Translate this page into:
Magnetically recyclable Schiff-based palladium nanocatalyst [Fe3O4@SiNSB-Pd] and its catalytic applications in Heck reaction
⁎Corresponding authors. lotfor@ums.edu.my (Md. Lutfor Rahman), shaheen.sarkar@tus.ie (Shaheen M. Sarkar)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
A magnetically separable palladium nanocatalyst has been synthesized through the immobilization of palladium onto 3-aminopropylphenanthroline Schiff based functionalized silica coated superparamagnetic Fe3O4 nanoparticles. The nanocatalyst (Fe3O4@SiNSB-Pd) was fully characterized using several spectroscopic techniques, such as FT-IR, HR-SEM, TEM, XRD, ICP, and XPS. The microscopic image of Fe3O4 showed spherical shape morphology and had an average size of 150 nm. The Pd-nanoparticles exhibited an average size 3.5 ± 0.6 nm. The successful functionalization of Fe3O4@SiNSB-Pd was identified by FT-IR spectroscopy and the appearance of palladium species in Fe3O4@SiNSB-Pd was confirmed by XRD analysis. While XPS has been utilized for the determination of the chemical oxidation state of palladium species in Fe3O4@SiNSB-Pd. Several activated and deactivated arene halides and olefines were employed for Mizoroki-Heck cross-coupling reactions in the presence of Fe3O4@SiNSB-Pd, each of which produced the respective cross-coupling products with excellent yields. The Fe3O4@SiNSB-Pd shows good reactivity and reusability for up to seven consecutive cycles.
Keywords
Magnetic nanoparticles
Silica coated
Phenanthroline
Palladium
Schiff-base
Heck reaction
1 Introduction
In recent decades, the Mizoroki-Heck C-C bond formation reaction of olefines with arene halides or arenediazonium salts has emerged as a novel method for the development of pharmaceuticals, natural products, modern materials, bioactive molecules, and a variety of other derivatives that are used as intermediate compounds in various industries (Trost et al., 2016; Biffis et al., 2018; Arghan et al., 2018; Parouch et al., 2020). Conventional Heck reactions employ highly soluble Pd-phosphine complexes and show high efficiency in cross-coupling reactions (Das et al., 2010; Sardarian et al., 2019; Parlett et al., 2013). The production of highly reactive stable Pd-ligand catalytic systems with minimal Pd leaching is highly desirable for industry, especially in the synthesis of sensitive organic biomolecules, pharmaceuticals, organoelectronic materials and the food industry (Veerakumar et al., 2017). Despite the high reactivity in the catalytic reaction, homogeneous catalytic reactions based on metals still suffer from several disadvantages such as thermal instability and complex and laboriousness process for the separation of the metal catalysts from the reaction mixture and final product. The high cost of the metal and the use of toxic and hazardous solvents limit their further practical application in the development of homogeneous catalysts (Polshettiwar et al., 2011; Esmaeilpour et al., 2014).
Several organometallic catalysts have been developed for Heck reactions, for example Cu (Calo et al., 2005), Ni (Zheng and Newman, 2019), Co (Kazemnejadi et al., 2019), and Pd (Khodaei and Dehghan, 2019; Esmaeilpour and Zahmatkesh, 2019; Mhaldar et al., 2020; Vibhute et al., 2020; Sarkar et al., 2015; Alamgholiloo et al., 2020). Of these, Pd-catalyzed cross-coupling reactions are the most effective synthetic route and have been studied extensively by researchers. In general, the catalytic activity of solid supported heterogeneous metal catalysts is not as efficient as that of homogeneous counter-catalysts due to their larger surface area and smaller centres (Bodaghifard, 2019). However, due to high prices and toxicity of the metal palladium, it is necessary to develop simple and inexpensive highly reactive heterogeneous-Pd catalysts (Lim and Lee, 2010; Garrett and Prasad, 2004) to minimize of palladium contamination and its harmful effects to the environment and reduce costs. From an economic, sustainable and ecological point of view, it is of great important to develop a highly active, reusable Pd-catalyst that prevents the loss of small amounts of metal and which can also be easily recovered from the reaction mixture. Conventional heterogeneous catalysts have several disadvantages, such as low efficiency, stability and metal bonding ability, and require high temperatures and harsh reaction conditions. An interesting approach to overcome these disadvantages is the immobilization of metal catalysts on nanoparticle-based solid supports, which provides an accumulation of advantages of both homo- and heterogeneous metal catalysts (Mofijur et al., 2020; Hong et al., 2020; Ahadi et al., 2019; Algarou et al., 2020). Palladium nanoparticles have attracted immense interest in the academic and industrial research field of fabrication because of their superior reactivity due to their huge surface area, which greatly improves the interaction of reactant and the catalytic active sites of the metal (Isfahani et al., 2013).
Therefore, a useful approach is the stabilization of a metal catalyst on a solid support, which increase the surface area, provides the properties of nanoparticles with a large number of active catalytic centres and facilitates agglomeration and prevents their coherent distribution (Yang et al., 2013; Li et al., 2013; Li et al., 2012). In recent years, much effort has been put into introducing a different catalyst that is more efficient, environmentally friendly, easier and more reliable by introducing palladium nanoparticles on a solid support. For example, carbon-based materials (Sun et al., 2013; Kamal et al., 2012; Shekarizadeh and Azadi, 2020), cellulose (Sarkar et al., 2017), silica (Sarkar et al., 2015), polymers (Sato et al., 2015), magnetic Fe3O4 (Choghamarani et al., 2019; Vinnik et al., 2021); MOF (Luo et al., 2019; Van Velthoven et al., 2020); carbon nanotubes (Zhou et al., 2017) composite materials (Madrahalli Bharamanagowda et al., 2020), Pd-NHCs complex in MIL-101 (Cr) (Niknam et al., 2021); fiber (Xiao et al., 2021) were investigated for the development of stabilized nanocatalysts based on palladium. However, they suffer from limitations such as high cost, the use of toxic solvents throughout catalyst preparation, and high consumption of chemicals.
