Translate this page into:
Facile preparation and properties of superhydrophobic nanocellulose membrane
⁎Corresponding author at: College of Chemical and Material Engineering, Quzhou University, Quzhou, Zhejiang 324000, China. liuguoqingmr@126.com (Guoqing Liu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Superhydrophobic nanocellulose membrane was prepared by synergistically modifying biodegradable nanocellulose with low-carbon perfluoroorganosiloxane and ethyl orthosilicate. The effects of four kinds of low-carbon perfluoroorganosiloxanes with different structures and their ratio to ethyl orthosilicate on the hydrophobic properties of nanocellulose membrane were investigated, and then FT-IR, XPS, XRD, SEM, TEM, AFM, TG and contact angle goniometer were used to characterize the structure and hydrophobic properties of nanocellulose membrane before and after modification. It is found that when the molar ratio of 1H,1H,2H,2H-perfluorooctyltrimethoxysilane (PFOTMS) to ethyl orthosilicate (TEOS) is 1, the modified nanocellulose membrane PFOTMS-TEOS-CNF is loaded with silica nanoparticles both inside and on its surface, and a micro-nano hierarchical rough morphology with low surface energy is constructed. At this point, the root-mean-square roughness (Rq) of nanocellulose membrane is 112 nm, and the static contact angle of water droplet is 153.5°, successfully realizing superhydrophobicity. In addition, compared to unmodified nanocellulose membrane, PFOTMS-TEOS-CNF with better thermal stability includes an additional maximum weight loss rate temperature (491.2 °C). The above advantages markedly improve the shortcomings of pristine nanocellulose, such as superhydrophilicity and insufficient thermal stability, and also broadens its high-value application in many fields.
Keywords
1H,1H,2H,2H-perfluorooctyltrimethoxysilane
Ethyl orthosilicate
Superhydrophobicity
Nanocellulose membrane
1 Introduction
The development and application of renewable biomass resources is of great significance to green and sustainable chemistry. Cellulose, as the most abundant, green and renewable biomass resource in nature, can be treated by physical and chemical processes to obtain nanocellulose with higher application value (Panchal et al., 2019; Kim et al., 2015). Compared with cellulose, nanocellulose has wider applications due to its nano-size effect, high elastic modulus, high mechanical strength, biocompatibility, regeneration, and degradability (Panchal et al., 2019; Kim et al., 2015; Jonoobi et al., 2015; Liu et al., 2018; Xie et al., 2019). In recent years, a series of novel nanocellulose-based composites with excellent properties have been developed in the fields of polymer materials (Li et al., 2020; Spinella et al., 2015; Xu et al., 2013; Xu et al., 2013), biomedicine (Dai and Si, 2019; Si and Xu, 2020; Jorfi and Foster, 2015; Si, 2019; Yang and Cranston, 2014), oil–water separation (Yang and Cranston, 2014; Bashar et al., 2017), and electronic devices (Kafy et al., 2016; Sadasivuni et al., 2015; Wang et al., 2016; Wang and Huang, 2021). However, nanocellulose has strong hydrophilic properties due to a large number of hydroxyl groups inherent in the structure, which largely limits its application in many practical fields. Therefore, to solve the problems, superhydrophobic modification of nanocellulose is necessary. Moreover, two conditions must be met to realize this purpose of superhydrophobicity (Wang and Jiang, 2007; Song and Rojas, 2013), one is the construction of a micro-nano rough surface structure, which is mainly based on the Wenzel and Cassie-Baxter model theory (Wenzel, 1936; Cassie and Baxter, 1944), and the other is the decrease of surface energy (Cheng et al., 2015).
At present, the research on nanocellulose-based superhydrophobic materials mainly includes three categories (Sun et al., 2021), nanocellulose-based superhydrophobic coatings (Tang et al., 2021; Liu et al., 2016), nanocellulose-based superhydrophobic membranes (Li et al., 2021; Zhang et al., 2019), and nanocellulose-based superhydrophobic aerogels (Hasan et al., 2019; Zuo et al., 2019; Chen et al., 2020). Among them, nanocellulose-based superhydrophobic membranes have recently attracted increasing attention and have become a research hotspot, and two kinds of methods have been developed for preparing the superhydrophobic membranes (Li et al., 2021; Wang et al., 2020; Shi et al., 2019; Ioannis et al., 2015; Baidya et al., 2017; Musikavanhu et al., 2019; Pegah et al., 2018). The first method is using organosilane compounds such as (1H,1H,2H,2H-perfluorodecyltrimethoxysilane, 1H,1H,2H,2H-perfluorodecyltrichlorosilane) to chemically modify nanocellulose, and then getting superhydrophobic nanocellulose membrane by spraying or vacuum filtration. However, C8 perfluorocompounds (including —(CF2)7CF3) involved in this method are difficult to degrade in the environment and have been completely banned internationally. Another method is by mixing nanocellulose with functionalized nanoparticles to build a rough surface structure to enhance the hydrophobicity of the membrane, and then by impregnating, spraying or chemical vapor deposition to graft low surface energy silane compounds onto the surface of the membrane to achieve superhydrophobicity. However, the complex preparation process, high equipment and environmental protection requirements greatly limit the popularization and application of the above two methods. Therefore, it is highly necessary to develop a simple, green and efficient method for the preparation of nanocellulose-based superhydrophobic membrane.
