Translate this page into:
Integration of in silico and in vitro approaches to evaluate antioxidant and anticancer properties of Tribulus terrestris extracts
⁎Corresponding authors. saimamuzammil83@gmail.com (Saima Muzammil), sulman.shafeeq@ki.se (Sulman Shafeeq)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Plants have been found useful in treating many human diseases caused by bacteria and viruses. The ability to synthesize compounds by plant secondary metabolism makes them an invaluable source of pharmaceutical and therapeutic products. The present study was designed to evaluate the phytochemical constituents, antioxidant, and anticancer activities of Tribulus terrestris seed extracts on HepG2 cell lines. TPC and TFC contents were 51 ± 0.7 mg GAE/g and 66.5 ± 0.4 mg QE/g, respectively. The antioxidant profile of the T. terrestris revealed that all the extracts have antioxidant potential and display the highest antiradical behavior in the pattern of methanolic > acetonic > chloroform > n-hexane, through DPPH, FRAP, OH radical scavenging, and NO radical scavenging assays. The antioxidant activity explored at the cellular level against H2O2-induced DNA damage showed a dose-dependent antioxidant effect of T. terrestris. Moreover, the methanolic extracts of all plant extracts showed notable thrombolytic potentials, the percentage of clot lysis accounted for T. terrestris was 33%, 27%, 17%, and 6% which indicated the significant clot lysis of methanolic and acetonic extracts in contrast to positive and negative standards. The genotoxicity was assessed through comet assay which exposed that T. terrestris at a low dose (0.5 mg/mL) is considered to be safe for effective treatment. MTT assay using HepG2 cell lines revealed that the highest tested concentration i.e., 100 μg/mL of the methanolic extract resulted in 86% cell viability compared to the control group. In silico study, from 14 selected compounds, three compounds, Heptacosane, Apiol, and Palmitic acid showed an affinity with target protein 51X0. The present findings may serve as a guideline for the standardization and validation of natural drugs containing the T. terrestris as an ingredient.
Keywords
T. terrestris
Zygophyllaceae
DPPH
Anticancer
In silico
Methanolic extract
1 Introduction
Medicinal plants are key components as a widely available primary treatment for healthcare remedies (Aminah et al., 2021). Most medicinal plants are used all over the world to treat various diseases, including inflammation, heart diseases, cancer, diabetes, aging, cardiac dysfunction and other degenerative disorders (Andleeb et al., 2020). The use of herbal remedies is now in demand because of their easy availability, cost-effectiveness, and safer use than contemporary pharmaceuticals (Maher et al., 2021). Because the quest for a cure is a lengthy process, experts have shifted their focus to prevention rather than treatment (Santhoshkumar et al., 2019). Almost all organisms have acquired antioxidant defense mechanisms and DNA repair processes to defend themselves from oxidative damage; however, these systems are insufficient to completely avoid the damage (Narayanaswamy and Balakrishnan, 2011). The two major elements of cells are DNA and membrane, which are essential for cell viability and proliferation·H2O2 was known to provoke DNA strand breaks by generating hydroxyl radicals (OH●). This might result in DNA instabilities, and mutations ultimately cause cancer (Halliwell & Gutteridge, 2015). Therapeutic plants and related compounds are directly linked to their ability to quench the radical substances by electron donation and eventually stop the radical chain reaction, thereby protecting the body against different diseases (Narayanaswamy and Balakrishnan, 2011). Diet rich in phenolic and flavonoid possesses antioxidant activity, which has attracted the attention of scientists all over the world (Tungmunnithum et al., 2018).
Oxidative stress, linked to several health problems like thromboembolic disorders such as pulmonary emboli, deep vein thrombosis, strokes and heart attacks are the main causes of morbidity and mortality which come from a shortage of endogenous antioxidant protection (Alamgir, 2017). An estimated 17.5 million people died in 2012 as a result of cardiovascular diseases (CVDs), which accounts for 31 percent of global fatalities, according to the World Health Organization. It is estimated that 7.4 million of these deaths were due to heart disease, with 6.7 million owing to stroke. In developing countries, synthetic anti-thrombolytic agents such as streptokinases (SC) or tissue plasminogen activators (t-PA) have increased popularity in the treatment of heart or thrombotic diseases due to their low cost (Uddin et al., 2019). However, in some medical cases, such as a nerve lesions case, gastrointestinal bleeding, or hypertension, the use of these substances is not effective. So, new research is moving towards the use of natural resources to evaluate safer and more effective antiplatelet, anticoagulant, and antithrombotic properties (Lu et al., 2017).
Natural antioxidants present in many medicinal plants are inhibiting the harmful effects of oxidative stress. Several bioactive chemicals found in plants act as free and oxidation-free radical scavengers (Phuyal et al., 2020). Natural phenolic compounds and flavonoids are secondary metabolites that embrace a tiny hydroxyl aromatic rings group (Lu et al., 2017). Plant polyphenols are reported to have anti-cancer activity and are also involved in reactive oxygen species (ROS) mediated pathways that inhibit oxidative stress and DNA damage implicated in mutagenesis, carcinogenesis, and premature aging.
Flavonoids are biologically active in anti-inflammatory, antioxidant, antibacterial, antiviral, antiplatelet, anti-carcinogenic, and DNA protection-like activities (Sudhakaran et al., 2014). They can reduce the availability of oxidants and other damaging compounds because of their ability to quench free radicals from oxygen by giving hydrogen or an electron, chelated redox-active metals, and inhibit lipooxygenases (Merghem et al., 2021).
Tribulus terrestris belongs to the Zygophyllaceae family. It is commonly known as puncture vine, yellow vine, hard thorn, gokharu, bhakdi or bhakra in Punjabi (Chhatre et al., 2014). It is widely distributed in tropical and mildly temperate regions like Pakistani, India, Panama, and South East Asia (Mathur and Sundaramoorthy, 2013). This plant is also described in the traditional medicine system for its use as an antidepressant and anti-inflammatory. It has been reported that the phytochemicals such as steroidal saponins and flavonoids have prominent anti-inflammatory and antiaging activities (Akram et al., 2011). T. terrestris was also reported to have antibacterial, antiviral, antioxidant, and antihyperglycaemic activities (Mushtaq et al., 2020).
The present study was designed to evaluate the phytochemical (total phenolics and flavonoid content), and in vitro biological activities like antioxidant properties, thrombolytic activity, anticancer potential against HepG2 cell line, and genotoxicity of indigenous plant Tribulus terrestris extracts.
2 Materials & methods
2.1 Chemicals and reagents
Following chemicals and reagnts have been used to conduct the study. Methanol, acetone, chloroform, n-hexane, Dimethyl sulfoxide (DMSO), Folin-Ciocalteau reagent (FCR), sodium carbonate (Na2CO3), gallic acid, aluminum chloride (AlCl3), sodium hydroxide (NaOH), sodium nitrite (NaNO2), quercetin, 2,2-diphenyl-1-picrylhydrazyl (DPPH), phosphate buffer, potassium ferric hexocynide K3Fe(CN)6, Trichloracetic acid (TCA), ferric chloride (FeCl3), thiobarbituric acid, Curcumin, clopidogrel, RPMI-1640, agarose gel, ethidium bromide, Dulbecco's Modified Eagle's Medium (DMEM), penicillin–streptomycin, fetal bovine serum, MTT solution.
