Translate this page into:
The synthesis of the P/N-type NdCoO3/g-C3N4 nano-heterojunction as a high-performance photocatalyst for the enhanced photocatalytic degradation of pollutants under visible-light irradiation
⁎Corresponding author. salavati@kashanu.ac.ir (Masoud Salavati-Niasari)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The treatment of industrial and domestic colored effluents and the use of photocatalysts have today attracted much attention among researchers. With the varied photocatalytic materials, the ones with narrow bandgap are thus of greater significance thanks to their acceptable performance in optical fields. The other obvious property of such materials is their lower costs because a large part of sunlight is in the visible region. In this work, the bare perovskite-type neodymium cobaltite and neodymium cobaltite/graphite carbon nitride (p/n-type NdCoO3/g-C3N4) was synthesized using sol-gel auto combustion. Different reductants were also employed during this fabrication for morphological engineerings, such as glucose (carbohydrate), L-valine (amino acid), and citric acid. As well, X-ray diffraction analysis (XRD), Fourier transform infrared spectroscopy (FTIR), and energy-dispersive X-ray spectroscopy (EDS) were utilized to prove the purity of the specimens, and then transmission electron microscopy (TEM) and field-emission scanning electron microscopy (FE-SEM) were applied to explore their morphology. The various properties of the product, such as the optical, magnetic, porosity, and surface features, were correspondingly checked by diffuse reflectance spectroscopy (DRS), photoluminescence (PL) spectroscopy, vibrating-sample magnetometry (VSM), and Brunauer-Emmett-Teller (BET) surface area analysis, respectively. Citric acid was thus selected as an optimal fuel because of the formation of finer particles and morphology. In addition, the NdCoO3 nanoparticles (NPs) were exploited as a catalyst for the degradation of anionic dyes (viz. erythrosine [Red No. 3], methyl orange [MO], and eriochrome black T [EBT]) and cationic ones (i.e., rhodamine B [RhB], acid red 14 [ACR14], and acid yellow 23 [AY23]) under visible-light irradiation. The study findings accordingly revealed that the heterostructures emerged at the interfaces between the p-type NdCoO3 and n-type g-C3N4, leading to the EBT decolorization, reached 95.8% after 120 min, and were higher than the photodegradation of the pristine NdCoO3 and g-C3N4.
Keywords
Carbon nitride
Eriochrome black T
Neodymium cobaltite
Photocatalysis
Sol-gel autocombustion
Visible-light irradiation
1 Introduction
Over recent decades, technological and scientific advancements along with researchers' efforts have significantly contributed to the treatment of industrial and domestic effluents and the improvement of drinking water (Zhang et al., 2019; Omrani and Nezamzadeh-Ejhieh, 2020; Zhang et al., 2020a,b; Zhang et al., 2021). Among the effective methods to resolve this dilemma is photodegradation, using ultraviolet (UV) or visible-light irradiation and nano-semiconductors as catalysts. This method is simple, green, and at approximately a low cost, so it has attracted much attention in the industry and among researchers (Ghanbari et al., 2016; Zhang et al., 2020a,b; Zhang et al., 2021). Nano-semiconductors can be thus assumed as helpful catalysts for photoactivity because of reducing particle size and high surface area (Di Mauro et al., 2015; Wu et al., 2015; Heidari-Asil et al., 2020; Zhou et al., 2021).
However, nanocatalysts have thus far solved many problems corresponding to photocatalytic ones due to their distinctive properties. Researchers are still constantly trying to find innovations; for example, the high rate of recombination of the photo-induced electron and the hole is one of the relevant problems. In addition to doping and loading metals, one solution is to create a heterojunction made by two semiconductors. This strategy, employing semiconductor conduction and valance bands, can accordingly assist in the better separation of electron-hole pairs (He et al., 2020; Xu et al., 2020; Ranjeh et al., 2021; Zhou et al., 2021). On the other hand, researchers have focused more on visible-light-driven semiconductors, perovskite-type oxides, and graphitic carbon nitride (g-C3N4), rather than UV ones. Both g-C3N4 and perovskite materials are a good choice in photocatalysis owing to their structural stability and being affordable and active under visible-light irradiation (particularly, narrow bandgap) (Luo et al., 2019; Monga and Basu 2019; Chen et al., 2020, Faisal et al., 2020, Rokesh et al., 2020).
The polymeric semiconductor, g-C3N4, also possesses two-dimensional (2D) aromatic sheets in the form of a tri-s-triazine structure with the highest stability compared with its other allotropes (Shi et al., 2014; Gaddam et al., 2020). Nonetheless, the low photoactivity of g-C3N4 stems from the low separation of the electron-hole pair in this semiconductor. Consequently, it is essential to enhance the photo performance of the n-type g-C3N4 couples with another semiconductor to produce p/n-type heterojunction, which is helpful to separate electrons and holes (Christoforidis et al., 2016a,b; Li et al., 2019; Ghane and Sadrnezhaad, 2020; Ismael, 2020; Melchionna and Fornasiero, 2020; Yang et al., 2021; Xu et al., 2022). Of note, g-C3N4 behaves as an n-type semiconductor because of the presence of electron donors in its structure (i.e., –NH/NH2 groups) (Fu et al., 2018). The reported bandgap for g-C3N4 is around 2.7 eV in the visible region. (Azami et al., 2020; Liao et al., 2020). Moreover, many perovskite materials (e.g., ABO3) in single, metal-doped, or composite forms, such as strontium stannate (SrSnO3) (Teixeira et al., 2019), strontium titanate/cobalt ferrite (SrTiO3/CoFe2O4), strontium cations doped in potassium tantalate (KTaO3) (Sudrajat et al., 2019), graphite carbon nitride/silver/lanthanum ferrite (g-C3N4g/AG/LaFeO3) (Gao et al., 2019), β-Fe2O3/g-C3N4 nanocomposite (Christoforidis et al., 2016a,b), TiO2/carbon nitride heterojunctions(Christoforidis et al., 2019) and palladium/cerium-manganese oxidase 3 (Pd-CeMnO3) (Yi et al., 2018) have been synthesized and applied as photocatalysts.
Until recently, there has been much interest in lanthanum cobaltite (LaCoO3), as a member of the multicopper oxidase 3 (MCoO3) perovskite family, synthesized via different methods, such as sol-gel auto combustion and coprecipitation (CPT) (Maulana and Nandiyanto, 2019). Furthermore, this special perovskite has been manufactured in various forms, e.g., silver phosphate/lanthanum cobaltite (Ag3PO4/LaCoO3) nanocomposite (Guo et al., 2017), iron (Fe) and bismuth (Bi) doping on LaCoO3 (Ajmal et al., 2019), La1-xSrxCoO3/Ag3PO4 (Chen et al., 2021), and LaCoO3/g-C3N4 (Luo et al., 2018). Lanthanide perovskites are also effective photocatalysts in the presence of visible illumination because of their high stability and environmentally friendly features; however, it is better to improve their photocatalytic performance by adding other materials to separate more electrons and holes. Therefore, neodymium cobaltite (NdCoO3) as an n-type lanthanide perovskite is appropriate for photocatalytic issues that have recently received less attention (Ali et al., 2011; Ling et al., 2016; Guo et al., 2019; Nakhostin Panahi et al., 2020).
This work, as the first attempt in this line, explores the role of synthesized NdCoO3 nanoparticles (NPs) using sol-gel auto combustion, as a catalyst for the decolorization of erythrosine [Red No. 3], methyl orange (MO), and eriochrome black T [EB] as anionic dyes and rhodamine B [RhB], acid red 14 [ACR14], and acid yellow 23 [AY23] as cationic ones. Finally, a comparison was made between the photoperformance efficiency of g-C3N4, NdCoO3, and NdCoO3/g-C3N4 heterojunction for the EBT degradation. To the best of the authors' knowledge, there was no study on photocatalytic dye degradation using NdCoO3/g-C3N4, PN-type heterojunction.
2 Experimental study
2.1 Materials
For the synthesis of the NdCoO3/g-C3N4 nanocomposite, all chemicals were used from the Merck & Co. (USA) in a pure form, including melamine (C3H6N6), cobalt (II) nitrate (Co (NO3)2)·6H2O, neodymium nitrate (Nd (NO3)3·6H2O), glucose (C6H12O6), citric acid (C6H8O7), and L-valine (C5H11NO2). In this work, the dyes utilized as water pollution factors, i.e., Red No. 3 (C20H6I4Na2O5), RhB (C28H31CIN2O3), EBT (C20H12N3O7SNa), ACR14 (C20H12N2Na2O7S2), MO (C14H14N3NaO3S), and AY23 (C16H9N4Na3O9S2) were also acquired from Sigma-Aldrich Corp. (USA).