Among the various nanoparticles, magnetite (Fe3O4) widely used as a solid support for palladium (Fatahi et al., 2021; Marandi and Koukabi, 2021) due to its unique physicochemical properties, low cost, non-hazardous, high chemical and thermal stability, and biocompatibility (Arco et al., 2016) and easy separation from the reaction mixture (Firouzabadi et al., 2014; Ma et al., 2018; Trukhanov et al., 2021). One of the issues associated with Fe3O4 nanoparticles is their poor stability, specifically under acidic conditions. This is because it aggregates over time to reduce surface area and tends to oxidize easily in normal atmospheres (Corma, 1997). To overcome these problems, efficient methods are needed for the preparation of nanoparticles that enhance their physicochemical stability against degradation in the course of or after the synthesis process. Recently, silicon dioxide has been used as a protective shell of Fe3O4, which can (1) inhibit the aggregation and formation of Fe3O4 (2) keeping the magnetic properties of the core with efficient separation for reuse (3) provides large surface Si-OH groups for further modifications and (4) well-defined distribution of active metal centres or intrinsic acid-basic reactive centres to forward the catalytic reaction smoothly (Du et al., 2012; Li et al., 2013).
This study introduces a recyclable silica-coated superparamagnetic iron oxide-based shift-based Fe3O4@SiNSB-Pd nanocatalyst and its catalytic application toward Mizoroki-Heck C-C bond formation reactions. The Fe3O4@SiNSB-Pd effectively facilitated Mizoroki-Heck and Heck-Matsuda reactions of arene halides and arene-diazonium salts with various olefins to give the corresponding olefinic products in excellent yields. The cross-coupling results indicated that the supported Fe3O4@SiNSB-Pd propagated the reaction efficiently and could be recovered from the reaction combination using an external magnet. The Fe3O4@SiNSB-Pd was successfully reused seven-times without any significant drop of its initial catalytic activity.
2 Experimental
2.1 Preparation of Fe3O4 nanoparticles
To a 300 ml beaker, 6.5 g of FeCl3·6H2O and 2.43 g sodium citate were dissolved in 75 ml of ethylene glycol for 1 h with vigorous magnetic stirring. To this solution, 12 g of NaOAc was added and the mixture was aggressively stirred for another 1 h. The reaction mixture was heated for 8 h at 200 °C in a Teflon-lined stainless-steel autoclave. After that, the autoclave was left to cool to ambient temperature naturally. A magnet was used to collect the black Fe3O4 magnetic nanoparticles (MNPs), which were then rinsed multiple times with an aqueous solution of ethanol before being oven-dried at 60 °C overnight.
2.2 Synthesis of silica coated [Fe3O4@Si] 1
A modified Stöber method was used to cover the SiO2 layer on Fe3O4 nanoparticles. In a typical synthesis process of core–shell Fe3O4@Si, 1 g of as-prepared Fe3O4 particles were added to a round-bottom flask and the particles were sonicated for 1 h. To this suspension, a mixture of 350 ml ethanol, 85 ml of deionized water, and 6 ml of concentrated ammonia were added. The resulting mixture was stirred and 3 ml of TEOS was added dropwise to the reaction flask and continued to stir for 6 h at room temperature. The magnetically coated [Fe3O4@Si] 1 product was washed with aqueous ethanol, dried at 60 °C and separated by using of an external magnate.
2.3 Amine functionalization [Fe3O4@Si-NH2] 2
The amine functionalized Fe3O4@Si-NH2 was synthesized by post-synthesis grafting method. The synthesized silica coated 1 (6 g) was dispersed in 45 ml of toluene for 10 min at 50 °C. Then to this suspension, 4 ml of 3-aminopropyltriethoxysilane (APTES) was added and the reaction mixture was refluxed for 12 h. The amino functionalized magnetic solid products were filtered off, washed several times with methanol and dried at 70 °C for 12 h. The magnetic [Fe3O4@Si-NH2] 2 was separated using an external permanent magnate (Fekri and Zeinali, 2020).
2.4 Preparation of magnetic Schiff-base [Fe3O4@SiNSB] 3
The amine functionalyzed [Fe3O4@SiO2-NH2] 2 (1 g) was dispersed in 30 ml of ethanol and 1 mmol of 1,10-phenanthroline-2,9-dicarbaldehyde was added to the reaction mixture. The resulting suspension was stirred at 60 °C for 12 h under a nitrogen atmosphere. The reaction mixture was cooled to room temperature, and the resulting 3 was filtered on a glass filter, washed three times with ethanol and dried in oven at 60 °C.
2.5 Preparation of [Fe3O4@SiNSB-Pd] 4
An aqueous solution of (NH4)2PdCl4 (280 mg, 45 ml of water) was added to a stirred suspension of [Fe3O4@SiNSB] 3 (1.5 g) in 50 ml of methanol. The resulted suspension was stirred at 25 °C for 1 h, and 0.75 ml of hydrazine monohydrate was added. The lite brown colour Fe3O4@SiNSB turned to dark brown and the mixture was continued to stir for another 1 h at 25 °C. The resulted magnetic silica coated palladium nanocatalyst [Fe3O4@SiNSB-Pd] 4 was filtered on a glass filter, washed with aqueous MeOH and dried in oven at 60° C for 2 h. ICP-AES analysis of 4 showed 0.015 mmol/g of palladium was contained and the average Pd-nanoparticle 3.5 ± 0.6 nm was determined by TEM analysis.
