Recent advances in the treatment of PAHs in the environment: Application of nanomaterial-based technologies
⁎Corresponding author. mohammad.alghouti@qu.edu.qa (Mohammad A. Al-Ghouti)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Polycyclic aromatic hydrocarbons (PAHs) are one of the most frequent petroleum hydrocarbons detected in the environment. PAHs are receiving global attention due to their toxicity, environmental persistence, and potential bioaccumulation. PAHs contamination occurs mainly due to anthropogenic sources related to the disposal of industrial and domestic run-offs or atmospheric emissions from plants or vehicles. Once PAHs are introduced into the environment, they undergo different processes either physical, chemical, or biological transformations, which are collectively known as weathering processes. These weathering processes are highly dependent on the nature and the physiochemical properties of PAHs molecules and the surrounding environment, which eventually will determine their transport, fate, and distribution in the environment either through adsorption to soil/sediments, or volatilization to the atmosphere, or dissolution in the water. Different treatment strategies for PAHs pollution have been introduced. Each remediation technology has its advantages and disadvantages. To choose a treatment, a full analysis of the case study should be provided, including the properties of the target pollutant, site description, and treatment procedure. As the treatment of PAHs contaminated sites is usually a difficult and expensive task, it would be a good choice to go for treatment, which is adaptive, sustainable, eco-friendly, efficient, and cost-effective. In this review, the transport, fate, and distribution processes of PAHs in the environment with their dependent factors were elaborated. In addition, this paper provided an overview of different traditional and advanced potential treatment approaches used to treat PAHs-contaminated sites such as thermal, chemical, electrokinetic, nano-adsorption, and biological treatments.
Keywords
PAHs
PAHs-contamination
Petroleum pollution
Treatments technologies
Nanomaterial
1 Introduction
The rapid increase of the world population has led to the growth of industrial activities specifically petroleum industries as they are the main energy sources, which support many aspects of human daily life and the world economy (Tornero and Hanke, 2016; Yim et al., 2007). Parallel with the increase of activities of the oil and gas industry and other petrochemical industries, different environmental matrices that include air, water, soil/sediments, as well as biota are receiving industrial pollutants during upstream and downstream processes (Ghosal et al., 2016). Wastes from petrochemical refineries have introduced a huge number of different pollutants; organic and inorganic pollutants, including hydrocarbons, heavy metals, oil, and grease, to the environment which are characterized by their potential impact on the environment and environmental persistence. This is dictated by their chemical stability and resistance to oxidation and reduction in nature (Chen et al., 2020). PAHs are a group of organic pollutants that are widely spread and frequently detected in the environment with different structures and varied biological toxicity (Eldos et al., 2020). PAHs are consisting of carbon and hydrogen assembled in two or more aromatic rings arranged in linear, angular, or cluster (Balmer et al., 2019) (Fig. 1). PAHs can be classified into different classes based on their sources: petrogenic, pyrogenic, or biogenic PAHs. Petrogenic PAHs are from petroleum products, pyrogenic PAHs are from combustion processes, and biogenic PAHs are from biological origins (Merhaby et al., 2019).
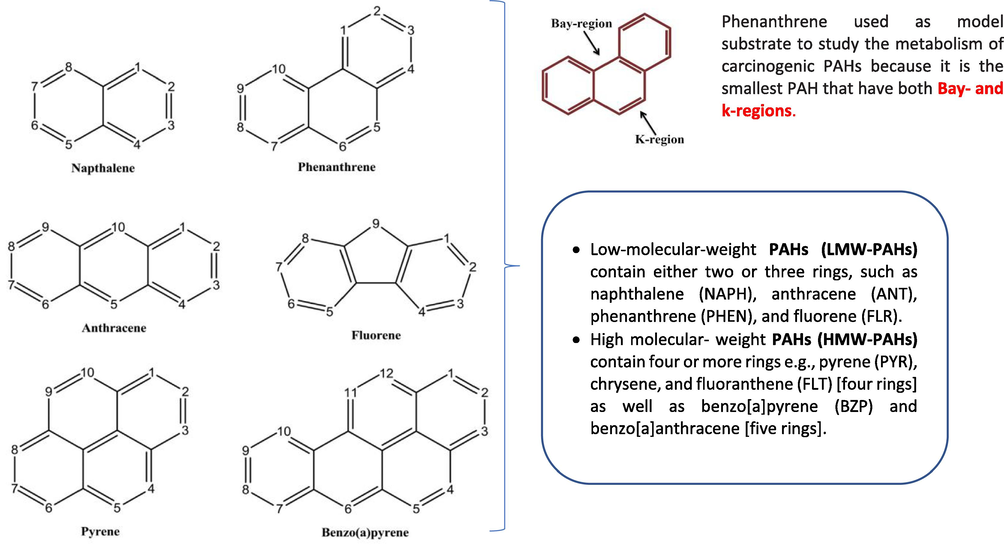
- PAHs Chemical structures along with the ring numbering (Nzila, 2018; Ghosal et al., 2016).
Qatar is an important producer of oil and gas and is characterized by its arid area and weather (AlKaabi et al., 2020). A report about oil and gas regulation in Qatar, 2019 stated that the oil and gas industry accounts for around 85% of the country’s export incomes and 60% of its gross domestic product (GDP). Hence, it always is seeking to raise the production capacity of liquefied natural gas (LNG) as most of its GDP depends on oil and gas industries (Mahmood and M. Earley and. S. Al-Abdul, , 2019). Hence, Qatar continually works on adopting the best practices during the activities of gas and oil extraction. Several initiative management strategies are conducted to stimulate energy security, sustain water resources, and protect the environment. Additionally, the country implements laws for the regulation of the release and discharge of wastewater. One of Qatar's vision 2030 is that promote a sustainable environment through the investment in advanced technologies to reduce the uncontrollable damage caused by economic projects. As a result, several research works are focusing on developing comprehensive strategies for wastewater management and treatments to allow their recycling and reusing and thus reducing the generated wastewater. However, this is a significant challenge for many petrochemical companies in Qatar due to the characteristics of this region, the amount and the characteristics of the wastewater, and the water scarcity. It is, indeed, critical to characterize the composition of the local petroleum industry effluents to propose an efficient, suitable, and sustainable treatment process.
2 Characteristics of (PAHs)
PAHs are one of the toxic contaminants that are frequently detected in the environment (Eldos et al., 2020). PAHs are classified into two groups based on the number of aromatic rings either low molecular weight (LMW) compounds consisting of less than four aromatic rings or high molecular weight (HMW) compounds consisting of four or more aromatic rings. These compounds are described as toxic, very stable, highly lipophilic, and hydrophobic which made them able to accumulate in biota, which increases the chance of exposure to other species and humans (Fig. 2) (D. J. Shilla and J. Routh, “Distribution, Behavior, and Sources of Polycyclic Aromatic Hydrocarbon in the Water Column, Sediments and Biota of the Rufiji Estuary, Tanzania,” Frontiers in Earth Science, vol. 6, 2018; Soliman et al., 2014). Due to their toxicity, genotoxicity, mutagenicity, and carcinogenicity, PAHs are strictly controlled by laws in industrial countries. Hence, 16 PAHs compounds are listed as priority pollutants by the US-EPA including; Naphthalene, Acenaphthylene, Acenaphthene, Fluorene, Phenanthrene, Anthracene, Fluoranthene, Pyrene, Benzo(a) anthracene, Chrysene, Benzo(b) fluoranthene, Benzo(k) fluoranthene, Benzo(a) pyrene, Indeno(1,2,3-cd) pyrene, Dibenzo(a,h) anthracene, and Benzo(ghi) perylene (Soliman et al., 2014). It is worth pointing out that, PAHs with higher molecular weight and a high number of aromatic rings in their structure have been characterized by high toxicity, chemical stability, and environmental persistency (Botsou and Hatzianestis, 2011). Most PAHs have low water solubility, low vapor pressure, and high melting and boiling point. The water solubility and vapor pressure tend to decrease with increasing molecular weight and therefore their resistance to oxidation and reduction increases. Thus, they are soluble in organic solvents as they are hydrophobic. Different physicochemical properties of individual PAH determine their mobility and partition between different compartments in the environment, including octanol–water partition coefficient (KOW), organic carbon partition coefficient (KOC), octanol–air partition coefficient (KOA), Henry’s law constant (H), water solubility (S), and vapor pressure (P) (Mackay and Callcott, 1998). Once PAHs are released into the environment, they undergo several processes such as physical, chemical, and biological reactions through metabolic and microorganisms’ pathways, which are collectively called weathering processes (Ossai and A. Ahmed and. A. Has, , 2020; Latimer and Zheng, 2003). The weathering processes are highly dependent on two abiotic factors, these are the nature of the environment and the structure of the pollutant. The weathering processes include adsorption of these recalcitrant pollutants to the soil, volatilization to the atmosphere, and dissolution in the water (Fig. 2) (Schwarzenbach et al., 2016). The physiochemical properties of individual PAH contribute to its fate, behavior, and transport in the environment. PAHs will vary greatly in their partition between environmental matrices based on their molecular properties and environmental conditions (e.g. temperature). Thus, when PAHs migrate to the environment their concentration differs from one site to another (Chen et al., 2020). Other environmental conditions and processes, such as soil aggregation, wetness, aeration, dryness, volatilization, biodegradation, transformation, leaching, and uptake by biota will affect the PAHs bioavailability in soil (Fig. 2). Therefore, PAHs with greater mobility and bioavailability have greater overall toxicity effects. Non-polar PAHs are more likely to exhibit chronic health effects than polar PAHs due to their structure.
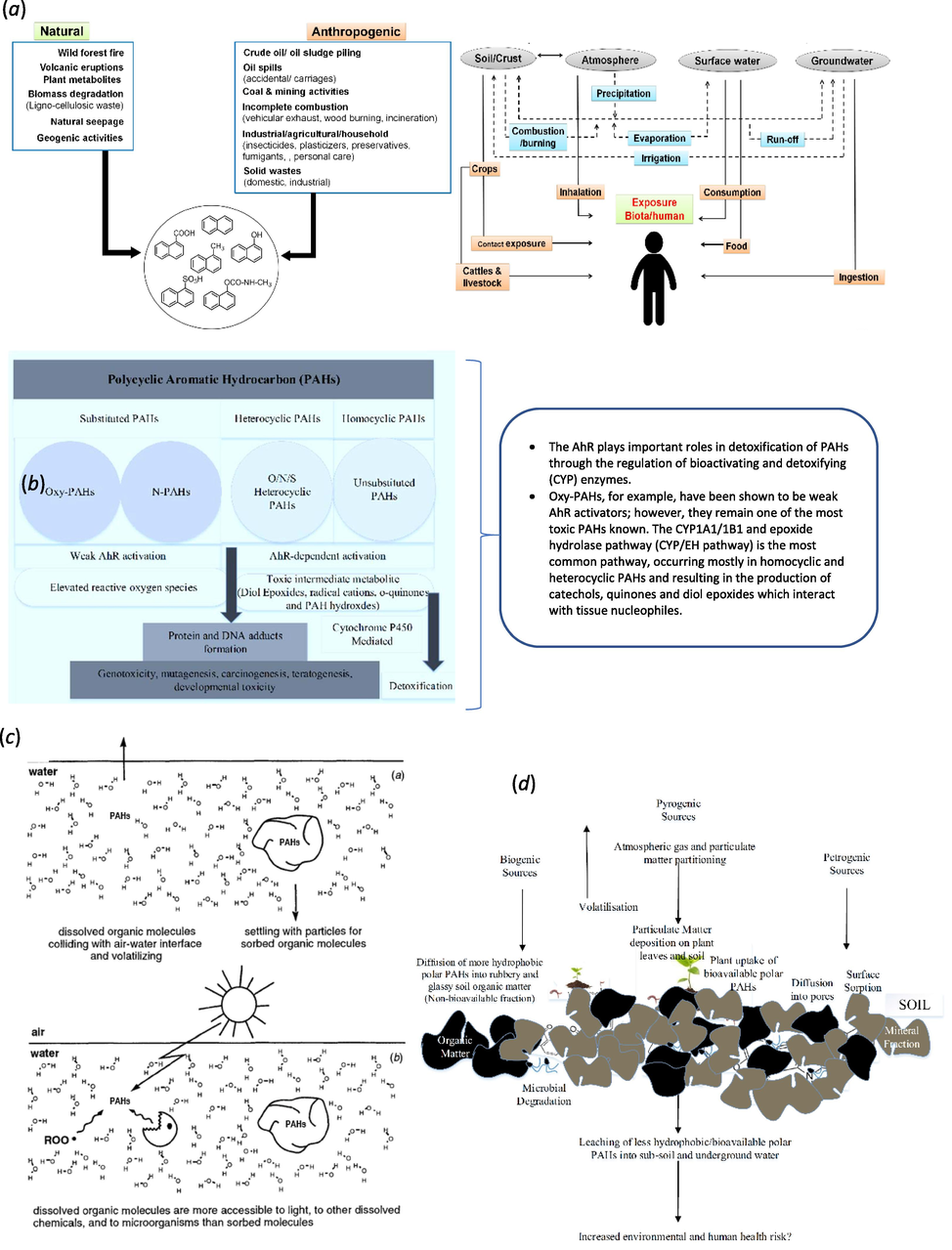
- (a) Sources and routes for entry of PAHs through various ecological compartments (Mohapatra and Phale, 2021), (b) PAH toxicity (Idowu et al., 2019), (c) Dissolved species of PAHs may directly participate in the air–water phase while sorbed species may settle or react at different rates, and dissolved species of PAHs may react at different rates as compared with their sorbed counterparts because of differential access of other dissolved and solid-phase, and (d) fate of polar PAHs in soil (Idowu et al., 2019).
The tendency of PAHs to partition between different matrices is quantified by several parameters, including air–water partition coefficient (KAW), KOA, KOW, KOC, H, S, and P (Mackay and Callcott, 1998). KAW is very important to estimate the movement of individual PAH across the air–water interface and it is a temperature-dependent parameter described below in Equations (1), 2, and 3.
Where
KOW can be used to estimate organic carbon–water partitioning and it is estimated indirectly through KOC by Equations (5) and (6).
KOC is a parameter to measure the ability of the pollutant to be sorbed onto organic carbon in the soil, sediments, and biota (Xu and Kropscott, 2014). It can be estimated by Equation (5).
