Translate this page into:
Circular RNA circRHOT1 contributes to platelet-derived growth factor BB-stimulated proliferation and migration of airway smooth muscle cells by targeting Tip60/TLR4
⁎Corresponding author. qianzhang@njmu.edu.cn (Qian Zhang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background
Asthma serves as a chronic inflammatory respiratory diseases disorder. It mainly occurs airway inflammation and remodeling. Circular RNA (circRNA) is mainly the control of cancer disease and asthma. In specific work, we were interested in the function of circRHOT1 in the advances in asthma.
Methods
Effects of platelet-derived growth factor BB (PDGF-BB) on airway smooth muscle cells (ASMCs) and remolded simulation cells were assessed. The expressions of circRHOT1, TLR4, and Tip60 were detected by qRT-PCR and the increase and decrease of cell number were analyzed by cell count-8 (CCK-8), Transwell or flow cytometry. At the same time, the levels of PCNA, IGF1R protein were determined by western blot approach. The correlation of circRHOT1, TLR4, and Tip60 was analyzed by qRT-PCR, western blotting, ChIP, and RNA pulldown.
Results
CircRHOT1 was significantly elevated in the serum collected from asthmatic patients. PDGF-BB had a positive effect on ASMCs with enhanced levels of circRHOT1, TLR4, and Tip60. Depletion of circRHOT1 avoided wider impact and migration but induced cell apoptosis. CircRHOT1 contributed PDGF-BB had a direct impact on the proliferation and migration of ASMCs by regulating TLR4. Mechanically, circRHOT1 could cooperate with acetyltransferase Tip60 in ASMCs. CircRHOT1 silencing was easy to have an adverse effect on the enrichment of Tip60 on TLR4 promoter in ASMCs. The depletion of circRHOT1 or Tip60 reduced h3k27ac and RNA polymerase II on the TLR4 promoter. Consistently, the inhibition of circRHOT1 or Tip60 reduced TLR4 expression in ASMCs. The deletion of circRHOT1 could reduce the expression level of TLR4, but the overexpression of Tip60 was easy to have a positive effect on the down-regulation of ASMCs. In addition, Tip60 could inhibit ASMCs proliferation and migration caused by PDGF-BB stimulation. CircRHOT1 could use PDGF-BB to stimulate ASMCs proliferation and migration when Tip60 regulation was implemented.
Conclusions
We can think that based on the related effects of Tip60/TLR4, circular RNA circRHOT1 with platelet-derived growth factor BB can stimulate airway smooth muscle cells to proliferate or migrate, and circRHOT1 is an important target for the treatment of asthma.
Keywords
Asthma
circRHOT1
Tip60
TLR4
Proliferation
Migration
1 Background
Asthma is a chronic airway inflammatory disease that involves multiple cells and tissue components (Lemanske and Busse, 1997; Mims, 2015; Sockrider and Fussner, 2020). Epidemiological analysis indicates that over 5% of adults and 10% of children suffer from asthma, and these numbers increase globally due to the increased pollution and deterioration of the environment (Islam, 2015). The characterization of asthma mainly includes airway hyperresponsiveness, airway inflammation and airway remodeling (Bousquet et al., 2010; Poon and Hamid, 2016). It is worth to be noted that airway inflammation is featured by the infiltration of immune cells, such as Th2 cells, mast cells, neutrophils, along with abnormal secretion including tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and IL-6, etc (Castillo et al., 2017). Studies also suggest that airway smooth muscle cells (ASMCs) has key impact on asthma (Bhalla et al., 2018; Liang et al., 2017; Mouraux et al., 2018). As the major, ASMCs are closely involved in inflammatory responses and airway remodeling during asthma, manifested by release of inflammatory platelet-derived growth factor (PDGF) or other factors (Castillo et al., 2017; Hartley et al., 2014). Therefore, PDGF-BB, the important member of PDGF, has been widely applied in cell model of asthma, owing to their ability to stimulate proliferation, migration and tracheal remodeling of ASMCs (Halayko et al., 2008).
Circular RNAs (circRNAs) are defined as noncoding RNAs with a special covalently closed looped RNA structure (Salzman et al., 2012). Studies have suggested the abnormal expression and participation of circRNAs during the progression of various diseases, including cancers and cardiovascular diseases (Vo et al., 2019). CircRNAs could function as nucleators or component of protein complex to modulate gene expression (Vo et al., 2019). CircRHOT1 is a recently spotted circular RNA that is upregulated in several cancers, mainly in hepatocellular carcinoma and pancreatic cancer. (Zhang et al., 2021; Ling et al., 2020), and participates in the regulation of tumor cell proliferation and death (Ling et al., 2020; Qu et al., 2019). Yet, the role of circRHOT1 in asthma is not clear. Lysine acetyltransferases (KATs) are able to modulate the acetylation of proteins, and reported to harbor therapeutic potential for diseases (Kwan et al., 2020; Dekker and Haisma, 2009). Besides, studies have proved the recruitment of KATs by circRNAs during gene regulation. Among the KAT family members, KAT5 (also known as Tip60) play prominent role in regulating gene expression, and silencing of Tip60 could be lethal (Gorrini et al., 2007).
