201
SSF
Shandong
190301
Hunan
190201
Shanxi
190301
Henan
190101
Hubei
181201
2.2 Methods
2.2.1 Determination of monosaccharide contents
2.2.1.1 Preparation of solutions
Preparation of sample solutions: Powder of Schisandra (2.0 g) was accurately weighed using electronic balance (AL204, Mettler Toledo Instruments Co., Ltd, Shanghai, China), added 20 mL pure water, and extracted at an ultrasonic cleaner (KQ2200DB, Kunshan Ultrasonic Instrument Co., Ltd, China) for 30 min. Then they were filtered to the sample solutions.
Preparation of standard solution: Fructose (40.08 mg) and glucose (44.52 mg) were accurately weighed using 1/100000 electronic analytical balance (AB135-S, Mettler Toledo Instruments Co., Ltd, Shanghai, China) and dissolved in 5.0 mL pure water to obtain the standard solution.
2.2.1.2 Chromatographic conditions
Monosaccharide contents were measured by HPLC (Waters Alliance e2695, Waters, Milford, MA, USA) on a YMC-Pack NH2/S-5 μm/12 nm column (250 × 4.6 mm, i.d.) at 30 °C. The mobile phase was acetonitrile–water (79:21) at a flow rate of 1.0 mL/min. Twenty microlitre of the samples was loaded respectively onto the column. The ELSD (2424, Waters, Milford, MA, USA) drift tube was 60 °C, neb heater was 60%, the carrier gas pressure was 35 psi, and the gain was set at 10.
2.2.1.3 Method validation
A HPLC-ELSD method was developed for determining monosaccharide contents of SCF and SSF. The analytical procedure was validated according to international guidelines for linearity, precision, repeatability, stability and recovery.
The logarithm of standard concentration was used as abscissa (X), and the logarithm of peak area was used as ordinate (Y) for linear regression.
The standard solution was measured six times continuously by HPLC-ELSD. The relative standard deviation (RSD) of peak area (after logarithm) was calculated for precision.
Sample solutions were prepared six copies in parallel, and the peak area was determined. Repeatability was evaluated by the RSD of peak area's logarithm.
Sample solutions were measured at different times (0, 2, 4, 8, 12 h) for stability. The RSD of peak area (after logarithm) was calculated.
Powder of SCF with known glucose and fructose contents was accurately weighed and added standard solution equivalent to the content of each component to validate the recovery.
2.2.1.4 Determination of fructose and glucose contents
Powder of five batches of SCF and five batches of SSF from various areas was weighed respectively, and sample solutions were prepared according to the method (Section 2.2.1.1). Samples were injected separately according to the chromatographic conditions (Section 2.2.1.2) to determine the peak area and calculate contents.
2.2.2 Determination of molecular weight
2.2.2.1 Extraction of polysaccharides
One gram of SCF and SSF powder from different areas was separately heated with 30 mL pure water to reflux for 3 h, then centrifuged (3750 r/min, 10 min). The supernatant was concentrated by rotary evaporation to 10 mL and mixed with four volumes of 95% ethanol. It was kept at 4 °C overnight. The precipitate was obtained by centrifugation (3750 r/min, 10 min), and heated to remove residual ethanol (60 °C). The dry precipitate was dissolved in 10 mL water (60 °C), then swirled and centrifuged (3750 r/min, 10 min). The supernatant was centrifuged (2220 r/min, 22 min) by ultrafiltration centrifuge tube (3 kDa intercalated molecular weight), and the solution was condensed using a rotary evaporator (FDU-2110, EYELA, Tokyo, Japan) to obtain polysaccharides (Deng et al., 2018; Niu et al., 2017).
2.2.2.2 Preparation of solutions
Preparation of sample solutions: Polysaccharides of SCF and SSF were weighed and added into the liquid to make a polysaccharide solution with a concentration of 2 mg/mL. By filtering through the 0.22 µm microporous membrane, the filtrate was used as test solution of samples.
Preparation of standard solution: Dextran (3 mg/mL, Mw: 40 kDa) was prepared as standard solution.
