Translate this page into:
Phytochemical profiling, in vitro biological activities, and in-silico molecular docking studies of Typha domingensis
⁎Corresponding authors. kashifur.rahman@iub.edu.pk (Kashif-ur-Rehman Khan), hati@ksu.edu.sa (Hanan Y. Aati),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
A comparative study between methanolic extract and n-hexane fraction of Typha domingensis (Typhaceae) was conducted for the evaluation of phytochemical potential, in vitro biological activities, and in-silico molecular docking studies. The phytochemical composition was estimated by total phenolic and total flavonoid contents, and by GC–MS analysis. Several biological activities were performed such as antioxidant assays (ABTS, FRAP, DPPH, & CUPRAC), enzyme inhibition activity (Tyrosinase, Acetylcholinesterase & Butyrylcholinesterase), thrombolytic activity, and antimicrobial activity (antibacterial & antiviral) to evaluate the medicinal importance of Typha domingensis. The results of the comparative study showed that methanolic extract has more total phenolic and total flavonoid contents (95.72 ± 5.76 mg GAE/g, 131.66 ± 7.92 mg QE/g, respectively) as compared to n-hexane fraction which confirms its maximum antioxidant potential (ABTS 114.31 ± 8.17, FRAP 116.84 ± 3.01, DPPH 283.54 ± 7.3 & CUPRAC 284.16 ± 6.5 mg TE/g). In the case of in vitro enzyme inhibition study and thrombolytic activity, better results were observed for methanolic extract. Almost similar antimicrobial patterns were observed for both methanolic extract and n-hexane fraction of Typha domingensis. The major bioactive phytochemicals identified by GC–MS were further analyzed for in-silico molecular docking studies to determine the binding affinity between ligands and the enzymes. The docking study indicated that most of the bioactive compounds showed a better binding affinity with enzymes as compared to the standard compounds (kojic acid & galantamine). The results of this study recommended that Typha domingensis has promising pharmaceutical importance and it should be further analyzed for the isolation of bioactive phytochemicals which may be useful for the treatment of several diseases.
Keywords
Molecular docking
Phytochemical Screening
Methanolic extract
Typha domingensis
Biological activities
1 Introduction
Phytochemicals such as phenolic compounds and flavonoids, commonly known as antioxidants are the major source of lead compounds and drugs (Carlson 2015). Many studies have reported that phytochemicals from medicinal plants are used for their potential applications in nutraceutical, cosmetic, pharmaceutical, and health-promoting e.g., antioxidant, antibacterial, antiviral, and anti-cancer effects, and also in neurological disorders (Dahibhate et al., 2019). The pharmaceutical and nutraceutical sector can benefit from understanding the antioxidant properties of plant extract/fractions to find new natural sources of antioxidants for supplements and therapeutic drugs. The literature review of antioxidant activity indicates that wild leaf plants with higher contents of phenolic compounds and flavonoids are an important source of natural antioxidants (Xiang et al., 2019). The pollens of Typha domingensis are a good source of bioactive secondary metabolites such as tannins, glycosides, alkaloids, saponins, polyphenols, and flavonoids (Karbon and Alhammer 2020).
T. domingensis, commonly named southern cattail belongs to the Typhaceae family, having a single genus. The T. domingensis, a familiar plant, is found in a variety of wetland ecosystems, including swamps, marshes, and lakeshores. This is spread in tropical and subtropical areas. The plant has a diverse morphology and is found in various countries throughout the world. In the Turkish traditional medicine system, the flowers of this plant are used for wound healing (Akkol et al., 2011). The leaves have analgesic, astringent, dehydrating, antioxidant, diuretic, and hemostatic effects (Duke and Ayensu 1985, Islam et al., 2015). It is used to treat nosebleeds, hematemesis, hematuria, uterine bleeding, dysmenorrhea, postpartum abdominal pain and gastralgia, scrofula, and abscesses. It is contraindicated for pregnant women (Yeung 1995). The pollens of T. domingensis are eaten in Pakistan owing to properties like antipyretic, increase flow of urine, and the treatment of injuries (Sardar et al., 2014). The people of Al Ahwar, the southern part of Iraq, use the powder of pollen to increase male fertility, named Viagra of Al Ahwar (Al-Kalifawi et al., 2017). It was observed that T. domingensis is efficacious in decreasing bacterial contamination and this plant also reduces enterobacteria, found in the intestine of humans which is responsible for the development of many diseases (He et al., 2020). The leaves of the plant have antimicrobial potential against gram-positive and gram-negative bacteria (Al-Kalifawi et al., 2017). The aqueous ethanol extract of T. domingensis showed bronchodilator, spasmolytic, and vasodilatation effects (Imran 2020) and it has also cytotoxic activity against breast cancer (Karbon and Alhammer 2020). Different solvent extractives of T.angustifolia revealed significant thrombolytic activity as compared to streptokinase as standard (Akkol et al., 2011). The leaves of T.angustifolia have revealed significant nootropic activity and they have also the potential for the treatment of Alzheimer's disease (Kumar et al., 2014).
The current study aimed to evaluate the bioactive phytochemicals, biological activities, and in-silico molecular docking studies of methanolic extract and n-hexane fraction of T. domingensis. The bioactive phytochemical screening was assessed by preliminary phytochemical, total polyphenol contents (total phenolic and total flavonoid contents), and GC–MS analysis. The biological activities were analyzed by antioxidant assays (ABTS, FRAP, DPPH, and CUPRAC), enzyme inhibition (tyrosinase, acetylcholinesterase, and butyrylcholinesterase) thrombolytic and antimicrobial activities (antibacterial and antiviral). The bioactive phytochemicals identified by GC–MS were also analyzed for binding with enzymes by molecular docking technique. To the best of our knowledge, there is no such comparative study between methanolic extract and n-hexane fraction of T. domingensis has ever been conducted.
2 Materials and methods
2.1 Sample collection and plant identification
The collection of mature plants was carried out in March 2019 from Multan, Pakistan, identified by the Herbarium Department of Botany, Life science Faculty, The Islamia University of Bahawalpur, and the botanical sample was submitted in the herbarium with reference number 412.
2.2 Extract preparation
The air-dried powdered plant material (4 kg) was soaked in 80% aqueous methanol (16 L) for 7 days at room temperature with occasional stirring. An aqueous methanol solvent system was used due to its efficient extraction of phenolics and flavonoids (Justine et al., 2019). It was initially filtered with muslin cloth followed by filtration through Whatman-1 filter paper. The filtrates were subjected to a rotary evaporator (Heidolph, Germany) under reduced pressure at 40 °C for concentrating the extract. This concentrated extract was further air-dried. The dried methanolic extract was dissolved in distilled water to form a uniform solution for liquid–liquid fractionation. This solution was fractionated with the n-hexane solvent by using a separating funnel. The fraction was further concentrated with the rotary evaporator at 40 °C, air-dried, and stored for further analysis (Ghalloo et al., 2022).
2.3 Phytochemical analyses
2.3.1 Preliminary phytochemical analysis
The sample solutions of T. domingensis were analyzed for their primary and secondary metabolites to confirm the presence of various primary metabolites, such as carbohydrates, amino acids, proteins, and secondary metabolites, such as alkaloids, tannins, phenols, saponins, steroids, glycosides, and resins, according to standard methods described in the literature (Geetha and Geetha 2014).
2.3.2 Estimation of total phenol contents
For total phenolic contents, 25 µL sample solution (1 mg/mL) was mixed with diluted 10 µL Folin-Ciocalteu reagent (1:9, v/v) and shaken vigorously. After 3 min, 75 µL sodium carbonate solution (1%) was added and the absorbance of the sample was measured at 760 nm after a 2 h incubation at 37 °C with a BioTek synergy HT microplate reader. The standard curve of gallic acid was plotted accordingly. The same procedure was repeated for negative control replacing fraction solutions with buffer solutions. Results were exhibited as milligrams of gallic acid equivalents (mg GAE/g DE) (Boeing et al., 2014).
2.3.3 Estimation of total flavonoid contents
The aluminum chloride method was used to measure the total flavonoid content. The solution was made by mixing 0.5 mL of plant extract/fraction solution (1 mg/mL), 2 mL of distilled water, and 0.15 mL of 5% sodium nitrite in a test tube. After 5 min of incubation at room temperature, 0.15 mL of 10% aluminum chloride was added and the solution was allowed to incubate for another 5 min. Then 1 mL of a 4% NaOH solution was added and diluted in 5 mL of pure water. At room temperature, the final solution was vortexed and incubated for 15 min. The standard used for the estimation of flavonoids was quercetin. A blank was made by mixing the sample solution (0.5 mL) with methanol (1 mL) but without AlCl3. The 250 µL volume of the mixture was taken in the 96-well plates and the absorbance was measured at the 420 nm wavelength with a BioTek HT synergy microplate reader (Obeng et al., 2020).
2.3.4 Gas Chromatography-Mass spectrometry analysis (GC–MS)
The methanolic extract and n-hexane fractions were analyzed by GC–MS Agilent (6890 series and Hewlett Packard, 5973 ground sensor). Blown barriers were removed on an HP-5MS column (30 m length × 250 µm diameter × 0.25 µm film thickness). GC–MS spectroscopy involves an electron ionization system that uses energy-intensive electricity (70 eV). The Helium gas was used as a carrier at the flow of 1.0 mL/min. The injector was operated at 250 °C and the oven temperature was set in such a way, 50 °C for 5 min, then gradually increased to 250 °C at 100 °C/min, and lastly to 3000 °C for 10 min at 70 °C/min. The identification was made using a standard scanning method ranging from 35 to 600 m/z, and the bioactive compounds were tentatively identified by the NIST 2011 library (Djouahri et al., 2013).
2.4 Bioactivity assays
2.4.1 Antioxidant activities
Radical scavenging (DPPH and ABTS) and reducing potency (FRAP and CUPRAC) assays were used to assess antioxidant activity. The sample solutions were made at 1 mg/mL in methanol.
2.4.1.1 Free Radical Scavenging (DPPH) Activity
The DPPH solution (0.267 mM 40 µL) was combined with 10 µL of the sample solution and incubated for 30 min before the absorbance was measured at 517 nm using a BioTek synergy HT microplate reader. The results were given in milligrams of Trolox equivalent per gram of dry extract (Marinova and Batchvarov 2011).
2.4.1.2 Radical Cation Scavenging (ABTS) Activity
The reaction of a 7 mM ABTS solution with 2.45 mM potassium persulfate resulted in the formation of the ABTS+ radical cation. 1 mL of sample solution was mixed with 2 mL of ABTS+ solution, and after 30 min, the absorbance was measured at 734 nm with a BioTek HT synergy microplate reader. Results are expressed as mg Trolox equivalent per gram of dry extract (He et al., 2012).
2.4.1.3 Cupric Ion Reducing (CUPRAC) Method
This technique was carried out by mixing a 0.5 mL sample and mixing it with CuCl2 (1 mL, 10 mM), nectarine (1 mL, 7.5 mM), and NH4Ac buffer (1 mL, 1 M, pH 7.0). The mixture was incubated at room temperature for 30 min. Then, the absorption of the mixture was measured at 450 nm with a BioTek Synergy HT microplate reader. Results were expressed in the mg of Trolox equivalent per gram of the dry extract (Apak et al., 2004).
2.4.1.4 Ferric Reducing Antioxidant Power (FRAP) Method
In this process, 0.1 mL of sample solution was added to a 2 mL reagent in acetate buffer (0.3 M, pH 3.6), 2,4,6-tris (2-pyridyl) - s-triazine (TPTZ) (10 mM) in 40 mM HCl, and ferric chloride (20 mM) in a 10: 1: 1 ratio (v /v /v) and the absorbance was measurements at 593 nm after 30 min with BioTek Synergy HT microplate reader. The results were presented in the mg of Trolox equivalent per gram of the dry extract (Benzie and Szeto 1999).
