Translate this page into:
Nephroprotective effects of 4-4(hydroxyl-3 methoxyphenyl)-2-butane against sodium tellurite induced acute kidney dysfunction by attenuating oxidative stress and inflammatory cytokines in rats
⁎Corresponding author. firozalam309@gmail.com (Mohammad Firoz Alam)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The aim of this study was to elucidate the protective action mechanism of 4-4(hydroxyl-3-methoxyphenyl)-2-butane against Sodium tellurite (ST) induced nephrotoxicity in rats. ST is a hazardous substance used in metallurgical and glassware industries, but its renal toxicities have not been well established before. Rats were distributed into four groups, six rats contain in each group. Normal control group given only vehicles only, toxic group given ST 8.5 mg/kg p o, treated groups given ST and 4-(hydroxyl-3-methoxyphenyl)-2-butane(100 mg/kg bwt), and positive control given only treatment drug 4-(hydroxyl-3-methoxyphenyl)-2-butane (100 mg/kg bwt) daily for 14 days. ST administration increases an alteration in biochemical, oxidative stress, cytokines markers, and morphological changes in toxic group. When it was treated with 4-(hydroxyl-3- methoxyphenyl)-2-butane significantly (p < 0.5) restores all these changes such as biochemical markers, antioxidant, inflammatory cytokines, and histopathological improvements in treated group as compared to toxic group. No significant (p > 0.05) changes have been seen in positive control as compared to normal control. In conclusion, 4(hydroxyl-3 methoxyphenyl)-2-butane successfully defended the kidney from oxidative stress, inflammatory cytokines and necrosis against ST intoxication. Thus, significant improvements were reflected and confirms with the improvement in histopathological changes.
Keywords
Nephrotoxicity
4(hydroxyl-3 methoxyphenyl)-2-butane
Sodium tellurite
Oxidative stress
Inflammatory cytokines
Histopathology

1 Introduction
Medicinal plants have always played a significant role in minimizing various health problems since prehistoric times due to minimal side effects and more satisfactoriness. The active ingredient of ginger is 4-4(hydroxyl-3-methoxyphenyl)-2-butane, also called Zengerone. It is a crystalline solid that is sparingly soluble in water and soluble in ether. Its chemical and structural formula is C11H14O3, molecular mass is 194.22 g/mole, melting point is 40–41 °C and boiling point is 187–188 °C (Fig. 1). It has a wide range of pharmacological activities such as anti-emetic, antidiabetic, antioxidant, anti-inflammatory, anti-apoptotic, and anti-cancer activity (Ahmad et al., 2015; Anwer et al., 2019). Zingerone plays a potential role in scavenging free radicals and protecting brain mitochondrial toxicity against sodium tellurite (Alam et al., 2018b). Alam et al. (2018b) also described the potential healing role of Vanillylacetone against carbon tetrachloride-induced hepatotoxicity through attenuating the free radicals, cytokines, and apoptosis in mice.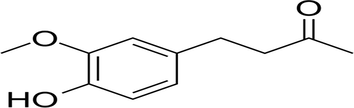
Chemical Structure of 4-(4-hydroxy-3-methoxyphenyl)-2-butatone.
Sodium Tellurite (ST) is an organic potential hazardous compound with white crystalline powder used in metallurgical and glassware industries. Its application is also growing day by day in various industrial processes like coating on iron, steel, aluminum, and batteries etc. (Borsetti et al., 2003). Continuous uses and repeated exposure of ST can increase the risk of health problems in daily workers by direct contact and breathe in dust particles (de Meio, 1947 and Steinberg et al., 1942). Therefore, ST is listed as a hazardous substance because of its high toxicity phenomena, and it is regulated by occupational safety and health administration (OSHA) in the USA (EPA, 1988). Several studies have been reported related to its toxicity in animal models (Schroeder and Mitchener, 1971; Srivastava et al., 1983). ST demonstrates properties of free radicals generation and lipid peroxides formation, which resulted in oxidative stress that causes cell death (Safhi et al., 2016). Oxidative stress activates the release of inflammatory mediators and cytokines. Cytokines are small proteins that regulate cell signaling and play an essential role in inflammations, growth, migration, etc. Inflammatory cytokines such as IL1β, IL-6 and TNFα mediates the inflammation by activation of macro phases (Hamid et al., 2017). Kaur et al. (2003) reported that ST induces neurotoxicity by declining lipid profile in mice's cerebrum, cerebellum, and brain stem. Similarly, Safhi et al. (2016) also reported the adverse effect of ST on liver enzymes through oxidative stress. There are several scientific reports are available for the ST toxicity on different organs except for kidney dysfunction that inspires us to investigate detailed toxicity mechanism in the rats model. We reported the preliminary studies on the effectiveness of Zingerone against sodium tellurite-induced nephrotoxicity on serum biochemical markers only (Alam et al., 2020). In continuation of the previous research, more emphasis has given to oxidative stress, inflammatory cytokines, and histopathological changes. Therefore, this study was designed to investigate the detailed mechanistic approach of 4-4(hydroxyl-3-methoxyphenyl)-2-butane against ST-induced renal toxicity in the rat model.
