Translate this page into:
Ecofriendly synthesis of silver nanoparticles using Kei-apple (Dovyalis caffra) fruit and their efficacy against cancer cells and clinical pathogenic microorganisms
⁎Corresponding authors. salemsalahsalem@azhar.edu.eg (Salem S. Salem), tabdelghany.201@azhar.edu.eg (T.M. Abdelghany)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Green fabrication has become a safe approach for producing nanoparticles. Plant-based biogenic synthesis of silver nanoparticles (AgNPs) has emerged as a possible alternative to traditional chemical production. In this paper, we provide a low-cost, green synthesis of AgNPs utilizing using Kei-apple (Dovyalis caffra) fruit extract. Ultraviolet–visible (UV–Vis) spectroscopy, Fourier Transform Infrared (FTIR), Transmission Electron Microscopy (TEM), X-Ray Diffraction (XRD), Scanning-Electron Microscope (SEM), and Dynamic Light Scattering (DLS) analyses were used to characterize green produced AgNPs. The formation of AgNPs was shown to have a surface resonance peak of 415 nm in UV–visible spectra, and FTIR spectra verified the participation of biological molecules in Synthesis of AgNPs. The TEM revealed that the biosynthesized AgNPs were mostly spherical in form, with size range of 12–53 nm. XRD diffractogram was used to demonstrate the face cubic centre (fcc) character of AgNPs. Excellent anticancer activity of AgNPs was recorded where more than 80% of Prostate Cancer (PC-3) cell lines was inhibited by 100–150 µg/mL of AgNPs, while 38% only was recorded using AgNO3 and 55.62% was recorded D. caffra fruit extract at 150 µg/mL. Destructions of PC-3 cell was observed as a result of exposed to AgNPs, followed by D. caffra fruit extract, while minor alterations were recorded as exposed to AgNO3. The 2,2-Diphenyl-1-picrylhydrazyl (DPPH) scavenging using AgNPs was three fold using fruit extract at 100 µg/mL indicating good antioxidant activity. Excellent inhibitory activity of AgNPs was recorded against Bacillus subtilis, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Candida albicans and Aspergillus fumigatus with inhibition diameter zone 28.22 ± 0.25 mm, 23.21 ± 0.35 mm, 27.25 ± 0.03 mm, 28.40 ± 0.15 mm, 29.23 ± 0.44 mm, and 9.52 ± 0.5 mm, respectively compared with AgNO3. D. caffra fruits considered a promising and safe source for fabrication of AgNPs with multi-biological functions.
Keywords
Silver nanoparticles
Kei-apple fruit
Anticancer
Antimicrobial activity

1 Introduction
The unique properties of nanoparticles (NPs) have attracted the attention of researchers in various fields (Hashem and Salem, 2021, Salama et al., 2021, Salem and Fouda, 2021). Nanoparticles' unique properties, such as low melting points, optical and magnetic properties, high surface area to volume ratios, and excellent mechanical strength, have been documented in numerous scientific reports, making them useful not only in biological fields like medicine, diagnostics, drug delivery, agriculture, but also in industrial mechanical applications (Burdușel et al., 2018, Ganash et al., 2018, Mathur et al., 2018, Alsharif et al., 2020, Salem et al., 2020, Badawy et al., 2021, Abdelaziz et al., 2022, Salem, 2022). Among NPs, silver nanoparticles (AgNPs) have received some attention due to their unique physicochemical and biological properties (Abdelghany et al., 2018; Salem et al., 2022; Sharaf et al., 2022). The biological synthesis of NPs has several advantages, including reduced production of toxic by-products, increased stability, and reduced toxicity to healthy cells (Hashem et al., 2021). Furthermore, biogenic synthesis is low-cost, allowing for the rapid production of effective NPs (Hammad et al., 2022; Mohamed et al., 2021). Plant extracts, fungus, bacteria, and algae have all been proposed as safe sources for the synthesis of NPs in the prior (Ghany et al., 2013, Mohamed 2013, Shaheen et al., 2021). When compared to chemical procedures, green approaches allow for the synthesis of NPs in a variety of sizes and forms, each with its own set of characteristics (Salem et al., 2023; Salem and Fouda, 2021). Plants are a good and quick source of various NPs (Mittal et al., 2013). However, the world literature comprises very little information about the bio-fabrication of NPs using Dovyalis caffra. D. caffra (Kei-apple) belongs to family Salicaceae (Qanash et al., 2022). There is still a need for a cost-effective, commercially viable, and ecologically friendly method of synthesizing AgNPs from novel plant sources. Despite the fact that various studies have confirmed the production of AgNPs from a variety of plants (Abdelghany et al., 2018). Kei-apple fruit extract was used to investigate the production and cytotoxic effects of Ag, Au, and Au-Ag bimetallic nanoparticles, which exhibited anticancer potential against breast cancer MCF7 cell line (Adeyemi et al., 2019a,b). Synthesis of ZnONPs by D.caffra fruits extract, and its photocatalytic evaluations was also evaluated (Adeyemi et al., 2019a,b). From previous literature, it is evident that the formation of NPs by plant extract is enabled by the presence of phenolic acids, terpenoids, alkaloids, and polysaccharides within the plant extract that can act as reducing and capping agents for NPs formation (Kuppusamy et al., 2016, Aboyewa et al., 2021). They exhibited good reservoir of these bioactive ingredients. Numerous biological activities of NPs stabilized with plant extracts have been studied. Green AgNPs have been used to combat drug resistance in a variety of applications, including anticancer and antibacterial (Wypij et al., 2021). Among some documents, Abdelghany et al. (Abdelghany et al., 2018), have recorded good antimicrobial and antitumor effect of AgNPs mediated by green approaches. Zhang et al. (Zhang et al., 2019) studied the antibacterial activity of AgNPs from plants, and showed food-born and human-pathogenic bacteria. The current research aimed to synthesis of AgNPs using D. caffra fruit extracts with studding their some biological activities including antitumor, antioxidant and antimicrobial activities.
2 Material and methods
2.1 Material
Silver nitrate (AgNO3) used in this study was purchased from Sigma-Aldrich, Louis, USA. Other chemicals, culture media and reagents used in this study were purchased from Modern Lab Co., India in analytical grade without any purification required.