Toll-like receptors (TLRs) are a famous participant in the host immune response model to distinguish the situation of the receptor (Shetab Boushehri and Lamprecht, 2018). Accumulating evidences have implied the correlation between TLRs and disease development. Among the TLRs family, the TLR4 signaling is widely reported to be associated with cancer progression and inflammatory response (Chen et al., 2018; Lau et al., 2020). Moreover, inhibition of TLR4 could alleviate asthma in mouse model (Hwang et al., 2019). In this work, we tried to decipher the role of circRHOT1 during asthma, demonstrated that circRHOT1 induced inflammation and cell-barrier injury to promote development of asthma through recruiting Tip60 and epigenetically regulating TLR4 expression. Our work would get new therapeutic targets of asthma.
2 Materials and methods
2.1 Clinical specimen
From May 2020 to June 2021, blood samples were collected from patients with asthma (n = 50) treated in Changzhou Second People’s Hospital Affiliated to Nanjing Medical University. Meanwhile, the control samples were collected from 50 healthy donors. All operations were carried out with the permission of the ethics committee of Changzhou Second People’s Hospital Affiliated to Nanjing Medical University, and the relevant participants gave informed consent.
2.2 Isolation and treatment of ASMCs
The ASMCs were isolated from Sprague Dawley rats (female, 80–100 g) following the preciously reported protocols (Salter et al., 2017). The trachea of anesthetized rats was separated and the epithelial fibrous tissue was removed. In addition, the remaining tissue was digested with elastase IV and collagenase I at 37 °C for half an hour. The ASMCs were centrifuged and cultured on Dulbecco's modified Eagle medium (DMEM) (clone, USA) combined with 10% fetal bovine serum (FBS) (clone, USA). In order to obtain the relevant asthma cell model, ASMCs needed to be treated with 25 ng/ml PDGF-BB for 24 h at 37 °C.
2.3 Cell transfection
The small interfering RNAs that target circRHOT1, Tip60, or TRL4 were synthesized by RiboBio (China). The overexpressing vectors of circRHOT1, Tip60, or TRL4 were obtained by inserting the CDS regions to pcDNA plasmids. Lipofectamine 2000 (Invitrogen) was introduced for cell transfection in accordance with the manufacturer introduced it, which mainly inoculated cells into 6-well plates overnight, then hatched with a mixture of 0.5 μg oligonucleotides and 5 μl Lipofectamine 2000 and for 48 h.
2.4 Cell viability and apoptosis
According to relevant instructions, cell counting kit-8 (CCK-8, beyotime) produced in China was used to evaluate the degree of cell viability, and ASMCs was placed in 96 well plate (5000 cells/well) after determined oligonucleotide transfection, and PDGF-BB was used for 48 h of treatment. Then CCK-8 reagent (10 μl) was added to one hour-incubation. Absorbance values were obtained by testing at 450 nm by a spectrophotometric detector (Thermo, USA). The apoptosis of ASMCs was assessed by annexin V/PI (Thermo, USA) following to manufacturer’s description.
2.5 Transwell assay
Transwell assay was adopted for evaluation of cell migration. ASMCs prohibition of freedom DMEM (200 μl) and placed in top chambers (Costar, USA), and the bottom chambers were filled with DMEM medium. After incubation for 48 h, the top chambers were washed with PBS and dyed by crystal violet for about twenty minutes. The migrated cells were photographed by a microscope.
2.6 Western blotting assay
Ripa lysis buffer (sigma) was mainly obtained by extracting total protein from cells, and the same amount of protein was separated by SDS-PAGE (40 μg) It was imprinted on the relevant PVDF membrane and incubated with PCNA primary antibody after blocking with 5% skimmed milk, MMP-9, TLR4, and GAPDH overnight at 4 °C. Next day, the blots were hatched with corresponding secondary antibodies and an ECL solution (Millipore, USA) for visualization. All antibodies were purchased from Abcam (USA).
2.7 Real-time PCR assay
Total RNA from ASMCs was extracted using Trizol reagent (Beyotime, China), and the first strand cDNA synthesis kit was implemented to effectively promote its recording as cDNA (Thermo). Quantification of circRHOT1, Tip60, and TRL4 was performed by using a SYBR Green SuperMix kit (Thermo), and was normalized to internal control β‐actin.
2.8 Chromatin immunoprecipitation (ChIP)
ASMCs were fixed with 1% formaldehyde for ten minutes, then lysed and sonicated to fragments around 200 to 500 bp. Specifically, anti Tip60 antibody was used for cell lysate floating incubation (Abcam) overnight at 4 °C, and subsequently conjugated with Cell signaling technology for 4 h. Then, the precipitants were eluted and quantified by qRT-PCR.
2.9 RNA pulldown
Cells were collected and lysed by using a specific lysate buffer (Beyotime) for 10 min. The cell lysate and anti-tip60 antibody (Abcam) were incubated overnight at 4 °C, and m-280 streptavidin magnetic beads (sigma) were incubated at 4 °C for 6 h. The pull-down products were detected by qRT-PCR evaluation of circRHOT1.
