Translate this page into:
Rhamnus pallasii subsp. sintenisii fruit, leaf, bark and root: Phytochemical profiles and biological activities
⁎Corresponding author. akramtaleghani@yahoo.com (Akram Taleghani) akramtaleghani@gonbad.ac.ir (Akram Taleghani)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
The genus Rhamnus has received a lot of interest as a source of phenolic chemicals. There have been no reports on the phytochemicals and biological activities of R. pallasii subsp. sintenisii various morphological components (fruit, leaf, bark, and root) in Iran to yet. Two crude ether petroleum (EP) and hydro-methanolic (HM) extracts were obtained from the separate parts. The antioxidant and antibacterial capabilities of the extracts, as well as their phytochemical screening (total phenolic, flavonoid, phenolic acid, and anthocyanin concentrations), were measured. Furthermore, the phytochemical profiles of EP and HM extracts were determined using GC–MS and LC-ESI–MS, respectively. LC-ESI-MS detected 59 chemicals in HM extracts, including flavonoids (62.71 %), phenolic acids (10.16 %), and anthraquinones (16.94 %). Furthermore, the predominant group components in EP extracts examined by GC–MS were fatty acids (58.82%), phenolic compounds (49.28%), and hydrocarbons (35.15 to 59.45 %). In terms of biological testing (DPPH radical scavenging and anti-bacterial activity), all examined extracts, particularly the fruit, had the highest activities in both assays (IC50: 7.52 to 22.39 µg/ml and MIC: 0.39 to 3.12 mg/ml), owing to their high phenolic content. As a result, individual morphological elements of the species might be thought of as natural antioxidant and antibacterial agents.
Keywords
Rhamnus pallasii subsp. sintenisii
Phenolic profiles
HPLC-ESI-MS
GC–MS
Antioxidant activity
Anti-bacterial activity
1 Introduction
Rhamnus is a genus known as buckthorns in the Rhamnaceae family, with over 150 recognised species of small trees or shrubs. Species (deciduous and evergreen) range in height from 1 to 10 m and are endemic to East Asia and North America. Leaves with serrate margins that are 3–15 cm long and grouped in opposing pairs or subopposite. The branches terminate in a woody spine. Fruits are berry-like, red or black, 2–4 stoned, and globose in shape. Male and female yellowish green flowers are on distinct plants. The seeds are oblong in shape and have a long, narrow furrow. Rhamnus pallasii subsp. sintenisii (Rech. f.) Browicz &. J. Zielinski is a spiny shrub native to Iran and Turkey that can grow to a height of 3 m. The leaves are simple, alternately arranged in opposite pairs, and smaller than those of other Rhamnus species (Akkemik et al., 2014).
Plants of the Rhamnus genus have been used in traditional medicine as antioxidants, radical scavengers, anti-inflammatory agents, and for the treatment of liver disorders, constipation, and laxatives (Zeouk and Bekhti, 2020; Nigussie et al., 2021; Nekkaa et al., 2021). Furthermore, various chemicals have been found in plants related to the Rhamnus genus, including quercetin, rhamnetin, kaempferol, kaempferide, rhamnazin, anthrones, isorhamnetin, rhamnocitrin, and naphthaline derivatives (Cuoco et al., 2014; Nigussie et al., 2021; Rocchetti et al., 2019). Sakushima et al. extracted a dihydroflavonol, 2,3-dihydromyricetin-4/-O-methyl ether, as well as seven recognized flavonoids from the bark of Turkish R. pallasii in 1983: kaempferol, quercetin, isorhamnetin, mearnsetin, aromadendrin, eriodictyol, and taxifolin (Sakushima et al., 1983). Coşkun et al. isolated an anthraquinone glycoside known as physcion-8-O-β-primeveroside and a naphthalide known as α-sorinin from the bark of the same sources later in 1984 (Coşkun et al., 1984). There have been no reports on the chemical profile or biological effects of R. pallasii to date. The objectives of this study were to characterize the phytochemical profile of R. pallasii extracts prepared with EP and HM (80%) solvents using gas chromatography–mass spectrometry (GC–MS) and liquid chromatography–electrospray ionization mass spectrometry (LC-ESI-MS), as well as to determine the antioxidant and anti-bacterial activities of these extracts, which had never been done before. In herbal medicine, LC-MS and GC–MS are sensitive technologies for identifying and profiling multi-components.
2 Material and methods
2.1 Plant material
R. pallasii subsp. sintenisii fruits, leaves, barks, and roots were collected in August 2020 from the Chakhmaqlu mountains of North Khorasan province, Iran (37°29′34′′N 56°56′52′′E) (Fig. 1). A voucher specimen (803893) has been deposited in the Gonbad Kavous University herbarium. The individual portions were dried at 30 °C in a well-ventilated room and stored in the dark until use.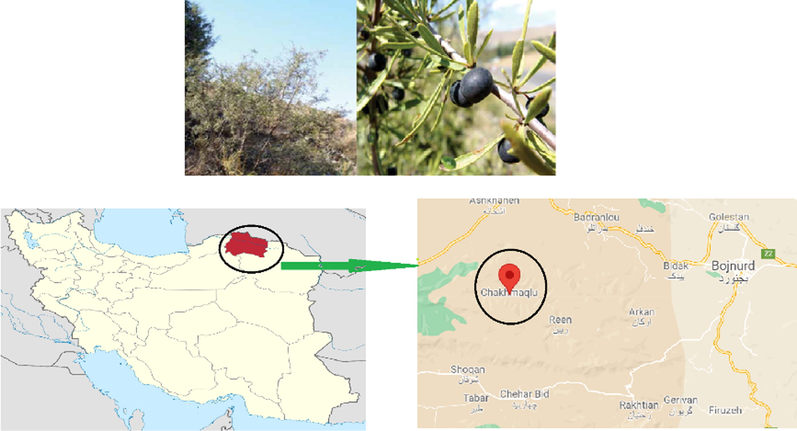
Rhamnus pallasii subsp. sintenisii and map showing the location of the sampling (Chakhmaqlu altitudes, North Khorasan, Iran).
2.2 Chemicals and reagents
Caffeic acid, gallic acid, quercetin, cyanidin-3-glucoside, butylated hydroxytoluene (BHT), sodium hydroxide, hydrochloric acid, sodium molybdate, sodium carbonate, sodium acetate, aluminum chloride, potassium acetate, potassium chloride, Folin-Ciocalteu reagent, 1,1-diphenyl-2-picrylhydrazyl (DPPH), ether petroleum, methanol and formic acid were purchased from Sigma Aldrich (USA). The other compounds that were employed were of analytical grade. Aqueous solutions were also prepared using deionized water. Microorganism cultures were obtained from Iranian microbial collections, Pasteur Institute of Iran. The cultures of Gram-positive Staphylococcus aureus (ATCC 9144) and Gram-negative Escherichia coli (ATCC 25922) were used for the study.
2.3 Preparation of the extracts
The dried powder of species' fruits, leaves, barks, and roots (2 g) were extracted separately with 20 ml of EP at room temperature for 24 h (three times), and residues were extracted with water-methanol under the same conditions (80%). All of these extracts were filtered using a vacuum pump, and the organic solvents were extracted using a rotary evaporator at 40 °C under decreased pressure. Finally, concentrated extracts were lyophilized to dryness in a freeze dryer and stored in darkness at +4 °C for further analysis. The extraction yields (w/w) for EP extracts ranged from 1.2% to 3.5% and 2.9% to 6.3% for HM extracts.
2.4 Quantification of total phenolic content
The total phenolic content (TPC) of HM extracts from species' fruits, leaves, bark, and roots was evaluated using the Folin-Ciocalteu spectrophotometric method described by Singleton et al. (1999), with minor modifications (Singleton et al., 1999). In brief, 200 µL of diluted extracts were combined with 0.25 M Folin–Ciocalteu reagent, 600 µL of H2O, and 1000 µL of 1.0 M Na2CO3. The absorbance of the solutions was measured at 760 nm after 1 h of incubation at room temperature in the dark. The findings were reported in milligrams of gallic acid equivalents (GAE) per gram of dried extract (mg GAE/g DE).
2.5 Quantification of total flavonoid content
The total flavonoid content (TFC) of all HM extracts was measured using the aluminum chloride colorimetric method described previously (Zhishen et al., 1999), with quercetin standard. In brief, diluted extracts or quercetin standard solutions were combined with 720 µL of distilled water, 90 µL of 5% NaNO2, 600 µL of NaOH, and 90 µL of AlCl3. The absorbance of reaction mixtures was measured at 510 nm after incubation at room temperature, and the TFC was reported as milligram of quercetin equivalents per gram of dry extract (mg QE/g E).
2.6 Quantification of total phenolic acid content
The total phenolic acid content (including hydroxycinnamic acid derivatives) was assessed using the Matkowski et al. (2008) method for determining the interaction of phenolic acids with sodium nitrite-sodium molybdate. Each extract (1 ml) was combined with 2 ml HCl (0.5 M), 2 ml Arnow reagent (10 g sodium molybdate and 10 g sodium nitrite diluted to 100 ml with deionized water), 2 ml NaOH (8.5 % w/v), and 3 ml water. The solutions were compared to a control mixture that did not contain Arnow reagent. The absorbance at 490 nm was measured, and the total hydroxycinnamic acid concentration was estimated using a caffeic acid calibration curve and represented as mg caffeic acid equivalent (CAE) per gram of dried extract (mg CAE/g DE).