In this work, a facile method for the preparation of nanocellulose-based superhydrophobic membrane was developed. Nanocellulose emulsion was used as starting material, and low-carbon perfluoroorganosiloxane was used as modification reagent to reduce the surface energy, with simultaneous addition of ethyl orthosilicate for pre-hydrolysis. The modified nanocellulose mixed solution was prepared by a one-pot method, and then the superhydrophobic nanocellulose membrane was obtained by vacuum filtration, drying and solidification.
2 Experimental section
2.1 Reagents
Nanocellulose emulsion (CNF, 1 wt%) was purchased from Jinjiahao green nanomaterials Co., Ltd.; 1H,1H,2H,2H-perfluorooctyltrimethoxysilane (PFOTMS, 97%), (1,1,2,2-Tetrahydrononafluorohexyl)trimethoxysilane (NFHTMS, ≥97%), trimethoxy(pentafluorophenyl)silane (PFPTMS, ≥97%), 3,3,3-(trifluoropropyl)trimethoxysilane (TFPTMS, ≥96%) and ethyl orthosilicate (TEOS, 99.99%) were purchased from Shanghai Macklin biochemical technology Co., Ltd.; glacial acetic acid (AR) was purchased from Xilong scientific company.
2.2 Preparation of superhydrophobic nanocellulose membrane
30 mL of nanocellulose (CNF) emulsion was added to a three-necked flask with a condenser and a thermometer, and then the emulsion was heated up to 60 °C under vigorous stirring. Afterwards, 2 mmol of low-carbon perfluoroorganosiloxane and a certain amount of ethyl orthosilicate were added dropwise, and glacial acetic acid was added to adjust the pH value (until pH = 4). After reacting for 6 h, the mixture was cooled to room temperature, and then a certain amount of the resultant mixture was poured into a sand core funnel for the vacuum filtration, and then a wet nanocellulose membrane was obtained. Finally, the wet membrane was placed in an oven at 100 °C for 24 h to get the superhydrophobic nanocellulose membrane. The schematic diagram of PFOTMS-TEOS-CNF membrane fabrication was shown in Scheme 1.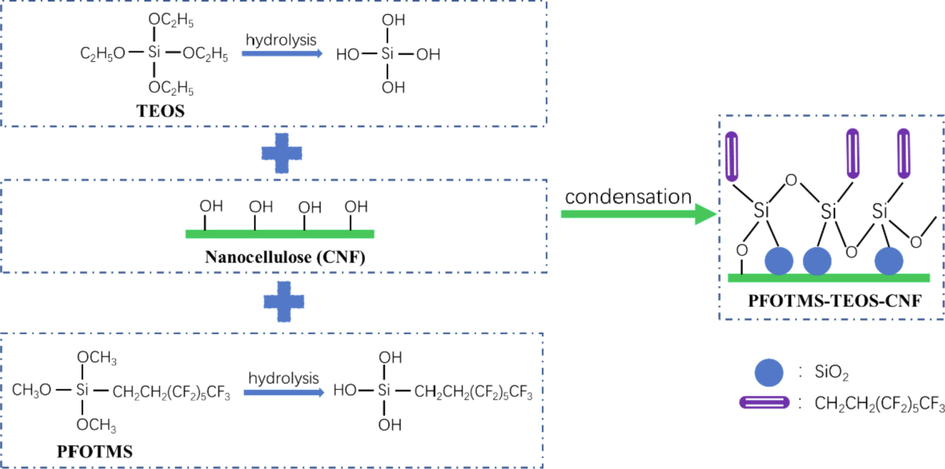
Schematic diagram of PFOTMS-TEOS-CNF membrane fabrication.
2.3 Characterization of samples
Fourier transform infrared spectroscopy (FTIR) of samples were recorded using an infrared spectrometer (Nicolet Is50, Thermo Fisher Scientific Co., Ltd.) in the wavenumber range of 4000–400 cm−1; The surface morphology of membranes were observed using a field emission scanning electron microscope (SU8010, Japan Hitachi Co., Ltd.) with the extra-high-tension (EHT) of 5.00 kV, while the samples were sputtered with a layer of Au before observation; Water contact angle (WCA) of membranes were measured using a contact angle goniometer (DSA30S, Kruss Scientific Instrument Co., Ltd.) with the deionized water droplet volume of 2 μL; The pyrolysis behaviors of samples were tested using a thermogravimetric analyzer (TGA2, Mettler-Toledo, Switzerland) with the temperature range of 30–600 °C at a heating rate of 10 °C/min under nitrogen atmosphere; Scanning within a 2θ range of 5–70°, X-ray diffraction patterns were obtained using an X-ray diffractometer (XRD-6100, Shimadzu, Japan) at a voltage of 40 kV, a current of 30 mA and a scan speed of 5°/min using Cu Kα radiation (λ = 0.154178 nm); The surface chemical composition and chemical bonding information of membranes were obtained by using a X-ray photoelectron spectrometer (K-Alpha, Thermo Fisher Scientific); The surface roughness of membranes were measured with a tapping mode by using a atomic force microscope (SPM-9700, Shimadzu, Japan); The micromorphology of cross section of the membranes were observed using a transmission electron microscope (JEM-2100F, JEOL, Japan) with an acceleration voltage of 200 kV, while the samples were pretreated by using ultrathin-section method before observation.