2.2 Fabrication of NdCoO3 NPs
As indicated in Fig. 1, the NdCoO3 NPs were created by various fuels, such as L-valine, citric acid, and glucose employed in this study. First, neodymium salt (0.075 g) and fuel (0.134 g glucose, 0.143 g citric acid, and 0.08 g L-valine) were separately dissolved in distilled water with a molar ratio of 1:4, respectively. Next, under a magnetic stirrer at 60 °C, the prepared fuel of the solution, as a reductant and morphological engineering agent, was added to the solution containing neodymium nitrate. After 30 min according to the molar ratio selected, 1:1 for Nd:Co, an aqueous solution of cobalt (II) nitrate (0.05 g) was added into the solution placed on the magnetic stirrer by heating the solution at 120 °C for 60 min, the solvent was evaporated, and hence the viscose gel was formed. Afterward, the gel obtained was placed in the oven at 70 °C for one day. Finally, the dried violet product was calcinated at 600 °C for 2 h. In this method, citric acid and other fuels were further used as reducing and capping agents. The schematic diagram of the formation of the NdCoO3 nanostructures by different fuels is shown in Fig. 1.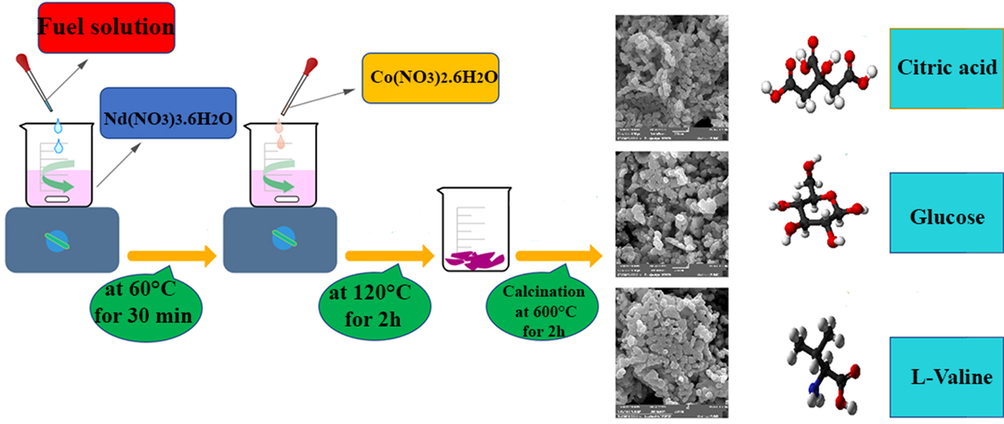
Schematic of the formation of NdCoO3 nanostructures by different fuels.
2.3 Fabrication of g-C3N4
To form g-C3N4, melamine powder was utilized thoroughly by direct heating. For this purpose, first, white melamine powder was placed into a crucible, and then, it was calcinated in the atmosphere at 550 °C for 4 h. Finally, the yellow g-C3N4 powder was formed.
2.4 Fabrication of NdCoO3/g-C3N4 nanocomposite
In this part, the NdCoO3/g-C3N4 nanocomposite was prepared in an ex-situ route. First, 0.1 g NdCoO3 was obtained by citric acid as an optimum fuel, as a morphologically optimal sample, with 0.3 g g-C3N4, and then sonicated in 30 ml ethanol for 40 min. Next, the suspension was stirred at room temperature for a day. Then, the suspension was heated at 50 °C under stirring to evaporate the solvent. Then after, the cement-shape sediment was dried at 70 °C for 24 h, and subsequently ground in a mortar. Finally, the NdCoO3/g-C3N4 sample with 25- wt% was synthesized. The preparation conditions of the products are listed below (Table 1), and the schematic diagram of the NdCoO3/g-C3N4 nanocomposite preparation is illustrated in Fig. 2.
Sample No.
Fuel
g-C3N4:NdCoO3
Calcination temperature (°C)
Products
1
Citric acid
0: 1
600
NdCoO3
2
Glucose
0: 1
600
NdCoO3
3
L-valine
0: 1
600
NdCoO3
4
Citric acid
1: 3
600
NdCoO3/g-C3N4
5
–
1: 0
550
g-C3N4
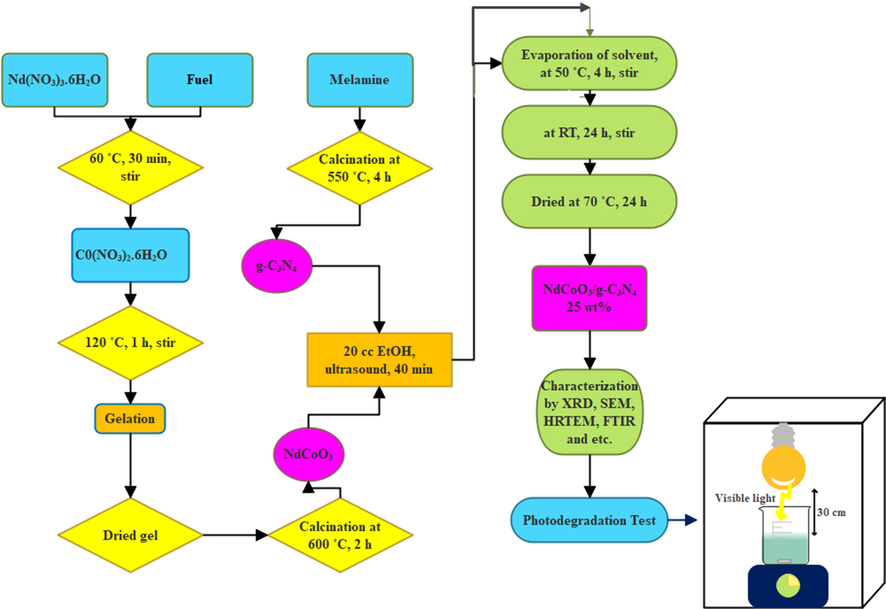
Schematic diagram of the preparation of NdCoO3/g-C3N4 nanocomposite.
2.5 Photocatalytic test
First, 0.05 g of NdCoO3 was weighed and added to 50 ml of 5-ppm dye-containing solution. Next, the sample placed on the stirrer magnetic was 15 cm outdistance from the light source. Then, the sample was stirred without light for half an hour before irradiating until it reached balancing adsorption-desorption. Subsequently, with the onset of light illumination, a certain amount of the sample was gathered every 20 min and then centrifuged. Finally, the solution adsorption was studied by UV–Vis spectrometry at a specific wavelength. The percentage of the destruction of the dyes was calculated as:
2.6 Characterization
To explore the crystallite size, purity, and phase composition of the synthesized samples, a diffractometer of Philips Co. (Netherlands, i.e., the X-ray diffraction analysis [XRD]) was used with copper K-α (Cu Kα) radiation. The crystallite size was thus computed using the full width at half maximum (FWHM) data from this technique and the Scherrer equation as follows:
Fourier transform infrared spectroscopy (FTIR; Shimadzu, FTIR-4300 spectrophotometer, Japan) on potassium bromide (KBr) pellets was also used to examine the chemical bonds of the prepared samples. Employing field emission-scanning electron microscopy (FE-SEM, LMU TESCAN BRNO-Mira3, Czech Republic) and transmission electron microscopy (TEM, Philips Co. EM208, Netherlands, with an accelerating voltage of 100 kV), the particle size and surface morphologies of the photocatalysts were studied, respectively. The specific surface area was further investigated by the Brunauer-Emmett-Teller (BET) surface area analysis (BELsorp mini II, Japan) for the thorough adsorption and desorption of nitrogen gas at its boiling point. The optical property of the nanostructures was then examined by exploiting a spectrophotometer (UV–vis, Shimadzu, UV-2550, Japan) and photoluminescence spectroscopy (PL, optical luminescence, Perkin Elmer Co., LS-55, USA). Utilizing the below equation and drawing the Tauc plot, the bandgap resulting from the absorption data was then acquired:
The magnetic feature was also explored with a vibrating-sample magnetometer (VSM, Meghnatis Kavir Kashan Co, Iran). Moreover, the synthesis of the NdCoO3/C3N4 nanocomposite was carried out by a sonicator (Bandelin Sonopuls HD 3200, Germany).