Where 0.41 is an empirical factor depending on the organic carbon nature, and
| PAH | Molecule formula | Weight (g/ mol) | Aqueous solubility (25 °C, mg/L) | Log KOW | Molar volume | Vapor pressure (Pa, 25 °C) |
Melting point (°C) | Boiling point (°C) | Molar dimensions |
|---|---|---|---|---|---|---|---|---|---|
| Naphthalene (Nap) | C10H8 | 128 | 31.7 | 3.30 | 148 | 11.14 | 80.2 | 218 | 9.1 × 7.3 × 3.8 |
| Acenaphthene (Ace) | C12H10 | 154 | 3.9 | 3.92 | 173 | 3.07 | 90–96 | 265–280 | 9.1 × 8.3 × 4.2 |
| Acenaphthylene (Acy) | C12H8 | 152 | 16.1 | 3.94 | 168* | 3.87 | 92–93 | 265–280 | NR |
| Fluorene (Flu) | C13H10 | 166 | 1.9 | 4.18 | 188 | 1.66 | 116–118 | 293–295 | 11.4 × 7.3 × 4.2 |
| Phenanthrene (Phe) | C14H10 | 178 | 1.15 | 4.46 | 199 | 1.06 × 10− 1 | 96–101 | 339–340 | 11.6 × 7.9 × 3.8 |
| Anthracene (Ant) | C14H10 | 178 | 0.0434 | 4.45 | 170.3 | 8.6 × 10− 4 | 216–219 | 340 | NR |
| Pyrene (Pyr) | C16H10 | 202 | 0.135 | 4.88 | 186 | 5.0 × 10− 5 | 150–156 | 360–404 | NR |
| Fluoranthene (Flua) | C16H10 | 202 | 0.26 | 5.16 | 187.7 | 8.61 × 10− 4 | 107–111 | 375–393 | NR |
| Benzao(a)anthracene (B [a]A) | C18H12 | 228 | 0.0094 | 5.76 | NR | 5.43 × 10− 4 | 157–167 | 435 | NR |
| Chrysene (Chr) | C18H12 | 228 | 0.002 | 5.81 | NR | 4.0 × 10− 6 | 252–256 | 441–448 | NR |
| Benzo(k)fluoranthene (B [k]F) | C20H12 | 252 | 0.0015 | 5.78 | NR | 5.0 × 10− 7 | 198–217 | 480–471 | NR |
| Benzo(b)fluoranthene (B [b]F) | C20H12 | 252 | 0.0008 | 6.11 | NR | 5.2 × 10− 8 | 167–168 | 481 | NR |
| Benzo(a)pyrene (B [a]P) | C20H12 | 252 | 0.00162 | 6.13 | NR | 6.0 × 10− 8 | 177–179 | 493–496 | NR |
| Dibenzo (a,h)anthracene (D[a,h] A) | C22H14 | 278 | 0.00249 | 6.75 | NR | 1.33 × 10− 8 | 266–270 | 524 | NR |
| Indeno (1,2,3-cd)pyrene (I [1,2,3-cd] P) | C22H12 | 276 | 0.00019 | 6.7 | NR | 1.27 × 10−7 | 162–163 | 530 | NR |
| Benzo (g,h,i)perylene (B [g,h,i]P) | C22H12 | 276 | 0.00026 | 6.63 | NR | 1.38 × 10− 8 | 275–278 | 525 | NR |
3 The behavior, fate, and transport of PAHs in the atmosphere, land, and marine environments
The physical properties of PAHs (e.g. chemical stability, hydrophobicity, and lipophobicity) allow their passage through the atmosphere for a long distance before they adsorb on suspended particles and sediments and accumulate in the biota (Ossai and A. Ahmed and. A. Has, , 2020). The atmospheric processes that contribute to PAHs accumulation in the lands or oceans mainly are (1) wet deposition, (2) dry deposition, and (3) air–water vapor exchange. Several important parameters that determine the dominant process are including temperature, wind speed, composition and organic matter content of matrices, salinity, density, precipitation rates, and the physicochemical properties of the pollutant (Latimer and Zheng, 2003). PAHs with low vapor pressures, i.e. HWM-PAHs tend to accumulate on sorbents at the solid/water phase more than LMW-PAHs. The variability of PAHs in the vapor pressure causes the different distribution of different concentrations in the vapor and other sorbent phases. Mainly, HMW-PAHs with high KOW and KOA will be highly partitioned to marine aerosols (AbdelShafy and Mansour, 2016). Dry deposition is a direct load of PAHs onto marine aerosols or soil. Settling of the PAHs particles is depending on particle and surface properties such as particle size and density, particle composition, humidity, and wind speed. For example, coarse particles tend to settle more by gravity and turbulence effect. In addition, wind speed increases the dry deposition of PAHs onto marine aerosols which are supported by high concentrations of dissolved PAHs on the water surface (I. Arslan-Alaton and. T. Olmez-Hanci, , 2013). Wet deposition is precipitating of PAHs as vapors from the atmosphere dissolve in raindrops and are attached to particles that are absorbed in the rain. The intensity of the rain and spatial and seasonal variations have an influence on the wet deposition process (Golomb, 2001). PAHs tend to accumulate in the organic matter in the soil/sediment rather than in water. PAHs profiles in marine sediments mainly consist of the more hydrophobic HMW-PAHs (Cai et al., 2016). The diffusive exchange between the air and water surface is the diffusing of PAHs in the air–water interface as vapors phase in the atmosphere and/or as dissolved particles in water. The direction of this diffusive transport depends on the fugacity of the PAHs in air and water. Fugacity is the escaping tendency of a substance from a phase and each PAH showed a specific fugacity from air and water depending on its chemical properties and conditions of the environment (Wang et al., 2011). It is worth mentioning the fugacity of the pollutant in both air and water phases increase with temperature. Hence, high temperature increases the rate of evaporation and decreases the rate of absorption and vice versa (Wania et al., 1998). Also if the atmosphere has a high degradation capacity it acts as a source of PAHs pollution to the land/marine environment and as a sink of these released PAHs. Many chemical reactions can affect PAHs concentration in the atmosphere such as photodegradation reaction with oxidative substances and radicals such as hydroxyl radicals or ozone. However, the degradation of PAHs in the atmosphere is involving mainly pollutants in the gas phase and it is dominated by the interaction with OH radicals (Merhaby et al., 2019). In brief, two processes determine the presence of PAHs in the atmosphere, which is the degradation or deposition that is controlled by the heat and presence of oxidative substances (e.g. OH-radical) (Merhaby et al., 2019).
PAHs move through the water column either vertically or horizontally based on the size and density of the particles and the flow of the water in the surrounding area it is laminar or turbulent (Curtosi et al., 2009). During the settling process, the difference in the settling velocity between different sizes of particles allows large ones to scavenge small particles. Also, small particles can be collided and cause aggregation which helps in the settling process. In opposite, resuspension of these particles can be induced by wave or current. Moreover, bubbles formation due to wave breaking or raindrop splashing, or massive photosynthesis reaction by marine algae can help in transferring PAHs from water to air phase (Adeniji et al., 2019). Microorganisms play a role in controlling and defining the fate of PAHs in the marine environment through the biological pump and microbial biodegradation. The biological pump has a vital role in the water surfaces and water-column trapping PAHs from atmospheric deposition or point sources and then transferring them to deep sediments (Duran and Cravo-Laureau, 2016; Shilla, 2018).
This exchange process between the atmospheric and water column can be affected by further factors such as octanol–air partition coefficient KOA, octanol–water partition coefficient KOW, particulate and dissolved organic carbon partition coefficient (KOC and KDOC) (Meylan and Howard, 2005; Andrade et al., 2019). However, all these processes are largely dependent on the PAHs' properties such as hydrophobicity, water solubility, vapor pressure, and biodegradability (Duran and Cravo-Laureau, 2016; Andrade et al., 2019). Thus, PAHs with the same physiochemical properties tend to have a similar trend in their pathways and fate in the environment. Whatever the source of pollution, marine sediments are considered the final sink of PAHs, especially for HMW-PAHs, and constitute PAH reservoirs (Duran and Cravo-Laureau, 2016).
For a given DOM (dissolved organic matter), KiDOC values (dissolved organic carbon partition coefficient of the pollutant) could be correlated with log KiOW values (octanol–water partition coefficient of the pollutant) as,
Considering the complex structural features of DOM, good correlations can be found only for compounds exhibiting similar molecular interaction features.
It is shown in Fig. 3 linear isotherms over the whole concentration range. The significant differences in KiDOC between PAHs exhibiting the same KiOW can be explained considering the stronger van der Waals interactions of the PAHs with the aromatic structures of the humic acid and/or polar interactions of the PAHs with H-donor groups (Schwarzenbach et al., 2016).
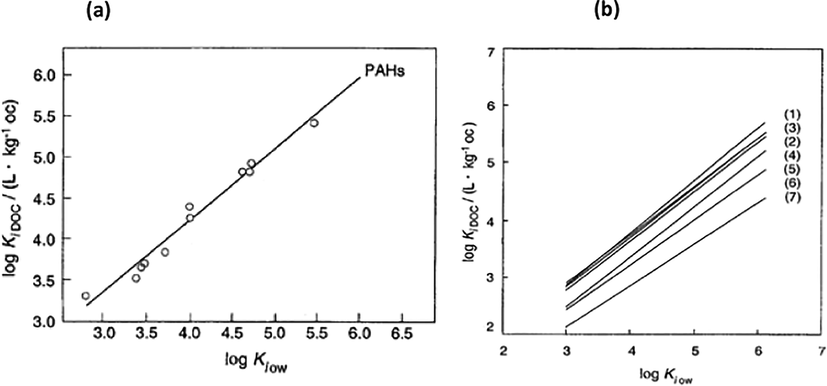
-
(a) Equilibrium sorption to a commercial humic acid and (b) log KiDOC and log KiOW of a set of PAHs for seven different humic or fulvic acids (Schwarzenbach et al., 2016).
In the terrestrial environment, these pollutants can enter through direct discharges or by accidental spillages and leaks, or air deposition. They can transport through the atmosphere by vapor or dust or particulate matter which has been sorbed with organic pollutants. Several important parameters that determine the dominant process are including temperature, wind speed, composition, and organic matter content of the soil (Zeng et al., 2018). For instance, PAHs have a high organic sorption tendency KOC tends to adsorb into the soil with high organic matter content. These pollutants are well known for their degradation resistance, however, once they introduce into the terrestrial environment, they can undergo delicate transformation. The transformations that could occur to these pollutants in soils and sediments by different processes are evaporation and loss to the atmosphere, leaching to groundwater and surface water, adsorption on solid organic particles, or loss through biodegradation (Ossai and A. Ahmed and. A. Has, , 2020). As described above, these processes are related to the pollutant’s physiochemical parameters and portioning parameters. For instance, when considering the leaching of PAHs from soil; the more soluble which are LMW-PAHs will have more potential to leach. Similar to the case of volatilization, LMW-PAHs are more volatile (Hussain et al., 2018). Hence, the soil matrix will be enriched with HMW-PAHs molecules due to the high organic sorption tendency. Another transformation that may occur in the soil environment is biodegradation mainly by microorganisms. Microorganisms have the potential to break down these pollutants to some extent and cause transformation or mineralization. The rate of biodegradation is highly affected by physical conditions of the soil like soil moisture, pH, oxygen and nutrient content, and type of microbial communities (Jaspers et al., 2014). Fig. 4 summarizes the main processes of PAHs in different compartments of the environment. Gao et al., (Gao et al., 2019) studied the distribution of PAHs in sunken oils with the presence of chemical dispersant and sediment. The oil sinking efficiency (OSE) of 16 priority PAHs in the sediment phase was examined with different amounts of dispersant. The results showed that HMW-PAHs have the tendency to partition to sediment more than the LMW-PAHs. The collective effect of sediment and chemical dispersant could enhance the OSE of HMW-PAHs in sunken oils, which could subsequently cause side effects for marine benthonic organisms.
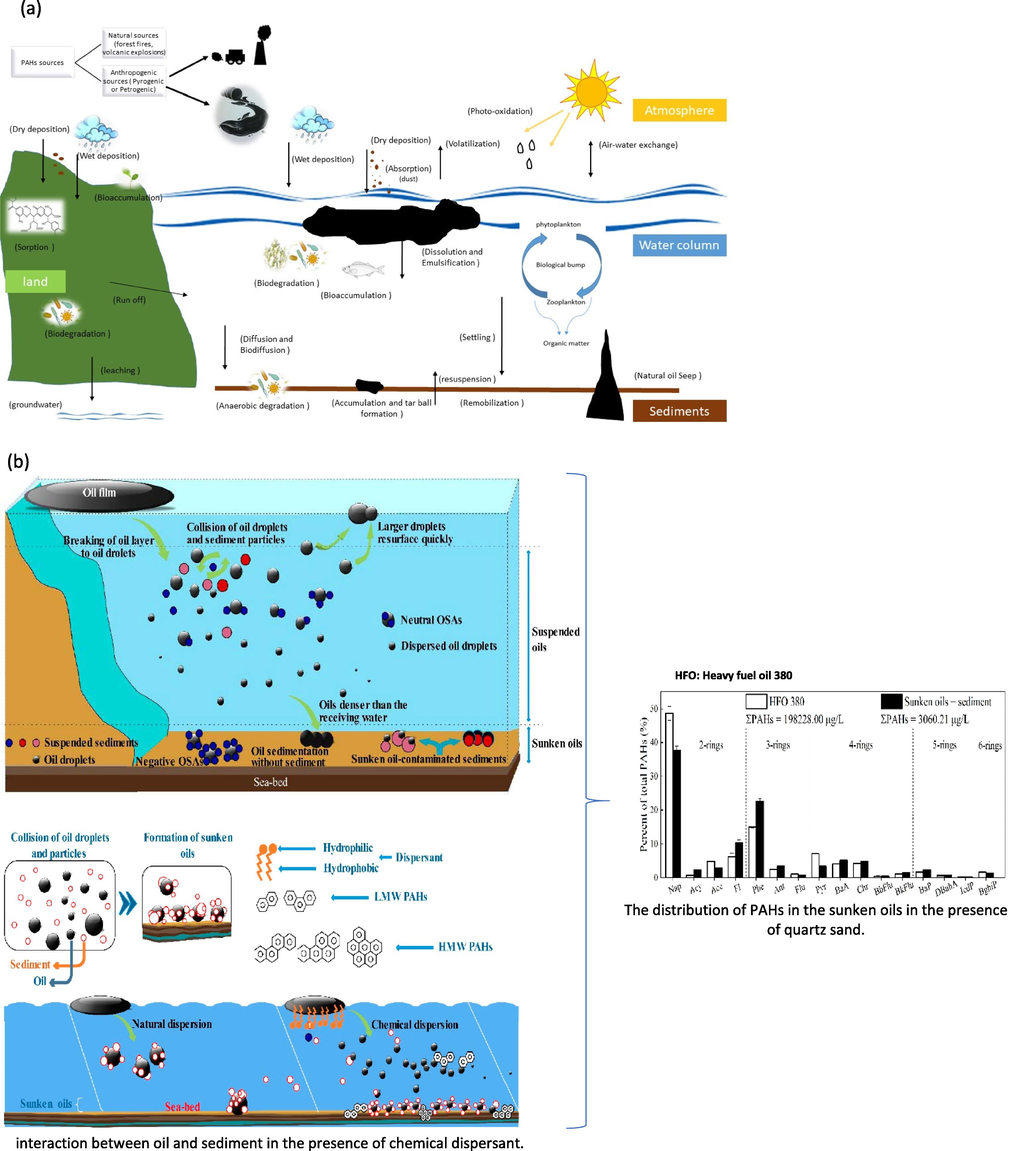
- (a) Weathering Process of PAHs in the environment and (b) Formation and the fate of sunken and suspended oils in the marine environment (Li et al., 2020).
4 Treatment techniques of PAHs contaminated sites
The treatment of the industrial effluents should be efficient and cost-effective for a sustainable environment and the development of industries (Mahmood and M. Earley and. S. Al-Abdul, , 2019). Many treatment methods have been introduced with different degradation mechanisms that can be classified into physical, chemical, and biological treatment (Ossai and A. Ahmed and. A. Has, , 2020). Each treatment has its advantages and disadvantages but selecting the appropriate treatment for a contaminated site needs a full analysis of the case including the pollutants' properties, site characteristics, and the potential corresponding treatment procedure. In general, the best decision to treat the contaminated site is using remediation technologies, which are adaptive, site-suitable, sustainable, efficient, quick, and cost-effective because treating an oil-contaminated site is usually a difficult and expensive task (Gitipour et al., 2018). Remediation technologies used to remove organic and inorganic pollutants from contaminated sites work on one or a combination of the following principles: extraction, transformation or degradation, and sequestration and immobilization such as sorption (Zhang et al., 2019). The common strengths and drawbacks of different classes of remediation technologies have been summarized by Eldos et al. (Eldos et al., 2020). For instance, physical treatment such as solvent extraction and electrokinetic remediation is easy to apply and usually needs non-toxic agents. However; the main drawback of this class of treatment is the requirement for secondary treatment. The main advantage of the chemical treatments such as chemical oxidation and catalytic degradation is the viability in degrading high molecular weight (HMW), persistent, and toxic pollutants. However, the formation of toxic by-products or derivatives is expected (Crini and Lichtfouse, 2018). On the other hand, the biological treatment is known as eco-friendly treatment with almost no generated waste. However, it requires a long duration for efficient degradation, besides the toxicity to microorganisms. Lastly, thermal treatments such as those using microwave heating and thermal desorption can degrade HMW hydrocarbons e.g. PAHs. However, they require high energy and secondary treatment for gaseous products. In the case of petroleum wastes containing hydrocarbons are required mainly oxidation approaches, while pollutants like heavy metal pollutants require reduction, precipitation, or adsorption approaches. These processes can be challenging by using a single technique for both types of pollutants (Zhang et al., 2019). Hence, there is a growing need to offer broader applicable and multi-functional technologies for the quick and effective removal of PAHs in petroleum wastes. In this review, several traditional and advanced remediation technologies such as thermal, chemical, electrokinetic, nano-adsorption, and microbial treatment are described in detail.
4.1 Thermal technologies
Thermal technologies are considered as successful technologies for remediation of PAHs contaminated sites due to their ability to remediate quickly and efficiently. The thermal process results in high removal efficiency of total petroleum hydrocarbon. Contrary to these advantages, thermal treatment requires high temperatures which can cause several complications. Heating contaminated sites to high temperatures require intensive energy that is considered costly (Vidonish et al., 2016). Site composition (i.e. organic matter) can be totally destroyed causing structural deterioration at high temperatures which limits restoring the ecosystem to its original state. Though it is clear that thermal technologies can effectively remove pollution, the effects of high temperatures on ecosystems (i.e. soil/sediment organisms) and restoring efforts are quite unexplored (Buttress et al., 2016). Therefore, there is a growing need to create a system to optimize thermal treatments with sustainable goals such as energy conservation and ecosystem protection. Developing a comprehensive approach toward thermal treatment is a must to make sure that environmental treatment efforts do not cause unnecessary damage to the environment but instead support global efforts to allow a sustainable future. Studies demonstrated that among different thermal techniques, microwave irradiation has been exploited as an effective tool in treating PAHs contaminated sites with high efficiency with low costs and time (Buttress et al., 2016; Robinson et al., 2012; Falciglia and Vagliasindi, 2017).