2.10 Statistical analysis
The data were expressed as mean ± standard deviation (SD) of three repetitions. SPSS 19.0 software was adopted for data analysis. Difference between all groups was evaluated by two-tailed unpaired when students were analyzed by t-test or one-way ANOVA. P < 0.05 was the main indication of statistical significance.
3 Results
3.1 Patients with asthma and ASMCs stimulated by PDGF-BB have increased levels of circRHOT1
By evaluating whether circRHOT1 expression was related to asthma and analyzing clinical samples, it could be concluded that circRHOT1 expression was higher in the serum of asthmatics (Fig. 1A). And enhanced expression of circRHOT1, TLR4, and Tip60 was occured in PDGF-BB stimulated ASMCs when compared with the non stimulated group (Fig. 1B-D), suggesting that key role of circRHOT1, TLR4, and Tip60 in the progression of asthma.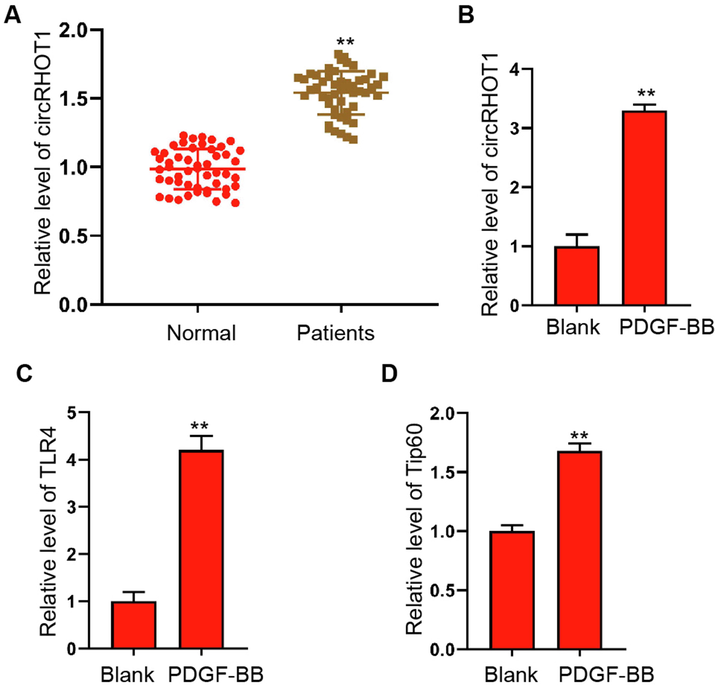
The expression of circRHOT1 is upregulated in the serum of patients with asthma and PDGF-BB-stimulated ASMCs. (A) QRT-PCR quantification of circRHOT1 level in the serum collected from patients with asthma (n = 50) and healthy donors (n = 50). (B-D) ASMCs were stimulated with PDGF-BB (25 ng/ml) for 24 h, the levels of circRHOT1, TLR4, and Tip60 were detected by qRT-PCR assay. **p < 0.01.
3.2 CircRHOT1 knockdown suppresses PDGF-BB stimulated ASMCs proliferation and migration
To evaluate specific function of circRHOT1 in ASMCs, we showed circRHOT1 knockdown practice research. Transfection with more shRNAs against circRHOT1 was able to effectively reduce expression of circRHOT1 in ASMCs (Fig. 2A), and we selected circRHOT1 shRNA-2 for the following experiments. The depletion of circRHOT1 remarkably repressed PDGF-BB stimulated ASMCs proliferation and migration (Fig. 2B-2D). Western blotting analysis further revealed the levels of PCNA and MMP-9, signs of diffusion and migration, were lower, after depletion of circRHOT1, relative to the PDGF-BB stimulated ASMCs (Fig. 2E). The knockdown of circRHOT1 promoted apoptosis of ASMCs under PDGF-BB stimulation (Fig. 2F). These results indicated that circRHOT1 could promote the proliferation and migration of PDGF-BB stimulated ASMCs.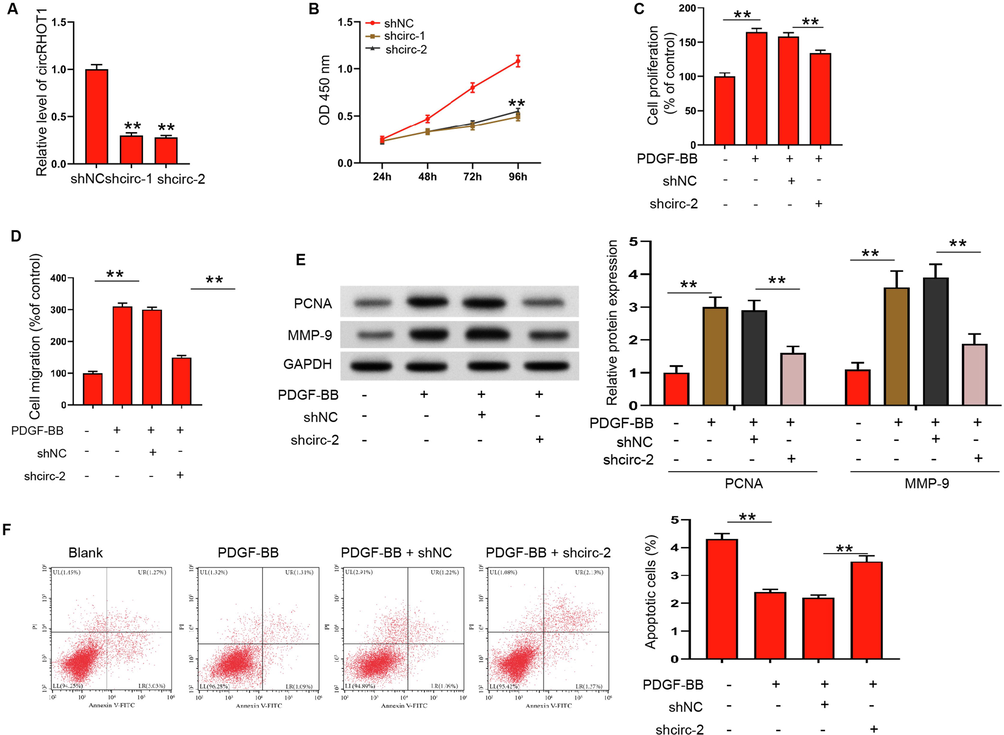