3 Results and discussion
3.1 XRD
The XRD patterns related to the fabricated samples, depicted in Fig. 3, confirm the successful formation of all specimens with high crystallinity. The g-C3N4 hexagonal phase also has a faint peak, and a strong peak at 2ϴ = 13.0°, corresponding to (1 0 0) plane and 2ϴ = 27.6° ascribed to (0 0 2) plane (Moakhar et al., 2020; Munusamy et al., 2021), respectively. The feeble peak is due to the in-plane structural packing motif of the tri-s-triazine units (d = 0.680 nm). The stronger one is also attributed to the interlayer stacking of the aromatic rings (d = 0.325 nm) (Zhao et al., 2014; Papailias et al., 2020). As for NdCoO3 with an orthorhombic structure, six characteristic peaks are obviously at 2ϴ = 23.6°, 33.6°, 41.5°, 48.2°, 60.0° and 70.5°, ascribed to (1 0 1), (1 2 1), (2 2 0), (2 0 2), (1 2 3) and (2 4 2) crystal planes, sequentially (Ateia et al., 2019). Thus, NdCoO3 is fabricated in a pure form with the help of all three fuels. Fig. 3d reveals the major diffraction peaks of NdCoO3 and g-C3N4, indicating a good interaction between them. Interestingly, the NdCoO3/g-C3N4 nanocomposite has a high crystallinity, too. Furthermore, the NdCoO3/g-C3N4 grain size measured by the Scherer equation, D = 0.9λ/βCosϴ, is obtained at 23 nm.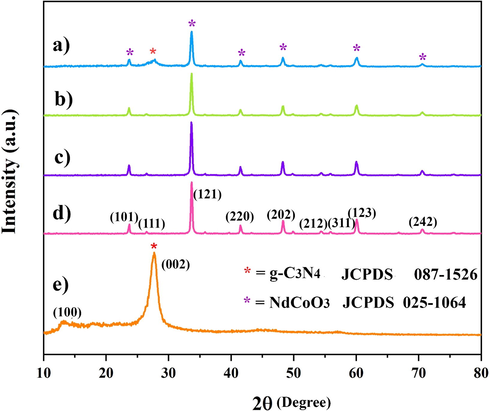
XRD pattern of (a) NdCoO3/g-C3N4, (b) NdCoO3 (valine), (c) NdCoO3 (glucose), (d) NdCoO3 (citric acid), (e) g-C3N4 powders.
3.2 FTIR
As shown in Fig. 4, the peaks of the bare NdCoO3, corresponding to the vibrating and stretching of Nd-Co-O and Co-O, are 577 and 473 cm−1, respectively, similar to DyCoO3 and LaCoO3 (Xiong et al., 1997; Michel et al., 2019; Phadtare et al., 2019). As for the g-C3N4, the peak located at 806 cm−1 is ascribed to the triazine (viz. the breathing vibration) (Wang et al., 2020). The wide range in the 3000–3500 cm−1 is further associated with the N-H and NH2 groups (namely, the stretching and bending vibration) (Zi et al., 2021). The bending and stretching vibration modes of water sitting on the NP surfaces are also observed at 1630 and 3430 cm−1, respectively.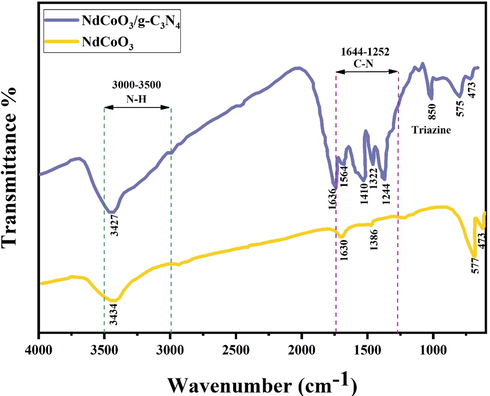
FT-IR spectrum of pure NdCoO3 nanostructure (sample No. 1), and NdCoO3/g-C3N4 nanocomposite.
3.3 TEM, FE-SEM, and EDS
Fig. 5 depicts the micro-images of NdCoO3 prepared through the reaction between metal nitrates as oxidizing and fuels as reducing agents. The fuels used for this purpose also have different chelating effects because of their functional (viz. hydroxyl and carboxyl) groups. Hence, the morphology and particle size of the synthesized nanostructures are shaped by the chemical structure of the fuel. The glucose structure also has five hydroxyl groups (OH–), whereas that of L-valine and citric acid has one and three carboxyl acid groups, respectively. Moreover, the hydroxyl group has a weaker electron-donating group than the carboxyl one. So, L-valine has a less chelating ability than glucose and citric acid (Yahya et al., 2018). For this reason, the synthesized samples with L-valine and glucose aggregated more than those with citric acid. Similarly, the average particle size calculated by the ImageJ software was around 20.7 nm (L-valine), 19.4 nm (glucose), and 17.4 nm (citric acid) (Fig. 5). Likewise, the sample produced by citric acid has been selected as an optimum because of better morphology and smaller average particle size.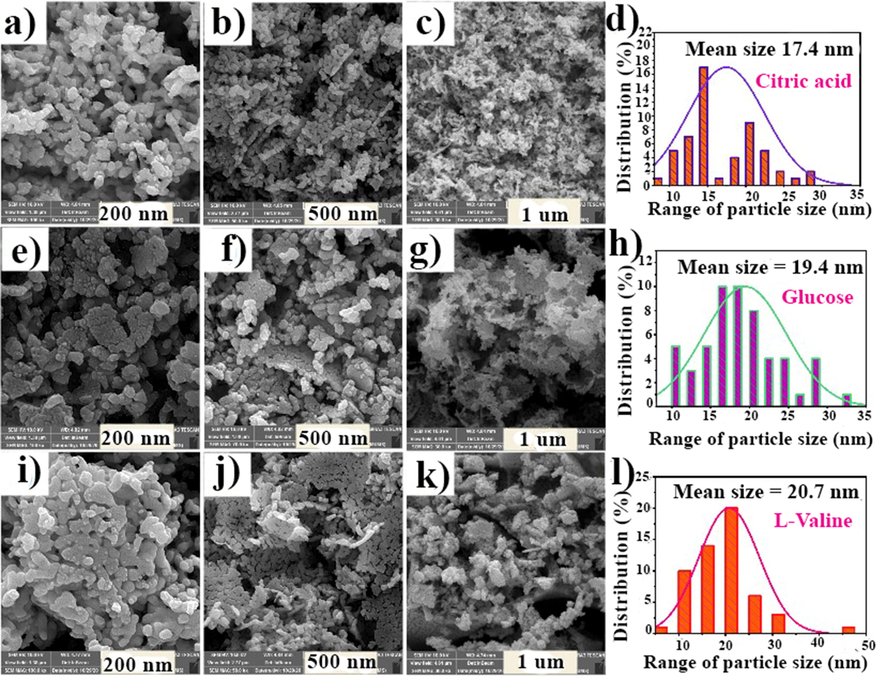
FE-SEM images of the fabricated NdCoO3 nanoparticles by various reductant and associated average particle size histogram: (a-d) citric acid, (e-h) glucose, (i-l) valine in different scales.
Fig. 6 illustrates the FE-SEM and TEM images of the prepared NdCoO3/g-C3N4 nanocomposite in the presence of optimum fuel (citric acid) in different scales. Both NdCoO3 and layered structure g-C3N4 are distinguishable in Fig. 6f. The resulting photos and the particle size distribution histogram reveal the increase in the size of the particles. The particle size received from TEM is usually smaller than that in FE-SEM due to the lack of gold coating of the surfaces in TEM and not SEM. Nevertheless, owing to the agglomerate phenomenon in the nanocomposite, this subject was reversed in this research. Moreover, the crystallite size calculated from the XRD pattern is 23 nm and smaller than the FE-SEM and TEM outcomes, which shows the particles are composed of crystallites.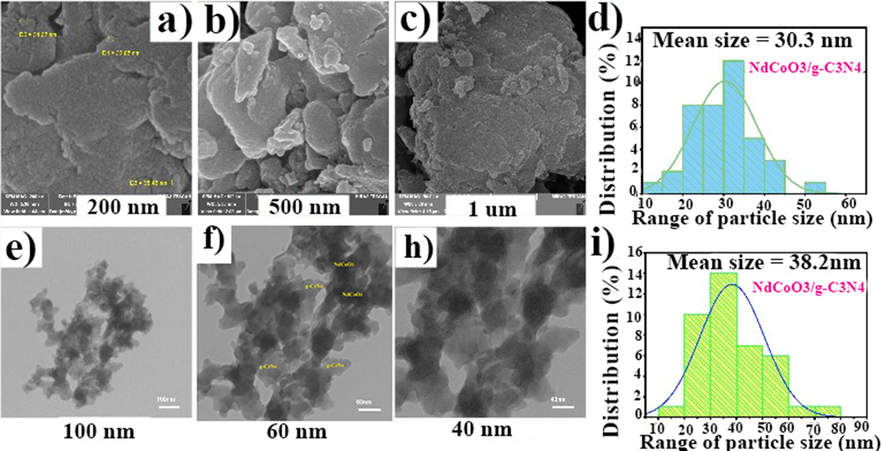
FE-SEM and TEM images of prepared NdCoO3/g-C3N4 nanocomposite corresponding average particle size histogram.