2.7 Quantification of anthocyanin content
The anthocyanin content of all HM extracts was measured using differential pH methods (Camelo-Méndez et al., 2013), two diluted solutions were prepared, one in 0.4 M sodium acetate buffer with a pH of 4.5; and the other in 0.025 M potassium chloride buffer with a pH of 1.0. At 510 and 700 nm, the absorbance was determined using a spectrophotometer. The absorbance was calculated as follows: A = (A510 − A700) pH1 − (A510 − A700) pH4.5.
The content of anthocyanins was determined using the absorbance of (A) and the molar absorptivity of cyanidin 3-glucoside (29,600). The TAC values were calculated as mg cyanidin-3-glucoside per gram dry extract. TAC = (A/e × L) × (449.2) × D/G × V × 100.
Where A is absorbance; e (26,900) is the molar extinction coefficient, of cyanidin 3-glucoside (Giusti and Jing, 2008); L (1 cm) is the cell length; 449.2 is anthocyanins molecular weight; D is dilution factor; V (ml) is final volume and G (mg) is the dry weight (dw) of samples.
2.8 DPPH radical-scavenging activity
The DPPH radical scavenging activities of HM extracts was monitored according to the method of Cavin et al. (1998). Five different concentrations of each extract were added to 915 μL methanol, then 200 μL DPPH solution in methanol (0.022%) were added. After 30 min incubation at room temperature in the dark, the reaction mixture's absorbance was measured at 517 nm. The absorbance of extracts was compared to that of methanol without DPPH as a blank.
DPPH radical-scavenging activity was determined by: % Inhibition rate = (A control − A sample)/A control × 100.
The effective concentration necessary to inhibit 50% of the DPPH radicals was expressed by IC50 value (half maximal inhibitory concentration).
2.9 Antibacterial activity
The antibacterial activity of R. pallasii extracts against Gram-positive Staphylococcus aureus (ATCC 9144) and Gram-negative Escherichia coli (ATCC 25922) was tested using the microdilution broth technique as described by Suffredini et al. (2006) with certain modifications. Strains were obtained from Iranian microbial collections, Pasteur Institute of Iran. Two-fold serial dilutions of the extracts of fruit, leaf, bark and root were prepared in Mueller Hinton Broth ranging from 25 to 0.195 mg/ml in 96-wells plates. Additionally, gentamicin discs and each fraction's solvents were employed as positive and negative controls, respectively. After 24 h of incubation, the plates inhibitory effect on bacteria growth was determined visually by examining the growth in each well. After incubation, the MIC value was determined as the lowest concentration of plates at which microorganisms displayed no observable growth. Additionally, the MBC value was calculated using the lowest concentration of plates that exhibited no bacterial growth. Each microorganism was subjected to three independent analyses.
2.10 HPLC-ESI-MS analysis
The HPLC-ESI-MS analysis was implemented by using the Waters Alliance 2695 HPLC system, which was connected with micro mass quattro micro API mass spectrometer with electrospray ion source (ESI). A standard solution containing uracil, 4-hydroxymethyl benzoate, 4-hydroxy ethyl benzoate and benzophenone was injected into the device in order to validate the reliability of the system.
2.10.1 HPLC analysis
All crude HM extracts (1 mg) were diluted in 1 ml methanol and filtered through a 0.45 m Millipore filter before being injected into the HPLC. HPLC separations were carried out on a Zorbax SB-C18 column (3, 2.1100 mm) using a gradient mobile phase composed of acetonitrile + 0.1 % formic acid (solvent A) and H2O + 0.1 % formic acid (solvent B). Gradient elution was performed as follows: 0–2 min, 10% A; 2–10 min, 10–50 % A; 10–16 min, 50% A; 16–20 min, 50–90% A; 20–24 min, 90% A; 24–26 min, 90–10 % A; 26–30 min, 10% A. The injection volume was set to 10 L and the column temperature to 35 °C. 0.2 ml/min flow rate was used.
2.10.2 ESI-MS analysis
Electrospray ionization (ESI, negative mode) was used to generate the ions, with nitrogen serving as the cone and desolvation gas. The following parameters were used in the spray chamber: capillary voltage, 3.5 kV; cone voltage, 25 V; extractor voltage, 2 V; collision energy, 30 eV; source temperature, 120C; desolvation temperature, 300C; gas flow, 200 L/h; and nebulizer pressure, 15 psi. Acquisitions of full scans were made in the 150–2000 m/z range. The extracted ion chromatograms (EIC) from total ion chromatograms were used to examine the samples (TIC). The MZmine analysis software program, version 2.3, was used to process the data.
2.11 GC–MS analysis
The GC–MS analyses were performed using an Agilent 6890 gas chromatograph linked to a 5973 MSD mass spectrometer and an HP-5 ms column with a 30 m 0.25 mm i.d. and 0.25 m film thickness. On the basis of Wiley 7n.L and NIST libraries, the chemical profiles of fruit, leaf, bark, and root were identified. Separation of the compounds occurred at a rate of 3°Celsius per minute along a temperature gradient extending from 50 to 280 °C. The instrumentation used a 250 °C analyzer and ion source, a split ratio of 1:20, a 1 μL injection volume, a 70 eV ionization potential, a helium carrier gas flowing at 1.0 ml/min, and a mass range of 50–550 m/z. The components were identified by comparing their mass spectra to those in the Wiley 7.0 mass spectral library and the literature (Adams, 2007). This procedure is similar to that described by Faizi et al., but with minor variations (Faizi et al., 2014).
2.12 Statistical analysis
Each test was conducted in triplicate. Results were expressed as a mean ± standard deviation. SPSS statistics version 20 software and ANOVA procedures were used for statistical analysis. A significance level of 0.05 was considered.
3 Result and discussion
The phytochemical composition of Rhamnus species varies greatly. It is well established that genetic (species, organ, and developmental stage) and environmental diversity contribute significantly to the nutritional quality and phytochemical content of plants. To our knowledge, no investigations on the phytochemical screening, chemical profile by LC-MS, GC–MS, or biological activity of R. pallasii have been published.
3.1 Total phenolics (TPC), total flavonoids (TFC), total phenolic acids (TFAC) and total anthocyanin contents (TAC)
Fig. 2a illustrates the phytochemical analysis of R. pallasii fruit, leaf, bark, and root extracts. The extracts contained a total of 69.8 1.4 to 232.8 2.5 mg GAE/g DE. The fruit extract had the greatest TPC concentration (232.8 1.5 mg GAE/g), followed by the leaf (208.6 2.3 mg GAE/g), the bark (124.3 1.8 mg GAE/g), and the root (69.8 1.4 mg GAE/g) extracts, respectively. The TPC of a methanolic extract of R. alaternus examined in the literature (Moussi et al., 2015). The leaves contained 77.8 mg GAE/g TPC, which was lower than the value observed in our investigation. Another study determined the TPC of a 60% ethanol extract of R. prinoides stems to be 228.21 ± 13 mg of GAE/g (Chen et al., 2020). Additionally, the TPC of R. lycioides leaves was 259.33 ± 4.95 mg of GAE/g, which was greater than the value found in our study (Benamar et al., 2019). A similar result was achieved when species total flavonoids content was determined. However, the fruit extract (187.03 ± 2.09 mg QE/g DE) had a higher TFC value than the leaf (98.6 ± 2.5 mg QE/g), bark (83.3 ± 1.2 mg QE/g), and root (46.8 ± 2.4 mg QE/g) extracts. The TFC values calculated in this study for the leaves extract were higher than those previously reported for R. alaternus collected in Algeria (30.11 ± 5.76 mg QE/g) (Moussi et al., 2015), but were lower than those previously reported for R. alaternus collected in Tunisia (283 ± 11 mg QE/g) (Ammar et al., 2007). In previous research, the TFC of methanol extracts of R. kurdica and R. lycioides leaves was determined to be 86.32 ± 2.98 mg catechin equivalent per mg plant and 74.08 ± 2.10 mg catechin equivalent per g dry extract, respectively (Gholivand and Piryaei, 2014; Benamar et al., 2019). Additionally, we determined the total anthocyanin concentration of fruit, leaf, bark, and root extracts (Fig. 2a). The fruit (75.14 ± 0.03 mg cyanidin 3-glucoside/g DE) and bark (51.76 ± 0.02 mg/g) extracts had the highest TAC values, followed by the leaf and root extracts at 42.21 ± 1.02 and 12.41 ± 2.3 mg/g, respectively. Gholivand discovered that R. kurdica flowers and leaves have a significant concentration of anthocyanin (21.53 ± 0.57 and 12.36 ± 0.84 g/100 mg fw, respectively) (Gholivand and Piryaei, 2014). The anthocyanin content of extracts varied considerably. These distinctions are related to the diversity of chemicals that make up plant pigments. Finally, the extracts total phenolic acid content (TPAC) was determined (25.01 ± 1.3 to 63.14 ± 2.2 mg CAE/g DE). The fruit extract (63.14 ± 2.2 mg/g) had the highest TPAC value, followed by the leaf (52.4 ± 1.5 mg/g), bark (41.3 ± 2.2 mg/g), and root (25.01 ± 1.3 mg/g) extracts, respectively.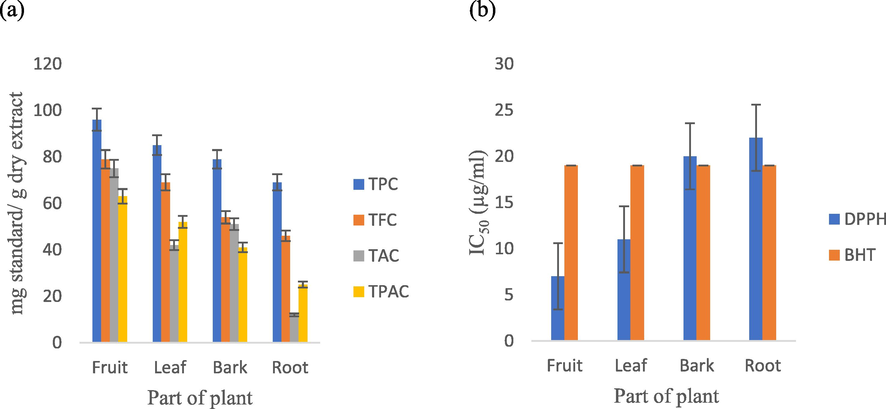
Total phenolics, total flavonoids, total anthocyanins and total phenolic acids contents (a) and antioxidant activities (b) of the HM extracts of R. pallasii.