2.2 Instruments
Following instruments have been used in the study. Soxhlet apparatus (Japson, SKU-JA21830-37, India), Rotary evaporator (Heidolph, model Laborata 4000, Schwabach, Germany), UV spectrophotometer (Halo DB-20S, Dynamica instruments, UK), Fluorescence microscope (Dewinter RSB-14, UK), Microplate reader (BioTek, Winooski, VT, USA).
2.3 Plant collection and identification
Clean, hygienic, and disease-free medicinal plant was collected from Soan Skesar Valley, near district Khushab, Punjab, Pakistan. The botanical identification of selected plants and their significant parts were authenticated by the Department of Botany, GCUF, using standard keys & descriptions (Dalziel, 1937).
2.4 Extract preparation
The plant extraction process was carried out according to the technique defined by (Dipankar and Murugan, 2012). The plant seeds were dried under the shade and then ground to make powder. Methanol, acetone, chloroform, and n-hexane solvents were used for soxhlet extraction. In a thimble, 20 g of dried fine plant powder was filled and inserted into the extraction chamber. The round bottom flask was loaded with approximately 250 mL of solvent and extraction was continued for 48 h. The liquid extracts obtained were subjected to a rotary evaporator individually to obtain respective solvent crude extract. Then extracts were weighed and stored in the refrigerator at 4 °C for further use.
2.5 Quantitative phytochemical analysis
2.5.1 Estimation of total phenolic contents (TPC)
Folin-Ciocalteu assay was used to evaluate TPCs of medicinal plant extracts followed by the protocol of Stanojević et al. (2016). A dilution of 1 mL of dimethyl sulfoxide (DMSO), and 1 mg of plant extract was used to prepare the stock solution. The Folin-Ciocalteau reagent (FCR) (100 µL) was added to 20 μL of each extract, thereafter, followed by three incubations of the dark mixture. The resulting mixture was then combined with 100 μL 10% Na2CO3 solution and then put in an area of darkness for 1 h. UV spectrophotometer (Halo DB-20S, Dynamica instruments, UK) was used to take absorbance at 765 nm. All procedures have been performed in triplicate. TPCs were determined based on a standard equivalent curve, expressed in gallic acid equivalents (GAE mg/g) of the plant extract using different gallic acid concentrations (100, 200, 300,400, and 500 μg/mL) (PE) (Hossain and Shah, 2015).
2.5.2 Estimation of total flavonoid contents (TFC)
The TFCs of plant extracts were determined by the aluminium chloride (AlCl3) colorimetric method followed by Bojar et al. (2016). Mix 1 mL of each plant extract with 0.1 mL of 5% NaNO2 and incubated for. 0.5.min. later, the mixture was treated with 0.6 mL of NaOH. Five ml of distilled H2O was added to the reaction mix and the UV spectrophotometer was used to take absorbance at 510 nm. For the standard curve of quercetin prepared the dilution of 100, 200, 400, 600, 800, and 1000 µ.g./mL. o.f quercetin. The findings were expressed as quercetin equivale.nt (Q.E mg./.g of P.E) (Medini et al., 2014).
2.6 In vitro antioxidant activities
2.6.1 DPPH free radical scavenging assay
The free radical scavenging assay of plant extracts was examined by the method followed by Sowndhararajan and Kang (2013) with slight modifications. Briefly, the DPPH solution (0.1 mM) was freshly made with extract concentrations 62.5, 125, 250, 500, and 1000 mg/mL. The dilutions were incubated in darkness for 30 min at 27 °C. The optical density was calculated at 517 nm after incubation. Without adding plant extract, a control experiment was conducted. DP.PH radical scavenging percent was computed according to the following equation.
2.6.2 Determination of reducing power
The reducing p.ower (R.P) o.f plant extract was calculated by the procedure of Sylvie et al. (2014). The concentration of the extract (6.25, 12.5, 25.0, 50.0 and 100.0 μg/mL) was mixed with freshly made phosphate buffer (0.2 M, pH 6.6) and 1 percent K3Fe(CN)6 for power reduction testing. The reaction mixture was heated for 20 min at 50 °C. TCA (10% w/v) was added and centrifuged after 20 min of incubation (10000 rpm for 10 min). The resultant supernatant was then diluted with deionized water and FeCl3 (0.1% w/v) freshly made. The reaction mixture’s optical density at 700 nm was measured.
2.6.3 Hydroxyl* radical* scaven*ging *activity
The hydroxyl radical scavenging potential of plant extracts was identified by evaluating the degradation of deoxyribose into thiobarbituric acid **reactive species* (T*BARS) * as defined by Bhat et al. (2018). Phosphate buffer (0.2 M, pH 7.0), 10 mM EDTA, 10 mM 2-deoxyribose (10 mM), H2O2 (10 mM), and plant extract were included in the reaction mixture. The solution mixture was incubated for 4 h at 37 °C. The TCA (2.8% w/v) and TBA (1% w/v in NaOH) was added to the reaction mix and cooked for 20 min, followed by room temperature cooling. Without adding plant extract, a control reaction mixture was created. To determine the production of TBARS, the optical density of the reaction mixture was evaluated at 532 nm. The efficacy of hydroxyl-radical scavenging of extracts was determined by the following equation:
Where AC = Absorbance of the control, AT = Absorbance of the treated sample.
2.6.4 Nitric oxide radical scavenging assay
The technique adopted by Ebrahimzadeh et al. (2008) was used to assess the scavenging ability of plant extracts against radical nitric oxide (NO). NO was produced from sodium nitroprusside and measured by the reaction of Greiss. Curcumin was taken as a standard. Curcumin prevents nitric oxide synthase activation and is a potent scavenger of nitric oxide that occurs naturally. This reduces the amount of nitrite created by sodium nitroprusside between oxygen and nitric oxide. The absorbance was estimated at 596 nm and the antioxidant activity percentage was determined using the equation formula.
2.7 Thrombolytic activity
Venous whole blood (6 mL) was collected from non-contraceptive and anticoagulant perceptive healthy volunteers (n = 10). In distinct pre-weighed, micro centrifugal micro-tubes (500 μL blood/tube and 10 tubes per plant extract), blood was transferred instantly. The two tubes were then combined well and incubated at 37 °C for 45 min to coat approximately 200 μL of 2 percentile calcium chloride. The thrombolytic activity was conducted using the protocol of Ali et al. (2016) to test the thrombolytic capacity of plant extracts. First, from collected blood, 0.5 mL of blood was transferred to 10 pre-weighed centrifuge tubes and at 2,000 rpm centrifuged for 5 min. The centrifuge tubes were then incubated in the heat-controlled incubator at a simulated body temperature, i.e. 37 °C for 45 min. At the bottom of every centrifuge tube, after 45 min, a blood clot was formed. In the later stages, the serum was withdrawn without breaking down the continuity of the micro-centrifugal tube. The weights of the micro centrifugal tubes containing the coagulation were then taken to measure the weight of the coagulation again. Then, 100 μL extracts from plants were added to each test tube and incubated at 37 °C for 90 min. The discharged fluid was withdrawn, and the tubes were weighed again after the lump ruptured to determine the weight difference. The distilled water and 100 µL of clopidogrel were used as negative and positive controls.