2.2.2.3 Determination of molecular weight
HPSEC-MALLS-RID is an efficient and powerful technique for analyzing molecular weight and distribution of natural polymers (Wu et al., 2015). Molecular weight of polysaccharides can be obtained through the Rayleigh Light Scattering Equation (Hu et al., 2013), which is an ideal method for determination of molecular weight. In this article, molecular weight and polydispersion index were determined by high performance size exclusion chromatography (LC-20AD, Shimadzu, Kyoto, Japan) coupled with multi-angle laser light scattering detector (DAWN8, WYATT Technologies, Inc., Santa Barbara, USA) and refractive index detector (RID-20A, Shimadzu, Kyoto, Japan) on a TSKgel GMPWXL column (7.8 mm × 30 cm, i.d.; 13 µm) at 35 °C. Samples were eluted with 0.7% Na2SO4 (containing 0.02% Proclin 300) at a flow rate of 0.6 mL/min. An injection volume of 100 µL was used. The MALLS was normalized by standard solution (Deng et al., 2018). And polydispersion index is equal to Mw divided by Mn.
2.2.3 Analysis of monosaccharide composition
2.2.3.1 Preparation of solutions
Preparation of sample solutions: Polysaccharides were hydrolyzed with 2.0 mol/L trifluoroacetic acid (TFA) at 100 °C for 6 h (mpolysaccharides: VTFA = 2:1). The hydrolysate, with excess TFA removed by repeated co-distillations with methanol, was dissolved with purified water (mpolysaccharides: VH2O = 2:1) (Li et al., 2014).
Two hundred microlitres of the above was taken to a tube, added 200 µL PMP-methanol solution (0.5 mol/L) and 200 µL sodium hydroxide solution (0.3 mol/L), respectively. After swirling, the hydrolysate was derivatized at 70 °C for 30 min, then added 200 µL hydrochloride solution (0.3 mol/L) after cooling to room temperature. Subsequently, the sample solution was added 1.0 mL chloroform, blended and centrifuged for 10 min at 8000 r/min to remove the excess PMP. The supernatant was repeated likewise three times with chloroform. HPLC was used to determine the final supernatant.
Preparation of standard solution: Substances of D-mannose (5.42 mg), D-galactose (10.36 mg), D-glucose (25.72 mg), L-arabinose (5.60 mg), and D-galacturonic acid (4.97 mg) were weighed precisely and dissolved into 10 mL pure water to prepare the mixed standard solution. The derivatization method of the mixed standard solution was the same as that of the polysaccharide hydrolysate.
2.2.3.2 Chromatographic conditions
Monosaccharide compositions were determined by HPLC (ACQUITY Arc, Waters, Milford, MA, USA) on a Kromasil 100–5 C18 column (5 µm, 4.6 mm × 250 mm) at 35 °C with a PDA detector (2998, Waters, Milford, MA, USA) at 250 nm. The mobile phase was made of acetonitrile (A) and 0.1 mol/L ammonium acetate solution (B) (A: B = 84:16, v/v) at a flow rate of 1.0 mL/min. An injection volume of 20 µL was used.
2.2.3.3 Method validation
Method validation was carried out to verify reliability and feasibility of the method which was used to determine monosaccharide composition of SCF and SSF polysaccharides.
The mixed standard solution was diluted to different multiples (2, 4, 8, 16, 32, 64, 128), then they were determined after derivatization. Linear regression was performed with standards concentration as abscissa (X) and chromatographic peak area as ordinate (Y).
Precision was assessed by measuring mixed standard solution after derivatization six times continuously. The RSD of peak area was calculated.
Repeatability of analysis of monosaccharide composition method was the same as that of determination of monosaccharide contents method. Only the sample solutions were replaced to that under Section 2.2.3.1.
SCF polysaccharides were made into sample solution according to Section 2.2.3.1. Then it was measured at different time (0, 2, 4, 6, 8, 10, 12 h) and analyzed the peak area. Stability was calculated by the RSD of peak area.
SSF polysaccharides were weighed and added standard solution equivalent to the content of each compound. Six copies were derived in parallel and measured the peak area to validate the recovery.