3.2 Antioxidant activity
For the first time, the antioxidant activities of several parts of R. pallasii were determined using the DPPH radical scavenging assay, and the results were reported as IC50 values (Fig. 2b). Among the R. pallasii extracts, the fruit and leaf extracts displayed the highest scavenging activity, with IC50 values of 7.52 ± 2.1 and 11.81 ± 1.06 g/ml, respectively, which are significantly more active than the positive control butylated hydroxytoluene (BHT) (IC50 = 19.3 ± 1.06 g/ml). The bark and root extracts had the lowest IC50 values, at 20.01 ± 2.5 and 22.39 ± 0.10 g/ml, respectively. The extracts free radical scavenging activity may be a result of their high TPC and TFC content, which have hydrogen-donating capabilities (Rice-Evans et al., 1997). Only a few publications in the literature have discussed the DPPH assay in relation to other species. Our values are lower than those previously reported for other species, including methanolic leaf extract of R. kurdica (IC50 of 21.04 ± 1.35 g/ml), 60 % ethanolic stem extract of R. prinoides (IC50 of 51.21% 0.046 g/ml), and methanolic leaf extract of R. lycioides (IC50 of 29.69 ± 0.33 g/ml) (Gholivand and Piryaei, 2014; Chen et al., 2020; Benamar et al., 2019).
3.3 Antibacterial activity
Table 1 summarizes the antimicrobial activity of EP and HM extracts of R. pallasii fruit, leaf, bark, and root. The extracts were antimicrobial against both microorganisms that cause food poisoning (Staphylococcus aureus and Escherichia coli). The results indicated that the extracts had significantly more antibacterial action against S. aureus than against E. coli, owing to the bacterial strains different cell wall structures. Gram-negative bacteria have an outer membrane composed of lipids and a polysaccharide component that serves as a barrier to antimicrobial drug penetration (Lambert, 2002). When compared to gentamicin, the antibacterial effects of the HM extract of fruit and EP extract of root were much stronger than those of other extracts (positive control). The extracts had MIC and MBC values of 0.39 to 3.12 and 0.78 to 6.25 mg/ml respectively. No investigations on the antibacterial activity of R. pallasii have been conducted to date, however various studies have reported on the antimicrobial activity of other species. Molla demonstrated that methanol and chloroform leaf extracts of R. prinoides were bactericidal against four bacterial strains with MIC values ranging from 8.13 to 32.5 mg/ml (Molla et al., 2016). EP and methanolic extracts of R. alaternus had no detectable inhibitory action on gram-negative and gram-positive bacteria until 6 mg/ml (Ben Ammar et al., 2007). Another study found that the MIC and MBC values of R. prinoides fruit and leaf extracts against S. aureus and E. coli were 1.3 to 5.23 and 2.08 to 8.33 g/ml, respectively (Kibret, 2019). Carranza et al. discovered that methanolic extracts of R. californica leaves had MIC values of 5.0 to 6.0 mg/ml against six bacterial species (Carranza et al., 2015). The results of this study suggest that the significant antibacterial activity of the EP and HM extracts of R. pallasii may be attributed to the presence of terpenes, phenols, and flavonoids, which act as antimicrobial agents via a variety of different mechanisms (Guimarães et al., 2019).
Plant part
S. aureus
E.coli
MIC
MBC
MIC
MBC
EP
HM
EP
HM
EP
HM
EP
HM
Fruit
3.12 ± 2.13
0.39 ± 1.32
6.25 ± 2.14
0.78 ± 3.16
3.12 ± 1.42
0.39 ± 2.94
3.12 ± 2.92
1.56 ± 3.12
Leaf
0.78 ± 1.09
1.56 ± 1.45
0.78 ± 1.65
3.12 ± 3.52
0.78 ± 1.24
0.78 ± 2.31
1.56 ± 2.81
0.78 ± 2.34
Bark
3.12 ± 3.12
1.56 ± 4.12
3.12 ± 2.34
3.12 ± 2.17
1.56 ± 2.41
0.78 ± 1.49
1.56 ± 1.56
3.12 ± 1.93
Root
0.39 ± 1.21
3.12 ± 3.16
0.78 ± 1.56
3.12 ± 2.41
0.78 ± 1.56
3.12 ± 1.11
1.56 ± 2.86
6.25 ± 3.32
Gentamicin
2.32 ± 1.49 (µg/ml)
32.12 ± 2.12 (µg/ml)
16.31 ± 1.29 (µg/ml)
128.21 ± 3.12 (µg/ml)
3.4 GC–MS analysis
GC–MS analysis was used to determine the chemical composition of an EP extract of R. pallasii (fruit, leaf, bark, and root). The extract yields were 3.5 % (fruit), 1.2 % (leaf), 3.1 % (bark), and 2.8 % (stem) based on the plant's dry weight. The chemicals that have been identified are listed in Table 2. A total of 68 chemicals were isolated from EP extracts of various plant sections. Our findings indicate that fruit extract contains 26 compounds that account for 93.62 % of its composition; leaf extract contains 29 compounds that account for 92.97 % of its composition; bark extract contains 38 compounds that account for 96.97 % of its composition; and root extract contains 28 compounds that account for 89.92 % of its composition. Each extract contained the following major compounds (in percentages): fruit (oleic acid 45.93 %, stigmastan-3,5-diene 9.76 %, -tocopherol 6.60 %); leaf (-tocopherol 44.28 %, hentriacontane 8.93 %, clionasterol 7.56 %); bark (clionasterol 12.99 %, -tocopherol 11.89 %, tridecane 6.43 %); and root (-tocop Generally, the extracts are a good source of biological components. Terpenes, phenolics, fatty acids, fatty esters, steroids, and hydrocarbons are all significant types of chemicals found in R. pallasii extracts (Table 2). A review of the literature indicated that no data on GC–MS studies of R. pallasii extracts were given, while data on the volatile components of other species were reported (Chouitah et al., 2012; Mekala et al., 2017).