The following equation calculated the %age of clot lysis:
2.8 Genotoxicity evaluation assay by comet assay
Comet analyses were used to quantify DNA damage in plant extracts, as described by Tice et al. (2000) using single-cell gel electrophoresis. From healthy nonsmoking volunteers, the blood was collected by venipuncture in a heparin vial. The whole blood (50 µL) combined with RPMI-1640 medium (950 µL) and 0.5 mg/ mL of plant extracts (50 µL). Whole blood + RPMI-1640 was used as negative control and whole blood + H2O2 (50 µM) + RPMI-1640) was used as a positive control. Cell suspensions were incubated for 2 h at 37 °C. After that, the suspension was centrifuged at 2000 rpm for 5 min. The cell suspensions were poured on slides covered with 1% normal melting point agarose gel. Then these slides were covered with low melting point agarose gel (1%). The loaded slides were transferred into a chilled lysing solution. Then these slides were placed for 20 min into the electrophoresis chamber to unwind DNA. Electrophoresis was done at 25 V, 300 mA. Then slides were neutralized and stained with ethiridium bromide. All the process was performed in dark to prevent photo arousal.
2.8.1 Estimation of comets by image analysis
A fluorescence microscope analysis of comets, and images with the camera attached to the microscope were carried out. Almost 50 images of a slide were analyzed by CASP 13.2b software to quantify the DNA damage caused by plant extracts on human blood cells. The standard descriptive measure like tail DNA, tail length, tail movement (TM), and olive tail movement (OTM) were observed.
2.9 Cell viability assay
The anticancer activities of the extracts were evaluated in the HepG2 cell line using MTT assay. In order to study whether the plant extract has any cytotoxic effects on normal cells, the retinal pigment epithelial (RPE) cell lines were used. Cells were seeded in Dulbecco's Modified Eagle's Medium (DMEM) with 10% fetal bovine serum and 1% penicillin–streptomycin at a concentration of 2x104 cells per well in 96 wells plate. The plate was incubated under optimized conditions i.e., 5% CO2 at 37 °C for 24 h. After incubation, the cells were treated with different concentrations (6.25, 12.5, 25.0, 50.0 and 100.0 μg/mL) of plant extracts, and the wells without any treatment were referred to as the control group. Cells were again incubated for 48 h. The medium was replaced with 100 µL of fresh serum-free medium and MTT solution (10 µL) followed by incubation for 4 h. The precipitates were dissolved in 150 µL of dimethyl sulfoxide (DMSO) and optical density was measured at 490 nm by using a microplate reader (BioTek, Winooski, VT, USA). Cell viability was measured using the following equation.
2.10 In silico analysis
2.10.1 Preparation of ligands
The SDF file of each compound of Tribulus terrestris was retrieved from PubChem. Such files could not be directly used to dock simulation, thus utilizing Chemdraw ultra, and therefore they were converted to m/mol2 format. Non-polar hydrogen atoms were merged, Gasteiger partial charges were added, and rotatable bonds were defined.
2.10.2 Ligand and energy minimization
For ligand energy minimization, the ChemDraw ultra 12.0 and chem 3D pro were used. The ligand atoms were given Gasteiger partial charges. Rotatable bonds were described by merging non-polar hydrogen atoms. The ligands were obtained from PubChem (https://pubchem.ncbi.nlm.nih.gov). GOLD considers the degree of freedom in the binding site that corresponds to the reorientation of hydrogen bond donor and acceptor groups. Despite accounting for a small fraction of the total conformational space available, this degree of freedom accounts for a significant difference in binding energy values.
2.10.3 Preparation of receptors
The X-ray crystal structures of the anti-liver cancer receptor were retrieved from the Protein Data Bank (PDB). The structure was edited to remove water, ligands, and heteroatoms (HETATM) using Discovery Studio Visualizer v 19.1.0 (BIOVIA, San Diego, CA, USA). All other parameters remained at default. Preparation of receptor files involves changing atom type, removing water molecules, adding polar hydrogen atoms, Gasteiger charges, and conversion into PDB format using GOLD v 5.3.0.
2.10.4 Ligand-protein docking
For predicting the binding affinities of a variety of ligands, molecular docking protocols are commonly used. The parameters of molecular docking software were used to conduct the experiments (https://www.ccdc.cam.ac.uk/). The Lamarckian genetic algorithm (LGA) and the Solis & Wets local search method were used to simulate docking. The initial positions, orientations, and torsions of the ligand molecules were chosen at random. Each docking experiment was divided into ten separate runs, each of which was set to end after a maximum of 1.5 energy evaluations.
2.10.5 Molecular docking
The molecular docking technique is used to calculate ligand-binding affinities and energies, which is essential in the structure-based drug design process. 3D protonation, energy minimization, and prediction of the active site for ligands were used to prepare the protein for molecular docking, with the parameters left at their defaults. Then, using GOLD version 5.3.0 software, ligands were docked with the target protein 5IXO. The grid box size was set for receptor, and the exhaustiveness was set to 12. As a result, differences in the position root-mean-square deviation<2 Å were clustered together. The results with the best conformation and energy were selected for further analysis. Discovery Studio Visualizer was used for the visualization and analysis of the protein–ligand complexes.
2.11 Statistical analysis
The data were expressed as mean ± S.E. One-way analysis of variance (ANOVA) was conducted for statistical analysis of data, employing SPSS version 22.0. For statistical significance, p < 0.05 was considered. Docking calculations were carried out by using GOLD version 5.3.0 and BIOVIA Discovery studio visualizer http://www.3dsbiovia.com, accessed on 15 July 2021.
3 Results
3.1 Quantitative phytochemical analysis of T. terrestris extracts
3.1.1 Total phenolic contents
TPCs were determined using the F.olin.–Ciocalteu. technique.. with ga.L.lic ac.id a.s the standard. The asorbance values obtained at various gallic acid d concent.rat.ions (10.0–1000 g./mL) were used. to create calibr.ation cur.ve. The findings were generated using the calibration curve's regression equation (Y = 0.0037x + 0.319; R2 = 0.993) of gallic acid and e.xpr.essed as m.g gal.lic acid equivalents (GAE) per g.ram of the plant ext.ract (mg/g). Our results showed that the methanolic extract of T. terrestris extracts showed the highest value for phenolic content as compared to other extracts (Table 1). Differences in the polarity of the extraction solvent. could lead to a large variance in the levelof extraction of bioactive chemicals. In this study, the maximum TPC values were recorded in the following order methanolic > acetonic > chloroform > n-hexane extracts as shown in Table 1.
T. terrestris
Plant extract
TPC (mg. GA.E/g)
TFC(m.g QE/g)
Methanolic extract
51 ± 0.7
66.5 ± 0.4
Acetonic extract
47 ± 1.5
52.5 ± 0.5
Chloroform extract
37 ± 1.2
43 ± 1.5
n-hexane extract
30.5 ± 0.3
32.7 ± 0.5
3.1.2 Total flavonoid contents
Flavonoid concentration in plant extracts was quantified using aluminium chloride in a colorimetric technique as a starting point. The.results. were derived from the calibration curve (Y = 0.0019x + 0.6157; R.2 = 0.9933) of quercetin (100–1000 µg/mL) and expressed as mg quercetin equivalents (QE) per gram of plant extract (mg/g). Similar tendencies with the values of TFC were seen in the case of TPC values. The maximum mean TFC values were shown by methanolic extracts of T. terrestris i.e., 66.5 ± 0.4 mg QE/g PE.