The EDS spectrum of the NdCoO3 NPs fabricated by diverse reductants, the NdCoO3/g-C3N4 nanocomposite, and the percentage of all elements are further displayed in Fig. 7(a-d). The EDS results also prove the purity of the products. This pattern demonstrates the existence of N, Co, O, Nd, and C elements in the fabricated nanocomposite.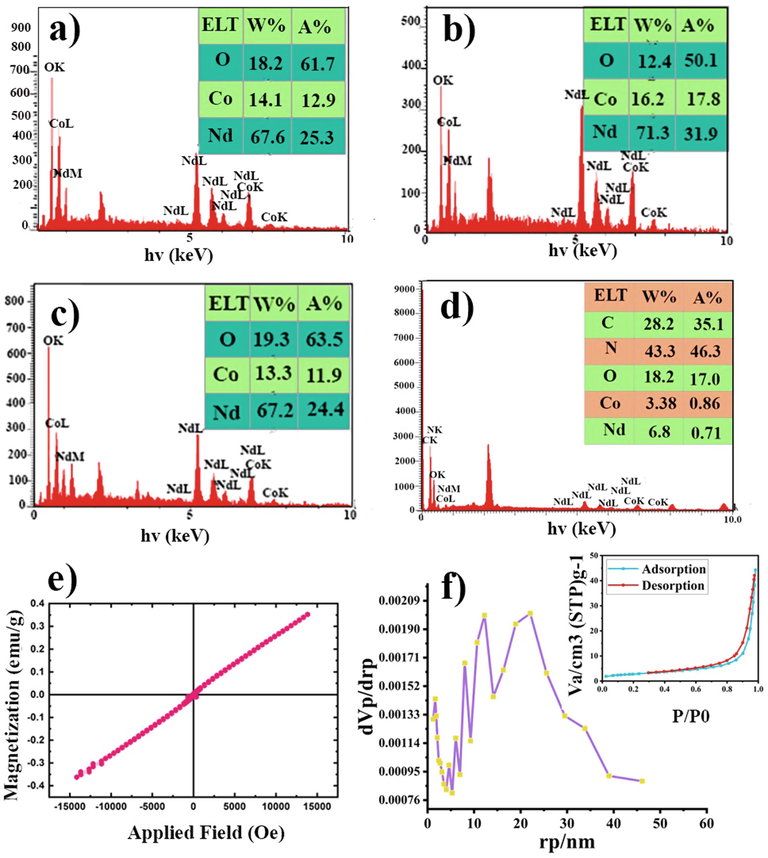
EDS spectrum related to the synthesized NdCoO3 by (a) citric acid, (b) valine, (c) glucose, (d) NdCoO3/g-C3N4, (e) VSM curve and (f) BJH and N2 absorption-desorption (inset) of the NdCoO3 (sample No.1).
3.4 VSM
The magnetic measurement of the NdCoO3/g-C3N4 nanocomposite was performed at the ambient temperature using a VSM. As shown in Fig. 7e, the linear H/M plot depicts the NdCoO3/g-C3N4 nanocomposite as a paramagnetic compound. Of note, the paramagnetic behavior is due to the presence of NdCoO3 in nanocomposites. The coercivity (Hc), remnant magnetization (Mr), saturation magnetization (Ms), and squareness (Mr/Ms) also have approximated around 189 Oe, 7.93 × 10-3 emu/g, 0.352 emu/g, and 0.022, sequentially.
3.5 BET surface area analysis
Fig. 7f demonstrates the BET surface area analysis of the NdCoO3/g-C3N4 nanocomposite. The adsorption-desorption isotherm curve is similar to the type-III and H3-type hysteresis in conformity with the International Union of Pure and Applied Chemistry (IUPAC) classification. The specific surface area of the nanocomposite was approximately 9.94 m2/g, and the total pore volume measured was around 2.284 cm3/g. On the other hand, the average pore diameter was 27 and 22 nm, according to the BET and Barrett-Joyner-Halenda (BJH) analysis data plots. Thereby, both results of the particle size perfectly matched the FE-SEM and TEM consequences.
3.6 UV–Vis absorbance spectral analysis
Using UV–Vis diffuse reflectance spectroscopy (DRS) helped measure the optical features of g-C3N4 and NdCoO3 NPs. As shown in Fig. 8(a-c), the suitable absorptions of both are observed in the range of about 200–450 nm, and the absorption of NdCoO3 is a little more than carbon nitride. On the other hand, the bandgap is obtained, using Eq. (3), and then the graph between (αhѵ)1/2 vs. the hѵ, Tauc plot. Likewise, the bandgaps are 2.73 and 2.79 eV for g-C3N4 and NdCoO3, indicating them as sufficient catalysts to photodegrade colored effluents under visible-light irradiation, especially when they are together as a composite.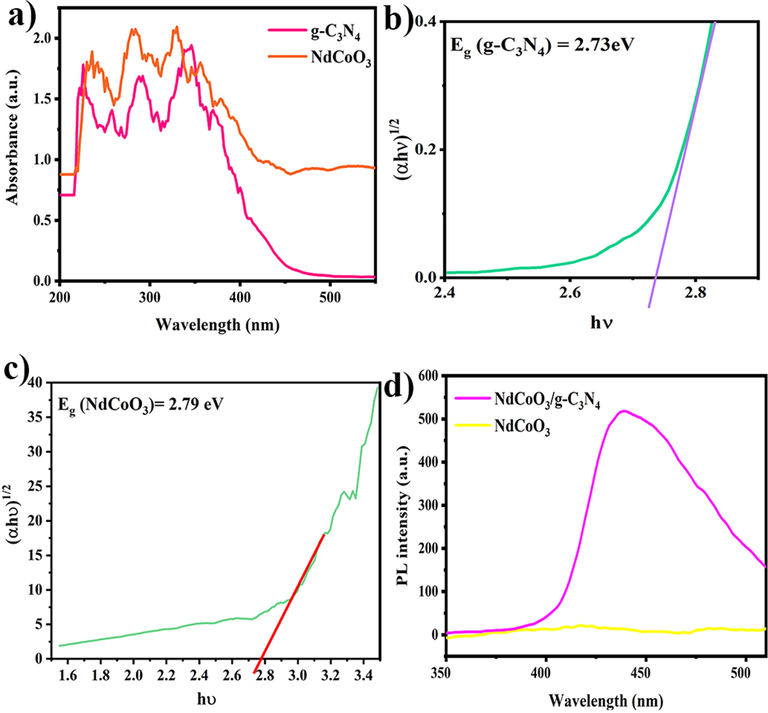
(a) UV–Vis DRS spectrum of g-C3N4 and NdCoO3 (sample No.1); the plot of bandgap energies for (b) g-C3N4 (sample No.5), and (c) NdCoO3 (sample No.1); (d) PL spectra of NdCoO3/g-C3N4 (sample No.4).
3.7 PL spectroscopy
The photoluminescence spectra of the bare NdCoO3 and g-C3N4/NdCoO3 are demonstrated in Fig. 8d. The PL of a single NdCoO3 does not show any PL signals. As reported by (Chen et al., 2014), the single g-C3N4, on account of the recombination of the photogenerated electron-hole pairs, can have a clear fluorescence emission peak at 455 nm. Thus, the observed fluorescence signal around 439 nm is associated with carbon nitride in the composite in the PL of the g-C3N4/NdCoO3 nanocomposite, establishing a good interaction between NdCoO3 and g-C3N4, which leads to a blue-shift in the PL spectra of the NdCoO3/g-C3N4 nanocomposite compared with the bare g-C3N4, at 455 nm (Jin et al., 2019a,b). Finally, the bandgap of the NdCoO3/g-C3N4 nanocomposite is estimated at 2.82 eV by the 1240/λ equation.