Compounds
Molecular
FormulaClassification
RT (min)
Percentage %
Fruit
Leaf
Bark
Root
Fruit
Leaf
Bark
Root
2-Methylheptan
C8H18
Aliphatic hydrocarbon
–
3.553
–
–
–
0.321%
–
–
3-Methylheptan
C8H18
Aliphatic hydrocarbon
–
3.696
–
–
–
0.328%
–
–
Octane
C8H18
Aliphatic hydrocarbon
–
4.208
–
–
–
0.206%
–
–
cis-1,2-Dimethyl cyclohexane
C8H16
Cycloalkane
–
4.871
–
–
–
0.084%
–
–
2-Ethylhexanol
C8H18O
Fatty Alcohols
–
4.999
–
–
–
0.086%
–
–
4-Methyloctane
C9H20
Aliphatic hydrocarbon
–
5.744
–
–
–
0.066%
–
–
3-Methyloctane
C9H20
Aliphatic hydrocarbon
–
5.947
–
–
–
0.058%
–
–
Nonane
C9H20
Aliphatic hydrocarbon
–
6.722
6.723
–
–
0.133%
0.167%
–
2,6,7-Trimethyldecane
C13H28
Aliphatic hydrocarbon
–
–
7.739
–
–
–
0.199%
–
3-Ethyl-2-methylheptane
C10H22
Aliphatic hydrocarbon
–
–
7.942
–
–
–
0.170%
–
1,1,2,3-Tetramethylcyclohexane
C10H20
Cycloalkane
–
–
8.364
–
–
–
0.196%
–
4-Methylnonane
C10H22
Aliphatic hydrocarbon
–
–
8.567
–
–
–
0.305%
–
2-Methylnonane
C10H22
Aliphatic hydrocarbon
–
–
8.650
–
–
–
0.221%
–
2,6-Dimethyloctane
C10H22
Aliphatic hydrocarbon
–
–
8.846
–
–
–
0.428%
–
1-Methyl-2-propylcyclohexane
C10H20
Cycloalkane
–
–
9.290
–
–
–
0.302%
–
Decane
C10H22
Aliphatic hydrocarbon
9.812
9.756
9.749
9.794
0.904%
0.441%
1.630%
0.220%
4-Methyldecane
C11H24
Aliphatic hydrocarbon
10.504
10.449
–
–
0.384%
0.192%
–
–
2-Methyldecane
C11H24
Aliphatic hydrocarbon
–
–
–
11.781
–
–
–
0.194%
Undecane
C11H24
Aliphatic hydrocarbon
12.928
12.835
12.858
12.888
1.621%
0.627%
3.282%
1.043%
Cycloundecene,1-methyl
C12H22
Cycloalken
–
–
–
15.778
–
–
–
0.679%
Naphthalene,decahydro-1,6-dimethyl
C12H22
Polycyclic hydrocarbon
15.857
–
15.944
16.305
1.096%
–
1.783%
2.621%
2,6-Dimethyldecalin
C12H22
Polycyclic hydrocarbon
16.007
–
–
16.004
0.748%
–
–
1.202%
Naphtalene,decahydro-2,3-dimethyl
C12H22
Polycyclic hydrocarbon
16.203
16.531
15.824
16.591
1.036%
0.684%
2.600%
3.108%
Cycloheptanon,2-(-2-methyl propylidene
C11H18O
Cyclic ketone
16.293
–
–
–
0.438%
–
–
–
4,8-decadien-3-ol,5,9dimethyl
C12H22O
Alcoholic compound
16.519
–
–
–
1.788%
–
–
–
Decahydro-1,2-dimethylnaphthalene
C12H22
Polycyclic hydrocarbon
–
–
16.562
–
–
–
3.799%
–
Naphtalene,decahydro-1,5-dimethyl
C12H22
Polycyclic hydrocarbon
16.760
16.689
16.712
16.749
2.656%
1.159%
1.630%
5.099%
Tridecane
C13H28
Aliphatic hydrocarbon
18.702
18.624
16.639
18.699
0.609%
0.299%
6.439%
2.861%
2-Methyltridecane
C14H30
Aliphatic hydrocarbon
–
–
–
20.393
–
–
–
0.825%
2,6,10-Trimethyltetradecane
C17H36
Aliphatic hydrocarbon
–
–
–
20.732
–
–
–
0.902%
farnesane
C15H32
Sesquiterpene
–
–
20.657
20.739
–
–
1.016%
1.469%
Tetradecane
C14H30
Aliphatic hydrocarbon
21.344
21.281
21.312
21.379
1.078%
0.572%
2.975%
2.006%
Pentadecane
C15H32
Aliphatic hydrocarbon
23.844
–
23.781
23.856
0.268%
–
0.778%
7.068%
Hexadecane
C16H34
Aliphatic hydrocarbon
26.230
–
26.144
26.242
0.381%
–
2.244%
1.948%
Neoisolongifolene,8-bromo
C15H23Br
Sesquiterpene
–
–
27.018
–
–
–
0.875%
–
Banzoic acid,4heptyl-4-cyanophenyl ester
C21H23NO2
Benzoic
acid derivatives27.103
–
–
27.108
0.298%
–
–
3.445%
Banzan,1,3,5tris(1-methyl propyl)
C18H30
Benzene derivatives
27.540
–
27.492
27.559
0.350%
–
1.190%
2.013%
Paullinic acid
C20H38O2
Fatty acid
–
–
28.538
28.591
–
–
1.596%
2.648%
Octadecane
C18H38
Aliphatic hydrocarbon
30.603
–
30.571
30.638
0.418%
–
3.026%
2.289%
Phytol
C20H40O
Diterpene
–
36.720
31.354
–
–
2.122%
0.934%
4.247%
Methyl palmitate
C17H34O2
Fatty acid aster
33.216
–
–
–
1.213%
–
–
–
palmitic acid
C16H32O2
Fatty acid
34.367
34.093
34.011
–
2.756%
2.921%
2.611%
–
Eicosane
C20H42
Aliphatic hydrocarbon
–
–
34.560
34.628
–
–
3.847%
4.730%
2-Methyl-1-hexadecanol
C17H36O
Alcoholic compound
–
–
36.141
–
–
–
1.386%
–
Methyl oleate
C19H36O2
Fatty acid ester
36.588
–
–
–
3.373%
–
–
–
Ethyl linoleate
C20H36O2
Fatty acid ester
–
–
37.278
–
–
–
4.054%
–
Oleic acid
C18H34O2
Fatty acid
38.252
37.466
–
–
45.934%
4.394%
–
–
Docosane
C22H4
Aliphatic hydrocarbon
–
–
38.204
–
–
–
3.752%
–
Acetyl tributyl citrate
C20H34O8
Fatty acid aster
–
39.423
39.416
–
–
1.323%
1.968%
–
Bis(2-ethylhexyl) adipate
C22H42O4
Fatty acid aster
–
41.591
–
–
–
1.637%
–
–
Octadecane,3-ethyl-5-(2-ethyl butyl)
C26H54
Aliphatic hydrocarbon
43.265
–
47.576
–
1.804%
–
3.611%
–
17-pentatriacontene
C35H70
Aliphatic hydrocarbon
–
–
48.118
41.629
–
–
1.664%
8.621%
Tribehenin
C69H134O6
Fatty acid aster
–
–
–
44.730
–
–
–
5.098%
Heptacosane
C27H56
Aliphatic hydrocarbon
–
46.108
41.591
38.294
–
1.143%
5.901%
4.461%
Erucamide
C22H43NO
Fatty amid
47.533
–
–
47.455
3.549%
–
–
7.418%
Squalene
C30H50
Triterpene
–
47.997
–
–
–
2.072%
–
–
Nonacosane
C29H60
Aliphatic hydrocarbon
49.084
48.976
44.678
–
6.516%
4.725%
4.192%
–
d-Allo-dec-2-enonic acid,5,8-anhydro2,3,4,9-tetradeoxy-8-c-(hydroxymethyl)3-methyl-7,8-O-(1-methylethylid)
C18H28O8
Carbohydrate derivatives
–
49.721
–
–
–
3.506%
–
–
Tetratetracontane
C44H90
Aliphatic hydrocarbon
–
–
50.406
–
–
–
3.125%
–
Hentriacontane
C31H64
Aliphatic hydrocarbon
–
52.205
–
–
–
8.934%
–
–
Stigmast-5-en-3-Ol,oleate
C47H82O2
Triterpenoids
–
–
–
52.295
–
–
–
7.356%
Stigmastan-3,5-diene
C29H48
Sterols
52.351
–
–
–
9.764%
–
–
α-Tocopherol (vitaminE)
C29H50O2
Tocopherol(Phenolics)
53.179
53.146
52.958
53.071
6.602%
44.282%
11.897%
10.084%
7,8-Epoxylanostan-11-ol, 3-acetoxy-
C32H54O4
Triterpenoid
55.437
–
51.099
–
1.416%
–
1.217%
–
Tritetracontane
C43H88
Aliphatic hydrocarbon
–
56.609
–
–
–
1.540%
–
–
Clionasterol
C29H50O
Sterol(Phytosterol)
–
57.264
57.302
–
–
7.561%
12.990%
–
β-sitosterol
C29H50O
Sterol(Phytosterol)
–
–
–
57.399
–
–
–
6.342%
α -Amyrin
C30H50O
Triterpenoid
–
59.371
–
–
–
3.583%
–
–
Major Grouped Compounds
Fruits
Leaves
Barks
Roots
Terpenes
1.416%
7.777%
4.042%
13.072%
Phenolics
6.602%
44.282%
11.897%
10.084%
Fatty acids,Fatty acid asters and fatty amides
54.825%
10.275%
9.229%
14.164%
Steroids
9.764%
7.561%
12.990%
6.342%
Hydrocarbons
18.51%
19.5%
57.45%
42.87%
Miscellaneous
2.524%
3.592%
1.386%
3.445%
Total Identified%
93.62%
92.97%
96.97%
89.92%
3.5 LC-ESI-MS analysis
The profile of bioactive chemicals in HM extracts of R. pallasii fruits, leaves, barks, and roots was published in this work for the first time using an LC-ESI-MS method in the negative ion mode. All extracts included 59 chemicals, including 24 flavonols, 6 flavones, 4 flavanones, 3 flavanonols, 6 phenolic acids, 10 anthraquinones, 3 naphthaenic lactone derivatives, 2 naphthalene derivatives, and 1 coumarin derivative (Table 3). Fig. 3A-H illustrates the total ion chromatogram (TIC) of extracts and instances of extracted ion chromatograms (EIC). Peaks were identified using molecular weights, retention times (Rt), complete ESI-MS, and matching mass adducts ([M−H]-, [2 M], [2 M−H], [M−2H], and [M−2H + Na]), as well as comparisons to published data. Only ten of the 59 compounds had been identified previously in R. pallasii, and they were all kaempferol, quercetin, isorhamnetin, mearnsetin, aromadendrin, taxifolin, eriodictyol, pallasiin, -sorinin, and physclon-8-O- β-primeveroside from the barks of Turkish species and leaves of Georgian species (Sakushima et al., 1983; Coşkun et al., 1984). There are no data on the phytochemical profiles of other components of this plant to our knowledge.
