Translate this page into:
MOF-253 immobilized Pd and Cu as recyclable and efficient green catalysts for Sonogashira reaction
⁎Corresponding author. cesxjw@foxmail.com (Jianwei Xie)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
MOF-253·Pd(OAc)2 and MOF-253·CuI were prepared, characterized, and evaluated firstly as heterogeneous co-catalysts, which showed high catalytic activity in Sonogashira coupling reaction of various substituted (hetero)aryl halides with terminal alkynes at 70–120 °C, and afforded the corresponding products in 45–99% yields with high TON (∼2722 for Pd). The best result was achieved with an extremely low Pd (0.036 mol%) and Cu (0.397 mol%) loading. Moreover, the catalysts can be reused at least five times without significantly reducing the activity. Besides, Hg(0) and PVP-poisoning experiments confirmed that the present catalysts were efficient and heterogeneous catalysts in this coupling reaction.
Keywords
MOF-253
Immobilized Pd and Cu catalysts
Sonogashira coupling reaction
Green heterogeneous catalyst
Terminal alkynes
1 Introduction
The Sonogashira reaction is one of the most efficient and straightforward methods for the construction of carbon (sp2)-carbon (sp) bonds via palladium/copper co-catalyzed coupling of aryl, heteroaryl, and vinyl halides or triflates with terminal alkynes in the presence of amine as the base (Sonogashira, 2002), which was firstly reported in 1975 (Sonogashira et al., 1975). Because of the broad application of alkynes in various agrochemicals, natural products, pharmaceuticals, and versatile material intermediates, the reaction has still been received extensive attention for the synthesis of substituted alkynes (Danilkina et al., 2015; Frigoli et al., 2005; Mansour, 2016; Platonova et al., 2014; Wagner and Comins, 2006). In addition, the di-substituted acetylenes can contribute to being further converted into other functional groups (Chinchilla and Nájera, 2007). Although the significant progress has been achieved in this transformation, by utilizing catalytic palladium combined with CuI as a co-catalyst under homogenous conditions (Chinchilla and Nájera, 2011), they inherently suffer from some problems, such as non-reusability and metal residues, etc., which cause to the low economic efficiency of catalysts and narrow application range of products, particularly in the preparation of pharmaceutical chemicals and optoelectronic devices. Many efforts have been made to develop heterogeneous catalysts to overcome the mentioned drawbacks (Arpad, 2011; Shi, 2013). For these purposes, different solid supports, such as polymers (Gholinejad et al., 2015; Navalon et al., 2013; Steel and Teasdale, 2004), magnesium oxide (Gholinejad et al., 2018a,b), silica (Bedford et al., 2005; Dehbanipour et al., 2017), graphene (Gholinejad et al., 2017; Wang et al., 2017), resins (Bakherad et al., 2014; Ciriminna et al., 2013; Sengupta et al., 2014) and naturally occurring polysaccharides (Gholinejad et al., 2016a,b), have been used to anchor palladium or copper species. Visible-light-mediated Songashira coupling reactions have also been reported under homogeneous or heterogeneous conditions (Li et al., 2019a,b; Protti et al., 2005; Song et al., 2018; Zhou and Zhao, 2022). However, there is still space for improvement in this field, such as catalyst preparation, low efficiency of recyclable and loss of actives of the catalysts. Copper-free palladium-catalyzed Sonogashira reactions have also been reported in recent years (Ciriminna et al., 2013; Dissanayake et al., 2019; Gao et al., 2010; Gholinejad et al., 2018a,b; Handa et al., 2015; Handa et al., 2019; Gholinejad et al., 2016a,b; Yu et al., 2016), but it has been proved by some groups in many cases that the cross-coupling reaction could be accelerated in the presence of copper under oxygen-free conditions to prevent the formation of diyne as homo-coupling products (Beccalli et al., 2014; Bellina and Lessi, 2012; Lamblin et al., 2010; Mas-Marzá et al., 2003; Tan et al., 2013).
Metal-organic frameworks (MOFs) are a unique class of porous and crystalline materials composed of metal cations (or metal clusters) and organic linkers. The employment of MOFs as heterogeneous catalysis is one of the best promising applications (Dhakshinamoorthy et al., 2015; Jiao et al., 2018; Lee et al., 2009; Li et al., 2019a,b; Liu et al., 2014; Xu et al., 2019), which is attributed to the large surface areas, good thermal stability and potential metal active sites (Castillo-Blas and Gandara, 2018; Liu et al., 2010; Wen et al., 2018). Among them, (Al(OH)(bpydc)) (bpydc = 2,2′-bipyridine-5,5′-dicarboxylate) is one of the well-studied MOFs, which was firstly reported in 2010 by Yaghi et al. and denominated the structure as “MOF-253” (Bloch et al., 2010). After then, because of possessing open N,N’-chelating sites in its structure, MOF-253 has been investigated as an excellent carrier-immobilized metal heterogeneous catalyst in several organic conversions (Chen et al., 2015; Deng et al., 2018; Deng et al., 2019; Liu et al., 2012; Long et al., 2012; Van Zeeland et al., 2016; Wang et al., 2012). Nonetheless, there is no report with MOF-253 immobilized Cu and Pd as the co-catalysts for Sonogashira carbon–carbon coupling reactions.
In continuation to develop environmentally friendly protocols for transition-metal catalyzed carbon-heteroatom bonds formation (Li et al., 2016; Ma et al., 2018; Wang et al., 2018; Zhou et al., 2015), in this paper, the aim is to study the direct coupling reaction of (hetero)aryl halides with terminal alkynes by using MOF-253·xPd(OAc)2 and MOF-253·yCuI (x or y, molar ratio of Pd(OAc)2 or CuI to bpy) as the heterogeneous bimetallic catalysts system.
2 Experimental section
2.1 General methods
All reagents were purchased from Adamas-beta and used as received unless otherwise noted. Anhydrous DMSO (99.7+%, extra dry, over molecular sieves) was purchased from Acros. All the reactions were carried out in N2 atmosphere under magnetic stirring. 1H NMR and 13C NMR were recorded on a Bruker Avance III HD 400 instrument using TMS as the internal standard and DMSO or CDCl3 as the solvent. Mass spectra were recorded on a GC–MS (Agilent 7890A/5975C) instrument in EI mode. AAS was obtained on the AA-7003 instrument of Beijing East-West Instrumental Analysis. Inductively coupled plasma-mass spectrometry (ICP-MS) was obtained on an Agilent 720 instrument, and the elemental analysis (C, H, N) was measured by Thermo Fisher Flash 2000 instrument. Powder X-ray diffraction (XRD) was measured by a Bruker D8 ADVANCE instrument. Fourier transform infrared spectroscopy (FT-IR) spectra was determined by a Bruker Vertex70 FTIR spectrometer (wavenumber range 500–4000 cm−1). X-ray photoelectron spectroscopy (XPS) was collected using a Kratos AMICUS spectrometer (Shimadzu, JP). N2 adsorption/desorption experiments were detected using a Brunauer-Emmett-Teller (BET) instrument. Column chromatography was performed with silica gel (200-–300 mesh) purchased from Qingdao Haiyang Chemical Co., Ltd.