2.4.2 Enzyme inhibition activities
2.4.2.1 Tyrosinase Inhibition Activity
The volume of 20 μL of 0.1 M potassium phosphate buffer having pH 6.8 and 40 μL extract/fraction solution (1 mg/mL) was mixed. Tyrosinase enzyme 40 μL (200 units/mL) was added and incubated for 15 min. The substrate l-DOPA 100 μL was added to the incubated mixture. This solution was further incubated for 20 min at room temperature. The absorbance was measured at 450 nm with a BioTek synergy HT microplate reader. The same procedure was adopted for negative control by adding 40 μL of buffer solution instead of extract/fraction. The kojic acid was used as the standard compound in tyrosinase inhibition activity (Grochowski et al., 2019).
2.4.2.2 Acetylcholinesterase (AChE) and Butyrylcholinesterase (BChE) Inhibition Assay
For AChE and BChE inhibition activity analysis, after 15 min of incubation at room temperature of the reaction mixture which is composed of 50 µL solution of the extract/fraction (1 mg/mL), 125 µL DTNB (3 mM), and 25 µL enzymes solution (0.265 U/mL for AChE or 0.026 U/mL for BChE) in Tris-HCl. The buffer having pH 8.0 was incubated for 15 min at ambient temperature. The volume of 5 µL acetylthiocholine iodide or butyrylthiocholine chloride (15 mM) as substrates were mixed into the incubated mixture. Then absorbance of the final solution was measured at 405 nm after 15 min. Also, blank solution (without extract/fraction or standard) was prepared in the same way and analyzed according to this approach. The galantamine was used as a standard agent in this assay (Pohanka et al., 2011).
2.4.3 Thrombolytic activity
The 5 mL blood samples of healthy volunteers (n = 05) with no history of antidepressants were used (according to guidelines approved by the ethical committee). The volume of 5 mL sterile water was added to the streptokinase vial (15,00,000 i.u) and stirred. The standard drug for thrombolytic activity was streptokinase injection.
The volume of 0.7 mL Blood samples was added to the previously weighed eppendorf tubes. The blood was incubated for clotting purposes, and the serum was removed once the clot had formed. These eppendorf tubes were weighed after removing serum. In these eppendorf tubes, 100 mL of sample solution (1 mg/mL) or standard was mixed and stored for 30 min. Finally, the clot's liquid part was removed and weighed. The weight difference was computed, and the weight loss was reported as a percentage of lytic activity of sample solutions or a standard. Instead of fractions solution, water was used as a negative control, and the same method was followed. After deducting the value of negative control activity, the results were calculated (Elnager et al., 2014).
2.4.4 Antimicrobial activity
2.4.4.1 Antibacterial Activity
Eight bacterial strains including Bacillus subtilis ATCC 1692, Micrococcus luteus ATCC 4925, Staphylococcus epidermidis ATCC 8724, Bacillus pumilus ATCC 13835, and Staphylococcus aureus ATCC 6538 (Gram-Positive) Escherichia coli ATCC 25922, Bordetella bronchiseptica ATCC 7319, and Pseudomonas aeruginosa ATCC 9027 (Gram-negative) bacteria were obtained by the DTL Bahawalpur (Punjab Pakistan). Inoculums were made by mixing a few colonies of the relevant bacteria from the 24-hour old cultures into a sterile nutrient broth medium of 10 mL. The turbidity was adjusted to 0.5 McFarland, which is equivalent to 108 CFU/mL of cell density (Zaman et al., 2016).
The procedure described in the literature with slight alteration was followed (Manyasree et al., 2018). In an autoclave, Petri dishes and nutrient agar media were sterilized. Agar nutrient was placed into Petri dishes and allowed to solidify in a laminar flow hood. Bacterial cultures were streaked on the agar surface, followed by the development of four 5 mm diameter holes in each petri dish. Using a micropipette, 60 μL of co-amoxiclav (1 mg/mL) and extract/fraction solutions (20, 10, and 5 mg/mL) were added to wells. All of these Petri plates were incubated for 18–24 h at 37 °C in an incubator. The zones of inhibition were evaluated after incubation to determine the antibacterial activity. The results were calculated by averaging three tests.
2.4.4.2 Antiviral activity
The four viral strains Avian Influenza virus H9N2 (AIV), New Castle Disease virus Lasoota (NDV), an Infectious bursal disease virus (IBDV), and Infectious bronchitis virus (IBV) were used in this antiviral analysis. All the viral strains were analyzed against methanolic extract and n-hexane fraction through In Ovo antiviral activity. Sterile eggs were acquired from the government-owned poultry farm, Model Town A Bahawalpur, and all viral strains mentioned above were inoculated in the chorioallantoic fluid of 9–11 days chick embryonated (CE) eggs. The eggs were sterilized by candling before and after inoculations. The broader ends of eggs were further swabbed with 70% alcohol for sterilization and transferred into cleaned trays in biosafety cabinet II. The sterile wide broader ends of eggs were pierced with a sterile needle. Then viral inoculum is injected through the sterile disposable 1 cc syringe. After the inoculation, a hole was wrapped with molten wax, and eggs were incubated at 37 °C. The eggs were harvested 72 h post-inoculation and allantoic fluids were collected and subjected to two types of assays. Haemagglutination (HA) or Indirect Hemagglutination (IHA) in case of IBDV test to check the titer of each virus. The whole activity was done in biosafety cabinet type II. Serial passages of viral strains were performed to increase titer before taking the antiviral analysis of the methanolic extract and n-hexane fraction of T. domingensis.
The first step is to perform a hemagglutination (HA) test. 20 mL of Alsever's solution is poured into a test tube and 5 mL is injected into the blood of the chickens. The volume of 10 mL of blood was injected at 4000 rpm for 7 min. This process was repeated three times to optimize and find the best solution. 1% RBC was prepared by adding 10 µL of packaged R.B.C to 1 mL of PBS solution (pH 7.4) in an eppendorf tube. The tube was gradually discarded to prevent rain (Musaddiq et al., 2020).
2.5 Molecular docking
Molecular docking is a useful tool in the development of molecular biology and computer-aided design. The goal of ligand–protein binding is to predict biological activity. For molecular docking, different tools auto Dock vina software, MGL Tools, Discovery Studio, PyRx, and Babel were used. The receptor molecule which was downloaded from the protein data bank was further prepared with the Discovery Studio. Ligand molecules were prepared with the Babel. These prepared receptors and ligands were uploaded in Vina which was embedded in PyRx. Finally, docking was performed with Vina. The results were visualized with Discovery Studio.
2.6 Statistical analysis
Each test was performed three times. Results were expressed as mean ± S.D. One-way ANOVA was performed with IBM SPSS Statistics version 20. P-value < 0.05 was considered significant.
3 Results and Discussion:
The manufacture of novel medications for the treatment of various ailments relies on a phytochemical analysis of medicinal plants (Viji and Murugesan 2010). Anticancer, antibacterial, antiviral, antidiabetic, antidiuretic, skin disorders, and anti-inflammation properties are found in medicinal plants (Gnanaraja et al., 2014).
T. domingensis is of commercial interest in research institutions and the pharmaceutical sector. We anticipate that the essential phytochemical qualities discovered in the research of the native T. domingensis plant will benefit in the fight against a variety of diseases in this region.
3.1 Phytochemical analysis of T. domingensis
Secondary metabolites are often species-specific and can be used in a variety of situations. However, most secondary metabolites are derived as by-products or intermediates of primary metabolism. Secondary metabolites are mainly produced under regulated stress for a specific function, such as defense against diseases and herbivores, enhanced tolerance to abiotic stressors, insect and mammal attraction for fertilization and/or seed dissemination, or repellence of undesirable feeders (Böttger et al., 2018). Alkaloids, flavonoids, phenols, saponins, steroids, and tannins are among secondary metabolites (Mungole and Chaturvedi 2011). The qualitative phytochemical screening of methanolic extract and n-hexane fraction was carried out for different constituents. Table.1 revealed the presence of carbohydrates, proteins, glycosides, terpenes, steroids, resins, tannins, and phenols while amino acid, saponin, resins, and alkaloids were absent. In the literature, it was observed that methanol extract showed the presence of alkaloids, phenols, flavonoids, tannins, saponins, and steroids. “TDm” methanolic extract, “TDnh” n-hexane fraction “-“Absent “+” Present.
Sr.no
Constituents
Test
TDm
TDnh
1
Carbohydrates
Molish test
+
+
2
Amino acid
Ninhydrin
–
–
3
Protein
Biuret
+
–
4
Tannins and Phenols
(a) FeCl3
(b) Lead Acetate+
++
+
5
Saponin
Frothing
–
–
6
Alkaloids
(a)Dragendorff
(b)Mayers
(c)Wagner+
-
-+
-
-
7
Glycosides
Borntrager
+
–
8
Steriods
Salkowki
+
+
9
Resins
Acetic Acid
+
+
3.1.1 Polyphenolic quantification (total phenolic and flavonoid contents)
Due to their antioxidant and other health-promoting characteristics, phenolic compounds are prevalent in most medicinal plants and constitute an important part of the human diet (Pandey and Rizvi 2009). The total phenolic and flavonoid content of methanolic extract and n-hexane fraction were analyzed as shown in Fig. 1 and Table 2. All the experiments were performed in triplicates (n = 3) “TDm” methanolic extract, “TDnh” n-hexane fraction, “TPC” total phenolic contents, “TFC” total flavonoid contents, “DPPH” 2,2-Diphenyl-1-Picrylhydrazyl, “ABTS” 2, 2'-Azino- Bis (3- Ethylbenzthia zoline-6-Sulfonic acid, “CUPRAC” Cupric Ion Reducing Antioxidant Capacity, and “FRAP” Ferric Reducing Antioxidant Power Assay.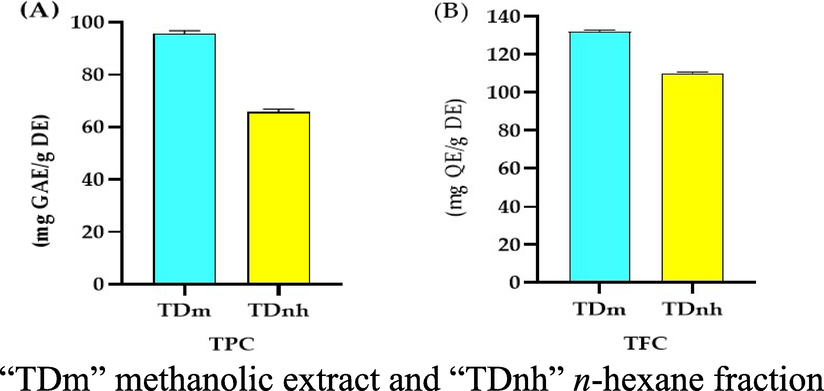
(A) “TPC” Total phenolic contents (mg GAE/g DE) of T. domingensis, (B) “TFC” Total Flavonoid Contents (mg QE/g DE) of T. domingensis.
Extract/Fraction name
TPC (mg GAE/g extract.)
TFC (mg QE/g extract.)
DPPH (mg TE/g extract.)
ABTS (mg TE/g extract.)
CUPRAC (mg TE/g extract.)
FRAP (mg TE/g extract.)
TDm
95.72 ± 5.76
131.66 ± 7.92
283.54 ± 7.3
114.31 ± 8.17
284.16 ± 6.5
116.84 ± 3.01
TDnh
65.91 ± 3.63
110.25 ± 6.57
140.53 ± 4.67
75.12 ± 5.02
140.14 ± 3.01
84.28 ± 4.01
The total phenolic contents (TPC) observed in the methanolic extract of T. domingensis was 95.72 ± 5.76 mg GAE/g DE while in n-hexane extract was 65.91 ± 3.63 mg/g DE. Previously reported a TPC value of 401.46 ± 5.77 mg GAE/g DE in methanolic extract of fruits of T. domingensis (Chai et al., 2015). This very significant difference between the TPC values revealed that there are different phytochemicals in plants or this difference may be due to different habitats of the plant. Because phenolic molecules are extremely reactive to free radicals, measuring them is also a good indicator of antioxidant activity (Ammar et al., 2014).