2.2.3.4 Monosaccharide composition of polysaccharides
Different sample solutions were prepared following the method (Section 2.2.3.1) individually. HPLC was used to measure monosaccharide composition of polysaccharides according to chromatographic conditions (Section 2.2.3.2).
2.2.4 Statistical analysis
The Origin 2019b software was used to perform PCA and HCA analysis. PCA analysis is used to study the similarity and difference between various samples. On the PCA graph, the closer the distribution points are, the more similar the samples are (Lv et al., 2018). The dimensionality reduction process was used to obtain two principal components. PCA analysis was performed on monosaccharide contents and its compositions of polysaccharides. Meanwhile, HCA was performed on the results of monosaccharide composition of polysaccharides, and hierarchical clustering was performed using the inter-group connection method and average Euclidean distance.
3 Results and discussion
3.1 Determination of monosaccharide contents
A quantitative analysis method for monosaccharide contents of SCF and SSF was established based on HPLC-ELSD, and its methodology was investigated. The precision of instrument, stability and repeatability of samples determination were all less than 0.2%. Meanwhile, the RSD of recovery were less than 5% (Table 2), indicating that this method was reliable and reproducible. It can be used to determine monosaccharide contents of SCF and SSF. The results were shown in Table 3. Note: “-” means that the measurement cannot be made accurately.
Herb
Monosaccharides
Regression Equation
R2
Linear Range (mg/mL)
Precision RSD%
Repeatability RSD%
Stability RSD%
Recovery RSD%
Schisandra
Fructose
Y = 1.23799X + 6.14241
0.9996
0.25050–8.016
0.05
0.14
0.09
1.41
Glucose
Y = 1.26135X + 5.95357
0.9992
0.27825–8.904
0.04
0.16
0.18
3.76
Regions
Fructose (mg/g)
Glucose (mg/g)
Shandong
69.02
59.84
Hunan
43.07
35.87
Shanxi
38.22
–
Henan
52.94
–
Hubei
34.92
29.42
Qingyuan, Liaoning
33.41
27.74
Fushun, Liaoning
30.04
28.18
Xiuyan, Liaoning
31.40
29.52
Changbaishan, Jilin
33.24
31.27
Benxi, Liaoning
44.23
44.04
The contents of glucose and fructose of SCF and SSF were analyzed by PCA. Principal components 1 and 2 were used to obtain PCA scatter plots. The cumulative variance contribution rate reached 100%, as shown in Fig. 2. It can be seen visually that monosaccharide contents among different regions were distinct. The contents of fructose and glucose of Schisandra were similar, but there were differences among different origins of SSF.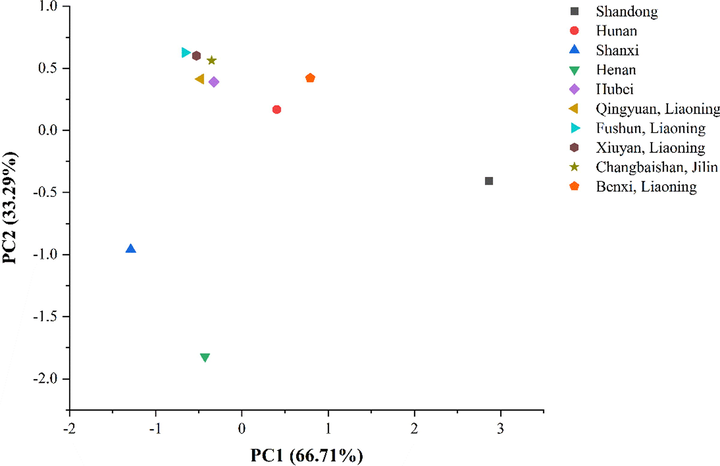
PCA diagram of monosaccharide contents.