2.2 General procedure for MOF-253·Pd(OAc)2/MOF-253·CuI-catalyzed the coupling reaction of (hetero)aryl halides with terminal alkynes
To a 25 mL of Schlenk tube were added MOF-253·Pd(OAc)2 (Pd 0.036 mol%), MOF-253·CuI (Cu 0.397 mol%), (hetero)aryl halide (0.50 mmol), K2CO3 (1.0 mmol) and DMSO (2.0 mL), then the mixture was vacuumed three times to replace with N2. The terminal alkyne (0.55 mmol) was added under N2 atmosphere, and finally, the reaction tube was sealed and reacted in the preheated IKA reaction module at 70–120 °C for 12–24 h. The reaction mixture was cooled to room temperature and extracted with ethyl acetate (20 mL × 3). The combined organic phase was washed with water and brine, dried over anhydrous Na2SO4, and concentrated in vacuo. The residue was purified by flash column chromatography on silica gel (ethyl acetate/ petroleum ether as the eluent) to afford the target products 3a-3au and 4a-4c.
2.3 Recycling of the catalysts MOF-253·Pd(OAc)2 and MOF-253·CuI
After completion of the first run, the reaction mixture was cooled to room temperature, and extracted with petroleum ether. The remaining petroleum ether in the reaction tube was steamed under reduced pressure at 80 °C. And then, the reaction liquid containing the catalyst was re-added with substrate, partial solvent (0.5 mL), and base (2.0 Equiv.) to start a new cycle. This process was then repeated 5 times.
2.4 Hg (0) and PVP poisoning experiment
At the beginning of the reaction, to a 25 mL of Schlenk tube was added MOF-253·Pd(OAc)2 (Pd 0.036 mol%), MOF-253·CuI (Cu 0.397 mol%), and Hg(0) (56 mg) or PVP-60000 (112 mg). The mixture was stirred at room temperature for 30 min, then added to other required materials under standard conditions, reacted under optimal conditions, cooled at room temperature, and worked up to obtain the isolated yield.
2.5 Metal leaching test of catalyst MOF-253·Pd(OAc)2 and MOF-253·CuI
After completion of the reaction, the reaction mixture was subjected to hot filtration under vacuum. The solid was washed with DMSO, and the liquid phase was analyzed by AAS.
3 Results and discussion
The synthetic method for the preparation of MOF-253 immobilized Pd and Cu catalysts is shown in Fig. 1. Oxidation of commercially available 5,5′-dimethyl-2,2′-bipyridine with H2SO4/K2Cr2O7 catalytic system provided 2,2′-bipyridine-5,5′-dicarboxylic acid. Followed by the hydrothermal method with AlCl3·6H2O and acetic acid to afford MOF-253 (Wang et al. 2012). In MOF-253, the 2,2′-bipyridyl (bpy) moiety does not coordinate with any metal ion and thus provides a platform for coordination chelation of the metal center. Therefore, the activated MOF-253 was immersed in the acetone solution of Pd(OAc)2 or in the acetonitrile solution of CuI to obtain MOF-253·xPd(OAc)2 and MOF-253·yCuI, respectively. The structures of MOF-253, MOF-253·xPd(OAc)2 (x = 0.02) and MOF-253·yCuI (y = 0.54) were characterized by XRD, FT-IR and BET analysis, which were matched with the reported literature (Figure S1-S3) (Wang et al. 2012), and the content of Pd and Cu were determined by ICP-MS and elemental analysis (Table 1). The intermolecular interactions between MOF-253 and Pd(OAc)2 or CuI were investigated by XPS, and the results were illustrated in Fig. 2. Compared to MOF-253, the binding energy of the N 1s peak of MOF-253·CuI and MOF-253·Pd(OAc)2 were shifted toward higher binding energy with the value of 0.8 eV and 1.4 eV, respectively, which indicated the existence of intermolecular coordination interaction between bpy unit of MOF-253 and CuI or Pd(OAc)2, resulting in a decrease in the electron density of N atoms.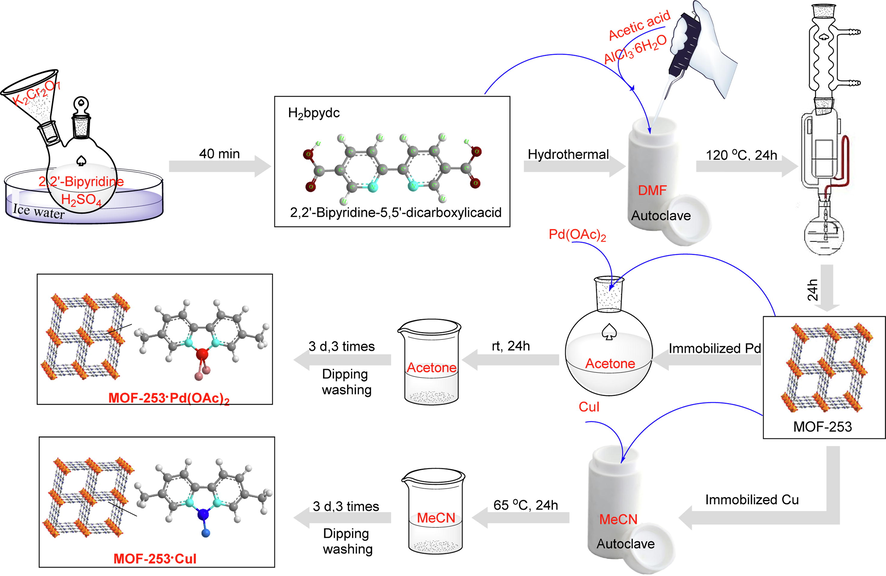
Catalyst preparation process (Bloch et al., 2010; Wang et al. 2012).