The total flavonoid contents (TFC) revealed by methanolic extract and n-hexane fraction of T. domingensis were 131.66 ± 7.92 and 110.25 ± 6.57 mg QE/g DE respectively. Chai et al. 2014 reported a TFC value of 103.38 ± 5.29 mg QE/g DE in an aqueous extract of fruits of T. domingensis (Chai et al., 2014). The results of the present study and reported in the literature revealed a significant difference because of different parts of plants and different solvents used for extraction and this difference may be also due to different atmospheric conditions.
3.1.2 Gas Chromatography-Mass Spectrometry (GC–MS)
Similarly, to have detailed knowledge about phytochemical composition, the GC–MS analysis of the methanolic extract and n-hexane fraction was performed and resulted in the tentative qualitative identification of numerous phytochemicals in the methanolic extract and n-hexane fraction, (Figs. 2 and 3, Tables 3 and 4). This tentative identification of the bioactive phytochemicals was performed by using the NIST 11 library database. The detailed list of these tentatively identified phytochemicals (with their tentatively identified names, molecular formula, molecular weight, retention time, % peak area, quality index, chemical class, and reported biological activity) in methanolic extract and n-hexane fraction are listed in Tables 3 and 4 has been taken which have quality index more than 90% respectively and the GC–MS spectra for both extract and a fraction showing different peaks of the tentatively identified compounds are shown in Figs. 2 and 3. The majority of these compound classes include benzene derivatives, fatty acids, alkanes, aldehydes, steroids, and terpenes, among others. The major tentatively identified bioactive phytochemicals were benzene, o-xylene, 8,11-octadecadienoic acid, 9,12,15-octadecatrienoic acid, phytol, N-acetoacetyl-deacetylcolchicine, sitosterol, phenol, 2,5-bis(1,1-dimethylethyl) phenol, tetradecanoic acid, octadecane, eicosane, n-hexadecanoic acid, Octadec-9-enoic acid, 1,2-Benzenedicarboxylic acid, Vitamin E and many other minor compounds.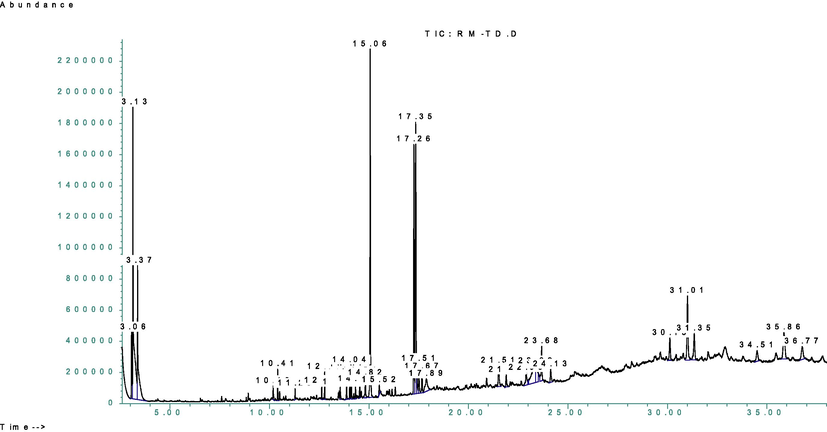
GC–MS spectra of the methanolic extract.
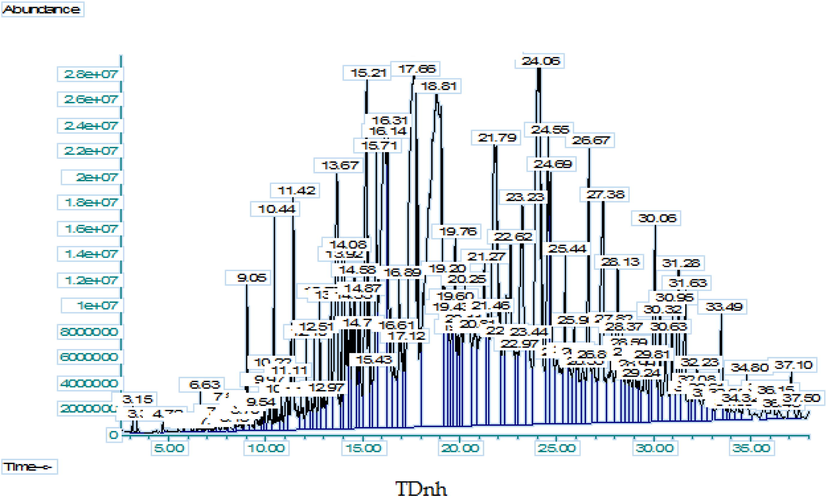
GC–MS spectra of n-hexane fraction of.
R.T.
Compound Name
Molecular formula
Molecular weight
Relative area (%)
Similarity index
Chemical class
Biological Activity
3.06
Ethylbenzene
C8H10
106.16
2.00
91
Benzenoids
Antimicrobial (Wang et al., 2013)
3.13
Benzene
C6H6
78.11
15.62
97
Aromatic hydrocarbon
Anti-inflammatory (Firke and Bari 2015)
3.37
o-Xylene
C8H10
106.16
7.59
97
Hydrocarbon
Catalytic (Wu et al., 2019)
10.42
2,4-bis(1,1-dimethylethl) phenol
C17H30
278.5
0.92
94
Phenol derivative
Antibacterial, Antifungal, Anticancer, Antioxidant (Devi et al., 2021)
11.29
1-Heptadecene
C17H34
238.45
0.26
94
Alkene
Antifungal (Yoon et al., 2011)
12.77
Methyl tetradecanoate
C15H30O2
242.4
0.77
97
Fatty Acid
Antimicrobial (Kalsoom et al., 2020)
13.55
Tetradecanoic acid
C14H28O2
228.37
0.44
94
Fatty Acid
Antioxidant (Sokmen et al., 2014)
14.82
9-Hexadecenoic acid
C16H30O2
254.41
0.88
99
Fatty Acid
Antimicrobial (Ubaid et al., 2016)
15.52
8,11-Octadecadienoic acid
C18H32O2
280.4
9.38
99
Fatty Acid
Antifungal (Khan and Javaid 2020)
17.35
9,12,15-Octadecatrienoic acid
C18H32O2
278.4
13.38
99
Fatty Acid
Antimicrobial, Anticancer, Antiinflamatory (Malathi and Ramaiah 2017)
17.35
8-Octadecenoic acid
C18H34O2
282.5
1.06
99
Fatty Acid
Antioxidant (Farhan et al., 2021)
17.51
Phytol
C20H40O
128.17
1.33
91
Acyclic Diterpenoids
Antioxidant, Anticancer (Ran and Han 2014)
17.67
Heptadecanoic acid
C17H34O2
270.45
0.97
97
Fatty Acid
Antioxidants (Ashley et al., 2014)
17.89
Oleic Acid
C18H34O2
282.47
1.97
95
Fatty Acid
Apoptotic (Van Evera 1998)
21.90
Phenol
C6H5OH
94.11
0.67
93
Phenolic compounds
Antimicrobial, Antioxidant (Amrani-Allalou et al., 2021, Morais et al., 2022)
23.39
N-Acetoacetyl-deacetylcolchicine
C20H23NO5
357.4
1.98
91
Alkaloid derivative
Anti-tubulin (Brossi 1991)
35.86
Sitosterol
C29H50O
414.7
3.09
90
Lipids
Analgesic, anti-inflammatory (Brossi 1991)
R.T.
Compounds
Molecular formula
Molecular weight(g/mol)
Relative area (%)
Similarity index
Chemical Class
Biological Activity
9.05
Tetradecane
C14H30
198.39
0.36
98
Alkane
Antimicrobial, Antipyretic, Bronchitis, Tuberculosis (Banakar and Jayaraj 2018)
10.22
Pentadecane
C15H32
212.42
0.19
96
Alkane
Antibacterial (Uma et al., 2010)
10.44
Phenol, 2,5-bis(1,1-dimethylethyl
C14H22O
206.32
0.54
95
Phenol
Antimicrobial, Antioxidant (Nyaberi et al., 2017)
11.11
Dodecanoic acid
C12H24O2
200.31
0.28
99
Fatty Acid
Antimicrobial (Chionis et al., 2016)
12.10
Hexadecane
C16H34
226.41
0.36
98
Alkane hydrocarbon
Antimicrobial (El-Sheshtawy and Doheim 2014)
12.38
Tridecanoic acid
C13H26O2
214.34
0.15
95
Long-chain fatty acid
Antimicrobial (Chowdhury et al., 2021)
12.51
Heptadecane
C17H36
240.47
0.33
97
Alkane
Antibacterial (Uma et al., 2010)
12.79
Tridecanoic acid
C13H26O2
241.34
0.38
96
Long-chain fatty acid
Antipersister (Jin et al., 2021)
13.36
Tetradecanoic acid
C14H28O2
228.37
1.03
95
Long-chain fatty acid
Antimicrobial (Abubakar and Majinda 2016)
13.67
Octadecane
C18H38
254.49
1.76
97
Alkane
Antioxidant, Antimicrobial (Jasim 2015)
13.92
Pentadecanoic acid
C15H30O2
242.39
0.35
98
Palmitic acid
Antioxidant (Adeyemi et al., 2017)
14.35
Octadecane, 2-methyl-
C19H40
268.5
0.45
93
Alkane
Antimutagenic (Lawal et al., 2015)
14.77
Nonadecane
C19H40
268.51
0.30
96
Alkane
Antimutagenic (Lawal et al., 2015)
14.87
9-Hexadecenoic acid
C16H30O2
254.40
0.51
99
Omega-7 fatty acid
Antifungal (Hameed et al., 2018)
15.71
Dibutyl phthalate
C16H24O4
278.34
1.41
91
Phthalates
Antifungal (Ahsan et al., 2017)
16.14
Eicosane
C20H42
282.54
4.83
99
Alkane
Antioxidant, Antimicrobial (El-Shahaby et al., 2019)
16.31
n-Hexadecanoic acid
C16H32O2
256.4
1.78
99
Fatty Acid
Antioxidant, Lubricant, Antiandrogenic, 5- alpha-reductase inhibitor (Lawal et al., 2015)
16.60
3-(3,5-Di-tert-butyl-4-hydroxyphenyl)propionic Acid
C17H26O3
278.39
0.70
96
Phenyl propanoid
Enzyme catalyst (Milaeva et al., 2006)
17.12
Cyclotetradecane
C14H28
196.37
0.33
91
Cyclic Alkane
Antimicrobial (Chuah et al., 2018)
17.66
9,12,15-Octadecatrienoic acid
C18H30O2
278.43
7.71
99
Fatty Acid
Antimicrobial (Abker 2021)
18.81
Octadec-9-enoic acid
C18H34O2
282.46
12.07
95
Fatty Acid
Anti-hyperglycaemic (Kapoor et al., 2019)
19.20
14-Methyl-8-hexadecyn-1-ol
C17H32O
252.4
1.20
90
Aliphatic hydrocarbon
Antimicrobial, Anticancer (Ahuchaogu et al., 2018)
19.43
Pentadec-7-ene
C15H30
210.4
0.55
90
Alkene
Antioxidant, Antimicrobial
19.60
E,E,Z-1,3,12-Nonadecatriene-5,14-diol
C19H34O2
294.5
0.59
95
Alcohol
Antiviral (Tassakka et al., 2021)
19.76
Docosane
C22H46
310.60
0.98
98
Alkane
Antifungal (Bierer et al., 1995)
19.90
Decanoic acid
C10H20O2
172.26
0.46
92
Fatty Acid
Antiseizure, Antim (Chang et al., 2016, Shen et al., 2021)
20.25
Octadecane
C18H38
254.49
2.17
96
Alkane
Antineoplastic Anticancer (Talbaoui et al., 2020)
20.81
Pyridine-3-carboxamide
C6H8N2O
140.14
1.09
91
Organoheterocyclic compound
Antibacterial (Narramore et al., 2019)
21.79
Tetracosane
C24H50
338.65
4.72
97
Alkane
Antimicrobial, Antioxidant (Asha et al., 2017)
22.23
2-Dodecen-1-yl(-)succinic anhydride
C16H26O3
266.38
0.54
93
Carboxylic anhydride
Antioxidant (Marzocchi et al., 2018)
22.62
Octacosane
C28H58
394.77
2.69
98
Alkane
Antimicrobial (Chelliah et al., 2017)
22.97
22-Tricosenoic acid
C23H44O2
352.6
0.50
90
Fatty Acid
Antimicrobial (Selvan and Velavan 2015)
24.06
1,2-Benzenedicarboxylic acid
C8H6O4
166.14
5.69
98
Benzenoid
