Translate this page into:
Synthesis, characterization and evaluation of biological properties of selenium nanoparticles from Solanum lycopersicum
⁎Corresponding author. mrimranqadir@hotmail.com (Muhammad Imran Qadir)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Synthesis of nanoparticles by green synthesis has a large number of biomedical applications worldwide. In this study, Selenium Nanoparticles (SeNPs) were synthesized by using sodium salt of selenium and Solanum lycopersicum (tomato) fruit juice and seeds extract. The plant extracts were used as a reducing agent in ratio 1:4 i.e. sodium selenite salt (Na2SeO3). SeNPs were characterized by UV–visible spectrophotometry, FTIR and Zeta Sizer analysis. The UV-graphs indicated the highest peak of absorbance at 350 nm. Whereas, FTIR analysis of SeNPs indicated absorbance bands at 3262.35–1633.72 cm−1. Zeta sizer analysis showed the average size of SeNPs for Fruit juice extract as 1020 d.nm. with PDI 0.432. In case of seeds extract, average size was 1155 d.nm. with PDI 0.761; and the PDI value for both extracts showed polyderse nature of these NPs. SeNPs possessed significant antimicrobial activity against selected strains of E. coli, S. aureus, M. luteus, S. enterica, B. subtilis, K. pneumoniae and P. aureginosa. The α-amylase inhibitory assay of these SeNPs indicated that they had antidiabetic role with IC50 value 24.4642 µg/mL. The DPPH assay showed that SeNPs of Solanum lycopersicum have antioxidant activity with IC50 value of 20.7398 µg/mL.
Keywords
Selenium nanoparticles (SeNPs)
Tomato fruit juice
Enzyme Inhibition
Antimicrobial
Antioxidant
1 Introduction
Nanotechnology is the branch of science that involves the synthesis and characterization of particles which are nanometers in size i.e. billionth part. Nanotechnology is the revolutionary field and it has large number of applications in all fields of life (Silva, 2004). The word nanotechnology itself is a new word but this field is not a new one. It is as old as life existence on earth. Nanoscience is the science that works behind that field. Nanotechnology has large number of beneficial aspects that can serve humanity and society (Poole and Owens, 2003). At the end of 20th century; the door of innovations has been opened by nanotechnology. Nanotechnology has a revolutionary power. This field has large number of applications in almost every field of life. Nanotechnology has practical applications in medical; industrial and environment etc. In previous time people used nanotechnology even without knowing it but this technology possess far distant background that exists in past (Bhushan, 2017). The term nanotechnology is associated with the Mr. R. Feynman’s first lecture. In 1959 he delivered a lecture at American Physical Society. There is a saying famous in past time that the ‘Small is beautiful’ but that statement is modified now that ‘Small is beautiful and powerful too’.
Nanoparticles are the particle that are 10-9 m in size. Nanoparticles from different sources holds different properties e.g. gold nanoparticles possess vivid colors because of this feature they are of great importance for the researchers. Other than their beauty these NPs have different properties (Dreaden et al., 2012). Nanoparticles looks like simple but not so simple. Sometimes they may cause toxicity but this problem can be solved by modifications of NPs. Nanoparticles are of many types the nanoparticles that are composed of metals are named as metallic nanoparticles. These NPs have large number of applications in various field of life like biotechnology, medicines and cosmetics etc. Single stranded DNA get adhere by NPs without getting any harm or damage that’s why NPs have many useful aspects in diagnostics and health. For example, NPs of CuO have application in industry and pesticides. These NPs are also utilized in antimicrobial industry (Thakkar et al., 2010). These NPs are synthesized by plants sources and contain many important properties. Metallic NPs are the center of attention for investigators because of their qualities and size specificities. It has been reported that gold NPs possess antimicrobial role (Zheng et al., 2006). Nanoparticles can be synthesized by physical, chemical and biological methods. The biological method involves the use of microbes like bacteria, fungi and plants. The production of nanoparticles by utilization of plant or its parts is known as green synthesis. This method is safe, economical and ecofriendly. It don’t involves the use of any harmful chemical or reducing agent, in fact the plant extract served as a reducing agent. The plant extract reduces the metal ions and NPs are produced. NPs such as copper, gold, silver and zinc etc. are produced by green synthesis and contain many useful properties like anti-tumor, antimicrobial and antioxidant role (Mittal et al., 2013). Selenium (Se) is a metalloid having both qualities of metals and non-metals. It is the 16th group element of periodic table. It has been reported that Se is a dietary element required by the human body for its proper functioning. It possesses large number of useful medical applications. In previous studies it has been reported that Se have anti-cancerous role. It has ability to reduce the risk of cancers like lungs and prostate cancer. It has also been investigated that probiotics enriched in selenium are capable of inhibiting pathogenic microbes such as E. coli (Tran and Webster, 2011). Selenium was discovered in 1817 and in the start researchers and scientists considered it as a toxic matter but later on in almost 1950′s it has been found that Se is a dietary supplement. Previous studies has proved that Se is involved in prevention of many diseases like cystic fibrosis, cardiovascular problems and stress treatment, etc (Skalickova et al., 2017).
Due to these qualities selenium salt has been selected and it can also fulfill the dietary requirement. Selenium nanoparticles (SeNPs) have large number of applications. SeNPs have capability to inhibit the pathogenic bacterial growth and fungus. Due to the size of NPs and their surface area, SeNPs can bind to bacterial membrane and kill them (Kong et al., 2014). Because of antimicrobial role SeNPs can be used for sterilization. It has been investigated that SeNPs have many useful qualities like antioxidant role, improves memory, Improves hair production, anti-parasitic role, stress relief and improves immune system, etc (Hosnedlova et al., 2018).
In this study Solanum lycopersicum plant common name Tomato is selected. This plant source has been selected due to the qualities it possesses. Tomatoes are rich in antioxidants and many nutritious compounds. These are the part of our routine diet. Tomatoes in bulk amount are utilized to produce juices and purees at industrial level. In literature, it has been reported that Solanum lycopersicum have anti-cancerous and antioxidant roles. There are some phenolic compounds that have relationship with antioxidant role. These compounds prevent cell damages by reducing free radicals in the body (Silva-Beltrán et al., 2015). The selection of tomato plant for this research project is all because of its nutritional values and qualities. It has been investigated that consumption of tomatoes has direct relationship with low risk of heart diseases. This plant contains various bioactive elements and compounds like flavonoids and vitamins, etc (Borguini et al., 2009; Silva-Beltrán et al., 2015, 2015).
In Diabetes mellitus, the blood sugar level becomes high in comparison with the cell. This disease resulted in long lasting effects or damages such as organs failure (Bala et al., 2015). Sugars breakdown in human body is maintained by enzymes. Alpha amylase is the enzyme that is responsible for the breakdown of starch into glucose. Any sort of dysfunction in this enzyme or its inhibition results in decreased blood glucose level. In this study, it has been demonstrated that SeNPs contain antidiabetic property (Liu et al., 2018).