2.6 Procedure for cross-coupling reaction
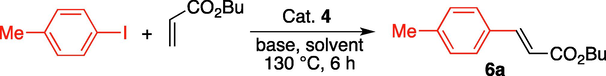
A 10 ml screw-cap glass vial was charged with 10 mg (0.00015 mmol 0.015 mol%) of 4, aromatic halide (1.0 mmol), olefine (1.5 mmol), Et3N (3 mmol) and 2 ml of DMA. The reaction vial was heated at 130 °C for 6 h. For Heck-Matsuda reaction, we just added catalyst, arenediazonium salt, olefine in ethanol and stirred at room temperature for 5 h. The reaction progress and the production of cross-coupling product was monitored by TLC and GC analyses. After completion of the reaction, the reaction vial was cold to room temperature, diluted with ethyl acetate and the solid 4 was separated with an external magnate. The organic layer was washed with water, dried over MgSO4 and the volatile materials were removed under reduced pressure. The crude product was purified on a short silica gel column chromatography using hexane and ethyl acetate as an eluent. 1H NMR for 6a: 500 MHz δ 7.67 (d, 1H, J = 16.0 Hz), 7.43 (d, 2H, J = 8.15 Hz), 7.19 (d, 2H, J = 8.0 Hz), 6.41 (d, 1H, J = 16.0 Hz), 4.20 (t, 2H, J = 6.75 Hz), 2.37 (s 3H), 1.71–1.66 (m 2H), 1.46–1.43 (m 2H), 0.96 (t, 3H, J = 7.45 Hz). 13C NMR (125 MHz) δ 144.52, 140.58, 131.72, 129.57, 128.02, 117.18, 64.32, 30.77, 21.42, 19.18, 13.73.
3 Results and discussion
The magnetic nanocatalyst was constructed based on the incorporation of palladium nanoparticles onto a silica coated Fe3O4 core–shell phenanthroline Schiff base structure. The synthesis steps for nanocatalyst are schematically illustrated in Scheme 1. Preparation of the Fe3O4 was carried out by the reaction of Fe3+ with an aqueous solution of sodium acetate, sodium citate in ethylene glycol at 200 °C. After successfully synthesis of magnetic Fe3O4 nanoparticles, SiO2 encapsulation was accomplished using tetraethylorthosilicate (TEOS) in aqueous ammonia solution. The resulted 1 core–shell was then treated with (3-aminopropyl)triethoxysilane (APTES) to afford amino functionalisation 2. To introduce phenanthroline chelating group onto the amino functionalyzed nanoparticles 2 first we treated 2,9-dimethyl-1,10-phenanthroline with selenium oxide (Johnson and Ji, 2018) to afford the corresponding dialdehyde 5 and the respective 1H NMR spectrum is presented in Fig. 1.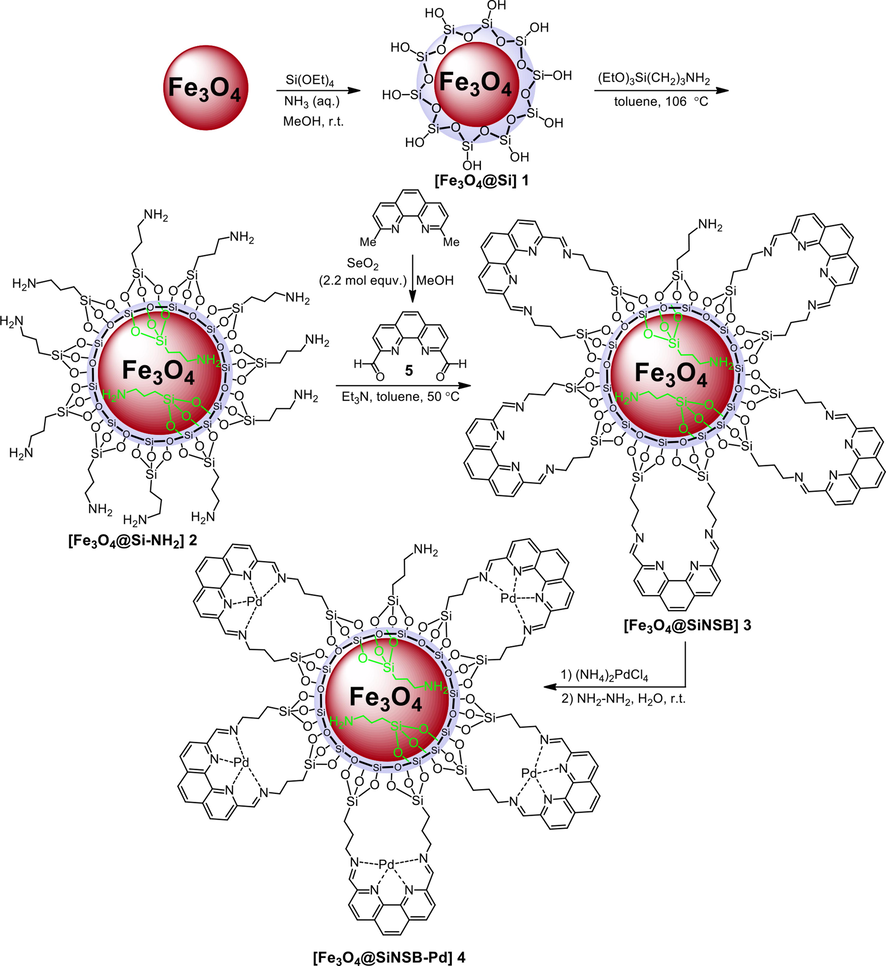
Synthesis of magnetically supported palladium nanocatalyst 4.
1H NMR spectrum of 5.
This dialdehyde 5 was then treated with 2 at 50 °C in toluene to give the corresponding phenanthroline Schiff base 3 and it has been used as Schiff base magnetic bidentate chelating ligand to the incorporation of palladium nanoparticles. To obtain the magnetically silica coated Schiff-based palladium nanocatalyst we treated 3 with an aqueous solution of ammonium tetrachloropalladate at room temperature. The resulting brown colored palladium complex was then treated with hydrazine hydrate to produce the nanocatalyst 4 as a dark brown solid powder. The content of palladium in the nanocatalyst 4 was 0.015 mmol/g. The synthesized material and the composition of the nanocatalyst 4 were examined using various spectroscopic methods.