Sample
Cu
(wt%)a
Pd
(wt%)a
C
(wt%)b
H
(wt%)b
N
(wt%)b
Value of x and y
MOF-253·xPd(OAc)2
—
0.75
43.38
3.12
8.82
0.02
MOF-253·yCuI
8.41
—
34.79
2.39
6.87
0.54
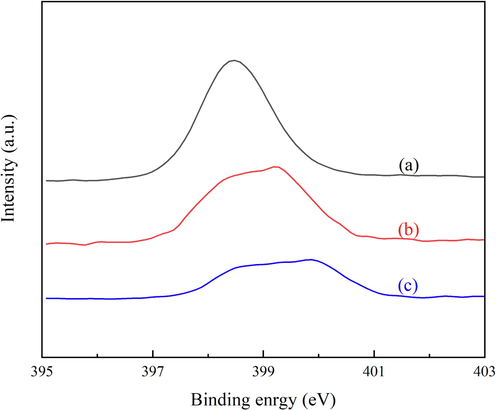
XPS spectra of the N 1s region for (a) MOF-253, (b) MOF-253·CuI and (c) MOF-253·Pd(OAc)2.
With the MOF-253 immobilized Pd and Cu solid catalysts prepared, 4-iodoanisole and phenylacetylene were selected as model substrates to optimize the Sonogashira coupling reaction conditions. Since the catalyst load greatly affects the economic benefits of the catalysts, the amount of the catalyst was firstly studied, and the results are shown in Fig. 3. When 0.073 mol% of MOF-253·Pd(OAc)2 in combination with 2.416 mol% of MOF-253·CuI (molar ratio of Cu to Pd was 33:1) were used as co-catalyst, Cs2CO3 as the base in DMSO at 80 °C, a good isolated yield (91%) of the target product (3a) was obtained after 12 h (entry 1). Fixed the molar content of Pd at 0.073 mol% and continuously reduced the molar content of Cu to 0.397 mol%, the yield reduced from 96% to 90% (entries 2 and 3), and the best molar ratio of Cu: Pd was 11:1. Besides, it was considered that the amounts of catalysts used were more than the actual demand, decreased the amount of the catalysts by half while maintaining the same ratio of Cu: Pd (11:1), an excellent yield of 90% was also obtained (entry 4 vs 2). The yield was significantly reduced to 48%, when 0.036% of MOF-253·Pd(OAc)2 was used alone with the same other reaction conditions (entry 5). Finally, the reaction carried out in the absence of palladium was used as a comparison, and only trace of the target product was detected (entry 6).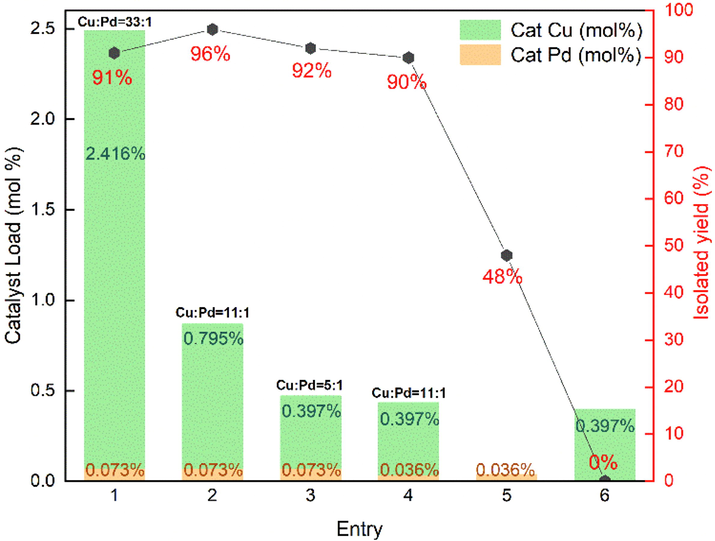
Effect of MOF-253 immobilized catalyst loading on the coupling reaction of 4-iodoanisole and phenylacetylene.
In order to test the effect of alkalis and solvents, Cu:Pd (11:1) with the smaller amounts of catalysts, instead of the highest yield one, was chosen as the starting catalyst system (entry 4 in Fig. 3 and entry 1 in Table 2). As shown in Table 2, various bases were examined, including K2CO3, Et3N, DABCO and t-BuOK, and the K2CO3 provided the best efficiency with a yield of 92% (entries 2–6). The control experiment indicated that the coupling reaction did not occur without a base (entry 7).
Entry
Base
Solvent
Temp. (°C)
Yield (%)b
TONc
TOF (h−1)c
1
Cs2CO3
DMSO
80
90
2500
208
2
K2CO3
DMSO
80
92
2556
213
3
Et3N
DMSO
80
47
1306
109
4
DABCO
DMSO
80
66
1833
153
5
K3PO4
DMSO
80
86
2389
199
6
t-BuOK
DMSO
80
trace
—
—
7
—
DMSO
80
—
0
0
8
K2CO3
DMF
80
53
1472
123
9
K2CO3
H2O
80
13
361
30
10
K2CO3
EtOH/H2O (1/1)
80
trace
—
—
11
K2CO3
CH3CN
80
36
1000
83
12
K2CO3
Dioxane
80
20
556
46
13
K2CO3
DMSO
90
93
2583
215
14
K2CO3
DMSO
70
98, 50d
2722
227
15
K2CO3
DMSO
60
55
1528
127
The reaction carried out in DMSO was faster than in DMF, H2O, EtOH/H2O, CH3CN or 1,4-dioxane (entries 8–12). When raised the reaction temperature from 80 °C to 90 °C only slightly increased the yield (entry 13). Surprisingly, the yield increased to 98% when the reaction temperature was reduced to 70 °C, but further lowering the temperature to 60 °C resulted in a dramatic decrease in the yield (entries 14 and 15). In the absence of MOF-253, the addition of Pd(OAc)2 and CuI only provided 50% yield (entry 14). Thus, the optimal coupling reaction conditions between 4 and iodoanisole and phenylacetylene are MOF-253·Pd(OAc)2 (0.036 mol%), MOF-253·CuI (0.397 mol%) and K2CO3 (2.0 equiv.) under N2 atmosphere in DMSO (2.0 mL) at 70 °C for 12 h. By calculating turnover number (TON) and turnover frequency (TOF) values, 0.036 mol% of Pd catalyst produced an extremely high TON of 2722 (entry 14), which was much higher than calculated values in other literatures (Ciriminna et al., 2013; Protti et al., 2005).