Antimicrobial, Anticancer, antitumor, Antioxidant, chemopreventive (Ezhilan and Neelamegam 2012)
25.05
1-Eicosene
C20H40
280.53
0.92
95
Hydrocarbon
Antimicrobial (Naser et al., 2019)
25.90
Triacontane
C30H62
422.82
0.68
96
Alkane
Antibacterial, Antidiabetic, Antitumor (Mallick and Dighe, 2014a, 2014b)
26.89
9-Hexacosene
C26H52
364.7
0.68
96
Alkene
Antinociceptive, Analgesic, Antiinflammatory (Naser et al., 2019)
27.38
Hentriacontane
C31H64
436.85
2.03
99
Alkane
Antiinflammatory, Antitumor, Antimicrobial (Khajuria et al., 2017)
28.58
Nonacosane
C29H60
408.6
0.46
99
Alkane
Antimicrobial (Palic et al., 2002)
28.78
Piperine
C17H19NO3
285.34
0.40
97
Piperine derivatives
Depression, Analgesic, Antipyretic, anti-inflammatory, Antioxidant (Okwute and Egharevba 2013)
30.06
Triacontane
C30H62
422.82
1.47
99
Alkane
Antitumor, Antidiabetic, Antibacteria (Mallick and Dighe, 2014a, 2014b)
30.63
Stigmasta-5
C29H48O
412.7
0.74
95
Steroids derivative
Antibacterial (Joycharat et al., 2013)
31.62
Stigmastan-3,5-diene
C29H48
396.7
1.00
99
Phenanthrene
Antimicrobial (Sánchez-Pérez et al., 2009)
32.22
Vitamin E
C29H50O2
430.71
0.41
98
Methylated phenol
Antioxidant (Vrolijk et al., 2015)
33.49
Triacontane
C30H62
422.82
0.84
99
Alkane
Antitumor, Antibacterial, Antidiabetic (Amudha et al., 2018)
3.2 In vitro biological activities
3.2.1 Antioxidants activity (mg Trolox Eq. Per gram of dry extract) of T. domingensis
Natural antioxidants have brought a lot of interest in recent years due to their beneficial role in many ailments associated with free radicals (Sen and Chakraborty 2011). Antioxidants of natural origin are gaining popularity because they can protect the human body from free radicals without causing toxicity (Altemimi et al., 2017). Consumption of antioxidants has long been linked to a lower risk of a variety of diseases in which oxidative stress may play a role, particularly chronic diseases like cardiovascular diseases, cancer, neurological disorders, and skin diseases. Natural antioxidants derived from plant components are currently gaining popularity as a replacement for synthetic antioxidants. This is because natural antioxidants are safer than synthetic antioxidants because they occur naturally in plant foods, and they are more desirable than their synthetic derivatives, thus there is a lot of interest in identifying natural antioxidants from plants (Gulcin 2020). The literature review of antioxidant activity indicates that wild leaf plants with higher contents of phenolic compounds and flavonoids are an important source of natural antioxidants (Xiang et al., 2019). Plants that have been shown to have high antioxidant activity can be considered to prevent toxic oxidation in food chemicals or drugs for treating cancers (Amadi et al., 2019). According to the literature, two-thirds of all plant species have therapeutic and antioxidant potential (Al Rashdi et al., 2021) (Fig. 4).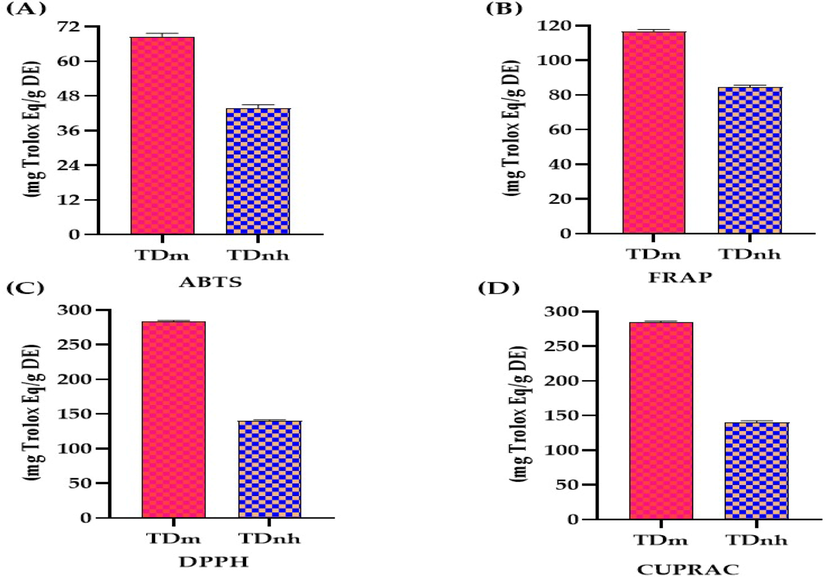
Antioxidant potential for methanolic extract “TDm” and n-hexane fraction “TDnh” of T. domingensis.
Antioxidant activity for the methanolic extract and n-hexane fraction of T. domingensis (Table 2 and Fig. 5) was determined by 2,2′-Azino-Bis(3-Ethylbenzthia zoline-6-Sulfonic acid (ABTS), Ferric Reducing Antioxidant Power Assay (FRAP), 2,2-Diphenyl-1-Picrylhydrazyl (DPPH), and Cupric Ion Reducing Antioxidant Capacity (CUPRAC). The antioxidant potential determined by ABTS, FRAP, DPPH, and CUPRAC methods revealed that methanolic extract exhibited maximum activity (114.31 ± 8.17, 116.84 ± 3.01, 283.54 ± 7.3, 284.16 ± 6.5 respectively) While n-hexane extract has ABTS, FRAP, DPPH, and CUPRAC (75.12 ± 5.02, 84.28 ± 4.01, 140.53 ± 4.67, 140.14 ± 3.01 respectively) antioxidant activity. The antioxidant potential in different extracts of fruit of T. domingensis by the DPPH method was previously reported (Chai et al., 2015). The antioxidant activity of this plant extract and fraction is attributed due to phenolic and flavonoid contents because of their ability to donate hydrogen atoms to free radicals for denaturation of these free radicals (Aryal et al., 2019). These results suggest that both methanolic extract and n-hexane of T. domingensis are excellent sources of antioxidants.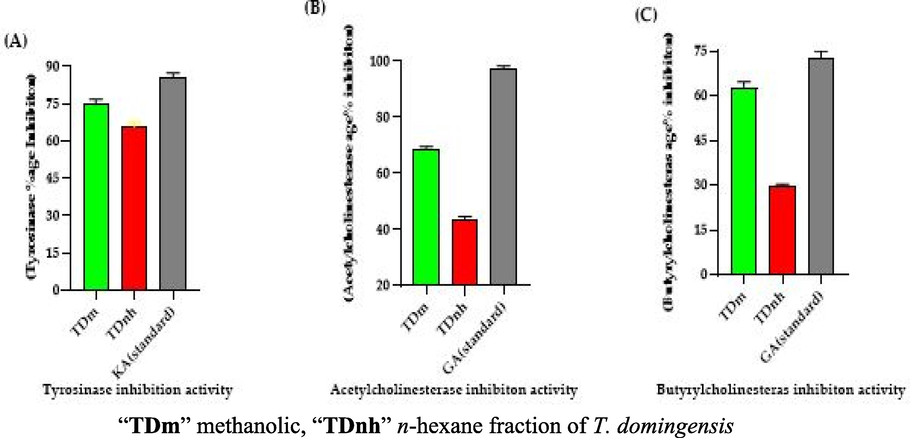
“A” Tyrosinase inhibition activity “KA” kojic acid (standard), “B” Acetylcholinesterase inhibition activity “GA” galantamine (standard) “C” Butyrylcholinesterase inhibition, galantamine (standard).
3.2.2 Enzyme inhibition activity of T. domingensis
Enzyme inhibitors are of clinically prodigious value in various areas of disease management. The tyrosinase, acetylcholinesterase, and butyrylcholinesterase inhibition activity of methanolic extract and n-hexane fraction of T. domingensis were analyzed.
3.2.2.1 Tyrosinase Inhibition Activity
Hyperpigmentation and browning of the skin are two common unwanted effects in humans, and tyrosinase is the enzyme involved in these effects humans (Roselan et al., 2021). Tyrosinase is known as polyphenol oxidase, and it is a copper-containing multifunctional enzyme, found in both plants and animals (Seo et al., 2003). Due to the high demand for tyrosinase inhibitors, researchers are working hard to identify, isolate, and synthesize novel moieties for use in the food, cosmetics, and pharmaceutical industries (Masum et al., 2019).
The results revealed that methanolic extract showed maximum tyrosinase inhibition activity (74.51 ± 3.49%) as compared to the n-hexane fraction (65.32 ± 2.64%) of T. domingensis (Table 5 and Fig. 5). The %age inhibition of tyrosinase expressed by kojic acid was (85.58 ± 0.85%). The tyrosinase inhibition and antioxidant potential of plant extracts are dependent upon the presence of polyphenolic compounds (Nerya et al., 2004). This activity was the first time performed on any part of T. domingensis. Each experiment was performed in triplicates (n = 3), “TDm” methanolic extract and “TDnh” n-hexane fraction.
SAMPLES
Tyrosinase Inhibition
Acetylcholinesterase Inhibition
Butyrylcholinesterase Inhibition
TDm
74.51 ± 3.49
68.25 ± 0.13
62.62 ± 1.43
TDnh
65.32 ± 2.64
43.72 ± 1.23
29.53 ± 1.17
Reference
85.58 ± 0.85a
97.11 ± 1.26b
72.88 ± 2.61b
3.2.2.2 Acetylcholinesterase and Butyrylcholinesterase Inhibition Activity
Cholinesterase inhibitors are agents that enhance cholinergic function by increasing the amount of acetylcholine in cholinergic synapses in the brain (Khan et al., 2018). Acetylcholine is the most common neurotransmitter in the human body, with roles in both the peripheral and central nervous systems. Cholinesterase inhibitors are most commonly used to treat dementia in Alzheimer's disease patients. Cholinesterase inhibitors have been demonstrated to have a minor impact on cognitive symptoms associated with dementia (Anand and Singh 2013). Numerous plant extracts have been analyzed for their potential role to counteract cognitive disorders and other neurological diseases (Chonpathompikunlert et al., 2010).
The methanolic extract revealed maximum acetylcholinesterase and butyrylcholinesterase inhibition activity (68.25 ± 0.13 and 62.62 ± 1.43% respectively) as compared to inhibition results of n-hexane extract (43.72 ± 1.23 and 29.53 ± 1.17%). The acetylcholinesterase and butyrylcholinesterase inhibition activities shown by galantamine were 97.11 ± 1.26 and 72.88 ± 2.61% respectively (Table 5 and Fig. 5). The cholinesterase inhibition activity is directly related to flavonoid contents in the plant extracts (Khan et al., 2018). The potential of T. domingensis for the neurological disorder was first time evaluated.