3.2 Determination of antioxidant activities
3.2.1 DPPH free radical scavenging assay
The result of antioxidant activity rationalized by DPPH indicated that the T. terrestris extracts showed a concentration-dependent response. At various concentrations (62.5, 150, 250, 500, and 1000 g/mL), the scavenging activity (in terms of % inhibition) of T. terrestriswas measured. The IC50 value of ascorbic acid for DPPH activity was calculated as 65.4 ± 13.8. All the extracts showed their maximum scavenging activity at 1000 μg/mL. The lowest activity was shown at 62.5 μg/mL concentration. Radical scavenging activity was observed in the following order: methanol > acetone > chloroform > n-hexane extract (Fig. 1A).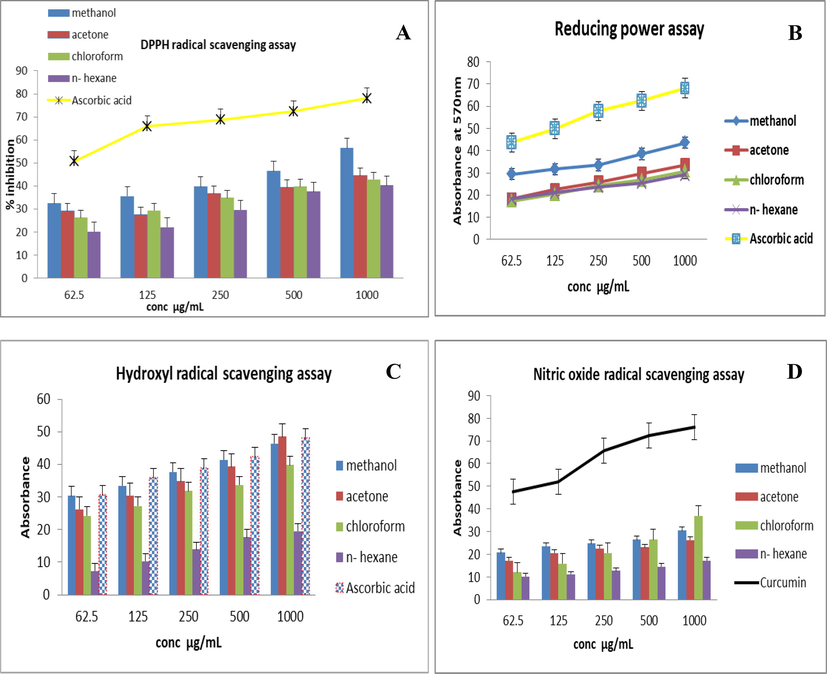
I.n vi.tro antiox.idant activ.ities of T. terrestris seeds ext.rac.ts.(A) DPPH free radical scavenging activity (B) Reducing power (C) Hydroxyl radical scavenging activity (D) Nitric oxide antioxidant activity.
3.2.2 Reducing power (RP) activity
The obtained results showed that the increasing reducing power (RP) of the T. terrestris extracts corresponds to the increase in the optical density. The IC50 value of ascorbic acid for RP activity was calculated as 56.3 ± 9.7 μg/mL. The order of reducing power of the T. terrestris extracts was methanol > acetone > n- hexane > chloroform (p < 0.05) (Fig. 1B). The highest RP potential showed by the methanolic extract was 18.25 ± 0.03 to 43.32 ± 1.7 from low 62.5 to 1000 µg/mL.
3.2.3 Hydroxyl radical scavenging activity
The analysis of OH scavenging activity also revealed the maximum scavenging activity was achieved by acetone extract of T. terrestris as shown in Fig. 1C. The IC50 value of ascorbic acid for OH● scavenging activity was calculated as 39.14 ± 6.5. Overall, all extracts of T. terrestris showed reducing power that was comparable with that of ascorbic acid.
3.2.4 Nitric oxide (NO) radical scavenging assay
The findings of the nitric oxide scavenging test were not comparable to the other assays. Overall, all extracts showed moderate nitric oxide activity as shown in Fig. 1D. IC50 value for curcumin was calculated as 62.7 ± 12.5 µg/mL. The highest activity was observed in the methanolic extract at 1000 μg/mL i.e. (36.4 ± 0.05) and the least activity was observed in n-hexane extract (10.23 ± 0.03). T. terrestris extracts showed the NO scavenging activity in similar order that is for DPPH and FRAP activities. The percentage inhibition was increased with the increasing concentration of the extract. However, activity of curcumin was more pronounced than that of T. terrestris extracts and it was also dose-dependent.
The percentage clot lysis of different extracts of T. terrestris and controls were presented in Fig. 2. The application of positive and negative control to the clots, namely clopidogrel and distilled water, showed 66 and 6.4% respectively of clot lysis activity. In contrast, the percentage of clot lysis accounted for T. terrestris was 33%, 27%, 17%, 6%. Significant (p > 0.05) clot lysis of methanolic, acetone extracts in contrast to positive and negative standards (Fig. 2).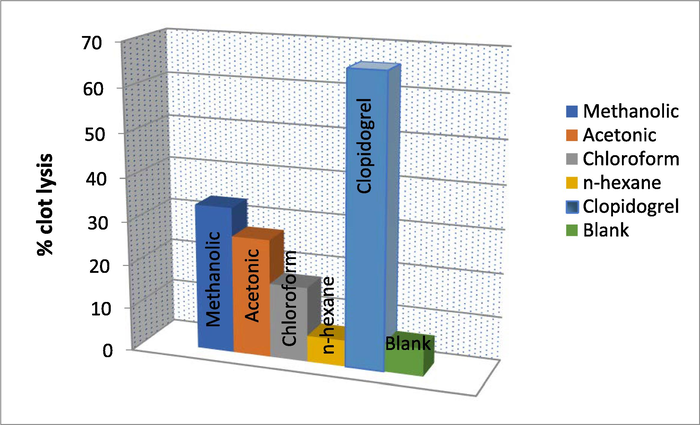
Percentage clot lysis of T. terrestris extracts with positive and negative controls.
3.3 Identification of DNA damage in T. terrestris treated blood cells
Analysis of variance of different parameters of DNA damage test caused by T. terrestris extract and control group are shown in Table 2. Results of one-way ANOVA showed that tail length DNA, TM and OTM are non-significantly different when compare between and within groups, which indicates that the extracts treated groups were not cause damage to DNA as compared to the control group. Non-significant = (P > 0.05); Significant = (P < 0.05); Highly significant = (P < 0.01).
Parameteres
Su..m o.f. Squ.are.s
df
M.ean S.q.u.are
.F.
S.ig.