After successfully establishing optimized reaction conditions (entry 14 in Table 2), the substrate tolerances for various aryl iodides and different terminal alkynes were evaluated. The results are summarized in Table 3. The coupling reaction proceeded well for most aryl iodides and aryl alkynes, and furnished the desired products in good to quantity yields upon isolation. It is worth noting that the electronic nature of the para-substituents on aryl iodides has almost no effect on catalytic efficiency. For example, para-substituents aryl iodides with an electron-neutral group (3a), an electron-donating group such as OMe (3b), or electron-withdrawing groups such as Ph, F, COOH, CN or NO2 (3i, 3j, 3k, 3m and 3p), provided the corresponding products in 92–98% yield. Steric hindrance has a certain effect on the reaction. 99% yield was obtained when 1-iodo-3,5-dimethylbenzene was reacted with phenylacetylene (3c), but the yield of ortho-substituted aryl iodides (Me, i-Pr, OMe and NH2) decreased approximately to 65–90% (3d-3g). The reaction of methyl 5-iodosalicylate with phenylacetylene proceeded relatively slowly, thus yielding a final yield of 68% (3h). The adaptation range of the substituted aryl terminal alkynes was tested, bearing different functional groups such as NH2, F and n-C5H11. Among them, the reaction of 1-iodo-3,5-dimethylbenzene with 4-pentylphenylacetylene only provided the 76% yield caused by the incomplete conversion of aryl iodide (3o), while the other cases could be offered excellent yields of 87%-98% (3l-3n, 3p, 3r, 3s and 3w). Interestingly, heteroaryl iodides were very amenable under the standard reaction conditions, which showed the desired products in 93–98% yield (3q-3w). a Reaction conditions: 1 (0.5 mmol), 2 (0.55 mmol), MOF-253·Pd(OAc)2 (0.036 mol%), MOF-253·CuI (0.397 mol%), K2CO3 (1.0 mmol), DMSO (2.0 mL), 70 °C, 12 h, N2 atmosphere; isolated yield; b 24 h; c 100 °C; d 90 °C.
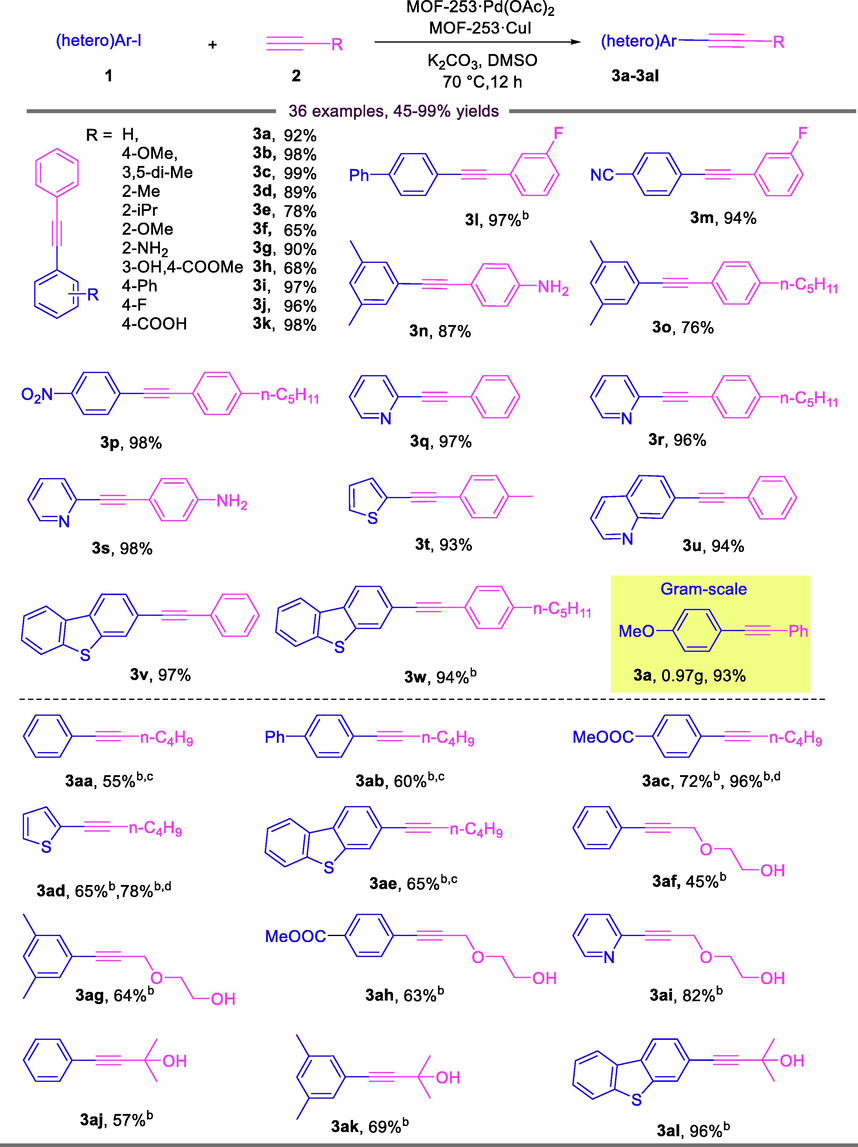
Due to the lower reactivity than aryl alkynes, alkynylation of aliphatic alkynes required prolonging the reaction time and/or elevating the temperature, and afforded relatively lower yields. For example, the reaction of 1-hexyne with aryl iodides gave moderate yields of 55%-72% (3aa-3ae). But the reaction of methyl 4-iodobenzoate and 2-iodothiophene with 1-hexyne can be significantly improved by raising the reaction temperature (3ac and 3ad). The same results were observed in the reactions between 2-(prop-2-yn-1-yloxy)ethan-1-ol or 2-methylbut-3-yn-2-ol with electron-donating or electron-withdrawing substituted aryl iodides (3af-3ah, 3aj and 3ak). The reactions involving of 2-iodopyridine and 2-iodo dibenzothiophene once again proved that they were perfect reaction substrates, and excellent yield of 82% and 96% were obtained, respectively (3ai and 3al).
To further verified the scalability and practicality of this present catalytic system, a scale-up reaction was carried out by taking 5.0 mmol of 1a and 5.5 mmol 2a under the standard conditions, and the expected product 3a was generated in 93% yield (Table 3).