2.2 Preparation of D. caffra extract for AgNPs synthesis
During the months of January to August 2021, fresh, healthy fruits were picked from the D. caffra plant. D. caffra plant was collected from Menoufia Governorate, Egypt (30° 62′8014″ N, 116° 31′ 070334″ E). The fruits were rinsed in a stream of running tap water to eliminate any dust, then pulped (skin and meat) in an electric mixer for additional extraction. The pulp was filtered using a 1 mm mesh to get smooth pulp free of skin and fibers. The purified extract was used to generate AgNPs. In a clean Erlenmeyer flask, 1 mL of aqueous D. caffra fruits extract and 9 mL of 1 mM AgNO3 solution were combined to make the appropriate reaction mixture. On the other hand, as a control, the identical experimental setup containing 1 mL aqueous D. caffra fruits extract with 9 mL distilled water was used. Both flasks were incubated in the rotary shaker for 2–4 h at room temperature in the dark. The generated AgNPs were then separated and purified using pure water and rapid centrifugation (9000 rpm, 20 min at 10 °C) SiGMA 2 k15, Germany. The AgNPs was separated and dried at 80 °C for 48 h. For further analysis and bioactivity evaluation, the dried AgNPs were stored at 4 °C.
2.3 Characterization of silver nanoparticles
During the incubation time, the formation of AgNPs was visually monitored by following the changes in suspension colour. The creation of Ag colloids by D. caffra extract was also frequently examined using UV–Vis spectra (JENWAY 6305 Spectrophotometer) at wavelengths of 200–800 nm to identify a strong absorption peak linked to surface plasmonic excitation. D. caffra extract without silver nitrate was used to symbolize as blanck. The formation of AgNPs in the absorbance range 400–450 nm was suggested by a strong peak in the UV visible spectrum. To identify functional groups relevant for reduction, regulating, and encapsulating AgNPs, FT-IR analysis was done on D. caffra fruit extract and bio generated AgNPs. Using a FT-IR spectrometer (Agilent system Cary 630 FT-IR model) and the potassium bromide (KBr) pellet method, the assessment was completed. The frequencies were recorded at a resolution of 4 cm−1 in the 4000–400 cm−1 range. TEM (JEOL 1010, Tokyo, Japan) was utilized to characterize AgNPs in order to identify their size and shape. The sample was made by dropping the AgNPs solution onto a carbon coated Cu-grid and placing it into a specimen holder. The AgNPs were analyzed using an X-ray diffractometer X'Pert Pro (Philips, Eindhoven, Netherlands), which was fitted with a ratio detector of Ni-filter/Cu-K radiation (=1.5405) and maintained at a voltage of 40 kV and a current of 30 mA. The crystalline-nature of AgNPs was studied at temperatures ranging from 10° to 80 °C. The collected peaks were allocated and compared to the Joint Committee on Powder-Diffraction Standards database (JCPDS). The surface morphology of Ag particles were confirmed by scanning-electron microscope (SEM) (JEOL- JSM.6360LA, Japan). DLS was used to study the dispersion of AgNPs in colloidal solution utilizing Zetasizer Nano equipment (Malvern Instruments, Worcestershire, UK). The AgNPs sample was re-suspended in purified water at 25 ppm and vortex-mixed to obtain a homogenous solution. Colloidal solution (1.5 mL) of AgNPs was then transferred to a square cuvette for detection. The measurements were done in the range of 0.1–1000 nm. Zetasizer software was used to examine the data of AgNPs sample.
2.4 Inhibitory activity of AgNPs against some bacteria and fungi
The inhibitory activity of AgNPs against bacteria and fungi was achieved using a disc-diffusion test as described previously [16] with some minor modifications. Four bacteria including Bacillus Subtilis (ATCC 6633), Staphylococcus aureus (ATCC 6538), Escherichia coli (ATCC 8739), Pseudomonas aeruginosa (ATCC 90274), one unicellular fungus Candida albicans (ATCC 10221) and one filamentous fungus Aspergillus fumigatus (ATCC 10845), that provided from Regional center for mycology and biotechnology (RCMB), Al-Azhar University, Egypt. Sterile petri dishes containing 20 mL of Mueller–Hinton agar medium were inoculated by test bacteria (0.1 mL bacterial suspensions, 108 cfu mL−1) via streaking method. Sterile petri dishes containing also 20 mL of Yeast Extract Peptone Dextrose agar were used as medium of fungi growth, streaked by 0.1 mL bacterial suspensions, 104 cfu mL−1. Two dilutions including 15 and 30 mg/mL of AgNPs were prepared from the stock solution of 30 mg/mL of AgNPs using DMSO. Filter paper (Whatman filter paper-3) discs with diameter 6 mm radius under aseptic conditions were immersed in each dilution, then dried, placed on the surface of the inoculated growth media. The inoculated plates were kept in refrigerator for 40 min. for proper diffusion of AgNPs, followed by incubation at 30 °C for 2–3 days for fungi and 37 °C for 1 day for bacteria. The visualized inhibition zone around each disc was measured. Discs loaded with Gentamycin (0.1 mg/mL) as antibiotic, Ketoconazole (0.1 mg/mL) as antifungal, were used as a positive control. Discs loaded with DMSO and AgNO3 (30 mg/ mL) was used as a negative control in separate streaked plates with tested organisms giving no any activity.
2.5 Antioxidant activity of AgNPs
To detect the antioxidant activity of AgNPs using DPPH free radical scavenging assay, 1 mL of AgNPs at different dilutions up to 800 µg/ml dissolved in 10 % DMSO was added to 1 mL of 0.3 mM DPPH (Abdelghany et al., 2021). The reaction mixture was kept in the dark for 30 min. At 540 nm, the absorbance was recorded as a result of reduction in color intensity of DPPH using UV–Vis spectrophotometer, model (UV-1800 Shimadzu). Ascorbic acid at different concentrations up to 40 µg/mL of was applied as reference. The following formula was used to calculate inhibition %.
2.6 Antitumor assay
PC-3 cell lines were obtained from Nawah Scientific Inc., Cairo, Egypt. Antibiotics (Penicillin 100 units/mL and streptomycin100 mg/mL) were added to the Dulbecco's Modified Eagle Medium and 10% of heat- inactivated fetal bovine serum was used for cancer cell preservation at 37 °C in humidified containing 5% (v/v) of CO2 atmosphere. Viability of cell was detected via Sulforhodamine B (SRB) assay, briefly the cells suspension (100 μL) containing 5 × 103 cells was poured 96-well plates containing treated medium by different levels of samples, then incubated for 24 h. After exposure of cells to treatments, it fixed via substituting media by 150 μL of 10 % Trichloroacetic acid (TCA) and preserved for 1 h at 4 °C, followed by TCA desiccant and washing the cells for 5 times by distilled water. Solution of TCA was detached, and the cells were washed 5 times using distilled water. The cells were immersed by 70 μL SRB solution (0.4% w/v) for 10 m in a dark at 25 °C, then the plates were washed by 1% of acetic acid for 3 times, the dried in air for 12 h. Dissolve protein bound SRB stain was performed using 150 μL TRIS base solution (10 mM, pH 10.5), using a BMG LABTECH®- FLUOstar Omega microplate reader (Ortenberg, Germany) the absorbance was reading at 540 nm.