No.
Compounds
Formula
[M−H]−(m/z)
Rt/ Intensity (En)
Parts reported in literature1
Ref.
Fruit
Root
Bark
Leaf
1
Quercetin
C15H10O7
301
19.1/9.9E4
18.9/3.8E5
–
18.5/8.7E5
F, L⁎, B⁎
(Sakushima et al., 1983; Chen et al., 2016; Ammar et al., 2009; Moussi et al., 2015)
2
Quercetin-3-O- glucoside (isoquercitrin)
C21H20O12
463
16.1/1.5E5
16.2/1.4E5
16.1/1.8E5
16.5/6.2E5
B
(Chen et al., 2016)
3
Quercetin-7-O-glucoside
C21H20O12
463
–
16.7/6.8E4
–
16.5/4.7E5
AP
(Marzouk et al., 1999)
4
Quercetin-3-O-robinobioside
C27H30O16
609
9.1/1.1E5
8.7/9.6E4
9.2/1.8E5
8.3/5.5E5
F
(Marzouk et al., 1999)
5
Quercetin-3-rhamninoside
C33H40O20
755
3.5/5.7E4
3.9/6.9E6
3.6/1.1E5
3.1/6.4E5
F
(Marzouk et al., 1999)
6
Quercetin-3-methyl ether-7-O-glucoside
C22H22O12
477
10.8/9.2E4
10.7/1E5
11/2.1E5
–
AP
(Marzouk et al., 1999)
7
Kaempferol
C15H10O6
285
20.9/2.1E5
21.3/4.4E5
21.5/4.7E5
21.2/5.4E5
F, L⁎, B⁎, AP
(Chen et al., 2016; Ammar et al., 2009; Moussi et al., 2015)
8
Kaempferol-7-O-glucoside
C21H20O11
447
17.5/1.4E5
17.7/1.9E5
17.7/1.7E5
17.5/1.4E5
B
(Chen et al., 2016)
9
Kaempferol-3-O-acetyl-rhamninoside
C35H42O20
781
8.5/1.8E5
8.6/1.1E5
8.6/1.3E5
8.4/4.6E4
F
(Cuoco, Mathe, and Vieillescazes, 2014)
10
Kaempferol-3-O-robinoside
C27H30O15
593
10.2/8.2E4
10.5/2.5E5
10/1.6E5
10.7/5.9E4
F
(Marzouk et al., 1999)
11
Kaempferol-3-O-rhamninoside
C33H40O19
739
7.5/2E5
–
6.9/7.5E4
7.3/6.8E5
F, AP
(Marzouk et al., 1999)
12
Kaempferol-4′-O-rhamninoside
C33H40O19
739
7.9/9.1E4
–
8.2/1.4E5
8.4/6.4E5
F
(Ammar et al., 2009)
13
Rhamnetin
C16H12O7
315
19.8/5.7E4
20.1/1.6E5
–
19.4/4.8E5
F, L, AP
(Cuoco, Mathe, and Vieillescazes, 2014; Marzouk et al., 1999; Ammar et al., 2009)
14
Isorhamnetin
C16H12O7
315
20.3/8.9E4
–
–
19.8/3.7E5
F, L⁎, B⁎, AP
(Cuoco, Mathe, and Vieillescazes, 2014; Sakushima et al., 1983; Marzouk et al., 1999)
15
Isorhamnetin-3-O-rhamninoside
C33H40O19
769
4.5/2.7E5
4.5/5.3E5
–
5.1/8.3E5
F
(Marzouk et al., 1999)
16
Rhamnetin-3-O-rhamninoside
C34H42O20
769
4.3/1.3E5
4.1/1.3E5
–
4.6/1.3E6
F, L, AP
(Marzouk et al., 1999; Benamar et al., 2019)
17
Rhamnazin
C17H14O7
329
21.5/1.1E5
20.9/9E4
21.3/3.2E5
20.6/8.5E4
F, L, AP
(Cuoco, Mathe, and Vieillescazes, 2014; Ammar et al., 2009)
18
Rhamnazin-3-O-acetyl- rhamninosid
C37H46O21
825
5.6/2.2E5
5.5/1.4E5
6.2/7.3E4
6.1/1.1E5
F
(Cuoco, Mathe, and Vieillescazes, 2014)
19
Rhamnazin-3-O-robinoside
C29H34O16
637
10.1/1.8E5
9.6/9.8E4
9.8/6.3E4
9.6/6.5E4
AP
(Marzouk et al., 1999)
20
Rhamnocitrin
C16H12O6
299
21.8/1.9E5
22.1/5.3E5
–
22.5/3.6E5
F, L, B, AP
(Nindi et al., 1999)
21
Rhamnocitrin-3-O-acetyl- rhamninoside
C36H44O20
795
5.6/7.7E4
4.9/9E4
–
5.2/5.4E4
F
(Cuoco, Mathe, and Vieillescazes, 2014)
22
Rhamnocitrin-4′-O-rhamninoside
C34H42O19
753
–
–
–
5.3/1.8E5
F
(Ammar et al., 2009)
23
Rhamnocitrin-3-O-rhamninoside
C34H42O19
753
5.7/7.8E4
5.2/1E5
5.2/1.1E5
5.1/5E5
F, AP
(Ammar et al., 2009)
24
Mearnsetin
C16H12O8
331
18.1/1.5E5
18.3/1.8E5
18/1.9E5
17.8/1.8E5
B⁎
(Sakushima et al., 1983)
25
Luteolin
C15H10O6
285
–
21.3/3.8E5
22.1/3.6E5
21.9/5.3E5
L, B
(Chen et al., 2016; Moussi et al., 2015; Benamar et al., 2019)
26
Apigenin
C15H10O5
269
23.9/1.4E5
24.1/1.1E6
24.5/1.5E6
24.1/3.3E6
L, B
(Chen et al., 2016; Ammar et al., 2009; Moussi et al., 2015)
27
Orientin
C21H20O11
447
15.5/2.2E5
15.7/7.6E4
–
15.3/5.8E4
B
(Chen et al., 2016)
28
Isoorientin
C21H20O11
447
15.6/1.9E5
16/1.3E5
–
–
B
(Chen et al., 2016)
29
Vitexin
C21H20O10
431
17.2/8.8E4
17.3/1.1E5
17.5/1.3E5
–
B
(Chen et al., 2016)
30
Diosmetin-7-O-glucoside
C22H22O11
461
18/6.8E4
18.1/2.7E5
17.9/1.4E5
18.3/7.8E4
B
(Chen et al., 2016)
31
Eriodictyol
C15H12O6
287
22.8/1.1E5
23.1/1.9E5
23.5/4.4E5
23.1/7.1E4
L, B⁎, AP
(Sakushima et al., 1983; Marzouk et al., 1999; Benamar et al., 2019)
32
Naringenin
C15H12O5
271
24.1/9.8E4
–
24.3/5.1E5