Free radicals are the one of the reason behind certain diseases like cancer and heart diseases. The free radicals can be produced when the body causes lysis of food or in case of smoking. Thus, antioxidants play a vital role in preventing such disorders that are results of free radicals. In this project antioxidant role of SeNPs was also tested. The basic aim of the project was to prepare such nanoparticles that possess antimicrobial, antioxidant and antidiabetic activities that may aid in treatment of many diseases like diabetes, many bacterial infections and free radicals associated diseases. As microbes are developing resistance against available antibiotics so, it is the need of the hour to develop efficient antimicrobial agents (Vyas and Rana, 2017). There are many infections reported in previous studies that are caused by bacterial species and for such infections available medicines are no more effective. So, SeNps may be used in future as a treatment for various diseases like bacteremia, meningitis, opthalmitis and ear infections etc.
2 Materials and methodology
2.1 Collection of Materials
The samples required are sodium salt of selenium, Solanum lycopersicum (Tomato) fruits and seeds. Bacterial cultures were collected from department of Biotechnology at Bahauddin Zakariya University Multan Pakistan.
2.1.1 Tomato plant collection
Solanum lycopersicum fresh fruits and seeds were purchased from nearby market in Multan, Pakistan.
2.1.2 Sodium selenite (Na2SeO3) salt
Sodium selenite salt of UNI-CHEM Company was purchased.
2.2 Plant extract preparation
Two types of Extracts were prepared from Tomato plant.
-
First, the Fruits extract.
-
Second, the Seeds Extract.
2.2.1 Preparation of fruit extract
Fresh tomato fruits were purchased and washed in order to remove all kind of dust. Washing step was performed 2, 3 times. After washing tomatoes were dried with tissue or towel. Then the tomatoes were cut into fine pieces and grinding step was done. The tomato paste was diluted and filtered (Sutradhar and Saha, 2016).
2.2.2 Tomato seeds extract preparation
Seeds extract was prepared by soaking the seeds in distilled water. After washing the seeds were dried and ground in mortar and pestle. Then centrifugation at 13000 rpm for 5-10mins was performed. Supernatant was diluted and filtered. The filtrate was used as The protocol of Behrouz Elahi and his coworker was followed with modifications (Elahi et al., 2019).
2.3 Preparation of salt solution
Different molar solutions were prepared for the synthesis of nanoparticles. 1 M stock solution was prepared by adding 172.98 g/L of distilled water, it was used as a stock solution. From that stock solution working solutions of molarity 8 mM, 10 mM, 12 mM and 14 mM were prepared.
2.4 Synthesis of SeNPs
The protocol of Nishant Srivastava was followed with some modifications. By following the green synthesis method for the preparation of SeNPs from Solanum lycopersicum, solutions having ratio 1:4 (salt: extract) were prepared. Same 1:4 reaction mixtures were prepared for 8 mM, 10 mM, 12 mM and 14 mM concentrations (Barman et al., 2013). The pH of the reaction mixture was checked and noted. Then the reaction mixtures were kept in dark condition on rotatory shaker at 75° C for 3 h at 200 rpm. The reaction mixture’s color was observed and noted. Then the reaction mixture was placed in incubator at 37° C for 96 h. After this incubation the color of reaction mixture was completely changed. This color change was an indication for the synthesis of nanoparticles (Zia et al., 2016). Same procedure was carried out with the seeds extract. The seeds extract and the salt solutions were mixed in 1:4 respectively. Same reaction mixtures were prepared for 8 mM, 10 mM, 12 mM and 14 mM. The reaction mixtures were placed in dark on rotatory shaker at 75° C for 3 h at 200 rpm. Then reaction mixtures were incubated at 37° C for 4 days and then the color of reaction mixture was noticed and recorded (Srivastava and Mukhopadhyay, 2015).
2.5 Optimization of conditions for SeNPs synthesis
Parameters like pH, time incubation and concentration were adjusted to find out the best conditions for synthesis in order to obtain maximum amount of nanoparticles.
2.5.1 Effect of pH
The pH of all reaction mixtures i.e. 10 mM, 12 mM and14mM were adjusted to 5, 7 and 8 by the help of hydrochloric acid (HCl) and sodium hydroxide (NaOH). 1 N solution of HCl and NaOH were used to maintain the pH then the UV–visible spectrophotometry was performed to analyse the production of SeNPs.
2.5.2 Effect of incubation time
After the reaction mixture was incubated for synthesis, the UV–visible spectrophotometry analysis of the reaction mixtures was performed and analyzed after 24, 48, 72 and 96 h respectively.
2.5.3 Effect of concentration of sodium selenite solution
Concentrations of sodium selenite (Na2SeO3) solution i.e. 8 mM, 10 mM, 12 mM and 14 mM were prepared respectively. The UV-spectrophotometry was performed to optimize the conditions for the maximum production of SeNPs.
2.5.4 Effect of temperature
Soon after mixing the extracts and salt solution the reaction mixtures were incubated at 75° C for 3 h. Then the reaction mixture was incubated at 37° C for 96 h and the color of the reaction mixture was observed (Wadhwani et al., 2017).
2.6 Characterization tests
The characterization of SeNPs was done by UV-spectrophotometry, Fourier Transform Infrared Spectroscopy and Zeta sizer analysis.
2.6.1 UV spectrophotometry
By the help of UV-spectrophotometer the absorbance of each sample of nanoparticles was measured. The absorption spectrum was noted in wavelength settings of 200 to 800 nm. The results were compared with reported values of spectrum peaks. Distilled water was used as a blank. 1 mL of each sample was used separately and placed in spectrophotometer. Double beam spectrophotometer was used. The peaks of SeNPs that were prepared from tomato juice and seeds extracts were analyzed (Prasad et al., 2013).
2.6.2 FTIR analysis
FTIR analysis was performed to find out the functional present on the nanoparticles. This technique involves use of infrared radiation (IR) to obtain the peaks of the samples. In spectrometry (FTIR) Infrared radiation passed 103 to 100 cm−1 times across the SeNPs sample (Gulmine et al., 2002). Some IR rays passes through the sample and some are transmitted. The absorbed IR rays by SeNPs was converted into energy. FTIR spectroscopy was done to measure how sample of nanoparticles absorbed light at particular wavelength (Kamnev et al., 2017). Results of FTIR indicates the peaks of absorbance and transmittance. Resulting spectrum can have the range that can be 4000 to 400 cm−1. FTIR is used to indicate the stabilization of the SeNPs. In case of SeNPs, the sample can be used both in dried and diluted form and results were recorded in range that resides 1000 to 4000 cm−1 (Hoo et al., 2008).
2.6.3 Dynamic light scattering (DLS) analysis
DLS also known as photon correlation spectroscopy (PCS). The average size of NPs can be determined by this technique. The sample used for DLS was in suspension form. The SeNPs had Brownian motion in solvent form. This technique works on the principle of light scattering i.e. the light scattered by the SeNPs and thus the average size of NPs was obtained with its polydispersity index (PDI) (MacLeod et al., 1954;68(6):680.).