The microwave irradiation application is based on dissipating electromagnetic field energy into the irradiated substances that adsorbed the microwave energy and then transferring this energy into heat which is used for organic pollutant removal mostly thermal desorption (Eldos et al., 2020). In MW heating, the penetration of electromagnetic fields into the contaminated matrix induces the rotation of dipoles of water and other polar substances present in the matrix such as PAHs. The intermolecular friction, which is caused by rotation results in the generation of heat. The microwave absorption capability of substances depends on their dielectric properties which is a critical factor for the success of the remediation process (Sivagami et al., 2019; Bientinesi et al., 2015; Vidonish et al., 2016). In microwave irradiation heating, when pollutant dielectric constants are higher than the supportive matrix, selective heating will be shown as the main mechanism of removal. While for pollutants with a low dielectric constant, the polarity of the matrix (i.e. water content and mineral composition) plays the main role in the heating removal process (Eldos et al., 2020; Vidonish et al., 2016). Soil/sediment moisture is gradually transformed into water vapor during MW heating, thus resulting in pollutant desorption by condensation, which contributes to the pollutant degradation mechanisms. The impacts of vaporization on the remediation process of PAHs contaminated sediment are incredibly important. In addition, the permeability of sediments also plays a significant role in PAHs vaporization as the permeability significantly affects the sediments' desorption interaction (Falciglia and Vagliasindi, 2017). On the other hand, the properties of PAHs specifically the polarity and size could affect the PAHs degradation and maximum temperature to be reached during microwave irradiation heating treatments in addition to the kinetics of PAHs removal. The degradation efficiency of PAHs contaminated sediments could be due to several mechanisms such as (a) evaporation by thermal desorption (b) degradation by breakdown of chemical bonds (c) stripping of PAHs vapor. (Fig. 5). Falcigila et al. (Falciglia et al., 2013) studied the effect of microwave heating on marine sediments contaminated with hydrocarbons under different heating and operation conditions (treatment time and power). The study demonstrated that microwave irradiation heating compared to conventional thermal remediation technology significantly reduces the energy costs and remediation times. It is worth mentioning that microwave irradiation heating highly depends on water content and therefore is more effective and sustainable in wet sites like marine sediments than in dry areas (Vialkova et al., 2016). Reducing treatment temperature is a must to save energy and decrease site damages and structural deterioration of the ecosystem. Microwave irradiation heating is an ideal treatment for PAHs contaminated sediments where water is abundant (Vidonish et al., 2016).
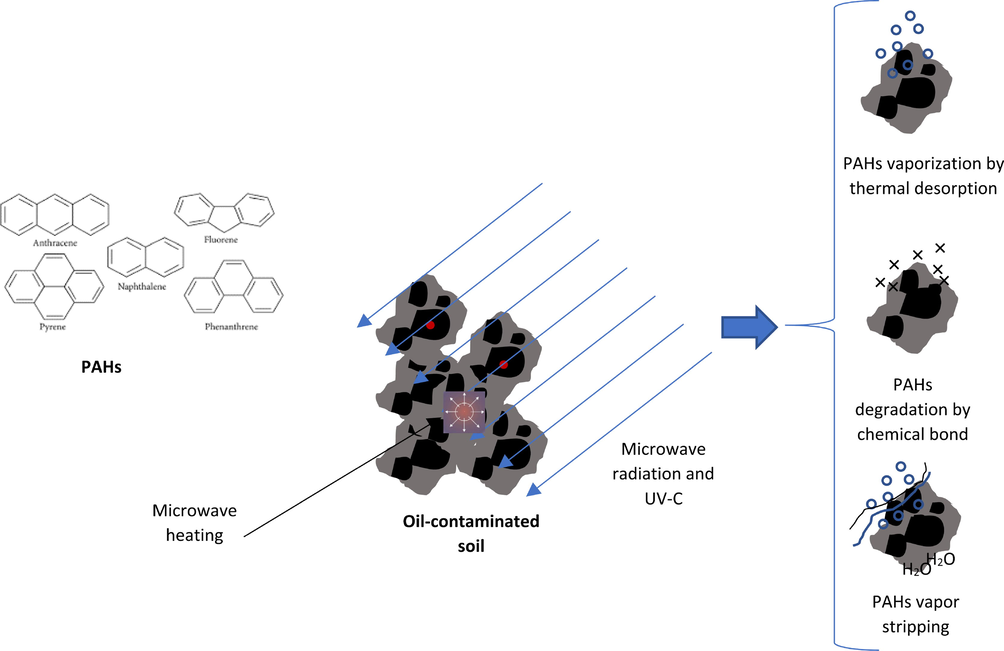
- Mechanisms of PAHs degradation during microwave irradiation heating (Eldos et al., 2020).
4.2 Chemical oxidation process
Another treatment technology, which is generally used to degrade recalcitrant organic pollutants (i.e. PAHs) is the chemical oxidation process. Conventional chemical oxidation processes i.e. ozonation, photolysis, and chlorination can degrade PAHs from contaminated water. However, this treatment itself can generate more toxic byproducts. For instance, chlorine as a classic oxidant can react with natural organic matter in the water forming toxic halogenated hydrocarbon. (Amor et al., 2019; Rubio-Clemente et al., 2014). Removal of PAHs from water by ozonation has been considered by several authors (Ates and Argun, 2018; Sakulthaew et al., 2015; Miller and Olejnik, 2001). Ozone oxidizes the pollutant through two mechanisms; (a) ozone directly oxidizes the pollutant through electron chemical bound, or atomic nuclei (b) indirect degradation of ozone by organic matter or metal oxide to produce radicals that oxidize pollutants to small molecules (Zhang et al., 2020). These degradation pathways are highly dependent on pH. The indirect pathway where O3 decomposes and generates OH radicals is facilitated at basic pH. In the case of acidic pH, a direct pathway is preferable where O3 becomes more stable and oxidizes the pollutant. Direct pathways between O3 and PAHs cause cleavage of the benzene ring generating intermediate compounds such as carboxylate and hydroxylated benzene. Later, intermediate compounds can go through direct or indirect pathways which can compete with target pollutants for O3 and OH radical (Rubio-Clemente et al., 2014). Limitations of oxidization such as the presence of OH scavengers in contaminated water and the formation of toxic byproducts can negatively affect PAHs degradation (Miller and Olejnik, 2001). Ozonation coupled with UV radiation and/or H2O2 has been used to enhance the performance of the O3 oxidation process (Bernal-Martinez et al., 2009).
4.3 Photolysis
Another chemical oxidation process is photolysis. PAHs in an aqueous system can naturally degrade through photolysis (Jacobs and L. Weavers and. Y.-P. Chin, , 2018). The main pathway of photolysis is the absorption of light by PAHs forming an exciting form of the target PAH. Later, the exciting form can either (a) return to the ground state causing dissipation of its energy or (b) transform into a radical form releasing electrons in the liquid (Miller and Olejnik, 2001).
Generally, degradation of PAHs by photolysis occurs when the emission spectrum of the introduced light overlaps the absorption spectrum of the target compound. The structure of the PAHs defined which wavelength will be absorbed. The absorption spectra of PAHs vary between 200 nm and 386 nm (Sanches et al., 2011). In addition to the absorption spectrum of the target pollutant, the photolysis process depends on the intensity of radiation, pH, temperature, and water composition. However, direct photolysis of PAHs in water which naturally occurs can produce some intermediate compounds that are toxic to marine life (Miller and Olejnik, 2001). The conventional chemical process appears to be less effective than other hybrid processes where different combinations of energy sources, oxidants, and catalysts are used (Guieysse et al., 2004).
4.4 Advanced oxidation processes (AOPs)
Advanced oxidation processes (AOPs) refer to chemical oxidative methods mainly based on the generation of intermediate free radicals which are less selective and high reactive mainly hydroxyl radicals (OH) using several hybrid treatment systems of energy sources, oxidants, and catalysts to oxidize organic pollutants (PAHs) (Fig. 6). AOPs involve all different catalytic and noncatalytic processes that use the high oxidizing capacity of oxidants (Rubio-Clemente et al., 2014). The redox potential (ORP) of different oxidative reagents used in AOPs is illustrated in Fig. 6 a. Hydroxyl radicals have a high standard oxidation potential that characterized them as strong oxidative reagents that efficiently oxidize several organic contaminants (Zhang et al., 2020).
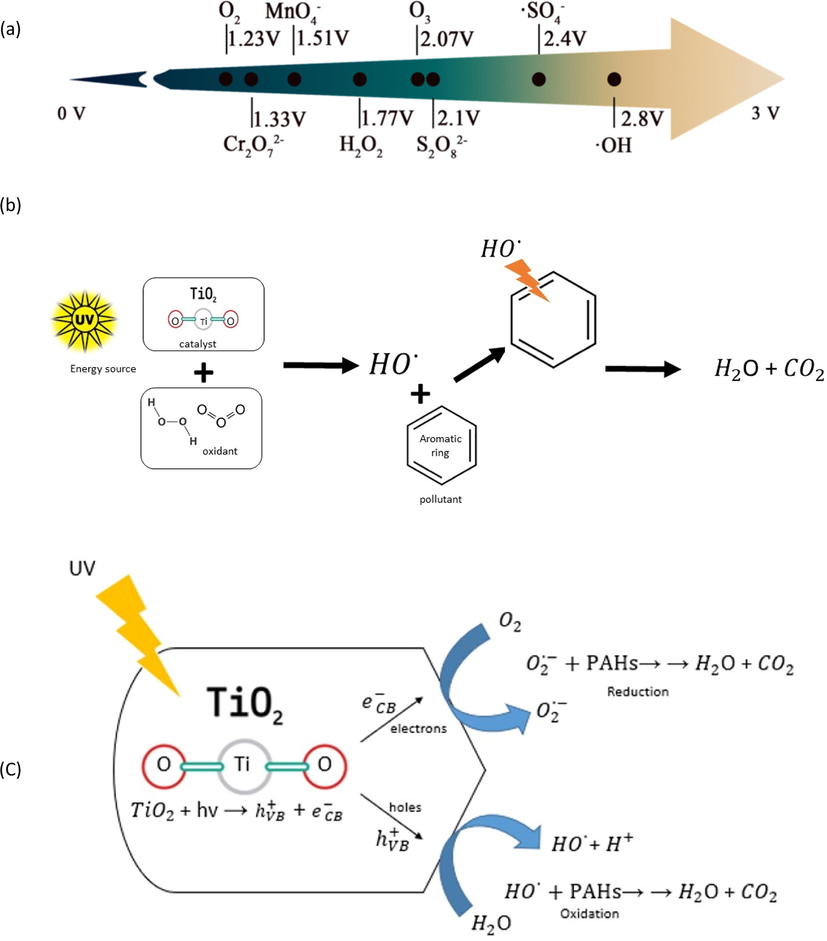
- (a) The redox potential (ORP) of different oxidative reagents used in AOPs (Zhang et al., 2020) (b) Generation of hydroxyl radical through AOPs (modified from (Cuerda-Correa and M. f. Alexandre-Franco and C. Fernández-González, , 2019). (c) Mechanisms of TiO2 photocatalysis degradation reaction (modified from (Zuluaga-Gomez et al., 2020).
It has been stated that AOPs can totally mineralize organic pollutants in contaminated water generating CO2 and H2O and inorganic ions by using hybrid treatment systems (Cuerda-Correa and M. f. Alexandre-Franco and C. Fernández-González, , 2019). AOPs can be classified into photochemical or non-photochemical processes depending on the way of generation of OH radicals. In the case of the photochemical process, a light source is applied i.e. UV light with catalysts or oxidizers. The generation of OH radical can be started up by oxidants such as H2O2, O3, UV light, or can be triggered by catalysts. The efficiency of AOPs depends on several factors including oxidant type and dose, the type and intensity of light, pH, the generation rate of free radicals, the concentration of pollutant and its properties, and water composition (Rubio-Clemente et al., 2014). AOPs consider relatively high operational costs due to the cost of chemicals i.e. Hydrogen peroxide and high consumption of energy. Also, OH radical is a highly non-selective species that can scavenge non-target pollutants present in the contaminated water. Photochemical AOPs are the most promising process and it has economic benefit in the case of using a solar light source (Comninellis et al., 2008).
Ozone-based AOPs; using UV radiation or oxidation i.e·H2O2 or UV combined with O3 can improve the degradation of some recalcitrant organic pollutants (Rubio-Clemente et al., 2014). In the UV/O3 hybrid process, the reaction starts with (a) the photolysis of O3 in water by UV which directly forms O2 and H2O2, (b) the reaction proceeds with the photolyze of H2O2 to OH radical or dissociates to HO2 anion initiating additional decomposition of residual of O3 into OH radical which in turn attack O3 and hydrogen peroxide molecules. In the case of the H2O2/O3 hybrid system; O3 decomposes by H2O2 generating OH radical and HO2 radical (Von Gunten, 2003). In a hybrid system like UV/O3 or H2O2/O3, when PAHs are introduced, they can go through oxidation by OH radical, HO2 radical, or O3– radical or by direct ozonation. Comparing hybrid systems UV/O3 or H2O2/O3 with H2O2/UV system, it is worth mentioning that the first two systems are less efficient in terms of the mean of energy (large quantities of OH radicals are generated by the H2O2/UV system) as a result of the low solubility of O3 in liquid compared to H2O2 (Rubio-Clemente et al., 2014). In H2O2/ UV hybrid system; the formation of OH radicals is generated by photolysis of H2O2 or HO2 anions by applying UV light. In presence of PAHs in water, OH radicals quickly interact with them causing their oxidation. Also, in the hybrid system, PAHs can go through direct photolysis under UV radiation (Sanches et al., 2011).
Photocatalysis is a series of light-based reduction–oxidation reactions occurring by a semiconductor. A semiconductor photocatalyst t is a material that is able to absorb light and transfer it to a higher energy level for the substance (i.e. organic pollutant) to initiate a chemical reaction. In another word, semiconductor reacts with light to produce reactive species which can oxidize organic pollutants (PAHs) (Rubio-Clemente et al., 2014). The photocatalytic reaction can be divided into homogeneous photocatalysis and heterogeneous photocatalysis. When both the reactant and the semiconductor are in the same phase (e.g. gas/ solid/ liquid) the reaction is classified as homogeneous photocatalysis. While if the reactant and the semiconductor are in different phases the reaction is classified as heterogeneous photocatalysis. To date, different semiconductors materials have been developed with fascinating properties such as efficient absorption of light and great physiochemical stability (Sharmaa et al., 2019).
Among the variety of metal oxides semiconductor, TiO2 has been chosen as a novel photocatalyst material due to its high absorption behavior, high photocatalytic degradation ability, high activity, and low cost (Zhang et al., 2020). TiO2 photocatalysis reaction initiates with TiO2 absorption of UV radiation which results in the generation of electron or positive holes in the conduction and valence band (Zuluaga-Gomez et al., 2020). The holes can either directly react with PAH or form an OH radical that can oxidize PAHs, In the other side, electrons can directly oxidize organic pollutants i.e. PAHs by consuming oxygen molecules through a reduction reaction (Fig. 6 c). Still, many semiconductors materials are developing to synthesize a material, which is not toxic, can be made from readily available precursors, and can be easily modified (Sharmaa et al., 2019). Graphitic carbon nitride has emerged as a potential semiconductor due to its excellent photocatalytic performance. Graphitic carbon nitride semiconductor comprised of carbon and nitrogen, the most two elements abundant on earth, it can be easy to adjust, has stable chemical and physical properties and its unique electronic band structure make it an exceptional semiconductor material for photocatalytic reaction. Kumar et al., (Kumar et al., 2020) discussed the application of graphitic carbon nitride photocatalysis system in degrading different pollutants including organic pollutants such as volatile organic compounds VOCs.
Accordingly, the main function of a photocatalyst is to initiate or accelerate redox reaction in presence of an irradiated semiconductor. To complete the redox process, the semiconductor photocatalyst must be irradiated with energy that is equivalent to or higher than the energy of the band gap of the semiconductor catalyst (Sonu et al., 2019). Using a single semiconductor in photocatalytic reaction led to facing a problem of recombination of electrons and holes because of the larger band gap and narrow diffusion length. Hence, researchers explore to try to overcome this challenge, which introduces the different types of photocatalysts hybridization such as metal-doping, metal loading, and heterojunction photocatalyst (Kumar et al., 2021). Heterojunction photocatalysis is the combining of a semiconductor with other semiconductors (s), which changes the interfacial interactions between the semiconductor resulting in a distinctive photocatalyst. The main advantage of heterojunction photocatalysis is the junction of electrons and holes to different semiconductors, simultaneously traveling to surfaces and going through a redox reaction. Accordingly, photocatalytic treatment considers one of the most attractive treatment techniques used in wastewater treatment. The advantages of photocatalytic treatment are the idea of photocatalyst hybridization, adequate preparation methods, degradation of different pollutants, and usage of a sustainable source of energy (Hasija et al., 2021).