3 Results and discussion
3.1 Optimization of the selection of low-carbon perfluoroorganosiloxane
According to the preparation method of 2.2, four kinds of low-carbon perfluoroorganosiloxanes (PFOTMS, NFHTMS, PFPTMS and TFPTMS) were used to modify nanocelluloses to prepare four membranes without adding ethyl orthosilicate and glacial acetic acid, which were denoted as PFOTMS-CNF, NFHTMS-CNF, PFPTMS-CNF and TFPTMS-CNF, respectively. Contact angle goniometer and scanning electron microscope were used to investigate the water contact angle and surface microstructure of the samples, and the results are shown in Figs. 1 and 2, respectively. It is found that PFOTMS-CNF and PFPTMS-CNF display good hydrophobic properties with water contact angles of 132.2° and 126.6°, respectively, which mainly results from the existence of cross-linking between PFOTMS/PFPTMS and nanocellulose to make the formation of compact membrane (as shown in SEM images). At the same time, the introduction of fluorine-containing groups reduces the surface energy of membrane (Cardellini et al., 2021; Joseph and Ellen, 2021). As for NFHTMS-CNF and TFPTMS-CNF, the water contact angles are respectively 89.8° and 15.3°, and the surfaces are hydrophilic. Moreover, it can be seen from Fig. 2c–e that the surface structure of the modified samples is almost unchanged compared with that of CNF. Therefore, considering cost and performance, PFOTMS is finally determined to be the modification monomer.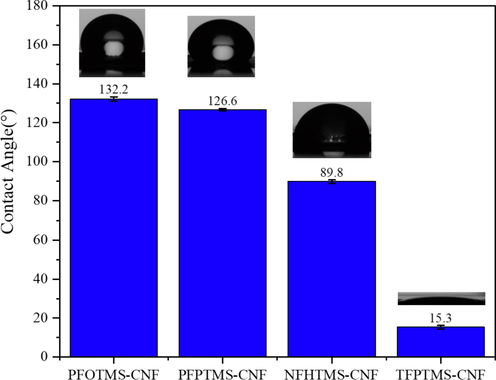
Contact angles of the prepared samples.
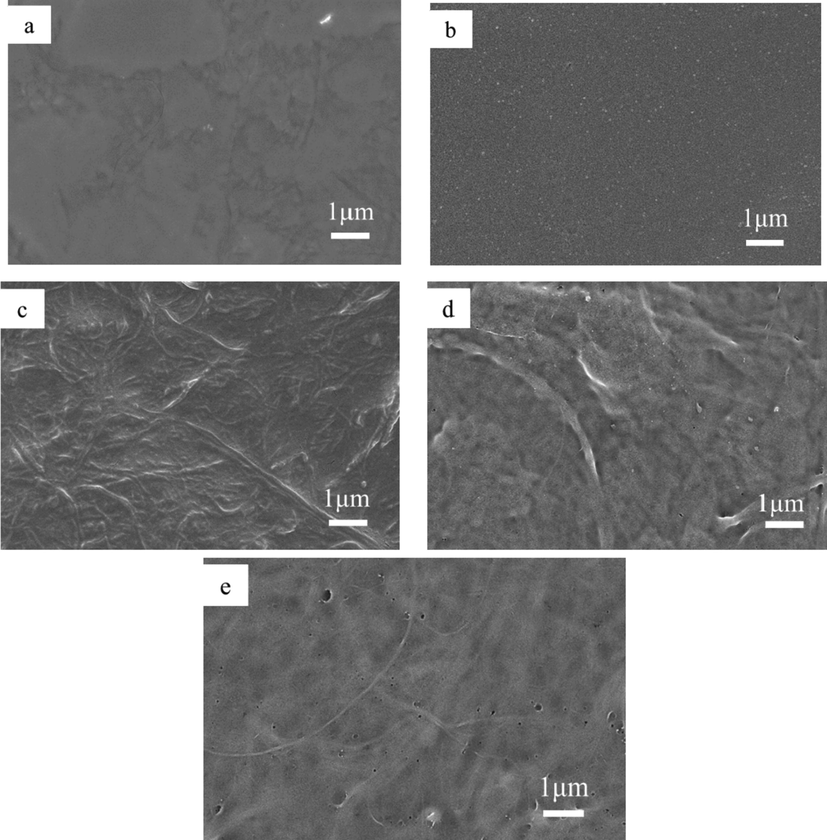
SEM images of (a) PFOTMS-CNF, (b) PFPTMS-CNF, (c) NFHTMS-CNF, (d) TFPTMS-CNF, (e) CNF.
3.2 Optimization of TEOS:PFOTMS molar ratio
According to the preparation method of 2.2, a series of hydrophobic nanocellulose membrane samples (PFOTMS-TEOS-CNF) were prepared by using PFOTMS as modification reagent, and the molar ratio of TEOS to PFOTMS was optimized. The static contact angle of water droplets and the macroscopic state of the surface of membrane were observed, and the results are shown in Table 1. The photographs and contact angle test pictures of samples are shown in Fig. 3. It is found that, when n (TEOS: PFOTMS) < 1, the water contact angle of the modified nanocellulose membrane gradually increases with the increasing amount of TEOS. When n (TEOS: PFOTMS) = 1, the modified nanocellulose membrane displays superhydrophobic property with a water contact angle of 153.5°, at which the surface of the sample is smooth. When n (TEOS: PFOTMS) = 1.25, the water contact angle is 156.9°, and the membrane still maintains superhydrophobic property, but the surface of the membrane is rough with some bulges. When n (TEOS: PFOTMS) = 1.5, the water contact angle is decreased to 143.4°, and the surface is rough and peeled off. Therefore, the generated silica will not be fully loaded on the nanocellulose with excess amount of TEOS, and the excess silica would cause bulge or falling off. So the molar ratio of TEOS to PFOTMS was determined to be 1, and all the PFOTMS-TEOS-CNF membranes in the following paragraphs refer to samples prepared under such condition.
n (TEOS: PFOTMS)
Contact angle (°)
Macroscopic State
0
132.2 ± 1.0
Smooth
0.75
145.3 ± 0.5
Smooth
1
153.5 ± 0.6
Smooth
1.25
156.9 ± 1.6
Rough and bulged
1.5
143.4 ± 2.1
Rough and peeled off

Photographs and contact angle test pictures of samples, (Note: inset 132.2° contact angle test picture is a duplication of inset in Fig. 1.).