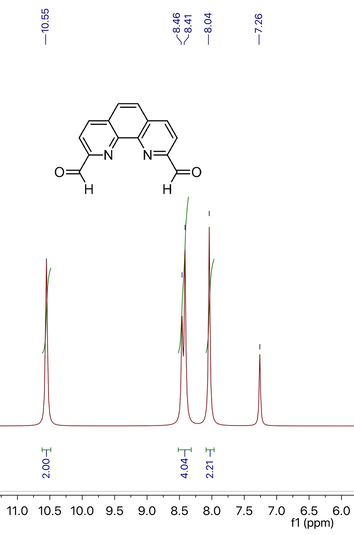
The FT-IR spectra for all synthesized materials are represented in Fig. 2. Vibration at 3037 cm−1 was associated with sp3 C-H stretching in 1 due to the entrapment of TEOS. After incorporating amino functionality in 1, the peaks of absorption were observed at 2850 cm−1 and 2955 cm−1 due to the aliphatic C-H (sp3) stretching. The peaks at 3333 cm−1 and 3366 cm−1 were observed (N-H stretching) for primary amine group attached onto 2. While, after incorporation of the phenanthroline chelating ligand onto 3 absorption peaks at 2845 cm−1, 2955 cm−1 and 2993 cm−1 were observed and N-H stretching peaks were disappeared. Furthermore, after metallic palladium coordination with the nitrogen atoms, the IR peaks were shifted toward the shorter wave number and 2856 cm−1, 2932 cm−1, 2956 cm−1 and 3042 cm−1 peaks were observed for 4.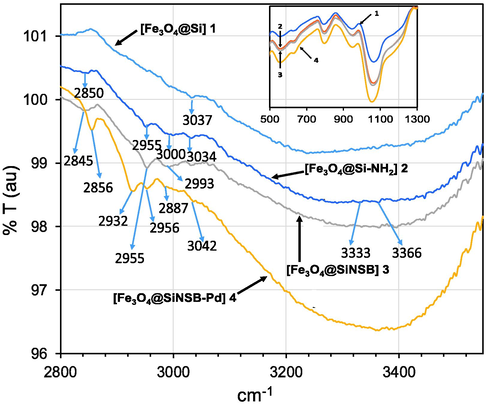
FT-IR images of synthesized materials.
The SEM image analysis of Fe3O4 showed that the synthesized magnetic nanoparticles were in spherical shape, well dispersed (Fig. 3a) and average diameter was 150 nm (Fig. 3b). SEM image of silica encapsulated core–shell 1 was also showed spherical morphology (Fig. 3c) and the magnified SEM image of 1 clearly indicated the successful encapsulation of Fe3O4 nanoparticles by silica (Fig. 3d). After incorporation of amino group with silanol moieties in 1, the SEM image of 2 showed greater diameter than 1 (Fig. 3e). Due to the incorporation of the organic phenanthroline chelating group with 2, the SEM image of 3 showed similar spherical structure with larger diameter than 2 (Fig. 3f). After introduction of palladium nanoparticles, the SEM image of 4 also showed rough morphologies on spherically shaped structures (Fig. 3g).
(a) SEM image of Fe3O4, (b) nano scale SEM image of Fe3O4, (c) SEM image of 1, (d) nano scale SEM image of 1, (e) SEM image of 2, (f) SEM image of 3, (g) SEM image of 4.
TEM image of 4 showed that the palladium nanoparticles were well dispersed onto the surface of Fe3O4 and the spherical shape of Fe3O4 nanoparticles were still maintained (Fig. 4a). The magnified TEM image of palladium nanoparticles in 4 were also showed spherical structure (Fig. 4b). About one hundred and twenty-five palladium nanoparticles were considered to determine the average diameter (Fig. 5) which was determined to be 3.5 ± 0.6 nm. Interestingly, the TEM image of 3rd recycle of 4 showed similar morphology with unused particles and were not aggregated after reused in the Heck reaction (Fig. 4c).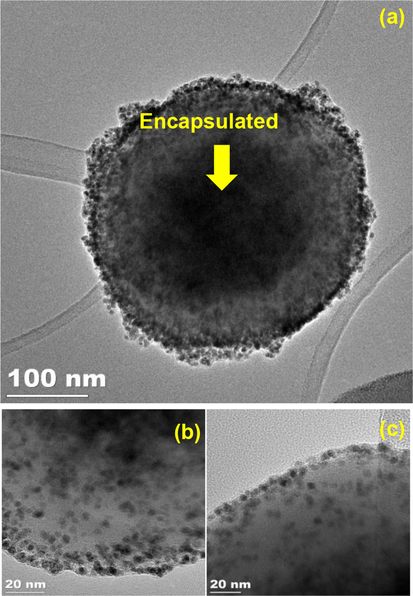
(a) TEM image of 4, (b) TEM image of magnified 4, (c)TEM image of 3rd recycled of 4.
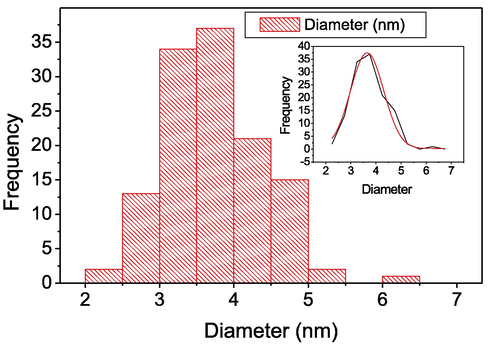
Palladium nanoparticles size distribution of 4.