3.2.3 Thrombolytic activity
Thrombolysis, also known as thrombolytic therapy, is a procedure that dissolves blood clots, improves blood flow, and prevents tissue and organ damage (Wardlaw et al., 2014). Thrombolytic therapy is used in patients with acute pulmonary embolism (PE) to eliminate the embolic burden efficiently and improve cardiorespiratory hemodynamics. Thrombolytic agents function by activating plasminogen, and it is subsequently transformed to plasmin, which is an enzyme that breaks down fibrin strands (Voros 2017). Five samples of blood were taken and were observed through thrombolytic activity by the use of Streptokinase as a standard. The current study revealed that the n-hexane fraction showed higher thrombolytic activity than methanolic extract (Table 6). Previously the aqueous, methanol, and chloroform extracts of leaves of T. angustifolia revealed 51.76 ± 2.5, 58 ± 2.32, and 18 ± 1.84% clot lysis activity respectively (Umesh et al., 2014). Methanolic extract of the entire plant of T. elephantina was analyzed for thrombolytic activity and serial concentrations of plant extracts were used (2, 4, 6, 8, 7, and 10 mg/mL) among these concentrations 4 mg/mL exhibited maximum thrombolytic activity (Singh et al., 2020). “TDm” methanolic extract, “TDnh” n-hexane fraction, and “SK” streptokinase.
Plant Extract and Fraction
Blood Sample 1
Blood Sample 2
Blood Sample 3
Blood Sample 4
Blood Sample 5
TDm
60.06
60.82
60.78
60.07
60.89
TDnh
66.62
66.96
67.27
67.58
67.41
SK (standard)
79.07
79.15
79.33
78.52
78.65
3.2.4 Antimicrobial activity
3.2.4.1 Antibacterial activity
Eight bacterial strains (Bacillus subtilis, Micrococcus luteus, Staphylococcus epidermidis, Bacillus pumilus, Staphylococcus aureus, Escherichia coli, Bordetella bronchi septica, and Pseudomonas aeruginosa) were used to check the antibacterial potential of methanolic extract and n-hexane fraction of T. domingensis and co-amoxiclav was used as a standard antibacterial agent for this assay. Both methanolic extract and n-hexane fraction revealed good antibacterial results (Table 7). Three different Conc. (5, 10, and 20 mg/mL) were used and observed zone of inhibition. This antibacterial potential was concentration-dependent. At higher concentrations (20 mg/mL), the zone of inhibition was very significant except for Pseudomonas aeruginosa (gram-negative). The findings of this assay suggest that these test samples are efficacious against most gram-positive bacteria and some gram-negative bacteria. Al-Kalifawi et al., 2017 determined the antimicrobial potential of some aqueous, methanol, chloroform, and petroleum ether extracts of T. domingensis (Al-Kalifawi et al., 2017). Various compounds in the GC–MS profile of methanolic extract and n-hexane fraction have reported antimicrobial and antibacterial potential. These compounds include 2,4-bis(1,1-dimethylethl) phenol (Yoon et al., 2011), 9,12,15-Octadecatrienoic acid (Malathi and Ramaiah 2017), Heptadecane (Uma et al., 2010), Tetradecanoic acid (Abubakar and Majinda 2016), and Triacontane (Mallick and Dighe, 2014a, 2014b).
Strain
Zone of inhibition (mm) of Standard (Co-Amoxiclav) (Conc. 1 mg/mL)
Conc. of sample (mg/mL)
Zone of Inhibition of TDm (mm)
Zone of Inhibition of TDnh (mm)
Bacillus subtilis
23
5
NA
NA
10
8
14
20
13
18
Micrococcus luteus
20
5
5
NA
10
11
13
20
18
18
Staphylococcus epidermidis
18
5
6
NA
10
11
14
20
20
17
Bacillus pumilus
21
5
7
NA
10
12
8
20
18
13
Staphylococcus aureus
21
5
NA
NA
10
10
13
20
17
18
Escherichia coli
24
5
6
NA
10
9
15
20
15
19
Bordetella bronchiseptica
25
5
NA
NA
10
8
11
20
13
16
Pseudomonas aeruginosa
6
5
NA
NA
10
NA
8
20
8
13
3.2.4.2 Antiviral Activity of T. domingensis
Due to the lack of antiviral agents and the emergence of new viral diseases, there is a demand for the screening of newer antiviral therapies. Four viral strains were used namely Influenza A viruses (IAVs), Infectious bronchitis virus (IBV), Newcastle disease virus (NDV), and Infectious bursal disease virus (IBDV). The results were expressed as HA titre count. Both methanolic and n-hexane extracts showed highly strong results against these tested viral strains (Table 8). Many GC–MS profiled compounds of both methanolic extract and n-hexane fraction have reported antimicrobial and antiviral activities such as E, E, Z-1,3,12-Nonadecatriene-5,14-diol (Tassakka et al., 2021), Dodecanoic acid (Chionis et al., 2016), Octadecane (Jasim 2015), Eicosane (El-Shahaby et al., 2019), and Hentriacontane (Khajuria et al., 2017). HA titre 0–8: highly strong, 16–32: strong, 64–128: moderate, 256–2048: not active (Musaddiq et al., 2020). “TDm” methanolic extract, “TDnh” n-hexane fraction, “IAVs” Avian Influenza A viruses, “IBV” Infectious bronchitis virus, “NDV” New Castle Disease virus, and “IBDV” Infectious Bursal Disease Virus.
Strain Name
Control
HA titre count treated with TDm
HA titre count treated with TDnh
IAVs
2048
04
04
IBV
1024
00
08
NDV
2048
00
08
IBDV
1024
08
02
3.3 Molecular docking studies
In addition, further improvements can be made to in vitro research methods for the rapid screening of enzyme inhibitors using molecular modeling. Therefore, a combination of bioinformatics simulation and in vitro analysis will be useful for the evaluation of extract and fraction for their biological activities (Sabe et al., 2021). Molecular docking is the study of how two or more molecular structures (such as drugs and enzymes or proteins) fit together. In a simple definition, docking is a molecular modeling technique used to predict how proteins (enzymes) interact with small molecules (binders or ligands) (Rudnitskaya et al., 2010). A detailed understanding of the protein–ligand interactions is therefore central to understanding biology at the molecular level. Moreover, knowledge of the mechanisms responsible for the protein–ligand interactions and binding will also facilitate the discovery, design, and development of drugs. In the binding of enzymes and ligands, binding affinity has prime importance. The least the binding affinity, the more will be better binding between the ligands and enzyme. The binding affinity in the positive (+) symbol represents that the ligand and the enzyme have no interaction. To estimate the binding affinities and binding interactions at the active sites, the molecular docking of major GC–MS-identified compounds was performed. The standard drugs utilized in these assays were also docked with respective enzymes. Piperine resulted in the best binding affinity (-10.2 kj/mol) for tyrosinase and the binding affinity for kojic acid (standard) was −5.4 kj/mol. The 3-(3,5-Di-tert-butyl-4-hydroxyphenyl)propionic acid showed the least binding affinity against both acetylcholinesterase and butyrylcholinesterase (-9.9 and −10.8 kj/mol respectively) while the binding affinity of galantamine (standard) for these both enzymes were −7.6 and −8.4 kj/mol respectively. The ligands interacting with weak intermolecular forces, like van der waals forces revealed better binding affinity than the ligands binding with conventional hydrogen bonding. In the docking results represented in the 2D figures (Figs. 6–8), it is clear that there is the least number of amino acids which are interacting with conventional hydrogen bonding than van der waals forces. This indicated that our ligands have better binding affinities with the enzyme (Table 9).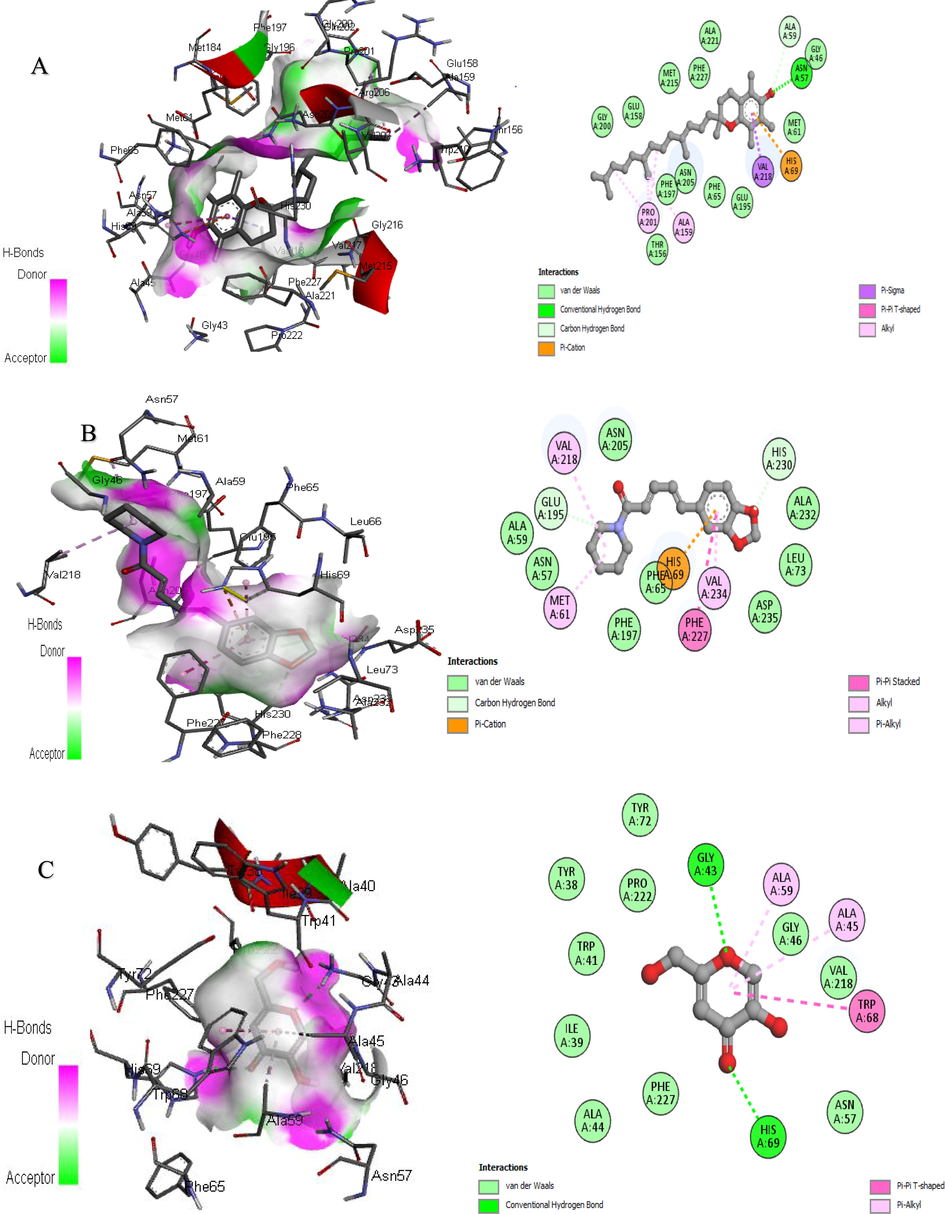
3D and 2D Interaction of tyrosinase with ligands. “A” Vitamin E, “B” Piperine, and “C” Kojic acid.
