Translate this page into:
The Biological and Chemical Ameliorative Effects of Bread Substituted with Dried Moringa Leaves
⁎Corresponding author. ahmed.abdelfatah@fsed.bu.edu.eg (Ahmed A. Aly)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
This study conducted to evaluate the effect of additional dried moringa leaves on bread and their effects on the chemical and biological of rats fed it. Wheat flour with dried moringa leaves powder (DMLB) is used at different levels (6 and 9%) to produce moringa bread. We performed a sensory evaluation of wheat bread substituted with dried moringa leaves, chemical properties (approximate composition and antioxidants), microbiology (total bacteria, spore-forming bacteria, fungi, and yeast) and biological analysis (Triglycerides, total cholesterol, HDL and LDL, ALT, AST, urea, creatinine). Therefore, histopathology examination for kidney and livers of male albino rats fed fortified bread with dried moringa leaves compared with the control sample. The results of the approximate analysis showed significant differences by adding moringa leaves and the percentage of ash, protein, fat, and fiber increased with the moisture content. Moreover, carbohydrates decreased from fortified bread with dried moringa leaves compared to the control sample. The best treatment was fortified bread with 6% dried moringa leaves for all sensory evaluations compared to the other samples and moringa bread (DMLB 9%) helped reduce the microbial load during storage. The effect of moringa bread on liver cirrhosis was evaluated in rats induced by carbon tetrachloride CCl4. We found out that the effect of moringa leaves on liver and kidney functions in a state of improvement, as for high-density lipoprotein (HDL) and low-density lipoprotein (LDL), the proportion of LDL was less than HDL compared to the control sample. At the same time, moringa leaves have a significant effect on reducing cholesterol, triglycerides, urea, and creatinine in the serum of rats, which may be attributed to the presence of biologically active plant components. It can be concluded that moringa leaves can improve biological and histological of rats feed it.
Keywords
Moringa Leaves
Chemical properties
Sensory evaluation
Microbial load
Biological
Histopathological
1 Introduction
The moringa plants are the most cultivated species belonging to the moringaceae family. This plant is native to northern India, Asia, and Africa and covers 13 species of tropical climates (Al_husnan & Alkahtani, 2016; Salaheldeen et al., 2014). While moringa oleifera is famous (the tree of life) because it contains multiple ingredients including vitamins, enzymes, amino acids, lipids, and minerals. As a result, it is considered a good source of nutrients for human use (Mensah et al., 2012). It is also possible to extract many usable oils with different combinations of fatty acids from the maringa oleifera tree. (Al Juhaimi et al., 2016) And also the moringa tree has the ability to provide humans with a torrent of nutrients that far exceeds all other animal and vegetable sources of minerals, vitamins, amino acids, and antioxidants, as the dried leaf powder contains 9 times the protein found in yogurt, 4 times the vitamin A found in carrots, 19 times the iron Found in spinach, 17 times the calcium, 4 times the fiber in oats, 4 times the potassium in bananas, 7 times the vitamin C found in oranges (Rockwood et al., 2013). However, moringa oleifera is used in the manufacture of animal feed, food fortification, and the treatment of arthritis. Some of the major and highly marketed moringa products are dried moringa leaf tea, moringa sausages, moringa biscuits, burgers, and moringa bread. moringa sauce for soup and moringa noodles; however, further investigations are needed to increase the nutrient quality and shelf life of all beneficial products (Mani et al., 2007), even in industrialized countries, it is one of the most important therapeutic benefits of this plant. It can be used as a treatment of anemia, diseases of the heart, brain, nerves, cancer, prevention of vision loss due to vitamin A deficiency, treatment of cystitis, treatment of prostatitis. The juice of leaves mixed with lemon is used to treat dropsy because it works as a diuretic, treats boils and pimples, treats diarrhea, treats liver and spleen, and treats skin diseases and rheumatism (Farooq et al., 2012; Abraham and Okon, 2014; Rahman et al., 2009; Abd-Rabou et al., 2017; Al-Asmari et al., 2015), anti-inflammatory (Abdull Razis et al., 2014), antifungal (Zaffer et al., 2015; Patel et al., 2014). Being a good storehouse of antioxidants (Vats and Gupta, 2017) Because of its high nutritional content and antibacterial qualities, all portions of the M. oleifera plant are used as a food fortifier in a variety of foods, including Cakes, cheese, bread, dairy, moringa tea, soups, and biscuits were also added to meat products (Oyeyinka and Oyeyinka, 2018). While that moringa increases the nutritional value of the dough and reduces the potential for it to retrograde. The crude protein level was boosted by 48 percent by adding 10% leaf powder, despite the color being poorly evaluated (He et al., 2013). The liver is a primary organ that processes crucial biochemical and physiological processes such as metabolism and detoxification of endogenous and exogenous chemicals such as medicines and xenobiotics, as well as homeostasis, growth, energy, and nutrient supply (Mahmood et al., 2014; Saleem et al., 2018). Cirrhosis is a very difficult disease that occurs when the liver becomes scarred, and the liver is a large organ that belongs to the digestive system (Tsukada et al., 2006; Török, 2008). Since a long time ago, herbal medicines have been used for treating liver problems, as many studies have proven the effectiveness of herbal medicines and their effect on cirrhosis at the medical level (Schuppan et al., 1999; Luk et al., 2007). Bread has been known to be a stable food all over the world for a long time. It is an excellent source of energy and easily digestible carbohydrates. It is also low in cost. Wheat flour with dried moringa leaf powder (DMLB) is used at different levels (6 and 9%) to produce moringa bread. This study aims to manufacture an affordable, low-cost product and advantage nutritional value, throw adding moringa leaves with 6 and 9 % to the formula of wheat bread. Moreover, studying the effect of moringa leaves on the functional of liver of male albino rats with cirrhosis and evaluate some parameters of biological analysis such as Triglycerides, total cholesterol, HDL and LDL, ALT, AST, urea, creatinine for male albino rats feed on fortified bread with moringa leaves.
2 Materials and method
2.1 Materials
Moringa oleifera leaves were obtained from trees planted in a region in Menoufia governorate, Egypt. Wheat flour was provided from the local market. As for the chemicals that were used, they were obtained from the Arab Company for Pharmaceuticals and Chemical Industries, Al-Amiriya, Cairo. Carbon tetrachloride (10%) was used to poison the liver.
2.1.1 Preparing moringa leaves
Branches of trees were collected and cleaned with water. The leaves were removed and dried at 45 ± 3 ˚C using Binder FED 53-UL Forced Convection Drying Oven in the university laboratory. After completion, they were ground and packed into thin polyethylene bags until further analyses (Fig. 1).