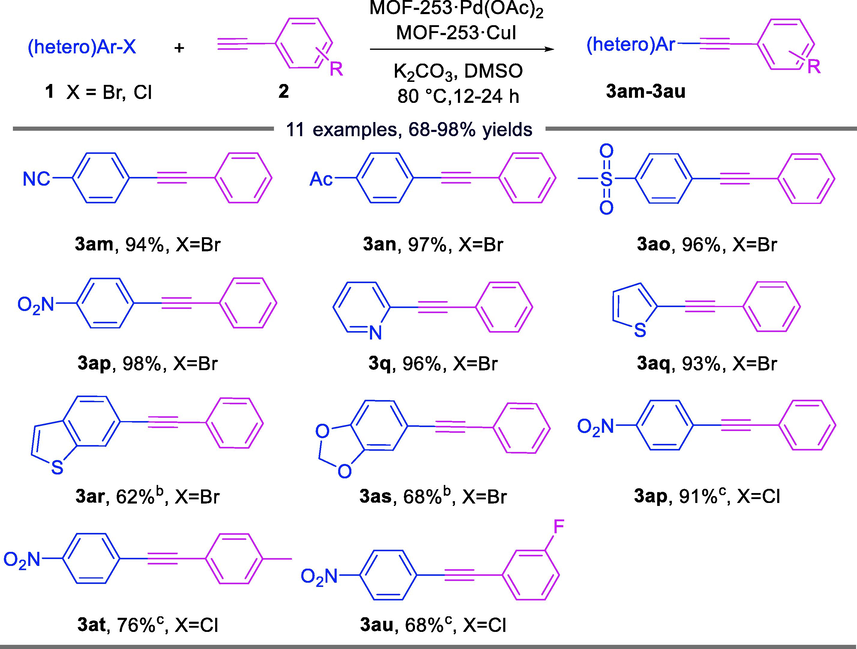
The catalytic system was also suitable for the reaction of different substituents of aryl bromides and phenylacetylene without changing other conditions, and only reacted at 80 °C for 24 h and achieved excellent yields of 94–98% (Table 4, 3am-3aq and 3q). For 5-bromobenzo[b]thiophene and 5-bromobenzo[d][1,3-dioxane], moderate yields of 62% and 68% were also obtained with the addition of 1.0 equivalent of tetrabutylammonium iodide (TBAI) and elevated temperature to 100 °C (3ar and 3as). The coupling of 4-chloronitrobenzene with phenylacetylenes occurred with poor conversion under optimized reaction conditions. Nevertheless, further higher temperature (120 °C) and the additive (2.0 equiv. of TBAB) were required, and 68%-91% yields were obtained (3ap-3au). a Reaction conditions: 1 (0.5 mmol), 2 (0.55 mmol), MOF-253·Pd(OAc)2 (0.036 mol%), MOF-253·CuI (0.397 mol%), K2CO3 (1.0 mmol), DMSO (2.0 mL), 80 °C, 24 h, N2 atmosphere, isolated yield; b 100 °C, 1.0 equiv. TBAI; c 120 °C, 2.0 equiv. TBAB.
Finally, the catalytic system was also applied to more complex drug-like substrates under the optimal conditions. As shown in Fig. 4, ethisterone, ethinylestradiol and norgestrel showed good efficiency, and estradiol had the best reactivity with a yield of 96% (4b). Meanwhile, 68% and 83% yields were obtained for ethisterone and norgestrel, respectively (4a and 4c).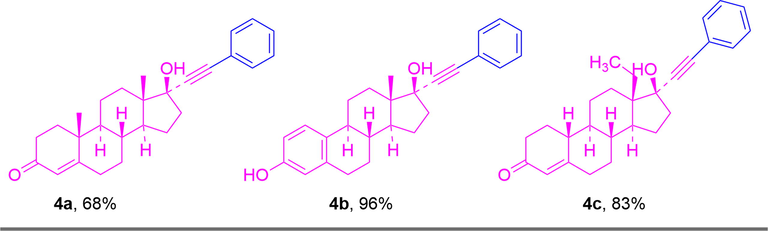
Coupling reactions of ethisterone, ethinylestradiol and norgestrel with iodobenzene. Reaction conditions: iodobenzene (0.5 mmol), ethisterone, ethinylestradiol and norgestrel (0.55 mmol), MOF-253·Pd(OAc)2 (0.036 mol%), MOF-253·CuI (0.397 mol%), K2CO3 (1.0 mmol), DMSO (2.0 mL), 100 °C, 24 h, N2 atmosphere; isolated yield.
The recoverability and reusability are important features of heterogeneous catalysts. Based on this, a recycling experiment with coupling reaction of 4-iodoanisole and phenylacetylene under the standard conditions was conducted to check the recyclability of the catalytic system. The catalysts of MOF-253·Pd(OAc)2 and MOF-253·CuI are fine powdery solids, and only 0.0026 g and 0.0015 g were used in the present experiment, respectively. In order to avoid the loss of catalyst caused in the separation process, after each reaction was completed, the reaction mixture was carefully extracted with petroleum ether several times, and the residual petroleum ether in the Schlenk tube was evaporated under reduced pressure at 80 °C. Then, the substances, partial solvent and base were re-added to the reaction tube and started a new cycle. The catalyst can be recycled at least five times without an obvious decrease in yield (Fig. 5).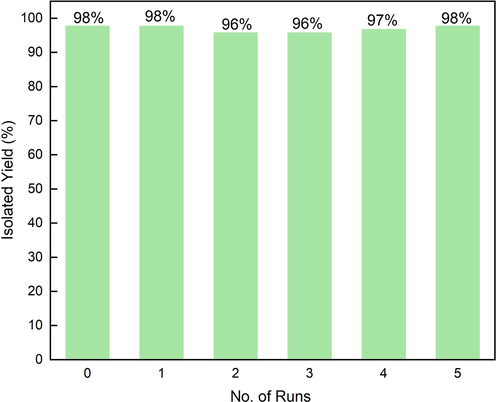
Reusability of MOF-253·Pd(OAc)2 and MOF-253·CuI for coupling reaction between 4-iodoanisole and phenylacetylene under the standard conditions.