3 Result and discussion
3.1 Synthesis and characterization of AgNPs
Plants are widely available and may be used to manufacture metal nanoparticles (M−NPs), particularly AgNPs. Plants convert metal ions to M−NPs at a far quicker pace than microbes (Vanlalveni et al., 2021). The crude extract of ripe fruit Kei-apple (Fig. 1) was evaluated for synthesis of AgNPs. The dissolving of the D. caffra extract resulted in a quick shift in colour from yellow to dark brown after 4 h, demonstrating the rapid reduction of Ag+ to Ag0 in AgNO3 solution (Fig. 2A), whereas the control samples showed no change. The primary and most visible confirmation for the creation of Ag NPs is the colour change in the solution caused by surface plasmonic excitement of AgNPs (Pirtarighat et al., 2019). Furthermore, UV–Visible optical findings validated the production of AgNPs in the mixture, with a surface plasmon-resonance (SRP) spectra at 415 nm of maximum absorption (Fig. 2B). The obtained results are analogous to those published in the literature, in which a Datura stramonium extract was employed to produce silver nanoparticles, and UV–Visible absorbance spectra indicated that the SPR band for Ag particles was in the 400–450 nm range (Gomathi et al., 2017). The aqueous extract of a previously collected batch of plant was utilized to validate the repeatability of AgNPs synthesis, which produced AgNPs when the optimal ratio of plant extract to Ag+ (1–9 mL) was applied (Gomathi et al., 2017, Aslam et al., 2021).
Ripe fruit Kei-apple (orang arrows) and extract (E).
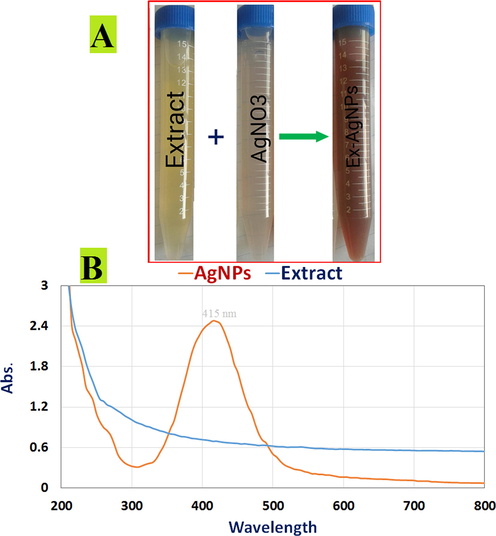
Biosynthesis of AgNPs confirmed. (A) Color changes in D. caffra extract after adding 1 mM aqueous AgNO3 solution for AgNP production; (B) UV–visible spectrum of AgNPs generated by D. caffra extract (Orange curve) and UV–visible spectrum of D. caffra extract.
Fig. 3 also depicts the FTIR spectra of biogenic AgNPs produced from D. caffra extract following interaction with Ag+, as well as a D. caffra extract control without Ag+. As seen in Fig. 3, the FTIR data shows a slight change in the peak location of spectra. The amount of functional biological groups responsible for NPs stabilization, which serve as capping or stabilizing agents, is revealed by spectral analysis. Different absorption peaks were found in FTIR measurements based on AgNPs mediated by D. caffra extract at 3659 cm−1, 3335 cm−1, 3098 cm−1, 2355 cm−1, 2068 cm−1, 1623 cm−1, 669 cm−1, 566 cm−1, and 482 cm−1. In the presence of phenols and alcohols with free —OH and —NH groups, absorption bands at 3659 cm−1, 3335 cm−1, and 3098 cm−1 emerge (Akinfenwa et al., 2021). The presence of symmetric stretching of —COO and alkanes, —C—N groups, is represented by the bands at 2355 cm−1 and 2068 cm−1. The absorption band at 1629 cm−1 is corresponded to the —NH (amide group) and —C⚌C— in aromatic materials (Aref and Salem, 2020). The —OH and —C—N stretching vibrations of amines are attributed to bands at 1407 cm−1, 1228 cm−1, and 1054 cm−1 (Krithiga et al., 2015). In the spectrum of AgNPs, additional bands at 669 cm−1, 566 cm−1, and 482 cm−1 were discovered, which might allude to the binding of hydroxyl of a plant's organic metabolite with AgNPs (Masum et al., 2019). Proteins that contain free carboxylate sites can interact to AgNPs and stabilize them. The location of emission spectra in the FTIR spectrum of D. caffra extract and biosynthesized Ag NPs differed only little. The presence of D. caffra extract components in AgNP biosynthesis was verified by shifting peaks (Taher et al., 2018). As a result, D. caffra extract substances such as NH, CO, and OH groups play an important role in the reduction and stability of AgNPs (Pirtarighat et al., 2019, Eid et al., 2020).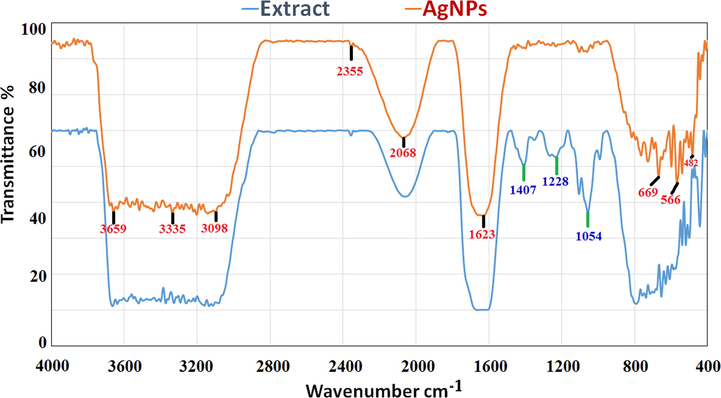
Fourier-transform infrared spectrum of biosynthesized AgNPs and extract of D. caffra.