CircRHOT1 knockdown suppresses PDGF-BB stimulated proliferation and migration of ASMCs. (A) Expressions of circRHOT1 in ASMCs transfected with circRHOT1 siRNA-1, circRHOT1 siRNA-2, or negative control (shNC) were detected by qRT-PCR experiment. (B-F) ASMCs were treated with PDGF-BB and depleted of circRHOT1. (B and C) Cell proliferation was detected by CCK-8 assay. (D) Cell migration was detected by Transwell assay. (E) The expression of PCNA and MMP-9 were measured by western blotting. (F) Apoptosis of ASMCs was measured by flow cytometry. **p < 0.01.
3.3 CircRHOT1 contributes to PDGF-BB stimulated ASMCs proliferation and migration by regulating TLR4
We then analyzed the correlation of circRHOT1 with TLR4 in the modulation of PDGF-BB can stimulate ASMC proliferation and migration. Effectiveness of TLR4 overexpression was validated in ASMCs (Fig. 3A). The cell viability of PDGF-BB stimulated ASMCs repressed by circRHOT1 depletion was rescued by the overexpression of TLR4 (Fig. 3B). Meanwhile, the overexpression of TLR4 promoted circRHOT1 knockdown-inhibited ASMCs migration (Fig. 3C). In addition, the circRHOT1 depletion-induced apoptosis of PDGF-BB stimulated ASMCs was blocked by the overexpression of TLR4 (Fig. 3D), suggesting that circRHOT1 contributed PDGF-BB easy to make ASMC proliferate and migrate by regulating TLR4.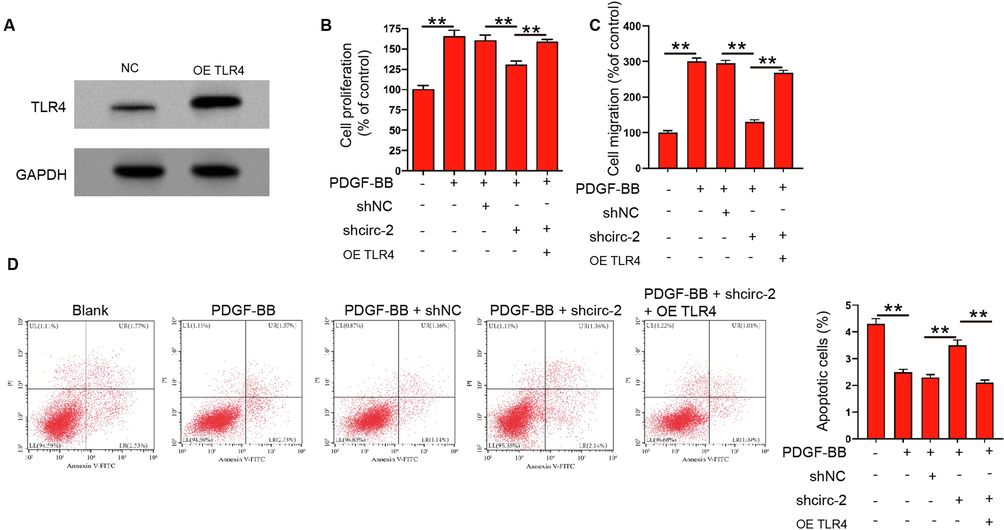
CircRHOT1 contributes to PDGF-BB -stimulated proliferation and migration of ASMCs by regulating TLR4. (A) The expression of TLR4 was measured by western blotting in ASMCs treated with TLR4 overexpressing plasmid. (B-D) ASMCs were treated with PDGF-BB, or co-treated with circRHOT1 siRNA and TLR4 overexpressing plasmid. (B) Cell proliferation was detected by CCK-8 assay. (C) Cell migration was detected by Transwell assay. (D) Apoptosis of ASMCs was measured by flow cytometry. **p < 0.01.