Fig. 6 showed a typical XRD diagram of Fe3O4, silica coated 1 and Schiff-based palladium nanocatalyst 4. The six characteristic peaks 2θ = 29.9 (2 2 0), 36.3 (3 1 1), 43.6 (4 0 0), 53.5 (4 2 2), 57.5 (5 1 1) and 63.4 (4 4 0) were observed for the Fe3O4 nanoparticles. The crystallinity patterns of the Pd-nanoparticles indicate five distinct reflections at 2θ = 40.2° (1 1 1), 45.5° (2 0 0), 68.2° (2 2 0), 81.86° (3 1 1) and 86.12° (2 2 2) (Khan et al., 2014). These attribute reflections were considered to the face centered cubic (fcc) structure of palladium (JCPDS: 87–0641, space group: Fm3m (2 2 5)) (Shankar et al., 2004). The strong reflection at (1 1 1), in assessment with the other four, can be attributed to the desired growth controlled of the nanocrystals. On the basis of the half-width reflection at (1 1 1), the average crystallite size of Pd-nanoparticles (∼3.56 nm) was determined using the Scherrer equation. The broad peak at 2θ of 20–28 corresponds to the amorphous structure of the silica layer (Safavi et al., 2013).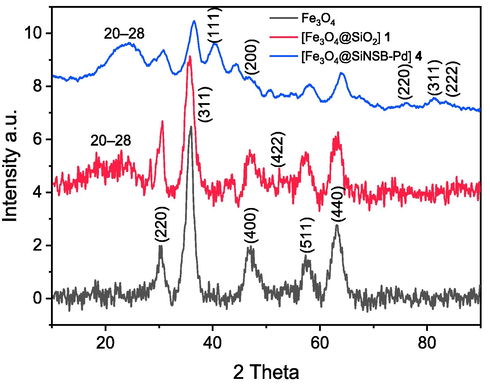
XRD spectra of synthesized materials.
The X-ray photoelectron spectra (XPS) of 4 showed in Fig. 7. For comparison of the oxidation states of palladium, we measure XPS of 4 and (NH4)2PdCl4. The narrow scan profile of 4 displayed two signals at 335.7 eV and 340.6 eV those can be assigned to Pd3d5/2 and Pd3d3/2 for Pd° species respectively (Zhang et al., 2017). However, signals at 337.52 eV and 342.82 eV were observed with respect to Pd3d5/2 and Pd3d3/2 for Pd(II) species in (NH4)2PdCl4 which indicated that the palladium was successfully reduced by hydrazine.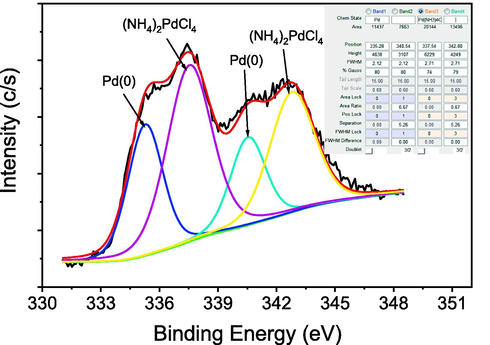
Narrow scan XPS image of Pd(0) in 4.
After successfully characterization, the catalytic applicability of 4 to the Mizoroki-Heck C-C bond formation reaction was investigated. The nanocatalyst 4 was applied for the construction of C-C bond by the utilization of alkenes and arene halides to afford the corresponding substituted alkenes. Reactions were performed under various conditions to identify the optimal conditions for Heck C-C bond formation. The reaction of butyl acrylate and 4-iodotoluene was carried out using a fixed catalytic dose (10 mg, 0.015 mol%), temperature (130 °C) and reaction time (6 h) where solvents and bases were varied. It was found that the desired product was obtained under all reaction conditions (Table 1). The yields of the product were lower when aqueous DMF and inorganic bases were used (entries 1–4). However, the yield was significantly increased when organic bases i.e. triethyl amine and diisopropylethyl amine were used (entries 5–6). Interestingly, the best result was achieved when the Heck C-C bond formation reaction was conducted with triethyl amine in N,N-dimethylacetamide (entry 8). The reaction was also performed using 0.0075 mol% of 4 (entry 9) and 64% yield was obtained. However, NMP and DMSO were not found to be good solvents for this catalytic system. Therefore, suitable reaction conditions were considered to consist of DMA as solvent, triethyl amine as base, 0.015 mol% of 4 nanocatalyst at 130 °C for 6 h (Table 1, Entry 8). [a]Reaction conditions: 4-iodotoluene (1 mmol), butyl acrylate (1.5 mmol), 4 (0.015 mol%), base 3 mmol and 2 ml of solvent for 6 h. [b]GC yields. [c]Isolated yield. [d]Reaction was conducted in presence of 0.0075 mol% of 4.