Several studies investigated the efficiency of using an integrated advanced oxidation system to degrade organic pollutants in contaminated water (Fernandes et al., 2019; Jiméneza et al., 2019; Liu et al., 2021). Jiméneza et al., (Jiméneza et al., 2019) studied the efficiency of different integrated AOPs to treat produced water including photocatalysis, Fenton-based processes, and ozonation. The results showed in all studied AOPs, benzene, toluene, ethylbenzene, xylene (BTEX), and naphthalene in produced water were removed, however, the removal efficiency in terms of total organic carbon (TOC) varied largely between the studied treatments. Photocatalysis was the less effective treatment for produced water where TOC was less than 20%. On the other side, ozonation with H2O2 showed the best results were all studied organic compounds were removed with 70 % percentage of acetic acid content. Using microwave radiation with UV light to assess TiO2 photocatalysis reaction can significantly contribute to the treatment of organic pollutants in contaminated water as AOPs (Horikoshi and Serpone, 2014; Serpone et al., 2010).
4.5 Electrokinetic (EK)
Electrokinetic (EK) remediation, is a physical treatment technology that is effective for non-biodegradable organic pollutants such as PAHs. The EK process is based on being applied in low levels of direct current in the contaminated matrix for the generation of electric fields (Kim et al., 2002). During the EK process, pollutants are induced by the electric field to be transported and mobilized towards the electrode (anode and cathode) mainly by the effect of electromigration and electroosmosis. The electrolytic reaction at the electrodes involves the generation of oxygen and H+ at the anode due to water oxidation while the generation of hydrogen gas and hydroxyl radicals at the cathode is due to water reduction (Gitipour et al., 2018). However, some limitations that negatively affect the PAHs removal are the low water solubility, hydrophobicity, and low dispersant rate of PAHs. EK treatment can be more effective, practical, and less expensive when it is combined with other proven remediation technologies as integrated systems such as electrokinetic-bioremediation, electrokinetic-thermal desorption, electrokinetic-oxidation reduction reaction, and electrokinetic-biosolubilization (Alcántara et al., 2018). Combining EK remediation with bioremediation has shown a strong promise as an effective developed treatment. The application of an electric field to the contaminated site during the EK process increases the mobilization of the pollutants which in turn increases their bioavailability to the microorganism. In other words, EK overcomes the limitation of bioremediation and increases its efficiency by making pollutants, nutrients and electron donors, and electron acceptors more available to catabolically active microorganisms. It is worth mentioning here that optimization of the strength of an electric field is needed to avoid its effect on the microbial community structure and activity (Cappello et al., 2019).
4.6 Emerging technologies - nanomaterial-based technologies
Among the emerging technologies, the development and application of nanomaterial-based technologies have been suggested as promising treatment alternatives for removing several pollutants from contaminated sites, at the same time. Consequently, numerous types of nanomaterials have lately been introduced into the market as nanosorbents to enhance the treatment of PAH- contaminated water before subsequent reuses (Russo et al., 2020).
In the literature, the adsorbent materials used for treatment are commonly evaluated by their characteristics related to the structure (surface and pores), the kinetics of adsorption, specificity of adsorption, and adsorption isotherms (Yousef et al., 2020). Characterization of the adsorbent system is important to understand the adsorption mechanisms of pollutants. An adsorbent with good adsorption capacity exhibits specific characteristics related to the surface area and pores size of the adsorbent (Nethaji et al., 2012). Several studies have described the adsorption kinetics process by performing time interval experiments that are dependent on extracting samples at certain time intervals and determining their respective concentrations. The obtained results are used to fit a suitable kinetics model that is able to describe the adsorption mechanisms (Largitte and Pasquier, 2016). The most common kinetics models observed in the petroleum effluents adsorption are pseudo-first-order and pseudo-second-order models (Revellame et al., 2020). The pseudo-first-order model and pseudo-second-order model are commonly described as adsorption mechanisms by physical forces and chemical means respectively (Narayanan, 2019). Additionally, the adsorption processes have been assessed by fitting the equilibrium data of the adsorption in terms of isotherm models (Yousef et al., 2020). The equilibrium association between the adsorbent and adsorbate is expressed by different isotherms that measure the amount of adsorbate (pollutants) adsorbed at a constant temperature. The most common isotherms models are the Freundlich model, Langmuir model, the Temkin model, the Sips model, and the Dubinin-Radushkevich (Wang and Guo, 2020). A wide range of adsorbent materials has been employed for the remediation process. Different sorbents composed of a broad range of functional groups and various geometrical surface structures are appropriate based on their application in the industry (Dąbrowski, 2001). Adsorbent materials can be classified as natural adsorbents including organic or/and inorganic adsorbents and synthetic adsorbents (Al-Jammal and Juzsakova, 2017). Natural sorbents can be generated from the earth or biological sources; plant-based or food wastes such as graphene, clay minerals, bentonite, vegetable fibers, sawdust, straw, date palm, etc. The strengths of these natural organic sorbents are that they are readily available and cheap materials compared to other sorbents. They have been used to maximize the adsorption and recycle the wastes into usable materials (sorbents) (Ndimele et al., 2018; De Gisi et al., 2016). However, the major drawback of their use is the complexity of collecting the adsorbent for disposal or reuse after their use in oil spill water (Crini et al., 2018). Synthetic sorbents which are widely used include lab-synthesized adsorbents or commercially available adsorbents such as activated carbon, nanotechnology-based adsorbents, zeolites, and carbon nanotube, etc.
Activated carbon (AC) adsorption is one of the best available technologies and has been recommended by the USEPA. Remediation of oil-contaminated water using AC is widely applied and approved to be technically feasible in the removal of a wide range of organic molecules (Okiel et al., 2011). However, the cost of AC is considered expensive, especially in developing countries. These synthetic adsorbents can absorb up to 100 times their weights in oil media because of their oleophilic and hydrophobic nature (Ndimele et al., 2018; De Gisi et al., 2016). They are known to be less biodegradable or non-biodegradable compared to other sorbents. To choose the efficient adsorbent, it is recommended to explore its applicability for usage by considering essential factors such as the adsorbent availability, the adsorbent processing, and the environmental and economic aspect. A review published by Yousef et al., (Yousef et al., 2020) discussed the adsorption processes of produced water treatment and listed the most pollutants and adsorbent types targeted by investigators in the literature. Fig. 7 shows hydrocarbons that are the most targeted pollutants and natural adsorbents which are the most utilized adsorbents based on the studies included in that review. Table 2 compares different studies that used different adsorbents in treating pollutants present in petroleum industry effluents.
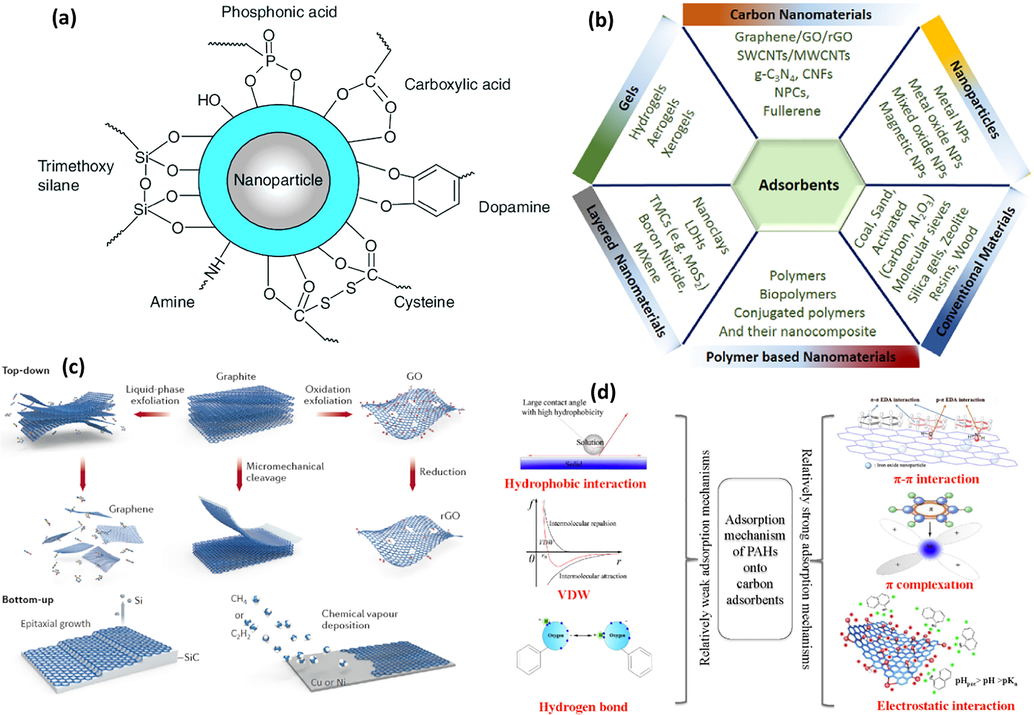
- (a) Surface multi-functionalities of metal oxide NPs (Nassar, 2013); (b) Variety of materials as adsorbents for water contamination remediation (Gusain et al., 2020) (c) Top-down processes or bottom-up processes of graphene fabrication (Gusain et al., 2020), and (d) adsorption mechanisms of PAHs on carbon adsorbents (Li et al., 2020).
| Adsorbent | Adsorbent type | Targeted pollutant | Adsorption capacity | Adsorption isotherms | Ref. |
|---|---|---|---|---|---|
| Diphenyldichlorosilane coated zeolite | Natural clinoptilolite zeolite | Toluene | 16.58 g of toluene/g of adsorbent | Langmuir | (Ma et al., 2020) |
| Chitosan modified biochar | Natural adsorbent (wheat straw biochar) | Dissolved organic matter (DOM) | 52% removal of DOM | NR | (Shi et al., 2020) |
| Dimethyl dioctadecyl ammonium bromide (DDAB) modified sepiolite | Natural sepiolite | Emulsified oil | 1013.5 mg of oil / of DDAB modified Sepiolite | Langmuir | (Zheng et al., 2020) |
| Powdered activated carbon | Synthetic adsorbent (Commercial). | Naphthalene contaminated saline water | 179.24 L/g | Freundlich | (Younker and Walsh, 2015) |
| Chitosan with coagulants (carboxymethyl cellulose) | Natural adsorbent (shrimp shells chitosan) | Oil in produced water | 96.35% removal of oil droplets | NR | (Hosny et al., 2016) |
| Resin (phenyl epoxy/PVP/Nano Fe3O4 particles) | Synthetic adsorbent | Oil in petroleum produced water | 99.9% removal of oil | ـــ NR | (Abdel-Shafy et al., 2020) |
| Amberlite XAD 7 (Acrylic ester) | Commercial synthetic resins | Oil in aqueous solution | > 98% removal | Freundlich | (Albatrni et al., 2019) |
| Lewatit AF 5 (Styrene-Divinylbenzene) | Commercial synthetic resins | Oil in aqueous solution | > 98% removal | Dubinin Radushkevich | (Albatrni et al., 2019) |
| Powdered activated carbon (AC) with coagulant | Commercial Adsorbent | Oil in produced water |
99% removal | NR | (Rosenblum et al., 2016) |
| Alumina nanoparticle functionalized with petroleum vacuum residue | Lab synthesized Adsorbent | Oil in saltwater emulsions | 193.77 mg of oil / g of adsorbent | BET model | (Franco et al., 2014) |
| Produced Carbon nanotubes (P-CNTs) | Commercial Adsorbent | Oil contaminated water | 97% removal | Freundlich | (Kayvani Fard et al., 2016) |
| Sludge-based AC | Lab synthesized Adsorbent | Hydrophobic organic compounds from stormwater | 2800 µg of HOC /g of AC | Langmuir | (K. Björklund and. L. Y. Li, , 2017) |
Nanomaterials are described as materials with structural elements measuring between 1 and 100 nm at least in one dimension. These nanomaterials are characterized by a higher surface-to-volume ratio and greater magnetic and catalytic properties than their bulk material (Kumari and Singh, 2016). These characteristics of nanomaterials e.g. higher surface area and larger surface reactivity allow them to remove pollutants from wastewaters at a faster rate and to decrease the number and quantity of toxic by-products (Bhattacharya et al., 2013). To date, several nanomaterials are being used for remediation purposes such as carbon nanotubes (CNTs), nanoscale zeolites, metal oxides, biopolymers, and metal–organic frameworks (MOF). The application of these nanoparticles (NPs) for petroleum industrial effluent remediation has been developed through different nanotechnology-based treatment approaches. Younis et al. (Younis et al., 2020) have divided these approaches based on the function of the used NPs; (1) nanosorbents (2) nano-membrane filtration, and (3) multi-functional approaches such as dispersant, oil absorption, and catalytic degradation. Generally, the application of nanotechnology approaches in wastewater treatment could be summarized in three approaches (i) nanoscale filtration techniques (ii) pollutant adsorption on NPs-nanoadsorbent, and (iii) pollutant degradation by nano-catalysts (Baruah et al., 2019). Nanosorbent based technologies involve using NPs in sorption processes. NPs have significant properties that enable them to be excellent adsorbents such as large specific surface and surface sorption multi functionalities (Fig. 7). These features make NPs not only effective adsorbents for different pollutants in wastewater but also time effective due to their small size which facilitates their dispersion and surface accessibility, leading to fast adsorption rate and high adsorption efficiency (Nassar, 2013).
Sorption is a surface phenomenon where NPs (sorbents) interact with the pollutants (adsorbate) in the media by either physical or chemical mechanisms. The physical and chemical adsorption can be named also sportive or reactive adsorption, respectively. In chemical adsorption, a real chemical reaction occurs forming a chemical bond between the sorbent and the sorbate. Regeneration of used sorbent materials can be viable by chemical methods. While physical adsorption involves the trapping of the sorbate by the sorbent through van der Waals forces within the sorbent pores. The used sorbent can be recovered by sonication, calcination, etc. (Dhaka et al., 2019). Generally, nanosorbents are characterized by unique surface features namely, high surface area, high porosity, surface-active functional sites, fast catalytic rate, and high efficiency for treating several pollutants (Ali et al., 2020). The performance of NPs in an adsorptive reaction is linked to the number of native or acquired active surface sites and high electronic density on NPs surfaces, which reactively interact with the pollutants (Jain et al., 2021). Improvements in nanosorbents facilitated the manufacturing of sorbent-based treatment systems such as solid-phase extractors of volatile organic compounds (VOCs). Additionally, the hydrophobicity features of some nanosorbents such CNTs; enable the free passages of the water molecules without any interaction, easing the selective trapping of most organic pollutants such as PAHs. Carbon nanomaterials (CNMs) with high hydrophobicity exhibit flexible functionality, high porosity, and good mechanical and chemical stability in wastewater treatment systems at a wide range of pH. Although these CNMs are known as relevant nanosorbents for the sorption of petroleum pollutants, some impurities can occur with CNMs after synthesizing them. This leads to impairing their adsorptive performance affecting their surface hydrophobicity. Hence, their chemical functionalization is considered to enhance the surface area, and increase the active sites, chemical reactivity, and stability of CNMs for petroleum industrial effluents remediation.
The introduction of surface functional groups (e.g., –OH, –COOH) enhances the surface functionality and liquid dispersibility. Yu et al., (Yu et al., 2016) examined the removal of phenols from the aqueous phase using graphene oxides (GOs). GOs chemically exfoliated with H2SO4 ــH3PO4 showed excellent uniform dispersion in the water phase. Besides, the functionalized GO exhibited a higher adsorption capacity for phenol and its derivatives. Different methods are used to synthesize nanosorbent particles and can be classified into top-down processes or bottom-up processes. The former process applies the traditional approach for nanosorbent synthesis followed by reducing the particle size via erosion, mechanical alloying, and high-energy ball milling (Khan et al., 2019). The latter process is based on the building of the particle from the bottom such as molecular self-assembly, chemical or physical vapor deposition. Each process has its own strengths and drawbacks. For instance, the main disadvantage of the top-down process is the destruction of crystallographic and surface structure during the reduction of particle size. On another hand, the bottom-up process requires chemical purification and large-scale production, which is also challenging. However, nowadays the bottom-up process is believed to be the ideal technology because it can generate particles with high specific properties designed to the remediation need (El-sayed, 2020). Sadegh et al., (Sadegh et al., 2017) identified the ideal criteria for the materials of nanosorbents to be used in remediation technologies namely: (i) the material should be nontoxic, (ii) the materials should have high sorption capacity and selectivity for removal of pollutants at low concentration, and (iii) the surface materials of the nanosorbent should be reactivated and functionalized easily. Hence, several developments have been addressed to remove pollutants from wastewater. Additionally, the adsorbent reusability in adsorption treatment is a very critical parameter from an environmental and economical point of view. Various methods are used for adsorbent recovery after the adsorption process such as solvent washing, chemical and thermal processes (Ahamad et al., 2019; Ahamad et al., 2020; Al-Kahtani et al., 2019). Ahmed et al., (Ahamad et al., 2019) fabricated Nitrogen and sulfur-doped magnetic carbon aerogel (N/S-MCA) nanocomposites from sugarcane bagasse cellulose and used them to adsorb bisphenol-A. The used nanocomposites after adsorption were regenerated by methanol washing to remove the absorbed bisphenol-A and they were efficient until the sixth cycle. Recent studies focused on one or more different classes of nanosorbents such as CNTs, zeolites, metal-based nanosorbents, and MOFs (Liu et al., 2021; Xiang et al., 2021; Ma et al., 2020; Shi et al., 2020; Zheng et al., 2020; Amin et al., 2014).