3.4 CircRHOT1 recruits Tip60 epigenetically in ASMCs to stimulate TLR4 expression.
Then, the potential mechanism of circRHOT1 mediated TLR4 upregulation was deeply discussed. RNA pull-down showed that circRHOT1 could act more directly with acetyltransferase Tip60 in ASMCs (Fig. 4A). It was also analyzed that Tip60 could fuse with the TLR4 promoter in ASMCs (Fig. 4B). The silencing of circRHOT1 had an adverse effect on the enrichment of Tip60 on the TLR4 promoter in ASMCs (Fig. 4C). In addition, it was determined that the deletion of circRHOT1 or Tip60 resulted in relatively less enrichment of h3k27ac and RNA polymerase II on the TLR4 promoter (Fig. 4D-4G). Always, inhibition of circRHOT1 or Tip60 decreased the expression of TLR4 in ASMCs cells (Fig. 4H and 4I). The deletion of circRHOT1 was easy to adversely affect the expression of TLR4, and the overexpression of Tip60 could better reverse the down-regulation of ASMCs (Fig. 4J), suggesting that circRHOT1 epigenetically induced TLR4 expression by recruiting Tip60 in ASMCs.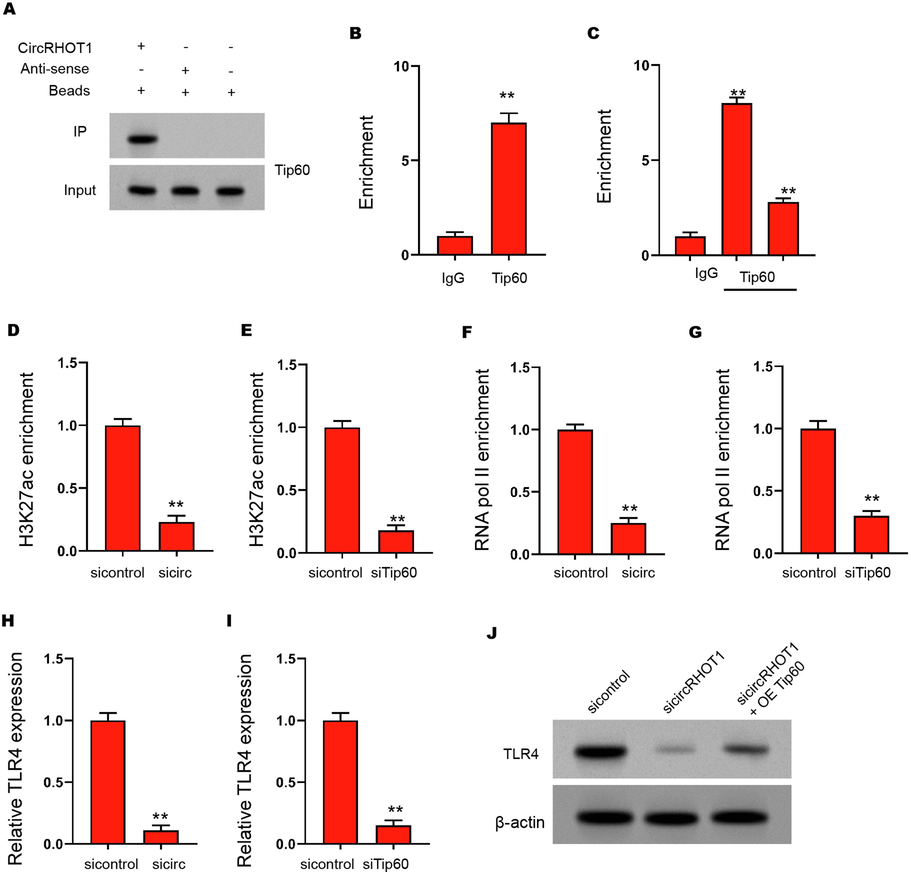
CircRHOT1 epigenetically induces TLR4 expression by recruiting Tip60 in ASMCs. (A) The interaction of circRHOT1 and Tip60 was measured by RNA pull-down in ASMCs. (B) The enrichment of Tip60 on the TLR4 promoter was analyzed by ChIP. (C) The enrichment of Tip60 on the TLR4 promoter was analyzed by ChIP in ASMCs treated with circRHOT1 siRNA. (D) The enrichment of H3K27ac on the TLR4 promoter was analyzed by ChIP in ASMCs treated with circRHOT1 siRNA. (E) The enrichment of H3K27ac on the TLR4 promoter was analyzed by ChIP in ASMCs treated with Tip60 siRNA. (F) The enrichment of RNA polymerase II on the TLR4 promoter was analyzed by ChIP in ASMCs treated with circRHOT1 siRNA. (G) The enrichment of RNA polymerase II on the TLR4 promoter was analyzed by ChIP in ASMCs treated with Tip60 siRNA. (H) The mRNA expression of TLR4 was tested by qPCR in ASMCs treated with circRHOT1 siRNA. (I) The mRNA expression of TLR4 was tested by qPCR in ASMCs treated with Tip60 siRNA. (J) The protein levels of TLR4 were determined by Western blot analysis in ASMCs co-treated with circRHOT1 siRNA and Tip60 overexpression vector. ** P < 0.01.