3.3 IR spectra analysis
Infrared spectroscopy was used to characterize and analyze the changes of functional groups before and after modification of the nanocellulose membrane (Fig. 4). It can be seen that the spectra have obvious differences, the characteristic absorption peak of nanocellulose at 3335 cm−1 for the O—H stretching vibration is significantly weakened after modification, indicating that most O—H groups of nanocellulose has undergone the modification reaction. The C—O—C stretching vibration absorption peak of the nanocellulose at 1034 cm−1 shows a bule shift after modification (Zuo et al., 2019), that is because the introduction of electron-withdrawing perfluorinated groups has reduced the electron density of the nanocellulose skeleton structure. Moreover, the characteristic absorption peaks in the range of 1034–1545 cm−1 are partially overlapped before and after modification. The absorption peaks of CNF are mainly attributed to the stretching vibrations of C—OH and C—C and the bending vibrations of O—H and C—H (Le et al., 2016), while the absorption peak of modified CNF is attributed to the stretching vibration of CF2, CF3, Si—O—Si (Zuo et al., 2019; Gao et al., 2020; Jiang et al., 2021). Besides, the newly added characteristic peaks in the range of 560–804 cm−1 and the characteristic peak at 436 cm−1 are assigned to silica, which are mainly attributed to the stretching vibration of Si—C and Si—O—C (Le et al., 2016; Gao et al., 2020; Jiang et al., 2021). Therefore, these results verify that a chemical modification reaction occurs between PFOTMS, TEOS and CNF.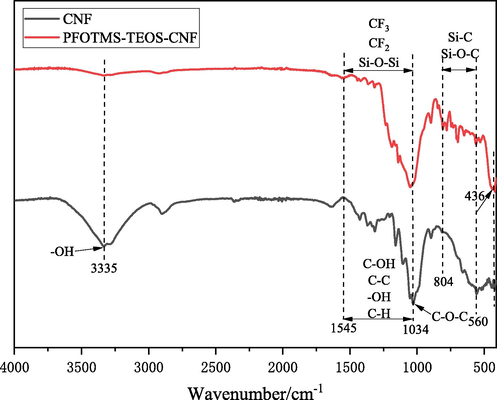
IR spectra of CNF and PFOTMS-TEOS-CNF.
3.4 XPS analysis
X-ray photoelectron spectroscopy (XPS) was used to characterize the surface element composition of the nanocellulose membranes before and after modification. Fig. 5a shows the XPS survey spectra of CNF and PFOTMS-TEOS-CNF, in addition to C 1s and O 1s characteristic peaks, the characteristic peaks of F 1s, Si 2s, and Si 2p are observed at the binding energies of 689, 154, and 102 eV, respectively, indicating that the incorporation of F and Si elements for the PFOTMS-TEOS-CNF. Fig. 5b, c, and d show the high-resolution XPS spectra of F 1s, Si 2p, and C 1s of PFOTMS-TEOS-CNF, respectively. Fig. 5c displays the peaks at 102.8, 102.1, and 101.5 eV, corresponding to Si—O, C—Si—O, and Si—C, respectively. The five characteristic peaks at 294.3, 292.0, 288.3, 286.8, and 285.6 eV in Fig. 5d are assigned to CF3, CF2, O—C—O, C—O, and C—C/C—H/C—Si (Piłkowski et al., 2021; Giner et al., 2019; Chen et al., 2021), respectively. The above results further demonstrate the successful modification of PFOTMS and TEOS for nanocellulose.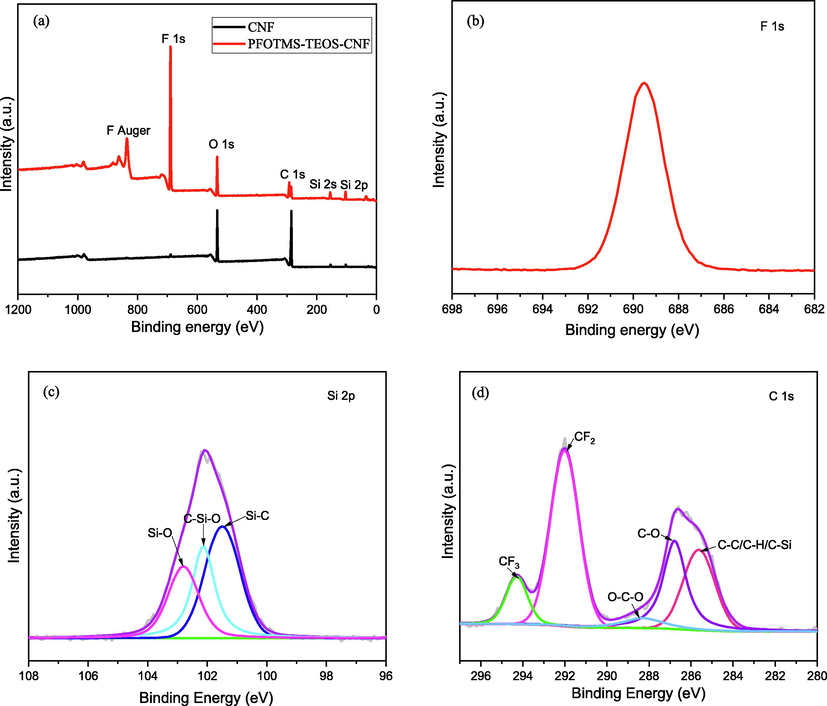
(a) XPS survey spectra of the prepared samples, high-resolution XPS spectra of (b) F 1s, (c) Si 2p and (d) C 1s.