4.6.1 Metal oxides NP
Iron, manganese, silicon, tungsten, and titanium are the most commonly used oxides as nanoadsorbent. Metal oxide nanoparticles have many advantages including low cost, are easily manufactured, and can be functionalized simply to adjust their adsorption capacity and selectivity (Namdeo, 2018). Recently, iron oxides-based nanosorbents have been explored for their ability to remove different organic pollutants and heavy metal ions. Additionally, the magnetic nature of these NPs facilitates their magnetic separation from the treated site through a magnetic field (Gutierrez et al., 2018). Nanoparticles-based tungsten oxide has shown a very high capacity for the adsorption of large organic dyes in water. Moreover, these metal oxide nanoparticles can be employed in adsorptive media filters or reactors. Nanometals can be compacted into porous pellets or used in powder form to ease their application in existing treatments (Gehrke et al., 2015). Namdeo (Namdeo, 2018) reported the impact of using nano- magnetite in water purification and simplified the synthesis, surface functionalization, application, and reuse of magnetite-based NPs in the removal of several pollutants.
4.6.2 Metal-organic framework (MOFs)
Other classes of advanced nanosorbents are metal–organic frameworks (MOFs) and nano-polymers, which are described as next-generation nanosorbents against petroleum wastewaters due to their potentiality in wastewater treatment. MOFs are polymers that consist of inorganic; metal ions or clusters; bridged through organic ligands forming a hybrid crystalline structure with high porosity (Russo et al., 2020; Wang et al., 2018).
By selecting the organic and inorganic compounds of MOFs, the crystalline structure and the chemical functionalities can be modulated which allows their use in several applications such as separation, catalysis, chemical sensing, surface adsorption, and drug delivery (Meng et al., 2020). Since their discovery, most of the MOF studies have concentrated on the bulk phases of MOF, but more recently huge work has been dedicated toward the understanding of MOF-NPs (Feng et al., 2019). Various properties have been observed such as accelerated sorption kinetics and improved bioavailability which are not relevant in bulk systems as a result of their nano-size and high surface area. Generally, MOF-NPs features include high surface area, large adsorption capacity, tunable porosity, large stability and recyclability, design flexibility, and surface functionality including catalytic activity and cellular uptake (Dhaka et al., 2019). The unique features of MOFs make them useful in a wide range of applications including biomedical, electrochemical, catalytic, sensing applications, water and air purification, and membrane technologies (Pettinari et al., 2017). Preparation and analysis costs are always considered critical issues for the applicability of any synthetic materials. MOFs are synthesized through different methods mainly hydrothermal and solvothermal reactions. The total cost of MOFs includes the reactor, the reagent, the services costs, and the cost of the separation and activation step. Table 3 shows the cost of the materials that are used for MOFs preparation (retrieved from (Liu et al., 2012). The reagents of MOFs consist of metal sources, organic linkers, and solvents for the reaction and exchange processes (Liu et al., 2012). Table 4 represents the advantages and disadvantages of available adsorbents including MOFs.
| Adsorbent | Cost / Kg (US $) |
|---|---|
| CuBTC (HKUST-1) | 20.08 |
| MIL-100 | 15.64 |
| MIL-101 | 4.57 |
| CoCo (Co3[Co(CN)6]2) | 35.14 |
| Silica gel | 1.00 |
| Zn/DOBDC (Zn-MOF-74) | 1.90 |
| MOF-5 (IRMOF-1) | 2.93 |
| Adsorbent | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Carbon-based adsorbent (e.g., Activated carbon). |
|
|
(Younis et al., 2020; Crini et al., 2018) |
| Natural adsorbent (e.g., zeolite, clay, chitosan, and wood) |
|
|
(Sing et al., 2018; Owlad et al., 2008) |
| Metal oxides based adsorbent | Effective adsorbent of a wide range of inorganic pollutants (e.g., heavy metals) Effective for surface modification. High chemical stability Stable at high temperatureEase of recycling and regeneration process (applying magnetic field) Applicable for membrane nanotechnology |
Low surface area and less hydrophobicity Cost around 5–13 $ per g Can be biocidal |
(Younis et al., 2020) |
| MOFs | Diversity of the structureEase of structure modification due to their inorganic and organic parts (production of the nanostructure is easy) Simple and highly porous adsorbents Great performance due to its heterogeneous adsorption and catalysis Recommend for effluents with a mixture of organic and inorganic pollutants due to their structure. Numerous active sites, good stability, and excellent selectivity. |
The range stability is limited to 300C. Less information on the reuse and recycling of MOF. Cost around 13$ per g |
(Adesina Adegoke et al., 2020; Younis et al., 2020) |
Methods to synthesize MOF-NPs and understand their structure–function relationships are very important for their applications but it is still in their infancy. Different methods are used to synthesize uniform MOF-NPs such as solvothermal, hydrothermal, electrochemical, ultrasound, and microwave.
MOFs are proved as good adsorbent materials to be used in the decontamination of wastewater from organic pollutants, because of the high porosity and the specific surface interaction. Moreover, the adsorption process itself is considered more effective than other wastewater treatment methods for the removal of organic and inorganic pollutants in terms of cost, simplicity of handling, the efficiency of sorbent, and regeneration. In recent years, MOFs received great attention for liquid-phase adsorptive removal of organic and inorganic pollutants due to their structural and functional properties. Different structures of MOFs have different adsorption capacities (Qe). Fig. 8 emphasizes different adsorption interaction mechanisms between the pollutants (adsorbates) and MOFs that have been reported in the literature such as electrostatic, hydrogen bonding, acid-base,
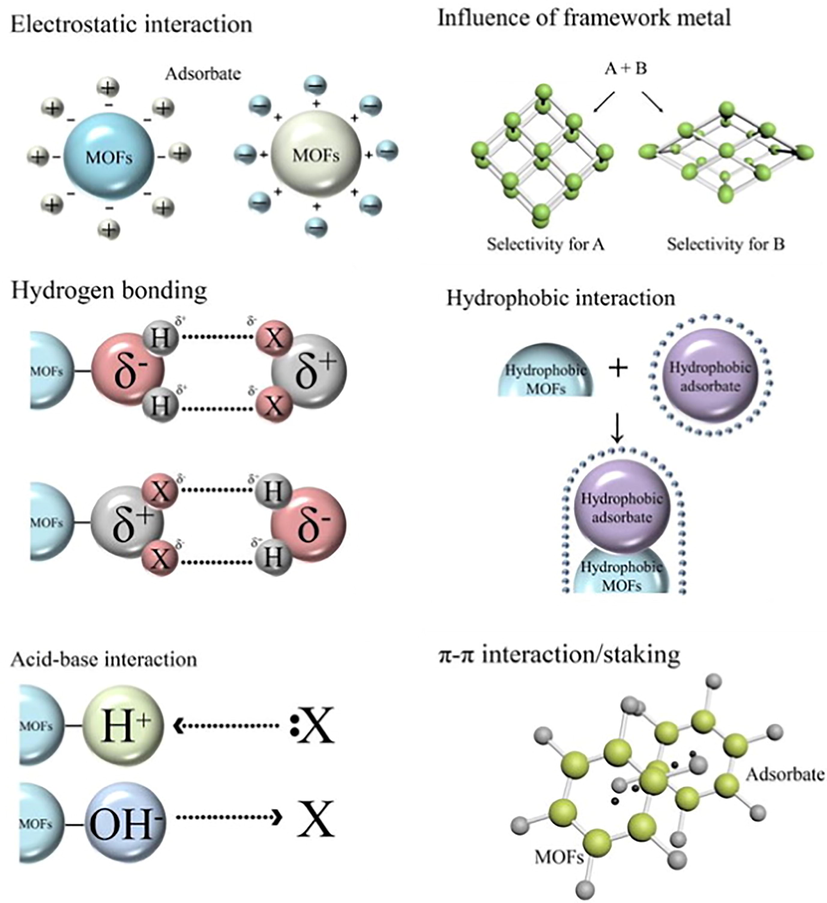
- Possible adsorption interaction mechanisms between MOF and pollutants (Dhaka et al., 2019).
| MOF | Metal | Organic Ligand | Pollutant | Adsorption capacity (mg g−1) | Type of interaction | References |
|---|---|---|---|---|---|---|
| UIO-67-30BA |
Zr (ZrCl4) |
4.4-biphenyl dicarboxylic acid | Dimethyl phthalate | 228.1 | π-π interaction | (Liu et al., 2021) |
| UIO-67-30BA |
Zr (ZrCl4) |
4.4-biphenyl dicarboxylic acid | Phthalic acid | 434.0 | electrostatic interaction and π-π interaction |
(Liu et al., 2021) |
| E. coli@UiO-67 composites | Zr (ZrCl4) |
4.4-biphenyl dicarboxylic acid | bisphenol A |
402.930 | electrostatic interaction and π-π interaction | (Xiang et al., 2021) |
| Imino-functionalized MIL-101 |
CrCr (NO3) |
terephthalic acid | bisphenol A |
460.8 | hydrogen bonding, π-π interaction, and electrostatic attraction | (Dapaah et al., 2021) |
| MIL-53 | Al (Al2O3) |
1,4‐benzene dicarboxylate | Dimethyl phthalate | 206 |
|
(Li and Y. n. Wu, J. Li, Y. Zhang, X. Zou and F. Li, , 2015) |
| ZIF-8 |
Zn (Zinc acetate) |
2-methylimidazole | Phthalic acid Diethyl phthalate | 654 ± 14 | Electrostatic interaction (at high pH)acid-base interaction (at low pH) |
(N. A. khan, B. K. Jung, Z. Hasan and S. H. Jhung, , 2015) |
| ZIF- 67 | Co (Cobalt nitrate hexahydrate) |
2-methylimidazole | Phenol | 378.89 | Electrostatic interaction |
(Pan et al., 2016) |
| Fe-MOF (mixture of MIL-53 & MIL-88B) |
Fe (iron oxide) |
Carbon composites | Oil/hydrocarbon | ـــ | van der Waals (vdW) interactions. |
(Banerjee et al., 2012) |
4.7 Combined adsorption photocatalysis remediation
One of the interesting methods in nanotechnology is the combination of adsorption methods and advanced oxidation processes to ensure high efficiency for trapping and degrading pollutants. Among the advanced oxidation processes, heterogeneous photocatalysis is commonly explored with nanoparticle adsorbents. In this method, the reactive oxygen species are produced from the nanostructured semiconductor catalyst stimulated by UV or visible light (Yahya et al., 2018). Nanoparticle which has two characteristics; adsorption affinity and photocatalysis will be a novel material that utilizes the advantages of both techniques for organic pollutant degradation. The most common photocatalytic adsorbents studied are metal oxide, metal–organic framework, and TiO2 nanocomposites. Iron oxide is widely used in catalytic processes and as an adsorbent in water treatment. It has a high adsorption capacity for organic and inorganic compounds due to its core chemical and electronic properties. Iron oxide is used as a semiconductor in photocatalysis processes related to its reduced band gap energy, which led to the absorption of visible radiation with an energy higher than water oxidization potential (Pawar and Lee, 2015). In addition, iron oxide materials are low cost and environment friendly with good stability. All these features that made the iron oxide nanoparticles are great potential candidates for strong adsorbents for pollutants removal from water (Shahrodin et al., 2020). Another common photocatalytic adsorbent that recently emerged as an exciting adsorbent is MOF due to the flexible modification in their porous structure and composition, topology, better surface area, and large visible-light absorption (Patial et al., 2021). Multivariate metal–organic frameworks (MTV-MOFs) are a new class of MOFs that consist of a large number of different components arranged in a single structure with photocatalysis application. MTV-MOFs possess mixed components which improve adsorption and photocatalysis performance due to their large surface area, defective sites, and synergetic effect between the components in adsorption and photocatalysis (Hasija et al., 2022). Park et al., (Park et al., 2019) discussed the photocatalytic and adsorption mechanisms of graphene-based nano-spinel ferrites (GNSFs). Additionally, the reusability of GNSFs is reported and it is stated that GNSFs are a great potential adsorbent/photocatalyst to treat organic and inorganic pollutants in the water environment.
4.8 Microbial bioremediation
Another available treatment is Microbial bioremediation which is the enhancement of the natural degradation process where microbes used their catabolic activities to degrade/eliminate or transform PAHs from the environment (Alegbeleye et al., 2017). Bioremediation is an environmentally friendly strategy that received great attention and high public acceptance for its potential to treat hydrocarbon contaminated sites (Ghosal et al., 2016). Microorganisms are capable of degrading PAHs such as bacteria, algae, yeast, and fungi by using the pollutant as the main energy and carbon source or through co-metabolism and commensalism (Al-Hawash et al., 2018). The biodegradation process involves the breakdown of PAHs into inorganic molecules, H2O, and CO2 (in case of aerobic degradation) and CH4 (in case of anaerobic degradation). Fig. 9. Shows different approaches to anaerobic biodegradation of organic compounds including PAHs.
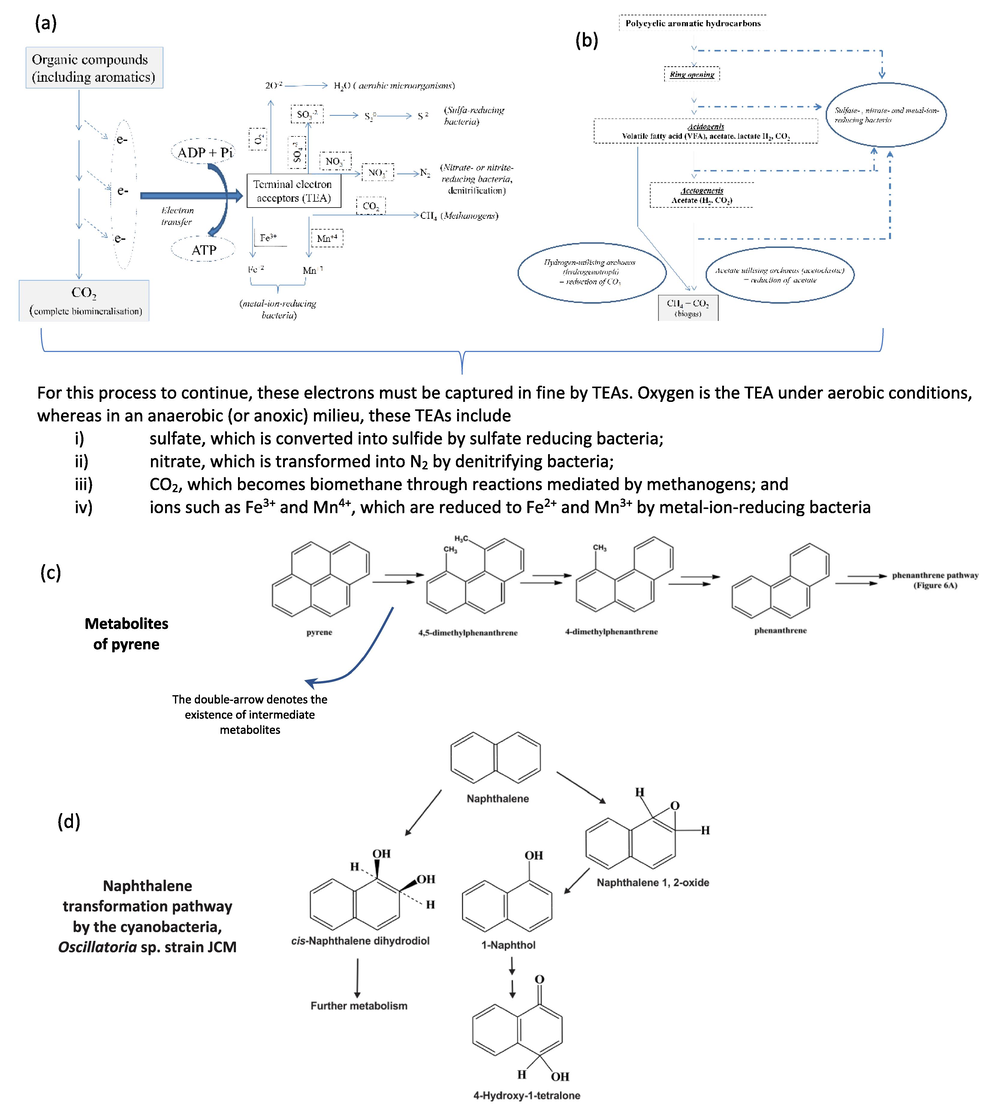
- (a) Strategies of anaerobic biodegradation of PAHs, (b) Summary of anaerobic biodegradation of PAHs up to biomethane formation, and (c) an example of Metabolites of pyrene (Nzila, 2018), (d) Naphthalene transformation pathway by the cyanobacteria, Oscillatoria sp. strain JCM (Ghosal et al., 2016).