Preparing of moringa leaves: show preparing of moringa leaves including collect the moringa leaves, and grind them before add to bread.
2.1.2 Baking technology of wheat bread
Ingredients consist of kilo wheat flour 72% ext. 700 ml of water, 10 g of dry yeast were mixed, and 10 of salt were added. And the same ingredients are added to prepare the moringa bread in addition, the dried moringa leaves were put in proportions of 6, 9%, After leaving the dough for 15 min to rest, it was cut into 175 g. Molds were placed with a layer of soft wheat rum and left to ferment for 45 min. It started to flatten to about 25 cm in diameter and bake on high. - Oven temperature (450–550 °C) from 1 to 3 min after baking in January 2020. Leave the bread to cool at (20 °C) and after finishing the sensory evaluation of the bread (Fig. 2).
Balady bread prepared using dried moringa leaves powder as a partial substitute for wheat flour.
2.2 Analytical methods
2.2.1 Chemical analysis of bread
Bread samples were distributed in Petri dishes and dried in the oven at 105–110 °C for moisture determination. The bread samples were divided into the crucible and placed in the ash oven) Muffle) to estimate the percentage of ash. The crude fat, protein and crude fiber were analyzed according to AOAC, (2019). For total carbohydrates, it was estimated by the difference method [100 - (moisture + ash + protein + fat + fiber %) according to Aly et al., (2021).
2.2.2 Antioxidant activity
The antioxidant activity of moringa oleifera leaves was evaluated by the radical scavenging activity (DPPH) method mentioned by Ismail et al., (2020).
2.2.3 Sensory evaluation of processed wheat bread
Sensory evaluation: A group of arbitrators (15 females) evaluated the bread samples in the university laboratory, Faculty of Specific Education in Benha. The values were recorded in the sensory evaluation form for food, which includes texture, color, smell, taste, and appearance, and this continued for a week. Aly et al., (2020).
2.2.4 Microbiological analysis
Microbial loads including total bacterial count, molds, yeasts, and spore-forming bacteria of bread supplemented with moringa leaves, were determined as methods described by Aly et al., (2021b).
2.2.5 Biological evaluation
2.2.5.1 Animals
In this study, twenty male albino rats (150 ± 10 g) were obtained through the Agricultural Research Center, Giza, Egypt. Rats were placed in wire cages under normal laboratory rats and fed on a standard diet for one week as an adaptation period. The rats were given a diet in special food cups to avoid loss feeding conditions. Also, the water was supplied by glass tubes supported on one side of the cage to the rats, as checked daily (Aly et al., (2021c).
2.2.5.2 Induction of liver intoxication in rats
Male albino rats were injected with the first dose at the beginning of the week and the second in the middle, subcutaneously with carbon tetrachloride (CCl4) and paraffin oil at 5 % V / V (2 ml/kg by weight). Liver cirrhosis occurs two weeks after injection, according to the method described by Aly et al., (2021b).
2.2.5.3 Experimental design
Rats were placed in wire cages at room temperature around 25 °C and the experiment was conducted at the Agricultural Research Center in Cairo, under normal hygienic conditions.
The rats' groups were divided as follows:
Group 1: (-ve) 5 rats fed on basal diet only, as a negative control.
Group 2: (+ve) 5 rats fed on basal diet.
Group 3: 5 rats feed on moringa-free bread (control).
Group 4: 5 rats feed on bread fortified with dried moringa powder at a rate of 9%.
2.2.5.4 Blood samples and organs
At the end of the experiment, blood samples were collected after 12 h of fasting. The rats were scratched under diethyl ether. Anesthetized blood samples were received. Then the samples were drawn through an artery in the eye and left to clot at room temperature and then placed in the middle for 10 min at 3000 cycles per minute to separate serum plasma was carefully separated, transferred to clean cuvette tubes, and stored frozen at −20 °C for analysis Simultaneously the organs: kidney, the liver was removed, washed in saline, dried with filter paper, weighed, and frozen in 10% formalin solution % for histopathological testing according to the described method, by Drury and Wallington, (1980) (Fig. 3).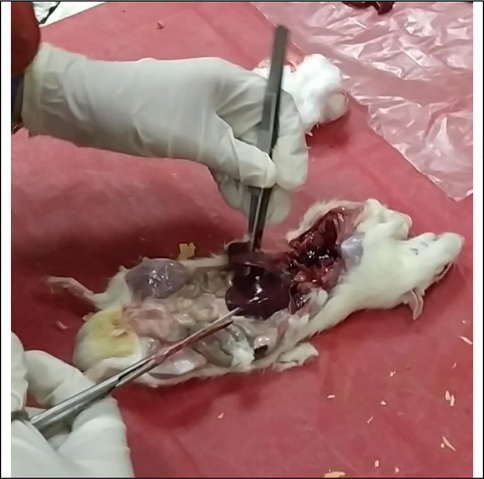
Collect organ from the animal model.
2.2.5.5 Biological analysis
Triglycerides, total cholesterol, HDL and LDL, ALT, AST, urea, creatinine for male albino rats feed on fortified bread with moringa leaves determined according to the method described by Aly et al. (2021a).
2.2.6 Histopathological investigation
Liver and kidney samples were collected and placed in formalin solution with different concentrations Aly et al., (2021d).
2.2.7 Statistical analysis
All experiments were repeated in triplicate and evaluated using one-way analysis of variance (ANOVA) and data averaging statistical differences at p < 0.05 considered significant. Ismail et al., (2020) Çoban, et al (2020).
3 Results d and dissection
3.1 Chemical composition of wheat bread and bread supplemented bread with moringa leaves
Table 1 show that the chemical composition of dried moringa leaves. It have been contain a high percentage of carbohydrates as the moisture content was lower compared to the other treatments due to the effect of the drying process by (5.53% ± 0.15) and it contained ash (6.40% ± 0.10), fibers (10.37% ± 0.15) and protein (26.13% ± 0.15) and these results are consistent with (Folin et al. 2011). It was observed that all nutritional parameters increased significantly except for the moisture content, which decreased as a result of drying. These results were confirmed by (Özcan, 2020) which showed that carbohydrates were higher compared to other treatments in moringa oleifera leaves While the chemical composition of dried moringa bread and bread prepared from wheat flour was 72%, showed that moisture content of moringa bread was lower than that of wheat bread, which was 22.10% ± 0.10 and 25.00% ± 0.10, respectively. (Al Juhaimi et al., 2016) reported that the moisture levels in moringa oleifera leaves low and thus helped reduce the moisture content in moringa bread, while moringa bread (prepared by dried leaf 9%) had a higher ratio of ash (2.00% ± 0.10) than wheat bread (0.63% ± 0.02). Also, moringa bread had a higher ratio of fat (2.74 %± 0.06), fiber (1.25% ± 0.02), and protein (10.10 %± 0.36) compared to the wheat flour while noting a lower proportion of carbohydrates. These results are consistent with Olaitan et al. (2014) The results were confirmed by (El-gammal et al., 2016) which indicated that with the increase in the proportions of moringa leaf powder, the content of crude protein, crude fat and ash gradually increased, while the content of moisture and carbohydrates gradually decreased. Therefore, the moisture content of any of the foods is a good indicator of the shelf life and the quality of foods, and it is necessary to measure the moisture levels in bakery products because of its potential impact on the chemical, microbial, sensory and physical properties of these products. Different letters (a,b,c) within row show significant differences between values (p < 0.05). Data are indicated as: average (For three replicates) ± standard deviation (SD); % on dry weight basis.*: Least significant differences.