24.5/2.8E5
B
(Chen et al., 2016)
33
Sakuranetin
C16H14O5
285
–
25.2/2.2E5
25.3/2.7E5
25.5/5.1E5
B
(Chen et al., 2016)
34
Sakuranetin dimer
C32H26O10
551
24.9/5.7E4
24.8/6.5E4
25.3/3.2E5
25.2/ 3.0E4
B
(Chen et al., 2016)
35
Aromadendrin
C15H12O6
287
–
23.3/1.7E5
–
23.5/5.9E4
B
(Sakushima et al., 1983; Chen et al., 2016)
36
Taxifolin
C15H12O7
303
19.3/1.4E5
–
19.0/1.0E5
–
L, B⁎, AP
(Sakushima et al., 1983; Chen et al., 2016; Benamar et al., 2019)
37
Pallasiin
C16H14O8
333
18.7/1.1E5
19/1.1E5
–
18.6/9.8E4
B⁎
(Sakushima et al., 1983)
38
Protocatechuic acid
C7H6O4
153
1.8/1.5E5
1.7/1.9E5
1.5/1.6E5
1.6/1.1E5
F, AP
(Marzouk et al., 1999; SATAKE et al., 1993)
39
p-hydroxybenzoic acid
C7H6O3
137
2.1/2E5
2.6/2E5
2.4/3.2E5
–
F
(SATAKE et al., 1993)
40
2–5-dihydroxybenzoic acid
C7H6O4
153
–
–
2.1/2.6E5
2.2/7.4E4
AP
(Marzouk et al., 1999)
41
Gallic acid
C7H6O5
169
1.8/1.6E5
1.6/2.3E5
1.5/3.1E5
–
L
(Ammar et al., 2009; Moussi et al., 2015)
42
ferulic acid
C10H10O4
193
–
2.1/1.3E5
2.4/2.5E5
–
L
(Ammar et al., 2009; Moussi et al., 2015)
43
p-Coumaric acid
C9H8O3
163
3.4/1.4E5
3.5/1.5E5
–
–
L
(Ammar et al., 2009; Moussi et al., 2015)
44
Physclon-8-O-β-primeveroside
C27H30O14
697
8.4/9.8E4
8.5/5.3E4
8.2/9.1E4
8.1/1.2E5
B⁎
(Coşkun et al., 1984)
45
Emodin
C15H10O5
269
20.1/1.1E5
20.6/7.2E4
20.4/1.3E6
20.9/2.5E6
F, L, B
(Benamar et al., 2019; Nindi et al., 1999; SATAKE et al., 1993)
46
Chrysophanol
C15H10O4
253
28.1/1E5
–
28.3/1.6E5
–
L
(Benamar et al., 2019; Nindi et al., 1999)
47
Physcion
C16H12O5
283
28.2/8.1E4
–
–
–
F, L, B
(Benamar et al., 2019; Nindi et al., 1999)
48
Physcion-8-O-glucoside
C22H22O10
445
16.7/1.2E5
16.8/1.2E5
16.3/9.2E4
16.1/6.6E5
B
(Chen et al., 2016)
49
Physcion-8-O-rutinoside
C22H22O10
591
13.1/1.1E5
–
–
12.8/6.9E4
B
(Chen et al., 2016)
50
Emodin-1-glucoside
C21H20O10
431
–
–
13.8/2.2E5
13.1/5.3E4
F, B
(SATAKE et al., 1993)
51
Emodin anthrone
C15H12O4
255
26.3/7.9E4
–
26.1/1.6E5
26.2/9.3E4
F, L
(Benamar et al., 2019)
52
Emodin bianthrone
C30H22O8
509
–
26.2/1.4E5
25.8/1.8E5
25.6/4E5
F
(Bezabih and Abegaz, 1998)
53
Prinoidin
C25H26O10
485
14/7.8E4
13.8/2.4E5
14.1/6.5E4
13.9/5.6E4
F
54
Sorigenin
C12H8O4
215
–
27.9/3.5E6
28.1/8.9E4
–
L, B⁎
(Nindi et al., 1999)
55
α-sorinin
C24H28O14
539
12.5/8.3E4
12.8/9.2E4
12.3/2.6E5
–
B
(Coşkun et al., 1984)
56
Geshoidin
C18H18O10
377
28.6/8.2E4
29.1/9.4E5
–
28.7/9.5E5
L
(Nindi et al., 1999)
57
Isofraxetin
C10H8O5
207
28.2/1.2E5
28.1/2.5E5
27.9/1.1E6
27.4/1.3E5
AP
(Marzouk et al., 1999)
58
Isotorachrysone
C14H14O4
245
27.9/8.3E4
27.5/1.7E6
–
–
B
(Hsiao et al., 1996)
59
Musizin
C13H12O3
215
–
26.3/2.5E6
–
–
L
(Nindi et al., 1999)
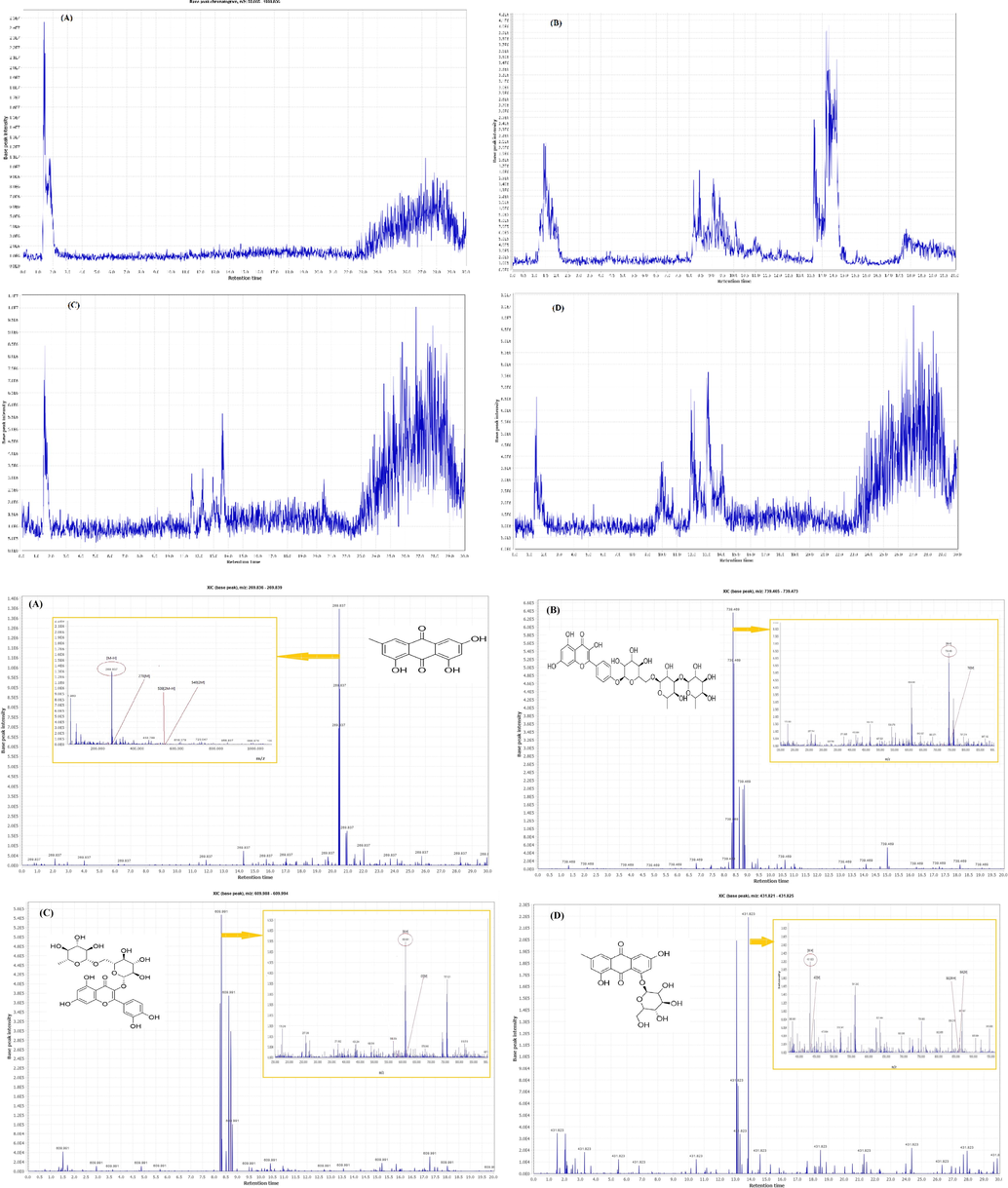
Chromatograms and corresponding mass adducts in the HM extracts of R. pallasii. (A) Total ion chromatogram (TIC) of fruit; (B) TIC of leaf; (C) TIC of bark; (D) TIC of root; (E) Emodin chromatogram (XIC) and mass adducts, m/z 269.837; (F) Kaempferol-4′-O-rhamninoside XIC and mass adducts, m/z 739.469; (G) Quercetin-3-O-robinobios XIC and mass adducts, m/z 609.991; (H) Emodin-1-glucoside XIC and mass adducts, m/z 431.823.
3.5.1 Flavonoids
The most abundant class of chemicals discovered were flavonoids. They are potent antioxidants composed of two phenyl rings and a heterocyclic ring. Plants include a variety of flavonoid classes, including flavones, flavanones, flavonols, and anthocyanins. The flavonoids found in Rhamnus species tested in this study were classified into four classes: flavonols, flavones, flavanones, and flavanonols, as shown in Table 3. Only eight of the 38 flavonoids discovered in R. pallasii have been previously detected: quercetin, kaempferol, isorhamnetin, mearnsetin, aromadendrin, taxifolin, eriodictyol, and pallasiin (Sakushima et al., 1983; Coşkun et al., 1984).