3.2 Determination of molecular weight
HPSEC-MALLS-RID was used to determine molecular weight and distribution of polysaccharides in SCF and SSF from different regions. The polysaccharide samples of SCF and SSF were eluted a variety of different molecules between 16.25 and 20.00 min. The Mw of peak 2 and peak 3 could not be accurately determined (Fig. 3). Because the molecular weight was large and its distribution of polysaccharides was wide. Meanwhile, the separation effect of gel chromatography column was poor. The Mw and polydispersion index of polysaccharide fractions (peak 1) were shown in Table 4. The results showed that the range of Mw for polysaccharide fractions (peak 1) of SCF was from 1.561 × 102 to 6.599 × 102 kDa, and that for polysaccharide fractions (peak 1) of SSF was from 8.524 × 102 to 1.7416 × 103 kDa. The Mw of SSF polysaccharides were higher than that of SCF. And polydispersion indexes of SSF polysaccharides were more similar and higher than that of SCF polysaccharides. This suggested that the distribution of molecular weight of SSF polysaccharides was wider than that of SCF polysaccharides. Note: “-” means that the measurement cannot be made accurately.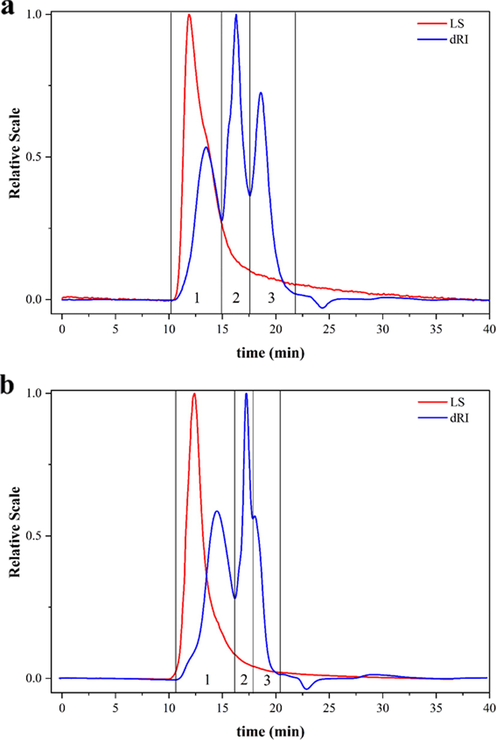
HPSEC-MALLS-RID chromatograms of SCF (a) and SSF (b) polysaccharides.
Regions
Peak 1 Mw (kDa) and Error
Mw/Mn and Error
Qingyuan, Liaoning
352.3 (±0.9%)
2.624 (±1.236%)
Benxi, Liaoning
659.9 (±1.8%)
1.838 (±2.878%)
Changbaishan, Jilin
156.1 (±1.4%)
2.342 (±2.466%)
Xiuyan, Liaoning
238.7 (±4.2%)
–
Fushun, Liaoning
304.1 (±4.9%)
–
Shanxi
1549.4 (±1.1%)
3.652 (±1.432%)
Hunan
852.4 (±1.0%)
3.191 (±1.313%)
Henan
1163.2 (±0.8%)
3.170 (±0.993%)
Hubei
1064.1 (±0.8%)
3.085 (±1.025%)
Shandong
1741.6 (±1.0%)
3.560 (±1.300%)
3.3 Analysis of monosaccharide composition
Pre-column derivatization combined with HPLC method was used to determine monosaccharide composition of SCF and SSF polysaccharides, and the methodological investigation was carried out. The RSDs of instrument precision were from 0.14% to 3.72%. That of samples repeatability and stability were 1.38%-3.26% and 0.38%-4.69%, all less than 5% (Table 5). The results showed that this method was reliable and repeatable. The method could be used to determine monosaccharide compositions. The monosaccharides were identified by the relative retention time. HPLC-PDA chromatograms of PMP derivatization were shown in Fig. 4. The results of monosaccharide composition were shown in Table 6. It showed that polysaccharides of SCF and SSF from different origins were mainly composed of mannose, galacturonic acid, glucose, galactose and arabinose. However, monosaccharide composition of polysaccharides from various regions was significantly different.