2 Experimental methodology
2.1 Chemicals and kits
High-grade chemicals like Sodium tellurite, 4-4(hydroxyl-3-methoxyphenyl)-2-butane were acquired from Sigma Aldrich USA. Elisa kits like Interleukin-6 (IL-6), Interleukin 1beta (IL-1β), and Tumor Necrosis Factor (TNFα) were purchased from Abcam, United Kingdom. Kidney markers as Blood Urea Nitrogen (BUN), Uric Acid (UA) and Creatinine (Cr) were obtained from Randox UK.
2.2 Experimental design
Male Wistar rats (200–220 g) were purchased from Medical Research Centre Jazan University Saudi Arabia, and animals were reserved in ideal laboratory condition (12 h dark/light cycle, temp 25 ± 2 °C, and humidity RH-45–55%) for acclimatization before commencing the experiment. The standing committee also approved this study design for scientific research ethics, Jazan University (Letter No 814/705//1440 IRREC). The rats were divided into four groups, with six animals in each group. First control group A (vehicles only), second kidney dysfunction group B (ST 8.3 mg/kg p.o) Safhi et al., 2016, third toxic + treatment group C (ST + 4-4(hydroxyl-3-methoxyphenyl)-2-butane 100 mg/kg treatment) and fourth treatment group was received only 4-4(hydroxyl-3-methoxyphenyl)-2-butane 100 mg/kg. Sodium tellurite was dissolved in water and given orally for 15 days while treatment with 4-4(hydroxyl-3-methoxyphenyl)-2-butane given orally two days before and till 15 days daily. At the end of the experiment, rats were sacrificed under anesthesia (chloral hydrate 200 mg/kg body weight), and kidney tissue was isolated from each group to prepare tissue homogenate. In brief, 10% homogenate of kidney tissue was prepared by using phosphate buffer (0.01 M, pH 7.0) under homogenizer, and it was further centrifuged at 800× g for 10 min at 4 °C to eliminate the cell rubbish. The supernatant was used for lipid peroxidation (LPO) assay, remaining supernatant was further centrifuged at 10 500× g for 30 min at 4 °C for post mitochondrial supernatant (PMS). This PMS was used for the analysis of BUN, UA, Cr, antioxidant enzyme like reduced glutathione (GSH), catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx), glutathione reductase (GR) and glutathione s- transferase (GST), protein estimation and inflammatory cytokines such as IL-1β, IL-6 and TNFα. Kidney tissue was taken from each group and preserved in formalin fixative solution for histopathological analysis.
2.3 Biochemical assay
The biochemical assays for BUN, UA and Cr were measured as per standard procedure of kits by using a spectrophotometer (UV-1800 Shimadzu, Japan).
Lipid peroxidation test was estimated as per Utley et al., 1967 to determine the TBARS (Thiobarbituric reactive substance) level in supernatant of kidney tissue. In detail, 0.1 ml supernatant of kidney homogenate (10%) was used for incubation at 37 °C in a metabolic shaker and another equal volume of it was incubated at 0 °C for 1hr. After that 0.5 ml of 5 % (w/v) chilled trichloroacetic acid (TCA) and 0.5 ml of 0.67%, thiobarbituric acid added and further centrifuged at 3000rmp for 10 min. Thus, different supernatant was collected in a test tube and placed in a boiling water bath for 10 min. After that, pink colour was developed, measured at 535 nm in a spectrophotometer. The TBARS value was calculated using the molar extinction coefficient of 1.56 × 105 M−1CM−1, and the value was represented in TBARS nmol formed per hour g−1 protein.
Glutathione test was estimated as described by the method of Jollow et al. (1974) with minor modification with Alam (2018a). In details, each sample was mixed the 4% sulfosalicylic acid (w/v) in 1:1 ratio (v/v). The samples were incubated at 4 °C for 1hr and centrifuged at 4000×g for 10 min at 4 °C. The further assay mixture was added 1 mM DTNB (5,5-dithio-bis-(2-nitrobenzoic acid) after that yellow colour was developed and measured at 412 nm wavelength in a spectrophotometer. The value was calculated using the molar extinction coefficient of 13.6 × 103 M−1 cm−1, and the value was represented as µmol /mg protein.