3.5.1.1 Characterization of flavonols
Flavonols were identified as quercetin, kaempferol, isorhamnetin, mearnsetin, rhamnazin, and rhamnocitrin aglycones and their derivatives at C-7 and/or C-3 locations. Compounds 1–6 with [M−H]- ions at m/z 301, 463, 609, 755, and 477 were identified as quercetin, quercetin-3-O-glucoside (isoquercitrin), quercetin-7-O-glucoside, quercetin-3-O-rhamnoside, quercetin-3-O-robinobioside, quercetin-3-rhamnino quercetin-3-methyl ether-7-O-glucoside, respectively, based on the comparison of data obtained with literature findings (Sakushima et al., 1983; Chen et al., 2016; Ammar et al., 2009; Moussi et al., 2015; Marzouk et al., 1999). Additionally, derivatives of kaempferol have been found in R. pallasii and other Rhamnus species. Compounds 7–10 were identified as kaempferol, kaempferol-7-O-glucoside, kaempferol-3-O-acetyl-rhamninoside, and kaempferol-3-O-robinoside, respectively, using [M−H]- ions at m/z 285, 447, 781, and 593. These chemicals have been isolated and identified from the fruit, leaf, and bark of more Rhamnus species, including R. davurica, R. saxatilis, R. disperma, and R. libanoticus (Chen et al., 2016; Ammar et al., 2009; Moussi et al., 2015; Sakushima et al., 1983; Marzouk et al., 1999). The ions found at m/z 739 were identified as kaempferol-O-rhamninoside isomers (kaempferol-3-O-rhamninoside and kaempferol-4′-O-rhamninoside) (Ammar et al., 2009; Marzouk et al., 1999). Two molecules, 13 and 14, were identified as rhamnetin and isorhamnetin at m/z 315. (Sakushima et al., 1983; Cuoco et al., 2014; Marzouk et al., 1999). Additionally, compounds 15 and 16 were identified as isorhamnetin-3-O-rhamninoside and rhamnetin-3-O-rhamninoside, respectively, due to their deprotonated molecules at m/z 769. Previously, these chemicals were discovered in the fruit and leaves of R.catharticus and R.disperma (Marzouk et al., 1999; Benamar et al., 2019). Rhamnazin 17 (m/z 329) and two rhamnazin-O-glycosides 18 (m/z 825) and 19 (m/z 637) were identified as rhamnazin-3-O-acetylrhamninosid and rhamnazin 3-O-D-robinoside, respectively. Compounds from other rhamnus species, including R. saxatilis, R.prinoides, R. alaternus, and R. disperma, were also given (Ammar et al., 2009; Cuoco et al., 2014; Marzouk et al., 1999). A [M−H]- at m/z 299 and 795 was used to identify rhamnocitrin 20 and its derivative, rhamnocitrin-3-O-acetyl-rhamninoside 21. For rhamnocitrin-4′-O-rhamninoside 22 and rhamnocitrin-3-O-rhamninoside 23, an identical pseudomolecular ion peak at m/z 753 was also found (Nindi et al., 1999; Cuoco et al., 2014; Ammar et al., 2009). Similarly, compound 24 was identified as mearnsetin, as previously described for R. pallasii (Sakushima et al., 1983).
3.5.1.2 Characterization of flavones
Eight compounds isolated from various sections of R. pallasii demonstrated flavone structural features. Five of these (25–29) were identified as the flavone aglycones luteolin, apigenin, orientin, isoorientin, and vitexin, respectively, using [M−H] ions at m/z 285, 269, 447, and 431. On the basis of comparisons to published data, one flavone glycoside (30) was identified from extracts as diosmetin-7-O-glucoside (m/z 461). Earlier this year, compounds with a similar pattern were found in R. davurica, R. alaternus, and R. lycioides (Moussi et al., 2015; Chen et al., 2016; Benamar et al., 2019; Ammar et al., 2009).
3.5.1.3 Characterization of flavanones
Four flavanone derivatives (31–34) were identified in the plant extracts with m/z values of 287, 271, 285 and 551 and were identified as eriodictyol, naringenin, sakuranetin, and sakuranetin dimer, respectively. For R. disperma, R. lycioides, and R. davurica, these chemicals were already mentioned in the literature (Sakushima et al., 1983; Marzouk et al., 1999; Benamar et al., 2019; Chen et al., 2016).
3.5.1.4 Characterization of flavanonols
Three flavanonol derivatives were found as previously published by R. davurica, R.disperma, and R.lycioides: aromadendrin 35 (m/z 287), taxifolin 36 (m/z 303), and pallasiin 37 (m/z 333). (Sakushima et al., 1983; Chen et al., 2016; Benamar et al., 2019).
3.5.2 Phenolic acids
Phenolic acids are another class of phenolic chemicals that are utilized to prevent heart disease. Additionally, they have an effect on the bitter and sour flavors of food plants (Rashmi and Negi, 2020). Essentially, hydroxybenzoic and hydroxycinnamic acids are two distinct subclasses of phenolic acids. Procatechuic acid 38 (m/z 153), p-hydroxybenzoic acid 39 (m/z 137), 2–5-dihydroxybenzoic acid 40 (m/z 153), and gallic acid 41 (m/z 169. 231). Ferulic acid 42 (m/z 193) and p-coumaric acid 43 (m/z 163) are hydroxycinnamic acid derivatives. These derivatives of R. thymifolius, R. disperma, and R. alaternus have already been reported in the literature (Marzouk et al., 1999; satake et al., 1993; Ammar et al., 2009; Moussi et al., 2015).
3.5.3 Anthraquinones
Natural pigment derivatives known as anthraquinones or anthracenedione are generated from anthracenes and include two keto groups on the central ring. They exhibit a broad range of biological properties, including antioxidant, antifungal, anticancer, and antibacterial properties (Malik and Müller, 2016). Compounds 44–46 were identified as physclon-8-O-β-primeveroside (m/z 697), aloe-emodin (m/z 269), and chrysophanol (m/z 253), respectively, based on the identical patterns stated previously. Previous works on R.prinoides, R.thymifolius, R.lycioides, and R.libanoticus have discussed the proposed structures (Benamar et al., 2019; Nindi et al., 1999; Satake et al., 1993; Coşkun et al., 1984). A molecule with m/z 283 was identified as physcion 47. This chemical was recently discovered in Rhododendron davurica, R. lycioides, R. prinoides, and R. nakaharai (Benamar et al., 2019; Nindi et al., 1999; Chen et al., 2016). Additionally, two anthraquinone glycosides, physcion 8-O-glucoside 48 (m/z 445) and physcion 8-O-rutinoside 49 (m/z 591), were discovered. Compound 50 was identified as emodin-1-glucoside at m/z 431. Our findings corroborated earlier research (Chen et al., 2016; Satake et al., 1993). Along with the anthraquinones, three anthrone derivatives were found, notably emodin athrone 51 (m/z 255), emodin bianthrone 52 (m/z 509), and prinoidin 53 (m/z 485). To our knowledge, these chemicals have been isolated from R. prinoides, R. lycioides, and R. nepalensis (Mai et al., 2001; Bezabih and Abegaz, 1998).
3.5.4 Other compounds
Three naphthaenic lactone compounds were identified in R. pallasii extracts. Sorigenin 54 possesses a deprotonated molecular ion peak at m/z 215 and was earlier found by R.prinoides (Nindi et al., 1999). In any case, chemicals 55 and 56 have been tentatively identified as α-sorinin (m/z 539) and geshoidin (m/z 377, respectively. These naphthalene glycosides are well-studied in R.prinoides (Coşkun et al., 1984; Nindi et al., 1999). A coumarin derivative was tentatively attributed to one of the compounds 57 with m/z 207. Isofraxetin was the name given to this chemical in the literature (Marzouk et al., 1999). Additionally, two naphthalene derivatives from R. pallasii were identified: isotorachrysone 58 (m/z 245.145) and musizin 59 (m/z 215.145). These chemicals were identified using previously published data on R. davurica, R. nakaharai, and R.prinoides (Chen et al., 2016; Hsiao et al., 1996; Nindi et al., 1999).
3.5.5 Comparison between phenolic compounds in different parts of R. Pallasii
The profile and relative intensity (En) of detected compounds varied according to the plant's morphology, as 49, 45, 41, and 48 compounds with varying intensities were discovered in fruit, leaf, bark, and root extracts, respectively. Flavonols (44.89 % of total phenols, 5.7E4 to 2.7E5) were found in the fruits, followed by anthraquinones (16.32 %, 4.7E4 to 1.2E5), flavones (10.20 %, 6.8E4-2.2E5), phenolic acids (8.16 %, 1.1E5 to 2.0 E5), flavanones (6.12 %, 5.7E4 to 1.1E5), flavanono (8.16 %, 8.2E4 to 1.2E5). In turn, flavonols (53.33 %, 4.6E4 to 1.3E6) were more abundant in leaves than anthraquinones (17.77 %, 4.0E4 to 2.5E6), flavanones (8.88 %, 3.0E4 to 5.1E5), flavones (6.66 %, 7.8E4-3.3E6), flavanonols (4.44 %, 5.9E4 to 9.8E4), phenolic acids (4.44 %, 1.3E5 to 9.5E5). However, flavonols were the most abundant class in the barks (39.02 %, 6.3E4 to 3.6E5), followed by anthraquinones (19.51 %, 6.5E4 to 1.3E6), phenolic acids (12.9 %, 1.6E5 to 3.2E5), flavanones (9.75 %, 2.7E5 to 5.1E5), flavones (9.75 %, 1.3E5-1.5E6), flavanon (12.5 %, 9.2E4 to 3.5E6). Among the phenolic chemicals found in plant components, the most abundant were quercetin-3-rhamninoside (flavonol, 6.9E6), sorigenin (naphthaenic lactone, 3.5E6), apigenin (flavone, 3.3E6), emodin (anthraquinone, 2.5E6), musizin and isotorachrysone (naphthalene derivatives, 1.7–2.5E6) and rhamnetin-3-O-rhamninoside (flavonol, 1.3E6). Overall, all samples, particularly the fruit extract, exhibited a high concentration of flavonoid and phenolic acid components, indicating that they were excellent natural sources of antioxidant and antibacterial agents.