To evaluate the catalytic effect of leached ions. The coupling reaction of 4-iodoanisole and phenylacetylene was subjected to a hot filtration test under optimized reaction conditions (Fig. 6d). After 1 h, the catalyst was separated by filtration at the reaction temperature, and the remaining mixture with another 2.0 equiv. K2CO3 was further stirred for 2 h, 3 h, 6 h, 9 h and 12 h, respectively. It was pointed out that the yield of desired product was not increased and stopped completely at 27% isolated yield after the removal of the catalyst, which indicated that the catalyst was a good heterogeneous catalyst for this type of reaction, and the reaction only occurred over the solid catalyst rather than in the solution.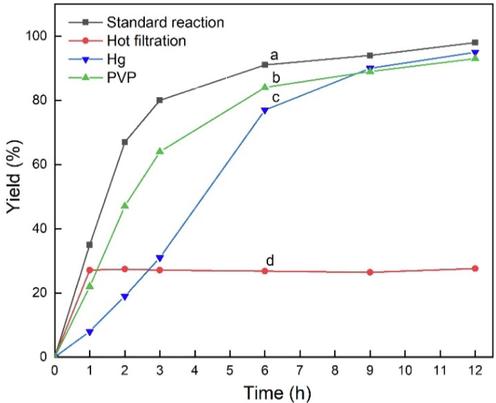
MOF-253·Pd(OAc)2 (Pd 0.036 mol%) and MOF-253·CuI (Cu 0.397 mol%) catalyzed the reaction of 4-iodoanisole with phenylacetylene: (a) reaction under optimized reaction conditions; (b) reaction in the presence of an excess amount of PVP; (c) reaction in the presence of an excess amount of Hg(0); (d) reaction under hot filtration conditions.
To further confirm the heterogeneous nature of the present catalytic system, the mercury-poisoning experiment was conducted with addition of excess Hg(0) to the reaction mixture as catalyst poison (1600-fold molar ratio relative to Pd). As shown in Fig. 6c, the reaction rate was slightly suppressed, but the final yield of 95% can still be achieved. It is well known that Hg(0) can poison heterogeneous catalysts always by fusing or adsorbing metal on the metal surface, which has been reported in many kinds of literature (Chernyshev et al., 2019; Gholinejad et al., 2016a,b; Richardson and Jones, 2006; Xie et al., 2018; Yu et al., 2005). However, different from common heterogeneous porous materials supported metal catalysts, in our catalytic system, MOF-253 immobilized Pd(OAc)2 or CuI were formed by the strong coordination between bpy and Pd or Cu, this proposal was consistent with the aforementioned higher binding energy of N 1s peak of the XPS spectrum. As we know, Hg(0) has no poisoning effect on homogeneous or ligand-protected Pd species, and due to the strong coordination between ligand with Pd/Cu, we reasonably believe that it was also fit to MOF-253·Pd(OAc)2 and MOF-253·CuI catalyst system. In addition, poly(vinyl pyridine) (PVP)-poisoning experiment was also carried out, which is another catalyst balancer and scavenger for soluble molecules Pd and soluble Pd(0) nanoparticles (Chernyshev et al., 2019; Gholinejad et al., 2016a,b; Richardson and Jones, 2006; Xie et al., 2018; Yu et al., 2005). Similar to the yield of standard conditions, 93% yield was obtained in the presence of PVP, which can be further confirmed the heterogeneous nature of our present co-catalyst (Fig. 6b).
In addition, the investigation was also performed to check the metal leaching of the catalyst for the model reaction. The liquid reaction solution was collected by hot filtration after the completion of the reaction and analyzed by AAS analysis. Only low than 0.18% of Pd and 0.19% of Cu content was detected in the reaction solution. The result proved again that the MOF-253 immobilized catalyst in this experiment was an excellent green solid catalyst.
Finally, we compared the activity of our catalysts with other reported palladium catalysts in the Sonogashira reaction (Table S1). It can be seen that the present catalysts exhibited a higher efficiency with higher yields, lower Pd loading and broad substrate scopes than other reported methods.
In general, the mechanism of Cu/Pd-co-catalyzed Sonogashira reaction is believed to take place through two catalytic cycles (Pd-cycle and Cu-cycle) and trans-metalation process. Considering the two metals both attached to the independent MOF, the traditional trans-metalation process would occur difficultly in the present process. In addition, we observed that 48% yield was still obtained, when 0.036% of MOF-253·Pd(OAc)2 was used alone under the same reaction conditions (entry 5 in Fig. 3). Therefore, all the results indicated that the Pd-cycle was the primary catalytic cycle for the present reaction. And the existence of MOF-253·CuI merely improved the reaction and conversion rate by promoting the formation of alkyne anions, which did not form an independent cycle process.
Based on these results, the reaction mechanism for this transformation was proposed and shown in Fig. 7. MOF-253·Pd(OAc)2 undergoes an oxidative addition reaction with an aryl halide to form an intermediate palladium complex (A). MOF-253·CuI reacts with terminal alkyne to form MOF-253·CuI π-alkyne complex (C), thus making the alkyne proton more acidic for more accessible abstraction (Camacho et al., 2017; Chinchilla and Nájera, 2007). Alkyne deprotonation by the K2CO3 would give the corresponding potassium acetylide (D), followed by a substitution reaction with complex (A) to form (B). Finally, reductive elimination reaction of (B) to provide the product and regenerate palladium catalyst.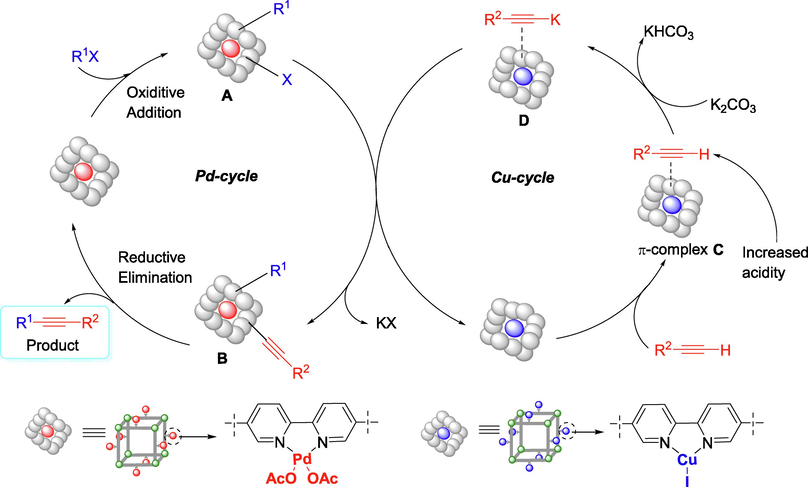
Plausible reaction mechanism.
4 Conclusion
In summary, two MOF-253 immobilized Pd and Cu catalysts were synthesized and evaluated as the heterogeneous co-catalyst for the Sonogashira reaction between (hetero)aryl halides with terminal alkynes. In this catalytic system, various (hetero)aryl iodides, bromides and chlorides can be coupled with aryl/aliphatic terminal alkynes smoothly, and provided the corresponding products in the range of 45–99% yields at 70–120 °C with low Pd loading (0.036 mol%) and high TON. The co-catalysts could be reused at least 5 times without significantly reducing activity. The thermal filtration testing, Hg(0) and PVP poisoning experiments demonstrated that the MOF-253 immobilized catalyst was a high-quality green heterogeneous catalytic system in this reaction. Further studies of other types of immobilized MOF catalytic reactions are currently underway in our laboratory and will be reported as appropriate.