Constituents (%)
Dried moringa leaves
Bread prepared by wheat flour 72%
Bread prepared by dried leaf 6%
Bread prepared by dried leaf 9%
L.S.D*0.05
Moisture
5.53 ± 0.15
25.00 ± 0.10a
22.80 ± 0.10b
22.10 ± 0.10c
0.19
Total lipids
6.17 ± 0.15
1.94 ± 0.08c
2.52 ± 0.03b
2.74 ± 0.06a
0.12
Ash
6.40 ± 0.10
0.63 ± 0.02c
1.68 ± 0.16b
2.00 ± 0.10a
0.20
Crud protein
26.13 ± 0.15
7.90 ± 0.20b
9.52 ± 0.44a
10.10 ± 0.36a
0.69
Fiber
10.37 ± 0.15
0.84 ± 0.02c
1.19 ± 0.02b
1.25 ± 0.02a
0.04
Total carbohydrates
45.47 ± 0.40
63.69 ± 0.21a
62.29 ± 0.37b
61.53 ± 0.46c
0.73
3.1.1 Antioxidant activity (DPPH)
Free radical scavenging activity (DPPH) of dried moringa leaves. The free radical scavenging activity of dried moringa leaves reached 86.9%, which indicates the ability of moringa to scavenge because it contains a high percentage of antioxidants, as it reduces free radicals. These results are consistent with (Pakade et al., 2013), where he referred to the ability of moringa leaves to scavenge and compared between moringa and a group of vegetables. The activity of antioxidants was twice as many as the vegetables. Nevertheless, moringa contains fewer free radicals. The proportions of the samples analyzed were 89.6, 88.2, and 89.9%. Ascorbic acid was used as an antioxidant. It is known for its effectiveness, but despite that, moringa had a higher ability to scavenge. These results were confirmed by (Al Juhaimiet al., 2017) who indicated that moringa oleifera contains a high percentage of antioxidants. (Ghafoor et al., 2020), who indicated that the antioxidants in the Moringa plant extend the shelf life of nutrients lately.
3.2 Sensory properties of balady bread prepared with wheat flour enriched with different levels of dried moringa leaves
Sensory evaluation of balady wheat bread and bread fortified with moringa leaves is presented in Table 2. At the beginning of the storage period (time zero), no individual differences were observed with statistical significance (P < 0.05) between the substituted and unsupported bread samples. A significant decrease was observed in all coefficients scores after 2 days, compared to the zero time but by a small percentage. We note a decrease in the organoleptic characteristics (appearance, taste, odor (texture and color) of the leaf-fortified bread samples, but all samples were generally acceptable, and the leaves contain a high percentage of antioxidants and flavonoids, which help prevent bacteria from growing for a longer period of time and storage. Results according to Sengev et al., (2013) stated that the sensory evaluation, despite the presence of a significant improvement (p < 0.05) in the nutritional composition, all bread samples were acceptable, but it decreased with the increase in the level of Moringa leaf powder supplementation. (Babiker et al., 2021) reported the importance of the sensory quality of wheat flour cookies and other additives that give a good chance to obtain a forum rich in bioactive compounds and also be satisfactory by consumers. Mean ± SD with the same latter in the same column are not significantly different (P ≤ 0.05) *: Least significant differences.
Parameters
Time of Storage
Moringa oleifera treatments
L.S.D*0.05
ControlDried moringa leaf bread 6%
Dried moringa leaf bread 9%
AppearanceZero time
9.25 ± 0.63a
8.55 ± 1.12a
8 ± 0.82a
0.80
3 days
8.5 ± 0.59a
7.45 ± 1.07b
7.1 ± 0.74b
0.75
6 days
6.35 ± 0.75a
6.1 ± 1.26a
5.45 ± 0.55a
0.83
ColorZero time
9.05 ± 0.72a
8.55 ± 0.72a
8.8 ± 0.63a
0.63
3 days
8.1 ± 0.74a
7.65 ± 0.53a
7.75 ± 0.59a
0.57
6 days
6.05 ± 0.80a
5.7 ± 0.59a
5.76 ± 0.60a
0.61
TextureZero time
9.15 ± 0.63a
8.85 ± 0.71a
9.05 ± 0.55a
0.58
3 days
8.15 ± 0.63a
7.85 ± 0.71a
8.05 ± 0.55a
0.56
6 days
6.15 ± 0.63a
5.85 ± 0.71a
6.05 ± 0.55a
0.55
TasteZero time
8.85 ± 0.63a
8.8 ± 0.67a
8.95 ± 0.64a
0.59
3 days
7.8 ± 0.67a
7.75 ± 0.68a
7.9 ± 0.61a
0.60
6 days
5.75 ± 0.72a
5.7 ± 0.63a
5.85 ± 0.63a
0.60
OdorZero time
9.25 ± 0.49a
8.3 ± 0.54b
8.63 ± 0.75b
0.55
3 days
8.15 ± 0.53a
7.4 ± 0.52a
7.65 ± 0.63a
0.51
6 days
6.2 ± 0.59a
5.35 ± 0.53b
5.6 ± 0.61b
0.52
3.3 Bacterial load of balady bread supplemented with moringa leaves during the 8-day storage period at (25 °C ± 2)
Initially, treats of balady wheat bread and mashed bread were prepared with dried moringa leaves. Samples that had been stored for 8 days at room temperature were analyzed. The results in Table 3 showed that there were no total bacteria counts in both fresh bread and bread supplemented with dried moringa leaves at time zero. Before the storage period, on the second day, the total bacterial count of balady bread (control treatment) was 2.8 × 103 cfu/g during the storage period until it reached 5.5 × 103 cfu/g on the 4 days. The proportion of bacteria clearly increased to the eye. It spoiled and was rejected after the sixth day. Meanwhile, the bread supplemented with dried 6% moringa leaves (DMLB), the percentage of (TBC) was 2.55 × 103 cfu/g on the second day, but on the sixth day, it was (8.2 × 103 cfu/g). While the bread was supplemented with dried 9% moringa