Herb
Saccharides
Regression Equation (n = 2)
R2
Linear Range (mg/mL)
Precision
RSD%
Repeatability
RSD%
Stability
RSD%
Recovery
RSD%
Schisan-dra
mannose
Y = 3.92936 × 107X −161305.99342
0.9998
0.004234–0.542
0.18
1.57
0.42
2.46
galacturonic acid
Y = 2.65277 × 107X
−7395.440740.9995
0.003883–0.497
3.72
3.26
4.69
13.08
glucose
Y = 2.57369 × 107X −164114.79357
0.9999
0.020094–2.572
0.21
1.50
0.38
3.97
galactose
Y = 4.25721 × 107X −144499.49303
0.9999
0.008094–1.036
0.15
1.38
1.46
3.82
arabinose
Y = 4.98127 × 107X −147001.78776
0.9999
0.004375–0.56
0.14
1.52
1.58
3.84
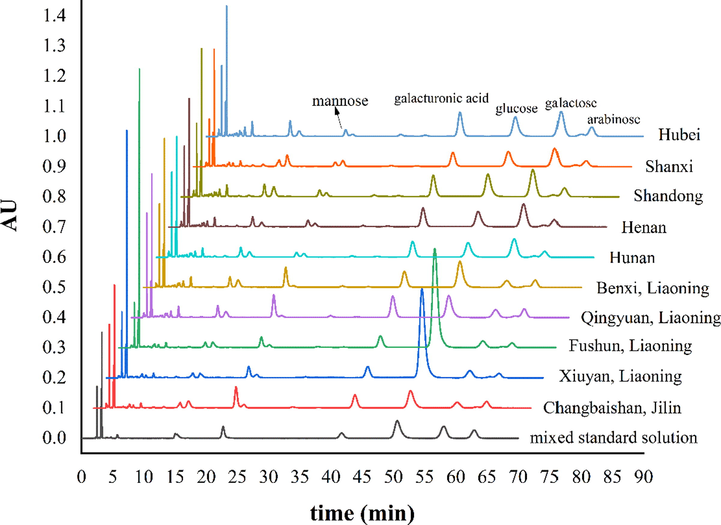
HPLC-PDA chromatograms of PMP derivatization of mixed standard solution and polysaccharide hydrolysate of SCF and SSF.
Regions
Mannose (mg/mL)
Galacturonic Acid (mg/mL)
Glucose (mg/mL)
Galactose (mg/mL)
Arabinose (mg/mL)
Hubei
0.0196
0.1421
0.1566
0.1258
0.0387
Shandong
0.0199
0.1253
0.1824
0.1373
0.0383
Henan
0.0199
0.1109
0.1288
0.1175
0.0309
Hunan
0.0155
0.0934
0.1238
0.0963
0.0253
Shanxi
0.0150
0.0849
0.1293
0.0947
0.0256
Benxi, Liaoning
0.0562
0.0967
0.2249
0.0362
0.0288
Qingyuan, Liaoning
0.0595
0.1301
0.1860
0.0415
0.0335
Fushun, Liaoning
0.0307
0.0685
0.8031
0.0361
0.0196
Xiuyan, Liaoning
0.0326
0.0649
0.7212
0.0390
0.0184
Changbaishan, Jilin
0.0572
0.0785
0.1531
0.0319
0.0267
3.3.1 The results of PCA
Using Origin 2019b software, PCA was performed on the monosaccharide composition of SCF and SSF polysaccharides after hydrolysis. The cumulative variance contribution rate of two principal components reached 89.97%. PCA scatter plots were obtained for principal components 1 and 2, as shown in Fig. 5. The samples of SCF from Xiuyan, Fushun (Liaoning) belonged to the first category. The samples from Benxi, Qingyuan (Liaoning) and Changbaishan (Jilin) were classified into the same category. The samples of SSF from Hubei, Shandong, Henan, Hunan and Shanxi provinces belonged to one category.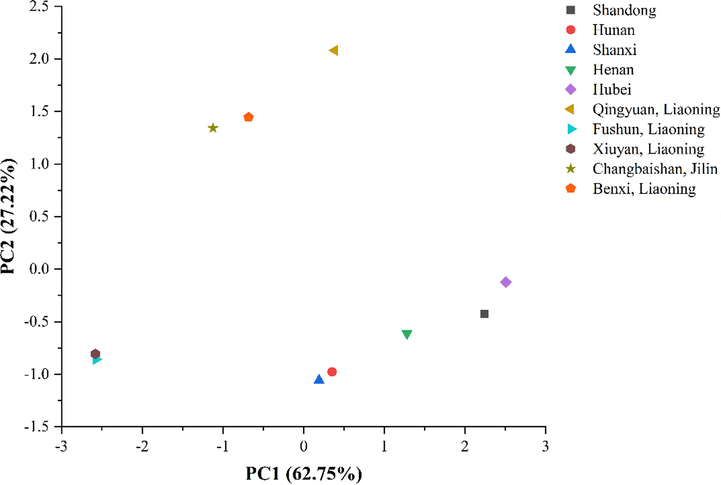
PCA diagram of SCF and SSF polysaccharides.