HeadLength
Betw.een grou.ps
23426.267
0.4
5856.567
144.726
0.000
With.in gro.ups
404.667
45
40.467
To.tal
23830.933
49
TailLenght
Be.tween g.roups
271.600
4
67.900
1.667
0.233
With.in grou.ps
407.333
45
40.733
Tot.al
678.933
49
CometLenght
Be.twe.en gro.ups
14745.733
4
3686.433
11.404
0.001
With.in grou.ps
3232.667
45
323.267
Tot.al
17978.400
49
HeadDNA
Betw.een gro.ups
165590.402
4
41397.601
42.058
0.000
With.in.group.s
9843.055
45
984.306
Tota.L
175433.457
49
TailDNA
Bet.ween g.roups
282.457
4
70.614
1.119
0.400
Wit.hin gro.ups
630.811
45
63.081
To.tal
913.268
49
TM
Betw.ee.n grou.ps
8.682
4
2.170
0.915
0.492
Wit.hin gro.ups
23.710
45
2.371
Tot.al
32.392
49
OTM
Be.tween gro.ups
31.219
4
7.805
1.985
0.173
W.ithin gro.ups
39.319
45
3.932
Tot.al
70.539
49
The highest mean value was shown by methanolic extract of T. terrestris 26.6 ± 2.8 for L head as compared to other groups and control. These values showed highly significant differences between groups. The maximum mean value for L tail showed 15 ± 8.6 and 15.6 ± 10.6 values for methanolic and chloroform extract respectively. L tail parameter showed a non-significant difference between groups. The maximum value showed for L comet by methanolic extract i.e., 69.3 ± 20.2, and showed a highly significant difference between groups. Head DNA and Tail DNA showed maximum values in the control group. Methanolic extract showed maximum values 2.47 ± 2.2 and 4.67 ± 2.9 for TM and OTM and both of these parameters showed non-significant differences between groups. Results of the comparison of mean values are depicted in Table 3. After one-way ANOVA, the different letters indicate a statistically significant difference in mean values.
Factors
Control
Methanolic Extract
Acetone extract
Chloroform extract
n-hexane extract
Mean ± S.D
Mean ± S.D
Mean ± S.D
Mean ± S.D
Mean ± S.D
L Head
11.1 ± 3.4a
26.6 ± 2.8b
21 ± 2.6b
22.3 ± 11.5b
11.3 ± 4.04b
L Tail
6.6 ± 0.56 a, b
15 ± 8.6 a, b
8.6 ± 1.1b
15.6 ± 10.6b
6.9 ± 3.4 a
L Comet
24.4 ± 1.7 a
69.3 ± 20.2b
51.6 ± 4.6b
61.3 ± 32.5b
29.6 ± 11.0b
Head DNA
88.4 ± 1.2 a
34.9 ± 10.1b
39.2 ± 9.4b
66.6 ± 67.9b
12.6 ± 11.1b
Tail DNA
13.6 ± 0.01b
9.96 ± 8.9 a
2.93 ± 0.07 a
12.04 ± 15.1a
2.30 ± 2.5 a
TM
1.29 ± 0.6 a
2.47 ± 2.2 a
0.65 ± 0.3 a
2.3 ± 2.1 a
0.86 ± 0.7 a
OTM
0.68 ± 0.01b
4.67 ± 2.9a
1.72 ± 0.5 a
3.82 ± 3.02 a
1.92 ± 1.1 a
3.4 Cell viability assay
The anticancer activities of different concentrations of extracts (6.25, 12.5, 25.0, 50.0, and 100.0 μg/mL on the dry weight basis of the seed) were determined using a HepG2 cell line. Results of the present study revealed that the percentage of cell death of HepG2 cells after applying different extracts at a concentration of 100.0 μg/mL was 38.567–47.301%, and the maximum activity was observed for methanolic extract followed by acetone extract of the plant. Whereas, at the lowest concentration (6.25 μg/mL) cytotoxic effect on cancer cell lines was found to be 10.546–13.553 %. As shown in Fig. 3B, methanolic extract of plant exhibited a cytotoxic effect in HepG2 cell lines by inducing oxidative stress and morphological variations have been observed in cells treated with the methanolic extract. Overall, the results displayed a dose-dependent cytotoxic effect in HepG2 cells (Fig. 3A & B).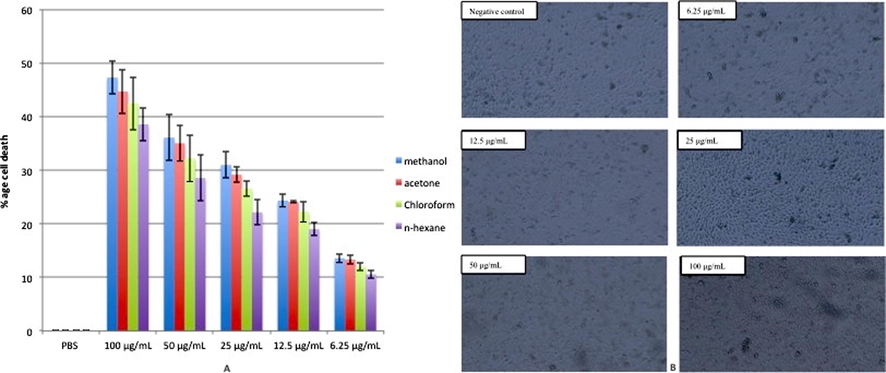
(A) Cytotoxic effects of different extracts of T. terrestris (6.25, 12.5, 25.0, 50.0 and 100.0 μg/mL on dry weight basis of the seed) against HepG2 cell lines. (B) Phase-contrast microscopy of HepG2 cell lines treated with different concentrations of methanolic extract of T. terrestris.
Results of the cell viability on normal cell lines i.e., RPE cells treated with different concentrations of plant extracts did not exhibit cell cytotoxicity as the cell viability remained 86–99.68%. Maximum cell viability was observed in the cells treated with the lowest concentration of the plant extracts (6.25 μg/mL). In case of methanolic extract, the highest tested concentration i.e., 100 μg/mL resulted in 86% cell viability compared to the control group, which was still under the safe range to use this plant for further biological activities.
3.5 In silico study
The molecular docking technique is used to calculate ligand-binding affinities and energies, which is essential in the structure-based drug design process. 3D protonation, energy minimization, and prediction of the active site for ligands were used to prepare the protein for molecular docking, with the parameters left at their defaults. Then, using GOLD version 5.3.0 software, ligands were docked with the target protein (5IX0). In our study, 16 secondary metabolites from Tribulus terrestris which was docked with inhibition of HDAC2 (5IX0). Table S1 contains a list of the compounds which are downloaded from PubChem ( https://pubchem.ncbi.nlm.nih.gov) in-silico by molecular docking method in order to.
Following the selection of these 16 compounds based on the literature survey ChemDraw ultra 12.0 and chem 3D pro was used in GOLD docking for energy minimization of ligands. The coordinate crystal structure of the 5IX0 protein was obtained from the Protein Data Bank and loaded into GOLD suite version 5.3.0 with a resolution of 0. GOLD 5.3.0 version was used to screen various docked complexes based on docking fitness and GOLD ratings. The binding compatibility, i.e., docking score and fitness, were used to test the results. The best drug was selected as the ligand molecule with the highest binding affinity with the receptor molecule. The number of hydrogen bonds formed and the bond distance between the active site and inhibit atomic coordinates were used to decide the final docked conformation for various chemicals. The GOLD docking scores for the phytochemicals are given in Table S1.
For each ligand, each docking routine returned the top ten rated docked poses. Compounds having maximum ligand-receptor binding energy and interactions with the receptor (<6 Å bond lengths) were predicted to be most effective.