The antioxidant enzymes like GPx, GR, GST, CAT and SOD were evaluated as per the standard procedure. The glutathione peroxidase (GPx) was estimated by Mohandas et al. (1984) procedure. The enzymatic activity was calculated as nmol (NADPH) oxidized/min/ mg protein using the molar extinction coefficient of 6.22 × 103 M−1cm–1. Glutathione reductase (GR) was estimated as described by Carlberg and Mannervik (1975). The enzymatic reaction was quantified by measuring the disappearance of NADPH, and the value was calculated nmol NADPH oxidized/min/mg protein using molar extinction coefficient 6.22 × 103 M−1 CM−1.
Glutathione-S-transferase (GST) was estimated by the procedure of Habig et al. In detail, each sample having reaction mixture of phosphate buffer, 0.1 ml of 20 mM reduced glutathione, 0.1 ml of 20 mM of CDNB(1-chloro-2,4-dinitrobenzene) and 0.1 ml of PMS. The changes in absorbance were noted at 340 nm per min for 3 min, and enzymatic activity was calculated as nmol CDNB conjugate formed/min/mg protein using molar extinction coefficient 9.6 × 103 m−1 cm−1.
Catalase (CAT) activity was estimated as described by Claiborne A (1985). In detail, 0.1 ml of 180 mM of hydrogen peroxide, phosphate buffer (0.1 M, pH7.4) and 0.05 ml of post mitochondrial supernatant (PMS). The changes were observed at 240 nm per min for 3 min. The catalase value was calculated using nmol H2O2 consumed/min/mg protein using molar extinction coefficient 43.6 × 103 M−1 cm−1.
The superoxide dismutase (SOD) activity was estimated as per Stevens et al. (2000) method by monitoring the auto-oxidation of (−)-epinephrine at pH10.4 for 3 min at 480 nm. In details the reaction mixture contained glycine buffer (50 mM, pH 10.4), 0.05 ml of 20 mM (−)-epinephrine and 0.2 ml of PMS. The enzyme activity was calculated in nmol (−)-epinephrine protected from oxidation/min/mg protein using the extinction coefficient of 4.02 × 103 M−1 cm−1. Estimation of protein in kidney PMS was performed according to Lowry et al. (1951).
The inflammatory cytokines (IL-1β, IL-6 and TNFα) were analyzed as per standard protocol by using Elisa reader (Elx800TM Biotech, USA).
2.4 Histopathological examination
The kidney tissue sample of each group was fixed in 10% formalin and was processed through gradient alcohol and xylene dehydration. After that, tissue was embedded in paraffin, and blocks were made. Further tissue was sectioned at 5 mm thickness using Leica microtome and then staining with hematoxylin and eosin for histological examination using a light microscope at 10× magnification.
2.5 Statistical analysis
The results were represented in mean ± sem of six replicates by using Graphpad prism 9 software USA. Analysis of variance (one way) and post hock Tukey's were used to calculate the significance among the groups. The value with p < 0.05 was considered to be statistically significant.
3 Results
3.1 Biochemical study
Table 1 represents the magnificently increase in renal markers (UA, BUN, and Cr) in PM of ST induced group B in contrast to normal control. It was restored in treated group C as compared to group B. Here, no significant variations were noticed in group D. Estimation of biochemical markers in ST induced renal toxicity and its treatments with 4-(4-hydroxy-3-methoxyphenyl)-2-butatone in rats. Values are expressed as mean ± SD (n = 6). ap < 0.001 vs group A, bp < 0.001 vs group B and cp > 0.05 vs group A.