3.8 Photocatalytic activity
The catalytic performance of the NdCoO3 NPs was surveyed for the decolorization of different dyes as effluents under visible-light irradiation (400 W). Afterward, the effect of diverse parameters such as the catalyst dosage, pH, scavenger, and the role of g-C3N4 added to the NdCoO3, was studied on the optimal dye in terms of higher photodegradation. A dark experiment in the absence of irradiation was performed for 30 min to test whether the absorption of the EBT caused the decolorization of EBT by the nanoparticles. EBT concentration did not decrease obviously, indicating that decolorizing of EBT is mostly due to photodegradation rather than adsorption. Summary performed photocatalytic result is tabled (Table 2).
Photocatalyst
Dye
Catalyst loading (g)
Initial pH
Scavenger
Degradation%
NdCoO3
RhB (cationic)
0.05 g
7
–
25.7%
NdCoO3
ACR14 (cationic
0.05 g
7
–
48.8%
NdCoO3
AY23 (cationic)
0.05 g
7
–
38.0%
NdCoO3
MO (anionic)
0.05 g
7
–
26.8%
NdCoO3
Erithrosine (anionic)
0.05 g
7
–
35.5%
NdCoO3
EBT (anionic)
0.05 g
7
–
52.8%
NdCoO3
EBT (anionic)
0.025
7
–
47.2%
NdCoO3
EBT (anionic)
0.075
7
–
70.2%
NdCoO3
EBT (anionic)
0.075
3
–
89.9%
NdCoO3
EBT (anionic)
0.075
3
EDTA
74.4%
NdCoO3
EBT (anionic)
0.075
3
Benzoquinone
90.6%
NdCoO3
EBT (anionic)
0.075
3
Benzoic acid
55.5%
g-C3N4
EBT (anionic)
0.075
3
–
81.8%
NdCoO3/g-C3N4
EBT (anionic)
0.075
3
–
95.8%
3.8.1 Impact of dye
Fig. 9 (a and b) illustrates the photocatalytic performance of NdCoO3 for 5 ppm of anionic organic dyes (via. MO, Red No. 3, and EBT) and the cationic organic ones (namely, RhB, ACR14, and AY23) as pollutants in industrial wastewater. The most decolorization of the anionic dyes is associated with EBT (58.2%), and this value is 26.8% and 35.5% for MO and Red No. 3, respectively. As shown in the photocatalyst plot, the highest degradation of the cationic dyes is related to ACR14 (48.8%). Sequentially, the percentage of the decolorization of RhB and AY23, the other cationic dyes, is 25.7% and 38.0%. Hence, it seems that NdCoO3 is a better catalyst for the EBT anionic dyes.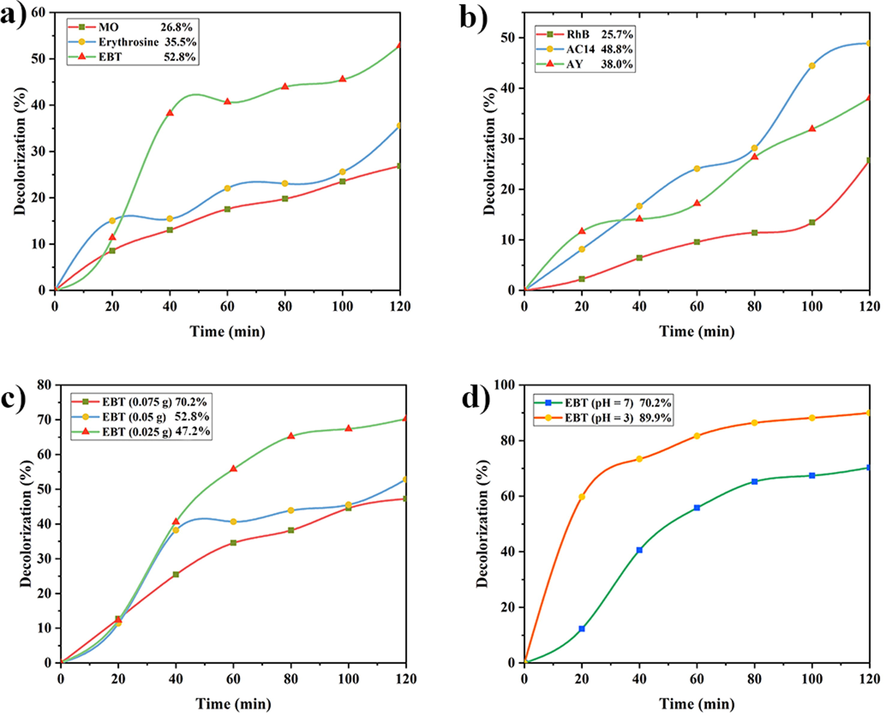
A comparison between the photocatalytic performances NdCoO3 (0.05 g, sample No.1) nanoparticle (pH = 7) in the presence of (a) anionic dyes and (b) cationic dyes (5 ppm). A comparison between the photocatalytic performances of NdCoO3 (sample No.1), for degradation of EBT (5 ppm) under visible-light irradiation in the different (a) catalyst dosage (pH = 7) and in the different (b) pH environments (0.07 g of NdCoO3, sample No.1).
3.8.2 Effect of catalyst loading
Fig. 9c demonstrates a comparison between the 0.025 g, 0.05 g, and 0.075 g of the nanocatalyst on the photocatalytic behavior. With the growing amount of NdCoO3, the catalytic performance increases. The leading cause of this incremental routine is aligned with the rise in the available surface area. As shown in the degradation plot, when the 0.025 g of the catalyst is used, the percentage of the decolorization of EBT is obtained as 47.2%. Once the 0.05 g and 0.07 g of the sample are utilized, the degradation efficiency is by 52.8% and 70.2%, respectively. Consequently, 0.075 g of NdCoO3 as a photocatalyst for the EBT contaminant was the ideal dosage.
3.8.3 Effect of Initial pH
In this section, the EBT decolorization was inspected in an acidic and natural environment. A comparison of the two results revealed the photocatalyst performance in an acidic medium (pH = 3), which was better than natural environment (pH = 7). This behavior was owned to the presence of H+ caused by the acidic environment, which could lead to more electrostatic attraction and, consequently, more relationship between the active surface of the catalyst and the pollutant. As denoted in Fig. 9d, 70.2% of EBT in the natural environment and 89.9% of EBT in the acidic environment is degraded by the NdCoO3 NPs within 120 min.