Heptacosane, Apiol, Palmitic acid showed the best interaction with HDAC2 (PDB ID, 51X0) protein having Gold fitness (74.08, 65.53, 56.50) and GOLD docking score is (-9.28, −8.30, −6.37) including forming Hydrogen bond as shown in Table S1. The ratio of the docking result is Hepatocosane > Apiole > Palmitic acid. These molecules showed exceptionally good interaction with HDAC2 protein and can be considered as potential molecules that may prove to be beneficial in anticancer activity through their direct action on liver cancer. Other of these compounds showed moderate binding affinity and GOLD docking score. 2D view of protein-ligand interactions from the best poses produced by Discovery studio showed all molecules exhibit the same binding mode, as shown in Fig. 4.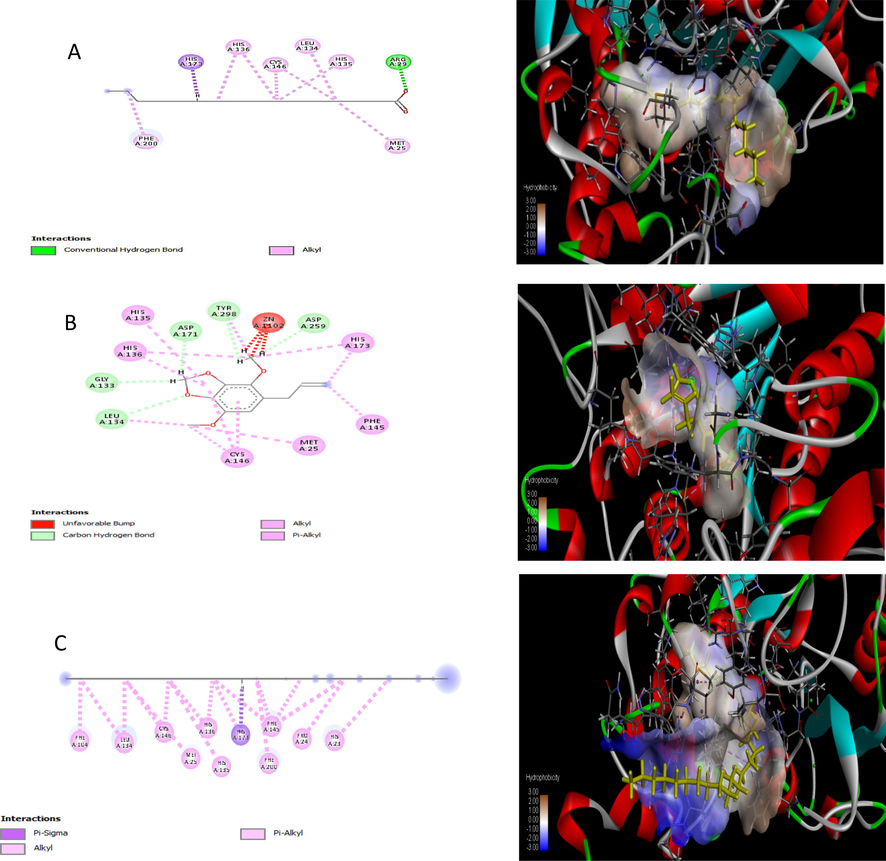
In silico molecular docking 2D and 3D structure of binding interactions between 51X0 proteins with screened compounds as ligand (A) Hepatocosane (B) Apiole (C) Palmitic acid.
4 Discussion
Modern medicine has gained popularity as a result of its accurate and fast response. Despite significant improvements in contemporary medicine, the majority of available drugs are expensive and harmful to the body, and hence, are not safe at all (Happy et al., 2019). Herbal medications are in high demand in poor nations these days, not only for their low cost, but also for their cultural acceptance, compatibility, and minimal or no adverse effects (Aminah et al., 2021).
The total phenolic contents of the medicinal plants were determined using Folin-Ciocalteu technique, with gallic acid serving as standard. The TPC yield from the Table. 1 expressed that the methanolic extract of T. terrestris showed the maximum TPC contents while the least quantity was shown by n-hexane extract. Results from Table 2, revealed that all the medicinal plants show variation in the yield of TPC according to the polarity of solvent used in the following order, methanolic > acetone > chloroform > n-hexane. The phenolic content results from our present investigation may differ from those found in the literature. This might be owing to the investigation of differences in the ability of investigated components, solvents, or extraction procedures, which could affect the number of phenolics in them (Bloesch et al., 2017). The present results are in the agreement with Elfalleh et al. (2012) who reported the highest values of total polyphenols and flavonoid contents in the methanolic extract of pomegranate in the order followed by peels > flowers > leaves > seeds, as compared to acetone and chloroform.
The total flavonoids (TFC) were estimated by AlCl3 colorimetric method as quercetin equivalents (QE) per gram (mg/g). Higher flavonoid contents were found in the methanolic extract of T. terrestris. Previously, Arshad et al. (2019) reported that the methanolic extracts of root > stem of T. terrestris exhibited a potent amount of TFC contents as compared to n-hexane extract. These data imply that the quantity of TFC varies greatly depending on the solvent, with different plants exhibiting varying TFC levels in the same solvent. The findings are consistent with earlier studies that indicated that polar solvents could be used to get higher yields of flavonoids (Nguyen et al., 2011).
Flavonoids and phenolics have long been renowned as therapeutically active chemicals that control critical physiological functions in the human body (Echendu and Dharmadasa, 2015). Because of the various health advantages documented in several epidemiological studies, research on flavonoids derived from plants has grown dramatically (Kumar and Pandey, 2013). Flavonoids exhibit potent antioxidant activity and have significant health and nutrition impacts (Burri et al., 2017). Polyphenolic and therapeutic plant flavonoids prevent the peroxidation of lipids and efficiently scavenge free radicals (Dai and Mumper, 2010). Phenolics have considerable attention as natural antioxidants among the most widespread metabolites in the plant kingdom (Vladimir-Knežević et al., 2012).
T. terrestris extracts depicted a good antioxidant ability with DPPH assay by methanolic extracts. The result of the present study showed that the in vitro free radical potential of the extract exhibited maximum free radical scavenging activity with a comparable IC50 value of the standards. All extracts showed the polarity-dependent pattern as the best DPPH activity showed by methanolic > acetone > chloroform > n-hexane extracts. Results are in line with Khan et al. (2020) who investigated the antioxidant activity of aerial parts of L. serriola extracts through DPPH and hydroxyl radical scavenging. They revealed that methanolic extract was found to be more effective than chloroform and n-hexane.
Among the four extracts, the maximum reducing power activity showed by the methanolic extract. Researchers found correlations between antioxidant activity and reduced plant extract power. The reduction characteristics are usually linked to the presence of reductones which serves as antioxidants by breaking the free radical chain, by a hydrogen atom donated (DeMets et al., 1990). Oxyradicals and lipid peroxidation have been demonstrated to generate Fe2+. As a result, reductions of Fe2+ levels would protect against oxidative damage in the Fenton process. Reductones also react with some peroxide precursors, inhibiting the production of peroxides (Singh and Rajini, 2004).
The maximum scavenging activity of hydroxyl radical was detected at 1000 µg/mL of T. terrestris acetone extract. Radical OH is a powerful species of active oxygen, which stimulates lipid peroxidation and causes cellular damage. Cellular DNA is known to be damaged by free radicals such as OH (Guha et al., 2011). In the OH radical scavenging assay, pharmacological molecules are tested for their ability to prevent OH radical production. Methanolic T. terrestris possesses significant OH radical scavenging action, and this activity may be related to the increased flavonoid and phenolic contents of the plant, according to the present study. Immanently, the use of methanolic T. terrestris in conjunction with other plants could be used to treat OH radical-induced disorders of lipid peroxidation.