4 Conclusion
This is the only study that we are aware of that examines the phytochemical profile, antioxidant activity, and antibacterial activity of several sections of R. pallasii subsp. sintenisii. LC-ESI-MS and GC–MS analyses were performed on individual morphological parts of species (fruit, leaf, bark, and root) in order to identify the chemicals responsible for their biological activity. The HM extracts of all samples, particularly the fruit and leaf, were high in polyphenols, including flavonols, flavones, flavanones, flavanonols, phenolic acids, and anthraquinones, and shown substantial antioxidant and antibacterial activity. The EP extract of the root, on the other hand, was a rich source of terpenes and had substantial antibacterial activity. These results may be explained by components of quercetin-3-rhamninoside, apigenin, emodin, quercetin, isorhamnetin-3-O-rhamninoside, and orientin discovered in this work. Additionally, the polar and nonpolar extracts of this species may provide valuable natural chemicals for the creation of novel medications. Additional research on the morphological characteristics of species is required to unravel the mechanism of antioxidant and antibacterial activity and to isolate bioactive components from extracts with higher therapeutic effects.
CRediT authorship contribution statement
Soghra Mahmoodi: Investigation, Methodology. Akram Taleghani: Conceptualization, Funding acquisition, Writing – original draft, Software. Reza Akbari: Conceptualization, Investigation, Formal analysis. Majid Mokaber-Esfahani: Investigation, Methodology.
Acknowledgments
The authors gratefully acknowledge the support of this work by the University of Gonbad Kavous Research Council.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Identification of essential oil components by gas chromatography/mass spectrometry. IL: Allured Publishing Corporation Carol Stream; 2007.
- ’Rhamnus L’, Türkiye’nin Doğal-Egzotik Ağaç ve Çalıları II. Ankara: Orman Genel Müdürlüğü Yayınları; 2014. p. :248-271.
- Antioxidant and free radical-scavenging properties of three flavonoids isolated from the leaves of Rhamnus alaternus L. (Rhamnaceae): A structure-activity relationship study. Food chem.. 2009;116:258-264.
- [Google Scholar]
- Transcriptional response of genes involved in cell defense system in human cells stressed by H2O2 and pre-treated with (Tunisian) Rhamnus alaternus extracts: Combination with polyphenolic compounds and classic in vitro assays. Chem. Biol. Interact.. 2007;168:171-183.
- [Google Scholar]
- Antibacterial and cytotoxic activities of extracts from (Tunisian) Rhamnus alaternus (Rhamnaceae) Ann. Microbiol.. 2007;57:453-460.
- [Google Scholar]
- Phytochemical profiles, antioxidant and anti-acetylcholinesterasic activities of the leaf extracts of Rhamnus lycioides subsp. oleoides (L.) Jahand. & Maire in different solvents. Nat. Prod. Res.. 2019;33:1456-1462.
- [Google Scholar]
- Glucofrangulin A diacetate from the fruits of Rhamnus prinoids. Bull. Chem. Soc. Ethiop.. 1998;12:45-48.
- [Google Scholar]
- Comparative study of anthocyanin and volatile compounds content of four varieties of Mexican roselle (Hibiscus sabdariffa L.) by multivariable analysis. Plant Foods Hum. Nutr.. 2013;68:229-234.
- [Google Scholar]
- Antibacterial activity of native California medicinal plant extracts isolated from Rhamnus californica and Umbellularia californica. Ann. clin. Microbiol.. 2015;14:1-6.
- [Google Scholar]
- Antioxidant and lipophilic constituents of Tinospora crispa. Planta medica.. 1998;64:393-396.
- [Google Scholar]
- Antioxidant, anti-inflammatory activities and polyphenol profile of Rhamnus prinoides. Pharmaceuticals.. 2020;13:55.
- [Google Scholar]
- Analysis of flavonoids in Rhamnus davurica and its antiproliferative activities. Molecules.. 2016;21:1275.
- [Google Scholar]
- Analysis of the Chemical Composition and Antimicrobial Activity of the Essential Oil from Rhamnus alaternus. J. Biol. Act. Prod. Nat.. 2012;2:302-306.
- [Google Scholar]
- An anthraquinone Glycoside from Rhamnus pallasii. Phytochemistry.. 1984;23:1485-1487.
- [Google Scholar]
- Liquid chromatographic analysis of flavonol compounds in green fruits of three Rhamnus species used in Stil de grain. Microchem. J.. 2014;115:130-137.
- [Google Scholar]
- GC/GCMS analysis of the petroleum ether and dichloromethane extracts of Moringa oleifera roots. Asian Pac. J. Trop. Biomed.. 2014;4:650-654.
- [Google Scholar]
- Total phenols, flavonoids, anthocyanins, ascorbic acid contents and antioxidant activity of Rhamnus kurdica Boiss for flower and leaves in flowering and pre-flowering stages. Afr. J. Biotechnol.. 2014;13:1131-1135.
- [Google Scholar]
- Giusti, M., Mónica, Jing, P., 2008. 'Analysis of anthocyanins'. Food Colorants: Chemical and Functional Properties. 6, 479-506.
- Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules.. 2019;24:2471.
- [Google Scholar]
- Antioxidant properties of isotorachrysone isolated from Rhamnus nakaharai. Biochim. Biophys. Acta. Proteins.. 1996;1298:119-130.
- [Google Scholar]
- Kibret, M., 2019. 'Antibacterial Activity of Rhamnus prinoides extracts against Staphylococcus aureus and Escherichia coli'. DOI: 10.21203/rs.2.17220/v1.
- Cellular impermeability and uptake of biocides and antibiotics in Gram-positive bacteria and mycobacteria. J. Appl. Microbiol.. 2002;92:46S-54S.
- [Google Scholar]
- Cytotoxicity of Rhamnosylanthraquinones and Rhamnosylanthrones from Rhamnus nepalensis. J. Nat. Prod.. 2001;64:1162-1168.
- [Google Scholar]
- Anthraquinones as pharmacological tools and drugs. Med. Res. Rev.. 2016;36:705-748.
- [Google Scholar]
- Antioxidant activity of herb extracts from five medicinal plants from Lamiaceae, subfamily Lamioideae. J. Med. Plant Res.. 2008;2:321-330.
- [Google Scholar]
- Mekala, A.B., Satyal, P., Setzer, W.N., 2017. 'Phytochemicals from the bark of Rhamnus caroliniana'. Nat. Prod. Commun. 12, 1934578X1701200324.
- Evaluation of the in vitro antibacterial activity of the solvent fractions of the leaves of Rhamnus prinoides L’Herit (Rhamnaceae) against pathogenic bacteria. Complement Altern. Med.. 2016;16:1-9.
- [Google Scholar]
- HPLC-DAD profile of phenolic compounds and antioxidant activity of leaves extract of Rhamnus alaternus L. Ind. Crops Prod.. 2015;74:858-866.
- [Google Scholar]
- Rhamnus alaternus Plant: Extraction of Bioactive Fractions and Evaluation of Their Pharmacological and Phytochemical Properties. Antioxidants.. 2021;10:300.
- [Google Scholar]
- Nigussie, G., Alemu, M., Ibrahim, F., Werede, Y., Tegegn, M., Neway, S. and ANNİSA, M.E., 2021. 'Phytochemistry, Ethnomedicinal Uses and Pharmacological Properties of Rhamnus prinoides: A Review'. Int. J. Second. Metab. 8, 136-51.
- Electrospray liquid chromatography–mass spectrometry of the leaf extract of Rhamnus prinoides. Phytochem. Anal.. 1999;10:69-75.
- [Google Scholar]
- Phenolic acids from vegetables: A review on processing stability and health benefits. Int. Food Res. J.. 2020;136:109298
- [Google Scholar]
- UHPLC-QTOF-MS phytochemical profiling and in vitro biological properties of Rhamnus petiolaris (Rhamnaceae) Ind. Crops. Prod.. 2019;142:111856
- [Google Scholar]
- Satake, T., Hori, K., Kamiya, K., Saiki, Y., Fujimoto, Y., Kimura, Y., Maksut, C., Mekin, T., 1993. 'Studies on the constituents of Turkish Plants. I. Flavonol Triglycosides from the fruit of Rhamnus thymifolius'. Chem. Pharm. Bull. 41, 1743-45.
- Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent' Meth. Enzymol.. 1999;299:152-178.
- [Google Scholar]
- Antibacterial and cytotoxic activity of Brazilian plant extracts-Clusiaceae. Memorias. Do. Instituto. Oswaldo. Cruz.. 2006;101:287-290.
- [Google Scholar]
- A critical overview of the traditional, phytochemical and pharmacological aspects of Rhamnus alaternus: a Mediterranean shrub. Adv. Tradit. Med.. 2020;20:1-11.
- [Google Scholar]
- The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food chem.. 1999;64:555-559.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.103924.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1
Supplementary data 2
Supplementary data 2