3.5 XRD analysis
X-ray diffraction was used to characterize and analyze the changes in the crystal phase structure of the nanocellulose membrane (CNF) before and after modification (Fig. 6). It can be seen that the CNF has the characteristic diffraction peaks at 2θ = 15.0, 16.5, 18.5 and 23.0°. And the modified PFOTMS-TEOS-CNF has a new characteristic diffraction peak at 2θ = 26.6°, which is assigned to (4 2 2) facet of crystalline silica. While the characteristic diffraction peaks at 2θ = 15.0°, 16.5° basically disappear and the characteristic diffraction peaks at 2θ = 18.5°, 23.0° obviously weaken, indicating that the crystallinity of the modified PFOTMS-TEOS-CNF significantly decreases, which is caused by the formation and incorporation of crystalline silica and cross-linking reaction between PFOTMS/TEOS and nanocellulose. All above destroy the crystal structure of nanocellulose to some extent and reduce its lattice order degree.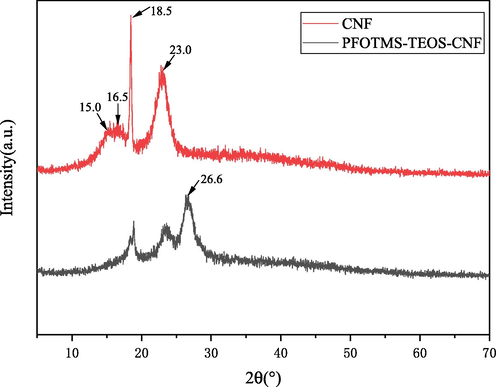
XRD patterns of CNF and PFOTMS-TEOS-CNF.
3.6 SEM and TEM analysis
Scanning electron microscopy was used to characterize and analyze the changes in the surface microstructure of the nanocellulose membranes before and after modification (Fig. 7). Fig. 7a and b are the SEM images of the original nanocellulose membrane, which displays a nanofibrillar structure, a relatively smooth surface, and a small number of nanopores. Fig. 6c and d show the SEM images of the modified PFOTMS-TEOS-CNF membrane, it is found that the PFOTMS-TEOS-CNF has a more obvious network structure formed by interweaving of nanofibrils. There are a large number of nanopores in the framework, and a large number of stacked silica nanoparticles evenly load on the surface, thereby a porous hierarchical structure with a good micro-nano rough surface is constructed. After modification, the surface morphology of the membrane changes from the Wenzel model state to the Cassie-Baxter model state, and the surface hydrophobicity is significantly improved, as shown in Fig. 8.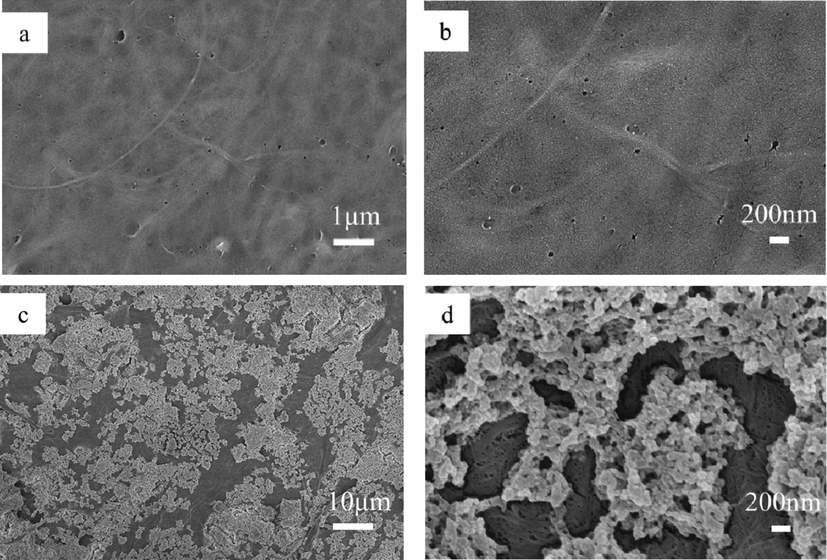
SEM images of (a, b) CNF and (c, d) PFOTMS-TEOS-CNF. (Note: (b) is a zoom of central area of (a). (d) is a zoom of central area of (c). (a) is a duplication of Fig. 2e.).

Image of superhydrophobicity and contact angle test of PFOTMS-TEOS-CNF. (Note: inset 153.5° contact angle test picture is a duplication of inset in Fig. 3.).