Enzymes
Group-A
Group-B
Group-C
Group-D
Blood Urea Nitrogen (mg/dl)
17.83 ± 1.17
34.17 ± 3.25a
21.67 ± 2.16b
18.67 ± 1.37c
Creatinine (mg/dl)
0.94 ± 0.11
1.88 ± 0.10a
1.11 ± 0.35b
0.99 ± 0.26c
Uric Acid (mg/dl)
5.29 ± 1.05
12.72 ± 1.42a
9.22 ± 0.80b
7.18 ± 0.76c
3.2 Oxidative stress
Lipid peroxidation level was measured significantly high (P < 0.001) in ST administered toxic group in contrast to normal control group-A and a significant decrease were also estimated in 4-4(hydroxyl-3-methoxyphenyl)-2-butane treated group C. No significant changes were measured in positive control group D (P > 0.05). Reduced glutathione (GSH) was significantly low (P < 0.001) in ST administered group B in contrast to normal control but the level was restored in 4-4(hydroxyl-3-methoxyphenyl)-2-butane treated group C. Similarly, antioxidant enzymes (GPx, GR, CAT, SOD and GST) content were significantly decreased in ST given kidney dysfunction group B as compared to normal control group A. It was also noticed that treatment with 4-4(hydroxyl-3-methoxyphenyl)-2-butane significant improved in these enzyme contents in the tissue of group C as compared to kidney dysfunction group B. Significant differences were not seen in positive control group D (Table 2). ST induce significant (**p < 0.001) changes in antioxidant markers (LPO, GSH, SOD, CAT, GPx, GR and GST) in Group B as compared to normal control. 4-(4-hydroxy-3-methoxyphenyl)-2-butatone treatment in group C improve these level significantly (*p < 0.01, **p < 0.001,) as compared to group B. No significant (nsp > 0.05) changes noticed in 4-(4-hydroxy-3-methoxyphenyl)-2-butatone treated group D as compared to Group A. a, b value in parentheses represent the percentage change vs control and vs all ST treated group. All values are represented in Mean ± SD (n = 6).
Antioxidant Markers
Group A
Group B
Group C
Group D
LPO (TBARS nmol/g tissue)
8.57 ± 0.73
22.96 ± 0.79** (167.91%)
11.16 ± 0.58** (−51.39%)
9.29 ± 0.84ns (8.40%)
GSH (µmol/mg protein)
16.25 ± 0.99
5.57 ± 0.41** (−65.72%)
12.72 ± 0.65** (128.36%)
17.25 ± 0.66ns (6.15%)
SOD (nmol epinephrine protected from oxidation/min/ mg protein)
53.9 ± 1.07
20.02 ± 1.47** (−62.85%)
34.42 ± 2.23** (71.92%)b
54.57 ± 1.59ns (1.24%)
CAT (nmol of H2O2consumed/min/ mg / protein)
22.5 ± 1.20
10.91 ± 1.28** (−51.51%)a
18.25 ± 2.77** (67.27%)b
23.81 ± 2.10ns (5.8%)
GPx (nmol NADPH oxidized/min/mg/protein)
36.36 ± 7.97
17.028 ± 3.20** (−53.19%)a
28.21 ± 3.48* (65.64%)b
37.37 ± 2.45ns (2.74%)
GR (nmol NADPH oxidized/min/mg/protein)
29.18 ± 3.61
15.36 ± 3.83** (−47.36%)a
24.96 ± 3.60** (62.39%)b
30.43 ± 3.10ns (4.28%)
GST (nmol CDNB conjugate/min/mg protein)
40.47 ± 1.03
19.69 ± 1.63** (−51.34%)a
29.72 ± 3.25* (50.93%)b
41.97 ± 1.94ns (3.70%)
3.3 Inflammatory cytokines
Figs. 2–4 represents the effects of sodium tellurite on inflammatory cytokines (IL-1β, IL-6 and TNFα). These cytokines were significantly higher in kidney dysfunction group B than in normal control group A. Treatment with 4-4(hydroxyl-3-methoxyphenyl)-2-butane attenuated these cytokines in group C as compared to kidney dysfunction group B. There were no significant variances were measured in positive control group D in contrast to normal control group A.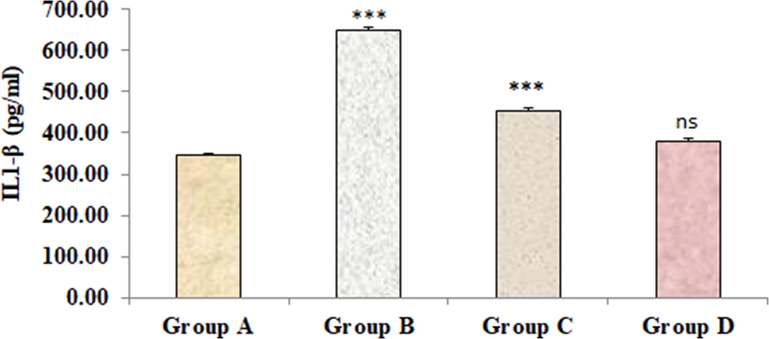
Effect of 4-(4-hydroxy-3-methoxyphenyl)-2-butatone treatment on renal inflammatory cytokine IL-1β (A) content induced by ST. Data represented as mean ± SD (n = 6), *p < 0.001 vs. Group A (control), #p < 0.001 vs. Group B (ST) and nsp > 0.05 Group D vs Group A.