Bacteria have been extensively studied for their capability to degrade recalcitrant organic pollutants. Bacteria are nature scavengers that use organic compounds i.e. PAHs as an energy source by several metabolic pathways. Bacteria are widely used in treating toxic PAHs due to their quick adaptability, ubiquitous spreading in a different environments, fast-growing, easy manipulation, and highly diverse in their catabolic pathways of hydrocarbons (Ghosal et al., 2016; Alegbeleye et al., 2017). In addition, bacteria are easily adapted through molecular changes leading to the expression of catabolic enzymes for the degradation of PAHs (Sakshi and Singh and A. K. Haritash, , 2020). Bacterial degradation of PAHs can be either aerobically or anaerobically, however, the aerobic pathways and their kinetics, enzymatic, and genetic regulations are more widely documented (Haritash and Kaushik, 2009). Aerobic biodegradation is occurred where oxygen is available or anaerobically where oxygen is limited or absent. Microorganisms get energy by breaking down and transferring electrons for the pollutant (electron donor) to an electron acceptor such as O2. Oxygen is the final electron acceptor in an aerobic catabolic reaction, whereas other substances like nitrate, sulfate, iron, carbon dioxide, or inorganic compounds can be the final electron acceptor in anaerobic catabolism (Aitken and Long, 2004). Several bacteria species are capable of aerobic degradation which are the most extensively documented due to their ability to degrade many PAHs. It is worth mentioning that the availability of electron acceptors is depending on the polluted environment (Díaz, 2004). The polluted environments are frequently anoxic where oxygen is limited such as in aquatic sediments and groundwater; in such environments, facultative microorganisms or anaerobes are dominant and able to carry out biodegradation using alternatively nitrate, sulfate, iron, carbon dioxide, or inorganic compounds. For instance, the reduction of ferric iron is the most dominant mechanism for PAHs oxidation in the subsurface environment while sulfate reduction is the main mechanism for PAHs oxidation in the marine environment because of the high concentration of sulfate in seawater (Díaz, 2004). In terms of energy, biodegradation using oxygen as the final electron acceptor generates the highest yield of energy followed by nitrate, Fe (III), and sulfate. When these electron acceptors are exhausted, bacteria can use organic molecules as electron donors and the acceptor is a metabolic pathway known as fermentation (Dhar et al., 2019). This process yields low redox potential of the reactions and the least amount of energy as only a small fraction of the organic pollutant can readily be utilized by the microorganism. It is worth mentioning in aerobic degradation of PAHs oxygen serves as a final electron acceptor and a co-substrate for hydroxylation and cleaving of an aromatic ring. On the other hand, anaerobic degradation of PAHs has a different mechanism to attack the benzene ring mainly depending on reductive reactions. Though the aerobic degradation of PAHs has been elaborated for several decades, the anaerobic catabolism of PAHs has recently been discovered and stills need more understanding (Ghosal et al., 2016). During aerobic degradation, the aromatic ring structure of PAHs is generally modified by oxygenase-mediated metabolism using monooxygenases or dioxygenases enzymes. Hence, the microbial degradation of PAHs is faster only when oxygen is available for the cleavage of the aromatic ring. Mainly bacteria prefer aerobic conditions for PAHs degradation depending on oxygenase-mediated metabolism using monooxygenase or dioxygenase enzymes (Imron et al., 2020). Aerobic degradation initiates with hydroxylation of the aromatic ring by monooxygenase or dioxygenase enzymes and the formation of cis-dihydrodiol. These cis-dihydrodiols are dehydrogenated to produce catechols. In the next step, the catechols ring will be cleaved by intradiol or extradiol ring-cleaving dioxygenases by an ortho-cleavage or meta-cleavage pathway which will cause the termination of the aromatic ring by oxygen molecules. The latter reaction will produce a liner hydrocarbon which can be converted to Acetyl-CoA from the β-oxidation process and eventually enters bacterial central pathways i.e. Krebs cycle (Imron et al., 2020). Under anoxic conditions, where the degradation pathways will not involve oxygen it might be difficult to anaerobically degrade some types of PAHs which have no functional groups. Thus, activation of hydrocarbons is important for anaerobic degradation, and four main enzymatic pathways are involved: (1) addition of fumarate to form aromatic substituted succinates by glycyl radical enzyme, (2) addition of methyl group to unsubstituted aromatic compounds, (3) hydroxylation of alkyl substituent by dehydrogenase enzyme, (4) and direct carboxylation reaction (Varjani and Upasani, 2017).
However, the rate of the bioremediation process is mainly depending on three factors; the natural environment, the type of microorganisms, and the pollutants being degraded. Intrinsic bioremediation can occur by natural attenuation but in general, it could take several years (Díaz, 2004). Therefore, various strategies have been introduced to enhance the natural microbial metabolism of these pollutants by changing several factors. Enhancement of microbial catabolism of PAHs has been achieved by either biostimulation or bioaugmentation (Mohapatra and Phale, 2021) (Fig. 10). Biostimulation involves the addition of any stimulatory materials to stimulate the catabolic activities of endogenous microorganisms such as nutrients or electron acceptors or biosurfactants. While bio-augmentation is the application of exogenous microorganisms with certain catabolic activities which have adapted and have shown the capability to degrade particular pollutants (Alegbeleye et al., 2017; Coates, 2004). One of the main limitations of biodegradation of PAHs is their low water solubility and dissolving rate thus limiting their contact with degradation microorganisms. Many scientists use biosurfactants which are molecules produced from microorganisms with surface activity that can enhance the degradation of PAHs by improving their solubility in the aqueous phase and increasing their bioavailability to microorganisms (Cameotra, 2008). Biosurfactants are amphiphilic molecules containing hydrophilic and hydrophobic parts. The hydrophobic part is commonly a hydrocarbon chain while the hydrophilic part has many variations.
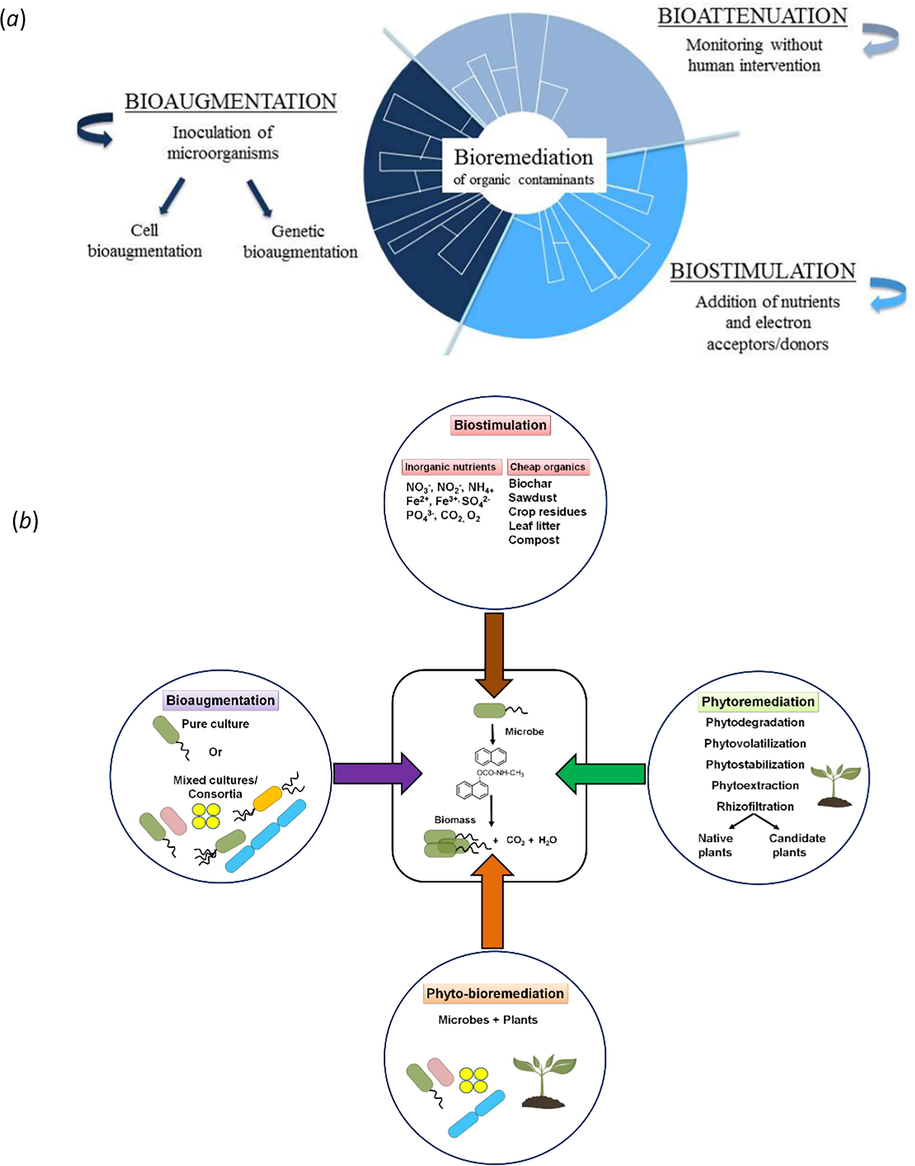
- (a) Bioremediation approaches of organic pollutants: bio-attenuation, bio-stimulation and bio-augmentation (Garbisu et al., 2017), (b) Bioremediation approaches for degradation/cleanup of xenobiotic aromatic pollutants (Mohapatra and Phale, 2021).
Microbial surfactants can be classified into two classes (1) low molecular weight (LMW) including glycolipids and lipopeptides, (2) high molecular weight (HWM) including polysaccharides, and lipoproteins. The structural variation between biosurfactants leads to different functionality. Generally, LMW biosurfactants are effective as a reducer of interfacial tension while HMW biosurfactants are more valuable as emulsion stabilizers (Johnson et al., 2021). LMW biosurfactants are significantly more utilized than HMW biosurfactants due to their high surface tension reduction (Femina Carolin et al., 2021). Common HWM biosurfactants are emulsan, alasan and biodisperan. The glycolipids class of LMW molecules are composed of carbohydrates such as rhamnose sophorose, or trehalose fused with lipopeptide or long-chain aliphatic acid-forming rhamnolipids, sophorolipids, and trehalolipids (Borah et al., 2021; Markande et al., 2021). Glycolipidic surfactants are mainly produced by Gram-negative bacteria such as Pseudomonas aeruginosa (Chebbi et al., 2017). The most common groups of lipopeptides are surfactins, iturins, polymyxin, and fengycins. Lipopeptide surfactants are mostly produced by Gram-positive bacteria such as Bacillus sp. (Yuliani et al., 2018). Biosurfactants possess diverse functional characteristics including dispersion, surface activity, emulsification, wetting, foaming, cleansing, etc. allowing them to be a suitable candidate for biotechnological applications like bioremediation, emulsion stabilizer, and microbial enhanced oil recovery (MEOR) (Adetunji and Olaniran, 2021). Table 6 shows the commonly studied biosurfactants with their producing microorganisms. The main limitation of biosurfactant production is the high cost compared to synthetic surfactant cost production despite the environmental benefits. Cost of the synthetic surfactant production is considered lower and yields higher since they use well-established processes and do not depend on the microbial culture for their production.
| Biosurfactant type | Microorganism | Carbon source | References |
|---|---|---|---|
| Lipopeptide | Bacillus subtilis- RSL2 | Crude oil | (Sharma and Pandey, 2020) |
| Lipopeptide | Bacillus licheniformis LRK1 | Several substrates | (Nayak et al., 2020) |
| Surfactin | Brevibacillus brevis | ـــ | (Krishnan et al., 2019) |
| Surfactin | Bacillus tequilensis MK 729,017 | Crude oil | (Datta et al., 2020) |
| Mono-rhamnolipid | Klebsiella sp. KOD36 | Soybean oil | (Ahmad et al., 2021) |
| Rhamnolipid | P. aeruginosa F-2 | Petroleum oil | (Yan et al., 2012) |
| Emulsan | Acinetobacter calcoaceticus PTCC1318 | Crude oil | (Amani and Kariminezhad, 2016) |
| Trehalolipids | Rhodococcus qingshengii strain FF | n‐hexadecane | (Wang et al., 2019) |
| Alasan | Acinetobacter sp. | Hydrocarbon | (Uzoigwe et al., 2015) |
However, a fair cost comparison should be considered including the total life cycle not only the cost of production. The total life cycle is involving the cost of environmental damage such as the removal of synthetic surfactants and possible fines for ecosystem damages. Hence, further investigation is required to conduct full lifecycle costs related to biosurfactant production and uses (Johnson et al., 2021). Biosurfactants have many unique advantages which enhance their uses and application in environmental remediation including, (i) structural diversity owning unique properties (Adebajo et al., 2018), (ii) stability under extreme conditions of temperature, pH, and salinity (Naughton et al., 2019), (iii) low toxicity, low irritancy, compatibility with human skin, and high biodegradability, and (iv) ability to be produced from cheap and renewable substrates owning a chance of cost-effective production (Markande et al., 2021).
Moreover, biosurfactants have been demonstrated to enhance PAH mobility and bioavailability (Dell'Anno et al., 2020). This facilitates catabolism and promotes biomass development, thereby increasing the biodegradation rate. Consequently, these biocompounds enhance cellular access to hydrophobic substrates, and they are a natural substitute for synthetic surfactants. Noteworthy, biosurfactants still have economic barriers due to their heavy production costs (Lopes et al., 2018).
Bioremediation treatments can be applied either in situ or ex-situ. Other examples of bioremediation techniques are bioventing, bio-sparging, bioslurry, land farming, and composting (Langenbach, 2013). Bioventing is the injection of air into the contaminated site to enhance the in-situ degradation process. Bio-sparging is used in the saturated zone where air/oxygen and nutrients are applied to enhance the oxygen concentration for better biological activities of the indigenous microorganisms. Bioslurry involves treating the contaminated site in a controlled bioreactor. These bioreactors are designed to control, monitor, and manipulate different factors such as temperature, and nutrients to reach maximal removal efficiency. Land farming involves spreading of the polluted soil over an area of land. It also required the addition of oxygen, moisture, and nutrients (i.e. fertilizers) as the biodegradation of the pollutant takes place at the oil–water interface. Composting is a controlled natural microbial degradation to change organic wastes into humic-like material called compost which can be used as soil fertilizers (Ossai and A. Ahmed and. A. Has, 2020).
5 Conclusion
PAHs generated either natural or by anthropogenic sources are considered hazardous pollutants due to their negative impact on the environment and human health. Due to serious concerns in the environment, remediation technologies are required to be assessed. In this review, the transport, fate, and distribution processes of PAHs in the environment are discussed. In addition, several traditional and advanced remediation technologies such as thermal, chemical, electrokinetic, nano-adsorption, and microbial treatment are thoroughly described and relevant findings are reported.
Different common strengths and drawbacks of various remediation methods are reported. To choose a treatment, a full study of the case study should be provided including the properties of the target pollutant, site description including climate conditions, and treatment procedure with the deficiencies and advantages. Removal rates of pollutants and other effective factors should be also included. Recently, combined or integrated treatment technologies are widely used to achieve maximum efficiency of the treatment. So, it is essential to apply innovative and site-specific treatment technology which can be feasible and efficient in the removal of PAHs from contaminated matrices (Vidonish et al., 2016). Finally, it is worth mentioning that there is no one exclusive treatment or combination of treatments by themselves that is suitable to remediate all contaminated sites. The selection of one or more treatment methods is key in decision-making due to the conflict of many parameters in nature. The site characteristic is also vital in choosing the most appropriate treatment technique(s). Generally, the selection criteria are based on the pollutant type and concentration, costs, difficulties, advantages, and site characteristics (Gitipour et al., 2018). It is a good choice to go for treatments, which are adaptive, sustainable, eco-friendly, efficient, quick, and cost-effective as treating PAHs contaminated sites is usually a difficult and expensive task.
Acknowledgment
This publication was made possible by grant # [QUEX-CAS-EMRQ-21/22-1] from ExxonMobil Research Qatar Limited (EMRQ). The findings achieved herein are solely the responsibility of the author[s]. Open Access funding provided by the Qatar National Library.
Conflict of interest statement
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Chemical contaminants entering the marine environment from sea-based sources: Areview with a focus on European seas. Mar. Pollut. Bull. 2016:17-38.