The micromorphology of cross section of the superhydrophobic PFOTMS-TEOS-CNF membrane was characterized by TEM, while the sample was pretreated by using ultrathin-section method before observation. The results are shown in Fig. 9. It is found that the modified PFOTMS-TEOS-CNF still retains the network structure of nanofibers, and the fibers of PFOTMS-TEOS-CNF are decorated with a large number of tiny silica nanoparticles. So combined with the SEM results, it can be concluded that the modified nanocellulose membrane PFOTMS-TEOS-CNF is loaded with silica nanoparticles both inside and on its surface.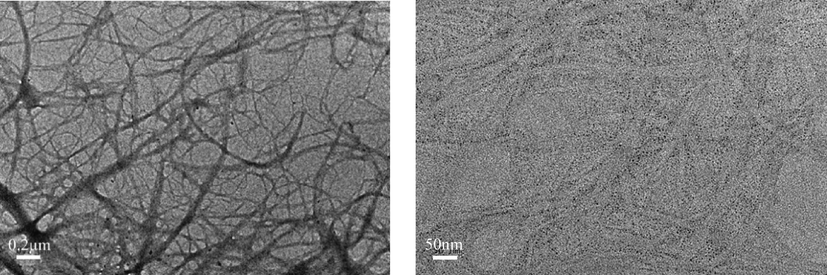
TEM images of PFOTMS-TEOS-CNF.
3.7 AFM analysis
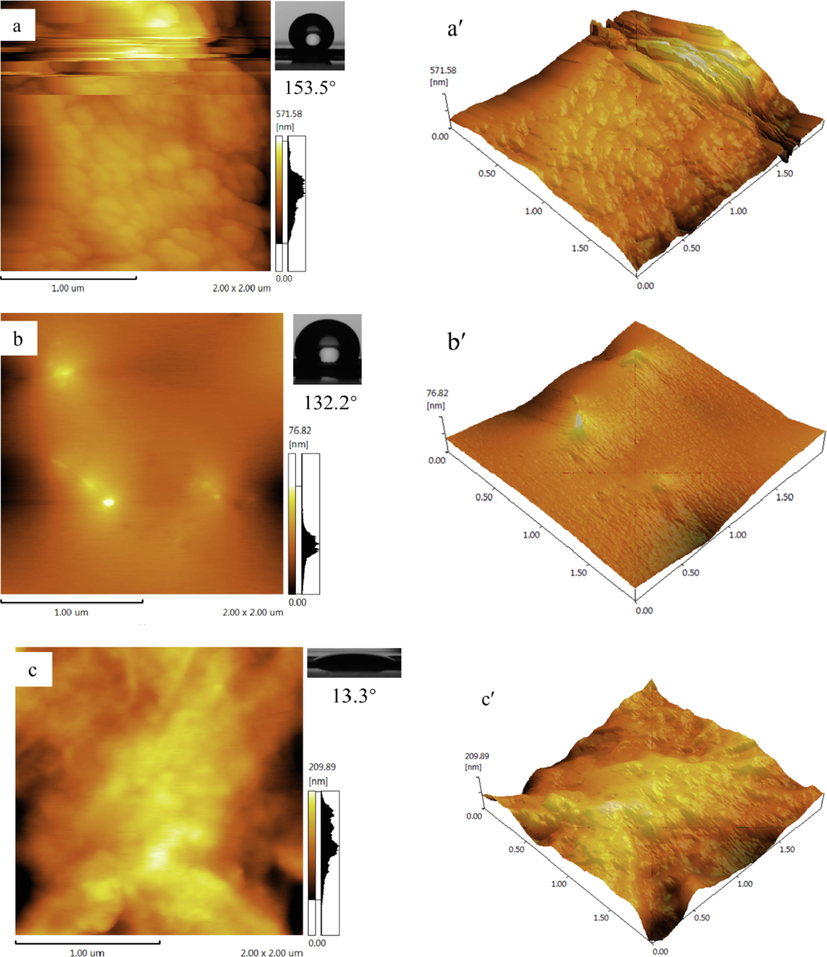
Surface roughness is one of the most important factors affecting the hydrophobicity of the membrane. Atomic force microscopy was used to characterize and analyze the surface roughness of PFOTMS-TEOS-CNF, PFOTMS-CNF, and CNF membranes, as shown in Fig. 10. The root-mean-square roughness (Rq) of PFOTMS-TEOS-CNF surface with superhydrophobicity is 112 nm, and the water contact angle is 153.5°. The root-mean-square roughness (Rq) of PFOTMS-CNF with hydrophobicity is 10 nm, and the water contact angle is 132.2°. The root-mean-square roughness (Rq) of the unmodified CNF is 45 nm, and it is highly wettable with the water contact angle of 13.3°. The surface roughness order of the three samples is: PFOTMS-TEOS-CNF > CNF > PFOTMS-CNF, which is consistent with the SEM images. Above results show that PFOTMS-CNF modified by single PFOTMS has good hydrophobicity because of its low surface energy, but the roughness is not enough to achieve superhydrophobicity. However, PFOTMS-TEOS-CNF modified by PFOTMS and TEOS both have low surface energy and good roughness, so its superhydrophobic performance is achieved.