2.7 Antimicrobial activity evaluation
Antimicrobial activity was performed by well diffusion assay.
2.7.1 Bacterial cultures used
The cultures that were selected and used were Escherichia coli (E. coli), Micrococcus luteus (M. luteus), Staphylococcus aureus (S. aureus), Klebsiella pneumoniae (K. pneumoniae), Bacillus subtilis (B. subtilis), Pseudomonas aeruginosa (P. aeruginosa), and Salmonella enterica (S. enterica) (Ahamed et al., 2014). Cultures were provided by Institute of Molecular Biology and Biotechnology department at Bahauddin Zakariya University Multan Pakistan. These microbes were selected because two reasons, first the infections caused by them. Secondly, these microbes has developed resistance against the present antibiobiotics.
2.7.2 Drug dilutions
The drug Ciprofloxacin was purchased and used as a standard for antimicrobial activity. Different dilutions such as 1%, 0.5% and 0.25% dilutions were prepared and used as a standard (Narayanan et al., 2012).
2.7.3 Well diffusion method
To check the antimicrobial activity of SeNPs agar well diffusion assay was used. Nutrient agar media plates were used. Bacterial cultures of selected strains were spread on the media plates with the help of sterilized cotton swabs. After spreading holes were bored with the help of 6 mm sterilized borer. The samples and the control were loaded in the wells. SeNPs of 10 mM, 12 mM and 14 mM concentration were loaded separately in each well in amount of 50 µl. same procedure was adopted for SeNPs prepared from tomato juice extract and seeds extract respectively. Then the plates were placed in incubator at 37° C for 24 h and the zone of inhibition (ZOI) was measured. All bacterial strains were used for lawn formation on separate plates and the ZOI was measured against 7 different pathogenic bacterial strains (Nickavar and Amin, 2011). Plant extract was also used as a standard (control).
2.8 Antioxidant activity (DPPH assay)
DPPH assay was performed to analyse the antioxidant role of SeNPs. 1000 µl of 0.1 M DPPH in methanol was prepared. In that already prepared solution 500 µl sample i.e. SeNPs of various dilutions like 10 mM, 12 mM and 14 mM were added saperately. The mixture was incubated at 37° C for 30 min. Then absorbance of all samples was measured at 517 nm. DPPH solution prepared above without sample served as a control (Kong et al., 2014). Then DPPH activity i.e. free radical scavenging activity was calculated. % DPPH radical scavenging = Acontrol-Asample/ Acontrol × 100
2.9 Antidiabetic activity (alpha amylase assay)
The potential of SeNPs as an antidiabetic agent was checked by Alpha amylase inhibitory assay with few modifications (Ariga et al., 2015). In this assay 100 µl of SeNPs with different diluions was mixed up with 200 µl of phosphate buffer 0.1 M with pH 6.9. 100 µl of enzyme i.e. alpha amylase was added in the same reaction mixture. Then the reaction mixture was added at 37° C for 10 min. 100 µl potato starch was added in the above reaction mixture and then incubated for 10mins at 30° C. 300 µl DNS (3, 5- dinitrosalicylic acid) was added up in above reaction mixture and then incubated at 95° C for 5 min. The absorbance of the reaction mixture was measured and analysed at 450 nm and compared with standard solution of acarbose. Percentage inhibition was calculated by using formula as follow; %age Inhibition = Acontrol-Asample/ Acontrol × 100
3 Results
3.1 Biosynthesis of SeNPs from Solanum lycopersicum (Fruit, Seeds) extracts
SeNPs were prepared by using two types of extracts freshly prepared tomato fruit extract and seeds extract. Salt solutions of sodium salt of selenite with molarity of 10 mM, 12 mM and 14 mM were used. Tomato fruits and seeds extract were mixed up with salt solutions in 1:4 ratios i.e. (Extract1: 4 Salt). The plant extract reduced the Se salt and thus nanoparticles of Se were produced after incubation. The colour of solution changed soon after mixing up the salt and plant extract solutions. After incubation the brick red colour indicates the production of SeNPs. Fig. 1 indicated the colour change and synthesis of SeNPs at different concentrations are shown in Fig. 2.
Slightly Yellowish Reaction Mixture of Na2SeO3 and Tomato Plant Extracts (a) Fruit Juice (b) Seeds Extract.
SeNPs Synthesized at (a) 10 mM, (b) 12 mM (c) 14 mM Concentrations of Na2SeO3.
3.2 Optimization
3.2.1 Effect of pH
By changing the pH clear colour change of the reaction mixture was noticed. The pH of reaction mixture was adjusted to 5,7 and 8 and UV-spectrophotometry analysis was performed. The range of wavelength was set at 200 to 800 nm and aborbance range was selected as 0 to 5 cm−1. The highest peak was observed at 350 nm that is a characteristic of SeNPs. Whereas, other two peaks were also characteristic peaks of SeNPs according to reported literature (Fig. 3).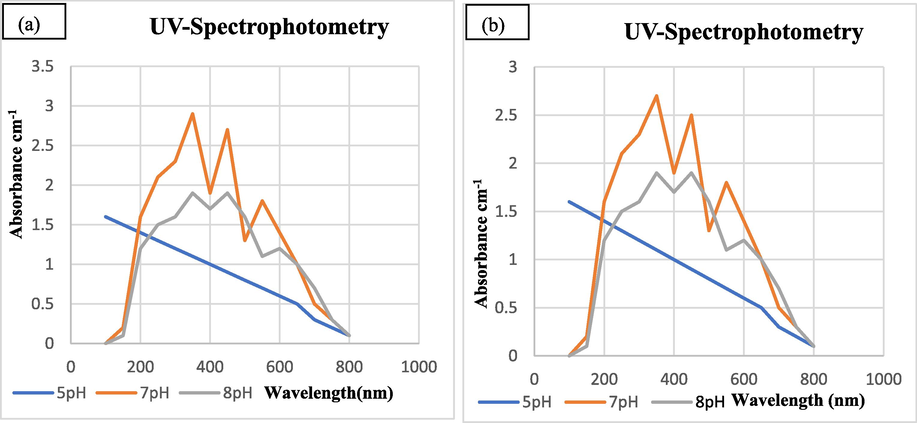
UV–visible Photometric Analysis of Biosynthesized SeNPs at Different pH from Tomato Plant (a) Juice Extract (b) Seeds Extract.