Nitric oxide is a free radical produced in mammalian cells involved in the regulation of various physiological processes. However, excess production of nitric oxide is associated with several diseases. Researchers believe phenolic compounds in plants may be responsible for nitric oxide scavenging effects on cells. NO reacts to highly reactive compounds like N2O4, N3O4, NO3, and NO2 with oxygen. The structure and functionality of several cellular components are affected by highly reactive NO molecules. Acute NOs exposure is harmful to tissues and causes vascular collapse with septic shock, according to Adinortey et al. (2018) while chronic NOs exposure has been linked to oxidative stress-related illnesses such as arthritis, and ulcerative colitis, and diabetes. NO can also react with superoxide to generate the highly reactive peroxynitrite anion (ONOO), which is more harmful to tissues. L. serriola methanolic extract was more effective at scavenging NO radicals. This could be attributed to the antioxidant principles and benefits of total phenolics in the extract, which compete with oxygen to react with NO, preventing the formation of nitrite. Thus, the methanolic and acetonic extracts of L. serriola could be employed to treat disorders caused by NO oxidative stress.
The graphical depictions demonstrated that all extracts T. tribulus have substantial free radical scavenging activity in a concentration-dependent pattern i.e., enhanced antioxidant capacity of plant extracts was observed at increasing concentrations of plant extracts. Many researchers have validated the dose-dependent effect of antioxidant capacity given by various plant extracts (Hina et al., 2017) and a similar pattern was found in our study as well. There has been an increase in the amounts of phenolic elements including phenolic acids, flavonoids, and phenolic diterpene to increase the capability of antioxidants. In these phenolic compounds with a high antioxidant capacity, several hydroxy groups, especially O-dihydroxy groups are present (Soni and Sosa 2013).
In the case of thrombolytic test, it can be demonstrated that our findings may have significant implications for cardiovascular health. A widely used thrombolytic agent clopidogrel and streptokinase act by converting additional plasminogen to plasmin. But these agents have several adverse effects which encouraged the researcher to discover alternative agents (Bhowmick et al., 2014). Therefore, in this study clot lysis activity was determined. The comparative study between positive and negative control clearly showed that the methanolic extracts of all plants showed the notable ability to break down the clot thus strengthening their thrombolytic potentials (Fig. 2). Previously, it has also been reported that flavonoids have thrombolytic activity (Adinortey et al., 2018), and quercetin, a flavonoid that is also a strong free radical terminator and can aid to reduce atherosclerosis that leads to heart attack and stroke (Koffuor et al., 2011).
Coumarin is derived from a variety of plants used as an anticoagulant. In the blood coagulation cascade, coumarin works by inhibiting calcium activity. Previously, it has been reported that coumarins have antithrombotic and thrombolytic activity, therefore antithrombotic activity of test plant extract might be due to its phenolic coumarins (Alamgir, 2017). Another study by Pawlaczyk et al. (2011) proved phenolic polysaccharides isolated from Erigeron canadensis as a potentially useful anticoagulant and antiplatelet agent.
The toxicity of substances or extracts derived from plants represents a major issue in the validation of herbal medicine, which requires evaluation in preclinical trials. The diversity of chemical compounds present in natural products calls for a thorough search of possible beneficial and/or deleterious biological actions. The balance between the therapeutic and toxicological effects of a compound is a very important measure of the usefulness of a pharmacological drug.
In the comet assay, it is possible to quantify and distinguish cells with different rates of DNA damage, thus the analysis of the average values of the scores for each treatment group was very important (Serpeloni et al., 2008). The comet assay has been established as a simple, rapid, cheap, flexible and, most importantly, sensitive method to detect DNA damage, which is also able to detect DNA damage in individual cells. The results of current research indicated that under the experimental conditions tested, T. terrestris extracts were not genotoxic at 0.5 mg/mL concentrations, so their use in folk medicine would be safe. All extracts showed statistically non-significant differences of p > 0.05 when compared with negative control. Conversely, the use of n- hexane showed little genotoxicity p < 0.01 toward human blood. Similar results were reported by Qari and El-Assouli. (2019), the aqueous extract of T. terrestris fruits did less damage to the target DNA at the lower concentration (10 mg/ mL) and has a genotoxic effect in the therapeutic protocols if used in high doses. Another report by (Konermann et al., 2015) showed that none of the methanolic and aqueous extracts possessed genotoxic activities at concentrations of 0–2400 µg/mL. Doucet et al. (2016) also determined the genotoxicity of three plants and found that both aqueous extracts of A. cavens and A. furcatispina showed no damage DNA at 0.1 and 10 mg/mL but, at 20 mg/mL induced DNA damage (p < 0.05), showing moderate level damage. On the other hand, P. torquata extract was strongly genotoxic at all concentrations tested. The present results obtained from the comet assay suggest that the methanolic extract T. terrestris with tested dose (0.5 µg/ mL) cannot induce genotoxicity over the cells subjected for two hours to the H2O2.
Nevertheless, these results cannot guarantee safety for therapeutic use. Whereas the complementation of all stages of pre-clinical and clinical trials for various chronic experiments, plus in vivo tests at varying concentrations, is warranted. Therefore, any safety recommendation would be premature, given that additional studies are needed to confirm efficacy, toxicity and safety.
The anticancer activities of different extracts of T. terrestris were examined against HepG2 cell lines and the cell cytotoxicity was also measured against normal cell lines (RPE). The results revealed that normal cell lines treated with different concentrations of plant extracts did not exhibit any significant toxic effects. However, different extracts of T. terrestris showed anticancer potential against HepG2 cell lines, and the percentage of cell death after applying different extracts at a concentration of 100.0 μg/mL was 38.567–47.301%. Moreover, variations in cell morphology were observed in HepG2 cells after treatment with different doses of plant extract depicting cell damage due to oxidative stress. The results of the current study demonstrated a dose-dependent cytotoxicity in cancer cells. Khalid et al. (2022), also reported significant anticancer activities of T. terrestris against three cancer cell lines (MCF7, A2780 and HT29) at different tested concentrations. Patel et al. (2019), also revealed the cytotoxic effects of methanolic and saponin extracts from leaves and seeds of T. terrestris against breast cancer cell lines (MCF7) and their results suggested that inhibitory activities were contributed to intrinsic and extrinsic apoptotic pathways.
5 Conclusion
This study showed that the methanolic extract of Tribulus terrestris presented significant in vitro antioxidant properties as well as dose-dependently DNA damage, thromobolytic and cytotoxic effect in HepG2 cells. Among the selected phytocompounds, for In silico study, Heptacosane, apiol and palmitic acid showed good interactions with the target protein while Heptacosane was observed as an ideal candidate for the production of a novel drug against lung cancer. It may conclude that T. terrestris is an important medicinal plant with a potent source of natural antioxidants as well as anticancer activity and justifies its traditional use in green therapeutics. Further studies could be taken on T. terrestris for drug discovery for the welfare of human beings.
Acknowledgement
Researchers Supporting Project number (RSP-2021/397), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- DNA damage protecting activity and antioxidant potential of Launaea taraxacifolia leaves extract. J. Nat. Sci. Biol. Med.. 2018;9:6.
- [Google Scholar]
- Therapeutic use of medicinal plants and their extracts:. Vol volume 1. Springer; 2017.
- Tukh-e-karhu (Lactuca sativa Linn.): pharmacological and phytochemical profile and uses in unani medicine. J. Pharm. Innov.. 2016;5:1-4.