Entry
Solvent
Base
Yield (%)[b]
1
DMF:H2O
Na2CO3
71(67)[c]
2
DMF:H2O
K2CO3
66
3
DMF:H2O
NaOMe
68
4
DMF:H2O
Na3PO4
70
5
DMF
DIPEA
85
6
DMF
Et3N
91
7
DMA
DIPEA
86
8
DMA
Et3N
95(91)[c]
9d
DMA
Et3N
71(64)[c]
10
NMP
Et3N
84
11
DMSO
Et3N
76
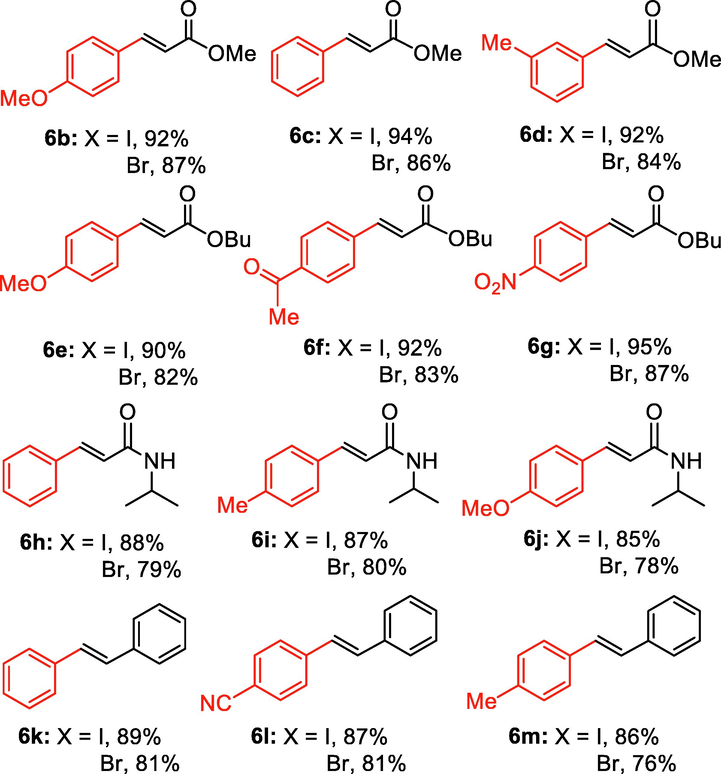
After screening of the Heck C-C bond formation reaction, we extended the application of 4 to a variety of substituted arene halides and olefins (Table 2). An olefine i.e. methyl acrylate was smoothly reacted with 4-iodoanisole, iodobenzene and 3-iodotoluene to afford the respective alkenes 6b-d with 92%, 94% and 92% yields respectively. The C-C bond formation reactions of arene bromides and olefins are in great demand because of the low cost of arene bromides, those are desirable material for industrial and pharmaceutical applications. By observing the excellent catalytic performance of 4 towards the arene iodides, we further investigated the scope of the Heck C-C bond formation reaction of electronically poor arene bromides with a variety of olefins. The nanocatalyst 4 showed excellent catalytic efficiency towards arene bromides and substituted arene bromides with a variety of olefines to afford the respective products 6b-d with 87%, 86%, and 84% yields. It was observed that arene bromides showed lower catalytic activity compared to arene iodide due to the slow oxidative addition rate of arene bromides with the active site of metal catalyst in the catalytic cycle. A variety of olefins, such as butyl acrylate and N-isopropyl acrylamide, were also readily formed C-C bond with arene halides to provide the corresponding alkenes in excellent yield (6e-j). The highest yield of the corresponding product (6g) was observed in the couplings of 4-iodonitrobenzene and butyl acrylate. The arene halides having electron-rich group showed higher catalytic activity compared to the electron-poor arene halides. The arene bromides were also smoothly afforded the respective products 6e-j in up to 85% yield. Interestingly, the sterically hindered, bulky and less reactive arene olefin i.e. styrene was also smoothly forwarded the Heck C-C bond formation reaction with arene halides to produce the corresponding stilbene derivatives 6km in high yield. Recently, Yamada et. al (Yamada et al., 2014) reported that palladium nanoparticles on silicon nanowire (SiNA-Pd, 0.3 mol%) catalyzed Heck C-C bond formation reaction at 140 °C in presence of 0.2–1 mol equiv of tetrabutylammonium bromide as an additive and they continued the reaction for 48 h which provided only 85% yield of 6f. Herein our catalytic system showed twenty times more reactive compared to their work. aReaction condition: arene halide (1 mmol), alkene (1.5 mol equiv), 0.015 mol% of 4, Et3N 3 mmol, 2 ml of DMA.
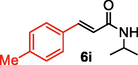
We further extended the catalytic applicability of 4 to the Heck-Matsuda C-C bond formation reaction of arenediazonium salt which is known as an excellent arene electrophile. (Andrus et al., 2002; Selvakumar et al., 2002). To investigate the catalytic efficiency of 4, 4-methoxyarenediazonium tetrafluoroborate was treated with methyl acrylate at room temperature for 5 h smoothly afforded the respective cross-coupling product 6b with 92% yield (Table 3). It is know that arene diazonium salt easily form an active cationic arene-Pd(II) species through oxidative addition during the catalytic cycle which enhance the rate of formation of product (Penafiel et al., 2012). Therefore, the Heck-Matsuda C-C bond formation can be carried out under mild reaction conditions compared to the traditional Mizoroki-Heck reaction. When phenyldiazonium salt was treated with olefine i.e. methyl acrylate the C-C bond formation reaction smoothly forwarded to give 6c in 91% yield. The electron pushing and electron withdrawing substituted arene diazonium salts were efficiently forwarded the Heck-Matsuda reaction with butyl acrylate to afford the respective cross-coupling products 6a, 6n and 6g with 87%, 88% and 93% yield respectively. N-isopropylacrylamide is relatively less reactive compared to methyl or butyl acrylate however N-isopropylacrylamide was also smoothly promoted Heck-Matsuda reaction to afford the respective products 6g and 6i in 86% and 83% yield respectively. Interestingly the coupling of sterically hindered styrene and aromatic diazonium salts was also observed under reaction conditions at room temperature and the yields of the corresponding coupling products 6l and 6m were 89% and 88%, respectively. aReaction condition: 1 mmol of arenediazonium tetrafluoroborate, 1.5 mmol of olefin, 0.015 mol% 4, 3 ml of ethanol, 5 h at r.t. bIsolated yield.