3.5 Tip60 knockdown represses PDGF-BB stimulated ASMCs proliferation and migration
We then assessed specific effect of Tip60 on ASMCs. The effectiveness of Tip60 knockdown by shRNAs was confirmed and we selected shRNA-2 in the subsequent analysis (Fig. 5A). The depletion of Tip60 remarkably repressed PDGF-BB stimulated ASMCs proliferation and migration (Fig. 5B-5D). The knockdown of Tip60 promoted apoptosis of ASMCs under PDGF-BB stimulation (Fig. 5E).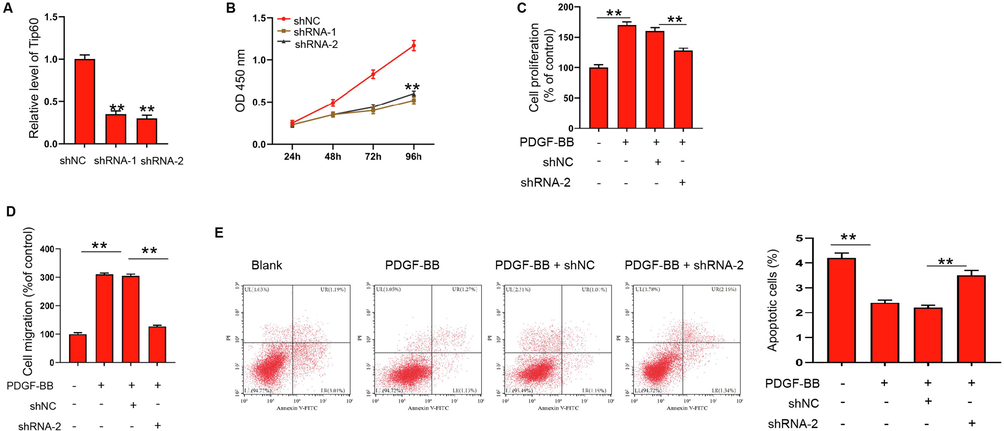
Tip60 knockdown represses PDGF-BB stimulated proliferation and migration of ASMCs. (A) Expressions of Tip60 in ASMCs transfected with Tip60 siRNA-1, Tip60 siRNA-2, or negative control (shNC) were detected by qRT-PCR experiment. (B-E) ASMCs were treated with PDGF-BB and depleted of Tip60. (B and C) Cell proliferation was detected by CCK-8 assay. (D) Cell migration was detected by Transwell assay. (E) Apoptosis of ASMCs was measured by flow cytometry. **p < 0.01.
3.6 CircRHOT1 contributes to PDGF-BB stimulated ASMCs proliferation and migration by regulating Tip60
Next, we explored the correlation of circRHOT1 with Tip60 in PDGF-BB stimulated proliferation and migration of ASMCs. Cell viability of PDGF-BB stimulated ASMCs inhibited by circRHOT1 depletion was reversed by the overexpression of Tip60 (Fig. 6A). Consistently, the overexpression of Tip60 promoted circRHOT1 knockdown-repressed migration of PDGF-BB stimulated ASMCs (Fig. 6B). Besides, the circRHOT1 kncodown-induced apoptosis was blocked by the overexpression of Tip60 (Fig. 6C and D), suggesting that circRHOT1 contributed to PDGF-BB stimulated proliferation and migration of ASMCs by regulating Tip60.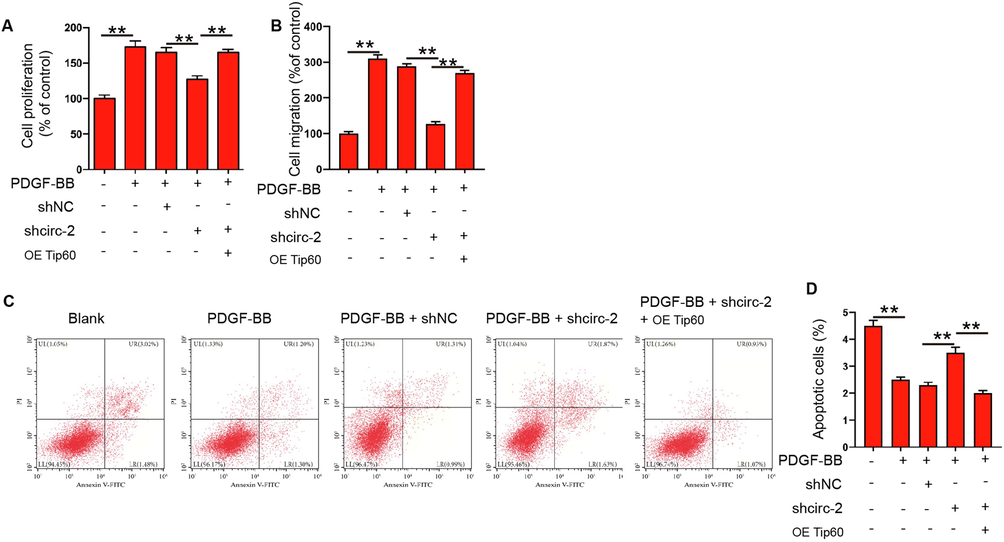
CircRHOT1 contributes to PDGF-BB stimulated proliferation and migration of ASMCs by regulating Tip60. (A-D) ASMCs were treated with PDGF-BB, or co-treated with circRHOT1 siRNA and Tip60 overexpressing plasmid. (A) Cell proliferation was detected by CCK-8 assay. (B) Cell migration was detected by Transwell assay. (C and D) Apoptosis of ASMCs was measured by flow cytometry. **p < 0.01.