The TEM image (Fig. 4A) clearly demonstrated that most AgNPs were significantly the spherical shape seems to be predominant, which was consistent with the SEM image (Fig. 4A). AgNPs were observed to have an average size of 12 nm to 53 nm. The brilliant circular spots in the area selected electron diffraction (SAED) pattern (Fig. 4B) indicated the (3 1 1), (2 2 0), (2 0 0), and (1 1 1) planes, as well as the crystalline structure of D. caffra's Ag particles. The XRD pattern of AgNPs is seen in Fig. 4C. In the first stage, the indexing was completed, and miller indices were allocated to each peak (Vishwasrao et al., 2019). AgNPs with face-centered cubic symmetry accounted for the whole of the reflection peaks (Shah et al., 2021). The AgNPs were extremely crystalline, as evidenced by the high intensity of peaks. Peaks at 38.2°, 45.66°, 64.42°, and 77.74° in the diffraction pattern of AgNPs were attributed to 111, 200, 220, and 311, respectively, of face-centered cubic pure AgNPs (JCPDS 87–0720). It confirmed that Ag metal was the main ingredient of AgNPs. The peaks' line widening is mostly owing to the tiny particle size (Jain and Mehata 2017). The AgNPs generated by the reduction of Ag+ ions by the D. caffra extract are crystalline in nature, according to the X-ray diffraction observations. The results showed that the phyto-synthesized AgNPs were made up of high-purity crystalline Ag particles.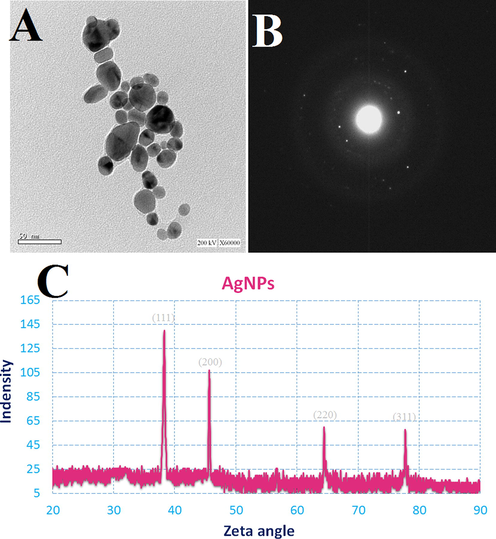
TEM image (A); SAED pattern (B); and XRD (C) of the synthesized AgNPs by D. caffra extract.
The AgNPs morphology was examined using a scanning electron microscope (SEM) image (Fig. 5A). The bulk of Ag particles were spherical in form, with a few oval AgNPs thrown in for good measure. The biosynthesized AgNPs had been evenly distributed throughout the solution. The findings of this study are consistent with prior research that found spherical AgNPs when synthesis was facilitated by plant extract (Ansari and Alzohairy 2018, Pirtarighat et al., 2019). Dynamic light scattering (DLS) analysis was used to determine the average diameter of total Ag particles. The bulk of the AgNPs had a diameter of 90 nm with average diameter 170 nm (Fig. 5B). However, the size derived via DLS is modified by metabolites of D. caffra deposited on NP surface, such as organic molecules attached as stabilizers, as well as the metal core of Ag particles (Aref and Salem 2020). In the SEM image, the biomolecule coating of the biosynthesized Ag NPs can be seen. The function of D. caffra extract metabolites in the production and stabilization of biosynthesized AgNPs is confirmed by this layer. They are molecules found in plant extracts that attach to the edge of AgNPs and function as chelating agents, according to published data (Taruna et al., 2016).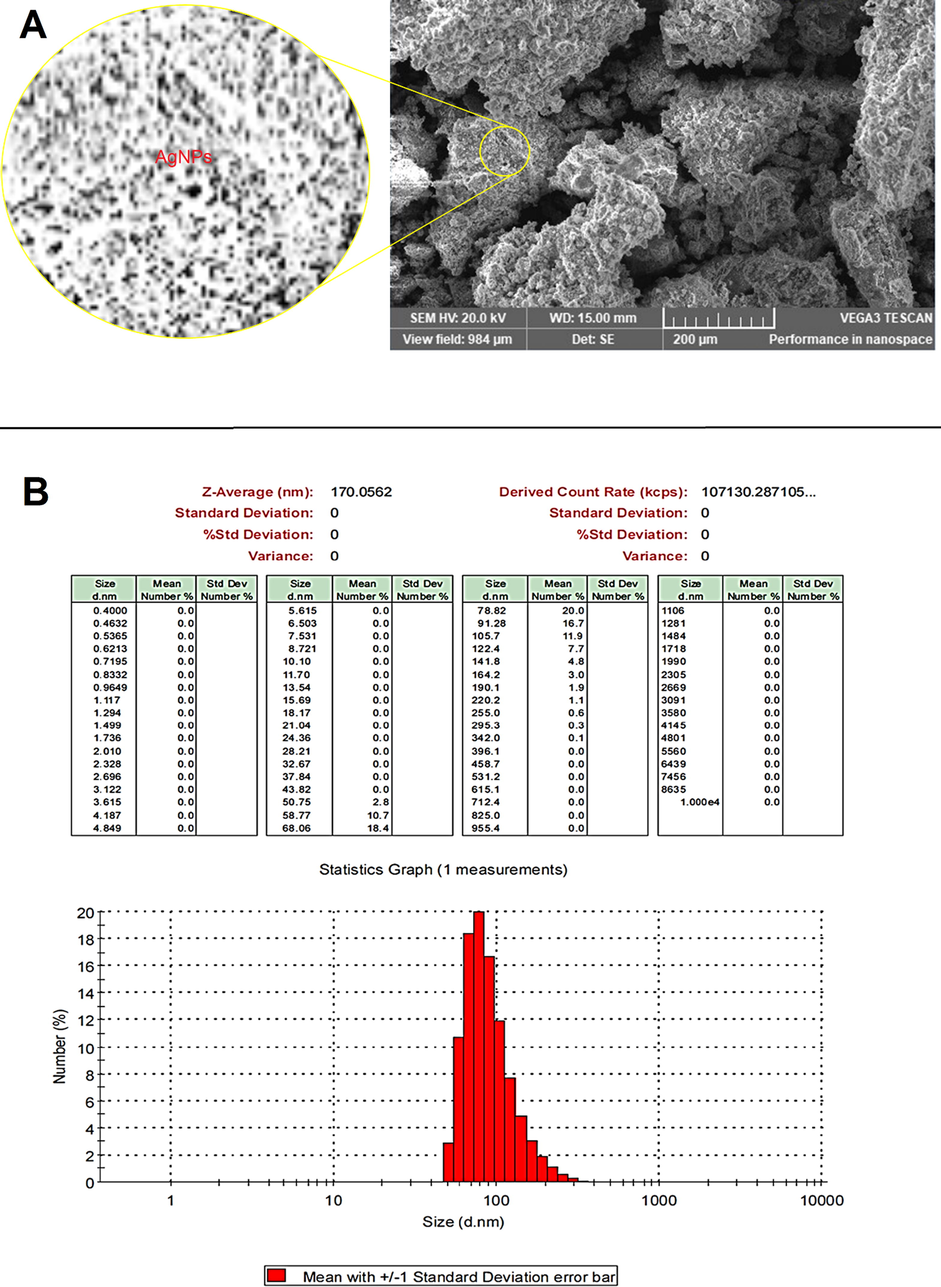
SEM image (A) and DLS analysis (B) of AgNPs synthesized by D. caffra extract.