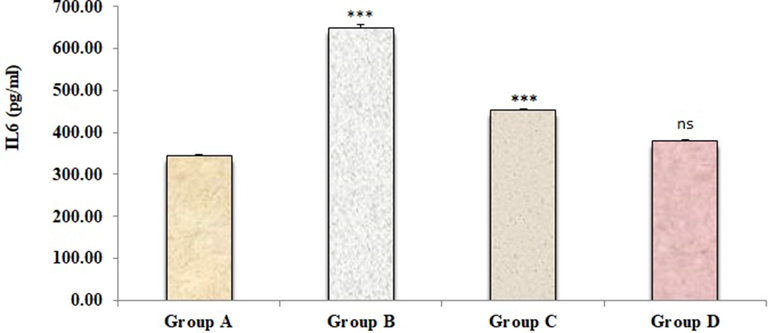
Effect of 4-(4-hydroxy-3-methoxyphenyl)-2-butatone treatment on renal inflammatory cytokine IL-6 (B) content induced by ST. Data represented as mean ± SD (n = 6), *p < 0.001 vs. Group A (control), #p < 0.001 vs. Group B (ST) and nsp > 0.05 Group D vs Group A.
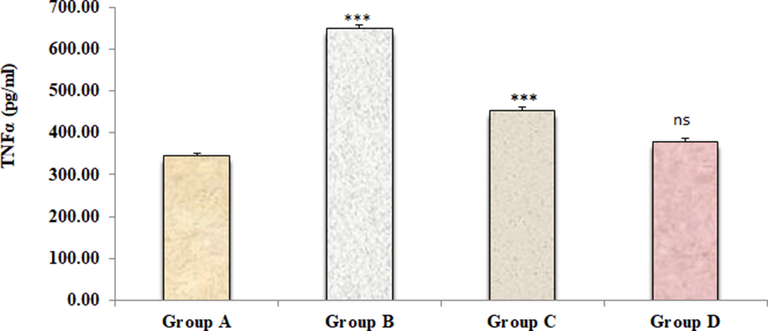
Effect of 4-(4-hydroxy-3-methoxyphenyl)-2-butatone treatment on renal inflammatory cytokine TNFα (C) content induced by ST. Data represented as mean ± SD (n = 6), *p < 0.001 vs. Group A (control), #p < 0.001 vs. Group B (ST) and nsp > 0.05 Group D vs Group A.
3.4 Histopathological examination
Microscopic examination of renal histology showed a normal structure with intact glomeruli and regular shape of renal tubules in normal control group A (Fig. 5A). However, the renal architecture was found disturbed with administration ST in kidney dysfunction group B. In this group, ST induced lesion in renal tubules and severe degenerative changes in the glomerular basement membrane. These lesions were observed as hemorrhage, congestion, vacuolation, coagulative necrosis, and edema in group B (Fig. 5B). However, these severities were observed to reduce after treatment with 4-4(hydroxyl-3-methoxyphenyl)-2-butane in group C vs group B. In this group improvement in the morphology of glomeruli, proximal, distal tubules and vacuolization have been noticed as compared to group B (Fig. 5C). A significant lesion was not observed in positive control group D, and its histology was similar to normal control without any changes (Fig. 5D).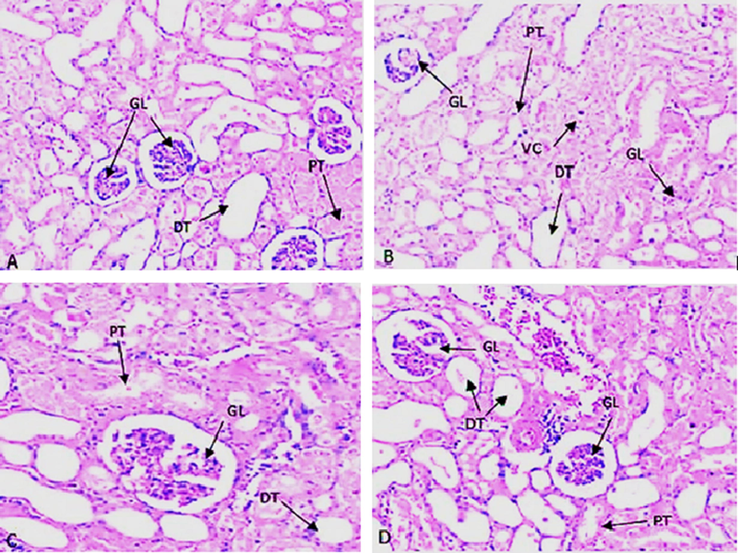
(A to D). Histopathological changes by H&E staining represents (A) Normal control with clear glomerular basement membrane (GL), normal proximal tubule (PT), Distal convoluted tubule (DT) and no vacuolization(VC); (B) ST group with clear damages in GL, PT, DT along with debris in duct and also seen Vacuolization(VC). (C) ST and drug treatment group (4-(4-hydroxy-3-methoxyphenyl)-2-butatone) with significant improve in GL, PT, DT and disappearing of Vacuolization. (D) Drug treatment (4-(4-hydroxy-3-methoxyphenyl)-2-butatone) with no significant changes in GL, PT, and DT etc.