3.3.2 The results of HCA
The monosaccharide contents of mannose, galacturonic acid, glucose, galactose and arabinose after hydrolysis were used as clustering variables, as shown in Fig. 6. The results of HCA showed that Xiuyan, Fushun (Liaoning) belonged to one type. Benxi, Qingyuan (Liaoning) and Changbaishan (Jilin) were another type. The monosaccharide composition from Xiuyan, Fushun (Liaoning) was quite different from that of other origins. The samples of SSF from Hubei, Shandong, Henan, Hunan, and Shanxi were the same category, and monosaccharide composition of their polysaccharides was similar. The result of HCA analysis was the same as that of PCA.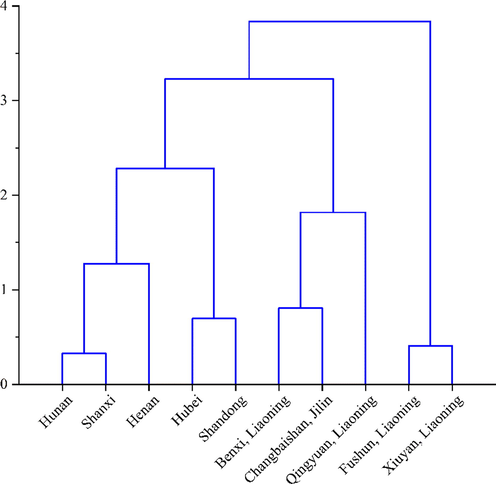
HCA results of SCF and SSF polysaccharides.
Schisandra polysaccharides have important application value in food, medicine or other fields, and their biological activities are often affected by contents and compositions. Therefore, saccharide contents and monosaccharide compositions of polysaccharides are one of the important indexes to measure the quality. However, saccharides are water-soluble, and chemical structures of polysaccharides are complex and difficult to determine, which leads to certain difficulties in the qualitative and quantitative analyses. In this study, we established a quantitative analysis method for monosaccharide contents of SCF and SSF based on HPLC-ELSD. HPSEC-MALLS-RID was used to determine the molecular weight of SCF and SSF polysaccharides. The monosaccharide compositions were detected by HPLC-PDA with PMP pre-column derivatization. PCA and HCA were used to compare the differences between SCF and SSF from various areas.
4 Conclusion
This study showed that SCF and SSF from ten producing areas had differences in saccharide contents and monosaccharide compositions of polysaccharides. The herbs could be divided into three categories according to methods we established. This suggests that sugar could be quality marker to evaluate the quality of phytomedicines with high sugar content. The results were similar for PCA and HCA. The methods developed in this paper were simple, accurate and stable. It provides fast and reliable methods for the identification and quality control of Schisandra. Furthermore, more quality control methods using sugar as marker are needed for distinguishing similar phytomedicines with high saccharide contents.
Acknowledgements
The authors would like to acknowledge those who contributed to this study. All authors are grateful for the projects which provide financial support.
Funding
This study was supported by the National Key R&D Program of China, Synthetic Biology Research (No. 2019YFA0905300), the National Natural Science Foundation of China (No. 82074276) and Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (No. ZYYCXTD-D-202002).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Research progress on the chemical constituents and pharmacological effects of Schisandra chinensis. Chin. Tradit. Patent Med.. 2019;41(09):2177-2183.
- [CrossRef] [Google Scholar]
- Quality evaluation of Lonicerae Japonicae Flos and Lonicerae Flos based on simultaneous determination of multiple bioactive constituents combined with multivariate statistical analysis. Phytochem. Anal.. 2021;32(2):129-140.