leaves (DMLB), the percentage of (TBC) was 2.47 × 103 cfu/g on the second day, but on the sixth day, it was 7.8 × 103 cfu/g. All treatments were rejected on the eighth day. Similarly, spore-forming bacteria increased during the storage period, balady bread contained more spore-forming bacteria (4.9 × 102, 7.5 × 102 cfu/g while bread supplemented with 6% dried moringa leaves (DMLB) were 4.15 × 102 to 6.75 × 102 cfu/g, but bread supplemented with 9% dried moringa leaves (DMLB) were 3.8 × 102 to 6.4 × 102 cfu/g after 2, 4, and 6 days of storage, respectively. This type is known as heat-loving or resistant bacteria, and therefore it is one of the reasons for the spoilage of healthy foods (André et al., 2017). The baladi wheat bread of the control sample contains greater numbers of fungi and yeasts from 6.2 × 102 cfu/g to 7.8 × 102 cfu/g on this arranged pattern after 2 and 4 days, while bread supplemented with 6% dried moringa leaves (DMLB) was 5.4 × 102 cfu/g on the 2 days and reached 8.7 × 102 cfu/g on the 6 days, but bread supplemented with 9% dried moringa leaves (DMLB) during storage days, as the percentages of fungi and yeasts decreased compared to the control bread as indicated (Batista et al., 2014). Prevent plant pathogenic fungi from causing disease and threatening economic crop production. Several reports indicate that moringa oleifera shows the ability to control fungal diseases in plants, and these results are somewhat similar (Mishra et al., 2021), which confirmed that the total number of bacteria and the number of fungi decreased with the increasing concentration of moringa leaf powder. DMLB: Dried Moringa Leaves Bread. Cfu, colony forming units; ND, Not detected; R*: Reject Data are presented as: average (for three replicates) ± standard deviation.
Microbial
parameters
(cfu/g)
Storage
PeriodMicrobial numbers of balady bread prepared with wheat flour Replaced with different levels of Moringa leaves
Control bread
Dried moringa leaf bread 6%
Dried moringa leaf bread 9%
Total bacterial
countZero Time
ND
ND
ND
2 days
2.8 × 103
2.55 × 103
2.47 × 103
4 days
5.5 × 103
5.25 × 103
5.17 × 103
6 days
8.2 × 103
7.9 × 103
7.8 × 103
8 days
R*
R*
R*
Spore forming
bacteriaZero Time
ND
ND
ND
2 days
4.9 × 102
4.15 × 102
3.8 × 102
4 days
6.0 × 102
5.25 × 102
4.9 × 102
6 days
7.5 × 102
6.75 × 102
6.4 × 102
8 days
R*
R*
R*
Mold
&
yeastZero Time
ND
ND
ND
2 days
6.2 × 102
5.4 × 102
5.0 × 102
4 days
7.8 × 102
7.0 × 102
6.6 × 102
6 days
9.9 × 102
9.1 × 102
8.7 × 102
8 days
R*
R*
R*
3.4 Biological analysis
3.4.1 Effect of feeding bread samples containing dried moringa leaves powder on liver enzymes (GPT/ALT) and (GOT/AST) for cirrhosis of rats
Table 4 shows the effect of moringa bread nutrition on liver enzymes. Glutamic pyruvic transaminase (GPT) and oxaloacetic transaminase (GOT) enzymes from cirrhosis of male albino rats, where it was found that the mean GPT value of the negative control was significantly lower compared to the positive control, which was 21.47 ± 1.55 and 120.33 ± 1.53 On the other hand, for G4, it shows the effect of moringa on the protective evaluation of the liver. We find that G4 that is fed on bread was replaced by 9% of the dried leaves of moringa, which reached 32.27 ± 2.57, and thus led to an improvement in GPT. While G3, which is fed on bread free from moringa leaves, we note that it did not positively affect GPT. The increase in moringa level further improved the activity of liver enzymes in G4. These data are in agreement with Bahashwan et al., (2015) and Gangarapu et al., (2014), who showed the same elevated hepatic enzyme. The data in Table 4 also showed that the mean value in GOT enzyme for the negative control group (27.68 ± 1.97 U/L) was much lower compared to the positive control (160.67 ± 2.08 U/L). It can be seen that the G3 group had the highest level of GOT enzyme in the blood. The G4 that was fed on bread supplemented with 9% of the moringa leaves reached 35.70 ± 1.15 units/liter, which showed an improvement in the level of GOT compared to G3, and therefore we note significant differences, and these results are in agreement with El- Meligy et al. (2014) showed the same rise in liver function. Some studies indicated that in clinical procedures, when monitoring and examining the development of diseases, including liver disease, liver function tests are used. The most common of these tests are aspartate aminotransferase (AST / GOT), alanine aminotransferase (ALT / GPT), albumin, and prothrombin. Mean ± SD with the same latter in the same column are not significantly different (P ≤ 0.05) *: Least significant differences. Group 1: The control group (-ve), in which normal rats were fed the basic diet and tap water. Group 2: the control group (+ve), in which rats with hepatotoxicity with CCl4 were fed a base diet and tap water. Group 3: Rats with abnormal liver function, treated with control bread. Group 4: Rats with liver defects treated with bread fortified with moringa leaves (prepared with 9% of moringa leaves). The trial period occurred for 28 days.