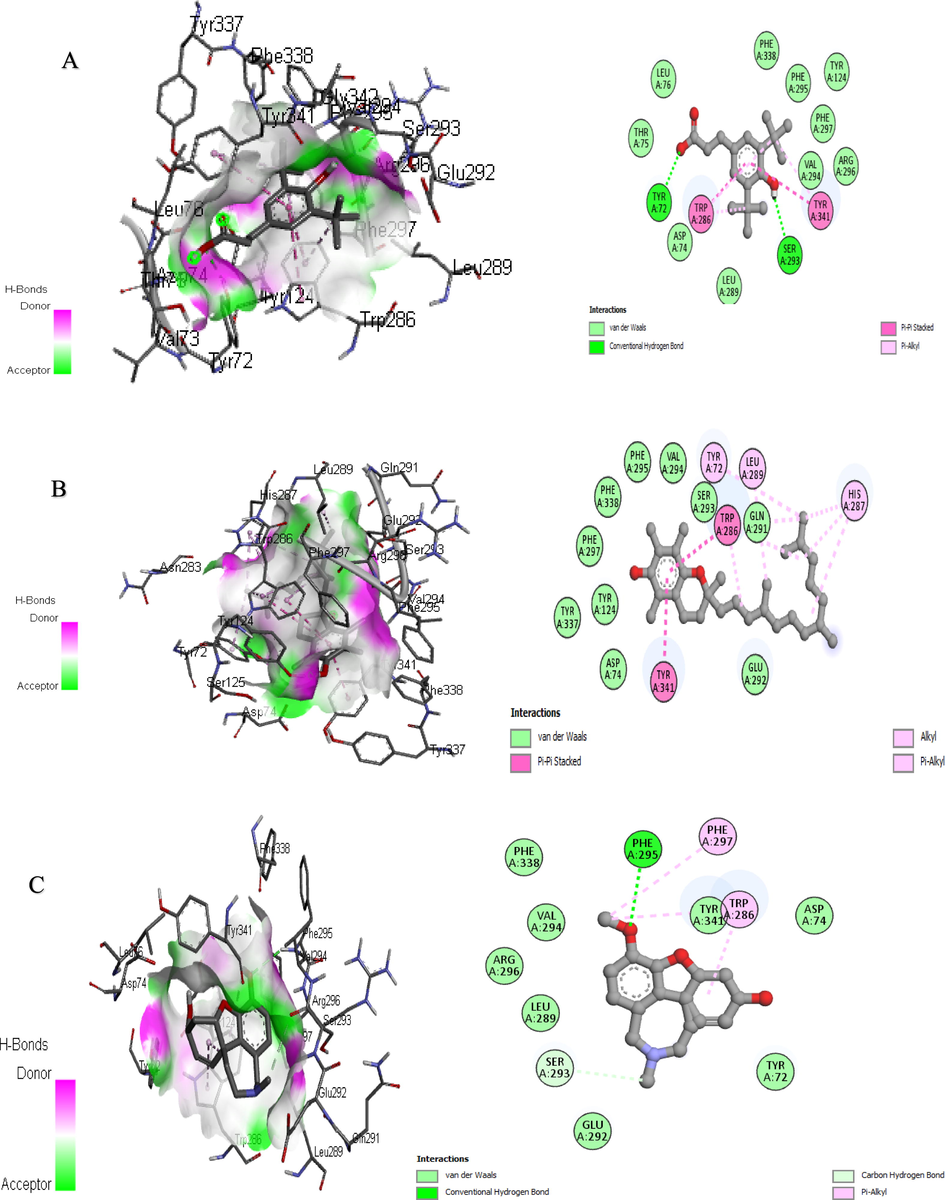
3D and 2D Interaction of acetylcholinesterase with ligands “A” 3-(3,5-Di-tert-butyl-4-hydroxyphenyl) propionic Acid, “B” Vitamin E, and “C” Galantamine.
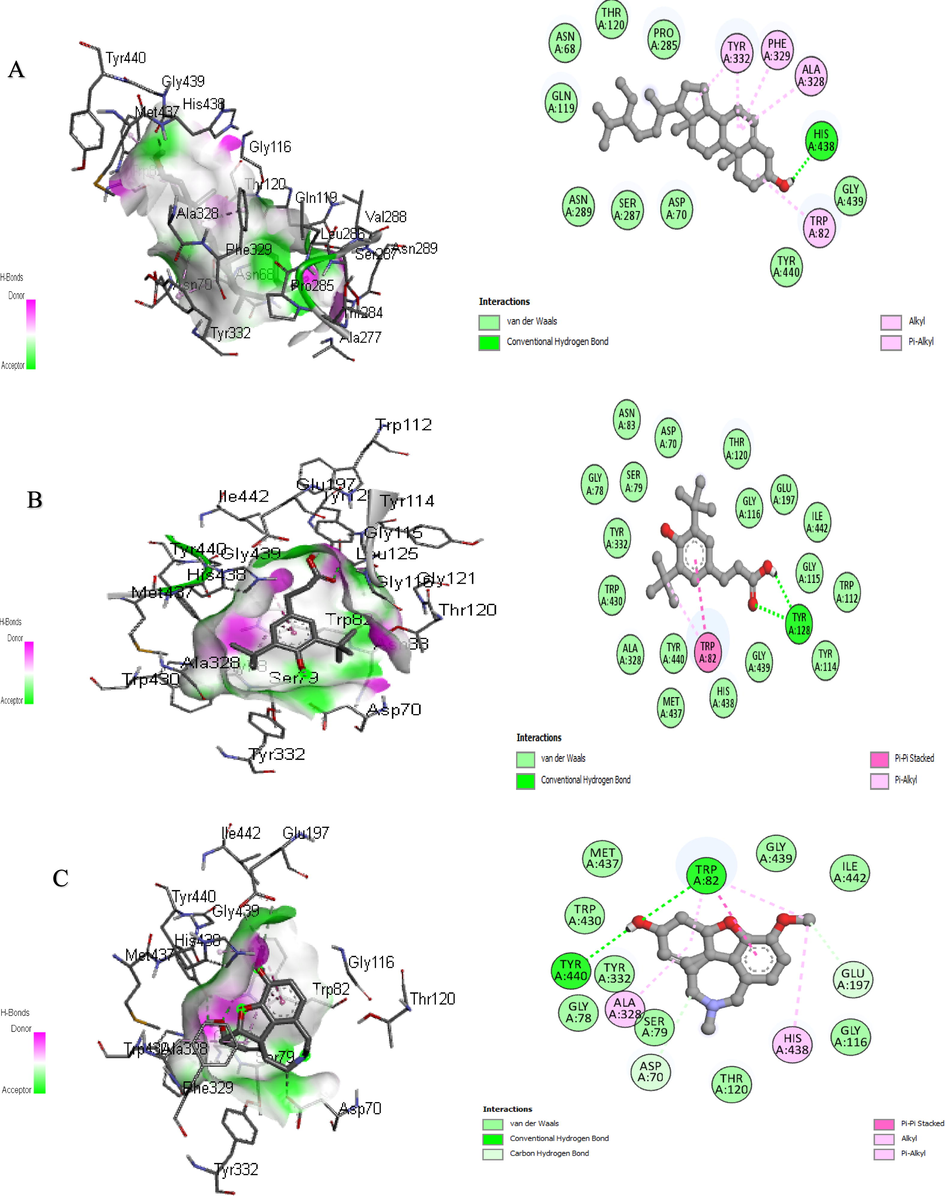
3D and 2D Interaction of butyrylcholinesterase with ligands “A” Sitosterol, “B” 3-(3,5-Di-tert-butyl-4-hydroxyphenyl) propionic Acid, and “C” Galantamine.
Sr.No
Compounds Name
TYR (Binding affinity)
Interacting Amino Acid Residues
AChE (Binding affinity)
Interacting Amino Acid Residues
BChE (Binding affinity)
Interacting Amino Acid Residues
1.
Vitamin E
−9.1
Gly46,Asn57,Ala59,Met61,Phe65,His69,Thr156, Glu158,Ala159,Glu195,Phe197,Gly200,Pro201,Asn205Met215,Val218,Ala221,Phe227,
−8.5
Tyr72,Asp74,Tyr124,Trp286,His287,Leu289,Gln291,Glu292,Ser293,Val294,Phe295,Phe297,Tyr337,Phe338,Tyr341
−8.4
Asp70,Trp82, Gly115,Gly116, Gly117,Thr120, Gly121,Leu125, Tyr128,Glu197, Pro285,Ala328, Phf329,Tyr332, Trp430,Met437, His438,Tyr440
2.
3-(3,5-Di-tert-butyl-4-hydroxyphenyl)propionic Acid
−5.6
Trp41,Arg75, Arg78,Trp269, Asn270,Thr271, Asp275,Asp275
−9.9
Tyr72,Asp74,Thr75,Leu76,Tyr124,Trp286,Leu289,Ser293,Val294,Phe295,Arg296Phe297,Phe338,Typ341
−10.8
Asp70,Gly78,Ser79,Asn83,Trp112,Tyr114,Gly115,Gly116,Thr120,Tyr128,Glu197,Ala328,Tyr332,Trp430,Met437,His438,Gly439,Tyr440,Ile442
3.
Sitosterol
−8.3
Asp36,Ile39, Ala40,Phe48, His49,Ile139, Gly143,Pro219,Tyr267,
--9.7
Ala377,Leu380, Tyr382,Asp404,Gln527,Ala528, Phe531,
−9.3
Asn68,Asp70,Trp82Thr120,Gln119,Pro285,Ser287,Asn289,Ala328,Phe329,Try332,Phe329,His438,Tyr440,
4.
Pyrrolidine 2,5-Cyclohexadiene-1,4-dione
−8.5
Asp36,Ile39, Lys47,Phe48, His49,Pro52, Ile139,Asp140 Glu141,Gly143, Tyr267
−7.4
Ala412,Gln413, Gly416,Arg417,Tyr503,Ala505,Gln508,Ala526,Gly529,Ala530,Asn533,
−6.9
Trp82,Gly115, Gly116,Glu197, Ala328,Phe329, Tyr332,His438, Gly439,Tyr440
5.
Piperine
−10.2
Asn57,Ala59,Met61,Phe65,His69,Glu195,Phe197,Leu73,Ans205,Val218,Phe227His230,,Ala232,Val234,Asp235,
−7.9
Ala377,Leu380, Tyr382,Thr383, Asp384,Ala397Asp404,Gln527Ala528,Phe531, Phe535
−6.8
Gly78,Trp82, Gly116,Gly117, Gln119,Thr120, Pro285,Leu286, Ser287,Val288, Ala328,Phe329, His428,Trp430, Met437,Gly439,Tyr440
6.
N-Acetoacetyl-deacetylcolchicine
−6.2
Trp41,Arg75, Glu71,Agr78, Trp269,Pro268, Asn270,
−8.4
Ala377,Val378, Leu380,Tyr382,Thr383,Gln527, Ala528,Phe531
−9.2
Asp70,Gly78, Ser79,Asn83, Gly115,Gly116, Gly117,Thr120, Gly121,Tyr128, Glu197,Ala199, Trp231,Tyr322, Phe329,Phe398, His438
7.
Phytol
−7.9
Asp36,Ile39, Ala40,Ala44, Lys47,Phe48, Pro52,Ile139, Glu141,Gly143,Tyr267
−6.9
Ala377,Val378, Leu380,Tyr382, Gln527,Ala528, Phe531,Phe535
−6.5
Trp82,Tyr114, Gly115,Gly116, Gly117,Thr120, Glu197,Ala199, Trp231,Leu286, Ala328,Phe329, Tyr332,Phe398, His438
8.
9,12,15-Octadecatrienoic acid
−7.7
Asp36,Ile39, Ala40,Lys47, Phe48,His49, Pro52,Ile139, Gln142,Gly143, Tyr267
−6.8
Ala377,Val378, Leu380,Tyr382, Gln527,Ala528, Phe531,Phe535
−6.9
Trp82,Gly115, Gly116,Gly117, Ala199,Trp231, Pro285,Leu286, Val288,Phe329, Phe398,His438, Gly439
9.
2-Dodecen-1-yl(-)succinic anhydride
−7.4
Ilu39,Ala40, Gly43,Lys47, Phe48,Ile139, Tyr267,
−6.9
Ala377,Val378, Leu380,Tyr382,Thr383,Gln527, Ala528,Phe531, Phe535
−7.0
Gly78,Trp82, Gly116,Gly117, Ala199,Trp231, Leu286,Ser287, Val288,Ala328, Phe329,Phe398,Trp430, His438
10.