3.2 Antimicrobial activity of AgNPs
To overcome the problems of antibiotic resistance bacteria and microbial infection development, discover or development safe antimicrobial compounds are necessary in the current todays. The green synthesized AgNPs at two concentrations (15 mg/mL and 30 mg/mL) were tested against some bacteria and fungi using agar disc diffusion protocol compared with AgNO3 and positive control. Good inhibitory activity of AgNPs was observed against tested microorganisms with inhibition diameter zone was 28.22 ± 0.25 mm, 23.21 ± 0.35 mm, 27.25 ± 0.03 mm, 28.40 ± 0.15 mm, 29.23 ± 0.44 mm, and 9.52 ± 0.5 mm against B. Subtilis, S. aureus, E. coli, P. aeruginosa, C. albicans and A. fumigatus, respectively (Fig. 6). Via disc diffusion assay, the antibacterial activity of AgNPs was confirmed against S. aureus, B. cereus and P. aeruginosa, E. coli (Chandrasekharan et al., 2022). In another recent study, different diameter of inhibition zones 13 mm, 17 mm, 18 mm and 14 mm were recorded against Listeria monocytogenes, E. coli, Shigella dysenteriae and Salmonella typhi, respectively as a result of AgNPs activity (Sharma et al., 2022). The obtained results indicated that AgNPs showed weak antifungal activity against filamentous fungus A. fumigatus unlike unicellular fungus C. albicans, but what supports the unique properties of AgNPs is that the AgNO3 did not give any inhibition at the same used concentration of AgNPs (Fig. 6). These observations were partially agreement with the results Ahmad et al. (Ahmad et al., 2022), where who mentioned that AgNPs showed excellent antibacterial activity, moderate antifungal potential against Alternaria alternate and minor antifungal potential against Fusarium gramium. The exact of the action mechanism of AgNPs against microorganisms still under researchers studies day up to day. Although some mechanisms were reported such as interference with microbial DNA, disruption of proteins (Abdel-Ghany et al., 2018, Sharma et al., 2022).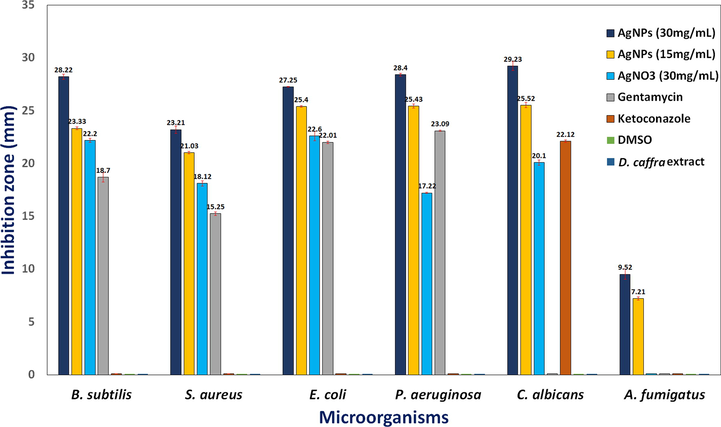
Antimicrobial activity of AgNPs synthesized by D. caffra extract against some pathogenic strains.
3.3 Antioxidant activity
Research for biological antioxidant compounds increase day by day, therefore the antioxidant characteristic of AgNPs was studied in some scientific papers. The obtained experiment reflected excellent antioxidant activity AgNPs followed by D. caffra fruit extract at the same utilized concentrations where the DPPH scavenging was 52.68 and 52.68 at 800 µg/ml. DPPH scavenging using AgNPs was three fold using fruit extract at 100 µg/ml (Fig. 7A). These results were compared to ascorbic acid as a potent antioxidant. Recently, Gecer et al. (Gecer et al., 2021) documented the antioxidant activity of AgNPs synthesized by Echinacea purpurea extract and recommended the application of AgNPs as additives in drugs and food. Antioxidant of D. caffra fruit was recorded in some studies due to the presence of phenolic and flavonoid compounds (Taher et al., 2018), and amino acids (Augustyn et al., 2018). DPPH scavenging % increment with increasing concentration AgNPs and fruit extract in dose depending manner. From the obtained results AgNPs displayed a promising antioxidant activity as compared to the standard, ascorbic acid (Fig. 7B). Good radical scavenging capability of AgNPs can be attributed to bioactive secondary metabolites present in the fungal cell filtrate which acts as a capping agent in nanoparticles.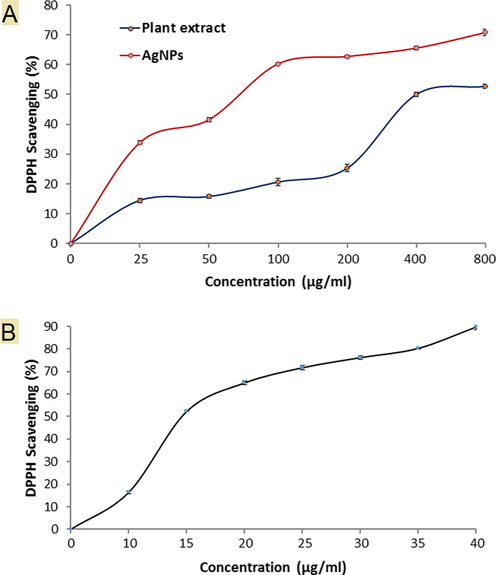
DPPH Scavenging (%) of AgNPs, plant extract (A) and ascorbic acid (B).