Parameters
Groups
L.S.D*0.05
G1
G2
G3
G4
GPT (U/L)
21.47 ± 1.55d
120.33 ± 1.53a
101.20 ± 3.36b
32.27 ± 2.57c
4.48
GOT (U/L)
27.68 ± 1.97d
160.67 ± 2.08b
180.90 ± 1.87a
35.70 ± 1.15c
3.4
Cholesterol (mg/dl)
62.17 ± 1.60c
173.33 ± 2.12a
164.43 ± 1.59b
65.53 ± 1.80c
3.37
Triglyceride (mg/dl)
78.53 ± 2.93c
225.50 ± 1.77a
223.40 ± 0.96a
82.59 ± 1.47b
3.62
HDL
52.33 ± 1.29a
23.52 ± 2.36c
26.33 ± 2.68c
48.19 ± 2.13b
4.09
LDL
22.03 ± 2.92c
95.14 ± 2.24a
96.37 ± 3.11a
33.44 ± 2.35b
5.04
Urea (mg/dl)
19.53 ± 1.12c
82.97 ± 2.77a
78.30 ± 2.40b
23.10 ± 1.67c
3.94
Creatinine (mg/dl)
0.69 ± 0.01c
1.77 ± 0.02a
1.74 ± 0.02b
0.67 ± 0.01c
0.03
3.4.2 Effect of feeding moringa bread samples containing leaves for 28 days on the levels of total cholesterol and triglycerides (mg/dl) on liver fibrosis of rats
Also, Table 4 showed the effect of moringa bread on the level of total cholesterol in the blood of rats with cirrhosis. The total cholesterol (TC) mean value for the negative control (62.17 ± 1.60 mg/dL) was significantly lower at (p < 0.05) compared with the group Positive control (173.33 ± 2.12 mg/dL). For the G4 fed with bread substituted with 9% moringa leaves, we observed a decrease in the percentage of TC in the blood compared with the control group fed with G3 free moringa bread. The mean value of triglycerides (TG) for the negative control was (78.53 ± 2.93 mg/dL) which showed a significant decrease in lipids compared to the positive control (C + ve) group (225.50 ± 1.77 mg/dL). G4 fed the substitute bread with moringa leaves, which was less valuable compared to the other groups. These results are in agreement with Mehta et al., (2003). It was found that the levels of cholesterol and triglycerides decreased in the levels of TG and TC among the rats fed moringa oleifera compared to the other groups. These results were confirmed by Aborhyem et al., (2016) and Ghasi et al., (2000), showing that the bioactive components present in moringa leaves helped lower blood cholesterol in infected mice and confirmed by Dubey et al., (2013). Chumark et al. (2008) observed that moringa extract lowered cholesterol and triglyceride levels by a very high rate and helped reduce the formation of atherosclerotic plaques after 12 weeks of feeding in rats fed a high proportion of cholesterol.
3.4.3 Effect of feeding moringa bread samples containing leaves for 28 days on HDL and LDL levels (mg/dl) of liver fibrosis of rats
Table 4 showed the effect of replacing balady bread with 9% moringa leaves fed rats with cirrhosis on high-density lipoprotein (HDL-C) levels. The mean HDL value of the negative control was significantly higher (p < 0.05) compared to the positive control and was 52.33 ± 1.29 mg/dL and 23.52 ± 2.36 mg/dL, respectively. G4 fed on bread substituted with moringa leaves had higher serum HDL compared to G3 (26.33 ± 2.68 mg/dL), while we find the effect of moringa bread on low-density lipoprotein (LDL) levels. The mean serum LDL value for the negative control group was (22.03 ± 2.92 mg/dL) which revealed a notable decrease (P < 0.05) compared to the positive control group (95.14 ± 2.24 mg/dL). Whereas the group fed with G4 moringa leaf bread had the lowest LDL level compared to the G3 fed on moringa-free bread that reached the highest percentage of 96.37 ± 3.11 mg/dL of LDL. These results agree with Jain et al., (2010), who found that moringa leaves had an effect on LDL and HDL, which helped reduce the proportion of low-density lipoprotein. On the contrary, it increased the high cholesterol lipoprotein present. Infer how effective moringa is in reducing lipids and cholesterol in the blood. These results were confirmed by Reddy et al., (2012), which showed that when rats were given powdered extracts of moringa leaves orally, it reduced the level of cholesterol (HDL-C) in the blood. It was also explained that moringa leaves are used to manufacture therapeutic drugs that fulfill the same purpose.
3.4.4 Effect of feeding moringa bread samples containing leaves for 28 days on urea and creatinine (mg/dl) for liver cirrhosis in rats.
Table 4 showed the effect of replacing balady bread with 9% of moringa leaves fed to rats with cirrhosis on serum urea and creatinine levels. The mean values of the urea negative control group were (19.53 ± 1.12 mg/dL), which showed a significant decrease at (p < 0.05) compared to the positive control group 82.97 ± 2.77 mg/dL., while G4 fed with moringa-free bread led to a decrease in urea that reached 23.10 ± 1.67 mg/dL compared to G3 that was fed on moringa-free bread (control) and the positive control group. As for creatinine level, the results presented in Table 4 explained the effect of eating bread substituted with 9% of moringa leaves on liver creatinine in rats. The positive control group was higher than the control group at (P < 0.05) and its scores were 1.77 ± 0.02 mg/dL and 0.69 ± 0.01 mg/dL. The G4 fed on bread that was replaced by 9% moringa leaves was 0.67 ± 0.01 mg/dl, which was decreased compared to the G3 fed on moringa-free bread. These results are consistent with Halabi et al., (2013), which showed that the level of urea nitrogen and creatinine gradually decreased according to the concentration of moringa oleifera. These results confirmed that moringa oleifera helps raise the nutritional value of what contains minerals, vitamins, and other elements and works to improve kidney and liver functions. On the contrary, a previous study by Ghebreselassie et al., (2011) indicated that the groups of rats treated with different doses of 600 and 900 mg/kg of body weight of aqueous extract of moringa leaves did not show a significant change in the level of creatine and urea compared to the control group, but it was reduced in the level of both.