4 Discussion
ST is enormously used in the metallurgical industry and is considered a hazardous substance due to high toxicity phenomena. Its continuous exposure to the occupation can lead to serious health problems related to kidney dysfunction in humans. A kidney is a vital organ that plays a crucial role in excusing waste chemicals and its metabolite from the body. The potential of ST toxicity depends on the accumulation of dimethyl telluride metabolite of ST in humans' liver, spleen, and kidney etc. which may be responsible for the reactive oxygen species production that resulted in oxidative stress and cell death (Keall et al., 1946; Amdur, 1947).
This study indicates that continuous oral exposure of ST increases the free radicals by increasing lipid peroxidation. Thus, increasing free radicals resulted in the production of reactive oxygen species (ROS) with high specificity to bind the cellular lipid and protein that degrade the lipid membrane. Lipid peroxidation is an important marker of oxidative stress. Different kinds of free radical generation occur that bind to intracellular protein and lipid of the cell membrane, resulting in cell toxicity or death. Results revealed that administration of ST elevated the lipid peroxidation or TBARS content due to the generation of reactive oxygen. Furthermore, the action mechanism of ST has reported a decrease in cellular content of glutathione with a consequent increase in the production of ROS (Tremaroli et al., 2007).
GSH is an essential endogenous antioxidant that helps control cellular damage due to free radicals and peroxide generation. It also plays a vital role in regulating antioxidant and anti-inflammatory genes (Slater, 1984; Gao et al., 2006). Glutathione inhibition increased the susceptibility of lipid membrane towards peroxide attack in the tissue. This study indicated that GSH content was reduced in ST administered group B.
The antioxidant metabolizing enzyme (GPx, GR, and GST) activity was reduced significantly in ST induced group B. GPx plays a key role in the antioxidant defence system by eradicating free radicals (Kurutas, 2016) through conversion of H2O2 in the water molecule and accepting proton that produces reduced glutathione (GSH) after oxidation. Similarly, GR is also an important antioxidant enzyme that converts the oxidized glutathione (GSSG) to reduced glutathione (GSH). Thus, GPx and GR help to recycle the reduced glutathione by normal redox catalytic reaction. Different scientists in different organ also reported these decreased activities of GPx and GR.
GST is also another metabolizing enzyme that plays an important role during the glutathione-s-conjugate formation, where GSH is consumed by the cell and total intracellular GSH content reduced, which causes toxicity to the cell. The GST content was also reduced in ST administered group B.
The activity SOD and CAT was also declined in ST administered group B. SOD catalyzes the very toxic superoxide into fewer toxic hydrogen peroxide (H2O2), and catalases detoxify the hydrogen peroxide in water and oxygen that is need of cell surviving. Thus ST has depleted the activities of these enzymes resulted in high content of hydrogen peroxide inside the cell that causes renal toxicity. Previous reports also support the lowest doses (0.5 mg/kg) of ST is responsive to changes in body weight and kidney problem in rat and rabbit (Taylor, 1996). Overall, increasing oxidant stress resulting in decreased antioxidant status (Taysi et al., 2008). Thus misbalancing of the above all enzymes creates oxidative stress inside the cell and decreasing the antioxidant enzymes resulted in kidney dysfunction by elevated renal markers in PMS.
Natural products are good sources of antioxidant properties that suppress the ROS production, and free radicals generation to prevent liver, kidney, and brain damages (Tirkey et al., 2005; Jayakumar et al., 2008). The effective repair of lipid peroxidation and enchantment of GSH content as well as all antioxidant enzymes were observed in 4-4(hydroxyl-3-methoxyphenyl)-2-butane treated group C. The significant protection and improvement in these enzymes GPx, GR, GST, CAT and SOD were noticed in 4-4(hydroxyl-3-methoxyphenyl)-2-butane treated group C.