3.4 Anticancer activity
Results of anticancer activity was documented against PC-3 cell lines at different concentrations of AgNPs compared with AgNO3 and D. caffra fruit extract (Fig. 8). Inhibition of cell viability % was concentration dependent manner from 25 up to 150 µg/mL. AgNPs was more effective at all applied concentrations than D. caffra fruit extract followed by AgNO3 and, for example the inhibition of cell viability at 25 µg/mL was 21.03, 12.21 and 11.06 %, respectively. Sharply cell viability inhibition more than 80 % was observed at 100–150 µg/mL of AgNPs, while at the same concentration of AgNO3, viability inhibition not reached to 38%. At 150 µg/mL of D. caffra fruit extract viability inhibition reached to 55.62%. Previous investigations have shown that biosynthesized AgNPs have a cytotoxic impact in vitro against PC-3, lung cancer cell lines, MCF-7, Hep-2 and HeLa cell lines (Raman et al., 2015, Al-Sheddi et al., 2018, Sangour et al., 2021, Chen et al., 2022). Although the anticancer potential of AgNPs was reported in numerous studies but the activity varies regarding size of AgNPs and source of synthesis. Rajawat et al. (Rajawat et al., 2016) observed that the proliferation of MCF-7 cancer cell lines were completely inhibited by 9 nm of AgNPs compared to 15 nm of AgNPs. Ovais et al. (Ovais et al., 2016) and Erdogan et al. (Erdogan et al., 2019) mentioned that the inhibitory mechanisms of AgNPs to cancer cells via mitochondria-mediated caspase apoptosis. The AgNPs showed cytotoxicity with IC50 of 40 μg/mL against A549 cells (Akther et al., 2019). The differences of AgNPs toxicity dependent on its size and morphology beside cancer cell type. Morphological changes were observed on cells exposed to AgNPs, followed by D. caffra fruit extract, while negligible changes were recorded on AgNO3 exposed cells (Fig. 7). In the same line Firdhouse and Lalitha (Firdhouse and Lalitha 2013) noted that the apoptosis rate was highly of PC-3 treated by biosynthesized AgNPs than exposed to silver ions. There is no clear differentiation among control cells (Fig. 9A) and treated by 100 μg/mL (Fig. 9B) and 150 μg/mL (Fig. 9C) of AgNO3. While relatively changes in the treated cells were observed including irregular clusters and floating cells using D. caffra fruit extract (Fig. 9D&E). On the other hand, membrane shrinkage, failure of cell adhesion, blebbing of cell membrane, lyses of cell membrane, appearance of unusual cellular crinkle and cell destruction were microscopically recorded at 100 μg/mL (Fig. 9F) and more observed at 150 μg/mL of AgNPs (Fig. 9G). The changes in the cells may be due to the interface of AgNPs with the functioning of PC-3 proteins. Most recognizable alterations in treated cells were consistent with recent report (Morais et al., 2021) viewing on efficacy of AgNPs against PC-3. Another mechanism reflect the efficacy of AgNPs through its induction to Fragmentation of PC-3 DNA that cause apoptosis in the cells (Zhang et al., 2019). The obtained finding demonstrated that the antiproliferative action of AgNPs mainly depends on the concentration, therefore, D. caffra fruit extract mediated AgNPs may offer as promising source for cancer treatment. Current finding suggested that the anticancer potential may be due to AgNPs may perhaps penetrate into the cell membrane of cancer cell leading to DNA damage as demonstrated by the DNA fragmentation (Kovács et al., 2022).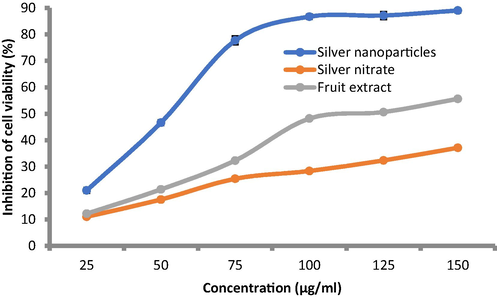
Anticancer activity of AgNPs synthesized by D. caffra extract compared to D.caffra fruit extract and AgNO3.
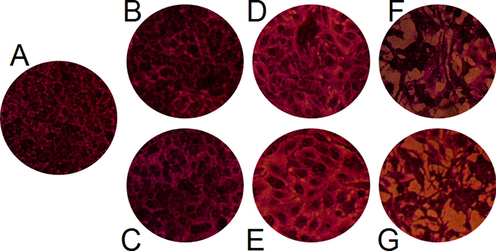
Morphological alteration of treated PC-3. Control (A without treatment), 100 μg/mL (B) and 150 μg/ mL (C) of AgNO3; 100 μg/ mL (D) and 150 μg/ mL (E) of D. caffra fruit extract; 100 μg/ mL (F) and 150 μg/ mL (G) of AgNPs.
4 Conclusion
The created AgNPs were characterized using UV–Vis spectra, FT-IR analysis, TEM, XRD, SEM and DLS. All these characterization confirmed the synthesis of AgNPs. Through TEM analysis, the average size of AgNPs was 12 nm to 53 nm in spherical forms. The results of the FTIR spectra showed that the functional groups present in D. caffra extract play a major role in the formation of AgNPs. D. caffra fruit extract is a well-known producer of AgNPs, which have antioxidant, antibacterial, and anticancer properties, and may be used to solve a variety of medicinal, nutritional, and industrial problems.
CRediT authorship contribution statement
Aisha M.H. Al-Rajhi: Conceptualization, Writing – review & editing. Salem S. Salem: Conceptualization, Methodology, Software, Validation, Writing – original draft, Writing – review & editing. Asmaa A. Alharbi: Writing – original draft. T.M. Abdelghany: Writing – original draft, Writing – review & editing.
Acknowledgments
To Princess Nourah bint Abdulrahman University for their grant through Researchers Supporting Project number PNURSP2022R217, Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Molecular characterization of Trichoderma asperellum and lignocellulolytic activity on barley straw treated with silver nanoparticles. BioResources.. 2018;13:1729-1744.
- [Google Scholar]
- Potential of biosynthesized zinc oxide nanoparticles to control Fusarium wilt disease in eggplant (Solanum melongena) and promote plant growth. Biometals 2022
- [CrossRef] [Google Scholar]
- Effect of Thevetia peruviana seeds extract for microbial pathogens and cancer control. Int. J. Pharmacol.. 2021;17:643-655.