3.5 Histopathological examination
3.5.1 Study of hepatic cells of rat by histological examination
Fig. 4 shows liver biopsy from rats of (_ve) group 1 showed that normal histological structure of hepatic lobules. But liver biopsy from group 2 show profilated fibroblast in the portal tract, new bile ductules are formed, focal hepatocellular necrosis and destruction surrounded by inflammatory cell then the fibrous capsule then biopsy from group 3 revealed hydropic degeneration of hepatocytes. By examination from group 4 showed mild hydropic degeneration of some hepatic cells, mild fibroplasia in the portal tract, and formation of newly bile ductules. These results are consistent with Taha et al., (2015). Histopathological research of the present study not only confirms the toxicity of CCl4 on hepatic cells but also the hepatic recovery after treatment of intoxicated rats with moringa oleifera extract. Because of improvement in hepatic architecture, severe hepatic fatty degeneration together by infiltrated inflammatory cells after moringa oleifera extract given.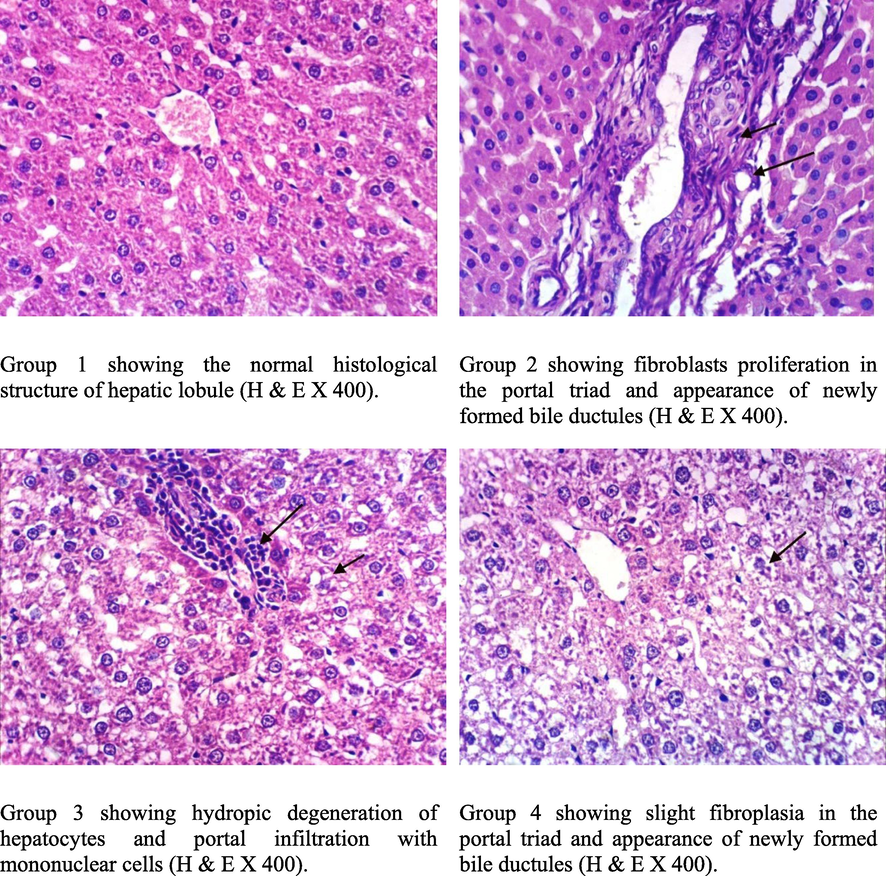
Liver of rat.
3.5.2 Study of rat renal cells by histological examination
Fig. 5 shows microscopic examination of the kidneys of rats from group 1 show renal parenchyma is normally histological structures. On the other side nephro histology of rats from group 2 had protein caseation material in the lumen of renal tubules. But, kidney s of rats from group 3 showed proteinaceous materials in the lumen of renal tubules, congested blood vessels of intertubules, and focal necrosis of renal tubules with inflammatory cells infiltration. At the same time, kidneys from group 4 showed proteinaceous material in the lumen of some renal tubules, mild vacuolation of epithelial lining some renal tubules, and mildly congested blood capillaries of intratubules, and these results are consistent with Halaby et al., (2013) which proved the effect of bread enriched with moringa in improving kidney functions.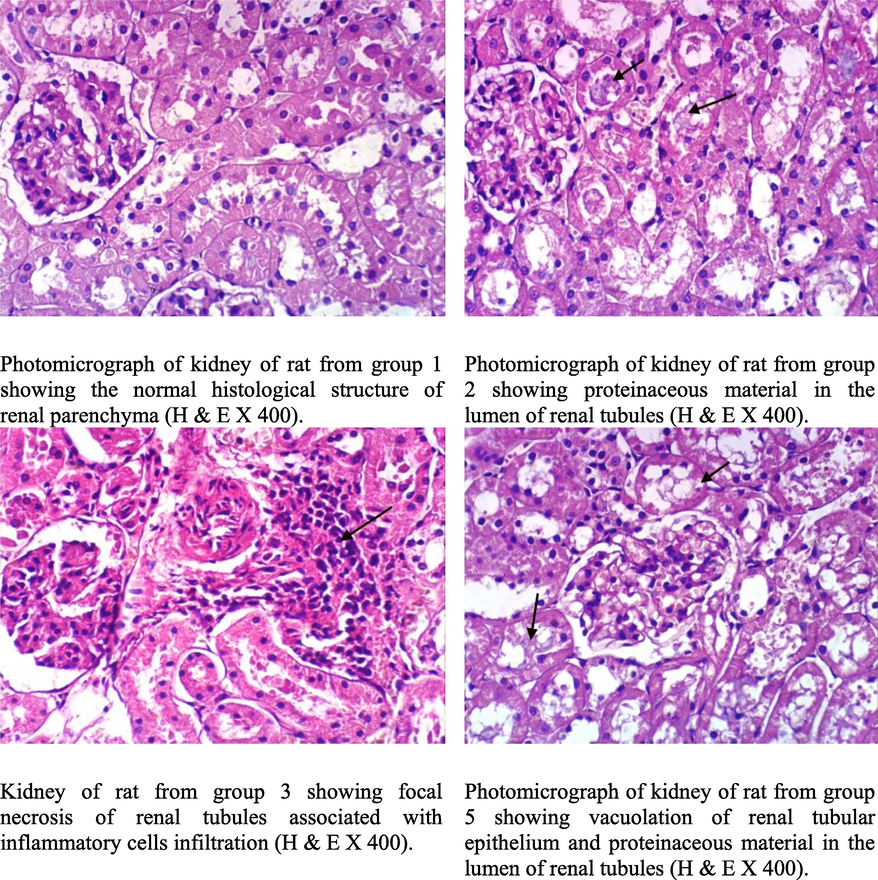
Kidney of rat.
4 Conclusions
This study showed that moringa leaves have high nutritional value, so in our study, we enriched with moringa leaves to improve the organoleptic properties and the fiber in the food product, enhancing its nutritional value. Also, moringa contains a high level of antioxidant activity, which has resulted in a significant decrease in the growth of fungi, yeasts, and bacteria in processed bread. In addition, the study shows that moringa leaves significantly influence the biological parameters in the serum of rats with cirrhosis liver by CCL4, it reduced liver and kidney enzymes GPT, GOT, cholesterol, triglycerides, high-density lipoprotein, low-density lipoprotein, HDL, LDL. Furthermore, the damaged liver and kidney tissues by CCL4 carbon tetrachloride are slightly improved. Finally, we should pay more attention to the high nutritional value of moringa leaves through advertising, product branding, and targeted communication. Also, a need to create many moringa leaves supplement products that are made with a lack of knowledge about the effect of treatment with dried moringa leaves on health and nutritional values.
Acknowledgements
The authors are grateful to Benha University, Egypt and University of Bisha, Also, the authors would like to thank the Deanship of Scientific Research at Umm Al-Qura University for supporting this work by Grant Code: (22UQU4280401DSR01).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abd-Rabou, A. A., Abdalla, A. M., Ali, N. A., & Zoheir, K. M. A. 2017. Moringa oleifera Root Induces Cancer Apoptosis more Effectively than Leave Nanocomposites and Its Free Counterpart. Asian Pacific journal of cancer prevention : APJCP, 18(8): 2141 214910.22034/APJCP.2017.18.8.2141https://pubmed.ncbi.nlm.nih.gov/28843248.