Acknowledgement
We are grateful to the National Natural Science Foundation of China (21868032) and Natural Science Foundation of Hunan Province (2021JJ30290) for their financial support.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Efficient, selective, and recyclable palladium catalysts in carbon-carbon coupling reactions. Chem. Rev.. 2011;111:2251-2320.
- [Google Scholar]
- A novel 1,2,4-triazine- functionalized polystyrene resin-supported Pd(II) complex, a copper- and solvent-free highly efficient catalyst for Sonogashira coupling reactions. J. Organomet. Chem.. 2014;749:405-409.
- [Google Scholar]
- Recent advances in heterobimetallic palladium(II)/copper(II) catalyzed domino difunctionalization of carbon-carbon multiple bonds. Org. Biomol. Chem.. 2014;12:6767-6789.
- [Google Scholar]
- Nanoparticulate palladium supported by covalently modified silicas: Synthesis, characterization, and application as catalysts for the Suzuki coupling of aryl halides. Chem. Mater.. 2005;17:701-707.
- [Google Scholar]
- Mild Pd/Cu-catalyzed sila-Sonogashira coupling of (hetero)aryl bromides with (hetero)arylethynylsilanes under PTC conditions. Synlett. 2012;23:773-777.
- [Google Scholar]
- Metal insertion in a microporous metal-organic framework lined with 2,2'-bipyridine. J. Am. Chem. Soc.. 2010;132:14382-14384.
- [Google Scholar]
- DNA-supported palladium nanoparticles as a reusable catalyst for the copper- and ligand-free Sonogashira reaction. Catal. Sci. Technol.. 2017;7:2262-2273.
- [Google Scholar]
- Metal-organic frameworks incorporating multiple metal elements. Isr. J. Chem.. 2018;58:1036-1043.
- [Google Scholar]
- Immobilization of Pd(II) on MOFs as a highly active heterogeneous catalyst for Suzuki-Miyaura and Ullmann-type coupling reactions. Catal. Today. 2015;245:122-128.
- [Google Scholar]
- Pd and Pt catalyst poisoning in the study of reaction mechanisms: what does the mercury test mean for catalysis? ACS Catal.. 2019;9:2984-2995.
- [Google Scholar]
- The Sonogashira reaction: A booming methodology in synthetic organic chemistry. Chem. Rev.. 2007;107:874-922.
- [Google Scholar]
- Heterogeneous Sonogashira coupling over nanostructured siliacat Pd(0) ACS Sustain. Chem. Eng.. 2013;1:57-61.
- [Google Scholar]
- Synthesis and chemosensing properties of cinnolinecontaining poly(arylene ethynylene)s. Beilstein J. Org. Chem.. 2015;11:373-384.
- [Google Scholar]
- Nano–silica supported palladium catalyst: synthesis, characterization and application of its activity in Sonogashira cross–coupling reactions. J. Organomet. Chem.. 2017;853:5-12.
- [Google Scholar]
- Construction of a stable Ru-Re hybrid system based on multifunctional MOF-253 for efficient photocatalytic CO2 reduction. Inorg. Chem.. 2018;57:8276-8286.
- [Google Scholar]
- MOF-253-supported Ru complex for photocatalytic CO2 reduction by coupling with semidehydrogenation of 1,2,3,4-tetrahydroisoquinoline (THIQ) Inorg. Chem.. 2019;58:16574-16580.
- [Google Scholar]
- Metal–organic frameworks catalyzed C-C and C–heteroatom coupling reactions. Chem. Soc. Rev.. 2015;44:1922-1947.
- [Google Scholar]
- A BODIPY-functionalized PdII photoredox catalyst for Sonogashira C-C cross- coupling reactions. Chem. Commun.. 2019;55:4973-4976.
- [Google Scholar]
- A practical and efficient process for the preparation of tazarotene. Org. Process Res. Dev.. 2005;9:646-650.
- [Google Scholar]
- Palladium nanoparticles supported on MOF-5: a highly active catalyst for a ligand- and copper-free Sonogashira coupling reaction. Appl. Catal. A: Gen.. 2010;388:196-201.
- [Google Scholar]
- Graphene quantum dot modified Fe3O4 nanoparticles stabilize PdCu nanoparticles for enhanced catalytic activity in the Sonogashira reaction. ChemCatChem. 2017;9:1442-1449.
- [Google Scholar]
- Magnesium oxide supported bimetallic Pd/Cu nanoparticles as an efficient catalyst for Sonogashira reaction. J. Catal.. 2018;363:81-91.
- [Google Scholar]
- Novel oxime-palladacycle supported on clay composite as an efficient heterogeneous catalyst for Sonogashira reaction. Inorg. Chim. Acta. 2018;483:262-270.
- [Google Scholar]
- A novel polymer containing phosphorus-nitrogen ligands for stabilization of palladium nanoparticles: an efficient and recyclable catalyst for Suzuki and Sonogashira reactions in neat water. Dalton Trans.. 2015;44:14293-14303.
- [Google Scholar]
- Agarose functionalized phosphorus ligand for stabilization of small-sized palladium and copper nanoparticles: efficient heterogeneous catalyst for Sonogashira reaction. Tetrahedron. 2016;72:2491-2500.
- [Google Scholar]
- Palladium supported on bis(indolyl)methane functionalized magnetite nanoparticles as an efficient catalyst for copper-free Sonogashira-Hagihara reaction. Appl. Catal. A. 2016;525:31-40.
- [Google Scholar]
- Sustainable Fe–ppm Pd nanoparticle catalysis of Suzuki-Miyaura cross-couplings in water. Science. 2015;349:1087-1091.
- [Google Scholar]
- Sonogashira couplings catalyzed by Fe nanoparticles containing ppm levels of reusable Pd, under mild aqueous micellar conditions. ACS Catal.. 2019;9:2423-2431.
- [Google Scholar]
- Metal–organic frameworks as platforms for catalytic applications. Adv. Mater.. 2018;30:1703663.
- [Google Scholar]
- Recyclable heterogeneous palladium catalysts in pure water: sustainable developments in Suzuki, Heck, Sonogashira and Tsuji-Trost reactions. Adv. Syn. Catal.. 2010;352:33-79.
- [Google Scholar]
- Metal–organic framework materials as catalysts. Chem. Soc. Rev.. 2009;38:1450-1459.