- [Google Scholar]
- Recent advances in green synthesis of silver nanoparticles and their applications: about future directions. A Review. BioNanoScience. 2018;8:5-16.
- [CrossRef] [Google Scholar]
- Green synthesis of metallic nanoparticles using some selected medicinal plants from Southern Africa and their biological applications. Plants (Basel).. 2021;10:1929.
- [CrossRef] [Google Scholar]
- ZnO nanoparticles mediated by aqueous extracts of Dovyalis caffra fruits and the photocatalytic evaluations. Mater. Res. Express.. 2019;6:125091
- [Google Scholar]
- Bio-inspired synthesis and cytotoxic evaluation of silver-gold bimetallic nanoparticles using Kei-Apple (Dovyalis caffra) fruits. Inorganic Chem. Commun.. 2019;109:107569
- [Google Scholar]
- Green Fabrication of Silver Nanoparticles using <i>Euphorbia serpens</i> Kunth Aqueous Extract, Their Characterization, and Investigation of Its <i>In Vitro</i> Antioxidative, Antimicrobial, Insecticidal, and Cytotoxic Activities. BioMed Res. Int.. 2022;2022:5562849.
- [CrossRef] [Google Scholar]
- Cytotoxic effects of phytomediated silver and gold nanoparticles synthesised from rooibos (Aspalathus linearis), and Aspalathin. Plants.. 2021;10:2460.
- [Google Scholar]
- Fungal-mediated synthesis of pharmaceutically active silver nanoparticles and anticancer property against A549 cells through apoptosis. Environ. Sci. Pollut. Res.. 2019;26:13649-13657.
- [CrossRef] [Google Scholar]
- Anticancer potential of green synthesized silver nanoparticles using extract of <i>Nepeta deflersiana</i> against human cervical cancer cells (HeLA) Bioinorg. Chem. Appl.. 2018;2018:9390784.
- [CrossRef] [Google Scholar]
- Multifunctional properties of spherical silver nanoparticles fabricated by different microbial taxa. Heliyon.. 2020;6
- [CrossRef] [Google Scholar]
- One-pot facile green synthesis of silver nanoparticles using seed extract of<i> Phoenix dactylifera</i> and their bactericidal potential against MRSA. Evidence-Based Complement. Alternative Med.. 2018;2018:1860280.
- [CrossRef] [Google Scholar]
- Bio-callus synthesis of silver nanoparticles, characterization, and antibacterial activities via Cinnamomum camphora callus culture. Biocatal. Agric. Biotechnol.. 2020;27
- [CrossRef] [Google Scholar]
- Phyto-extract-mediated synthesis of silver nanoparticles using aqueous extract of Sanvitalia procumbens, and characterization, optimization and photocatalytic degradation of Azo dyes orange G and direct blue-15. Molecules. 2021;26:6144.
- [Google Scholar]
- A preliminary study on the chemical characteristics of Kei apple (Dovyalis caffra), an undervalued South African fruit. S. Afr. J. Bot.. 2018;117:268-275.
- [CrossRef] [Google Scholar]
- Efficacy assessment of biosynthesized copper oxide nanoparticles (CuO-NPs) on stored grain insects and their impacts on morphological and physiological traits of wheat (Triticum aestivum L.) plant. Biology.. 2021;10:233.
- [Google Scholar]
- Biomedical applications of silver nanoparticles: an up-to-date overview. Nanomaterials (Basel).. 2018;8:681.
- [CrossRef] [Google Scholar]
- Sustainable phyto-fabrication of silver nanoparticles using Gmelina arborea exhibit antimicrobial and biofilm inhibition activity. Sci. Rep.. 2022;12:156.
- [CrossRef] [Google Scholar]
- Citrus sinensis leaf aqueous extract green-synthesized silver nanoparticles: Characterization and cytotoxicity, antioxidant, and anti-human lung carcinoma effects. Arabian J. Chem.. 2022;15:103845
- [CrossRef] [Google Scholar]
- Endophytic streptomyces laurentii mediated green synthesis of Ag-NPs with antibacterial and anticancer properties for developing functional textile fabric properties. Antibiotics.. 2020;9:1-18.
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles via Cynara scolymus leaf extracts: The characterization, anticancer potential with photodynamic therapy in MCF7 cells. PLoS ONE. 2019;14:e0216496
- [Google Scholar]
- Biosynthesis of silver nanoparticles using the extract of Alternanthera sessilis—antiproliferative effect against prostate cancer cells. Cancer Nanotechnol.. 2013;4:137-143.
- [CrossRef] [Google Scholar]
- Morphological and biomolecules dynamics of Phytopathogenic Fungi under stress of silver nanoparticles. BioNanoScience.. 2018;8:566-573.
- [CrossRef] [Google Scholar]
- Gecer, E. N., R. Erenler, C. Temiz, et al., 2021. Green synthesis of silver nanoparticles from Echinacea purpurea (L.) Moench with antioxidant profile. Particulate Sci. Technol., 1-8.
- Silver nanoparticles biosynthesis by Fusarium moniliforme and their antimicrobial activity against some food-borne bacteria. Mycopath.. 2013;11:1-7.
- [Google Scholar]
- Green synthesis of silver nanoparticles using Datura stramonium leaf extract and assessment of their antibacterial activity. Resour.-Effic. Technol.. 2017;3:280-284.
- [CrossRef] [Google Scholar]
- Purpureocillium lilacinum mediated biosynthesis copper oxide nanoparticles with promising removal of dyes. Biointerface Research in Applied Chemistry. 2022;12(2):1397-1404. In this issue
- [CrossRef] [Google Scholar]
- Biomedical Applications of Mycosynthesized Selenium Nanoparticles Using Penicillium expansum ATTC 36200. Biological Trace Element Research. 2021;199(10):3998-4008. In this issue
- [CrossRef] [Google Scholar]
- Green and ecofriendly biosynthesis of selenium nanoparticles using Urtica dioica (stinging nettle) leaf extract: Antimicrobial and anticancer activity. Biotechnol. J. 2021
- [CrossRef] [Google Scholar]
- Medicinal plant leaf extract and pure flavonoid mediated green synthesis of silver nanoparticles and their enhanced antibacterial property. Sci. Rep.. 2017;7:15867.