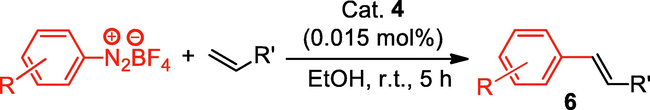
Entry
Olefine
Product
Yield (%)b
1
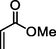
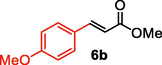
92
2
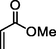
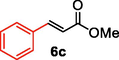
91
3
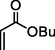
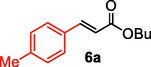
87
4
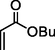
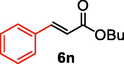
88
5
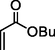
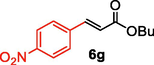
93
6
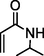
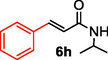
86
7
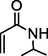
83
8
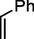
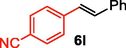
89
9

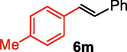
88
The current catalyst is compared between the previously reported catalyst to evaluate the performance and applicability of the developed catalyst 4. For improved comparison, the results of the iodobenzene in the hack reaction was selected and the time of product formation and catalyst loading were compared with the catalyst mentioned earlier in Table 4 (Li et al., 2018; Naghipour and Fakhri 2016; Banazadeh et al., 2015; Wang et al., 2006; Nasrollahzadeh et al., 2014; Wang et al., 2014; Zhang et al., 2011; Zolfigol et al., 2014). The catalyst used in this Hack C-C bond formation reactions does not have serious issues such as high acceleration, large dosage, low catalytic efficiency, high reaction temperature, and longer reaction time. In addition, the reusability and recycling of this new catalyst is more convenient and faster than those previously reported. In addition, the small catalyst (0.0075 mol%) of nanocatalyse 4 is another advantage of the current catalyst compared to others (Table 1, entry 9).
Catalyst
Conditions
Time (h)
Yield (%)
Ref.
0.1-PdAu0.16/Fe3O4@LDH, (0.05 %)
DMF/H2O, K2CO3, 120 °C
1.5
89.7
(Li et al., 2018)
Fe3O4@CS-Schiff base, (1 %)
DMF, Et3N
0.3
98
(Naghipour and Fakhri, 2016)
Fe3O4@(A-V)-silica-PdMNPs, (0.4%)
K2CO3, DMF, 130 °C.
2.5
95
(Banazadeh et al., 2015)
MNPs-TDA-Pd (20 mg)
K2CO3, DMF, 100 ◦C,
8 h
93
(Bodaghifard, 2019)
Fe3O4@SiO2-HPGOPPh2-PNP, (25 mg)
K2CO3, DMF, 100 °C,
1 h
87
(Du et al., 2012)
Fe3O4@SiO2-NH2-Pd (30 mg)
CH3CN/H2O, NaOAc, reflux
12 h
71
(Wang et al., 2006)
TiO2@Pd NPs (1%)
DMF, Et3N, 140 °C
10
93
(Nasrollahzadeh et al., 2014)
HMMS-NH2-Pd (4%)
NMP, K2CO3, 130 °C
8
98
(Wang et al., 2014)
Fe3O4-NH2-Pd (5%)
NMP, K2CO3, 130 °C
10
99
(Zhang et al., 2011)
SMNPs-DF-Pd (1%)
Solvent free, DABCO, 140 °C
2
92
(Zolfigol et al., 2014)
Fe3O4@SiNSB-Pd (0.015%)
DMA, Et3N, 130 °C
6
94
This report
The reusability of the heterogeneous catalyst has significant interest from n economical and sustainable viewpoint. Therefore the recoverability and potential reuse of nanocatalysy 4 was investigated. After the first cycle of Heck reactions (Table 2, 6g, X = I), a strong magnet was appled outside of the reaction vessel and carefully remove the reaction mixture (Fig. 8).
Recovery of 4 by using external magnate.
The magnetic nanocatalyst was cleaned with ethyl acetate, dried at 50 °C for 30 min and used it to the next catalytic cycle. The magnetically recyclable and separable palladium nanocatalyst 4 was repeatedly used seven times and found excellent catalytic performance with no significant drop in its catalytic performance. A slight decrease of catalytic performance has been seen due to loss of 4 during its recovery process (Fig. 9). The TEM image of 3rd recycles of 4 is presented in Fig. 4c which clearly indicated that after the Heck reaction, palladium nanoparticles were not aggregated. Therefore, silica coated magnetic phenanthroline based palladium nanocatalyst 4 exhibited high stability and catalytic performance in the cross-coupling reactions.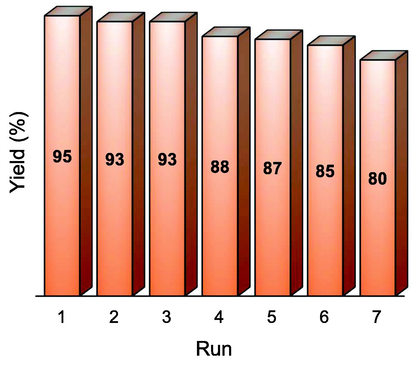
Recycle of 4.
Heterogeneous catalysts have an important aspect in the stability and heterogeneity of the supported metal catalyst during the reaction. Therefore, a thermal filtration experiment was conducted to evaluate whether the reaction would proceed under the conditions of a heterogeneous reaction (Fig. 10). We considered the hot filtration experiment using 4-iodotoluene with butyl acrylate under optimized reaction conditions (Table 1, entry 8). After two hours of reaction, the magnetic nano catalyst was removed with an external magnet under hot conditions and the reaction vial was heated again under the same reaction conditions.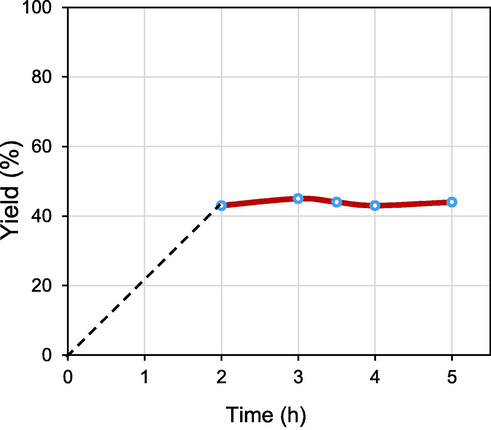
Heterogeneity test of 4 in the Mizoroki-Heck reaction.