4 Discussion
Asthma is a chronic inflammatory respiratory disease, which poses a serious threat to people’s health and belongs to a serious public problem (Mims, 2015; Sockrider and Fussner, 2020). Airway inflammation and remodeling are asthma-related cases, which are easy to cause unconventional phenomena in human ASMCs (Bousquet et al., 2010; Huang et al., 2019; Zhang et al., 2019). More and more evidences show that PDGF, FGF, TGF, EGF and other growth factors have a certain relationship closely related to airway remodeling and the degree of asthma (Shang et al., 2020). PDGF-BB itself belongs to an important aspect of PDGF family. It is reported that it can promote ASMCs to become a highly proliferative and migratory phenotype (Halayko et al., 2008). Therefore, it is used by cell model to simulate ASMCs remodeling. Previous studies have confirmed that circRNAs has a significant impact on asthma regulation. It is reported that circ_ U0005519 is a potential biomarker of asthma and fully regulates IL-6 and IL-13, and is abnormal in CD4+ T cells induced by let-7a-5p (Huang et al., 2019). Targeting circRNAs may be a new way to treat asthmatic diseases. In relevant studies, we further identified the significant increase of circRHOT1 in the serum collected from asthmatic patients, and PDGF-BB could fully stimulate the expression of ASMCs and enhance the level of circRHOT1, TLR4, and Tip60. The deletion of circRHOT1 had an effect on the changes of cells and made cells apoptosis. CircRHOT1 caused PDGF-BB stimulated ASMCs proliferation by regulating TLR4. We presented new evidence of the crucial effect of circRNAs on asthma and the clinical significance of circRHOT1 should be validated in future investigations.
TLR4 plays a critical role in the progression of asthma. It has been reported that the repression of TLR4 and HMGB1 reduces DINP-stimulated asthma in vivo (Hwang et al., 2019). Resveratrol attenuates asthma-induced airway remodeling and inflammation by suppressing TLR4/NF-κB signaling (Zhang et al., 2019). HMGB1 is negatively modulated by HSF1 and regulates TLR4/MyD88/NF-κB signaling in asthma (Shang et al., 2020). Moreover, it has been reported that circRHOT1 contributes to the progression of hepatocellular carcinoma by regulating NR2F6 expression through targeting Tip60 (Wang et al., 2019), indicating the effect of circRHOT1 on Tip60. Meanwhile, histone deacetylase inhibitor SAHA represses post-seizure hippocampal microglia TLR4 signaling and suppresses TLR4 gene expression by histone acetylation (Hu and Mao, 2016). Our mechanism study showed that circRHOT1 could directly interact with acetyltransferase Tip60 in ASMCs. The silencing of circRHOT1 could affect the enrichment of Tip60 on the TLR4 promoter in ASMCs, and the deletion of circRHOT1 or Tip60 could also adversely affect the enrichment of h3k27ac and RNA polymerase II on the TLR4 promoter. Fully inhibit circRHOT1 or Tip60 reduced the expression of TLR4 in ASMCs cells. The deletion of circRHOT1 easily affected the expression of TLR4, and the overexpression of Tip60 could reverse the down-regulation of ASMCs to a greater extent. In addition, Tip60 knockdown inhibited PDGF-BB stimulated ASMCs proliferation. CircRHOT1 could promote the proliferation and migration of PDGF-BB stimulated ASMCs under the regulation of Tip60. Our findings are mainly that circRHOT1 has more relatively perfect insights into the mechanism of promoting asthma by targeting Tip60/TLR4 signal transduction.
5 Conclusions
In conclusion, circular RNA circRHOT1 with platelet-derived growth factor BB can stimulate airway smooth muscle cells to proliferate or migrate based on Tip60/TLR4. CircRHOT1 is a key target for the treatment of asthma.
Funding
This study was carried out with the support of Jiangsu social projects (BE2020651 to Q.Z.); “333 talents” project of Jiangsu Province (BRA2020015 to Q.Z.); Changzhou science and technology plan (CE20205023 to Q.Z.); Changzhou science and technology plan (CJ20200094 to M.L.).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Airway Eosinophilopoietic and Autoimmune Mechanisms of Eosinophilia in Severe Asthma. Immunol. Allergy Clin. North Am.. 2018;38:639-654.