- [CrossRef] [Google Scholar]
- Physicochemical properties, antioxidant activity and immunological effects in vitro of polysaccharides from Schisandra sphenanthera and Schisandra chinensis. Int. J. Biol. Macromol.. 2019;131:744-751.
- [CrossRef] [Google Scholar]
- Structural elucidation of three antioxidative polysaccharides from Tricholoma lobayense. Carbohydr. Polym.. 2017;157:484-492.
- [CrossRef] [Google Scholar]
- Quality distinguish of Red Ginseng from different origins by HPLC–ELSD/PDA combined with HPSEC–MALLS–RID, focus on the sugar-markers. Separations. 2021;8(11):198.
- [CrossRef] [Google Scholar]
- Metabolic mechanism of a polysaccharide from Schisandra chinensis to relieve chronic fatigue syndrome. Int. J. Biol. Macromol.. 2016;93(Pt A):322-332.
- [CrossRef] [Google Scholar]
- Qualitation and quantification of water soluble non-starch polysaccharides from Pseudostellaria heterophylla in China using saccharide mapping and multiple chromatographic methods. Carbohydr. Polym.. 2018;199:619-627.
- [CrossRef] [Google Scholar]
- Hypoglycemic effect of acidic polysaccharide from Schisandra chinensis on T2D rats induced by high-fat diet combined with STZ. Biol. Pharm. Bull.. 2019;42(8):1275-1281.
- [CrossRef] [Google Scholar]
- Quality evaluation and species differentiation of Rhizoma coptidis by using proton nuclear magnetic resonance spectroscopy. Anal. Chim. Acta.. 2012;747:76-83.
- [CrossRef] [Google Scholar]
- Extraction, structure and bioactivities of the polysaccharides from Ginkgo biloba: A review. Int. J. Biol. Macromol.. 2020;162:1897-1905.
- [CrossRef] [Google Scholar]
- Comparative studies on Schisandra chinensis and Schisandra sphenanthera. Shanghai, China: Fudan University; 2003. PhD
- Research on the methods for relative molecular weight determination of polysaccharides from Ophiopogon japonicus. Chin. Tradit. Patent Med.. 2013;35(12):2684-2689.
- [Google Scholar]
- Andrographis paniculata (Burm.f.) Nees: Traditional uses, phytochemistry, pharmacological properties and quality control/quality assurance. J. Ethnopharmacol.. 2021;275:114054
- [CrossRef] [Google Scholar]
- Li, W., Xia, Q., Sun, C.R., et al, 2014. Analysis of monosaccharide compositions of various kinds of Liquorice polysaccharide by HPLC pre-column derivatization. J. Liaoning Univ. Tradit. Chin. Med. 16 (01), 56-58. http://doi.org/10.13194/j.issn.1673-842x.2014.01.020
- A review of polysaccharides from Schisandra chinensis and Schisandra sphenanthera: properties, functions and applications. Carbohydr. Polym.. 2018;184:178-190.
- [CrossRef] [Google Scholar]
- Liu, J., Xu, J., Guo, J.T., 2019. Review of active constituents and pharmacological activities of Schisandrae chinensis fructus. Chin. J. Exp. Med. Formul. 25 (11), 206-215. http://doi.org/10.13422/j.cnki.syfjx.20190911
- Correlation evaluation of antioxidant properties on the monosaccharide components and glycosyl linkages of polysaccharide with different measuring methods. Carbohydr. Polym.. 2011;86(1):320-327.
- [CrossRef] [Google Scholar]
- Chemical comparison of Ophiopogonis radix and Liriopes radix based on quantitative analysis of multiple components by HPLC coupled with electrospray ionization tandem triple quadrupole mass spectrometry. J. AOAC Int.. 2020;103(4):1148-1159.
- [CrossRef] [Google Scholar]
- Determination and PCA analysis of ten chemical components in Chysanthemi flos by UPLC-UV. China Pharm.. 2018;21(08):1374-1378.