3.8.4 Scavenger-quenching impact
This step aimed to recognize the effective active species (i.e., h+, •OH, and .O2–) in the EBT decolorization. Therefore, three scavengers, including benzoic acid, EDTA, and benzoquinone, were exploited for trapping •OH, h+, and .O2–, respectively (Zhang et al., 2019). According to Fig. 10a, the influence of benzoquinone on the photocatalytic process is similar to the no-scavenger sample, and the EBT destruction is at a maximum (90%). Hence, the superoxide radical is not used in the photocatalytic process. On the other hand, the addition of the hole (EDTA) and the hydroxyl radical (BA) agents reduced the percentage of decolorization to 74.4% and 55.5%, respectively. The descending decolorization rates also demonstrated that h+ played a minor role and •OH was the prominent active radical in the EBT degradation.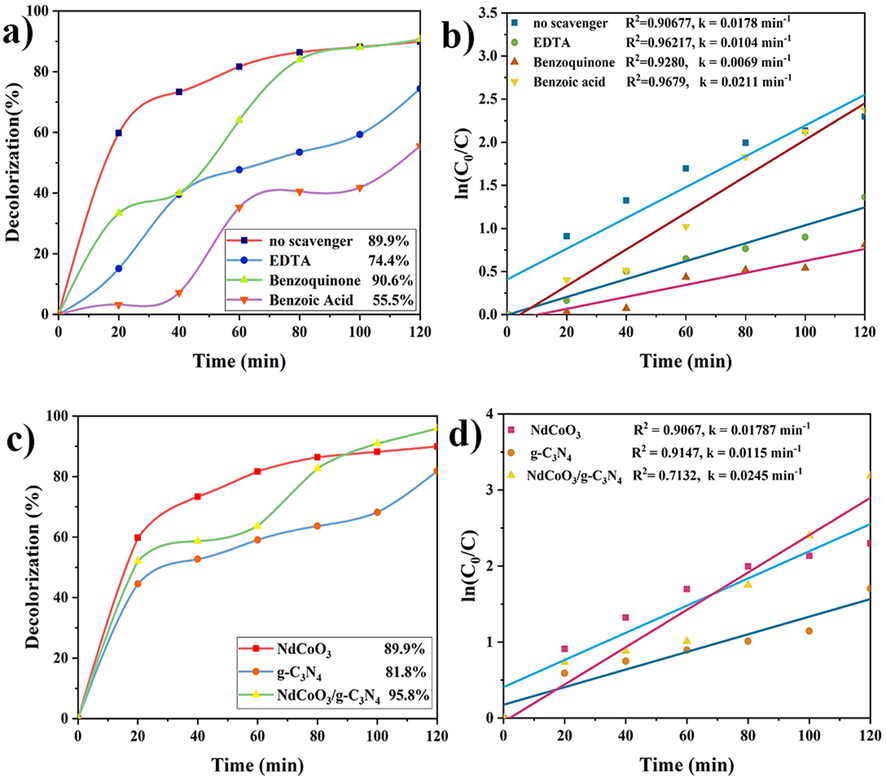
The photocatalytic performances of (a) NdCoO3 nanoparticles (0.05 g, sample No.1) for degradation of EBT (5 ppm, pH = 3) in the presence of different scavengers and (c) NdCoO3 (sample No.1) compared with g-C3N4 and NdCoO3/g-C3N4 (0.05 g of catalyst, 5 ppm, pH = 3). (b and d) Plots of ln(C/C0) vs. time.
3.8.5 Synergetic performance
The photocatalytic behavior of NdCoO3, g-C3N4, and NdCoO3/g-C3N4 was assessed under visible-light irradiation for 120 min. As exhibited in Fig. 10c, the single phases show a lower decolorization percentage than nanocomposite. Moreover, this value is 89.9% for NdCoO3 and 81.8% for g-C3N4. Because of adding g-C3N4 to NdCoO3, the electron-hole separation is enhanced. Thereby, these parts together as a composite ameliorate the catalytic activity. The final percentage of the EBT decolorization by the NdCoO3/g-C3N4 nanocomposite also reached 95.8%.
3.8.6 Photodegradation pathway
Creating a p/n-type heterojunction between NdCoO3 (p-type, Ef located near the valence band, VB) and g-C3N4 (n-type, Ef located near conduction band, CB) creates an electric field between their contact interfaces. The CB and VB potential of NdCoO3 (p-type) and g-C3N4 (n-type) are thus indispensable to ascertain a possible mechanism between them. Accordingly, the ECB (viz. the conduction band edge) and EVB (namely, the valance band edge) for the semiconductors can be computed utilizing the following empirical formula:
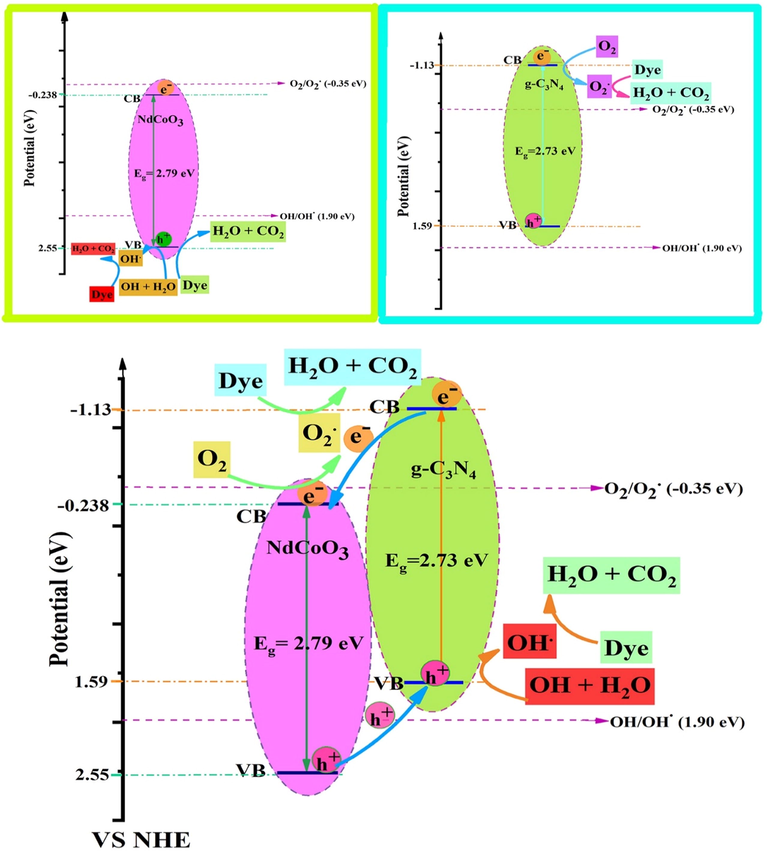
Photocatalytic mechanism scheme of bare NdCoO3 (sample No.1), g-C3N4 (sample No.5), and NdCoO3/g-C3N4 (sample No.4), heterojunction nanocomposites under visible light irradiation.
Moreover, the EBT photodegradation mechanism by the NdCoO3/g-C3N4 nanocomposite is shown in Fig. 12. The photocatalytic properties of nanocomposites based on g-C3N4 for environmental pollution are reviewed in Table 3. The catalytic activity of all nanocomposites is affected by the precursors and fabrication methods, even though they all have g-C3N4 as the main component. Compared to singular materials, coupled nanostructures have higher photocatalytic efficiency. As a result of heterojunctions being formed in hybridized materials, there is a cooperative effect to be accomplished.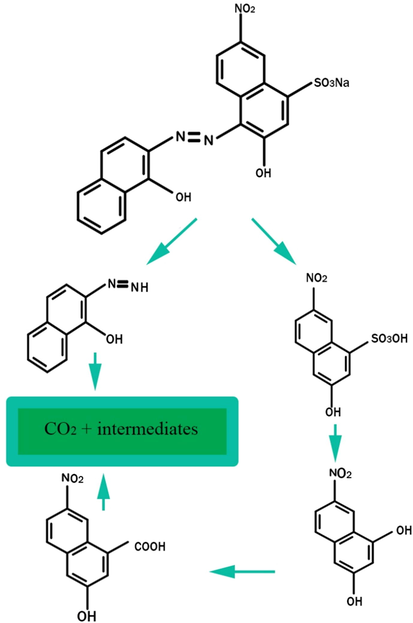
Intermediate photoproducts formed and plausible pathway for the EBT photodegradation.
Materials
Optimum C3N4 loading
Fabrication method
Degradation efficiency
Ref.
NdCoO3/g-C3N4
25%
Sol-gel combustion
95.8% in 120 min/Vis EBT
This work
LaCoO3/g-C3N4
60%
Citric acid sol–gel method-sonication
85% in 5 h/Vis phenol
(Jin et al., 2019a,b)
LaFeO3/g-C3N4
15%
Polyacrylamide gel
97.4% in 120 min/Vis RhB
(Ye et al., 2018)
Cu doped ZnO/g-C3N4
–
Hydrothertmal
100% in 90 min/Vis EBT
(Ahmad and Technology 2020)
TiSnI3/g-C3N4
100%
Coprecipitation-sonication
84.6% in 120 min/Vis RhB
(Yousefzadeh et al., 2022)
TiO2/g-C3N4
66.7%
Solvothermal
99.3% in 120 min/Vis RhB
(Jiang et al., 2016)
CuI/g-C3N4
100%
Coprecipitation-sonication
78.6% in 120 min/Vis MO
(Ghanbari et al., 2021)
AgI/g-C3N4
100%
Hydrothermal
94.1% in 120 min/Vis MO
(Ghanbari et al., 2021)
3.8.7 Kinetic study
The integral method was utilized to ascertain the order of the photocatalytic reaction. According to some surveys, the dye degradation process follows the first-order kinetics model as per:
By plotting the ln(C0/C) vs. degradation time (120 min), the linear plot is acquired with the constant slope (k), which is the first-order rate constant (min−1) associated with photocatalytic activity. At the constant temperature and pH, the value of k indeed depends only on the photocatalytic activity. As denoted in Fig. 10 (b and d), the effect of the manifold parameters on the constant rate of the photodegradation behavior was investigated, revealing that the higher the constant rate, the more acceptable the photocatalytic performance. The constant rate of NdCoO3, g-C3N4, and NdCoO3/g-C3N4 was further determined by 0.0178 min−1, 0.0115 min−1, and 0.0245 min−1, respectively. Thereby, the integration of the g-C3N4 phase into the NdCoO3 NPs improved the photocatalytic process.