- Health benefits of Moringa oleifera. Asian Pac. J. Cancer Prev.. 2014;15(20):8571-8576.
- [CrossRef] [Google Scholar]
- Aborhyem, S., Ismail, H., Agamy, N., & Tayel, D. 2016. Effect of Moringa Oleifera on Lipid Profile in Rats. Journal of High Institute of Public Health, 46(1): 8-1410.21608/jhiph.2016.20201https://jhiphalexu.journals.ekb.eg/article_20201_9c74205f3978d397d62520a89a22f234.pdf.
- Antibacterial effect of Moringa oleifera extracts on bacteria associated with urinary tract infection. International Journal of Research. 2014;1
- [Google Scholar]
- Al-Asmari, A. K., Athar, M. T., Al-Shahrani, H. M., Al-Dakheel, S. I., & Al-Ghamdi, M. A. 2015b. Efficacy of Lepidium sativum against carbon tetra chloride induced hepatotoxicity and determination of its bioactive compounds by GC MS. Toxicology reports, 2: 1319-132610.1016/j.toxrep.2015.09.006https://pubmed.ncbi.nlm.nih.gov/28962474 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5598273/.
- Fatty acid composition of three different Moringa leave oils. Rivista Italiana delle Sostanze Grasse. 2016;93(2):111-113.
- [Google Scholar]
- The biochemical composition of the leaves and seeds meals of moringa species as non-conventional sources of nutrients. J. Food Biochem.. 2016;41
- [Google Scholar]
- Comparative study of mineral and oxidative status of Sonchus oleraceus, Moringa oleifera and Moringa peregrina leaves. J. Food Meas. Charact.. 2017;11:1-7.
- [Google Scholar]
- Chemical, microbial and biological studies on fresh mango juice in presence of nanoparticles of zirconium molybdate embedded chitosan and alginate. Arabian J. Chem.. 2021;14(4) 10.1016/j.arabjc.2021.103066https://www.sciencedirect.com/science/article/pii/S1878535221000812
- [Google Scholar]
- Addition of Whole Barley Flour as a Partial Substitute of Wheat Flour to Enhance the Nutritional Value of Biscuits. Arabian J. Chem.. 2021;14(5) 10.1016/j.arabjc.2021.103112https://www.sciencedirect.com/science/article/pii/S1878535221001271
- [Google Scholar]
- Aly, A. A., Refaey, M. M., Hameed, A. M., Sayqal, A., Abdella, S. A., Mohamed, A. S., M.A.A, H., & Ismail, H. A. 2020. Effect of addition sesame seeds powder with different ratio on microstructural and some properties of low fat Labneh. Arabian Journal of Chemistry, 13(10): 7572-758210.1016/j.arabjc.2020.08.032https://www.sciencedirect.com/science/article/pii/S1878535220303233.
- Effect of Thyme Addition on some Chemical and Biological Properties of Sunflower Oil. Arabian J. Chem.. 2021;14(11) 10.1016/j.arabjc.2021.103411https://www.sciencedirect.com/science/article/pii/S1878535221004263
- [Google Scholar]
- The Impact of Addition Oats (Avena sativa) and Cinnamon on Cookies and their Biological Effects on Rats Treated with Cirrhosis by CCL4. Saudi Journal of Biological Sciences. 2021;28(12):7142-7151. 10.1016/j.sjbs.2021.08.010https://www.sciencedirect.com/science/article/pii/S1319562X21006835
- [Google Scholar]
- Spore-forming bacteria responsible for food spoilage. Res. Microbiol.. 2017;168(4):379-387.
- [CrossRef] [Google Scholar]
- Official Methods of Analysis (19th Edition). Washington: International; 2019.
- Bioactive compounds, nutritional and sensory properties of cookies prepared with wheat and tigernut flour. Food Chem.. 2021;349 129155
- [Google Scholar]
- Crocin mitigates carbon tetrachloride-induced liver toxicity in rats. Journal of Taibah University Medical Sciences. 2015;10(2):140-149.
- [CrossRef] [Google Scholar]
- Batista, A. B., Oliveira, J. T. A., Gifoni, J. M., Pereira, M. L., Almeida, M. G. G., Gomes, V. M., Da Cunha, M., Ribeiro, S. F. F., Dias, G. B., Beltramini, L. M., Lopes, J. L. S., Grangeiro, T. B., & Vasconcelos, I. M. 2014. New insights into the structure and mode of action of Mo-CBP3, an antifungal chitin-binding protein of Moringa oleifera seeds. PloS one, 9(10): e111427e11142710.1371/journal.pone.0111427https://pubmed.ncbi.nlm.nih.gov/25347074.
- Chumark, P., Khunawat, P., Sanvarinda, Y., Phornchirasilp, S., Morales, N. P., Phivthong-Ngam, L., Ratanachamnong, P., Srisawat, S., & Pongrapeeporn, K. U. 2008. The in vitro and ex vivo antioxidant properties, hypolipidaemic and antiatherosclerotic activities of water extract of Moringa oleifera Lam. leaves. J Ethnopharmacol, 116(3): 439-44610.1016/j.jep.2007.12.010.
- Fatty acid composition, mineral contents and glysemic index values of chips produced with different cooking methods and lupine (Lupinus albus L.) flour formulations. J. Food Process. Preserv.. 2020;45
- [Google Scholar]
- Dubey, D., Dora, J., Kumar, A., Kumar, R., & Gulsan. 2013. Review Article A Multipurpose Tree- Moringa oleifera.
- Drury, R. A., and Wallington, E. &. (1980): Carlton's Histological Technique 5 Ed Oxford University.
- Protective role of Cynanchum acutum L. extracts on carbon tetrachloride-induced hepatotoxicity in rat. Int. J. Chem. Appl. Biological Sci.. 2014;1:8-13.
- [CrossRef] [Google Scholar]
- Effect of Moringa Leaves Powder (Moringa oleifera) on Some Chemical and Physical Properties of Pan Bread 2016
- Farooq, F. B., Rai, M., Tiwari, A., Khan, A. A., & Farooq, S. 2012. Medicinal properties of Moringa oleifera: An overview of promising healer.
- Combined effect of curcumin and vitamin E against CCl 4 induced liver injury in rats Combined Effect of Curcumin and Vitamin E against CCl 4 Induced Liver Injury in Rats. Am. J. Life Sci. 2014 110.11648/j.ajls.20130103.17
- [Google Scholar]
- Evaluation of the antioxidant activity of some plant extracts (rosemary, sage and savory, summer) on stability of moringa oil. J. Food Process. Preserv.. 2020;45
- [Google Scholar]
- Hypocholesterolemic effects of crude extract of leaf of Moringa oleifera Lam in high-fat diet fed wistar rats. J. Ethnopharmacol.. 2000;69(1):21-25.