- [Google Scholar]
- Distribution and characteristics of PAHs in sediments from the marine environment of Korea. Chemosphere. 2007;68(1):85-92.
- [Google Scholar]
- Current State of Knowledge in Microbial Degradation of Polycyclic Aromatic Hydrocarbons (PAHs): A Review. Front. Microbiol.. 2016;7
- [Google Scholar]
- Distribution, sources, and behavior of PAHs in estuarine water systems exemplified by Salt River, Taiwan. Mar. Pollut. Bull.. 2020;154:111029
- [Google Scholar]
- Rapid assessment of the impact of microwave heating coupled with UV-C radiation on the degradation of PAHs from contaminated soil using FTIR and multivariate analysis. Arabian J. Chem.. 2020;13(11):7609-7625.
- [Google Scholar]
- Sources and environmental fate of pyrogenic polycyclic aromatic hydrocarbons (PAHs) in the Arctic. Emerging Contaminants. 2019;5:128-142.
- [Google Scholar]
- Overview of sediments pollution by PAHs and PCBs in mediterranean basin: Transport, fate, occurrence, and distribution. Mar. Pollut. Bull.. 2019;149:110646
- [Google Scholar]
- Biodegradation of high-molecular-weight polycyclic aromatic hydrocarbons under anaerobic conditions: Overview of studies, proposed pathways and future perspectives. Environ. Pollut.. 2018;239:788-802.
- [Google Scholar]
- Potential for native hydrocarbon-degrading bacteria to remediate highly weathered oil-polluted soils in Qatar through self-purification and bioaugmentation in biopiles. Biotechnol. Rep,. 2020;28:e00543
- [Google Scholar]
- S. Mahmood , . M. Earley and . S. Al-Abdul, “Oil and gas regulation in Qatar: overview,” 2019.
- D. J. Shilla and J. Routh, “Distribution, Behavior, and Sources of Polycyclic Aromatic Hydrocarbon in the Water Column, Sediments and Biota of the Rufiji Estuary, Tanzania,” Frontiers in Earth Science, vol. 6, 2018.
- Concentration, composition and sources of PAHs in the coastal sediments of the exclusive economic zone (EEZ) of Qatar, Arabian Gulf. Mar. Pollut. Bull.. 2014;85(2):542-548.
- [Google Scholar]
- Polycyclic aromatic hydrocarbons (PAHs) in marine sediments of the Hellenic coastal zone, eastern Mediterranean: levels, sources and toxicological significance. J. Soils Sediments. 2011;12(2):265-277.
- [Google Scholar]
- D. Mackay and D. Callcott, “Partitioning and Physical Chemical Properties of PAHs,” The Handbook of Environmental Chemistry, pp. 325-345, 1998.
- I. C. Ossai, . A. Ahmed and . A. Has, “Remediation of soil and water contaminated with petroleum hydrocarbon: A review,” Environmental Technology & Innovation, vol. 17, p. 100526, 2020.
- “The sources, transport, and fate of PAHs in the marine environment,” PAHs: An Ecotoxicological. Perspective 2003:7-33.
- [Google Scholar]
- Environmental organic chemistry. John Wiley & Sons; 2016.
- B. Mohapatra and P. S. Phale, “Microbial degradation of naphthalene and substituted naphthalenes: Metabolic diversity and Genomic insight for bioremediation,” Frontiers in Bioengineering and Biotechnology, vol. 9, 2021.
- O. Idowu , K. T. Semple, . K. Ramadass, . W. O'Connor, . P. Hansbro and . P. Thavamani, “Beyond the obvious: Environmental health implications of polar polycyclic aromatic hydrocarbons,” Environment International, vol. 123, pp. 543-557, 2019.
- Environmental organic chemistry. John Wiley & Sons; 2003.
- Estimating octanol–air partition coefficients with octanol–water partition coefficients and Henry’s law constants. Chemosphere. 2005;61(5):640-644.
- [Google Scholar]
- The octanol-air partition coefficient KOA as a predictor of gas-particle partitioning of polycyclic aromatic hydrocarbons and polychlorinated biphenyls at industrial and urban sites. J. Serb. Chem. Soc.. 2011;76(3):447-458.
- [Google Scholar]
- Evaluation of the three-phase equilibrium method for measuring temperature dependence of internally consistent partition coefficients (K OW, K OA, and K AW) for volatile methylsiloxanes and trimethylsilanol. Environ. Toxicol. Chem.. 2014;33(12):2702-2710.
- [Google Scholar]
- Partitioning of polycyclic aromatic hydrocarbons (PAH) to water-soluble soil organic matter. Eur. J. Soil Sci.. 1995;46(2):193-204.
- [Google Scholar]
- S. Mathews and N. P. Sithebe, “The role of bacteria in the breakdown of carcinogenic substances (PCBs) in wastewater for safe recycling purposes – A review,” International Journal of Environment and Sustainability, vol. 3, no. 3, 2015.
- Distribution, fate and risk assessment of PAHs in water and sediments from an aquaculture- and shipping-impacted subtropical lake, China. Chemosphere. 2018;201:612-620.
- [Google Scholar]
- Fate of polycyclic aromatic hydrocarbons in seawater from the Western Pacific to the Southern Ocean (17.5 N to 69.2 S) and their inventories on the Antarctic Shelf. Environ. Sci. Technol.. 2016;50(17):9161-9168.
- [Google Scholar]
- Applications of carbonaceous adsorbents in the remediation of polycyclic aromatic hydrocarbon-contaminated sediments: A review. J. Cleaner Prod.. 2020;255:120263
- [Google Scholar]
- Distribution of polycyclic aromatic hydrocarbons in sunken oils in the presence of chemical dispersant and sediment. Journal of Marine Science and Engineering. 2019;7(9):282.
- [Google Scholar]
- K. Hussain, R. R. Hoque, S. Balachandran, S. Medhi, M. G. Idris, M. Rahman and F. L. Hussain, “Monitoring and risk analysis of PAHs in the environment,” Handbook of Environmental Materials Management, pp. 1-35, 2018.
- Review on characteristics of PAHs in atmosphere, anthropogenic sources and control technologies. Sci. Total Environ.. 2017;609:682-693.
- [Google Scholar]
- A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet.. 2016;25(1):107-123.
- [Google Scholar]
- I. Arslan-Alaton and . T. Olmez-Hanci, “Sources of Environmental Pollution: Persistent Organic Pollutants,” New and Future Developments in Catalysis , pp. 303-398, 2013.
- Atmospheric deposition of polycyclic aromatic hydrocarbons near New England coastal waters. Atmos. Environ.. 2001;35(36):6245-6258.
- [Google Scholar]
- Fugacity approach to evaluate the sediment–water diffusion of polycyclic aromatic hydrocarbons. J. Environ. Monit.. 2011;37(7):1589.
- [Google Scholar]
- A review of processes involved in the exchange of persistent organic pollutants across the air–sea interface. Environ. Pollut.. 1998;102(1):3-23.
- [Google Scholar]
- Distribution of PAHs in the water column, sediments and biota of Potter Cove, South Shetland Islands, Antarctica. Antarct. Sci.. 2009;21(04):329.
- [Google Scholar]
- Levels of Polycyclic Aromatic Hydrocarbons in the Water and Sediment of Buffalo River Estuary, South Africa and Their Health Risk Assessment. Arch. Environ. Contam. Toxicol.. 2019;76(4):657-669.
- [Google Scholar]
- Role of environmental factors and microorganisms in determining the fate of polycyclic aromatic hydrocarbons in the marine environment. FEMS Microbiol. Rev.. 2016;40(6):814-830.
- [Google Scholar]
- Influence of sediment parameters on the distribution and fate of PAHs in an estuarine tropical region located in the Brazilian semi-arid (Jaguaribe River, Ceará coast) Mar. Pollut. Bull.. 2019;146:703-710.
- [Google Scholar]
- V. Jaspers , D. Megson and G. O’Sullivan, “POPs in the Terrestrial Environment,” Environmental Forensics for Persistent Organic Pollutants, pp. 291-356, 2014.
- S. Gitipour, G. A. Sorial, S. Ghasemi and M. Bazyari, “Treatment technologies for PAH-contaminated sites: a critical review,” Environmental Monitoring and Assessment, vol. 190, no. 9, 2018.
- T. Zhang , G. Lowry , N. Capiro, J. Chen , W. Chen , Y. Chen, D. D. Dionysiou , D. W. Elliott , S. Ghoshal , T. Hofmann , T. Hofmann, H. Hsu-Kim, J. Hughes, . C. Jiang, G. Jiang , C. Jing, M. Kavanaugh, Q. Li ,, . S. Liu, J. Ma , B. Pan , T. Phenrat, X. Qu, . X. Quan, N. Saleh, . P. . J. Vikesland , Q. Wang , P. Westerhoff, . M. S. Wong , T. Xia , B. Xing , B. Yan , L. Zhang, D. Zhou and P. J. J. Alvarez , “In situ remediation of subsurface contamination: opportunities and challenges for nanotechnology and advanced materials,” Environmental Science: Nano, vol. 6, no. 5, pp. 1283-1302, 2019.
- Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett.. 2018;17(1):145-155.
- [Google Scholar]
- Thermal Treatment of Hydrocarbon-Impacted Soils: A Review of Technology Innovation for Sustainable Remediation. Engineering. 2016;2(4):426-437.
- [Google Scholar]
- Development and evaluation of a continuous microwave processing system for hydrocarbon removal from solids. Chem. Eng. J.. 2016;283:215-222.
- [Google Scholar]
- Microwave remediation of hydrocarbon-contaminated soils – Scale-up using batch reactors. Sep. Purif. Technol.. 2012;96:12-19.
- [Google Scholar]
- P. P. Falciglia and F. G. Vagliasindi, “microwave heating-mediated remediation of hydrocarbon-polluted soils: Theoretical background and techno-economic considerations,” Enhancing cleanup of Environmental Pollutants, pp. 75-95, 2017.
- Microwave (MW) remediation of hydrocarbon contaminated soil using spent graphite – An approach for waste as a resource. J. Environ. Manage.. 2019;230:151-158.
- [Google Scholar]
- Radio Frequency heating for oil recovery and soil remediation. IFAC-PapersOnLine. 2015;48(8):1198-1203.
- [Google Scholar]
- Microwave heating remediation of soils contaminated with diesel fuel. J. Soils Sediments. 2013;13(8):1396-1407.
- [Google Scholar]
- Effective Sediment Treatment of Urban Sewage by Microwave Radiation. Mater. Sci. Forum. 2016;871:223-232.
- [Google Scholar]
- Application of Advanced Oxidation Processes for the Treatment of Recalcitrant Agro-Industrial Wastewater: A Review. Water. 2019;11(2):205.
- [Google Scholar]
- Removal of polycyclic aromatic hydrocarbons in aqueous environment by chemical treatments: a review. Sci. Total Environ.. 2014;478:201-225.
- [Google Scholar]
- Removal of PAHs from leachate using a combination of chemical precipitation and Fenton and ozone oxidation. Water Sci. Technol.. 2018;78(5):1064-1070.
- [Google Scholar]
- Removing PAHs from urban runoff water by combining ozonation and carbon nano-onions. Chemosphere. 2015;141:265-273.
- [Google Scholar]
- Photolysis of polycyclic aromatic hydrocarbons in water. Water Res.. 2001;35(1):233-243.
- [Google Scholar]
- T. Zhang , Y. Liu , S. Zhong and . L. Zhang, “AOPs-based remediation of petroleum hydrocarbons-contaminated soils: Efficiency, influencing factors and environmental impacts,” Chemosphere , vol. 246, p. 125726, 2020.
- Removal of polycyclic aromatic hydrocarbons (PAH) during anaerobic digestion with recirculation of ozonated digested sludge. J. Hazard. Mater.. 2009;162(2–3):1145-1150.
- [Google Scholar]
- L. Jacobs , L. Weavers and . Y.-P. Chin, “DIRECT AND INDIRECT PHOTOLYSIS OF POLYCYCLIC AROMATIC HYDROCARBONS IN NITRATE-RICH SURFACE WATERS,” Environmental Toxicology and Chemistry, vol. 27, no. 8, p. 1643, 2018.
- Direct photolysis of polycyclic aromatic hydrocarbons in drinking water sources. J. Hazard. Mater.. 2011;192(3):1458-1465.
- [Google Scholar]
- E. M. Cuerda-Correa, M. f. Alexandre-Franco and C. Fernández-González, “Advanced Oxidation Processes for the Removal of Antibiotics from Water. An Overview,” Water, vol. 12, no. 1, p. 102, 2019.
- Techniques for water disinfection, decontamination and desalinization: a review. DESALINATION AND WATER TREATMENT. 2020;181:47-63.
- [Google Scholar]
- Advanced oxidation processes for water treatment: advances and trends for R&D. J. Chem. Technol. Biotechnol.. 2008;83(6):769-776.
- [Google Scholar]
- Ozonation of drinking water: Part I. Oxidation kinetics and product formation. Water Res.. 2003;37(7):1443-1467.
- [Google Scholar]
- Recent advances in enhanced photocatalytic activity of bismuth oxyhalides for efficient photocatalysis of organic pollutants in water: A review. J. Ind. Eng. Chem.. 2019;20:1-78.
- [Google Scholar]
- Perspective and status of polymeric graphitic carbon nitride based Z-scheme photocatalytic systems for sustainable photocatalytic water purification. Chem. Eng. J.. 2020;391:123496
- [Google Scholar]
- Sonu, V. Dutta, S. Sharma, P. Raizada, A. Hosseini-Bandegharaei, V. K. Gupta and P. Singh, “Review on augmentation in photocatalytic activity of CoFe2O4 via heterojunction formation for photocatalysis of organic pollutants in water,” Journal of Saudi Chemical Society, vol. 23, no. 8, pp. 1119-1136, 2019.
- A. Kumar, P. Raizada, A. Hosseini-Bandegharaei, V. K. Thakur, V.-H. Nguyen and P. Singh, “C-, N-Vacancy defect engineered polymeric carbon nitride towards photocatalysis: viewpoints and challlenges,” Journal of Materials Chemistry A, vol. 9, no. 1, pp. 111-153, 2021.
- Step-scheme heterojunction photocatalysts for solar energy, water splitting, CO2 conversion, and bacterial inactivation: A review. Environ. Chem. Lett.. 2021;19(4):2941-2966.
- [Google Scholar]
- Integrated photocatalytic advanced oxidation system (TiO2/UV/O3/H2O2) for degradation of volatile organic compounds. Sep. Purif. Technol.. 2019;224:1-14.
- [Google Scholar]
- Produced water treatment by advanced oxidation processes. Sci. Total Environ.. 2019;666:12-21.
- [Google Scholar]
- Photocatalytic ozonation of offshore produced water by TiO2 nanotube arrays coupled with UV-LED irradiation. J. Hazard. Mater.. 2021;402:123456
- [Google Scholar]
- On the influence of the microwaves’ thermal and non-thermal effects in titania photoassisted reactions. Catal. Today. 2014;224:225-235.
- [Google Scholar]
- Microwaves in advanced oxidation processes for environmental applications. A brief review. J. Photochem. Photobiol., C. 2010;11(2–3):114-131.
- [Google Scholar]
- Electrokinetic remediation strategy considering ground strate: A review. Geoscience Journal. 2002;6(1):57-75.
- [Google Scholar]
- Electrokinetic remediation of PAH mixtures from kaolin. J. Hazard. Mater.. 2018;179(1–3):1156-1160.
- [Google Scholar]
- Combining electrokinetic transport and bioremediation for enhanced removal of crude oil from contaminated marine sediments: Results of a long-term, mesocosm-scale experiment. Water Res.. 2019;157:381-395.
- [Google Scholar]
- V. Russo, M. Hmoudah, F. Broccoli, M. R. Iesce, O.-S. Jung and D. S. Martino, “Applications of Metal Organic Frameworks in Wastewater Treatment: A Review on Adsorption and Photodegradation,” Frontiers in Chemical Engineering, vol. 2, 2020.
- Adsorption as a Process for Produced Water Treatment: A Review. Processes. 2020;8(12):1657.
- [Google Scholar]
- Adsorption isotherms, kinetics and mechanism for the adsorption of cationic and anionic dyes onto carbonaceous particles prepared from Juglans regia shell biomass. Int. J. Environ. Sci. Technol.. 2012;10(2):231-242.
- [Google Scholar]
- A review of the kinetics adsorption models and their application to the adsorption of lead by an activated carbon. Chem. Eng. Res. Des.. 2016;109:495-504.
- [Google Scholar]
- Adsorption kinetic modeling using pseudo-first order and pseudo-second order rate laws: A review. Cleaner Engineering and Technology. 2020;1:100032
- [Google Scholar]
- Kinetic and Equilibrium Adsorption Studies of Methylene Blue from Aqueous Solution Using Low-Cost Adsorbent. International Journal of Psychosocial Rehabilitation. 2019;23(4):1722-1738.