4 Conclusion
In this study, the pristine p/n perovskite-type NdCoO3 and NdCoO3/g-C3N4 heterojunction catalysts were fabricated through sol-gel auto combustion in the presence of citric acid, L-valine, and glucose as reductants for morphological engineering. Then, the prepared samples were characterized by XRD, FTIR, EDS, FE-SEM, TEM, BET, DRS, PL, and VSM. The products were further applied as catalysts under visible-light irradiation for 2 h. Besides, various dyes were used as effluents. The outcome denoted the acidic pH and the catalyst dosage had positive effects on the efficiency degradation. On the other hand, creating a heterostructure by the NdCoO3/g-C3N4 nanocomposite with a low bandgap could lead to an ascending trend in the photocatalytic rate and it has degraded 95.8% of EBT in 120 min. Likewise, it is possible to recuperate the photocatalytic performance by changing the phase percentage in the nanocomposite composition. Though the slight agglomeration of the prepared heterostructure may decline the optical degradation process, as highlighted in the related studies and observations, it seems that the NdCoO3/g-C3N4 nanocomposite can upgrade the dye degradation efficiency.
CRediT authorship contribution statement
Salimeh Kianipour: Methodology, Investigation, Software, Formal analysis. Fatemeh Sadat Razavi: Writing – original draft, Visualization, Formal analysis, Software. Morteza Hajizadeh-Oghaz: Software, Formal analysis, Conceptualization, Software. Waleed K. Abdulsahib: Writing – review & editing, Data curation, Investigation. Makarim A. Mahdi: Writing – review & editing, Data curation, Investigation. Layth S. Jasim: Writing – review & editing, Data curation, Funding acquisition. Masoud Salavati-Niasari: Formal analysis, Writing – review & editing, Writing – original draft, Conceptualization, Methodology, Supervision, Project administration, Investigation, Data curation, Validation, Resources, Visualization, Funding acquisition.
Acknowledgments
The authors hereby extend their gratitude to the Research Council of Iran National Science Foundation (INSF, 97017837) and the University of Kashan, Kashan, Iran, for supporting this work by grant No. 159271/SK1.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ahmad, I.J.S., Technology, P., 2020. Comparative study of metal (Al, Mg, Ni, Cu and Ag) doped ZnO/g-C3N4 composites: efficient photocatalysts for the degradation of organic pollutants. 251, 117372.
- Effect of Fe and Bi doping on LaCoO3 structural, magnetic, electric and catalytic properties. J. Mater. Res. Technol.. 2019;8:4831-4842.
- [Google Scholar]
- Ali, Z., Ahmad, I., Amin, B., et al., 2011. Theoretical studies of structural and magnetic properties of cubic perovskites PrCoO3 and NdCoO3. 406, 3800-3804.
- Armstrong, D.A., Huie, R.E., Koppenol, W.H., et al., 2015. Standard electrode potentials involving radicals in aqueous solution: inorganic radicals (IUPAC Technical Report). 87, 1139-1150.
- Synthesis, characterization of NdCoO 3 perovskite and its uses as humidity sensor. Appl. Phys. A. 2019;125:1-9.
- [Google Scholar]
- Tuning of the electronic band structure of fibrous silica titania with g-C3N4 for efficient Z-scheme photocatalytic activity. Appl. Surf. Sci.. 2020;512:145744
- [Google Scholar]
- Perovskite LaNiO3/TiO2 step-scheme heterojunction with enhanced photocatalytic activity. Appl. Surf. Sci.. 2020;503:144287
- [Google Scholar]
- Enhanced photocatalytic activity of La1-xSrxCoO3/Ag3PO4 induced by the synergistic effect of doping and heterojunction. International Ceramics 2021
- [Google Scholar]
- In situ template-free ion-exchange process to prepare visible-light-active g-C3N4/NiS hybrid photocatalysts with enhanced hydrogen evolution activity. J. Phys. Chem. C. 2014;118:7801-7807.
- [Google Scholar]
- Christoforidis, K., Melchionna, M., Montini, T., et al., 2016. Solar and visible light photocatalytic enhancement of halloysite nanotubes/gC 3 N 4 heteroarchitectures. 6, 86617-86626.
- Christoforidis, K.C., Montini, T., Bontempi, E., et al., 2016. Synthesis and photocatalytic application of visible-light active β-Fe2O3/g-C3N4 hybrid nanocomposites. 187, 171-180.
- Christoforidis, K.C., Montini, T., Fittipaldi, M., et al., 2019. Photocatalytic hydrogen production by boron modified TiO2/carbon nitride heterojunctions. 11, 6408-6416.
- Effect of Pt nanoparticles on the photocatalytic activity of ZnO nanofibers. Nanoscale Res. Lett.. 2015;10:1-7.
- [Google Scholar]
- Au nanoparticles-doped g-C3N4 nanocomposites for enhanced photocatalytic performance under visible light illumination. Ceram. Int.. 2020;46:22090-22101.
- [Google Scholar]
- Fu, J., Yu, J., Jiang, C., et al., 2018. g‐C3N4‐Based heterostructured photocatalysts. 8, 1701503.
- Graphitic carbon nitride (g-C3N4) reinforced polymer nanocomposite systems—A review. Polym. Compos.. 2020;41:430-442.
- [Google Scholar]
- A plasmonic Z-scheme three-component photocatalyst g-C3N4/Ag/LaFeO3 with enhanced visible-light photocatalytic activities. Opt. Mater.. 2019;88:229-237.
- [Google Scholar]
- Ghanbari, D., Sharifi, S., Naraghi, A., et al., 2016. Photo-degradation of azo-dyes by applicable magnetic zeolite Y–Silver–CoFe2O4 nanocomposites. 27, 5315-5323.
- Ghanbari, M., Salavati-Niasari, M.J.E., Safety, E., 2021. Copper iodide decorated graphitic carbon nitride sheets with enhanced visible-light response for photocatalytic organic pollutant removal and antibacterial activities. 208, 111712.
- Combustion synthesis of g-C3N4/Fe2O3 nanocomposite for superior photoelectrochemical catalytic performance. Appl. Surf. Sci.. 2020;534:147563
- [Google Scholar]
- Synthesis and characterization of Ag3PO4/LaCoO3 nanocomposite with superior mineralization potential for bisphenol A degradation under visible light. J. Alloy. Compd.. 2017;696:226-233.
- [Google Scholar]
- Guo, J., Li, P., Yang, Z.J.C.C., 2019. A novel Z-scheme g-C3N4/LaCoO3 heterojunction with enhanced photocatalytic activity in degradation of tetracycline hydrochloride. 122, 63-67.
- Enhanced photocatalytic H2-production activity of WO3/TiO2 step-scheme heterojunction by graphene modification. Chin. J. Catal.. 2020;41:9-20.
- [Google Scholar]
- Heidari-Asil, S.A., Zinatloo-Ajabshir, S., Amiri, O., et al., 2020. Amino acid assisted-synthesis and characterization of magnetically retrievable ZnCo2O4–Co3O4 nanostructures as high activity visible-light-driven photocatalyst. 45, 22761-22774.
- A review on graphitic carbon nitride (g-C3N4) based nanocomposites: synthesis, categories, and their application in photocatalysis. J. Alloy. Compd.. 2020;156446
- [Google Scholar]
- Jiang, Z., Zhu, C., Wan, W., et al., 2016. Constructing graphite-like carbon nitride modified hierarchical yolk–shell TiO 2 spheres for water pollution treatment and hydrogen production. 4, 1806-1818.
- One-step impregnation method to prepare direct Z-scheme LaCoO3/g-C3N4 heterojunction photocatalysts for phenol degradation under visible light. Appl. Surf. Sci.. 2019;491:432-442.
- [Google Scholar]
- Jin, Z., Hu, R., Wang, H., et al., 2019. One-step impregnation method to prepare direct Z-scheme LaCoO3/g-C3N4 heterojunction photocatalysts for phenol degradation under visible light. 491, 432-442.