- [Google Scholar]
- Uniform definition of asthma severity, control, and exacerbations: document presented for the World Health Organization Consultation on Severe Asthma. J. Allergy Clin. Immunol.. 2010;126:926-938.
- [Google Scholar]
- Asthma Exacerbations: Pathogenesis, Prevention, and Treatment. J. Allergy Clin. Immunol. Pract.. 2017;5:918-927.
- [Google Scholar]
- The Cancer Prevention, Anti-Inflammatory and Anti-Oxidation of Bioactive Phytochemicals Targeting the TLR4 Signaling Pathway. Int. J. Mol. Sci.. 2018;19
- [Google Scholar]
- Histone acetyl transferases as emerging drug targets. Drug Discov. Today.. 2009;14:942-948.
- [Google Scholar]
- Tip60 is a haplo-insufficient tumour suppressor required for an oncogene-induced DNA damage response. Nature. 2007;448:1063-1067.
- [Google Scholar]
- Phenotype and functional plasticity of airway smooth muscle: role of caveolae and caveolins. Proc. Am. Thorac. Soc.. 2008;5:80-88.
- [Google Scholar]
- Severe asthma: novel advances in the pathogenesis and therapy. Pol. Arch. Med. Wewn.. 2014;124:247-254.
- [Google Scholar]
- Histone deacetylase inhibitor SAHA attenuates post-seizure hippocampal microglia TLR4/MYD88 signaling and inhibits TLR4 gene expression via histone acetylation. BMC Neurosci.. 2016;17:22.
- [Google Scholar]
- Hsa_circ_0005519 increases IL-13/IL-6 by regulating hsa-let-7a-5p in CD4(+) T cells to affect asthma. Clin. Exp. Allergy. 2019;49:1116-1127.
- [Google Scholar]
- Inhibitions of HMGB1 and TLR4 alleviate DINP-induced asthma in mice. Toxicol. Res. (Camb). 2019;8:621-629.
- [Google Scholar]
- Increasing Incidence of Infants with Low Birth Weight in Oman. Sultan Qaboos Univ. Med. J.. 2015;15:e177-e183.
- [Google Scholar]
- Depletion of TRRAP Induces p53-Independent Senescence in Liver Cancer by Down-Regulating Mitotic Genes. Hepatology. 2020;71:275-290.
- [Google Scholar]
- Paclitaxel Induces Immunogenic Cell Death in Ovarian Cancer via TLR4/IKK2/SNARE-Dependent Exocytosis. CancerImmunol. Res.. 2020;8:1099-1111.
- [Google Scholar]
- Asthma. JAMA. 1997;278:1855-1873.
- Inhibition of airway remodeling and inflammation by isoforskolin in PDGF-induced rat ASMCs and OVA-induced rat asthma model. Biomed. Pharmacother.. 2017;95:275-286.
- [Google Scholar]
- CircRHOT1 mediated cell proliferation, apoptosis and invasion of pancreatic cancer cells by sponging miR-125a-3p. J. Cell Mol. Med.. 2020;24:9881-9889.
- [Google Scholar]
- Asthma: definitions and pathophysiology. Int. Forum Allergy Rhinol.. 2015;5(Suppl. 1):S2-S6.
- [Google Scholar]
- Airway microbiota signals anabolic and catabolic remodeling in the transplanted lung. J. Allergy Clin. Immunol.. 2018;141 718-29 e7
- [Google Scholar]
- Severe Asthma: Have We Made Progress? Ann. Am. Thorac. Soc.. 2016;13(Suppl. 1):S68-S77.
- [Google Scholar]
- Circular RNA circRHOT1 is upregulated and promotes cell proliferation and invasion in pancreatic cancer. Epigenomics. 2019;11:53-63.
- [Google Scholar]
- Regulation of human airway smooth muscle cell migration and relevance to asthma. Respir. Res.. 2017;18:156.
- [Google Scholar]
- Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS One. 2012;7:e30733.
- [Google Scholar]
- HMGB1 was negatively regulated by HSF1 and mediated the TLR4/MyD88/NF-kappaB signal pathway in asthma. Life Sci.. 2020;241:117120.
- [Google Scholar]
- TLR4-Based Immunotherapeutics in Cancer: A Review of the Achievements and Shortcomings. Mol. Pharm.. 2018;15:4777-4800.
- [Google Scholar]
- Circular RNA circRHOT1 promotes hepatocellular carcinoma progression by initiation of NR2F6 expression. Mol. Cancer. 2019;18:119.
- [Google Scholar]
- Circular RNA RHOT1 promotes progression and inhibits ferroptosis via mir-106a-5p/STAT3 axis in breast cancer. Aging (Albany NY). 2021;13
- [Google Scholar]
- Resveratrol Inhibits MMP3 and MMP9 Expression and Secretion by Suppressing TLR4/NF-kappaB/STAT3 Activation in Ox-LDL-Treated HUVECs. Oxid. Med. Cell Longev.. 2019;2019:9013169.
- [Google Scholar]