The present finding also emphasizes the inflammatory cytokines directly or indirectly associated with oxidative stress that increases the production of inflammatory cytokines and vice versa. There are several cellular and molecular factors that influence kidney dysfunction. ST metabolite (dimethyl telluride) may be accountable for the development of ROS and oxidative stress that activates the transcription factor NF-kB to the nucleus that mediates the inflammatory changes and renal expression of TNFα. TNFα plays a major role in many inflammatory diseases because of this role as a master regulator of inflammatory cytokines production (Maini et al., 1995). Thus, TNFα promotes inflammatory cytokines such as IL-1β and IL-6 (Ramesh et al., 2007; Volarevic et al., 2019). These cytokines are released by leukocytes in tubular cells and are directly associated with inflammation in kidney tissue (Elmarakby and Sullivan, 2012). In this study, these cytokines (IL-1β, IL-6 and TNFα) were increased after oral exposure to ST in group B. However, these cytokines' significant protection and improvements were noticed in 4-4(hydroxyl-3-methoxyphenyl)-2-butane treated group C. Thus 4-4(hydroxyl-3-methoxyphenyl)-2-butane showed potential renal protection against sodium tellurite administration.
The above findings were also supported by histopathological evaluation of the kidney section. Thus, ST administration indicates kidney dysfunction in rats by visible histopathological changes in glomeruli, tubular damages, vacuolization, etc., compared to normal control. However, the treatment with 4-4(hydroxyl-3-methoxyphenyl)-2-butane protected the kidney from these damages and helped to improve glomerular texture, disappearing vacuolization, tubular regeneration etc. Thus, the clinical significance of this study will be more effective in managing nephrotoxicity, especially for those who work in the metallic industry, where there are more chances of sodium tellurite exposure. Therefore, zinger can be a preventive measure to avoid any renal complication/dysfunction due to ST.
5 Conclusion
This study approves that ST induces kidney toxicity by accelerating oxidative stress, inflammatory cytokines and by depleting the antioxidant enzymes in tissues. 4-4(hydroxyl-3-methoxyphenyl)-2-butane treatment also confirms the significant attenuation of oxidative stress, inflammatory cytokines and improves the antioxidant enzymes. In this way, 4-4(hydroxyl-3-methoxyphenyl)-2-butane may better protect against ST-induced renal toxicity by regenerating kidney cells.
Acknowledgement
Not applicable.
Author contribution
Conceptualization, M. F. A; methodology, M.F.A., S. Al. S and T. A. Dosing and sample collection, E. A. A. , M. A. A. and A. A. Al. S; formal analysis and investigation, G. K ,A. K and M. F.A; Elisa, S. S. M. and M. F.A; Histopathology, M.F.A. K.T.A.and G. K., Data analysis, T.A, M.F.A and A.K.; writing and reviewing, M.F.A and S. Al. S. All authors have read and agreed to publish the manuscript.
Data availability statement
The data used to support the findings of this study are included within the article.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Review on pharmacological properties of Zingerone (4-(4-hydroxy-3 methoxyphenyl)-2-butanone) Sci. World J.. 2015;1–6
- [CrossRef] [Google Scholar]
- Zingerone ameliorates tellurium-induced nephrotoxicity by abating elevated serum markers in the rats. Environ. Conserv. J.. 2020;21(1 & 2):125-129.
- [CrossRef] [Google Scholar]
- Neuroprotective effects of Zingerone against carbon tetrachloride (CCl4) induced brain mitochondrial toxicity in Swiss albino mice. J. Nat. Appl. Sci.. 2018;10(2):548-552.
- [CrossRef] [Google Scholar]
- Therapeutic potential of Vanillylacetone against CCl4 induced hepatotoxicity by suppressing the serum marker, oxidative stress, inflammatory cytokines, and apoptosis in Swiss albino mice. Exp. Mol. Pathol.. 2018;105(1):81-88.
- [CrossRef] [Google Scholar]
- Modulatory effect of Zingerone against STZ-nicotinamide induced type-2 diabetes mellitus in rats. Arch. Physiol. Biochem.. 2019;127(4):304-310.
- [CrossRef] [Google Scholar]
- Tellurite uptake by cells of the facultative phototroph Rhodobacter capsulatus is a Delta pH-dependent process. FEBS Lett.. 2003;554(3):315-318.
- [CrossRef] [Google Scholar]
- Catalase activity. In: Greenwald R.A., ed. Handbook of Methods for Oxygen Radical Research. Boca Raton, Florida: CRC Press; 1985. p. :283-284.
- [Google Scholar]
- Tellurium effect of ascorbic acid on the tellurium breath. J. Indust. Hyg. Toxicol.. 1947;29(6):393-395.
- [CrossRef] [Google Scholar]
- Relationship between oxidative stress and inflammatory cytokines in diabetic nephropathy. Cardiovasc. Ther.. 2012;30(1):49-59.
- [CrossRef] [Google Scholar]
- Resveratrol reduces the elevated level of MMP-9 induced by cerebral ischemia-reperfusion in mice. Life. Sci.. 2006;78(22):2564-2570.