Standard
−5.4a
Tyr38,Ile39,Trp41,Gly43,Ala44,Ala45,Gly46,Asn57,Ala59,Trp68,His69,Tyr72,Val218,Pro222,Phe227
−7.6b
Tyr72,Asp74,Trp296,Leu289,Glu292,Ser293,Val294,Phe295,Arg296,Phe297,Phe338,Tyr341
−8.4b
Asp70,Gly78, Ser79,Trp82, Gly116,Thr120, Glu197,Ala328, Tyr332,Trp430, Met437,His438,Gly439,Tyr440, Ile442
4 Conclusion
The current comparative study was focused on phytochemical profiling and in vitro biological activities for methanolic extract and n-hexane fraction of T. domingensis. The methanolic extract exhibited the maximum polyphenolic contents (TPC and TFC), antioxidant potential and tyrosinase, acetylcholinesterase, and butyrylcholinesterase inhibition activity. The GC–MS analysis of the methanolic extract and n-hexane fraction resulted in the tentative identification of several secondary metabolites. The enzyme inhibition activity for methanolic extract and n-hexane fraction of T. domingensis was further confirmed by in-silico molecular docking studies. n-Hexane fraction resulted in better thrombolytic activity than methanolic extract. Methanolic extract and n-hexane fraction of T. domingensis exhibited significant and almost similar antibacterial and antiviral activity against the tested strains. The phytochemical and biological potential of this plant highlighted its value for further isolation of bioactive compounds which is currently in progress.
Author contributions
Conceptualization, R.D. and B.A.G.; methodology, K.U.R.; software, H.T., and A.E.S.; validation, H.Y.A., and J.H.A.; formal analysis, A.B.; investigation, M.H.; resources, H.Y.A; data curation, M.A.; writing—original draft preparation, R.D. and B.A.G.; writing—review and editing, B.A.G And R.D.; visualization, S.A.; supervision, K.U.R, And S.A.; project administration, J.H.A.; funding acquisition, H.Y.A. And J.H.A. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful to the King Saud University, Riyadh, Saudi Arabia for funding this study through Project number RSP2022R504.
Institutional review board statement
All the trials were carried out following the NIH guidelines and were approved by the Department of Pharmaceutical chemistry’s concerned committee (1009/AS & RB/12/07/2021).
Informed consent statement
Not applicable.
Acknowledgments
The authors are thankful to Researchers Supporting Project number (RSP2022R504), King Saud University, Riyadh, Saudi Arabia. As well, the authors are grateful to Dr. Saeed Ahmed, Chairperson Department of Pharmaceutical Chemistry for his technical support, suggestions, and laboratory facilities.
References
- The GC-MS Analysis and Antimicrobial Activity of Oils from Potential Medicinal Herbs. Sudan University of Science and Technology; 2021.
- GC-MS analysis and preliminary antimicrobial activity of Albizia adianthifolia (Schumach) and Pterocarpus angolensis (DC) Medicines. 2016;3:3.
- [Google Scholar]
- Phytochemical analysis and GC-MS determination of Lagenaria breviflora R. fruit. Int. J. Pharmacogn. Phytochem. Res.. 2017;9:1045-1050.
- [Google Scholar]
- Extraction and identification of bioactive compounds (eicosane and dibutyl phthalate) produced by Streptomyces strain KX852460 for the biological control of Rhizoctonia solani AG-3 strain KX852461 to control target spot disease in tobacco leaf. AMB Express.. 2017;7:1-9.
- [Google Scholar]
- GC-MS Analysis of Bioactive Compounds from Whole Plant Chloroform Extract of Ageratum conyzoides. Int. J. Med. Plants Nat. Prod.. 2018;4:13-24.
- [Google Scholar]
- The potential role of female flowers inflorescence of Typha domingensis Pers. in wound management. J. Ethnopharmacol.. 2011;133:1027-1032.
- [Google Scholar]
- Antioxidant and antibacterial activities of leaves crude extracts of Adenium obesum grown in Oman National Botanical Garden. Adv. Biomarker Sci. Technol.. 2021;3:8-14.
- [Google Scholar]
- Al-Kalifawi, E. J., Y. J. Al-Azzawi, K. kat Al-Fartousi, et al., 2017. Physicochemical, phytochemical profiling, and Biological activities of leaves extract of Bardy (Typha domingensis Pers.) from Al-Chibayish marshes in southern Iraq. مؤتمرات الآداب والعلوم الانسانية والطبيعية.
- Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants. 2017;6:42.
- [Google Scholar]
- Natural antidotes and management of metal toxicity. Environ. Sci. Pollut. Res.. 2019;26:18032-18052.
- [Google Scholar]
- In vitro antioxidant activity and total phenolic and flavonoid contents of Alhydwan (Boerhavia elegana Choisy) seeds. J. Food Nutr. Res.. 2014;2:215-220.
- [Google Scholar]
- Phenolic compounds from an Algerian medicinal plant (Pallenis spinosa): Simulated gastrointestinal digestion, characterization, and biological and enzymatic activities. Food Funct.. 2021;12:1291-1304.
- [Google Scholar]
- Identification of bioactive components in Enhalus acoroides seagrass extract by gas chromatography-mass spectrometry. Asian J. Pharm. Clin. Res.. 2018;11:131-137.
- [Google Scholar]
- A review on cholinesterase inhibitors for Alzheimer’s disease. Arch. Pharmacal Res.. 2013;36:375-399.
- [Google Scholar]
- Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food. Chem.. 2004;52:7970-7981.
- [Google Scholar]
- Total phenolic content, flavonoid content, and antioxidant potential of wild vegetables from Western Nepal. Plants. 2019;8:96.
- [Google Scholar]
- GC-MS Analysis of the Ethanolic Extract of the whole Plant Drosera Indica L. J. Pharmacogn. Phytochem. Res.. 2017;9:685-688.
- [Google Scholar]
- Spread of artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med.. 2014;371:411-423.
- [Google Scholar]
- GC-MS analysis of bioactive compounds from ethanolic leaf extract of Waltheria Indica Linn. and their pharmacological activities. Int. J. Pharm. Sci. Res.. 2018;9:2005-2010.
- [Google Scholar]
- Total antioxidant capacity of teas by the ferric reducing/antioxidant power assay. J. Agric. Food. Chem.. 1999;47:633-636.
- [Google Scholar]
- Isolation, structure elucidation, and synthesis of irlbacholine, 1, 22-Bis [[[2-(trimethylammonium) ethoxy] phosphinyl] oxy] docosane: A novel antifungal plant metabolite from Irlbachia alata and Anthocleista djalonensis. J. Org. Chem.. 1995;60:7022-7026.
- [Google Scholar]
- Evaluation of the solvent effect on the extraction of phenolic compounds and antioxidant capacities from the berries: application of principal component analysis. Chem. Cent. J.. 2014;8:1-9.
- [Google Scholar]
- Plant secondary metabolites and their general function in plants. Lessons on caffeine, cannabis & co. Springer; 2018. p. :3-17.
- Mammalian alkaloids: conversions of tetrahydroisoquinoline-1-carboxylic acids derived from dopamine. Planta Med.. 1991;57:S93-S99.
- [Google Scholar]
- Carlson, E., 2015. Biol., 2010, 5, 639 CrossRef CAS PubMed;(b) A. Harvey, R. Edrada-Ebel and R. Quinn. Nat. Rev. Drug Discovery. 14, 111.
- Alpha-glucosidase inhibitory and antioxidant activity of solvent extracts and fractions of Typha domingensis (Typhaceae) fruit. Trop. J. Pharm. Res.. 2015;14:1983-1990.
- [Google Scholar]
- Antioxidant, iron-chelating, and anti-glucosidase activities of Typha domingensis Pers (Typhaceae) Trop. J. Pharm. Res.. 2014;13:67-72.
- [Google Scholar]
- Seizure control by decanoic acid through direct AMPA receptor inhibition. Brain.. 2016;139:431-443.
- [Google Scholar]
- Nutritional quality of Moringa oleifera for its bioactivity and antibacterial properties. Int. Food Res. J.. 2017;24:825.
- [Google Scholar]
- Synthesis and biological activity of lipophilic analogs of the cationic antimicrobial active peptide anoplin. J. Pept. Sci.. 2016;22:731-736.
- [Google Scholar]
- Piperine, the main alkaloid of Thai black pepper, protects against neurodegeneration and cognitive impairment in an animal model of cognitive deficit like the condition of Alzheimer’s disease. Food Chem. Toxicol.. 2010;48:798-802.
- [Google Scholar]
- Isolation of antimicrobial Tridecanoic acid from Bacillus sp. LBF-01 and its potentization through silver nanoparticles synthesis: a combined experimental and theoretical studies. J. Nanostruct. Chem.. 2021;11:573-587.
- [Google Scholar]
- Eicosane, pentadecane, and palmitic acid: The effects in in vitro wound healing studies. Asian Pac. J. Trop. Biomed.. 2018;8:490.
- [Google Scholar]
- Mangrove plants as a source of bioactive compounds: A review. Natural Prod. J.. 2019;9:86-97.
- [Google Scholar]
- Antifungal Activity and Molecular Docking of Phenol, 2, 4-Bis (1, 1-Dimethylethyl)-Produced By Plant Growth-Promoting Mangrove Actinobacteria. Kutzneria Sp. Strain TSII; 2021.
- Effect of extraction method on chemical composition, antioxidant and anti-inflammatory activities of essential oil from the leaves of Algerian Tetraclinis articulata (Vahl) Masters. Ind. Crops Prod.. 2013;44:32-36.
- [Google Scholar]
- Medicinal plants of China. Reference Publications; 1985.
- Elnager, A., W. Z. Abdullah, R. Hassan, et al., 2014. In vitro whole blood clot lysis for fibrinolytic activity study using d-dimer and confocal microscopy. Advances in hematology. 2014,
- Evaluation of the biological activity of Capparis spinosa var. aegyptiaca essential oils and fatty constituents as Anticipated Antioxidant and Antimicrobial Agents. Progress Chem. Biochem. Res.. 2019;2:211-221.
- [Google Scholar]
- Selection of Pseudomonas aeruginosa for biosurfactant production and studies of its antimicrobial activity. Egypt. J. Pet.. 2014;23:1-6.
- [Google Scholar]
- GC-MS analysis of phytocomponents in the ethanol extract of Polygonum chinense L. Pharmacogn. Res.. 2012;4:11.
- [Google Scholar]
- The Antibacterial and Antioxidant Activity of Moringa Oleifera Seed Oil Extract Against Some Foodborne Pathogens. Indian J. Forensic Med. Toxicol.. 2021;15:2529.
- [Google Scholar]
- Synthesis, biological evaluation, and docking study of maleimide derivatives bearing benzenesulfonamide as selective COX-2 inhibitors and anti-inflammatory agents. Bioorg. Med. Chem.. 2015;23:5273-5281.
- [Google Scholar]
- Phytochemical screening, quantitative analysis of primary and secondary metabolites of Cymbopogan citratus (DC) Stapf. leaves from Kodaikanal hills, Tamilnadu. Int. J. Pharmtech. Res.. 2014;6:521-529.
- [Google Scholar]
- Phytochemical Profiling, In Vitro Biological Activities, and Silico Molecular Docking Studies of Dracaena reflexa. Molecules. 2022;27:913.
- [Google Scholar]
- Qualitative and quantitative phytochemicals analysis of selected fabaceae medicinal plants from Allahabad region. Pharma Innov. J.. 2014;3:53-56.
- [Google Scholar]
- In vitro antioxidant and enzyme inhibitory properties of Rubus caesius L. Int. J. Environ. Health Res.. 2019;29:237-245.
- [Google Scholar]
- Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol.. 2020;94:651-715.