- [Google Scholar]
- Adsorption isotherm models: Classification, physical meaning, application and solving method. Chemosphere. 2020;258:127279
- [Google Scholar]
- Adsorption — from theory to practice. Adv. Colloid Interface Sci.. 2001;93(1–3):135-224.
- [Google Scholar]
- Review on the effectiveness of adsorbent materials in oil spills clean up. Sea. 2017;25:36.
- [Google Scholar]
- P. E. Ndimele, A. O. Saba, D. O. Ojo, C. C. Ndimele, M. A. Anetekhai and E. S. Erondu, “Remediation of Crude Oil Spillage,” The Political Ecology of Oil and Gas Activities in the Nigerian Aquatic Ecosystem, pp. 369-384, 2018.
- Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustainable Mater.Technol.. 2016;9:10-40.
- [Google Scholar]
- Adsorption-Oriented Processes Using Conventional and Non-conventional Adsorbents for Wastewater Treatment. Environ. Chem. Sustainable World. 2018;17(1):195-213.
- [Google Scholar]
- Treatment of oil–water emulsions by adsorption onto activated carbon, bentonite and deposited carbon. Egypt. J. Pet.. 2011;20(2):9-15.
- [Google Scholar]
- Hydrocarbon adsorption performance and regeneration stability of diphenyldichlorosilane coated zeolite and its application in permeable reactive barriers: Column studies. Microporous Mesoporous Mater.. 2020;294:109843
- [Google Scholar]
- Dissolved organic matter (DOM) removal from biotreated coking wastewater by chitosan-modified biochar: Adsorption fractions and mechanisms. Bioresour. Technol.. 2020;297:122281
- [Google Scholar]
- Study of STAB- and DDAB-modified sepiolite tructures and their adsorption performance for emulsified oil in produced water. Colloid Interface Sci. Commun.. 2020;34:100231
- [Google Scholar]
- Impact of salinity and dispersed oil on adsorption of dissolved aromatic hydrocarbons by activated carbon and organoclay. J. Hazard. Mater.. 2015;229:562-569.
- [Google Scholar]
- Treatment of the oily produced water (OPW) using coagulant mixtures. Egypt. J. Pet.. 2016;25(3):391-396.
- [Google Scholar]
- Integrated treatment for oil free petroleum produced water using novel resin composite followed by microfiltration. Sep. Purif. Technol.. 2020;234:116058
- [Google Scholar]
- Hydraulic fracturing wastewater treatment by coagulation-adsorption for removal of organic compounds and turbidity. J. Environ. Chem. Eng.. 2016;4(2):1978-1984.
- [Google Scholar]
- Removal of oil from oil-in-saltwater emulsions by adsorption onto nano-alumina functionalized with petroleum vacuum residue. J. Colloid Interface Sci.. 2014;433:58-67.
- [Google Scholar]
- Outstanding adsorption performance of high aspect ratio and super-hydrophobic carbon nanotubes for oil removal. Chemosphere. 2016;164:142-155.
- [Google Scholar]
- K. Björklund and . L. Y. Li, “Adsorption of organic stormwater pollutants onto activated carbon from sewage sludge,” Journal of environmental management, vol. 197, pp. 490-497, 2017.
- A review on multifaceted application of nanoparticles in the field of bioremediation of petroleum hydrocarbons. Ecol. Eng.. 2016;97:98-105.
- [Google Scholar]
- Potential of ceramic microfiltration and ultrafiltration membranes for the treatment of gray water for an effective reuse. Desalin. Water Treat.. 2013;51(22–24):4323-4332.
- [Google Scholar]
- Nanotechnology-based sorption and membrane technologies for the treatment of petroleum-based pollutants in natural ecosystems and wastewater streams. Adv. Colloid Interface Sci.. 2020;257:102071
- [Google Scholar]
- A. Baruah, . V. Chaudhary, R. Malik and V. K. Tomer, “Nanotechnology Based Solutions for Wastewater Treatment,” Nanotechnology in Water and Wastewater Treatment, pp. 337-368, 2019.
- N. Nassar, “The application of nanoparticles for wastewater remediation,” Applications of Nanomaterials for Water Quality, pp. 52-65, 2013.
- Metal–organic frameworks (MOFs) for the removal of emerging contaminants from aquatic environments. Coord. Chem. Rev.. 2019;380:330-352.
- [Google Scholar]
- Nanoadsorbents for wastewater treatment: next generation biotechnological solution. Int. J. Environ. Sci. Technol.. 2020;17(9):4095-4132.
- [Google Scholar]
- Nanotechnology in Wastewater Management: A New Paradigm Towards Wastewater Treatment. Molecules. 2021;26(6):1797.
- [Google Scholar]
- Removal of Phenols from Aqueous Solutions by Graphene Oxide Nanosheet Suspensions. J. Nanosci. Nanotechnol.. 2016;16(12):12426-12432.
- [Google Scholar]
- Nanoparticles: Properties, applications and toxicities. Arabian J. Chem.. 2019;12(7):908-931.
- [Google Scholar]
- Nanoadsorbents for water and wastewater remediation. Sci. Total Environ.. 2020;739:139903
- [Google Scholar]
- H. Sadegh, G. A. Ali, V. K. Gupta, A. S. Makhlouf, R. Shahryari-ghoshekandi, M. N. Nadagouda, M. Sillanpää and . E. Megiel, “The role of nanomaterials as effective adsorbents and their applications in wastewater treatment,” Journal of Nanostructure in Chemistry, vol. 7, no. 1, pp. 1-14, 2017.
- T. Ahamad, M. Naushad, Ruksana, A. N. Alhabarah and S. M. Alshehri, “N/S doped highly porous magnetic carbon aerogel derived from sugarcane bagasse cellulose for the removal of bisphenol–A,” International Journal of Biological Macromolecules, vol. 132, pp. 1031-1038, 2019.
- Preparation of chitosan based magnetic nanocomposite for tetracycline adsorption: Kinetic and thermodynamic studies. Int. J. Biol. Macromol.. 2020;147:258-267.
- [Google Scholar]
- A. A. Al-Kahtani, S. M. Alshehri, M. Naushad, Ruksana and T. Ahamad, “Fabrication of highly porous N/S doped carbon embedded with ZnS as highly efficient photocatalyst for degradation of bisphenol,” International Journal of Biological Macromolecules, vol. 121, pp. 415-423, 2019.
- Defective UiO-67 for enhanced adsorption of dimethyl phthalate and phthalic acid. J. Mol. Liq.. 2021;321:114477
- [Google Scholar]
- E. coli@UiO-67 composites as a recyclable adsorbent for bisphenol A removal. Chemosphere. 2021;270:128672
- [Google Scholar]
- A Review of Removal of Pollutants from Water/Wastewater Using Different Types of Nanomaterials. Adv. Mater. Sci. Eng.. 2014;2014:1-24.
- [Google Scholar]
- ecent advances in carbon nanomaterial-based adsorbents for water purification. Coord. Chem. Rev.. 2020;405:213111
- [Google Scholar]
- M. Namdeo, “Magnetite Nanoparticles as Effective Adsorbent for Water Purification: A Review,” Advances in Recycling & Waste Management, vol. 02, no. 03, 2018.
- “Recent advances on iron oxide magnetic nanoparticles as sorbents of organic pollutants in water and wastewater treatment,” nano. Online 2018
- [Google Scholar]
- Innovations in nanotechnology for water treatment. Nanotechnology, Science and Applications. 2015;8:1.
- [Google Scholar]
- Simultaneously efficient adsorption and photocatalytic degradation of tetracycline by Fe-based MOFs. J. Colloid Interface Sci.. 2018;519:273-284.
- [Google Scholar]
- Advances in metal–organic framework coatings: versatile synthesis and broad applications. Chem. Soc. Rev.. 2020;49(10):3142-3186.
- [Google Scholar]
- Controllable Synthesis of Metal-Organic Frameworks and Their Hierarchical Assemblies. Matter. 2019;1(4):801-824.
- [Google Scholar]
- Progress in adsorption-based CO 2 capture by metal–organic frameworks. Chem. Soc. Rev.. 2012;41(6):2308-2322.
- [Google Scholar]
- Water purification by using adsorbents: a review. Environ. Technol. Innovation. 2018;11:187-240.
- [Google Scholar]
- Removal of Hexavalent Chromium-Contaminated Water and Wastewater: A Review. Water Air Soil Pollut.. 2008;200(1–4):59-77.
- [Google Scholar]
- Metal-organic frameworks as adsorbents for sequestering organic pollutants from wastewater. Mater. Chem. Phys.. 2020;253:123246
- [Google Scholar]
- Adsorption of organic compounds from aqueous solution by pyridine-2-carboxaldehyde grafted MIL-101(Cr)-NH2 metal-organic frameworks. J. Environ. Chem. Eng.. 2021;9(4):105275
- [Google Scholar]
- Z. Li, Y. n. Wu, J. Li, Y. Zhang, X. Zou and F. Li, “The Metal-Organic Framework MIL-53(Al) Constructed from Multiple Metal Sources: Alumina, Aluminum Hydroxide, and Boehmite,” Chemistry - A European Journal, vol. 21, no. 18, pp. 6913-6920, 2015.
- N. A. khan, B. K. Jung, Z. Hasan and S. H. Jhung, “Adsorption and removal of phthalic acid and diethyl phthalate from water with zeolitic imidazolate and metal–organic frameworks,” Journal of Hazardous Materials, vol. 282, pp. 194-200, 2015.
- Adsorptive removal of phenol from aqueous solution with zeolitic imidazolate framework-67. J. Environ. Manage.. 2016;169:167-173.
- [Google Scholar]
- MOF derived porous carbon–Fe3O4 nanocomposite as a high performance, recyclable environmental superadsorbent. J. Mater. Chem.. 2012;22(37):19694.
- [Google Scholar]
- A review of integrated photocatalyst adsorbents for wastewater treatment. J. Environ. Chem. Eng.. 2018;6(6):7411-7425.
- [Google Scholar]
- R. C. Pawar and C. S. Lee, “Nanomaterial-based photocatalysis,” Heterogeneous Nanocomposite-Photocatalysis for Water Purification, pp. 25-41, 2015.
- Superparamagnetic iron oxide as Photocatalyst and adsorbent in wastewater treatment – A review. Micro and Nanosystems. 2020;12(1):4-22.
- [Google Scholar]
- Recent advances in photocatalytic multivariate metal organic frameworks-based nanostructures toward renewable energy and the removal of environmental pollutants. Mater. Today Energy. 2021;19:100589
- [Google Scholar]
- Covalent organic frameworks promoted single metal atom catalysis: Strategies and applications. Coord. Chem. Rev.. 2022;452:214298
- [Google Scholar]
- Potential utility of graphene-based nano spinel ferrites as adsorbent and photocatalyst for removing organic/inorganic contaminants from aqueous solutions: A mini review. Chemosphere. 2019;221:392-402.
- [Google Scholar]
- Polycyclic Aromatic Hydrocarbons: A Critical Review of Environmental Occurrence and Bioremediation. Environ. Manage.. 2017;60(4):758-783.
- [Google Scholar]
- Principles of microbial degradation of petroleum hydrocarbons in the environment. The Egyptian Journal of Aquatic Research. 2018;44(2):71-76.
- [Google Scholar]
- Sakshi , S. K. Singh and A. K. Haritash, “Evolutionary Relationship of Polycyclic Aromatic Hydrocarbons Degrading Bacteria with Strains Isolated from Petroleum Contaminated Soil Based on 16S rRNA Diversity,” Polycyclic Aromatic Compounds, pp. 1-14, 2020.
- Biodegradation aspects of Polycyclic Aromatic Hydrocarbons (PAHs): A review. J. Hazard. Mater.. 2009;169(1–3):1-15.
- [Google Scholar]
- Biotransformation, Biodegradation, and Bioremediation of Polycyclic Aromatic Hydrocarbons. Soil Biology 2004:83-124.
- [Google Scholar]
- Bacterial degradation of aromatic pollutants: a paradigm of metabolic versatility. International Microbiology. 2004;7:173-180.
- [Google Scholar]
- Anaerobic Microbial Degradation of Polycyclic Aromatic Hydrocarbons: A Comprehensive Review. Rev. Environ. Contam. Toxicol. 2019:25-108.
- [Google Scholar]
- Future challenges in diesel biodegradation by bacteria isolates: A review. J. Cleaner Prod.. 2020;251:119716
- [Google Scholar]
- A new look on factors affecting microbial degradation of petroleum hydrocarbon pollutants. Int. Biodeterior. Biodegrad.. 2017;120:71-83.
- [Google Scholar]
- Biosurfactant—Enhanced bioremediation of hydrophobic pollutants. J. Biotechnol.. 2008;136:S678.
- [Google Scholar]
- Plasmid-Mediated Bioaugmentation for the Bioremediation of Contaminated Soils. Front. Microbiol.. 2017;8
- [Google Scholar]
- Effect of synthetic surfactants on the environment and the potential for substitution by biosurfactants. Adv. Colloid Interface Sci.. 2021;288:102340
- [Google Scholar]
- Sustainable strategy for the enhancement of hazardous aromatic amine degradation using lipopeptide biosurfactant isolated from Brevibacterium casei. J. Hazard. Mater.. 2021;408:124943
- [Google Scholar]
- D. Borah, A. Chaubey, A. Sonowal, B. Gogoi and R. Kumar, “Microbial Biosurfactants and Their Potential Applications: An Overview,” Environmental and Microbial Biotechnology, pp. 91-116, 2021.
- A review on biosurfactants: properties, applications and current developments. Bioresour. Technol.. 2021;330:124963
- [Google Scholar]
- Rhamnolipids fromPseudomonas aeruginosa strain W10; as antibiofilm/antibiofouling products for metal protection. J. Basic Microbiol.. 2017;57(5):364-375.
- [Google Scholar]
- Antimicrobial activity of biosurfactant derived from Bacillus subtilis C19. Energy Procedia. 2018;153:274-278.
- [Google Scholar]
- Production and potential biotechnological applications of microbial surfactants: An overview. Saudi Journal of Biological Sciences. 2021;28(1):669-679.
- [Google Scholar]
- Biosurfactants producing bacteria from oil-polluted soil in Abeokuta, Ogun State. Ife Journal of Science. 2018;20(2):287-297.
- [Google Scholar]
- Microbial biosurfactants: current trends and applications in agricultural and biomedical industries. J. Appl. Microbiol.. 2019;127(1):12-28.
- [Google Scholar]
- Production of biosurfactant by Bacillus subtilis RSL-2 isolated from sludge and biosurfactant mediated degradation of oil. Bioresour. Technol.. 2020;307:123261
- [Google Scholar]
- Biosurfactant production and engine oil degradation by marine halotolerant Bacillus licheniformis LRK1. Biocatalysis and Agricultural Biotechnology. 2020;29:101808
- [Google Scholar]
- Investigation of antifungal activity of surfactin against mycotoxigenic phytopathogenic fungus Fusarium moniliforme and its impact in seed germination and mycotoxicosis. Pestic. Biochem. Physiol.. 2019;155:101-107.
- [Google Scholar]
- Oil washing proficiency of biosurfactant produced by isolated Bacillus tequilensis MK 729017 from Assam reservoir soil. J. Petrol. Sci. Eng.. 2020;195:107612
- [Google Scholar]
- Production, functional stability, and effect of rhamnolipid biosurfactant from Klebsiella sp. on phenanthrene degradation in various medium systems. Ecotoxicol. Environ. Saf.. 2021;207:111514
- [Google Scholar]
- Oil recovery from refinery oily sludge using a rhamnolipid biosurfactant-producing Pseudomonas. Bioresour. Technol.. 2012;116:24-28.
- [Google Scholar]
- Study on emulsification of crude oil in water using emulsan biosurfactant for pipeline transportation. Pet. Sci. Technol.. 2016;34(3):216-222.
- [Google Scholar]
- Characterization of trehalose lipids produced by a unique environmental isolate bacterium Rhodococcus qingshengii strain FF. J. Appl. Microbiol.. 2019;127(5):1442-1453.
- [Google Scholar]
- Bioemulsifiers are not biosurfactants and require different screening approaches. Front. Microbiol.. 2015;6:245.
- [Google Scholar]
- Assessing the efficiency and eco-sustainability of bioremediation strategies for the reclamation of highly contaminated marine sediments. Marine Environmental Research. 2020;162:105101
- [Google Scholar]
- Biosurfactants in Improving Bioremediation Effectiveness in Environmental Contamination by Hydrocarbons. Microbial Action on Hydrocarbons 2018:21-34.
- [Google Scholar]
- T. Langenbach, “Persistence and Bioaccumulation of Persistent Organic Pollutants (POPs),” Applied Bioremediation - Active and Passive Approaches, 2013.