Interestingly, the Heck C-C bond formation reaction was not preceded even 5 h after removing of the catalyst. In addition, ICP-AES analysis of the filtrate showed no palladium specie was leached out into the reaction mixture. Thus, it is reasonable to state that the Heck C-C bond formation reactions were proceed under heterogeneous metal catalysed conditions.
4 Conclusions
In summary, we have successfully synthesized and characterized a recyclable palladium nanocatalyst based on silica-coated magnetic core shell phenanthroline. The magnetic palladium nanocatalyst was well stabilized by the phenonthrolide ligand and showed excellent catalytic performance in the Mizoroki-Heck C-C bond formation reaction of arene iodides/bromides with various types of olefins to provide the corresponding olefinic products in excellent yield. The magnetic catalyst was also efficiently promoted Heck-Matsuda C-C bond formation reaction of arene diazonium tetrafluoroborate salts with a variety of olefins to afford the respective olefinic products at room temperature. The magnetic palladium nanocatalyst showed high stability in the reaction medium and it was easy to recover from the reaction mixture with an external magnet. Moreover, the heterogeneous nanocatalyst could be reused in seven consecutive cycles without significant decrease of initial catalytic efficiency.
Acknowledgments
The authors are thankful to the Research Management Centre of Universiti Malaysia Sabah for support to this work.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Chem. Rev.. 2016;116:15035.
- Chem. Rev.. 2018;118:2249.
- Appl. Organo Chem.. 2018;32:4346.
- Res. Chem. Intermed.. 2020;46:3295.
- Tetrahedron Lett.. 2010;51:1479.
- Dalton Trans.. 2019;48:3132.
- Chem. Soc. Rev.. 2013;42:3876.
- Chem. Eng.. 2017;5:8475.
- Chem. Rev.. 2011;111:3036.
- J. Mol. Catal. A Chem.. 2014;393:18.
- Org. Lett.. 2005;7:617.
- Angew. Chem. Int. Ed.. 2019;58:18159.
- Green Chem.. 2019;21:1718.
- Appl. Organomet. Chem.. 2019;33:4618.
- Inorg. Nano-Met Chem.. 2019;49:267.
- React. Funct. Polym.. 2020;152:104586
- Tetrahedron Lett.. 2020;61:151801
- RSC Adv.. 2015;5:19630.
- H. Alamgholiloo, S. Rostamnia, N. Noroozi Pesyan, Appl. Organomet. Chem. 2020, 34, 5452.
- J. Organomet. Chem.. 2019;886:57.
- Nano Today. 2010;5:412.
- Adv. Synth. Catal.. 2004;346:889.
- Nano Mater.. 2020;3:2070.
- Catalysts. 2019;9:140.
- J. Mater. Res. Technol.. 2020;9:5858-5870.
- Adv. Synth. Catal.. 2013;355:957.
- Green Chem.. 2013;15:3429.
- Appl. Catal. A. Gen.. 2013;459:65.
- Catal. Commun.. 2012;17:179.
- Catal. Today. 2013;212:206.
- Green Chem.. 2012;14:2513.
- Appl. Organomet. Chem.. 2020;34:5775.
- J. Cat. 2017;350:103-110.
- J. Chin. Chem. Soc.. 2015;62:33-40.
- ChemCatChem. 2015;7:2141-2148.
- Mater. Today Chem.. 2021;20:100460
- Micropor. Mesopor. Mat.. 2019;284:366.
- ACS Catal.. 2020;10:5077.
- Mater. Inter.. 2019;11:32579.
- Chem. Eng.. 2017;6:2103.
- M. Madrahalli Bharamanagowda, R. K. Panchangam, Appl. Organomet. Chem. 2020, 34, 5837.
- Organomet. Chem.. 2021;935:121676
- React. Funct. Poly.. 2021;161:104843
- Colloids Surf A Physicochem Eng Asp.. 2021;621:126597
- J. Organomet. Chem. 2021;936:121711
- Nanoscale. 2016;8:8138.
- S. V. Trukhanov, T. I. Zubar, V. A. Turchenko, An. V. Trukhanov, T. Kmjec, J. Kohout, L. Matzui, O. Yakovenko, D. A. Vinnik, A. Yu. Starikov, V. E. Zhivulin, A. S. B. Sombra, D. Zhou, R. B. Jotania, C. Singh, A. V. Trukhanov, Mater. Sci. Eng. B 2021, 272, 115345.
- RSC Adv.. 2014;4:17060.
- New J. Chem.. 2018;42:4748.
- Chem. Rev.. 1997;97:2373.
- Tetrahedron. 2012;68:3577.
- L. Z. Fekri, S. Zeinali. Appl Organomet. Chem. 2020, 5629.
- MOJ Biorg Org Chem.. 2018;2:154.
- Dalton Trans.. 2014;43:9026.
- Nat. Mater.. 2004;3:482.
- Anal. Chim. Acta. 2013;796:115.
- Appl. Surf. Sci.. 2017;396:812.
- Angew. Chem.. 2014;126:131.
- Chem. Eur. J.. 2002;8:3901.
- Org. Lett.. 2002;4:2079.
- Eur. J. Org. Chem. 2012:3151.
- J. Organomet. Chem.. 2018;878:84.
- Cat. Commun.. 2016;73:39.
- Inorg. Chim. Acta. 2015;429:132.
- Colloids Surf. A Physicochem. Eng. Asp.. 2006;276:116.
- J. Mol. Catal. A Chem.. 2014;394:205.
- New J. Chem.. 2014;38:1138.
- Green Chem.. 2011;13:1238.
- RSC Adv.. 2014;4:40036.
Appendix A
The NMR spectra for the synthesized coupling products can be found in the supplementary information section.
Appendix B
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.103914.
Appendix B
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