3.8 TG analysis
Thermogravimetric analyzer was used to characterize the thermal stability of the nanocellulose membrane before and after modification, Fig. 11a and b are the TG and DTG curves, respectively. It can be seen that with the increase of temperature, CNF undergoes a rapid thermal decomposition weight loss process at 240 °C, and the maximum weight loss rate occurs at 339.5 °C, and then it tends to slow thermal decomposition after 390 °C. The same phenomenon is observed in the temperature range of 240–390 °C for PFOTMS-TEOS-CNF, which indicates that thermal weight loss at this stage mainly results from the decomposition of a small amount of unmodified CNF (Le et al., 2016; Abraham et al., 2011; Liu et al., 2016). Combined with the DTG results, the thermal behavior of the modified PFOTMS-TEOS-CNF has undergone significant changes with two maximum weight loss rate temperatures: 339.5 and 491.2 °C, and also it is found that the rapid thermal decomposition process still occurs after 390 °C. The thermal stability of PFOTMS-TEOS-CNF is significantly improved, which further indicates that most of the hydroxyl groups on the nanocellulose are modified to form a certain degree of chemical cross-linking.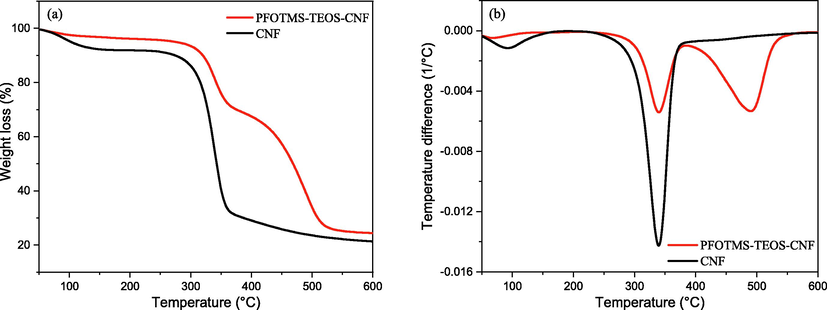
(a) TG and (b) DTG curves of CNF and PFOTMS-TEOS-CNF.
4 Conclusions
In conclusion, the superhydrophobic nanocellulose membrane was successfully prepared by using both low-carbon perfluoroorganosiloxane and ethyl orthosilicate as modification reagents with a one-pot method. PFOTMS was screened as the low-carbon perfluoroorganosiloxane modification monomer. When the molar ratio of TEOS to PFOTMS is 1, the water contact angle of the prepared modified nanocellulose membrane PFOTMS-TEOS-CNF reaches 153.5°, and superhydrophobic property is achieved. The modified nanocellulose membrane PFOTMS-TEOS-CNF retains the crystalline network structure of nanocellulose and loads a large amount of silica nanoparticles both inside and on its surface. In the work, PFOTMS is responsible for the decrease of surface energy, and TEOS is responsible for formation of silica nanoparticles to construct a micro-nano rough surface structure, which synergistically create the typical rough surface with low surface energy to obtain superhydrophobicity, while the water droplet is in a Cassie-Baxter state on the membrane surface. Furthermore, PFOTMS-TEOS-CNF displays better thermal stability compared with CNF. The prepared superhydrophobic nanocellulose membrane is a promising candidate for the superhydrophobic environmental protection composite materials, which will greatly expand its potential applications in the areas of self-cleaning, energy storage, and waterproof packaging materials.
Acknowledgements
This work was supported by Basic Public Welfare Research Project of Zhejiang Province (LGG21B060001), and Science and Technology Project of Institute of Zhejiang University-Quzhou (IZQ2019-KJ-026).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Extraction of nanocellulose fibrils from lignocellulosic fibres: A novel approach. Carbohyd. Polym.. 2011;86:1468-1475.
- [Google Scholar]
- Organic solvent-free fabrication of durable and multifunctional superhydrophobic paper from waterborne fluorinated cellulose nanofiber building blocks. ACS Nano. 2017;11(11):11091-11099.
- [Google Scholar]
- Superhydrophobic surfaces with fluorinated cellulose nanofiber assemblies for oil-water separation. RSC Adv.. 2017;7:37168-37174.
- [Google Scholar]
- Integrated molecular dynamics and experimental approach to characterize low-free-energy perfluoro-decyl-acrylate (PFDA) coated silicon. Mate. Design. 2021;208:109902
- [Google Scholar]
- Feasibility Study of Dielectric Barrier Discharge Jet-Patterned Perfluorodecyltrichlorosilane-Coated Paper for Biochemical Diagnosis. ECS J. Solid State SC.. 2021;10:037005
- [Google Scholar]
- Enhanced Oil Adsorption and Nano-Emulsion Separation of Nanofibrous Aerogels by Coordination of Pomelo Peel-Derived Biochar. Ind. Eng. Chem. Res.. 2020;59(18):8825-8835.
- [Google Scholar]
- Surface adhesive forces: A metric describing the drag-reducing effects of superhydrophobic coatings. Small. 2015;11:1665-1671.
- [Google Scholar]
- Recent advances on cellulose-based nano-drug delivery systems: design of prodrugs and nanoparticles. Curr. Med. Chem.. 2019;26(14):2410-2429.
- [Google Scholar]
- Preparation and characterization of hydrophobic cellulose nanofibril aerogels. J. Funct. Mater.. 2020;51(2):02107-02112.
- [Google Scholar]
- Water adsorption and capillary bridge formation on silica micro-particle layers modified with perfluorinated organosilane monolayers. Appl. Surf. Sci.. 2019;475:873-879.
- [Google Scholar]
- Robust Superhydrophobic Cellulose Nanofiber Aerogel for Multifunctional Environmental Applications. Polymers. 2019;11(3):495.
- [Google Scholar]
- Facile Method to Prepare Superhydrophobic and Water Repellent Cellulosic Paper. J. Nanomater. 2015:219013.
- [Google Scholar]
- Preparation of the Temperature-Responsive Superhydrophobic Paper with High Stability. ACS Omega. 2021;6:16016-16028.
- [Google Scholar]
- Different preparation methods and properties of nanostructured cellulose from various natural resources and residues: A review. Cellulose. 2015;22:935-969.