3.2.2 Effect of incubation time
To check the stability of synthesized SeNPs UV-spectrometry analysis was done. The results indicated a clear highest peak at 350 nm with maximum absorbance. Soon after mixing the plant extract and salt solution, the reaction mixture was incubated for 3 h at high temperature of 75° C and then placed at 37° C for 24, 48, 72 and 96 h. The results indicated that NPs of selenium prepared from tomato plant have robust stability. Highest peak obtained at 350 nm after incubation of 96 h (Fig. 4).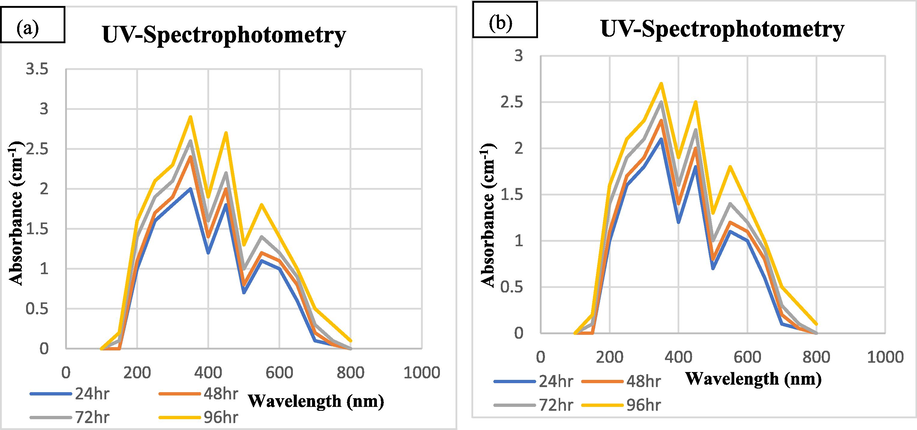
UV–visible photometric analysis of biosynthesized SeNPs from Tomato Plant at different time incubations (a Juice Extract (b) Seeds Extract.
3.2.3 Effect of different concentrations
In order to synthesize SeNPs in maximum amount, sodium selenite (Na2SeO3) concentration was optimized. At concentration of 1 mM, 2 mM, 3 mM, 4 mM and 5 mM, there was no change in colour of reaction mixture. At 6 mM concentration of salt a slight colour change was observed. For the sake of having maximum amount of SeNPs 10 mM, 12 mM and 14 mM concentrations were selected and clear colour change i.e. colourless to brick red was obtained. UV–Vis spectrophotometry results showed that the characteristic peak obtained with maximum absorbance was at 350 nm (Fig. 5).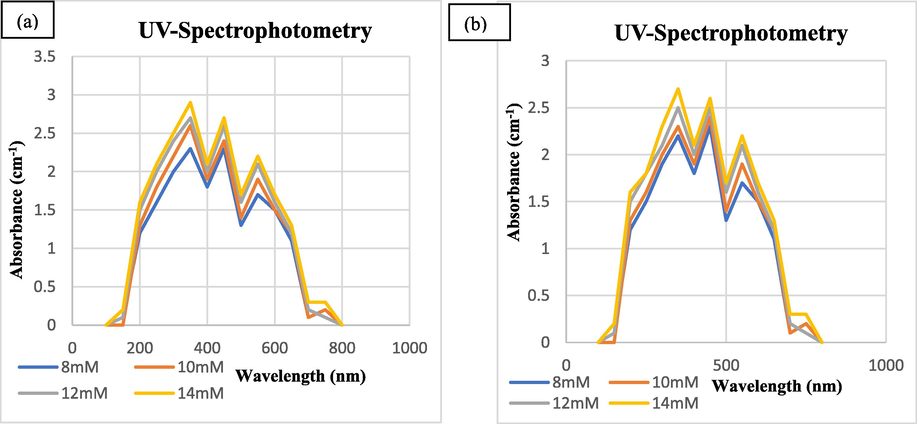
UV–visible Photometric Analysis of Biosynthesized SeNPs at Different Concentrations from Tomato Plant (a) Juice Extract (b) Seeds Extract.
3.2.4 Effect of temperature
The reaction mixture was incubated at 75° C at rotatory shaker for 3 h. Then further incubated at 37° C for 96 h in order to have maximum quantity of SeNPs (Fig. 6).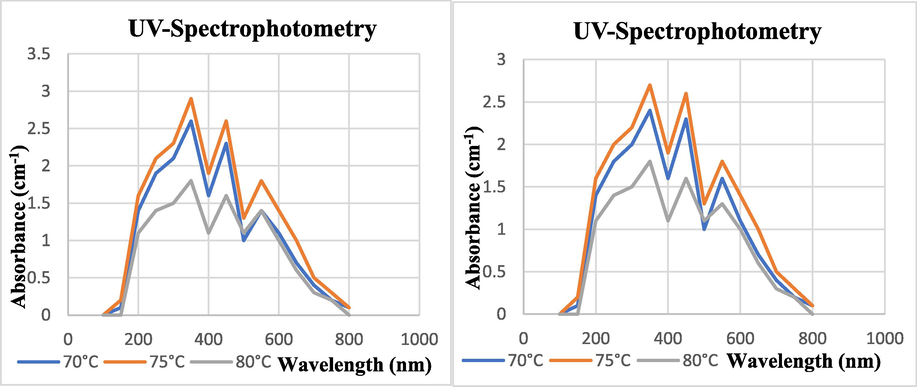
UV–visible Photometric Analysis of Biosynthesized SeNPs at Different Temperatures fot Tomato Plant (a) Juice Extract (b) Seeds Extract.
3.3 Characterization tests
There are different test used for nanoparticles characterization. Following tests were performed for selenium nanoparticles characterization.
3.3.1 UV- spectrophotometry analysis
SeNPs synthesis was confirmed by UV spectrophotometry. Se nanoparticles that were synthesized from Tomato fruit extract and seeds extract were analyzed by setting the wavelength range between 100 and 800 nm. 3 different peaks of absorbance were obtained at 350 nm, 450 nm and at 550 nm respectively. The highest peak of maximum absorbance was obtained at 350 nm for both extracts (Fig. 7).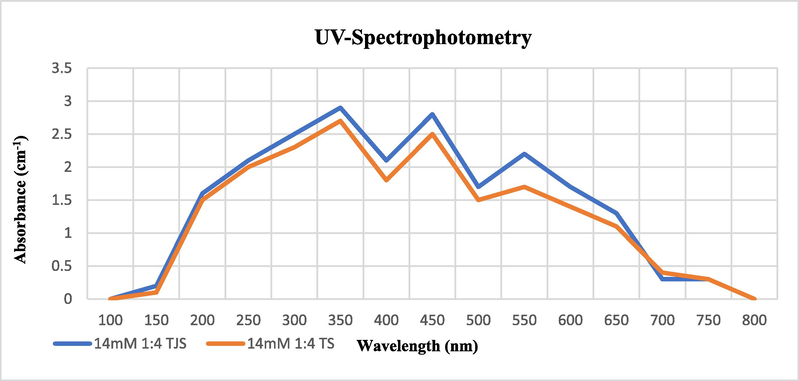
UV- Spectrum of SeNPs Synthesized from Solanum lycopersicum at 14 mM Concentration at Standard Conditions.