- [Google Scholar]
- Chemical constituents and their biological activities from Taunggyi (Shan state) medicinal plants. Heliyon.. 2021;7:e06173
- [Google Scholar]
- In-vitro antibacterial and antifungal properties of the organic solvent extract of Argemone mexicana L. J. King Saud Univ. Sci.. 2020;32:2053-2058.
- [Google Scholar]
- Bhat, A., K. Dar, M. Sofi, et al., 2018. Rheum spiciforme Royle—the medicinal herb with positive modulatory effect on controlled in vitro oxidative stress.
- In vivo analgesic, antipyretic, and anti-inflammatory potential in Swiss albino mice and in vitro thrombolytic activity of hydroalcoholic extract from Litsea glutinosa leaves. Biol. Res.. 2014;47:1-8.
- [Google Scholar]
- Iterated extended Kalman filter based visual-inertial odometry using direct photometric feedback. Int J Rob Res.. 2017;36:1053-1072.
- [Google Scholar]
- Results of the wmt16 metrics shared task. In: Proceedings of the First Conference on Machine Translation: Volume 2, Shared Task Papers. 2016.
- [Google Scholar]
- Antioxidant capacity and major phenol compounds of horticultural plant materials not usually used. J. Funct. Foods.. 2017;38:119-127.
- [Google Scholar]
- Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15:7313-7352.
- [Google Scholar]
- Dalziel, J.M, 1937. The Useful Plants of West Tropical Africa Crown Agents for Oversea Governments and Administration; Crown Agents for the Colonies: London, UK, 1955; p. 147
- The green synthesis, characterization and evaluation of the biological activities of silver nanoparticles synthesized from Iresine herbstii leaf aqueous extracts. Colloids Surf. B: Biointerfaces. 2012;98:112-119.
- [Google Scholar]
- Thermochemical processing of a South African ultrafine coal fly ash using ammonium sulphate as extracting agent for aluminium extraction. Hydrometallurgy. 2016;166:174-184.
- [Google Scholar]
- Iron chelating activity, phenol and flavonoid content of some medicinal plants from Iran. Afr. J. Biotechnol.. 2008;7
- [Google Scholar]
- Graded-bandgap solar cells using all-electrodeposited ZnS, CdS and CdTe thin-films. Energies. 2015;8:4416-4435.
- [Google Scholar]
- Total phenolic contents and antioxidant activities of pomegranate peel, seed, leaf and flower. J. Med. Plant Res.. 2012;6:4724-4730.
- [Google Scholar]
- Antioxidant Activity of Lawsonia inermis Extracts Inhibits Chromium(VI)-Induced Cellular and DNA Toxicity, Evid-Base Compl. Alter. Med.. 2011;2011:1-9.
- [CrossRef] [Google Scholar]
- Halliwell, B. and Gutteridge, J.M.C. (2015) Free Radicals in Biology and Medicine. 5th Edition, Oxford University Press, New York. http://dx.doi.org/10.1093/acprof:oso/9780198717478.001.0001.
- Phyto-assisted synthesis of zinc oxide nanoparticles using Cassia alata and its antibacterial activity against Escherichia coli. Biochem. Biophys. Rep.. 2019;17:208-211.
- [Google Scholar]
- In vitro antioxidant, hepatoprotective potential and chemical profiling of Syzygium aromaticum using HPLC and GC-MS. Sci: Pak. J. Pharm; 2017. p. :30.
- A study on the total phenols content and antioxidant activity of essential oil and different solvent extracts of endemic plant Merremia borneensis. Arab. J. Chem.. 2015;8:66-71.
- [Google Scholar]
- Amelioration of salt induced toxicity in pearl millet by seed priming with silver nanoparticles (AgNPs): the oxidative damage, antioxidant enzymes and ions uptake are major determinants of salt tolerant capacity. Plant Physiol. Biochem.. 2020;156:221-232.
- [Google Scholar]
- Immunomodulatory and erythropoietic effects of aqueous extract of the fruits of Solanum torvum Swartz (Solanaceae) Pharmacogn. Mag.. 2011;3:130.
- [Google Scholar]
- Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature.. 2015;517:583-588.
- [Google Scholar]
- Kumar, S. and A. K. Pandey, 2013. Chemistry and biological activities of flavonoids: an overview. “Sci. World J. 2013,
- JAK-STAT-mediated chronic inflammation impairs cytotoxic T lymphocyte activation to decrease anti-PD-1 immunotherapy efficacy in pancreatic cancer. Oncoimmunology. 2017;6:e1291106
- [Google Scholar]
- Mathur, M. and S. Sundaramoorthy, 2013. Economic assessment and conservation priorities of the Indian Thar desert medicinal plants.
- Total phenolic, flavonoid and tannin contents and antioxidant and antimicrobial activities of organic extracts of shoots of the plant Limonium delicatulum. J. Taibah Univ. Sci.. 2014;8:216-224.
- [Google Scholar]
- Acute toxicity study of sinapis alba aqueous extract in swiss albino mice. Plant Cell BiotechMolecuBiol.. 2021;531–536
- [Google Scholar]
- Synthesis of polyamide-imides with different monomer sequence and effect on transparency and thermal properties. Polymer. 2020;190:122218
- [Google Scholar]
- Evaluation of some medicinal plants for their antioxidant properties. Int. J. Pharm. Tech. Res.. 2011;3:381-385.
- [Google Scholar]
- The expanding scope of antimicrobial peptide structures and their modes of action. Trends Biotechnol.. 2011;29:464-472.
- [Google Scholar]
- Anticoagulant and anti-platelet activity of polyphenolic-polysaccharide preparation isolated from the medicinal plant Erigeron canadensis L. Thromb. Res.. 2011;127:328-340.
- [Google Scholar]
- Total phenolic, flavonoid contents, and antioxidant activities of fruit, seed, and bark extracts of Zanthoxylum armatum DC. World J: Sci; 2020. p. :2020.
- Toxicology evaluation and antidermatophytic activity of silver nanoparticles synthesized using leaf extract of Passiflora caerulea. S. Afr. J. Chem. Eng.. 2019;29:17-23.
- [Google Scholar]
- In vivo assessment of DNA damage and protective effects of extracts from Miconia species using the comet assay and micronucleus test. Mutagenesis. 2008;23:501-507.
- [Google Scholar]
- Free radical scavenging activity of an aqueous extract of potato peel. Food Chem.. 2004;85:611-616.
- [Google Scholar]
- Phytochemical analysis and free radical scavenging potential of herbal and medicinal plant extracts. J. Pharmacogn. Phytochem.. 2013;2:22-29.
- [Google Scholar]
- Free radical scavenging activity from different extracts of leaves of Bauhinia vahlii Wight & Arn Saudi. J. Biol. Sci.. 2013;20:319-325.
- [Google Scholar]
- FMRP and Ataxin-2 function together in long-term olfactory habituation and neuronal translational control. PNAS. 2014;111:E99-E108.
- [Google Scholar]
- Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen. 2000;35:206-221.
- [Google Scholar]
- Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: an overview. Medicines. 2018;5:93.
- [Google Scholar]
- Nootropic and anti-Alzheimer’s actions of medicinal plants: molecular insight into therapeutic potential to alleviate Alzheimer’s neuropathology. Mol. Neurobiol.. 2019;56:4925-4944.
- [Google Scholar]
- Vladimir-Knežević, S., B. Blažeković, M. B. Štefan, et al., 2012. Plant polyphenols as antioxidants influencing the human health, IntechOpen.