- [Google Scholar]
- Recent advances in nanocellulose for biomedical applications. J. Appl. Polym. Sci.. 2015;132(14):41719.
- [Google Scholar]
- Recent advances in the preparation of semifluorinated polymers. Polym. Chem.. 2021;12(45):6515-6526.
- [Google Scholar]
- Cellulose nanocrystal/graphene oxide composite film as humidity sensor. Sensor. Actuat. A-Phys.. 2016;247:221-226.
- [Google Scholar]
- Review of Nanocellulose for Sustainable Future Materials. Int. J. Pr. Eng. Man-gt.. 2015;2:197-213.
- [Google Scholar]
- Preparing hydrophobic nanocellulose-silica film by a facile one-pot method. Carbohyd. Polym.. 2016;153:266-274.
- [Google Scholar]
- Strong and Superhydrophobic Wood with Aligned Cellulose Nanofibers as a Waterproof Structural Material. Chinese J. Chem.. 2020;38(8):823-829.
- [Google Scholar]
- Preparation methods and research progress of superhydrophobic paper. Coordin. Chem. Rev.. 2021;449:214207
- [Google Scholar]
- Mussel-inspired cellulose-based nanocomposite fibers for adsorption and photocatalytic degradation. ACS Sustain. Chem. Eng.. 2018;6(11):15756-15763.
- [Google Scholar]
- Recent Progress in Fabrication and Applications of Superhydrophobic Coating on Cellulose-Based Substrates. Materials. 2016;9(3):124.
- [Google Scholar]
- Preparation, modification and its application of nanocrystalline cellulose in barrier coating of cellulosic paper. J. Funct. Mater.. 2016;10(47):10239-10244.
- [Google Scholar]
- Facile method for the preparation of superhydrophobic cellulosic paper. Appl. Surf. Sci.. 2019;496:143648.
- [Google Scholar]
- Trends in advanced functional material applications of nanocellulose. Processes. 2019;7(1):10.
- [Google Scholar]
- Superhydrophobic paper from nanostructured fluorinated cellulose esters. ACS Appl. Mater. Inter.. 2018;10(13):11280-11288.
- [Google Scholar]
- Environmental testing of hydrophobic fluorosilane-modified substrates. Surf. Interfaces. 2021;23
- [Google Scholar]
- Transparent and Flexible Cellulose Nanocrystal/Reduced Graphene Oxide Film for Proximity Sensing. Small. 2015;11(8):994-1002.
- [Google Scholar]
- Fabrication of transparent and superhydrophobic nanopaper via coating hybrid SiO2/MWCNTs composite. Carbohyd. Polym.. 2019;225:11522.
- [Google Scholar]
- The development of lignocellulosic biomass in medicinal applications. Curr. Med. Chem.. 2019;26(14):2408-2409.
- [Google Scholar]
- Recent advances in bio-medicinal and pharmaceutical applications of bio-based materials. Curr. Med. Chem.. 2020;27(28):4581-4583.
- [Google Scholar]
- Approaching super-hydrophobicity from cellulosic materials: A Review. Nord. Pulp Pap. Res. J.. 2013;28(2):216-238.
- [Google Scholar]
- Polylactide/cellulose nanocrystal nanocomposites: Efficient routes for nanofiber modification and effects of nanofiber chemistry on PLA reinforcement. Polymer. 2015;65:9-17.
- [Google Scholar]
- Recent Advances in Hydrophobic Modification of Nanocellulose. Cur. Org. Chem.. 2021;25(3):417-436.
- [Google Scholar]
- Superhydrophobic Hierarchical Structures from Self-Assembly of Cellulose-Based Nanoparticles. ACS Sustain. Chem. Eng.. 2021;9:14101-14111.
- [Google Scholar]
- Transparent, conductive and superhydrophobic cellulose films for flexible electrode application. RSC Adv.. 2021;11:36607-36616.
- [Google Scholar]
- Superhydrophobic paper fabricated via nanostructured titanium dioxide-functionalized wood cellulose fibers. J. Mater. Sci.. 2020;55:7084-7094.
- [Google Scholar]
- Tailoring percolating conductive networks of natural rubber composites for flexible strain sensors via a cellulose nanocrystal templated assembly. Soft Matter. 2016;12:845-852.
- [Google Scholar]
- Resistance of solid surfaces to wetting by water. Ind. Eng. Chem.. 1936;28(8):988-994.
- [Google Scholar]
- Preparation of thermally stable and surface-functionalized cellulose nanocrystals via mixed H2SO4/Oxalic acid hydrolysis. Carbohyd. Polym.. 2019;223:115116
- [Google Scholar]
- Mechanical and thermal properties of waterborne epoxy composites containing cellulose nanocrystals. Polymer. 2013;54(24):6589-6598.
- [Google Scholar]
- Cellulose Nanocrystals vs. Cellulose Nanofibrils: A Comparative Study on Their Microstructures and Effects as Polymer Reinforcing Agents. ACS Appl. Mater. Inter.. 2013;5:2999-3009.
- [Google Scholar]
- Chemically cross-linked cellulose nanocrystal aerogels with shape recovery and superabsorbent properties. Chem. Mater.. 2014;26:6016-6025.
- [Google Scholar]
- Reactive superhydrophobic paper from one-step spray-coating of cellulose-based derivative. Appl. Surf. Sci.. 2019;497:143816
- [Google Scholar]
- Superamphiphobic nanocellulose aerogels loaded with silica nanoparticles. Cellulose. 2019;26:9661-9671.
- [Google Scholar]