- [CrossRef] [Google Scholar]
- The effects of Moringa stenopetala on blood parameters and histopathology of liver and kidney in mice. Ethiopian J. Health Development. 2011;25:51-57.
- [Google Scholar]
- Halaby, M. S., Elmetwaly, E. M., & Omar, A. 2013. Effect of Moringa Oleifera on serum lipids and kidney function of hyperlipidemic rats 1.
- Antihypertensive and free radical scavenging properties of enzymatic rapeseed protein hydrolysates. Food Chem.. 2013;141(1):153-159.
- [CrossRef] [Google Scholar]
- Rheological, physio-chemical and organoleptic characteristics of ice cream enriched with Doum syrup and pomegranate peel. Arabian J. Chem.. 2020;13(10):7346-7356.
- [CrossRef] [Google Scholar]
- Hypolipidemic activity of Moringa oleifera Lam., Moringaceae, on high fat diet induced hyperlipidemia in albino rats. Revista Brasileira De Farmacognosia-brazilian Journal of Pharmacognosy. 2010;20:969-973.
- [Google Scholar]
- Traditional Chinese herbal medicines for treatment of liver fibrosis and cancer: from laboratory discovery to clinical evaluation. Liver Int. 2007;27(7):879-890.
- [CrossRef] [Google Scholar]
- Amelioration of Paracetamol-Induced Hepatotoxicity in Rat by the Administration of Methanol Extract of <i>Muntingia calabura</i> L Leaves. BioMed Research International. 2014;2014 695678
- [CrossRef] [Google Scholar]
- Optimization of Solvent Extraction of Moringa (Moringa Oleifera) Seed Kernel Oil Using Response Surface Methodology. Food Bioprod. Process.. 2007;85:328-335.
- [Google Scholar]
- Effect of fruits of Moringa oleifera on the lipid profile of normal and hypercholesterolaemic rabbits. J. Ethnopharmacol.. 2003;86(2–3):191-195.
- [Google Scholar]
- Phytochemical, nutritional and antibacterial properties of dried leaf powderof Moringa oleifera (Lam) from Edo Central Province, Nigeria. J. Natural Product Plant Resources. 2012;2:107-112.
- [Google Scholar]
- Evaluation of Nutritional and Microbial Properties of Bread Developed by Incorporating Moringa oleifera Leaves and Fenugreek Leaves. Asian Food Science J. 2021 93–10110.9734/afsj/2021/v20i730325
- [Google Scholar]
- Quality evaluation of complementary food formulated from Moringa Oleifera leaf powder and pearl millet (Pennisetum Glaucum) flour. Int. J. Eng. Science (IJES). 2014;3:59-63.
- [Google Scholar]
- Oyeyinka, A. T., & Oyeyinka, S. A. 2018. Moringa oleifera as a food fortificant: Recent trends and prospects. Journal of the Saudi Society of Agricultural Sciences, 17(2): 127 13610.1016/j.jssas.2016.02.002https://www.sciencedirect.com/science/article/pii/S1658077X15301235.
- Moringa spp: Composition and bioactive properties. S. Afr. J. Bot.. 2020;129:25-31.
- [Google Scholar]
- Pakade, V., Cukrowska, E., & Chimuka, L. 2013. Comparison of antioxidant activity of Moringa oleifera and selected vegetables in South Africa. South African Journal of Science, 10910.1590/sajs.2013/1154.
- Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science. 2014;344(6190):1396-1401.
- [CrossRef] [Google Scholar]
- Antibacterial Activity of Leaves Juice and Extracts of Moringa oleifera Lam. (2n = 28) against Some Human Pathogenic Bacteria. Chiang Mai University J. Natural Sciences. 2009;8:219-227.
- [Google Scholar]
- Reddy, V., F. Ahmed, A. Urooj, .,: 2510-2516. . 2012. Inhibition of 3-Hydroxy-3-Methylglutaryl Coenzyme A (HMG Co-A) Reductase in Liver Microsomes by Moringa Oleifera L. Polyphenols. . IJPSR, 3((7)): 2510-2516.
- Rockwood, J., Anderson, B. G., & Casamatta, D. A. 2013. POTENTIAL USES OF Moringa oleifera AND AN EXAMINATION OF ANTIBIOTIC EFFICACY CONFERRED BY M. oleifera SEED AND LEAF EXTRACTS USING CRUDE EXTRACTION TECHNIQUES AVAILABLE TO UNDERSERVED INDIGENOUS POPULATIONS.
- An evaluation of Moringa peregrina seeds as a source for bio-fuel. Ind. Crops Prod.. 2014;61:49-61.
- [CrossRef] [Google Scholar]
- Comparative Protective Effects of N-Acetylcysteine. N-Acetyl Methionine, and N-Acetyl Glucosamine against Paracetamol and Phenacetin Therapeutic Doses-Induced Hepatotoxicity in Rats.. 2018;2018:7603437.
- [CrossRef] [Google Scholar]
- Herbal products for liver diseases: a therapeutic challenge for the new millennium. Hepatology. 1999;30(4):1099-1104.
- [CrossRef] [Google Scholar]
- Effect of Moringa oleifera Leaf Powder Supplementation on Some Quality Characteristics of Wheat Bread. Food and Nutrition Sciences. 2013;443036:270-275.
- [Google Scholar]
- Taha1, N. R., , S. O. R., , S. A. S., & Mograby4, a. M. M. 2015. Effect of Moringa oleifera Leaves on Diclofenac Sodium Induced Hepatic Injury in Albino Rats: Ultrastructural and Immunohistochemical Studies. J. Cytology & Histology, 10: 2157-7099.
- Recent advances in the pathogenesis and diagnosis of liver fibrosis. J. Gastroenterol.. 2008;43(5):315-321.
- [CrossRef] [Google Scholar]
- Vats, S., & Gupta, T. 2017. Evaluation of bioactive compounds and antioxidant potential of hydroethanolic extract of Moringa oleifera Lam. from Rajasthan, India. Physiol Mol Biol Plants, 23(1): 239-24810.1007/s12298-016-0407-6.
- Zaffer, M., Ganie, S., Gulia, S. S., Yadav, S., Singh, R., & Ganguly, S. 2015. Antifungal Efficacy of Moringa Oleifera Lam. Am. J. Phytomedicine Clinical Therapeutics, 03: 0 2 8-0 3 3.