3.3.2 FTIR analysis
FTIR analysis was done to find out the functional groups present on SeNPs (Fig. 8). Absorbance was noted taken for all samples synthesized from both extracts i.e. fruits and seeds extract. The absorbance range was set at 1000 to 350 cm−1. Selenium nanoparticles of juice extract indicated absorbance of 3262.35 cm−1, 2118.74 cm−1, 1633.85 cm−1. Whereas, nanoparticles of Se that were prepared from seeds extract showed absorbance peaks from 3200 to 330 cm−1 range. These absorbance values have confirmed the presence of Hydroxyl group (OH bond), Alcohols, Phenols, and N-H bond stretches (Amide group). For peaks in 1600 to 170 cm−1 region C-N stretches i.e. Amides such as amides I and II with C-H bonds due to alkenes presence was obtained.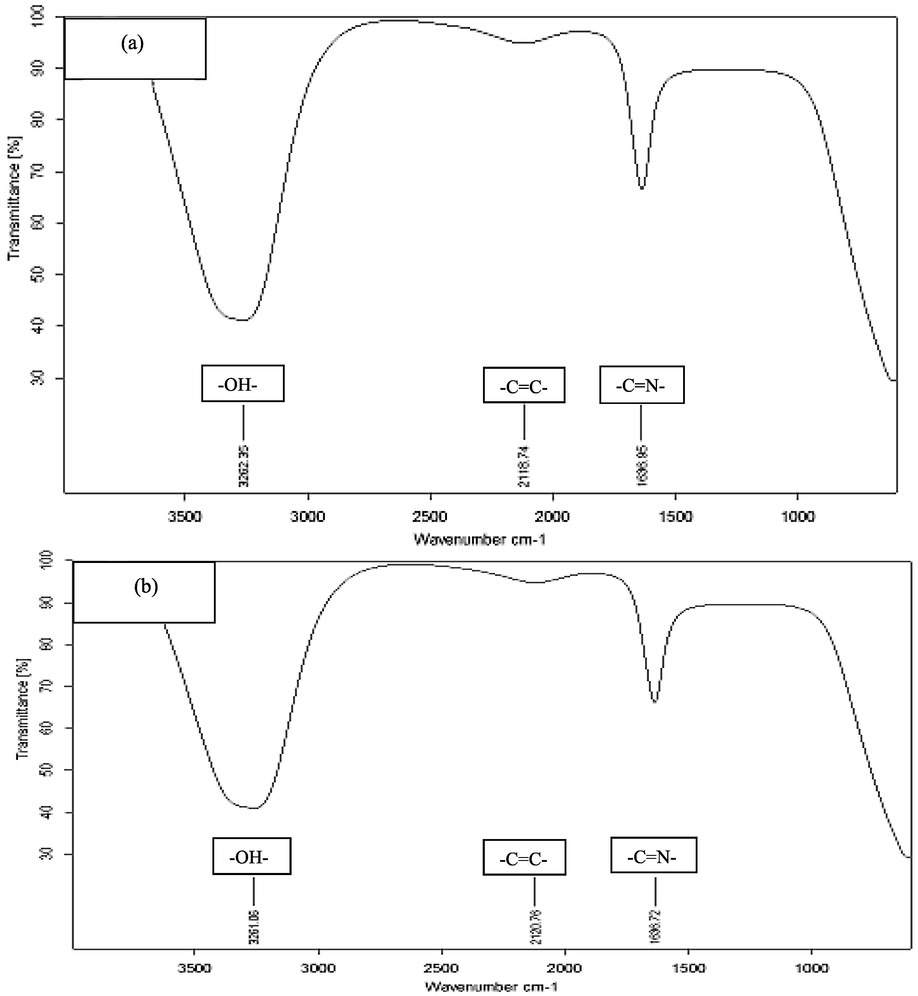
Spectroscopic FTIR analysis SeNPs prepared from Tomato Plant (a) Juice Extract (b) Seeds Extract.
3.3.3 Dynamic light scattering (DLS) analysis
Graphs obtained for Se nanoparticles were mentioned in Fig. 9. This analysis helped us to find the zeta average size of nanoparticles of Se. Results obtained from DLS analysis for tomato juice extract were 2 model peaks 1st at 989.5 d.nm and 2nd at 151.7 d.nm with PDI value 0.432. The average size obtained was 1020 d.nm. There was only single model peak obtained for seed extract NPs at 330.8 d.nm and with PDI value 0.761. The average size resulted was 1155 d.nm. This analysis indicated polydispersity of SeNPs.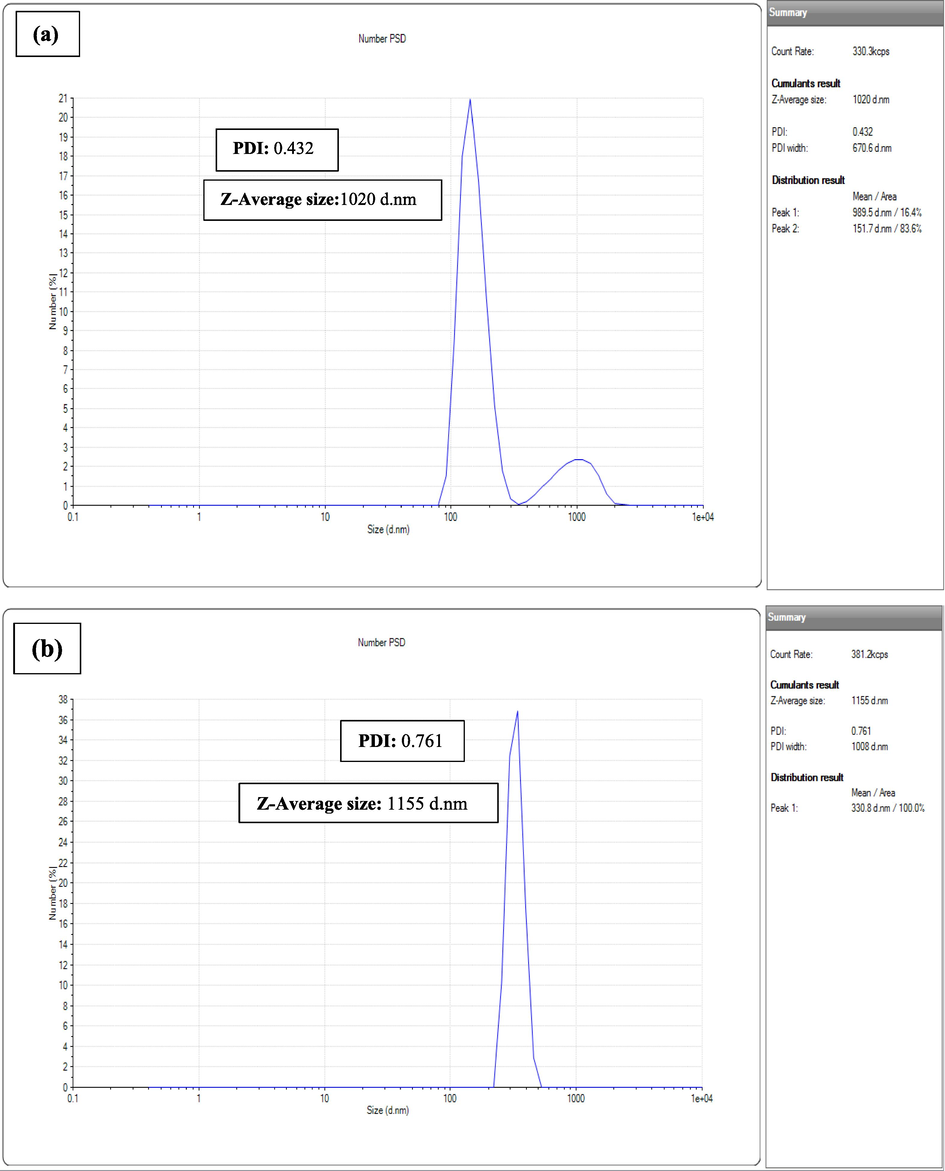
Size Distribution Patterns of SeNPs Synthesized by Tomato Plant (a) Juice Extract (b) Seeds Extract.