- [Google Scholar]
- Analysis of secondary metabolites released by Pseudomonas fluorescens Using GC-MS technique and determination of its anti-fungal activity. Indian J. Public Health. 2018;9
- [Google Scholar]
- Endophyte-assisted phytoremediation: mechanisms and current application strategies for soil mixed pollutants. Crit. Rev. Biotechnol.. 2020;40:31-45.
- [Google Scholar]
- Subcritical water extraction of phenolic compounds from pomegranate (Punica granatum L.) seed residues and investigation into their antioxidant activities with HPLC–ABTS+ assay. Food Bioprod. Process.. 2012;90:215-223.
- [Google Scholar]
- Pharmacological studies pertaining to spasmolytic, bronchodilator and vasodilating effect of Typha domingensis An evidence-based approach. Pakistan J. Pharm. Sci. 2020
- [Google Scholar]
- Evaluation of antioxidant, analgesic, and cytotoxic activities of Typha angustata L. root. Dhaka University J. Pharm. Sci.. 2015;14:55-59.
- [Google Scholar]
- Effect of soil sulfur fertilizer and some foliar fertilizers on growth and yield of broccoli in saline soil. Annales of West University of Timisoara. Series Biol.. 2015;18:123.
- [Google Scholar]
- Undecanoic acid, lauric acid, and N-tridecanoic acid inhibit Escherichia coli persistence and biofilm formation. J. Microbiol. Biotechnol.. 2021;31:130.
- [Google Scholar]
- Antibacterial substances from Albizia myriophylla wood against cariogenic Streptococcus mutans. Arch. Pharmacal. Res.. 2013;36:723-730.
- [Google Scholar]
- Effect of drying methods and extraction solvents on phenolic antioxidants and antioxidant activity of Scurrula ferruginea (Jack) Danser (Loranthaceae) leaf extracts. Sains Malaysiana. 2019;48:1383-1393.
- [Google Scholar]
- Phytochemical analysis and antifungal activity of some medicinal plants against Alternaria specie isolated from an onion. J. Animal Plant Sci.. 2020;30:1-7.
- [Google Scholar]
- Synthesis, ADME, docking studies, and in vivo anti-hyperglycaemic potential estimation of novel Schiff base derivatives from octadec-9-enoic acid. Bioorg. Chem.. 2019;84:478-492.
- [Google Scholar]
- Cytotoxic effect of aqueous-ethanol extract of Typha domingensis pers. (pollen) against human breast cancer cells in vitro. System. Rev. Pharm.. 2020;11:1158-1161.
- [Google Scholar]
- Anti-inflammatory potential of hentriacontane in LPS stimulated RAW 264.7 cells and mice model. Biomed. Pharmacother.. 2017;92:175-186.
- [Google Scholar]
- Flavonoids as acetylcholinesterase inhibitors: Current therapeutic standing and future prospects. Biomed. Pharmacother.. 2018;101:860-870.
- [Google Scholar]
- Comparative antifungal potential of stem extracts of four quinoa varieties against Macrophomina phaseolina. Int. J. Agric. Biol.. 2020;24:441-446.
- [Google Scholar]
- Evaluation of memory enhancement activity of leaf extract of Typha angustata. Int J. Phytopharmacol.. 2014;15:218-220.
- [Google Scholar]
- Lawal, B., O. K. Shittu, T. AbdulRasheed-Adeleke, et al., 2015. GC-MS determination of bioactive constituents of giant African Snail (Archachatina maginata) Haemolymph.
- Ethyl iso-allocholate from a medicinal rice Karungkavuni inhibits dihydropteroate synthase in Escherichia coli: A molecular docking and dynamics study. Indian J. Pharm. Sci.. 2017;78:780-788.
- [Google Scholar]
- Detection and estimation of alpha-Amyrin, beta-Sitosterol, Lupeol, and n-Triacontane in two medicinal plants by high-performance thin-layer chromatography. Adv Chem.. 2014;2014:143948
- [Google Scholar]
- Detection and estimation of alpha-amyrin, beta-sitosterol, lupeol, and n-triacontane in two medicinal plants by high performance thin layer chromatography. Adv. Chem. 2014:2014.
- [Google Scholar]
- Characterization and antibacterial activity of ZnO nanoparticles synthesized by co-precipitation method. Int. J. Appl. Pharmaceutics 2018:224-228.
- [Google Scholar]
- Evaluation of the methods for determination of the free radical scavenging activity by DPPH. Bulgarian J. Agric. Sci.. 2011;17:11-24.
- [Google Scholar]
- Enzymatic alkylsuccinylation of tyrosol: Synthesis, characterization, and property evaluation as a dual-functional antioxidant. Food Chem.. 2018;246:108-114.
- [Google Scholar]
- Tyrosinase inhibitors from natural and synthetic sources as skin-lightening agents. Rev. Agric. Sci.. 2019;7:41-58.
- [Google Scholar]
- Milaeva, E. R., V. Y. Tyurin, Y. A. Gracheva, et al., 2006. Protective effect of meso-tetrakis-(3, 5-di-tert-butyl-4-hydroxyphenyl) porphyrin on the in vivo impact of trimethyltin chloride on the antioxidative defense system. Bioinorganic Chemistry and Applications. 2006,
- Morais, L. V. F. d., J. R. D. d. Luz, T. E. S. d. Nascimento, et al., 2022. Phenolic composition, toxicity potential, and antimicrobial activity of licania rigida benth (chrysobalanaceae) leaf extracts. J. Med. Food. 25, 97-109.
- Hibiscus sabdariffa L. a rich source of secondary metabolites. Int. J. Pharm. Sci. Rev. Res.. 2011;6:83-87.
- [Google Scholar]
- Thiazolidines: Potential anti-viral agents against avian influenza and infectious bronchitis viruses. Urmia, Iran: Veterinary Research Forum, Faculty of Veterinary Medicine, Urmia University; 2020.
- New insights into the binding mode of pyridine-3-carboxamide inhibitors of E. coli DNA gyrase. Bioorg. Med. Chem.. 2019;27:3546-3550.
- [Google Scholar]
- Antibacterial activity and phytochemical investigation of leaves of Calotropis procera plant in Iraq by GC-MS. IJPSR. 2019;10:1988-1994.
- [Google Scholar]
- Chalcones as potent tyrosinase inhibitors: the effect of hydroxyl positions and numbers. PhytoChem. 2004;65:1389-1395.
- [Google Scholar]
- Nyaberi, M., C. Onyango, F. Mathooko, et al., 2017. Profiling active phytochemical compounds of Ziziphus abyssinica herb responsible for antioxidant and antimicrobial activity.
- Antioxidant, total phenols, and proximate constituents of four tropical leafy vegetables. Sci. Afr.. 2020;7:e00227.
- [Google Scholar]
- Piperine-type amides: review of the chemical and biological characteristics. Int. J. Chem.. 2013;5:99.
- [Google Scholar]
- Chemical composition and antimicrobial activity of the essential oil and CO2 extracts of the oriental tobacco. Prilep. Flavour Fragrance J.. 2002;17:323-326.
- [Google Scholar]
- Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxid. Med. Cell. Longevity. 2009;2:897484
- [CrossRef] [Google Scholar]
- Assessment of acetylcholinesterase activity using indoxylacetate and comparison with the standard Ellman’s method. Int. J. Mol. Sci.. 2011;12:2631-2640.
- [Google Scholar]
- A study on the Effects of Fiscal Policy on agricultural production management intention and behavior. In: 2014 International Conference on Management Science & Engineering 21th Annual Conference Proceedings. IEEE; 2014.
- [Google Scholar]
- In vitro cytotoxicity assay, mushroom tyrosinase inhibitory activity and release analysis of kojic monooleate nanodelivery system and in silico molecular docking study against 2Y9X target enzyme. J. Drug Delivery Sci. Technol.. 2021;66:102764.
- [Google Scholar]
- Molecular docking of enzyme inhibitors: A computational tool for structure-based drug design. Biochem. Mol. Biol. Educ.. 2010;38:261-265.
- [Google Scholar]
- Current trends in computer-aided drug design and a highlight of drugs discovered via computational techniques: A review. Eur. J. Med. Chem.. 2021;224:113705.
- [Google Scholar]
- Root extracts from Mexican avocado (Persea americana var. drymifolia) inhibit the mycelial growth of the oomycete Phytophthora cinnamomi. Eur. J. Plant Pathol.. 2009;124:595-601.
- [Google Scholar]
- In vitro antioxidant potential and free radical scavenging activity of various extracts of pollen of Typha domigensis Pers. Pakistan J. Pharm. Sci.. 2014;27
- [Google Scholar]
- Analysis of bioactive compounds in methanol extract of cissus vitiginea leaf using GC-MS. Rasayan J. Chem.. 2015;8:443.
- [Google Scholar]
- The role of antioxidants in human health. In: Oxidative stress: diagnostics, prevention, and therapy. ACS Publications; 2011. p. :1-37.
- [Google Scholar]
- Decanoic acid modification enhances the antibacterial activity of PMAP-23RI-Dec. Eur. J. Pharm. Sci.. 2021;157:105609.
- [Google Scholar]
- Typha elephantina Roxb.: A review on ethanomedicinal, morphological, phytochemical and pharmacological perspectives. Res. J. Pharm. Technol.. 2020;13:5546-5550.
- [Google Scholar]
- Some monohydroxy tetradecanoic acid isomers as novel urease and elastase inhibitors and as new antioxidants. Appl. Biochem. Biotechnol.. 2014;172:1358-1364.
- [Google Scholar]
- Chemical composition, in vitro cytotoxic, and antibacterial activities of Moroccan medicinal plants Euphorbia resinifera and Marrubium vulgare. Biointerface Res. Appl. Chem.. 2020;10:7343-7355.
- [Google Scholar]
- Potential bioactive compounds as SARS-CoV-2 inhibitors from extracts of the marine red alga Halymenia durvillei (Rhodophyta)–A computational study. Arabian J. Chem.. 2021;14:103393.
- [Google Scholar]
- Analysis of bioactive compounds of Tribolium castaneum and evaluation of antibacterial activity. Int. J. Pharm. Clin. Res.. 2016;8:1192-1198.
- [Google Scholar]
- Optimization of extraction parameters of total phenolic compounds from henna (Lawsonia inermis) leaves. Sains Malaysiana. 2010;39:119-128.
- [Google Scholar]
- Evaluation of in vitro anti-thrombolytic activity and cytotoxicity potential of Typha angustifolia L leaves extracts. Int J. Pharm. Pharm. Sci.. 2014;6:81-85.
- [Google Scholar]
- Phytochemical analysis and antibacterial activity of medicinal plant Cardiospermum halicacabum Linn. J. Phytol.. 2010;2:68-77.
- [Google Scholar]
- Studies on tissue plasminogen activator: efficiency of thrombolysis in the presence of iodinated contrast media and development of a novel targeted t-PA delivery system. Hungary: University of Pecs, Medical School; 2017.
- The shifting perception on antioxidants: The case of vitamin E and β-carotene. Redox Biol.. 2015;4:272-278.
- [Google Scholar]
- Effects of surfactant and Zn (II) at various concentrations on microbial activity and ethylbenzene removal in biotricking filter. Chemosphere. 2013;93:2909-2913.
- [Google Scholar]
- Insight into the enhanced activity of Ag/NiOx-MnO2 for catalytic oxidation of o-xylene at low temperatures. Appl. Surf. Sci.. 2019;479:1262-1269.
- [Google Scholar]
- Profile of phenolic compounds and antioxidant activity of finger millet varieties. Food Chem.. 2019;275:361-368.
- [Google Scholar]
- Handbook of Chinese herbs and formulas. Institute Chinese Medicine; 1995.
- Antifungal activity of polyacetylenes isolated from Cirsium japonicum roots against various phytopathogenic fungi. Ind. Crops Prod.. 2011;34:882-887.
- [Google Scholar]
- Configuring two-algorithm-based evolutionary approach for solving dynamic economic dispatch problems. Eng. Appl. Artif. Intell.. 2016;53:105-125.
- [Google Scholar]