- [Google Scholar]
- Structural characterization, α-amylase and α-glucosidase inhibitory activities of polysaccharides from wheat bran. Food Chem.. 2021;341(Pt 1):128218
- [CrossRef] [Google Scholar]
- Rapid quantification and quantitation of alkaloids in Xinjiang Fritillaria by ultra performance liquid chromatography-quadrupole time-of-flight mass spectrometry. Molecules. 2017;22(5):719.
- [CrossRef] [Google Scholar]
- In vitro antioxidant activities and anti-diabetic effect of a polysaccharide from Schisandra sphenanthera in rats with type 2 diabetes. Int. J. Biol. Macromol.. 2017;94(Pt A):154-160.
- [CrossRef] [Google Scholar]
- Rapid analysis of nine lignans in Schisandra chinensis by supercritical fluid chromatography using diode array and mass spectrometric detection. J. Pharm. Biomed. Anal.. 2020;185:113254
- [CrossRef] [Google Scholar]
- Determination of the chemical constituents of the different processed products of Anemarrhena asphodeloides Rhizomes by high-performance liquid chromatography quadrupole time-of-flight mass spectrometry. Biomed. Chromatogr.. 2016;30(4):508-519.
- [CrossRef] [Google Scholar]
- Protective effect of Schisandra chinensis polysaccharides against the immunological liver injury in mice based on Nrf2/ARE and TLR4/NF-κB signaling pathway. J. Med. Food. 2019;22(9):885-895.
- [CrossRef] [Google Scholar]
- Preparation of different molecular weight polysaccharides from Porphyridium cruentum and their antioxidant activities. Int. J. Biol. Macromol.. 2009;45(1):42-47.
- [CrossRef] [Google Scholar]
- Population genetic differentiation of Schisandra chinensis and Schisandra sphenanthera as revealed by ISSR analysis. Biochem. Syst. Ecol.. 2010;38(3):257-263.
- [CrossRef] [Google Scholar]
- Distinguishing Radix Angelica sinensis from different regions by HS-SFME/GC-MS. Food Chem.. 2015;186:200-206.
- [CrossRef] [Google Scholar]
- Characteristics and antioxidant activity of lignans in Schisandra chinensis and Schisandra sphenanthera from different locations. Chem. Biodivers.. 2018;15(6):e1800030
- [CrossRef] [Google Scholar]
- An evaluation system for characterization of polysaccharides from the fruiting body of Hericium erinaceus and identification of its commercial product. Carbohydr. Polym.. 2015;124:201-207.
- [CrossRef] [Google Scholar]
- Yang, S.Y., Yuan, C.H., 2021. Schisandra chinensis: A comprehensive review on its phytochemicals and biological activities. Arabian J. Chem. 14(9). 10.1016/j.arabjc.2021.103310
- Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym.. 2018;183:91-101.
- [CrossRef] [Google Scholar]
- Application of identification and evaluation techniques for ethnobotanical medicinal plant of genus Panax: A review. Crit. Rev. Anal. Chem.. 2021;51(4):373-398.
- [CrossRef] [Google Scholar]
- Molecular structural characteristics of polysaccharide fractions from Canarium album (Lour.) Raeusch and their antioxidant activities. J. Food Sci.. 2015;80(11):H2585-H2596.
- [CrossRef] [Google Scholar]
- Schisandra chinensis fructus and its active ingredients as promising resources for the treatment of neurological diseases. Int. J. Mol. Sci.. 2018;19(7):1970.
- [CrossRef] [Google Scholar]
- Schisandra polysaccharide evokes immunomodulatory activity through TLR 4-mediated activation of macrophages. Int. J. Biol. Macromol.. 2014;65:33-40.
- [CrossRef] [Google Scholar]
- Qualitative and quantitative analysis of major triterpenoids in Alismatis Rhizoma by high performance liquid chromatography/diode-array detector/quadrupole-time-of-flight mass spectrometry and ultra-performance liquid chromatography/triple quadrupole mass spectrometry. Molecules. 2015;20(8):13958-13981.
- [CrossRef] [Google Scholar]
- Chemotaxonomic study on Schisandraceae and resources utilization of Chinese medicinal plants from Schisandra michx. Beijing, China: Peking Union Medical College; 2002. PhD