3.4 Antimicrobial activity (well diffusion assay)
There were 7 bacterial cultures against which antimicrobial activity was assessed (Table 1 & 2). Bacterial cultures used were some gram negative i.e. Escherichia coli, Pseudomonas aeruginosa, Salmonella enterica and some gram positive cultures i.e. Staphylococcus aureus, Micrococcus luteus, Bacillus subtilis, Klebsiella pneumoniae. Results indicated that NPs of Se had zone of inhibition (ZOI) that inhibit microbial growth. SeNPs indicated significant ZOI and size of zone increases with the concentration of SeNPs. In comparison of results, it was found that NPs from Tomato juice extract had greater ZOI than seeds extract. More significant results were shown by SeNPs prepared at 14 mM concentration. Plant extracts i.e. juice and seeds extract were also loaded on plates as a control but no (ZOI) was recorded, it means that the juice and seeds of the plant do not possess antibacterial activity.
Bacteria
Molecules
Replicates
B. subtilis
S. aureus
M. luteus
ZOI mm
Standard
R1
36.3
36.3
33.8
R2
36.1
36.9
35.9
R3
35.6
34.8
35.3
Mean ± STD
36 ± 0.360555
36 ± 1.081665
35 ± 1.081665
10 mM
R1
17.5
18.3
17.6
R2
18
17.6
18.1
R3
18.5
18.1
18.3
Mean ± STD
18 ± 0.5
18 ± 0.360555
18 ± 0.360555
12 mM
R1
18.8
20.3
19.6
R2
20.9
19.5
20.1
R3
20.3
20.2
20.3
Mean ± STD
20 ± 1.081665
20 ± 0.43589
20 ± 0.360555
14 mM
R1
22.2
21.4
21.8
R2
23.1
23
23.9
R3
23.7
21.6
23.3
Mean ± STD
23 ± 0.754983
22 ± 0.87178
23 ± 1.081665
Bacteria
Molecules
Replicates
E. coli
S. enterica
P. aeruginosa
K. pneumoniae
ZOI mm
Standard
R1
36
36.3
36.4
36.7
R2
38
36.1
35
37.5
R3
37
35.6
36.6
36.8
Mean ± STD
37 ± 1
36 ± 0.360555
37 ± 0.87178
37 ± 0.43589
10 mM
R1
17.4
17.8
17.8
18.6
R2
18.8
17.4
19.9
19.3
R3
17.8
18.8
19.3
19.1
Mean ± STD
18 ± 0.72111
18 ± 0.72111
19 ± 1.081665
19 ± 0.360555
12 mM
R1
20.1
19.2
20.4
21.8
R2
20.7
20.1
22
20.2
R3
19.2
20.7
20.6
21
Mean ± STD
20 ± 0.754983
20 ± 0.754983
21 ± 0.87178
21 ± 0.8
14 mM
R1
23.3
22.6
22.8
24.3
R2
22.9
24
24.9
23.5
R3
22.8
22.4
24.3
24.2
Mean ± STD
23 ± 0.264575
23 ± 0.87178
24 ± 1.081665
24 ± 0.43589
3.5 Antioxidant activity (DPPH assay)
DPPH assay was employed in order to measure the free radical scavenging activity. Results are shown in Table 3. The results demonstrated that antioxidant activity of selenium NPs was enhanced with the increase in concentration. Results showed that SeNPs contain free radical scavenging role with IC50 value of 20.7398 µg/mL.
Conc. µg/mL
%Inhibition
R1
R2
R3
Mean ± Std
IC50 µg/mL
10
30.535
29.935
30.935
30.735
30.53 ± 0.52
24.76
20
42.9385
44.1885
41.9385
42.6885
42.93 ± 1.14
30
45.9649
45.4798
45.6159
46.799
45.96 ± 0.72
40
48.3333
47.7333
48.7333
48.5333
48.33 ± 0.52
50
53.3771
52.7771
53.5771
53.7771
53.37 ± 0.52
60
54.1315
54.9315
53.5315
53.9315
54.13 ± 0.72
70
58.2456
57.8456
58.0456
58.8456
58.24 ± 0.52
80
58.5964
57.3464
59.2314
59.2114
58.59 ± 1.08
90
60.4385
61.3385
59.9385
60.0385
60.43 ± 0.78
100
61.6666
62.4666
61.1666
61.3666
61.66 ± 0.7
Control
2.28
3.6 Antidiabetic activity (alpha amylase assay)
The antidiabetic activity of SeNPs from Solanum lycopersicum was checked by alpha amylase assay by using concentrations 10 to 100 μg/mL. The results demonstrated that SeNPs have antidiabetic role with IC50 value of 24.4642 µg/mL. Table 4 showed the results. This activity of SeNPs has not been reported before by alpha amylase inhibitory assay and it is reported for the 1st time.
Conc.
µg/mL
%Inhibition
R1
R2
R3
Mean ± Std
IC50 µg/mL
10
30.44642857
31.34642857
29.84642857
30.14642857
30.44 ± 0.79
22.07
20
41.42857143
40.17857143
42.42857143
41.67857143
41.42 ± 1.14
30
43.30357143
44.10357143
42.60357143
43.20357143
43.30 ± 0.75
40
52.14285714
51.54285714
52.64285714
52.24285714
52.14 ± 0.55
50
57.14285715
55.14285715
58.64285715
57.64285715
57.14 ± 1.80
60
58.01785714
58.41785714
57.71785714
57.91785714
58.01 ± 0.36
70
59.82142857
59.52142857
59.22142857
60.72142857
59.82 ± 0.79
80
60.92857143
62.12857143
59.92857143
60.72857143
60.92 ± 1.11
90
61.60714286
62.30714286
61.30714286
61.20714286
61.60 ± 0.60
100
66.2
64.2
67.4
67
66.2 ± 1.74
control
1.12
4 Discussion
In present time nanotechnology is becoming the most emerging field of interest because of its applications in life. Literature has evidences that NPs have many useful applications. NPs are prepared from different materials like polymers, ceramics and lipids etc. NPs with various sizes carry antimicrobial, antioxidant and anticancer activities etc. There are different methods used for NPs synthesis like physical, chemical and biological (Sozer and Kokini, 2009). But there are many issues regarding physical and chemical methods regarding to their toxicity and not being eco-friendly. So, a method which is more safe and non-toxic required. Biological method for the synthesis of NPs is preferred. It has many useful and beneficiary aspects as compared to other methods. It is safe, natural, cheap and ecofriendly (Sanvicens and Marco, 2008). From all these synthesis methods green synthesis is most beneficial and safe way. Previous literature indicates that nanoparticles are produced by large number of metals such as gold, silver etc (Gracia et al., 2021;10(2):217.). The first prepared NPs were of gold and silver. Now the NPs from metalloids are also being synthesized and they behold various beneficial properties like drug delivery role in case of cancer. Large numbers of metals and metalloids sources are available and it is an important task to select the best one source with least or no toxicity. Se is selected because of its useful qualities and its requirement by the human body. It carries many useful properties which belongs to different fields of life (Alagesan and Venugopal, 2019).