- [CrossRef] [Google Scholar]
- Glutathione-S-transferase. The first enzymatic step in mercapturic acid formation. J. Biol. Chem.. 1974;249(22):7130-7139.
- [Google Scholar]
- Amelioration of CCl4-induced liver injury in rats by selenizing Astralagus polysaccharides: Role of pro-inflammatory cytokines, oxidative stress, and hepatic stellate cell. Res. Vet. Sci.. 2017;114:202-211.
- [CrossRef] [Google Scholar]
- Pleurotus ostreatus, an oyster mushroom, decreases the oxidative stress induced by carbon tetrachloride in rat kidneys, heart, and brain. Chem. Biol. Interact.. 2008;176(2–3):108-120.
- [CrossRef] [Google Scholar]
- Bromobenzene-induced liver necrosis: Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology.. 1974;11(3):151-169.
- [CrossRef] [Google Scholar]
- Tellurium-induced dose-dependent impairment of antioxidant status: differential effect in cerebrum, cerebellum and brain stem of mice. Biol. Trace. Elem. Res.. 2003;94(3):247-258.
- [CrossRef] [Google Scholar]
- A report of three cases of accidental poisoning by sodium tellurite. Br. J. Ind. Med.. 1946;3:175-176. PMC1035750
- [Google Scholar]
- The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr. J.. 2016;15(1):71.
- [CrossRef] [Google Scholar]
- Protein measurement with the Folin phenol reagent. J. Biol. Chem.. 1951;193(1):265-275.
- [Google Scholar]
- Beneficial effects of tumour necrosis factor-alpha (TNF-alpha) blockade in rheumatoid arthritis (RA) Clin. Exp. Immunol.. 1995;101(2):207-212.
- [CrossRef] [Google Scholar]
- Low activities of glutathione-related enzymes as factors in the genesis of urinary bladder cancer. Cancer Res. 1984;44:5086-5091.
- [Google Scholar]
- Endotoxin and cisplatin synergistically induce renal dysfunction and cytokine production in mice. Am. J. Physiol. Renal Physiol.. 2007;293(1):325-332.
- [CrossRef] [Google Scholar]
- Repeated exposure of Sodium Tellurite on the rat liver and on the potential mechanisms of the metalloid-induced hepatotoxicity. Acta. Pol. Pharm.. 2016;73(3):675-682.
- [Google Scholar]
- Selenium and tellurium in rats: effect on growth, survival, and tumors. J. Nutr.. 1971;101(11):1531-1540.
- [CrossRef] [Google Scholar]
- Effect of organo-tellurium compounds on the enzymatic alterations in rats. Toxicol. Lett.. 1983;16(3–4):311-316.
- [CrossRef] [Google Scholar]
- Industrial exposure to tellurium: Atmospheric studies and clinical evaluations. J. Ind. Hyg. Toxicol.. 1942;24(7):183-192.
- [CrossRef] [Google Scholar]
- Effects of DL-alpha-lipoic acid on peripheral nerve conduction, blood flow, energy metabolism and oxidative, blood flow, energy metabolism, and oxidative stress in experimental diabetic neuropathy. Diabetes. 2000;49(6):1006-1015.
- [CrossRef] [Google Scholar]
- Increased oxidant stress and decreased antioxidant status in erythrocytes of rats fed with zinc-deficient diet. Biol. Trace Elem. Res.. 2008;123(1–3):161-167. Epub 2008 Feb 14. PMID: 18273565
- [CrossRef] [Google Scholar]
- Hesperidin, a citrus bioflavonoid, decreases the oxidative stress produced by carbon tetrachloride in rat liver and kidney. BMC Pharmacol.. 2005;5(1):127-135.
- [CrossRef] [Google Scholar]
- Evidence for a tellurite-dependent generation of reactive oxygen species and absence of a tellurite-mediated adaptive response to oxidative stress in cells of Pseudomonas pseudoalcaligenes KF707. Arch Microbiol.. 2007;187(2):127-135.
- [CrossRef] [Google Scholar]
- U.S. Environmental Protection Agency, Washington, DC, 1988, EPA/600/6-87/008 (NTIS PB88179874). https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=34855.
- Effect of sulfhydryl reagent on peroxidation in microsomes. Arch. Biochem. Biophys.. 1967;118(1):29-32.
- [CrossRef] [Google Scholar]
- Molecular mechanisms of cisplatin induced nephrotoxicity: a balance on the knife edge between Reno protection and tumor toxicity. J. Biomed. Sci.. 2019;26(1):25.
- [CrossRef] [Google Scholar]