- [CrossRef] [Google Scholar]
- Cancer therapy by silver nanoparticles: fiction or reality? Int. J. Mol. Sci.. 2022;23:839.
- [Google Scholar]
- Krithiga, N., Rajalakshmi, A., Jayachitra, A., 2015. Green synthesis of silver nanoparticles using leaf extracts of Clitoria ternatea and Solanum nigrum and study of its antibacterial effect against common nosocomial pathogens. J. Nanosci. 2015,
- Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications – An updated report. Saudi Pharmaceut. J.. 2016;24:473-484.
- [CrossRef] [Google Scholar]
- Biogenic synthesis of silver nanoparticles using Phyllanthus emblica fruit extract and its inhibitory action against the pathogen Acidovorax oryzae strain RS-2 of rice bacterial brown stripe. Front. Microbiol.. 2019;10:820.
- [Google Scholar]
- Pharmaceutical aspects of silver nanoparticles. Artif. Cells Nanomed. Biotechnol.. 2018;46:115-126.
- [CrossRef] [Google Scholar]
- Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv.. 2013;31:346-356.
- [CrossRef] [Google Scholar]
- Stachybotrys chartarum: a novel biological agent for the extracellular synthesis of silver nanoparticles and their antimicrobial activity. Indonesian J. Biotechnol.. 2013;18:75-82.
- [Google Scholar]
- Eco-friendly Mycogenic Synthesis of ZnO and CuO Nanoparticles for In Vitro Antibacterial, Antibiofilm, and Antifungal Applications. Biological Trace Element Research. 2021;199(7):2788-2799. In this issue
- [CrossRef] [Google Scholar]
- Starch-capped AgNPs’ as potential cytotoxic agents against prostate cancer cells. Nanomaterials.. 2021;11:256.
- [Google Scholar]
- Green synthesis of silver nanoparticles via plant extracts: beginning a new era in cancer theranostics. Nanomedicine.. 2016;12:3157-3177.
- [Google Scholar]
- Green synthesis of silver nanoparticles using the plant extract of Salvia spinosa grown in vitro and their antibacterial activity assessment. J. Nanostruct. Chem... 2019;9:1-9.
- [CrossRef] [Google Scholar]
- Anticancer, antioxidant, antiviral and antimicrobial activities of Kei Apple (Dovyalis caffra) fruit. Scientific Reports 20225914
- [CrossRef] [Google Scholar]
- Study of anti-cancer properties of green silver nanoparticles against MCF-7 breast cancer cell lines. Green Process. Synth,. 2016;5:173-181.
- [Google Scholar]
- Mycosynthesis and characterization of silver nanoparticles from Pleurotus djamor var. roseus and their in vitro cytotoxicity effect on PC3 cells. Process Biochem.. 2015;50:140-147.
- [CrossRef] [Google Scholar]
- Nickel oxide nanoparticles application for enhancing biogas production using certain wastewater bacteria and aquatic macrophytes biomass. Waste Biomass Valoriz.. 2021;12:2059-2070.
- [CrossRef] [Google Scholar]
- Bio-fabrication of selenium nanoparticles using Baker’s yeast extract and its antimicrobial efficacy on food borne pathogens. Appl. Biochem. Biotechnol. 2022
- [CrossRef] [Google Scholar]
- Pseudomonas indica-mediated silver nanoparticles: antifungal and antioxidant biogenic tool for suppressing Mucormycosis Fungi. Journal of Fungi.. 2022;8
- [CrossRef] [Google Scholar]
- Bactericidal and in-vitro cytotoxic efficacy of silver nanoparticles (Ag-NPs) fabricated by endophytic actinomycetes and their use as coating for the textile fabrics. Nanomaterials.. 2020;10:1-20.
- [CrossRef] [Google Scholar]
- Green synthesis of metallic nanoparticles and their prospective biotechnological applications: an overview. Biol. Trace Elem. Res.. 2021;199:344-370.
- [CrossRef] [Google Scholar]
- A Comprehensive Review of Nanomaterials: Types, Synthesis, Characterization, and Applications. Biointerface Research in Applied Chemistry. 2023;13(1):41 In this issue
- [CrossRef] [Google Scholar]
- Effect of Ag nanoparticles on viability of MCF-7 and Vero cell lines and gene expression of apoptotic genes. Egyptian J. Med. Human Genet.. 2021;22:9.
- [CrossRef] [Google Scholar]
- Synthesis of silver nanoparticles using Plantago lanceolata extract and assessing their antibacterial and antioxidant activities. Sci. Rep.. 2021;11:20754.
- [CrossRef] [Google Scholar]
- Current Advances in Fungal Nanobiotechnology: Mycofabrication and Applications. In: Lateef A., Gueguim-Kana E.B., Dasgupta N., eds. Microbial Nanobiotechnology: Principles and Applications. Singapore: Springer Singapore; 2021. p. :113-143.
- [Google Scholar]
- A new strategy to integrate silver nanowires with waterborne coating to improve their antimicrobial and antiviral properties. Pigment and Resin Technology 2022
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles and its antibacterial activity using fungus Talaromyces purpureogenus isolated from Taxus baccata Linn. Micro Nano Syst. Letters.. 2022;10:2.
- [CrossRef] [Google Scholar]
- Phytochemical constituents, antioxidant activity and safety evaluation of Kei-apple fruit (Dovyalis caffra) Food Chem.. 2018;265:144-151.
- [CrossRef] [Google Scholar]
- Green synthesis and physico-chemical study of silver nanoparticles extracted from a natural source Luffa acutangula. J. Mol. Liq.. 2016;224:991-998.
- [CrossRef] [Google Scholar]
- Green synthesis of silver nanoparticles using plant extracts and their antimicrobial activities: a review of recent literature. RSC Adv.. 2021;11:2804-2837.
- [Google Scholar]
- Green synthesis of silver nanoparticles using Sapota fruit waste and evaluation of their antimicrobial activity. Waste Biomass Valorization. 2019;10:2353-2363.
- [CrossRef] [Google Scholar]
- Green synthesized silver nanoparticles: antibacterial and anticancer activities, biocompatibility, and analyses of surface-attached proteins. Front. Microbiol.. 2021;12
- [CrossRef] [Google Scholar]
- Synthesis of silver nanoparticles (AgNPs) from leaf extract of Salvia miltiorrhiza and its anticancer potential in human prostate cancer LNCaP cell lines. Artif. Cells Nanomed. Biotechnol.. 2019;47:2846-2854.
- [Google Scholar]