To prepare NPs, Se salt of sodium (Na2SeO3), Selenous acid (H2SeO3) and sodium selenosulfate (Na2SeSO3) has been reported in previous literature (Panahi-Kalamuei et al., 2014). SeNPs were synthesized by microwave irradiation method. But this method has several disadvantages like high power usage for generation of microwave irradiations. It has been investigated that NPs of selenium were also by chemical methods also. But these protocols involves the usage of chemicals as a reducing agent that may cause some sort of toxicity e.g. Cytochrome c3 dehydrogenase (Charbgoo et al., 2017).
Similarly, NPs of Se were prepared by hydrothermal method but extreme conditions of temperature and pressure like 1–10,000 atm required for it. Biological method is more preferable and safe as compared to other methods. Green synthesis comes under biological method and it is the most economical and safe way (Rafique et al., 2017). Different NPs of various sizes were reported by green synthesis with size of 140–200 nm and 180 to 190 nm (Ramamurthy et al., 2013).
In this project green synthesis method is adapted and NPs of Se were prepared by Solanum lycopersicum plant extracts i.e. fruits juice and seeds extract. Freshly prepared extracts were mixed up with salt solutions of different concentrations. Tomato extracts reduces the salt i.e. Na2SeO3 and results in SeNPs. The synthesis of SeNPs was indicated by the change in colour of the mixture. After mixing salt and extract the colour of the mixture was colourless and later it turns to brick red. Different molar solution of 10 mM, 12 mM and 14 mM were used. Na2SeO3 serves as a precursor for Se. The proper conditions were given to reaction mixtures i.e. 75° C and for 3 h. After that mixture incubated at 37° C in dark and brick red SeNPs were produced (Srivastava and Mukhopadhyay, 2015). After the synthesis UV spectrophotometry analysis was carried out and absorbance was recorded. The maximum absorbance peaks were obtained between 300 and 600 nm. The peaks obtained confirmed the synthesis of SeNPs. In order to find the functional groups on NPs surface FTIR analysis was performed. The results lie in the range of 1000 to 350 cm−1. All samples resulted the peaks of maximum absorbance at 1600 cm−1 to 1700 cm−1 and 3200 cm−1 to 330 cm−1. These absorbance ranges indicated the OH bond alcohols, phenols, N-H amide bond stretches, proteins, amides i.e. I and II, C = C etc. NPs of selenium carried antimicrobial role at significant level. DLS analysis was carried out to calculate the average size of SeNPs that were synthesized from both extracts separately. The average size obtained for fruit extract NPs of Se was 1020 d.nm and for seeds extract was 1155 d.nm. The results showed that the NPs were polydisperse in nature.
There are many antibiotics available like streptomycin, amoxicillin, ampicillin but they have some limitations. These antibiotics are expensive and cause microbes to be resistant to them. Massive usage is one of the main causes of microbial resistance against drugs. Keeping an eye on this problem, it is the need to find or develop a source which contain antimicrobial role. In this study nanoparticles of selenium were synthesized and their antimicrobial activity was evaluated. Well diffusion assay was assessed. Ciprofloxacin was served as a standard drug. Microbial cultures of gram positive and gram negative bacteria were used and ZOI was measured. The zones of inhibition obtained were of a range from 21 mm to 24 mm. Previous investigation of Shanti Ratnakomala and his team supports the results of our project (Chand et al., 2019). Zone size depends on the concentration and amount of SeNPs loaded in the wells of nutrient agar media plate. Thus, SeNPs have antimicrobial role and it inhibits the bacterial growth by causing disruption in the bacterial membrane. The presence of various functional groups on the NPs surface cause the absorption of them into the bacterial cell results in cell lysis (Esquivias et al., 2021).
NPs of Se have antioxidant property and that is reported in past papers (Kong et al., 2014). DPPH assay indicated that these NPs have free radical scavenging activity. In this study project it has been found that nanoaprticles of Se prepared from Solanum lycopersicum contain antidiabetic role with IC50 value 20.7398 µg/mL. Previous studies indicated that our results are more significant and our particles are more effective. The antidiabetic activity was assessed by alpha amylase inhibitory assay and the results showed that our nanoparticles have effective role as an antidiabetic agent with IC50 value of 24.4642 µg/mL. This has not been reported before.
SeNPs have ability to replace the antibiotics and antidiabetic medicines in future. These NPs have antioxidant activity as well and can be used in future for free radicals scavenging and can be used in cosmetics industry as well.
SeNps from tomato are novel and holds unique qualities with efficiency. In previous studies Nps of selenium from different sources were reported with different useful activities but Nps from Solanum lycopersicum are unique and newly synthesized [43]. The antimicrobial results against various bacterial species are highly significant. Moreover, the antidiabetic and antioxidant qualities of these Nps are also marvelous. As, the microbes are developing resistance against the antibiotics that are in our markets so, these nanoparticles may serve in future for this purpose and also for skin diseases and diabetes treatment.
5 Conclusion
Selenium nanoparticles from Solanum lycopersicum i.e. Tomato plant were prepared successfully. The nanoparticles of Se were synthesized by using Tomato fruit juice and seeds extract. Many methods are available for the synthesis of SeNPs and from all these methods green synthesis is most feasible, cheap, non-toxic and environment friendly, due to all these aspects green synthesis is adopted. Optimized conditions of temperature, concentration of salt and pH resulted in SeNPs synthesis successfully in maximum quantity. SeNPs were characterized by UV-spectrophotometry, DLS and FTIR analysis. Average size determined by the DLS was 1020 d.nm for fruit extract and for seeds extract was 1155 d.nm. SeNPs were polydisperse in nature and have antimicrobial role against selected strains of bacteria. Moreover, SeNPs have antidiabetic and antioxidant qualities due to which they may replace antibiotics and antidiabetic medicines as well and be used in treatment and prevention of diseases caused by free radicals.