- Construction of heterostructured CuFe2O4/g-C3N4 nanocomposite as an efficient visible light photocatalyst with peroxydisulfate for the organic oxidation. Appl. Catal. B. 2019;244:974-982.
- [Google Scholar]
- Nitrogen defect structure and NO+ intermediate promoted photocatalytic NO removal on H2 treated g-C3N4. Chem. Eng. J.. 2020;379:122282
- [Google Scholar]
- Ling, F., Anthony, O.C., Xiong, Q., et al., 2016. PdO/LaCoO3 heterojunction photocatalysts for highly hydrogen production from formaldehyde aqueous solution under visible light. 41, 6115-6122.
- Origin of high photocatalytic efficiency in monolayer g-C3N4/CdS heterostructure: a hybrid DFT study. J. Phys. Chem. C. 2015;119:28417-28423.
- [Google Scholar]
- Utilization of LaCoO3 as an efficient co-catalyst to boost the visible light photocatalytic performance of g-C3N4. Sep. Purif. Technol.. 2018;201:309-317.
- [Google Scholar]
- Interfacial coupling effects in g-C3N4/SrTiO3 nanocomposites with enhanced H2 evolution under visible light irradiation. Appl. Catal. B. 2019;247:1-9.
- [Google Scholar]
- Maulana, M.I., Nandiyanto, A.B.D., 2019. Economic Evaluation of Different Solvents in the Production of LaCoO3 Nanoparticles Prepared by the Co-precipitation Method.
- Melchionna, M., Fornasiero, P.J.A.C., 2020. Updates on the Roadmap for Photocatalysis. 10, 5493-5501.
- Michel, C.R., Lopez-Alvarez, M.A., Martínez-Preciado, A.H., et al., 2019. Novel UV Sensing and Photocatalytic Properties of DyCoO3. 2019.
- One-pot microwave synthesis of hierarchical C-doped CuO dandelions/g-C3N4 nanocomposite with enhanced photostability for photoelectrochemical water splitting. Appl. Surf. Sci.. 2020;530:147271
- [Google Scholar]
- Enhanced photocatalytic degradation of industrial dye by g-C3N4/TiO2 nanocomposite: Role of shape of TiO2. Adv. Powder Technol.. 2019;30:1089-1098.
- [Google Scholar]
- Physicochemical and electrochemical characterization of CdO/g-C3N4 nanocomposite for the photoreforming of petrochemical wastewater. Mater. Today:. Proc.. 2021;42:15-21.
- [Google Scholar]
- Nakhostin Panahi, P., Rasoulifard, M.H., Gholami, Z., et al., 2020. Synthesis of modificated lanthanide nanoperovskites for photocatalytic removal of azo dyes under visible light irradiation. 1-17.
- BiVO 4/WO 3 nano-composite: characterization and designing the experiments in photodegradation of sulfasalazine. Environ. Sci. Pollut. Res.. 2020;27:44292-44305.
- [Google Scholar]
- Phadtare, D., Kondawar, S., Athawale, A., et al., 2019. Crystalline LaCoO3 perovskite as a novel catalyst for glycerol transesterification. 475, 110496.
- Porcu, S., Castellino, M., Roppolo, I., et al., 2020. Highly efficient visible light phenyl modified carbon nitride/TiO2 photocatalyst for environmental applications. 531, 147394.
- Ranjeh, M., Masjedi-Arani, M., Amiri, O., et al., 2021. Pechini sol-gel synthesis of Ni (II) doped LiMnBO3/Li2MnO3/Li2B2O4 nano-photocatalyst under UV–Vis irradiation. 46, 10324-10336.
- Calcium bismuthate (CaBiO3): a potential sunlight-driven perovskite photocatalyst for the degradation of emerging pharmaceutical contaminants. ChemPhotoChem. 2020
- [Google Scholar]
- Influence of g-C3N4 nanosheets on thermal stability and mechanical properties of biopolymer electrolyte nanocomposite films: a novel investigation. ACS Appl. Mater. Interfaces. 2014;6:429-437.
- [Google Scholar]
- Electron population and water splitting activity controlled by strontium cations doped in KTaO3 Photocatalysts. J. Phys. Chem. C. 2019;123:18387-18397.
- [Google Scholar]
- SrSnO3 perovskite obtained by the modified Pechini method—Insights about its photocatalytic activity. J. Photochem. Photobiol., A. 2019;369:181-188.
- [Google Scholar]
- Wang, R., Ye, C., Wang, H., et al., 2020. Z-Scheme LaCoO3/g-C3N4 for efficient full-spectrum light-simulated solar photocatalytic hydrogen generation. 5, 30373-30382.
- Biological effect of ultraviolet photocatalysis on nanoscale titanium with a focus on physicochemical mechanism. Langmuir. 2015;31:10037-10046.
- [Google Scholar]
- Xiong, G., Zhi, Z.L., Yang, X., et al., 1997. Characterization of perovskite-type LaCoO3 nanocrystals prepared by a stearic acid sol--gel process. 16, 1064-1068.
- Xu, C., Zhou, Q., Huang, W.-Y., et al., 2022. Constructing Z-scheme β-Bi2O3/ZrO2 heterojunctions with 3D mesoporous SiO2 nanospheres for efficient antibiotic remediation via synergistic adsorption and photocatalysis. 1-14.
- Insight into enhanced visible-light photocatalytic activity of SWCNTs/g-C3N4 nanocomposites from first principles. Appl. Surf. Sci.. 2020;530:147181
- [Google Scholar]
- Effects of the citric acid addition on the morphology, surface area, and photocatalytic activity of LaFeO3 nanoparticles prepared by glucose-based gel combustion methods. Ind. Eng. Chem. Res.. 2018;58:609-617.
- [Google Scholar]
- Yang, L., Guo, J., Yang, T., et al., 2021. Self-assembly Cu2O nanowire arrays on Cu mesh: A solid-state, highly-efficient, and stable photocatalyst for toluene degradation under sunlight. 402, 123741.
- Ye, Y., Yang, H., Wang, X., et al., 2018. Photocatalytic, Fenton and photo-Fenton degradation of RhB over Z-scheme g-C3N4/LaFeO3 heterojunction photocatalysts. 82, 14-24.
- Novel synthesis of Pd-CeMnO3 perovskite based on unique ultrasonic intervention from combination of Sol-Gel and impregnation method for low temperature efficient oxidation of benzene vapour. Ultrason. Sonochem.. 2018;48:418-423.
- [Google Scholar]
- Yousefzadeh, F., Yousif, Q.A., Ghanbari, M., et al., 2022. Fabrication of TlSnI3/C3N4 nanocomposites for enhanced photodegradation of toxic contaminants below visible light and investigation of kinetic and mechanism of photocatalytic reaction. 118443.
- Zhang, D., Liang, S., Yao, S., et al., 2020. Highly efficient visible/NIR photocatalytic activity and mechanism of Yb3+/Er3+ co-doped Bi4O5I2 up-conversion photocatalyst. 248, 117040.
- Zhang, D., Liu, H., Su, C., et al., 2019. Combustion synthesis of highly efficient Bi/BiOBr visible light photocatalyst with synergetic effects of oxygen vacancies and surface plasma resonance. 218, 1-7.
- Zhang, D., Liu, X., Wang, S., et al., 2021. Enhanced charges separation to improve hydrogen production efficiency by organic piezoelectric film polarization. 869, 159390.
- Zhang, D., Tang, Y., Qiu, X., et al., 2020. Use of synergistic effects of the co-catalyst, pn heterojunction, and porous structure for improvement of visible-light photocatalytic H2 evolution in porous Ni2O3/Mn0. 2Cd0. 8S/Cu3P@ Cu2S. 845, 155569.
- Fabrication of atomic single layer graphitic-C3N4 and its high performance of photocatalytic disinfection under visible light irradiation. Appl. Catal. B. 2014;152:46-50.
- [Google Scholar]
- Zhou, Q., Huang, W., Xu, C., et al., 2021. Novel hierarchical carbon quantum dots-decorated BiOCl nanosheet/carbonized eggshell membrane composites for improved removal of organic contaminants from water via synergistic adsorption and photocatalysis. 420, 129582.
- A facile route to prepare TiO2/g-C3N4 nanocomposite photocatalysts by atomic layer deposition. J. Alloy. Compd.. 2021;855:157446
- [Google Scholar]