- [Google Scholar]
- Palladium catalysis for aerobic oxidation systems using robust metal–organic framework. Angew. Chem. Int. Ed.. 2019;58:17148-17152.
- [Google Scholar]
- Visible-light-initiated Sonogashira coupling reactions over CuO/TiO2 nanocomposites. Catal. Sci. Technol.. 2019;9:377-383.
- [Google Scholar]
- Efficient and recyclable copper-based MOF-catalyzed N-arylation of N-containing heterocycles with aryl iodides. Org. Biomol. Chem.. 2016;14:10861-10865.
- [Google Scholar]
- Transition-metal-free highly chemo- and regioselective arylation of unactivated arenes with aryl halides over recyclable heterogeneous catalysts. Chem. Commun.. 2012;48:2033-2035.
- [Google Scholar]
- Applications of metal-organic frameworks in heterogeneous supramolecular catalysis. Chem. Soc. Rev.. 2014;43:6011-6061.
- [Google Scholar]
- Engineering homochiral metal-organic frameworks for heterogeneous asymmetric catalysis and enantioselective separation. Adv. Mater.. 2010;22:4112-4135.
- [Google Scholar]
- Activation of molecular oxygen by a metal–organic framework with open 2,2′-bipyridine for selective oxidation of saturated hydrocarbons. Chem. Commun.. 2012;48:12109-12111.
- [Google Scholar]
- Heterogeneous amorphous Cu-MOF-74 catalyst for C-N coupling reaction. ChemistrySelect. 2018;3:10694-10700.
- [Google Scholar]
- Tazarotene copper complexes: synthesis, crystal structure, DFT and biological activity evaluation. Polyhedron. 2016;109:99-106.
- [Google Scholar]
- Improved Sonogashira C-C coupling through clay supported palladium complexes with tridentate pincer bis-carbene ligands. Tetrahedron Lett.. 2003;44:6595-6599.
- [Google Scholar]
- Polymer- and ionic liquid-containing palladium: recoverable soluble cross-coupling catalysts. ChemCatChem. 2013;5:3460-3480.
- [Google Scholar]
- Zn(II)-bisthienylethynylbipyridine complex: Preparation, characterization and vapochromic behaviour in polymer films. Dyes Pigm.. 2014;110:249-255.
- [Google Scholar]
- Photo-cross-coupling reaction of electron-rich aryl chlorides and aryl esters with alkynes: a metal-free alkynylation. Angew. Chem. Int. Ed.. 2005;44:5675-5678.
- [Google Scholar]
- Poly(4-vinylpyridine) and quadrapure TU as selective poisons for soluble catalytic species in palladium-catalyzed coupling reactions–application to leaching from polymer-entrapped palladium. Adv. Synth. Catal.. 2006;348:1207-1216.
- [Google Scholar]
- Pd/Cu bimetallic nanoparticles embedded in macroporous ion-exchange resins: an excellent heterogeneous catalyst for the Sonogashira reaction. J. Mater. Chem. A. 2014;2:3986-3992.
- [Google Scholar]
- On the synergetic catalytic effect in heterogeneous nanocomposite catalysts. Chem. Rev.. 2013;113:2139-2181.
- [Google Scholar]
- Visible-light-assisted cobalt-2-(hydroxyimino)-1-phenylpropan-1-one complex catalyzed Pd/Cu-free Sonogashira-Hagihara cross-coupling reaction. ChemCatChem. 2018;10:758-762.
- [Google Scholar]
- Development of Pd–Cu catalyzed cross-coupling of terminal acetylenes with sp2-carbon halides. J. Organomet. Chem.. 2002;653:46-49.
- [Google Scholar]
- A convenient synthesis of acetylenes: catalytic substitutions of acetylenic hydrogen with bromoalkenes, iodoarenes and bromopyridines. Tetrahedron Lett.. 1975;16:4467-4470.
- [Google Scholar]
- Polymer supported palladium N-heterocyclic carbene complexes: long lived recyclable catalysts for cross coupling reactions. Tetrahedron Lett.. 2004;45:8977-8980.
- [Google Scholar]
- Continuous flow Sonogashira C-C coupling using a heterogeneous palladium–copper dual reactor. Org. Lett.. 2013;15:65-67.
- [Google Scholar]
- MOF-253-Pd(OAc)2: a recyclable MOF for transition-metal catalysis in water. RSC Adv.. 2016;6:56330-56334.
- [Google Scholar]
- Expedient five-step synthesis of SIB-1508Y from natural nicotine. J. Org. Chem.. 2006;71:8673-8675.
- [Google Scholar]
- Reduced graphene oxide supported Cu2O nanoparticles as an efficient catalyst for Sonogashira coupling reaction. Catal. Commun.. 2017;101:36-39.
- [Google Scholar]
- Ligand-free coupling of phenols and alcohols with aryl halides by a recyclable heterogeneous copper catalyst. RSC Adv.. 2012;2:5528-5530.
- [Google Scholar]
- 2,6-Bis(2-methylhydrazine-1- carbonyl)pyridine 1-oxide as an efficient ligand for copper-catalyzed C-N coupling reaction in water. Catal. Lett.. 2018;148:1142-1149.
- [Google Scholar]
- Pore surface engineering of metal-organic frameworks for heterogeneous catalysis. Coord. Chem. Rev.. 2018;376:248-276.
- [Google Scholar]
- Bronsted acidic ionic liquid-promoted amidation of quinoline N-oxides with nitriles. ACS Sustain. Chem. Eng.. 2018;6:7989-7994.
- [Google Scholar]
- Functional metal-organic frameworks for catalytic applications. Coord. Chem. Rev.. 2019;388:268-292.
- [Google Scholar]
- Evidence that SCS pincer Pd(II) complexes are only precatalysts in Heck catalysis and the implications for catalyst recovery and reuse. Adv. Synth. Catal.. 2005;347:161-171.
- [Google Scholar]
- Gram-scale preparation of Pd@PANI: a practical catalyst reagent for copper-free and ligand-free Sonogashira couplings. Org. Process Res. Dev.. 2016;20:2124-2129.
- [Google Scholar]
- 2-Pyrrolecarbaldiminato-Cu(II) complex catalyzed three-component 1,3-dipolar cycloaddition for 1,4-disubstituted 1,2,3-triazoles synthesis in water at room temperature. RSC Adv.. 2015;5:6661-6665.
- [Google Scholar]
- Glaser coupling- and Sonogashira coupling-control over CuxO nanoparticles/carbon nanotube by switching visible-light off and on. Appl. Catal. B: Environ.. 2022;300:120721.
- [Google Scholar]