References
- Introduction to nanotechnology and its applications to medicine. Surg. Neurol. Int.. 2004;61(3):216-220.
- [Google Scholar]
- Introduction to Nanotechnology. Canada: John Wiley & Sons; 2003.
- Springer Handbook of Nanotechnology. U.S.: Springer; 2017.
- The golden age: gold nanoparticles for biomedicine. Chem. Soc. Rev.. 2012;41(7):2740-2779.
- [Google Scholar]
- One-step one-phase synthesis of monodisperse noble-metallic nanoparticles and their colloidal crystals. J. Am. Chem. Soc.. 2006;128(20):6550-6551.
- [Google Scholar]
- Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv.. 2013;31(2):346-356.
- [Google Scholar]
- Selenium nanoparticles inhibit Staphylococcus aureus growth. Int. J. Nanomedicine. 2011;6:1553.
- [Google Scholar]
- Synthesis and antioxidant properties of gum arabic-stabilized selenium nanoparticles. Int. J. Biol. Macromol.. 2014;65:155-162.
- [Google Scholar]
- Nano-selenium and its nanomedicine applications: a critical review. Int. J. Nanomed.. 2018;13:2107.
- [Google Scholar]
- Chemical constitution and effect of extracts of tomato plants byproducts on the enteric viral surrogates. Int. J. Environ. Health Res.. 2015;25(3):299-311.
- [Google Scholar]
- Tomatoes and tomato products as dietary sources of antioxidants. Food Rev. Int.. 2009;25(4):313-325.
- [Google Scholar]
- Total phenolic, flavonoid, tomatine, and tomatidine contents and antioxidant and antimicrobial activities of extracts of tomato plant. Int. J. Anal. Chem. 2015:1-10.
- [Google Scholar]
- Green synthesis of zinc oxide nanoparticles using Hibiscus subdariffa leaf extract: effect of temperature on synthesis, anti-bacterial activity and anti-diabetic activity. RSC Adv.. 2015;5(7):4993-5003.
- [Google Scholar]
- Synthesis and antidiabetic activity of selenium nanoparticles in the presence of polysaccharides from Catathelasma ventricosum. Int. J. Biol. Macromol.. 2018;114:632-639.
- [Google Scholar]
- Antioxidant activity and green synthesis of selenium nanoparticles using allium sativum extract. Int. J. Phytomed.. 2017;9(4):634.
- [Google Scholar]
- Green synthesis of zinc oxide nanoparticles using tomato (Lycopersicon esculentum) extract and its photovoltaic application. J. Exp. Nanosci.. 2016;11(5):314-327.
- [Google Scholar]
- Preparation of cerium oxide nanoparticles in Salvia Macrosiphon Boiss seeds extract and investigation of their photo-catalytic activities. Ceram. Int.. 2019;45(4):1-27.
- [Google Scholar]
- Bio-fabrication of gold nanoparticles using aqueous extract of red tomato and its use as a colorimetric sensor. Nanoscale Res. Lett.. 2013;8(1):181.
- [Google Scholar]
- Green synthesis of silver nanoparticles from grape and tomato juices and evaluation of biological activities. IET Nanobiotechnol.. 2016;11(2):193-199.
- [Google Scholar]
- Green synthesis and structural characterization of selenium nanoparticles and assessment of their antimicrobial property. Bioproc. Biosyst. Eng.. 2015;38(9):1723-1730.
- [Google Scholar]
- Green synthesis of selenium nanoparticles using Acinetobacter sp. SW30: optimization, characterization and its anticancer activity in breast cancer cells. Int. J. Nanomed.. 2017;12:6841-6855.
- [Google Scholar]
- Biosynthesis of Se nanoparticles and its effect on UV-induced DNA damage. Colloids Surf. B.. 2013;103:261-266.
- [Google Scholar]
- FTIR spectroscopic studies of selenite reduction by cells of the rhizobacterium Azospirillum brasilense Sp7 and the formation of selenium nanoparticles. J. Mol. Struct.. 2017;1140:106-112.
- [Google Scholar]
- A comparison of atomic force microscopy (AFM) and dynamic light scattering (DLS) methods to characterize nanoparticle size distributions. J. Nanopart. Res.. 2008;10(1):89-96.
- [Google Scholar]
- MacLeod, R.A., Onofrey, E., Norris, M.E., 1954. Nutrition and metabolism of marine bacteria I.: survey of nutritional requirements. J Bacteriol. 68(6), 680.
- Synthesis, characterization, and antimicrobial activity of copper oxide nanoparticles. J. Nanomater.. 2014;2014:1-4.
- [Google Scholar]
- Synthesis, characterization, and antimicrobial activity of zinc oxide nanoparticles against human pathogens. J. Bionanosci.. 2012;2(4):329-335.
- [Google Scholar]
- Enzyme assay guided isolation of an α-amylase inhibitor flavonoid from Vaccinium arctostaphylos leaves. Iran. J. Pharm. Res.. 2011;10(4):849-853.
- [Google Scholar]
- Nanoarchitectonics: a new materials horizon for nanotechnology. Mater. Horiz.. 2015;2(4):406-413.
- [Google Scholar]
- Nanotechnology and its applications in the food sector. Trends Biotechnol.. 2009;27(2):82-89.
- [Google Scholar]
- Multifunctional nanoparticles–properties and prospects for their use in human medicine. Trends Biotechnol.. 2008;26(8):425-433.
- [Google Scholar]
- Green synthesis of selenium nanoparticle using leaves extract of withania somnifera and its biological applications and photocatalytic activities. Bionanoscience. 2019;9(1):105-116.
- [Google Scholar]
- Facile microwave synthesis, characterization, and solar cell application of selenium nanoparticles. J Alloys Compd.. 2014;617:627-632.
- [Google Scholar]
- Cerium oxide nanoparticles: green synthesis and biological applications. Int. J. Nanomed.. 2017;12:1401.
- [Google Scholar]
- A review on green synthesis of silver nanoparticles and their applications. Artif Cells Nanomed. Biotechnol.. 2017;45(7):1272-1291.
- [Google Scholar]
- Green synthesis and characterization of selenium nanoparticles and its augmented cytotoxicity with doxorubicin on cancer cells. Bioproc. Biosyst Eng.. 2013;36(8):1131-1139.
- [Google Scholar]
- Green synthesis characterization and antimicrobial activity against Staphylococcus aureus of silver nanoparticles using extracts of neem, onion and tomato. RSC Adv.. 2019;9(30):17002-17015.
- [Google Scholar]
- A review of the antimicrobial activity of selenium nanoparticles. J. Nanosci. Nanotechnol.. 2021;21(16):5383-5398.
- [Google Scholar]
- Gracia, Y.C., Alvarez, C.C., Pliego, G.C., Mendoza, A.B., Fuente de al, C.M., Rangel, A.S., Reyna, J.V., Maldonado, A.J., 2021. Effect of three nanoparticles (se ,si and cu) on bioactive compounds of bell peper fruits under saline stress. Plant J. 10(2), 217.







